Abstract
Serial femtosecond crystallography (SFX) is a powerful technique that utilizes X-ray free electron lasers (XFEL) to determine structures of biomolecular complexes. Specifically, it benefits the study of atomic resolution structures of large membrane protein complexes and time-resolved reactions with crystallography. One major drawback of SFX studies with XFELs is the consumption of large amounts of protein crystal sample to collect a complete X-ray diffraction data set for high-resolution crystal structures. This increases the time and resources required for sample preparation and experimentation. A major reason why such large amounts of sample are required, is the intrinsic pulsed nature of all current X-ray sources. Any crystal sample that is delivered in the path of the X-ray beam during its ‘off-time’ is wasted. To address this large sample consumption issue, we developed a 3D-printed microfluidic system with integrated metal electrodes for water-in-oil droplet generation to dynamically create and manipulate aqueous droplets. We demonstrate on-demand droplet generation using DC potentials and the ability to tune the frequency of droplet generation through the application of AC potentials. More importantly, to assist with the synchronization of droplets and XFEL pulses, we show that the device can induce a phase shift in the base droplet generation frequency. This novel approach to droplet generation has the potential to reduce sample waste by more than 95% for SFX experiments with XFELs and can operate under low and high-pressure liquid injection systems.
Keywords: microfluidic, droplet, oil, aqueous, crystallography, electrowetting, 3D printing
Graphical Abstract
INTRODUCTION
Serial femtosecond crystallography (SFX) is an emerging crystallography technique and among the most powerful tools to determine structures of proteins. SFX allows for an atomic level analysis of challenging protein structures, time-resolved diffraction at room temperature, and the study of sub-micrometer and micrometer sized protein crystals, which are considered too small for conventional crystallography.1-5 In most cases, SFX is performed by employing a high intensity femtosecond X-ray free-electron laser (XFEL). The XFEL pulses irradiate ideally one protein crystal at a time and the resulting diffraction pattern is acquired before the protein crystal is destroyed.4-6 Since many thousands of such diffraction patterns are required to construct an electron density map of a protein structure, SFX experiments with XFELs commonly use a continuous sample delivery method, which is realized with a gas dynamic virtual nozzle (GDVN).2,7 Using this delivery method, protein crystals are jetted into the path of a pulsed X-ray beam, either in a vacuum chamber8 or under atmospheric pressure.9 This continuous sample delivery method coupled with the intrinsic pulsed nature of XFELs is the primary reason why SFX experiments consume a large amount (up to grams) of protein sample. With the current XFEL repetition rates and the flow rates required for a stable liquid jet injection through a GDVN, nearly 95-99% of injected protein crystals are wasted in between laser pulses. This is particularly problematic for proteins that can only be produced in small quantities.9 Any wastage of sample increases costs and experimentation time enormously.
To circumvent the problem of large sample loss due to the inherent pulsed nature of XFELs, several methods for sample delivery have been proposed including reduction of the speed of sample delivery, drop on demand injection, interrupting the jet of a GDVN or breaking the continuous sample delivery into droplets through a segmented flow approach.10 Reduced sample speed approaches rely on slowing down the speed of the jet extruding the crystal slurries. Weierstall et al. developed a viscous lipidic cubic phase (LCP) injector that extrudes sample at low speeds with reduction in sample waste by a factor of 20.11 With other viscous media, such as agarose, the sample waste could be reduced by up to two orders of magnitude.9 However, the LCP injector approach is not suited for all SFX experiments because many protein crystals cannot be grown in or are unstable when mixed into LCP or other viscous media. In addition, large background X-ray scattering is induced because of the large diameter of the LCP jet.8,11,12 Furthermore, to populate an undamaged crystal for each pulse, XFELs that operate at MHz X-ray pulse frequencies require a high velocity jet (>50 m/s).13 One such example is the European XFEL (EuXFEL), which delivers pulse trains that repeat at 10Hz, whereas each train may contain several hundred MHz frequency pulses.14 The microfluidic electrokinetic sample holder (MESH) is another reduced sample consumption technique with continuous sample introduction at low flow rates.15 Drawbacks of the technique include the high voltages required to jet the crystal suspensions and the necessary addition of cryoprotectant which has been addressed with the concentric MESH (CoMESH) injector surrounding the sample jet with the cryoprotectant to prevent sample dehydration and freezing in vacuum.16
An alternative approach consists of droplet injection. For example, Mafuné et al. developed a setup that introduced pulsed liquid droplets containing protein crystals in the path of X-rays17 delivering sample via a piezoelectric element where the droplet release is stimulated by an external trigger.18 This injector has been used at the SPring-8 Angstrom Compact free-electron LAser (SACLA) for SFX studies on lysozyme18 and bacteriorhodopsin.19 Other approaches employed acoustic droplet ejection (ADE). Roessler et al. have generated crystals-containing droplets on demand and have demonstrated the intersection of the majority of generated droplets with the X-ray pulses.20 This approach is however affected by crystal settling, generates fairly large droplets and introduces large background scattering. ADE has also been combined with a conveyer belt system for time-resolved crystallography where droplets are ejected acoustically onto the conveyor belt. This approach is also known as droplet on tape (DOT) and facilitates the time-resolved study of photoinitiated and gas-initiated reactions.21,22 However, the delivery of droplets on a tape is not suitable for time-resolved studies where substrates are in solution.
Segmented sample delivery for XFELs has been proposed on microfluidic platforms previously by Echelmeier et al.23 based on established two phase droplet manipulation for droplet generation, transport, sorting, coalescence and splitting on microfluidic devices.24-26 Conventionally, by introducing an immiscible fluid into another, droplets or a segmented flow can be produced passively for sample delivery.27,28 We recently developed a method based on this segmented-flow approach to generate droplets carrying crystals and conserve sample at SFX experiments.29 In order to conserve substantial sample amounts, the droplet generation and the X-ray pulses at the XFEL must not only be of identical frequency, but also in phase. It is also important to ensure that the frequency of the segmented droplet approach is compatible with current XFEL repetition frequencies ranging from 10 up to 120 Hz. Thus, precise control of the droplet generation frequency and phase is necessary, which can be established with an external stimuli such as electrical, magnetic, centrifugal, optical, thermal, or mechanical approaches.27,28 Among these, electrical stimuli have been demonstrated to be useful for controlling droplet generation by tuning of electrowetting characteristics when a direct current (DC) or an alternating current (AC) potential is applied between sets of electrodes.30-32
In this paper, we present a 3D-printed microfluidic device with integrated metal electrodes to dynamically create and manipulate aqueous droplets. This approach is designed to address the limitations of continuous flow protein crystal sample delivery methods in SFX experiments. Based on the type and duration of the electrical potential applied between a set of electrodes, we were able to generate droplets in various modes. These include i) on-demand droplets where a droplet is generated upon application of an electrical pulse, ii) inducing a phase shift in a stream of continuous droplets by the application of an external electrical trigger signal and iii) tunable frequency modulation, where the frequency of a continuous stream of droplets is changed for the duration of the applied electrical pulse. These three modes may be suitable for the synchronization of the pulsed XFELs with sample delivery in picoliter to nanoliter droplets. We further relate the observed phenomena with physical interface phenomena allowing for the operation in the three triggering modes. The presented microfluidic device can be fully integrated into SFX injection systems and is expected to reduce protein sample consumption for SFX experiments significantly in the future.
EXPERIMENTAL SECTION
All materials and chemicals used as well as data acquisition details are described in the Supporting Information.
Preparation of 3D-printed Device
The droplet generator device was designed using Autodesk Fusion 360 (Autodesk, CA, USA) and 3D-printed using a Photonic Professional GT Printer (Nanoscribe GmbH, Germany) as described previously.29 In brief, IP-S photoresist was used to print via dip-in laser lithography and two-photon polymerization. The printed device was developed in SU-8 developer, washed with isopropyl alcohol. Polished fused silica capillaries, 30 cm in length, were inserted in the fluidic channels of the droplet generator and glued permanently with epoxy. Both oil and aqueous channels were then coated with Novec 1720 as described in previous work.33
To insert electrodes, the liquid Gallium was loaded into a 1μL glass syringe and immediately injected into electrode channels of the 3D printed device through the inlets34 while vacuum was applied to the electrode channel outlets with a vacuum pump (Model 2027, Welch, Prospect, IL, USA). Subsequently, 5 cm long Ni-Cr wires (diameter = 320 μm) were attached to each metal electrode channel and connected to an external power source. An epoxy adhesive was then applied to the metal channel inlets and outlets and cured at room temperature for 1 h. Schematic drawings of the device are presented in Figure 1.
Figure 1.
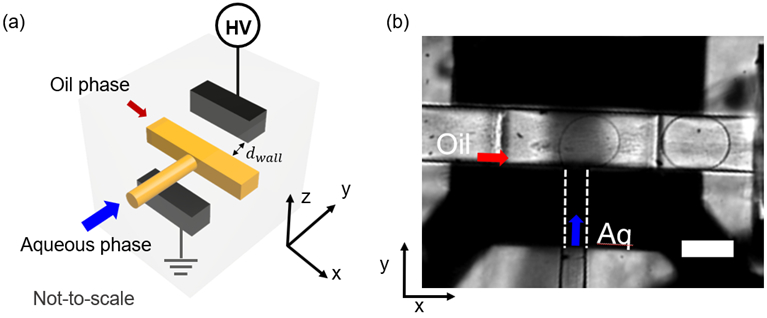
(a) 3D schematic representation of the droplet generator with fluidic T-junction (yellow) and two inlets for the aqueous phase and the oil phase. The diagonally arranged metal electrodes (black) were connected to an external power source (not-to-scale). The arrow shows the distance between the electrode and the fluidic channel along the y-axis. (d) A microscopic image shows a droplet generated at the T-junction. The flow directions of oil (red) and aqueous phase (blue) are depicted with arrows. The dashed lines indicate the portion of the aqueous channel masked by the solid electrodes. All scale bars represent 100 μm.
Fluidic Setup
A mixture of PFD and PFO (PFD/PFO, v/v = 10/1) was used as the oil phase. Two mother liquor crystallization suspensions for proteins, termed buffer 1 and 2 were used as aqueous phase. Buffer 1 for crystallizing KDO8PS (3-deoxy-D-manno-octulosonate 8-phosphate synthase) contained 46 mM KCl, 8mM Tris-HCl, 7.2 w/v% 5K PEGME.35 Buffer 2 for crystallizing photosystem I contained 5 mM MES at pH 6.4 and 0.02 v% of β-DDM.9
For experiments at low-pressure (<14 psi) a flow controller (MFCS-EZ, Fluigent, France) delivered liquid through aqueous and oil reservoirs of Fluiwells (Fluigent, France) to the device through 40 cm long fused silica capillaries (100 μm inner diameter (ID) and 360 μm outer diameter (OD)). For high-pressure experiments (>14 psi), HPLC pumps (LC-20AD, Shimadzu Co., Japan) were connected to reservoirs filled with oil or aqueous phase as described previously to displace liquid towards the droplet generator.29 Liquid flow sensors SLI-0430 and SLG-0075 (Sensirion, Switzerland) monitored the flow rates after the reservoirs. PEEK tubings (250 μm ID and 1/16-in OD) with fittings and ferrules were used to connect the HPLC pumps to the reservoirs and sensors, while fused-silica capillaries and PicoClear unions were used downstream from the droplet generator. After any adjustment of the flow rates, the system was allowed to equilibrate for 5-10 min until pressures stabilized within the system. The aqueous flow rate (Qa) range was between 0.2 μL/min and 5 μL/min and the oil flow rate (Qo) ranged between 5 μL/min and 25 μL/min.
RESULTS AND DISCUSSION
Delivering droplet suspensions at a defined frequency and synchronized with an XFEL laser will reduce the sample amount required for SFX experiments dramatically. Our approach to reduce the amount of sample wasted in SFX experiments consists of a microfluidic droplet generator fabricated using a high-resolution 3D printing technique. Droplet generation control is attained by applying a potential between embedded electrodes. Under pressure driven flow, the shear forces induced by the oil phase acting on the aqueous phase elongate the aqueous droplets leaving the T-junction downstream, until eventually the droplets get pinched off.28 The frequency of the resulting droplet generation is a complex interplay between the fluid viscosities, the surface tension of the involved interfaces, as well as the flow rates and pressures acting in the system.
A representative schematic of the droplet generation device with integrated electrodes is shown in Figure 1 (a). Figure S1 (a) depicts a computer-aided rendition of the device used for 3D printing. This figure illustrates the T-junction, the micro-electrodes, and their respective inlets and outlets for the melted metal in close proximity to the fluidic channels. The distance between the electrode and the fluidic channel along the y-axis is indicated as dwall in Figure 1 (a). This distance was varied from 50 μm to 5 μm and could withstand experimental pressures ranging from a few up to several hundred psi and flow rates of 0.5-20 μL/min. Figure S1 (b) shows the top view of an assembled droplet generator with inserted capillaries and integrated electrodes. A representative image of droplets generated at Qo = 8 μL/min and Qa = 1 μL/min is shown in Figure 1 (b).
Three different droplet generation modes based on DC or AC electric potentials were investigated, as shown in Figure 2. The top row represents the applied trigger signal (green), and the bottom row represents the droplet signal obtained from the droplet detector (photodiode). In Mode 1 no droplets are generated unless a potential is applied, serving as a “drop-on-demand” method. Each DC electrical pulse generates a single droplet. Mode 1 generates droplets on-demand when a DC voltage with an amplitude, UDC (210 V ≤ UDC ≤ 1000 V) is applied for a duration, tw,DC (≥ 300 ms). In mode 2, an AC trigger potential with a peak amplitude (UAC), a frequency (fu), and a short duration (tw,AC) is applied to induce a phase shift (Δϕ) in an already established droplet train with a stable frequency (fb), as shown in Figure 2 (b). Characteristic for mode 2 is the recovery of fb* after the application of the electrical trigger. Finally, mode 3 employs an AC electrical trigger signal with an amplitude UAC (250 V ≤ UAC < 400 V) and fu (100 Hz ≤ UAC < 400 Hz) applied for tw,AC (> 100 ms) as shown in Figure 2 (c). During the application of the applied electrical stimulus, fb (blue) increases to a frequency ft (red). After the electrical stimulus, the droplet generation frequency returns to fb* (blue), which as in mode 2 is similar to fb. The parameters we investigated for the operation of these three modes are summarized in Table S-1 (See Supporting Information).
Figure 2.
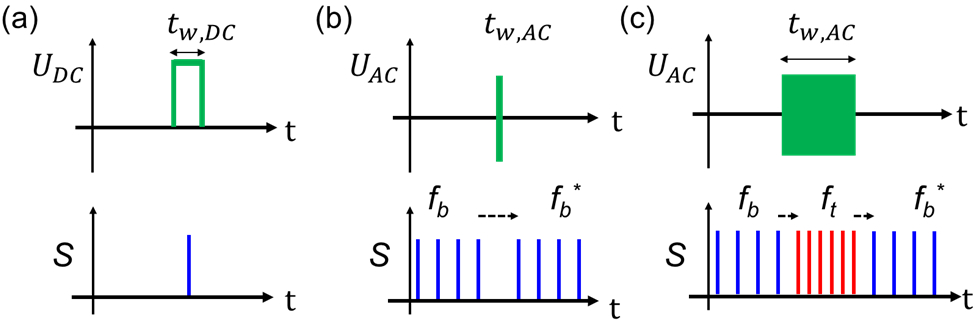
Overview of the three triggering modes. Applied DC or AC potentials with duration (tw) and amplitude are shown in green in the top row. The corresponding droplet detector signal (S) is shown below each trace. The blue and red droplet detector signals correspond to the droplet generation frequency (prior (fb) and after (fb*) the trigger) and increased frequency (ft), respectively. (a) In Mode 1, on-demand droplet generation is induced by a DC pulse of amplitude UDC for a duration tw,DC. (b) In Mode 2, a short pulse (tw,AC = 10 ms) of an AC potential with amplitude UAC leads to a phase shift (Δϕ) in a continuous stream of generated droplets without affecting fb. (c) In Mode 3, a longer AC trigger signal (tw,AC > 100 ms) accelerates fb (blue), into a faster droplet generation frequency ft (red) for the duration of the applied signal.
Mode 1: Drop on-demand
The capability of a device to generate droplets on-demand is important as it allows a single aqueous droplet to be dispensed in the microfluidic chip with precise controllability over trigger timing. Thus, mode 1 can be used to synchronize the injection of protein crystal suspension droplets with XFEL pulses. To explore this mode, a low-pressure setup (<14 psi) with the PFD/PFO oil mixture as the oil phase and buffer 1 as the aqueous phase was employed. A DC potential in the 200 V ≤ UDC ≤ 1000 V range with a duration of tw,DC = 300 ms was used as electrical stimulus. It was important to establish an equilibrium at the aqueous-oil interface in the microfluidic T-junction achieved by optimizing the pressures applied to both phases such that no droplets were generated while allowing the aqueous medium to protrude slightly into the oil channel, as depicted in Figure 3 (a). Once this was achieved, DC trigger pulses of variable amplitudes but fixed tw,DC were tested until eventually a droplet was generated. This potential was characterized as the threshold potential, UDC,Th. Below UDC,Th, the aqueous-oil interface fluctuated and no droplets were formed. Above UDC,Th, one droplet was formed per stimulus pulse.
Figure 3.
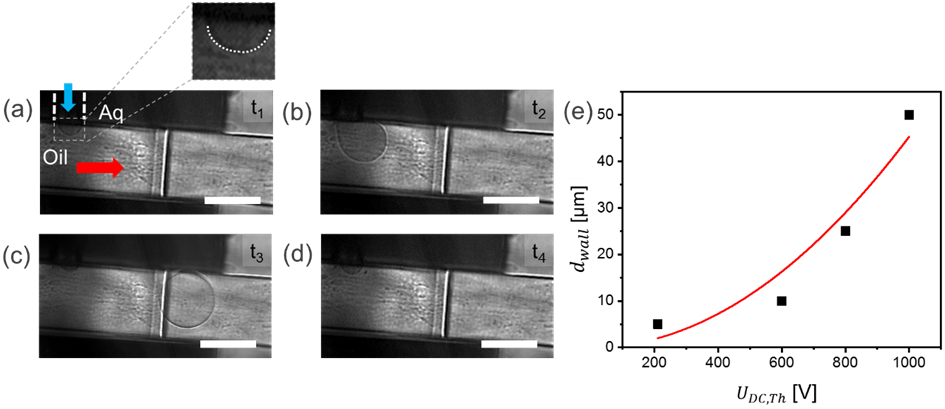
(a – d) Sequential droplet images at the T-junction in mode 1. (a) Snapshot of a stable interface (t = 0) between the oil (PFD:PFO 10/1, v/v) phase and aqueous phase (Buffer 1) at the T-junction; (b) A droplet composed of the aqueous phase medium is pulled out into the oil phase after a short DC pulse was applied (UDC = 210 V, tw,DC = 300 ms); (c) The formed droplet leaving the T-junction, and (d) droplet generated is no longer in field of view. Po was 197 mbar and Pa was 190 mbar. The flow in the fluidic channel proceeds from left to right. All scale bars represent 100 μm. (e) Relationship between dwall and the threshold voltage for droplet generation. Origin software was used to create a quadratic (red line) for the relationship between dwall and UDC,Th.
Figure 3 (a-d) depicts typical sequential images recorded for on-demand droplet generation using a high-speed camera. Milliseconds after the trigger pulse started, a droplet was generated as observed in Figure 3. A single droplet was successfully generated each time the stimulus was above UDC,Th. Supporting Information Video S-1 shows a series of single droplet on-demand generation events using a DC trigger signal (UDC,Th = 1000 V and tw,DC = 300 ms) in the device (dwall = 5 μm).
We further explored the relation between UDC,Th and dwall (see Figure 3 e). As dwall was decreased from 50 to 5 μm, the required threshold potential, UDC,Th, decreased from nearly 1000 V to 210 V. When the applied potential was below the threshold, or the duration of the trigger was too short, tw,DC < 300 ms, the aqueous phase only fluctuated at the aqueous-oil interface and no droplet was released. Experiments showed that a DC pulse of at least 300 ms was required to induce droplets (data not shown). Each stimulus with a duration from 300 ms to 60 seconds resulted in a single droplet release. There was a minimum time interval required between two consecutive droplet generation events, which was observed to be around 300 ms. In conclusion, by controlling UDC and tw,DC, mode 1 can be used to trigger droplets on-demand in the device, with potential for synchronizing each droplet with an XFEL pulse. With our current experimental setup, the maximum frequency of on-demand droplet generation in this mode is about 1.7 Hz. While this droplet generation is below the typical XFEL repetition rates, improved design layouts and further studies will allow drop on-demand generation to reach a repetition frequency of at least 10 Hz. This would thus allow exploiting X-ray crystallography, taking advantage of the MHz pulse repetition rate within the 10 Hz trains generated at the EuXFEL.
Mode 2: Inducing a phase shift
The significance of mode 2 is underlined by the required synchronization of a droplet generation base frequency with the non-tunable XFEL repetition rate. We thus explored the potential to synchronize the phase of the droplet generation frequency with the XFEL repetition rate, without changing the oil or aqueous flow rates by use of electrical stimulation in a device generating droplets at fb and retard the droplet release for a defined time at the T-intersection to achieve a required Δϕ. This was explored via the application of a short AC trigger signal (tw,AC = 10 ms) to induce a delay in fb. We chose to investigate mode 2 for the pulse train repetition frequency matching the EuXFEL of 10 Hz. A high-pressure set-up as described in the methods section was used to mimic the XFEL facility requirements. A representative trace of the droplet frequency (fb = 10.9 ± 0.3 Hz) is shown in Figure 4 (a), where Qo = 6.5 and Qa = 0.21 μL/min. While droplets where passively produced at the T-junction, a short AC trigger signal (UAC = 250 V at fu = 100 Hz, tw,AC = 10 ms) was applied causing droplet generation to stop for a brief silent time (ts = 54 ms), as shown in Figure 4 (a), after which droplet generation resumed at the base frequency (Figure 4c) within error margins. The phase shift can thus be characterized by the following equation:
| (1) |
where n represents the number of droplets that would be generated during ts at fb without the stimulus. The stimulus can be repeated as needed, as shown for a total of 5 consecutive stimuli (UAC = 250 V at fu = 100 Hz with tw,AC = 10 ms) exemplarily in Figure 4 (b). We further investigated whether the droplet volume varied before and after a trigger signal as shown in the boxplot in Figure 4 (c). This figure shows that the droplet volumes are not significantly different before and after the trigger. Similar findings apply to fb and fb*.
Figure 4.
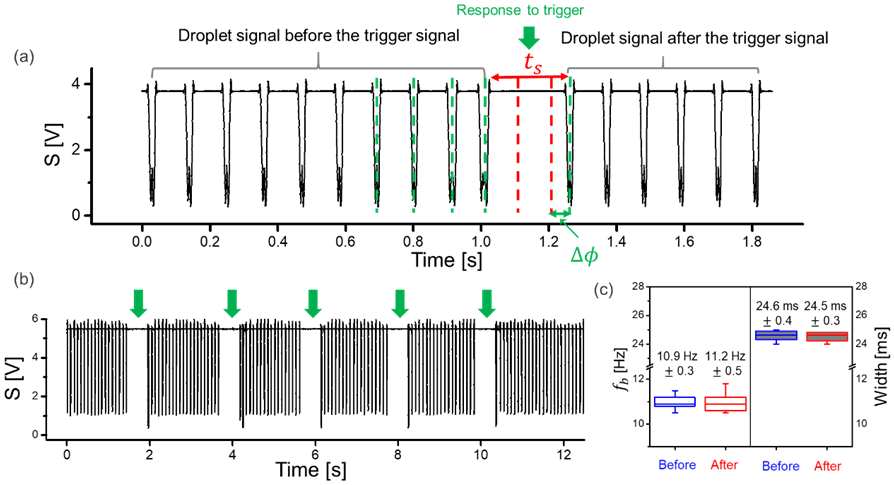
(a) A trace of stable droplet generation frequency (fb) in mode 2. Upon application of an AC potential (UAC = 250 V at fu = 100 Hz, tw = 10 ms), the droplet generation is shortly interrupted (response to trigger). This leads to a Δϕ, without change in fb. The dashed red lines during ts are representative of the droplets that would have been generated at fb without a stimulus. (b) Example droplet trace showing multiple trigger signals (UAC = 250 V at fu = 100 Hz, tw,AC = 10 ms), all generating a similar response to the trigger (silent time), whereas the frequency fb* recovers to fb after each electrical trigger. The droplet signals were acquired using the droplet detector and recorded with Powerlab and Labchart (see Supplementary Information for details). (c) Analysis of the droplet frequency and width over 10 seconds before and after the trigger. The base frequency and droplet length (in time) are consistent before and after the trigger with errors. In the box plots, boxes extend from the 25th to the 75th percentiles with a line at the median. Whiskers extend to the max/min data points.
Further, we investigated how Δϕ varied with repetitions of the same stimulus (UAC = 250 V at 100 Hz, tw,AC = 10 ms). Out of 14 consecutive stimuli the resulting Δϕ values ranged from 3 to 90 ms. Since the droplets have a finite reproducible size, this range of phase shift is suitable for phase synchronization between the droplet and the XFEL pulse. In summary, mode 2 showed that short AC triggers were able to cause a phase shift in fb without affecting fb* after the stimulus. We also showed that the size of the droplets remained stable before and after the phase change. This mode has potential for synchronizing droplets that are out of phase but at the same frequency as the pulsed XFELs for SFX experiments.
Mode 3: Tuning the droplet frequency
In mode 3, we explored whether an electrical trigger can induce a change in the frequency of a continuous stream of droplets to improve synchronization with an XFEL for the duration of the applied trigger without altering flow rates. Even if flow rate control instrumentation would allow fine-tuning of the droplet generation frequency, any adjustment in flow conditions requires hydrostatic pressure changes, which induce instability in the droplet generation and generate droplets irregularly with a large variation in droplet size.36 Droplet generation may take up to 30 min to stabilize, depending on the flow conditions, and lost time is always disadvantageous for an XFEL experiment. Thus, increasing the droplet generation frequency (ft), without changing flow rates and affecting adjacent flow streams, is a useful technique.
To operate the droplet generator in mode 3, a continuous stream of droplets is generated at fb by holding Qo and Qa constant for a range of AC potentials (100 ≤ UAC ≤ 400 V) with a frequency (fu = 100 Hz) for tw,AC > 100 ms. We characterized the relationship between UAC and ft at the T-junction using high and low-pressure systems, as demonstrated in Figure 5 (a). Before the trigger, fb was 49.1 ± 5.7 Hz and 5.6 ± 0.3 Hz for high- and low-pressure settings, respectively. By increasing UAC, fb increased to ft. The increase in ft as compared to fb is not very evident between 0 and 240 V, but becomes more significant after 250 V for both pressure systems with a maximum of an approx. 4-fold increase. Video S-2 (Supporting Information) shows acceleration of droplet frequency under various AC trigger signals in the device.
Figure 5.
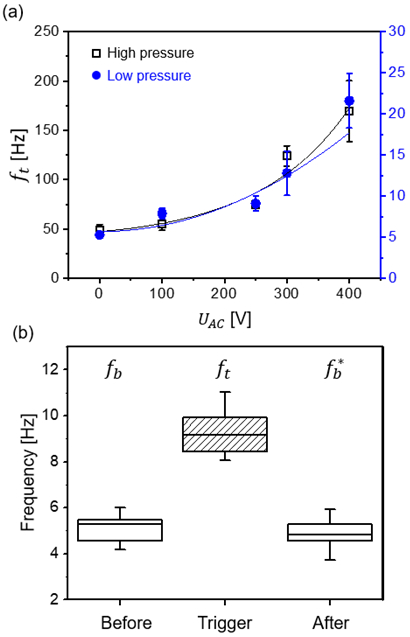
(a) Dependence of the achieved droplet generation frequency (ft) on the applied AC amplitude (UAC) at fu = 100 Hz in both high-pressure (black) and low-pressure (blue) systems. The base frequency was 49.1 ± 5.7 Hz and 5.6 ± 0.3 Hz for high and low pressure, respectively. The lines represent quadratic curve fits. Error bars represent the standard deviation. Some error bars in (a) are obscured by the size of the symbol. (b) Analysis of fb 2 min before and after the trigger signal for UAC = 250 V at fu = 100 Hz, tw,AC = 1 s. Droplet generation frequencies, fb, ft and , were 5.0 ± 0.4 Hz, 9.2 ± 0.7 Hz and 4.7 ± 0.5 Hz, respectively. The droplet generation frequency is accelerated during tw,AC and fb* is similar to fb, within error margins. In the box plots, boxes extend from the 25th to the 75th percentiles with a line at the median. Whiskers extend to the max/min data points.
In addition to this, we also tested the stability of the droplet generation related to fb and fb*. As shown in Figure 5 (b), we observed that ft settled to fb* after the electrical stimulus was stopped and fb* is similar to fb within error margins. This demonstrates that the droplet generation frequency only changes during the application of an electrical stimulus and remains unaffected otherwise. We also tested mode 3 for a duration of 5 minutes and successfully observed the change from fb to ft for the entire duration. In summary, mode 3 allows frequency increments in both, high- and low-pressure systems, indicating that the increase in droplet generation is independent of the fluidic setup. While the tuning of the droplet frequency was demonstrated with mode 3, the droplet generation frequency becomes unstable if the applied stimulus is above a certain intensity UAC or frequency fu. We attribute this to electrical instability, which is illustrated in Figure S2. Above a certain UAC (or fu) however, the droplet size and frequency becomes unstable, leading to an “electro-spraying” regime, as illustrated in Figure S2. Clearly, in extreme cases, fb can no longer be increased to a stable ft during triggering, but rather results in irregularly sized droplets and irregular frequencies. It is also evident that the wetting properties change, as the aqueous droplets contact and wet the microchannel walls.
Based on the achieved electrically induced triggering, we investigated two major phenomena previously described in literature, that could be responsible for the here demonstrated effects37, namely electrowetting on dielectrics (EWOD) and dielectrophoresis (DEP). Electrowetting on dielectrics (EWOD) refers to changes in contact angle of a dielectric material initiated upon application of a voltage. The control of wettability on a dielectric surface is allowed by controlling the interfacial energy changes between the liquid and the surface.30 Similarly, in the droplet generator, the interfacial energies of the involved surfaces may be altered leading to the release of aqueous droplets. The major force (Fw) acting on the aqueous liquid on a dielectric substrate is given as 37,38
| (2) |
where the pre-factor, α, is the ratio of involved permittivity and a correction factor .38,39 Here, ε0 denotes the permittivity of vacuum and εd, the dielectric constant of the insulator (i.e. the photoresist employed to fabricate the 3D-printed device), U is the applied voltage and dwall is the thickness of the dielectric layer. A larger Fw is thus induced through larger applied potentials and a shorter dwall. This was observed in our study, as lower threshold voltage amplitudes were required for decreased dwall as observed in mode 1 (Figure 3 (e)). Moreover, the study conducted in mode 3 showed that increased voltage amplitudes lead to acceleration of the droplet frequency. This can also be explained with increased Fw as demonstrated in Figure 5 (a) and (b). In addition, analysis of the video sequences demonstrated wetting of the hydrophobic inner channel walls with the aqueous droplets, when being released from the T-junction upon triggering. This effect is dependent on employed flow rates, droplet sizes and applied voltage amplitudes, but is a strong indication for EWOD effects during electrical triggering. Similar arguments may be used for the effect of DEP on droplet release, as the dielectrophoretic force scales with the applied potential squared.40,41 In DEP, a polarizable particle or a polarizable interface experiences an attractive or repulsive force under a non-uniform electric field.37,42 In our case, the electrode geometry used in the droplet generator induces a non-uniform electric field. While the detailed mechanism of DEP at the T-junction needs further study, it seems reasonable that DEP forces may push the aqueous-oil interface into the T-junction thereby facilitating droplet release. However, previous reports have shown that DEP forces are typically weaker than EWOD effects in the frequency regime studied here.30,37 Therefore, it seems likely that electrowetting forces dominate the droplet release in the presented device. A more detailed study however, needs to be conducted to quantitatively compare the magnitude of EWOD and DEP forces.
CONCLUSION
We demonstrated a 3D-printed microfluidic platform with embedded gallium electrodes to enhance control of aqueous droplet generation in a stream of oil. The microfluidic T-junction with embedded gallium electrodes can be operated in three different modes to generate droplets. First, on-demand droplets can be generated through programmable DC potentials. We demonstrated on-demand droplet generation with a maximum frequency of 1.7 Hz. Future optimization and device design may likely allow an increase in the on-demand droplet generation frequency to match current XFEL repetition rates. Second, the phase of the droplet generation frequency can be tuned under short AC signals that can be employed to assist the synchronization of the aqueous droplets with the XFEL pulses. Third, the droplet generation frequency can be increased by AC trigger signals without hampering droplet generation stability, before and after the trigger. In addition, we discuss the origin of the observed phenomena and conclude that electrowetting is predominantly responsible for the observed droplet triggering effects. This innovative approach of controlling droplet production and delivery in the path of X-rays can be utilized to improve synchronization of droplets and the X-ray beam and consequently play a crucial role in saving protein crystal sample in SFX experiments. In addition, 3D printing technology will allow for future integration of the 3D printed droplet generator with 3D-printed GDVN injectors recently developed for XFEL sample delivery.
Supplementary Material
ACKNOWLEDGEMENT
We thank Dr. Richard Kirian and Dr. Uwe Weierstall from the Department of Physics at Arizona State University for the use of the high-speed camera. This work was supported by the STC Program of the National Science Foundation (NSF) through BioXFEL under Agreement No. 1231306 and the National Institutes of Health Award No. R01GM095583.
Footnotes
Supporting Information
Materials and Chemicals, Droplet observation and data analysis, Supplementary Figure and Table (PDF)
Droplet on-demand generation events using a DC trigger signal (AVI)
Acceleration of droplets under the various AC trigger signal (AVI)
REFERENCES
- (1).Spence JCH XFELs for structure and dynamics in biology, IUCrJ 2017, 4, 322–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Boutet S; Lomb L; Williams GJ; Barends TRM; Aquila A; Doak RB; Weierstall U; DePonte DP; Steinbrener J; Shoeman RL; Messerschmidt M; Barty A; White TA; Kassemeyer S; Kirian RA; Seibert MM; Montanez PA; Kenney C; Herbst R; Hart P, et al. High-Resolution Protein Structure Determination by Serial Femtosecond Crystallography, Science 2012, 337, 362–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Liu W; Wacker D; Gati C; Han GW; James D; Wang D; Nelson G; Weierstall U; Katritch V; Barty A; Zatsepin NA; Li D; Messerschmidt M; Boutet S; Williams GJ; Koglin JE; Seibert MM; Wang C; Shah STA; Basu S, et al. Serial Femtosecond Crystallography of G Protein–Coupled Receptors, Science 2013, 342, 1521–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Kupitz C; Basu S; Grotjohann I; Fromme R; Zatsepin NA; Rendek KN; Hunter MS; Shoeman RL; White TA; Wang D; James D; Yang J-H; Cobb DE; Reeder B; Sierra RG; Liu H; Barty A; Aquila AL; Deponte D; Kirian RA, et al. Serial time-resolved crystallography of photosystem II using a femtosecond X-ray laser, Nature 2014, 513, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Martin-Garcia JM; Conrad CE; Coe J; Roy-Chowdhury S; Fromme P Serial femtosecond crystallography: A revolution in structural biology, Arch. Biochem. Biophys 2016, 602, 32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Frank M; Carlson DB; Hunter MS; Williams GJ; Messerschmidt M; Zatsepin NA; Barty A; Benner WH; Chu K; Graf AT; Hau-Riege SP; Kirian RA; Padeste C; Pardini T; Pedrini B; Segelke B; Seibert MM; Spence JCH; Tsai C-J; Lane SM, et al. Femtosecond X-ray diffraction from two-dimensional protein crystals, IUCrJ 2014, 1, 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Chapman HN; Fromme P; Barty A; White TA; Kirian RA; Aquila A; Hunter MS; Schulz J; DePonte DP; Weierstall U; Doak RB; Maia FRNC; Martin AV; Schlichting I; Lomb L; Coppola N; Shoeman RL; Epp SW; Hartmann R; Rolles D, et al. Femtosecond X-ray protein nanocrystallography, Nature 2011, 470, 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Johansson LC; Stauch B; Ishchenko A; Cherezov V A Bright Future for Serial Femtosecond Crystallography with XFELs, Trends Biochem. Sci 2017, 42, 749–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Conrad CE; Basu S; James D; Wang D; Schaffer A; Roy-Chowdhury S; Zatsepin NA; Aquila A; Coe J; Gati C; Hunter MS; Koglin JE; Kupitz C; Nelson G; Subramanian G; White TA; Zhao Y; Zook J; Boutet S; Cherezov V, et al. A novel inert crystal delivery medium for serial femtosecond crystallography, IUCrJ 2015, 2, 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Boutet S, Fromme Petra, Hunter Mark S. X-ray Free Electron Lasers; Springer International Publishing: Switzerland, 2018. [Google Scholar]
- (11).Weierstall U; James D; Wang C; White TA; Wang D; Liu W; Spence JCH; Bruce Doak R; Nelson G; Fromme P; Fromme R; Grotjohann I; Kupitz C; Zatsepin NA; Liu H; Basu S; Wacker D; Won Han G; Katritch V; Boutet S, et al. Lipidic cubic phase injector facilitates membrane protein serial femtosecond crystallography, Nat. Commun 2014, 5, 3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Liu W; Wacker D; Wang C; Abola E; Cherezov V Femtosecond crystallography of membrane proteins in the lipidic cubic phase, Philos. Trans. R. Soc. Lond., B, Biol. Sci 2014, 369, 20130314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Wiedorn MO; Oberthür D; Bean R; Schubert R; Werner N; Abbey B; Aepfelbacher M; Adriano L; Allahgholi A; Al-Qudami N; Andreasson J; Aplin S; Awel S; Ayyer K; Bajt S; Barák I; Bari S; Bielecki J; Botha S; Boukhelef D, et al. Megahertz serial crystallography, Nat. Commun 2018, 9, 4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Altarelli M; Mancuso Adrian P Structural biology at the European X-ray free-electron laser facility, Philos. Trans. R. Soc. Lond., B, Biol. Sci 2014, 369, 20130311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Sierra RG; Laksmono H; Kern J; Tran R; Hattne J; Alonso-Mori R; Lassalle-Kaiser B; Glockner C; Hellmich J; Schafer DW; Echols N; Gildea RJ; Grosse-Kunstleve RW; Sellberg J; McQueen TA; Fry AR; Messerschmidt MM; Miahnahri A; Seibert MM; Hampton CY, et al. Nanoflow electrospinning serial femtosecond crystallography, Acta Cryst. D 2012, 68, 1584–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Sierra RG; Gati C; Laksmono H; Dao EH; Gul S; Fuller F; Kern J; Chatterjee R; Ibrahim M; Brewster AS; Young ID; Michels-Clark T; Aquila A; Liang M; Hunter MS; Koglin JE; Boutet S; Junco EA; Hayes B; Bogan MJ, et al. Concentric-flow electrokinetic injector enables serial crystallography of ribosome and photosystem II, Nat. Methods 2015, 13, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Mafune F; Miyajima K; Tono K; Takeda Y; Kohno J.-y.; Miyauchi N; Kobayashi J; Joti Y; Nango E; Iwata S; Yabashi M Microcrystal delivery by pulsed liquid droplet for serial femtosecond crystallography, Acta Cryst. D 2016, 72, 520–523. [DOI] [PubMed] [Google Scholar]
- (18).Tono K Fluid sample injectors for x-ray free electron laser at SACLA, High Power Laser Sci. Eng 2017, 5, e7. [Google Scholar]
- (19).Kubo M; Nango E; Tono K; Kimura T; Owada S; Song C; Mafune F; Miyajima K; Takeda Y; Kohno J.-y.; Miyauchi N; Nakane T; Tanaka T; Nomura T; Davidsson J; Tanaka R; Murata M; Kameshima T; Hatsui T; Joti Y, et al. Nanosecond pump-probe device for time-resolved serial femtosecond crystallography developed at SACLA, J. Synchrotron Radiat 2017, 24, 1086–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Roessler Christian G.; Agarwal R; Allaire M; Alonso-Mori R; Andi B; Bachega José F. R.; Bommer M; Brewster Aaron S.; Browne Michael C.; Chatterjee R; Cho E; Cohen Aina E.; Cowan M; Datwani S; Davidson Victor L.; Defever J; Eaton B; Ellson R; Feng Y; Ghislain Lucien P., et al. Acoustic Injectors for Drop-On-Demand Serial Femtosecond Crystallography, Structure 2016, 24, 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Fuller FD; Gul S; Chatterjee R; Burgie ES; Young ID; Lebrette H; Srinivas V; Brewster AS; Michels-Clark T; Clinger JA; Andi B; Ibrahim M; Pastor E; de Lichtenberg C; Hussein R; Pollock CJ; Zhang M; Stan CA; Kroll T; Fransson T, et al. Drop-on-demand sample delivery for studying biocatalysts in action at X-ray free-electron lasers, Nat. Methods 2017, 14, 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Grünbein ML; Nass Kovacs G Sample delivery for serial crystallography at free-electron lasers and synchrotrons, Acta Cryst. D 2019, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Echelmeier A; Nelson G; Abdallah BG; James D; Roy-Chowdhury S; Tolstikova A; Mariani V; Kirian RA; Oberthüer D; Dörner K; Fromme P; Chapman HN; Weierstall U; Spence JCH; Ros A Biphasic droplet-based sample delivery of protein crystals for serial femtosecond crystallography with an X-ray free electron laser, μTAS Proceedings 2015, 1374–1376. [Google Scholar]
- (24).Chen J-S; Jiang J-H Droplet Microfluidic Technology: Mirodroplets Formation and Manipulation, Chinese J. Anal. Chem 2012, 40, 1293–1300. [Google Scholar]
- (25).Gu H; Duits MHG; Mugele F Droplets Formation and Merging in Two-Phase Flow Microfluidics, Int. J. Mol. Sci 2011, 12, 2572–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Baroud CN; Gallaire F; Dangla R Dynamics of microfluidic droplets, Lab Chip 2010, 10, 2032–2045. [DOI] [PubMed] [Google Scholar]
- (27).Pit AM; Duits MHG; Mugele F Droplet Manipulations in Two Phase Flow Microfluidics, Micromachines 2015, 6, 1768–1793. [Google Scholar]
- (28).Zhu P; Wang L Passive and active droplet generation with microfluidics: a review, Lab Chip 2017, 17, 34–75. [DOI] [PubMed] [Google Scholar]
- (29).Echelmeier A; Villarreal JC; Kim D; Gandhi S; Egatz-Gomez A; Quintana S; Coe J; Brehm G; Messerschmidt M; Meza-Aguilar JD; Weinhaussen B; Mills G; Vagovic P; Kim Y; Schultz J; Döner K; Mancuso A; Weierstall U; Spence JCH; Chapman HN, et al. Segmented Flow Generator for Serial Crystallography at X-Ray Free Electron Lasers, Manuscript in preparation.
- (30).Mugele F; Baret JC Electrowetting: From basics to applications, J. Phys.: Condens. Matter 2005, 17, 705–774. [Google Scholar]
- (31).Mugele F; Duits M; van den Ende D Electrowetting: A versatile tool for drop manipulation, generation, and characterization, Adv. Colloid Interface Sci 2010, 161, 115–123. [DOI] [PubMed] [Google Scholar]
- (32).Pollack MG; Shenderov AD; Fair RB Electrowetting-based actuation of droplets for integrated microfluidics, Lab Chip 2002, 2, 96–101. [DOI] [PubMed] [Google Scholar]
- (33).Echelmeier A; Kim D; Villarreal JC; Coe J; Quintana S; Brehm G; Egatz-Gomez A; Sierra R; Zatsepin N; Kirian R; Grant TD; Fromme P; Ros A 3D Printed Droplet Generation Devices for Serial Femtosecond Crystallography Enabled by Surface Coating, J. Appl. Crystallogr 2019, Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Lin Y; Gordon O; Khan MR; Vasquez N; Genzer J; Dickey MD Vacuum filling of complex microchannels with liquid metal, Lab Chip 2017, 17, 3043–3050. [DOI] [PubMed] [Google Scholar]
- (35).Coe J; Fromme P; Sayres S; Mujica V; Redding K Life In Motion: Visualizing Biomacromolecules By Time-Resolved Serial Femtosecond Crystallography. Doctoral Dissertation, Arizona State University; 2018. [Google Scholar]
- (36).Chong ZZ; Tan SH; Gañán-Calvo AM; Tor SB; Loh NH; Nguyen NT Active droplet generation in microfluidics, Lab Chip 2016, 16, 35–58. [DOI] [PubMed] [Google Scholar]
- (37).Jones TB On the relationship of dielectrophoresis and electrowetting, Langmuir 2002, 18, 4437–4443. [Google Scholar]
- (38).Kang KH How Electrostatic Fields Change Contact Angle in Electrowetting, Langmuir 2002, 18, 10318–10322. [Google Scholar]
- (39).Jones TB More about the electromechanics of electrowetting, Mech. Res. Commun 2009, 36, 2–9. [Google Scholar]
- (40).Jones TB Electromechanics of Particles; Cambridge University Press: New York, 1995, p 265. [Google Scholar]
- (41).Pohl HA Dielectrophoresis : the behavior of neutral matter in nonuniform electric fields; Cambridge University Press: Cambridge; New York, 1978. [Google Scholar]
- (42).Kim D; Sonker M; Ros A Dielectrophoresis: From Molecular to Micrometer-Scale Analytes, Anal. Chem 2019, 91, 277–295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


