Abstract
Background:
Converging lines of evidence point to hippocampal dysfunction in schizophrenia. It is thought that hippocampal dysfunction spreads across hippocampal subfields and to cortical regions by way of long-range efferent projections. Importantly, abnormalities in the excitation/inhibition balance could impair the long-range modulation of neural networks.
The goal of this project was twofold. First, we sought to identify replicable patterns of hippocampal dysconnectivity in patients with a psychosis spectrum disorder, and second we aimed to investigate a putative link between glutamatergic metabolism and hippocampus connectivity alterations.
Methods:
We evaluated resting state hippocampal functional connectivity alterations in two cohorts of patients with a psychosis spectrum disorder: the first consisted of 55 medication-naïve first episode psychosis (FEP) subjects and 41 matched healthy controls (HC), and the second consisted of 42 unmedicated patients with schizophrenia and 41 matched controls. We also acquired measurements of glutamate + glutamine (Glx) in the left hippocampus using magnetic resonance spectroscopy for 42 FEP and 37 HC from our first cohort.
Results:
We observed a pattern of hippocampal functional hypoconnectivity to regions of the default mode network (DMN) and hyperconnectivity to lateral occipital cortex in both cohorts. We also show that, in HC, greater hippocampal Glx levels predicted greater hippocampal functional connectivity to the anterior DMN. Furthermore, this relationship was reversed in medication-naïve FEP subjects.
Conclusion:
These results suggest that an alteration in the relationship between glutamate and functional connectivity may disrupt the dynamic of major neural networks.
Keywords: psychosis, first episode, resting state functional connectivity, hippocampus, default mode network, magnetic resonance spectroscopy, glutamate
INTRODUCTION
Converging lines of evidence from cognitive assessments (1–4), multimodal brain imaging (5–8) and postmortem (9, 10) studies point to hippocampal dysfunction in schizophrenia. Meta-analyses show reduced hippocampal volume in patients with chronic schizophrenia (11, 12) and in first episode psychosis (FEP) patients (13), suggesting the presence of hippocampal pathology across illness stages. There is evidence that hippocampal dysfunction begins in the CA1 region (5, 6, 14) followed by a spread to other hippocampal subfields (6, 14, 15) and to cortical regions by way of long-range efferent projections (15).
Resting state functional connectivity (FC) measures the temporal coherence of spontaneous neural activity between brain regions (16, 17), allowing for the identification of large-scale networks that interact in a well-characterized manner (18). A pattern of dysconnectivity of the hippocampus to a number of cortical regions that comprise one of the major large-scale brain networks, the default mode network (DMN), has been reported in patients with psychosis spectrum disorders (19–23), which is consistent with the idea that hippocampal dysfunction extends to cortical regions.
A possible mechanism underlying this dysfunction is a disruption in the excitation/inhibition balance due to N-methyl-D-aspartate (NMDA) receptor hypofunction on GABA interneurons (24–26), which are tasked with the regulation of easily excitable glutamatergic neurons (27, 28). This is especially relevant for the hippocampus, as its pyramidal layers are tightly packed with glutamatergic neurons which have a low firing threshold, ensuring a high level of neuroplasticity (28). Using proton magnetic resonance spectroscopy (MRS), we previously reported elevated glutamate levels in the hippocampus in unmedicated patients with schizophrenia (SZ) (22), consistent with a disruption in the excitation/inhibition balance. Furthermore, two studies, including ours, have identified an association between glutamate measured in the anterior cingulate cortex (ACC) and the blood oxygen level dependent (BOLD) response in posterior regions of the DMN, an association that was reversed in patients with schizophrenia (29–31). These results support the role of glutamate in the long-range modulation of neural networks, as well as the proposition that this modulation is impaired in patients with schizophrenia.
The goal of this project was twofold. First, we sought to identify patterns of hippocampal dysconnectivity in patients with a psychosis spectrum disorder, and second we aimed to investigate the role of glutamatergic metabolism in hippocampus connectivity alterations. We evaluated hippocampal functional connectivity in two cohorts of subjects with psychosis spectrum disorders: a cohort of medication-naïve FEP and matched healthy controls (HC), and a cohort of unmedicated SZ and matched HC. In the first cohort, we also measured a marker of glutamatergic metabolism, glutamate + glutamine (Glx), with MRS.
Based on our previous work, we hypothesized that we would identify decreased hippocampal FC in regions of the DMN in both cohorts. We further hypothesized that we would find evidence of excess Glx in medication naïve FEP and that the relationship between hippocampal functional connectivity and hippocampal Glx would be altered.
METHODS
Subjects
Here, we included data from two independent cohorts of patients with schizophrenia spectrum disorders recruited from the emergency room, inpatient units, and outpatient clinics at the University of Alabama at Birmingham (UAB). The first group consisted of antipsychotic drug-naïve FEP with no more than five days of lifetime antipsychotic medication exposure, the second group consisted of SZ who were off antipsychotic medications for at least two weeks at the time of enrolment. The UAB Institutional Review Board gave approval for these studies. Written informed consent was obtained prior to enrolment and after subjects were deemed to have capacity to provide consent (32).
Exclusion criteria were major neurological or medical conditions, a history of head trauma with loss of consciousness, substance use disorders (excluding nicotine [and cannabis in the first cohort only]) within one month of imaging, pregnancy or breastfeeding, or MRI contraindications. Diagnoses were established by review of medical records and consensus of two board certified psychiatrists (ACL and NVK). The Brief Psychiatric Rating Scale (BPRS) was used to assess symptom severity (33). Cognitive function was characterized using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (34).
We also recruited healthy controls (HC) for each cohort who were matched on age, gender, and parental socioeconomic status (SES). In addition to above outlined criteria, HCs with a personal history or a family history of a psychiatric illness in a first-degree relative were also excluded.
Data acquisition parameters
Dataset 1
Participants were scanned on a whole-body 3T Siemens MAGNETOM Prisma MRI scanner using a 20 channel head coil.
Anatomical scans were acquired via a T1-weighted MPRAGE (TR/TE = 2400/2.22 ms, flip angle 8°, 0.8 mm isotropic voxels).
Two resting state scans were acquired in opposing phase encoding directions (A > P and P > A; TR/TE = 1550/37.80 ms, flip angle = 71°, 2 mm isotropic voxels, 72 axial slices, 225 acquisitions in each direction). During the scan, subjects were instructed to keep their eyes open and stare passively ahead.
After placing a spectroscopy voxel in the left hippocampus (voxel size: 15 × 27 ×10 mm), we used automated and manual shimming to optimize field homogeneity across the voxel and suppressed the water signal with chemical shift selective (CHESS). Data were acquired with a PRESS sequence (TE/TR = 80/2000 ms; flip angle = 90°, vector size 1024, 192 averages; these parameters optimize the glutamate signal and minimize macromolecule contribution (35)) followed by 8 averages of unsuppressed water scans for reference.
Dataset 2
Participants were scanned on a head-only 3T Siemens MAGNETOM Allegra MRI scanner with a circularly polarized transmit/receive head coil.
Anatomical scans were acquired via a T1-weighted MPRAGE (TR/TE = 2300/3.93 ms, flip angle = 12°, 1 mm isotropic voxels).
Resting state scans were acquired with a 5-minute gradient recalled echo-planar imaging sequence (TR/TE = 2000/30 ms, flip angle = 70°, 6-mm slice thickness, 1 mm gap, 30 axial slices, 225 acquisitions). During the scan, subjects were instructed to keep their eyes open and stare passively ahead.
Data preprocessing
Resting state fMRI
We removed the first ten frames separately for each run in dataset 1 to allow for signal equilibration and then merged data acquired in opposite phase encoding directions as well as field inhomogeneity correction with FSL’s topup (36). Then, for both datasets, data were slice timing corrected, realigned, co-registered, normalized, and spatially smoothed with a 6mm Gaussian kernel in the CONN toolbox version 18a (37). Physiological and other spurious sources of noise were estimated with aCompcor, and the first five principal temporal components each from white matter and CSF were removed. No global signal regression was performed. Residual data was band-pass filtered (0.008 < f < 0.08 Hz).
Motion outliers detected by the artifact detection (ART) toolbox were censored (framewise displacement > .9 mm and mean global signal change > 5), and excluded from analyses. The entire time series was excluded if > 50% of frames were contaminated by movement, or if mean framewise displacement exceeded 0.9 mm.
The first eigenvariate of the BOLD time series from a left hippocampus seed region, defined by the AAL atlas, was extracted and correlated to the time series of all other voxels to create seed-to-voxel correlation maps for each subject (units of Pearson’s r correlation). Maps were then converted to normally distributed values using Fisher’s r-to-z transform.
MRS Data
Spectra were analyzed in LCModel (version 6.3–1N) (38) using a standard basis set that included alanine, aspartate, ascorbate, creatine (Cr), GABA, glucose, glutamine (Gln), glutamate (Glu), glycerophospho-choline (GPC), glutathione (GSH), myo-Inositol (mI), scyllo-Inositol, lactate, phosphocreatine (PCr), phosphocholine (PCh), phosphorylethanolamine, N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG), and taurine. Spectra were fitted between 0.2 and 4.0 ppm, using baseline corrections. Fitted spectra were visually inspected for the presence of residual water, lipid contamination, and spurious echoes.
We segmented the anatomical image into grey matter, white matter and cerebrospinal fluid. Here, we quantified Glx, referenced to internal water, in institutional units (IU) and corrected measurements for partial volume effects according to Gasparovic and colleagues including T1 and T2 relaxation times (39, 40).
Spectra were excluded from analyses if the signal to noise ratio (SNR) was < 3 or full width at half maximum (FWHM) of the NAA peak was > 0.1 ppm (41), or if the fit of the spectrum was more than +/− 2 SD outside mean of the absolute CRLB of our control sample (42).
Statistical analysis
Group-level functional connectivity maps for each subject were obtained by performing one-sample t-tests on each group’s participant-level functional connectivity maps. Group differences (between FEP and matched HC in dataset 1, and between SZ and matched HC in dataset 2) in functional connectivity were assessed using two-sample t-tests on the groups’ participant-level functional network connectivity maps. Regression analyses were performed to assess the relationship between resting state connectivity and Glx in dataset 2 for each group separately. This was followed by a whole-brain regression analysis to test for the interaction between group, hippocampal connectivity, and Glx. All analyses were corrected using voxel (p < 0.05, uncorrected) and cluster level correction (p < 0.05, FDR corrected). Age, sex, and framewise displacement (also see Table 1) were entered into the models as covariates of no interest as appropriate.
Table 1.
Demographics, clinical measures, and covariates1
| Data Set 1 | ||||
|---|---|---|---|---|
| FEP (n = 55) | HC (n = 41) | t/χ 2 | p value | |
| Gender (%male) | 65.4 | 65.8 | 0.002 | .968 |
| Age | 24.18 (6.27) | 24.73 (6.29) | 0.425 | .672 |
| Socioeconomic status2 | 5.80 (4.83)3 | 4.12 (4.03) | 12.349 | .499 |
| Smoking (packs per day) | 0.23 (0.34)4 | 0.03 (0.10)4 | −4.020 | < .001 |
| BPRS Total | 51.64 (11.89) | |||
| Positive | 11.78 (3.47) | |||
| Negative | 6.20 (3.36) | |||
| RBANS5 Total | 75.67 (15.04) | 94.83 (11.14) | 6.368 | < .001 |
| Immediate Memory | 83.02 (18.37) | 102.11 (16.17) | 4.914 | < .001 |
| Visuospatial/Constructional | 76.40 (17.20) | 84.94 (13.85) | 2.421 | .018 |
| Language | 83.73 (16.21) | 99.46 (15.17) | 4.483 | < .001 |
| Attention | 81.56 (16.38) | 102.57 (17.49) | 5.608 | < .001 |
| Delayed Memory | 79.69 (13.26) | 93.11 (9.28) | 5.139 | < .001 |
| Resting state fMRI | ||||
| % of volumes retained after scrubbing | 93.77 (6.97) | 97.32 (3.63) | −3.232 | .002 |
| Framewise displacement (mm) | 0.32 (0.19) | 0.22 (0.10) | −3.279 | .002 |
| MRS6 | ||||
| Glx | 10.87 (2.56) | 10.19 (1.42) | −1.492 | .140 |
| NAA | 15.61 (1.19) | 15.91 (1.50) | 1.003 | .319 |
| Signal to noise ratio | 9.40 (1.70) | 9.73 (1.52) | 0.923 | .359 |
| Full width at half maximum | 0.06 (0.01) | 0.06 (0.00) | −1.787 | .078 |
| Glx Cramér–Rao lower bound | 14.41 (4.37) | 13.92 (3.84) | −0.523 | .602 |
| NAA Cramér–Rao lower bound | 1.96 (0.58) | 2.00 (0.38) | 1.003 | .319 |
| Voxel gray matter content | 60.21 (4.04) | 59.43 (4.79) | −0.790 | .432 |
| Voxel white matter content | 35.25 (5.05) | 36.01 (5.65) | 0.635 | .527 |
| Data Set 2 | ||||
| SZ (n = 42) | HC (n = 41) | t/χ 2 | p value | |
| Gender (%male) | 71.4 | 75.6 | .186 | .666 |
| Age | 27.64 (9.61) | 28.73 (9.37) | .522 | .603 |
| Socioeconomic status2 | 6.62 (5.81)3 | 5.27 (4.23) | −15.790 | .261 |
| Smoking (packs per day) | 0.32 (0.48) | 0.26 (0.47) | −.534 | .595 |
| APD naïve (yes/ no) | 27/15 | |||
| BPRS Total | 49.67 (9.18) | |||
| Positive | 9.74 (3.49) | |||
| Negative | 7.52 (9.18) | |||
| RBANS5 Total | 70.70 (14.87) | 91.61 (13.20) | 6.697 | < .001 |
| Immediate Memory | 77.90 (17.06) | 97.39 (14.33) | 5.573 | < .001 |
| Visuospatial/Constructional | 70.28 (15.81) | 84.37 (17.73) | 3.772 | < .001 |
| Language | 83.65 (11.67) | 98.78 (15.10) | 5.038 | < .001 |
| Attention | 77.10 (21.91) | 95.80 (18.51) | 4.155 | < .001 |
| Delayed Memory | 73.83 (20.22) | 92.71 (9.30) | 5.378 | < .001 |
| Resting state fMRI | ||||
| % of volumes retained after scrubbing | 93.11 (7.64) | 96.96 (3.81) | −2.919 | .005 |
| Framewise displacement (mm) | 0.29 (0.14) | 0.36 (.19) | −1.949 | .055 |
Abbreviations: FEP first episode psychosis patients; SZ patients with schizophrenia; HC healthy controls; APD antipsychotic drug; NAA N-acetylaspartate; BPRS Brief Psychiatric Rating Scale (positive subscale included conceptual disorganization, hallucinatory behavior, and unusual thought content; negative subscale included emotional withdrawal, motor retardation, and blunted affect); RBANS Repeatable Battery for the Assessment of Neuropsychological Status; MRS magnetic resonance spectroscopy; Glx glutamate + glutamine; NAA N-acetylaspartate; fMRI functional magnetic resonance imaging.
Mean (standard deviation) unless indicated otherwise.
Parental socioeconomic ranks determined from Diagnostic Interview for Genetic Studies (1–18 scale); higher rank (lower numerical value) corresponds to higher socioeconomic status.
Data Set 1: Data not available for 10 FEP subjects; n=45; Data Set 2: Data not available for 2 SZ subjects; n=39
Data Set 1: Data not available for 4 FEP subjects or 2 HC; FEP n=51, HC n = 39.
Data Set 1: Data not available for 7 FEP subjects or 6 HC; FEP n=48, HC n = 35; Data Set 2: Data not available for 1 SZ subject; SZ n=40.
Data not available for 12 FEP subjects or 4 HC; FEP n=43, HC n = 37
In dataset 1, 58 FEP and 41 HC completed the resting state portion of the scan, and 50 FEP and 38 HC completed the MRS portion of the scan. A total of 3 resting state datasets (3 FEP and 0 HC) were excluded, and 8 spectra (7 FEP and 1 HC) were excluded from final analysis due to poor data quality. In dataset 2, 46 SZ and 41 HC completed the resting state scan. A total of 4 resting state datasets were excluded (4 SZ and 0 HC).
Overall, final analyses included 55 FEP and 41 HC in dataset 1 and 42 SZ and 41 HC in dataset 2 for group comparisons in hippocampal connectivity, as well as 43 FEP and 37 HC in dataset 1 for examining the relationships between hippocampal connectivity and Glx.
RESULTS
Demographics and Clinical Data
No significant group differences were observed for gender, age, or parental SES in either dataset. As expected, HC scored higher on the RBANS (including all subscales) than patients in both datasets. All demographic and clinical measures can be found in Table 1.
Hippocampal Connectivity
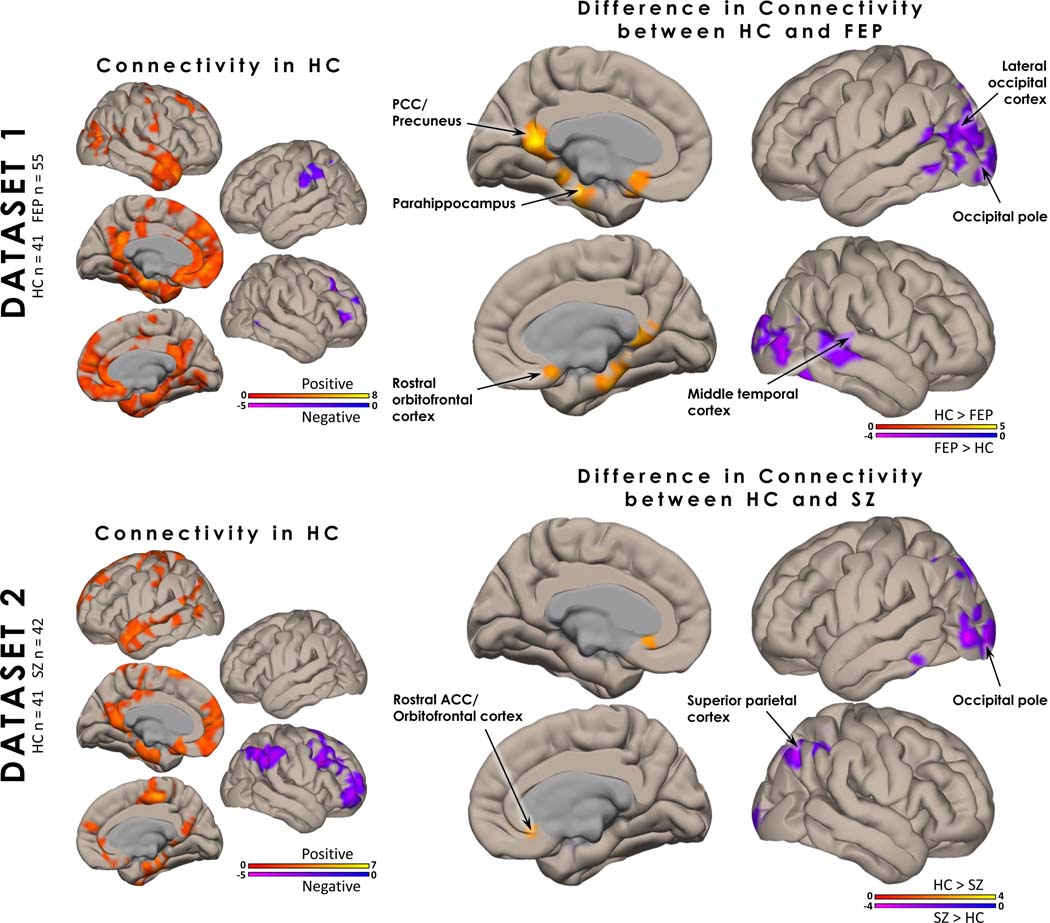
In both datasets controls showed consistent patterns of positive hippocampal functional connectivity to regions of the DMN, including the posterior cingulate cortex (PCC)/precuneus, medial prefrontal cortex (mPFC), parahippocampus, superior temporal gyrus and temporal pole, as well as negative hippocampal connectivity to the dorsolateral prefrontal cortex, the precuneus and supramarginal gyrus (Figure 1; Table 2).
Figure 1. Hippocampal Functional Connectivity:
In healthy controls (HC), hippocampal functional connectivity (FC) from datasets 1 and 2 showed consistent patterns of positive hippocampal functional connectivity to regions of the default mode network, including the posterior cingulate cortex (PCC)/precuneus, medial prefrontal cortex (mPFC), parahippocampus, superior temporal gyrus and temporal pole. Negative FC patterns were seen in the dorsolateral PFC and supramarginal gyrus. Group differences in FC between medication-naïve first episode psychosis patients and HC (dataset 1) were seen in the PCC/precuneus, mPFC, parahippocampus, visual cortex, lateral PFC, and middle temporal cortex. Group differences between the unmedicated patients with schizophrenia and HC (dataset 2) were seen in more restricted, but overlapping regions. All analyses were corrected using voxel (p < 0.05, uncorrected) and cluster level correction (p < 0.05, FDR corrected). Age, sex, and mean framewise displacement were included as covariates of no interest.
Table 2:
Clusters of Significance
| Analysis | Brain regions | Voxels in cluster | Hempishpere | Voxels in regiona | Peak coordinatesb | Peak t | P FDR c | ||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||||
| Hippocampal Connectivity | |||||||||
| Data Set 1 | |||||||||
| HC>FEP | |||||||||
| Cluster 1 | 5696 | −14 | −22 | −22 | 6.14 | ||||
| Thalamus | B | 511 | |||||||
| Parahippocampus | B | 477 | |||||||
| Hippocampus | B | 382 | |||||||
| Precuneus | B | 362 | |||||||
| PCC | B | 256 | |||||||
| mPFC/ACC (subcallosal) | B | 193 | |||||||
| Fusiform Gyri | B | 149 | |||||||
| Orbitofrontal Cortex | L | 110 | |||||||
| Nucleus Accumbens | B | 127 | |||||||
| Pallidum | B | 78 | |||||||
| Putamen | B | 86 | |||||||
| FEP>HC | |||||||||
| Cluster 1 | 8566 | −22 | −66 | −4 | −5.26 | ||||
| Lateral Occipital Cortex | B | 1855 | |||||||
| Occiptal Pole | B | 1248 | |||||||
| Fusiform Gyri | B | 787 | |||||||
| Lingual Gyri | B | 638 | |||||||
| Middle Temporal Cortex | B | 589 | |||||||
| Calcarine Sulcus | B | 484 | |||||||
| Precuneus | B | 269 | |||||||
| Angular Gyrus | L | 222 | |||||||
| Fusiform Gyri | B | 212 | |||||||
| Cuneus | B | 201 | |||||||
| Supramarginal Gyri | B | 74 | |||||||
| Data Set 2 | |||||||||
| HC>SZ | |||||||||
| Cluster 1 | 2971 | 24 | 32 | 8 | 6.08 | ||||
| Frontal Pole | R | 149 | |||||||
| mPFC/ACC (subcallosal) | B | 127 | |||||||
| SZ>HC | |||||||||
| Cluster 1 | 2608 | −44 | −84 | −4 | −7.60 | ||||
| Occiptial Pole | B | 858 | |||||||
| Lateral Occiptal Cortex | B | 802 | |||||||
| Lingual Gyrus | B | 156 | |||||||
| Precuneus | B | 127 | |||||||
| Superior Parietal Cortex | L | 102 | |||||||
| Cluster 2 | 1750 | −44 | −52 | −30 | −5.37 | ||||
| Inferior Temporal Cortex | L | 98 | |||||||
| Cluster 3 | 1601 | 32 | −64 | 44 | −4.82 | ||||
| Lateral Occipital Cortex | R | 897 | |||||||
| Angular Gyrus | R | 201 | |||||||
| Supramarginal Gyrus | R | 174 | |||||||
| Superior Parietal Cortex | R | 151 | |||||||
| Cluster 4 | 1493 | 24 | −12 | −4 | −4.71 | ||||
| Thalamus | R | 300 | |||||||
| Hippocampus | R | 113 | |||||||
| Putamen | R | 100 | |||||||
| Lingual Gyrus | R | 89 | |||||||
| Cluster 5 | 1237 | 6 | −22 | 30 | −4.32 | ||||
| PCC | B | 656 | |||||||
| Hippocampal Connectivity and Glutamate | |||||||||
| Group Interaction | |||||||||
| Cluster 1 | 3129 | 8 | 22 | −16 | 5.94 | ||||
| Frontal Pole | B | 561 | |||||||
| vmPFC | B | 291 | |||||||
| mPFC/ACC (subcallosal) | B | 199 | |||||||
| Putamen | B | 134 | |||||||
| Parahippocampus | L | 98 | |||||||
| Orbitofrontal Cortex | B | 77 | |||||||
| Caudate | B | 52 | |||||||
Abbreviations: HC, healthy controls; FEP first episode psychosis patients; SZ unmedicated patients with schizophrenia; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; mPFC, medial prefrontal cortex; vmPFC, ventral medial prefrontal cortex; L, left; R, right; B, bilateral.
Regions with ≥ 50 voxels were included in the table.
Reported in Montreal Neurological Institute coordinates (X, Y and Z).
All comparisons were FDR-corrected (PFDR ≤ .05).
Contrasting hippocampal connectivity between groups, patients demonstrated greater connectivity to the cuneus/precuneus and lingual gyrus than HC in both datasets. Additionally, HC showed greater hippocampal connectivity to the PCC/precuneus, parahippocampus, and rostral orbitofrontal cortex compared to patients in dataset 1 and greater connectivity to the rostral ACC/orbitofrontal cortex and part of the middle frontal gyrus compared to patients in dataset 2. FEP also showed greater connectivity to the middle temporal gyrus and lateral occipital cortex. All hippocampal connectivity results can be found in Table 2.
To determine if there was any cognitive or clinical association with our hippocampal connectivity results we extracted beta weights from the group difference clusters in both cohorts (Figure 1). Our hippocampal connectivity results did not significantly correlate with RBANS (total, immediate memory, and delayed memory subscales) or BPRS (negative and positive subscales) in either cohort.
Relationship between hippocampal Connectivity and Glx
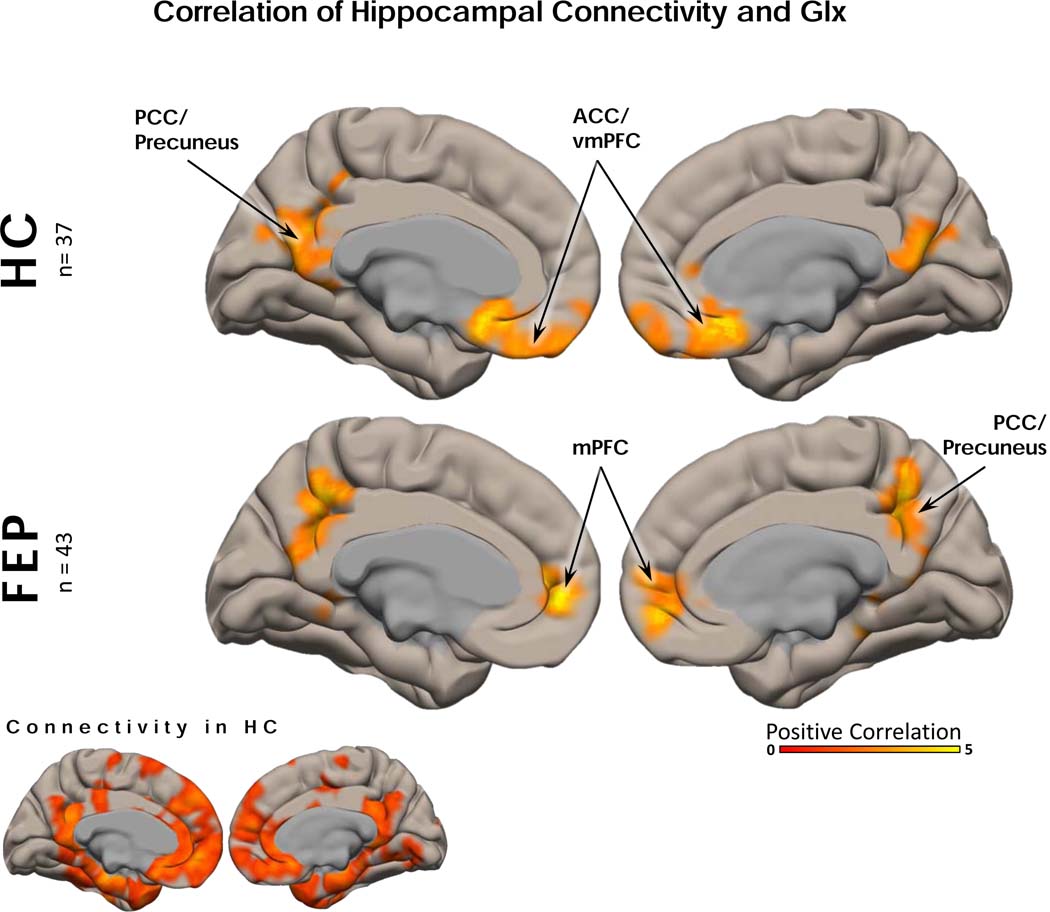
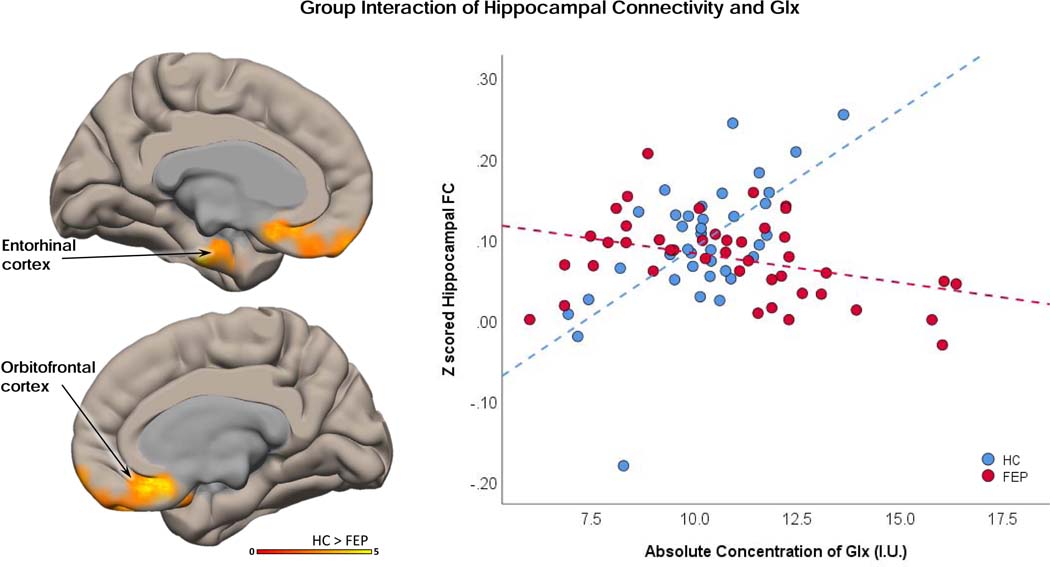
The following analyses pertain to dataset 1 only. There were no group differences in Glx levels (when we accounted for smoking as a covariate of no interest we also found no group difference in Glx), NAA levels, signal to noise, full width at half maximum, Cramér–Rao lower bound measures, or gray/white voxel composition (Table 1, Figure 2). We also found that hippocampal Glx did not correlate with RBANS (total, immediate memory, and delayed memory subscales) or BPRS (negative and positive subscales) in either cohort. In both HC and FEP subjects, greater hippocampal connectivity was correlated with greater hippocampal Glx levels in regions of the anterior and posterior DMN (Figure 3). There was a significant interaction between the correlation of Glx to hippocampal connectivity and group (Figure 3). Regions showing a significant interaction included the entorhinal and orbitofrontal cortices. In those regions, higher hippocampal Glx levels predicted greater functional connectivity in HC, and the opposite was true in FEP (Figure 4). All hippocampal connectivity by hippocampal Glx results can also be found in Table 2.
Figure 2: Hippocampus Spectroscopy.
(A) Example of magnetic resonance spectroscopy (MRS) voxel placement in the left hippocampus (2.7×1.5×1cm). The image is displayed in radiological convention (right side of image is subject’s left side). (B) Example spectrum. The black line is a spectrum obtained from the left hippocampus voxel, the red line is an overlay of spectral fit. Arrows indicate peaks for glutamate + glutamine (Glx) and N-acetylaspartate (NAA). Spectra are measured in parts per million (ppm). (C) Scatterplot of individual glutamate+glutamine (Glx) measures for first episode psychosis patients (FEP) and healthy controls (HC). Dots represent individual measurements, the line represents the group mean.
Figure 3. Hippocampal Functional Connectivity and Hippocampal Glx:
In healthy controls (HC), hippocampal functional connectivity (FC) was positively correlated with glutamate + glutamine (Glx) in precuneus/posterior cingulate cortex and ventromedial prefrontal cortex. The same pattern was seen in medication-naïve first episode psychosis patients. All analyses were corrected using voxel (p < 0.05, uncorrected) and cluster level correction (p < 0.05, FDR corrected). Age, sex, and mean framewise displacement were included as covariates of no interest.
Figure 4. Interaction Between Groups of Functional Hippocampal Connectivity and Hippocampal Glx:
An interaction between groups, hippocampal functional connectivity (FC), and hippocampal glutamate + glutamine (Glx) was seen in the orbitofrontal and entorhinal cortex. Z-scored FC beta weight values from the interaction region of interest ROI were extracted and plotted against hippocampal Glx levels. Healthy controls showed a positive correlation between hippocampal FC and Glx. Medication naïve first episode psychosis patients showed an opposite, negative correlation between FC and Glx.
DISCUSSION
To our knowledge, this is the first replication study of hippocampal dysconnectivity in two cohorts of antipsychotic-naïve/medication free patients with a psychosis spectrum disorder. In both patient cohorts, we report a pattern of hippocampal hypoconnectivity to regions of the DMN and hyperconnectivity to the lateral occipital cortex. Contrary to our hypothesis, Glx levels in FEP were not significantly different from those of HC. Nonetheless, in regions of the anterior DMN, higher hippocampal Glx levels predicted greater functional connectivity in HC, while the opposite was true in FEP. These results suggest that, in FEP, hippocampal glutamatergic metabolism may have a role in the disruption of DMN connectivity.
In HC, we found a consistent pattern of hippocampal functional connectivity to anterior and posterior regions of the DMN across both cohorts, which is also strikingly similar to other reports of hippocampal functional connectivity measured in healthy volunteers (43, 44). Not only does the consistency of the network’s spatial extent (45) across multiple studies acquired on different scanners and analyzed with different methods support this as a potential biomarker candidate, the observation that both our patient cohorts share a pattern of hippocampal hypoconnectivity to the DMN underscores its potential clinical relevance. Others have also reported hippocampal hypoconnectivity to regions of the DMN, including in medication-naïve FEP (46), unmedicated patients (21), medicated patients (20) and in patients across the psychosis spectrum (19). In FEP, decreased hippocampal FC encompassed both the anterior and posterior DMN, but in unmedicated SZ, this was observed only in the anterior DMN. It is not clear whether this difference is the result of illness chronicity or prior antipsychotic medication exposure or if it is the result of scanner and acquisition differences. In addition, we observed a consistent pattern of hyperconnectivity to regions of the lateral occipital cortex. Interestingly, hippocampal hyperconnectivity to visual areas (47) and to the thalamus (48) have been reported in association with visual (47) and auditory hallucinations (48). Based on these reports, we explored relationships between hallucinations and hippocampal connectivity. In our study, we failed to find an association between observed hippocampal hyperconnectivity to occipital areas and hallucinations (visual, auditory or both, data not shown). The occipital cortex provides an important input into the hippocampus (49). Speculatively, this pattern of hyperconnectivity could represent a compensatory mechanism for the hypoconnectivity to regions of the DMN.
Contrary to our hypothesis, we found no significant differences in levels of Glx between medication-naïve FEP and HC in left hippocampus. This is in agreement with another study in a small sample of medication-naïve FEP (n=15) where no group difference in Glx levels was identified in the medial temporal cortex (50). However, it is in contrast to our prior study where we reported elevated hippocampus Glx levels in a group of unmedicated SZ (51). A subset of patients in our previous study (51) had prior exposure to antipsychotic medication which could in part explain discrepancies in findings. The considerably larger variance in Glx values in FEP compared to HC in this study also points towards heterogeneity in glutamatergic metabolism in patients, suggesting that only a subgroup of patients may present with excess glutamate metabolism. To which extent these results could be leveraged for prediction of clinically relevant variables such as treatment response or long term functional outcomes has yet to be determined. Nonetheless, our findings underscore the importance of taking heterogeneity into consideration when characterizing psychosis spectrum patients in the early illness stages.
In both HC and FEP, hippocampal Glx levels predicted the patterns of hippocampal functional connectivity to regions of the anterior and posterior DMN. It is important to note that there is a spatial similarity seen across the two groups. However, visual inspection also indicates that there were differences between the patterns of functional connectivity associated with hippocampal Glx in the medial and rostral prefrontal cortex, parahippocampal cortex and temporal poles. In these regions, hippocampal Glx levels differentially predicted hippocampal functional connectivity in patients and HC; higher hippocampal Glx levels predicted greater functional connectivity in HC, while the opposite was true in FEP. Taken in concordance with our previous findings (29, 31), these results support a putative role of glutamate metabolism in the long-range modulation of neural networks, and this modulation may be affected in patients with schizophrenia.
Strengths and Limitations
The major strength of this study is the investigation of hippocampus connectivity across two independent datasets, obtained at two different scanners with different data acquisition protocols. Another important strength is that both cohorts were not exposed to antipsychotic medication at the time of the scan. Importantly, the first dataset included exclusively FEP patients, thus mitigating the confounds of illness chronicity, and the second dataset consisted of about 2/3 antipsychotic-naïve patients. We used similar parameters for data preprocessing and applied the same data quality control parameters in both resting state datasets to minimize the variance in data. However, it is important to note that because of the more advanced acquisition parameters in dataset 1, it is possible that the superior signal to noise ratio allowed us to detect some abnormalities in resting state connectivity patterns that were below detectable thresholds in the second dataset. For spectroscopy data, we chose a sequence that is optimized for detection of glutamate. With these acquisition parameters, approximately 80–85% of the signal quantified reflects glutamate, while glutamine makes a relatively small contribution (35). Since creatine was still the gold standard used as a reference for metabolite quantification at the time the data were collected on the Siemens Allegra scanner, we could not calculate institutional units for glx in the second data set. It would therefore be important to replicate our finding of an abnormal relationship between hippocampal connectivity and Glx in an independent cohort. Additionally, head motion in functional connectivity data differed between groups in both cohorts, which is not surprising given the patient population studies, but could confound connectivity data. Ideally, subjects would have been matched on motion parameters, but this was not feasible in this context. For correlations between Glx and connectivity, it should be noted that it is possible we were underpowered to detect the accurate magnitude of our reported correlations; Monte-Carlo simulations to determine the critical sample size from which a magnitude of a correlation is expected to be stable has been estimated to be at about 250 subjects (52).
Conclusions
In two patient cohorts, we observed a pattern of hippocampal functional hypoconnectivity to regions of the DMN and hyperconnectivity to lateral occipital cortex. In addition, we show that, in HC, there is an empirical link between hippocampal Glx levels and hippocampal functional connectivity to the DMN, and that this relationship is abnormal in medication-naïve FEP subjects. The balance between brain networks is crucial for proper brain function. It is tempting to speculate that the altered relationship between glutamate and functional connecitivity may disrupt the dynamic of major neural networks, possibly affecting cognition.
ACKNOWLEDGMENTS
We would like to thank the patients and their families for their participation in this study.
Funding: This work was supported by the National Institutes of Health grants R01MH081014, R01MH102951, R01MH113800 (ACL) and K23MH106683 (NVK). The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. This study was presented in part at the Schizophrenia International Research Society (SIRS) Conference; April 13th, 2019; Orlando, Florida.
Footnotes
DISCLOSURES
All authors report no relevant biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weiss AP, Schacter DL, Goff DC, Rauch SL, Alpert NM, Fischman AJ, et al. (2003): Impaired hippocampal recruitment during normal modulation of memory performance in schizophrenia. Biol Psychiatry. 53:48–55. [DOI] [PubMed] [Google Scholar]
- 2.Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, et al. (1998): Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1:318–323. [DOI] [PubMed] [Google Scholar]
- 3.Öngür D, Cullen TJ, Wolf DH, Rohan M, Barreira P, Zalesak M, et al. (2006): The neural basis of relational memory deficits in schizophrenia. Arch Gen Psychiatry. 63:356–365. [DOI] [PubMed] [Google Scholar]
- 4.Achim AM, Bertrand M-C, Sutton H, Montoya A, Czechowska Y, Malla AK, et al. (2007): Selective abnormal modulation of hippocampal activity during memory formation in first-episode psychosis. Arch Gen Psychiatry. 64:999–1014. [DOI] [PubMed] [Google Scholar]
- 5.Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, et al. (2009): Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Archives of General Psychiatry. 66:938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, et al. (2013): Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 78:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talati P, Rane S, Kose S, Blackford JU, Gore J, Donahue MJ, et al. (2014): Increased hippocampal CA1 cerebral blood volume in schizophrenia. NeuroImage: Clinical. 5:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutcheson NL, Reid MA, White DM, Kraguljac NV, Avsar KB, Bolding MS, et al. (2012): Multimodal analysis of the hippocampus in schizophrenia using proton magnetic resonance spectroscopy and functional magnetic resonance imaging. Schizophrenia Research. 140:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitt A, Steyskal C, Bernstein HG, Schneider-Axmann T, Parlapani E, Schaeffer EL, et al. (2009): Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathol. 117:395–407. [DOI] [PubMed] [Google Scholar]
- 10.Benes FM (1999): Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biol Psychiatry. 46:589–599. [DOI] [PubMed] [Google Scholar]
- 11.Nelson MD, Saykin AJ, Flashman LA, Riordan HJ (1998): Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 55:433–440. [DOI] [PubMed] [Google Scholar]
- 12.Wright IC, Rabe-Hesketh S, Woodruff PWR, David AS, Murray RM, Bullmore ET (2000): Meta-analysis of regional brain volumes in schizophrenia. American Journal of Psychiatry. 157:16–25. [DOI] [PubMed] [Google Scholar]
- 13.Baglivo V, Cao B, Mwangi B, Bellani M, Perlini C, Lasalvia A, et al. (2018): Hippocampal subfield volumes in patients with first-episode psychosis. Schizophr Bull. 44:552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho NF, Iglesias JE, Sum MY, Kuswanto CN, Sitoh YY, De Souza J, et al. (2017): Progression from selective to general involvement of hippocampal subfields in schizophrenia. Molecular psychiatry. 22:142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heckers S, Konradi C (2015): GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophrenia Research. 167:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance Medicine. 34:537–541. [DOI] [PubMed] [Google Scholar]
- 17.Damoiseaux JS, Rombouts SARB, F. B, Scheltens P, Stam CJ, Smith SM, et al. (2006): Consistent resting-state networks across healthy subjects. PNAS. 103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox MD, Raichle ME (2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 8:700–711. [DOI] [PubMed] [Google Scholar]
- 19.Samudra N, Ivleva EI, Hubbard NA, Rypma B, Sweeney JA, Clementz BA, et al. (2015): Alterations in hippocampal connectivity across the psychosis dimension. Psychiatry Research: Neuroimaging. 233:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Shu N, Liu Y, Song M, Hao Y, Liu H, et al. (2008): Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr Res. 100:120–132. [DOI] [PubMed] [Google Scholar]
- 21.Kraguljac NV, White DM, Hadley N, Hadley JA, Ver Hoef L, Davis E, et al. (2016): Aberrant hippocampal connectivity in unmedicated patients with schizophrenia and effects of antipsychotic medication: a longitudinal resting state functional MRI study. Schizophrenia Bulletin. 42:1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraguljac NV, White DM, Hadley J, Reid MA, Lahti AC (2014): Hippocampal-parietal dysconnectivity and glutamate abnormalities in unmedicated patients with schizophrenia. Hippocampus. 24:1524–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutcheson NL, Sreenivasan KR, Deshpande G, Reid MA, Hadley J, White DM, et al. (2015): Effective connectivity during episodic memory retrieval in schizophrenia participants before and after antipsychotic medication. Human Brain Mapping. 36:1442–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. (2008): Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends in Neurosciences. 31:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bickel S, Javitt DC (2009): Neurophysiological and neurochemical animal models of schizophrenia: focus on glutamate. Behav Brain Res. 204:352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraguljac NV, Frölich MA, Tran S, White DM, Nichols N, Barton-Mcardle A, et al. (2017): Ketamine modulates hippocampal neurochemistry and functional connectivity: A combined magnetic resonance spectroscopy and resting-state fMRI study in healthy volunteers. Molecular Psychiatry. 22:562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freund TF, Buzsáki G (1996): Interneurons of the hippocampus. Hippocampus. 6:347–470. [DOI] [PubMed] [Google Scholar]
- 28.Tamminga CA, Stan AD, Wagner AD (2010): The hippocampal formation in schizophrenia. American Journal of Psychiatry. 167:1178–1193. [DOI] [PubMed] [Google Scholar]
- 29.Overbeek G, Gawne TJ, Reid MA, Salibi N, Kraguljac NV, White DM, et al. (2019): Relationship between cortical excitation and inhibition and task-induced activation and deactivation: a combined magnetic resonance spectroscopy and functional magnetic resonance imaging study at 7T in first-episode psychosis. Biol Psychiatry Cogn Neurosci Neuroimaging. 4:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falkenberg LE, Westerhausen R, Specht K, Hugdahl K (2012): Resting-state glutamate level in the anterior cingulate predicts blood-oxygen level-dependent response to cognitive control. PNAS. 109:5069–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cadena EJ, White DM, Kraguljac NV, Reid MA, Maximo JO, Nelson EA, et al. (2018): A longitudinal multimodal neuroimaging study to examine relationships between resting state glutamate and task related BOLD response in schizophrenia. Front Psychiatry. 9:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpenter WT, Gold JM, Lahti AC, Queern CA, Conley RR, Bartko JJ, et al. (2000): Decisional capacity for informed consent in schizophrenia research. Archives of General Psychiatry. 57:533–538. [DOI] [PubMed] [Google Scholar]
- 33.Overall JE, Gorham DR (1962): The brief psychiatric rating scale. Psychological Reports. 10:799–812. [Google Scholar]
- 34.Randolph C, Tierney MC, Mohr E, Chase TN (1998): The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. Journal of Clinical and Experimental Neuropsychology. 20:310–319. [DOI] [PubMed] [Google Scholar]
- 35.Schubert F, Gallinat J, Seifert F, Rinneberg H (2004): Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 tesla. NeuroImage. 21:1762–1771. [DOI] [PubMed] [Google Scholar]
- 36.Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. (2013): The minimal preprocessing pipelines for the human connectome project. Neuroimage. 80:105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitfield-Gabrieli S, Nieto-Castanon A (2012): Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2:125–141. [DOI] [PubMed] [Google Scholar]
- 38.Provencher SW (1993): Provencher estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic resonance in medicine. 30:672–679. [DOI] [PubMed] [Google Scholar]
- 39.Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, et al. (2006): Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med. 55:1219–1226. [DOI] [PubMed] [Google Scholar]
- 40.Gussew A, Erdtel M, Hiepe P, Rzanny R, Reichenbach JR (2012): Absolute quantitation of brain metabolites with respect to heterogeneous tissue compositions in 1 H-MR spectroscopic volumes. Magn Reson Mater Phy. 25:321–333. [DOI] [PubMed] [Google Scholar]
- 41.Wilson M, Andronesi O, Barker PB, Bartha R, Bizzi A, Bolan PJ, et al. (2019): Methodological consensus on clinical proton MRS of the brain: review and recommendations. Magn Reson Med. 82:527–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreis R (2016): The trouble with quality filtering based on relative cramer-rao lower bounds. Magn Reson Med. 75:15–18. [DOI] [PubMed] [Google Scholar]
- 43.Vos de Wael R, Lariviere S, Caldairou B, Hong SJ, Margulies DS, Jefferies E, et al. (2018): Anatomical and microstructural determinants of hippocampal subfield functional connectome embedding. PNAS. 115:10154–10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Flores R, Mutlu J, Bejanin A, Gonneaud J, Landeau B, Tomadesso C, et al. (2017): Intrinsic connectivity of hippocampal subfields in normal elderly and mild cognitive impairment patients. Hum Brain Mapp. 38:4922–4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raichle ME (2015): The brain’s default mode network. Annu Rev Neurosci. 38:433–447. [DOI] [PubMed] [Google Scholar]
- 46.Blessing EM, Murty VP, Zeng B, Wang J, Davachi L, Goff DC (2019): Anterior hippocampal-cortical functional connectivity distinguishes antipsychotic naive first-episode psychosis patients from controls and may predict response to second-generation antipsychotic treatment. Schizophr Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ford JM, Palzes VA, Roach BJ, Potkin SG, van Erp TG, Turner JA, et al. (2015): Visual hallucinations are associated with hyperconnectivity between the amygdala and visual cortex in people with a diagnosis of schizophrenia. Schizophr Bull. 41:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amad A, Cachia A, Gorwood P, Pins D, Delmaire C, Rolland B, et al. (2014): The multimodal connectivity of the hippocampal complex in auditory and visual hallucinations. Mol Psychiatry. 19:184–191. [DOI] [PubMed] [Google Scholar]
- 49.Tsanov M, Manahan-Vaughan D (2008): Synaptic plasticity from visual cortex to hippocampus: systems integration in spatial information processing. Neuroscientist. 14:584–597. [DOI] [PubMed] [Google Scholar]
- 50.Wood SJ, Berger GE, Wellard RM, Proffitt T, McConchie M, Velakoulis D, et al. (2008): A 1 H-MRS investigation of the medial temporal lobe in antipsychotic-naive and early-treated first episode psychosis. Schizophr Res. 102:163–170. [DOI] [PubMed] [Google Scholar]
- 51.Kraguljac NV, White DM, Reid MA, Lahti AC (2013): Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry. 70:1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schönbrodt FD, Perugini M (2013): At what sample size do correlations stabilize? Journal of Research in Personality. 47:609–612. [Google Scholar]