Abstract
Introduction:
The tumor-draining lymph node (TDLN) plays a role in tumor immunity. Intratumorally administered microspheres (MS) that encapsulate immunomodulatory agents have emerged as a treatment strategy capable of causing profound changes in the tumor microenvironment (TME) and eliciting potent antitumor effects. We hypothesized that local delivery of MS to the TME may also drain to and therefore target the TDLN to initiate antitumor immune responses.
Methods:
Fluorescent MS were injected into orthotopically implanted murine pancreatic tumors, and tissues were examined by whole mount microscopy and imaging flow cytometry. The role of the TDLN was investigated for mice treated with intratumoral interleukin-12 (IL-12)-encapsulated MS in combination with stereotactic body radiotherapy (SBRT) by cytokine profile and ablation.
Results:
Fluorescent AF-594 MS delivered intratumorally were detected in the tumor, peritumoral lymphatics, and the TDLN two hours after injection. Phagocytic cells were observed with internalized fluorescent MS. SBRT + IL-12 MS induced upregulation of Th1 and antitumor factors IL-12, IFN-γCXCL10, and granzyme B in the TDLN, and excision of the TDLN partially abrogated treatment efficacy.
Conclusions:
Our results demonstrate that intratumorally administered MS not only target the TME, but also drain to the TDLN. Furthermore, MS encapsulated with a potent antitumor cytokine, IL-12, induce an antitumor cytokine profile in the TDLN, which is essential to treatment efficacy.
Keywords: tumor-draining lymph node (TDLN), pancreatic ductal adenocarcinoma (PDA), interleukin-12 (IL-12), immunotherapy, microparticle
Introduction
The latest advances in cancer immunotherapies to improve treatment efficacy have sparked interest in the tumor-draining lymph node (TDLN). In current clinical oncology, the TDLN is primarily evaluated for metastasis to determine the clinical stage of the tumor and the corresponding tumor-specific treatment regimen (Brierley et al., 2017; Nathanson et al., 2015; Jones et al., 2018). Recent evidence has demonstrated that the TDLN is not only a key clinical prognostic factor, but also a critical component in establishing the tumor immune microenvironment (Munn and Mellor, 2006). Within the TDLN, professional antigen presenting cells (APCs), including dendritic cells (DCs) and macrophages, can present tumor-specific antigens for effective activation and maturation of tumor-specific cytotoxic T lymphocytes (CTLs) (Salmon et al., 2016; Roberts et al., 2016; Banchereau J et al., 2000; Broz et al., 2014). Conversely, prolonged exposure to tumor-derived factors can suppress APC function in the TDLN, preventing CTL priming and eventually leading to tumor progression and metastasis (Bandola-Simon and Roche, 2019; Matsuda et al., 2019; Watanabe et al., 2008; Cochran et al., 2006; Hoon et al., 1987). Promoting antitumor CTL stimulation and proliferation in the TDLN may be key to converting immunologically “cold” tumors to “hot.”
Therapeutic targeting of the TDLN has been investigated for malignancies such as melanoma and breast cancer for which the sentinel lymph node biopsy serves as standard of care for staging (Nathanson et al., 2015; Rotman et al., 2019). A previous study in breast cancer revealed minimal drug concentrations in axillary TDLNs following standard systemic infusion of chemotherapy, whereas local chemotherapy administration adjacent to the tumor resulted in significantly increased concentrations in the TDLNs (Chen et al., 2004). These results demonstrate that systemic therapies may bypass the TDLN and only local administration of drug will result in appreciable accumulation in the TDLN. Furthermore, clinical trials with intratumoral administration of granulocyte-macrophage colony-stimulating factor (GM-CSF) and CpG oligodeoxynucleotide have demonstrated improved tumor control and recurrence-free survival (Vuylsteke et al., 2004; Molenkamp et al., 2007; Sluijter et al., 2015). In these studies the intratumoral therapy not only modulated the tumor microenvironment (TME), but also potentiated the antitumor immune reserve in the TDLN with increased frequency of mature DCs and enhanced reactivity of tumor-specific CTLs. Additionally, preclinical studies in various murine cancer models demonstrated that antitumor immune responses following PD-1/PD-L1 checkpoint therapy was abrogated by the surgical excision of the TDLN (Chamoto et al., 2017; Fransen et al., 2018). These clinical and preclinical studies provide evidence that targeting the TDLN via intratumoral drug delivery can be an effective immunotherapeutic strategy.
For more effective therapeutic targeting of the TDLN, novel nano- and microparticles have been introduced as drug delivery vehicles. These particles can facilitate immunotherapy access to the TDLN by direct lymphatic flow or by engulfment in migratory phagocytic cells when particles are greater than 200 nm (Batty et al., 2018; Manolova et al., 2008; Champion et al., 2008). In addition to particle size, other material properties can influence the rate and extent of phagocytosis as well as rate of drug release, which can be critical for therapeutic agents that are short-lived or systemically toxic (Beningo and Wang, 2002; Palomba et al., 2018; Riley et al., 2019; Zhang et al., 2018). Biodegradable polylactic acid polymer microspheres (MS) 1–5 μm in diameter are one example of controlled-release particles that can provide local, sustained delivery of bioactive contents for days to weeks (Sharma et al., 2004). When loaded with pleiotropic proinflammatory cytokine interleukin-12 (IL-12) and delivered intratumorally, IL-12 MS therapy in combination with stereotactic body radiotherapy (SBRT) has been shown to repolarize the tumor immune microenvironment and promote systemic antitumor immunity in preclinical models of pancreatic ductal adenocarcinoma (PDA) (Mills et al., 2019). The local delivery and controlled-release of IL-12 eliminated systemic toxicities that have been the main limitation for clinical application of this cytokine (Tugues et al., 2015; Atkins et al., 1997; Leonard et al., 1997). Although this study demonstrates the potential for achieving antitumor immune responses by targeted delivery to the TME, the contribution of the TDLN was not assessed and will be the focus of this manuscript.
To further characterize MS-delivered immunotherapy in the PDA TDLN, MS loaded with fluorescent AF-594 molecules were developed to examine MS trafficking to the TDLN following intratumoral injection. Both free and engulfed fluorescent MS were found in the TDLN by fluorescence microscopy and imaging flow cytometry. Based on these observations, we further investigated the possible mechanistic role of the TDLN in generating antitumor immunity for the SBRT + IL-12 MS combination therapy. IL-12 and downstream mediators such as IFN-γ, CXCL10, and granzyme B were elevated in the TDLN following SBRT and intratumoral IL-12 MS administration. More importantly, surgical excision of the TDLN abrogated the SBRT + IL-12 MS antitumor response, suggesting that the TDLN is essential in mediating therapeutic efficacy.
Results
Fluorescent MS are visible in the tumor following intratumoral injection.
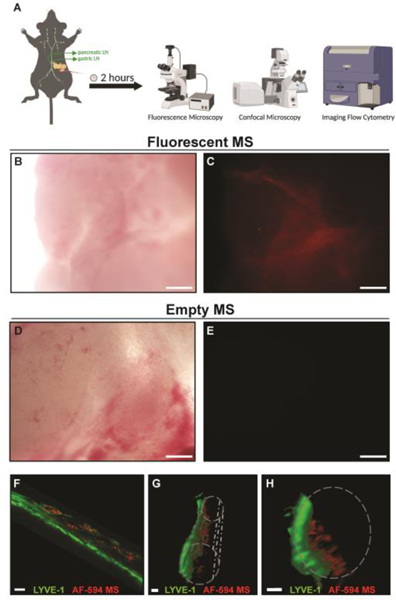
To track the dissemination of intratumoral MS in a preclinical model of PDA, we effectively administered 2 mg of fluorescent MS into established KCKO tumors, and examined the tumor parenchyma by whole mount fluorescence microscopy as previously described (Gerber et al., 2003) (Figure 1A). Fluorescent MS were observed throughout the tumor parenchyma (Figure 1B, C), whereas Empty MS controls were undetectable (Figure 1D, E), indicating no MS autofluorescence and validating our ability to specifically identify MS using fluorescence. Our previous data established that intratumorally-administered MS were not detected in the blood circulation (Mills et al., 2019). However, while visualizing fluorescent MS in the tumor (Figure 1), we noticed the MS followed a vessel-like pattern in the adjacent normal pancreatic tissue. Confocal whole mount analysis revealed fluorescent MS closely associated with LYVE-1+ lymphatic vessels in the peritumoral pancreas parenchyma. (Figure 1F). The three-dimensional rendering of the LYVE-1+ lymphatic vessels confirmed that the fluorescent MS were inside the vessel structures (Figure 1G, H). These data suggested that although most MS are retained in the tumor following injection, a proportion of MS enter adjacent lymphatics.
Figure 1. Injection of Orthotopic PDAC Tumors with Labeled MS.
2×105 KCKO tumor cells were implanted orthotopically in the pancreas of C57BL/6J mice. Established tumors were directly injected with 2 mg (20 μl) of either empty or fluorescent AF-594 MS on Day 10 and mice were sacrificed 2 hours following injection as shown in the schematic outlining the experimental design (A). Tumor tissue from mice injected with fluorescent MS (B: bright field and corresponding field of view to C: fluorescence) or empty MS (D: bright field and corresponding field of view to E: fluorescence) was examined by fluorescence microscopy. Whole tissue tumor-bearing pancreas with intratumoral fluorescent MS was stained by immunofluorescence for LYVE-1 and evaluated by confocal microscopy. Merged images of approximately 50 μm into a vessel showing co-localization of fluorescent MS (red) and LYVE-1 (green) (F). The entire z-stack is shown as a 3D rendered longitudinally (G) or as a ventral cross-section (H) demonstrating one side of the vessel wall (green) with luminal MS (red). Approximation of the entire vessel is shown in gray. n = 4. Scale bar = 200 μm in A-E; 14 μm in F; 10 μm in G-H.
MS are abundant in the pancreatic TDLN following intratumoral injection.
Lymphatics are conduits that allow both passive and active unidirectional transport of cells, molecules, and other particulates from tissue to lymph nodes (Swartz and Lund, 2012). Here, we assessed whether MS could be visualized in TDLNs two hours following intratumoral injection. While fluorescent MS were not observed in the non-draining pyloric lymph node (Figure 2A, B), abundant MS were distributed throughout the pancreatic TDLN, predominantly in the structural pattern of subcapsular and cortical sinuses (Figure 2C, D). The afferent lymphatic vessel, which was dissected with the TDLN, also contained numerous fluorescent MS (Figure 2E, gray arrows). Higher magnification demonstrated small, punctate fluorescent spheres co-localized along a vessel structure (Figure 2F). Collectively, these data suggest that MS injected locally into the pancreatic tumor traffic to the TDLN via lymphatics.
Figure 2. Labeled MS Injected Intratumorally can be Visualized in the TDLN.
2 × 105 KCKO tumor cells were implanted orthotopically in the pancreas of C57BL/6J mice. Established tumors were directly injected with 2 mg (20 μl) of fluorescent MS on Day 10 and mice were sacrificed 2 hours following injection. The non-draining pyloric lymph node (A: bright field and corresponding field of view to B: fluorescence) and the pancreatic-draining lymph node (C: bright field and corresponding field of view to D: fluorescence) were imaged by whole mount fluorescence microscopy for the presence or absence of fluorescent MS. The afferent lymphatic vessel was dissected along with the draining lymph node and imaged by whole mount fluorescence microscopy in E (see gray arrows), and further magnified in F. Note the presence of fluorescent MS associated with the vessel. n = 4. Scale bar = 500 μm in A-D; 100 μm in E; 50 μm in F.
Both free and engulfed MS are detected in the PDA TDLN.
Multiple cell types are capable of microparticle uptake, and these processes are driven by several properties including particle size, surface charge, and stiffness (Batty et al., 2018). To assess the localization of microspheres within the TDLN, imaging flow cytometry was used to visualize and quantify both free and engulfed MS. PDA tumors were injected with fluorescent MS and the TDLN and non-draining pyloric lymph nodes were removed two hours later. Nodes were dissociated and stained with antibodies to phenotype immune phagocytic subsets most abundant in the TME. As expected, MS were not detected in the non-draining pyloric lymph node (data not shown). Conversely, fluorescent MS (AF-594+) were readily observed in the TDLN and could be detected as free MS or engulfed by CD45+ immune cells (Figure 3A). Fluorescent MS+ immune cells were classified into four discrete populations: 1) CD11b+F4/80−Ly6G−MHCIIhi, 2) CD11b−F4/80−Ly6G−MHCIIhi, 3) CD11b+F4/80−Ly6G−MHCIIlo, and 4) CD11b−F4/80−Ly6G−MHCIIlo (Figure 3B, C). Furthermore, levels of MS uptake were found to vary between cell types with the CD11b−MHCII+ population demonstrating the highest level of MS fluorescence (Figure 3D). Surprisingly, less than 13% of all AF-594+ myeloid cells were determined to be CD11b+F4/80+ macrophages (Figure S1A, B) and less than 6% were CD11b+Gr-1hi granulocytes (Figure S1A, C).
Figure 3. Free and Engulfed Microspheres Reside in the TDLN Following Intratumoral Injection.
Draining lymph nodes were harvested from one KCKO tumor-bearing mouse 2 hours after intratumoral fluorescent MS injection. Cell suspensions were stained for CD45, CD11b, and MHCII prior to imaging flow cytometry (ISX) analysis collecting approximately 10,000 events. (A) Gating of fluorescent+ events. (B) Representative ISX images of AF-594+ events. (C) Phenotypic distribution (percentage) of fluorescent+ cells. (D) Average fluorescent MS stain intensity (geometric mean intensity) within identified populations.
Intratumoral delivery of IL-12-encapsulated MS alters the TDLN cytokine profile.
MS can encapsulate cytokines allowing for a sustained, local release in the tumor microenvironment over days to weeks (Sharma et al., 2004). We previously demonstrated that the combination of SBRT with subsequent intratumoral injection of IL-12 MS completely eliminated primary PDA tumors and resulted in prolonged survival (Mills et al., 2019). As Figure 2 illustrates that fluorescent MS traffic to the TDLN, we addressed if IL-12 protein could be detected in the pancreatic TDLN following SBRT and intratumoral IL-12 MS administration. KCKO tumor-bearing mice were left unirradiated or treated with SBRT (6 Gy over 4 consecutive days) followed by an intratumoral injection of Empty or IL-12 MS (Figure 4A). The TDLN was removed at 6 and 24 hours after MS injection, and examined by Luminex for various cytokine proteins. IL-12 was elevated in TDLN at 6 hours in mice treated with UT + IL-12 MS and SBRT + IL-12 MS (Figure 4B). We also examined two downstream mediators of IL-12, CXCL10 and IFN-γ. Both of these factors are strongly induced in the presence of IL-12 and assist in driving a Th1 immune response (Dufour et al., 2002). Minimal induction of CXCL10 and IFN-γ was observed at 6 hours (data not shown); however, by 24 hours, CXCL10 and IFN-γ (Figure 4C, D) were upregulated in the TDLN isolated from the UT + IL-12 MS and SBRT + IL-12MS treated mice. We next quantified granzyme B, an effector protein commonly associated with enhanced cytolytic function of CD8+ T cells and NK cells (Chowdhury and Lieberman, 2008). Granzyme B was strongly induced in the TDLN specifically at the 24-hour timepoint and only in the SBRT + IL-12 MS group (Figure 4E). These data suggest that the combination of SBRT and intratumoral IL-12 MS modulates the PDA TDLN by upregulating factors commonly associated with Th1 immune responses and enhanced antitumor effector function.
Figure 4. IL-12 encapsulated in MS + SBRT polarizes the TDLN.
2 × 105 KCKO tumor cells were implanted orthotopically in the pancreas of C57BL/6J mice. On Day 6 after initial tumor inoculation, established tumors were left untreated or treated with SBRT (6 Gy each day for 4 consecutive days) followed by an intratumoral injection of 2 mg (20 μl) of Empty MS or IL-12 MS on Day 10 (A). At 6- or 24-hours following MS injection, the TDLN was removed and processed for protein analysis by Luminex. The presence of IL-12p70 (B), biological mediators of IL-12, CXCL10 (C) and IFN-γ (D), and an effector protein granzyme B (E) are shown after normalizing for protein concentration. Significance determined by Kruskal-Wallis multiple comparisons test (p < 0.05). n = 5/experimental group for each time point.
TDLN ablation reduces therapeutic efficacy of SBRT + IL-12 MS.
To assess the role of the TDLN in mediating SBRT + IL-12 MS efficacy, lymphadenectomy of the pancreatic node or a sham procedure (control) was performed concurrent to KCKO PDA tumor implantation. SBRT + IL-12 MS therapy occurred as described in Figure 4. As expected from our previous studies, treated control mice with the TDLN rejected the primary pancreatic tumor (Figure 5A, green line) and demonstrated 100% survival (Figure 5B, green line). However, removal of the TDLN resulted in the acceleration of primary tumor growth (Figure 5A, red line) and a decrease in survival to 44% (Figure 5B, red line). These data suggest that the TDLN plays an essential role in mediating the antitumor effects of SBRT + IL-12 MS therapy in PDA, providing primary tumor control and significant survival advantage.
Figure 5. Removal of the TDLN abrogates SBRT + IL-12 MS Efficacy.
2 × 105 luciferase-expressing KCKO tumor cells were implanted orthotopically in the pancreas of C57BL/6J mice and, in the same surgery, one group of mice also received a lymphadenectomy of the pancreatic draining lymph node. On Day 6 after initial tumor inoculation, established tumors were left untreated or treated with SBRT (6 Gy each day for 4 consecutive days) followed by an intratumoral injection of 2 mg (20 μl) of Empty MS or IL-12 MS on Day 10. Mice were assessed for tumor burden by IVIS (A), along with survival (B). Significance for (A) determined by Kruskal-Wallis test (SBRT + IL-12 MS + LN is statistically different from both the other 2 groups) and for (B) by Mantel-Cox test (p < 0.05) of 2 independent experiments. n = 9.
Discussion
Immunosuppression is a hallmark of cancer that is studied primarily in the TME. Despite evidence that the TDLN is a reservoir of immune potential (Munn and Mellor, 2006), it is often overlooked during immunotherapy development. Tumor-derived factors drive APCs in the TDLN toward immature and immunosuppressive phenotypes that prevent optimal priming of T cells against tumor neoantigens (Bandola-Simon and Roche, 2019; Watanabe et al., 2008; Cochran et al., 2006). Whereas few systemically-administered immunomodulatory drugs have been reported to reach the TDLN (Rotman et al., 2019), we report the distribution of polymer MS to the PDA TME and TDLN following intratumoral injection and successful delivery of the proinflammatory cytokine IL-12 to modulate the tumor and TDLN immune microenvironments. Proportionate increases in the IL-12 effector molecules IFN-γ and CXCL10 in the TDLN confirmed successful MS transit, IL-12 release from MS, and TDLN polarization toward a proinflammatory Th1-like state. Engaging the IL-12 receptors on naïve CD4+ helper T cells with exogenous IL-12 upregulates the transcription of T-bet, which in turn promotes the production of the Th1 effector molecules (e.g. IFN-γ) and silences classically Th2 cytokines (IL-4, IL-13) (Trinchieri, 1995). However, even with the proper activation signals, IL-12 MS alone did not eliminate tumors. Only in combination with SBRT did we observe complete responses and prolonged survival (Mills et al., 2019). In the TDLN, elevated IFN-γ and CXCL10 were detected after both IL-12 MS monotherapy and SBRT + IL-12 MS combination therapy. Yet, granzyme B, a cytotoxic serum protease commonly found in CTLs (Lieberman, 2003), was expressed at higher levels only after SBRT + IL-12 MS, which argues that IL-12 alone, like SBRT, is not sufficient to initiate the cascade of events necessary to stimulate tumor-specific CTLs. This observation in addition to the loss of treatment efficacy in the absence of the TDLN suggests that the radiation and the MS each makes a unique contribution to support efficient antigen presentation via local activation and repolarization of the TME and CTL stimulation specifically in the TDLN, generating the robust antitumor effector response appreciated with SBRT + IL-12 MS.
In general, nano- and micro-scale particles travel through the lymphatics to reach the TDLN. We noted fluorescent MS inside of pancreatic lymphatic vessels, the afferent lymphatic vessel associated with the TDLN, and distribution throughout the subcapsular and cortical sinuses. These findings strongly suggest that intratumoral MS reach the TDLN via lymphatic trafficking, and previous work from our group demonstrating the absence of MS in the blood and peritoneal cavity further supports this premise (Mills et al., 2019). It has been reported that PDA tumors contain a paucity of lymphatic vessels with architecture that is irregularly distributed and disconnected from peritumoral vessels (Olszewski et al., 2012). Additionally, the desmoplastic stroma common to PDA causes high interstitial pressures that affect the distribution of therapeutics (Provenzano and Hingorani, 2013). It is plausible that these TME characteristics may dispel some MS out of the tumor core and into adjacent normal tissue, where access to intact lymphatic drainage is available to the TDLN. It is possible that removal of the TDLN disrupts local lymph drainage similar to observations by Kataru et al. following local lymphatic ablation (Kataru et al., 2019). However, we did not observe overt edema in the pancreas or tumor, which suggests that collateral lymph vessels potentially compensates for dysfunction in lymphatic drainage following lymphadenectomy. These data further highlight the importance of the TDLN in generating potent, long-lasting antitumor responses following SBRT + IL-12 MS, which was diminished in the absence of the TDLN. In addition, our data in Figure 3 demonstrated both extracellular (free) and intracellular (engulfed) fluorescent MS in the TDLN. Therefore, it is possible that both passive and active transport of MS, via cellular uptake and migration, route through lymphatics to the TDLN.
Intracellular MS were observed in four discrete cell phenotypes in the TDLN. Although granulocytes and macrophages commonly phagocytose microparticles (Batty et al., 2018; Manolova et al., 2008; Champion et al., 2008), a paucity of Gr-1hi and F4/80+ cells were found to be AF-594+ suggesting that these subtypes are not the main drivers of immune activation within the TDLN. The CD11b+MHCIIhi, CD11b−MHCIIhi, and CD11b−MHCIIlo groups closely resemble antigen-presenting conventional DC (cDC) type 2 (cDC2) and type 1 (cDC1), and phagocytic plasmacytoid DCs, respectively (Eisenbarth, 2019; Tel et al., 2010). Furthermore, the CD11b+MHCIIlo population is characteristic of the subcapsular sinus macrophage (SSM) phenotype as they mostly present antigen to B cells and do not express MHC molecules (Louie and Liao, 2019; Gray and Cyster, 2012). Several elements of SSM biology also advocate a role in MS uptake, as they function to sample incoming antigens that drain into the subcapsular sinus from the afferent lymphatic vessel (Moran et al., 2019). Despite SSMs being the first cells to encounter the MS in the TDLN, the greatest number and highest fluorescence of MS+ cells were found to be CD11b−MHCIIhi. This population most closely resembles cDC1 cells that may have engulfed MS in the tumor before migrating to the TDLN or resident cells that engulfed the free MS that reached the TDLN (Eisenbarth, 2019). Just as free MS release biologically active IL-12 into the extracellular matrix to induce immune stimulation, IL-12 MS internalization could be a critical component in activating host cell function and ultimately the potent antitumor effect elicited by SBRT + IL-12 MS therapy and warrants further investigation. APC engulfment indicates a spatial proximity between MS and cells, and thus the delivery of IL-12 MS would result in the accumulation of IL-12 at sites of T cell priming with cDCs and promote enhanced effector cell proliferation and stimulation. Furthermore, IFN-γ release by activated T cells could further support the maturation and activation of APCs as a positive feedback loop, perpetuating IL-12 production and reinforcing the Th1 antitumor response (Pan et al., 2004).
Particle-based drug delivery systems are commonly used as therapeutic vehicles to improve the delivery and distribution of anti-cancer agents to impervious tumor microenvironments. Beyond this, we show that polymer microspheres loaded with the IL-12 cytokine traffic from pancreatic tumors to the TDLN and activate the TDLN immune microenvironment. At its core, our data suggest that direct immune stimulation in the PDA TME can induce an antitumor response for partial therapeutic effect, but the TDLN has an essential role in promoting potent, lasting antitumor therapeutic responses. These findings advocate the continued development of microparticle delivery systems containing immune-modulating agents, and warrant further studies elucidating the cellular and molecular mechanisms of IL-12 MS in the TDLN.
Materials and Methods
In vivo Animal Studies
All experiments were approved by the University Committee on Animal Resources, and were performed in compliance with both the NIH and University of Rochester approved guidelines for the care and use of animals. Six- to eight-week old age-matched female C57BL/6J mice (Jackson Laboratory) were used. All mice were subjected to a 12-hour light/dark cycle and kept in individually ventilated cages with bedding and nesting material.
Cell Cultures
The KCKO cell line stably expressing firefly luciferase (KCKO-luc) was obtained from Dr. David DeNardo (Zhu et al., 2014). This cell line is syngeneic to the C57BL/6J background. Cell cultures were maintained in RPMI (GIBCO) supplemented with 5% fetal bovine serum (GIBCO) and 1% penicillin/streptomycin (ThermoFisher Scientific) at 37°C and 5% CO2 in a humidified incubator.
Orthotopic Tumor Implantation
Mice were anesthetized using an isoflurane anaesthetic vaporizer (Scivena Scientific) and hair was removed from the incision site using a topical hair depilatory cream. The surgical area was sterilized with a povidone-iodine prep pad and a 10-mm laparotomy incision was made to expose both the spleen and pancreas. Cell lines were detached with 0.25% Trypsin/EDTA (GIBCO) and re-suspended in a 1:1 PBS:Matrigel (BD Biosciences) solution. 2×105 cells (50 μL) were injected into the pancreatic tail, and two 4-mm titanium fiducial markers (Horizon) were implanted flanking the injected tumor cells to assist in SBRT targeting. A cotton swab was placed over the injection site for one minute to prevent peritoneal leakage and to allow gelation of Matrigel. IVIS bioluminescent imaging (described below) was used to verify successful implantations with no direct seeding into the peritoneal cavity and also for providing baseline measurements for standardizing pre-treatment groups and tumor growth.
Lymphadenectomy
Lymphadenectomy was performed at the time of tumor implantation. Mice were anesthetized as described above and a 10-mm laparotomy incision was made to expose the spleen, pancreas, and pancreatic draining lymph node (TDLN). The pancreas-draining lymph nodes were excised using curved surgical forceps. Sham excisions (used for control) were performed by locating and exposing the pancreatic-draining lymph node and placing it gently back into the retro-peritoneum.
Radiation Therapy
All radiation was delivered using the Small Animal Radiation Research Platform (SARRP, XStrahl) using a 5-mm collimator. Mice were anesthetized with vaporized isoflurane for the duration of all radiation treatments. Stereotactic body radiation therapy (SBRT) was administered to tumor-bearing mice following a schedule of 4 fractions of 6 Gy radiation each on days 6–9 post tumor implantation, yielding a BED of 38.4 for tumor and 72 for normal tissue (assuming an α/β ratio of 10 for tumor and 3 for normal). Localized delivery was performed using visualization of previously mentioned titanium fiducial markers via computed tomography (CT) scans. The dosing isocenter was positioned using a beam angle designed to avoid major organs. In each case, a dose volume histogram (DVH) was generated to confirm full dose deposition to the tumor and negligible amounts to surrounding organs (e.g., liver and TDLN).
Bioluminescent Imaging
KCKO-luc tumor growth was measured in vivo using the IVIS Spectrum Imaging System (IVIS, PerkinElmer). Mice were anesthetized via vaporized isoflurane and injected subcutaneously with D-luciferin (2.5 mg/100 μL PBS, Invitrogen). Mice were placed in the right lateral recumbent position and a series of images measuring photon emissions were collected at 2-minute intervals for 24 minutes. Bioluminescence (p/sec/cm2/sr) was calculated within circular regions of interest (ROIs) manually placed over tumors. Peak intensity was recorded for each tumor upon two sequential measurements demonstrating signal decay.
Microsphere Injection
Poly-lactic acid microspheres were manufactured using phase inversion as previously described (Egilmez et al., 2000). Lyophilized microspheres were resuspended in PBS prior to intratumoral delivery. Twenty-four hours following the final SBRT fraction (day 10), mice were anesthetized with vaporized isoflurane and a 10-mm laparotomy incision was made to expose pancreas tumors. Empty MS control (2 mg beads) or IL-12 MS (2 mg beads containing 0.5 μg recombinant IL-12) or fluorescent MS (2 mg beads containing AF-594-conjugated BSA) were injected intratumorally using a 30.5-gauge Hamilton syringe (20 μL per mouse tumor).
Whole Mount Microscopy
Whole mount microscopy was performed by excising tumors and lymph nodes from mice at endpoint. The afferent lymphatic vessel was dissected intact with the TDLN. Tissues were then placed on glass slides with two drops of PAB (1L PBS, 1g sodium azide, 10 g BSA). A coverslip was placed on top of the tissue and gently pressed down, excess PAB was removed by blotting. The tissue was then visualized via bright field and fluorescence microscopy of the same field of view. For confocal microscopy, tissues were prepared as above and the following antibodies were used for identification of blood and lymphatic vessels: CD31-BV421 (390, BioLegend) and LYVE-1-AF-488 (ALY7, eBioscience). Surface markers were stained for 30 minutes at 4°C in the dark, and washed two times with 2 mL of PAB for 2 minutes prior to mounting on slides. Images were captured using a Nikon A1R HD laser scanning confocal microscope using the high-speed resonant and galvano (non-resonant) scanner. All images were analyzed using ImageJ.
Imaging Flow Cytometry
Following sacrifice, the TDLN was mechanically dissociated between two frosted slides and filtered using 40-μm filters. Cells were resuspended in PAB (1L PBS, 1g sodium azide, 10 g BSA) at approximately 1 × 106 cells per sample. The following antibodies were used for identification of cell subsets: CD45-PE (30-F11, eBioscience), CD11b-BV450 (M1/70, eBioscience), F4/80-FITC (BM8, eBioscience), IA/IE-PerCP-Cy5.5 (M5/114.15.2, BD), Gr1-APC-Cy7 (RB6-8C5, BioLegend). Surface markers were stained for 30 minutes at 4°C in the dark. Cells were fixed for 20 minutes using Cytofix/Cytoperm (BD), washed and resuspended in PAB. All samples were run on an Amnis ImageStream GenX (Luminex Corporation). 2–5 × 104 events/sample were collected and analyzed using FlowJo software (FlowJo).
Luminex Analyte Assay
Following sacrifice, TDLNs were dissociated with a tissue homogenizer (Branson) in 150 μL 0.5x Cell Lysis Buffer 2 (R&D Systems, diluted in PBS), containing 1x Halt Protease Inhibitor Cocktail and 1x Halt Phosphatase Inhibitor Cocktail (ThermoFisher Scientific). Magnetic Luminex Assays were performed using a Mouse Premixed Custom Designed Cytokine/Chemokine Multi-Analyte Kit (R&D Systems). Assay procedures were carried out following manufacturer’s instructions. Microplates were run on a Bio-Plex 200 system (Bio-Rad) collecting 50–100 beads per target with less than 20% aggregate. Pierce BCA Protein Assays (ThermoFisher Scientific) were performed on remaining lysates following manufacturer’s instructions. Total protein concentrations for each sample were used for analyte normalization into pg/mg protein values.
Statistical Analysis
Statistical analysis was performed using Graphpad Prism 8 software. Data are presented as mean +/− SEM. For multiple group comparisons, significance was determined using the Kruskal-Wallis test (p<0.05). Significance for tumor survival were determined using the Mantel-Cox test (p<0.05).
Supplementary Material
Acknowledgements:
Supported by grants from the NIH (R01CA230277 to S.A.G.; R01CA168863 to D.C.L.; R01CA28332 to E.M.L. and S.A.G.; T90DE021985 to University of Rochester; and S10OD021548-01 to Jacqueline Williams, Training Grant to B.J.H.). We thank the core facilities at the University of Rochester Medical Center, specifically Eric Hernady and Dr. Carl Johnston of the Small Animal Irradiation Core, the Flow Cytometry Shared Resource Core, and the Confocal and Conventional Microscopy Core.
References
- Atkins MB, Robertson MJ, Gordon M, Lotze MT, DeCoste M, DuBois JS, Ritz J, Sandler AB, Edington HD, Garzone PD, Mier JW, Canning CM, Battiato L, Tahara H, Sherman ML. 1997. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res 3: 409–417. [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. 2000. Immunobiology of dendritic cells. Annu Rev Immunol 18: 767–811. [DOI] [PubMed] [Google Scholar]
- Bandola-Simon JM, Roche PA. 2019. Dendritic cells dysfunction in tumor-draining lymph nodes. J Immunol. 202: 135.16. [Google Scholar]
- Batty CJ, Tiet P, Bachelder EM, Ainslie KM. 2018. Drug delivery for cancer immunotherapy and vaccines. Pharm Nanotechnol 6: 232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo KA, Wang Y. 2002. Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J Cell Sci 115: 849–856. [DOI] [PubMed] [Google Scholar]
- Brierley JD, Gospodarowicz MK, Wittekind C (2017). UICC: TNM Classification of Malignant Tumours, 8th Edition. Wiley-Blackwell. [Google Scholar]
- Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, Amigorena S, Van’t Veer LJ, Sperling AI, Wolf DM, Krummel MF. 2014. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26: 638–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoto K, Chowdhury PS, Kumar A, Sonomura K, Matsuda F, Fagarasan S, Honjo T. 2017. Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependen antitumor activity. Proc Natl Acad Sci U S A 114: E761–E770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion JA, Walker A, Mitragotri S. 2008. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res 25: 1815–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wang L, Yao Q, Ling R, Li K, Wang H. 2004. Drug concentrations in axillary lymph nodes after lymphatic chemotherapy on patients with breast cancer. Breast Cancer Res 6: R474–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury D, Lieberman J. 2008. Death by a thousand cuts: granzyme pathways of programmed cell death. Annu Rev Immunol 26: 389–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran AJ, Huang RR, Lee J, Itakura E, Leong SP, Essner R. 2006. Tumour-induced immune modulation of sentinel lymph nodes. Nat Rev Immunol 6: 659–670. [DOI] [PubMed] [Google Scholar]
- Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. 2002. IFN-γ-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a roel for IP-10 in effector T cell generation and trafficking. J Immunol 168: 3195–3204. [DOI] [PubMed] [Google Scholar]
- Egilmez NK, Jong YS, Sabel MS, Jacob JS, Mathiowitz E, Bankert RB. 2000. In situ tumor vaccination with interleukin-12-encapsulated biodegradable microspheres: induction of tumor regression and potent antitumor immunity. Cancer Res 60: 3832–3837. [PubMed] [Google Scholar]
- Eisenbarth SC. 2019. Dendritic cell subsets in T cell programming: location dictates function. Nat Rev Immunol 19: 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen MF, Schoonderwoerd M, Knopf P, Camps MGM, Hawinkels LJAC, Kneilling M, van Hall T, Ossendorp F. 2018. Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight 3: e124507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EE, Cyster JG. 2012. Lymph node macrophages. J Innate Immun 4: 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber SA, Moran JP, Frelinger JG, Frelinger JA, Fenton BM, Lord EM. 2003. Mechanism of IL-12 mediated alterations in tumour blood vessel morphology: analysis using whole-tissue mounts. Br J Cancer 88: 1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon SB, Korn EL, Cochran AJ. 1987. Variations in functional immunocompetence of individual tumor-draining lymph nodes in humans. Cancer Res 47: 1740–1744. [PubMed] [Google Scholar]
- Jones D, Pereira ER, Padera TP. 2018. Growth and immune evasion of lymph node metastasis. Front Oncol 8: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataru RP, Ly CL, Shin J, Park HJ, Baik JE, Rehal S, Ortega S, Lyden D, Mehrara BJ. 2019. Tumor lymphatic function regulates tumor inflammatory and immunosuppressive microenvironments. Cancer Immunol Res 7: 1345–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, Sosman JA, Dutcher JP, Vogelzang NJ, Ryan JL. 1997. Blood 90: 2541–2548. [PubMed] [Google Scholar]
- Lieberman J 2003. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol 3: 361–370. [DOI] [PubMed] [Google Scholar]
- Louie DAP, Liao S. 2019. Lymph node subcapsular sinus macrophages as the frontline of lymphatic immune defense. Font Immunol 10: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. 2008. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol 38: 1404–1413. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Miyauchi E, Hsu YW, Nagayama S, Kiyotani K, Zewde M, Park JH, Kato T, Harada M, Matsui S, Ueno M, Fukuda K, Suzuki N, Hazama S, Nagano H, Takeuchi H, Vigneswaran WT, Kitagawa Y, Nakamura Y. 2019. TCR sequencing analysis of cancer tissues and tumor draining lymph nodes in colorectal cancer patients. Oncoimmunology 8: e1588085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills BN, Connolly KA, Ye J, Murphy JD, Uccello TP, Han BJ, Zhao T, Drage MG, Murthy A, Qui H, Patel A, Figueroa NM, Johnston CJ, Prieto PA, Egilmez NK, Belt BA, Lord EM, Linehan DC, Gerber SA. 2019. Stereotactic body radiation and interleukin-12 combination therapy eradicates pancreatic tumors by repolarizing the immune microenvironment. Cell Rep 29: 406–421.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenkamp BG, van Leeuwen PA, Meijer S, Sluijter BJ, Wijnands PG, Baars A, van den Eertwegh AJ, Scheper RJ, de Gruijl TD. 2007. Intradermal CpG-B activates both plasmacytoid and myeloid dendritic cells in the sentinel lymph node of melanoma patients. Clin Cancer Res 13: 2961–2969. [DOI] [PubMed] [Google Scholar]
- Moran I, Grootveld AK, Nguyen A, Phan TG. 2019. Subcapsular sinus macrophages: the seat of innate and adaptive memory in murine lymph nodes. Trends Immunol 40: 35–48. [DOI] [PubMed] [Google Scholar]
- Munn DH, Mellor AL. 2006. The tumor-draining lymph node as an immune-privileged site. Immunol Rev 213: 146–158. [DOI] [PubMed] [Google Scholar]
- Nathanson SD, Shah R, Rosso K. 2015. Sentinel lymph node metastases in cancer: causes, detection and their role in disease progression. Semin Cell Dev Biol 38: 106–116. [DOI] [PubMed] [Google Scholar]
- Olszewski WL, Stanczyk M, Gewartowska M, Domaszewska-Szostek A, Durlik M. 2012. Lack of functioning intratumoral lymphatics in colon and pancreas cancer tissue. Lymphat Res Biol 10: 112–117. [DOI] [PubMed] [Google Scholar]
- Palomba R, Palange AL, Rizzuti IF, Ferreira M, Cervadoro A, Barbato MG, Canale C, Decuzzi P. 2018. Modulating phagocytic cell sequestration by tailoring nanoconstrct softness. ACS Nano 12: 1433–1444. [DOI] [PubMed] [Google Scholar]
- Pan J, Zhang M, Wang J, Wang Q, Xia D, Sun W, Zhang L, Yu H, Liu Y, Cao X. 2004. Interferon-gamma is an autocrine mediator for dendritic cell maturation. Immunol Lett 94: 141–151. [DOI] [PubMed] [Google Scholar]
- Provenzano PP, Hingorani SR. 2013. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br J Cancer 108: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RS, June CH, Langer R, Mitchell MJ. 2019. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov 18: 175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, Kaisho T, Bogunovic D, Bhardwaj N, Krummel MF. 2016. Critical role for CD103(+)/CD141(+) dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell 30: 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman J, Koster BD, Jordanova ES, Heeren AM, de Gruijl TD. 2019. Unlocking the therapeutic potential of primary tumor-draining lymph nodes. Cancer Immunol Immunother 68: 1681–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, Casanova-Acebes M, Khudoynazarova M, Agudo J, Tung N, Chakarov S, Rivera C, Hogstad B, Bosenberg M, Hashimoto D, Gnjatic S, Bhardwaj N, Palucka AK, Brown BD, Brody J, Ginhoux F, Merad M. 2016. Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 44: 924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Harper CM, Hammer L, Nair RE, Mathiowitz E, Egilmez NK. 2004. Characterization of cytokine-encapsulated controlled-release microsphere adjuvants. Cancer Biother Radiopharm 19: 764–769. [DOI] [PubMed] [Google Scholar]
- Sluijter BJR, van den Hout MFCM, Koster BD, van Leeuwen PAM, Schneiders FL, van de Ven, Molenkamp BG, Vosslamber S, Verweij CL, van den Tol MP, van den Eertwegh AJM, Scheper RJ, Scheper RJ, de Gruijl TD. 2015. Arming the melanoma sentinel lymph node through local administration of CpG-B and GM-CSF: recruitment and activation of BDCA3/CD141(+) dendritic cells and enhanced cross-presentation. Cancer Immunol Res 3: 495–505. [DOI] [PubMed] [Google Scholar]
- Swartz MA, Lund AW. 2012. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat Rev Cancer 12: 210–219. [DOI] [PubMed] [Google Scholar]
- Tel J, Lambeck AJ, Cruz LJ, Tacken PJ, de Vries IJ, Figdor CG. 2010. Human plasmacytoid dendritic cells phagocytose, process, and present exogenous particulate antigen. J Immunol 184: 4276–4283. [DOI] [PubMed] [Google Scholar]
- Trinchieri G 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol 13: 251–276. [DOI] [PubMed] [Google Scholar]
- Tugues S, Burkhard Sh, Vrohlings M, Nussbaum K, vom Berg J, Kulig P, Becher B. 2015. New insights into IL-12-mediated tumor suppression. Cell Death and Differentiation 22: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuylsteke RJCLM Molenkamp BG, Gietema HA, van Leeuwen Pam, Wijnands PGJTB, Vos W, van Diest PJ, Scheper RJ, Meijer S, de Gruijl TD. 2004. Local administration of granulocyte/macrophage colony-stimulating factor increases the number and activation state of dendritic cells in the sentinel lymph node of early-stage melanoma. Cancer Res 64: 8456–8460. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Deguchi K, Zheng R, Tamai H, Wang L, Cohen PA, Shu S. 2008. Tumor-induced CD11b+Gr-1+ myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J Immunol 181: 3291–3300. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li N, Suh H, Irvine DJ. 2018. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun 9: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. 2014. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 74: 5057–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.