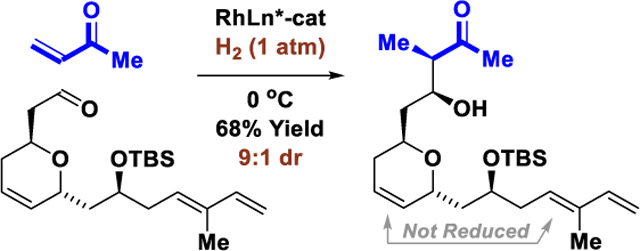
Abstract
Catalytic reductive coupling of enone, acrylate or vinyl heteroaromatic pronucleophiles with carbonyl or imine partners offers an alternative to base-mediated enolization in aldol and Mannich type reactions. In this monograph, direct catalytic reductive aldol and Mannich reactions are exhaustively catalogued on the basis of metal or organocatalyst. Step-wise processes involving enone conjugate reduction to form discrete enol or (metallo)enolate derivatives followed by introduction of carbonyl or imine electrophiles and aldol reactions initiated via enone conjugate addition are not covered.
Graphical Abstract
1. Introduction: Historical Perspective and Scope of Review
With initial observations by Kane (1838),1,2 but attributed to independent reports by Borodin (1869)3,4 and Würtz (1872),5,6 the aldol reaction is the Proteus of enolate-mediated C-C bond formations and persists as one of the most broadly utilized transformations in chemical synthesis. Core physical organic and stereochemical principles associated with the aldol reaction,7–13 applications of the aldol reaction in the total synthesis of natural products,14–19 and catalytic enantioselective aldol reactions20–24 have been reviewed. Indeed, the maturation of organic chemistry as a field, from its very inception to the current state-of-the-art, may be viewed through the lens of the aldol reaction and the diverse issues of selectivity posed by this fundamental transformation. The development of methods for base-mediated enolization of carbonyl compounds to furnish structurally defined (metallo)enolates had a pronounced impact on the field of aldol chemistry. Discrete formation of lithium enolates was first reported by Hauser (1951) using lithium amide.25–28 Wittig (1963) later described the use of lithium diisopropylamide (LDA) in deprotonations of aldimines in so-called “Wittig directed aldol condensations.”29–31 More detailed studies into dialkylamide bases ensued, which defined methods for stereoselective enolization under kinetically controlled conditions, as understood by Irelands model (1976),32 or under thermodynamic conditions. These advances, combined with the observations of Dubois (1967)33–35 and Heathcock (1980)36 that (Z)- and (E)-enolates undergo carbonyl addition stereospecifically through closed “Zimmerman-Traxler”37 transition structures to provide syn- and anti-addition products, respectively, laid the foundation for absolute stereocontrol, as exemplified by the use of Evan’s auxiliary (1981).38 Finally, in parallel with progress on stereocontrolled aldol additions of lithium12 and boron13,14,39 enolates, alternate, mechanistically distinct strategies for stereoselective aldol addition arose. The Mukaiyama aldol reaction (1973)40,18 intermolecular variants of the Hajos-Parrish-Eder-Sauer-Wiechert reaction (1971),41–43 metal-catalyzed asymmetric aldol additions reported by Hayashi and Ito (1986)44 and “direct” metal-catalyzed asymmetric aldol additions reported by Shibasaki (1997)45,46 offered powerful complementary approaches to stereoselective aldol addition (Figure 1).
Figure 1.
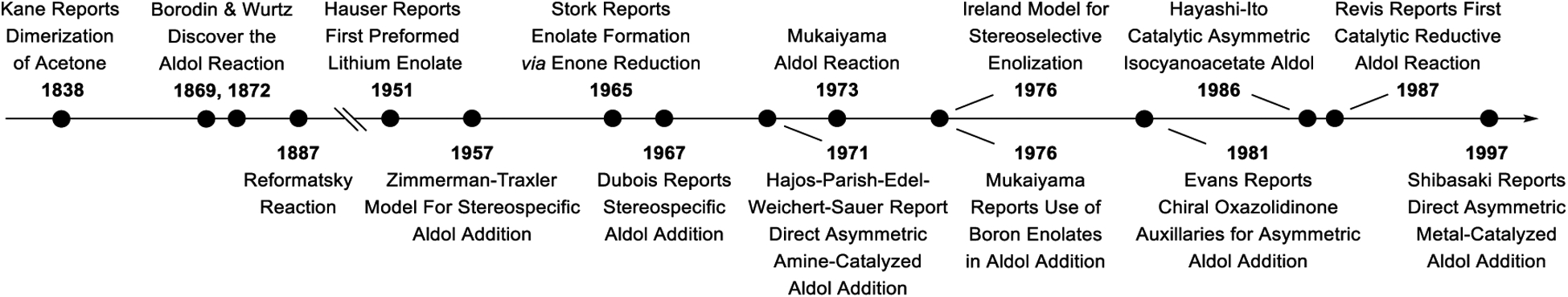
Selected milestones in aldol addition and enolization chemistry.
Along with this expansion in scope in aldol chemistry, certain limitations were brought to light. For example, whereas regioselective enolization is readily achieved for nonsymmetric ketones possessing different degrees of substitution at the α-positions, such as methylcyclohexanone, upon deprotonation under kinetically or thermodynamically controlled conditions,47,48 divergent regioselectivity is seldom attained in enolizations of non-symmetric ketones that possess identical degrees of substitution at the α-positions. The deprotonation of cholesterane-3-one represents a classic case.49–53 Thermodynamically controlled enolization delivers the Δ3-enolate with good isomer selectivity. In contrast, the Δ2-enolate cannot be formed selectively via deprotonation under kinetic or thermodynamic conditions. Further, introduction of 7,8-unsaturation results in an inversion of regioselectivity. An overwhelming thermodynamic preference in favor of the Δ2-enolate is observed, and the Δ3-enolate cannot be formed selectivity under kinetically or thermodynamically controlled deprotonation conditions. Reductive enolate generation, initially realized in the context of the Reformatsky reaction (1887),54 enables regiospecific formation of enolate isomers that are often inaccessible via base-mediated deprotonation. In what may be viewed as a prelude to the catalytic reductive aldol reaction, reductive enolization based on the dissolving metal reduction (Li/NH3) of conjugated enones was reported by Stork (1965) (Scheme 1).55,56
Scheme 1.
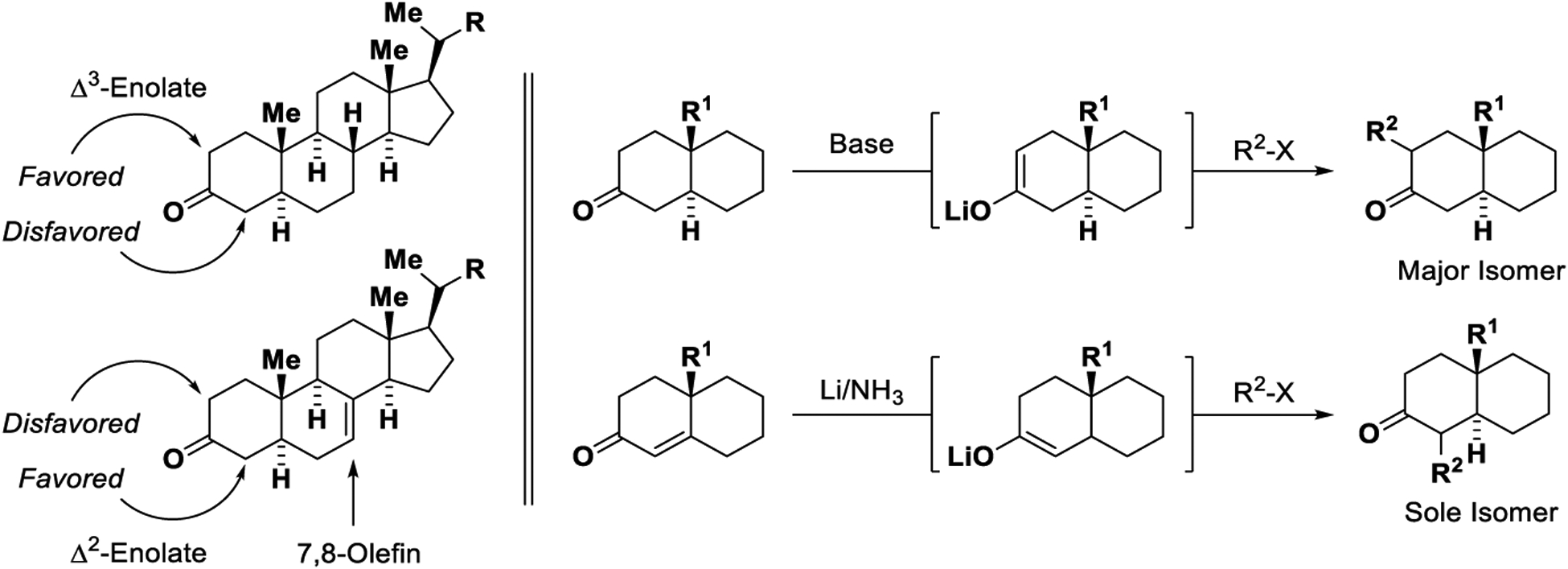
Classic studies highlighting the challenge of regioselective enolization.
Following Stork’s seminal studies, a diverse array of metal catalysts for the conjugate reduction of α,β-unsaturated carbonyl compounds were developed utilizing molecular hydrogen,57–63 silanes or borohydrides.64–71 This work encompasses enantioselective conjugate reductions of α,β-unsaturated carbonyl compounds catalyzed by ruthenium,72–80 rhodium,81–93 iridium,94–109 palladium,110–113 copper114–120 and cobalt complexes,121–128 as well as enantioselective Lewis base-catalyzed conjugate reductions.129–131,226 Additionally, preformation of enol derivatives in the context of tandem 1,4-reduction-carbonyl addition sequences have been disclosed.132–146 As described in the review literature, this abundance of prior art laid the foundation for catalytic reductive couplings of α,β-unsaturated carbonyl compounds partners with carbonyl electrophiles, termed “reductive aldol reactions.”147–162 Discovered over 30 years ago by Revis (1987),163 catalysts for reductive aldol coupling based on rhodium,163–189 cobalt,190–195 iridium,175,196 ruthenium,197–199 palladium,200 nickel,201–203 platinum,204 copper,205–220 zinc221 and indium222–225 have been described. Additionally, Lewis base-catalyzed reductive aldol additions have been described.226–229 Related catalytic reductive Mannich reactions230–237 and reductive couplings of vinyl heteroaromatic pronucleophiles to carbonyl and imines partners were developed in parallel.238–241
As shown herein, the catalytic reductive aldol reaction complements the scope of preexisting protocols for aldol addition. One advantage of the reductive aldol reaction resides in the ability to directly deploy feedstock pronucleophiles such as acrylates and methyl vinyl ketone, which enhances step-economy and minimizes mass-intensity (Figure 2).242 That is, for chiral auxiliary-based aldol additions, for example, the Evans aldol reaction,38,243 multiple steps are required for auxiliary synthesis and attachment, enolization and auxiliary removal, with each step utilizing sacrificial reagents that generate stoichiometric byproducts. Another advantage of the reductive aldol reaction relates to its regiospecificity, and the ability to access aldol isomers that are otherwise difficult to prepare (Scheme 2). For example, in direct metal-catalyzed45,46,244,245 or secondary amine-catalyzed aldol additions,246–250 the nonsymmetric ketone 2-butanone undergoes C-C coupling at the less substituted enolizable position. In contrast, enantioselective rhodium-catalyzed reductive aldol reactions of methyl vinyl ketone provide the corresponding branched isomers with complete levels of regiocontrol.188
Figure 2.
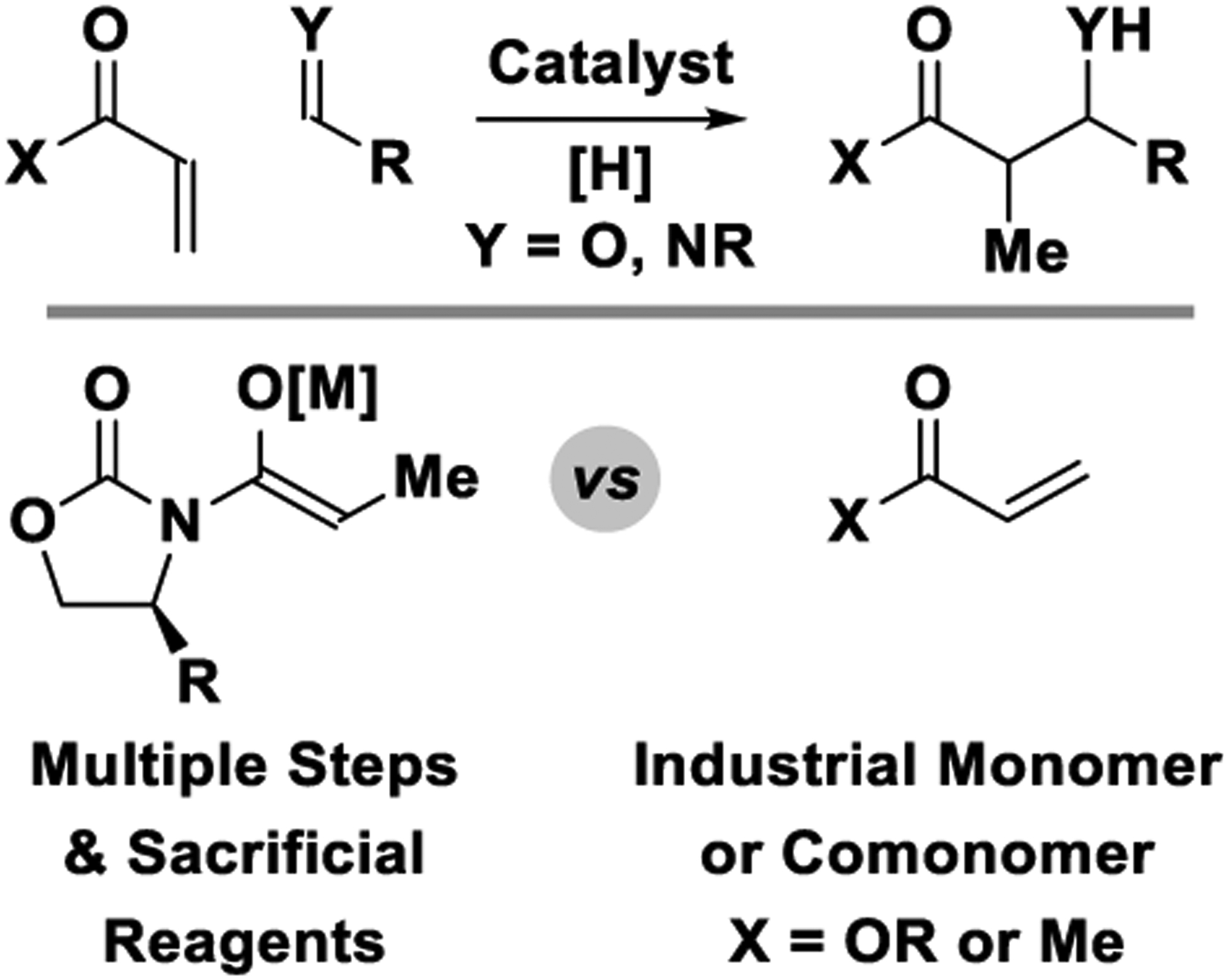
Catalytic reductive aldol and Mannich additions using abundant feedstock pronucleophiles.
Scheme 2.
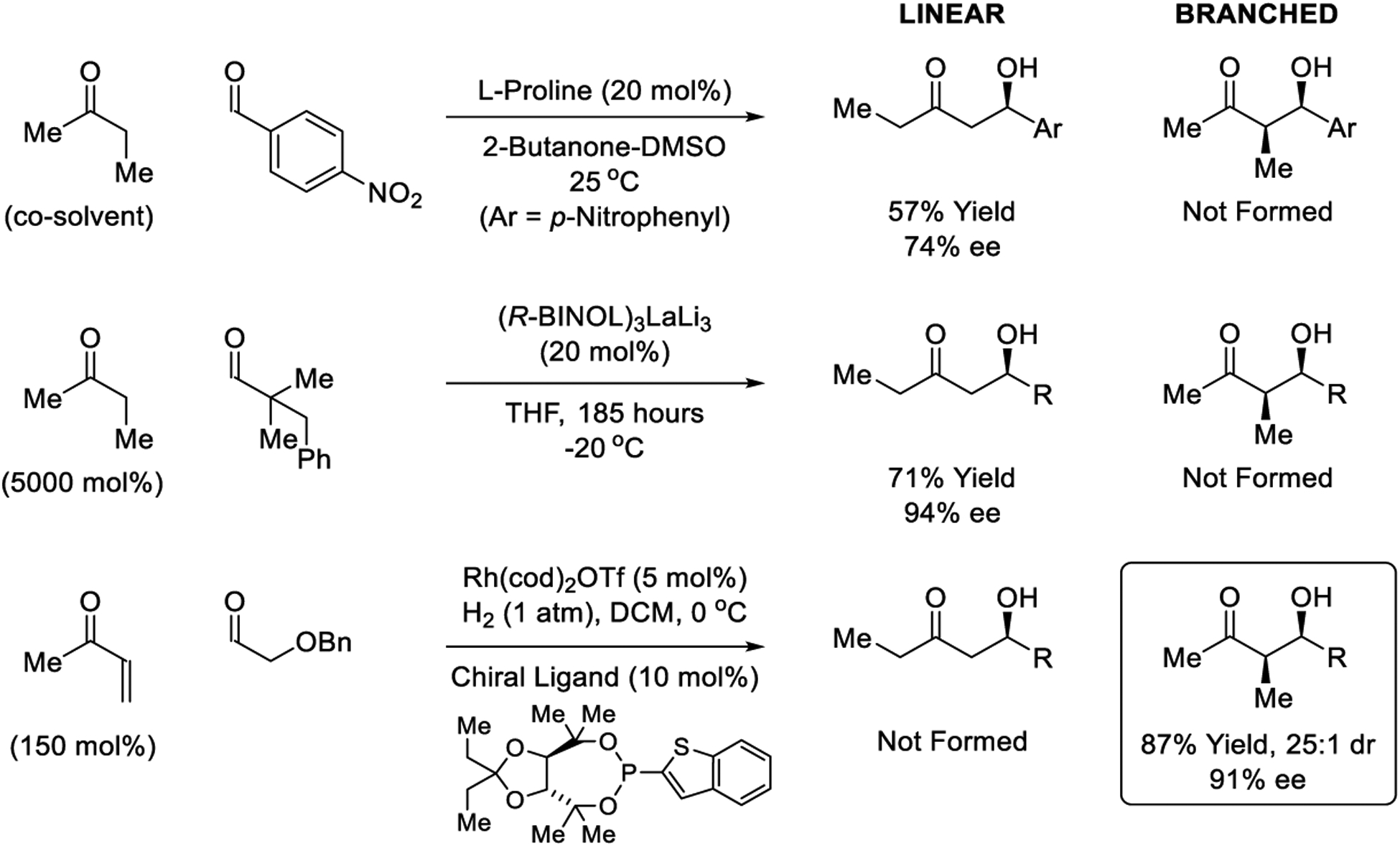
Catalytic reductive coupling enables regiospecific formation of branched aldol isomers.
In this review, catalytic reductive coupling of enone, acrylate and vinyl heteroaromatic pronucleophiles to carbonyl and imine partners are exhaustively catalogued on the basis of metal catalyst or organocatalyst.147–241 Catalytic reductive Michael reactions are described elsewhere.191,192,204,251–254 Step-wise processes involving conjugate reduction to form discrete (metallo)enolate derivatives followed by introduction of carbonyl or imine electrophiles,132–146 and aldol reactions initiated via conjugate addition255–262 have been reviewed elsewhere.263–266 For enone-C=X (X = O, NR) reductive couplings that result in functionalization at the β-position of the α,β-unsaturated pronucleophile, the reader is referred to the review literature.267–270 The catalytic reductive couplings described herein contribute to a departure from the use of premetalated reagents in carbonyl addition.242, 271–281
2. Catalytic Reductive Aldol and Mannich Reactions
2.1. General Catalytic Mechanisms
To streamline the discussion of mechanism, representative pathways for metal-catalyzed reductive aldol coupling are shown for rhodium-catalyzed reactions that employ the generic terminal reductant “H-Y”, where, for example, Y = SiR3 or H (Scheme 3). Organocatalyzed reductive aldol couplings are less common and their mechanisms will be discussed ad hoc. Catalytic cycle A is initiated via H-Y oxidative addition to rhodium(I) to form the rhodium(III) hydride I. Oxidative additions of hydrosilane (Y = SiR3)282 or hydrogen (Y = H) to neutral283–285 or cationic286–289 rhodium(I) complexes have been reviewed.290–292 Insertion of the aldehyde carbonyl into the Rh-Y bond to deliver complex II finds precedent in aldehyde silylformylations (Y = SiR3) catalyzed by cobalt293 and rhodium,294,295 but is unknown for other reductants. Enone or acrylate hydrometalation forms complex III, which upon C-C reductive elimination provides the aldol adduct (as the silyl ether for Y = SiR3) with regeneration of low valent rhodium to close the catalytic cycle. In catalytic mechanism B, enone or acrylate hydrometalation mediated by complex I furnishes rhodium(III) enolate IV, which upon aldehyde addition provides the rhodium(III) aldolate V. Reductive elimination of “H-Y”296,297 releases the aldol adduct (as the silyl ether for Y = SiR3) and low valent rhodium to close the catalytic cycle. This pathway finds precedent in aldol additions of preformed late transition metal enolates.298–302 Though not depicted, oxygen-silicon reductive elimination296,297 from enolate IV formation followed by Mukaiyama-type aldol addition also may affect formation of aldolate V. In catalytic cycle C, oxidative coupling of the reactants delivers oxarhodacyclopentane VI.303 σ-Bond metathesis with hydrosilane (Y = SiR3)304–306 or hydrogen (Y = H)307 provides complex VII, which upon C-H reductive elimination affords the aldol adduct and low valent rhodium to close the catalytic cycle. Finally, for catalytic cycle D, a rhodium(I) hydride, which in the case of elemental hydrogen as reductant is derived upon formal heterolytic hydrogen activation,308–310 promotes enone or acrylate hydrometalation to form rhodium(I) enolate VIII. Aldol addition affords aldolate IX, which upon hydrogen oxidative addition and O-H reductive elimination delivers aldol product.
Scheme 3.
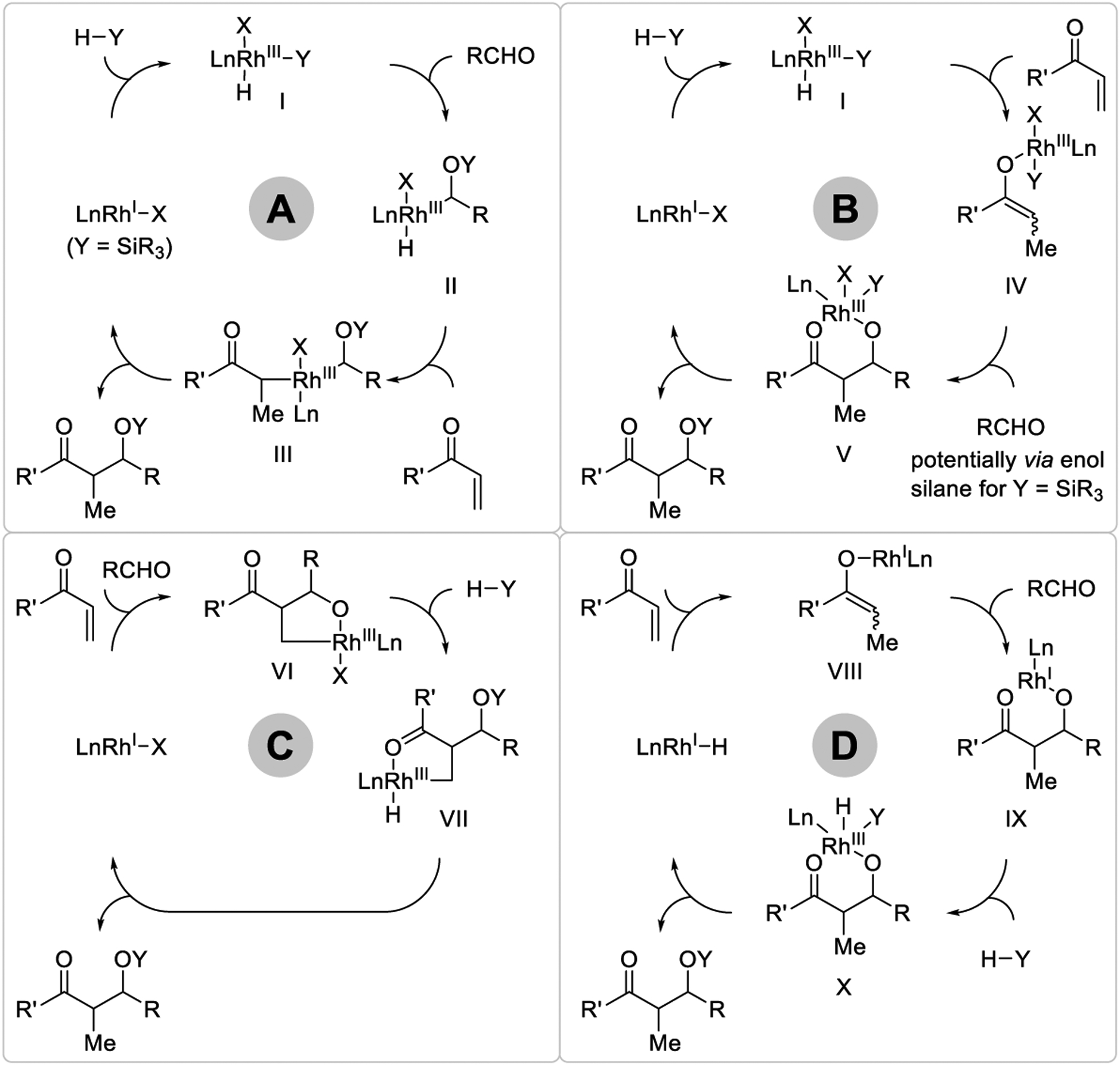
General mechanisms for metal-catalyzed reductive aldol addition using silane as terminal reductant.
2.2. Rhodium
2.2.1. Reductant ≠ Hydrogen
In 1987, Revis reported the first examples of reductive aldol coupling (Scheme 4).163 Upon exposure to substoichiometric quantities of RhCl3•3H2O in combination with Me3SiH as terminal reductant, methyl methacrylate (and related α,β-unsaturated esters) undergoes reductive coupling to both aldehyde and ketone partners. Aldol products that incorporate contiguous fully substituted carbon centers are formed in good yields at ambient temperature at exceptionally low catalyst loading. The silyl ketene acetal derived from methyl methacrylate could be detected in crude reaction mixtures, and attempted reductive coupling of methyl vinyl ketone to acetone instead resulted in enol silane formation. These data are consistent with intervention of catalytic mechanism B (Scheme 3). Using the rhodium catalyst derived from Rh4(CO)12 and MePh2P, Matsuda reports enone-aldehyde reductive couplings mediated by Et2MeSiH.164 Modest levels of syn-diastereoselectivity are observed for certain reaction products. Participation of β,β-disubstituted enone pronucleophiles is a notable feature of this catalytic system. Like Revis’ catalytic system, enol silanes are detected as byproducts. For the processes developed by both Revis and Matsuda, exposure of preformed silyl enol ethers to the carbonyl electrophile under the reaction conditions does not result C-C coupling. These data implicate rhodium enolates as reactive intermediates in the C-C bond forming event, and that Mukaiyama aldol pathways are not operative.
Scheme 4.
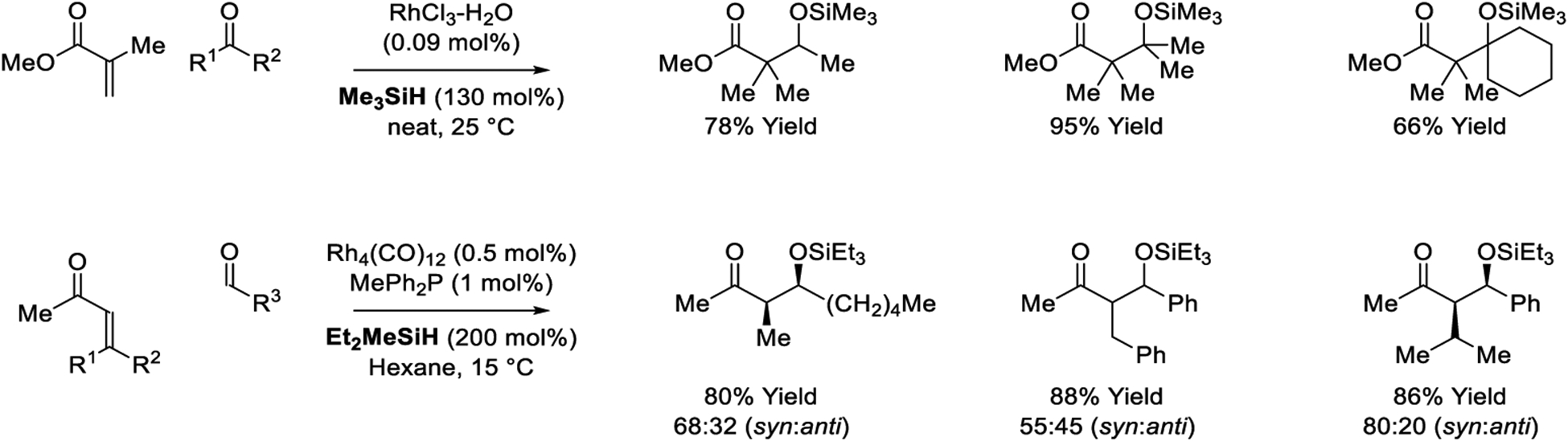
Rhodium-catalyzed reductive aldol reactions reported by Revis and Matsuda.
Rhodium-catalyzed reductive aldol cyclizations were first reported by Motherwell (Scheme 5).165,166 Diastereoselectivity in these processes is catalyst-dependent. Cyclizations catalyzed by Wilkinson’s complex, RhCl(PPh3)3, display a modest preference for formation of the cis-diastereomer. For cyclizations catalyzed by RhH(PPh3)4, a more pronounced preference for the anti-diastereomer is observed. The reaction is restricted to aldehyde electrophiles, as attempted cyclization onto a tethered ketone catalyzed results in olefin isomerization-enone hydrosilylation to form the indicated enol silane. The observed divergence in diastereoselectivity may reflect intervention of distinct catalytic pathways. For example, cyclizations catalyzed by RhCl(PPh3)3 vs RhH(PPh3)4 may occur through catalytic cycles B and D (Scheme 3), respectively; the latter involving a low-valent rhodium hydride. These different catalytic cycles might, in turn, possess different kinetic preferences for formation (E)- vs (Z)-enolates, which would be anticipated to undergo stereospecific addition.33–36
Scheme 5.
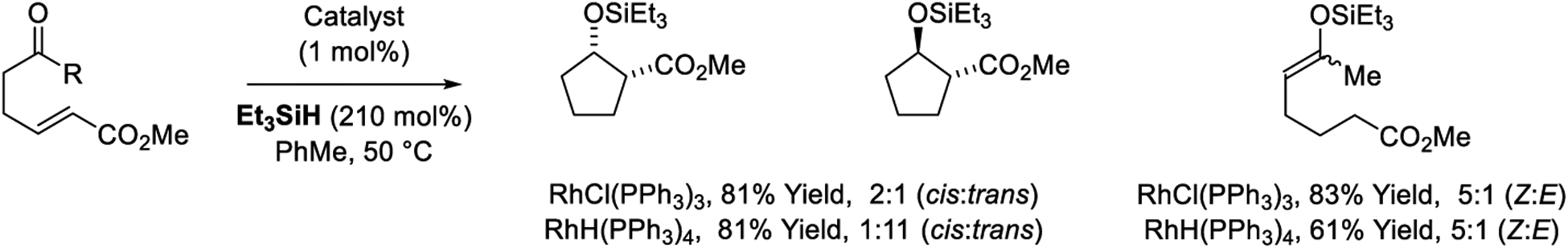
Rhodium-catalyzed reductive aldol cyclizations reported by Motherwell.
The first diastereo- and enantioselective reductive aldol couplings were developed by Morken (Scheme 6).167–169 Using an arrayed catalyst screening method, 192 independent catalytic systems were evaluated, which revealed a strong interdependence of reaction variables.167 Based on this approach, conditions for the diastereoselective reductive coupling of methyl acrylate and benzaldehyde were identified. Using the catalyst assembled from [Rh(cod)Cl]2 and Me-DuPhos in combination with Cl2MeSiH as terminal reductant, the aldol was obtained in good yield with exceptional syn-diastereoselectivity. Good levels of syn-diastereoselectivity are persevered upon application of these conditions to alternate reactants, but use of enolizable aldehydes results in lower isolated yields. Although these reactions employ a chiral ligand, racemic products are obtained. As subsequently determined, the reductant, Cl2MeSiH, promotes formation of silyl ketene acetals that spontaneously participate in carbonyl addition.169 Use of Et2MeSiH as reductant prevents such racemic background reactions, enabling enantioselective aldol addition of phenyl acrylate, albeit with lower levels of diastereocontrol.168 Under these conditions, but in the absence of aldehyde, less than 5% of the silyl ketene acetal was detected after 24 hours. Additionally, exposure of preformed silyl ketene acetal to aldehyde under these conditions in the absence of the acrylate led to the formation of the reductive aldol product in < 5% yield. Reactions employing PhMe2SiD as reductant result in partial deuteration at the former acrylate β-position with complete regiocontrol, consistent with reversible acrylate hydrometalation.
Scheme 6.
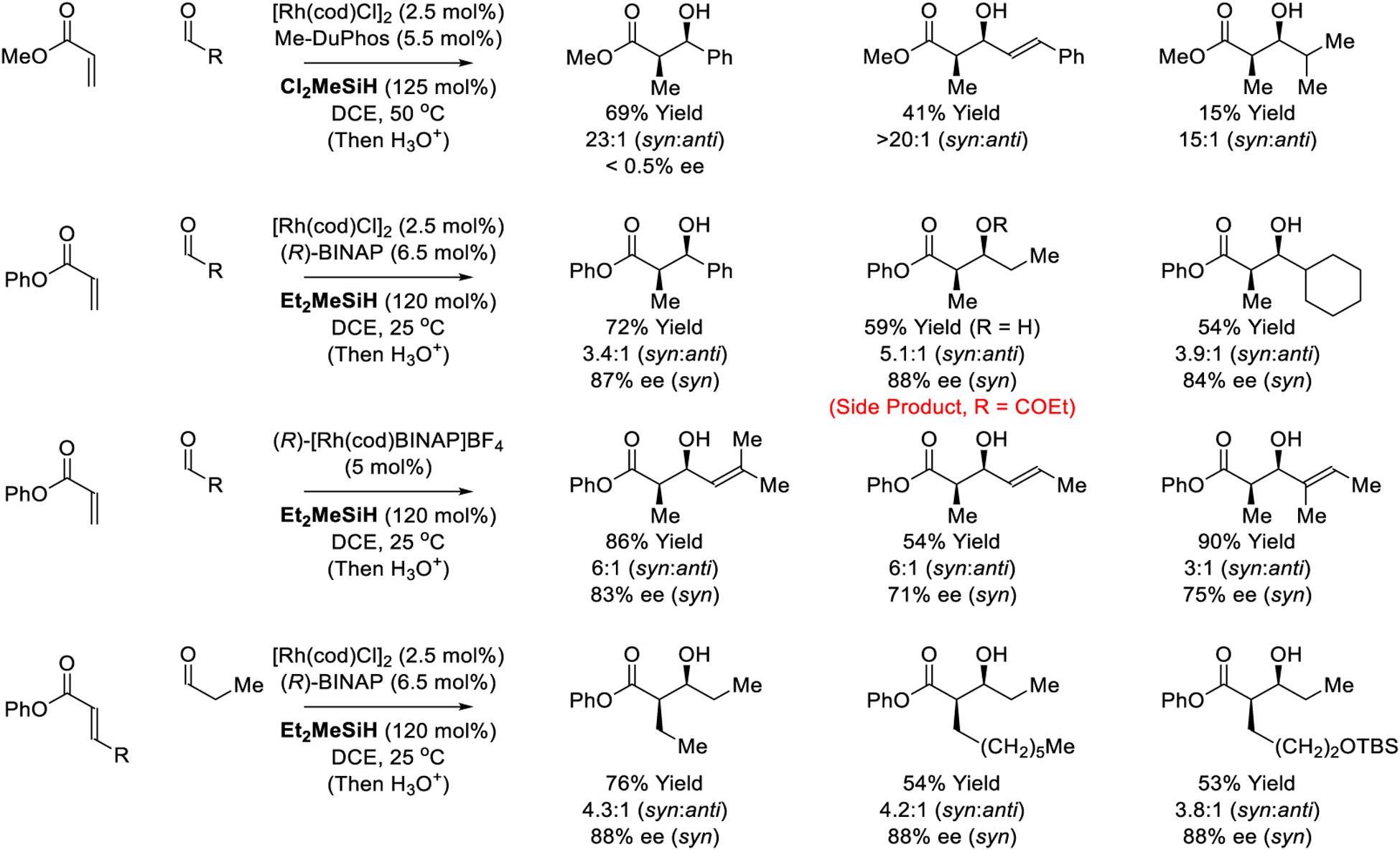
Diastereo- and enantioselective rhodium-catalyzed reductive aldol reactions reported by Morken.
The preceding enantioselective aldol additions of phenyl acrylate are accompanied by oxidative esterification side-products, which form with identical levels of diastereo- and enantioselectivity.170 Studies into the reaction mechanism led to several significant observations. Very little of the rhodium precatalyst entered the catalytic cycle, instead pooling as the chloro-bridged dimer, [Rh(BINAP)Cl]2. Moreover, upon exposure to hydrosilane, the dimer [Rh(BINAP)Cl]2 was converted to a species with NMR spectral characteristics consistent with the corresponding hydride-bridged dimer, [Rh(BINAP)H]2. To facilitate formation of the rhodium(I) hydride and, therefrom, entry into catalytic cycle B (Scheme 3), it was posited that cationic rhodium(I) precatalysts would be beneficial. Indeed, the authors found that using precatalyst (R)-[Rh(cod)BINAP]BF4 an expansion of reaction scope to encompass enal electrophiles could be realized (Scheme 6). Alternatively, by simply using a greater excess silane, the reaction of β-substituted acrylate pronucleophiles could be achieved.170,172 In further studies, the authors demonstrate that comparable yields and selectivities are obtained in the formation of the silyl-protected aldol adducts upon use of iPrMe2SiH as reductant (not shown).171
In 2005, Nishiyama disclosed a remarkably efficient Rh(phebox) catalyst for asymmetric silane-mediated reductive aldol addition (Scheme 7).173 Using tert-butyl acrylate as pronucleophile, uniformly high anti-diastereo- and enantioselectivities were observed across diverse aldehyde electrophiles. The level of enantiomeric enrichment is highly dependent upon the choice of silane, suggesting the silyl group is present during the enantiodetermining event, which is consistent with catalytic cycle B (Scheme 3). Although attempts to modify the structure of the phebox ligand did not avail significant expansion of scope (not shown),175,176 it was subsequently shown that ketones are competent electrophilic partners in reactions of β-substituted acrylate pronucleophiles catalyzed by the parent Rh(phebox) complex.177 High levels of stereoselectivity were observed in reductive aldol additions to the chiral α-stereogenic aldehyde, 2-phenyl propionaladehyde, when the enantiofacial bias of the catalyst matches the Felkin-Anh preference the aldehyde.179 Finally, cyclic enones were shown to be effective pronucleophiles.180 These transformations are conducted at 50°C with slow addition of hydrosilane. anti-Diastereo- and enantioselective aldol addition is postulated to occur through a chair-like transition state37 by way of the (E)-enolate in accord with the indicated stereochemical model.
Scheme 7.
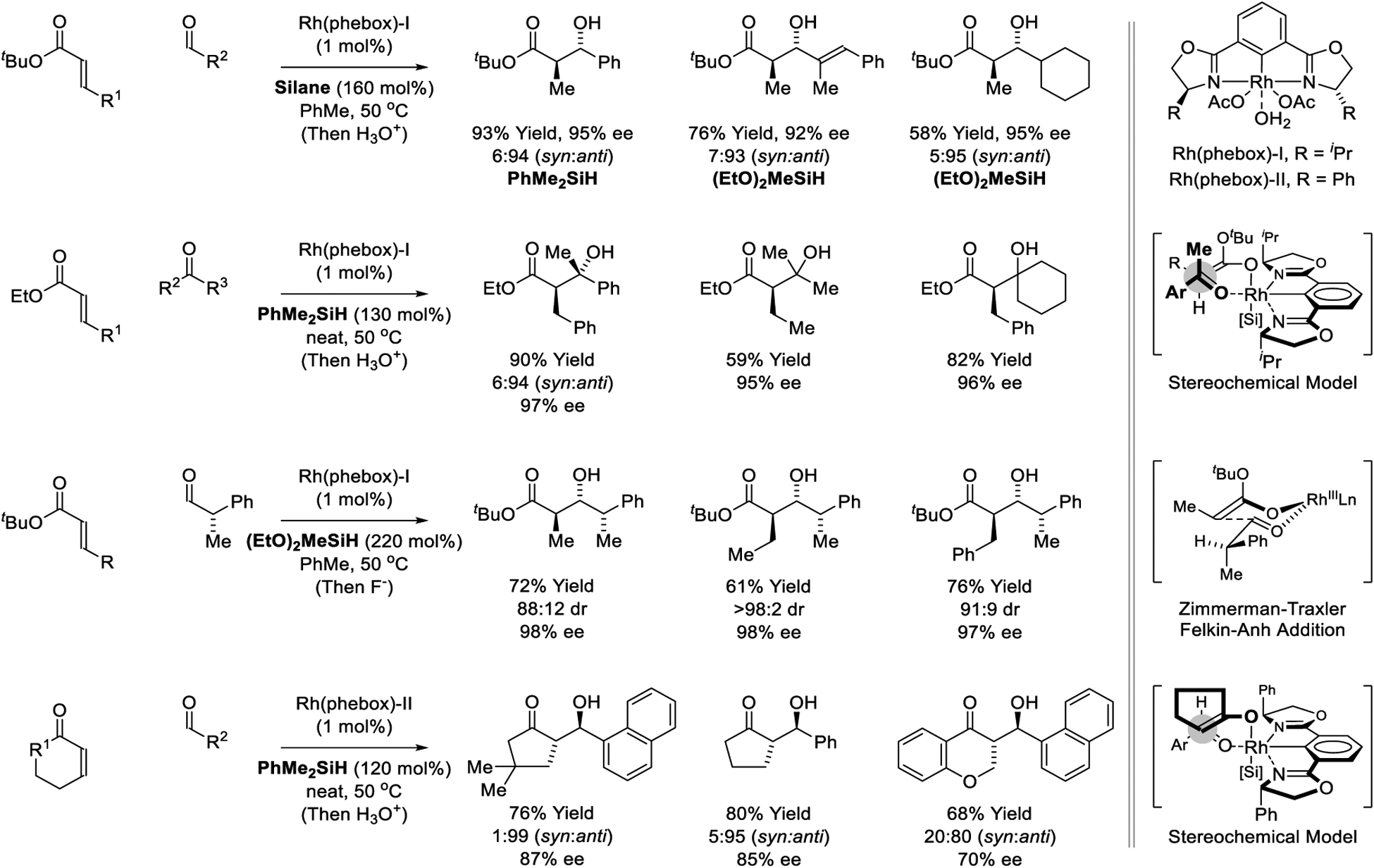
Diastereo- and enantioselective Rh(phebox)-catalyzed reductive aldol reactions reported by Nishiyama.
In 2005, Willis developed a variant of the catalytic reductive aldol reaction wherein β-sulfido-aldehydes serve dually as carbonyl electrophiles and reductants.171 As initially observed by Bendorff,311 the β-sulfide directs aldehyde C-H oxidative addition to form a chelated acylrhodium hydride. Enone hydrometalation provides a rhodium enolate. Carbonyl addition to a second β-sulfido-aldehyde provides an aldolate, which upon C-O reductive elimination delivers the O-acyl aldol with concomitant catalyst regeneration. Thus, oxidative esterification balances reductive aldol addition. The authors later demonstrated that crossed three-component reductive aldol addition could be achieved upon use of tert- butyl glyoxalate as the electrophilic partner.178 Although these reactions proceed in excellent yield, the lack of diastereo- and enantiocontrol diminishes their preparative utility (Scheme 8).
Scheme 8.
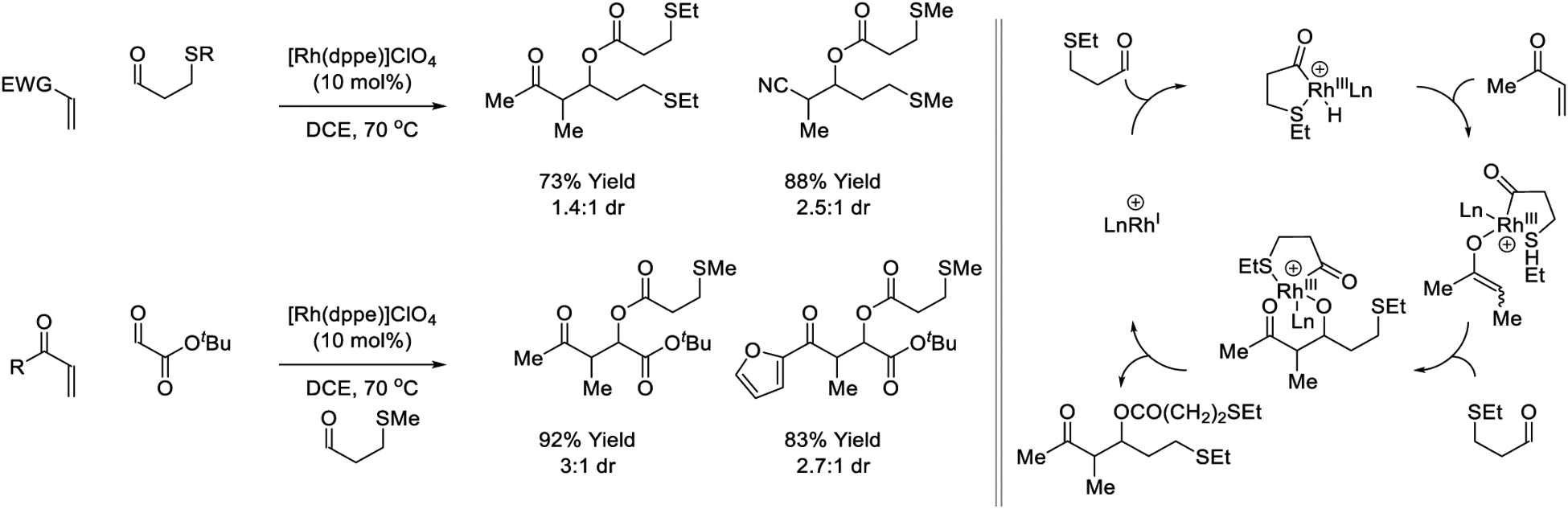
Rhodium-catalyzed reductive aldol reactions reported by Willis.
In 2002, Matsuda reported the first rhodium-catalyzed reductive Mannich reaction (Scheme 9).230 Exposure of methyl acrylate to N-tosyl or N-phenyl imines in the presence of Et2MeSiH and substoichiometric quantities of [Rh(cod){P(OPh)3}2]OTf led to good yields of the Mannich products, however, modest levels of diastereoselectivity were observed. A Rh(phebox)-catalyzed reductive Mannich reaction of tert-butyl acrylate and N-phenyl imines mediated by Et2MeSiH was subsequently reported by Nishiyama (Scheme 9).233 Good yields were accompanied by uniformly good levels of anti-diastereoselectivity. Finally, using Et2Zn as terminal reductant, Ando developed a reductive Mannich reaction of methyl acrylate with N-benzyl or N-p-methoxyphenyl imines (Scheme 9).236 In this process, anti-diastereoselective imine addition delivers a zinc amides that undergoes cyclization to furnish cis-β- lactams in good to excellent yields and high levels of relative stereocontrol. The authors applied this method to the synthesis of the β-lactam cholesterol absorption inhibitor (±)-ezetimib (not shown).237
Scheme 9.
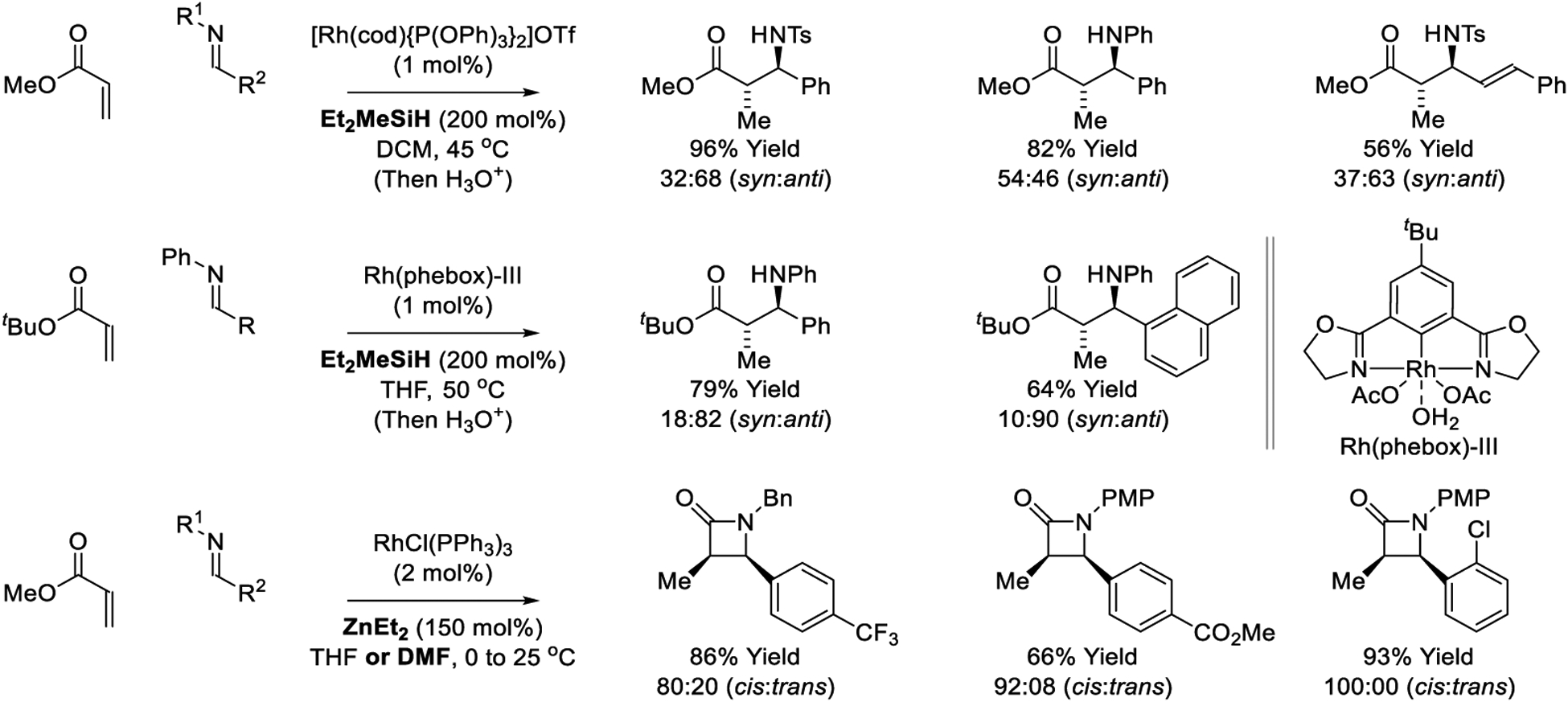
Rhodium-catalyzed reductive Mannich reactions reported by Matsuda, Nishiyama and Ando.
2.2.2. Reductant = Hydrogen
Hydroformylation, the prototypical C-C bond forming hydrogenation, is a longstanding method for industrial chemical manufacture.312–317 As described in the review literature,154,273,277,278,318,319 the Krische laboratory developed the first hydrogen-mediated reductive couplings beyond carbon monoxide, including reductive aldol additions.181–189 Using cationic rhodium catalysts, hydrogenation of enone-aldehydes promotes syn-diastereoselective aldol cyclization to form 5- and 6-membered rings (Scheme 10).181–183 Enones bearing tethered ketones also undergo aldol cyclization with complete levels of syn-diastereoselectivity, however, variable quantities of competing enone hydrogenation are observed (indicated parenthetically).182 In related cyclizations of enones bearing tethered 1,3-diketones, bicyclic ring systems that incorporate three contiguous stereocenters are formed and competing enone hydrogenation is not observed.182 The aldol addition of aldehyde enolates to ketones represents a particularly challenging transformation due to the reversibility of carbonyl addition.11,320 Rhodium-catalyzed hydrogenation of enal-ketones delivers the aldol adducts in good yield with a preference for the syn-diastereomer, but competitive enal hydrogenation is again evident.183
Scheme 10.
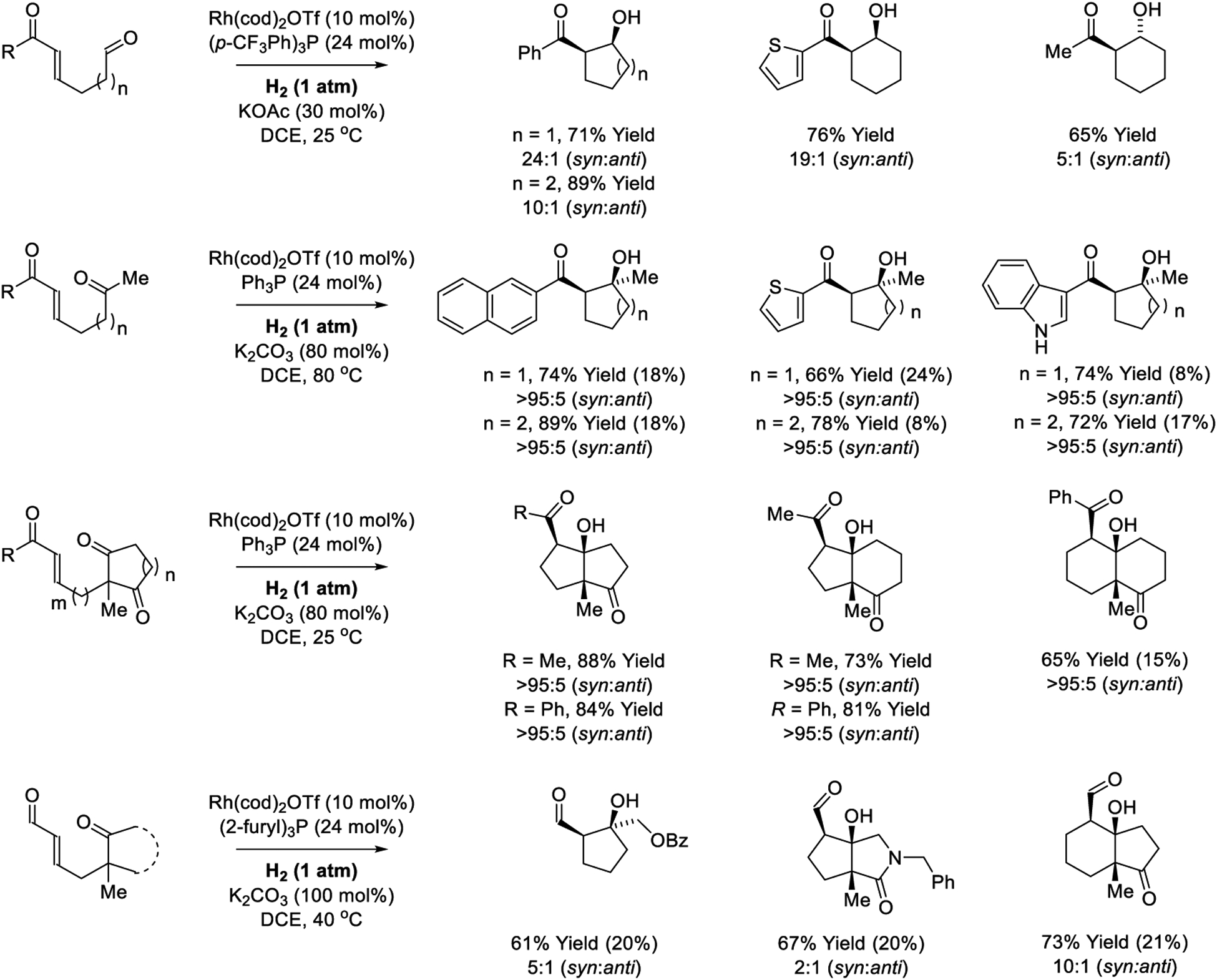
Reductive aldol cyclizations via rhodium-catalyzed hydrogenation reported by Krische.
Initially developed intermolecular variants of the hydrogen-mediated reductive aldol reaction gave the desired products as diastereomeric mixtures (not shown).181 It was later found that cationic rhodium complexes modified by tri-2-furylphosphine321,322 catalyzed intermolecular hydrogen-mediated reductive aldol addition of methyl vinyl ketone or ethyl vinyl ketone with excellent levels of syn-diastereoselectivity (Scheme 11).185 Notably, diverse reducible functional groups (alkynes, alkenes, benzylic ethers, nitroaryl and aromatic bromides) were tolerated under the reductive coupling conditions. Additionally, more highly functionalized enone pronucleophiles, such as crotyl vinyl ketone, were found to undergo chemoselective aldol reductive coupling at the less substituted vinyl moiety with good levels of syn-diastereoselectivity and without competing hydrogenation of the crotyl substructure.186 Substrate-directed asymmetric induction is achieved in intermolecular hydrogen-mediated reductive aldol additions of vinyl ketones to N-Boc-α-aminoaldehydes.187 Complete levels of syn-diastereoselectivity are accompanied by complete levels of anti-Felkin-Anh control due to intramolecular NH-O hydrogen-bonding in the low dielectric reaction medium. As determined by HPLC analysis, enantiomeric purity of the configurationally labile α-aminoaldehydes is fully preserved under the essentially neutral hydrogenation conditions. Acrolein and higher enals participate in hydrogenative aldol coupling with α-ketoaldehydes.184 The resulting β-hydroxy-γ-ketoaldehydes are unstable, but are amenable to condensation with hydrazine to furnish 3,5-disubstituted pyridazines in good yield (not shown).
Scheme 11.
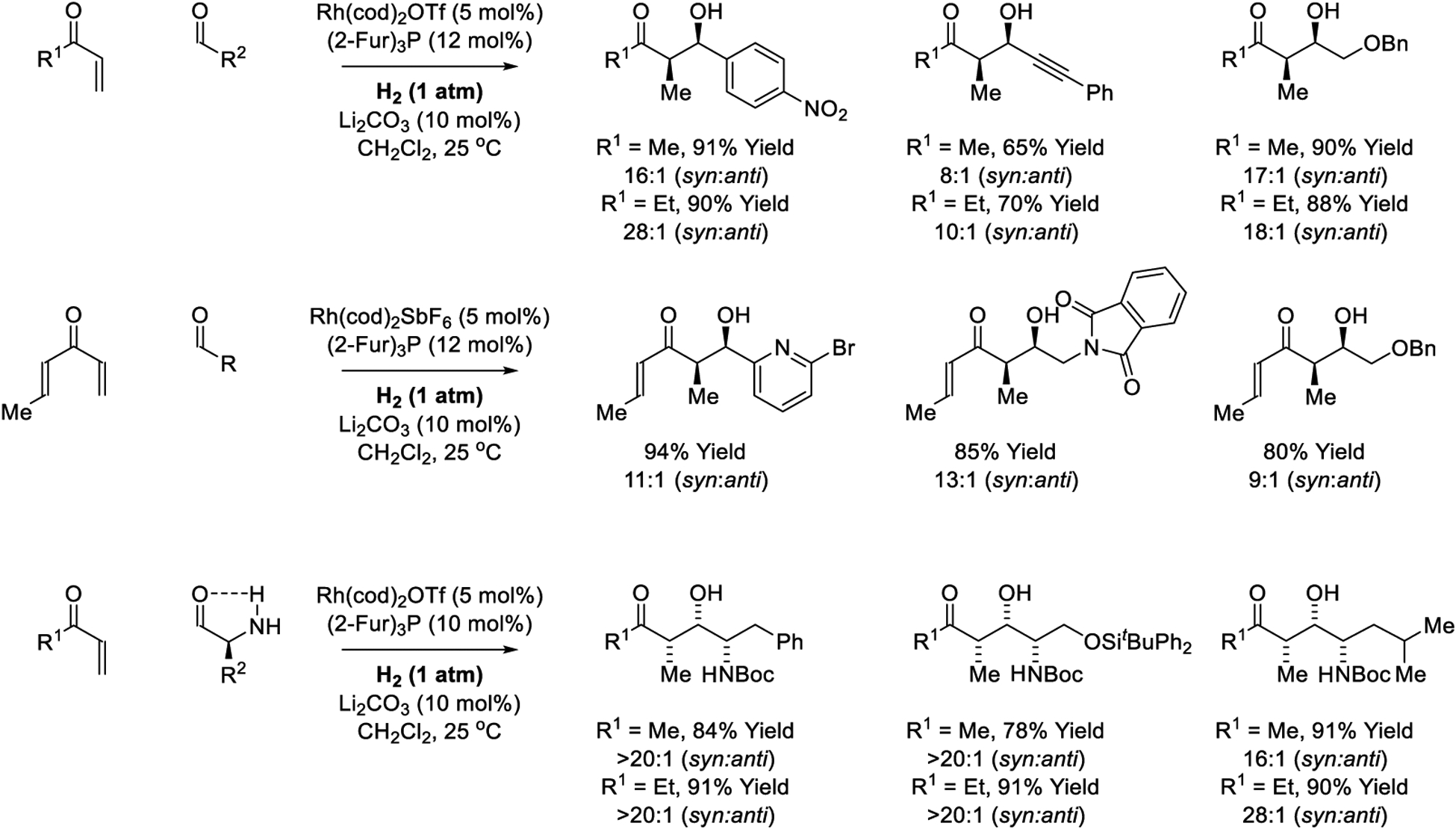
syn-Diastereoselective reductive aldol reactions via rhodium-catalyzed hydrogenation reported by Krische.
Highly diastereo- and enantioselective intermolecular hydrogen-mediated reductive aldol additions required the design of a chiral congener of tri-2-furylphosphine ligand; a formidable task as use of chelating phosphine ligands led to catalytically inactive rhodium complexes. Ultimately, a benzothiophene-substituted TADDOL-like phosphonite ligand was identified through modular ligand design, in which the P-aryl, ketal and carbinol substituents of the TADDOL-like scaffold were independently varied to illuminate key structure-selectivity trends. The ligand AP-I (“AbbasPhos-I”), which combines all three optimal substructures, provided the highest yields and stereoselectivities. Using the preformed cationic rhodium phosphonite complex, [Rh(cod)(AP-I)2]OTf, hydrogenation of commercially available methyl vinyl ketone or ethyl vinyl ketone in the presence of aliphatic aldehydes provided the aldol adducts with excellent control of relative and absolute stereochemistry (Scheme 12).188 The reactions are operationally facile and are conducted at 0 °C simply using balloons of hydrogen gas. A stunning application of the asymmetric intermolecular hydrogen-mediated reductive aldol reaction is found in the total synthesis of the actin-binding marine polyketide swinholide A, a macrodiolide bearing 30 stereogenic centers (Scheme 13).188 Hydrogenative aldol addition occurs chemoselectively in the presence of alkene and diene functional groups with good levels of catalyst-directed diastereoselectivity.
Scheme 12.
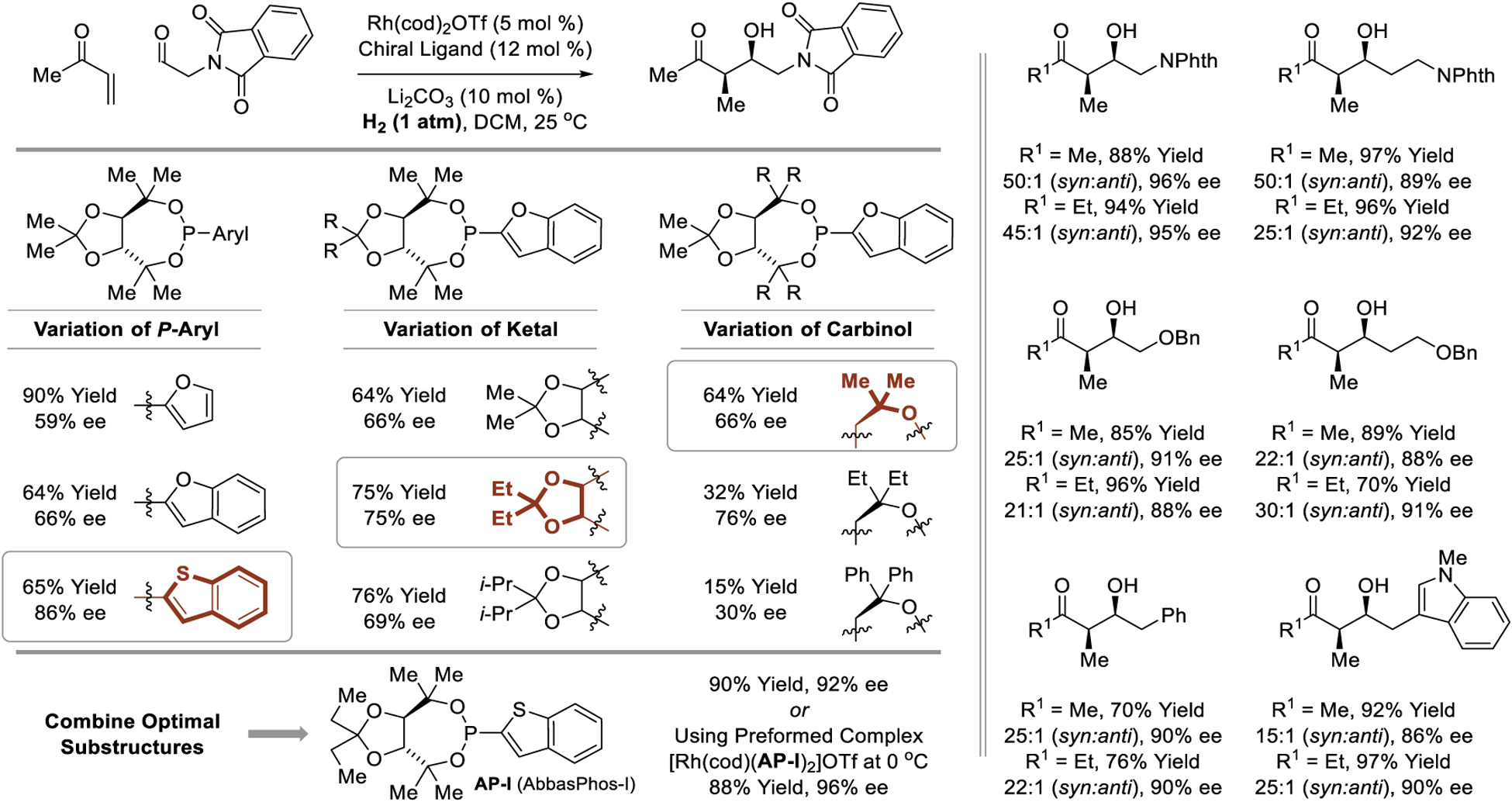
syn-Diastereo- and enantioselective reductive aldol reactions via rhodium-catalyzed hydrogenation reported by Krische.
Scheme 13.
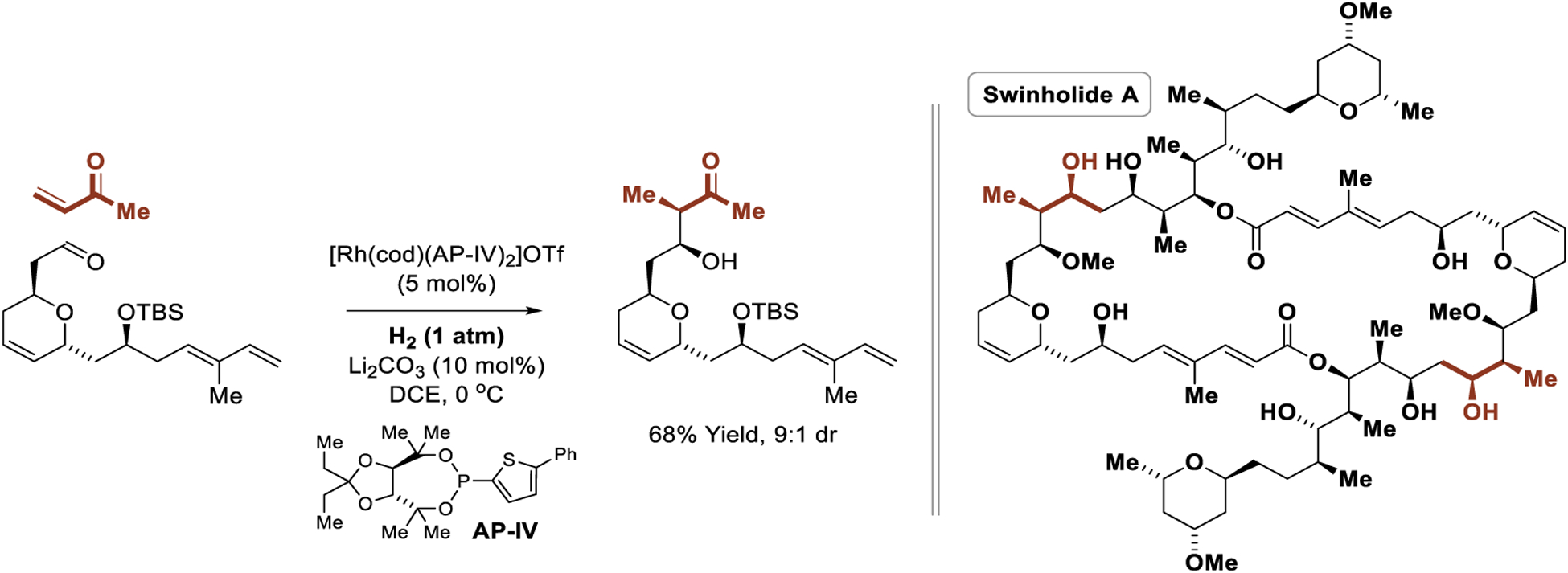
Application of the asymmetric intermolecular hydrogen-mediated reductive aldol reaction in Krische’s total synthesis of swinholide A.
Regarding the mechanism of the hydrogen-mediated reductive aldol reaction, cationic rhodium-catalysts are required, as neutral rhodium complexes promote simple enone hydrogenation. Additionally, basic additives such as lithium carbonate incur a small but significant increase in isolated yield (ca 20%). The acidity of cationic rhodium hydrides323 along with the improvement in yield upon introduction of substoichiometric base suggests heterolytic hydrogen activation (H2 + RhI-X → RhI-H + HX)308–310 and entry into catalytic cycles involving low valent rhodium monohydrides, that is, catalytic cycle D (Scheme 3). Here, syn-diastereoselectivity would require enone hydrometalation to form the (Z)-rhodium enolate as the major isomer. An alternate interpretation is based on the following observations. Unlike neutral rhodium(I) complexes,283–285 hydrogen oxidative addition is often turn-over-limiting for cationic rhodium(I) complexes.286–289 Cationic rhodium(I) complexes also have an additional vacant coordination site, which facilitates simultaneous coordination of both enone and aldehyde reactants. These effects may conspire to promote enone-aldehyde oxidative coupling to form rhodium(III) oxametalacycles303 as in catalytic cycle C (Scheme 3). To gain insight into the reaction mechanism, the intermolecular reductive coupling of methyl vinyl ketone was conducted under an atmosphere of elemental deuterium (Scheme 14).185 Precisely one deuterium atom was incorporated into the aldol product exclusively at the former enone β-position. While this result alone cannot differentiate catalytic cycles C and D, alkene hydrometalation is often reversible and typically does not occur with complete regioselectivity, suggesting oxidative coupling pathways may be operative. The rhodium complex [Rh(cod)(AP-I)2]OTf has been characterized single crystal X-ray diffraction analysis. A related model to account for diastereo- and enantiodetermining oxidative coupling is herewith proposed (Scheme 14).
Scheme 14.
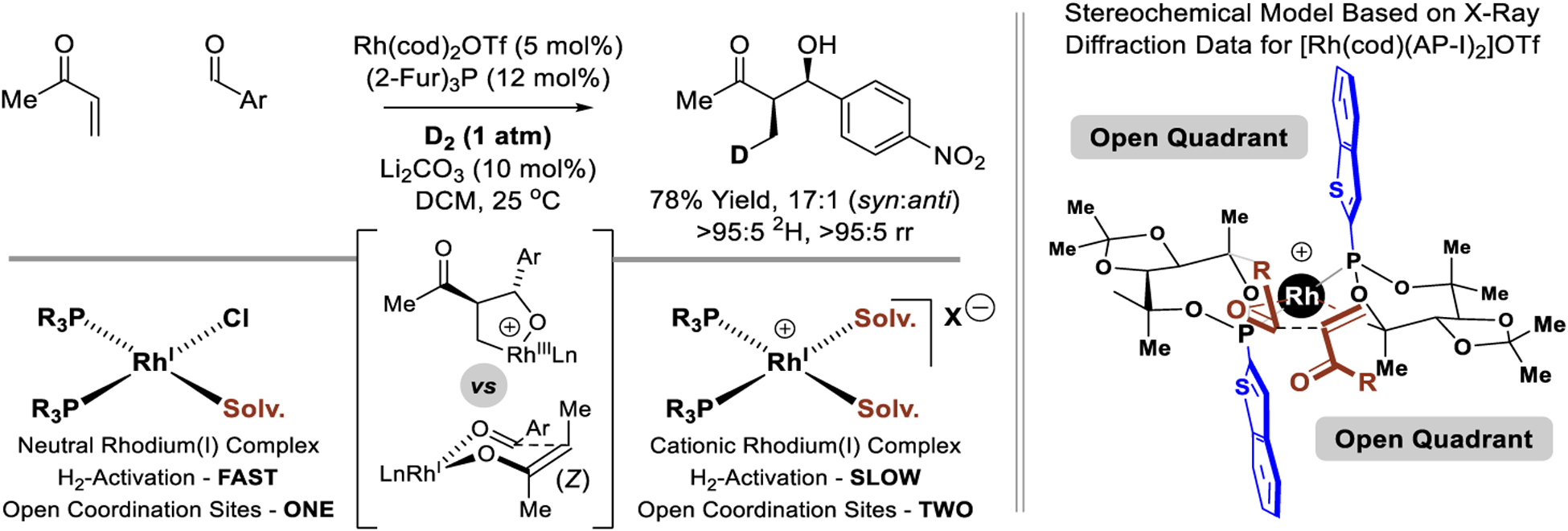
Deuterium labelling study and stereochemical model for syn-diastereo- and enantioselective reductive aldol reactions via rhodium-catalyzed hydrogenation.
Related hydrogen-mediated reductive Mannich-type reactions of enone and vinyl azine pronucleophiles have been developed (Scheme 15).232,238 Rhodium-catalyzed hydrogenation of methyl vinyl ketone or ethyl vinyl ketone in the presence of electron deficient N-(o-nitrophenylsulfonyl)imines delivers reductive Mannich-type products with good levels of syn-diastereoselectivity.232 N-Arylimines also participate in reductive coupling, but lower levels of syn-diastereoselectivity are evident. Similarly, hydrogenation of 2-vinyl azines in the presence of N-arylsulfonyl imines at ambient temperature and pressure employing cationic rhodium catalysts results in regioselective reductive coupling to furnish branched products of imine addition.238 Under an atmosphere of elemental deuterium, the reductive coupling product incorporates a single deuterium atom exclusively at the former β-position of the vinyl moiety, which is consistent with vinyl azine-imine oxidative coupling to furnish a cationic aza-rhodacyclopentane, as described in catalytic mechanism C (Scheme 3).
Scheme 15.
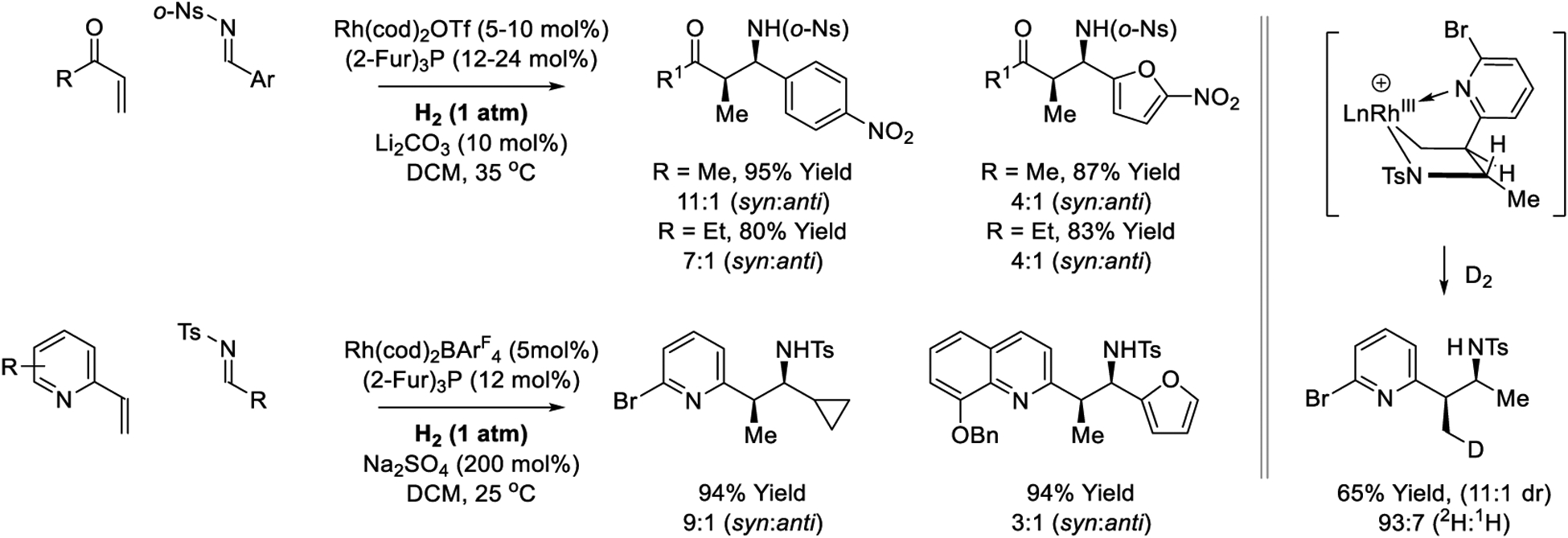
Reductive Mannich-type reactions via rhodium-catalyzed hydrogenation reported by Krische.
2.3. Cobalt
The first cobalt catalysts for reductive aldol coupling were described by Mukaiyama in 1989.190 A cobalt(II) precatalyst modified by dipivaloylmethane (dpm) was used in combination with phenylsilane as reductant (Scheme 16). Diverse pronucleophiles participate in the reductive coupling, including α,β-unsaturated nitriles, amides and esters. The aldol adducts are formed in good yield, but as diastereomeric mixtures. Related aldol cyclizations occur with complete levels of syn-diastereoselectivity,191 which may be explained on the basis of the indicated stereochemical model, which involves aldol addition by way of the (Z)-cobalt enolate through a closed transition structure.37 Unlike the intermolecular reactions, attempted aldol cycloreduction of α,β-unsaturated nitriles, amides and esters resulted in simple conjugate reduction.
Scheme 16.
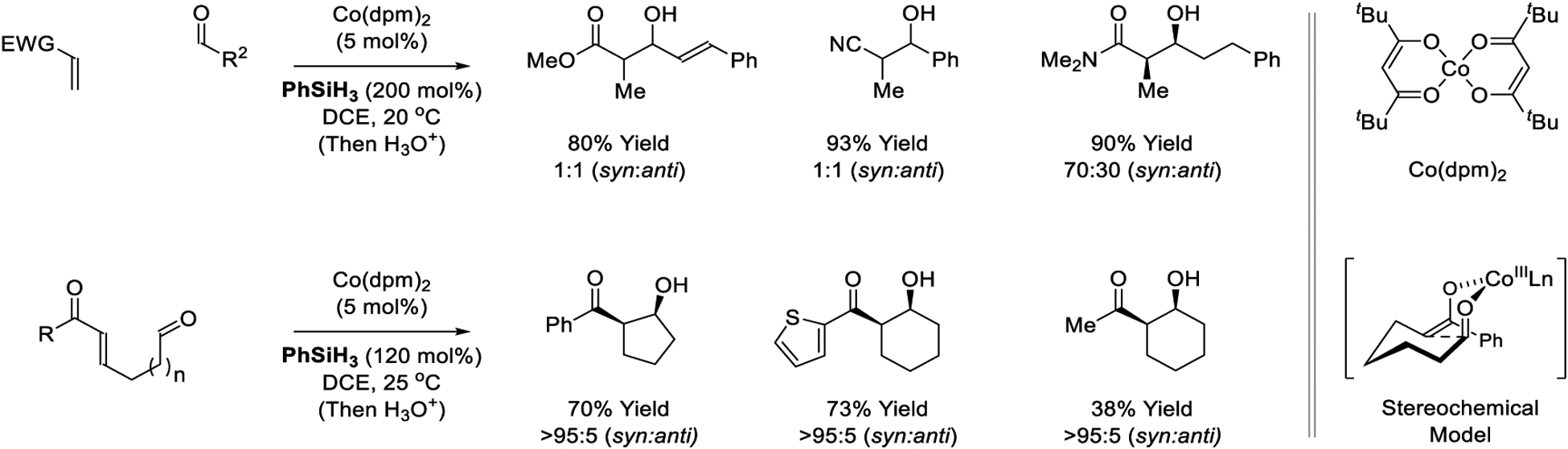
Inter- and intramolecular cobalt-catalyzed reductive aldol reactions reported by Mukaiyama and Krische, respectively.
Investigations into the reaction mechanism reveal silane-dependent partitioning of hydrometalative vs anion radical pathways (Scheme 17).192 Aldol cyclization mediated by d3-phenylsilane results in incorporation of a single deuterium at the former enone β-position as a 1:1 epimeric mixture, inferring rapid isomerization of the kinetic cobalt enolate in advance of carbonyl addition. For bis(enone) substrates, the silane source was found to influence competing Michael cycloreduction and [2 + 2] cycloaddition manifolds.192,324 Tetrahedral d7-metal complexes such as Co(dpm)2 are paramagnetic and participate in single electron oxidative addition.325 Co(II) complexes are also subject to disproportionation.326 As illustrated in equations A and B (Scheme 16), these reaction manifolds enable access to cobalt(I) complexes, which exist in equilibrium with the corresponding cobalt(III) silyl hydrides. Whereas use of PhSiH3, for which oxidative addition occurs readily, triggers hydrometalation en route to products of reductive cyclization, use of PhMeSiH2 stabilizes cobalt(I) to promote catalytic anion radical [2 + 2] cycloaddition. Intervention of anion radical intermediates is corroborated by [2 + 2]cycloadditions induced via cathodic reduction327 or single electron transfer from arene anion radicals.328
Scheme 17.
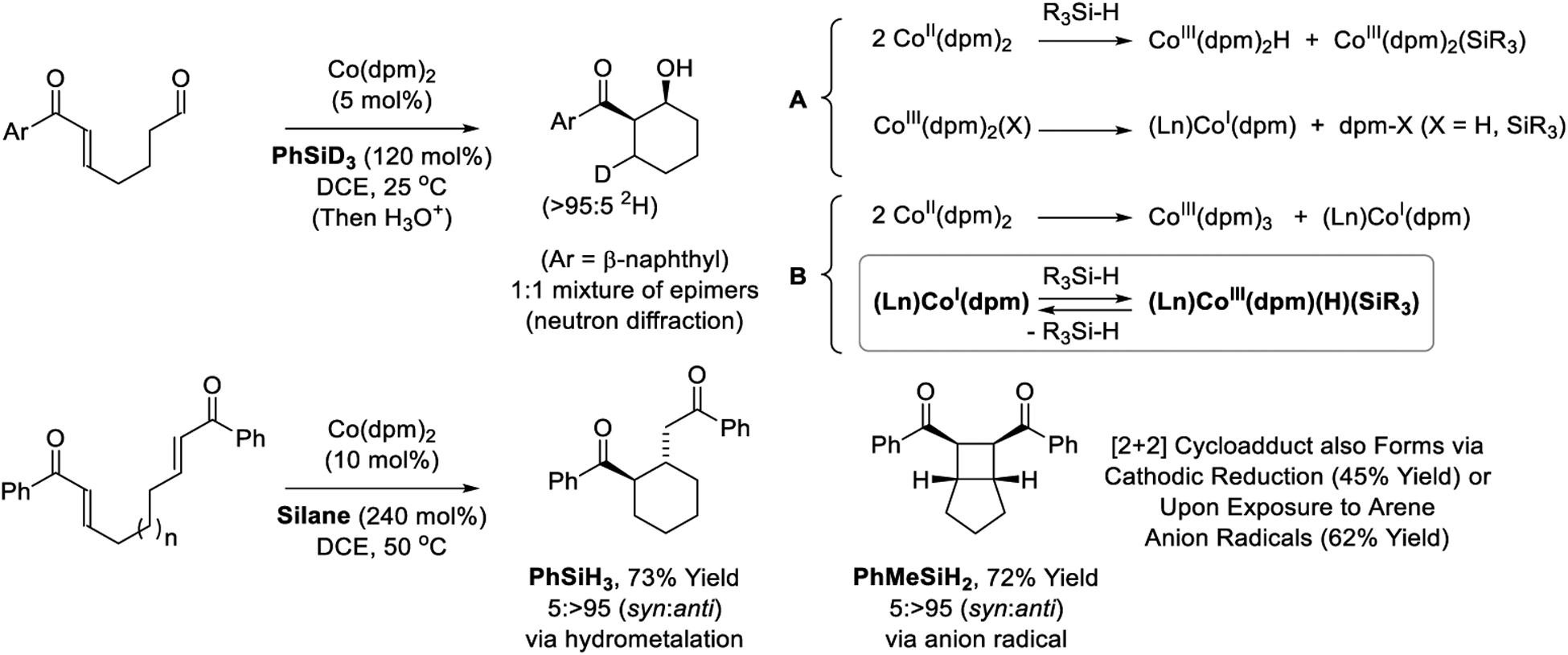
Silane-dependent partitioning of hydrometalative vs anion radical pathways in silane mediated reactions of cobalt(II) diketonates.
In a powerful extension of scope, the laboratory of Lam reports cobalt-catalyzed reductive aldol additions of α,β-unsaturated amides to ketone electrophiles mediated by Et2Zn (Scheme 17).193–195 The authors initial report describes aldol cyclizations to form 5- and 6-membered rings.193 Excellent levels of syn-diastereoselectivity were observed. Intermolecular reactions were developed subsequently.194 Here, acrylamides as well as fumaric amides undergo syn-diastereoselective aldol addition to ketones. Notably, the fumaric amides deliver aldol adducts as single regioisomers. Attempted enantioselective reactions using chiral ligands led to racemic products, suggesting aldol addition occurs through the (Z)-zinc(II) enolate. Hence, to induce asymmetry an N-acryloyloxazolidine pronucleophile is employed.195 The mechanism for zinc(II) enolate formation is based on related Lewis acid-assisted oxidative additions of transition metals to enones to form oxy-π-allylmetal species.329–333 Specifically, β-hydride elimination from the indicated ethyl substituted oxy-π-allylcobalt intermediate followed by C-H reductive elimination delivers the zinc(II) enolate (Scheme 18). The ethylzinc(II) enolates that arise via cobalt-catalyzed conjugate reduction of acryloylmorpholine can also be captured via imine addition to furnish Mannich products with good levels of anti-diastereoselectivity (Scheme 19).235
Scheme 18.
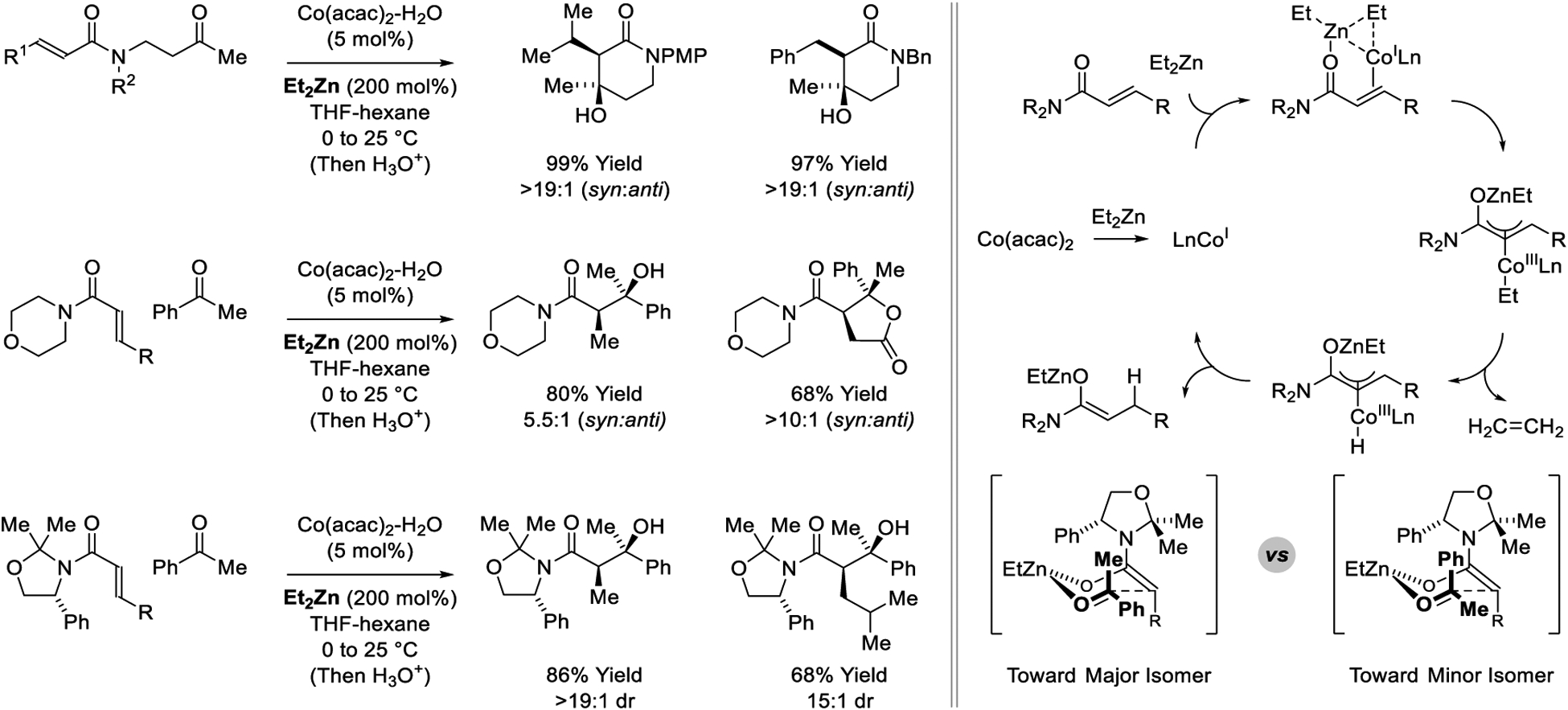
Cobalt-catalyzed reductive aldol reactions reported by Lam.
Scheme 19.
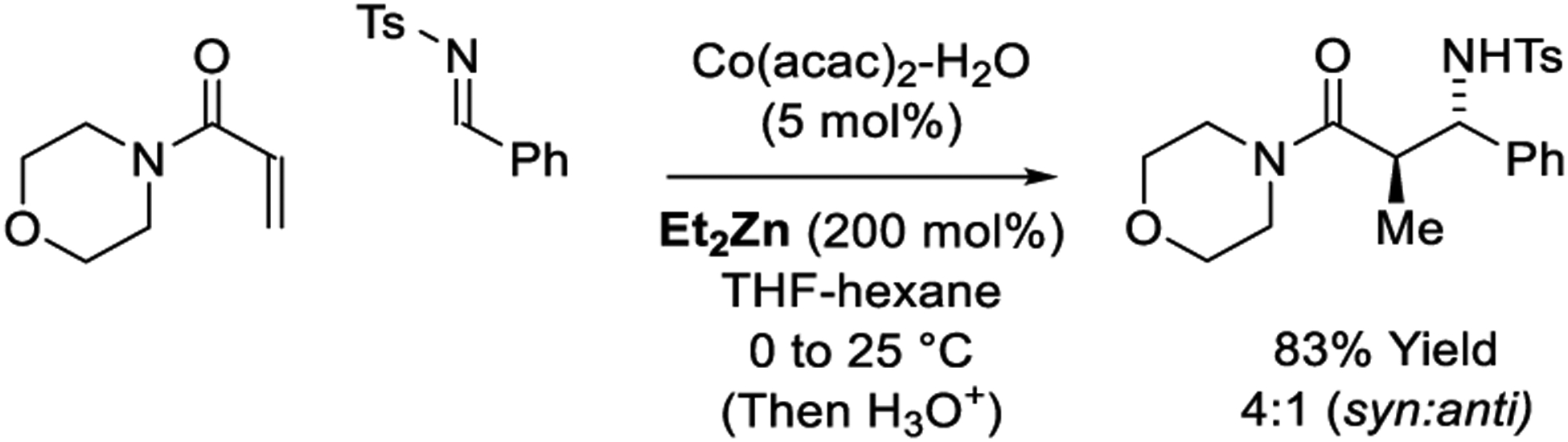
Cobalt-catalyzed reductive Mannich reaction reported by Lam.
2.4. Iridium
Highly syn-diastereo- and enantioselective iridium-catalyzed reductive aldol couplings were reported by Morken using an Ir(pybox) catalyst and Et2MeSiH as terminal reductant (Scheme 20).196 Inductively activated aldehydes that incorporate adjacent alkoxy groups are required as the parent aliphatic aldehydes do not participate in coupling. Closely related Ir(phebox) complexes investigated by Nishiyama catalyze anti-diastereo- and enantioselective reductive aldol reactions of tert-butyl acrylate and benzaldehyde, representing a ligand-dependent inversion in diastereoselectivity.175 Excellent yields and stereoselectivities are observed at relatively low loadings of the catalyst (1 mol%) (Scheme 20).
Scheme 20.
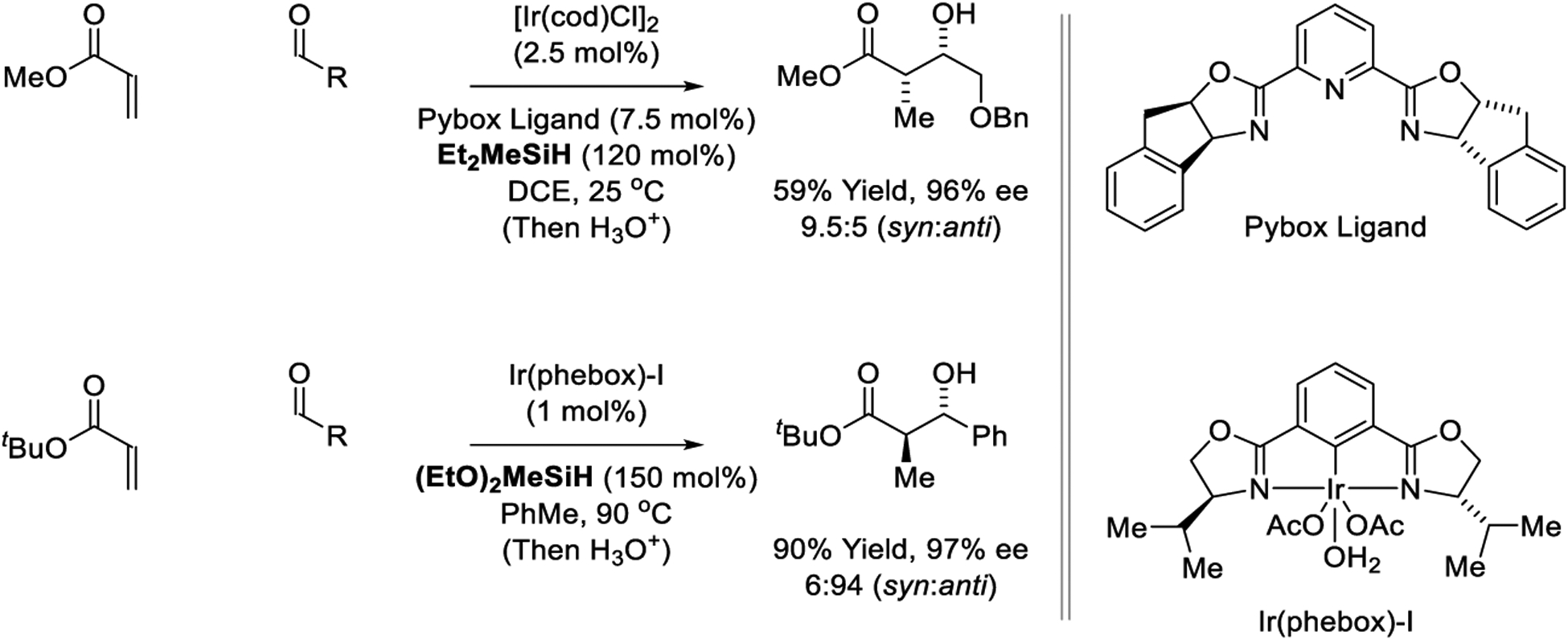
Diastereo- and enantioselective iridium-catalyzed reductive aldol reactions reported by Morken and Nishiyama.
As described by Morken, iridium-catalyzed reductive couplings of pentafluorophenyl acrylates with N-aryl imines mediated by Et2MeSiH provide Mannich products, which spontaneously cyclize to deliver β-lactams (Scheme 21).231 Competing hydrosilylation of alkene and alkyne functional groups is not observed. A linear Hammett correlation involving both the imine N-aryl moiety and the acrylate aryloxy group implicate rate-determining cyclization. Interestingly, whereas Ando’s rhodium-catalyzed reductive Mannich reactions deliver the cis-β-lactams (Scheme 9),237 the iridium-catalyzed reactions display a pronounced preference for the corresponding trans-isomers. This divergence in diastereoselectivity may be attributed to intervention of zinc(II) vs iridium(III) enolates, respectively.
Scheme 21.
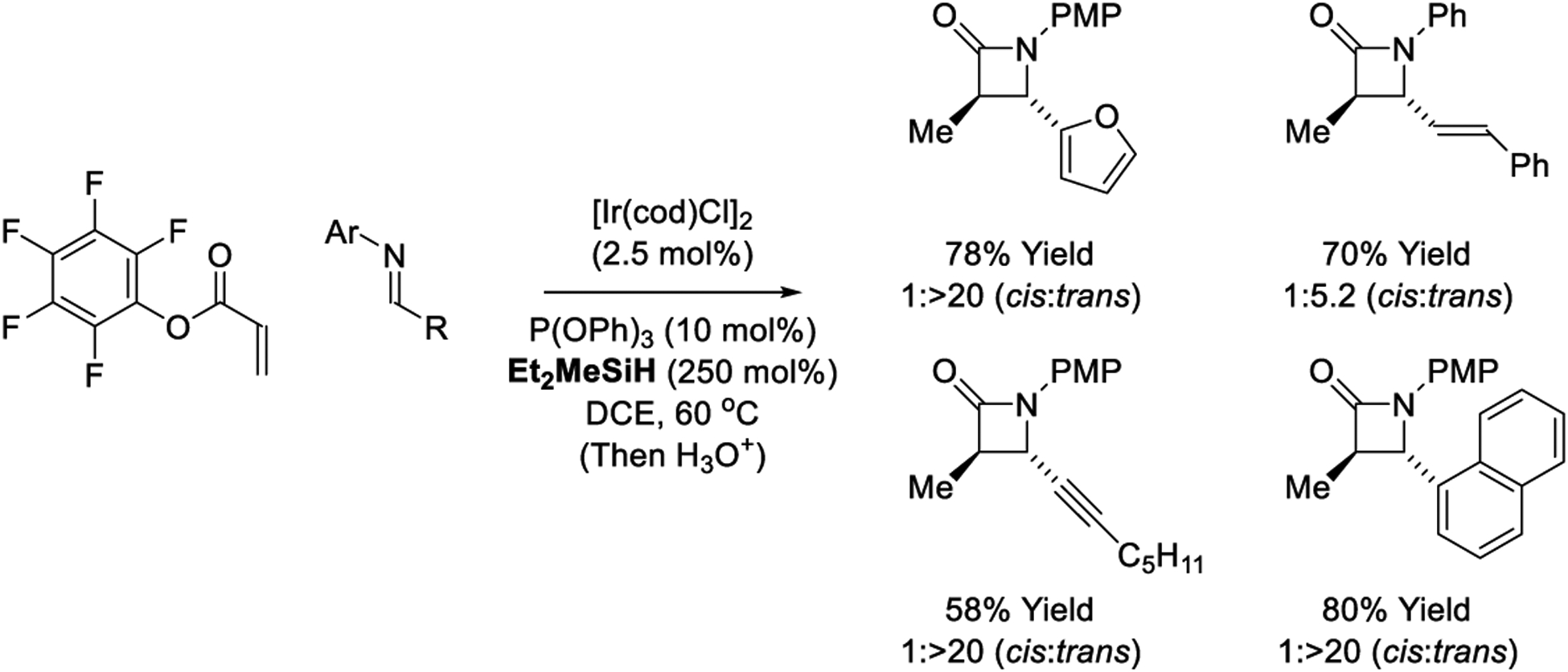
Diastereoselective iridium-catalyzed reductive Mannich-type reactions reported by Morken.
2.5. Ruthenium
Despite enormous progress in the area of ruthenium-catalyzed carbonyl reductive coupling,275,334–336 true reductive aldol couplings have not been reported. However, closely related transformations have been described by Ryu, who reports a 2-propanol-mediated reductive homo-coupling of enals catalyzed by RuHCl(CO)(PPh3)3 (Scheme 22).197 This transformation involves tandem reductive aldol addition-redox isomerization-aldehyde reduction and exploits 2-propanol as the terminal reductant. The catalytic mechanism is initiated by enal hydroruthenation to furnish a ruthenium(II) enolate. Aldehyde addition provides an aldolate, which upon internal redox isomerization delivers a transient β-ketoaldehyde. 2-Propanol-mediated reduction of the formyl group provides the reaction product. Related redox-neutral cross-couplings have been described wherein alcohols serve dually as reductants and aldehyde proelectrophiles (Scheme 22).199
Scheme 22.
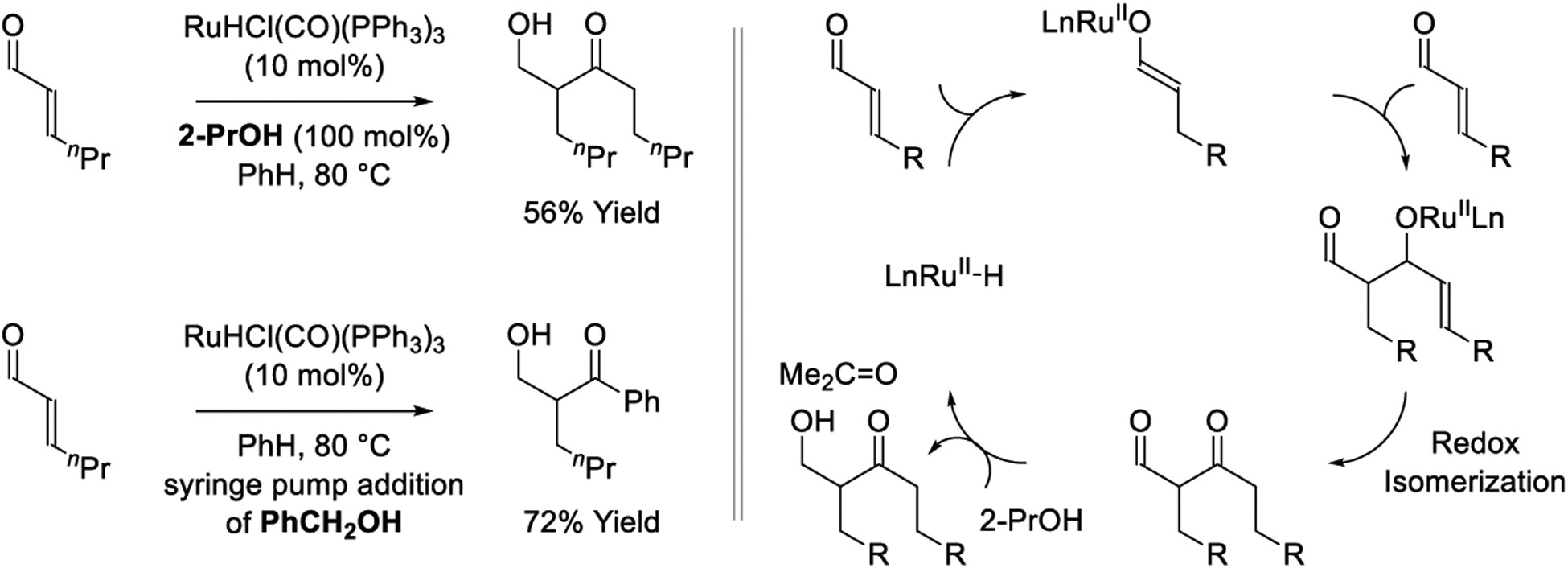
Ruthenium-catalyzed reductive aldol-type reactions reported by Ryu.
2.6. Palladium
In 1998, a palladium-catalyzed reductive aldol coupling was reported by Kiyooka (Scheme 23).200 The reaction employs tetrakis(triphenylphosphine)palladium as precatalyst and Cl3SiH as reductant. Using N,N-dimethyl acrylamide as pronucleophiles, the aldol product is generated in good yield but with modest anti-diastereoselectivity. The corresponding reaction of tert-butyl acrylate occurs in low yield and modest syn-diastereoselectivity. The reaction mechanism is postulated to involve oxidative addition of the trichlorosilane to palladium(0), followed by hydropalladation of the α,β-unsaturated compound. However, given the fact that Cl3SiH functions as a reductant in the presence of Lewis base (vide infra, Section 2.11.), it is possible that palladium is not required for the reported transformations.
Scheme 23.
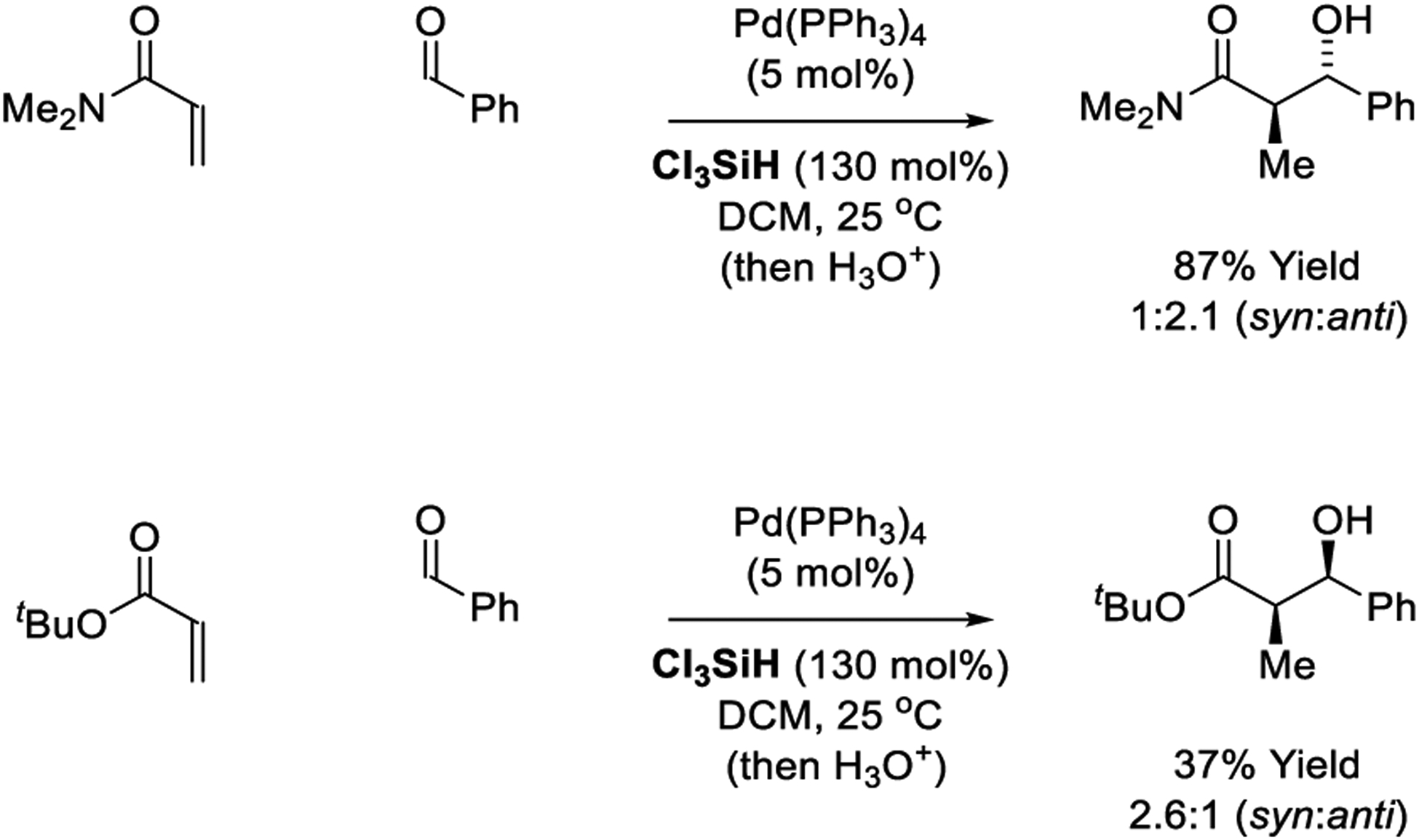
Palladium-catalyzed reductive aldol reactions reported by Kiyooka.
2.7. Nickel
Triethylborane-mediated reductive aldol additions catalyzed by nickel were developed in the laboratory of Montgomery (Scheme 24).201 Surprisingly, sterically unencumbered aryl iodides are required for initiation of the catalytic cycle. Specifically, oxidative addition of iodobenzene to nickel(0) with subsequent coordination of acrylate and triethylborane triggers formation of an ethyl(iodo)nickel complex and a β-aryl boron enolate (as evinced by conjugate addition-aldol addition byproducts). Entry into the catalytic cycle occurs from the ethyl(iodo)nickel complex, which upon elimination of ethylene forms a nickel hydride. Simultaneous coordination of acrylate and triethylborane results in acrylate hydrometalation, likely by way of an oxy-allylnickel(II) intermediate,329–333 to form a boron enolate with regeneration of the key ethyl(iodo)nickel complex to close the catalytic cycle. The boron enolate undergoes spontaneous aldol addition with good levels of syn-diastereoselectivity. Corroborating the authors mechanistic hypothesis, nickel(II) precatalysts promote an alternate reaction pathway that involves ethyl transfer to the acrylate followed by aldol addition.
Scheme 24.
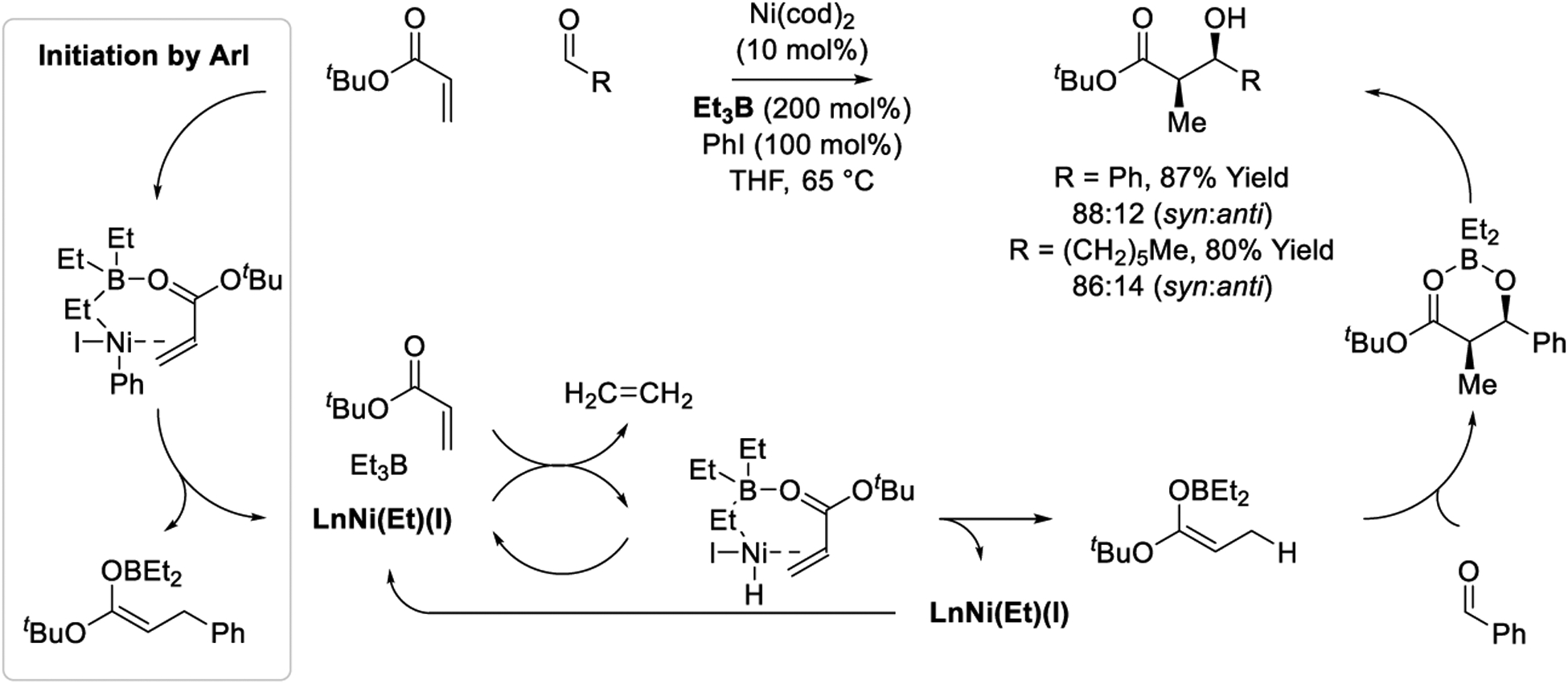
Nickel-catalyzed reductive aldol reactions reported by Montgomery.
The Lam laboratory reports highly diastereoselective nickel-catalyzed reductive aldol cyclizations onto tethered ketones mediated by Et2Zn (Scheme 25).202 Esters and amides undergo cycloreduction to form lactone and lactam products, respectively. The mechanism was probed by deuterium labelling studies, but these were inconclusive (not shown). However, when the ketone electrophile is tethered to the α,β-unsaturated carbonyl through the β-carbon the indicated homoaldol adduct is obtained. This result suggests intervention of catalytic cycle C (Scheme 3), wherein oxametalacycles formation occurs by of oxy-π-allylnickel intermediates.329–333 The nickel-catalyzed reductive aldol cyclization was used by the Lam laboratory to complete the formal synthesis of salinosporamide A (Scheme 25).203
Scheme 25.
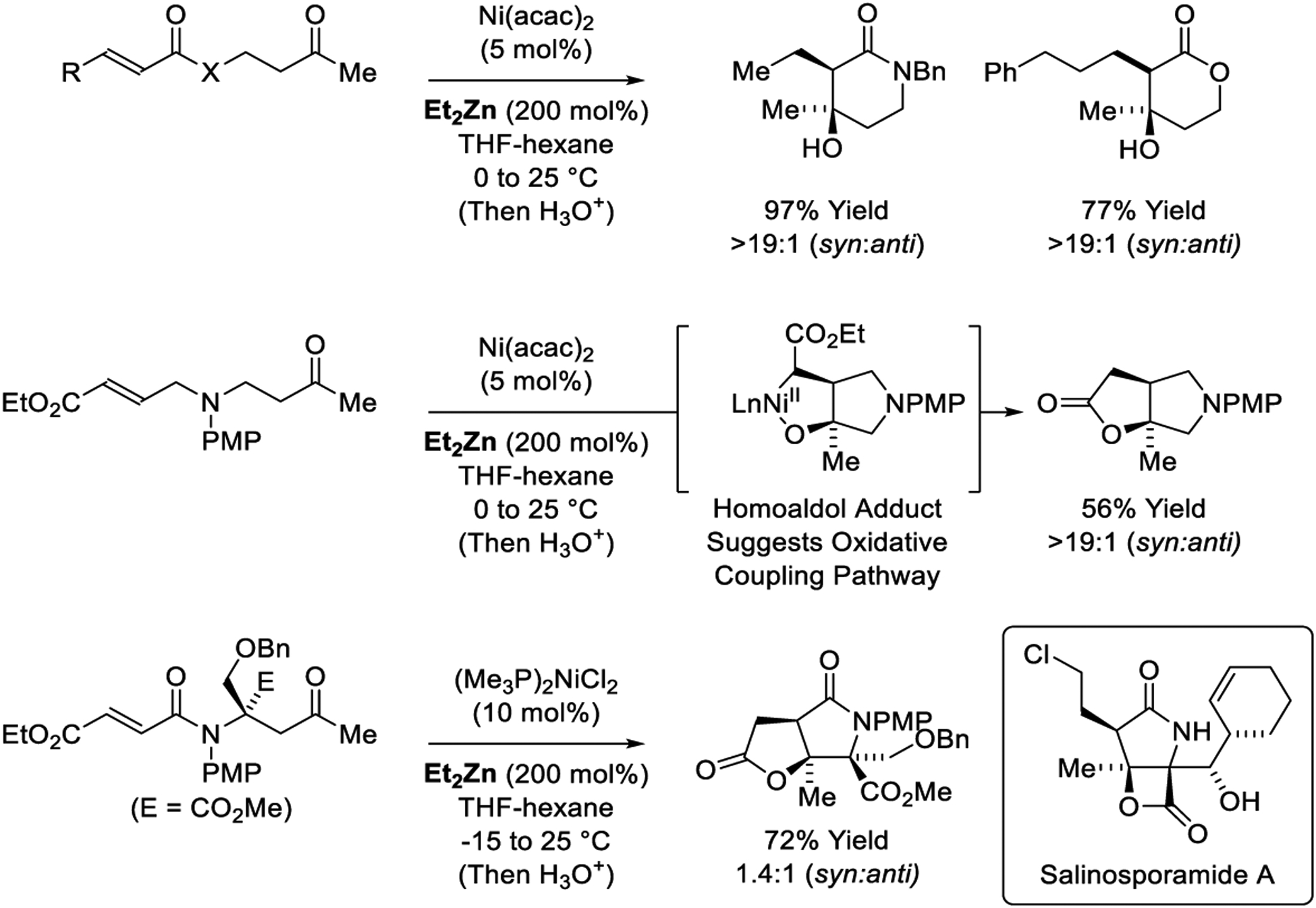
Nickel-catalyzed reductive aldol cyclizations reported by Lam.
2.8. Platinum
Platinum complexes modified by SnCl2 are effective catalysts for alkene hydrogenation.337–339 Jang and coworkers have demonstrated that the PtCl2-SnCl2 catalyst system promotes inter- and intramolecular hydrogen-mediated reductive aldol reactions (Scheme 26).204 Addition occurs with good to complete levels of syn-diastereoselectivity for intermolecular couplings and corresponding cycloreductions, respectively. Reactions mediated by triethylsilane also were explored but lower efficiencies were observed (not shown). Under a deuterium atmosphere, incomplete incorporation of 2H at the former enone β-position is evident, corroborating reversible enone hydrometalation in advance of C-C coupling. A monohydride mechanism involving LnPtD(SnCl3) was proposed (see Scheme 3, Cycle D).
Scheme 26.
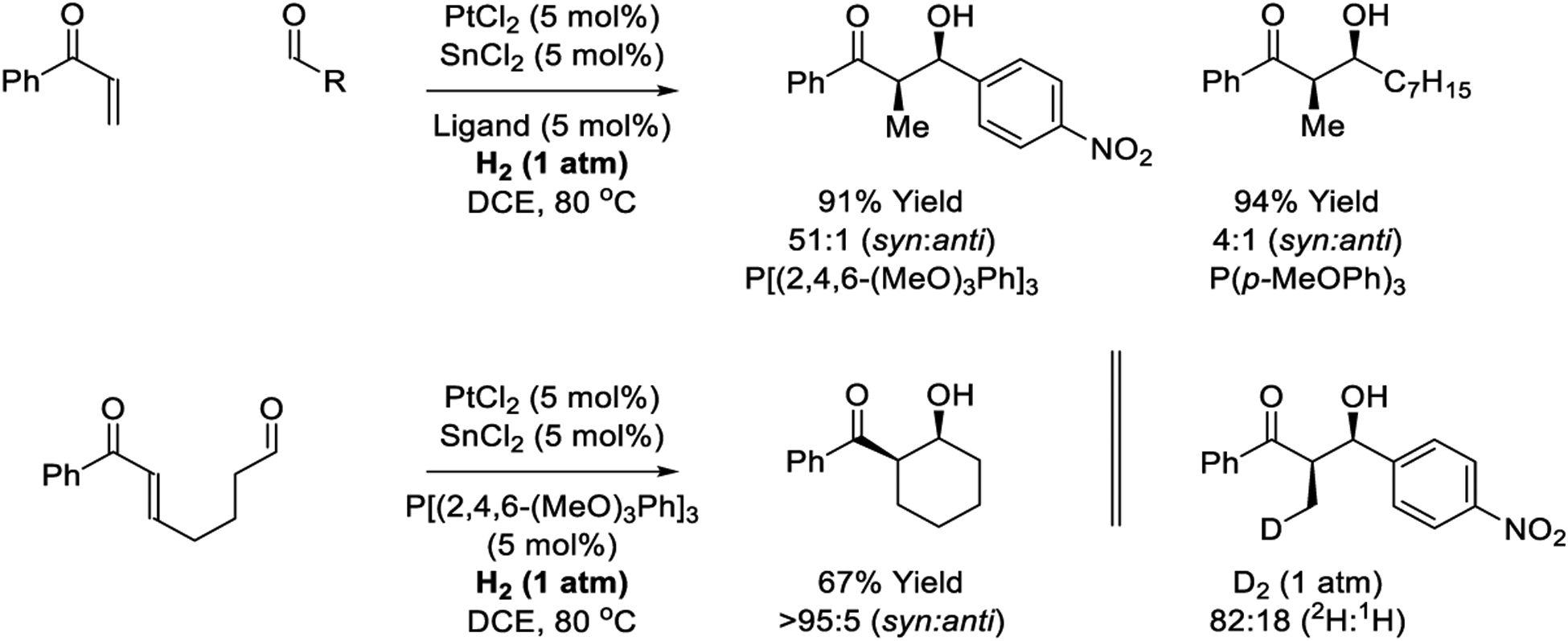
Inter- and intramolecular platinum-catalyzed reductive aldol reactions reported by Jang.
2.9. Copper
Reductive aldol cyclizations promoted by stoichiometric quantities of Stryker’s reagent, [Ph3PCuH]6, have been described.205–207 Additionally, Stryker’s reagent was reported to catalyze hydrosilane-mediated conjugate reduction of enones to form enol silanes that engage in aldol reactions with carbonyl electrophiles.135–136 While true reductive aldol reactions catalyzed by Stryker’s reagent remain undeveloped, related ynone-ketone cycloreductions catalyzed by Stryker’s reagent and mediated by hydrosilane have been reported by Chui (Scheme 27).207
Scheme 27.
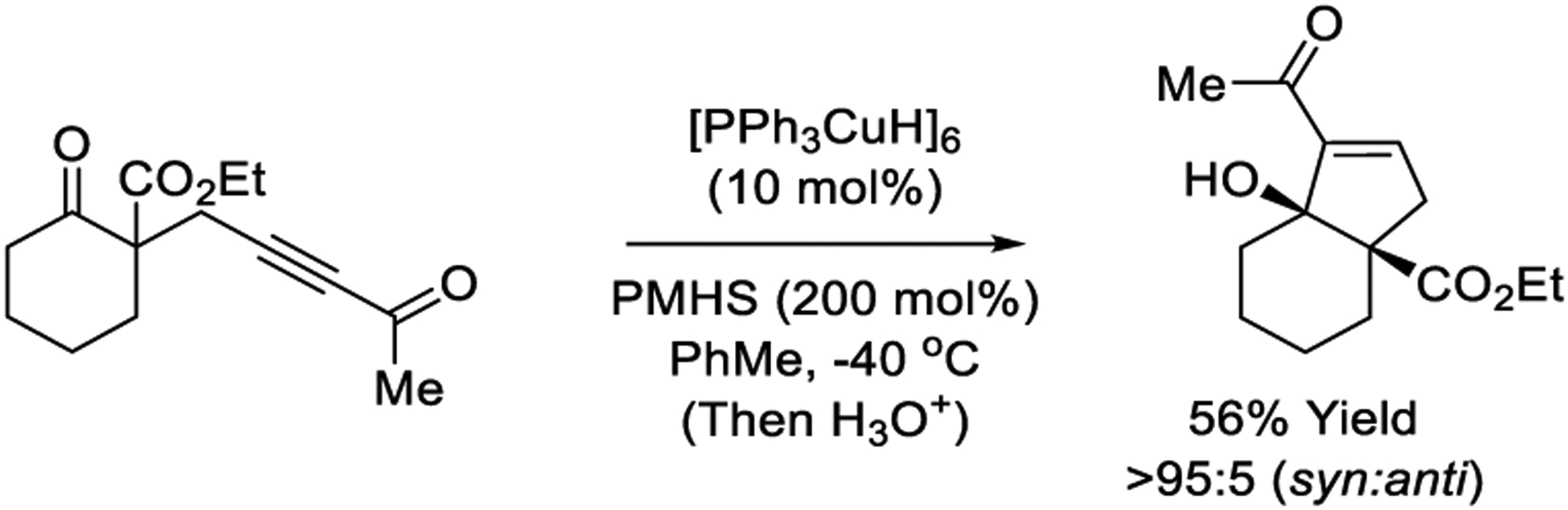
Copper-catalyzed reductive aldol cyclization of acetylenic ketones reported by Chui.
The first copper-catalyzed reductive aldol reaction was reported by Maruoka in 1999.208 The reaction mechanism is believed to involve copper(I) chloride initiated enone hydrostannation to form a tin enolate. The reaction does not proceed in the presence of galvinoxyl (5 mol%), which implicates a catalytic mechanism involving radical species. The authors speculate that copper serves a dual role in catalyzing tin enolate formation and subsequent Mukaiyama aldol addition. This process is applicable to the addition of vinyl ketone pronucleophiles to aliphatic, α,β-unsaturated or aromatic aldehydes. Good isolated yields are accompanied by modest levels of syn-diastereoselectivity (Scheme 28).
Scheme 28.
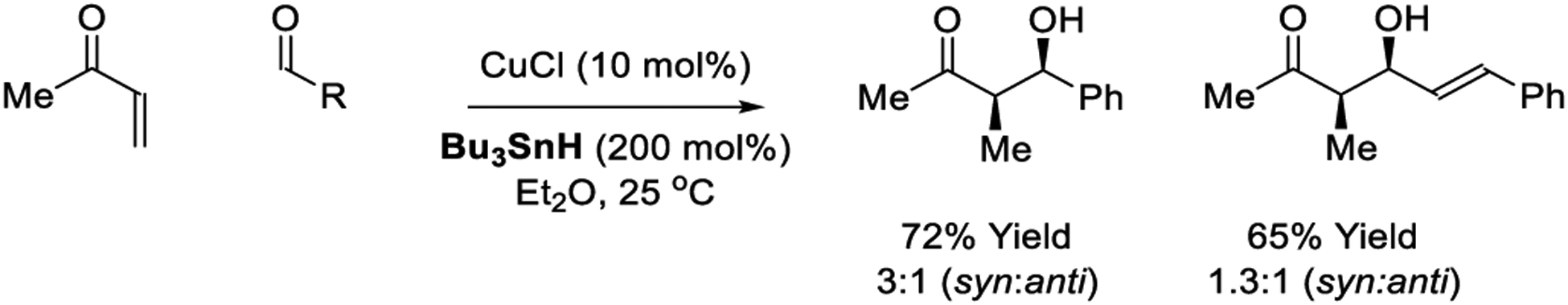
Copper-catalyzed reductive aldol reactions reported by Maruoka.
Copper-catalyzed reductive aldol cyclizations mediated by silane were reported by Lam (Scheme 29).209 Upon exposure of keto-enones to 1,1,3,3-tetramethylhydroxysiloxane (TMDS) in the presence of copper catalysts modified by MeO-BIPHEP, β-hydroxylactone formation occurs with complete syn-diastereoselectivity and moderate enantioselectivity.209 In related work, reductive cyclization of α,β-unsaturated keto-amides was found to provide 4-hydroxypiperdin-2-ones with high levels of substrate-directed asymmetric induction.183 The authors propose a mechanism akin to catalytic cycle D (Scheme 3), in which Cu(OAc)2•H2O is converted to a copper(I) monohydride complex, which hydrometallates the α,β-unsaturated ester or amide to form a copper enolate. Intramolecular ketone addition delivers a copper aldolate, which upon σ-bond metathesis with siloxane304–306 forms the aldol product with regeneration of the copper(I) monohydride.
Scheme 29.
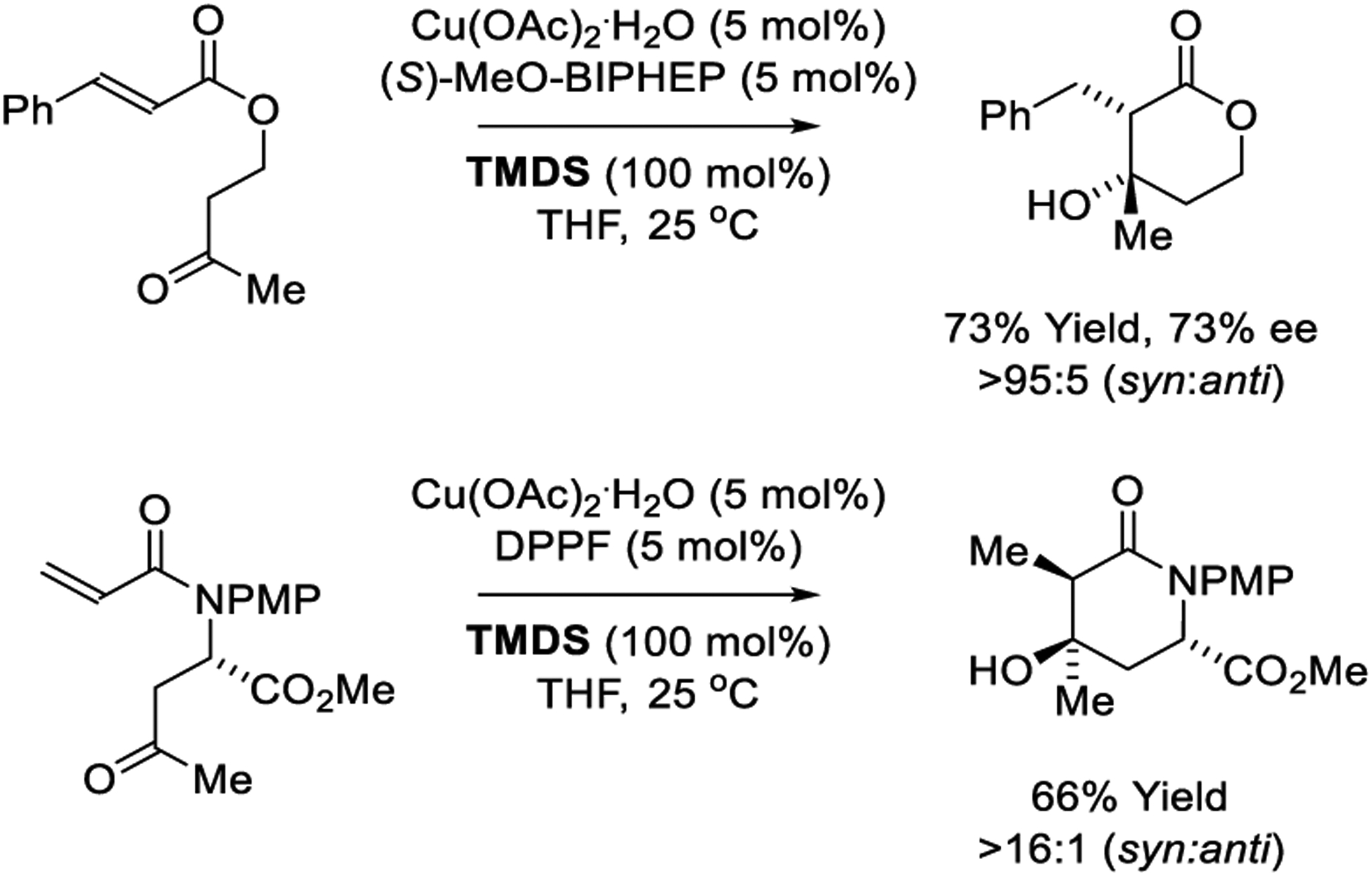
Copper-catalyzed reductive aldol cyclizations reported by Lam.
An impressive diastereo- and enantioselective intermolecular reductive aldol coupling of acrylate pronucleophiles to unactivated ketones was reported by Riant (Scheme 30).211 Using a copper complex derived from [CuF(PPh3)3]·2MeOH and a “TANIAPHOS” ligand in combination with phenylsilane as reductant, exceptional levels of anti-diastereo- and enantioselectivity are observed. Whereas corresponding aldehyde additions conducted under these conditions exhibit modest levels of diastereoselectivity and uneven levels of enantioselectivity (not shown),212,215 related cyclizations of enone-diones provide bicyclic aldol adducts that incorporate 3-contiguous stereogenic centers with excellent control of relative and absolute stereochemistry.217 Copper-catalyzed acrylate-ketone reductive aldol coupling using [CuF(PPh3)3]·2MeOH as precatalyst has been explored further by Fukuzawa218 and Li;219 however, improved selectivities were not observed (not shown). Later, Li reported high yielding reductive aldol reactions of dimethyl maleate with acetophenones that deliver racemic lactone products as diastereomeric mixtures (not shown).220
Scheme 30.
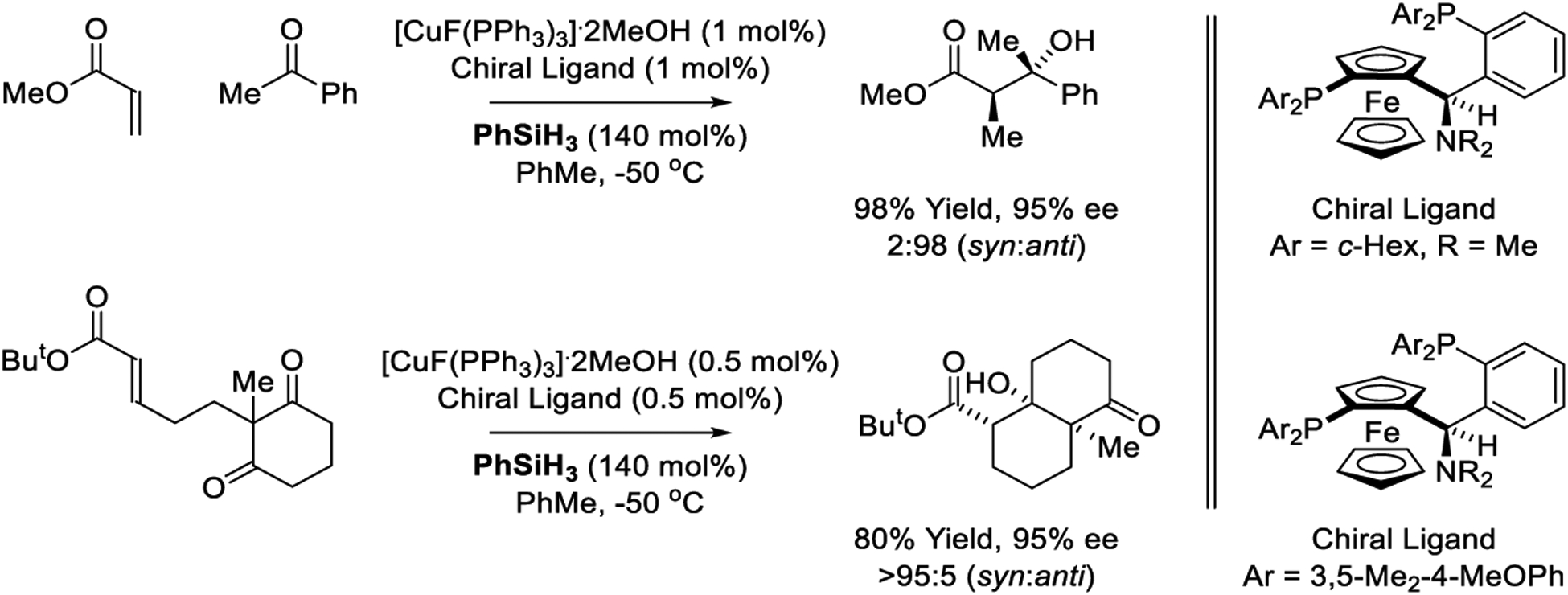
Inter- and intramolecular copper-catalyzed reductive aldol reactions reported by Riant.
Shibasaki and Kanai explored the intermolecular reductive aldol coupling of acrylates, β-substituted acrylates and allenic esters using [CuF(PPh3)3]•2EtOH as precatalyst and triethoxysilane as the stoichiometric reductant, which displayed modest levels of relative and absolute stereocontrol (not shown).213 However, in subsequent work using copper catalysts modified by a “TANIAPHOS” ligand and pinacolborane as reductant, enhanced stereoselectivities were observed in reductive aldol couplings of allenic esters to acetophenone (Scheme 31).214 Furthermore, using (R)-DTBM-SEGPHOS as ligand in combination with Cy3P as an additive, the same reactants form products of vinylogous reductive aldol addition with high levels of enantiocontrol and complete alkene (Z)-stereoselectivity (Scheme 31). As in the preceding examples (Schemes 29 and 30), these processes are postulated to proceed by way of copper(I) monohydrides through catalytic cycle D (Scheme 3).
Scheme 31.
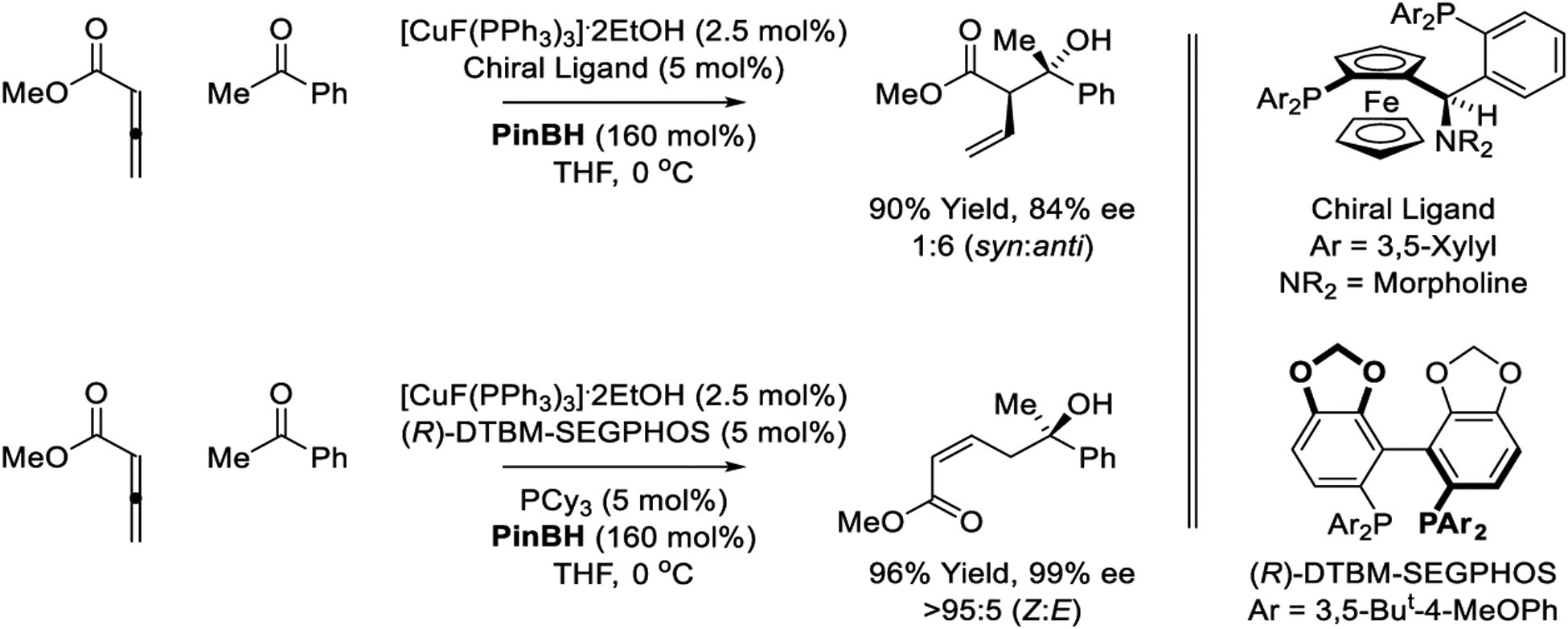
Copper-catalyzed reductive aldol reactions reported by Shibasaki and Kanai.
Lipshutz reports a copper-catalyzed reductive aldol cyclization of β,β-disubstituted enone pronucleophiles using a copper catalyst modified by a “JOSIPHOS” ligand (Scheme 32).216 In this process, enantiodetermining enone reduction triggers aldol cyclization to form products that embody 3-contiguous stereogenic centers with excellent levels of diastereo- and enantioselectivity. As the initially formed stereocenter guides the stereochemistry of carbonyl addition, the reactions are stereospecific with (E)- and (Z) enones providing enantiomeric products under otherwise identical conditions. Specifically, pseudo-equatorial disposition of the methyl-bearing stereocenter that arises via conjugate reduction enforces the indicated chair-like conformation from which carbonyl addition occurs by way of the (Z)-enolate.
Scheme 32.
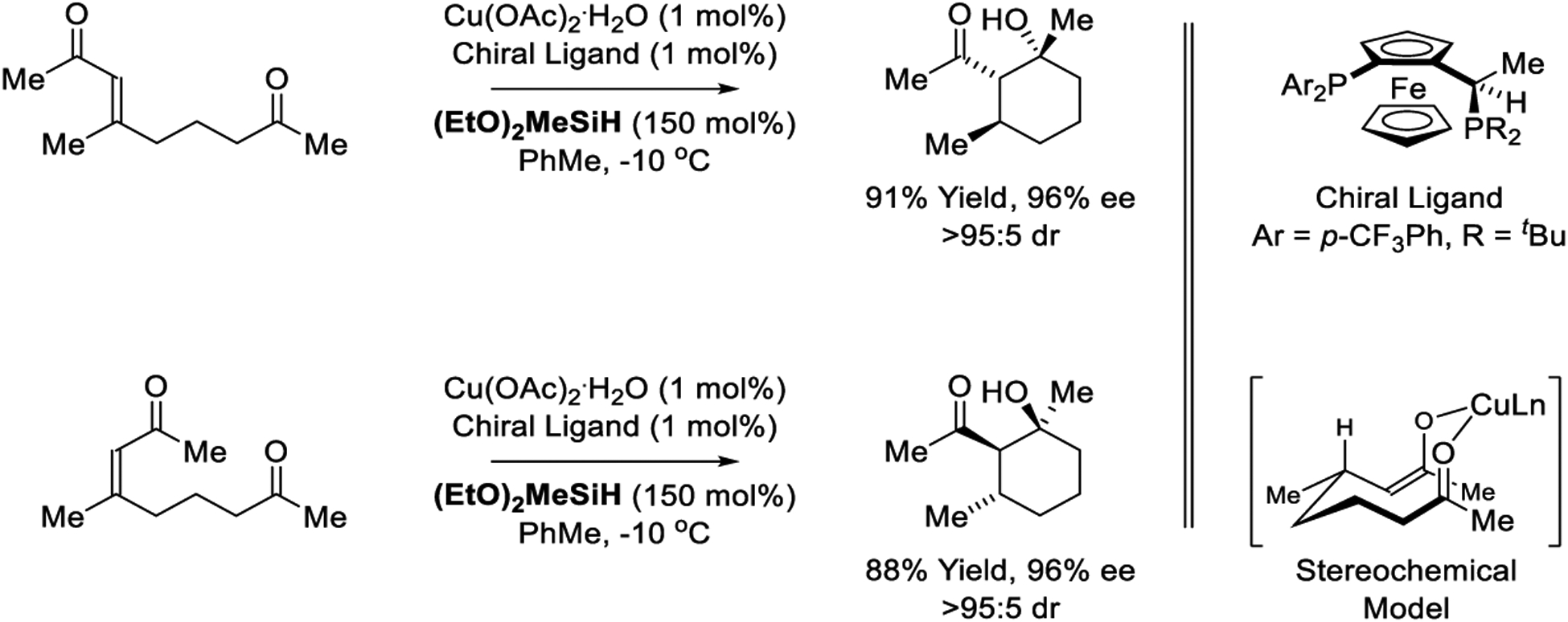
Copper-catalyzed reductive aldol cyclizations reported by Lipshutz.
In 2008, Shibasaki and Kanai reported copper-catalyzed reductive Mannich reactions of acrylate pronucleophiles with N-diphenylphosphinoyl ketimines mediated by pinacol borane (Scheme 33).234 Using a CuOAc-PPh3 catalyst, the Mannich products are generated in good yield with excellent diastereocontrol for ketimines bearing aromatic, aliphatic and vinyl substituents. In an initial evaluation of chiral ligands using borane reductants, significant levels of enantioselectivity proved to be elusive. However, using (EtO)3SiH as reductant highly diastereo- and enantioselective copper-catalyzed reductive Mannich reactions could be achieved.
Scheme 33.
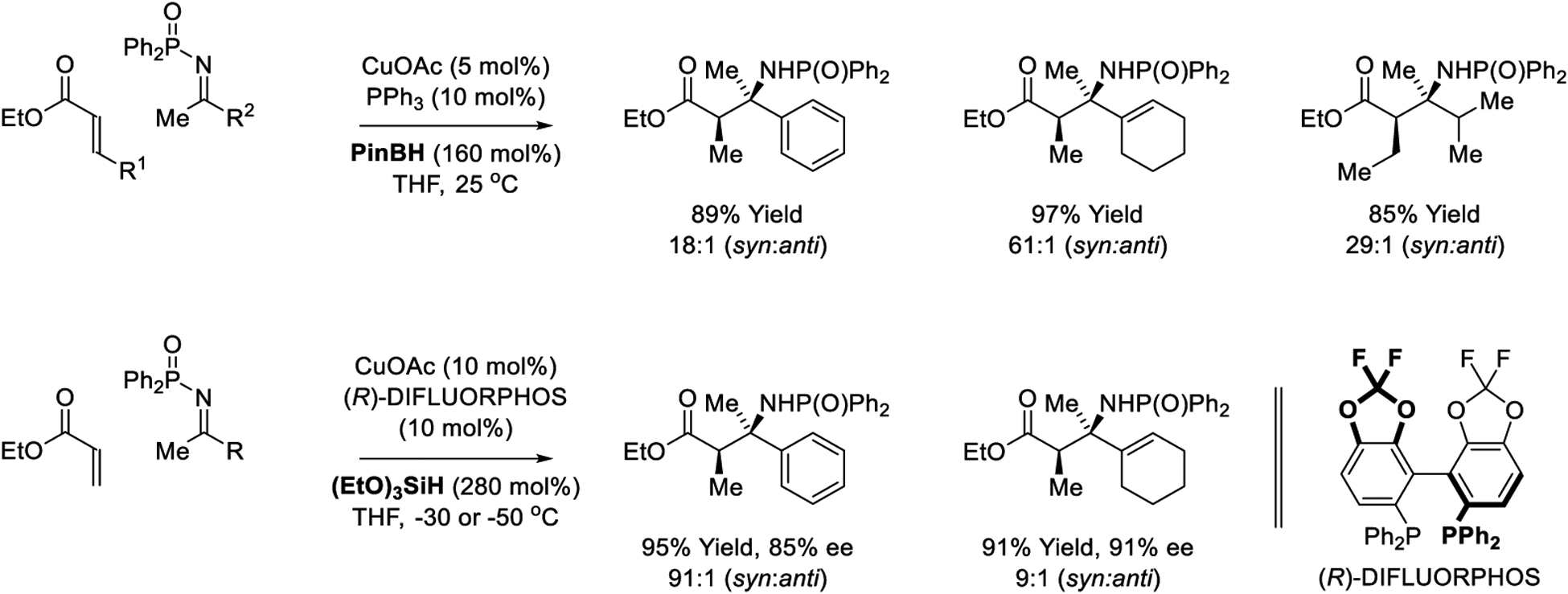
Copper-catalyzed reductive Mannich reactions reported by Shibasaki and Kanai.
Based on the concept of using azaarenes as activating groups,238,340 Lam developed copper-catalyzed reductive aldol reactions of vinyl-substituted heteroaromatic pronucleophiles with ketones (Scheme 34).239 The absolute stereochemistry of the indicated isoquinoline-containing product is opposite to that of the pyrimidine-containing product, even though the same enantiomer of chiral ligand was employed used. The authors developed related diastereo- and enantioselective reductive coupling of vinylazaarenes with N-Boc aldimines mediated by 1,1,3,3-tetramethylhydroxysiloxane (TMDS). The Mannich-type products are generated with good levels of anti-diastereo- and enantioselectivity.
Scheme 34.
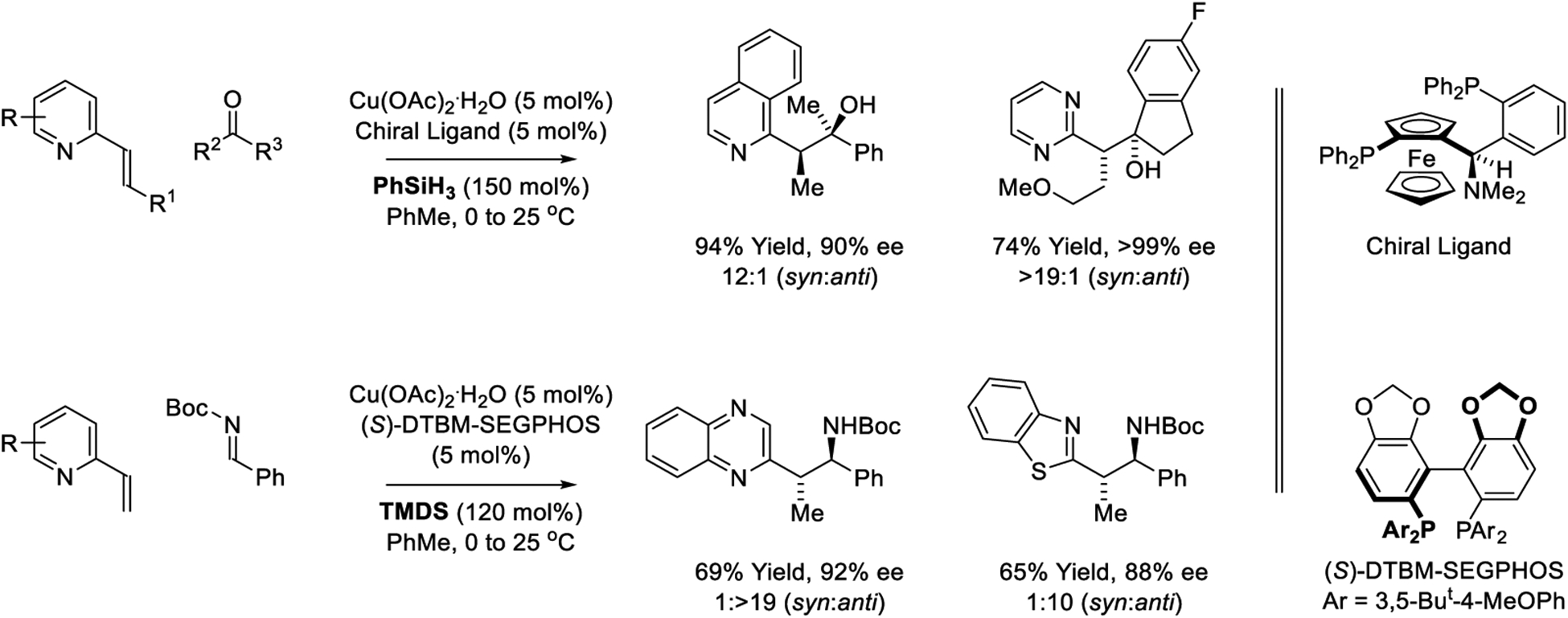
Copper-catalyzed reductive aldol and Mannich-type reactions of vinyl-substituted heteroaromatic pronucleophiles reported by Lam.
2.10. Zinc
Very recently, a zinc-catalyzed acrylate-ketone reductive aldol coupling was reported by Mlynarski (Scheme 35).221 Using the catalyst derived from Zn(OAc)2 and the indicated chiral diamine ligand, good yields of aldol product were obtained. Although modest stereoselectivities were observed, this catalyst system merits further investigation due to its cost-effectiveness. The reducing agent, (EtO)3SiH, readily forms ate-complexes with Lewis basic compounds, and control experiments conducted in the absence of metal salts were not disclosed. As described in the review literature,341 the proposed intervention of zinc vs silicon hydride intermediates is unclear.
Scheme 35.
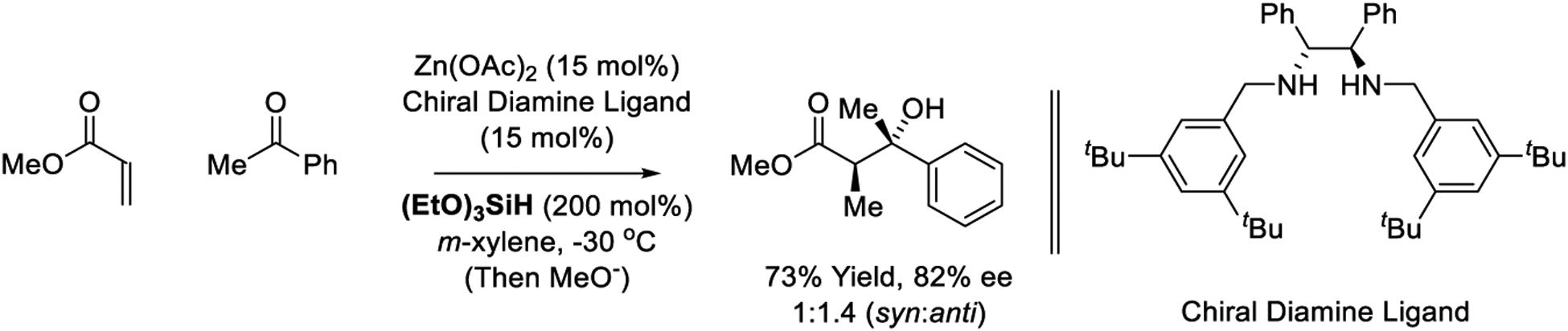
Zinc-catalyzed reductive aldol reaction reported by Mlynarski.
2.11. Indium
Following reports of stoichiometric InBr3-Bu3SnH promoted reductive aldol additions by Baba and Shibata,222 catalytic processes were developed employing hydrosilane as reductant (Scheme 36).223 This catalyst system is applicable to the syn-diastereoselective coupling of enone pronucleophiles with aldehydes. The authors propose that the catalytic cycle is initiated via transmetallation between InBr3 and Et3SiH to form HInBr2 and Et3SiBr, enabling entry to a monohydride-based catalytic cycle (see Scheme 6, Cycle D). syn-Diastereoselectivity is believed to arise through addition of the (Z)-indium enolate, which is formed upon hydrometalation of the enone s-cis-conformer through a 6-centered transition structure,133,134 followed by stereospecific carbonyl addition through a Zimmerman-Traxler type transition structure.37 A contemporaneous study by Hosomi and Miura utilizes PhSiH3 as reductant for intermolecular indium-catalyzed reductive aldol coupling and, as shown, corresponding reductive cyclizations.224 In subsequent work, Shibata conducted intermolecular indium-catalyzed enone-ketone reductive aldol couplings that proceed with high levels of syn-diastereoselectivity.225 Remarkably, the latter process is applicable to sensitive α-bromoketones.
Scheme 36.
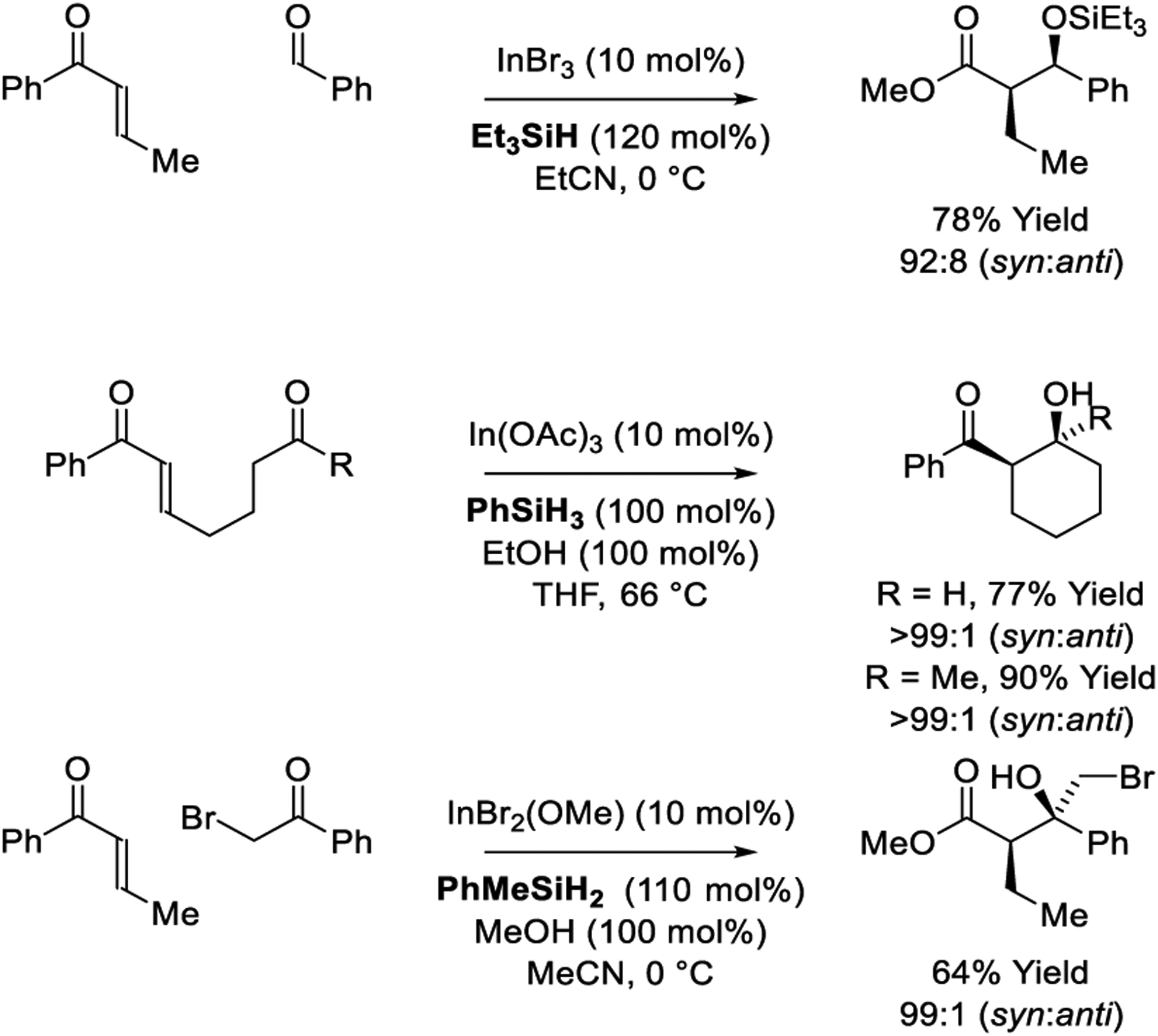
Inter- and intramolecular indium-catalyzed reductive aldol reactions reported by Baba, Shibata, Hosomi and Miura.
2.12. Organocatalyzed Reactions
Beyond metal-catalyzed processes, significant progress on organocatalytic reductive aldol additions has been made.226–229 Lewis basic catalysts in combination with Cl3SiH as reductant have proven particularly effective, as initially illustrated by Nakajima and Sugiura (Scheme 37).226 Specifically, using substoichiometric quantities Ph3P=O, enone-aldehyde reductive coupling occurs in good yield but with low levels of diastereocontrol. Enone reduction from the s-cis-conformer was postulated to occur through the indicated boat-like transition structure. In subsequent work, the same authors disclosed highly syn-diastereo- and enantioselective reductive aldol reactions using (S)-BINAPO as catalyst.227 Asymmetric additions to aromatic and α,β-unsaturated aldehydes were described. Continued studies by Nakajima and Kotani demonstrate that tertiary amines can serve as reductants in syn-diastereo- and enantioselective reductive aldol reactions.228 These processes likely involve hydride transfer from the amine to Cl3SiOTf by virtue of the so-called tert-amino effect; a topic that has recently been reviewed.342 Schindler recently reported a variant of the organocatalyzed reductive aldol reaction mediated by Cl3SiH that exploits α,α-disubstituted pronucleophiles.229 This process delivers aldol adducts bearing quaternary carbon stereocenters. The authors propose a boat-like transition structure to account for relative stereochemistry. Diastereoselectivity was dependent on the steric demand of the aldehyde with pivaldehyde and, as illustrated, ortho-substituted benzaldehydes providing the highest levels of relative stereocontrol. A secondary amine-catalyzed tandem conjugate reduction-Mannich addition mediated by the Hantzsch ester has been described, but this method is a step-wise process in which the imine is added to the reaction mixture after the enal is reduced (not shown).343
Scheme 37.
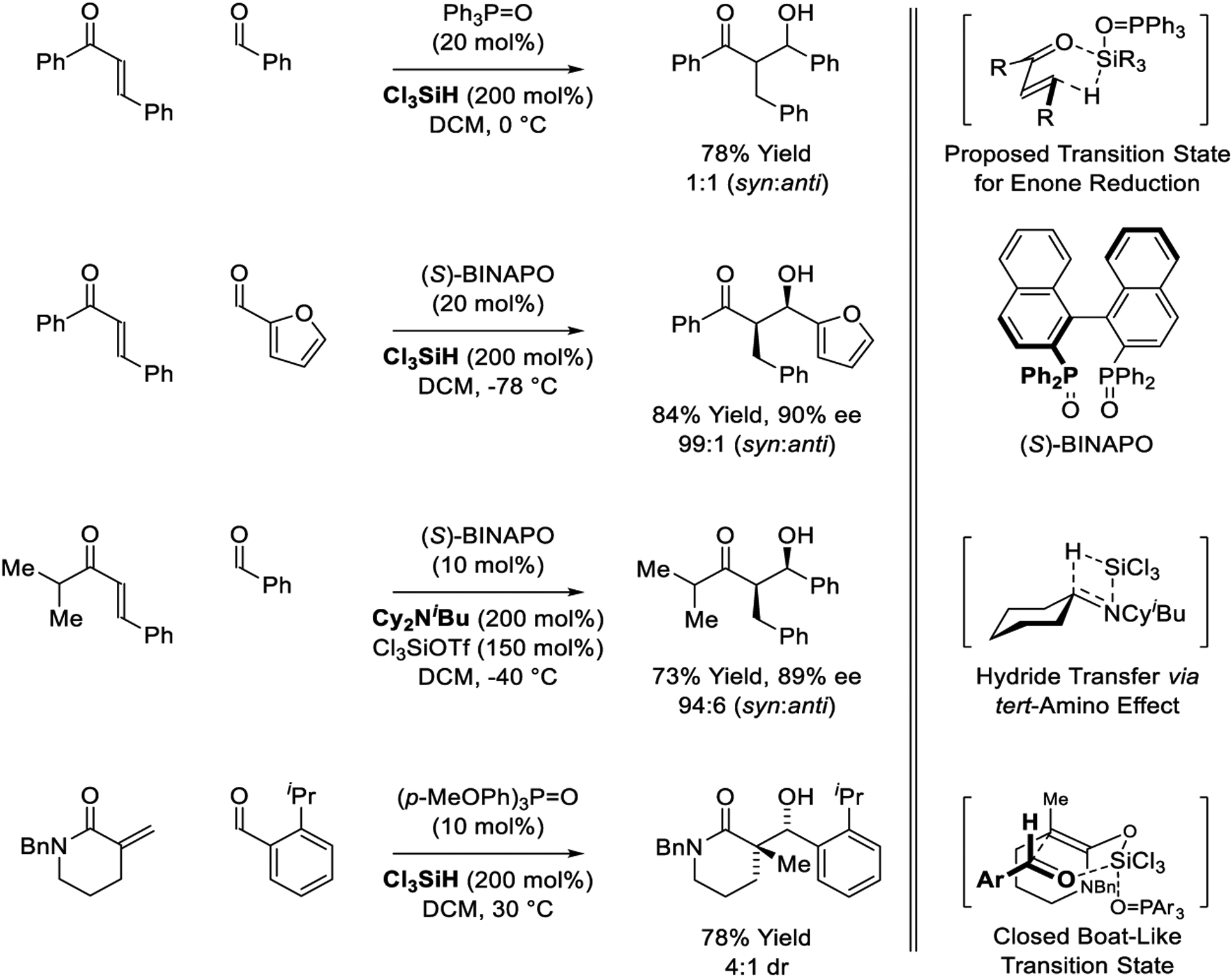
Organocatalyzed reductive aldol reactions reported by Nakajima, Sugiura, Kotani and Schindler.
3. Conclusion and Outlook
Chemical synthesis in the 20th century to the present day has largely been reliant on the use of non-native structural elements to manage issues of reactivity and selectivity. More ideal chemical synthesis requires direct deployment of basic chemical feedstocks in atom-efficient transformations for which issues of isomer-selectivity and functional group compatibility are catalyst controlled.242 While concepts pertaining to efficiency in chemical synthesis are longstanding344,345 and perhaps self-evident, the frequency with which they are discussed far exceeds the instances in which they are realized. Nevertheless, progress on step- and atom-efficient complex molecule synthesis has been made.346,347 In this context, the aldol reaction, which itself may be viewed as a bellwether for organic chemistry as a field, continues to pose unmet challenges in efficiency and selectivity. The reductive aldol reaction, discovered just over 30 years ago, addresses many of these unresolved issues: (a) the regiospecific nature of the reductive aldol reaction enables formation of aldol isomers that are otherwise inaccessible; (b) highly diastereo- and enantioselective additions to ketone electrophiles is possible; (c) the parent acrylate and enone pronucleophiles are inexpensive chemical feedstocks; and (d) in the case of hydrogen-mediated reductive aldol couplings,181–189 diastereo- and enantioselective reactions occur in the absence of stoichiometric byproducts. Many future challenges remain. For example, the majority of reductive aldol reactions employ hydrosilanes as reductants, which are relatively expensive and deliver adducts that incorporate silyl ether moieties. Adapting these catalytic systems to more cost-effective, less mass-intensive reductants, such as formate, carbon monoxide, 2-propanol or hydrogen, and the use of inexpensive base-metal catalysts, would increase the suitability of these methods vis-a-vis large-volume chemical manufacture. It is the authors’ hope that the chemistry and concepts put forth in this and other monographs242,276,277,279,280 will accelerate progress toward these goals and, more broadly, further shift the paradigm for C-C bond formation away from premetalated reagents toward processes that directly exploit abundant, inexpensive and renewable feedstocks.
ACKNOWLEDGMENTS
The Robert A. Welch Foundation (F-0038) and the NIH-NIGMS (RO1-GM069445) are acknowledged for financial support.
Biographies
Professor Michael J. Krische obtained a B.S. degree in Chemistry from the University of California at Berkeley (1989), where he performed research with Professor Henry Rapoport. After a year abroad as a Fulbright Fellow, he initiated doctoral studies at Stanford University with Professor Barry Trost as a Veatch Graduate Fellow. Following receipt of his Ph.D. degree (1996), he joined the laboratory of Professor Jean-Marie Lehn at the Université Louis Pasteur as an NIH Post-Doctoral Fellow. In 1999, he joined the faculty at the University of Texas at Austin. He was promoted directly to the rank of full professor (2004) and shortly thereafter appointed the Robert A. Welch Chair in Science (2007). Professor Krische has pioneered a new class of C-C bond formations that merge the characteristics of carbonyl addition and catalytic hydrogenation. Professor Krische’s research has garnered numerous awards, including the NSF-CAREER Award (2000), Cottrell Scholar Award (2002), Eli Lilly Granteeship for Untenured Faculty (2002), Frasch Award in Chemistry (2002), Dreyfus Teacher-Scholar Award (2003), Sloan Fellowship (2003), Johnson & Johnson Focused Giving Award (2005), Solvias Ligand Prize (2006), Presidential Green Chemistry Award (2007), ACS Corey Award (2007), Dowpharma Prize (2007), Novartis Lectureship (2008), Tetrahedron Young Investigator Award (2009), Humboldt Senior Research Award (2009–2011), Mukaiyama Award (2010), Glaxo-Smith-Kline Scholar Award (2011), ACS Cope Scholar Award (2012), and JSPS Fellow (2013), Eun Lee Lectureship, Korea (2015), Royal Society of Chemistry, Pedlar Award (2015), AAAS Fellow (2017), ACS Award for Creative Work in Synthetic Organic Chemistry (2020).
Cole C. Meyer obtained a B.S. in chemistry with honors from the University of California at Los Angeles (2018), where he conducted research under the tutelage of Professor Patrick G. Harran and received the Dean’s Prize Medal of Excellence. In Fall 2018, he entered the doctoral degree program at the University of Texas at Austin in the laboratory of Professor Michael J. Krische, where he is developing enantioselective metal-catalyzed carbonyl reductive couplings of π-unsaturated feedstocks.
Eliezer Ortiz obtained a B.S. in biochemistry with honors from the University of Texas at San Antonio (2019), where he conducted research under the instruction of Professor Doug E. Frantz and received the Dr. Thyagarajan award for excellence in organic chemistry. After spending a summer as a Postbaccalaureate Research Fellow at UTSA, he entered the doctoral degree program at the University of Texas at Austin in the laboratory of Professor Michael J. Krische, in the Fall of 2019, where he is developing enantioselective metal-catalyzed carbonyl reductive couplings of π-unsaturated feedstocks.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Kane R Ueber eine aus dem Essiggeist Entspringende Reihe con Verbindungen. Ann. Phys. Chem., Ser. 2 1838, 44, 473–494. [Google Scholar]
- (2).Kane R Ueber den Essiggeist und einige davon Abgeleitete Verbindungen. J. Prakt. Chem 1838, 15, 129–155. [Google Scholar]
- (3).Von Richter V. V. aus St. Petersburg am 17. October 1869. Ber. Deut. Chem. Ges 1869, 2, 552–553. [Google Scholar]
- (4).Borodin A Ueber einen Neuen Abkömmling des Valerals. Ber. Dtsch. Chem. Ges 1873, 6, 982–985. [Google Scholar]
- (5).Würtz CA Sur un Aldéhyde-Alcool. Bull. Soc. Chim. Fr 1872, 17, 436–442. [Google Scholar]
- (6).Würtz CA Ueber einen Aldehyd-Alkohol. J. Prakt. Chem 1872, 5, 457–464. [Google Scholar]
- (7).Heathcock CH Acyclic Stereocontrol through the Aldol Condensation. Science 1981, 214, 395–400. [DOI] [PubMed] [Google Scholar]
- (8).Heathcock CH Acyclic Stereoselection via the Aldol Condensation. ACS Symp. Ser 1982, 185, 55–72. [Google Scholar]
- (9).Evans DA; Nelson JV; Taber TR Stereoselective Aldol Condensations. Top. Stereochem 1982, 13, 1–115. [Google Scholar]
- (10).Heathcock CH The Aldol Addition Reaction. Asymmetic Synthesis 1984, 3, 111–212. [Google Scholar]
- (11).Heathcock CH The Aldol Reaction: Acid and General Base Catalysis In Comprehensive Organic Synthesis. Trost BM, Fleming I, Eds.; Pergamon Press: New York, NY, 1991; Vol. 2; Chapter 1.5; pp 133–279. [Google Scholar]
- (12).Heathcock CH The Aldol Reaction: Group I and Group II Enolates In Comprehensive Organic Synthesis; Trost BM, Fleming I, Eds.; Pergamon Press: New York, NY, 1991; Vol. 2; Chapter 1.6; pp 181–238. [Google Scholar]
- (13).Kim BM; Williams SF; Masamune S The Aldol Reaction: Group III Enolates In Comprehensive Organic Synthesis; Trost BM, Fleming I, Eds.; Pergamon Press: New York, NY, 1991; Vol. 2; Chapter 1.7; pp 239–275. [Google Scholar]
- (14).Paterson I; Doughty VA; Florence G; Gerlach K; McLeod MD; Scott JP; Trieselmann T Asymmetric Aldol Reactions Using Boron Enolates: Applications to Polyketide Synthesis. ACS Symposium Series 2001, 783, 195–206. [Google Scholar]
- (15).Schetter B; Mahrwald R Modern Aldol Methods for the Total Synthesis of Polyketides. Angew. Chem., Int. Ed 2006, 45, 7506–7525. [DOI] [PubMed] [Google Scholar]
- (16).Brodmann T; Lorenz M; Schaeckel R; Simsek S; Kalesse M Highly Stereoselective Aldol Reactions in the Total Syntheses of Complex Natural Products. Synlett 2009, 174–192. [Google Scholar]
- (17).Paterson I Total Synthesis of Polyketides Using Asymmetric Aldol Reactions In Asymmetric Synthesis, 2 nd ed.; Christmann M, Brase S, Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2008; pp 293–298. [Google Scholar]
- (18).Kan SBJ; Ng KK-H; Paterson I The Impact of the Mukaiyama Aldol Reaction in Total Synthesis. Angew. Chem., Int. Ed 2013, 52, 9097–9108. [DOI] [PubMed] [Google Scholar]
- (19).Hosokawa S Asymmetric Aldol Reactions in the Total Syntheses of Natural Products In Stereoselective Synthesis of Drugs and Natural Products; Andrushko V, Andrushko N, Eds.; John Wiley & Sons: New York, NY, 2013; Vol. 1; pp 215–247. [Google Scholar]
- (20).Modern Aldol Reactions; Rainer M, Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; Vol. 1 and 2. [Google Scholar]
- (21).Palomo C; Oiarbide M; Garcia JM Current Progress in the Asymmetric Aldol Addition Reaction. Chem. Soc. Rev 2004, 33, 65–75. [DOI] [PubMed] [Google Scholar]
- (22).Trost BM; Brindle CS The Direct Catalytic Asymmetric Aldol Reaction. Chem. Soc. Rev 2010, 39, 1600–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Dias LC; de Lucca EC Jr.; Ferreira MAB; Polo EC Metal-Catalyzed Asymmetric Aldol Reactions. J. Braz. Chem. Soc 2012, 23, 2137–2158. [Google Scholar]
- (24).Yamashita Y; Yasukawa T; Yoo W-J; Kitanosono T; Kobayashi S Catalytic Enantioselective Aldol Reactions. Chem. Soc. Rev 2018, 47, 4388–4480. [DOI] [PubMed] [Google Scholar]
- (25).Hauser CR; Puterbaugh WH β-Hydroxy Esters From Ketones and Esters. J. Am. Chem. Soc 1951, 73, 2972. [Google Scholar]
- (26).Hauser CR; Puterbaugh WH Aldol Condensation of Esters with Ketones or Aldehydes to Form β- Hydroxy Esters by Lithium Amide. Comparison with the Reformatsky Reaction. J. Am. Chem. Soc 1953, 75, 1068–1072. [Google Scholar]
- (27).Hauser CR; Lindsay JK Aldol Condensation of Ethyl Acetate with Ketones to Form β-Hydroxy Esters by Lithium Amide. J. Am. Chem. Soc 1955, 77, 1050–1051. [Google Scholar]
- (28).Dunnavant WR; Hauser CR Synthesis of β-Hydroxy Esters from Ethyl Acetate and Ketones or Aldehydes by Means of Lithium Amide. Some Results with Other Esters. J. Org. Chem 1960, 25, 503–507. [Google Scholar]
- (29).Wittig G; Frommeld HD; Suchanek P Über Gezielte Aldolkondensation. Angew. Chem 1963, 75, 978–979. [Google Scholar]
- (30).Wittig G; Reiff H Directed Aldol Condensations. Angew. Chem., Int. Ed 1968, 7, 7–14. [Google Scholar]
- (31).Wittig G On Directed Aldol Condensation. Rec. Chem. Prog 1967, 28, 45–60. [Google Scholar]
- (32).Ireland RE; Meuller RH; Willard AK The Ester Enolate Claisen Rearrangement. Stereochemical Control through Stereoselective Enolate Formation. J. Am. Chem. Soc 1976, 98, 2868–2877. [Google Scholar]
- (33).Dubois J-E; Dubois M Role des Interactions dans les Etats de Transition de l’Aldolisation. Controle Cinetique et Thermodynamique de la Cetolisation Mixte. Tetrahedron Lett. 1967, 23, 4215–4219. [Google Scholar]
- (34).Dubois J-E; Fort J-F Stereochemistry of Aldolization—XX. Study of the Stereochemical Composition versus Time: Quantitative Determination of Kinetic and Thermodynamic Stereoselectivities. Tetrahedron 1972, 28, 1653–1663. [Google Scholar]
- (35).Dubois J-E; Fort J-F Stereochemistry of Aldolization—XXI. Definition of the “Restoring Energy” of a System of Reversible Competitive Reactions. Tetrahedron 1972, 28, 1665–1675. [Google Scholar]
- (36).Heathcock CA; Buse CT; Kleschick WA; Pirrung MC; Sohn JE; Lampe J Acyclic Stereoselection. 7. Stereoselective Synthesis of 2-Alkyl-3-hydroxy Carbonyl Compounds by Aldol Condensation. J. Org. Chem 1980, 45, 1066–1081. [Google Scholar]
- (37).Zimmerman HE; Traxler MD The Stereochemistry of the Ivanov and Reformatsky Reactions. I. J. Am. Chem. Soc 1957, 79, 1920–1923. [Google Scholar]
- (38).Evans DA; Bartroli J; Shih TL Enantioselective Aldol Condensations. 2. Erythro-Selective Chiral Aldol Condensations via Boron Enolates. J. Am. Chem. Soc 1981, 103, 2127–2129. [Google Scholar]
- (39).Mukaiyama T; Inoue T New Cross-Aldol Reaction via Vinyloxyboranes. Chem. Lett 1976, 5, 559–562. [Google Scholar]
- (40).Mukaiyama T; Narasaka K; Banno K New Aldol Type Reaction. Chem. Lett 1973, 2, 1011–1014. [Google Scholar]
- (41).Hajos ZG; Parrish DR Asymmetric Synthesis of Optically Active Polycyclic Organic Compounds. German Patent DE 2102623, July 29, 1971. [Google Scholar]
- (42).Eder U; Sauer G; Wiechert R New Type of Asymmetric Cyclization to Optically Active Steroid CD Partial Structures. Angew. Chem., Int. Ed. Eng 1971, 10, 496–497. [Google Scholar]
- (43).Hajos ZG; Parrish DR Asymmetric Synthesis of Bicyclic Intermediates of Natural Product Chemistry. J. Org. Chem 1974, 39, 1615–1621. [Google Scholar]
- (44).Ito Y; Sawamura M; Hayashi T Catalytic Asymmetric Aldol Reaction: Reaction of Aldehydes with Isocyanoacetate Catalyzed by a Chiral Ferrocenylphosphine-Gold(I) Complex. J. Am. Chem. Soc 1986, 108, 6405–6406. [Google Scholar]
- (45).Yamada YMA; Yoshikawa N; Sasai H; Shibasaki M Direct Catalytic Asymmetric Aldol Reactions of Aldehydes with Unmodified Ketones. Angew. Chem., Int. Ed. Eng 1997, 36, 1871–1873. [Google Scholar]
- (46).Yoshikawa N; Yamada YMA; Das J; Sasai H; Shibasaki M Direct Catalytic Asymmetric Aldol Reaction. J. Am. Chem. Soc 1999, 121, 4168–4178. [Google Scholar]
- (47).House HO; Czuba LJ; Gall M; Olmstead HD Chemistry of Carbanions. XVIII. Preparation of Trimethylsilyl Enol Ethers. J. Org. Chem 1969, 34, 2324–2336. [Google Scholar]
- (48).Kraft ME; Holton RA Regiospecific Preparation of Thermodynamic Silyl Enol Ethers Using Bromomagnesium Dialkylamides. Tetrahedron Lett. 1983, 24, 1345–1348. [Google Scholar]
- (49).Velluz L; Valls J; Nomine G Recent Advances in the Total Synthesis of Steroids. Angew. Chem., Int. Ed 1965, 4, 181–200. [Google Scholar]
- (50).Corey EJ; Sneen RA Calculation of Molecular Geometry by Vector Analysis. Application to Six- membered Alicyclic Rings. J. Am. Chem. Soc 1955, 77, 2505–2509. [Google Scholar]
- (51).Berkoz B; Chavez EP; Djerassi C 249. Steroids. Part CLXXII. Factors Controlling the Direction of Enol Acetylation of 3-Oxo-steroids. J. Chem. Soc 1962, 1323–1329. [Google Scholar]
- (52).Mazur Y; Sondheimer F Synthesis and Reactions of Ring A Methylated Saturated Steroids. J. Am. Chem. Soc 1958, 80, 5220–5229. [Google Scholar]
- (53).Mazur Y; Sondheimer F Synthesis of 4α-Methyl-Δ7-steroids. The Interrelationship of Cholesterol, Citrostadienol and Lophenol. J. Am. Chem. Soc 1958, 80, 6296–6299. [Google Scholar]
- (54).Reformatsky S Neue Synthese Zweiatomiger Einbasischer Säuren aus den Ketonen. Ber. Dtsch. Chem. Ges 1887, 20, 1210–1211. [Google Scholar]
- (55).Stork G; Rosen P; Goldman NL The α-Alkylation of Enolates from the Lithium-Ammonia Reduction of α,β-Unsaturated Ketones. J. Am. Chem. Soc 1961, 83, 2965–2966. [Google Scholar]
- (56).Stork G; Rosen P; Goldman N; Coombs RV; Tsuji J Alkylation and Carbonation of Ketones by Trapping the Enolates from the Reduction of α,β-Unsaturated Ketones. J. Am. Chem. Soc 1965, 87, 275–286. [Google Scholar]
- (57).Haskel A; Keinan E Palladium-Catalyzed 1,4-Reduction (Conjugate Reduction) In Handbook of Organopalladium Chemistry for Organic Synthesis; Negishi E-I, Ed.; John Wiley & Sons: New York, NY, 2002; Vol. 2; Chapter 7.2.3; pp 2767–2782. [Google Scholar]
- (58).Farina V; Reeves JT; Senanayake CH; Song JJ Asymmetric Synthesis of Active Pharmaceutical Ingredients. Chem. Rev 2006, 106, 2734–2793. [DOI] [PubMed] [Google Scholar]
- (59).Jäkel C; Paciello R High-Throughput and Parallel Screening Methods in Asymmetric Hydrogenation. Chem. Rev 2006, 106, 2912–2942. [DOI] [PubMed] [Google Scholar]
- (60).Shimizu H; Nagasaki I; Matsumura K; Sayo N; Saito T Developments in Asymmetric Hydrogenation from an Industrial Perspective. Acc. Chem. Res 2007, 40, 1385–1393. [DOI] [PubMed] [Google Scholar]
- (61).Khumsubdee S; Burgess K Comparison of Asymmetric Hydrogenations of Unsaturated Carboxylic Acids and Esters. ACS Catal. 2013, 3, 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Verendel JJ; Pamies O; Dieguez M; Andersson PG Asymmetric Hydrogenation of Olefins Using Chiral Crabtree-type Catalysts: Scope and Limitations. Chem. Rev 2014, 114, 2130–2169. [DOI] [PubMed] [Google Scholar]
- (63).Zhu Shou-Fei; Zhou Qi-Lin, Iridium-Catalyzed Asymmetric Hydrogenation of Unsaturated Carboxylic Acids. Acc. Chem. Res 2017, 50, 988–1001. [DOI] [PubMed] [Google Scholar]
- (64).Keinan E; Greenspoon N Partial Reduction of Enones, Styrenes and Related Systems In Comprehensive Organic Synthesis; Trost BM, Fleming I, Eds.; Pergamon Press: Oxford, England, 1991; Vol. 8; Chapter 3.5; pp 523–578. [Google Scholar]
- (65).Sibi MP; Manyem S Enantioselective Conjugate Additions. Tetrahedron 2000, 56, 8033–8061. [Google Scholar]
- (66).Shibata I Recent Advances in Conjugate Reduction of Unsaturated Carbonyl Compounds. Organomet. News 2004, 2, 53. [Google Scholar]
- (67).Rendler S; Oestreich M Polishing a Diamond in the Rough: “Cu—H” Catalysis with Silanes. Angew. Chem., Int. Ed 2007, 46, 498–504. [DOI] [PubMed] [Google Scholar]
- (68).Deutsch C; Krause N; Lipshutz BH CuH-Catalyzed Reactions. Chem. Rev 2008, 108, 2916–2927. [DOI] [PubMed] [Google Scholar]
- (69).Díez-Gonazález S; Nolan SP Copper, Silver, and Gold Complexes in Hydrosilylation Reactions. Acc. Chem. Res 2008, 41, 349–358. [DOI] [PubMed] [Google Scholar]
- (70).Lipshutz BH Rediscovering Organocopper Chemistry through Copper Hydride. It’s All About the Ligand. Synlett 2009, 509–524. [Google Scholar]
- (71).Malkov AV Change of Direction: Enantioselective CuH-Catalyzed 1,2-Reduction of α,β-Unsaturated Ketones. Angew. Chem., Int. Ed 2010, 49, 9814–9815. [DOI] [PubMed] [Google Scholar]
- (72).Massonneau V; Le Maux P; Simonneaux G Catalytic Asymmetric Syntheses. II. Hydrogenation of α,β-Unsaturated Ketones Using Chiral Ruthenium Complexes. J. Organomet. Chem 1987, 327, 269–273. [Google Scholar]
- (73).Massonneau V; Le Maux P; Simonneaux G Catalytic Asymmetric Hydrogenation of α-β- Unsaturated Ketones Using Chiral Ruthenium Hydride Complexes. Tetrahedron Lett. 1987, 27, 5497–5498. [Google Scholar]
- (74).Le Maux P; Massonneau V; Simonneaux G Catalytic Asymmetric Syntheses: Part III. Asymmetric Hydrogenation of Piperitenone Catalysed by Chiral Ruthenium Hydrides : An Example of a Catalytic Kinetic Resolution. Tetrahedron 1988, 44, 1409–1412. [Google Scholar]
- (75).Dobbs DA; Vanhessche KPM; Brazi E; Rautenstrauch V; Lenoir JY; Genet JP; Wiles J; Bergens SH Industrial Synthesis of (+)-cis-Methyl Dihydrojasmonate by Enantioselective Catalytic Hydrogenation; Identification of the Precatalyst [Ru((−)-Me-DuPHOS)(H)-(ɳ6-1,3,5-cyclooctatriene)](BF4). Angew. Chem., Int. Ed 2000, 39, 1992–1995. [DOI] [PubMed] [Google Scholar]
- (76).Molinaro C; Shultz S; Roy A; Lau S; Trinh T; Angelaud R; O’Shea PD; Abele S; Cameron M; Corley E et al. A Practical Synthesis of Renin Inhibitor MK-1597 (ACT-178882) via Catalytic Enantioselective Hydrogenation and Epimerization of Piperidine Intermediate. J. Org. Chem 2011, 76, 1062–1071. [DOI] [PubMed] [Google Scholar]
- (77).Christensen M; Nolting A; Shevlin M; Weisel M; Maligres PE; Lee J; Orr RK; Plummer CW; Tudge MT; Campeau L-C; Ruck RT Enantioselective Synthesis of α-Methyl-β- cyclopropyldihydrocinnamates. J. Org. Chem 2016, 81, 824–830. [DOI] [PubMed] [Google Scholar]
- (78).Ohta T; Miyake T; Seido N; Kumobayashi H; Takaya H Asymmetric Hydrogenation of Olefins with Aprotic Oxygen Functionalities Catalyzed by BINAP-Ru(II) Complexes. J. Org. Chem 1995, 60, 357–363. [Google Scholar]
- (79).Fehr MJ; Consiglio G; Scalone M; Schmid R Asymmetric Hydrogenation of Substituted 2- Pyrones. J. Org. Chem 1999, 64, 5768–5776. [Google Scholar]
- (80).Xue D; Chen Y-C; Cui X; Wang Q-W; Zhu J; Deng J-G Transfer Hydrogenation of Activated C=C Bonds Catalyzed by Ruthenium Amido Complexes: Reaction Scope, Limitation, and Enantioselectivity. J. Org. Chem 2005, 70, 3584–3591. [DOI] [PubMed] [Google Scholar]
- (81).Scheuermann née Taylor CJ; Jaekel C Enantioselective Hydrogenation of Enones with a Hydroformylation Catalyst. Adv. Synth. Catal 2008, 350, 2708–2714. [Google Scholar]
- (82).Ohshima T; Tadaoka H; Hori K; Sayo N; Mashima K Highly Enantio‐ and s‐Trans C=C Bond Selective Catalytic Hydrogenation of Cyclic Enones: Alternative Synthesis of (−)‐Menthol. Chem. Eur. J 2008, 14, 2060–2066. [DOI] [PubMed] [Google Scholar]
- (83).Calvin JR; Frederick MO; Laird DLT; Remacle JR; May SA Rhodium-Catalyzed and Zinc(II) -Triflate-Promoted Asymmetric Hydrogenation of Tetrasubstituted α,β-Unsaturated Ketones. Org. Lett 2012, 14, 1038–1041. [DOI] [PubMed] [Google Scholar]
- (84).Wen J; Jiang J; Zhang X Rhodium-Catalyzed Asymmetric Hydrogenation of α,β-Unsaturated Carbonyl Compounds via Thiourea Hydrogen Bonding. Org. Lett 2016, 18, 4451–4453. [DOI] [PubMed] [Google Scholar]
- (85).Zhang T; Jiang J; Yao L; Geng H; Zhang X Highly Efficient Synthesis of Chiral Aromatic Ketones via Rh-Catalyzed Asymmetric Hydrogenation of β,β-Disubstituted Enones. Chem. Comm 2017, 53, 9258–9261. [DOI] [PubMed] [Google Scholar]
- (86).Ojima I; Kogure T Selective Asymmetric Reduction of α,β-Unsaturated Ketones via Hydrosilylation Catalyzed by Rhodium(I) Complexes with Chiral Phosphine Ligands. Chem. Lett 1975, 985–988. [Google Scholar]
- (87).Hayashi T; Yamamoto K; Kumada M Asymmetric Hydrosilylation of α,β-Unsaturated Carbonyl Compounds. Tetrahedron Lett. 1975, 16, 3–6. [Google Scholar]
- (88).Tsuchiya Y; Kanazawa Y; Shiomi T; Kobayashi K; Nishiyama H Asymmetric Conjugate Reduction of α,β-Unsaturated Esters with Chiral Rhodium(bisoxazolinylphenyl) Catalysts. Synlett 2004, 2493–2496. [DOI] [PubMed] [Google Scholar]
- (89).Kanazawa Y; Nishiyama H Conjugate Reduction of α,β-Unsaturated Aldehydes with Rhodium(bis-oxazolinylphenyl) Catalysts. Synlett 2006, 3343–3345. [Google Scholar]
- (90).Kanazawa Y; Tsuchiya Y; Kobayashi K; Shiomi T; Itoh J; Kikuchi M; Yamamoto Y; Nishiyama H Asymmetric Conjugate Reduction of α,β-Unsaturated Ketones and Esters with Chiral Rhodium(2,6- bisoxazolinylphenyl) Catalysts. Chem. Eur. J 2006, 12, 63–71. [DOI] [PubMed] [Google Scholar]
- (91).Itoh K; Tsuruta A; Ito J.-i.; Yamamoto Y; Nishiyama H. Enantioselective Synthesis of Optically Active 3,3-Diarylpropanoates by Conjugate Hydrosilylation with Chiral Rh-bis(oxazolinyl)phenyl Catalysts. J. Org. Chem 2012, 77, 10914–10919. [DOI] [PubMed] [Google Scholar]
- (92).Naganawa Y; Kawagishi M; Ito J -i.; Nishiyama, H. Asymmetric Induction at Remote Quaternary Centers of Cyclohexadienones by Rhodium-Catalyzed Conjugate Hydrosilylation. Angew. Chem., Int. Ed 2016, 55, 6873–6876. [DOI] [PubMed] [Google Scholar]
- (93).Yang H; Weng G; Fang D; Peng C; Zhang Y; Zhang X; Wang Z Enantioselective Conjugate Hydrosilylation of α,β-Unsaturated Ketones. RSC Adv. 2019, 9, 11627–11633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Hilgraf R; Pfaltz A Chiral Bis(N-sulfonylamino)phosphine- and TADDOL-Phosphite-Oxazoline Ligands: Synthesis and Application in Asymmetric Catalysis. Adv. Synth. Catal 2005, 347, 61–77. [Google Scholar]
- (95).Lu S-M; Bolm C Highly Enantioselective Synthesis of Optically Active Ketones by Iridium-Catalyzed Asymmetric Hydrogenation. Angew. Chem., Int. Ed 2008, 47, 8920–8923. [DOI] [PubMed] [Google Scholar]
- (96).Lu S-M; Bolm C Highly Chemo- and Enantioselective Hydrogenation of Linear α,β-Unsaturated Ketones. Chem. Eur. J 2008, 14, 7513–7516. [DOI] [PubMed] [Google Scholar]
- (97).Lu W-J; Chen Y-W; Hou X-L Iridium-Catalyzed Highly Enantioselective Hydrogenation of the C=C Bond of α,β-Unsaturated Ketones. Angew. Chem., Int. Ed 2008, 47, 10133–10136. [DOI] [PubMed] [Google Scholar]
- (98).Lu W-J; Hou X-L Highly Enantioselective Construction of the α-Chiral Center of Amides via Iridium- Catalyzed Hydrogenation of α,β-Unsaturated Amides. Adv. Synth. Catal 2009, 351, 1224–1228. [Google Scholar]
- (99).Zhao J; Burgess K Synthesis of Vicinal Dimethyl Chirons by Asymmetric Hydrogenation of Trisubstituted Alkenes J. Am. Chem. Soc 2009, 131, 13236–13237. [DOI] [PubMed] [Google Scholar]
- (100).Lu W-J; Chen Y-W; Hou X-L Highly Enantioselective Iridium-Catalyzed Hydrogenation of Trisubstituted Olefins, α,β-Unsaturated Ketones and Imines with Chiral Benzylic Substituted P,N Ligands. Adv. Synth. Catal 2010, 352, 103–107. [Google Scholar]
- (101).Tian F; Yao D; Liu Y; Xie F; Zhang W Iridium-Catalyzed Highly Enantioselective Hydrogenation of Exocyclic α,β-Unsaturated Carbonyl Compounds. Adv. Synth. Catal 2010, 352, 1841–1845. [Google Scholar]
- (102).Woodmansee DH; Mueller M-A; Troendlin L; Hoermann E; Pfaltz A Asymmetric Hydrogenation of α,β-Unsaturated Carboxylic Esters with Chiral Iridium N,P Ligand Complexes. Chem. Eur. J 2012, 18, 13780–13786. [DOI] [PubMed] [Google Scholar]
- (103).Mazuela J; Pamies O; Dieguez M Expanded Scope of the Asymmetric Hydrogenation of Minimally Functionalized Olefins Catalyzed by Iridium Complexes with Phosphite-Thiazoline Ligands. ChemCatChem 2013, 5, 2410–2417. [Google Scholar]
- (104).Li Q; Wan P; He Y; Zhou Y; Li L; Chen B; Duan K; Cao R; Zhou Z; Qiu L Enantioselective Hydrogenation of the Double Bond of Exocyclic α,β-Unsaturated Carbonyl Compounds Catalyzed by Iridium/H8-BINOL-Derived Phosphine-Oxazoline Complexes. Asian J. Org. Chem 2014, 3, 774–783. [Google Scholar]
- (105).Biosca M; Paptchikhine A; Pamies O; Andersson PG; Dieguez M Extending the Substrate Scope of Bicyclic P-Oxazoline/Thiazole Ligands for Ir-Catalyzed Hydrogenation of Unfunctionalized Olefins by Introducing a Biaryl Phosphoroamidite Group. Chem. Eur. J 2015, 21, 3455–3464. [DOI] [PubMed] [Google Scholar]
- (106).Biosca M; Magre M; Coll M; Pamies O; Dieguez M Alternatives to Phosphinooxazoline (t- BuPHOX) Ligands in the Metal-Catalyzed Hydrogenation of Minimally Functionalized Olefins and Cyclic β- Enamides. Adv. Synth. Catal 2017, 359, 2801–2814. [Google Scholar]
- (107).Zheng Z; Cao Y; Chong Q; Han Z; Ding J; Luo C; Wang Z; Zhu D; Zhou Q-L; Ding K Chiral Cyclohexyl-Fused Spirobiindanes: Practical Synthesis, Ligand Development, and Asymmetric Catalysis. J. Am. Chem. Soc 2018, 140, 10374–10381. [DOI] [PubMed] [Google Scholar]
- (108).Kerdphon S; Ponra S; Yang J; Wu H; Eriksson L; Andersson PG Diastereo- and Enantioselective Synthesis of Structurally Diverse Succinate, Butyrolactone, and Trifluoromethyl Derivatives by Iridium-Catalyzed Hydrogenation of Tetrasubstituted Olefins. ACS Catal. 2019, 9, 6169–6176. [Google Scholar]
- (109).Biosca M; Pamies O; Dieguez M Giving a Second Chance to Ir/Sulfoximine-Based Catalysts for the Asymmetric Hydrogenation of Olefins Containing Poorly Coordinative Groups. J. Org. Chem 2019, 84, 8259–8266. [DOI] [PubMed] [Google Scholar]
- (110).Fogassy G; Tungler A; Lévai A; Tóth G Enantioselective Hydrogenation of Exocyclic α,β- Unsaturated Ketones: Part II. Hydrogenation in the Presence of (S)-Proline. J. Mol. Catal. A. Chem 2002, 179, 101–106. [Google Scholar]
- (111).Thorey C; Bouquillon S; Helimi A; Hénin F; Muzart J, Palladium on Charcoal plus Enantiopure Amino Alcohols as Catalytic Systems for the Enantioselective 1,4-Reduction of α-Substituted α,β- Unsaturated Ketones. Eur. J. Org. Chem 2002, 2151–2159. [Google Scholar]
- (112).Tsuchiya Y; Hamashima Y; Sodeoka M A New Entry to Pd-H Chemistry: Catalytic Asymmetric Conjugate Reduction of Enones with EtOH and a Highly Enantioselective Synthesis of Warfarin. Org. Lett 2006, 8, 4851–4854. [DOI] [PubMed] [Google Scholar]
- (113).Monguchi D; Beemelmanns C; Hashizume D; Hamashima Y; Sodeoka M Catalytic Asymmetric Conjugate Reduction with Ethanol: A More Reactive System Pd(II)–iPr-DUPHOS Complex with Molecular Sieves 4A. J. Organomet. Chem 2008, 693, 867–873. [Google Scholar]
- (114).Appella DH; Moritani Y; Shintani R; Ferreira EM; Buchwald SL Asymmetric Conjugate Reduction of α,β-Unsaturated Esters Using a Chiral Phosphine-Copper Catalyst. J. Am. Chem. Soc 1999, 121, 9473–9474. [Google Scholar]
- (115).Hughes G; Kimura M; Buchwald SL Catalytic Enantioselective Conjugate Reduction of Lactones and Lactams. J. Am. Chem. Soc 2003, 125, 11253–11258. [DOI] [PubMed] [Google Scholar]
- (116).Lipshutz BH; Servesko JM; Petersen TB; Papa PP; Lover AA Asymmetric 1,4-Reductions of Hindered β-Substituted Cycloalkenones Using Catalytic SEGPHOS-Ligated CuH. Org. Lett 2004, 6, 1273–1275. [DOI] [PubMed] [Google Scholar]
- (117).Lipshutz BH; Servesko JM; Taft BR Asymmetric 1,4-Hydrosilylations of α,β-Unsaturated Esters. J. Am. Chem. Soc 2004, 126, 8352–8353. [DOI] [PubMed] [Google Scholar]
- (118).Rainka MP; Aye Y; Buchwald SL Copper-Catalyzed Asymmetric Conjugate Reduction as a Route to Novel β-Azaheterocyclic Acid Derivatives. Proc. Nat. Acad. Sci. U. S. A 2004, 101, 5821–5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Shimizu H; Nagano T; Sayo N; Saito T; Ohshima T; Mashima K Asymmetric Hydrogenation of Heteroaromatic Ketones and Cyclic and Acyclic Enones Mediated by Cu(I)–Chiral Diphosphine Catalysts. Synlett 2009, 3143–3146. [Google Scholar]
- (120).Rupnicki L; Saxena A; Lam HW Aromatic Heterocycles as Activating Groups for Asymmetric Conjugate Addition Reactions. Enantioselective Copper-Catalyzed Reduction of 2-Alkenylheteroarenes. J. Am. Chem. Soc 2009, 131, 10386–10387. [DOI] [PubMed] [Google Scholar]
- (121).Leutenegger U; Madin A; Pfaltz A Enantioselective Reduction of α,β-Unsaturated Carboxylates with NaBH4 and Catalytic Amounts of Chiral Cobalt Semicorrin Complexes. Angew. Chem., Int. Ed. Engl 1989, 28, 60–61. [Google Scholar]
- (122).Misun M; Pfaltz A Enantioselective Reduction of Electrophilic C=C Bonds with Sodium Tetrahydroborate and ‘Semicorrin’ Cobalt Catalysts. Helv. Chim. Acta 1996, 79, 961–972. [Google Scholar]
- (123).von Matt P; Pfaltz A Enantioselective Conjugate Reduction of α,β-Unsaturated Carboxamides with Semicorrin Cobalt Catalysts. Tetrahedron: Asymmetry 1991, 2, 691–700. [Google Scholar]
- (124).Yamada T; Ohtsuka Y; Ikeno T Enantioselective Borohydride 1,4-Reduction of α, β-Unsaturated Carboxamides Using Optically Active Cobalt(II) Complex Catalysts. Chem. Lett 1998, 1129–1130. [Google Scholar]
- (125).Ohtsuka Y; Ikeno T; Yamada T Catalytic Enantioselective Protonation of Cobalt-Enolate Equivalents Generated by 1,4-Reduction with Borohydride. Tetrahedron: Asymmetry 2003, 14, 967–970. [Google Scholar]
- (126).Geiger C; Kreitmeier P; Reiser O Cobalt(II)-Azabis(oxazoline)-Catalyzed Conjugate Reduction of α,β-Unsaturated Carbonyl Compounds. Adv. Synth. Catal 2005, 347, 249–254. [Google Scholar]
- (127).Aldea L; Fraile JM; García-Marín H; García JI; Herrerías CI; Mayoral JA; Pérez I Study of the Recycling Possibilities for Azabis(oxazoline)-Cobalt Complexes as Catalysts for Enantioselective Conjugate Reduction. Green Chem. 2010, 12, 435–440. [Google Scholar]
- (128).Shuto Y; Yamamura T; Tanaka S; Yoshimura M; Kitamura M Asymmetric NaBH4 1,4-Reduction of C3-Disubstituted 2-Propenoates Catalyzed by a Diamidine Cobalt Complex. ChemCatChem 2015, 7, 1547–1550. [Google Scholar]
- (129).Han ZS; Zhang L; Xu Y; Sieber JD; Marsini MA; Li Z; Reeves JT; Fandrick KR; Patel ND; Desrosiers J-N et al. Efficient Asymmetric Synthesis of Structurally Diverse P-Stereogenic Phosphinamides for Catalyst Design. Angew. Chem., Int. Ed 2015, 54, 5474–5477. [DOI] [PubMed] [Google Scholar]
- (130).Yang H; Weng G; Fang D; Peng C; Zhang Y; Zhang X; Wang Z Enantioselective Conjugate Hydrosilylation of α,β-Unsaturated Ketones RSC Adv. 2019, 9, 11627–11633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Sugiura M; Ashikari Y; Takahashi Y; Yamaguchi K; Kotani S; Nakajima M Lewis Base-Catalyzed Enantioselective Conjugate Reduction of β,β-Disubstituted α,β-Unsaturated Ketones with Trichlorosilane: E/Z-Isomerization, Regioselectivity, and Synthetic Applications. J. Org. Chem 2019, 84, 11458–11473. [DOI] [PubMed] [Google Scholar]
- (132).Evans DA; Fu GC Conjugate Reduction of α,β-Unsaturated Carbonyl Compounds by Catecholborane. J. Org. Chem 1990, 55, 5678–5680. [Google Scholar]
- (133).Boldrini GP; Mancini F; Tagliavini E; Trombini C; Umani-Ronchi A A new approach to (Z)- Vinyloxyboranes via 1,4 Hydroboration of (E)-α,β-Unsaturated Ketones. Synthesis of syn-Aldols. J. Chem. Soc. Chem. Commun 1990, 1680–1681. [Google Scholar]
- (134).Boldrini GP; Bortolotti M; Mancini F; Tagliavini E; Trombini C; Umani-Ronchi A A New Protocol for Regio- and Stereocontrolled Aldol Reactions Through the Conjugate Addition of Dialkylboranes to α,β-Unsaturated Ketones J. Org. Chem 1991, 56, 5820–5826. [Google Scholar]
- (135).Kawakami T; Miyatake M; Shibata I; Baba A Organotin Iodide Hydride: Chemoselective 1,4- Hydrostannations of Conjugated Enones in the Presence of Aldehydes and Subsequent Intermolecular Aldol Reactions. J. Org. Chem 1996, 61, 376–379. [Google Scholar]
- (136).Chrisman W; Nosson K; Papa P; Sclafani JA; Vivian RW; Keith JM; Lipshutz BH Copper Hydride-Catalyzed Tandem 1,4-Reduction/Alkylation Reactions. Tetrahedron 2000, 56, 2779–2788. [Google Scholar]
- (137).Lipshutz BH; Papa P Copper-Catalyzed Reductive Alkylations of Enones: A Novel Transmetalation Protocol. Angew. Chem., Int. Ed 2002, 41, 4580–4582. [DOI] [PubMed] [Google Scholar]
- (138).Huddleston RR; Cauble DF; Krische MJ Borane-Mediated Aldol Cycloreduction of Monoenone Monoketones: Diastereoselective Formation of Quaternary Centers. J. Org. Chem 2003, 68, 11–14. [DOI] [PubMed] [Google Scholar]
- (139).Ghosh AK; Xu X; Kim J-H; Xu C-X Enantioselective Total Synthesis of Peloruside A: A Potent Microtubule Stabilizer. Org. Lett 2008, 10, 1001–1004. [DOI] [PubMed] [Google Scholar]
- (140).Ghosh AK; Kass J; Anderson DD Xu; Marian C L-Selectride-Mediated Highly Diastereoselective Asymmetric Reductive Aldol Reaction: Access to an Important Subunit for Bioactive Molecules. Org. Lett 2008, 10, 4811–4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Ghosh AK; Dawson ZL Synthesis of Bioactive Natural Products by Asymmetric syn- and anti- Aldol Reactions. Synthesis 2009, 2992–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Kim H; Yun J Copper-Catalyzed Asymmetric 1,4-Hydroboration of Coumarins with Pinacolborane: Asymmetric Synthesis of Dihydrocoumarins. Adv. Synth. Catal 2010, 352, 1881–1885. [Google Scholar]
- (143).Deschamp J; Hermant T; Riant O An Easy Route Toward Enantio-Enriched Polycyclic Derivatives via an Asymmetric Domino Conjugate Reduction-Aldol Cyclization Catalyzed by a Chiral Cu(I) Complex. Tetrahedron 2012, 68, 3457–3467. [Google Scholar]
- (144).Nuhant P; Allais C; Roush WR Diisopinocampheylborane-Mediated Reductive Aldol Reactions: Highly Enantio- and Diastereoselective Synthesis of syn-Aldols from N-Acryloylmorpholine. Angew. Chem., Int. Ed 2013, 52, 8703–8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Allais C; Tsai AS; Nuhant P; Roush WR. Generation of Stereochemically Defined Tetrasubstituted Enolborinates by 1,4-Hydroboration of α,β-Unsaturated Morpholine Carboxamides with (Diisopinocampheyl)borane. Angew. Chem., Int. Ed 2013, 52, 12888–12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Allais C; Nuhant P; Roush WR (Diisopinocampheyl)borane-Mediated Reductive Aldol Reactions of Acrylate Esters: Enantioselective Synthesis of anti-Aldols. Org. Lett 2013, 15, 3922–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Motherwell WB Curiosity and Simplicity in the Invention and Discovery of New Metal-Mediated Reactions for Organic Synthesis. Pure Appl. Chem 2002, 74, 135–142. [Google Scholar]
- (148).Huddleston RR; Krische MJ Enones as Latent Enolates in Catalytic Processes: Catalytic Cycloreduction, Cycloaddition and Cycloisomerization. Synlett 2003, 1, 12–21. [Google Scholar]
- (149).Jang H-Y; Huddleston RR; Krische MJ Nucleophilic Activation of Enones via Homogeneous Catalytic Hydrogenation: Catalytic Reductive C—C Bond Formation under Hydrogenation Conditions. Chemtracts 2003, 16, 554–559. [Google Scholar]
- (150).Jang H-Y; Krische MJ Catalytic Hydrogen-Mediated Cross-Coupling of Enones and Carbonyl Compounds: Aldol Condensation by Hydrogenation. Eur. J. Org. Chem 2004, 3953–3958. [Google Scholar]
- (151).Jang H-Y; Krische MJ Catalytic C-C Bond Formation via Capture of Hydrogenation Intermediates. Acc. Chem. Res 2004, 37, 653–661. [DOI] [PubMed] [Google Scholar]
- (152).Chiu P Organosilanes in Copper-Mediated Conjugate Reductions and Reductive Aldol Reactions. Synthesis 2004, 2210–2215. [Google Scholar]
- (153).Krische MJ; Jang H-Y Metal-Catalyzed Reductive Carbocyclization (C=C, C≡C, C=O Bonds) In Comprehensive Organometallic Chemistry III; Mingos M; Crabtree R, Eds.; Elsevier: Oxford, England, 2006; Vol. 10; Chapter 10.10; pp 493–536. [Google Scholar]
- (154).Iida H; Krische MJ Catalytic Reductive Coupling of Alkenes and Alkynes to Carbonyl Compounds and Imines Mediated by Hydrogen. Top. Curr. Chem 2007, 279, 77–104. [Google Scholar]
- (155).Nishiyama H; Shiomi T Reductive Aldol, Michael, and Mannich Reactions. Top. Curr. Chem 2007, 279, 105–137. [Google Scholar]
- (156).Ngai M-Y; Kong J-R; Krische MJ Hydrogen-Mediated C-C Bond Formation: A Broad New Concept in Catalytic C-C Coupling. J. Org. Chem 2007, 72, 1063–1072. [DOI] [PubMed] [Google Scholar]
- (157).Krische MJ; Cho C-W Hydrogen-Mediated Carbon-Carbon Bond Formation Catalyzed by Rhodium In Handbook of Homogeneous Hydrogenation; de Vries JG, Elsevier CJ, Eds.; Wiley-VCH: Weinheim, Germany, 2007; Chapter 22; pp 713–741. [Google Scholar]
- (158).Bower JF; Krische MJ Hydrogenation for C-C Bond Formation In Handbook of Green Chemistry; Anastas P, Ed.; Wiley-VCH: Weinheim, 2007; Vol. 1; Chapter 8; pp 205–254. [Google Scholar]
- (159).Garner S; Han SB; Krische MJ Metal Catalyzed Reductive Aldol Coupling In Modern Reduction Methods; Andersson P, Munslow I, Eds.; Wiley-VCH: Weinheim, Germany, 2008; Chapter 16; pp 387–408. [Google Scholar]
- (160).Han SB; Hassan A; Krische MJ Diastereo- and Enantioselective Reductive Aldol Addition of Vinyl Ketones via Catalytic Hydrogenation. Synthesis 2008, 2669–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Patman RL; Bower JF; Kim IS Krische MJ Formation of C–C Bonds via Catalytic Hydrogenation and Transfer Hydrogenation: Vinylation, Allylation, and Enolate Addition. Aldrichim. Acta 2008, 41, 95–104. [PMC free article] [PubMed] [Google Scholar]
- (162).Jang H-Y; Krische MJ Metal-Catalyzed Reductive Aldol Coupling In Comprehensive Chirality; Yamamoto H, Carreira EM, Eds.; Elsevier: Oxford, England, 2012; Vol. 4; pp 100–121. [Google Scholar]
- (163).Revis A; Hilty TK Novel Synthesis of β-Siloxy Esters by Condensation of Carbonyls and Trimethylsilane with α,β-Unsaturated Esters Catalyzed by RhCl3. Tetrahedron Lett. 1987, 28, 4809–4812. [Google Scholar]
- (164).Matsuda I; Takahashi K; Sato S Rhodium Catalyzed Direct Coupling of α,β-Unsaturated Ketone, Aldehyde, and Trialkylsilane: An Easy Access to Regio-Defined Aldol Derivatives. Tetrahedron Lett. 1990, 31, 5331–5334. [Google Scholar]
- (165).Emiabata-Smith D; McKillop A; Mills C; Motherwell WB; Whitehead AJ Some Preliminary Studies on a Novel Rhodium(I)-Catalysed Tandem Hydrosilylation-Intramolecular Aldol Reaction. Synlett. 2001, 1302–1304. [Google Scholar]
- (166).Freiría M; Whitehead AJ; Tocher DA; Motherwell WB Further Observations on the Rhodium (I)-Catalysed Tandem Hydrosilylation-Intramolecular Aldol Reaction. Tetrahedron 2004, 60, 2673–2692. [Google Scholar]
- (167).Taylor SJ; Morken JP Catalytic Diastereoselective Reductive Aldol Reaction: Optimization of Interdependent Reaction Variables by Arrayed Catalyst Evaluation. J. Am. Chem. Soc 1999, 121, 12202–12203. [Google Scholar]
- (168).Taylor SJ; Duffey MO; Morken JP Rhodium-Catalyzed Enantioselective Reductive Aldol Reaction. J. Am. Chem. Soc 2000, 122, 4528–4529. [Google Scholar]
- (169).Zhao C-X; Bass J; Morken JP Generation of (E)-Silylketene Acetals in a Rhodium-DuPhos Catalyzed Two-Step Reductive Aldol Reaction. Org. Lett 2001, 3, 2839–2842. [DOI] [PubMed] [Google Scholar]
- (170).Russell AE; Fuller NO; Taylor SJ; Aurriset P; Morken JP Investigation of the Rh-Catalyzed Asymmetric Reductive Aldol Reaction. Expanded Scope Based on Reaction Analysis. Org. Lett 2004, 6, 2309–2312. [DOI] [PubMed] [Google Scholar]
- (171).Fuller NO; Morken JP Direct Formation of Synthetically Useful Silyl-Protected Aldol Adducts via the Asymmetric Reductive Aldol Reaction. Synlett 2005, 1459–1461. [Google Scholar]
- (172).Fuller NO; Morken JP Studies on the Synthesis of the Inostamycin Natural Products: A Reductive Aldol/Reductive Claisen Approach to the C10−C24 Ketone Fragment. Org. Lett 2005, 7, 4867–4869. [DOI] [PubMed] [Google Scholar]
- (173).Nishiyama H; Shiomi T; Tsuchiya Y; Matsuda I High Performance of Rh(Phebox) Catalysts in Asymmetric Reductive Aldol Reaction: High Anti-Selectivity. J. Am. Chem. Soc 2005, 127, 6972–6973. [DOI] [PubMed] [Google Scholar]
- (174).Willis MC; Woodward RL Rhodium-Catalyzed Reductive Aldol Reactions Using Aldehydes as the Stoichiometric Reductants. J. Am. Chem. Soc 2005, 127, 18012–18013. [DOI] [PubMed] [Google Scholar]
- (175).Ito JI; Shiomi T; Nishiyama H Efficient Preparation of New Rhodium- and Iridium- [Bis(oxazolinyl)-3,5-dimethylphenyl] Complexes by C-H Bond Activation: Applications in Asymmetric Synthesis. Adv. Synth. Catal 2006, 348, 1235–1240. [Google Scholar]
- (176).Shiomi T; Ito J-I; Yamamoto Y; Nishiyama H 4-Substituted-Phenyl(bisoxazoline)-Rhodium Complexes: Efficiency in the Catalytic Asymmetric Reductive Aldol Reaction. Eur. J. Org. Chem 2006, 5594–5600. [Google Scholar]
- (177).Shiomi T; Nishiyama H Intermolecular Asymmetric Reductive Aldol Reaction of Ketones as Acceptors Promoted by Chiral Rh(Phebox) Catalyst. Org. Lett 2007, 9, 1651–1654. [DOI] [PubMed] [Google Scholar]
- (178).Osborne JD; Willis MC Rhodium-catalysed hydroacylation or reductive aldol reactions: a ligand dependent switch of reactivity. Chem. Commun 2008, 5025–5027. [DOI] [PubMed] [Google Scholar]
- (179).Hashimoto T; Ito J.-i.; Nishiyama H Felkin–Anh selectivity in Rh(bisoxazolinylphenyl)-catalyzed reductive aldol coupling reaction: asymmetric synthesis of stereotriads. Tetrahedron 2008, 64, 9408–9412. [Google Scholar]
- (180).Shiomi T; Adachi T; Nishiyama H Intermolecular Antiselective and Enantioselective Reductive Coupling of Enones and Aromatic Aldehydes with Chiral Rh(Phebox) Catalysts. Org. Lett 2009, 11, 1011–1014. [DOI] [PubMed] [Google Scholar]
- (181).Jang HY; Huddleston RR; Krische MJ Reductive Generation of Enolates from Enones Using Elemental Hydrogen: Catalytic C-C Bond Formation under Hydrogenative Conditions. J. Am. Chem. Soc 2002, 124, 15156–15157. [DOI] [PubMed] [Google Scholar]
- (182).Huddleston RR; Krische MJ Enolate Generation under Hydrogenation Conditions: Catalytic Aldol Cycloreduction of Keto-Enones. Org. Lett 2003, 5, 1143–1146. [DOI] [PubMed] [Google Scholar]
- (183).Koech PK; Krische MJ Catalytic Addition of Metallo-Aldehyde Enolates to Ketones: A New C-C Bond-Forming Hydrogenation. Org. Lett 2004, 6, 691–694. [DOI] [PubMed] [Google Scholar]
- (184).Marriner GA; Garner SA; Jang HY; Krische MJ Metallo-Aldehyde Enolates via Enal Hydrogenation: Catalytic Cross Aldolization with Glyoxal Partners As Applied to the Synthesis of 3,5-Disubstituted Pyridazines. J. Org. Chem 2004, 69, 1380–1382. [DOI] [PubMed] [Google Scholar]
- (185).Jung CK; Garner SA; Krische MJ Hydrogen-Mediated Aldol Reductive Coupling of Vinyl Ketones Catalyzed by Rhodium: High syn-Selectivity through the Effect of Tri-2-furylphosphine. Org. Lett 2006, 8, 519–522. [DOI] [PubMed] [Google Scholar]
- (186).Han SB; Krische MJ Reductive Aldol Coupling of Divinyl Ketones via Rhodium-Catalyzed Hydrogenation: syn-Diastereoselective Construction of β-Hydroxyenones. Org. Lett 2006, 8, 5657–5660. [DOI] [PubMed] [Google Scholar]
- (187).Jung CK; Krische MJ Asymmetric Induction in Hydrogen-Mediated Reductive Aldol Additions to α-Amino Aldehydes Catalyzed by Rhodium: Selective Formation of syn-Stereotriads Directed by Intramolecular Hydrogen-Bonding. J. Am. Chem. Soc 2006, 128, 17051–17056. [DOI] [PubMed] [Google Scholar]
- (188).Bee C; Han SB; Hassan A; Iida H; Krische MJ Diastereo- and Enantioselective Hydrogenative Aldol Coupling of Vinyl Ketones: Design of Effective Monodentate TADDOL-Like Phosphonite Ligands. J. Am. Chem. Soc 2008, 130, 2746–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (189).Shin I; Hong S; Krische MJ Total Synthesis of Swinholide A: An Exposition in Hydrogen-Mediated C-C Bond Formation. J. Am. Chem. Soc 2016, 138, 14246–14249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (190).Isayama S; Mukaiyama T Cobalt(II) Catalyzed Coupling Reaction of α,β-Unsaturated Compounds with Aldehydes by the Use of Phenylsilane. New Method for Preparation of β-Hydroxy Nitriles, Amides, and Esters. Chem. Lett 1989, 18, 2005–2008. [Google Scholar]
- (191).Baik TG; Luis AL; Wang LC; Krische MJ Diastereoselective Cobalt-Catalyzed Aldol and Michael Cycloreductions. J. Am. Chem. Soc 2001, 123, 5112–5113. [DOI] [PubMed] [Google Scholar]
- (192).Wang LC; Jang H-Y; Roh Y; Lynch V; Schultz AJ; Wang X; Krische MJ Diastereoselective Cycloreductions and Cycloadditions Catalyzed by Co(dpm)2-Silane (dpm = 2,2,6,6-tetramethylheptane-3,5-dionate): Mechanism and Partitioning of Hydrometallative versus Anion Radical Pathways. J. Am. Chem. Soc 2002, 124, 9448–9453. [DOI] [PubMed] [Google Scholar]
- (193).Lam HW; Joensuu PM; Murray GJ; Fordyve EAF; Prieto O; Luebbers T Diastereoselective Cobalt-Catalyzed Reductive Aldol Cyclizations Using Diethylzinc as the Stoichiometric Reductant. Org. Lett 2006, 8, 3729–3732. [DOI] [PubMed] [Google Scholar]
- (194).Lumby RJR; Joensuu PM; Lam HW Diastereoselective Intermolecular Cobalt-Catalyzed Reductive Aldol Reactions of α,β-Unsaturated Amides with Ketones. Org. Lett 2007, 9, 4367–4370. [DOI] [PubMed] [Google Scholar]
- (195).Lumby RJR; Joensuu PM; Lam HW Racemic and Asymmetric Cobalt-Catalysed Reductive Aldol Couplings of α,β-Unsaturated Amides with Ketones. Tetrahedron 2008, 64, 7729–7740. [Google Scholar]
- (196).Zhao CX.; Duffey MO; Taylor SJ; Morken JP. Enantio- and Diastereoselective Reductive Aldol Reactions with Iridium-Pybox Catalysts. Org. Lett 2001, 3, 1829–1831. [DOI] [PubMed] [Google Scholar]
- (197).Doi T; Fukuyama T; Minamino S; Ryu I RuHCl(CO)(PPh3)3-Catalyzed Reductive Dimerization of α,β-Unsaturated Aldehydes Leading to α-Hydroxymethyl Ketones. Synlett 2006, 3013–3016. [Google Scholar]
- (198).Doi T; Fukuyama T; Minamino S; Husson G; Ryu I An Unusual Dimerization of Primary Unsaturated Alcohols Catalyzed by RuHCl(CO)(PPh3)3. Chem. Commun 2006, 1875–1877. [DOI] [PubMed] [Google Scholar]
- (199).Denichoux A; Fukuyama T; Doi T; Horiguchi J; Ryu I Synthesis of 2-Hydroxymethyl Ketones by Ruthenium Hydride-Catalyzed Cross-Coupling Reaction of α,β-Unsaturated Aldehydes with Primary Alcohols. Org. Lett 2010, 12, 1–3. [DOI] [PubMed] [Google Scholar]
- (200).Kiyooka SI; Shimizu A; Torii S A Mild Aldol Reaction of Aryl Aldehydes through Palladium- Catalyzed Hydrosilation of α,β-Unsaturated Carbonyl Compounds with Trichlorosilane. Tetrahedron Lett. 1998, 39, 5237–5238. [Google Scholar]
- (201).Chrovian CC; Montgomery J Surprising Role of Aryl Halides in Nickel-Catalyzed Reductive Aldol Reactions. Org. Lett 2007, 9, 537–540. [DOI] [PubMed] [Google Scholar]
- (202).Joensuu PM; Murray GJ; Fordyce EAF; Luebbers T; Lam HW Diastereoselective Nickel-Catalyzed Reductive Aldol Cyclizations Using Diethylzinc as the Stoichiometric Reductant: Scope and Mechanistic Insight. J. Am. Chem. Soc 2008, 130, 7328–7338. [DOI] [PubMed] [Google Scholar]
- (203).Margalef IV; Rupnicki L; Lam HW Formal Synthesis of Salinosporamide A Using a Nickel-Catalyzed Reductive Aldol Cyclization-Lactonization as a Key Step. Tetrahedron 2008, 64, 7896–7901. [Google Scholar]
- (204).Lee H; Jang M-S; Song Y-J; Jang H-Y Platinum-Catalyzed Reductive Aldol and Michael Reactions. Bull. Korean Chem. Soc 2009, 30, 327–333. [Google Scholar]
- (205).Chiu P; Chen B; Cheng KF A Conjugate Reduction-Intramolecular Aldol Strategy Toward the Synthesis of Pseudolaric Acid A. Tetrahedron Lett. 1998, 39, 9229–9232. [Google Scholar]
- (206).Chung WK; Chiu P Reductive Intramolecular Henry Reactions Induced by Stryker’s Reagent. Synlett 2005, 55–58. [Google Scholar]
- (207).Chiu P; Leung SK Stoichiometric and Catalytic Reductive Aldol Cyclizations of Alkynediones Induced by Stryker’s Reagent. Chem. Commun 2004, 2308–2309. [DOI] [PubMed] [Google Scholar]
- (208).Ooi T; Doda K; Sakai D; Maruoka K. Unique Property of Copper(I) Chloride as a Radical Initiator as Well as a Lewis Acid: Application to CuCl-Catalyzed Aldol Reaction of α,β-Unsaturated Ketones with Bu3SnH. Tetrahedron Lett. 1999, 40, 2133–2136. [Google Scholar]
- (209).Lam HW; Joensuu PMA Cu(I)-Catalyzed Reductive Aldol Cyclizations: Diastereo- and Enantioselective Synthesis of β-Hydroxylactones. Org. Lett 2005, 7, 4225. [DOI] [PubMed] [Google Scholar]
- (210).Lam HW; Murray GJ; Firth JD Diastereoselective Synthesis of 4-Hydroxypiperidin-2-ones via Cu(I)-Catalyzed Reductive Aldol Cyclization. Org. Lett 2005, 7, 5743–5746. [DOI] [PubMed] [Google Scholar]
- (211).Deschamp J; Chuzel O; Hannedouche J; Riant O Highly Diastereo- and Enantioselective Copper- Catalyzed Domino Reduction/Aldol Reaction of Ketones with Methyl Acrylate. Angew. Chem., Int. Ed 2006, 45, 1292–1297. [DOI] [PubMed] [Google Scholar]
- (212).Chuzel O; Deschamp J; Chauster C; Riant O Copper(I)-Catalyzed Enantio- and Diastereoselective Tandem Reductive Aldol Reaction. Org. Lett 2006, 8, 5943–5946. [DOI] [PubMed] [Google Scholar]
- (213).Zhao D; Oisaki K; Kanai M; Shibasaki M Catalytic Enantioselective Intermolecular Reductive Aldol Reaction to Ketones. Tetrahedron Lett. 2006, 47, 1403–1407. [Google Scholar]
- (214).Zhao D; Oisaki K; Kanai M; Shibasaki M Dramatic Ligand Effect in Catalytic Asymmetric Reductive Aldol Reaction of Allenic Esters to Ketones. J. Am. Chem. Soc 2006, 128, 14440–14441. [DOI] [PubMed] [Google Scholar]
- (215).Welle A; Diez-Gonzalez S; Tinant B; Nolan SP; Riant OA Three-Component Tandem Reductive Aldol Reaction Catalyzed by N-Heterocyclic Carbene-Copper Complexes. Org. Lett 2006, 8, 6059–6062. [DOI] [PubMed] [Google Scholar]
- (216).Lipshutz BH; Amorelli B; Unger JB CuH-Catalyzed Enantioselective Intramolecular Reductive Aldol Reactions Generating Three New Contiguous Asymmetric Stereocenters. J. Am. Chem. Soc 2008, 130, 14378–14379. [DOI] [PubMed] [Google Scholar]
- (217).Deschamp J; Riant O Efficient Construction of Polycyclic Derivatives via a Highly Selective CuI-Catalyzed Domino Reductive-Aldol Cyclization. Org. Lett 2009, 11, 1217–1220. [DOI] [PubMed] [Google Scholar]
- (218).Kato M; Oki H; Ogata K; Fukuzawa S.-i. Copper-ClickFerrophos-Complex-Catalyzed Enantioselective Reductive Aldol Reaction. Synlett 2009, 1299–1302. [Google Scholar]
- (219).Li Z; Jiang L; Li Z; Chen H Copper Hydride-Catalyzed Conjugate Reduction-Aldol Addition Domino Reaction of α,β-Unsaturated Carboxylates with Ketones. Chin. J. Chem 2013, 31, 539–544. [Google Scholar]
- (220).Li Z; Zhang Z; Yuan L; Jiang L; Li Z; Li Z Copper Hydride Catalyzed Reductive Aldol Addition/Lactonization Domino Reactions of α,β-Unsaturated Diester. Synlett 2014, 724–728. [Google Scholar]
- (221).Weglarz I; Szewczyk M; Mlynarski J Zinc Acetate Catalyzed Enantioselective Reductive Aldol Reaction of Ketones. Adv. Synth. Catal 2020, DOI: 10.1002/adsc.201. [DOI] [Google Scholar]
- (222).Inoue K; Ishida T; Shibata I; Baba A Remarkable Dependence of Diastereoselectivity on Anhydrous or Aqueous Solvent in the Indium Hydride Promoted Reductive Aldol Reaction of α,β-Unsaturated Ketones. Adv. Synth. Catal 2002, 344, 283–287. [Google Scholar]
- (223).Shibata I; Kato H; Ishida T; Yasuda M; Baba A Catalytic Generation of Indium Hydride in a Highly Diastereoselective Reductive Aldol Reaction. Angew. Chem., Int. Ed 2004, 43, 711–714. [DOI] [PubMed] [Google Scholar]
- (224).Miura K; Yamada Y; Tomita M; Hosomi A Indium(III) Acetate-Catalyzed 1,4-Reduction and Reductive Aldol Reactions of α-Enones with Phenylsilane. Synlett 2004, 1985–1989. [Google Scholar]
- (225).Ieki R; Miyamoto S; Tsunoi S; Shibata I Indium Hydride Catalyzed Chemo- and Diastereoselective Reductive Aldol Reactions. J. Organomet. Chem 2014, 751, 471–474. [Google Scholar]
- (226).Sugiura M; Sato N; Kotani S; Nakajima M Lewis Base-Catalyzed Conjugate Reduction and Reductive Aldol Reaction of α,β-Unsaturated Ketones Using Trichlorosilane. Chem. Commun 2008, 4309–4311. [DOI] [PubMed] [Google Scholar]
- (227).Sugiura M; Sato N; Sonoda Y; Kotani S; Nakajima M Diastereo- and Enantioselective Reductive Aldol Reaction with Trichlorosilane Using Chiral Lewis Bases as Organocatalysts. Chem. Asian J 2010, 5, 478–481. [DOI] [PubMed] [Google Scholar]
- (228).Osakama K; Sugiura M; Nakajima M; Kotani S Enantioselective Reductive Aldol Reaction Using Tertiary Amine as Hydride Donor. Tetrahedron Lett. 2012, 53, 4199–4201. [Google Scholar]
- (229).DePorre YC; Annand JR; Bar S; Schindler CS Lewis-Base-Catalyzed Reductive Aldol Reaction To Access Quaternary Carbons. Org. Lett 2018, 20, 2580–2584. [DOI] [PubMed] [Google Scholar]
- (230).Maruoka T; Kamiya S.-i.; Matsuda I; Itoh K Rhodium-Catalyzed Approach to Mannich-Type Products Using Aldimine, α,β-Unsaturated Ester, and Hydrosilane. Chem. Commun 2002, 1284–1285. [DOI] [PubMed] [Google Scholar]
- (231).Townes JA; Evans MA; Queffelec J; Taylor SJ; Morken JP Stereoselective Synthesis of trans β-Lactams through Iridium-Catalyzed Reductive Coupling of Imines and Acrylates. Org. Lett 2002, 4, 2537–2540. [DOI] [PubMed] [Google Scholar]
- (232).Garner SA; Krische MJ Rhodium-Catalyzed Reductive Mannich Coupling of Vinyl Ketones to N- Sulfonylimines Mediated by Hydrogen. J. Org. Chem 2007, 72, 5843–5846. [DOI] [PubMed] [Google Scholar]
- (233).Nishiyama H; Ishikawa J; Shiomi T Diastereoselective Reductive Mannich-Type Coupling of Acrylates and Aldimines with Rh(Phebox) Catalyst. Tetrahedron Lett. 2007, 48, 7841–7844. [Google Scholar]
- (234).Du Y; Xu L-W; Shimizu Y; Oisaki K; Kanai M; Shibasaki M Asymmetric Reductive Mannich Reaction to Ketimines Catalyzed by a Cu(I) Complex. J. Am. Chem. Soc 2008, 130, 16146–16147. [DOI] [PubMed] [Google Scholar]
- (235).Prieto O; Lam HW Cobalt-Catalyzed Reductive Mannich Reactions of 4-Acryloylmorpholine with N-Tosyl Aldimines. Org. Biomol. Chem 2008, 6, 55–57. [DOI] [PubMed] [Google Scholar]
- (236).Isoda M; Sato K; Funakoshi M; Tokonishi S; Omura K; Omote M; Ando A Diastereoselective Synthesis of syn-β-Lactams Using Rh-Catalyzed Reductive Mannich-Type Reaction of α,β-Unsaturated Esters. J. Org. Chem 2015, 80, 8398–8405. [DOI] [PubMed] [Google Scholar]
- (237).Isoda M; Sato K; Kunugi Y; Tokonishi S; Tarui A; Omote M; Minami H; Ando A Rh-Catalyzed Reductive Mannich-Type Reaction and its Application towards the Synthesis of (±)-Ezetimibe. Bielstein J. Org. Chem 2016, 12, 1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (238).Komanduri V; Grant CD; Krische MJ Branch-Selective Reductive Coupling of 2-Vinyl Pyridines and Imines via Rhodium Catalyzed C-C Bond Forming Hydrogenation. J. Am. Chem. Soc 2008, 130, 12592–12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (239).Saxena A; Choi B; Lam HW Enantioselective Copper-Catalyzed Reductive Coupling of Alkenylazaarenes with Ketones. J. Am. Chem. Soc 2012, 134, 8428–8431. [DOI] [PubMed] [Google Scholar]
- (240).Choi B; Saxena A; Smith JJ; Churchill GH; Lam HW Enantioselective Copper-Catalyzed Reductive Coupling of Vinylazaarenes with N-Boc Aldimines. Synlett 2015, 350–351. [Google Scholar]
- (241).Yang Y; Perry IB; Buchwald SL Copper-Catalyzed Enantioselective Addition of Styrene-Derived Nucleophiles to Imines Enabled by Ligand-Controlled Chemoselective Hydrocupration. J. Am. Chem. Soc 2016, 138, 9787–9790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (242).Doerksen RS; Meyer CC; Krische MJ Feedstock Reagents in Metal-Catalyzed Carbonyl Reductive Coupling: Minimizing Preactivation for Efficiency in Target-Oriented Synthesis. Angew. Chem., Int. Ed 2019, 58, 14055–14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (243).Heravi MM; Zadsirjan V Oxazolidinones as Chiral Auxiliaries in Asymmetric Aldol Reactions Applied to Total Synthesis. Tetrahedron: Asymm. 2013, 24, 1149–1188. [Google Scholar]
- (244).Shibasaki M; Matsunaga S; Kumagai N Direct Catalytic Asymmetric Aldol Reaction Using Chiral Metal Complexes In Modern Aldol Reactions; Mahrwald R, Ed.; Wiley-VCH: Weinheim, Germany, 2004; Vol. 2; Chapter 6; pp 197–228. [Google Scholar]
- (245).Trost BM; Bartlett MJ ProPhenol-Catalyzed Asymmetric Additions by Spontaneously Assembled Dinuclear Main Group Metal Complexes. Acc. Chem. Res 2015, 48, 688–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (246).List B Amine-Catalyzed Aldol Reactions. In Modern Aldol Reactions; Mahrwald R, Ed.; Wiley-VCH: Weinheim, Germany, 2004, Vol. 1, Chapter 4; pp 161–200. [Google Scholar]
- (247).Bisai V; Bisai A; Singh VK Enantioselective Organocatalytic Aldol Reaction Using Small Organic Molecules. Tetrahedron 2012, 68, 4541–4580. [Google Scholar]
- (248).Sakthivel K; Notz W; Bui T; Barbas CF III Amino Acid Catalyzed Direct Asymmetric Aldol Reactions: A Bioorganic Approach to Catalytic Asymmetric Carbon-Carbon Bond-Forming Reactions. J. Am. Chem. Soc 2001, 123, 5260–5267. [DOI] [PubMed] [Google Scholar]
- (249).Tang Z; Yang Z-H; Chen X-H; Cun L-F; Mi A-Q; Jiang Y-Z; Gong L-Z A Highly Efficient Organocatalyst for Direct Aldol Reactions of Ketones with Aldedydes. J. Am. Chem. Soc 2005, 127, 9285–9289. [DOI] [PubMed] [Google Scholar]
- (250).Luo S; Lu H; Li J; Zhang L; Cheng J-P A Simple Primary-Tertiary Diamine-Brønsted Acid Catalyst for Asymmetric Direct Aldol Reactions of Linear Aliphatic Ketones. J. Am. Chem. Soc 2007, 129, 3074–3075. [DOI] [PubMed] [Google Scholar]
- (251).Kamenecka TM; Overman L E.; Sakata, S. K. L. Construction of Substituted Cyclohexanones by Reductive Cyclization of 7-Oxo-2,8-alkadienyl Esters. Org. Lett 2002, 4, 79–82. [DOI] [PubMed] [Google Scholar]
- (252).Yang JW; Hechavarria MT; List B Catalytic Asymmetric Reductive Michael Cyclization. J. Am. Chem. Soc 2005, 127, 15036–15037. [DOI] [PubMed] [Google Scholar]
- (253).Lee H; Jang M-S; Hong J-T; Jang H-Y Platinum-Catalyzed Reductive Coupling of Activated Alkenes Under Hydrogenation Conditions. Tetrahedron Lett. 2008, 49, 5785–5788. [Google Scholar]
- (254).Oswald CL; Peterson JA; Lam HW Enantioselective Copper-Catalyzed Reductive Michael Cyclizations. Org. Lett 2009, 11, 4504–4507. [DOI] [PubMed] [Google Scholar]
- (255).Kitamura M; Miki T; Nakano K; Noyori R Conjugate Addition of Diorganozincs to α,β- Unsaturated Ketones Catalyzed by a Copper(I)-Sulfonamide Combined System. Tetrahedron Lett. 1996, 37, 5141–5144. [Google Scholar]
- (256).Arnold LA; Naasz R; Minnaard AJ; Feringa BL Catalytic Enantioselective Synthesis of Prostaglandin E1 Methyl Ester Using a Tandem 1,4-Addition-Aldol Reaction to a Cyclopenten-3,5-dione Monoacetal. J. Am. Chem. Soc 2001, 123, 5841–5842. [DOI] [PubMed] [Google Scholar]
- (257).Alexakis A; Trevitt GP; Bernardinelli G Tandem Enantioselective Conjugate Addition: Electrophile Trapping Reactions. Application in the Formation of Syn or Anti Aldols. J. Am. Chem. Soc 2001, 123, 4358–4359. [DOI] [PubMed] [Google Scholar]
- (258).Agapiou K; Cauble DF; Krische MJ Copper-Catalyzed Tandem Conjugate Addition-Electrophilic Trapping: Ketones, Esters, and Nitriles as Terminal Electrophiles. J. Am. Chem. Soc 2004, 126, 4528–4529. [DOI] [PubMed] [Google Scholar]
- (259).Bocknack BM; Wang L-C; Krische MJ Desymmetrization of Enone-Diones via Rhodium Catalyzed Catalytic Diastereo- and Enantioselective Tandem Conjugate Addition-Aldol Cyclization. Proc. Nat. Acad. Sci. U.S.A 2004, 101, 5421–5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (260).Howell GP; Fletcher SP; Geurts K; ter Horst B; Feringa BL Catalytic Asymmetric Synthesis of Acyclic Arrays by Tandem 1,4-Addition-Aldol Reactions. J. Am. Chem. Soc 2006, 128, 14977–14985. [DOI] [PubMed] [Google Scholar]
- (261).Li C; Tu S; Wen S; Li S; Chang J; Shao F; Lei X Total Synthesis of the G2/M DNA Damage Checkpoint Inhibitor Psilostachyin C. J. Org. Chem 2011, 76, 3566–3570. [DOI] [PubMed] [Google Scholar]
- (262).Ubukata S; Ito J -i.; Oguri, R.; Nishiyama, H. Asymmetric Three-Component Coupling Reaction of Alkyne, Enone, and Aldehyde Catalyzed by Chiral Phebox Ruthenium Catalysts. J. Org. Chem 2016, 81, 3347–3355. [DOI] [PubMed] [Google Scholar]
- (263).Guo H-C; Ma J-A Catalytic Asymmetric Tandem Transformations Triggered by Conjugate Additions. Angew. Chem., Int. Ed 2006, 45, 354–366. [DOI] [PubMed] [Google Scholar]
- (264).Galestokova Z; Sebesta R Domino Reactions Initiated by Enantioselective Cu-Catalyzed Conjugate Addition. Eur. J. Org. Chem 2012, 6688–6695. [Google Scholar]
- (265).Schneider C; Boomhoff M Aldol Reactions in Domino Processes In Domino Reactions; Tietze LF, Ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2014; Chapter 8; pp. 267–294. [Google Scholar]
- (266).Vargova D; Nemethova I; Plevova K; Sebesta R Asymmetric Transition-Metal Catalysis in the Formation and Functionalization of Metal Enolate. ACS Catal. 2019, 9, 3104–3143. [Google Scholar]
- (267).Ikeda S-I Nickel-Catalyzed Intermolecular Domino Reactions. Acc. Chem. Res 2000, 33, 511–519. [DOI] [PubMed] [Google Scholar]
- (268).Montgomery J Nickel-Catalyzed Cyclizations, Couplings, and Cycloadditions Involving Three Reactive Components. Acc. Chem. Res 2000, 33, 467–473. [DOI] [PubMed] [Google Scholar]
- (269).Montgomery J Nickel-Catalyzed Reductive Cyclizations and Couplings. Angew. Chem., Int. Ed 2004, 43, 3890–3908. [DOI] [PubMed] [Google Scholar]
- (270).Streuff J; Gansaeuer A Metal-Catalyzed β-Functionalization of Michael Acceptors through Reductive Radical Addition Reactions. Angew. Chem., Int. Ed 2015, 54, 14232–14242. [DOI] [PubMed] [Google Scholar]
- (271).Krische MJ, Jang H-Y Metal Catalyzed Reductive Cyclization (C=C, C≡C, C=O Bonds) In Comprehensive Organometallic Chemistry III; Mingos M, Crabtree R, Eds.; Elsevier: Oxford, 2006; Vol. 10; pp 493–536. [Google Scholar]
- (272).Metal Catalyzed Reductive C-C Bond Formation Topics in Current Chemistry; Krische MJ, Ed.; Springer-Verlag Berlin Heidelberg: Germany, 2007; Vol. 279. [Google Scholar]
- (273).Kimura M; Tamaru Y Nickel-Catalyzed Reductive Coupling of Dienes and Carbonyl Compounds. Top. Curr. Chem 2007, 279, 173–207. [Google Scholar]
- (274).Jeganmohan M; Cheng C-H Cobalt- and Nickel-Catalyzed Regio- and Stereoselective Reductive Coupling of Alkynes, Allenes, and Alkenes with Alkenes. Chem. Eur. J 2008, 14, 10876–10886. [DOI] [PubMed] [Google Scholar]
- (275).Shibahara F; Krische MJ Formation of C-C Bonds via Ruthenium Catalyzed Transfer Hydrogenation: Carbonyl Addition from the Alcohol or Aldehyde Oxidation Level. Chem. Lett 2008, 37, 1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (276).Bower JF; Kim IS; Patman RL; Krische MJ Catalytic Carbonyl Addition through Transfer Hydrogenation: A Departure from Preformed Organometallic Reagents. Angew. Chem., Int. Ed 2009, 48, 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (277).Hassan A; Krische MJ Unlocking Hydrogenation for C-C Bond Formation: A Brief Overview of Enantioselective Methods. Org. Proc. Res. Devel 2011, 15, 1236–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (278).Moragas T; Correa A; Martin R Metal-Catalyzed Reductive Coupling Reactions of Organic Halides with Carbonyl-Type Compounds. Chem. Eur. J 2014, 20, 8242–8258. [DOI] [PubMed] [Google Scholar]
- (279).Ketcham JM; Shin I; Montgomery TP; Krische MJ Catalytic Enantioselective C-H Functionalization of Alcohols by Redox-Triggered Carbonyl Addition: Borrowing Hydrogen, Returning Carbon. Angew. Chem., Int. Ed 2014, 53, 9142–9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (280).Nguyen KD; Park BY; Luong T; Sato H; Garza VJ; Krische MJ Metal Catalyzed Reductive Coupling of Olefin-Derived Nucleophiles: Reinventing Carbonyl Addition. Science 2016, 354, aah5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (281).Holmes M; Schwartz LA; Krische MJ Intermolecular Metal-Catalyzed Reductive Coupling of Dienes, Allenes and Enynes with Carbonyl Compounds and Imines. Chem. Rev 2018, 118, 6026–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (282).Riener K; Hoegerl MP; Gigler P; Kuehn FE Rhodium-Catalyzed Hydrosilylation of Ketones: Catalyst Development and Mechanistic Insights. ACS Catal. 2012, 2, 613–621 and references cited therein. [Google Scholar]
- (283).Tolman CA; Meakin PZ; Lindner DL; Jesson JP Triarylphosphine, Hydride and Ethylene Complexes of Rhodium(I) Chloride. J. Am. Chem. Soc 1974, 96, 2762–2774. [Google Scholar]
- (284).Halpern J; Wong CS Hydrogenation of Tris(triphenylphosphine)chlororhodium(I). Chem. Commun 1973, 629–630. [Google Scholar]
- (285).Halpern J; Okamoto T; Zakhariev A Mechanism of the Chlorotris(triphenylphosphine) Rhodium(I)-Catalyzed Hydrogenation of Alkenes. The Reaction of Chlorodihydidotris(triphenylphosphine)rhodium(III) with Cyclohexene. J. Mol. Catal 1976, 2, 65–68. [Google Scholar]
- (286).Landis CR; Halpern J Asymmetric Hydrogenation of Methyl-(Z)-α-Acetamidocinnamate Catalyzed by {1,2-Bis((phenyl-o-anisoyl)phosphine)ethane}rhodium(I): Kinetics, Mechanism, and Origin of Enantioselection. J. Am. Chem. Soc 1987, 109, 1746–1754. [Google Scholar]
- (287).Chan ASC; Halpern J Interception and Characterization of a Hydridoalkylrhodium Intermediate in a Homogeneous Catalytic Hydrogenation Reaction. J. Am. Chem. Soc 1980, 102, 838–840. [Google Scholar]
- (288).Halpern J; Riley DP; Chan ASC; Pluth JJ Novel Coordination Chemistry and Catalytic Properties of Cationic 1,2-Bis(diphenylphosphino)ethanerhodium(I) Complexes. J. Am. Chem. Soc 1977, 99, 8055–8057. [Google Scholar]
- (289).Halpern J Mechanism and Stereoselectivity of Asymmetric Hydrogenation. Science 1982, 217, 401–407. [DOI] [PubMed] [Google Scholar]
- (290).Halpern J Asymmetric Catalytic Hydrogenation: Mechanism and Origin of Enantioselection. Asymm. Synth 1985, 5, 41–69. [Google Scholar]
- (291).Landis CR; Brauch TW Probing the Nature of H2 Activation in Catalytic Asymmetric Hydrogenation. Inorg. Chim. Acta 1998, 270, 285–297. [Google Scholar]
- (292).Gridnev ID; Imamoto T On the Mechanism of Stereoselection in Rh-Catalyzed Asymmetric Hydrogenation: A General Approach for Predicting the Sense of Enantioselectivity. Acc. Chem. Res 2004, 37, 633–644. [DOI] [PubMed] [Google Scholar]
- (293).Murai S; Kato T; Sonoda N; Seki Y; Kawamoto K Catalytic Conversion of Aldehydes into Higher α-Siloxy Aldehydes by Hydrosilane and Carbon Monoxide. Angew. Chem., Int. Ed. Engl 1979, 18, 393–394. [Google Scholar]
- (294).Wright ME; Cochran BB Rhodium-Catalyzed Silylformylation of Aldehydes: A Mild and Efficient Catalytic Route to α-Silyloxyaldehydes. J. Am. Chem. Soc 1993, 115, 2059–2060. [Google Scholar]
- (295).Wright ME; Cochran BB Silylformylation of Carbonyl Compounds: A Study of Substrate, Catalyst, and Reaction Conditions. Organometallics 1996, 15, 317–324. [Google Scholar]
- (296).Schneider N; Finger M; Haferkemper C; Bellemin-Laponnaz S; Hofmann P; Gade LH Multiple Reaction Pathways in Rhodium-Catalyzed Hydrosilylations of Ketones. Chem. Eur. J 2009, 15, 11515–11529. [DOI] [PubMed] [Google Scholar]
- (297).Zhao L; Nakatani N; Sunada Y; Nagashima H; Hasegawa J -y. Theoretical Study on the Rhodium- Catalyzed Hydrosilylation of C=C and C=O Double Bonds with Tertiary Silane. J. Org. Chem 2019, 84, 8552–8561. [DOI] [PubMed] [Google Scholar]
- (298).Doney JJ; Bergman RG; Heathcock CH Synthesis, Structure, and Carbon-Carbon Bond-Forming Reactions of Carbon-Bound Molybdenum, Tungsten, and Rhenium Enolates. Detection of an η3-Oxaallyl Intermediate. J. Am. Chem. Soc 1985, 107, 3724–3726. [Google Scholar]
- (299).Heathcock CH; Doney JJ; Bergman RG Synthesis and Carbon-Carbon Bond-Forming Reactions of Tungsten, Molybdenum, and Rhenium Enolates. Pure Appl. Chem 1985, 57, 1789–1798. [Google Scholar]
- (300).Burkhardt ER; Doney JJ; Slough GA; Stack JM; Heathcock CH; Bergman RG Carbon-Carbon Bond Forming Reactions of Organotransition Metal Enolate Complexes. Pure Appl. Chem 1988, 60, 1–6. [Google Scholar]
- (301).Slough GA; Bergman RC; Heathcock CH Synthesis of η1 Oxygen-Bound Rhodium Enolates. Applications to Catalytic Aldol Chemistry. J. Am. Chem. Soc 1989, 111, 938–949. [Google Scholar]
- (302).Burkhardt ER; Bergman RG; Heathcock CH Synthesis and Reactions of Nickel and Palladium Carbon-Bound Enolate Complexes. Organometallics 1990, 9, 30–44. [Google Scholar]
- (303).Godard C; Duckett SB; Parsons S; Perutz RN Dipyridylketone Binding and Subsequent C–C Bond Insertion Reactions at Cyclopentadienylrhodium. Chem. Commun 2003, 2332–2333. [DOI] [PubMed] [Google Scholar]
- (304).For σ-bond metathesis of analogous nickel alkoxides with hydrosilane, see:; Haynes MT II; Liu P; Baxter RD; Nett AJ; Houk KN; Montgomery J. Dimer Involvement and Origin of Crossover in Nickel- Catalyzed Aldehyde-Alkyne Reductive Couplings. J. Am. Chem. Soc 2014, 136, 17495–17504. [DOI] [PMC free article] [PubMed] [Google Scholar]; See also ref. 305 and 306.
- (305).Jackson EP; Montgomery J Regiocontrol in Catalytic Reductive Couplings through Alterations of Silane Rate Dependence. J. Am. Chem. Soc 2015, 137, 958–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (306).Liu T; Bi S Impact of Ligand and Silane on the Regioselectivity in Catalytic Aldehyde-Alkyne Reductive Couplings: A Theoretical Study. Organometallics 2016, 35, 1114–1124. [Google Scholar]
- (307).Liu P; Krische MJ; Houk KN Mechanism and Origins of Regio- and Enantioselectivities in RhI- Catalyzed Hydrogenative Couplings of 1,3-Diynes and Activated Carbonyl Partners: Intervention of a Cumulene Intermediate. Chem. Eur. J 2011, 17, 4021–4029 and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (308).Schrock RR; Osborn JA Catalytic Hydrogenation Using Cationic Rhodium Complexes. I. Evolution of the Catalytic System and the Hydrogenation of Olefins. J. Am. Chem. Soc 1976, 98, 2134–2143. [Google Scholar]
- (309).Schrock RR; Osborn JA Catalytic Hydrogenation Using Cationic Rhodium Complexes. II. The Selective Hydrogenation of Alkynes to Cis Olefins. J. Am. Chem. Soc 1976, 98, 2143–2147. [Google Scholar]
- (310).Schrock RR; Osborn JA Catalytic Hydrogenation Using Cationic Rhodium Complexes. 3. The Selective Hydrogenation of Dienes to Monoenes. J. Am. Chem. Soc 1976, 98, 4450–4455. [Google Scholar]
- (311).Bendorf HD; Colella CM; Dixon EC; Marchetti M; Matukonis AN; Musselman JD; Tiley TA Chelation-Assisted Intramolecular Hydroacylation: Synthesis of Medium Ring Sulfur Heterocycles Tetrahedron Lett. 2002, 53, 7031–7034. [Google Scholar]
- (312).Beller M; Cornils B; Frohning CD; Kohlpaintner CW Progress in hydroformylation and carbonylation. J. Mol. Catal. A 1995, 104, 17–85. [Google Scholar]
- (313).Frohning CD; Kohlpaintner CW; Bohnen H-W In Applied Homogeneous Catalysis with Organometallic Compounds; Cornils B, Herrmann WA, Eds.; Wiley-VCH: Weinheim, Germany, 1996; Vol. 1; pp 29–104. [Google Scholar]
- (314).Rhodium Catalyzed Hydroformylation; van Leeuwen PWNM; Claver C, Eds.; Kluwer Academic Publishers: Norwell, MA, 2000. [Google Scholar]
- (315).Breit B; Seiche W Recent Advances on Chemo-, Regio- and Stereoselective Hydroformylation. Synthesis 2001, 1–36. [Google Scholar]
- (316).Weissermel K; Arpe H-J Industrial Organic Chemistry, 4th ed.; Wiley-VCH: Weinheim, Germany, 2003; pp 127–144. [Google Scholar]
- (317).Homogeneous Catalysis: Understanding the Art; van Leeuwen PWNM, Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004. [Google Scholar]
- (318).Ngai M-Y; Kong J-R; Krische MJ Hydrogen-Mediated C-C Bond Formation – A Broad New Concept in Catalytic C-C Coupling. J. Org. Chem 2007, 72, 1063–1072. [DOI] [PubMed] [Google Scholar]
- (319).Jang H-Y; Krische MJ Catalytic C-C Bond Formation via Capture of Hydrogenation Intermediates. Acc. Chem. Res 2004, 37, 653–661. [DOI] [PubMed] [Google Scholar]
- (320).Yachi K; Shinokubo H; Oshima K Reaction of Titanate-Type Aldehyde Enolate with Ketones To Provide 3-Hydroxyaldehydes. J. Am. Chem. Soc 1999, 121, 9465–9466. [Google Scholar]
- (321).Farina V New Perspectives in the Cross-Coupling Reactions of Organostannanes Pure Appl. Chem 1996, 68, 73–78. [Google Scholar]
- (322).Anderson NG; Keay BA 2-Furyl Phosphines as Ligands for Transition-Metal-Mediated Organic Synthesis. Chem. Rev 2001, 101, 997–1030. [DOI] [PubMed] [Google Scholar]
- (323).Kristjánsdóttir SS; Norton JR Acidity of Hydrido Transition Metal Complexes in Solution In Transition Metal Hydrides; Dedieu A Ed.; VCH: New York, NY, 1992; Chapter 9; pp 309–357. [Google Scholar]
- (324).Baik T-G; Wang L-C; Luiz A-L; Krische MJ A Diastereoselective Metal-Catalyzed [2 + 2] Cycloaddition of Bis-enones. J. Am. Chem. Soc 2001, 123, 6716–6717. [DOI] [PubMed] [Google Scholar]
- (325).Halpern J Oxidative-Addition Reactions of Transition Metal Complexes. Acc. Chem. Res 1970, 3, 386–392. [Google Scholar]
- (326).Socol SM; Verkade JG Steric and Electronic Effects in Cobalt(II) Disproportionation with Phosphorus Ligands. Inorg. Chem 1986, 25, 2658–2663 and references therein. [Google Scholar]
- (327).Roh Y; Jang H-Y; Bauld NL; Krische MJ Anion Radical Chain Cycloaddition of Tethered Enones: Intramolecular Cyclobutanation and Diels-Alder Cycloaddition. Org. Lett 2002, 4, 611–613. [DOI] [PubMed] [Google Scholar]
- (328).Yang J; Felton GAN; Bauld NL; Krische M Chemically Induced Anion Radical Cycloadditions: Intramolecular Cyclobutanation of bis(Enones) via Homogeneous Electron Transfer. J. Am. Chem. Soc 2004, 126, 1634–1635. [DOI] [PubMed] [Google Scholar]
- (329).Johnson JR; Tully PS; MacKenzie PB; Sabat MA Practical Reversed-Polarity Alternative to Organocuprate Conjugate Addition Chemistry. Halocarbon Coupling Reactions of Enal- and Enone-Derived Allylnickel Reagents. J. Am. Chem. Soc 1991, 113, 6172–6177. [Google Scholar]
- (330).Grisso BA; Johnson JR; MacKenzie PB Nickel-Catalyzed, Chlorotrialkylsilane-Assisted Conjugate Addition of Alkenyltributyltin Reagents to α,β-Unsaturated Aldehydes. Evidence for a [1- [(trialkylsilyl)oxy]allyl]nickel(II) Mechanism. J. Am. Chem. Soc 1992, 114, 5160–5165. [Google Scholar]
- (331).Ogoshi S; Yoshida T; Nishida T; Morita M; Kurosawa H Coordination of Lewis Acid to η2- Enonepalladium(0) Leading to Continuous Structure Variation from η2-Olefin Type to η3-Allyl Type. J. Am. Chem. Soc 2001, 123, 1944–1950. [DOI] [PubMed] [Google Scholar]
- (332).Ogoshi S; Tomiyasu S; Morita M; Kurosawa H Palladium/Me3SiOTf-Catalyzed Bis-silylation of α,β-Unsaturated Carbonyl Compounds Without Involving Oxidative Addition of Disilane. J. Am. Chem. Soc 2002, 124, 11598–11599. [DOI] [PubMed] [Google Scholar]
- (333).Hratchian HP; Chowdhury SK; Gutiérrez-García VM; Amarasinghe KKD; Heeg MJ; Schlegel HB; Montgomery J Combined Experimental and Computational Investigation of the Mechanism of Nickel-Catalyzed Three-Component Addition Processes. Organometallics 2004, 23, 4636–4646. [Google Scholar]
- (334).Moran J; Krische MJ Formation of C-C Bonds via Ruthenium Catalyzed Transfer Hydrogenation. Pure Appl. Chem 2012, 84, 1729–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (335).Perez F; Oda S; Geary LM; Krische MJ Ruthenium Catalyzed Transfer Hydrogenation for C-C Bond Formation: Hydrohydroxyalkylation and Hydroaminoalkylation via Reactant Redox Pairs. Top. Curr. Chem 2016, 374, 365–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (336).Sato H; Turnbull BWH; Fukaya K; Krische MJ Ruthenium(0) Catalyzed Cycloaddition of 1,2- Diols, Ketols or Diones via Alcohol-Mediated Hydrogen Transfer. Angew. Chem., Int. Ed 2018, 57, 3012–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (337).Cramer RD; Jenner EL; Lindsey RV; Stolberg UG Homogeneous Hydrogenations with Platinum-Tin Chloride Complexes. J. Am. Chem. Soc 1963, 85, 1691–1692. [Google Scholar]
- (338).Adams RW; Batley GE; Bailer JC Jr. Homogeneous Catalysis in the Reactions of Olefinic Substances. XI. Homogeneous Catalytic Hydrogenation of Short-Chain Olefins with Dichlorobis(triphenylphosphine) Platinum(II)—Tin(II) Chloride Catalyst. J. Am. Chem. Soc 1968, 90, 6051–6056. [Google Scholar]
- (339).Host MS; Wilson WL; Nelson JH Transition Metal-Tin Chemistry. Chem. Rev 1989, 89, 11–49. [Google Scholar]
- (340).Best D; Lam, Hon W. C=N-Containing Azaarenes as Activating Groups in Enantioselective Catalysis. J. Org. Chem 2014, 79, 831–845. [DOI] [PubMed] [Google Scholar]
- (341).Enthaler S Rise of the Zinc Age in Homogeneous Catalysis? ACS Catal. 2013, 3, 150–158. [Google Scholar]
- (342).Wang L; Xiao J Hydrogen-Atom Transfer Reactions. Top. Curr. Chem 2016, 374, 1–55. [DOI] [PubMed] [Google Scholar]
- (343).Zhao G-L; Córdova A Direct Organocatalytic Asymmetric Reductive Mannich-Type Reactions Tetrahedron Lett. 2006, 47, 7417–7421. [Google Scholar]
- (344).Hendrickson JB Systematic Synthesis Design. IV. Numerical Codification of Construction Reactions. J. Am. Chem. Soc 1975, 97, 5784–5800. [Google Scholar]
- (345).Trost BM Selectivity: A Key to Synthetic Efficiency. Science 1983, 219, 245–250. [DOI] [PubMed] [Google Scholar]
- (346).Feng J; Kasun ZA; Krische MJ Enantioselective Alcohol C-H Functionalization for Polyketide Construction: Unlocking Redox-Economy and Site-Selectivity for Ideal Chemical Synthesis. J. Am. Chem. Soc 2016, 138, 5467–5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (347).Schwan J; Christmann M Enabling Strategies for Step Efficient Syntheses. Chem. Soc. Rev 2018, 47, 7985–7995. [DOI] [PubMed] [Google Scholar]


