Abstract
Background
Inflammatory bowel disease [IBD] is characterised by abnormal host-microbe interactions. Proinflammatory cytokine IFNγ and a novel tumour necrosis factor [TNF] superfamily member, TL1A, have been implicated in epithelial barrier dysfunction. The divergent regulatory mechanisms of transcellular versus paracellular hyperpermeability remain poorly understood. Intestinal epithelia express two splice variants of long myosin light chain kinase [MLCK], of which the full-length MLCK1 differ from the shorter isoform MLCK2 by an Src kinase phosphorylation site. The aim of this study was to investigate the roles of MLCK splice variants in gut barrier defects under proinflammatory stress.
Methods and Results
Upregulated expression of TL1A, IFNγ, and two MLCK variants was observed in human IBD biopsy specimens. The presence of intraepithelial bacteria preceded tight junction [TJ] damage in dextran sodium sulphate-treated and TL1A-transgenic mouse models. Lack of barrier defects was observed in long MLCK[-/-] mice. TL1A induced MLCK-dependent terminal web [TW] contraction, brush border fanning, and transepithelial bacterial internalisation. The bacterial taxa identified in the inflamed colonocytes included Escherichia, Enterococcus, Staphylococcus, and Lactobacillus. Recombinant TL1A and IFNγ at low doses induced PI3K/Akt/MLCK2-dependent bacterial endocytosis, whereas high-dose IFNγ caused TJ opening via the iNOS/Src/MLCK1 axis. Bacterial internalisation was recapitulated in MLCK-knockout cells individually expressing MLCK2 but not MLCK1. Immunostaining showed different subcellular sites of phosphorylated MLC localised to the TJ and TW in the MLCK1- and MLCK2-expressing cells, respectively.
Conclusions
Proinflammatory cytokines induced bacterial influx through transcellular and paracellular routes via divergent pathways orchestrated by distinct MLCK isoforms. Bacterial transcytosis induced by TL1A may be an alternative route causing symptom flares in IBD.
Keywords: Host-microbiota interplay, chronic inflammation, bacterial translocation, intraepithelial microbes, brush border fanning, perijunctional contraction
1. Introduction
Abnormal host-microbe interactions are linked to the pathogenesis of inflammatory bowel disease [IBD], ie, Crohn’s disease and ulcerative colitis.1,2 A high density of mucosa-associated bacteria was found in the biopsy samples of patients with recent onset IBD but not in healthy individuals.3,4 Elevated intestinal permeability, as well as bacteraemia and endotoxaemia, has also been reported preceding symptom relapse in IBD patients.5–7 These observations indicated that epithelial barrier dysfunction accompanied by bacterial influx may drive the initiation and perpetuation of chronic gut inflammation.
The intestinal barrier comprises monolayered epithelia with ultrastructural brush borders [BBs] and tight junctions [TJs]. The densely packed microvilli rooted on the terminal web [TW] are composed of cytoskeletal proteins [eg, actin, myosin, and fodrin] and prevent bacterial contact with the cellular soma and limit transcellular bacterial penetration.8–11 The laterally expressed TJ proteins, such as occludin and claudins connecting to cytoplasmic zonula occludens [ZO] and the perijunctional actinomyosin ring, prevent bacteria from passing through the paracellular space under physiological conditions.12–14 However, intra-epithelial bacteria15–17 and altered TJ expression18,19 were observed in biopsy samples of IBD patients in cross-sectional studies. Despite long-term observation of coexisting transcellular and paracellular hyperpermeability in inflamed gut tissues, the temporal relationship and molecular mechanisms critical to the different routes of epithelial barrier defects remain obscure.
Elevated proinflammatory cytokines (eg, interferon gamma [IFNγ] and tumor necrosis factor α [TNFα]) and hyperactivation of myosin light chain kinase [MLCK] are involved in the mechanisms of epithelial barrier defects in IBD.20,21 Previous work demonstrated that high-dose IFNγ or IFNγ mixed with TNFα caused TJ disruption through MLCK-mediated phosphorylation of the myosin light chain [MLC] in the perijunctional actinomyosin ring, which was independent of cell apoptosis.22–25 The cytokine mixtures also induced cell death-dependent TJ destruction, resulting in bacterial translocation to extraintestinal viscera.26,27
Another line of evidence produced by our laboratory and others showed that low-dose IFNγ triggered bacterial transcytosis without TJ alteration in epithelial cells; in this case, the cytokine induced MLCK-dependent TW contraction and BB fanning that facilitated bacterial endocytosis through membranous lipid rafts.28–30 Recently, a novel TNF superfamily member, TNF-like 1A [TL1A/TNFSF15], was observed in the inflamed gut tissues with high levels, and its gene polymorphism was linked to IBD susceptibility.31,32 TL1A increased Th1/Th17 polarisation and IFNγ production, and activated downstream PI3K/Akt and NFκB signals.33–36 Apart from its proinflammatory role, TL1A may stimulate epithelial hyperpermeability, but evidence remains elusive. To date, how cytokines activate divergent MLCK-dependent pathways to compromise paracellular and/or transcellular barriers remains poorly understood.
Epithelial cells expressed long MLCK, which is a non-muscle form of MLCK with a high molecular weight of 210 kD. There are five splice variants of long MLCK encoded by a single MYLK gene.37 The gut epithelia express only two splice variants: the full-length MLCK1 and the shorter isoform MLCK2, which differ by a 207-base pair sequence [exon 11] corresponding to 69 amino acids with an Src kinase phosphorylation site.38 MLCK1 is predominantly located in the perijunctional actinomyosin ring that regulates TJ permeability in villous epithelia.38,39 However, the biological function of MLCK2 remains unclear. We speculate that the alternative splicing of the MLCK isoforms may account for myosin light chain [MLC] phosphorylation at different subcellular sites, ie, TW versus TJ, and causes distinct modes of barrier defects. The aims of the current study were to investigate whether proinflammatory cytokines induce divergent pathways of epithelial hyperpermeability and to decipher the relative roles of the MLCK variants in the regulation of transepithelial bacterial influx.
2. Materials and Methods
2.1. Human specimen collection
Colonic mucosal biopsy samples were collected from control subjects and patients with Crohn’s disease [CD] or ulcerative colitis [UC]. Written consent was obtained from all study subjects, and approval for this study was granted by the Research Ethics Committee of National Taiwan University Hospital [201802090RINB]. Samples were analysed by polymerase chain reaction [PCR] using primer pairs [Supplementary Table 1; and Supplementary Table 2, available as Supplementary data at ECCO-JCC online].
2.2. Animals
Specific pathogen-free male mice, including C57BL/6, mice deficient in long 210-kD MLCK (MLCK[-/-]),22,30 and transgenic mice with lymphocyte overexpression of TL1A [TL1A-Tg]40,41 on a B6 background, were bred and housed in the NTUCM animal Center. The mice were raised in a temperature-controlled room [20 ± 2°C] with a 12-h light-dark cycle, and fed regular mouse chow and water. Homozygous mice were single-housed in individual cages. All procedures were approved by the Animal Care and Use Committee.
2.3. Reagents
Dextran sodium sulphate [DSS] was purchased from MP Biomedicals [Taiwan]. Pharmacological inhibitors, including ML-7 [an MLCK inhibitor targeting the ATPase domain], a membrane permeant inhibitor of myosin light chain kinase PIK [a specific MLCK inhibitor targeting the kinase domain], LY294002 [a PI3K inhibitor], Akt-inh [an Akt inhibitor], Src inhibitor-1 [a Src kinase inhibitor], and L-Nil [an iNOS inhibitor] were purchased from Sigma-Aldrich [St. Louis, MO]. PIK was a gift from the Turner Laboratory. Neutralising anti-TL1A antibodies and the rat IgG1 isotype were purchased from R&D Systems. Recombinant TL1A and IFNγ were purchased from R&D Systems.
2.4. Mouse model of chemical-induced enterocolitis
Wild-type and MLCK[-/-] mice were given 2.5% dextran sodium sulphate [DSS] in drinking water for 0, 1, 4, and 7 days. In another setting, mice were administered ML-7 [1 mg/kg twice in a 12-h interval] or neutralising anti-TL1A [100 μg per mouse] via intraperitoneal [i.p.] injection before drinking DSS. Intestinal tissues were collected for barrier functional tests and PCR analysis using primer pairs [Supplementary Table 3, available as Supplementary data at ECCO-JCC online].
2.5. Mouse model of spontaneous ileitis
The TL1A-Tg mice were raised to the age of 4, 6, and 8 months for experiments. In another setting, TL1A-Tg mice were administered ML-7 via i.p. injection [1 mg/kg every 12 h for 3 days] before tissue collection.
2.6. Mouse model of intestinal obstruction
Mice were fasted overnight but allowed to drink water ad libitum before surgery for intestinal obstruction [IO] as previously described.23,30
2.7. Quantification of intraepithelial bacteria by gentamicin resistance assay
Intestinal epithelial cells were isolated using a previously established protocol.30 The viability and purity of the isolated epithelial cells were determined to be 92.7 ± 0.3% and 91.1 ± 1.2% by trypan blue exclusion and flow cytometry, respectively.30,42 The isolated epithelial cells [2 × 106] were incubated with 300 μg/mL gentamicin [Sigma] for 1 h with gentle shaking. After washing twice with PBS, the cells were lysed with 1% Triton X [TX]-100 in PBS for 10 min on ice. The cell lysate [200 μl] was plated onto fresh blood agar plates for overnight aerobic bacterial culturing at 37°C. The number of bacterial colonies was normalised to the number of trypan blue-negative viable epithelial cells and presented as log10 CFU per 106 cells. The individual bacterial colonies were selected for DNA extraction and sequencing by 16S ribosomal DNA sequencing and classified using a ribosomal project database as described.30 Each bacterial strain was tested for gentamicin susceptibility and showed a minimum inhibitory concentration of 2 μg/ml.
2.8. Ussing chamber studies and macromolecular permeability assay
The intestinal epithelial permeability was determined by the mucosal-to-serosal flux rate of dextran-FITC [MW 4000].12,23,30
2.9. Analysis of terminal web contraction and brush border BB fanning
Intestinal epithelial sheets were isolated for the measurement of TW contraction and BB fanning following previously described protocols.30 The width [X] of the TW and the height [Y] of the arc were measured on digitised images of a total of 100 epithelial cells from five mice in each group. The calculated ratio of Y to X represents the extent of TW contraction. Electron micrographs of longitudinal and cross-sectional views of the epithelial cells with BBs were taken at magnifications of 6000×, 10 000 × , and 30 000×. The extent of BB disarray and fanning was determined by quantifying the intermicrovillous space and packing angles in fixed-size windows on cross-sectional electron micrographs following the method of Tyska et al.11
2.10. Fluorescence in situ hybridisation
Intestinal tissues were fixed in Carnoy’s solution and processed using FITC-labelled universal bacterial probe.30,42
2.11. Cell culture studies
Human Caco-2 cells were treated with TL1A or IFNγ at various doses for 48 h.43,44 In some experiments, pharmacological inhibitors and gene silencing of MLCK was performed before cytokine treatment in cells.
2.12. Knockdown or knockout of the MLCK gene in human cell cultures
Caco-2 cells were transfected with siRNA oligonucleotides that target all MLCK variants or MLCK1.45,46 The pool of siMLCK1 targeted the exon 11 sequence [nucleotides 1746–1796] in the human MYLK gene [GenBank: NM_053025.4],37,39 the exon missing in the splice variant MLCK2. Custom-designed siRNAs were generated to target the bridging domain between exons 10 and 12 of the human MYLK gene for silencing MLCK2. The oligonucleotides were designed based on the human MLCK2 messenger RNA [mRNA] sequence [GenBank: NM_053026.4].
For a separate experiment, all MLCK variants were deleted in Caco-2 cells with CRISPR/Cas9 techniques for gene knockout [KO] at the First Research Core of National Taiwan University College of Medicine. The nucleotide sequence for MLCK1 or MLCK2 in pcDNA3.1 plasmids was transfected into KO cells before the bacterial internalisation assay.
2.13. Bacterial internalisation assay of epithelial cocultures
The amount of bacterial internalisation in Caco-2 cells was determined as previously described.29,30,42 Briefly, non-pathogenic E. coli strain BL21 was added at a multiplicity of infection [MOI] of 100 to the apical chamber of a Transwell plate and incubated with Caco-2 cells for 1 h, and the number of bacteria was normalised to that of trypan blue-negative epithelial cells using a gentamicin resistance assay.
2.14. Paracellular permeability studies in epithelial cell cultures
Caco-2 cells grown on Transwell plates were assessed for transepithelial electrical resistance [TER] using an electrovoltohmeter and by apical-to-basal transport of a dextran-FITC probe [MW 3000].
2.15. Immunofluorescent staining and confocal microscopy
Cell monolayers exposed to fluorescent E. coli were stained with anti-pMLC [1:100, Cell Signalling], anti-fodrin [1:100, Abcam], or anti-ZO-1 [1:100, Invitrogen]. The images were captured and reconstructed using a laser-scanning confocal microscope.
2.16. Statistical analysis
All data are expressed as the means ± standard error of the mean [SEM], except that dot plots are used for human specimens. The data were compared by ANOVA with Newman‐Keuls post-hoc test or by unpaired t test [GraphPad Prism v.5]. When comparing the difference among groups or between two groups of bacterial CFU, the non-parametric Kruskal‐Wallis test or Mann‐Whitney U test was used. A probability value <0.05 was considered to be significant. Detailed methods are included in the Supplementary Material and Methods, available as Supplementary data at ECCO-JCC online.
3. Results
3.1. Overexpression of proinflammatory cytokines and MLCK variants in IBD biopsy samples
Colonic biopsy samples were obtained from patients with IBD, including those with Crohn’s disease [CD] or ulcerative colitis [UC] [Table 1], to evaluate the expression of proinflammatory cytokines and long MLCK splice variants by semiquantitative and quantitative PCR. Higher mucosal levels of TL1A and IFNγ were observed in actively inflamed tissues from the CD and UC patients compared with non-inflamed tissues from the control subjects. The expression levels of both splice variants MLCK1 and MLCK2 were also significantly increased in actively inflamed tissues of the CD and UC patient samples [Figure 1].
Table 1.
Characteristics of control subjects and patients with Crohn’s disease [CD] and ulcerative colitis [UC].
| Control | CD | UC | |
|---|---|---|---|
| Number of patients | 19 | 17 | 18 |
| Age, years [mean] | 44–94 [71.42] | 19–75 [40.29] | 17–77 [46.17] |
| Gender, male; female | 11; 8 | 13; 4 | 7; 11 |
Figure 1.
Overexpression of TL1A, IFNγ, and two MLCK splice variants in colonic biopsy specimens of IBD patients. Biopsy specimens of actively inflamed tissues were collected from patients with Crohn’s disease [CD] and ulcerative colitis [UC] compared with non-inflamed [NI] tissues of control subjects. The tissue RNA was extracted and reverse-transcribed for semiquantitative and quantitative PCR. [A] Representative images of semiquantitative PCR bands for TL1A, IFNγ, and long MLCK variants in mucosal biopsies. The higher band [1400 bp] represents MLCK1 and the lower band [1200 bp] represents MLCK2. [B] Quantitative results of real-time PCR for [a] TL1A, [b] IFNγ, [c] MLCK1, and [d] MLCK2. Each dot represents one male [▲] or female [•] patient, n = 17–18/group. *p <0.05 vs NI. The data were compared by ANOVA followed with Kruskal‐Wallis test. IBD, inflammatory bowel disease; PCR, polymerase chain reaction.
3.2. Bacterial internalisation preceded tight junction damage in mice with chemical-induced enterocolitis
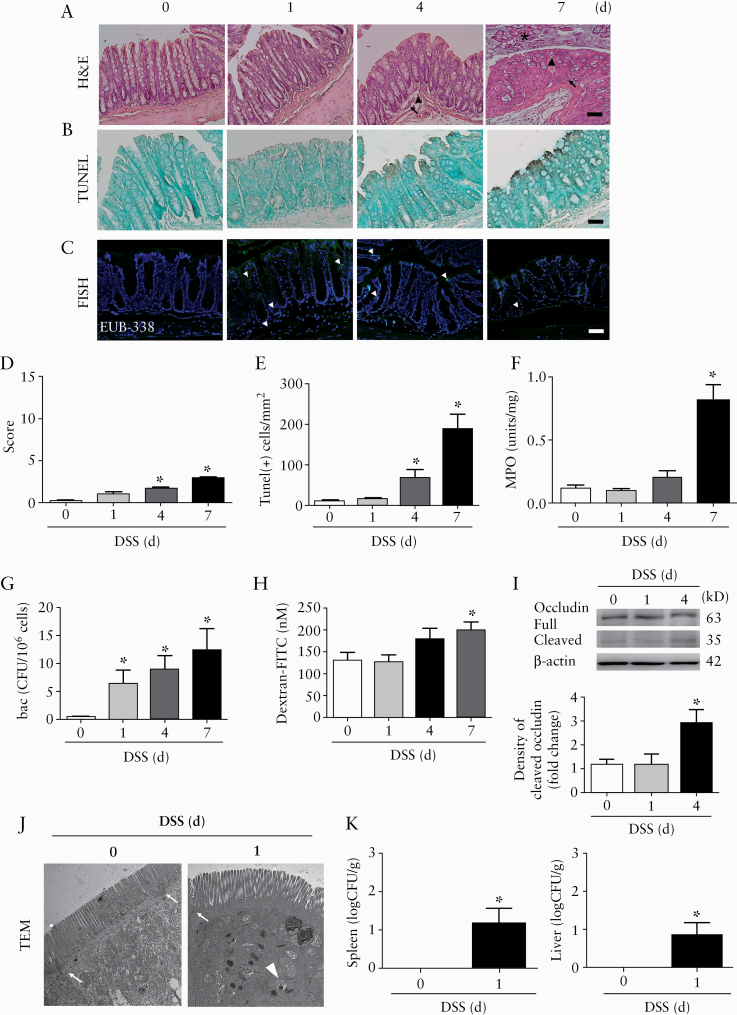
The relative timing of transcellular versus paracellular barrier defects was examined in a mouse model of IBD by giving 2.5% dextran sodium sulphate [DSS]. Mice displayed body weight loss and shortening of colon length, associated with increased histopathological scores, epithelial apoptosis, and myeloperoxidase activity in the ileal and colonic tissues 4–7 days after drinking DSS water [Figure 2A‐F, and Supplementary Figure 1A and B, available as Supplementary data at ECCO-JCC online]. Bacterial penetration to the intestinal mucosa was visualised by in situ hybridisation 1 day after mice initially started drinking DSS [DSS-1d], before detectable mucosal inflammation [Figure 2C]. An increase in intracellular bacterial counts in the colonic epithelial cells was found starting on DSS-1d [Figure 2G]. The intraepithelial bacteria were identified as Escherichia, Enterococcus, Staphylococcus, Lactobacillus, and Sporosarcina. On the other hand, tight junction [TJ] damage, such as occludin cleavage and increased macromolecular flux, in the intestinal tissues was noted 4–7 days after the mice started drinking DSS water [Figure 2H and I, and Supplementary Figure 1C‐H]. Electromicrographs confirmed the presence of bacteria inside the colonic epithelial cells, and the extraintestinal bacterial counts were determined in the liver and spleen tissues on DSS-1d [Figure 2J and K]. Therefore, further mechanistic studies of transcellular and paracellular barrier defects were conducted at the DSS-1d and DSS-4d time points, since high levels of epithelial cell death and denudation were readily observed on DSS-7d.
Figure 2.
Longitudinal study of epithelial barrier functions and histopathological features in a mouse model of chemical-induced colitis. Mice were administered 2.5% dextran sodium sulphate [DSS] in drinking water for 1, 4, and 7 days, and the following parameters were assessed in the colonic tissues. [A] Histological images by H&E staining. Presence of granulocyte infiltration [arrows], hyperaemia [arrowheads], and epithelial denudation [asterisks] was shown. [B] Photoimages of TUNEL staining. [C] Photoimages of fluorescent in situ hybridisation with probes [EUB338, green] targeting universal bacteria [arrowheads] and counterstained for cell nucleus with a Hoechst dye [blue]. Bar: 50 μm. n = 10/group. Images are representative of three independent experiments. [D] Histopathological score. [E] Numbers of TUNEL-positive apoptotic cells per mucosal area. [F] Myeloperoxidase activity. Ten sections per mouse groups were quantified and normalised per mm2. [G] Intracellular bacterial counts in colonic epithelial cells using a gentamicin resistance assay. n = 10/group. *p <0.05 vs DSS-0d [Day 0]. [H] Luminal-to-serosal macromolecular flux in colonic tissues. [I] Western blots showing occludin cleavage in colonic mucosal samples. Representative immunoblot images are shown in the upper panel, and densitometric analysis of cleaved occludin levels in the lower panel. n = 5/group. *p <0.05 vs DSS-0d. [J] Transmission electron micrographs [TEM] showing internalised bacteria [arrowhead] in the absence of tight junctional changes [arrow]. Magnification: 3000x. Bar: 1 μm. [K] Extraintestinal bacterial counts in the spleen and liver tissues of mice on DSS-1d. *p ≤0.05 vs DSS-0d. n = 10/group. The data were compared by ANOVA followed with Newman‐Keuls post-hoc test. When comparing the difference among groups of bacterial CFU, the Kruskal‐Wallis test was used. H&E, haematoxylin and eosin.
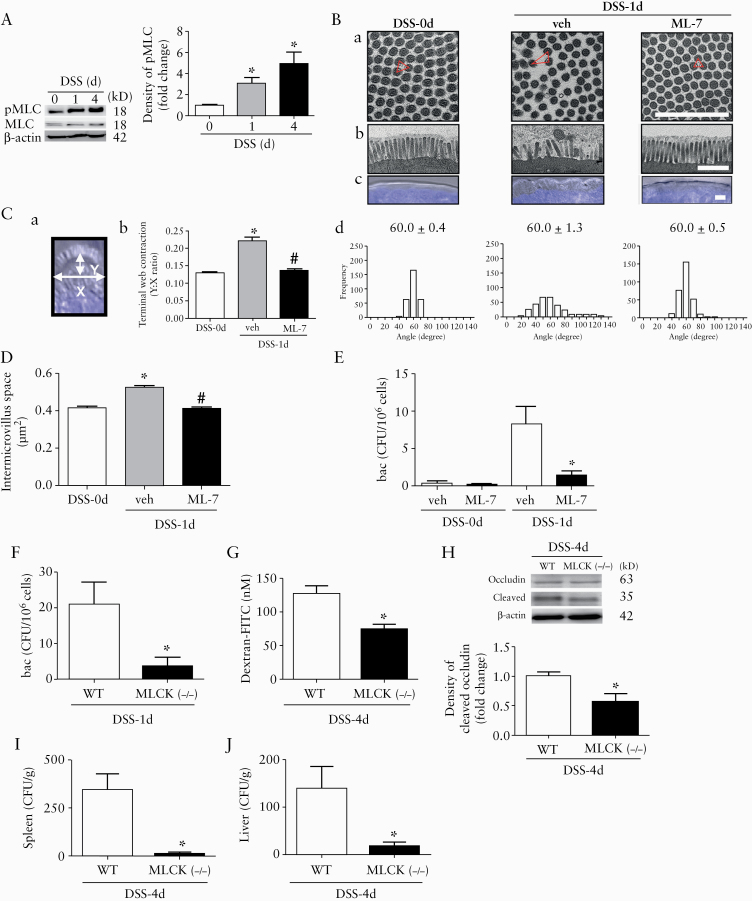
The dependency of epithelial barrier defects on long MLCK was examined using pharmacological inhibitors and gene-deficient mouse models. Increased mucosal MLC phosphorylation was observed in mice after drinking DSS for 1 and 4 days [Figure 3A]. Administration of ML-7 [an MLCK inhibitor] reduced the ultrastructural changes in the epithelial cells on Day 1, including terminal web [TW] contraction associated with brush border [BB] fanning and intermicrovillous space expansion [Figure 3B‐D]. The intracellular bacterial counts in mouse colonocytes were also significantly decreased by ML-7 [Figure 3E]. The changes in barrier defects at later time points were assessed using mice deficient of long MLCK-210 (MLCK [-/-]). In contrast to the observations made in the wild-type [WT] mice, bacterial internalisation and TJ damage were not observed in the colonic tissues of MLCK[-/-] mice on Day 1 and Day 4 after DSS drinking [Figure 3F‐H]. Moreover, the absence of barrier defects in the ileal tissues of MLCK[-/-] mice was also confirmed [Supplementary Figure 1I‐K]. Finally, the amount of bacteria translocated to the liver and spleen was reduced in the MLCK[-/-] mice compared with that in the WT mice on DSS-4d [Figure 3I and J].
Figure 3.
Myosin light chain kinase [MLCK]-dependent bacterial internalisation preceded TJ damage in colonic epithelia of DSS mice. [A] Western blots showing increased phosphorylation of myosin light chain [pMLC] in colonic mucosa of mice after DSS drinking for 1 and 4 days. Representative immunoblot images are shown in the left panel, and densitometric analysis of pMLC normalised to total MLC in the right panel. n = 5/group. *p <0.05 vs DSS-0d. [B] Administration of ML-7 [a MLCK inhibitor] prevented the epithelial ultrastructural changes in mouse colon on DSS-1d. Representative TEM images of epithelial brush borders [BBs]. Bar: 1 μm. [a] Cross-sectional views of BBs [mag: 30000x]. Packing angles are depicted in red colour. [b] Longitudinal views of BBs showing presence of bacteria [mag: 10000x]. [c] Differential interference contrast images showing the terminal web [TW] arc formation. Bar: 5 μm. [d] Quantification of packing angles from windows of the cross-sectional images. The average value of the packing angles is shown. [C] The extent of TW contraction was quantified by the ratio of the height [Y] to the width [X] of the TW arc. Schema of the width and height of the TW arc [panel a]. The extent of TW contraction was quantified by the ratio of Y to X [panel b]. *p <0.05 vs DSS-0d. #p <0.05 vs vehicle. A total of 100 windows for each group from 5 mice per group were analysed. [D] Intermicrovillous space was quantified from windows of the cross-sectional images in panel Ba. [E] Administration of ML-7 decreased the intracellular bacterial counts in mouse colonocytes on DSS-1d. n = 10/group. *p <0.05 vs vehicle. [F to J] The intestinal barrier defects in wild-type [WT] and MLCK[-/-] mice were compared after DSS drinking for 1 and 4 days. Compared with WT mice, MLCK[-/-] mice exhibited lower levels of [F] intraepithelial bacterial counts on DSS-1d, [G] Luminal-to-serosal macromolecular flux on DSS-4d, [H] occludin cleavage on DSS-4d, and bacterial translocation to [I] spleen and [J] liver tissues on DSS-4d. n = 10/group. *p <0.05 vs WT. The data were compared by ANOVA followed with Newman‐Keuls post-hoc test or unpaired t test. When comparing the difference among groups or between two groups of bacterial CFU, the Kruskal‐Wallis test or Mann‐Whitney U test was used. TJ, tight junction; DSS-0d, dextran sodium sulphate Day 0.
3.3. Proinflammatory cytokine TL1A induced MLCK-dependent bacterial endocytosis
Proinflammatory cytokines [ie, IFNγ and TNFα] were involved in MLCK-dependent TJ disruption in experimental models of colitis22,25; however, the cytokines that promote intraepithelial bacterial transcytosis in vivo remain poorly defined. A novel TNF superfamily member TL1A/TNFSF15 was observed in IBD specimens,31,32,36 and its role in transcellular epithelial hyperpermeability was investigated here. The levels of TL1A, IFNγ, and TNFα were elevated in the colonic mucosa 1 day after the mice started drinking DSS [Figure 4A]. Constitutive expression of DR3 [a TL1A receptor] was observed in the colonic epithelial cells [Figure 4A]. The downstream signalling pathways were also evaluated in the mucosal samples, showing heightened phosphorylation of Akt [Ser473] on Days 1 and 4, whereas Src [Tyr416] phosphorylation was not augmented in the colonic mucosa until Day 4 after DSS drinking [Figure 4B].
Figure 4.
Bacterial internalisation in DSS-treated and TL1A-transgenic mice was dependent on TL1A-induced Akt/MLC phosphorylation. Mucosal cytokine levels and signalling pathways were determined in DSS mice. [A] Colonic mucosal expression of [a] TL1A, [b] IFNγ, and [c] TNFα, and epithelial expression of [d] DR3 [a TL1A receptor] in mice after drinking DSS for 1 and 4 days. The gel blot of semiquantitative PCR bands was shown in the left panel. n = 5/group. *p <0.05 vs DSS-0d. [B] Western blots showing phosphorylation levels of Akt and Src in colonic mucosa of mice after drinking DSS for 1 and 4 days. [a] Representative immunoblot images and [b and c] densitometric analysis of phosphorylated Akt [pAkt] and phosphorylated Src [pSrc] normalised to total Akt and Src, respectively. n = 6/group. *p <0.05 vs DSS-0d. [C] Mice were administered neutralising anti-TL1A [100 μg per mouse] or IgG isotype antibody before DSS drinking, and the colonic mucosal samples were analysed on DSS-1d. [a] Representative immunoblot images and [b and c] densitometric analysis of pAkt and pSrc were shown. n= 6/group. *p <0.05 vs IgG. [D] Neutralisation of TL1A decreased the MLC phosphorylation in colonic mucosa on DSS-1d. [E] Neutralisation of TL1A reduced the intraepithelial bacterial counts in colonocytes on DSS-1d. n = 5/group. *p <0.05 vs IgG. [F, G, and H] Longitudinal study of intestinal barrier defects in a TL1A-transgenic [Tg] mouse model with spontaneous ileitis. Bacterial internalisation in ileal epithelial cells started at the age of 6 months, whereas increased macromolecular flux and MPO activity were noted in the ileal tissues at 8 months old. n= 10/group. *p <0.05 vs 4 mon. [I] Western blots showing MLC phosphorylation in the ileal mucosa of TL1A-Tg mice with increasing age. n = 6/group. *p <0.05 vs 4 months old. [J, K, and L] Administration of ML-7 in TL1A-Tg mice [8 months old] attenuated bacterial internalisation and ileal inflammation, but not macromolecular flux. n = 10/group. *p <0.05 vs PBS vehicle. The data were compared by ANOVA followed with Newman‐Keuls post-hoc test. When comparing the difference among groups of bacterial CFU, the Kruskal‐Wallis test was used. DSS-0d, dextran sodium sulphate-Day 0; PCR, polymerase chain reaction; MPO, myeloperoxidase.
Mice were administered neutralising anti-TL1A before DSS drinking in additional experiments to assess its role on bacterial internalisation. Neutralisation of TL1A significantly decreased the phosphorylation of Akt and MLC in colonic mucosa, and reduced the intracellular bacteria counts in colonocytes on DSS-1d [Figure 4C‐E]. No effect on Src phosphorylation levels in colonic mucosa was seen after giving anti-TL1A [Figure 4C]. The ileal mucosa also displayed TL1A-dependent Akt and MLC phosphorylation and bacterial internalisation on DSS-1d [Supplementary Figure 2A‐D, available as Supplementary data at ECCO-JCC online]. The effect of TL1A on paracellular hyperpermeability was not tested at later time points in the colitis model due to its known role as a T cell costimulatory factor for IFNγ synthesis.
The involvement of TL1A during early onset of bacterial transcytosis was also examined in a mouse model with an intestinal obstruction [IO] created by loop ligation. Previous work showed the BB fanning and bacterial internalisation in ileal epithelial cells 6 h after IO, which preceded TJ damage identified at the 24-h time point.23,30 Here, bacteria internalisation 6 h after IO induction and TJ damage, with extraintestinal bacterial translocation 24 h after IO induction, were attenuated by the enteral administration of a membrane permeant inhibitor of myosin light chain kinase [PIK, a specific inhibitor targeting the kinase enzymatic domain47] [Supplementary Figure 3, available as Supplementary data at ECCO-JCC online]. Moreover, administration of neutralising anti-TL1A decreased the BB fanning and intracellular bacterial counts in the enterocytes 6 h after IO induction [Supplementary Figure 3].
3.4. MLCK-dependent bacterial endocytosis in the TL1A-transgenic mice with spontaneous ileitis
The role of TL1A in MLCK-dependent epithelial barrier defects was next evaluated in a chronic model of IBD. A transgenic [Tg] mouse model with constitutive lymphocytic TL1A expression developed spontaneous ileitis with features similar to those of Crohn’s disease.40,41,48 The barrier functions of ileal tissues were assessed in the TL1A-Tg mice of various ages. The intracellular bacterial counts were increased in the enterocytes of the 6-month-old TL1A-Tg mice [Figure 4F]. The bacteria internalised in the enterocytes included Staphylococcus, Lactobacillus, Stenotrophomonas, and Flavobacteriaceae. The onset of bacterial internalisation occurred before TJ disruption and myeloperoxidase activity increase in the ileal tissues of the 8-month old TL1A-Tg mice [Figure 4G and H]. Hyperphosphorylation of MLC in the ileal mucosa was observed in the 6- and 8-month old mice [Figure 4I]. Administration of ML-7 reduced the intraepithelial bacterial counts and diminished the mucosal inflammation in the ileal tissues of the TL1A-Tg mice [Figure 4J‐L].
3.5. MLCK-activated epithelial hyperpermeability after cytokine treatment in vitro
Human intestinal epithelial Caco-2 cells were treated with proinflammatory cytokines to determine the molecular mechanisms of barrier alteration. The cytokine-treated cells were exposed to non-pathogenic E. coli BL21 to assess bacterial internalisation. TL1A administered at various doses [10–300 ng/ml] caused an increase in bacterial endocytosis without TJ disruption [Figure 5A‐C]. Low-dose IFNγ [10 IU/ml] also caused bacterial internalisation without TJ damage, but high-dose IFNγ [3000 IU/ml] triggered a drop in the transepithelial electrical resistance [TER] associated with increased dextran flux [Figure 5D‐F]. Previous work demonstrated that IFNγ treatment upregulated the expression of MLCK21,30 and inducible nitric oxide synthase [iNOS]49,50 to induce cell death-dependent or -independent barrier loss. Here, a dose response of iNOS expression was observed by IFNγ treatment, whereas no sign of iNOS was seen for all doses of TL1A tested [Figure 5G and H]. The TL1A treatment at low and high doses induced Akt [Ser473] phosphorylation but had no effect on Src phosphorylation [Figure 5I]. In contrast, high-dose IFNγ increased Src [Tyr416] phosphorylation, which was prevented by a selective iNOS inhibitor L-NIL [Figure 5J].
Figure 5.
Loss of transcellular and paracellular barrier functions after TL1A and IFNγ treatment in human intestinal epithelial cells. Caco-2 cells were treated with TL1A and IFNγ at various doses for 48 h, and the epithelial barrier functions were examined. [A] Intracellular bacterial counts in TL1A-treated cells after exposure to a non-pathogenic laboratory strain of E. coli for 1 h at multiplicity-of-infection [MOI] of 100. [B and C] Transepithelial electrical resistance [TER] and apical-to-basolateral dextran flux in TL1A-treated cells. [D] Intracellular bacterial counts in IFNγ-treated cells. [E and F] The TER and dextran flux in IFNγ-treated cells. [G and H] Western blots showing dose-dependent expression of inducible nitric oxide synthase [iNOS] protein following IFNγ, but not TL1A, treatment for 48 h. [I] Increased phosphorylation levels of Akt, but not Src, was noted in Caco-2 cells after TL1A treatment for 1 h. Densitometric analysis of [a] phosphorylated Akt [pAkt] and [b] phosphorylated Src [pSrc] were normalised to total Akt and Src, respectively. [J] Increased phosphorylation of Src was observed in cells after IFNγ treatment for 24 h, which was inhibited by pretreatment with L-Nil [a specific iNOS inhibitor]. n = 4–5/group. *p <0.05 vs 0. Representative data from two individual experiments. The data were compared by ANOVA followed with Newman‐Keuls post-hoc test. When comparing the difference among groups of bacterial CFU, the Kruskal‐Wallis test was used.
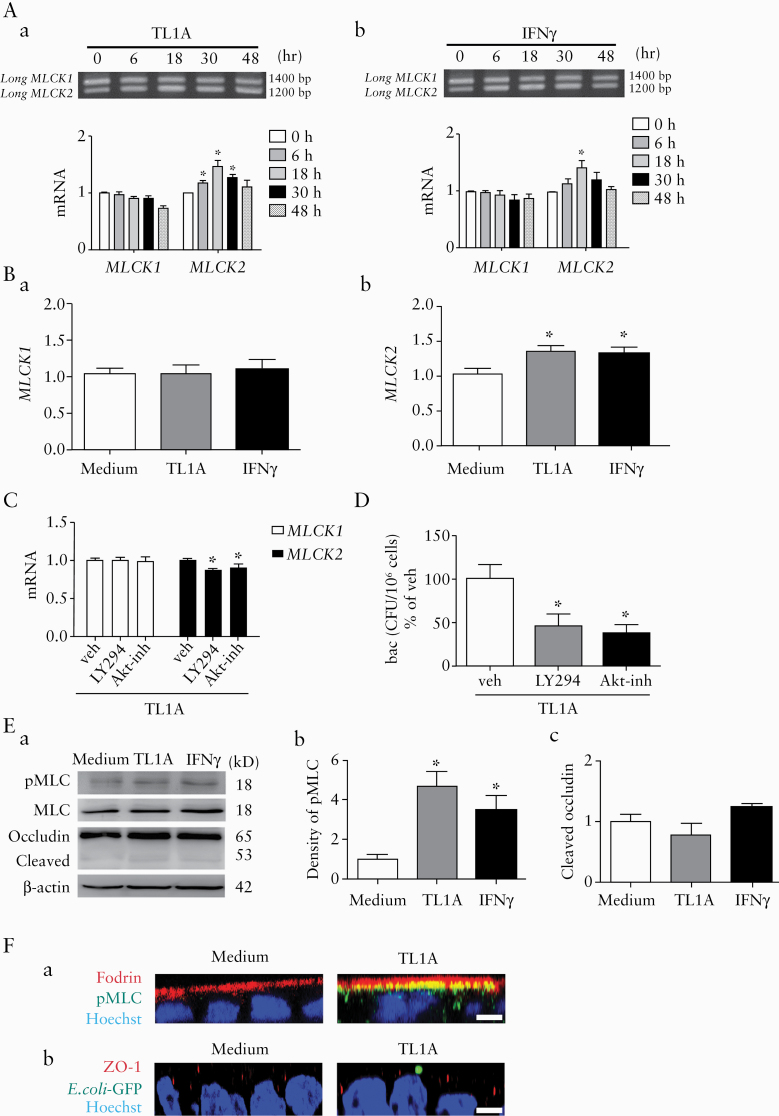
Two alternatively spliced variants of long MLCK encoded by a single MYLK gene were expressed by the intestinal epithelial cells. These long MLCK splice variants differ by a single exon, which is present only in MLCK1 but is missing in MLCK2.38,39 To evaluate the relative roles of these MLCK variants, the mRNA levels of MLCK1 and MLCK2 were determined in the cells after low-dose treatment with TL1A [10 ng/ml] and IFN [10 IU/ml] by semiquantitative PCR [Figure 6A] and quantitative real-time PCR [Figure 6B]. The treatment with low-dose cytokines upregulated the MLCK2 but not the MLCK1 transcripts [Figure 6A and B]. The cytokine-induced increase in MLCK2 transcripts and bacterial endocytosis were inhibited by LY294002 [a PI3K inhibitor] and Akt-inh [an Akt inhibitor] [Figure 6C and D], suggesting that PI3K/Akt was involved in the signalling pathways of MLCK2 upregulation. The western blotting results also confirmed the increase in total MLCK proteins after low-dose cytokine treatment, but the antibody used was not able to distinguish the proteins derived from the two splice variants [Supplementary Figure 4A, available as Supplementary data at ECCO-JCC online]. In addition, no difference in the expression levels of the MLC isoforms encoded by Myl12a or Myl12b was observed after cytokine treatment [Supplementary Figure 4B].
Figure 6.
TL1A increased the MLCK2 transcripts via PI3K/Akt pathways and phosphorylated the terminal web MLC to induce bacterial endocytosis. Human Caco-2 cells were treated with low doses of TL1A [10 ng/ml] and IFNγ [10 IU/ml] to assess changes in MLCK splice variants by semiquantitative and quantitative PCR. [A] The mRNA levels of MLCK1 and MLCK2 splice variants after treatment with low-dose cytokines, [a] TL1A and [b] IFNγ. Representative gel images of two independent experiments. n = 4/group. *p <0.05 v. 0 h. [B] The transcript levels of splice variants [a] MLCK1 and [b] MLCK2 following low-dose cytokine treatment, measured by real-time PCR. n = 6/group. *p <0.05 vs medium. [C] Pretreatment of LY294002 [a PI3K inhibitor] and Akt-inh [an Akt inhibitor] blocked the elevation of MLCK2 mRNA induced by low-dose TL1A. [D] Bacterial endocytosis induced by low-dose TL1A was prevented by LY294002 and Akt-inh. n = 5/group. *p <0.05 vs vehicle. [E] Western blots showing increased levels of phosphorylated MLC [pMLC] in the absence of occludin cleavage after low-dose cytokine treatment. [a] Representative immunoblot images. [b and c] Densitometric analysis of pMLC and cleaved occludin levels. n= 5/group. *p <0.05 vs medium. [F] Caco-2 cells treated with low-dose TL1A were exposed to a green fluorescent protein [GFP]-tagged bacteria to assess for subcellular structures and bacteria internalisation. [a] Confocal Z-axis serial imaging of TW fodrin [red], pMLC [green], and nuclear staining with a Hoechst dye [blue]. Co-localisation of pMLC with fodrin was observed in cells treated with TL1A but not medium. [b] Confocal Z-axis serial imaging of zonula occludens [ZO]-1 [red], E. coli-GFP [green], and cell nuclei [blue]. Presence of bacteria on apical region was observed in TL1A-treated cells without altering the orderly distribution of ZO-1 structure. Bar: 5 μm. Images were representative of three independent experiments. n = 3/group. The data were compared by ANOVA followed with Newman‐Keuls post-hoc test. When comparing the difference among groups of bacterial CFU, the Kruskal‐Wallis test was used. TW, terminal web.
Low-dose cytokine treatment increased MLC phosphorylation without changing the permeability or the occludin cleavage level in Caco-2 cells [Figure 6E]. The subcellular location of phosphorylated MLC [pMLC] in TL1A-treated cells was visualised by confocal microscopy. Immunostaining of fodrin [a TW protein] was diffusely distributed within the apical cytoplasm with no sign of pMLC in the medium-treated cells [Figure 6F]. Enhanced immunoreactivity to pMLC was found commensurate with the diffusely distributed fodrin in the cells treated with low-dose TL1A [Figure 6F], a finding similar that after low-dose IFNγ treatment, as reported in our previous study.30 The TL1A-treated cells were exposed to fluorescence-tagged non-pathogenic E. coli and stained for ZO-1 structures to verify the subcellular location of the bacteria. The ZO-1 stain was distributed in an orderly manner in a contiguous beaded pattern in the cells treated or not with low-dose TL1A [Figure 6F]. The fluorescent bacteria were observed in the apical region of the TL1A-treated cells between the perijunctional ZO-1 stained areas in the X-Z plane images [Figure 6F].
3.6. Divergent mechanisms of epithelial barrier defects were regulated by MLCK1 and MLCK2
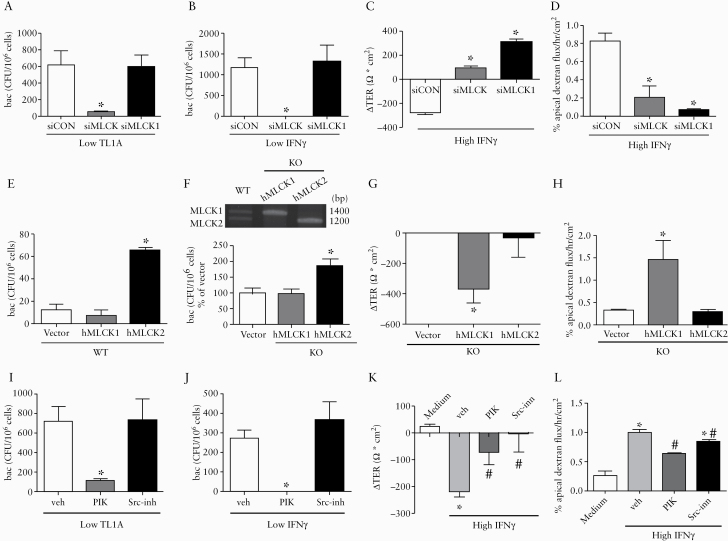
The alternative splice variants of MLCK involved in transcellular and paracellular permeability were evaluated using small interfering [si] RNA. Commercially available siRNA pools targeting all MLCK variants or MLCK1, and custom-designed siRNA strands targeting MLCK2, were tested in Caco-2 cells. The knockdown efficiency of siMLCK was verified by reduction in both the MLCK1 and MLCK2 transcripts [Supplementary Figure 5A, available as Supplementary data at ECCO-JCC online]. The specificity and silencing efficiency of siMLCK1 was also confirmed in the transfected cells, in which the siRNAs were designed to target exon 11 [207 bp] of the human MYLK gene in the full-length MLCK1, which is lacking in the MLCK2 variant [Supplementary Figure 5B and C]. However, none of the siRNAs targeting the bridging sequence connecting exons 10 and 12 in MLCK2 were able to silence the MLCK2 transcript [Supplementary Figure 5D] and therefore were not applied in further experiments. The bacterial endocytosis induced by TL1A and IFNγ at low doses was prevented by gene silencing of all MLCK variants but not MLCK1 alone [Figure 7A and B]. In contrast, the decreased TER level and augmented dextran flux triggered by high-dose IFNγ was inhibited by knocking down all MLCK variants or MLCK1 [Figure 7C and D].
Figure 7.
Bacterial endocytosis was dependent on MLCK2, whereas TJ disruption was mediated by Src/MLCK1. Caco-2 cells were gene-silenced to verify the individual roles of MLCK splice variants on transcellular and paracellular epithelial barrier defects. [A and B] Bacterial endocytosis induced by low-dose TL1A and IFNγ was decreased by knockdown of MLCK [including both variants] but not by gene silencing of MLCK1 alone. [C and D] The decrease of transepithelial electrical resistance [ΔTER] and increase of dextran flux caused by high-dose IFNγ were prevented by knockdown of MLCK or MLCK1. [E] Wild-type [WT] Caco-2 cells were transfected with plasmids encoding human MLCK1 or MLCK2. Overexpression of hMLCK2 but not hMLCK1 increased bacterial endocytosis compared with those transfected with a vector plasmid. [F] Caco-2 cells knock-out [KO] of MLCK gene were transfected with plasmids encoding human MLCK1 or MLCK2. Transfection efficiency was confirmed by mRNA levels [upper panel]. Expression of hMLCK2 but not hMLCK1 in the KO cells increased bacterial endocytosis [lower panel]. n = 6/group. *p <0.05 vs vector. [G and H] Expression of hMLCK1 but not hMLCK2 in the KO cells caused TER drop and increased dextran flux. n= 6/group. *p <0.05 v. vector. [I and J] Pretreatment with PIK [a specific MLCK inhibitor] but not Src-inh [a Src kinase inhibitor] attenuated the bacterial endocytosis by TL1A and IFNγ at low doses. n = 6/group. *p <0.05 vs vehicle. [K and L] Pretreatment with PIK and Src-inh blocked the changes in TER and dextran flux caused by high-dose IFNγ. n = 6/group. *p <0.05 vs medium. #p <0.05 vs vehicle. The data were compared by ANOVA followed with Newman‐Keuls post-hoc test. When comparing the difference among groups of bacterial CFU, the Kruskal‐Wallis test was used. TJ, tight junction.
To clarify the relative roles of the splice variants, wild-type [WT] Caco-2 cells were transfected with plasmids for human MLCK1 or MLCK2 overexpression before bacterial exposure. Higher intracellular bacterial counts were found in cells overexpressing hMLCK2 than hMLCK1, with the number in the hMLCK1 group similar to that in the vector group [Figure 7E]. Moreover, the Caco-2 cells in which total MLCK was deleted by CRISPR/Cas9-induced knockout [KO] techniques were transfected with plasmids carrying either hMLCK1 or hMLCK2, in a gain-of-function experiment. The KO cells that expressed only hMLCK2 showed an increase in bacterial endocytosis, whereas those expressing only hMLCK1 did not show this increase [Figure 7F]. In contrast, the KO cells expressing hMLCK1 but not hMLCK2 exhibited a decrease in TER accompanied by augmented dextran flux [Figure 7G and H].
The pharmacological blockade experiments showed that pretreatment with PIK [a specific MLCK inhibitor] but not Src-inh [a Src kinase inhibitor] decreased the number of bacteria endocytosed upon low-dose TL1A and IFNγ treatment [Figure 7I and J]. In contrast, the TER decrease and dextran flux enhancement triggered by high-dose IFNγ were prevented by PIK and Src-inh [Figure 7K and L].
Finally, the extent of bacterial uptake and the subcellular location of pMLC in the KO cells expressing either hMLCK1 or hMLCK2 were visualised by immunofluorescence staining. Fluorescent bacteria were observed inside the cells expressing hMLCK2 but not hMLCK1 [Figure 8A]. The pMLC stain was localised to different subcellular structures, ie, ZO-1 and fodrin, respectively, in the hMLCK1- and hMLCK2-expressing KO cells [Figure 8B and C]. Overall, the results indicated that MLCK1 and MLCK2 mediated the phosphorylation of MLC at TJ and TW sites, respectively, to regulate the paracellular and transcellular permeability of the epithelial cells.
Figure 8.
Phosphorylated MLC was localised to different subcellular sites after individually expressing MLCK1 and MLCK2 in transfected cells. Caco-2 cells knockout [KO] of MLCK gene were transfected with vector plasmids carrying human MLCK1 or MLCK2. [A] Transfected cells were exposed to GFP-tagged bacteria for visualisation of subcellular structures and bacterial internalisation. [a] Superimposed confocal X–Z images of cell nuclei by Hoechst staining [blue] merged with differential interference contrast images to show cell orientation and arc formation of BB. [b] E. coli-GFP [green, arrowheads] was observed inside the transfected cells expressing hMLCK2 but not hMLCK1. [c] Images of ZO-1 [red] and E. coli-GFP [green] with nuclear staining [blue] by focus stacking. Presence of intracellular bacteria [arrowheads] and orderly distribution of ZO-1 structures were observed after expression of hMLCK2 in cells. In contrast, absence of ZO-1 staining was noted in some area after expression of hMLCK1. Bar: 5 μm. [B] Images of ZO-1 [red] and pMLC [green] staining in cells. [a] The X–Y plane images showing honeycomb pattern of ZO-1 structures in vector-transfected and hMLCK2-expressing cells, but focal disruption or disappearance of ZO-1 was observed in hMLCK1-expressing cells. The pMLC staining was concentrated to junctional sites in the hMLCK1-expressing cells, whereas diffusely distributed pMLC staining was found in the hMLCK2-expressing cells. [b] Z-axis serial images of ZO-1 and pMLC merged with nuclei [blue] for cell orientation. Bar: 5 μm. [C] Representative images of fodrin [red] and pMLC [green] staining in cells. [a] The X–Y plane images showing diffusely distributed pMLC staining localised to TW fodrin in hMLCK2-expressing cells. [b] The Z-axis serial images of fodrin and pMLC merged with nuclei [blue] for cell orientation. Bar: 5 μm. Images were representative of three independent experiments. TW, terminal web; BB, brush border; GFP, green fluorescent protein.
4. Discussion
This study showed upregulation of cytokines TL1A and IFNγ and two MLCK splice variants in the colonic biopsy samples from IBD patients. A novel role for TL1A was observed in the induction of early-onset bacterial internalisation [uncoupled to TJ damage] prior to histopathological lesions. Bacterial internalisation was triggered by low-dose TL1A via the PI3K/Akt/MLCK2 pathway, and TJ opening was caused by high-dose IFNγ via the iNOS/Src/MLCK1 axis. Our data provide mechanistic details on how different cytokines regulate MLCK splice variants to regulate bacterial influx through transcellular and paracellular routes. The findings indicated that the intraepithelial passage of bacteria induced by TL1A may be an alternative route that leads to symptom flares in IBD.
Transcellular bacterial influx is a long-neglected aspect of the ‘leaky gut’, which often accompanies the junctional defects in epithelial cells. Differential regulatory mechanisms for the two forms of epithelial hyperpermeability have been poorly defined because most studies have examined changes at a single time point in a cross-sectional fashion, rather than through a longitudinal approach. Many studies have examined the mechanisms of TJ defects which are associated with epithelial cell death at late stages of colitis. Researchers, including those in our group and others, have previously demonstrated that bacteria are internalised through cholesterol-based lipid rafts on intermicrovillous clefts in a caveolin 1-dependent manner,30,42 although the intraepithelial bacterial counts in DSS-induced and TL1A-Tg mice [5–20 CFU per one million epithelial cells] were much lower than the in vitro cell line data following cytokine stimulation [≃1000 CFU per one million epithelial cells]. We suspect that the mixture of multiple cytokines in gut mucosa may impair the epithelial integrity, rendering partial permeation of gentamicin into the isolated epithelial cells which could result in an underestimation of intracellular bacterial counts in vivo. Overall, the temporal separation approach in our experimental models revealed that TL1A triggers MLCK-activated TW contraction and BB fanning, leading to bacterial internalisation in gut epithelial cells which could be distinguished from leakage through damaged TJs.
Distinct MLCK splice variants upregulated by cytokines exert divergent actions on the transcellular and paracellular barriers. Previous studies indicated that IFNγ/TNFα treatment induces the recruitment of MLCK1 to the perijunctional region and increases TJ permeability.38,39 Here, we showed that TL1A activated the PI3K/Akt signals and upregulated the expression of MLCK2, which mediated MLC phosphorylation at TW regions, leading to BB fanning and bacterial endocytosis in epithelial cells. Although the passage of macromolecules has also been documented on Peyer’s patches on M cells,51 these cells represent only a small fraction of the surface area compared with that of epithelium. The proinflammatory cytokine TL1A is a novel player in IBD pathogenesis and acts as a costimulatory factor for Th1/Th17 polarisation and IFNγ production.33–35 Here, the in vivo studies provided evidence that TL1A served as an initiating factor to stimulate transepithelial bacterial transcytosis, for which epithelial barrier loss preceded immune cell activation. Clinical studies indicated that a single nucleotide polymorphism in TL1A is linked to Crohn’s susceptibility.48,52,53 Taken together, these data suggest that TL1A may play a key role in the initiation and perpetuation of chronic inflammation through diverse actions on epithelial and immune cells.
This study demonstrated differential regulatory mechanisms of transcellular and paracellular permeability in gut epithelia. Numerous reports have shown that high-dose IFNγ [1000–3000 IU/ml] induces TJ disruption,24,29 and others have documented low-dose IFNγ [10–100 IU/ml] triggering an increase in bacterial transcytosis in epithelial cells.29,30,54 Perturbed mitochondrial function also caused internalisation of non-invasive bacteria into epithelial cell lines.55,56 Cytokine mixtures of IFNγ and TNFα increase paracellular permeability by upregulating TNFα receptor expression and TNFα-dependent MLCK promoter activity via AP1 and NFkB signalling.57,58 These in vitro findings depicting the individual modes of barrier loss have been difficult to recapitulate in animal models because of the gradual increase in cytokine levels following colitis induction. The approach of temporal separation of transcellular and paracellular defects in our mouse models helped unravel the divergent controls of the two pathways. The data further suggest that low-dose IFNγ upregulates MLCK2-dependent bacterial internalisation through a mechanism similar to that of TL1A, but that high-dose IFNγ caused TJ opening in a iNOS/Src/MLCK1-dependent manner. Previous reports also showed that Src kinase was activated downstream of IFNγ and nitric oxide stimuli.59,60 The phenomenon of iNOS production stimulated by IFNγ but not by TL1A explained the discrepancy in the ability of the two cytokines to control paracellular permeability.
The genetically modified mice and background wild-type mice used in this study were bred by homozygous parents, which harbours different gut microbiota. Further studies using heterozygous parents for breeding of knockout or transgenic mice to compare with wild types would be crucial to understand whether specific microbiota compositions also contribute to transepithelial bacterial penetration under inflammation. Recent studies implicated compositional changes in mucus microbiota or presence of dysbiotic bacteria as instigators of intestinal inflammation.61,62 Considering that opportunistic microbes may actively invade the epithelium, the possibility of pathobionts affecting our model was also discussed. Our animal model was based on wild-type mice harbouring a normal microflora for DSS-induced colitis, suggesting that the intraepithelial microbes observed at early time points [≃24 h] were passively endocytosed, as triggered by low-dose cytokine treatments. The bacterial taxa identified in the inflamed colonocytes mostly belong to the Firmicutes or Proteobacteria phylum, suggesting that the dominant microbiota may be either passively taken up or actively invade the host epithelial cells under proinflammatory stress. A recent study demonstrated that infliximab [a chimeric antibody to human TNFα] alleviated the transepithelial transport of adherent-invasive E. coli by blocking epithelial lipid rafts, suggesting that attenuation of bacterial endocytosis into epithelial cells partly contribute to its clinical efficacy in Crohn’s disease.63 Nevertheless, it cannot be ruled out that invasive pathobionts may have emerged from commensals by selective pressure for intracellular survival. The possibility of invasive pathobionts driving microbiota dysbiosis would also be worthy of investigation.64 In summary, this finding further supports that intraepithelial microbe penetration is an essential phenomenon underlying uncontrollable inflammation in IBD patients.
In conclusion, proinflammatory cytokines such as TL1A and IFNγ invoked bacterial internalisation before the onset of TJ damage and the appearance of histopathological lesions. Distinct splice variants of MLCK1 and MLCK2 were critical for regulation of paracellular and transcellular permeability. Based on our findings, first-line defence strategies can be developed to inhibit TL1A-mediated MLCK2-dependent bacterial transcytosis, which may serve as a therapeutic means to prevent inflammation relapse.
Funding
This study was funded by Ministry of Science and Technology [MoST 107-2320-B-002-041-MY3, 106-2320-B-002-017, 105-2320-B-002-063], National Health Research Institute [NHRI-EX108-10823BI, NHRI-EX109-10823BI, NHRI-EX110-10823BI], and National Taiwan University [NTU-CDP-105R7798, NTU-CCP-106R890504, NTU-CC-109L893102] to LCY, and National Institutes of Health [R01-DK068271] to JRT.
Confict of Interest
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
The authors thank the Animal Center, and the Imaging facility and Biomedical Resource Core of the First Core Laboratory of NTUCM, for the technical assistance. The authors thank the expertise of Dr Wei-Hao Peng, Graduate Institute of Anatomy and Cell Biology, NTUCM, for acquiring the transmission electron microscopic images. This article was subsidised by Ministry of Science and Technology and National Taiwan University [NTU], Taiwan.
Author Contributions
Guarantor of integrity of entire study, LCY; study concepts and design: LCY; data acquisition: YCP, LTW, SCW, LLW; data analysis/interpretation: YCP, LTW, SCW, LLW; statistical analysis: YCP, LTW; material and technical support: SCW, DWS, and JRT; obtained funding: LCY; manuscript drafting or revision for important intellectual content, literature research, manuscript editing, and manuscript final version approval: all authors.
References
- 1. Michielan A, D’Incà R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm 2015;2015:628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu LC Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: exploring a common ground hypothesis. J Biomed Sci 2018;25:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol 2005;43:3380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014;15:382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nazareth N, Magro F, Machado E, et al. Prevalence of Mycobacterium avium subsp. paratuberculosis and Escherichia coli in blood samples from patients with inflammatory bowel disease. Med Microbiol Immunol 2015;204:681–92. [DOI] [PubMed] [Google Scholar]
- 6. Pastor Rojo O, López San Román A, Albéniz Arbizu E, de la Hera Martínez A, Ripoll Sevillano E, Albillos Martínez A. Serum lipopolysaccharide-binding protein in endotoxemic patients with inflammatory bowel disease. Inflamm Bowel Dis 2007;13:269–77. [DOI] [PubMed] [Google Scholar]
- 7. Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology 2000;119:15–22. [DOI] [PubMed] [Google Scholar]
- 8. Shifrin DA Jr, McConnell RE, Nambiar R, Higginbotham JN, Coffey RJ, Tyska MJ. Enterocyte microvillus-derived vesicles detoxify bacterial products and regulate epithelial-microbial interactions. Curr Biol 2012;22:627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Danielsen EM, Hansen GH. Lipid rafts in epithelial brush borders: atypical membrane microdomains with specialized functions. Biochim Biophys Acta 2003;1617:1–9. [DOI] [PubMed] [Google Scholar]
- 10. Crawley SW, Mooseker MS, Tyska MJ. Shaping the intestinal brush border. J Cell Biol 2014;207:441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tyska MJ, Mackey AT, Huang JD, Copeland NG, Jenkins NA, Mooseker MS. Myosin-1a is critical for normal brush border structure and composition. Mol Biol Cell 2005;16:2443–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee TC, Huang YC, Lu YZ, Yeh YC, Yu LC. Hypoxia-induced intestinal barrier changes in balloon-assisted enteroscopy. J Physiol 2018;596:3411–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zareie M, Johnson-Henry K, Jury J, et al. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 2006;55:1553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang YJ, Pai YC, Yu LC. Host-microbiota interaction and intestinal epithelial functions under circadian control: implications in colitis and metabolic disorders. Chin J Physiol 2018;61:325–40. [DOI] [PubMed] [Google Scholar]
- 15. Lu C, Chen J, Xu HG, et al. MIR106B and MIR93 prevent removal of bacteria from epithelial cells by disrupting ATG16L1-mediated autophagy. Gastroenterology 2014;146:188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sadaghian Sadabad M, Regeling A, de Goffau MC, et al. The ATG16L1-T300A allele impairs clearance of pathosymbionts in the inflamed ileal mucosa of Crohn’s disease patients. Gut 2015;64:1546–52. [DOI] [PubMed] [Google Scholar]
- 17. Swidsinski A, Ladhoff A, Pernthaler A, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology 2002;122:44–54. [DOI] [PubMed] [Google Scholar]
- 18. Yamamoto-Furusho JK, Mendivil EJ, Mendivil-Rangel EJ, Fonseca-Camarillo G. Differential expression of occludin in patients with ulcerative colitis and healthy controls. Inflamm Bowel Dis 2012;18:E1999. [DOI] [PubMed] [Google Scholar]
- 19. Zeissig S, Bürgel N, Günzel D, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 2007;56:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest 2006;86:191–201. [DOI] [PubMed] [Google Scholar]
- 21. Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol 2005;166:409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Su L, Nalle SC, Shen L, et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology 2013;145:407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu LL, Chiu HD, Peng WH, et al. Epithelial inducible nitric oxide synthase causes bacterial translocation by impairment of enterocytic tight junctions via intracellular signals of Rho-associated kinase and protein kinase C zeta. Crit Care Med 2011;39:2087–98. [DOI] [PubMed] [Google Scholar]
- 24. Eun CS, Kim YS, Han DS, Choi JH, Lee AR, Park YK. Lactobacillus casei prevents impaired barrier function in intestinal epithelial cells. APMIS 2011;119:49–56. [DOI] [PubMed] [Google Scholar]
- 25. Wang F, Schwarz BT, Graham WV, et al. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology 2006;131:1153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gitter AH, Bendfeldt K, Schulzke JD, Fromm M. Leaks in the epithelial barrier caused by spontaneous and TNF-alpha-induced single-cell apoptosis. FASEB J 2000;14:1749–53. [DOI] [PubMed] [Google Scholar]
- 27. Sappington PL, Han X, Yang R, Delude RL, Fink MP. Ethyl pyruvate ameliorates intestinal epithelial barrier dysfunction in endotoxemic mice and immunostimulated caco-2 enterocytic monolayers. J Pharmacol Exp Ther 2003;304:464–76. [DOI] [PubMed] [Google Scholar]
- 28. Smyth D, Phan V, Wang A, McKay DM. Interferon-γ-induced increases in intestinal epithelial macromolecular permeability requires the Src kinase Fyn. Lab Invest 2011;91:764–77. [DOI] [PubMed] [Google Scholar]
- 29. Clark E, Hoare C, Tanianis-Hughes J, Carlson GL, Warhurst G. Interferon gamma induces translocation of commensal Escherichia coli across gut epithelial cells via a lipid raft-mediated process. Gastroenterology 2005;128:1258–67. [DOI] [PubMed] [Google Scholar]
- 30. Wu LL, Peng WH, Kuo WT, et al. Commensal bacterial endocytosis in epithelial cells is dependent on myosin light chain kinase-activated brush border fanning by interferon-γ. Am J Pathol 2014;184:2260–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prehn JL, Thomas LS, Landers CJ, Yu QT, Michelsen KS, Targan SR. The T cell costimulator TL1A is induced by FcgammaR signaling in human monocytes and dendritic cells. J Immunol 2007;178:4033–8. [DOI] [PubMed] [Google Scholar]
- 32. Shih DQ, Kwan LY, Chavez V, et al. Microbial induction of inflammatory bowel disease associated gene TL1A [TNFSF15] in antigen presenting cells. Eur J Immunol 2009;39:3239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takedatsu H, Michelsen KS, Wei B, et al. TL1A [TNFSF15] regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology 2008;135:552–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kamada N, Hisamatsu T, Honda H, et al. TL1A produced by lamina propria macrophages induces Th1 and Th17 immune responses in cooperation with IL-23 in patients with Crohn’s disease. Inflamm Bowel Dis 2010;16:568–75. [DOI] [PubMed] [Google Scholar]
- 35. Jin S, Chin J, Seeber S, et al. TL1A/TNFSF15 directly induces proinflammatory cytokines, including TNFα, from CD3+CD161+ T cells to exacerbate gut inflammation. Mucosal Immunol 2013;6:886–99. [DOI] [PubMed] [Google Scholar]
- 36. Lee SH, Kim EJ, Suk K, Kim IS, Lee WH. TL1A induces the expression of TGF-β-inducible gene h3 [βig-h3] through PKC, PI3K, and ERK in THP-1 cells. Cell Immunol 2010;266:61–6. [DOI] [PubMed] [Google Scholar]
- 37. Mascarenhas JB, Tchourbanov AY, Danilov SM, Zhou T, Wang T, Garcia JGN. The splicing factor hnRNPA1 regulates alternate splicing of the MYLK gene. Am J Respir Cell Mol Biol 2018;58:604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clayburgh DR, Rosen S, Witkowski ED, et al. A differentiation-dependent splice variant of myosin light chain kinase, MLCK1, regulates epithelial tight junction permeability. J Biol Chem 2004;279:55506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Graham WV, He W, Marchiando AM, et al. Intracellular MLCK1 diversion reverses barrier loss to restore mucosal homeostasis. Nat Med 2019;25:690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barrett R, Zhang X, Koon HW, et al. Constitutive TL1A expression under colitogenic conditions modulates the severity and location of gut mucosal inflammation and induces fibrostenosis. Am J Pathol 2012;180:636–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shih DQ, Barrett R, Zhang X, et al. Constitutive TL1A [TNFSF15] expression on lymphoid or myeloid cells leads to mild intestinal inflammation and fibrosis. PLoS One 2011;6:e16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu LC, Shih YA, Wu LL, et al. Enteric dysbiosis promotes antibiotic-resistant bacterial infection: systemic dissemination of resistant and commensal bacteria through epithelial transcytosis. Am J Physiol Gastrointest Liver Physiol 2014;307:G824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang CY, Huang CY, Pai YC, et al. Glucose metabolites exert opposing roles in tumor chemoresistance. Front Oncol 2019;9:1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang CY, Yu LC. Distinct patterns of interleukin-12/23 and tumor necrosis factor α synthesis by activated macrophages are modulated by glucose and colon cancer metabolites. Chin J Physiol 2020;63:7–14. [DOI] [PubMed] [Google Scholar]
- 45. Kuo WT, Lee TC, Yang HY, et al. LPS receptor subunits have antagonistic roles in epithelial apoptosis and colonic carcinogenesis. Cell Death Differ 2015;22:1590–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kuo WT, Lee TC, Yu LC. Eritoran suppresses colon cancer by altering a functional balance in toll-like receptors that bind lipopolysaccharide. Cancer Res 2016;76:4684–95. [DOI] [PubMed] [Google Scholar]
- 47. Owens SE, Graham WV, Siccardi D, Turner JR, Mrsny RJ. A strategy to identify stable membrane-permeant peptide inhibitors of myosin light chain kinase. Pharm Res 2005;22:703–9. [DOI] [PubMed] [Google Scholar]
- 48. Jacob N, Jacobs JP, Kumagai K, et al. Inflammation-independent TL1A-mediated intestinal fibrosis is dependent on the gut microbiome. Mucosal Immunol 2018;11:1466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chavez AM, Menconi MJ, Hodin RA, Fink MP. Cytokine-induced intestinal epithelial hyperpermeability: role of nitric oxide. Crit Care Med 1999;27:2246–51. [DOI] [PubMed] [Google Scholar]
- 50. Vignoli AL, Srivastava RC, Stammati A, Turco L, Tanori M, Zucco F. Nitric oxide production in Caco-2 cells exposed to different inducers, inhibitors and natural toxins. Toxicol In Vitro 2001;15:289–95. [DOI] [PubMed] [Google Scholar]
- 51. Hollander D, Kaunitz JD. The “leaky gut”: tight junctions but loose associations? Dig Dis Sci 2020;65:1277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tung CC, Wong JM, Lee WC, et al. Combining TNFSF15 and ASCA IgA can be used as a predictor for the stenosis/perforating phenotype of Crohn’s disease. J Gastroenterol Hepatol 2014;29:723–9. [DOI] [PubMed] [Google Scholar]
- 53. Wei SC, Ni YH, Yang HI, et al. A hospital-based study of clinical and genetic features of Crohn’s disease. J Formos Med Assoc 2011;110:600–6. [DOI] [PubMed] [Google Scholar]
- 54. McKay DM, Watson JL, Wang A, et al. Phosphatidylinositol 3’-kinase is a critical mediator of interferon-gamma-induced increases in enteric epithelial permeability. J Pharmacol Exp Ther 2007;320:1013–22. [DOI] [PubMed] [Google Scholar]
- 55. Lewis K, Lutgendorff F, Phan V, Söderholm JD, Sherman PM, McKay DM. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm Bowel Dis 2010;16:1138–48. [DOI] [PubMed] [Google Scholar]
- 56. Wang A, Keita ÅV, Phan V, et al. Targeting mitochondria-derived reactive oxygen species to reduce epithelial barrier dysfunction and colitis. Am J Pathol 2014;184:2516–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Graham WV, Wang F, Clayburgh DR, et al. Tumor necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signaling events. Characterization of the human long myosin light chain kinase promoter. J Biol Chem 2006;281:26205–15. [DOI] [PubMed] [Google Scholar]
- 58. Ye D, Ma TY. Cellular and molecular mechanisms that mediate basal and tumour necrosis factor-alpha-induced regulation of myosin light chain kinase gene activity. J Cell Mol Med 2008;12:1331–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chang YJ, Holtzman MJ, Chen CC. Interferon-gamma-induced epithelial ICAM-1 expression and monocyte adhesion. Involvement of protein kinase C-dependent c-Src tyrosine kinase activation pathway. J Biol Chem 2002;277:7118–26. [DOI] [PubMed] [Google Scholar]
- 60. Maa MC, Chang MY, Chen YJ, et al. Requirement of inducible nitric-oxide synthase in lipopolysaccharide-mediated Src induction and macrophage migration. J Biol Chem 2008;283:31408–16. [DOI] [PubMed] [Google Scholar]
- 61. Glymenaki M, Singh G, Brass A, et al. Compositional changes in the gut mucus microbiota precede the onset of colitis-induced inflammation. Inflamm Bowel Dis 2017;23:912–22. [DOI] [PubMed] [Google Scholar]
- 62. Keita ÅV, Alkaissi LY, Holm EB, et al. Enhanced E. coli LF82 translocation through the follicle-associated epithelium in Crohn’s disease is dependent on long polar fimbriae and CEACAM6 expression, and increases paracellular permeability. J Crohns Colitis 2020;14:216–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yakymenko O, Schoultz I, Gullberg E, et al. Infliximab restores colonic barrier to adherent-invasive E. coli in Crohn’s disease via effects on epithelial lipid rafts. Scand J Gastroenterol 2018;53:677–84. [DOI] [PubMed] [Google Scholar]
- 64. Yu LC Commensal bacterial internalisation by epithelial cells: an alternative portal for gut leakiness. Tissue Barriers 2015;3:e1008895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.