Abstract
Background
Clinicians cannot reliably predict complications of acute hematogenous osteomyelitis (AHO).
Methods
Consecutive cases of AHO from 2 pediatric centers in the United States were analyzed retrospectively to develop clinical tools from data obtained within 96 hours of hospitalization to predict acute and chronic complications of AHO. Two novel composite prediction scores derived from multivariable logistic regression modeling were compared with a previously published severity of illness (SOI) score, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) using area under the receiver operating characteristic curve analyses.
Results
The causative organisms were identified in 73% of 261 cases. Bacteremia (45%), abscesses (38%), and associated suppurative arthritis (23%) were relatively common. Acute or chronic complications occurred in 24% and 11% of patients, respectively. Multivariable logistic regression identified bone abscess (odds ratio [OR], 2.3 [95% confidence interval {CI}, 1.0–5.2]), fever > 48 hours (OR, 2.7 [95% CI, 1.2–6.0]), suppurative arthritis (OR, 3.2 [95% CI, 1.3–7.5]), disseminated disease (OR, 4.6 [95% CI, 1.5–14.3]), and delayed source control (OR, 5.1 [95% CI, 1.4–19.0]) as strong predictors of acute complications. In a separate model, CRP ≥ 100 mg/L at 2–4 days after antibiotics (OR, 2.7 [95% CI, 1.0–7.3]), disseminated disease (OR, 3.3 [95% CI, 1.1–10.0]), and requirement for bone debridement (OR, 6.7 [95% CI, 2.1–21.0]) strongly predicted chronic morbidity. These variables were combined to create weighted composite prediction scores for acute (A-SCORE) and chronic (C-SCORE) osteomyelitis, which were superior to SOI, CRP, and ESR and had negative predictive values > 90%.
Conclusions
Two novel composite clinical scores were superior to existing tools to predict complications of pediatric AHO.
Keywords: hematogenous osteomyelitis, child, predict, complication, score
Two novel composite scores derived from simple clinical and laboratory parameters observed within 96 hours of hospitalization can rule out complications of acute hematogenous osteomyelitis in children and may help guide early transition from parenteral to oral antibiotics.
Acute hematogenous osteomyelitis (AHO) has an estimated incidence of 2–20/100 000 children in well-resourced countries [1, 2]. The rate of pediatric AHO has remained relatively constant over the past 2 decades, although some investigators have reported an increasing incidence, which may reflect improved diagnostic capabilities [1, 2]. Children typically have favorable outcomes provided that the diagnosis is not delayed, antimicrobial therapy is appropriate, surgical debridement is performed when indicated, and patient adherence is ensured [3]. Nevertheless, up to 9% of children with AHO experience serious long-term sequelae such as avascular necrosis, chronic osteomyelitis, appendicular growth arrest, and pathologic fractures [2, 4]. Furthermore, the early course of infection may be complicated by persistent bacteremia, prolonged fever, sepsis, thrombophlebitis, and abscess formation necessitating 1 or more surgical procedures, longer hospital stays, and a prolonged course of parenteral antibiotics [5].
Guidelines for the management of pediatric AHO have been published by European, Canadian, and Australasian pediatric infectious disease societies [6–9] as well as an orthopedic center in the United States (US) [10]. In addition, the Infectious Diseases Society of America has provided recommendations for treatment of methicillin-resistant Staphylococcus aureus (MRSA) osteomyelitis in children [11]. However, in the absence of randomized controlled trials and a consensus guideline for the management of pediatric AHO in the US, therapeutic interventions across institutions have varied [5].
Although the optimal therapeutic approach has not yet been determined [12, 13], multiple retrospective and prospective studies over the past 4 decades have demonstrated favorable outcomes in children with uncomplicated AHO who were treated with early transition from parenteral to oral antibiotics and short duration of hospitalization [5, 14–23]. Currently, the decision to switch to oral antibiotics is predicated on resolution of fever, improvement in physical signs, and decline in C-reactive protein (CRP) [22, 24]. However, there are certain circumstances when a prolonged course of parenteral antibiotics may be justified, such as complex focal or metastatic pyogenic infection, endovascular infection, persistent bacteremia, or antimicrobial resistance to or intolerance of oral agents.
In an attempt to identify predictors of acute complications of AHO, a number of studies have evaluated the utility of certain individual variables [25–28], but only a single center developed a composite scoring system, based on length of stay as a surrogate for acute complications [29]. Another group of investigators identified individual risk factors for chronic complications caused specifically by S. aureus [4]. Therefore, there is a compelling need to develop novel tools for clinicians to predict acute and chronic complications of AHO in children.
The objective of this study was to develop scoring systems that could accurately identify children with AHO within 96 hours of admission who were at high risk of developing acute or chronic complications. We reasoned that early identification of such patients could potentially improve outcomes by indicating the need for high-resolution diagnostic imaging, an extended course of parenteral antibiotics, surgical interventions, and anticipatory counseling.
METHODS
Research Setting
The study was conducted at 2 tertiary pediatric care centers, Hasbro Children’s Hospital (HCH) in Providence, Rhode Island (87 beds), and Nationwide Children’s Hospital (NCH) in Columbus, Ohio (527 beds) (see Supplementary Methods).
Study Population
This study was a retrospective analysis of consecutive eligible subjects who were identified by searching billing databases at HCH and NCH for codes indicative of skeletal infections (see Supplementary Methods). Databases were queried for the periods from 1 January 2006 to 31 December 2016 at HCH and 1 January 2013 to 30 September 2016 at NCH to provide an approximate balance in number of patients from each hospital. We selected cases of documented acute osteomyelitis presumably caused by hematogenous spread of pyogenic bacteria. Patients were included if the final clinical diagnosis of AHO was supported by microbiological, histopathological, and/or radiographic evidence of infection; there was no preceding traumatic inoculation or contiguous spread of infection from extraskeletal sites; and the duration of symptoms at presentation was ≤ 14 days. See Supplementary Methods for exclusion criteria.
Data Management
Investigators at each center reviewed medical records systematically and collected demographic, clinical, laboratory, and radiographic data. See Supplementary Methods for data management.
Definition of Terms
Delayed source control referred to surgical intervention after hospital day 3. Antibiotic nonadherence was inferred if patients or their guardians reported missing doses, or if they failed to refill a prescription. Treatment failure was defined as persistence of symptoms and/or signs leading to readmission or change of antibiotics within 6 weeks of initiation of treatment despite apparent antibiotic adherence. Patients were considered lost to follow-up if they missed 1 or more scheduled clinic appointments during or after completing the antibiotic course. Additional definitions of terms are described in the Supplementary Methods.
Derivation of Composite Scores to Predict Adverse Outcomes
Our objective was to derive prediction scores that could be calculated at or near the time of admission to accurately identify children with AHO who were at greater risk of an acute complicated course or chronic morbidity. We used a previously described composite score, the severity of illness (SOI) score [29, 30], as well as erythrocyte sedimentation rate (ESR) and CRP, as reference measures to predict adverse outcomes of AHO (see Supplementary Methods). In the absence of a standardized definition for acute complicated course, we defined this as treatment failure within 6 weeks of initiation of antibiotic therapy, ≥ 2 bone debridements, or hospitalization > 14 days. The indication for surgical intervention was determined by the attending orthopedic surgeon in consultation with infectious disease and/or hospital medicine physicians on the basis of radiographic and clinical features.
Chronic morbidity [4] was defined as growth arrest or limb length discrepancy, pathologic fracture, avascular necrosis, frozen joint, chronic dislocation, or chronic osteomyelitis defined as persistence or recurrence of attributable symptoms and signs associated with a sequestrum, involucrum, or osteosclerosis on a plain radiograph, requiring antibiotics for at least 12 weeks.
We described peripherally inserted central catheter (PICC) complications as being either minor (occlusion of the line requiring treatment with tissue plasminogen activator, dislodgement, dermatitis, or localized superficial infection) or major (central line–associated bloodstream infection, central line–associated abscess, or thrombus).
Statistical Analyses
See the Supplementary Methods for detailed description of statistical methods. Because PICC use was not randomized in this study, we carefully balanced groups of patients with or without PICC use using propensity score matching to ensure similar baseline clinical characteristics and markers of disease severity to determine whether PICCs were independently associated with adverse effects (Supplementary Methods) [31].
Covariates with a P value < .2 from bivariate analyses were entered in backward stepwise binary logistic regression models to determine which variables independently predicted complicated outcomes (Supplementary Methods). We operationalized and simplified the resulting statistical models by deriving novel composite prediction scores for acute and chronic complications. We defined the Acute Score for Complications of Osteomyelitis Risk Evaluation (A-SCORE) and the Chronic Score for Complications of Osteomyelitis Risk Evaluation (C-SCORE) as combinations of numerical values that we assigned to significant predictor variables derived from the regression models for acute complications and chronic morbidity, respectively. The assigned value for each predictor was approximately proportionate to the odds ratio (OR) from the logistic regression model. Predicted probabilities for all patients were derived from each logistic regression model and used to construct receiver operating characteristic (ROC) curves showing the true positive rate (sensitivity) vs the false positive rate (1-specificity). We compared the diagnostic accuracy of the results from (1) the regression models, (2) the derived A-SCORE and C-SCORE, (3) admission ESR, (4) admission CRP, and (5) SOI score [29, 30] by analyzing the area under the ROC curve (AUC).
A 2-tailed P < .05 was considered statistically significant.
Ethics Statement
The institutional review board at each hospital provided ethics approval for this study and exemption from informed consent.
RESULTS
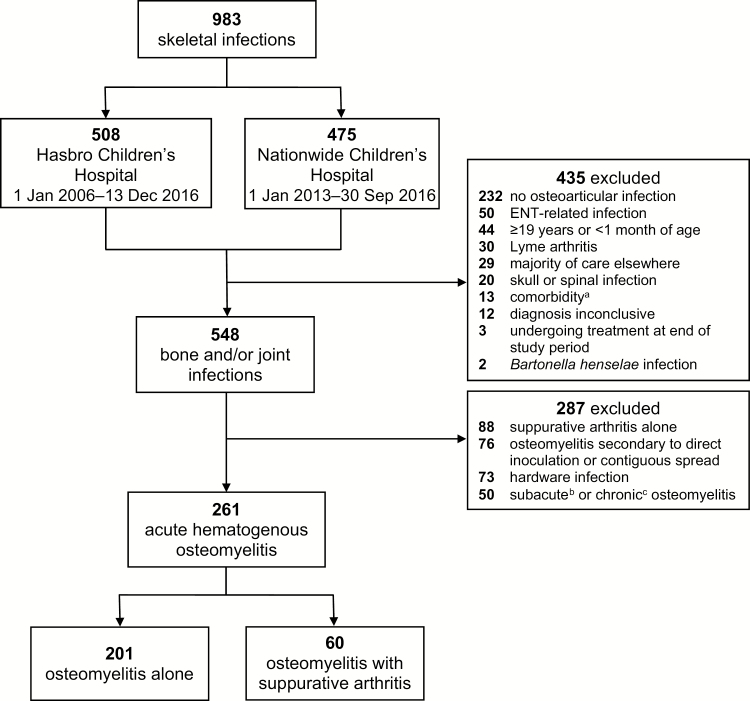
Nine hundred eighty-three unique cases of skeletal infections were identified at the 2 study sites during the defined periods (Figure 1). Of these, 261 cases (133 at HCH, 128 at NCH) met the inclusion criteria for AHO. Overall, 201 (77%) patients had only osteomyelitis whereas 60 (23%) patients had osteomyelitis associated with suppurative arthritis.
Figure 1.
Flow diagram of patients identified for inclusion in the study. aSickle cell disease, immunosuppression, or chronic vasculitis. bDuration of symptoms at presentation > 2 weeks and ≤ 12 weeks. cDuration of symptoms at presentation > 12 weeks. Abbreviation: ENT, ear, nose, and throat.
Demographic and Clinical Characteristics
The median age of patients was 9.0 years and the male-to-female ratio was 1.6:1. Patients from HCH and NCH had similar demographic characteristics except that self-reported Hispanic ethnicity was more frequent at HCH than NCH (P < .001) (Table 1). All children had preceding or current fever and bone pain at the time of admission. The median ESR on admission was slightly higher at HCH compared with NCH (48 mm/hour vs 43 mm/hour, P = .002), but white blood cell count and CRP values on admission were similar. Magnetic resonance imaging (MRI) was performed in 250 (96%) patients. Osteomyelitis was confirmed by plain radiographs (n = 9) or bone scans (n = 2) in the other children.
Table 1.
Demographic and Clinical Characteristics of Patients Enrolled in the Study
| Total | HCH | NCH | |||
|---|---|---|---|---|---|
| Characteristic | (N = 261) | (n = 133) | (n = 128) | Missing | P Value |
| Demographics | |||||
| Age, median (IQR), y | 9.0 (4.2–12.0) | 10.2 (5.8–12.4) | 8.6 (3.7–11.8) | … | .10 |
| Female sex | 102 (39) | 52 (39) | 50 (39) | … | 1.00 |
| Hispanic ethnicity | 29 (11) | 26 (20) | 3 (2) | … | < .001 |
| Race | 1 | .50 | |||
| White | 185 (71) | 94 (71) | 91 (71) | … | |
| African American | 40 (15) | 17 (13) | 23 (18) | … | |
| Asian | 3 (1) | 2 (2) | 1 (1) | … | |
| Other | 32 (12) | 19 (14) | 13 (10) | … | |
| Clinical findings | |||||
| Preceding duration of symptoms, median (IQR), d | 4 (2–7) | 4 (3–7) | 4 (2–7) | … | .22 |
| Location of infectiona | |||||
| Upper extremity long bones | 26 (10) | 14 (11) | 12 (9) | … | .76 |
| Lower extremity long bones | 126 (48) | 63 (47) | 63 (49) | … | .77 |
| Pelvis or sacrum | 49 (19) | 29 (22) | 20 (16) | … | .20 |
| Bones of the hands | 13 (5) | 8 (6) | 5 (4) | … | .43 |
| Bones of the feet | 36 (14) | 17 (13) | 19 (15) | … | .63 |
| Otherb | 8 (3) | 7 (5) | 1 (1) | … | .07 |
| Multifocal infectionc | 12 (5) | 9 (7) | 3 (2) | … | .14 |
| Disseminated infection | 30 (12) | 15 (11) | 15 (12) | … | .91 |
| Admitted to hospital | 257 (99) | 130 (98) | 127 (99) | … | .62 |
| Admitted to ICU | 16 (6) | 10 (8) | 6 (5) | … | .34 |
| Tmaxd, median (IQR), °C | 38.8 (37.739.8) | 38.9 (37.839.9) | 38.7 (37.739.8) | 1 | .07 |
| Duration of fevers in the hospital, median (IQR), d | 2.6 (1.1–4.6) | 2.7 (1.4–4.9) | 2.4 (0.9–4.5) | 1 | .35 |
| Laboratory and radiographic results | |||||
| WBC on admission, median (IQR), ×1000 cells/mL | 10.9 (8.0–15.3) | 10.7 (8.2–15.3) | 10.9 (7.9–15.4) | 19 | .94 |
| ESR on admission, median (IQR), mm/h | 46 (27–58) | 48 (31–68) | 43 (25–52) | 18 | .002 |
| CRP on admission, median (IQR), mg/L | 62 (26–146) | 69 (28–147) | 50 (26–146) | 15 | .87 |
| Suppurative arthritise | 60 (23) | 31 (23) | 29 (23) | … | .90 |
| Plain radiograph | 251 (96) | 128 (96) | 123 (96) | … | 1.00 |
| Magnetic resonance imaging | 250 (96) | 130 (98) | 120 (94) | … | .13 |
| Bone scan | 11 (4) | 8 (6) | 3 (2) | … | .22 |
| Bone abscess | 99 (38) | 46 (35) | 53 (41) | … | .26 |
| Bacteremia | 118 (45) | 60 (45) | 58 (45) | … | .97 |
| Causative organism identifiedf | 191 (73) | 94 (71) | 97 (76) | … | .35 |
| Staphylococcus aureus isolated | 152/191 (80) | 78/94 (83) | 74/97 (76) | … | .89 |
| MRSA isolated | 45/152 (30) | 18/78 (23) | 27/74 (36) | … | .11 |
| Severity of illness scoreg, median (IQR) | 1 (0–5) | 1 (0–5) | 1 (0–5) | 30 | .96 |
| Interventions | |||||
| Any surgical intervention | 165 (63) | 77 (58) | 88 (69) | … | .07 |
| ≥1 bone debridement | 102 (39) | 47 (35) | 55 (43) | … | .21 |
| ≥ 2 bone debridements | 37/102 (36) | 15/47 (32) | 22/55 (40) | … | .40 |
| PICC placed | 97 (37) | 81 (61) | 16 (13) | … | < .001 |
| Duration of antibiotic therapy, median (IQR), d | 33 (29–42) | 35 (30–46) | 32 (28–38) | 14 | .004 |
| Outcomes | |||||
| LOS, median (IQR), d | 4.9 (3.6–7.1) | 5.1 (3.8–7.8) | 4.8 (3.1–6.7) | 4 | .04 |
| Treatment failureh | 27 (10) | 13 (10) | 14 (11) | 1 | .76 |
| Readmission due to adverse events | 17 (7) | 12 (9) | 5 (4) | 1 | .13 |
| Acute complicated courseh | 62 (24) | 29 (23) | 33 (26) | 5 | .45 |
| Chronic morbidityh | 27 (11) | 16 (13) | 11 (9) | 8 | .36 |
Data are presented as no. (%) unless otherwise indicated. Bold values indicate significant results.
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HCH, Hasbro Children’s Hospital; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; MRSA, methicillin-resistant Staphylococcus aureus; NCH, Nationwide Children’s Hospital; PICC, peripherally inserted central catheter; WBC, white blood cell.
aTotal > 100% secondary to multifocal infections.
bClavicle, scapula, sternum, or patella.
cInvolvement of ≥2 noncontiguous bones.
dMaximum temperature recorded within the first 48 hours of presentation.
eSynovial fluid with positive culture, positive Gram stain for bacteria, nucleated cell count ≥ 10 000 cells/mL, or moderate-many neutrophils if nucleated cell count not done (4 cases).
fIn addition to S. aureus, organisms included Streptococcus pyogenes (n = 18 [9%]), Streptococcus pneumoniae (n = 6 [3%]), Kingella kingae (n = 4 [2%]), Salmonella spp (n = 3 [2%]), Streptococcus agalactiae (n = 3 [2%]), and 1 each of Clostridium perfringens, Escherichia coli, group C Streptococcus, Serratia marcescens, and Streptococcus anginosus.
gAs described by Athey et al [30].
hDefined in Methods section.
Overall, more patients at HCH were managed with PICCs compared with patients at NCH (61% vs 13%; P < .001). However, the time periods for comparison differed substantially between sites (HCH, 11 years; NCH, 3.75 years) and the average rate of PICC use at HCH declined significantly during the study period (87% during 2006–2011 vs 39% during 2012–2016; P < .001). Children treated at HCH had slightly greater median length of stay (5.1 days vs 4.8 days; P = .04) and median duration of treatment (35 days vs 32 days; P = .004) (Table 1).
The causative organism was identified in 191 (73%) patients. Of these, 152 (80%) infections were caused by S. aureus, of which 45 (30%) were MRSA. The other causative organisms are listed in Table 1. Infections of lower extremities (62%) were far more frequent than other sites (Table 1).
All patients received parenteral antimicrobials at the time of hospitalization. The most common intravenous antibiotics used alone or in combination for initial therapy were clindamycin (50%), vancomycin (32%), cefazolin (28%), and nafcillin (10%) (Supplementary Table 1A). The most common antibiotics used alone or in combination for definitive therapy included clindamycin (36%), cephalexin (30%), and cefazolin (13%) (Supplementary Table 1B). Sixty-six percent (n = 172) of patients were discharged on oral antibiotics, 37% (n = 97) had PICC lines inserted, and 34% (n = 89) were discharged with a PICC for parenteral antibiotics. Of the 89 patients discharged with a PICC, 42 patients transitioned to oral antibiotics as an outpatient and the remaining patients received parenteral therapy for the entire course. The decision to switch from parenteral to oral antibiotics was individualized and predicated on improvement in clinical symptoms, signs of inflammation, and inflammatory indices.
Surrogates of Severity
There was a relatively high frequency of associated bacteremia (n = 118 [45%]), bacteremia that lasted > 2 days (n = 19 [7%]), and subperiosteal or intraosseus abscesses (n = 99 [38%]) diagnosed by MRI (n = 98) or surgical exploration (n = 1); all abscesses were surgically drained (Table 1). A similar proportion of patients who had undergone a single drainage procedure underwent 1 or more additional debridements at each hospital (HCH, 32% vs NCH 40%; P = .4) (Table 1), which are within the range of reported rates for multiple debridements (14%–48%) [26, 32, 33]. Multifocal osteomyelitis and disseminated disease occurred in 5% (n = 12) and 12% (n = 30), respectively. The overall rate of acute complications of AHO occurred in 62 (24%) patients, 27 of whom were considered to have failed therapy. The proportion of patients who failed therapy and required subsequent surgical drainage was substantial at both hospitals (6/13 [46%] at HCH vs 10/14 [71%] at NCH; P = .25). Chronic morbidity (n = 27 [11%]) was attributable to ≥ 1 of the following: chronic osteomyelitis (n = 18), growth arrest (n = 7), pathologic fracture (n = 4), or frozen joint (n = 1). The median duration of antibiotics in patients who had acute complications (45 days) was significantly longer than in those without complications (31 days; P < .001), and duration of antibiotics in patients with chronic complications (67 days) was significantly longer than in those without chronic complications (32 days; P < .001).
Characteristics of Patients With or Without PICCs
In unadjusted analyses, the group of children with PICCs had evidence of significantly more severe disease in multiple domains (see “Markers of Severity” in Table 2) at or near the time of admission, as well as more acute complications, and longer hospitalization and antibiotic duration compared to those without PICCs. However, we speculated that the absence of standardized guidelines for PICC use may have led to confounding by indication, because physicians may have preferentially inserted PICCs in patients with more severe disease. Therefore, to determine if PICCs contributed to acute complications independent of disease severity, we selected the largest possible subsets of patients with or without PICCs matched for surrogates of disease severity (n = 136) using propensity score matching (Table 2). The adjusted analyses confirmed well-balanced matching between groups, except for ESR on admission, which was slightly higher in the PICC group. The significant unadjusted association between PICC use and an acute complicated course disappeared after propensity score matching. However, PICC use was significantly associated with subsequent adverse events necessitating a change of antibiotics, emergency department visit, or readmission. Notably, children managed without PICCs were more likely to be lost to follow-up (Table 2).
Table 2.
Comparison of Demographics, Clinical Characteristics, and Outcomes of Patients Stratified by Peripherally Inserted Central Catheter Use
| Full Cohort | Propensity Score–Matched Cohorta | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | No PICC (n = 164) | PICC (n = 97) | P Value | Missing | No PICC (n = 65) | PICC (n = 71) | P Value | Missing |
| Demographics | ||||||||
| Age, median (IQR), y | 8.9 (4.0–11.8) | 10.2 (5.4–12.5) | .25 | … | 9.7 (6.0–11.6) | 9.2 (4.8–12.3) | .93 | … |
| Study site, HCH | 52 (32) | 81 (84) | < .001 | … | 19 (29) | 61 (86) | < .001 | … |
| Sex, female | 62 (38) | 40 (41) | .58 | … | 23 (35) | 29 (41) | .51 | … |
| Location of infectionb | ||||||||
| Upper extremity long bone | 15 (9) | 11 (11) | .57 | … | 8 (12) | 7 (10) | .65 | … |
| Lower extremity long bone | 78 (48) | 48 (50) | .76 | … | 35 (54) | 32 (45) | .31 | … |
| Pelvis or sacrum | 22 (13) | 27 (28) | .004 | … | 13 (20) | 21 (30) | .20 | … |
| Hands | 9 (6) | 4 (4) | .77 | … | 2 (3) | 4 (6) | .68 | … |
| Feet | 28 (17) | 8 (8) | .05 | … | 8 (12) | 7 (10) | .65 | … |
| Otherc | 4 (2) | 4 (4) | .48 | … | 3 (5) | 2 (3) | .58 | … |
| Multifocal infectiond | 3 (2) | 9 (9) | .01 | … | 3 (5) | 3 (4) | 1 | … |
| Markers of severity | ||||||||
| Admitted to ICU | 6 (4) | 10 (10) | .03 | … | 3 (5) | 6 (9) | .50 | … |
| Tmaxe, median (IQR), °C | 38.6 (37.6–39.6) | 39.3 (38.4–40.0) | < .001 | 1 | 39.3 (38.6–39.9) | 39.0 (38.1–39.9) | .39 | 1 |
| Fever after > 48 h of antibiotic therapy | 42 (26) | 48 (50) | < .001 | … | 30 (46) | 30 (42) | .65 | … |
| Admission WBC, median (IQR), ×1000 cells/mL | 10.4 (7.8–13.9) | 12.7 (8.3–16.0) | .02 | 19 | 11.4 (7.4–16.0) | 11.5 (8.3–15.7) | .68 | 10 |
| Admission ESR, median (IQR), mm/h | 40 (22–52) | 55 (43–70) | < .001 | 18 | 46 (27–52) | 53 (38–69) | .003 | 7 |
| Admission CRP, median (IQR), mg/L | 46 (24–129) | 88 (34–178) | .001 | 15 | 72 (36–201) | 75 (28–151) | .41 | 4 |
| Suppurative arthritisf | 28 (17) | 32 (33) | .003 | … | 14 (22) | 21 (30) | .28 | … |
| Disseminated infectiong | 11 (7) | 19 (20) | .002 | … | 6 (9) | 7 (10) | .90 | … |
| Bone abscess (any) | 59 (36) | 40 (41) | .43 | … | 26 (40) | 26 (37) | .69 | … |
| CRP ≥ 100 mg/L after 2–4 d of anti- biotics | 19 (13) | 32 (39) | < .001 | 32 | 17 (26) | 20 (29) | .71 | 2 |
| Severity of illness scoreh | 1 (0–3) | 2 (0–7) | < .001 | 30 | 2 (0–5) | 1 (0–6) | .43 | 9 |
| Bacteremia | 62 (38) | 56 (58) | .002 | … | 45 (69) | 40 (56) | .12 | … |
| MRSA recovered | 24 (15) | 21 (22) | .15 | … | 15 (23) | 13 (18) | .49 | … |
| Treatment course | ||||||||
| Surgical intervention (any) | 98 (60) | 67 (69) | .13 | … | 38 (59) | 46 (65) | .45 | … |
| Delayed source control | 9 (6) | 15 (16) | .01 | … | 2 (3) | 8 (11) | .10 | … |
| Bone debridement | 59 (36) | 43 (44) | .18 | … | 26 (40) | 27 (38) | .81 | … |
| Multiple debridements | 22 (13) | 15 (16) | .65 | … | 12 (19) | 4 (6) | .03 | … |
| Length of stay, median (IQR), d | 4.3 (3.1–5.8) | 6.5 (4.5–11.1) | < .001 | 4 | 4.9 (3.9–7.0) | 5.9 (4.1–8.9) | .059 | 3 |
| Time to normal ESR, median (IQR), d | 21 (11–31) | 30 (18–43) | < .001 | 76 | 26 (18–33) | 27 (16–39) | .75 | 41 |
| Time to normal CRP, median (IQR), d | 18 (10–25) | 18 (11–28) | .37 | 29 | 21 (14–28) | 17 (9–29) | .045 | 15 |
| Duration of IV antibiotics, median, d (IQR) | 4 (2–5) | 35 (21–46) | < .001 | 9 | 4 (3–7) | 34 (17–45) | < .001 | 4 |
| Duration of antibiotics, median, d (IQR) | 28 (24–30) | 20 (14–28) | < .001 | 62 | 28 (24–31) | 19 (14–28) | .001 | 43 |
| Total duration of antibiotics, median, d (IQR) | 31 (28–35) | 42 (33–58) | < .001 | 14 | 31 (28–36) | 41 (31–51) | < .001 | 9 |
| Therapy-related AEs | ||||||||
| Gastrointestinal | 20 (13) | 16 (17) | .33 | 9 | 6 (10) | 12 (18) | .18 | 7 |
| Rash or allergy | 5 (3) | 11 (12) | .006 | 9 | 1 (2) | 7 (10) | .06 | 7 |
| Minor PICC-relatedi | … | 40 (43) | 9 | … | 30 (45) | 7 | ||
| Major PICC-relatedj | … | 2 (2) | 9 | … | 1 (2) | 7 | ||
| PICC removed due to AE | … | 19 (20) | 9 | … | 14 (21) | 7 | ||
| Severe neutropeniak | 1 (1) | 4 (4) | .06 | 9 | 0 (0) | 3 (5) | .25 | 7 |
| Nephrotoxicityl | 1 (1) | 3 (3) | .14 | 9 | 0 (0) | 3 (5) | .25 | 7 |
| Liver toxicitym | 0 (0) | 1 (1) | .37 | 9 | 0 (0) | 1 (1) | 1 | 7 |
| Antibiotic changed due to AE | 6 (4) | 17 (18) | < .001 | 9 | 1 (2) | 11 (16) | .01 | 7 |
| ED visit due to AE | 2 (1) | 27 (28) | < .001 | 1 | 1 (2) | 21 (30) | < .001 | 1 |
| ED visit specifically due to PICC AE | … | 16 (17) | 1 | … | 13 (19) | 1 | ||
| ED visit due to recurrence of infection symptoms | 12 (7) | 7 (7) | > .99 | 1 | 3 (5) | 2 (3) | .67 | 1 |
| Readmitted due to AE | 3 (2) | 14 (15) | < .001 | 1 | 1 (2) | 9 (13) | .02 | 1 |
| Readmitted specifically due to PICC AE | … | 5 (5) | 1 | … | 3 (4) | 1 | ||
| Readmitted due to recurrence of in- fection symptoms | 13 (8) | 13 (14) | .15 | 1 | 3 (5) | 7 (10) | .33 | 1 |
| Adherence to therapy | ||||||||
| Medication adherence issuesn | 10 (6) | 4 (4) | .58 | 3 | 3 (5) | 2 (3) | .67 | 3 |
| Lost to follow-upo | 26 (16) | 5 (5) | .01 | 4 | 9 (14) | 4 (6) | .14 | 4 |
| Outcomes | ||||||||
| Acute complicated coursep | 28 (17) | 34 (36) | .001 | 5 | 12 (19) | 16 (24) | .50 | 4 |
| Chronic morbidityp | 12 (8) | 15 (16) | .03 | 8 | 1 (2) | 8 (12) | .04 | 8 |
Data are presented as no. (%) unless otherwise indicated. Bold values indicate significant results.
Abbreviations: AE, adverse event (therapy-related only); CRP, C-reactive protein; ED, emergency department; ESR, erythrocyte sedimentation rate; HCH, Hasbro Children’s Hospital; ICU, intensive care unit; IQR, interquartile range; IV, intravenous; MRSA, methicillin-resistant Staphylococcus aureus; PICC, peripherally inserted central catheter; Tmax, maximum temperature; WBC, white blood cell.
aPropensity scores were estimated using binary logistic regression with the following covariates: fever > 48 hours after antibiotics, suppurative arthritis, disseminated infection, bone abscess, CRP ≥ 100 mg/L after 2–4 days of antibiotics, delayed source control, bone debridement.
bTotal > 100% secondary to multifocal infections.
cClavicle, scapula, sternum, or patella.
dTwo or more noncontiguous bones.
eIn the first 48 hours from presentation.
fSynovial fluid with positive culture, positive Gram stain for bacteria, nucleated cell count ≥ 10 000 cells/mL, or moderate-many neutrophils if nucleated cell count not done (4 cases).
gMultifocal infection, pneumonia, septic pulmonary embolism, deep vein thrombosis, or endocarditis.
hAs described by Athey et al [30].
iTreatment with tissue plasminogen activator dislodgement, dermatitis, local infection.
jCentral line–associated bloodstream infection or thrombus demonstrated by imaging.
kAbsolute neutrophil count < 500 cells/mL.
lIncrease in the serum creatinine concentration > 150% of the baseline value.
mAlanine aminotransferase > 150 IU/L.
nReceived ≤80% of the prescribed antibiotic doses.
oPatient did not return to clinic despite continued documented need.
pDefined in Methods section.
Prediction of Adverse Outcomes
We investigated clinical variables and laboratory results at or within 96 hours of admission to identify the optimal combination of factors that could reliably predict short- and long-term adverse outcomes. Bivariate statistical analyses revealed several significant differences between groups of patients with or without acute or chronic complications (Table 3). These covariates as well as PICC use, a potential confounder, were entered into multivariable binary logistic regression models. Five covariates independently predicted an acute complicated course (n = 225; Hosmer and Lemeshow P = .99): bone abscess (OR, 2.3 [95% CI, 1.0–5.2]), fever > 48 hours (OR, 2.7 [95% CI, 1.2–6.0]), associated suppurative arthritis (OR, 3.2 [95% CI, 1.3–7.5]), disseminated disease (OR, 4.6 [95% CI, 1.5–14.3]), and delayed source control (OR, 5.1 [95% CI, 1.4–19.0]). Bone debridement was not included as a potential predictor because it was incorporated in the definition.
Table 3.
Bivariate Analyses of Potential Predictors of Adverse Outcomes
| Acute Complicated Coursea | Chronic Morbiditya | |||||||
|---|---|---|---|---|---|---|---|---|
| Potential Predictor | Outcome Absent (n = 194) | Outcome Present (n = 62) | Missing | P Value | Outcome Absent (n = 226) | Outcome Present (n = 27) | Missing | P Value |
| Hospital site: HCH | 100 (52) | 29 (47) | 5 | .51 | 110 (49) | 16 (59) | 8 | .30 |
| Age, median (IQR), y | 9.3 (4.0–12.0) | 8.5 (5.4–12.3) | 5 | .98 | 9.0 (4.2–11.9) | 10.1 (5.6–12.3) | 8 | .52 |
| Sex, female | 67 (35) | 32 (53) | 5 | .02 | 89 (39) | 9 (33) | 8 | .54 |
| Location of infectionb | 5 | |||||||
| Upper extremity long bone | 17 (9) | 10 (16) | .10 | 22 (10) | 5 (19) | 8 | .18 | |
| Lower extremity long bone | 91 (47) | 39 (62) | .03 | 117 (52) | 13 (48) | 8 | .72 | |
| Hands | 8 (4) | 4 (7) | .49 | 10 (4) | 2 (7) | 8 | .37 | |
| Feet | 31 (16) | 6 (10) | .22 | 32 (14) | 2 (7) | 8 | .55 | |
| Pelvis or sacrum | 48 (25) | 8 (13) | .05 | 49 (22) | 7 (26) | 8 | .616 | |
| Otherc | 6 (3) | 2 (3) | 1 | 6 (3) | 2 (7) | 8 | .21 | |
| Multifocal infectiond | 5 (3) | 7 (11) | 5 | .01 | 7 (3) | 4 (15) | 8 | .02 |
| Disseminated infectione | 10 (5) | 20 (32) | 5 | < .001 | 19 (8) | 9 (33) | 8 | < .001 |
| Duration of preceding symptoms, median (IQR), d | 4 (2–7) | 4 (2–7) | 5 | .45 | 4 (2–7) | 4 (2–7) | 8 | .85 |
| Tmaxf, median (IQR), °C | 38.7 (37.639.6) | 39.4 (38.440.0) | 5 | < .001 | 38.8 (37.739.8) | 39.4 (38.440.0) | 11 | .06 |
| Fever after > 48 h of antibiotic therapy | 50 (26) | 40 (65) | 5 | < .001 | 72 (32) | 15 (56) | 8 | .01 |
| WBC on admission, median (IQR), ×1000 cells/mL | 10.4 (7.8–14.9) | 13.4 (10.1–16.5) | 18 | .002 | 10.7 (7.8–15.2) | 12.3 (10.3–15.7) | 28 | .07 |
| ESR on admission, median (IQR), mm/h | 45 (24–56) | 52 (40–65) | 17 | .002 | 46 (27–58) | 45 (35–66) | 28 | .26 |
| CRP on admission, median (IQR), mg/L | 47 (21–130) | 129 (61–220) | 14 | < .001 | 57 (25–136) | 138 (65–221) | 25 | .002 |
| CRP ≥ 50 mg/L after 2–4 d of antibiotics | 52 (31) | 36 (68) | 41 | < .001 | 70 (37) | 16 (67) | 47 | .004 |
| CRP ≥ 100 mg/L after 2–4 d of antibiotics | 25 (15) | 26 (49) | 36 | < .001 | 36 (18) | 13 (54) | 32 | < .001 |
| CRP ≥ 150 mg/L after 2–4 d of antibiotics | 15 (9) | 21 (40) | 32 | < .001 | 26 (13) | 9 (38) | 38 | .002 |
| Admitted to ICU | 8 (4) | 8 (13) | 5 | .01 | 13 (6) | 2 (7) | 8 | .67 |
| Suppurative arthritisg | 34 (18) | 25 (40) | 5 | < .001 | 47 (21) | 10 (37) | 8 | .06 |
| Bacteremia | 84 (43) | 33 (53) | 5 | .17 | 101 (45) | 15 (56) | 8 | .28 |
| Bone abscess | 59 (30) | 38 (61) | 5 | < .001 | 80 (35) | 18 (67) | 8 | .002 |
| Causative organism recovery | 130 (67) | 59 (95) | 5 | < .001 | 163 (72) | 24 (89) | 8 | .07 |
| Staphylococcus aureus recovered | 107 (55) | 42 (68) | 5 | .08 | 129 (57) | 19 (70) | 8 | .19 |
| MRSA recovered as causative organism | 21 (11) | 22 (36) | 5 | < .001 | 37 (16) | 7 (26) | 8 | .22 |
| Any surgical intervention | 103 (53) | 58 (94) | 5 | < .001 | 135 (60) | 24 (89) | 8 | .003 |
| Bone debridement | 52 (27) | 50 (81) | 5 | < .001 | 79 (35) | 20 (74) | 8 | < .001 |
| Delayed source control (excluding IR) | 7 (4) | 15 (24) | 5 | < .001 | 18 (8) | 6 (22) | 8 | .017 |
| Delayed source control (including IR) | 14 (7) | 16 (26) | 5 | < .001 | 25 (11) | 6 (22) | 8 | .095 |
| Multiple bone debridements | … | 35 (57) | 5 | 24 (11) | 11 (41) | 8 | < .001 | |
| Severity of illness scoreh, median (IQR) | 1 (0–3) | 6 (1–7) | 28 | < .001 | 1 (0–4) | 6 (1–7) | 40 | .001 |
Data are presented as no. (%) unless otherwise indicated. Bold values indicate significant results.
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HCH, Hasbro Children’s Hospital; ICU, intensive care unit; IQR, interquartile range; IR, drainage performed by Interventional Radiologist; MRSA, methicillin-resistant Staphylococcus aureus; Tmax, maximum temperature; WBC, white blood cell.
aDefined in Methods section.
bTotal > 100% secondary to multifocal infections.
cClavicle, scapula, sternum, or patella.
dDefined as the involvement of ≥2 noncontiguous bones.
eIndicated by the presence of multifocal infection, pneumonia, septic pulmonary embolism, deep vein thrombosis, or endocarditis.
fIn the first 48 hours from presentation.
gSynovial fluid with positive culture, positive Gram stain for bacteria, nucleated cell count ≥ 10 000 cells/mL, or moderate-many neutrophils if nucleated cell count not done (4 cases).
hAs described by Athey et al [30].
In a separate model (n = 220, Hosmer and Lemeshow P = .71), 3 variables independently predicted chronic morbidity: CRP ≥ 100 mg/L at 2–4 days after admission (OR, 2.7 [95% CI, 1.0–7.3]), disseminated disease (OR, 3.3 [95% CI, 1.1–10.0]), and requirement for bone debridement (OR, 6.7 [95% CI, 2.1–21.0]). Bone abscess was also a potential predictor but its effect size was smaller than that of bone debridement.
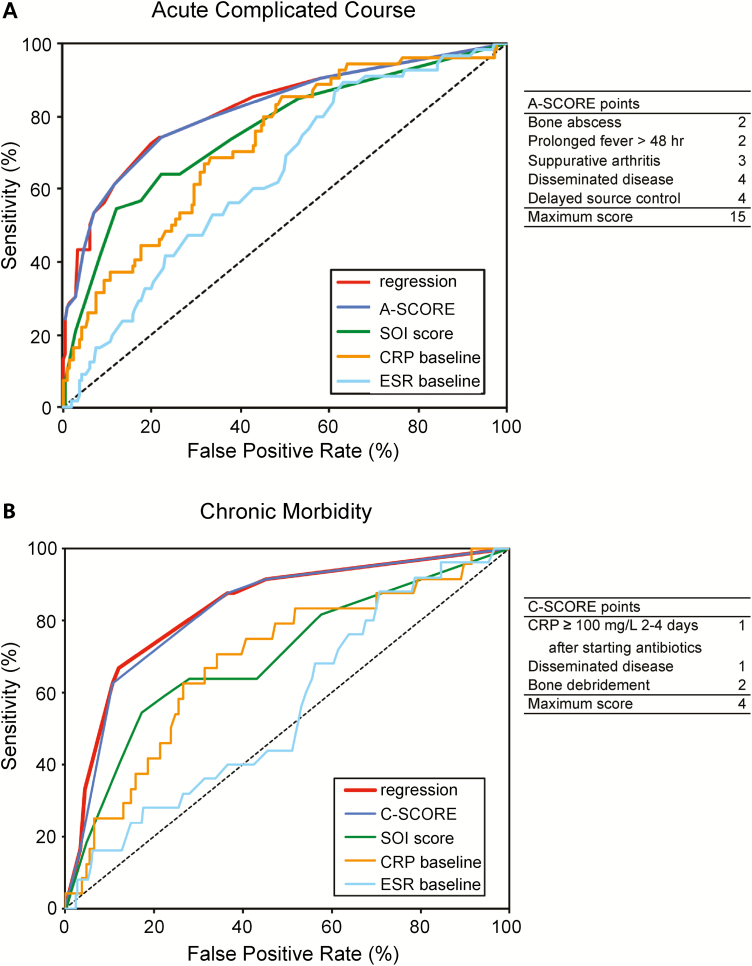
We then derived 2 composite scores, the A-SCORE and C-SCORE, from the results of the multivariable regression models. We hypothesized that these scores would accurately predict the risk of acute complications of AHO or chronic morbidities, respectively. The calculation of the scores is shown in Figure 2. ROC curves were generated to compare results of the logistic regression models, A- and C-SCORE, SOI score, admission ESR, and admission CRP as predictors of adverse outcomes (Figure 2). The A-SCORE (AUC, 0.82 [95% CI, .77–.86]) performed significantly better than the SOI score (AUC, 0.76 [95% CI, .70–.81]), CRP (AUC, 0.72 [95% CI, .66–.78]), and ESR (AUC, 0.64 [95% CI, .57–.70]) in predicting acute complications. At a cutoff = 4 (Youden index), the A-SCORE had a sensitivity of 74%, specificity of 78%, positive predictive value (PPV) of 52%, negative predictive value (NPV) of 91%, positive likelihood ratio (PLR) of 3.4, and negative likelihood ratio (NLR) of 0.3. The C-SCORE (AUC, 0.83 [95% CI, .77–.88]) performed significantly better than the SOI score (AUC, 0.70 [95% CI, .64–.76]), CRP (AUC, 0.69 [95% CI, .63–.75]), and ESR (AUC, 0.56 [95% CI, .49–.62]) in predicting chronic morbidity. At a cutoff = 3 (Youden index), the C-SCORE had a sensitivity of 63%, specificity of 89%, PPV of 42%, NPV of 95%, PLR of 5.9, and NLR of 0.4.
Figure 2.
Receiver operating characteristic (ROC) curves comparing potential predictors of adverse outcomes. ROC curves of clinical scores and laboratory values were compared to determine their ability to accurately predict acute complications (A) and chronic morbidity (B). The areas under the ROC curve (AUC) for Acute Score of Complications of Osteomyelitis Risk Evaluation (A-SCORE) and Chronic Score of Complications of Osteomyelitis Risk Evaluation (C-SCORE) were significantly greater than AUC for severity of illness (SOI) score [29], baseline C-reactive protein (CRP), and baseline erythrocyte sedimentation rate (ESR) in both models. The regression curve was derived from a backward stepwise binary logistic regression model described in the Methods.
DISCUSSION
This study is a retrospective observational analysis of 261 consecutively hospitalized children with documented, nontraumatic, pyogenic AHO of varying severity, in the absence of comorbidities. The study was conducted at 2 disparate academic pediatric centers with different hospital sizes and rates of PICC use for AHO, which partly reflect the diversity of institutions in the US. We demonstrated that 2 novel evidence-based composite scores, the A-SCORE and C-SCORE, derived from simple clinical parameters and common laboratory tests performed at 48–96 hours after admission, were significantly better than existing tools to predict acute and chronic complications of pediatric AHO [4, 30]. Notably, if the A-SCORE was ≤ 4, the corresponding NPV was ≥ 91%, and if the C-SCORE was ≤ 3, the corresponding NPV was ≥ 95%. These cutoff values potentially could be used by clinicians to rule out the risk of complications with relative confidence and to guide early transition from parenteral to oral antibiotics.
This study encompassed a wide spectrum of disease severity that was reflected by relatively high rates of bacteremia (45%), abscess formation (38%), disseminated disease (12%), persistent bacteremia (7%), and multifocal osteomyelitis (5%). Fourteen percent of cases with abscesses had multiple drainage procedures. Overall, 10% of patients required readmission for various indications, especially PICC complications, and 11% developed chronic morbidities.
The microbiological etiology was identified in almost three-quarters of patients. Of these isolates, S. aureus was responsible for 80% of cases, of which almost one-third was caused by MRSA. Our finding that MRSA was not associated with worse outcomes after adjustment for potential confounding is in agreement with some investigators [4, 27] but not others [32]. However, this analysis was limited by small sample size.
The constellation of abnormal clinical findings and laboratory results that constitute the A-SCORE and C-SCORE reflect exuberant proinflammatory host immune responses that are believed to contribute to the pathogenesis of AHO and its complications [34]. Pathogen-associated virulence determinants such as hemolysins, leukocidins, and phenol soluble modulins produced by S. aureus [35–37] are also considered important mediators of AHO complications. However, as there are no accurate clinically validated molecular biomarkers for pediatric AHO, novel predictive clinical models are urgently needed to guide therapy.
Widespread use of PICCs for AHO has been associated previously with complications [5, 38]. We confirmed that children with PICCs had substantial adverse events even after adjustment for possible indication bias. On the other hand, patients discharged from hospital on oral antibiotics were more likely to be lost to follow-up compared with those receiving parenteral therapy, and we were not able to determine their long-term outcomes.
This study has several limitations. The analyses were retrospective in nature and will require independent validation in prospective multicenter trials. Patients were enrolled over a longer period at one of the hospitals, during which time changes in clinical management, declining use of PICCs at HCH, and unmeasured secular trends in microbial virulence factors could have affected the results despite statistical adjustments. Eight (3%) children who did not have an MRI or surgical exploration may have had an undetected bone abscess, but that was unlikely because they did not experience an acute complicated course. Considering that the duration of follow-up varied, development of chronic complications may have been underestimated. On the other hand, the relatively large size of our cohort and wide variety of microbiological causes of AHO in children at 2 pediatric centers of different sizes, with diverse patient demographics and management approaches, provided us with a unique platform to generate a novel paradigm for risk prediction.
Based on the superior performance characteristics of the A-SCORE and C-SCORE, we propose that these measures, if validated, could be used in combination with widely accepted clinical criteria and improvement of CRP levels to indicate the optimal timing for transition from parenteral to oral antibiotics [22]. These new scoring systems could provide clinicians with a set of evidence-based parameters to rule out potential complications of AHO with great confidence, and to help select low-risk children who could benefit from early transition to enteral antibiotics, a practice that has been shown to be safe in various settings during the past 4 decades [5, 13–23, 39, 40].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Z. A. and I. C. M. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept, design, and supervision: Z. A., M. K., P. J. S., and I. C. M. Acquisition, analysis, or interpretation of data: Z. A., M. E., S. P., B. F., B. L., and I. C. M. Drafting of the manuscript: Z. A. and I. C. M. Critical revision of the manuscript for important intellectual content: Z. A., M. E., S. P., B. F., B. L., K. C., M. K., P. J. S., and I. C. M. Administrative, technical, or material support: Z. A. M. E., K. C., M. K., P. J. S., and I. C. M.
Acknowledgments. The authors thank Dr Penelope H. Dennehy for her assistance with the study and Dr John D. Nelson and Dr George H. McCracken, Jr for their mentorship and innovative research on osteomyelitis that inspired this study.
Financial support. This work was supported by the Department of Pathology and Laboratory Medicine, Laboratory of Clinical Microbiology, and the Department of Pediatrics, Division of Infectious Diseases, Rhode Island Hospital and Warren Alpert Medical School of Brown University. I. C. M. received support from the National Institutes of Health/National Institute of Allergy and Infectious Diseases (R25AI140490).
Potential conflicts of interest. P. J. S. reports grant support from Merck. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: IDWeek 2016, New Orleans, Louisiana, 26–30 October 2016 Abstract 59347.
References
- 1. Dodwell ER Osteomyelitis and septic arthritis in children: current concepts. Curr Opin Pediatr 2013; 25:58–63. [DOI] [PubMed] [Google Scholar]
- 2. Funk SS, Copley LA. Acute hematogenous osteomyelitis in children: pathogenesis, diagnosis, and treatment. Orthop Clin North Am 2017; 48:199–208. [DOI] [PubMed] [Google Scholar]
- 3. Peltola H, Pääkkönen M. Acute osteomyelitis in children. N Engl J Med 2014; 370:352–60. [DOI] [PubMed] [Google Scholar]
- 4. McNeil JC, Vallejo JG, Kok EY, Sommer LM, Hultén KG, Kaplan SL. Clinical and microbiologic variables predictive of orthopedic complications following Staphylococcus aureus acute hematogenous osteoarticular infections in children. Clin Infect Dis 2019; 69:1955–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keren R, Shah SS, Srivastava R, et al. Pediatric Research in Inpatient Settings Network Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr 2015; 169:120–8. [DOI] [PubMed] [Google Scholar]
- 6. Lorrot M, Gillet Y, Gras Le Guen C, Launay E, Cohen R, Grimprel E. Antibiotic therapy of bone and joint infections in children: proposals of the French Pediatric Infectious Disease Group. Arch Pediatr 2017; 24:36–41. [DOI] [PubMed] [Google Scholar]
- 7. Saavedra-Lozano J, Falup-Pecurariu O, Faust SN, et al. Bone and joint infections. Pediatr Infect Dis J 2017; 36:788–99. [DOI] [PubMed] [Google Scholar]
- 8. Le Saux N Diagnosis and management of acute osteoarticular infections in children. Paediatr Child Health 2018; 23:336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McMullan BJ, Andresen D, Blyth CC, et al. ANZPID-ASAP Group Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis 2016; 16:e139–52. [DOI] [PubMed] [Google Scholar]
- 10. Copley LA, Kinsler MA, Gheen T, Shar A, Sun D, Browne R. The impact of evidence-based clinical practice guidelines applied by a multidisciplinary team for the care of children with osteomyelitis. J Bone Joint Surg Am 2013; 95:686–93. [DOI] [PubMed] [Google Scholar]
- 11. Liu C, Bayer A, Cosgrove SE, et al. Infectious Diseases Society of America Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–55. [DOI] [PubMed] [Google Scholar]
- 12. Michelow IC, Mandell JG. Sequential intravenous oral antibiotic therapy for osteomyelitis: how short is long enough? JAMA Pediatr 2015; 169:698–9. [DOI] [PubMed] [Google Scholar]
- 13. Cortes-Penfield NW, Kulkarni PA. The history of antibiotic treatment of osteomyelitis. Open Forum Infect Dis 2019; 6:ofz181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tetzlaff TR, McCracken GH Jr, Nelson JD. Oral antibiotic therapy for skeletal infections of children. II. Therapy of osteomyelitis and suppurative arthritis. J Pediatr 1978; 92:485–90. [DOI] [PubMed] [Google Scholar]
- 15. Syrogiannopoulos GA, Nelson JD. Duration of antimicrobial therapy for acute suppurative osteoarticular infections. Lancet 1988; 1:37–40. [DOI] [PubMed] [Google Scholar]
- 16. Jaberi FM, Shahcheraghi GH, Ahadzadeh M. Short-term intravenous antibiotic treatment of acute hematogenous bone and joint infection in children: a prospective randomized trial. J Pediatr Orthop 2002; 22:317–20. [PubMed] [Google Scholar]
- 17. Le Saux N, Howard A, Barrowman NJ, Gaboury I, Sampson M, Moher D. Shorter courses of parenteral antibiotic therapy do not appear to influence response rates for children with acute hematogenous osteomyelitis: a systematic review. BMC Infect Dis 2002; 2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zaoutis T, Localio AR, Leckerman K, Saddlemire S, Bertoch D, Keren R. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics 2009; 123:636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jagodzinski NA, Kanwar R, Graham K, Bache CE. Prospective evaluation of a shortened regimen of treatment for acute osteomyelitis and septic arthritis in children. J Pediatr Orthop 2009; 29:518–25. [DOI] [PubMed] [Google Scholar]
- 20. Paakkonen M, Kallio MJ, Kallio PE, Peltola H. Shortened hospital stay for childhood bone and joint infections: analysis of 265 prospectively collected culture-positive cases in 1983–2005. Scand J Infect Dis 2012; 44:683–8. [DOI] [PubMed] [Google Scholar]
- 21. Arnold JC, Bradley JS. Osteoarticular infections in children. Infect Dis Clin North Am 2015; 29:557–74. [DOI] [PubMed] [Google Scholar]
- 22. Chou AC, Mahadev A. The use of C-reactive protein as a guide for transitioning to oral antibiotics in pediatric osteoarticular infections. J Pediatr Orthop 2016; 36:173–7. [DOI] [PubMed] [Google Scholar]
- 23. McNeil JC, Kaplan SL, Vallejo JG. The influence of the route of antibiotic administration, methicillin susceptibility, vancomycin duration and serum trough concentration on outcomes of pediatric Staphylococcus aureus bacteremic osteoarticular infection. Pediatr Infect Dis J 2017; 36:572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arnold JC, Cannavino CR, Ross MK, et al. Acute bacterial osteoarticular infections: eight-year analysis of C-reactive protein for oral step-down therapy. Pediatrics 2012; 130:e821–8. [DOI] [PubMed] [Google Scholar]
- 25. Roine I, Arguedas A, Faingezicht I, Rodriguez F. Early detection of sequela-prone osteomyelitis in children with use of simple clinical and laboratory criteria. Clin Infect Dis 1997; 24:849–53. [DOI] [PubMed] [Google Scholar]
- 26. Martin AC, Anderson D, Lucey J, et al. Predictors of outcome in pediatric osteomyelitis: five years experience in a single tertiary center. Pediatr Infect Dis J 2016; 35:387–91. [DOI] [PubMed] [Google Scholar]
- 27. Gijon M, Bellusci M, Petraitiene B, et al. Factors associated with severity in invasive community-acquired Staphylococcus aureus infections in children: a prospective European multicentre study. Clin Microbiol Infect 2016; 22:643.e1–6. [DOI] [PubMed] [Google Scholar]
- 28. Mignemi ME, Benvenuti MA, An TJ, et al. A novel classification system based on dissemination of musculoskeletal infection is predictive of hospital outcomes. J Pediatr Orthop 2016; 38:279–86. [DOI] [PubMed] [Google Scholar]
- 29. Copley LA, Barton T, Garcia C, et al. A proposed scoring system for assessment of severity of illness in pediatric acute hematogenous osteomyelitis using objective clinical and laboratory findings. Pediatr Infect Dis J 2014; 33:35–41. [DOI] [PubMed] [Google Scholar]
- 30. Athey AG, Mignemi ME, Gheen WT, Lindsay EA, Jo CH, Copley LA. Validation and modification of a severity of illness score for children with acute hematogenous osteomyelitis. J Pediatr Orthop 2016; 39:90–7. [DOI] [PubMed] [Google Scholar]
- 31. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70:41–55. [Google Scholar]
- 32. Davis WT, Gilbert SR. Comparison of methicillin-resistant versus susceptible Staphylococcus aureus pediatric osteomyelitis. J Pediatr Orthop 2018; 38:e285–91. [DOI] [PubMed] [Google Scholar]
- 33. Kok EY, Vallejo JG, Sommer LM, et al. Association of vancomycin MIC and molecular characteristics with clinical outcomes in methicillin-susceptible Staphylococcus aureus acute hematogenous osteoarticular infections in children. Antimicrob Agents Chemother 2018;62:e00084–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Benvenuti M, An T, Amaro E, et al. Double-edged sword: musculoskeletal infection provoked acute phase response in children. Orthop Clin North Am 2017; 48:181–97. [DOI] [PubMed] [Google Scholar]
- 35. Bocchini CE, Hulten KG, Mason EO Jr, Gonzalez BE, Hammerman WA, Kaplan SL. Panton-Valentine leukocidin genes are associated with enhanced inflammatory response and local disease in acute hematogenous Staphylococcus aureus osteomyelitis in children. Pediatrics 2006; 117:433–40. [DOI] [PubMed] [Google Scholar]
- 36. Josse J, Velard F, Gangloff SC. Staphylococcus aureus vs. osteoblast: relationship and consequences in osteomyelitis. Front Cell Infect Microbiol 2015; 5:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang B, Wang Y, Feng Z, et al. Panton-Valentine leucocidin (PVL) as a potential indicator for prevalence, duration, and severity of Staphylococcus aureus osteomyelitis. Front Microbiol 2017; 8:2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barrier A, Williams DJ, Connelly M, Creech CB. Frequency of peripherally inserted central catheter complications in children. Pediatr Infect Dis J 2012; 31:519–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peltola H, Pääkkönen M, Kallio P, Kallio MJ; Osteomyelitis-Septic Arthritis Study Group Short- versus long-term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: prospective, randomized trial on 131 culture-positive cases. Pediatr Infect Dis J 2010; 29:1123–8. [DOI] [PubMed] [Google Scholar]
- 40. Bachur R, Pagon Z. Success of short-course parenteral antibiotic therapy for acute osteomyelitis of childhood. Clin Pediatr (Phila) 2007; 46:30–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.