Abstract
Background:
Cancer-related fatigue (CRF) is a common side effect impacting breast cancer survivors. Research points to a relationship between obesity and CRF in breast cancer survivors, related to elevated systemic inflammation and metabolic alterations.
Methods:
This cross-sectional study examined the relationship of obesity to CRF, inflammatory markers and serum lipids through a secondary analysis of a nationwide randomized controlled trial. Breast cancer survivors with CRF were categorized based on BMI category. Symptoms of CRF, inflammatory markers and serum fatty acids were assessed among groups.
Results:
There were 105 breast cancer survivors in the analysis. BMI was positively associated with CRF based on MFSI General (p=0.020; 95% C.I. 0.024, 0.273) and MFSI Physical (p=0.013; 95% C.I. 0.035, 0.298) subscales. TNF-α (p=0.007; 95% C.I. 0.007, 0.044) and IL-6 (p=0.020; 95% C.I. 0.006, 0.073) were elevated in the obese. Monounsaturated fatty acid levels (p=0.047; 95% C.I. 0.000, 0.053) and the omega-6 to omega-3 fatty acid ratio were associated with obesity (p=0.047; 95% C.I. 0.002, 0.322).
Conclusions:
Obese breast cancer survivors had greater levels of CRF, inflammatory markers and certain fatty acids. Inflammatory markers and fatty acids were not found to have any mediating or positive association with CRF variables in this analysis. NCT02352779.
Keywords: Obesity, BMI, breast cancer, IL-6, cancer-related fatigue
Introduction
Cancer-related fatigue (CRF) is a persistent and debilitating symptom of cancer and its treatment and commonly affects patients with breast cancer (1–3). Symptoms of CRF include a subjective sense of physical, emotional, and/or cognitive tiredness that is not proportional to recent activity. CRF is an ongoing feeling of exhaustion that cannot be alleviated by sleep or rest and leads to loss of function and diminished quality of life (QOL) (3–6). Treatment options for CRF remain inadequate (1). Up to one-third of breast cancer survivors experience CRF for over ten years after treatment (7–9). Additionally, health status and pretreatment comorbidities, may impact the severity of CRF in a breast cancer survivor (10), including elevated body mass index (BMI), which is associated with increased CRF in patients with breast cancer (10–12).
Etiological factors for CRF may originate from alterations in metabolic pathways associated with chronic inflammation, although mechanisms of CRF from cancer and cancer treatment are still not clear (13, 14). In cancer, there is an increased systemic inflammatory response resulting in elevated levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8, IL-10 and other pro-inflammatory molecules (15–17). Xiao et al. (2017) identified a relationship between C-reactive protein (CRP) and other pro-inflammatory cytokines and fatigue, where elevated levels of inflammatory biomarkers correlated with greater CRF in patients with breast cancer (18, 19). Although cancer itself promotes inflammation, patients receiving chemotherapy treatment generally experience greater inflammation and symptoms of CRF long-term, due to the increased generation of inflammatory cytokines such as TNF-α from immune cells (20).
Obesity, as the result of the accumulation of excessive adipose tissue, is also associated with chronic low-grade inflammation (21, 22). As higher levels of white adipose tissue interacts with macrophages, both adipocytes and macrophages were found to produce exponentially more pro-inflammatory cytokines, including TNF-α and IL-1β (23, 24). Increasing levels of IL-1β stimulate production of IL-6, which may in turn increase symptoms of CRF in cancer survivors (23, 25).
Obesity also stimulates metabolic changes that are pro-inflammatory (26). Free fatty acid (FFA) levels often become elevated in obese individuals due to release from adipose tissue and reduced FFA clearance. FFAs are known to travel through the bloodstream and create low-grade inflammation in skeletal muscle and other regions of the body (27, 28). Elevated FFAs also inhibit insulin, including insulin’s anti-lipolytic action, promoting FFA release into the bloodstream (27, 28). Rates of insulin resistance are higher in the skeletal muscle of breast cancer survivors with obesity (29). Elevated levels of plasma free fatty acids promote defects in insulin signaling and insulin resistance (27, 30). Insulin resistance and elevated blood glucose levels further increase inflammation. Insulin resistance can decrease energy supply to muscle, is tied to fatigue and impaired mitochondrial function (22, 31). Taken together, insulin resistance in obese breast cancer survivors may contribute to CRF through higher inflammation levels, lower energy to muscle and mitochondrial dysfunction.
Dyslipidemia, common in obesity, is a condition where triglycerides and low-density lipoprotein (LDL) cholesterol are elevated while high density lipoprotein (HDL) levels are below healthy cutoffs (26). In patients with obesity, uncontrolled fatty acid release from adipose tissue, especially visceral adipose tissue, increases fatty acid delivery to the liver and synthesis of very-low-density lipoprotein (VLDL), further promoting hypertriglyceridemia or high serum triglycerides. Hypertriglyceridemia is associated with elevated IL-6 and TNF-α (28). What potential role, if any, elevated FFAs, dyslipidemia and chronic low-grade inflammation play in CRF remains unclear (28). However, due to these factors, the obese breast cancer patient may be burdened with a heavier symptom-load of CRF for a longer period of time (13).
In previous studies, excess adiposity appeared to be detrimental to the health and long-term prognosis of breast cancer survivors (32, 33). Many patients with breast cancer are overweight or obese at the time of diagnosis and obesity is a known risk factor for breast cancer (11, 34, 35). In addition, weight gain post-treatment is common for both premenopausal and postmenopausal breast cancer survivors (10, 11, 13, 36, 37).
Based on these previous findings, we hypothesized that the physiological etiology of CRF in breast cancer survivors may be directly linked to obesity and altered metabolic pathways resulting from increased adiposity. The primary objective of this cross-sectional study was to examine the strength of the relationship between obesity (based on BMI categories) and CRF symptoms in breast cancer survivors. We also investigated the relationship of BMI on metabolic factors such as blood lipids and inflammatory markers as potential mediators of CRF in this population. To our knowledge, this study is one of the first studies to assess the relationship of obesity on CRF, inflammation and serum lipids in breast cancer survivors.
Materials and Methods
Study Population
This study is a secondary analysis of a nationwide multicenter randomized controlled trial investigating the impact of fish oil vs. soybean oil supplementation on symptoms such as CRF in breast cancer survivors (38). Breast cancer survivors were recruited by clinical research coordinators during regularly scheduled oncologic visits. Eligibility included a confirmed diagnosis of breast cancer (stage 0-III), completion of chemotherapy, surgery and/or radiation therapy (on-going hormonal therapy allowed) within 4–36 months, ≥ 18 years of age and the presence of CRF. CRF was classified as a score ≥ 4 on the Symptom Inventory, an 11-point scale where “0” = no fatigue and “10” = as bad as you can imagine. Exclusion criteria included previous confirmed diagnosis of chronic fatigue syndrome.
The study was conducted through the University of Rochester Cancer Control NCI Community Oncology Research Program (NCORP) Research Base. A total of five NCORP sites obtained institutional review board approvals for participation in the randomized controlled trial. The study was activated November 2014 and closed to accrual in June 2015. This secondary analysis is a cross-sectional evaluation of all the participants prior to an intervention of lipid supplement administration. A complete description of methods was described previously (38).
Demographic and Medical Data
All eligible participants completed study specific forms for demographic information. Clinical data were collected by clinical research coordinators from medical charts. Height and weight, obtained from the clinical record, were used to calculate BMI. Participants were then classified based on BMI category to determine obesity status: normal weight (18.5–24.9 kg/m2; n=17), overweight (25.0–29.9 kg/m2; n=28), class I obesity (30.0–34.9 kg/m2; n=31), class II obesity (35.0–39.9 kg/m2; n=16) or class III obesity (≥ 40.0 kg/m2; n=13).
Karnofsky Performance Status (KPS) was obtained from the medical record. KPS Index is an assessment tool for functional impairment and prognosis in cancer survivors (39, 40). Ranging from 0 [dead] to 100 [normal activity, healthy], with a high score considered to be between 80–100 (41, 42). Race and exercise status came from self-reported information on demographic forms. Exercise status was assessed based on whether or not participants stated that they exercised weekly on a regular basis over the past six months.
Cancer-Related Fatigue Measures
Many researchers assess CRF as a multidimensional symptom of patient functioning, therefore, we used multiple tools for symptom assessment (43). Tools in this study examined the effect of CRF across several domains, including physical and socio-emotional functioning. Symptoms related to CRF were evaluated by the Brief Fatigue Inventory (BFI), Multidimensional Fatigue Symptom Inventory Short Form (MFSI-SF) and a fatigue question from the Symptom Inventory (SI) questionnaire. For all survey instruments except the MFSI Vigor subscale, a higher score indicates greater CRF. A lower score on the MFSI Vigor subscale is related to lower energy levels and mood state (44). The BFI is a 9-item, psychometrically validated instrument (45). Both the MFSI-SF and SI are reliable and validated in patients with cancer and breast cancer survivors (46, 47). The MFSI is a 30-item short form of the MFSI that yield scores for the empirically derived subscales. The subscales are designed to assess general, physical, emotional, behavioral and mental aspects of fatigue (48). The SI questionnaire asks patients to rate the severity of 13 disease and cancer treatment-related symptoms on an 11-point scale (49). The fatigue question was the only item included in this analysis.
Biomarkers
Serum was collected in vacutainers from a fasting blood draw at baseline. Protein levels of inflammatory markers (TNF-α, IFN-γ, IL-4, IL-5, IL-6, IL-8, IL-10) were assessed; samples were run in duplicate and quantified using a Luminex Magpix. The median of 50 beaded reactions per well was used to determine concentration per participant in picograms (pg)/mL. Pre-mixed customized MILLIPLEX MAP human cytokine and cytokine receptor magnetic bead immunoassay kits (catalog numbers: HCYTMAG-60K (Interleukins, TNF-α, IFN-γ)) were used for the analysis per manufacturer’s protocol (Millipore, Corp., Burlington MA).
Serum Fatty Acid and Lipid Analysis
Long-chain fatty acid and lipid concentrations were measured using capillary gas chromatography and electron-capture negative ion-mass spectrometry with use of methods previously described (Mayo Medical Laboratories, Rochester, MN).(50)
Statistical Analyses
Distribution of baseline characteristics was evaluated. The mean value and standard deviation were calculated for continuous variables and number (n) and proportion (%) of participants were reported for categorical measures. The magnitude and patterns of missing data were assessed for each variable across all groups of participants. No essential patterns of missing data were determined is this exploratory secondary analysis, and so we reported the complete case analysis.
The distribution of variables was assessed. Logarithmic transformations (log2) were applied to inflammatory markers to achieve a closer fit to normal distribution. Other variables did not require transformation. Initially, inflammatory markers, serum fatty acids, lipids and fatigue measures were evaluated by the mean of each of the five BMI categories. Then for the formal statistical inference, we evaluated BMI as a continuous variable. The associations of the measures with BMI were evaluated in bivariate analysis (Pearson’s correlations) and subsequently in multivariate linear regression analyses controlling for age, education level, time since diagnosis and exercise. The collinearity among covariates was assessed. Mediation analysis technique was used to assess the potential mediating effect of inflammatory markers and fatty lipids on the relation between obesity and CRF.(51) The analysis was adjusted by controlling for age, education level, time since diagnosis and exercise. P-values < 0.05 considered statistically significant. All analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC).
Results
Demographic and Medical Data
In the original randomized controlled trial there were 108 participants. However, three participants were excluded from this study due to not having weight records needed to calculate BMI, leaving 105 in the analysis. In this analysis, 105 women with breast cancer were evaluated for factors related to CRF, inflammation and lipids based on obesity status. The proportion of pre-menopausal patients was highest among participants with normal weight (29%). There were no marked differences among BMI categories in terms of race, education, cancer stage, exercise, previous cancer treatment or hormone status (see Table 1). The BMI assessment did not identify any breast cancer survivors who were underweight in this study. KPS scores were negatively correlated (p<0.001; 95% C.I. −0.667, −0.264) with BMI so that the participants in the class II and class III obesity groups had the lowest scores.
Table 1.
Descriptive statistics among groups, N=105.
| Variable | Normal n=17 | Overweight n=28 | Obese Class I n=30 | Obese Class II n=16 | Obese Class III n=13 | P-value |
|---|---|---|---|---|---|---|
|
| ||||||
| Age (mean, SD) | 57±14.5 | 61.6±8.7 | 58.7±10.5 | 60.3±9.7 | 59±10.8 | 0.703 |
| Body mass index (mean, SD) | 22.5±1.9 | 27.6±1.4 | 32.4±1.5 | 36.4±1.3 | 45.2±7.4 | <0.001 |
| Race (n, %) | 0.313 | |||||
| Caucasian | 16 (94%) | 24 (86%) | 30 (100%) | 15 (94%) | 12 (92%) | |
| Other | 1 (6%) | 4 (14%) | 0 | 1 (6%) | 1 (8%) | |
| Education | 0.214 | |||||
| Graduate | 4 (23.5%) | 6 (21%) | 3 (10%) | 1 (6%) | 1 (8%) | |
| College | 3 (17.5%) | 10 (36%) | 18 (60%) | 10 (63%) | 5 (38%) | |
| High School/GED | 9 (53%) | 9 (32%) | 8 (27%) | 4 (25%) | 6 (46%) | |
| No high School | 0 | 1 (4%) | 0 | 0 | 0 | |
| Unknown | 1 (6%) | 2 (7%) | 1 (3%) | 1 (6%) | 1 (8%) | |
| Menopausal status | 0.070 | |||||
| Premenopausal | 5 (29%) | 1 (4%) | 2 (7%) | 3 (19%) | 3 (23%) | |
| Postmenopausal | 12 (71%) | 27 (96%) | 28 (93%) | 13 (81%) | 10 (77%) | |
| Time from diagnosis (months) (mean, SD) | 21.1±8.0 | 18.0±9.3 | 24.9±9.9 | 16.3±7.0 | 20.0±9.8 | 0.015 |
| Cancer stage | 0.462 | |||||
| Stage 0 | 2 (12%) | 1 (4%) | 1 (3%) | 0 | 1 (8%) | |
| Stage 1 | 7 (41%) | 14 (50%) | 11 (37%) | 9 (56%) | 3 (23%) | |
| Stage 2 | 3 (17.5%) | 11 (39%) | 15 (50%) | 4 (25%) | 6 (46%) | |
| Stage 3 | 3 (17.5%) | 2 (7%) | 3 (10%) | 2 (13%) | 3 (23%) | |
| Unknown | 2 (12%) | 1 (6%) | ||||
| KPS (mean, SD) | 92.4±4.4 | 93.6±5.6 | 91.3±5.7 | 89.4±6.8 | 85.4±9.7 | 0.003 |
| Exercise (n, %) | 7 (41%) | 10 (36%) | 7 (21%) | 3 (19%) | 2 (15%) | 0.257 |
| Current Hormonal Therapy (n, %) | 12 (71%) | 24 (86%) | 20 (67%) | 13 (81%) | 11 (85%) | 0.593 |
| Previous Hormone Therapy (n, %) | 0 | 7 (25%) | 5 (17%) | 4 (25%) | 1 (8%) | 0.158 |
| Chemotherapy (n, %) | 7 (41%) | 12 (43%) | 15 (50%) | 8 (50%) | 8 (61%) | 0.766 |
| Radiation (n, %) | 14 (82%) | 18 (64%) | 19 (63.3%) | 13 (81%) | 9 (69%) | 0.379 |
| Surgery (n, %) | 16 (94%) | 25 (89%) | 28 (93.3%) | 15 (94%) | 11 (84%) | 0.704 |
| ER status (positive) | 14 (82%) | 24 (86%) | 26 (87%) | 12 (75%) | 12 (92%) | 0.751 |
| PR status (positive) | 11 (65%) | 23 (82%) | 21 (70%) | 11 (69%) | 10 (77%) | 0.705 |
| HER2 status (positive) | 4 (23.5%) | 4 (14%) | 6 (20%) | 2 (12.5%) | 3 (23%) | 0.773 |
SD, standard deviation; KPS, Karnofsky Performance Status; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
P-value was based on ANOVA for continuous variables and Chi-square for all categorical variables.
Cancer-Related Fatigue Measures
Obesity, based on BMI was associated with greater severity of CRF as measured by the MFSI General (p=0.020; 95% C.I. 0.024, 0.273) and MFSI Physical (p=0.013; 95% C.I. 0.035, 0.298) subscales, where the obese categories had the highest scores. MFSI Vigor (p=0.108; 95% C.I. −0.218, 0.022) and the MFSI-SF Total (p=0.055; 95% C.I. −0.009, 0.873) showed a trend towards significance, indicating a higher BMI may be associated with less vigor and more overall fatigue (see Table 2).
Table 2.
Differences in cancer-related fatigue measures among groups (N=105, mean ± standard deviation per group).
| Variable | Normal n=17 | Overweight n=28 | Obese Class I n=31 | Obese Class II n=16 | Obese Class III n=13 | P-value* | Adjusted P-value** |
|---|---|---|---|---|---|---|---|
| MFSI-SF Total | 19.00±15.75 | 22.42±12.46 | 29.00±17.30 | 25.27±16.33 | 32.58±16.14 | 0.051 | 0.055 |
| MFSI General | 14.00±5.15 | 13.42±3.96 | 16.07±4.28 | 13.60±4.48 | 18.42±2.84 | 0.043 | 0.020 |
| MFSI Physical | 5.93±4.30 | 8.12±4.55 | 7.10±4.97 | 7.87±4.36 | 11.42±5.26 | 0.040 | 0.013 |
| MFSI Emotional | 4.80±4.33 | 3.85±3.85 | 5.86±4.95 | 4.53±5.19 | 5.08±5.23 | 0.344 | 0.467 |
| MFSI Mental | 4.73±4.61 | 6.77±5.00 | 7.97±4.77 | 7.27±4.62 | 5.08±4.42 | 0.779 | 0.646 |
| MFSI Vigor | 10.47±4.61 | 9.73±4.34 | 8.00±4.30 | 8.00±4.94 | 7.42±3.65 | 0.044 | 0.108 |
| SI Fatigue | 6.00±2.48 | 5.85±1.43 | 6.76±1.86 | 6.20±1.66 | 7.08±1.73 | 0.371 | 0.481 |
| BFI Total | 4.67±1.58 | 4.97±1.56 | 5.37±1.93 | 5.78±2.03 | 5.62±1.90 | 0.125 | 0.184 |
MFSI, Multidimensional Fatigue Symptom Inventory-Short Form; SI, Symptom Inventory; BFI, Brief Fatigue Inventory.
P-value for bivariate association with BMI as a continuous variable.
P-value for association of markers with BMI as a continuous variable from multivariate linear regression controlling for age, education level, time since diagnosis and exercise.
Inflammatory Markers
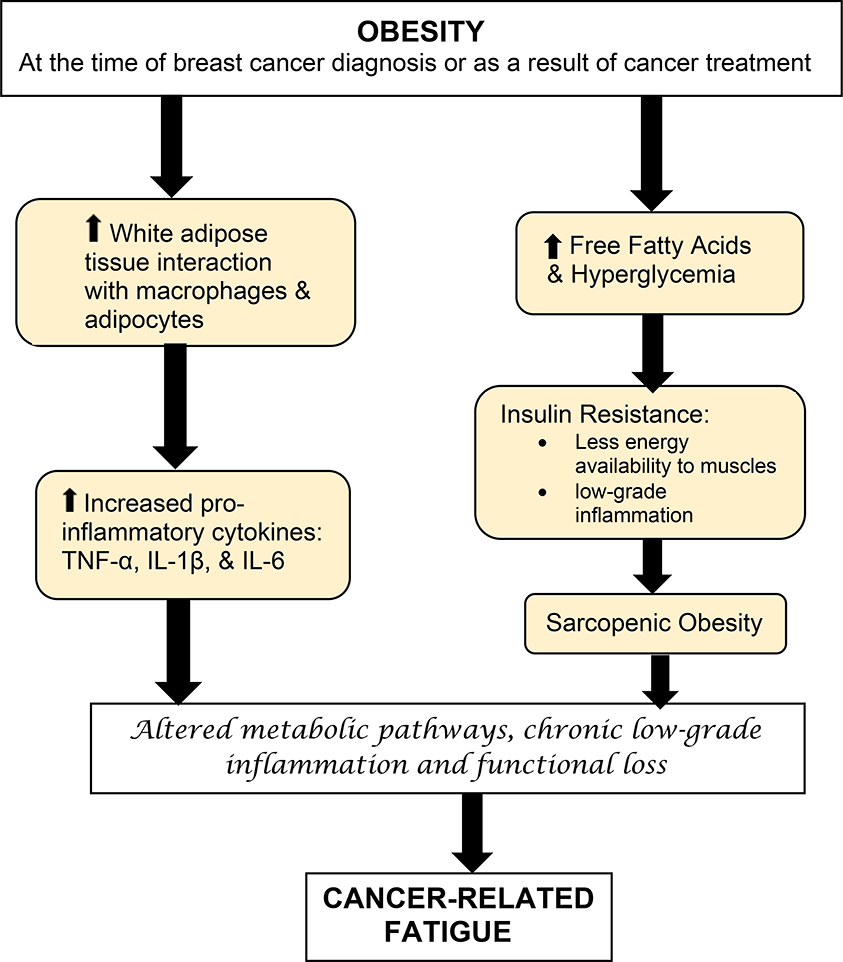
Among inflammatory cytokine levels, TNFα (p=0.007; 95% C.I. 0.007, 0.044) and IL-6 (p=0.020; 95% C.I. 0.006, 0.073) were elevated in the obese (see Figure 1); obese classes II and III had the highest levels and the normal weight participants had the lowest levels of these cytokines (see Table 3).
Figure 1.
Factors that contribute to cancer-related fatigue in breast cancer survivors with obesity.
Table 3.
Differences in inflammatory cytokines among groups (N=105, mean ± standard deviation).
| Variable§ | Normal n=17 | Overweight n=28 | Obese Class I n=31 | Obese Class II n=16 | Obese Class III n=13 | P-value* | Adjusted P-value** |
|---|---|---|---|---|---|---|---|
| TNFα, pg/ml | 2.10±1.19 | 2.17±0.53 | 2.48±0.45 | 2.56±0.60 | 2.51±0.68 | 0.030 | 0.007 |
| IL-1β, pg/ml | −0.13±1.62 | −0.13±1.62 | −0.19±1.55 | 0.02±1.81 | 0.99±2.01 | 0.175 | 0.219 |
| IL-2, pg/ml | 0.22±1.41 | 0.22±1.41 | −0.18±1.29 | −0.02±1.22 | 0.68±1.78 | 0.696 | 0.659 |
| IL-4, pg/ml | 2.05±1.68 | 2.05±1.68 | 2.44±2.16 | 1.42±2.36 | 2.92±2.08 | 0.231 | 0.364 |
| IL-5, pg/ml | 0.85±2.29 | 0.17±1.91 | 0.50±1.77 | 0.61±2.47 | 0.69±1.47 | 0.966 | 0.904 |
| IL-6, pg/ml | 0.60±1.61 | 1.23±1.17 | 1.20±1.17 | 1.42±0.72 | 1.88±0.91 | 0.020 | 0.020 |
| IL-8, pg/ml | 3.04±2.07 | 2.97±1.20 | 3.01±0.81 | 3.31±0.99 | 3.09±0.91 | 0.677 | 0.402 |
| IL-10, pg/ml | 2.19±1.67 | 2.31±1.45 | 2.52±1.44 | 2.39±1.44 | 2.93±1.30 | 0.342 | 0.365 |
| IFNγ, pg/ml | 2.10±1.19 | 2.17±0.53 | 2.48±0.45 | 1.77±1.76 | 2.78±1.35 | 0.192 | 0.112 |
Inflammatory markers were transformed by logarithm (log2).
TNF, tumor-necrosis factor; IL, interleukin; IFNγ, interferon-gamma.
P-value for bivariate association with BMI as a continuous variable.
P-value for association of markers with BMI as a continuous variable from multivariate linear regression controlling for age, education level, time since diagnosis and exercise.
Fatty Acid and Lipid Profiles
In the serum lipid analysis, only monounsaturated fatty acid levels (p=0.047; 95% C.I. 0.000, 0.053) and the omega-6 to omega-3 fatty acid ratio were significantly associated with obesity (p=0.047; 95% C.I. 0.002, 0.322) (see Table 4). Oleic acid trended towards significance among groups (p=0.053; 95% C.I. −0.241, 40.627). There was no mediating variable such as an inflammatory marker or fatty acid associated with both BMI and CRF simultaneously, leading researchers to conclude that the relationship of BMI to inflammation and inflammation to CRF is more complicated in this dataset. For the mediation analysis, biomarkers that were associated with BMI (IL-6,TNF-a, MUFA, Oleic Acid, Omega-3/Omega-6 ratio) were tested to further explain the relationship between obesity and CRF. None of the markers associated with BMI were also significantly associated with CRF in the mediation analysis.
Table 4.
Differences in serum fatty acids (μmol/L) and lipids among groups (N=85, mean ± standard deviation).
| Variable | Normal n=10 | Overweight n=24 | Obese Class I n=27 | Obese Class II n=15 | Obese Class III n=9 | P-value* | Adjusted P-value** |
|---|---|---|---|---|---|---|---|
| EPA † | 52.7±26.31 | 60.63±27.39 | 72.70±43.85 | 65.27±43.87 | 55.22±18.89 | 0.894 | 0.827 |
| DHA | 135.70±32.92 | 140.25±47.80 | 124.07±49.00 | 128.13±65.02 | 139.33±54.90 | 0.198 | 0.184 |
| α-Linolenic Acid | 67.60±27.43 | 74.67±27.94 | 97.59±44.13 | 78.93±29.88 | 78.22±36.44 | 0.525 | 0.860 |
| Linoleic Acid | 3245.10±526.37 | 3112.75±513.70 | 3499.15±711.39 | 3333.27±495.86 | 3513.11±671.19 | 0.360 | 0.569 |
| Lauroleic Acid | 2.10±0.64 | 2.21±0.47 | 2.60±0.82 | 2.49±0.52 | 2.47±0.81 | 0.175 | 0.469 |
| Lauric Acid | 22.50±13.90 | 17.63±8.40 | 24.52±11.90 | 21.47±6.91 | 20.44±17.88 | 0.515 | 0.977 |
| Myristoleic Acid | 8.50±3.69 | 13.38±6.85 | 19.37±10.45 | 15.27±5.73 | 15.00±9.75 | 0.119 | 0.301 |
| Myristic Acid | 119.60±33.68 | 142.17±50.19 | 182.44±72.66 | 159.60±43.22 | 165.00±127.34 | 0.121 | 0.346 |
| Oleic Acid | 1646.20±281.12 | 1822.25±395.71 | 2041.63±641.02 | 2000.07±534.50 | 2353.00±1100.99 | 0.027 | 0.053 |
| Arachidonic Acid | 749.00±187.61 | 814.67±200.80 | 813.78±298.62 | 788.60±279.87 | 862.78±232.98 | 0.995 | 0.874 |
| Palmitic Acid | 2213.70±346.88 | 2399.42±511.02 | 2768.33 ±690.88 | 2607.27±462.54 | 2973.89±1180.33 | 0.041 | 0.150 |
| Stearic Acid | 802±130.66 | 835.46±166.76 | 889.26±181.47 | 864.00±105.78 | 929.22±326.64 | 0.329 | 0.761 |
| Total Fatty Acids (mM) | 10.10±1.52 | 10.57±1.88 | 11.86±2.77 | 11.29±1.98 | 12.43±4.04 | 0.102 | 0.251 |
| SFA (mM) | 3.35±0.52 | 3.58±0.71 | 4.06±0.95 | 3.83±0.63 | 4.27±1.66 | 0.080 | 0.261 |
| MUFA (mM) | 2.19±0.33 | 2.43±0.53 | 2.79±0.83 | 2.71±0.68 | 3.12±1.41 | 0.019 | 0.047 |
| PUFA (mM) | 4.54±0.77 | 4.55±0.76 | 5.00±1.13 | 4.73±0.85 | 5.01±1.05 | 0.528 | 0.798 |
| Total ω3 (mM) | 0.30±0.07 | 0.31±0.10 | 0.33±0.12 | 0.29±0.13 | 0.30±0.11 | 0.522 | 0.355 |
| Total ω6 (mM) | 4.23±0.70 | 4.20±0.66 | 4.63±1.03 | 4.40±0.75 | 4.70±0.96 | 0.431 | 0.684 |
| ω6/ω3 ratio | 14.53±2.99 | 14.56±4.76 | 14.90±3.66 | 16.89±5.82 | 17.54±7.18 | 0.073 | 0.047 |
| Triene/tetraene ratio | 0.03±0.01 | 0.03±0.01 | 0.03±0.01 | 0.03±0.01 | 0.03±0.01 | 0.470 | 0.437 |
EPA, eicosapentaenoic acid; DHA, docosahexanoic acid; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; ω3, omega-3 fatty acids; ω6, omega-6 fatty acids.
P-value for bivariate association with BMI as a continuous variable.
P-value for association of markers with BMI as a continuous variable from multivariate linear regression controlling for age, education level, time since diagnosis and exercise.
Discussion
To our knowledge, this analysis is one of the first studies to assess the association of obesity on CRF, inflammation and serum lipids in breast cancer survivors. In this cohort of breast cancer survivors, we found a positive association between BMI and CRF levels, markers of systemic inflammation and serum lipids. CRF levels based on the MFSI-SF Physical and General subscales were highest in obese breast cancer survivors in this analysis. MFSI-SF Total scores demonstrated a trend towards significance with increasing obesity class. Inflammatory markers TNF-α and IL-6 were positively associated with BMI, with the highest levels in those with class II and class III obesity. Serum FFA levels were similar among obesity classes, although levels of monounsaturated fatty acids and the omega-6/omega-3 ratio were elevated in the obese breast cancer survivors.
Based on the MFSI General and MFSI Physical, the obese groups experienced more severe CRF. In a recent study, CRF levels as measured by MFSI-SF subscales were directly related to QOL in patients with resected lung cancer (52). Research by Chan et al. identified a minimal clinically important difference (MCID) for the MFSI-SF based on a within patient deterioration of 4.50–10.79 points based on anchor and distribution-based methods (53). In this study all the obese groups on average scored 6.27– 13.58 points higher on the MFSI-SF total than the normal weight group. Those in the class III obesity group continued to score above the MCID for MFSI Physical than the normal weight group. Although the MCID calculated by Chan et al. compared a within-patient difference, the greater scores for those in the obese groups may be a further indication for clinicians and researchers that the obese suffer greater fatigue (53, 54).
Previous studies reported that inflammatory markers IL-6 and TNF-α were elevated in patients with CRF. Van Vulpen et al. found IL-6 to be associated with CRF in a randomized controlled trial evaluating patients with breast cancer over the course of treatment (55). In a recent study of patients with acute myeloid leukemia, both men and women also demonstrated a positive relationship between IL-6 with CRF (56). Lastly, in a separate study evaluating single nucleotide polymorphisms (SNPs) in patients with breast cancer, Kühl et al. followed 1389 patients for 6.2 years post-diagnosis and 950 patients 11.7 years post-diagnosis; they identified a significant association between a SNP variant of TNF-α and long-lasting CRF post-chemotherapy (57). In this study elevated TNF-α and IL-6 levels and CRF were both positively associated with BMI and greater in participants in obese categories.
Obesity promotes chronic inflammation, greater concentrations of inflammatory cytokines and reduced adiponectin levels (10, 58). In obesity, macrophages are more common and their properties become altered so that the more abundant adipose tissue macrophages become pro-inflammatory (59). These pro-inflammatory macrophages produce tumor-promoting cytokines including TNF-α and IL-6 (59). Due to chronic inflammation, the obese breast cancer patient may be burdened with a higher symptom load, including greater CRF levels, over a longer period of time (10, 13). Future research examining the association of chronic inflammation due to obesity on CRF is needed in cancer survivors.
In this analysis, serum FFA levels were examined extensively among groups. Previous studies show a relationship with obesity and higher levels of FFAs (60, 61). Although serum FFA levels appeared to increase with obesity status, this difference was not significant for most fatty acids studied in this analysis. Only total monounsaturated fatty acids had a significant association with BMI. Myristoleic acid, oleic acid, palmitic acid and total fatty acid trended toward being higher in the obese groups, but the differences were not significant. In a recent study in Lebanon by Yammine et al., higher levels of serum monounsaturated fatty acids were significantly positively associated with BMI, dietary saturated fatty acids and endogenous lipogenesis in women (62). In another recent study looking at women with class III obesity, serum monounsaturated fatty acids and saturated fatty acids were positively associated with inflammation. As discussed earlier, increased inflammation is associated with CRF (63). The omega-6/omega-3 fatty acid ratio was also positively associated with higher BMI. A higher ratio of omega-6/omega-3 fatty acids in the peripheral blood leads to overproduction of pro-inflammatory cytokines and appears to promote higher rates of inflammatory chronic disease (64). Higher levels of omega-6 to omega-3 is also associated with obesity levels in previous research (65). Therefore, based on previous findings, the obese groups may have further inflammation due to elevated serum fatty acid levels. Increased inflammation may further increase CRF and other symptoms.
We conducted a mediation analysis with the goal of finding a variable significantly tied to both CRF and inflammation. None of the inflammatory markers (IL-6; TNF-α) or fatty acids (MUFA, Oleic Acid, Omega-3/Omega-6) which were associated with BMI were found to correlate to any CRF variables. A systematic review by Eyob et al., found inflammatory cytokines such as IL-6 to be associated with CRF in patients with cancer who received chemotherapy (66). Khosravi recently found IL-6 to be positively correlated with CRF in newly diagnosed leukemia patients (56). Sha et al., evaluated lung cancer patients and showed that TNF- α was elevated in patients with greater CRF (67). However, other research found that inflammatory cytokines such as TNF- α are no longer elevated after chemotherapy (68). The explanation as to why inflammatory markers were not directly tied to CRF in this study could be due to the fact that these breast cancer survivors were 4–36 months post-treatment. Although inflammation from cancer and cancer treatment leads to an increase in inflammation, this inflammatory response decreases over time post-treatment, even though the patient continues to experience CRF (12). The exact etiology of CRF is still not understood, and potentially, inflammation does not have to be present continually to alter mechanisms that lead to fatigue in cancer survivors. Furthermore, the small sample size of this study may not be sufficient to answer the question of whether inflammatory markers mediate the relationship between BMI and CRF. Only a modest numbers of studies are published on fatty acids and CRF with mixed findings on the influence of various fatty acids in breast cancer survivors (8, 38). More research is needed to determine whether an intake of a lower fat diet or more anti-inflammatory fatty acids reduce CRF.
Strengths for this study include a multicenter dataset and inclusion of variables related to both obesity and CRF such as inflammatory markers. This increases generalizability across female breast cancer survivors in the United States.
There are also a number of limitations to consider for this study. This analysis is cross-sectional and therefore cannot establish temporality. The sample was also limited in that most participants were Caucasian, post-menopausal and early stage (stage 0/I/II) breast cancer survivors. There were no data on lean mass and functional measures such as grip strength which may also relate to CRF and/or BMI. For fatty acids, data was missing for 19% of participants. This study also did not have data on chemotherapy dose, which may be somewhat increased in obese cancer patients and therefore contribute to symptoms.
Conclusion
In this study, CRF as measured by the MFSI General and MFSI Physical, was higher in obese breast cancer survivors, based on BMI. BMI was also positively correlated with inflammatory markers TNF-α and IL-6. Those with class II and class III obesity levels had the greatest levels of these markers. Blood lipids associated with inflammation, such as the omega-6 /omega-3 ratio, were also elevated in those with greater obesity levels based on BMI. These findings point to a potential combined role of obesity on CRF levels, systemic inflammation and serum lipids in breast cancer survivors. Interventions to help reduce long-term symptoms in breast cancer survivors should include ways to reduce obesity and to target inflammatory pathways associated with cancer, obesity and CRF. Awareness of the added burden of obesity in breast cancer survivorship may lead to better clinical outcomes in the medical and community settings.
Acknowledgments
We thank the participants of this study and all staff at the University of Rochester Cancer Center NCORP Research Base and our NCORP affiliate sites that recruited and observed participants. We also thank the staff of the Cancer Control Behavioral Medicine Research Unit and the Cancer Control and Psychoneuroimmunology Lab.
Funding
This work was supported by NIH Grants R03-CA175599, UG1-CA189961, K07-CA168911, NCI T32CA102618 and R25-CA102618.
Footnotes
Declaration of Interest Statement
The authors report no conflict of interest.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the University of Rochester. Informed consent was obtained from all participants. All mandatory laboratory health and safety procedures have been complied with in the course of conducting any experimental work reported in this paper.
Availability of data and Materials
Clinical Trials registration number: NCT02352779
References
- 1.Peoples AR, Roscoe JA, Block RC, Heckler CE, Ryan JL, et al. : Nausea and disturbed sleep as predictors of cancer-related fatigue in breast cancer patients: a multicenter NCORP study. Support Care Cancer 25, 1271–1278, 2017. doi: 10.1007/s00520-016-3520-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin PJ, Kleckner IR, Loh KP, Inglis JE, Peppone LJ, et al. : Influence of Yoga on Cancer-Related Fatigue and on Mediational Relationships Between Changes in Sleep and Cancer-Related Fatigue: A Nationwide, Multicenter Randomized Controlled Trial of Yoga in Cancer Survivors. Integr Cancer Ther 18, 1534735419855134, 2019. doi: 10.1177/1534735419855134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mustian KM, Lin P, Loh KP, Kleckner IR: Fatigue. In: Handbook of Cancer Suvivorship (2nd ed.) pp. 129–144. Springer: Cham, Switzerland, 2018. [Google Scholar]
- 4.Palesh OG, Mustian KM, Peppone LJ, Janelsins M, Sprod LK, et al. : Impact of paroxetine on sleep problems in 426 cancer patients receiving chemotherapy: a trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Sleep Med 13, 1184–90, 2012. doi: 10.1016/j.sleep.2012.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mustian KM, Roscoe JA, Palesh OG, Sprod LK, Heckler CE, et al. : Polarity Therapy for cancer-related fatigue in patients with breast cancer receiving radiation therapy: a randomized controlled pilot study. Integr Cancer Ther 10, 27–37, 2011. doi: 10.1177/1534735410397044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, et al. : Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol 32, 1840–50, 2014. doi: 10.1200/jco.2013.53.4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mortimer JE, Waliany S, Dieli-Conwright CM, Patel SK, Hurria A, et al. : Objective physical and mental markers of self-reported fatigue in women undergoing (neo)adjuvant chemotherapy for early-stage breast cancer. Cancer 123, 1810–1816, 2017. doi: 10.1002/cncr.30426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zick SM, Colacino J, Cornellier M, Khabir T, Surnow K, et al. : Fatigue reduction diet in breast cancer survivors: a pilot randomized clinical trial. Breast Cancer Res Treat 161, 299–310, 2017. doi: 10.1007/s10549-016-4070-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabrizi FM, Alizadeh S: Cancer Related Fatigue in Breast Cancer Survivors: in Correlation to Demographic Factors. Maedica (Buchar) 12, 106–111, 2017 [PMC free article] [PubMed] [Google Scholar]
- 10.Sheng JY, Sharma D, Jerome G, Santa-Maria CA: Obese Breast Cancer Patients and Survivors: Management Considerations. Oncology (Williston Park) 32, 410–7, 2018 [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitz KH, Neuhouser ML, Agurs-Collins T, Zanetti KA, Cadmus-Bertram L, et al. : Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. J Natl Cancer Inst 105, 1344–54, 2013. doi: 10.1093/jnci/djt223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inglis JE, Janelsins MC, Culakova E, Mustian KM, Lin PJ, et al. : Longitudinal assessment of the impact of higher body mass index on cancer-related fatigue in patients with breast cancer receiving chemotherapy. Support Care Cancer, 2019. doi: 10.1007/s00520-019-04953-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar NB, Fink A, Levis S, Xu P, Tamura R, et al. : Thyroid function in the etiology of fatigue in breast cancer. Oncotarget 9, 25723–25737, 2018. doi: 10.18632/oncotarget.25438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inglis JE, Lin PJ, Kerns SL, Kleckner IR, Kleckner AS, et al. : Nutritional Interventions for Treating Cancer-Related Fatigue: A Qualitative Review. Nutr Cancer, 1–20, 2019. doi: 10.1080/01635581.2018.1513046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMillan DC: The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 39, 534–40, 2013. doi: 10.1016/j.ctrv.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 16.Lippitz BE: Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol 14, e218–28, 2013. doi: 10.1016/s1470-2045(12)70582-x [DOI] [PubMed] [Google Scholar]
- 17.Lippitz BE, Harris RA: Cytokine patterns in cancer patients: A review of the correlation between interleukin 6 and prognosis. Oncoimmunology 5, e1093722, 2016. doi: 10.1080/2162402x.2015.1093722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao C, Miller AH, Felger J, Mister D, Liu T, et al. : Depressive symptoms and inflammation are independent risk factors of fatigue in breast cancer survivors. Psychol Med 47, 1733–1743, 2017. doi: 10.1017/s0033291717000150 [DOI] [PubMed] [Google Scholar]
- 19.Lacourt TE, Heijnen CJ: Mechanisms of Neurotoxic Symptoms as a Result of Breast Cancer and Its Treatment: Considerations on the Contribution of Stress, Inflammation, and Cellular Bioenergetics. Curr Breast Cancer Rep 9, 70–81, 2017. doi: 10.1007/s12609-017-0245-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang S, Chu S, Gao Y, Ai Q, Liu Y, et al. : A Narrative Review of Cancer-Related Fatigue (CRF) and Its Possible Pathogenesis. Cells 8, 2019. doi: 10.3390/cells8070738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kvehaugen AS, Aasbrenn M, Farup PG: Anti-Saccharomyces cerevisiae antibodies (ASCA) are associated with body fat mass and systemic inflammation, but not with dietary yeast consumption: a cross-sectional study. BMC Obes 4, 28, 2017. doi: 10.1186/s40608-017-0164-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee BC, Lee J: Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta 1842, 446–62, 2014. doi: 10.1016/j.bbadis.2013.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuggetta MP, Zonfrillo M, Villiva C, Bonmassar E, Ravagnan G: Inflammatory Microenvironment and Adipogenic Differentiation in Obesity: The Inhibitory Effect of Theobromine in a Model of Human Obesity In Vitro. Mediators Inflamm 2019, 1515621, 2019. doi: 10.1155/2019/1515621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y: Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci 13, 851–863, 2017. doi: 10.5114/aoms.2016.58928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu JM, Yang HT, Ho TW, Shun SC, Lin MT: Association between Interleukin-6 Levels and Perioperative Fatigue in Gastric Adenocarcinoma Patients. J Clin Med 8, 2019. doi: 10.3390/jcm8040543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller WM, Nori-Janosz KE, Lillystone M, Yanez J, McCullough PA: Obesity and lipids. Curr Cardiol Rep 7, 465–70, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Boden G: Obesity and free fatty acids. Endocrinol Metab Clin North Am 37, 635–46, viii-ix, 2008. doi: 10.1016/j.ecl.2008.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung UJ, Choi MS: Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci 15, 6184–223, 2014. doi: 10.3390/ijms15046184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cantarero-Villanueva I, Galiano-Castillo N, Fernandez-Lao C, Diaz-Rodriguez L, Fernandez-Perez AM, et al. : The influence of body mass index on survival in breast cancer patients. Clin Breast Cancer 15, e117–23, 2015. doi: 10.1016/j.clbc.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 30.Gastaldelli A, Gaggini M, DeFronzo RA: Role of Adipose Tissue Insulin Resistance in the Natural History of Type 2 Diabetes: Results From the San Antonio Metabolism Study. Diabetes 66, 815–822, 2017. doi: 10.2337/db16-1167 [DOI] [PubMed] [Google Scholar]
- 31.Suzuki R, Tamura Y: [Aging and homeostasis. Aging of skeletal muscle.]. Clin Calcium 27, 925–932, 2017. doi: CliCa1707925932 [PubMed] [Google Scholar]
- 32.Bradshaw PT, Cespedes Feliciano EM, Prado CM, Alexeeff S, Albers KB, et al. : Adipose Tissue Distribution and Survival Among Women with Nonmetastatic Breast Cancer. Obesity (Silver Spring), 2019. doi: 10.1002/oby.22458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayoub NM, Yaghan RJ, Abdo NM, Matalka II, Akhu-Zaheya LM, et al. : Impact of Obesity on Clinicopathologic Characteristics and Disease Prognosis in Pre- and Postmenopausal Breast Cancer Patients: A Retrospective Institutional Study. J Obes 2019, 3820759, 2019. doi: 10.1155/2019/3820759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azrad M, Blair CK, Rock CL, Sedjo RL, Wolin KY, et al. : Adult weight gain accelerates the onset of breast cancer. Breast Cancer Res Treat, 2019. doi: 10.1007/s10549-019-05268-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uhley VE, Jen K-LC: Nutrition and Weight Management. In: Handbook of Cancer Survivorship (2nd ed.) pp. 309–328. Springer: Cham, Swizerland, 2018. [Google Scholar]
- 36.Gandhi A, Copson E, Eccles D, Durcan L, Howell A, et al. : Predictors of weight gain in a cohort of premenopausal early breast cancer patients receiving chemotherapy. Breast 45, 1–6, 2019. doi: 10.1016/j.breast.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 37.Alacacioglu A, Kebapcilar L, Gokgoz Z, Oztekin O, Bozkaya G, et al. : Leptin, insulin and body composition changes during adjuvant taxane based chemotherapy in patients with breast cancer, preliminary study. Indian J Cancer 53, 39–42, 2016. doi: 10.4103/0019-509x.180836 [DOI] [PubMed] [Google Scholar]
- 38.Peppone LJ, Inglis JE, Mustian KM, Heckler CE, Padula GDA, et al. : Multicenter Randomized Controlled Trial of Omega-3 Fatty Acids Versus Omega-6 Fatty Acids for the Control of Cancer-Related Fatigue Among Breast Cancer Survivors. JNCI Cancer Spectr 3, pkz005, 2019. doi: 10.1093/jncics/pkz005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khatri D, Jaiswal A, Das KK, Pandey S, Bhaisora K, et al. : Health-related quality of life after surgery in supratentorial gliomas. Neurol India 67, 467–475, 2019. doi: 10.4103/0028-3886.257998 [DOI] [PubMed] [Google Scholar]
- 40.Quyen TC, Angkatavanich J, Thuan TV, Xuan VV, Tuyen LD, et al. : Nutrition assessment and its relationship with performance and Glasgow prognostic scores in Vietnamese patients with esophageal cancer. Asia Pac J Clin Nutr 26, 49–58, 2017. doi: 10.6133/apjcn.122015.02 [DOI] [PubMed] [Google Scholar]
- 41.Khalid MA, Achakzai IK, Ahmed Khan S, Majid Z, Hanif FM, et al. : The use of Karnofsky Performance Status (KPS) as a predictor of 3 month post discharge mortality in cirrhotic patients. Gastroenterol Hepatol Bed Bench 11, 301–305, 2018 [PMC free article] [PubMed] [Google Scholar]
- 42.Tandon P, Reddy KR, O’Leary JG, Garcia-Tsao G, Abraldes JG, et al. : A Karnofsky performance status-based score predicts death after hospital discharge in patients with cirrhosis. Hepatology 65, 217–224, 2017. doi: 10.1002/hep.28900 [DOI] [PubMed] [Google Scholar]
- 43.Jean-Pierre P, Figueroa-Moseley CD, Kohli S, Fiscella K, Palesh OG, et al. : Assessment of cancer-related fatigue: implications for clinical diagnosis and treatment. Oncologist 12 Suppl 1, 11–21, 2007. doi: 10.1634/theoncologist.12-S1-11 [DOI] [PubMed] [Google Scholar]
- 44.Asvat Y, Malcarne VL, Sadler GR, Jacobsen PB: Validity of the multidimensional fatigue symptom inventory-short form in an African-American community-based sample. Ethn Health 19, 631–44, 2014. doi: 10.1080/13557858.2014.885933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, et al. : The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 85, 1186–96, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Stein KD, Jacobsen PB, Blanchard CM, Thors C: Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage 27, 14–23, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones D, Zhao F, Fisch MJ, Wagner LI, Patrick-Miller LJ, et al. : The validity and utility of the MD Anderson Symptom Inventory in patients with prostate cancer: evidence from the Symptom Outcomes and Practice Patterns (SOAPP) data from the Eastern Cooperative Oncology Group. Clin Genitourin Cancer 12, 41–9, 2014. doi: 10.1016/j.clgc.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitehead L: The measurement of fatigue in chronic illness: a systematic review of unidimensional and multidimensional fatigue measures. J Pain Symptom Manage 37, 107–28, 2009. doi: 10.1016/j.jpainsymman.2007.08.019 [DOI] [PubMed] [Google Scholar]
- 49.Mendoza TR, Williams LA, Keating KN, Siegel J, Elbi C, et al. : Evaluation of the psychometric properties and minimally important difference of the MD Anderson Symptom Inventory for malignant pleural mesothelioma (MDASI-MPM). J Patient Rep Outcomes 3, 34, 2019. doi: 10.1186/s41687-019-0122-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lagerstedt SA, Hinrichs DR, Batt SM, Magera MJ, Rinaldo P, et al. : Quantitative determination of plasma c8-c26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders. Mol Genet Metab 73, 38–45, 2001. doi: 10.1006/mgme.2001.3170 [DOI] [PubMed] [Google Scholar]
- 51.Baron RM, Kenny DA: The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 51, 1173–82, 1986. doi: 10.1037//0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- 52.Chen HL, Liu K, You QS: Self-efficacy, cancer-related fatigue, and quality of life in patients with resected lung cancer. Eur J Cancer Care (Engl) 27, e12934, 2018. doi: 10.1111/ecc.12934 [DOI] [PubMed] [Google Scholar]
- 53.Chan A, Yo TE, Wang XJ, Ng T, Chae JW, et al. : Minimal Clinically Important Difference of the Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF) for Fatigue Worsening in Asian Breast Cancer Patients. J Pain Symptom Manage 55, 992–997.e2, 2018. doi: 10.1016/j.jpainsymman.2017.10.014 [DOI] [PubMed] [Google Scholar]
- 54.Donovan KA, Stein KD, Lee M, Leach CR, Ilozumba O, et al. : Systematic review of the multidimensional fatigue symptom inventory-short form. Support Care Cancer 23, 191–212, 2015. doi: 10.1007/s00520-014-2389-7 [DOI] [PubMed] [Google Scholar]
- 55.van Vulpen JK, Schmidt ME, Velthuis MJ, Wiskemann J, Schneeweiss A, et al. : Effects of physical exercise on markers of inflammation in breast cancer patients during adjuvant chemotherapy. Breast Cancer Res Treat 168, 421–431, 2018. doi: 10.1007/s10549-017-4608-7 [DOI] [PubMed] [Google Scholar]
- 56.Khosravi M, Taghvaye Masoumi H, Gholami K, Vaezi M, Hadjibabaei M, et al. : The Relationship between Fatigue and Cytokine Levels in Patients with Acute Myeloid Leukemia. Int J Hematol Oncol Stem Cell Res 12, 318–321, 2018 [PMC free article] [PubMed] [Google Scholar]
- 57.Kuhl T, Behrens S, Jung AY, Obi N, Thone K, et al. : Validation of inflammatory genetic variants associated with long-term cancer related fatigue in a large breast cancer cohort. Brain Behav Immun 73, 252–260, 2018. doi: 10.1016/j.bbi.2018.05.009 [DOI] [PubMed] [Google Scholar]
- 58.De Lorenzo A, Gratteri S, Gualtieri P, Cammarano A, Bertucci P, et al. : Why primary obesity is a disease? J Transl Med 17, 169, 2019. doi: 10.1186/s12967-019-1919-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Font-Burgada J, Sun B, Karin M: Obesity and Cancer: The Oil that Feeds the Flame. Cell Metab 23, 48–62, 2016. doi: 10.1016/j.cmet.2015.12.015 [DOI] [PubMed] [Google Scholar]
- 60.Feng R, Luo C, Li C, Du S, Okekunle AP, et al. : Free fatty acids profile among lean, overweight and obese non-alcoholic fatty liver disease patients: a case - control study. Lipids Health Dis 16, 165, 2017. doi: 10.1186/s12944-017-0551-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernandez-Navarro T, Diaz I, Gutierrez-Diaz I, Rodriguez-Carrio J, Suarez A, et al. : Exploring the interactions between serum free fatty acids and fecal microbiota in obesity through a machine learning algorithm. Food Res Int 121, 533–541, 2019. doi: 10.1016/j.foodres.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 62.Yammine SG, Naja F, Tamim H, Nasrallah M, Biessy C, et al. : Association between Serum Phospholipid Fatty Acid Levels and Adiposity among Lebanese Adults: A Cross-Sectional Study. Nutrients 10, 2018. doi: 10.3390/nu10101371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaska L, Mika A, Stepnowski P, Proczko M, Ratnicki-Sklucki K, et al. : The relationship between specific Fatty acids of serum lipids and serum high sensitivity C- reactive protein levels in morbidly obese women. Cell Physiol Biochem 34, 1101–8, 2014. doi: 10.1159/000366324 [DOI] [PubMed] [Google Scholar]
- 64.Simopoulos AP: The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 56, 365–79, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Simopoulos AP: An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 8, 128, 2016. doi: 10.3390/nu8030128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eyob T, Ng T, Chan R, Chan A: Impact of chemotherapy on cancer-related fatigue and cytokines in 1312 patients: a systematic review of quantitative studies. Curr Opin Support Palliat Care 10, 165–79, 2016. doi: 10.1097/spc.0000000000000205 [DOI] [PubMed] [Google Scholar]
- 67.Sha F, Zhuang S, Zhou L, Zhang L, Yang Y, et al. : Biomarkers for cancer-related fatigue and adverse reactions to chemotherapy in lung cancer patients. Mol Clin Oncol 3, 163–166, 2015. doi: 10.3892/mco.2014.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang S, Zeng D, Peng Y, Yang Y, Zhuang X, et al. : Cancer-related fatigue and chemotherapy-associated adverse effects: correlation with TNF-alpha, IL-1 and 17-hydroxycorticosteroids. Future Oncol 10, 1619–26, 2014. doi: 10.2217/fon.14.15 [DOI] [PubMed] [Google Scholar]