Abstract
Essential oils (EOs) have been utilized as a growth inhibitor of microorganisms. This study was aimed to recognize the composition, antioxidative, antibacterial, and time-kill activities of Origanum vulgare, Zataria multiflora, Syzygium aromaticum; and Cinnamomum verum EOs against Listeria monocytogenes, Escherichia coli O157:H7, Shewanella putrefaciens and Pseudomonas fluorescens. Gas chromatography-mass spectrometry was used to determine the chemical composition of EOs. Disc diffusion, minimum inhibitory concentration, minimum bactericidal concentration, and time-kill methods were used to determine the antibacterial activity of EOs. The antioxidative activity of EOs were determined by 2, 20-diphenyl-1-picrylhydrazyl radical scavenging and ferric reducing antioxidative power methods. All EOs exhibited antibacterial activity, however, Z. multiflora EO was the most effective followed by O. vulgare EO. The lowest antibacterial activity was observed in C. verum EO. The most sensitive among tested bacteria to Z. multiflora and O. vulgare EOs was E. coli O157:H7 and to S. aromaticum; and C. verum EOs were S. putrefaciens and P. fluorescens, respectively. Z. multiflora and O. vulgare EOs were able to kill 85.00% and 80.00% of the E. coli O157: H7 and S. putrefaciens cells in 4 hr, respectively. The highest antioxidative activity was observed in Z. multiflora EO. The tested EOs showed the highest antioxidative activity at a concentration of 2.00 g L-1. Ferric reducing antioxidant power value of Z. multiflora, O. vulgare, S. aromaticum and C. verum was 2.01 ± 0.03, 1.47 ± 0.04, 1.01 ± 0.03, and 0.66 ± 0.34, respectively. High concentrations of tested EOs showed a decrease in antioxidative activity.
Key Words: Antibacterial activity, Antioxidative assay, Essential oil, Minimum bactericidal concentration, Minimum inhibitory concentration
Introduction
Essential oils (EOs) are oily liquids achieved from plants. Several methods can be used to obtain EOs, but the steam technique is usually applied for the commercial fabrication of EOs.1 The researches on the properties of food and the effect of EOs against an extensive range of microorganisms; have been intensified.2,3 It is common knowledge that EOs are characterized by changes in their chemical composition.4 Origanum vulgare (OEO), Zataria multiflora (ZEO), Syzygium aromaticum (ZEO), Cinnamomum verum (CEO) EOs have been used in food preservation. Origanum vulgare is an herb belonging to the Lamiaceae family; found throughout Asia; and Europe.5,6 The OEO is recognized for its anti-oxidative and antibacterial activities. Reportedly, OEO has an antioxidative effect.7-9 Zataria multiflora is a popular spice and thyme-like EOs-bearing plant belonging to the Lamiaceae family.10,11 Recent in vitro studies have shown that the CEO effectively inhibits food spoilage and bacterial growth.12 Besides its antibacterial activity, this EO has multiple useful impacts on health.13,14 Therefore, it is considered an effective alternative food preservative agent. Studies on the influence of the EOs on Listeria monocytogenes and Escherichia coli O157:H7 and specific spoilage organisms (SSOs) including Shewanella putrefaciens and Pseudomonas fluorescens are limited; therefore, researches on the comprehensive assessment of the inhibitory effects of EOs on microflora are needed. Some researchers have evaluated the performance of EOs; however, there has been no comprehensive and comparative research on the chemical composition, anti-bacterial and antioxidative properties of the tested EOs.
This study was aimed to (1) identify chemical compositions of OEO, ZEO, CEO; and SEO by gas chromatography-mass spectrometry (GC-MS), (2) study the antibacterial activity of these EOs using agar disc diffusion and broth microdilution assay as well as kill-time assay against L. monocytogenes, E. coli O157:H7 and SSOs including S. putrefaciens and P. fluorescens; and (3) measure antioxidative capacities of tested EOs using outcomes of 2,20-dipheny-l-picrylhydrazyl (DPPH) and ferric reducing antioxidant power (FRAP) assays.
Materials and Methods
Plant material. Plant materials were collected in the geographical area of Mashhad (36.2605° N, 59.6168° E), Iran; during summer 2017, classified and recognized by the Institute of Medicinal Plants, Karaj, Iran. The EOs were prepared using the dried aerial parts.
Essential oils extraction. The EOs were extracted with a Clevenger-type device for steam distillation. The chemical compositions of the EOs were then determined by GC-MS.15 The dry herbs were crushed into powders (particle size less than 250 μm); and 600 g of each powder and 6.00 L of water were placed into a 10.00-L round-bottom flask. Herbal powders were extracted for 8 hr via steam distillation; and the EOs were collected, dehydrated; and dried using anhydrous sodium sulfate; and kept at – 20.00 ˚C.
Chemical analysis of EOs. The analysis of EOs was performed on a gas chromatograph (6890; Agilent, Palo Alto, USA) interfaced to a mass selective detector (5973N; Agilent). A vaporization injector working in the split mode (1:50) equipped with a fused silica capillary column (30.00 m length × 0.32 mm internal diameter × 0.25 μm film thickness; HP-5MS; 5.00% diphenyl, 95.00% dimethyl polydimethylsiloxane; Agilent) was used at 250 ˚C. The temperature of the oven was programmed at 45.00 ˚C for 1 min, raised to 250 ˚C with a speed of 5.00 ˚C per min, and maintained at 250 ˚C for 5 min. Helium was utilized as a carrier gas with an injection speed of 30 cm per sec and an injection volume of 1.00 μL. The transfer line temperature was set at 280 ˚C, ion source temperature was maintained at 230 ˚C, and the temperature of the quadrupole analyzer was kept at; 150 ˚C. A turbomolecular pump with a pressure of 10-5 Torr was utilized. Electron ionization mass spectra between 40.00 - 400 were recorded in the full-scan mode at 70.00 eV. A solvent delay was set at 3 min. The acquisition data and device control were carried out using the MSD Chem Station Software (version C.00.00; Agilent). The identity of all compounds was allocated through comparing their retention index with a standard mixture of n-alkanes, 14 and through comparing with the mass spectra characteristic properties acquired using Wiley’s library spectral data bank (G1035B, Rev D.02.00; Agilent).
Antibacterial activity. Antibacterial performance corresponding to the EOs was investigated against two important food-borne pathogens including L. mono-cytogenes ATCC 7644; and E. coli O157:H7 NCTC 12900 and SSOs including S. putrefaciens NCTC 10762 and P. fluorescens NCTC 10038. The bacterial strains were prepared from the Iranian Biological Resource Center and Department of Food Hygiene, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran. Bacterial suspensions were prepared for culturing the lyophilized bacteria in 9.00 mL of brain heart infusion (BHI) broth (Sigma-Aldrich, St. Louis, USA) and incubated at a temperature of 37.00 ˚C for 24 hr. Next, bacterial culture was enriched in the Mueller Hinton broth (MHB; Merck, Darmstadt, Germany) at 37.00 ˚C for 24 hr. The antibacterial susceptibility and EOs antibacterial activity tests were also carried out.
Disc diffusion method. The antibacterial performance of four EOs was studied by the disc diffusion technique. Isolates were grown from freezer stocks overnight in BHI broth (Sigma-Aldrich) at 37.00 ˚C, adjusted to 0.50 McFarland standard (1.50 × 108 CFU mL-1); and swabbed in Mueller Hinton agar (MHA; Merck). Paper discs with 6.00 mm diameter soaked with 5.00, 10.00, 15.00; and 20.00 μL of pure OEO, ZEO, SEO, and CEO were placed on the surface of MHA medium. The plates were incubated at 37.00 ˚C for 24 hr. The diameter corresponding to the bacterial growth inhibition zones was measured in millimeter.16 Gentamicin (10.00 µg per disc) was applied as a positive reference standard for determining and comparing the sensitivity of strain in analyzed microbial species. Disc without samples was utilized as a negative control. All measurements were carried out three times.
Determination of minimum inhibitory concentration (MIC). Susceptibility of pathogenic organisms (L. monocytogenes; and E. coli O157:H7) and SSOs including (S. putrefaciens and P. fluorescens) to test EOs (OEO, ZEO, SEO; and CEO) was investigated by broth microdilution technique.17 Broth microdilution technique was performed in sterile U-bottom microtiter plates (Spektar, Čačak, Serbia). The inoculum density was set to 0.50 McFarland, diluted 10 times in sterile saline and 5.00 μL of this suspension was inoculated in 0.10 mL of cation adjusted MHB (CAMHB; Becton and Dickinson Co., Sparks, USA) to reach final inoculum of 5.00 × 104 CFU per well. The active substance was diluted in aqueous dimethyl sulphoxide (DMSO; Sigma-Aldrich) and added to CAMHB from 0.01 mg mL-1 to 40.96 mg mL-1 by two-fold dilution in 96-well microtiter plates. To assess all probable pollutions corresponding to the culture medium only MHB with EOs was applied as a negative control; and the media with bacterial suspensions were utilized as positive controls. Incubation of plates at 37.00 ˚C was carried out for 24 hr. The MIC was determined as a minimum level of an antibacterial agent inhibiting the visible growth of a microorganism in the broth dilution susceptibility test.
Determination of minimum bactericidal concentration (MBC). The MBC was carried out based on the technique developed by Duarte et al.18 with modifications. From the wells showed inhibition of microorganisms in the MIC test, aliquots of 10.00 μL were collected, transferred to MHA medium, and incubated at 37.00 ˚C for 24 hr and less than five colonies were considered as MBC as they signify kill ratio of over 99.97%. Amikacin (Sigma-Aldrich) in the range of 0.03 - 64.00 μg mL-1 was utilized as a control. In cases where there was no bacterial growth in the culture medium, it can be observed that the tested EOs showed bactericidal effects, but not bacteriostatic ones.
Time-kill studies. Time-kill kinetics were determined by some remaining viable bacteria at different time points following exposure to the tested EOs. Kill time study was conducted with the MIC value obtained formerly. The enzyme-linked immunosorbent assay (ELISA) reader method was used. To determine the survival curves, sterile 96 well microplates were used with BHI. Sterile freshly prepared BHI broth (180 µL) was inserted into the wells. Twenty µL of EOs were added to the media (with 0.50% Tween 20) into the wells. To each well, 100 µL of the organism in BHI was added; thus, each well finally contained 300 µL. After every 4 hr, 10.00 µL of the mixture from each well was added to a new plate and the volume was adjusted to 200 µL with sterile distilled water and using the ELISA reader at 590 nm, the optical density was read. The experiment was repeated each 4 hr for 2 days.19
Antioxidative activity. The DPPH assay was carried out for measurement of the free radical scavenging activity as described previously.20 The DPPH radical scavenging assay is an applied tool for accessing the antioxidative performance of materials due to the relatively cheap cost and speed of completion. Four EOs in DMSO (0.01 - 20.00 mg mL-1) were combined with 0.10 mM DPPH solution was prepared by dissolving 4.00 mg of DPPH in 100 mL of methanol. The absorbance was read at 517 nm following incubation for 30 min at ambient temperature. The reduction of the DPPH solution absorption following the addition of an anti-oxidant was measured. Butylated hydroxyl toluene (BHT) was utilized as a positive control for comparison and 90.00 μM DPPH solution was considered as a blank. The percentage of DPPH inhibition was computed by the following equation:
I (%) = 100 × (A blank -A sample ) / A blank
where, Ablank represents the absorbance corresponding to the control (comprising all reagents except the test compound) and Asample refers to the absorbance corresponding to the test compound. The EC50 value (μg mL-1) refers to the effective concentration at which DPPH radicals are removed by 50.00%. This was achieved through interpolation and utilizing linear regression analysis. The BHT was applied as a control.
Determination of FRAP. This is a sensitive technique used to evaluate the antioxidative performance of biological fluids; and diet components. Reducing power is frequently applied to assess a substance’s antioxidant activity.21 Reagents included 300 mM L-1 acetate buffer (pH: 3.60), 3.10 g C2H3NaO2.3H2O (Sigma-Aldrich), and 16.00 mL C2H4O2 (Sigma-Aldrich) per liter of buffer solutions); 10.00 mmol L-1 2,4,6- tripyridyl-s-triazine (TPTZ; Fluka Chemicals, Switzerland) in 40.00 mmol L-1 hydrochloric acid (BDH Laboratory Supplies, Poole, UK); and 20.00 mmol L-1 FeCl3.6H2O (BDH Laboratory Supplies). Working FRAP reagent was freshly prepared through combining 25.00 mL acetate buffer, 2.50 mL TPTZ solution; and 2.50 mL FeCl3.6H2O solution. Three hundred μL freshly prepared FRAP reagent was warmed to 37.00 ˚C, a reagent blank reading was taken (M1) at 593 nm; and 10.00 μL of the various levels of the EOs (0.50 - 3.00 g L-1), using anhydrous ethanol, were added. A linear calibration curve for FeSO4.7H2O in the concentration range over 1000, 500, 250, 125, 62.50; and 31.25 μM FeCl2 was prepared. The corresponding regression calibration equation was:
A = 0.0019c + 0.1027
where, A is the absorbance at 593 nm, and c is the concentration of FeSO4.7H2O (R2 = 0.9998).
The procedure for preparation of the calibration curve was as follows: Buffer concentrate 1:10 was assayed by the addition of one portion of the concentrate to nine portions of distilled water. Standards were prepared with labeling test tubes from 1.00 to 6.180 μL of diluted assay buffer being added to tube 1 and 100 μL of diluted assay buffer being poured into the rest of the tubes. Twenty μL of the ferrous chloride standard stock was carefully added to tube 1 and vortex. One hundred μL of the solution from tube 1 was poured into tube 2 and vortex completely. The serial dilutions were repeated for tubes 3 through 6. The level of ferrous chloride in tubes 1 to 6 was 1000, 500, 250, 125, 62.50; and 31.25 μM FeCl2, respectively. The BHT was utilized as a positive control (0.50 - 3.00 g L-1). Experiments were carried out in triplicate. The graph was plotted with the average of three determinations. The resulting mixture was vigorously shaken and then incubated at 37.00 ˚C for 4 min and the increase in absorbance at 593 nm was determined and compared with the standard absorbance (A) readings taken at 593 nm. The 4-min readings were chosen for the calculation of FRAP values.
Statistical analysis. Analyses were run in triplicates. Analysis of data was carried out using SAS statistical software (version 9.0; SAS Institute, Cary, USA). Data were reported as mean ± SD using ANOVA. A statistical difference at (p ≤ 0.05) was considered significant.
Results
Essential oils chemical composition. Active components in EOs recognized with GC-MS are listed in Table 1. Major components of ZEO were thymol (40.00%) followed by carvacrol (31.12%) and in SEO was eugenol (87.10%). Major components of OEO were thymol (35.18%) followed by carvacrol (34.00%) and in CEO was e-cinnamaldehyde (36.06%). The ZEO and OEO contain monoterpene phenolic compounds; including carvacrol and thymol, whose antioxidant and antibacterial effects are related to these isomers. Carvacrol and thymol were not detected in the contents of SEO and CEO.
Table 1.
Essential oils composition (%) identified by gas chromatography-mass spectrometry
| Compound | ZEO | SEO | OEO | CEO | RI |
|---|---|---|---|---|---|
| alpha-Thujene | 0.19 | - | 0.14 | - | 930 |
| alpha-Pinene | 4.26 | 0.05 | 0.08 | 0.27 | 937 |
| Camphene | - | - | 0.23 | 0.16 | 952 |
| Sabinene | - | - | 0.40 | 0.14 | 976 |
| Bete-Pinene | 0.62 | - | 0.65 | 1.49 | 980 |
| Myrcene | 0.85 | - | 0.96 | 0.05 | 992 |
| alph-Terpinene | - | - | 0.61 | 0.08 | 1018 |
| Eucaliptol | 3.37 | - | - | - | 1023 |
| p-Cymene | - | 0.05 | 0.20 | 0.54 | 1028 |
| Limonene | - | - | 0.59 | 1032 | |
| 1,8-Cineole | - | 0.01 | 1.25 | 0.77 | 1039 |
| O-cymene | - | 0.27 | 0.16 | - | 1051 |
| gamma-Terpinene | 7.34 | - | 1.06 | 0.37 | 1063 |
| Terpinolene | - | - | 0.36 | 0.64 | 1092 |
| Linalool | 0.63 | 0.27 | 5.92 | 2.36 | 1098 |
| Menthone | - | - | - | 0.50 | 1154 |
| Borneol | - | - | 0.99 | 0.90 | 1177 |
| Terpineol | - | - | 0.53 | 0.89 | 1198 |
| Cinnamaldehyde | - | - | 2.37 | 1232 | |
| Pulegone | - | - | 0.52 | 1.96 | 1250 |
| Thymol | 40.00 | - | 35.18 | - | 1289 |
| E-Cinnamaldehyde | - | - | - | 36.06 | 1308 |
| Carvacrol | 31.12 | - | 34.00 | - | 1333 |
| Piperitenone | - | - | - | 11.18 | 1366 |
| Eugenol | - | 87.10 | - | 2.44 | 1382 |
| alpha-Copaene | - | 0.10 | - | 4.69 | 1393 |
| Trans-Caryophyllene | 2.73 | 2.70 | 0.36 | 3.40 | 1437 |
| beta-Farnesene | - | 0.40 | 0.42 | 0.32 | 1458 |
| Cinnamyl acetate | - | - | - | 2.54 | 1462 |
| alpha-Humulene | - | 0.40 | - | 0.58 | 1473 |
| Germacrene D | - | - | 0.99 | 0.72 | 1496 |
| alpha-Muurolene | - | - | - | 3.12 | 1516 |
| Eugenyl acetate | - | 8.01 | - | - | 1526 |
| delta-Cadinene | - | - | 0.11 | 5.96 | 1541 |
| Total identified | 90.92 | 99.36 | 85.33 | 85.09 | - |
ZEO: Zataria multiflora essential oil; SEO: Syzygium aromaticum essential oil; OEO: Origanum vulgare essential oil; CEO: Cinnamomum verum essential oil; RI: Retention index.
Antibacterial activity of EOs. The results regarding the disc diffusion technique varied at a great extent (Table 2). The majority of bacteria were sensitive to four applied EOs. The ZEO produced a zone of inhibition in the disk diffusion test greater than the zones of inhibition for the others; therefore, ZEO had the highest inhibitory effect against L. monocytogenes, E. coli O157:H7, S. putrefaciens, and P. fluorescens. The E. coli O157:H7 was the most sensitive bacterium to ZEO. Different volumes of ZEO (5.00, 10.00, 15.00; and 20.00 μL per disc) were determined by disk diffusion method and inhibition zones (12.00 ± 0.80 mm, 15.00 ± 1.00 mm, 19.00 ± 0.50 mm; and 24.00 ± 1.10, respectively) were measured. After that, OEO was more effective than other EOs against these bacteria. The most sensitive among these bacteria to OEO was S. putrefaciens. Different volumes of OEO (5.00, 10.00, 15.00; and 20.00 μL per disc) were determined by disk diffusion method and inhibition zones were measured (11.00 ± 0.70 mm, 13.00 ± 0.50, 17.00 ± 1.20, 22.00 ± 0.80 mm, respectively).
Table 2.
The inhibition zones (mm; mean ± SD) of four essential oils (EOs) detected by the disc diffusion method
| EOs | Tested bacteria |
EO concentration (µL per disc)
|
Gentamycin
(10.00 µg per disc) |
|||
|---|---|---|---|---|---|---|
| 5.00 | 10.00 | 15.00 | 20.00 | |||
| ZEO | E. coli O157:H7 | 12.00 ± 0.80d | 15.00 ± 1.01d | 19.00 ± 0.50d | 24.00 ± 1.17d | 22.00 ± 0.50b |
| L. monocytogenes | 9.00 ± 0.70b | 12.00 ± 1.01b | 16.00± 1.20b | 17.00 ± 0.50b | 21.00 ± 1.09a | |
| P. fluorescens | 8.00 ± 0.52a | 11.00 ± 1.01a | 13.00 ± 0.40a | 16.00 ± 0.70a | 23.00 ± 0.70c | |
| S. putrefaciens | 11.00 ± 0.30c | 14.00 ± 0.60c | 18.00 ± 1.10c | 23.00 ± 1.00c | 24.00 ± 1.10d | |
| OEO | E. coli O157:H7 | 11.00 ± 0.50b | 14.00 ± 0.70d | 16.00 ± 1.11c | 21.00 ± 0.40c | 22.00 ± 0.51b |
| L. monocytogenes | 8.00 ± 0.71a | 11.00 ± 1.20b | 15.00 ± 0.70b | 17.00 ± 0.54b | 21.00 ± 1.01a | |
| P. fluorescens | 8.00 ± 0.80a | 10.00 ± 0.10a | 13.00 ± 0.40a | 16.00 ± 0.71a | 23.00 ± 0.70c | |
| S. putrefaciens | 11.00 ± 0.70b | 13.00 ± 0.50c | 17.00 ± 1.21d | 22.00 ± 0.80d | 24.00 ± 1.10e | |
| SEO | E. coli O157:H7 | 7.00 ± 0.10a | 8.00± 0.40b | 8.00 ± 0.50a | 9.00 ± 0.70a | 22.00 ± 0.50b |
| L. monocytogenes | 7.00 ± 0.43a | 7.00 ± 0.53a | 8.00 ± 0.50a | 9.00 ± 0.50a | 21.00 ± 1.08a | |
| P. fluorescens | 7 .00± 0.01a | 8.00 ± 0.10b | 9.00 ± 0.41b | 10.00 ± 1.11b | 23.00 ± 0.70c | |
| S. putrefaciens | 7 .00± 0.01a | 8.00 ± 0.70b | 10.00 ± 0.50c | 13.00 ± 1.21c | 24.00 ± 1.13d | |
| CEO | E. coli O157:H7 | 7.00± 0.13a | 9.00 ± 0.50a | 12.00 ± 1.10b | 14.00 ± 0.80b | 22.00 ± 0.50b |
| L. monocytogenes | 8.00± 0.50b | 9.00 ± 0.73a | 11.00 ± 0.43a | 13.00 ± 1.20a | 21.00 ± 1.00a | |
| P. fluorescens | 9.00 ± 0.41c | 11.00 ± 0.50b | 14.00 ± 1.30c | 17.00 ± 1.10d | 23.00 ± 0.70c | |
| S. putrefaciens | 9.00 ± 0.57c | 12.00 ± 0.70c | 14.00 ± 0.50c | 16.00 ± 1.20c | 24.00 ± 1.10d | |
OEO: Origanum vulgare essential oil; ZEO: Zataria multiflora essential oil; SEO: Syzygium aromaticum essential oil; CEO: Cinnamomum verum essential oil. abcde Different superscript letters within the same EOs column and concentrations are significantly different (p < 0.05).
Determination of MIC. Antibacterial efficiency of EOs was quantified by the MIC method (Table 3). All tested bacterial strains were susceptible to four EOs. The MIC of four EOs for all of the tested bacteria was in the range of 0.64 - 40.96 mg mL-1. The ZEO showed strong antibacterial activities at MIC ≤ 1.28 mg mL-1. The MIC of ZEO for all of the bacteria was minimized. The bacterial growth was also inhibited by OEO at MIC ≤ 2.56 mg mL-1. There were no differences between the antibacterial activity of ZEO and OEO (p ≥ 0.05).
Table 3.
Antibacterial activity of four essential oils expressed as a minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) in mg mL1 against tested organisms
| Tested bacteria |
CEO
|
|
|
ZEO
|
|
SEO
|
|
OEO
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |||||
| Escherichia coli O157:H7 | 20.48b | 20.48b | 0.64a | 0.64a | 5.12b | 10.24b | 0.64a | 0.64a | ||||
| Listeria monocytogenes | 40.96c | 40.96c | 1.28b | 1.28b | 5.12b | 20.48c | 1.28b | 2.56b | ||||
| Pseudomonas fluorescens | 20.48b | 20.48b | 1.28b | 1.28b | 2.56a | 5.12a | 1.28b | 2.56b | ||||
| Shewanella putrefaciens | 10.24a | 10.24a | 0.64a | 0.64a | 2.56a | 5.12a | 0.64a | 0.64a | ||||
OEO: Origanum vulgare essential oil; ZEO: Zataria multiflora essential oil; SEO: Syzygium aromaticum essential oil; CEO: Cinnamomum verum essential oil. abc Different superscript letters indicate significant differences within a column (p < 0.05).
Determination of MBC. The MBC of EOs was determined based on Duarte et al.18 with modifications (Table 3). The MBCs of four EOs were in the range of 0.64 - 40.96 mg mL-1. The ZEO showed strong antibacterial activities at MBC ≤ 1.28 mg mL-1. There were no differences between the antibacterial activity of ZEO and OEO (p ≥ 0.05). The most bactericidal effect of ZEO was observed against E. coli O157:H7 and S. putrefaciens. The lowest bactericidal efficacy of EOs was observed in CEO.
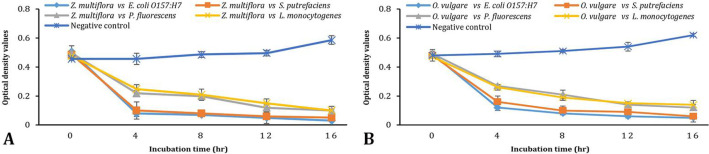
Killing kinetics of the bacterial cells. The two EOs (ZEO and OEO) indicating the MBC values less than 3.00 mg mL-1 decreased the number of organisms after 4 hr. The ZEO could kill 85.00% of the E.coli O157:H7 and S.putrefaciens cells within 4 hr (Fig. 1A). It could kill only 60.00% of the P. fluorescens and L. monocytogenes cells within 4 hr; then following 12 hr, it killed almost 70.00% of the P. fluorescens and L. monocytogenes cells. The OEO could kill about 80.00% of the E. coli O157:H7 and S. putrefaciens cells within 4 hr (Fig. 1B). It could kill only 50.00% of the P. fluorescens and L. monocytogenes cells.
Fig. 1.
A) Killing curves of Zataria multiflora essential oil (ZEO) against foodborne pathogenic and spoilage organisms indicated by the variation of optical density (590 nm) at 16 hr incubation time, B) Killing curves of Origanum vulgare essential oil (OEO) against foodborne pathogenic and spoilage organisms indicated by the variation of optical density (590 nm) at 16 hr incubation time
Antioxidative activity. The EOs exhibited anti-oxidative effects and inhibition of DPPH as the percentage increased with an increasing amount of EOs concentration (0.01 - 20.00 mg mL-1), which was compared with BHT, used as a standard at the concentration of 0.01 - 20.00 mg mL-1. Inhibitions of DPPH as percentage in 0.01 - 20 mg mL-1 EOs concentration for ZEO, SEO, OEO, and CEO were respectively 9.00-97.00%, 4.00-92.00%, 6.00-95.00%, and 0.50-80.00% but for BHT were 10.00 - 98.00%. According to the results, ZEO exhibited the strongest inhibition of DPPH (97.00%). This activity was followed by OEO95.00%) and SEO (92.00%). The CEO had the lowest DPPH free-radical scavenging activity (80.00%). The specimen level providing 50.00% inhibition (IC50) was computed from the graph corresponding to the inhibition percentage versus specimen concentration. The IC50 of BHT, OEO, SEO, ZEO, and CEO was 0.10 ± 0.00, 0.14 ± 0.03, 0.16 ± 0.03, 0.13 ± 0.04 and 6.00 ± 0.02 mg mL-1, respectively.
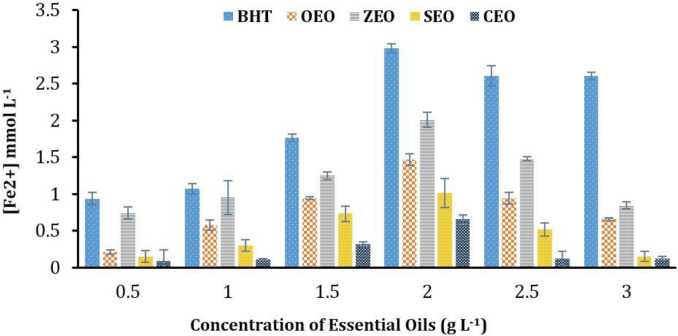
Ferric reducing antioxidative power. Ferric reducing antioxidative abilities of EOs and BHT were assessed with standard protocol and outcomes were reported in μM equivalent to FeSO4.7H2O. Four studied EOs were capable of chelating iron (II). Absorbance (at 593 nm) of various concentrations of Fe2SO4 including 31.25, 62.50, 125, 250, 500; and 1000 mmol L-1) in FRAP assay were 0.15, 0.22, 0.33, 0.59, 1.07; and 2.00, respectively. The FRAP assay is based on the reduction of colorless Fe3+ compounds into Fe2+ tripiridyltriazine in the existence of the antioxidant.22 The antioxidative capacity in the different concentrations of EOs is presented in Figure 1B. The FRAP values of BHT, OEO, ZEO, SEO, and CEO were respectively 2.98 ± 0.02, 1.47 ± 0.04, 2.01 ± 0.04, 1.01 ± 0.03; and 0.66 ± 0.34, and the tested EOs showed the highest antioxidative activity at 2.00 g L-1 (Fig. 2).
Fig. 2.
The antioxidative capacity in the different concentrations of essential oils determined using the ferric reducing antioxidative power assay. BHT: Butylated hydroxytoluene; OEO: Origanum vulgare essential oil; ZEO: Zataria multiflora essential oil; SEO: Syzygium aromaticum essential oil; CEO: Cinnamomum verum essential oil
Discussion
This part of the study was designed to evaluate the chemical components of four EOs using GC-MS. Components in tested EOs are listed in Table 1. The composition of EOs depends on several factors including harvesting seasons and geographical sources. These can explain differences between results obtained from different researches in which variation in amounts is significant, as this chemical compound can be found in traces or makeup to 80.00% of the EO’s composition.23,24 The ZEO and OEO exhibited good antibacterial properties and proved to be better due to their phenolic compounds content which also provided anti-oxidant capacity.
There are numerous studies on the antibacterial property of EOs against an extensive range of micro-organisms.25,26 Our results regarding the antibacterial performance of four EOs tested by the disc diffusion (Table 2) showed that the EOs could be useful against L. monocytogenes, E. coli O157:H7 and SSOs including S. putrefaciens and P. fluorescens. There were considerable differences between the antibacterial performance of ZEO and; CEO. Ličina et al. have prepared many extracts of oregano with high antibacterial performance. 27
Benavides et al. have reported that films produced with OEO show the largest inhibition zones for Gram-positive bacteria and the smallest inhibition zones for Gram-negative bacteria.28 Pelissari et al. have shown that fractions of OEO activity against species of bacteria; including S. aureus and E. coli O157:H7,29 that we found generally similar results and OEO showed strong antibacterial effects against tested bacterial strains.
Table 3 shows the MIC values corresponding to the EOs against tested bacterial strains. In antibacterial assay of EOs through MIC determination, our findings were following the outcomes obtained by our findings of the disc diffusion method and four EOs showed good inhibition activity against bacteria. The best antibacterial effects were exhibited by ZEO and OEO against bacteria; such as E. coli O157:H7, L. monocytogenes, P. fluorescens; and S. putrefaciens. This finding was per the results of Seydim and Sarikus; and the results of another study have shown that OEO is successful against E. coli O157:H7, which is similar to our results.26 The EOs used in this study exhibited significant antibacterial effects and showed great inhibitory activity against L. monocytogenes, E. coli O157:H7, and SSOs, but greater levels of these were required to obtain bactericidal effects in food.7
The MBC is the lowest broth dilution of antibacterial compound preventing the growth of the organism on the medium. It has been recognized that some EOs have antibacterial activities.30-32 This study showed that application of EOs was effective in inhibiting S. putrefaciens, P. fluorescens, L. monocytogenes; and E. coli O157:H7 (Table 3). The best antibacterial activity was exhibited by ZEO and OEO against E. coli O157:H7 and S. putrefaciens. There were no differences between the MBC of ZEO and OEO.
Research into time-kill kinetics assay of EOs, especially their antibacterial activity, has attracted attention recently. Through killing time study,33 only a few EOs were described for their antibacterial activities. This section of the study aimed to define the antibacterial effects of two EOs using the ELISA reader method. The two EOs (ZEO and OEO) decreased the number of an organism after 4 hr, but ZEO indicated consistent antibacterial activity more than OEO.
The DPPH radical scavenging method and FRAP can be cited as relatively simple methods that can be utilized for measuring the antioxidative activity of EOs.22 The DPPH assay is popular in natural product antioxidative studies. Bleaching the purple-colored methanolic solution of DPPH was used to measure the electrons donation potential of the relating extracts and several pure compounds. The antioxidative activity corresponding to EOs was showed with their DPPH radical scavenging efficiency relating to the content of EOs. The lower absorbance of the reaction solution indicates higher free radical scavenging performance.34 The DPPH radical-scavenging activities of four EOs are displayed in results. The highest antioxidative activity was observed in ZEO. The major compound of ZEO was thymol (40.00%) followed by carvacrol (31.12%) (Table 1). The metabolic mechanism of the carvacrol and thymol creation starts with the autoxidation of c-terpinene to p-cymene and continues by hydroxylation to thymol.35,36
Ruberto and Baratta have confirmed that thymol and carvacrol molecules are indeed responsible for the anti-oxidative performance of many thymol- and carvacrol-containing EOs.37 Strong antioxidative activity was also detected in OEO and SEO. Increasing of EOs’ concentration results in DPPH inhibition elevation. OEO, SEO, ZEO, and CEO were able to reduce the stable DPPH radical to a 50.00% reduction. A lower IC50 value shows more anti-oxidant potential. The highest antioxidative effect and the most active radical scavenging of EOs were obtained by ZEO followed by OEO. The CEO showed a weak antioxidative effect.
The studied EOs were capable of chelating iron (II). The ZEO had the highest FRAP value followed by OEO. All of the EOs showed interesting antioxidative activities. The standard anti-oxidant (BHT) had a higher FRAP value than natural anti-oxidant and the best reducing abilities. The outcomes showed that ZEO and OEO have good reductive power and may thus possess significant antioxidative activity. The FRAP of EOs revealed that EOs had lower FRAP values than the reference anti-oxidant.34 The SEO showed a higher FRAP value than that of the CEO. High concentrations of tested EOs showed a decrease in antioxidative activity.
According to the results, the antioxidative activities of EOs depend on the contents of carvacrol and thymol in the EOs and are amongst the natural compositions that can have several useful properties protecting foods against oxidation and increasing lifetime of food.7 The studied EOs showed varying degrees of antibacterial and antioxidative effects, but the highest activity was found in ZEO and strong antioxidative activity was also detected in OEO and SEO. The EOs show great potential for applicability in food conservation to improve the quality of food.
The study showed that the application of EOs was effective in antibacterial and antioxidative activities. It was generally observed in our results that ZEO and OEO exhibited the highest antioxidative activity and anti-bacterial effect against tested organisms. Additional in vitro and in vivo studies would be needed to characterize the active principles and to evaluate the potential toxicity of the four EOs.
Acknowledgments
This research was financially supported by a PhD grant No. 40023 from the Research Council of the Ferdowsi University of Mashhad, Mashhad, Iran.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Hossain MK, Dewan MW, Hosur M, et al. Mechanical performances of surface modified jute fiber reinforced biopol nanophased green composites. Compos Part B Eng. 2011;42:1701–1707. [Google Scholar]
- 2.Mittal M, Gupta N, Parashar P, et al. Phytochemical evaluation and pharmacological activity of Syzygium aromaticum: A comprehensive review. Int J Pharm Pharm Sci. 2014;6(8):67–72. [Google Scholar]
- 3.Mohammadi A, Hashemi M, Hosseini SM. The control of botrytis fruit rot in strawberry using combined treatments of chitosan with Zataria multiflora or Cinnamomum zeylanicum essential oil. J Food Sci Technol. 2015;52(11):7441–7448. [Google Scholar]
- 4.Govaris A, Solomakos N, Pexara A, et al. The anti-microbial effect of oregano essential oil, nisin and their combination against Salmonella Enteritidis in minced sheep meat during refrigerated storage. Int J Food Microbiol. 2010;137(2-3):175–180. doi: 10.1016/j.ijfoodmicro.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Gruenwald J, Brendler T, Jaenicke C. PDR for Herbal Medicines. Medical Economic: Montvale, USA; 2000. pp. 783–785. [Google Scholar]
- 6.Teixeira B, Marques A, Ramos C, et al. Chemical composition and bioactivity of different oregano (Origanumvulgare) extracts and essential oil. J Sci Food Agric. 2013;93(11):2707–2714. doi: 10.1002/jsfa.6089. [DOI] [PubMed] [Google Scholar]
- 7.Burt S. Essential oils: their antibacterial properties and potential applications in foods- a review. Int J Food Microbiol. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Karakaya S, Nehir El S, Karagözlü N, et al. Antioxidant and antimicrobial activities of essential oils obtained from oregano (Origanumvulgare ssp hirtum) by using different extraction methods. J Med Food. 2011;14(6):645–652. doi: 10.1089/jmf.2010.0098. [DOI] [PubMed] [Google Scholar]
- 9.Sarikurkcu C, Zengin G, Oskay M, et al. Composition, antioxidant, antimicrobial and enzyme inhibition activities of two Origanum vulgare subspecies (subsp vulgare and subsp hirtum) essential oils. Ind Crops Prod. 2015;70:178–184. [Google Scholar]
- 10.Shaffiee A, Javidnia K. Composition of essential oil of Zataria multiflora. Planta Med. 1997;63(4):371–372. doi: 10.1055/s-2006-957707. [DOI] [PubMed] [Google Scholar]
- 11.Sajed H, Sahebkar A, Iranshahi M. Zataria multiflora Boiss (Shirazi thyme)-an ancient condiment with modern pharmaceutical uses. J Ethnopharmacol. 2013;145(3):686–698. doi: 10.1016/j.jep.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Singh S, Lee MH, Park I, et al. Antimicrobial seafood packaging: a review. Food Sci Technol. 2016;53:2505–2518. doi: 10.1007/s13197-016-2216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edris AE. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res. 2007;21(4):308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 14.Mishra A, Bhatti R, Singh A, et al. Ameliorative effect of the cinnamon oil from Cinnamomum zeylanicum upon early stage diabetic nephropathy. Planta Med. 2010;76(5):412–417. doi: 10.1055/s-0029-1186237. [DOI] [PubMed] [Google Scholar]
- 15.Kayode RMO, Afolayan AJ. Cytotoxicity and effect of extraction methods on the chemical composition of essential oils of Moringa oleifera seeds. J Zhejiang Univ Sci B. 2015;16(8):680–689. doi: 10.1631/jzus.B1400303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CLSI. Performance standards for antimicrobial susceptibility testing (approved standard-eleventh edition). CLSI M100‐S21. Wayne, USA ; 2012. p. 144. [Google Scholar]
- 17.Doughari JH, El-mahmood AM, Manzara S. Studies on the antibacterial activity of root extracts of Carica papaya L. Afr J Microbiol Res. 2007:037–041. [Google Scholar]
- 18.Duarte S, Pascoal C, Cássio F, et al. Aquatic hyphomycete diversity and identity affect leaf litter decomposition in microcosms. Oecologia. 2006;147:658–666. doi: 10.1007/s00442-005-0300-4. [DOI] [PubMed] [Google Scholar]
- 19.Samie A, Nefefe T, Gundidza M, et al. Antimicrobial activities of essential oils from Southern Africa against selected bacterial and fungal organisms. Afr J Biotechnol. 2012;11(89):15560–15568. [Google Scholar]
- 20.Hussain AI, Anwar F, Sherazi STH, et al. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008;108(3):986–995. doi: 10.1016/j.foodchem.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: the FRAP Assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 22.Politeo O, Botica I, Bilusić T. et al. Chemical composition and evaluation of acetylcholinesterase inhibition and antioxidant activity of essential oil from Dalmatian endemic species Pinus nigra Arnold ssp. dalmatica (Vis.) Franco. J Med Plant Res. 2011;5(30):6590–6596. [Google Scholar]
- 23.Boskovic M, Zdravkovic N, Ivanovic J, et al. Antimicrobial activity of thyme (Tymus vulgaris) and oregano (Origanum vulgare) essential oils against some food-borne microorganisms. Procedia Food Sci. 2015;5:18–21. [Google Scholar]
- 24.De Falco E, Roscigno G, Landolfi S, et al. Growth, essential oil characterization, and antimicrobial activity of three wild biotypes of oregano under cultivation condition in Southern Italy. Ind Crop Prod. 2014;62:242–249. [Google Scholar]
- 25.Santos MIS, Martins SR, Veríssimo CSC, et al. Essential oils as antibacterial agents against food-borne pathogens: Are they really as useful as they are claimed to be? J Food Sci Technol. 2017;54:4344–4352. doi: 10.1007/s13197-017-2905-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seydim AC, Sarikus G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res Int. 2006;39(5):639–644. [Google Scholar]
- 27.Ličina BZ, Stefanovic O, Vasič S, et al. Biological activities of the extracts from wild growing Origanum vulgare L. Food Control. 2013;33:498–504. [Google Scholar]
- 28.Benavides S, Villalobos-Carvajal R, Reyes JE. Physical, mechanical and antibacterial properties of alginate film: Effect of the crosslinking degree and oregano essential oil concentration. J Food Eng. 2012;110(2):232–239. [Google Scholar]
- 29.Pelissari FM, Grossmann MVE, Yamashita F, et al. Antimicrobial, mechanical, and barrier properties of cassava starch-chitosan films incorporated with oregano essential oil. J Agric Food Chem. 2009;57(16):7499–7504. doi: 10.1021/jf9002363. [DOI] [PubMed] [Google Scholar]
- 30.Otoni CG, Pontes , SFO , Medeiros , EAA Edible films from methylcellulose and nanoemulsions of clove bud (Syzygiumaromaticum) and oregano (Origanumvulgare) essential oils as shelf life extenders for sliced bread. J Agric Food Chem. 2014;62(22):5214–5219. doi: 10.1021/jf501055f. [DOI] [PubMed] [Google Scholar]
- 31.Ashraf SA, Al-Shammari E, Hussain T, et al. In-vitro antimicrobial activity and identification of bioactive components using GC–MS of commercially available essential oils in Saudi Arabia. J Food Sci Technol. 2017;54(12):3948–3958. doi: 10.1007/s13197-017-2859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chorianopoulos NG, Nychas GJE, Haroutounian SA. Essential oils of Lamiaceae family taxa as natural preservatives of food preparations. Food. 2007;1(2):202–215. [Google Scholar]
- 33.Halcón L, Milkus K. Staphylococcusaureus and wounds: a review of tea tree oil as a promising antimicrobial. Am J Infect Control. 2004;32(7):402–408. doi: 10.1016/S0196655304003657. [DOI] [PubMed] [Google Scholar]
- 34.Thaipong K, Boonprakob U, Crosby K, et al. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compost Anal. 2006;19(6-7):669–675. [Google Scholar]
- 35.Alizadeh L. Essential oil constituents, phenolic content and antioxidant activity in Iranian and British Thymus vulgaris. Int J Agric Crop Sci. 2013;6(4):213–218. [Google Scholar]
- 36.Dashipour A, Razavilar V, Hosseini H, et al. Antioxidant and antimicrobial carboxymethyl cellulose films containing Zatariamultiflora essential oil. Int J Biol Macromol. 2015;72:606–613. doi: 10.1016/j.ijbiomac.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Ruberto G, Baratta MT. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000;69(2):167–174. [Google Scholar]