Significant natural variation was found in rice flag leaves for photosynthetic traits measured in steady- and non-steady-state conditions, with implications for improving water use efficiency and photosynthesis in this key crop.
Keywords: Atmospheric change, crop improvement, flag leaves, food security, natural variation, photosynthetic induction, rice, rice breeding, Rubisco activation, water use efficiency
Abstract
Several breeding initiatives have sought to improve flag leaf performance as its health and physiology are closely correlated to rice yield. Previous studies have described natural variation of photosynthesis for flag leaves; however, none has examined their performance under the non-steady-state conditions that prevail in crop fields. Photosynthetic induction is the transient response of photosynthesis to a change from low to high light. Rice flag leaf photosynthesis was measured in both steady- and non-steady-state conditions to characterize natural variation. Between the lowest and highest performing accession, there was a 152% difference for average CO2 assimilation during induction (Ā300), a 77% difference for average intrinsic water use efficiency during induction (iWUEavg), and a 185% difference for the speed of induction (IT50), indicating plentiful variation. No significant correlation was found between steady- and non-steady-state photosynthetic traits. Additionally, measures of neither steady-state nor non-steady-state photosynthesis of flag leaves correlated with the same measures of leaves in the vegetative growth stage, with the exception of iWUEavg. Photosynthetic induction was measured at six [CO2], to determine biochemical and diffusive limitations to photosynthesis in vivo. Photosynthetic induction in rice flag leaves was limited primarily by biochemistry.
Introduction
In cereals, the flag leaf is defined as the last leaf to emerge on a mature flowering stem. The flag leaf has a higher photosynthetic capacity relative to lower canopy leaves due to its position at the top of the canopy, which allows for greater interception of light (Adachi et al., 2017). Additionally, high rice yields are closely correlated with the size and health of the flag leaf as they contribute some 50% of assimilates utilized for grain filling (Yoshida, 1981; Ishii, 1993; Nakano et al., 1995; Li et al., 1998). This large contribution of photosynthates is partially due to the proximity of the flag leaf to the grain, as the sink can more easily attract assimilates from closer sources (Sicher, 1993). The rest of the photosynthates used in grain filling are supplied by the leaf immediately below the flag leaf, and by remobilization of stored carbohydrates in leaf sheaths and older senescing leaves (Li et al., 2017; Lin et al., 2018). Agronomic strategies are typically aimed to protect the flag leaf, since its destruction during grain filling is associated with yield losses of up to 45% in rice (Abou-Khalifa et al., 2008).
Consequently, great effort has been dedicated to understanding and optimizing the flag leaf to improve yields (Li et al., 1998). For example, flag leaf size and area can significantly influence grain yield (Li et al., 1998; Zhang et al., 2015). In rice, flag leaves with larger areas are significantly correlated with greater yields and have become a target for breeding programs seeking to achieve an ideal phenotype or ideotype (Zhang et al., 2015). Quantitative trait loci (QTLs) for larger flag leaves have been identified with the purpose of improving yield (Zhang et al., 2015). Additionally, rice yields have been increased by up-regulating NAL1, a gene that affects both flag leaf area and photosynthetic rates (Fabre et al., 2016). Increasing rice flag leaf photosynthesis, through either delaying senescence or a more acute leaf angle, has led to higher yields (Mantilla-Perez et al., 2017). Early flag leaf senescence significantly reduces seed-setting rate, 100-grain weight, and yield (Lin et al., 2018). Conversely, delaying senescence prolongs the period in which the flag leaf is photosynthetically active, resulting in more photosynthates that can fill grain (Ishii, 1993; Kobata et al., 2015; Leng et al., 2017). In previous studies, more vertical flag leaf angles resulted in 13% higher photosynthetic rates, reduced photoinhibition, delayed leaf senescence, and 15% higher yields (Chen et al., 2002; Mantilla-Perez et al., 2017).
Open-air elevation of [CO2] using free-air CO2 enrichment technology is an artificial means of enhancing photosynthesis season-long in field-grown rice and observing the yield response. Modern high-yielding rice lines that have larger single-grain sizes and are able to produce larger panicles show a greater response to this elevation of photosynthesis, in contrast to older lines which appear partially sink limited. The fact that even under these circumstances only 70–80% of spikelets ripen—and less than in the older rice lines—suggests that these high-yielding lines are actually strongly source limited (Hasegawa et al., 2013; Zhu et al., 2015; Sakai et al., 2019; Ainsworth and Long, 2020). These findings suggest that increasing the photosynthetic capacity of the flag leaf would be particularly valuable for these high-yielding cultivars.
Photosynthetic induction is the process by which leaves begin to assimilate CO2 upon a transition from low light into high light, and is characterized by a lag in photosynthetic efficiency. Previously, examination of rice leaves in the vegetative growth stage found greater variation for photosynthetic traits during non-steady-state lighting compared with steady state (Acevedo-Siaca et al., 2020). Despite the focus on flag leaf improvement, no studies have characterized the response of flag leaf photosynthesis in non-steady-state conditions or explored the possibility that there is variation in this character that could be utilized for improvement. Recently, it was shown that rice flag leaves are subject to different endogenous aging programs compared with other leaves (Lee et al., 2017). Given its proximity to the major sink in the panicle, feedback would also be expected through non-structural carbohydrate-driven ‘feast’ and ‘famine’ gene expression response networks (Paul et al., 2017). It is therefore likely that photosynthetic properties of flag leaves will differ from those of leaves formed in the vegetative growth stage.
Non-steady-state photosynthesis is important in a field agricultural setting where light is never constant. At the top of the canopy, flag leaves are subject to light fluctuations due to intermittent cloud cover and shadowing by other flag leaves or panicles as wind and sun angles change (Taylor and Long, 2017; Wang et al., 2020). In wheat, time taken for photosynthetic efficiency to recover from transient shadowing over the course of the day in the field was calculated to cost 21% of potential flag leaf assimilation (Taylor and Long, 2017). Improving the rate of recovery in this rarely measured photosynthetic parameter of flag leaves has the potential to increase yield. Limitations to the speed of photosynthetic induction on such shade to sun transitions are due to four main processes: the photoactivation of enzymes involved in the regeneration and production of ribulose-1,5-bisphosphate (RuBP); the buildup of intermediates of carbon metabolism; the activation of Rubisco by Rubisco activase; and stomatal opening (Pearcy et al., 1994; Mott and Woodrow, 2000; Slattery et al., 2018).
This study analyzed the performance of flag leaf photosynthesis in both steady-state and non-steady-state conditions. The objectives were limited to rice flag leaves and aimed to (i) determine the extent of variation between accessions in non-steady-state and steady-state photosynthetic parameters relating to productivity and resource use efficiency across rice accessions; (ii) examine the response of photosynthetic induction of rice flag leaves at different [CO2] to understand limitations to induction; and (iii) compare the response of photosynthetic induction in rice flag leaves between accessions and with induction in leaves in the vegetative growth stage of the same accessions as reported in Acevedo-Siaca et al. (2020).
Materials and methods
Growing conditions and germplasm
Six accessions were selected from the 3000 Rice Genome Project (3K RGP) held at the Germplasm Resources Center at the International Rice Research Institute (IRRI) in Los Baños, Philippines. Accessions were selected on nucleotide mismatches for the gene encoding Rubisco activase, which plays a central role in photosynthetic induction. Seeds were maintained at 50 °C for 1 week to break dormancy and then sown into soil from the IRRI Upland Farm in small pots (4.5 cm diameter×12 cm) and fertilized using 0.4 g–1 Osmocote Plus 15-9-12 (The Scotts Company Ltd, Thorne, UK). Seedlings were transferred to larger individual pots (21.5 cm diameter×21.5 cm, 6 liters) after the emergence of the second leaf. These were then placed in a screen house, a type of greenhouse with a glass roof and screen-meshed walls, with no additional lighting or temperature control at IRRI, during the Philippines dry season from March to May 2017. Each pot was kept flooded using a drip irrigation system to simulate paddy conditions (Supplementary Fig. S1).
Gas exchange measurements
Photosynthetic induction
After anthesis, the flag leaf of the main stem was placed in the cuvette of an open gas exchange system (LI-6400XT, LI-COR, Lincoln, NE, USA). Light was provided by an integrated LED head (2×3 LED, LI-6400-02B). Within the cuvette, air temperature was 28 °C to approximate ideal growing conditions, flow rate was 400 µmol s–1, and [CO2] was maintained at 400 µmol mol–1, and water vapor pressure deficit (VPD) at 1.3–1.7 kPa. Prior to measuring photosynthetic induction, rice plants were dark adapted for at least 1 h.
For induction, leaves were first allowed to reach a steady state in low light with a photosynthetically photon flux density (PPFD) of 50 µmol m–2 s–1 (‘shade’) for 300 s followed by 720 s at 1700 µmol m–2 s–1 (‘sun’). Gas exchange measures were recorded every 10 s for the duration of the experiment. Measurements were repeated for all six accessions (n=8 plants per accession) over 4 d to minimize any age effects. Plants were selected by a randomized design and measured from 08.00 h to 12.00 h, to avoid confounding accessions with any diurnal influences. Net CO2 uptake (A), stomatal conductance (gs), intercellular CO2 concentration (Ci), transpiration (E), and intrinsic water use efficiency (iWUE) were calculated following the equations of Farquhar et al. (1981). For a summary of all traits measured, see Table 1.
Table 1.
A summary of all traits measured and mentioned in the text
| Light condition | Trait | Description | Unit |
|---|---|---|---|
| Steady-state | A sat | Leaf net CO2 uptake in saturating light | µmol m–2 s–1 |
| g s | Stomatal conductance | mol m–2 s–1 | |
| C i | Intercellular CO2 concentration | µmol mol–1 | |
| iWUE | Intrinsic water-use efficiency (iWUE=A/ gs) | µmol CO2 mol H2O–1 | |
| V c, max | Maximum rate of carboxylation | µmol m–2 s–1 | |
| Jmax | Maximum rate of electron transport | µmol m–2 s–1 | |
| CE | Carboxylation efficiency | mol m–2 s–1 | |
| Γ | Compensation point | µmol m-2 s-1 | |
| Φ | Quantum yield | Unitless (0–1) | |
| Non-steady-state | Ā 300 | Average A during first 300 s of induction | µmol m–2 s–1 |
| Average gs during first 300 s of induction | mol m–2 s–1 | ||
| Average Ci during first 300 s of induction | µmol mol–1 | ||
| Average iWUE during first 300 s of induction | µmol CO2 mol H2O–1 | ||
| IT50 | Time to 50% induction | Seconds | |
| IT90 | Time to 90% induction | Seconds | |
| A Max | Maximum A during induction | µmol m–2 s–1 | |
| A 300 | A at the end of 300 s | µmol m–2 s–1 | |
| A* | A corrected for stomatal limitation | µmol m–2 s–1 | |
| 1/ τ | Rate constant of Rubisco activation | Seconds | |
| τ | Time to activation of photosynthesis | Seconds | |
| C Loss | Forgone assimilation | µmol m–2 |
Units and light conditions are included.
Two accessions, previously reported to show very different rates of induction for their leaves during the vegetative phase of growth, IR64-21 and AUS 278, were selected for further analysis of flag leaf induction at different [CO2] (Acevedo-Siaca et al., 2020). Induction was measured following the protocol described above for induction, but at a cuvette [CO2] of either 100, 200, 300, 400, 600, or 800 µmol mol–1 during induction. The order of cuvette [CO2] treatments for each individual leaf was randomized to avoid confounding [CO2] with time. Leaves were dark adapted for a minimum of 1 h between measurements at the different [CO2] at which they would later be measured. To determine limitations through induction, A was plotted against Ci for different time points, following the procedure of Soleh et al. (2016) and of Acevedo-Siaca et al. (2020). Five time points were selected for further analysis: 60, 180, 300, 360, and 700 s from the initiation of induction by transfer from darkness to high light (1700 µmol m–2 s–1). This allowed determination, at each time point, as to whether photosynthesis within the mesophyll was limited by the apparent maximum rates of RuBP regeneration (Jmax) or RuBP carboxylation by Rubisco (Vc,max).
Steady-state measurements
For steady-state measurements, flag leaves were allowed to reach constant rates of A and stomatal conductance (gs) at 1700 µmol m–2 s–1 PPFD. Cuvette conditions for steady-state measurements were as described above for photosynthetic induction.
Calculations
The rate constant of Rubisco activation, time to activation of photosynthesis, and forgone assimilation
The rate constant of Rubisco activation (1/τ) was calculated by fitting the slope of the linear phase of the natural log of corrected photosynthetic induction [ln(Af*–A*)] as described in Woodrow and Mott (1989). A* is photosynthesis at a point in time during induction corrected to a Ci value of 300 µmol mol–1 to remove limitation from stomata. Af* is the corrected value for photosynthesis at a Ci of 300 µmol mol–1 at the end of the induction. The correction to Ci was made following the methods of Soleh et al. (2016):
| (1) |
The time required to complete the activation of photosynthesis (τ) was calculated by taking the reciprocal of the rate constant of Rubisco activation (Woodrow and Mott, 1989).
The integrated amount of CO2 uptake foregone due to the lower rates through induction compared with steady-state (C Losst) was calculated as in Acevedo-Siaca et al. (2020):
| (2) |
where A is the steady-state rate of uptake and Āt the average rate across the measured time period from the start of the induction (t), 300 s and 700 s, respectively.
Statistical analyses
All statistical analyses and model fitting used R (version 3.5.2, R-project) (R Core Team, 2020). Normal distribution and homogeneity of variances were tested by the Shapiro–Wilk test and Brown–Forsythe test, respectively. Assumptions were met and ANOVA was performed followed by Tukey’s mean discrimination analysis, using the R-Project: ‘agricolae: Statistical Procedures for Agricultural Research’ package. Pearson correlation coefficients between different photosynthetic measures were calculated using accession mean values (R; ‘corrplot’ and ‘Hmisc’).
Results
Characterizing photosynthesis in flag leaves
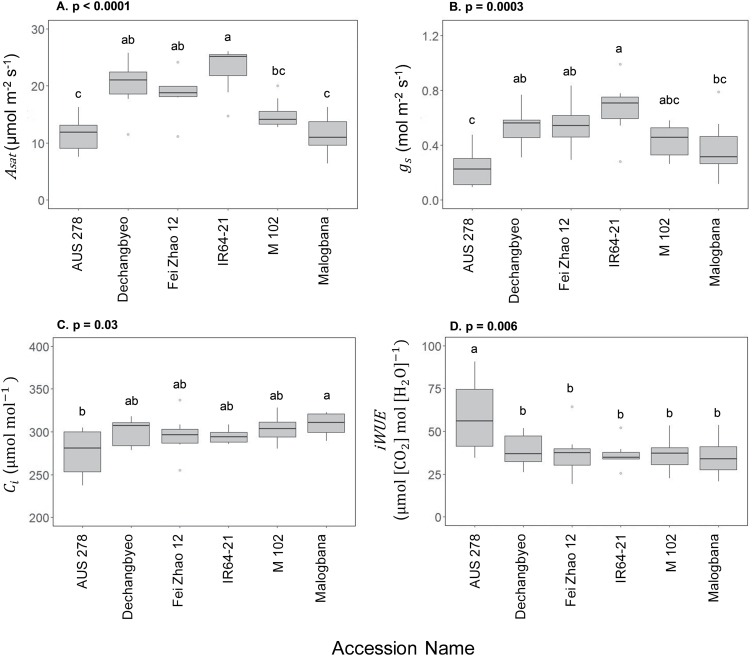
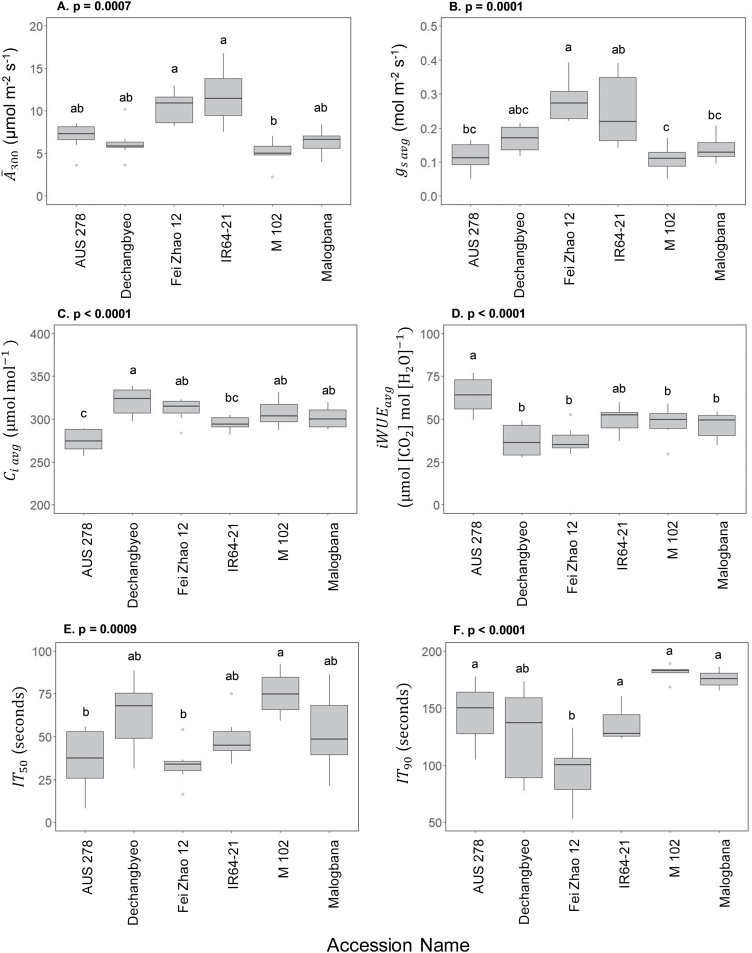
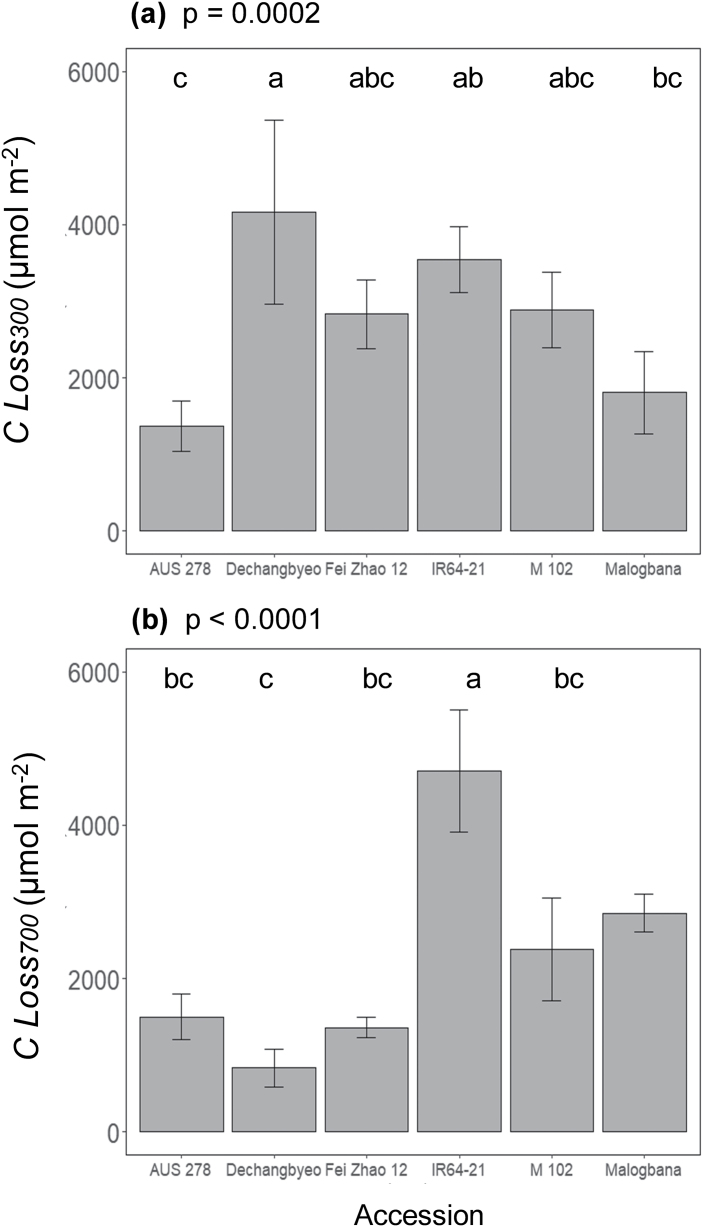
Significant variation was found across accessions for photosynthetic traits in both non-steady-state and steady-state conditions (Figs 1, 2). Significant differences were seen in steady-state parameters such as CO2 uptake in saturating light (Asat, P<0.0001), stomatal conductance (gs, P=0.0003), intercellular CO2 concentration (Ci, P=0.03), and intrinsic water use efficiency (iWUE, P=0.006) (Fig. 2). Between the highest and lowest performing accessions, during induction there was a 152% difference for average CO2 assimilation (Ā300) (M 102, 4.1 µmol m–2 s–1 versus IR64-21, 10.4 µmol m–2 s–1), 77% difference for average iWUE (iWUEavg) (Fei Zhao 12, 36.2 µmol CO2 mol H2O–1; AUS 278, 64.1 µmol CO2 mol H2O–1), and a 185% difference for the time to 50% induction (IT50) (Fei Zhao 12, 34.3 s; M 102, 98.2 s) (Fig. 3). Significant differences were also found among accessions for C Loss300 and C Loss700 (Fig. 4).
Fig. 1.
The response of flag leaves from six rice accessions during photosynthetic induction. (A) Net leaf CO2 assimilation (A), (B) stomatal conductance (gs), (C) intercellular CO2 concentration (Ci), and (D) intrinsic water use efficiency (iWUE=A/gs) with time (t) of induction upon change at 0 s from low light to high light (50 µmol m–2 s–1 to 1700 µmol m–2 s–1). The measurement was taken at an ambient [CO2] of 400 µmol mol–1. Two accessions, AUS 278 (red) and IR64-21 (black), were selected for further study at varied [CO2]. The other four accessions are Dechangbyeo, Fei Zhao 12, M 102, and Malogbana. Each point is the mean ±SE) of eight plants (n=8).
Fig. 2.
Mean and variation for flag leaf steady-state photosynthetic performance in six rice accessions. (A) Leaf CO2 assimilation (A), (B) stomatal conductance (gs), (C) intrinsic water use efficiency (iWUE=A/gs), and (D) intercellular CO2 concentration (Ci). Accessions are ordered by median performance. Letters are indicative of a significant difference between accessions.
Fig. 3.
Mean and variation for flag leaf non-steady-state photosynthetic performance in six rice accessions. (A) CO2 assimilation during the first 300 s of induction (Ā300), (B) average stomatal conductance during the first 300 s of induction (gs avg), (C) average intrinsic water use efficiency (iWUEavg=Ā300/gs avg), (D) average intercellular CO2 concentration (Ci avg), (E and F) time at which A reached 50% and 90% of A300 (IT50 and IT90, respectively). Accessions are ordered by median performance. Letters are indicative of a significant difference between accessions.
Fig. 4.
The integra1 of CO2 uptake forgone due to the lower than steady-state rates through the first (A) 300 s and (B) 700 s of induction compared with steady state (C Loss300 and C Loss700, respectively).
Understanding biochemical limitations to photosynthetic induction in flag leaves
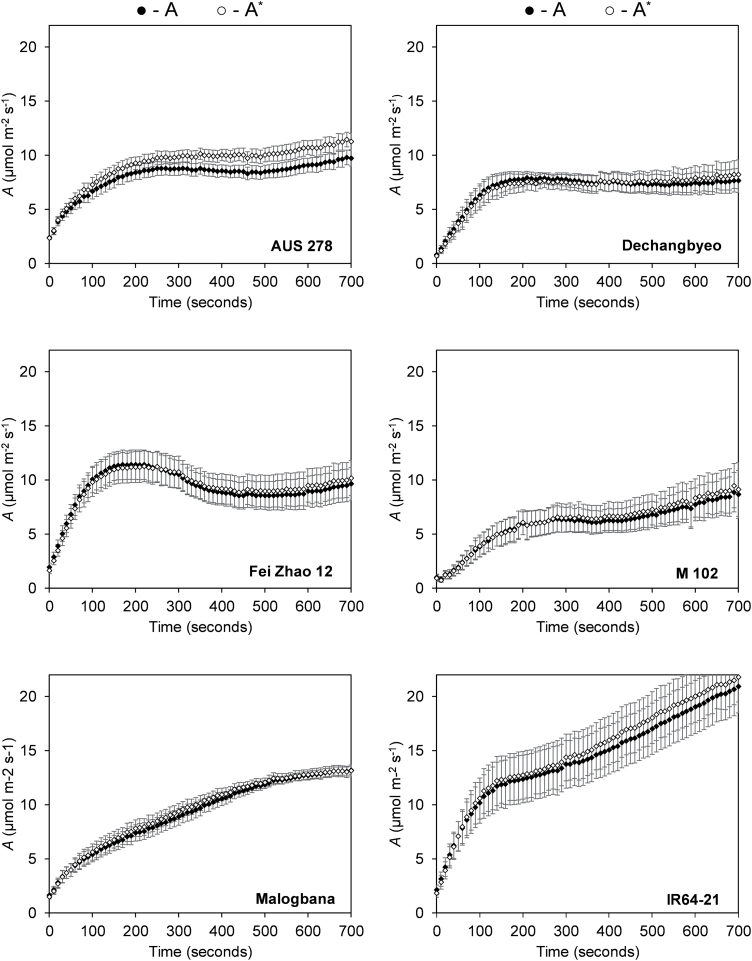
Photosynthetic induction in flag leaves was limited primarily by biochemistry, rather than stomata, during the first 300 s of induction (Fig. 5). The rate of photosynthesis corrected to remove stomatal limitation (A*) did not differ significantly from the uncorrected A values, suggesting that biochemical limitation dominated for these accessions (Fig. 5). This was also evident by the fact that Ci was higher than at steady state through most of the induction (Fig. 1C). A* showed only small differences from the corresponding A (~3–4%), except for AUS 278 which had an average difference of ~13% between A and A* (Fig. 5).
Fig. 5.
The response of uncorrected leaf CO2 assimilation (A; filled circles) and the response of leaf CO2 assimilation corrected for stomatal limitation (A*; open circles) over time in flag leaves of six rice accessions. The first line at 100 s indicates the mean time for the activation of Rubisco (τ) per accession. Each point represents the mean of at least six plants ±SE (n=6–8).
Photosynthetic induction was measured at six different [CO2] values in two selected accessions, AUS 278 and IR64-21. As expected, as [CO2] increased, A increased in both accessions (Supplementary Fig. S3). Also as expected, stomatal conductance decreased with increased [CO2] (Supplementary Fig. S3). As time after the beginning of induction increased, the amount of CO2 assimilated increased as well (Fig. 6). This response to increasing CO2 assimilation over time was seen in both selected accessions (Fig. 6).
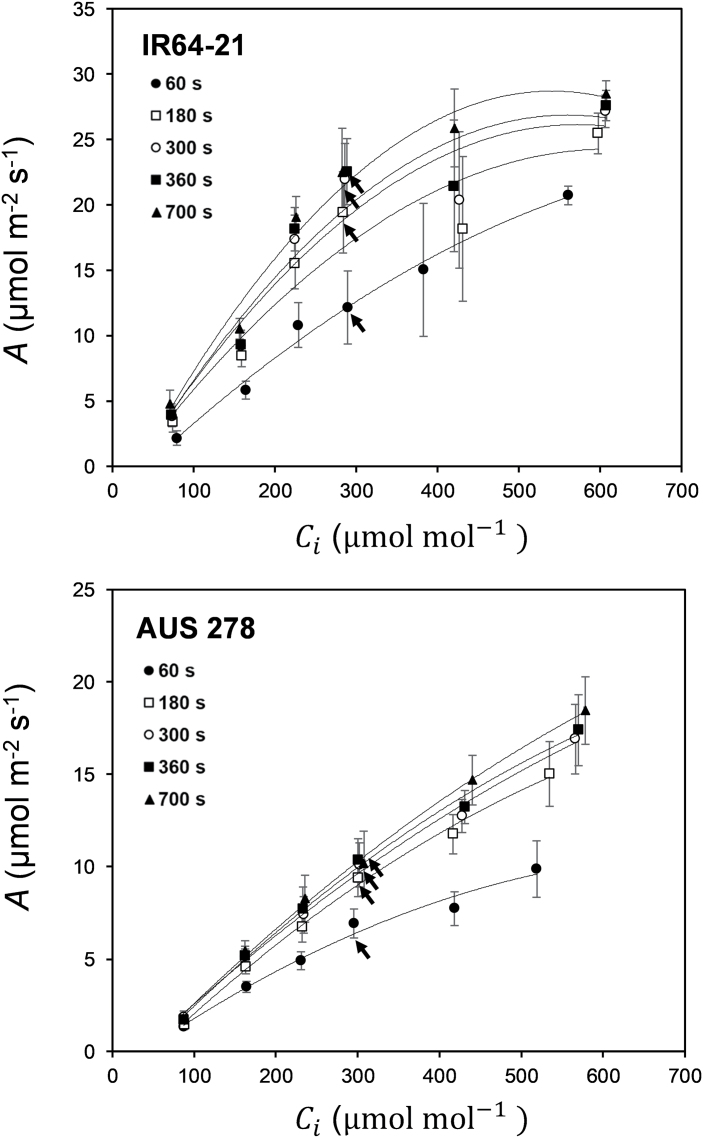
Fig. 6.
The responses of leaf CO2 assimilation (A) to intercellular [CO2] (Ci) at different points in time after the beginning of photosynthetic induction for IR64-21 and AUS 278. Times after induction were: 60 s (filled circles), 180 s (open squares), 300 s (open circles), 360 s (filled squares), and 700 s (filled triangles) from the start of induction. The operating point of each curve at 400 µmol mol–1 atmospheric [CO2] (Ca) is indicated with a black arrow. Each point is the mean (±SE) of four plants of each rice accession.
Utilizing these data, the operating point at an ambient [CO2] (Ca) of 400 µmol mol–1 was calculated for each curve. The operating point fell on the initial slope of the A/Ci curve, indicating limitation by Rubisco for both accessions at all time points (Fig. 6). Photosynthesis was corrected for stomatal limitation at the different [CO2] (Supplementary Figs S4, S5) to calculate the rate constant for Rubisco activation, τ, and forgone assimilation (F). The rate constant for Rubisco activation and τ was not significantly influenced by [CO2] (Supplementary Figs S6, S7) while C Loss300 increased significantly with [CO2] (Supplementary Fig. S7).
Finally, the photosynthetic response curves to PPFD, measured at low [CO2] (≤300 µmol mol–1), were used to infer the response of Vc,max to a PPFD in vivo. These results indicated a significant difference between AUS 278 and IR64-21, with AUS 278 being more strongly limited by the rate of carboxylation (Supplementary Fig. S8). This contrasts with induction at ambient [CO2], where AUS 278 and IR64-21 did not vary significantly in photosynthetic induction traits Ā300, gs avg, Ci avg, iWUEavg, IT50, and IT90 (Fig. 3).
Comparing steady- and non-steady-state photosynthetic performance
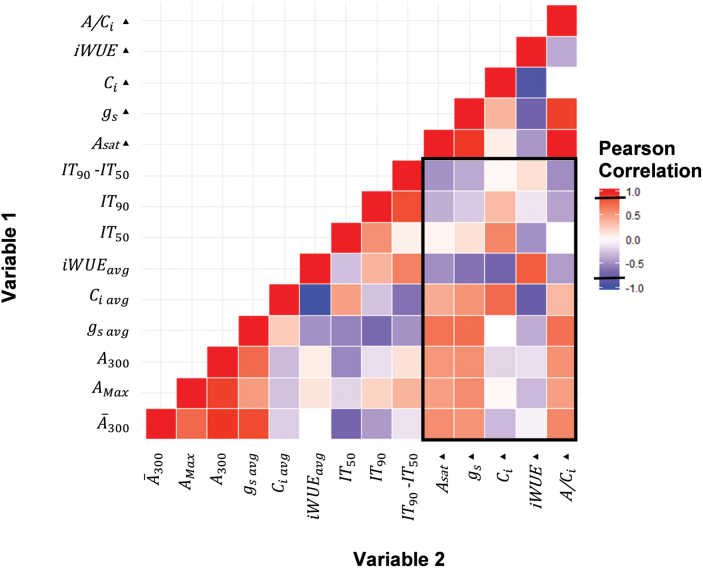
The photosynthetic performance of accessions in non-steady-state and steady-state conditions was compared using Pearson’s correlation coefficient. Significant (P<0.05) correlations were found between the different photosynthetic traits measured in steady-state and between the different traits measured in non-steady-state conditions (Fig. 7). However, there were no significant correlations between traits measured at steady state and their corresponding traits measured at non-steady state (Fig. 7). Additionally, as was previously found in vegetative-phase leaves, there was a significant correlation (P<0.05) between Ā300 and time at which A reached 50% induction (IT50) (Fig. 7).
Fig. 7.
Pearson correlation (R) of all measured dynamic and steady-state (filled tiangles) photosynthetic traits measured in rice flag leaves. Negative correlations are in blue, positive correlations are in red. Traits at steady-state are: intrinsic water-use efficiency (iWUE=A/gs), transpiration (E), intercellular CO2 concentration (Ci), stomatal conductance (gs), and net CO2 assimilation in saturating light (Asat). Traits at non-steady state over the first 300 s of induction are: the time at which A reached 50% and 90% of A300 (IT50 and IT90, respectively), average Ci during first 300 s of induction (Ci avg), average intrinsic water use efficiency (iWUEavg=Ā300/gs avg), average gs, the maximum A during induction (AMax), A at the end of this period (A300), and the average A (Ā300). A significant R value is marked by a black line on the scale (0.8).
Comparing photosynthetic induction performance between flag leaves and vegetative-phase leaves
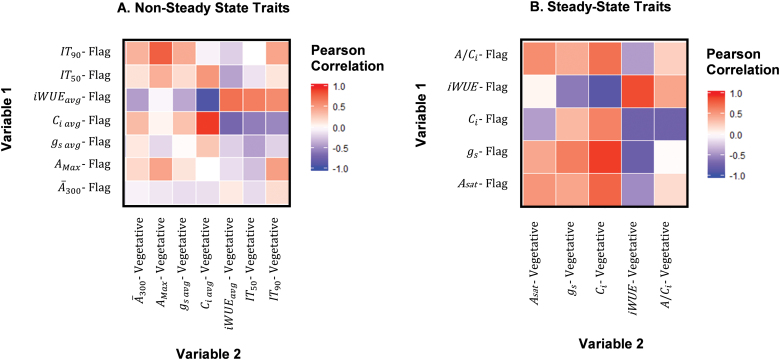
The photosynthetic traits measured here for flag leaves were compared with those made for leaves in the vegetative growth stage of the same accessions in a previous study (Acevedo-Siaca et al., 2020). Significant correlations were found between steady-state Ci in leaves in the vegetative growth stage and iWUE in flag leaves, Ci in leaves in the vegetative growth stage and gs in flag leaves, and iWUE in leaves in the vegetative growth phase and flag leaves (Fig. 8A). In non-steady-state conditions, significant correlations were found between Ci avg in leaves in the vegetative growth stage and both Ci avg and iWUEavg in flag leaves (Fig. 8). However, there was no significant correlation between any measure of CO2 assimilation between flag leaves and leaves during the vegetative growth phase.
Fig. 8.
Pearson correlation analysis between photosynthetic traits measured in flag leaves for the six cultivars measured here with the values obtained on leaves in the vegetative growth stage for the same cultivars in a previous study (Acevedo-Siaca et al. 2020). (A) Non-steady state; (B) steady state. Traits are as defined in Fig. 3.
Discussion
Currently, no study has aimed to characterize the photosynthetic induction response in rice flag leaves. With respect to the objectives as outlined in the Introduction, the following key answers were obtained. (i) There were substantial differences among the six accessions of ~150% between leaf CO2 uptake over the course of induction, with smaller differences in the light-saturated steady state (Figs 2, 3). This suggests significant scope for exploiting germplasm to increase rice photosynthesis in this key leaf for grain filling. (ii) Analysis of the responses of CO2 uptake to intercellular CO2 concentration showed that the in vivo activity of Rubisco (Vc,max) limited photosynthesis throughout induction (Fig. 6), suggesting that breeding or bioengineering increased Rubisco content and activity as a means to increase rice productivity. (iii) When the results obtained here for flag leaves were compared with those for the same accessions in the earlier study of leaves in the vegetative growth stage (Acevedo-Siaca et al., 2020), there was little correspondence between photosynthetic parameters for the two growth stages, with the exception of iWUE (Fig. 8). This suggests that breeding efforts to improve crop photosynthesis would need to address selection at both growth stages, although improved WUE could be selected at the vegetative stage alone, making this more tractable. The following discussion places these new findings in the context of prior studies and potential for increasing rice photosynthetic capacity, efficiency of resource use, and productivity.
Significant variation for photosynthetic traits in rice flag leaves
Previously, it was suggested that natural variation for photosynthesis was more prevalent in the flag leaves than for leaves in the vegetative growth stage of cereal crops, a finding confirmed here for rice (Dunstone et al., 1973; Bansal et al., 1993). Previous studies have focused on either steady-state or more recently non-steady-state photosynthesis, and demonstrated diversity within crop germplams (Flood et al., 2011; Gu et al., 2012; Driever et al., 2014; Soleh et al., 2016, 2017; Acevedo-Siaca et al., 2020; De Souza et al., 2020; Taniyoshi et al., 2020). Here, we found that significant natural variation exists for photosynthetic traits in both steady- and non-steady-state conditions (A 6.4–25.9 µmol m–2 s–1; Ā300 2.3–16.7 µmol m–2 s–1) in the flag leaf. Indeed, 43, 12, and 68% more variation was found here between flag leaves when compared with leaves in the vegetative growth stage of the same accessions (Acevedo-Siaca et al., 2020) for Ā300, iWUEavg, and IT50, respectively. This is significant given the key role of the flag leaf.
Further evidence for the lack of correlation between steady- and non-steady-state measurements
Consistent with studies of soybean, cowpea, and leaves in the vegetative growth stage of rice, no significant correlations were found between parameters of photosynthetic CO2 uptake in steady- and non-steady-state light in rice flag leaves (Soleh et al., 2016; Acevedo-Siaca et al., 2020; De Souza et al., 2020). These studies, together with the present study, provide compelling evidence for a reconsideration of when during crop development we measure and how we measure photosynthesis when considering crop improvement. In particular, clear evidence that steady-state measurements do not indicate photosynthetic efficiency under the non-steady-state fluctuating light conditions that can be dominant in the field should be noted. However, the greater between-accession variability of non-steady-state photosynthesis highlights a greater opportunity for increasing net crop CO2 uptake that could meet the apparent strong source limitation of modern high-yielding cultivars (Hasegawa et al., 2013; Zhu et al., 2015; Sakai et al., 2019).
It is possible that a balance for the distribution of resources between photosynthetic proteins underlies the differences between steady-state and non-steady-state photosynthesis. Previous studies have suggested a trade-off between maximum rates of photosynthesis in steady-state saturating light versus the speed of induction due to limited amounts of nitrogen invested and divided between Rubisco and Rubisco activase content (Woodrow and Mott, 1989; Mott and Woodrow, 2000). It is hypothesized that plants grown in fluctuating light environments that do not experience steady-state conditions frequently would benefit from having higher Rubisco activase content to be able to respond more quickly to changes in light (Mott and Woodrow, 2000; Yamori et al., 2012; Carmo-Silva and Salvucci, 2013; Kaiser et al., 2016). However, leaves that experience fewer sunflecks and have more exposure to direct sunlight would benefit from investing a higher proportion of resources in Rubisco to sustain higher photosynthetic rates at steady state (Mott and Woodrow, 2000). Such a trade-off is supported by the finding that antisense down-regulation of Rubisco activase increased Rubisco content in rice leaves (Jin et al., 2006). This trade-off between Rubisco and Rubisco activase content could help to partially explain the lack of correlation between steady- and non-steady-state photosynthesis, as an increase in the protein that helps the leaf excel in induction acts to the detriment of the protein needed at steady state. The trade-off between steady- and non-steady-state photosynthesis was shown clearly in IR64-21, which had the highest steady-state Asat yet was among the slowest to reach 90% of its steady-state level (IT90 A) during induction (Figs 2, 3).
Photosynthetic induction is primarily limited by biochemistry in flag leaves
At ambient [CO2], rice flag leaves are predominantly limited by biochemistry, specifically the maximum activity of Rubisco (Figs 5, 6). Differences between A and A* at ambient [CO2] were generally small, indicating little limitation by stomata (Fig. 5). This is likely to be due to the shape and small size of rice stomata allowing fast responses (McAusland et al., 2016), but also the evolutionary history of rice, which was domesticated from emergent aquatic progenitors and then bred in paddy conditions where water would not be limiting to the plant (Nay-Htoon et al., 2018).
Calculation of the operating point at ambient [CO2] (Ca=400 µmol mol–1) in AUS 278 and IR64-21 showed that photosynthesis during induction was predominantly limited by Rubisco, and not affected by either the capacity for regeneration of RuBP or triose phosphate utilization, throughout the induction and into steady state. The operating point is on the initial slope of the A/Ci curve, throughout (Fig. 6). This parallels the previous findings for rice leaves in the vegetative growth stage (Acevedo-Siaca et al., 2020) and suggests that the predominant limitation to photosynthetic CO2 assimilation at steady state and non-steady state throughout the life cycle of rice is consistently due to the in vivo capacity and, presumably, amount of Rubisco. This is also consistent with the recent observation that transgenic up-regulation of Rubisco in rice significantly increases paddy yield (Long, 2020; Yoon et al., 2020). One caveat is that this analysis is based on Ci and not the CO2 concentration at Rubisco (Cc). Ease of movement of CO2 from the intercellular space to Rubisco is governed by mesophyll conductance (gm), which was not measured here. So, it is possible that activation of gm, as well as Rubisco, could be a limiting factor. However, prior work with other species has suggested that activation of gm is likely to be faster than activation of Rubisco (Deans et al., 2019).
Photosynthetic performance shows little correlation between flag and vegetative-phase leaves
The only significant correlations found between flag and leaves in the vegetative growth stage were for Ci and iWUE (Fig. 8). These results suggest that iWUE is not be affected by rice developmental stage and could be consistent throughout the lifetime of these rice plants. Water availability is the biggest limitation to agricultural production worldwide and is expected to pose an even greater limitation with climate change and population growth (Wassmann et al., 2009; Ort and Long, 2014; Oladosu et al., 2019). If iWUE is consistent throughout the life cycle of rice, it could allow breeders to screen for high iWUE early in development, saving time and resources in selecting germplasm in breeding more water use-efficient plants.
Otherwise, no significant correlations were found between the photosynthetic performance of flag leaves and leaves in the vegetative growth stage in both steady- and non-steady-state conditions (Fig. 7). For example, elite cultivar IR64-21 was outperformed by other accessions in measurements of photosynthesis in leaves in the vegetative growth stage during steady- and non-steady-state conditions (Acevedo-Siaca et al., 2020). However, IR64-21 flag leaves had the highest photosynthetic rates during photosynthetic induction and at steady state, significantly outperforming the other accessions (Figs 1, 2; Supplementary Fig. S3). This suggests that increased flag leaf CO2 uptake may have been inadvertently improved through conventional breeding selection. This corresponds with other evidence that flag leaf photosynthesis has been improved unintentionally through breeding for higher yield potential. Newer and higher yielding rice varieties have flag leaves with higher photosynthetic rates per unit area than older varieties (Ishii, 1993). Additionally, flag leaves in Oryza sativa were found to maintain higher photosynthetic rates for longer relative to wild Oryza species (Ishii, 1993), which is curiously in contrast to wheat (Dunstone et al. 1973). These studies suggest that deliberate breeding for increased flag leaf photosynthesis might be a fertile avenue for a further increase in rice yield potential.
However, while an increased emphasis on flag leaf photosynthesis can lead to higher yields (Fabre et al., 2016), there should still be a focus on improving photosynthetic efficiency throughout the life cycle. Photosynthesis in leaves during the vegetative stage is important in establishing the plant and developing a robust root system and tillers capable of becoming reproductive. Improved photosynthesis in leaves in the vegetative growth stage results in increases in non-storage carbohydrates in leaves and stems, which can subsequently be remobilized, for grain filling. The expected ideotype would therefore be an accession which shows high capacity and efficiency during both growth phases.
Non-steady-state photosynthesis—a practical target for breeding?
Non-steady-state photosynthesis has received little attention in breeding, largely because the gas exchange methods required to effectively phenotype traits are not practical for large-scale testing. However, it was recently shown in wheat that large-scale screening of non-steady-state photosynthesis could be achieved effectively with excised leaves using modulated chlorophyll fluorescence imaging (McAusland et al., 2019). This high-throughput technique could make selection and breeding for improved efficiency under non-steady-state conditions practical, and probably more effective than selecting for improvement under steady-state conditions. Additionally, these methods could allow for the improvement of iWUE under non-steady-state conditions. As noted above, current methods of breeding paddy rice for yield may have lowered WUE. This study has revealed considerable variation—even within the limited germplasm examined—and a correlation in WUE between vegetative and reproductive growth. This finding suggests that selection of improved WUE could be achieved by screening during early growth. Furthermore, the development of integrated thermal and modulated fluorescence imaging of instantaneous WUE would now allow high-throughput phenotyping of this trait (McAusland et al., 2013). Accelerated breeding of rice lines requiring less water would help address the rising pressures on water supplies in many paddy rice-growing regions (Schyns et al., 2019). These benefits that go beyond increased yield are imperative for creating more sustainable agricultural systems that utilize this planet’s limited resources with more discretion, especially in the face of global climate change and a growing human population.
Supplementary data
The following supplementary data are available at JXB online.
Fig. S1. Growing conditions at the IRRI used in the study.
Fig. S2. Corrected photosynthesis for time to activation of photosynthesis (τ).
Fig. S3. Induction of leaf CO2 uptake (A) and stomatal conductance (gs) at six [CO2] in IR64-21 and AUS 278.
Fig. S4. Leaf CO2 uptake (A) and leaf CO2 uptake corrected for stomatal limitation (A*) in AUS 278.
Fig. S5. Leaf CO2 uptake (A) and leaf CO2 uptake corrected for stomatal limitation (A*) in IR64-21.
Fig. S6. Corrected photosynthesis for time to activation of photosynthesis (τ) in AUS 278 and IR64-21.
Fig. S7. Rate constant of Rubisco activation (1/τ), time to activation of photosynthesis (τ), and forgone assimilation in six [CO2] in AUS 278 and IR64-21.
Fig. S8. Vc,max during photosynthetic induction in AUS 278 and IR64-21.
Table S1. Description of accessions used in this study.
Acknowledgements
We thank Jacqueline Dionora, Irma Canicosa, and the entire C4 Rice Center for their support, as well as the IRRI Germplasm Resource Center (GRC) for access to the germplasm used in this project. This work was supported by the project Realizing Increased Photosynthetic Efficiency (RIPE), that is funded by the Bill & Melinda Gates Foundation, Foundation for Food and Agriculture Research (FFAR), and the UK Department Foreign, Commonwealth and Development Office under grant number OPP1172157. LAS was supported by U.S. Borlaug Fellows in Global Food Security Fellowship and funding through the Office of International Programs (OIP) in the College of Agricultural, Consumer, and Environmental Sciences (ACES) at the University of Illinois at Urbana-Champaign.
Author contributions
LGA and SPL planned the research. SPL and WPQ supervised the project. LGA and RC conducted the experimental work, LGA analyzed the data. LGA and SPL wrote the manuscript with the input of all the other authors.
Data availability
The data that support the findings of this study are openly available in the University of Illinois Data Bank at https://doi.org/10.13012/B2IDB-3596430_V1 (Acevedo-Siaca, Liana; Long, Stephen (2020): Photosynthetic induction of rice flag leaves. University of Illinois at Urbana-Champaign.)
References
- Abou-Khalifa ABA, Misra N, El-Azeem A, Salem KM. 2008. Effect of leaf cutting on physiological traits and yield of two rice cultivars. African Journal of Plant Science 2, 147–150. [Google Scholar]
- Acevedo-Siaca LG, Coe R, Wang Y, Kromdijk J, Quick WP, Long SP. 2020. Variation in photosynthetic induction between rice accessions and its potential for improving productivity. New Phytologist 227, 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi S, Yoshikawa K, Yamanouchi U, Tanabata T, Sun J, Ookawa T, Yamamoto T, Sage RF, Hirasawa T, Yonemaru J. 2017. Fine mapping of Carbon Assimilation Rate 8, a quantitative trait locus for flag leaf nitrogen content, stomatal conductance and photosynthesis in rice. Frontiers in Plant Science 8, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth EA, Long SP. 2020. 30 years of free-air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Global Change Biology doi: 10.1111/gcb.15375. [DOI] [PubMed] [Google Scholar]
- Bansal KC, Uprety DC, Abrol YP. 1993. Genetic variation in photosynthetic characteristics in wheat: causes and consequences. In: Abrol YP, Mohanty P, Govindjee, eds. Photosynthesis: photoreactions to plant productivity. Dordrecht: Springer Science + Business Media, 527–547. [Google Scholar]
- Carmo-Silva E, Salvucci ME. 2013. The regulatory properties of rubisco activase differ among species and affect photosynthetic induction during light transitions. Plant Physiology 161, 1645–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Zhang Y, Li X, Jiao D. 2002. Photosynthetic characteristics and assimilate distribution in super hybrid rice Liangyoupeijiu at late growth stage. Acta Agronomica Sinica 28, 777–782. [In Chinese, with an English abstract]. [Google Scholar]
- Deans RM, Farquhar GD, Busch FA. 2019. Estimating stomatal and biochemical limitations during photosynthetic induction. Plant, Cell & Environment 42, 3227–3240. [DOI] [PubMed] [Google Scholar]
- De Souza AP, Wang Y, Orr DJ, Carmo-Silva E, Long SP. 2020. Photosynthesis across African cassava germplasm is limited by Rubisco and mesophyll conductance at steady state, but by stomatal conductance in fluctuating light. New Phytologist 225, 2498–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever SM, Lawson T, Andralojc PJ, Raines CA, Parry MA. 2014. Natural variation in photosynthetic capacity, growth, and yield in 64 field-grown wheat genotypes. Journal of Experimental Botany 65, 4959–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstone RL, Gifford RM, Evans LT. 1973. Photosynthetic characteristics of modem and primitive wheat species in relation to ontogeny and adaptation to light. Australian Journal of Biological Sciences 26, 295–307. [Google Scholar]
- Fabre D, Adriani DE, Dingkuhn M, Ishimaru T, Punzalan B, Lafarge T, Clément-Vidal A, Luquet D. 2016. The qTSN4 effect on flag leaf size, photosynthesis and panicle size, benefits to plant grain production in rice, depending on light availability. Frontiers in Plant Science 7, 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GE, von Caemmerer S, Berry JA. 1981. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90. [DOI] [PubMed] [Google Scholar]
- Flood PJ, Harbinson J, Aarts MG. 2011. Natural genetic variation in plant photosynthesis. Trends in Plant Science 16, 327–335. [DOI] [PubMed] [Google Scholar]
- Gu J, Yin X, Stomph TJ, Wang H, Struik PC. 2012. Physiological basis of genetic variation in leaf photosynthesis among rice (Oryza sativa L.) introgression lines under drought and well-watered conditions. Journal of Experimental Botany 63, 5137–5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Sakai H, Tokida T, et al. 2013. Rice cultivar responses to elevated CO2 at two free-air CO2 enrichment (FACE) sites in Japan. Functional Plant Biology 40, 148–159. [DOI] [PubMed] [Google Scholar]
- Ishii R 1993. Leaf photosynthesis in rice in relation to grain yield. In: Abrol YP, Mohanty P, Govindjee, eds. Photosynthesis: photoreactions to plant productivity. Dordrecht: Springer Science + Business Media, 561–570. [Google Scholar]
- Jin SH, Hong J, Li XQ, Jiang DA. 2006. Antisense inhibition of Rubisco activase increases Rubisco content and alters the proportion of Rubisco activase in stroma and thylakoids in chloroplasts of rice leaves. Annals of Botany 97, 739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser E, Morales A, Harbinson J, Heuvelink E, Prinzenberg AE, Marcelis LFM. 2016. Metabolic and diffusional limitations of photosynthesis in fluctuating irradiance in Arabidopsis thaliana. Scientific Reports 6, 31252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobata T, Shinonaga M, Yoshida H, Tomisaka K, Akai K. 2015. Stay-green trait assessment using the leaf incubation method to examine the maintenance of assimilation rates under high temperature conditions during the grain-filling period in rice. Plant Production Science 18, 254–266. [Google Scholar]
- Lee S, Jeong H, Lee S, et al. 2017. Molecular bases for differential aging programs between flag and second leaves during grain-filling in rice. Scientific Reports 7, 9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Ye G, Zeng D. 2017. Genetic dissection of leaf senescence in rice. International Journal of Molecular Breeding 18, 2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Pinson SRM, Stansel JW, Paterson AH. 1998. Genetic dissection of the source–sink relationship affecting fecundity and yield in rice (Oryza sativa L.). Molecular Breeding 4, 419–426. [Google Scholar]
- Li Z, Wang F, Lin W, Zhao Q, Liu J, Cheng F. 2017. Carbon reserve and remobilization in leaf sheaths during the grain-filling stage in response to leaf early senescence. Acta Physiologiae Plantarum 38, 10. [Google Scholar]
- Lin W, Guo X, Pan X, Li Z. 2018. Chlorophyll composition, chlorophyll fluorescence, and grain yield change in esl mutant rice. International Journal of Molecular Sciences 19, 2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP 2020. Photosynthesis engineered to increase rice yield. Nature Food 1, 105–105. [DOI] [PubMed] [Google Scholar]
- Mantilla-Perez MB, Salas Fernandez MG. 2017. Differential manipulation of leaf angle throughout the canopy: current status and prospects. Journal of Experimental Botany 68, 5699–5717. [DOI] [PubMed] [Google Scholar]
- McAusland L, Atkinson JA, Lawson T, Murchie EH. 2019. High throughput procedure utilising chlorophyll fluorescence imaging to phenotype dynamic photosynthesis and photoprotection in leaves under controlled gaseous conditions. Plant Methods 15, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAusland L, Davey PA, Kanwal N, Baker NR, Lawson T. 2013. A novel system for spatial and temporal imaging of intrinsic plant water use efficiency. Journal of Experimental Botany 64, 4993–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAusland L, Vialet-Chabrand S, Davey PA, Baker NR, Brendel O, Lawson T. 2016. Effects of kinetics of light-induced stomatal responses on photosynthesis and water-use efficiency. New Phytologist 211, 1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott KA, Woodrow IE. 2000. Modelling the role of Rubisco activase in limiting non-steady-state photosynthesis. Journal of Experimental Botany 51, 399–406. [DOI] [PubMed] [Google Scholar]
- Nakano H, Makino A, Mae T. 1995. Effects of panicle removal on the photosynthetic characteristics of the flag leaf of rice plants during the ripening stage. Plant & Cell Physiology 36, 653–659. [Google Scholar]
- Nay-Htoon B, Xue W, Lindner S, Cuntz M, Ko J, Tenhunen J, Werner C, Dubbert M. 2018. Quantifying differences in water and carbon cycling between paddy and rainfed rice (Oryza sativa L.) by flux partitioning. PLoS One 13, e0195238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladosu Y, Rafii MY, Samuel C, Fatai A, Magaji U, Kareem I, Kamarudin ZS, Muhammad I, Kolapo K. 2019. Drought resistance in rice from conventional to molecular breeding: a review. International Journal of Molecular Sciences 20, 3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort DR, Long SP. 2014. Limits on yields in the corn belt. Science 344, 484–485. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Oszvald M, Jesus C, Rajulu C, Griffiths CA. 2017. Increasing crop yield and resilience with trehalose 6-phosphate: targeting a feast–famine mechanism in cereals for better source–sink optimization. Journal of Experimental Botany 68, 4455–4462. [DOI] [PubMed] [Google Scholar]
- Pearcy RW, Chazdon RL, Gross LJ, Mott KA. 1994. Photosynthetic utilization of sunflecks: a temporally patchy resource on a timescale of seconds to minutes. In: Caldwell MM, Pearcy RW, eds. Exploration of environmental heterogeneity by plants. San Diego: Academic Press, 175–208. [Google Scholar]
- R Core Team 2020. R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Sakai H, Tokida T, Usui Y, Nakamura H, Hasegawa T. 2019. Yield responses to elevated CO2 concentration among Japanese rice cultivars released since 1882. Plant Production Science 22, 352–366. [Google Scholar]
- Schyns JF, Hoekstra AY, Booij MJ, Hogeboom RJ, Mekonnen MM. 2019. Limits to the world’s green water resources for food, feed, fiber, timber, and bioenergy. Proceedings of the National Academy of Sciences, USA 116, 4893–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicher, R 1993. Assimilate partitioning within leaves of small grain cereals. In: Abrol YP, Mohanty P, Govindjee, eds. Photosynthesis: photoreactions to plant productivity. Dordrecht: Springer Science + Business Media, 351–360. [Google Scholar]
- Slattery RA, Walker BJ, Weber APM, Ort DR. 2018. The impacts of fluctuating light on crop performance. Plant Physiology 176, 990–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleh MA, Tanaka Y, Kim SY, Huber SC, Sakoda K, Shiraiwa T. 2017. Identification of large variation in the photosynthetic induction response among 37 soybean [Glycine max (L.) Merr.] genotypes that is not correlated with steady-state photosynthetic capacity. Photosynthesis Research 131, 305–315. [DOI] [PubMed] [Google Scholar]
- Soleh MA, Tanaka Y, Nomoto Y, Iwahashi Y, Nakashima K, Fukuda Y, Long SP, Shiraiwa T. 2016. Factors underlying genotypic differences in the induction of photosynthesis in soybean [Glycine max (L.) Merr.]. Plant, Cell, & Environment 39, 685–693. [DOI] [PubMed] [Google Scholar]
- Taniyoshi K, Tanaka Y, Shiraiwa T. 2020. Genetic variation in the photosynthetic induction response in rice (Oryza sativa L.). Plant Production Science 23, 513–521. [Google Scholar]
- Taylor SH, Long SP. 2017. Slow induction of photosynthesis on shade to sun transitions in wheat may cost at least 21% of productivity. Philosophical Transactions of the Royal Society B: Biological Sciences 372, 20160543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Burgess SJ, de Becker EM, Long SP. 2020. Photosynthesis in the fleeting shadows: an overlooked opportunity for increasing crop productivity? The Plant Journal 101, 874–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassmann R, Jagadish SVK, Sumfleth K, Pathak H, Howell G, Ismail A, Serraj R, Redona E, Singh RK, Heuer S. 2009. Regional vulnerability of climate change impacts on asian rice production and scope for adaptation. Advances in Agronomy 102, 91–133. [Google Scholar]
- Woodrow IE, Mott KA. 1989. Rate limitation of non-steady-state photosynthesis by ribulose-1,5-bisphosphate carboxylase in spinach. Functional Plant Biology 16, 487–500. [Google Scholar]
- Yamori W, Masumoto C, Fukayama H, Makino A. 2012. Rubisco activase is a key regulator of non-steady-state photosynthesis at any leaf temperature and, to a lesser extent, of steady-state photosynthesis at high temperature. The Plant Journal 71, 871–880. [DOI] [PubMed] [Google Scholar]
- Yoon DK, Ishiyama K, Suganami M, et al. 2020. Transgenic rice overproducing Rubisco exhibits high yields with high nitrogen use efficiency in a paddy field. Nature Food 1, 134–139. [DOI] [PubMed] [Google Scholar]
- Yoshida, S 1981. Physiological analysis of rice yield. In: Fundamentals of rice crop science. Makati City, Philippines: International Rice Research Institute, 231–251. [Google Scholar]
- Zhang B, Ye W, Re D, et al. 2015. Genetic analysis of flag leaf size and candidate genes determination of a major QTL for flag leaf width in rice. Rice 8, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Xu X, Wang D, Zhu J, Liu G. 2015. An indica rice genotype showed a similar yield enhancement to that of hybrid rice under free air carbon dioxide enrichment. Scientific Reports 5, 12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available in the University of Illinois Data Bank at https://doi.org/10.13012/B2IDB-3596430_V1 (Acevedo-Siaca, Liana; Long, Stephen (2020): Photosynthetic induction of rice flag leaves. University of Illinois at Urbana-Champaign.)