Intrinsically stretchable light-emitting diodes were developed by designing constituent materials to be mechanically stretchable.
Abstract
Soft and conformable optoelectronic devices for wearable and implantable electronics require mechanical stretchability. However, very few researches have been done for intrinsically stretchable light-emitting diodes. Here, we present an intrinsically stretchable organic light-emitting diode, whose constituent materials are all highly stretchable. The resulting intrinsically stretchable organic light-emitting diode can emit light when exposed to strains as large as 80%. The turn-on voltage is as low as 8 V, and the maximum luminance, which is a summation of the luminance values from both the anode and cathode sides, is 4400 cd m−2. It can also survive repeated stretching cycles up to 200 times, and small stretching to 50% is shown to substantially enhance its light-emitting efficiency.
INTRODUCTION
Soft electronics that flex and stretch have been rapidly developed and show potential for notably affecting our daily lives (1–3). A stretchable organic light-emitting diode (OLED) is one of the key components of stretchable displays, enabling expandable and foldable smartphones, rollable or collapsible wallpaper-like televisions, and neurological or epidermal medical devices (1, 3, 4). All of the materials and components of such OLEDs need to be stretchable and mechanically robust (5–7). The current technology for stretchable OLED display panels combines elastic interconnects with rigid LEDs (5, 8, 9). As an alternative, an intrinsically stretchable (is-) OLEDs in which all constituent materials are inherently stretchable will enable higher mechanical deformability and robustness, higher device density, and improved skin or tissue compatibility (10, 11). Various strategies have been suggested for designing stretchable conjugated polymeric materials for is-transistors (5, 6, 12). However, the production of is-OLEDs is still largely in its infancy (1, 13, 14).
The first is-organic light-emitting device, and perhaps the first is-organic electronic device of any kind, was an organic light-emitting electrochemical (LEC) device reported in 2011 (14). The electrodes were based on carbon nanotubes, and the blue emissive polymer used was PF-B, a polyfluorene copolymer (14). This structure has long alkyl side chains, consistent with the level of possible deformability, and was mixed with the ionic conductor poly(ethylene oxide) dimethacrylate ether and lithium trifluoromethane sulfonate (14). The authors later showed a yellow emissive device using the poly(phenylenevinylene) (PPV) derivative, “Super Yellow (SY),” which achieved an impressive extensibility of 120% (1, 13). However, the authors did not investigate the mechanical properties and the stretchability mechanism of the constituent materials for is-OLEDs. Since then, little has followed the previous work.
RESULTS
To develop is-OLEDs with superior mechanical stretchability, the unique is-constituent materials were designed in this work. First, for the is-emissive material layer (is-EML), a small-molecule nonionic surfactant (Triton X) was mixed as a plasticizer with a commercial emissive material (SY). The chemical structures of SY and Triton X are shown in Fig. 1A. The plasticizer can increase the free volume of the conjugated polymer by reducing the interchain interactions (15), thus making SY become softer and more stretchable.
Fig. 1. Design of is-OLEDs and characterization of is-EML.
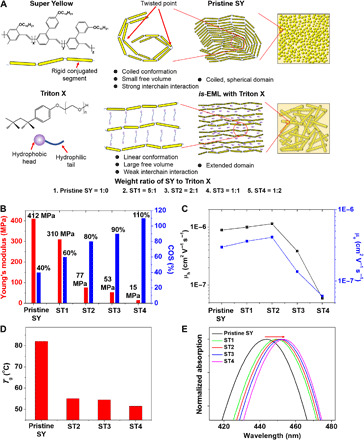
(A) Schematics comparing the microstructures of pristine SY and is-EMLs designed in this study. (B) Mechanical properties of pristine SY and is-EML thin films. (C) Carrier mobility of pristine SY and is-EMLs measured through the hole- and electron-only devices. (D) Tg of pristine SY and is-EMLs measured by DMA. (E) UV-vis absorption spectra of pristine SY and is-EMLs. On the basis of the various analysis results, by adding Triton X into SY, the SY chains become extended. Hence, a fibrillar microstructure with elongated domains is formed, which is favorable for stretchable electronics.
To verify our strategy, the mechanical properties [Young’s modulus, crack density, and crack onset strain (COS)] of pristine SY and is-EMLs (ST1 to ST4 in Fig. 1A) were investigated, with the results shown in Fig. 1B, figs. S1 and S2, and table S1. As expected, is-EML with a small amount of Triton X (ST1 in Fig. 1A) exhibits a 25% lower Young’s modulus than that of pristine SY. As the weight ratio of Triton X increases, the Young’s modulus further decreases to 15 MPa, as shown in Fig. 1B. In addition, the crack density and COS in is-EML were substantially reduced and improved, respectively, with the increase of the Triton X weight content as compared with that of pristine SY (Fig. 1B and fig. S2C). The change in Young’s modulus, crack density, and COS verified that Triton X can effectively modulate the mechanical properties of the light-emitting conjugated polymers.
The major challenge faced in developing is-EML lies in simultaneously maintaining good electrical and mechanical properties (6). As Triton X is an electrical insulator, its effect on the electrical properties of is-EMLs was also investigated. The carrier mobilities (hole mobility, μh, and electron mobility, μe) of is-EMLs were analyzed by fabricating hole- and electron-only devices (16). The detailed device structure and current density (J)–voltage (V) curves are shown in figs. S3 and S4, respectively. As shown in Fig. 1C, μh and μe values of pristine SY are approximately 9 × 10−7 and 3 × 10−7 cm−2 V−1 s−1, respectively (17, 18). Both μh and μe were notably decreased as the weight of Triton X added to SY increases to 1:1 (ST3) and 1:2 (ST4) due to the electrically insulating nature of Triton X. However, when the weight ratio of SY to Triton X is 5:1 (ST1) and 2:1 (ST2), both μh and μe become slightly higher than that of pristine SY, which confirms that, at the optimized weight ratio, Triton X improves the electrical properties of is-EML by changing the conformation of the SY chains. On the basis of the electrical and mechanical analysis in Fig. 1 (B and C), ST2 was chosen as is-EML for is-OLED.
To explain how Triton X affects the conformation and microstructure of SY chains, dynamic mechanical analysis (DMA) was performed to analyze the glass transition temperature (Tg) of pristine SY and ST2 to ST4 (19). When a small-molecule plasticizer is blended in polymer matrices, it reduces the interchain interaction of the polymer chains (20). Hence, the chain mobility and free volume of the polymer chains are increased, thus decreasing Tg of the polymer matrices (12). According to Fig. 1D and fig. S5, Tg of pristine SY is 82.1°C (21). However, Tg of ST2 is substantially reduced to 55.2°C, and increasing the weight ratio of Triton X, Tg further decreases to 51.6°C. Hence, Triton X addition reduced the interchain interaction between the SY chains, thereby making SY softer and more stretchable. However, such results contrast with the carrier mobility analysis shown in Fig. 1C. The charge carrier mobilities of the conjugated polymers tend to decrease due to reduced interchain charge carrier transport (22). Hence, there should be another mechanism underlying the carrier mobility increase in is-EMLs.
The chain conformation of pristine SY and is-EMLs was also analyzed by ultraviolet-visible (UV-vis) absorption (23–25). According to the UV-vis absorption spectra in Fig. 1E, the peak wavelength of is-EMLs is slightly redshifted with increasing Triton X, which indicates that the conjugation length of the SY chains of is-EMLs is extended (24–26). On the basis of this, we can presume that the conformation of the SY chain is changed from a coiled to a linear structure. To further verify the chain conformation change, Raman spectroscopy was performed. According to the detailed Raman spectra shown in fig. S6B, the main peaks are observed at 1112, 1270, 1310, 1585, and 1625 cm−1, which are similar to those of poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] (24, 27).
As shown in fig. S6C and table S2, the intensity ratio of 1580 to 1625 cm−1 (I1580∙I1625−1) tends to increase with Triton X, which also strongly confirms that the conjugation length of the SY chain increases with the addition of Triton X (23, 27). This explains the high carrier mobility of ST2 in Fig. 1C. In polymer chains with a coiled conformation, electron delocalization is hindered at the twisted point (25). Hence, the carrier mobility of the conjugated polymers tends to increase as the conjugation length increases via polymer chain extension (26, 28).
On the basis of our DMA, UV-vis absorption, and Raman spectroscopy results, the microstructure of is-EMLs and pristine SY from the molecular scale to the macroscale can be presumed to be as schematically shown in Fig. 1A. The SY chains in is-EMLs are extended at the molecular level due to the decreased interchain interaction arising from the plasticizing effect of Triton X. As a result, an extended domain is formed, as shown in Fig. 1A, which results in a fibrous structure with increased stretchability. The presumed microstructures described in Fig. 1A were further verified by atomic force microscopy (AFM). As shown in fig. S7, the microstructure of is-EMLs is changed from spherical domains to a fibrous structure, which strongly supports our assumption of a conformational change of the SY chains in is-EMLs. Although the surface roughness (Rq) of is-EMLs slightly increased as the amount of Triton X increased, Rq of is-EMLs was still lower than 1 nm, which is suitable for the is-OLED device fabrication. Such conformation change of the SY chains by the addition of Triton X also explained the electrical mobility increase in ST1 and ST2 because the increase of the intrachain conjugation also increases the carrier mobility of the conjugated polymers through enhancement in the intrachain carrier transport (29).
To obtain an is-hole transport layer (is-HTL), Triton X was added to poly(3,4-ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS; Clevios AI 4083, Heraeus). There are two types of PEDOT:PSS: highly conductive PEDOT:PSS (commonly PH1000, Heraeus), which is used mostly for electrodes in transistors (30–32), and highly resistive PEDOT:PSS, which is used for HTL in optoelectronic devices (1, 33–35). The difference between the two types of PEDOT:PSS lies in the weight ratio between PEDOT and PSS. To date, several studies conducted to improve the stretchability of PEDOT:PSS were limited to the modification of highly conductive PEDOT:PSS (31, 32).
When added to AI 4083, Triton X can prevent strong electrostatic interactions between PEDOT and PSS, inducing phase separation in PEDOT:PSS films (30). Hence, a nanofibrous PEDOT-rich domain is formed as in the highly conductive PEDOT:PSS films during the film formation process, as shown in Fig. 2A, which is favorable for mechanical stretchability (30, 31). The weight percent of Triton X was optimized on the basis of the hole transport property of is-HTL evaluated through the use of HTL-only devices [indium tin oxide (ITO)/is-HTL/Au] and OLEDs with conventional structures (fig. S8). As shown in fig. S8A, when is-HTL with 5 weight % (wt %) Triton X was used, ITO/is-HTL/Au devices showed the highest J value, and OLEDs showed performances comparable to those of OLEDs with pristine AI 4083, as shown in fig. S8B.
Fig. 2. Design of other is-functional layers and characterization.
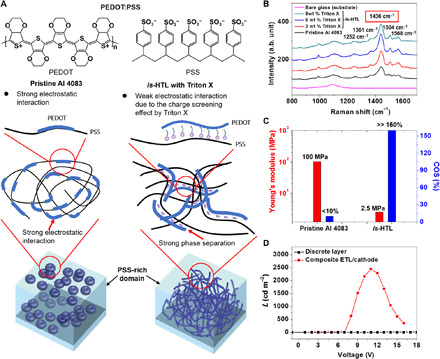
(A) Schematics of the microstructures of pristine HTL and is-HTL designed in this study. (B) Raman spectra of pristine AI 4083, is-HTL with various wt % of Triton X, and a glass substrate. (C) Mechanical properties of pristine HTL and is-HTL thin films. The conformation of the PEDOT chains is changed from coiled to linear with the addition of Triton X, which results in improved electrical and mechanical properties of is-HTL. (D) Luminance (L)–V plot of OLEDs with the solution-processed is-ETL and is-cathode with a discrete layer and a composite-like structure.
The change in the charge transport property of is-HTL with the addition of Triton X was analyzed using Raman spectroscopy. As shown in Fig. 2B, the Raman peak centered at 1436 cm−1 of is-HTL is broader and more intense than that of pristine AI 4083. Furthermore, the intensity of 1436 cm−1 peak was the highest when 5 wt % of Triton X was added to AI 4083. In PEDOT:PSS, the peak centered at 1436 cm−1 is assigned to the Cα = Cβ symmetric stretching vibration (33). The intensity increase at 1436 cm−1 indicates that the conformation of the PEDOT chains is changed from a benzoid (coiled) to a quinoid (linear) structure (33, 36). Hence, the Raman spectroscopy results correspond well to the hole transport property measured in fig. S8.
The mechanical properties of pristine AI 4083 and is-HTL were analyzed using the mechanical buckling method (12) and COS analysis. As shown in Fig. 2C, the Young’s modulus of is-HTL with 5 wt % Triton X is substantially reduced from 100 to 2.5 MPa. In addition, on the basis of the COS measurements, pristine AI 4083 shows severe cracks even at strains below 10% (fig. S9A). However, is-HTL does not show any cracks even when stretched up to 160%. This mechanical property change is due to the conformational change of the PEDOT:PSS films after the addition of Triton X (31, 32).
To further verify the change in the conformation of is-HTL, x-ray photoelectron spectroscopy (XPS) and AFM analyses were performed. According to the XPS surface analysis results in fig. S9C, the intensity ratio between the S2p bands from PEDOT and PSS increases with the addition of Triton X, which also supports the conformational change of the PEDOT chains in is-HTL (33). The conformational change of the PEDOT chains was also verified by microstructural analysis by AFM. As shown in fig. S9D, the PEDOT-rich domain is changed from a spherical to a fibrous structure (31, 32). The linear conformation is more favorable for efficient charge transport.
For is-ETL, doped polyethyleneimine ethoxylated (d-PEIE) and ZnO nanoparticles were used together. In previous works by others, the strong dipole layer formed by the amine moiety in PEIE could substantially reduce the work function of the cathode material, improving the electrical injection from the cathode (37). In addition, in our previous work, when a small amount of an n-type dopant (Cs2CO3 in this work) was added to PEIE, the work function of the cathode was further decreased (38). Moreover, PEIE was expected to be mechanically stretchable due to its low Tg of −25°C and strong hydrogen bonding arising from the amine functional moiety (39, 40). Among various nanoparticles, ZnO nanoparticles were selected because of their superior electron injection properties (41). According to the field emission scanning electron microscopy (FE-SEM) analysis in fig. S10A, is-ETL does not show any cracks during and after stretching to 100%.
For is-cathode and is-anode, one-dimensional silver nanowire (AgNW) networks were used. is-ETL was coated on AgNWs to form a composite structure. OLEDs with such a composite structure of is-ETL/AgNWs show very stable operation and good performance with an Lmax of 2500 cd m−2, as shown in Fig. 2D and fig. S10B. The significant improvement in the OLEDs’ performance was due to the efficient electron injection of the composite is-ETL/AgNWs/is-ETL structure. When AgNWs were coated on is-ETL, the interfacial contact area between AgNW and is-ETL was very small, as shown in fig. S10B. Such poor interfacial contact resulted in the inefficient electron injection from the cathode, as schematically shown in fig. S10C. Hence, OLEDs based on this type of cathode did not operate at all, as shown in Fig. 2D. To overcome this limitation, another is-ETL was coated again on the AgNW cathode to form the composite structure. As shown in fig. S10B, AgNWs were fully embedded in is-ETL. In this composite structure, the interfacial contact area between AgNWs and is-ETL notably improved, which enhanced electron injection behavior, as schematically shown in fig. S10C.
Static and cyclic stretching test results for the composite-type cathode are shown in fig. S11 (B and C). These results indicate that the composite structured cathode could maintain good electrical conductivity even stretched up to 100% strain and repeatedly stretched for hundreds of cycles. Furthermore, the composite structured cathode did not show any cracks on the surface after static and cyclic stretching tests (fig. S11D). Such stretching tests and surface analysis verified the good stretchability of composite structured cathode.
For is-anode and substrate, AgNWs and a silica aerogel composite were co-embedded in elastomeric polydimethylsiloxane (PDMS) (42). We have shown in our previous work that silica aerogels substantially improve the stretchability of AgNWs by enhancing the adhesion between AgNWs and PDMS matrices (42).
Last, is-OLEDs composed of is-anode, is-HTL, is-EML, is-ETL, and is-cathode were fabricated. Schematic descriptions of the structures and fabrication process for is-OLEDs are shown in Fig. 3A and fig. S12, respectively. Before fabricating the devices, the effects of solvents (isopropyl alcohol, 2-methoxyethanol, and toluene) used in this study on the thin films were analyzed first. As shown in fig. S13, SY did not show any change in its surface morphology and UV-vis absorption spectrum after the spin coating of either pure isopropyl alcohol or 2-methoxyethanol used as the solvents in the coating of is-ETL and is-cathode. Hence, there was no damage or conformational changes that occurred in SY during the consecutive solution-based coating processes.
Fig. 3. Fabrication and characterization of is-OLEDs.
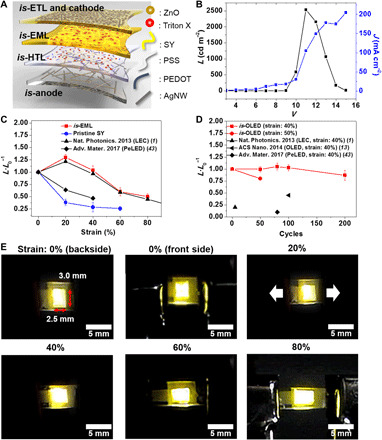
(A) Schematic structure of is-OLEDs fabricated in this study. (B) J-V-L curve of is-OLEDs. (C) Relative L change (L·L0−1) of is-OLEDs based on pristine SY and is-EML during the static stretching tests. (D) L·L0−1 changes of is-OLEDs during cyclic stretching tests. (E) Optical images of is-OLEDs with an original emission area of 3.0 × 2.5 mm2, operated under various strains at applied V of 9.5 V for 0, 20, and 40% strain and 9.8 V for 60 and 80% strain. All data were measured under ambient atmospheric conditions. L was measured on the basis of the emission from the cathode (front side). Photo credits (E): Jin-Hoon Kim, Yonsei University.
There was a slight change in the UV-vis absorption spectrum of is-HTL when spin-coated with pure toluene, which was the solvent of is-EML (fig. S14A). Raman spectroscopy and COS analysis were performed on is-HTL to determine the origin of this change. As shown in fig. S14B, there was no change in Raman spectra of both pristine AI 4083 and is-HTL after they were spin-coated of pure toluene. Hence, the spin coating of toluene does not vary the conformation in the PEDOT chains. Furthermore, as shown in fig. S14C, is-HTL still showed good stretchability after the spin coating of toluene, thus verifying the absence of any conformational change in is-HTL. Consequently, the slight change in the UV-vis absorption spectrum of is-HTL in fig. S14A could be interpreted as the removal of excess Triton X from is-HTL. The optimized is-HTL had a very large amount of Triton X mixed with AI 4083 solution at 5 wt %. Although excess Triton X was removed during the spin coating of the toluene-dissolved is-EML, it did not seriously affect the performances of is-OLEDs, as it did not cause any conformation and stretchability change of the PEDOT chain in is-HTL.
Figure 3B shows J-V-L curves of is-OLEDs for the front (cathode) side. The J-V-L curves of is-OLEDs with different EMLs (pristine SY, ST1, ST3, and ST4) are shown in fig. S15. is-OLED using ST2 showed the highest L value, which showed a similar trend with charge carrier mobility analysis shown in Fig. 1C. As the cathode and anode are transparent, is-OLEDs are semitransparent (as shown in fig. S16A), emitting light from both sides. As shown in Fig. 3B, is-OLEDs show an Lmax of 2500 cd m−2 at 11 V with a turn-on V (Von) of 8.3 V for the cathode (front) side, and the Lmax on the back (anode) side is 1900 cd m−2 at 11 V, as shown in fig. S16B. Such Lmax is the highest among the values reported for is-LECs and is-OLEDs (1, 13). Moreover, in other works based on is-LECs and is-OLEDs, Lmax was obtained at a voltage as high as 21 V (1, 13).
The stretchability of is-OLEDs was investigated by static and cyclic stretching tests. As shown in Fig. 3C, is-OLEDs based on pristine SY show poor stretchability owing to the highly brittle nature of pristine SY. However, is-OLEDs based on is-EML show superior stretchability owing to the all-is-constituent materials designed in this work. As shown in Fig. 3C, the L·L0−1 value of is-OLEDs is maintained at 1, even when OLEDs are stretched up to 40% strain, and even increases by 30% at 20% strain. Such phenomena were also observed in is-LECs reported by Liang et al. (1), which could be explained by an improvement in the interfacial charge injection and transport behavior due to the Poisson contraction during mechanical stretching. In addition, is-OLEDs maintain an L·L0−1 of 0.5 even when stretched up to 80% strain. As shown in fig. S16C, is-OLEDs show an Lmax of 1167 cd m−2 at 12 V and a Von of 9 V even when stretched up to 80% strain. The L·L0−1 values obtained from the static stretching test are the highest compared to those of other is-LEDs, including is-LECs (1) and is-perovskite LEDs (is-PeLEDs) (43) reported by others.
Such superior mechanical stretchability of is-OLEDs leads to a marked improvement in the cyclic stretching tests. As shown in Fig. 3D, is-OLEDs show almost constant L·L0−1 during cyclic stretching at 40% strain. Furthermore, more than 90% of the initial L of is-OLEDs is maintained even after 200 cycles at 40% strain. Moreover, is-OLEDs show an L·L0−1 of 0.8 for 50 cycles at 50% strain. In contrast, as shown in Fig. 3D, is-LEDs reported by others show severe degradation even after a few stretching cycles (1, 13, 43). For is-LECs, is-PeLEDS, and is-OLEDs, L·L0−1 substantially decreases to 0.2, 0.1, and 0.45 after only 5, 80, and 100 cycles of stretching at 40% strain, respectively (1, 13, 43).
The current efficiency (εc) of is-OLEDs based on is-EML and pristine SY is about 1.6 and 1.7 cd A−1, respectively. Such a similar εc value is due to the increased J value in is-OLEDs with is-EML, as shown in fig. S15. As shown in fig. S16H, the εc value of is-OLEDs slightly increases from 1.6 to 2.0 cd A−1 with stretching cycles. The increase in εc of is-OLEDs after cyclic stretching originated from the formation of the buckling waves during the cyclic stretching. As shown in fig. S17A, several buckling wave patterns were observed on the surface of is-OLEDs after the cyclic stretching due to the mismatch in the Young’s moduli of the is-OLED functional layers. These buckling wave patterns could enhance the light output by effectively extracting the trapped light in the devices (44). Optical images of is-OLEDs stretched up to various strains are shown in Fig. 3E. Uniform light emission is observed even up to 80% strain.
Cross-sectional transmission electron microscopy (TEM) analysis was used to analyze the structure of is-OLEDs and determine whether any delaminations occurred in is-OLEDs after mechanical stretching. Before stretching, is-OLEDs showed discrete interfaces between their functional layers, with each layer clearly distinguished through energy-dispersive spectroscopy (EDS) mapping shown in fig. S17B. Consequently, no observable delamination was seen in is-OLEDs even after being stretched up to 80% strain. Hence, all layers used in is-OLEDs could maintain good adhesion even during mechanical stretching.
Because the all-is-constituent materials used in this work were solution processable, the size of is-OLEDs is highly scalable. As shown in Fig. 4A, is-OLEDs with device areas up to 6 × 9 mm2 and 5 × 5 mm2 were successfully fabricated, and uniform emission throughout the area (cathode side) is observed with an Lmax of 1800 cd m−2 at 10 V. In addition, is-OLEDs can be stretched up to 60% strain with an L·L0−1 of over 0.5. Also, the biaxial stretchability could be verified by poking is-OLEDs with a sharp tip. As shown in Fig. 4B and movie S1, is-OLEDs showed stable operation even when poked by the tip of a ballpoint pen with a tip radius of 0.7 mm. is-OLEDs based on red-, green-, and blue-emitting polymers were fabricated to verify the applicability of is-OLEDs in real displays (45). To design is-EMLs, Triton X was added to commercial polymers emitting three colors. The performance of is-OLEDs with red-, green-, and blue-emitting polymers was slightly increased with the addition of Triton X. Such trend was analogous to is-OLEDs based on SY. As shown in Fig. 4C, red, green, and blue is-OLEDs could be fabricated and stretched up to 60% strain with an L·L0−1 of over 0.5.
Fig. 4. Various applications of is-OLEDs in deformable displays.
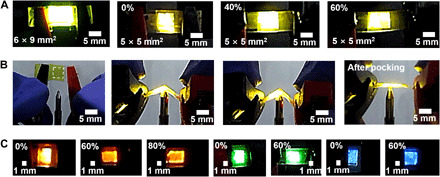
(A) Optical photographs of is-OLEDs with a large device area at applied V of 9.5 V. (B) Optical photographs of is-OLEDs being poked by a ballpoint pen with a tip radius of 0.7 mm at applied V of 9.5 V. The dashed line defines the device area. (C) Optical photographs of is-OLEDs based on red (at applied V of 12 V), green (at applied V of 12 V), and blue light-emitting polymers (at applied V of 8 V). The possibility of applying is-OLEDs to deformable displays with various form factors is shown. Photo credits (A to C): Jin-Hoon Kim, Yonsei University.
DISCUSSION
In this work, we presented a framework for designing is-constituent materials including is-emitting materials for is-OLEDs. Through various analyses, the small molecular nonionic surfactant was verified to be capable of modifying the mechanical properties of conjugated polymers via the plasticizing effect. The electrical properties of is-EMLs and is-HTL were also confirmed to change little with the highly improved mechanical properties. is-OLEDs based on these is-constituent materials showed superior mechanical stretchability compared to previously reported is-LEDs. The performances of is-OLEDs did not change even after being stretched for 200 cycles at 40% strain. In addition, is-OLEDs could be fabricated in a large area and be three-dimensionally stretchable. Last, we showed that our design strategy can be extended to materials emitting in various colors. The material design and fabrication process suggested in this work can provide a new paradigm for stretchable optoelectronics including three-dimensional displays, wearable biomedical information displays, photovoltaics, photodetectors, and various other LEDs.
The stability of is-OLEDs should be improved for practical applications. As shown in fig. S18A, the time it takes L of is-OLED to drop from L0 of 100 cd m−2 down to 50% of L0 (LT50) was around 27 s. Furthermore, when is-OLEDs were stretched up to 40 and 60% strain, LT50 was about 12 s. After 100 and 200 cycles of stretching at 40% strain, LT50 was about 24 s. The LT50 measurement results indicate that is-OLEDs were not stable during the operation. There are several reasons for the performance instability of is-OLEDs. Because there were no suitable stretchable encapsulation materials for is-OLEDs, is-OLEDs should undergo severe degradation due to exposure to oxygen and moisture during device operation. Hence, encapsulation materials for stretchable electronics should be developed to be applied in real applications.
Another reason for the is-OLED performance instability was the very high operational V of is-OLEDs shown in Fig. 3A. Such high operating V resulted into very high operation temperature, as shown in fig. S18B, which should cause severe thermal degradation of organic layers in is-OLEDs. Hence, the operating V of is-OLEDs also needs to be lowered by further optimizing the thickness of the active layers and effective energy level alignment between is-constituent materials. Last, the nanoscale protrusions were observed on the surface of is-anode, as shown in fig. S18C. Such protrusions evolved during the release of the embedded is-anodes from a sacrificial layer. These protrusions could lead to a catastrophic failure during the device operation because electrical currents could concentrate on these protrusions, resulting in severe degradation of organic layers in is-OLEDs. Hence, these issues should be resolved to improve the stability of is-OLEDs.
MATERIALS AND METHODS
Preparation of is-EML and is-HTL solutions
For preparation of the is-EML solution, Triton X (Sigma-Aldrich) was first dissolved in toluene at a certain concentration (from 1 to 10 mg ml−1). Then, SY (Sigma-Aldrich) was dissolved in the Triton X–dissolved toluene at a concentration of 5 mg ml−1. For pristine SY, SY was dissolved in pure toluene at a concentration of 5 mg ml−1. This solution was stirred using a magnetic stirrer at 300 rpm for 6 hours at room temperature.
For the is-HTL solution, various weight ratios of Triton X (from 3 to 8 wt %) were dissolved in a PEDOT:PSS solution (AI 4083, from Heraeus). Then, isopropyl alcohol was added to the is-HTL solution at a weight ratio of 1:1. This solution was stirred using a magnetic stirrer at 450 rpm for 1 hour at room temperature.
Mechanical characterization of is-EMLs and is-HTL
For COS analysis, PDMS film was prepared by mixing the base and a curing agent (Sylgard 184 elastomer kit from Dow Corning) at a weight ratio of 10:1. This liquid mixture was spin-coated at 500 rpm for 30 s on a polyethylene terephthalate (PET) substrate and cured at 120°C for 12 hours. Then, the cured PDMS film was peeled off from the PET substrate.
For COS analysis of is-HTL, is-HTL solution with 5 wt % Triton X was spin-coated on the PDMS film at 1000 rpm for 60 s. Then, is-HTL was annealed at 90°C for 10 min. For is-EML analysis, the is-EML solution was spin-coated on the is-HTL–coated PDMS substrate at 1500 rpm for 30 s and annealed at 90°C for 5 min.
Prepared specimens were cut into rectangular shapes of 25 × 5 mm2. These specimens were loaded on a homemade stretching jig. Strain from 0 to 120% for is-EML and from 0 to 160% for is-HTL was applied, and crack formation was observed at every 10% strain using optical microscopy (OM). For the crack density analysis of is-EML, the number of cracks was counted while the strain was maintained at 100%.
For Young’s modulus analysis, the mechanical buckling method was used. First, a self-assembled monolayer (SAM) was formed on a glass substrate using a silane solution (Sigma-Aldrich). For the SAM treatment, a glass substrate was placed in a vacuum chamber, and the silane solution was dropped near the glass substrate. Then, vacuum pumping was performed for 30 min, followed by thermal annealing at 130°C for 30 min. Pristine SY and is-EML were coated on this SAM-treated glass at 1500 rpm for 30 s and thermally annealed at 90°C for 5 min. Then, prestretched PDMS was conformally attached on the films. Last, PDMS was detached from the glass substrate, and the prestrain was released. During this process, pristine SY and is-EML were transferred to PDMS, and mechanical buckling was formed because of the mechanical property mismatch between the active layer and PDMS. By measuring the wavelength of the buckling formed on the thin films, the Young’s modulus of the thin films could be calculated by the following equation (6)
where Ef, Es, νf, νs, λ, and df are the Young’s moduli of the thin film and substrate, Poisson’s ratios of the thin film and substrate, wavelength of the buckling, and thickness of the thin film, respectively (46–48).
Tg of is-EMLs was analyzed by DMA. DMA was conducted following the ASTM D4065 standard. The size of the specimens was 40 mm long, 6 mm wide, and 50 μm thick. The temperature range was −50° to 100°C with a temperature increase rate of 2°C min−1. The oscillation strain was 0.1% with a frequency of 1 Hz. Tg of pristine SY and is-EMLs was located at the peak position of the loss tangent.
Electrical characterization of is-EMLs and is-HTL
To characterize is-EMLs, hole- and electron-only devices were fabricated as schematically described and shown in figs. S3 and S4, respectively. For the hole-only device, ITO with a thickness of 100 nm was deposited on a glass substrate using a DC magnetron sputtering system. Then, pristine HTL was spin-coated on the ITO-deposited glass at 4000 rpm for 60 s and annealed at 120°C for 10 min. After that, the samples were transferred to a N2-filled glove box. On top of HTL, pristine SY or an is-EML was spin-coated at 1500 rpm for 30 s. Then, the samples were thermally annealed at 90°C for 5 min. After annealing, the samples were transferred to a thermal evaporation chamber to deposit 100-nm-thick Au.
For the electron-only device, Ag with a thickness of 80 nm was deposited on the glass substrate using the thermal evaporator. d-PEIE was then used to modify the Ag cathode (38). For the d-PEIE solution, 0.25 wt % of PEIE (Sigma-Aldrich) and Cs2CO3 (Sigma-Aldrich) were co-dissolved in 2-ethoxyethanol (the weight ratio of PEIE to Cs2CO3 was 10:1). d-PEIE was spin-coated at 5000 rpm for 30 s and annealed at 100°C for 5 min. Then, the samples were transferred to the N2 gas–filled glove box. On top of d-PEIE/Ag, pristine SY or is-EML was spin-coated at 1500 rpm for 30 s and thermally annealed at 90°C for 5 min. After that, d-PEIE was spin-coated on EML at 5000 rpm for 30 s, followed by thermal annealing at 90°C for 5 min. Last, the samples were transferred to the thermal evaporation chamber to deposit 100-nm-thick Al cathodes.
The current density (J) and voltage (V) of the hole-only devices were measured using a Keithley 2400 source meter. μh and μe of the materials for EML were calculated on the basis of the space charge–limited current (SCLC) model. In the SCLC model, μ can be calculated by the Mott-Gurney equation (16)
where εr is the relative dielectric constant of the EML material, ε0 is the permittivity of free space, d is the thickness of EML, and V is the effective applied voltage in the device (V = Vapplied – Vbi – Vr, where Vapplied is the applied voltage, Vbi is the built-in voltage, which is the relative work function difference between the anode and cathode, and Vr is the voltage applied by the series and contact resistance potential drop).
OLEDs and HTL-only devices were fabricated to analyze the hole transport properties of is-HTL. The schematic structures of the HTL-only device and conventional OLED are shown in fig. S8, A and B, respectively. For the HTL-only device, 100-nm-thick ITO was deposited on a glass substrate using the DC magnetron sputter. Pristine HTL or is-HTL was then spin-coated on ITO at 1000 rpm for 60 s and annealed at 120°C for 10 min. Last, 100-nm-thick Au was deposited using the thermal evaporation system. For the conventional OLEDs, ITO with a thickness of 100 nm was deposited on a glass substrate using the DC magnetron sputter. Then, pristine HTL or is-HTL was spin-coated on ITO at 4000 rpm for 60 s. Then, the samples were thermally annealed at 120°C for 10 min. After that, the samples were transferred to the N2-filled glove box. For EML, pristine SY was spin-coated at 1500 rpm for 30 s and annealed at 90°C for 5 min. For ETL, d-PEIE was spin-coated at 5000 rpm for 30 s and annealed at 100°C for 5 min. After that, the samples were transferred to the thermal evaporation chamber to deposit a 100-nm-thick Al cathode. J, V, and L (luminance) of OLEDs were measured using a source meter and a Konica Minolta Cs-200 chromameter.
Other characterization methods of is-EMLs and is-HTL
For UV-vis absorption analysis, pristine SY and is-EMLs were spin-coated on glass substrates at 1500 rpm for 30 s in the N2-filled glove box. For Raman spectroscopy analysis of EML, pristine SY and is-EMLs were spin-coated on Si wafer substrates at 1500 rpm for 30 s in the N2-filled glove box. In addition, Triton X dissolved in toluene at a concentration of 5 mg ml−1 was also spin-coated on a Si wafer as a reference. The wavelength of the laser was 785 nm with an exposure time of 100 s.
For Raman spectroscopy analysis of HTL, pristine HTL and is-HTL were spin-coated on glass substrates at 1000 rpm for 60 s. The wavelength of the laser was 532 nm with an exposure time of 60 s. For XPS analysis, pristine HTL and is-HTL were spin-coated on a Si wafer. XPS analysis was performed using a monochromated Al Kα x-ray source. For microstructural analysis, tapping mode AFM (Park Systems) was used.
For the solvent orthogonality tests, solvents used in the fabrication of is-OLEDs were spin-coated on the surface of EML or HTL. For EML, pure isopropyl alcohol or 2-methoxyethanol was spin-coated on the EML-coated glass and annealed at 90°C for 5 min. For HTL, pure toluene was spin-coated on the HTL-coated glass and annealed at 90°C for 5 min. UV-vis absorption, OM, and Raman spectroscopy analysis were then used to determine the variations in the functional layers.
Preparation and characterization methods of is-ETL and is-cathode
For is-ETL, a ZnO nanoparticle dispersion and a d-PEIE solution were prepared. ZnO nanoparticles with a diameter of 110 nm (Sigma-Aldrich) were diluted to 3 wt % using isopropyl alcohol. The ZnO nanoparticle solution was sonicated using an ultrasonication bath before use. For the d-PEIE solution, 3 wt % PEIE (Sigma-Aldrich) and Cs2CO3 (Sigma-Aldrich) were co-dissolved in 2-methoxyethanol (the weight ratio of PEIE to Cs2CO3 was 10:1). The d-PEIE solution was stirred at 80°C for 6 hours.
For microstructural characterization, ZnO dispersion and d-PEIE solution were spin-coated on AgNW network–embedded PDMS. The detailed preparation process for the AgNW-embedded PDMS is described below (38). Before spin coating, the AgNW-embedded PDMS was O2 plasma–treated at 140 W for 90 s. ZnO dispersion was spin-coated at 1000 rpm for 30 s and annealed at 90°C for 5 min. On the ZnO nanoparticles, d-PEIE was spin-coated at 5000 rpm for 30 s and annealed at 90°C for 5 min. These processes were performed under ambient air conditions. The microstructure of is-ETL was analyzed using FE-SEM.
For electrical characterization, OLEDs with a discrete layer of is-ETL and OLEDs with a composite structure were fabricated. A schematic structure of each OLED is shown in fig. S10B. For OLEDs, ITO, is-HTL, and pristine SY were formed on a glass substrate as previously mentioned. On top of pristine SY, ZnO dispersion was spin-coated at 1000 rpm for 30 s in the N2-filled glove box and annealed at 90°C for 5 min. The d-PEIE solution was spin-coated at 5000 rpm for 30 s in the N2-filled glove box and annealed at 90°C for 5 min. After annealing d-PEIE, the samples were transferred to ambient atmosphere. Masking tape (Kapton tape) was used to define the cathode area. The AgNW solution (with a diameter of 30 nm and a length of 30 μm; Novarials) at a concentration of 2.5 mg ml−1 was spin-coated two times at 500 rpm for 30 s to form the cathode. AgNWs were annealed at 90°C for 5 min. For the composite structure, is-ETL was coated on AgNWs following the same process mentioned above.
Static and cyclic stretching tests were performed on is-cathode with composite structure. PDMS films were prepared by mixing the base and curing agent (Sylgard 184 elastomer kit from Dow Corning) at a weight ratio of 10:1. This liquid mixture was spin-coated at 500 rpm for 30 s on a PET substrate and cured at 120°C for 12 hours. The cured PDMS films were then peeled off from the PET substrate. On the PDMS films, ZnO nanoparticles were spin-coated at 1000 rpm for 30 s and annealed at 90°C for 5 min. Before spin coating ZnO nanoparticles, the surface of PDMS was O2 plasma–treated at 140 W for 90 s. On ZnO, d-PEIE was spin-coated at 5000 rpm for 30 s and annealed at 90°C for 5 min. A AgNW dispersion with a concentration of 2.5 mg ml−1 was then spin-coated three times at 500 rpm for 30 s and annealed at 90°C for 5 min. On the AgNW cathode, ZnO nanoparticles were spin-coated at 500 rpm for 30 s and annealed at 90°C for 5 min. Last, the d-PEIE solution was coated at 1000 rpm for 30 s, followed by annealing at 90°C for 10 min.
For static stretching tests, the strain was applied from 0 to 100% with a stretching rate of 10% s−1, and electrical resistance (R) was measured using a digital multimeter (DMM). The cyclic stretching test was performed at 20 and 40% strain at a stretching rate of 20 and 40% s−1, respectively. The change in R was measured using DMM. After stretching tests, the surface of the composite structured cathode was analyzed using FE-SEM.
Fabrication of is-OLEDs and characterization methods
Schematic descriptions for the fabrication processes of is-OLEDs were shown in fig. S12. For is-anode, AgNWs and aerogel nanoparticles (JIOS Aerogel) were co-embedded in a PDMS matrix. A AgNW concentration of 5 mg ml−1 was spin-coated two times on a PET substrate at 500 rpm for 30 s and annealed at 100°C for 5 min. The anode area was defined using a cotton swab. On AgNWs, an aerogel dispersion with 4 wt % ethanol was spin-coated at 1000 rpm for 30 s, followed by annealing at 100°C for 10 min. Then, a liquid mixture of PDMS was spin-coated at 300 rpm for 30 s, and PDMS was cured at 120°C for 12 hours. After curing PDMS, the AgNW-embedded PDMS was released from the PET substrate.
On is-anode, the is-HTL solution was spin-coated. Before coating is-HTL, is-anode was O2 plasma–treated at 140 W for 90 s. is-HTL was spin-coated at 1000 rpm for 60 s and annealed at 90°C for 10 min. Then, the samples were transferred to the N2-filled glove box. is-EML was spin-coated at 1500 rpm for 30 s, followed by annealing at 90°C for 5 min. For is-OLEDs with various colors, a red-orange light-emitting PPV-based copolymer (Sigma-Aldrich) dissolved in toluene at a concentration of 5 mg ml−1 for the red is-OLED, a green light-emitting spiro-based copolymer (Sigma-Aldrich) dissolved in toluene at a concentration of 8 mg ml−1 for the green is-OLED, and poly(9,9-di-n-octylfluorenyl-2,7,diyl) (Sigma-Aldrich) dissolved in toluene at a concentration of 5 mg ml−1 for the blue is-OLED were used. ZnO nanoparticles were spin-coated at 1000 rpm for 30 s and annealed at 90°C for 5 min. On ZnO, d-PEIE was spin-coated at 5000 rpm for 30 s and annealed at 90°C for 5 min. After annealing d-PEIE, the samples were moved to ambient atmosphere, and the cathode area was defined using masking tape. Then, a AgNW dispersion with a concentration of 2.5 mg ml−1 was spin-coated two times at 500 rpm for 30 s and annealed at 90°C for 5 min. On the AgNW cathode, ZnO nanoparticles were spin-coated at 500 rpm for 30 s and annealed at 90°C for 5 min. Last, the d-PEIE solution was coated at 1000 rpm for 30 s, followed by annealing at 90°C for 10 min. Then, the masking tape was removed, and the anode was exposed by removing the is-ETL layer.
J, V, and L of is-OLEDs were measured using a source meter and a chromameter. For electrical contact, liquid metal (InGa eutectic, Sigma-Aldrich) was applied on both the anode and cathode of is-OLEDs. For mechanical characterization, static and cyclic stretching tests were performed. For the static stretching test, V was applied during stretching up to 20, 40, 60, and 80% strain. The stretching rate was 80% s−1. The cyclic stretching test was performed at 20, 40, and 50% strain with a stretching rate of 80% s−1 for various stretching cycles.
is-OLEDs were analyzed using cross-sectional TEM analysis. For cross-sectional analysis, the surface of is-OLEDs was milled using focused ion beam to extract the cross section of is-OLEDs. The cross section of is-OLEDs was observed, and the atomic components were analyzed through EDS mapping. For lifetime analysis, is-OLEDs were operated at a voltage of 9.3 V, and J-V-L characteristics were regularly measured every 3 s.
Acknowledgments
Funding: J.-H.K. and J.-W.P. acknowledge the support from the National Research Foundation of Korea (NRF), the Ministry of Science, ICT and Future Planning (grant number 2018R1A2B6001390). This research was also supported by the Graduate School of Yonsei University Research Scholarship Grants in 2019. Author contributions: J.-H.K. and J.-W.P. designed the project. J.-H.K. carried out the experiments and analysis. J.-H.K. and J.-W.P. wrote the paper. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/9/eabd9715/DC1
REFERENCES AND NOTES
- 1.Liang J. J., Li L., Niu X. F., Yu Z. B., Pei Q. B., Elastomeric polymer light-emitting devices and displays. Nat. Photonics 7, 817–824 (2013). [Google Scholar]
- 2.Kim D.-H., Lu N., Ma R., Kim Y.-S., Kim R.-H., Wang S., Wu J., Won S. M., Tao H., Islam A., Yu K. J., Kim T.-i., Chowdhury R., Ying M., Xu L., Li M., Chung H.-J., Keum H., McCormick M., Liu P., Zhang Y.-W., Omenetto F. G., Huang Y., Coleman T., Rogers J. A., Epidermal electronics. Science 333, 838–843 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Yokota T., Zalar P., Kaltenbrunner M., Jinno H., Matsuhisa N., Kitanosako H., Tachibana Y., Yukita W., Koizumi M., Someya T., Ultraflexible organic photonic skin. Sci. Adv. 2, e1501856 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larson C., Peele B., Li S., Robinson S., Totaro M., Beccai L., Mazzolai B., Shepherd R., Highly stretchable electroluminescent skin for optical signaling and tactile sensing. Science 351, 1071–1074 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Sekitani T., Nakajima H., Maeda H., Fukushima T., Aida T., Hata K., Someya T., Stretchable active-matrix organic light-emitting diode display using printable elastic conductors. Nat. Mater. 8, 494–499 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Oh J. Y., Rondeau-Gagné S., Chiu Y. C., Chortos A., Lissel F., Wang G. J. N., Schroeder B. C., Kurosawa T., Lopez J., Katsumata T., Xu J., Zhu C. X., Gu X. D., Bae W. G., Kim Y., Jin L. H., Chung J. W., Tok J. B. H., Bao Z. N., Intrinsically stretchable and healable semiconducting polymer for organic transistors. Nature 539, 411–415 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Sekitani T., Noguchi Y., Hata K., Fukushima T., Aida T., Someya T., A rubberlike stretchable active matrix using elastic conductors. Science 321, 1468–1472 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Hong J. H., Shin J. M., Kim G. M., Joo H., Park G. S., Hwang I. B., Kim M. W., Park W. S., Chu H. Y., Kim S., 9.1-inch stretchable AMOLED display based on LTPS technology. J. Soc. Inf. Display 25, 194–199 (2017). [Google Scholar]
- 9.Kim R.-H., Kim D.-H., Xiao J., Kim B. H., Park S.-I., Panilaitis B., Ghaffari R., Yao J., Li M., Liu Z., Malyarchuk V., Kim D. G., Le A.-P., Nuzzo R. G., Kaplan D. L., Omenetto F. G., Huang Y., Kang Z., Rogers J. A., Waterproof AlInGaP optoelectronics on stretchable substrates with applications in biomedicine and robotics. Nat. Mater. 9, 929–937 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Wang S. H., Xu J., Wang W. C., Wang G. J. N., Rastak R., Molina-Lopez F., Chung J. W., Niu S. M., Feig V. R., Lopez J., Lei T., Kwon S. K., Kim Y., Foudeh A. M., Ehrlich A., Gasperini A., Yun Y., Murmann B., Tok J. B. H., Bao Z. A., Skin electronics from scalable fabrication of an intrinsically stretchable transistor array. Nature 555, 83–88 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Kim J., Salvatore G. A., Araki H., Chiarelli A. M., Xie Z., Banks A., Sheng X., Liu Y., Lee J. W., Jang K.-I., Heo S. Y., Cho K., Luo H., Zimmerman B., Kim J., Yan L., Feng X., Xu S., Fabiani M., Gratton G., Huang Y., Paik U., Rogers J. A., Battery-free, stretchable optoelectronic systems for wireless optical characterization of the skin. Sci. Adv. 2, e1600418 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J., Wang S. H., Wang G. J. N., Zhu C. X., Luo S. C., Jin L. H., Gu X. D., Chen S. C., Feig V. R., To J. W. F., Rondeau-Gagne S., Park J., Schroeder B. C., Lu C., Oh J. Y., Wang Y. M., Kim Y. H., Yan H., Sinclair R., Zhou D. S., Xue G., Murmann B., Linder C., Cai W., Tok J. B. H., Chung J. W., Bao Z. N., Highly stretchable polymer semiconductor films through the nanoconfinement effect. Science 355, 59–64 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Liang J., Li L., Tong K., Ren Z., Hu W., Niu X., Chen Y., Pei Q., Silver nanowire percolation network soldered with graphene oxide at room temperature and its application for fully stretchable polymer light-emitting diodes. ACS Nano 8, 1590–1600 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Yu Z., Niu X., Liu Z., Pei Q., Intrinsically stretchable polymer light-emitting devices using carbon nanotube-polymer composite electrodes. Adv. Mater. 23, 3989–3994 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Root S. E., Savagatrup S., Printz A. D., Rodriquez D., Lipomi D. J., Mechanical properties of organic semiconductors for stretchable, highly flexible, and mechanically robust electronics. Chem. Rev. 117, 6467–6499 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Chen S., Jung S., Cho H. J., Kim N.-H., Jung S., Xu J., Oh J., Cho Y., Kim H., Lee B., An Y., Zhang C., Xiao M., Ki H., Zhang Z.-G., Kim J.-Y., Li Y., Park H., Yang C., Highly flexible and efficient all-polymer solar cells with high-viscosity processing polymer additive toward potential of stretchable devices. Angew. Chem. Int. Ed. 57, 13277–13282 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Gambino S., Bansal A. K., Samuel I. D. W., Comparison of hole mobility in thick and thin films of a conjugated polymer. Org. Electron. 11, 467–471 (2010). [Google Scholar]
- 18.Tseng S. R., Chen Y. S., Meng H. F., Lai H. C., Yeh C. H., Horng S. F., Liao H. H., Hsu C. S., Electron transport and electroluminescent efficiency of conjugated polymers. Synth. Met. 159, 137–141 (2009). [Google Scholar]
- 19.Zheng Y., Wang G.-J. N., Kang J., Nikolka M., Wu H.-C., Tran H., Zhang S., Yan H., Chen H., Yuen P. Y., Mun J., Dauskardt R. H., McCulloch I., Tok J. B. H., Gu X., Bao Z., An intrinsically stretchable high-performance polymer semiconductor with low crystallinity. Adv. Funct. Mater. 29, 1905340 (2019). [Google Scholar]
- 20.Wang G. J. N., Gasperini A., Bao Z. A., Stretchable polymer semiconductors for plastic electronics. Adv. Electron. Mater. 4, 1700429 (2018). [Google Scholar]
- 21.Burns S., MacLeod J., Trang Do T., Sonar P., Yambem S. D., Effect of thermal annealing Super Yellow emissive layer on efficiency of OLEDs. Sci. Rep. 7, 40805 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen T.-Q., Martini I. B., Liu J., Schwartz B. J., Controlling interchain interactions in conjugated polymers: The effects of chain morphology on exciton−exciton annihilation and aggregation in MEH−PPV films. J. Phys. Chem. B 104, 237–255 (2000). [Google Scholar]
- 23.Mulazzi E., Ripamonti A., Wery J., Dulieu B., Lefrant S., Theoretical and experimental investigation of absorption and Raman spectra of poly(paraphenylene vinylene). Phys. Rev. B 60, 16519–16525 (1999). [Google Scholar]
- 24.Feng L., Wang F., Niu M.-S., Zheng F., Bi P.-Q., Yang X.-Y., Xu W.-L., Hao X.-T., Structural and optical properties of conjugated polymer and carbon-based non-fullerene material blend films for photovoltaic applications. Opt. Mater. Express 7, 687–697 (2017). [Google Scholar]
- 25.Traiphol R., Sanguansat P., Srikhirin T., Kerdcharoen T., Osotchan T., Spectroscopic study of photophysical change in collapsed coils of conjugated polymers: Effects of solvent and temperature. Macromolecules 39, 1165–1172 (2006). [Google Scholar]
- 26.Kim J., Swager T. M., Control of conformational and interpolymer effects in conjugated polymers. Nature 411, 1030–1034 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Bruevich V. V., Makhmutov T. S., Elizarov S. G., Nechvolodova E. M., Paraschuk D. Y., Raman spectroscopy of intermolecular charge transfer complex between a conjugated polymer and an organic acceptor molecule. J. Chem. Phys. 127, 104905 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Prins P., Grozema F. C., Siebbeles L. D. A., Efficient charge transport along phenylene−vinylene molecular wires. J. Phys. Chem. B 110, 14659–14666 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Prodhan S., Qiu J., Ricci M., Roscioni O. M., Wang L., Beljonne D., Design rules to maximize charge-carrier mobility along conjugated polymer chains. J. Phys. Chem. Lett. 11, 6519–6525 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Oh J. Y., Kim S., Baik H.-K., Jeong U., Conducting polymer dough for deformable electronics. Adv. Mater. 28, 4455–4461 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Teo M. Y., Kim N., Kee S., Kim B. S., Kim G., Hong S., Jung S., Lee K., Highly stretchable and highly conductive PEDOT:PSS/ionic liquid composite transparent electrodes for solution-processed stretchable electronics. ACS Appl. Mater. Interfaces 9, 819–826 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Wang Y., Zhu C., Pfattner R., Yan H., Jin L., Chen S., Molina-Lopez F., Lissel F., Liu J., Rabiah N. I., Chen Z., Chung J. W., Linder C., Toney M. F., Murmann B., Bao Z., A highly stretchable, transparent, and conductive polymer. Sci. Adv. 3, e1602076 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu B., Gopalan S.-A., Gopalan A.-I., Muthuchamy N., Lee K.-P., Lee J.-S., Jiang Y., Lee S.-W., Kim S.-W., Kim J.-S., Jeong H.-M., Kwon J.-B., Bae J.-H., Kang S.-W., Functional solid additive modified PEDOT:PSS as an anode buffer layer for enhanced photovoltaic performance and stability in polymer solar cells. Sci. Rep. 7, 45079 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng H., Zheng Y., Liu N., Ai N., Wang Q., Wu S., Zhou J., Hu D., Yu S., Han S., Xu W., Luo C., Meng Y., Jiang Z., Chen Y., Li D., Huang F., Wang J., Peng J., Cao Y., All-solution processed polymer light-emitting diode displays. Nat. Commun. 4, 1971 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Armin A., Jansen-van Vuuren R. D., Kopidakis N., Burn P. L., Meredith P., Narrowband light detection via internal quantum efficiency manipulation of organic photodiodes. Nat. Commun. 6, 6343 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Ouyang J., Xu Q., Chu C.-W., Yang Y., Li G., Shinar J., On the mechanism of conductivity enhancement in poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) film through solvent treatment. Polymer 45, 8443–8450 (2004). [Google Scholar]
- 37.Zhou Y., Fuentes-Hernandez C., Shim J., Meyer J., Giordano A. J., Li H., Winget P., Papadopoulos T., Cheun H., Kim J., Fenoll M., Dindar A., Haske W., Najafabadi E., Khan T. M., Sojoudi H., Barlow S., Graham S., Brédas J.-L., Marder S. R., Kahn A., Kippelen B., A universal method to produce low–work function electrodes for organic electronics. Science 336, 327–332 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Kim J.-H., Park J.-W., Designing an electron-transport layer for highly efficient, reliable, and solution-processed organic light-emitting diodes. J. Mater. Chem. C 5, 3097–3106 (2017). [Google Scholar]
- 39.Clark S. L., Hammond P. T., The role of secondary interactions in selective electrostatic multilayer deposition. Langmuir 16, 10206–10214 (2000). [Google Scholar]
- 40.Ohisa S., Kato T., Takahashi T., Suzuki M., Hayashi Y., Koganezawa T., McNeill C. R., Chiba T., Pu Y.-J., Kido J., Conjugated polyelectrolyte blend with polyethyleneimine ethoxylated for thickness-insensitive electron injection layers in organic light-emitting devices. ACS Appl. Mater. Interfaces 10, 17318–17326 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Dai X., Zhang Z., Jin Y., Niu Y., Cao H., Liang X., Chen L., Wang J., Peng X., Solution-processed, high-performance light-emitting diodes based on quantum dots. Nature 515, 96–99 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Kim J., Park J., Jeong U., Park J.-W., Silver nanowire network embedded in polydimethylsiloxane as stretchable, transparent, and conductive substrates. J. Appl. Polym. Sci. 133, 43830 (2016). [Google Scholar]
- 43.Bade S. G. R., Shan X., Hoang P. T., Li J., Geske T., Cai L., Pei Q., Wang C., Yu Z., Stretchable light-emitting diodes with organometal-halide-perovskite–polymer composite emitters. Adv. Mater. 29, 1607053 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Koo W. H., Jeong S. M., Araoka F., Ishikawa K., Nishimura S., Toyooka T., Takezoe H., Light extraction from organic light-emitting diodes enhanced by spontaneously formed buckles. Nat. Photonics 4, 222–226 (2010). [Google Scholar]
- 45.Han T.-H., Choi M.-R., Jeon C.-W., Kim Y.-H., Kwon S.-K., Lee T.-W., Ultrahigh-efficiency solution-processed simplified small-molecule organic light-emitting diodes using universal host materials. Sci. Adv. 2, e1601428 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim J.-H., Kim S.-R., Kil H.-J., Kim Y.-C., Park J.-W., Highly conformable, transparent electrodes for epidermal electronics. Nano Lett. 18, 4531–4540 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Du J., Anye V. C., Vodah E. O., Tong T., Zebaze Kana M. G., Soboyejo W. O., Pressure-assisted fabrication of organic light emitting diodes with MoO3 hole-injection layer materials. J. Appl. Phys. 115, 233703 (2014). [Google Scholar]
- 48.Dogru S., Aksoy B., Bayraktar H., Alaca B. E., Poisson’s ratio of PDMS thin films. Polym. Test 69, 375–384 (2018). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/9/eabd9715/DC1


