Abstract
Objectives
The goal of this study is to determine the incidence, predictors, and outcomes of atrial fibrillation (AF) or atrial flutter (AFL) in patients hospitalized with coronavirus disease-2019 (COVID-19).
Background
COVID-19 results in increased inflammatory markers previously associated with atrial arrhythmias. However, little is known about their incidence or specificity in COVID-19 or their association with outcomes.
Methods
This is a retrospective analysis of 3,970 patients admitted with polymerase chain reaction–positive COVID-19 between February 4 and April 22, 2020, with manual review performed of 1,110. The comparator arm included 1,420 patients with influenza hospitalized between January 1, 2017, and January 1, 2020.
Results
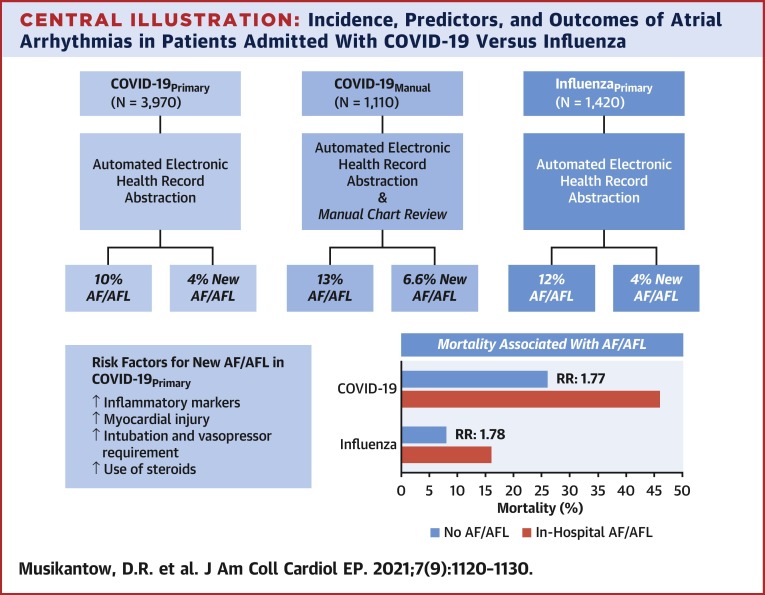
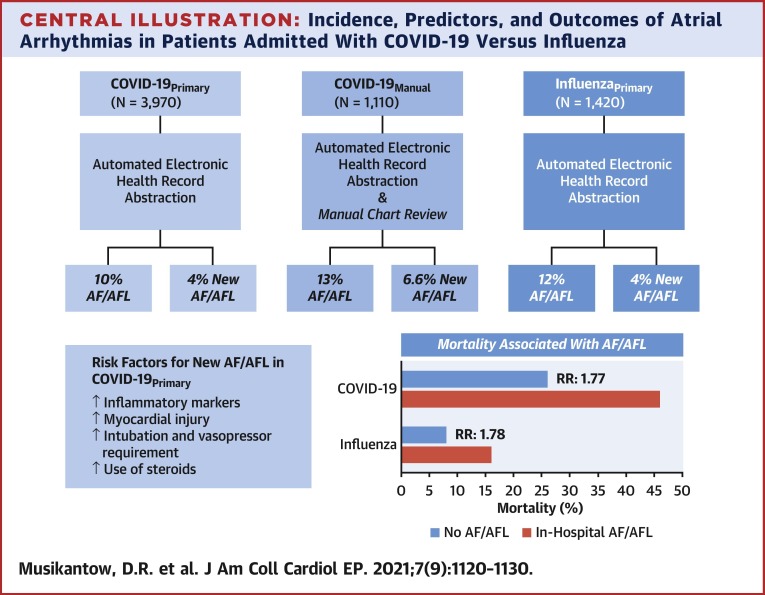
Among 3,970 inpatients with COVID-19, the incidence of AF/AFL was 10% (n = 375) and in patients without a history of atrial arrhythmias it was 4% (n = 146). Patients with new-onset AF/AFL were older with increased inflammatory markers including interleukin 6 (93 vs. 68 pg/ml; p < 0.01), and more myocardial injury (troponin-I: 0.2 vs. 0.06 ng/ml; p < 0.01). AF and AFL were associated with increased mortality (46% vs. 26%; p < 0.01). Manual review captured a somewhat higher incidence of AF/AFL (13%, n = 140). Compared to inpatients with COVID-19, patients with influenza (n = 1,420) had similar rates of AF/AFL (12%, n = 163) but lower mortality. The presence of AF/AFL correlated with similarly increased mortality in both COVID-19 (relative risk: 1.77) and influenza (relative risk: 1.78).
Conclusions
AF/AFL occurs in a subset of patients hospitalized with either COVID-19 or influenza and is associated with inflammation and disease severity in both infections. The incidence and associated increase in mortality in both cohorts suggests that AF/AFL is not specific to COVID-19, but is rather a generalized response to the systemic inflammation of severe viral illnesses.
Key Words: atrial fibrillation, atrial flutter, coronavirus disease-2019, influenza, ischemic stroke
Abbreviations and Acronyms: AF, atrial fibrillation; AFL, atrial flutter; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease-2019; ECG, electrocardiogram; ICD, International Classification of Disease; IL, interleukin; TIA, transient ischemic attack
Central Illustration
As of September 10, 2020, there have been 28 million patients with coronavirus disease-2019 (COVID-19) infections worldwide and more than 900,000 deaths (1). The pathophysiology of the severe acute respiratory syndrome coronavirus 2 viral infection appears driven by an inflammatory immune response with several markers of inflammation, such as C-reactive protein and the cytokine interleukin (IL)-6, correlating with disease severity and mortality (2,3).
Even before the COVID-19 pandemic, atrial fibrillation (AF) and atrial flutter (AFL) had been linked to conditions characterized by elevated inflammatory markers (4,5). Hence, it is not surprising that a high incidence of AF/AFL has been reported with COVID-19 (6, 7, 8). However, as available studies have been limited in scope and specificity, the true incidence of AF/AFL in this population is unknown. Also uncertain is whether the inflammatory milieu of COVID-19 is uniquely responsible for AF/AFL, or whether these arrhythmias reflect part of a nonspecific byproduct of severe viral respiratory illness.
Beyond inflammation, COVID-19 has been associated with both an elevated incidence of myocardial injury, and an increased risk of thrombotic events such as venous thromboembolism and ischemic stroke (9, 10, 11, 12). Accordingly, it is possible both that AF/AFL may correlate with cardiac injury, and, in the context of a prothrombotic state, contribute to the increased risk of thromboembolic events such as ischemic stroke.
We performed a retrospective analysis of a large cohort of hospitalized patients afflicted with COVID-19 (n = 3,970) to assess the incidence, predictors, and outcomes of AF/AFL. To address the unusual clinical environment occurring during the New York City COVID-19 pandemic, in a subset of this cohort, we also performed a manual chart review of primary patient data including electrocardiograms (ECGs) and telemetry to assess for under-representation of arrhythmias in clinical coding. Finally, we compared these observations to patients hospitalized with influenza to assess whether these atrial arrhythmias uniquely result from COVID-19, or whether they reflect a response to acute respiratory illness.
Methods
Study populations
This multicenter retrospective cohort study included consecutive adult patients (≥18 years of age) with laboratory-confirmed COVID-19 infection, admitted to 5 hospitals within the Mount Sinai Health System. We studied 3 patient cohorts—of which 2 overlapped. 1) The principal automated electronic record abstraction cohort (COVID-19Primary) included all patients with laboratory-confirmed COVID-19 admitted to the hospitals between February 4 and April 22, 2020. 2) The manually adjudicated patient cohort (COVID-19Manual) was drawn from the same population of patients, but only included consecutive patients admitted until March 28, 2020, and excluded patients who tested positive for COVID-19 more than 1 week into hospitalization. This exclusion was done because the manual cohort included a disproportionate amount of patients diagnosed at onset of the pandemic including several who had prolonged hospitalizations with unrelated conditions and contracted COVID-19 while an inpatient. 3) The automated electronic record abstraction influenza cohort (InfluenzaPrimary) included all patients with polymerase chain reaction–positive influenza A or B from January 1, 2017, until January 1, 2020; there was no temporal overlap with the COVID-19 population. All patient data were de-identified before analysis, and data abstraction was approved by the Mount Sinai Institutional Review Board.
Data collection
Data were abstracted from the electronic health records including baseline demographics, laboratory measurements, inpatient medications, and outcomes. Using the International Classification of Disease, version 9/10 (ICD 9/10) billing codes, comorbidities were identified; these included congestive heart failure (CHF), hypertension, diabetes, prior stroke/transient ischemic attack (TIA), chronic kidney and liver disease, HIV, chronic obstructive pulmonary disease (COPD), asthma, and obstructive sleep apnea. An analysis was then performed using ICD 9/10 codes for the occurrence of in-hospital ischemic stroke or TIA, and AF/AFL.
The COVID-19Manual cohort included laboratory data, baseline demographics, and hospital medications abstracted from the electronic health record and then manually reviewed. Baseline comorbidities, pre-hospital medications, and in-hospital events (including neurological events) were obtained from available clinical records. All available ECGs were independently reviewed by a cardiologist or electrophysiologist and chart documentation was assessed for atrial arrhythmias.
Statistical analysis
Continuous variables were summarized as median and interquartile range or means and standard deviations, as appropriate. Categorical variables were summarized as counts or percentages. No imputation was made for missing data. Median/Mann-Whitney U test, Fisher exact test, or chi square test was used to compare data where appropriate. A 2-tailed p value ≤ 0.05 was considered statistically significant. In the comparison of patients with influenza versus COVID-19 with new AF/AFL, we included only variables which were available in at least 75% of patients in both groups. We then plotted Kaplan-Meier curves for in-hospital mortality stratified by the presence of in-hospital AF/AFL. As our follow-up only included the duration of the hospitalization, patients discharged from the hospital were considered to have survived for the purposes of these curves. Separate Kaplan-Meier curves were also created in which discharged patients were censored (Supplemental Figure S1)
A multivariable logistic regression was performed examining predictors of new-onset AF. As not all laboratory values of interest were available for each patient, each value was included in an individual model along with other predictors of new-onset AF (age, race, hypertension, diabetes, and prior history of neurological event). Finally, a sensitivity analysis was performed comparing the relative risk (RR) of developing in-hospital and new-onset AF for patients admitted with COVID-19 versus those admitted with influenza. Models were constructed to adjust for differences in baseline demographics represented by hypertension, diabetes, prior stroke/TIA, chronic kidney disease, and COPD as well as severity of illness as represented by need for mechanical ventilation or vasopressors.
Statistical analysis was performed using SPSS version 25.0 (IBM Corp) as well as STATA version 16.1 (StataCorp LLC).
Results
Incidence and predictors of AF/AFL in COVID-19 patients
In the COVID-19Primary cohort, 3,970 patients admitted with polymerase chain reaction–confirmed COVID-19 were identified and incorporated into the analysis. The overall incidence of AF/AFL occurring during hospitalization was 10% (n = 375 patients). As shown in Table 1 , patients with AF/AFL were older (median 77 vs. 65 years of age; p < 0.01) and with more baseline comorbidities, including hypertension (56% vs. 32%; p < 0.01), diabetes (33% vs. 24%; p < 0.01), and CHF (25% vs. 5%, p < 0.01). Most patients with inpatient AF/AFL (61%) had a history of atrial arrhythmias, and of those with a history of atrial arrhythmias, 71% manifested AF/AFL during hospitalization. The overall incidence of AF/AFL in patients without a history of atrial arrhythmias (new-onset AF/AFL) was 4% (n = 146).
Table 1.
Patient Characteristics of the COVID-19Primary Cohort Stratified by In-Hospital AF/AFL and New AF/AFL
| All Patients (N = 3,970) | n | No AF/AFL (n = 3,595) | In-Hospital AF/AFL |
||||
|---|---|---|---|---|---|---|---|
| All AF/AFL (n = 375) | p Value | New-Onset AF/AFL (n = 146) | p Value | ||||
| Baseline demographics | |||||||
| Age, yrs | 66 (55–77) | 3,970 | 65 (54–76) | 77 (68–85) | <0.01 | 74 (68-84) | <0.01 |
| Male | 2,288 (57.6) | 3,970 | 2,063 (57.4) | 225 (60.0) | 0.33 | 90 (61.6) | 0.31 |
| Race/ethnicity | 3,970 | <0.01 | <0.01 | ||||
| Caucasian | 946 (23.8) | 792 (22.0) | 154 (41.1) | 55 (37.7) | |||
| African-American | 1,107 (27.9) | 1,025 (28.5) | 82 (21.9) | 33 (22.6) | |||
| Asian | 208 (5.2) | 189 (5.3) | 19 (5.1) | 9 (6.2) | |||
| Hispanic | 1,240 (31.2) | 1,154 (32.1) | 86 (22.9) | 30 (20.6) | |||
| Other or unknown | 469 (11.8) | 435 (12.1) | 34 (9.1) | 19 (13.0) | |||
| Obesity | 1,312 (33.1) | 3,970 | 1,177 (32.7) | 135 (36.0) | 0.20 | 50 (34.3) | 0.70 |
| Body mass index, kg/m2 | 27.8 (24.2–32.6) | 3,580 | 27.9 (24.3–32.6) | 27.4 (23.4–32.9) | 0.38 | 27.4 (23.8-32.4) | 0.93 |
| CHF | 271 (6.8) | 3,970 | 177 (4.9) | 94 (25.1) | <0.01 | 6 (4.11) | 0.66 |
| Prior atrial arrhythmias | 3,970 | ||||||
| Atrial fibrillation or flutter | 339 (8.5) | 110 (3.1) | 229 (61.1) | <0.01 | |||
| Atrial flutter | 57 (1.4) | 13 (0.4) | 44 (11.7) | <0.01 | |||
| Atrial fibrillation | 326 (8.2) | 104 (2.9) | 222 (59.2) | <0.01 | |||
| CAD | 410 (10.4) | 3,970 | 320 (9) | 90 (24) | <0.01 | 13 (8.9) | 0.68 |
| Hypertension | 1,367 (34.4) | 3,970 | 1,159 (32.2) | 208 (55.5) | <0.01 | 50 (34.3) | 0.61 |
| Diabetes | 976 (24.6) | 3,970 | 851 (23.7) | 125 (33.3) | <0.01 | 36 (24.7) | 0.78 |
| Prior stroke/TIA | 160 (4.0) | 3,970 | 123 (3.4) | 37 (9.9) | <0.01 | 7 (4.8) | 0.38 |
| Chronic kidney disease | 446 (11.2) | 3,970 | 368 (10.2) | 78 (20.8) | <0.01 | 20 (13.7) | 0.18 |
| Chronic liver disease | 79 (2.1) | 3,970 | 74 (2.1) | 5 (1.3) | 0.34 | 0 (0.0) | 0.12a |
| HIV | 68 (1.7) | 3,970 | 61 (1.7) | 7 (1.9) | 0.81 | 1 (0.7) | 0.52a |
| COPD | 157 (4.0) | 3,970 | 122 (3.4) | 35 (9.3) | <0.01 | 3 (2.1) | 0.49a |
| Asthma | 185 (4.7) | 3,970 | 166 (4.6) | 19 (5.1) | 0.70 | 3 (2.1) | 0.22a |
| OSA | 70 (1.8) | 3,970 | 57 (1.6) | 13 (3.5) | 0.01 | 1 (0.7) | 0.73a |
| Smoking | 3,970 | <0.01 | 0.35 | ||||
| Current | 152 (3.8) | 135 (3.8) | 17 (4.5) | 8 (5.5) | |||
| Past | 815 (20.5) | 696 (19.4) | 119 (31.7) | 23 (15.8) | |||
| Never | 3,003 (75.6) | 2,764 (76.9) | 239 (63.7) | 115 (78.8) | |||
| Hospitalization vital signs | |||||||
| Peak heart rate, beats/min | 96.0 (84.0-110.0) | 3,942 | 96.0 (84.0-110.0) | 96.0 (84.0-110.0) | 0.07 | 99.5 (82.0-117.0) | 0.13 |
| Max temperature, °F | 101.0 (99.8-102.4) | 3,942 | 101.0 (99.8-102.4) | 101.1 (99.8-102.5) | 0.87 | 101.5 (99.9-102.8) | 0.11 |
| Laboratory data | |||||||
| White blood cell count, × 10⁹/l | 7.6 (5.5-10.6) | 3,965 | 7.6 (5.5-10.5) | 7.9 (5.6-11.4) | 0.24 | 8.7 (5.8-12.0) | 0.13 |
| Neutrophil count, • 10⁹/l | 6.3 (4.1-9.7) | 2,550 | 6.2 (4.1-9.6) | 6.6 (4.1-10.5) | 0.75 | 7.8 (4.4-11.6) | 0.04 |
| Lymphocyte count, • 10⁹/l | 0.90 (0.6-1.2) | 2,158 | 0.9 (0.6-1.2) | 0.8 (0.5-1.1) | 0.01 | 0.8 (0.5-1.0) | 0.02 |
| Hemoglobin, g/l | 13.4 (11.9-14.6) | 2,067 | 13.4 (12.0-14.6) | 13.1 (10.7-14.6) | 0.09 | 13.3 (11.0-14.9) | 0.63 |
| Platelet count, • 10⁹/l | 207.0 (159.0-272.0) | 3,963 | 209.0 (160.0-274.0) | 196.0 (150.0-256.0) | 0.02 | 195.5 (150.0-259.0) | 0.14 |
| Nadir platelet count, • 10⁹/l | 175.0 (130.0-234.0) | 3,963 | 178.0 (134.0-236.0) | 146.0 (110.0-204.0) | <0.01 | 146.5 (112.0-191.0) | <0.01 |
| Albumin, g/l | 3.2 (2.9-3.6) | 3,855 | 3.2 (2.9-3.6) | 3.1 (2.7-3.4) | <0.01 | 3.1 (2.8-3.5) | <0.01 |
| ALT, U/l | 31.0 (19.0-54.0) | 3,835 | 31.0 (19.0-54.0) | 27.0 (17.0-47.0) | 0.02 | 29.0 (19.0-57.0) | 0.32 |
| AST, U/l | 44.0 (30.0-72.0) | 3,840 | 44.0 (30.0-71.0) | 41.0 (28.0-74.5) | 0.34 | 47.0 (31.0-80.0) | 0.33 |
| Lactate dehydrogenase, U/l | 436.0 (331.0-588.0) | 3,298 | 435.0 (332.0-587.0) | 440.0 (325.0-605.0) | 0.69 | 481.5 (371.0-648.0) | 0.07 |
| Serum creatinine, mg/dl | 1.06 (0.80-1.64) | 3,925 | 1.03 (0.80-1.61) | 1.27 (0.91-1.99) | <0.01 | 1.2 (0.9-1.9) | 0.05 |
| D-dimer | |||||||
| On admission, μg/ml | 1.54 (0.86-2.90) | 3,046 | 1.53 (0.85-2.87) | 1.61 (0.93-3.04) | 0.43 | 1.9 (1.0-3.3) | 0.02 |
| Peak level, μg/ml | 2.28 (1.22-5.10) | 2,653 | 2.25 (1.20-5.00) | 2.65 (1.54-6.24) | 0.01 | 3.7 (1.8-8.1) | 0.02 |
| Troponin-I | |||||||
| On admission, ng/ml | 0.03 (0.02-0.11) | 2,588 | 0.03 (0.02-0.10) | 0.06 (0.03-0.17) | <0.01 | 0.1 (0.0-0.2) | <0.01 |
| Peak level, ng/ml | 0.07 (0.02-0.25) | 2,253 | 0.06 (0.02-0.23) | 0.14 (0.05-0.53) | <0.01 | 0.2 (0.1-0.6) | <0.01 |
| Brain natriuretic peptide, pg/ml | 69.3 (27.2-214.4) | 2,321 | 56.4 (25.4-174.8) | 200.1 (86.8-557.5) | <0.01 | 124.8 (48.8-289.9) | <0.01 |
| Serum ferritin, μg/ml | 767.0 (359.0-1826.5) | 3,396 | 768.0 (360.0-1827.0) | 729.0 (340.0-1795.0) | 0.63 | 832.0 (447.0-2012.0) | 0.54 |
| C-reactive protein | |||||||
| On admission, mg/l | 117.1 (58.9-198.8) | 2,007 | 118.1 (59.0-198.8) | 106.3 (58.2-195.9) | 0.24 | 111.4 (59.3-213.4) | 0.43 |
| Peak level, mg/l | 176.3 (94.1-271.2) | 2,002 | 175.1 (93.2-270.4) | 186.5 (101.9-284.6) | 0.34 | 232.4 (157.9-312.1) | 0.01 |
| Procalcitonin, ng/ml | 0.20 (0.08-0.66) | 3,236 | 0.20 (0.08-0.63) | 0.25 (0.09-0.84) | 0.06 | 0.3 (0.1-0.9) | 0.09 |
| IL-6, pg/ml | 68.5 (34.2-137.0) | 2,197 | 67.8 (33.6-135.2) | 83.2 (39.4-154.0) | 0.02 | 93.5 (56.2-198.2) | 0.01 |
| Erythrocyte sedimentation rate, mm/h | 62.0 (38.0-88.0) | 1,903 | 63.0 (38.0-88.0) | 58.5 (35.0-85.0) | 0.09 | 60.0 (36.0-95.0) | 0.43 |
| Nadir PaCO2, mm Hg | 33.0 (28.0-38.8) | 1,123 | 33.0 (28.3-39.0) | 31.3 (26.0-37.9) | 0.05 | 30.4 (28.0-38.7) | 0.01 |
Values are median (interquartile range) or n (%).
AF = atrial fibrillation; AFL = atrial flutter; ALT = alanine aminotransferase; AST = aspartate aminotransferase; CAD = coronary artery disease; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; COVID-19 = coronavirus disease-2019; IL = interleukin; OSA = obstructive sleep apnea; PaCO2 = partial pressure of carbon dioxide; TIA = transient ischemic attack.
Fisher exact test. Values in bold indicate a p value ≤ 0.05.
Aside from age (median 74 vs. 66 years; p < 0.01) and race, patients with newly detected AF/AFL did not differ significantly in terms of baseline characteristics from those who did not develop AF/AFL. However, there were differences in certain laboratory values, including an increase in peak inflammatory markers: C-reactive protein (median 232 mg/dl vs. 175 mg/dl; p < 0.01) and IL-6 (median 93.5 mg/dl vs. 67.8 mg/dl; p < 0.01). There were also increases in other previously described markers of disease severity, including peak troponin (median 0.2 ng/ml vs. 0.07 ng/ml; p < 0.01), peak D-dimer (median 3.7 μg/ml vs. 2.3 μg/ml; p < 0.01), and B-type-natriuretic peptide (median 125 pg/ml vs. 56 pg/ml; p < 0.01).
A multivariate logistic regression model was constructed including individual laboratory values and in-hospital treatment along with comorbidities found to be predictive of developing new AF (Table 2 ). No admission laboratory value showed significant predictive value in this analysis; however, in-hospital markers of peak inflammation including C-reactive protein and platelet nadir, along with evidence of myocardial injury (troponin ≥0.03 ng/ml) maintained predictive value. Use of steroids and mechanical ventilation were also associated with a higher incidence of new AF in this analysis.
Table 2.
Individual Multivariable Logistic Regression Analyses of Predictors of New-Onset AF/AFL
| Odds Ratio | 95% Confidence Interval |
p Value | ||
|---|---|---|---|---|
| Lower Limit | Upper limit | |||
| Admission laboratory data | ||||
| IL-6, pg/ml | 1.000 | 0.999 | 1.000 | 0.66 |
| Serum creatinine, mg/dl | 1.054 | 0.991 | 1.121 | 0.09 |
| Brain natriuretic peptide, pg/ml | 0.999 | 0.999 | 1.000 | 0.94 |
| Hemoglobin, g/l | 1.023 | 0.920 | 1.138 | 0.68 |
| Platelet count, • 10⁹/l | 0.999 | 0.997 | 1.001 | 0.21 |
| Lymphocyte count, • 10⁹/l | 0.913 | 0.669 | 1.245 | 0.56 |
| Erythrocyte sedimentation rate, mm/h | 0.998 | 0.991 | 1.005 | 0.57 |
| Serum ferritin, μg/ml | 1.000 | 0.999 | 1.000 | 0.06 |
| C-reactive protein pg/ml | 1.002 | 0.999 | 1.005 | 0.08 |
| D-Dimer, μg/ml | 1.025 | 0.971 | 1.083 | 0.37 |
| Hospital course laboratory data | ||||
| Platelet nadir, • 10⁹ per l | 0.996 | 0.994 | 0.998 | <0.01 |
| Peak D-Dimer, μg/ml | 1.054 | 1.011 | 1.099 | 0.01 |
| Peak C-reactive protein, pg/ml | 1.004 | 1.002 | 1.006 | <0.01 |
| Peak troponin, ng/ml | 1.003 | 0.998 | 1.001 | 0.18 |
| Hospitalization treatment and outcomes | ||||
| Myocardial injury (peak troponin ≥0.03 ng/ml) | 2.489 | 1.245 | 4.977 | 0.01 |
| Severe myocardial injury (peak troponin ≥0.09 ng/ml) | 3.842 | 2.083 | 7.087 | <0.01 |
| Vasopressors administered | 3.456 | 2.380 | 5.015 | <0.01 |
| Steroids | 2.350 | 1.671 | 3.304 | <0.01 |
| Intubation | 3.687 | 2.587 | 5.284 | <0.01 |
Each individual laboratory test was compared in a multivariate analysis with several baseline characteristics including: age, hypertension, diabetes, race, and prior stroke. Bold indicates p ≤ 0.05.
Abbreviations as in Table 1.
Comparison to manually abstracted data
To perform a manual review of the COVID-19Primary dataset, a consecutive subset of patients, the COVID-19Manual cohort (n = 1,110) was screened for both baseline and outcome characteristics, including atrial arrhythmias. There were substantially higher rates of certain common comorbidities identified in the COVID-19Manual group compared to the corresponding COVID-19Primary group such as hypertension (63% vs. 35%; p < 0.01) and diabetes (38% vs. 24%; p < 0.01) (Supplemental Table S1). Of those with a pre-existing history of atrial arrhythmias, 43% were considered paroxysmal, 25% persistent, and the remaining could not be determined. Most importantly, including both ECG-confirmed and reported AF/AFL, the overall incidence was higher than the 10% captured in the COVID-19Primary analysis—the AF/AFL in COVID-19Manual was instead 13% (n = 140) with 6.6% of patients showing new AF/AFL.
Similar to the larger COVID-19Primary cohort, AF/AFL in this COVID-19Manual cohort was associated with increases in baseline comorbidities such as HF, hypertension, and age (Supplemental Table S2). In this COVID-19Manual cohort, the pre-admission medications of the AF/AFL patients included more frequent use of anticoagulant, lipid-lowering, and antihypertensive medications, but no significant difference in use of angiotensin-converting enzyme inhibitors (20% vs. 14%; p = 0.07), or angiotensin receptor blockers (16% vs. 17%; p = 0.81).
Management and outcomes of AF/AFL
In the COVID-19Primary cohort, patients with AF/AFL were slightly less often treated with hydroxychloroquine (68% vs. 76%; p = 0.03). Use of IL-6 inhibitors was similar compared to those without AF/AFL (Table 3 ). Corticosteroid use differed significantly between groups (40% vs. 28%; p < 0.01), and this association was stronger in patients with new-onset AF/AFL (47% vs. 28%; p < 0.01). In-hospital treatment of AF/AFL frequently included therapeutic anticoagulation with either parenteral heparin or oral anticoagulants (78%). Although there was no significant difference in peak hospitalization heart rates, AF patients frequently received antiarrhythmic drugs (25%), predominately amiodarone (86 of 95 patients, 91%).
Table 3.
Treatment and Outcomes Associated With AF/AFL
| All Patients (N = 3,970) | No AF/AFL (n = 3,595) | All AF/AFL (n = 375) | p Value | New-Onset AF/AFL | p Value | |
|---|---|---|---|---|---|---|
| Hospital treatments | ||||||
| Hydroxychloroquine | 2,970 (75) | 2,714 (76) | 256 (68) | <0.01 | 113 (77) | 0.60 |
| Azithromycin | 2,726 (69) | 2,479 (69) | 247 (66) | 0.22 | 105 (72) | 0.45 |
| Remdesivir | 59 (2) | 49 (1) | 10 (3) | 0.07 | 6 (4) | 0.01 |
| Interleukin-6 directed drugs | 248 (6) | 220 (6) | 28 (8) | 0.32 | 19 (13) | <0.01 |
| Tocilizumab | 211 (5) | 189 (5) | 22 (6) | 15 (10) | 0.01 | |
| Sarilumab | 37 (1) | 31 (1) | 6 (2) | 4 (3) | 0.02 | |
| Antiarrhythmic drugs | 182 (5) | 87 (3) | 95 (25) | <0.01 | 51 (35) | <0.01 |
| Amiodarone | 164 (4) | 78 (2) | 86 (23) | <0.01 | 50 (34) | <0.01 |
| Therapeutic anticoagulation | 1,587 (40) | 1,319 (37) | 268 (72) | <0.01 | 114 (78) | <0.01 |
| Prophylactic anticoagulation | 3,150 (79) | 2,948 (82) | 202 (54) | <0.01 | 105 (72) | <0.01 |
| Steroids | 1,173 (30) | 1,022 (28) | 151 (40) | <0.01 | 68 (47) | <0.01 |
| Intubation | 650 (16) | 550 (15) | 100 (27) | <0.01 | 55 (38) | <0.01 |
| Number of vasopressors | 0.21 ± 0.61 | 0.20 ± 0.60 | 0.35 ± 0.78 | <0.01 | 0.51 ± 0.85 | <0.01 |
| Outcomes | ||||||
| Ischemic stroke or TIA | 29 (1) | 23 (1) | 6 (2) | 0.05 | 4 (3) | <0.01 |
| Hemorrhagic stroke | 5 (0.1) | 5 (0.1) | 0 (0.0) | 0.47 | 0(0.0) | 0.65 |
| Death | 1,104 (28) | 933 (26) | 171 (46) | <0.01 | 80 (55) | <0.01 |
| Hospital length of stay, days | 7 (4–11) | 7 (3–11) | 8 (4–13) | <0.01 | 9 (5-17) | 0.09 |
Values are n (%), mean ± SD, or median (interquartile range). Bold values indicates p ≤ 0.05.
Abbreviations as in Table 1.
Overall, the presence of AF/AFL was associated with worse outcomes, including higher rates of intubation (27% vs. 15%, RR: 1.8; p < 0.01), ischemic stroke (1.6% vs. 0.6%, RR: 2.7; p = 0.05), and mortality (46% vs. 26%, RR: 1.78; p < 0.01).
AF/AFL in influenza versus COVID-19
To understand the specificity of observed atrial arrhythmias in COVID-19, we studied a cohort of 1,420 influenza patients (InfluenzaPrimary group). Comorbidities occurred more frequently in patients hospitalized with influenza than COVID-19 (Table 4 ). The incidence of in-hospital AF/AFL was higher in the InfluenzaPrimary than the COVID-19Primary cohort (12% vs. 10%, p = 0.03), but the incidence of new-onset AF/AFL was similar (4% vs. 4%; p = 0.93). Not surprisingly, despite more frequent comorbidities, the InfluenzaPrimary cohort had a substantially lower incidence of in-hospital mortality (9% vs. 29%, p < 0.01).
Table 4.
Baseline Demographics of Patients Hospitalized With COVID-19 Versus Influenza
| All Patients | COVID-19 (n = 3,970) | Influenza (n = 1,420) | p Value | |
|---|---|---|---|---|
| Baseline demographics | ||||
| Age, yrs | 66 (55–78) | 66 (55–77) | 67 (56–80) | <0.01 |
| Male | 2,882 | 2,288 (59) | 594 (42) | <0.01 |
| Race/ethnicity | <0.01 | |||
| Caucasian | 1,347 (25) | 946 (24) | 401 (28) | |
| African-American | 1,509 (28) | 1,107 (28) | 402 (28) | |
| Asian | 263 (5) | 208 (5) | 55 (4) | |
| Hispanic | 1,649 (31) | 1,240 (31) | 409 (29) | |
| Other | 459 (9) | 358 (9) | 131 (9) | |
| Unknown | 133 (3) | 111 (3) | 22 (2) | |
| Obesity | 1,762 (33) | 1,312 (33) | 450 (32) | 0.35 |
| Body mass index, kg/m2 | 27.5 (23.9–32.5) | 27.8 (24.2–32.6) | 26.8 (23.1–31.8) | <0.01 |
| CHF | 523 (10) | 271 (7) | 252 (18) | <0.01 |
| Atrial arrhythmias | 537 (10) | 339 (9) | 198 (14) | <0.01 |
| CAD | 686 (13) | 410 (10) | 276 (19) | <0.01 |
| Hypertension | 2,000 (37) | 1,367 (35) | 633 (45) | <0.01 |
| Diabetes | 1,331 (25) | 976 (25) | 355 (25) | 0.77 |
| Prior stroke/TIA | 281 (5) | 160 (4) | 121 (9) | <0.01 |
| Chronic kidney disease | 700 (13) | 446 (11) | 254 (18) | <0.01 |
| Chronic liver disease | 133 (3) | 79 (2) | 54 (4) | <0.01 |
| HIV | 131 (3) | 68 (2) | 63 (5) | <0.01 |
| COPD | 343 (6) | 157 (4) | 186 (13) | <0.01 |
| Asthma | 425 (8) | 185 (5) | 240 (17) | <0.01 |
| OSA | 128 (2) | 70 (2) | 58 (4) | <0.01 |
| Smoking | <0.01 | |||
| Current | 327 (6) | 152 (4) | 175 (12) | |
| Past | 1,267 (24) | 815 (21) | 452 (32) | |
| Never | 3,796 (71) | 3,003 (76) | 793 (56) | |
| Outcomes | ||||
| In-hospital AF/AFL | 538 (10) | 375 (10) | 163 (12) | 0.03 |
| New-onset in-hospital AF/AFL | 197 (4) | 146 (4) | 51 (4) | 0.88 |
| Hemorrhagic stroke | 9 (0.2) | 5 (0.1) | 4 (0.3) | 0.25 |
| Ischemic stroke or TIA | 29 (0.7) | 12 (0.8) | 0.67 | |
| Death | 1,234 (23) | 1,104 (28) | 130 (9) | <0.01 |
| Hospital length of stay, days | 6 (3–11) | 7 (4–11) | 5 (3–8) | <0.01 |
Values are median (interquartile range) or n (%). Bold values indicates p ≤ 0.05.
Abbreviations as in Table 1.
Similar to the COVID-19 patients, influenza patients with in-hospital AF/AFL were older and had higher rates of comorbidities including HF and hypertension (Supplemental Tables S3 and S4). The levels of inflammatory markers were not significantly different in AF/AFL patients in the InfluenzaPrimary cohort; however, they had increased markers of cardiac injury (median 0.08 ng/ml vs. 0.05 ng/ml; p < 0.01). Use of corticosteroids was similar in those with in-hospital AF/AFL (39% vs. 41%, p = 0.73). As in COVID-19 patients, in-hospital AF/AFL in the InfluenzaPrimary cohort was associated with more frequent intubation (14% vs. 7%; p < 0.01) and death (16% vs. 10%; p < 0.01).
We performed sensitivity analyses to address the potential impact of differences in acuity of illness and baseline characteristics in these 2 groups (Supplemental Table S5). After correcting for differences in age, sex, race, and various comorbidities, these analyses revealed similar rates of atrial arrhythmias in both the COVID-19Primary and InfluenzaPrimary cohorts for both the all in-hospital AF/AFL and the new-onset AF/AFL groups. After adjustment for severity of illness, the COVID-19Primary cohort showed a lower risk of all in-hospital AF/AFL (odds ratio: 0.79, 95% confidence interval: 0.65 to 0.98). When the analysis focused on new-onset AF/AFL, there was no significant difference between groups (odds ratio: 0.94; 95% confidence interval: 0.67 - 1.32).
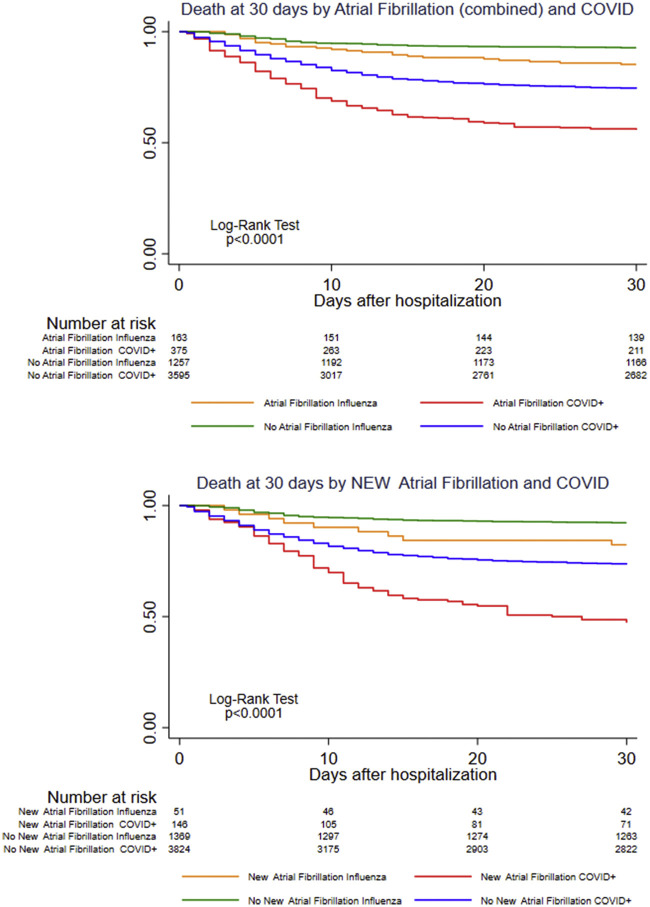
Kaplan-Meier analyses (Figure 1 , Supplemental Figure S1) show an early increase in mortality in patients with in-hospital AF/AFL regardless of whether the arrhythmia was new or preceded by a history of atrial arrhythmias. The association of AF/AFL with mortality was similar with both influenza (RR: 1.78) and COVID-19 (RR: 1.77) (Central Illustration ).
Figure 1.
Survival Stratified by AF/AFL in COVID-19 Versus Influenza
Survival estimates based on the days since hospital admission stratified by COVID-19 versus influenza status as well as the occurrence of in-hospital or new-onset atrial fibrillation. For the purpose of this analysis, patients discharged from the hospital were considered to have survived. AF = atrial fibrillation; COVID = coronavirus disease.
Central Illustration.
Incidence, Predictors, and Outcomes of Atrial Arrhythmias in Patients Admitted With COVID-19 Versus Influenza
AF = atrial fibrillation; AFL = atrial flutter; COVID-19 = coronavirus disease-2019; RR = relative risk.
Discussion
In this study, we examined the incidence, predictors, and outcomes of patients hospitalized with COVID-19 across 5 hospitals during the height of the pandemic in New York City. Our analysis involved an exceptionally ill cohort, as 16% required intubation and mechanical ventilation and 28% died during hospitalization. The incidence of AF/AFL reached 10% to 13%, of which 4% to 6.6% of patients exhibited a new diagnosis of atrial arrhythmia. On the other hand, similar rates of in-hospital all and new-onset AF/AFL were found in the influenza group collected from the same New York hospitals suggesting that these atrial arrhythmias occurred as a nonspecific response to severe viral respiratory illness. Several strengths unique to this study include: 1) a large diverse patient dataset across 5 hospitals comprising several potential risk factors for AF/AFL; 2) incorporation of a panel of influenza patients for comparison; and 3) validation of our automated analysis with comprehensive chart review.
Whereas in-hospital AF/AFL in COVID-19 patients occurred more often in those with pre-existing comorbidities, new-onset AF/AFL was largely irrespective of baseline patient characteristics. Rather, new-onset AF/AFL was influenced most by: 1) markers of inflammation such as IL-6 and C-reactive protein, which have previously been associated with AF; 2) laboratory markers such as troponin and D-dimer, which correlate with COVID-19 disease severity; and 3) administration of corticosteroids which, aside from potential drug effect, are frequently targeted toward those manifesting the largest hyper-inflammatory response. Together, these factors suggest a potential mechanistic link between inflammation and new atrial arrhythmias in patients with COVID-19.
This is the first study involving a large cohort of patients to address the incidence of in-hospital AF in COVID-19 patients. A previous analysis involving a manual review of 115 inpatients for various cardiac arrhythmias reported an AF incidence of 16.5%, all of which occurred in intensive care units (13). In a nationwide study in Denmark, there was a 47% decrease in the incidence of new-onset AF compared with the corresponding weeks in 2019 (14). Although this most likely resulted from underreporting and lower health care use, it suggests that COVID-19 patients overall do not develop atrial arrhythmias at a greater frequency than other acutely ill hospitalized patients.
Although the incidence of AF/AFL in patients with COVID-19 is not exceedingly high, the occurrence of in-hospital AF/AFL appears impactful to a patient’s clinical course, as indicated by the frequent use of antiarrhythmic therapy. Despite the cumulative risk potentially anticipated by combining the prothrombotic state of COVID-19 with the stasis of blood flow during AF/AFL, there was only a modest (1%) absolute increase in ischemic stroke in patients with atrial arrhythmias, perhaps due to concurrent use of therapeutic anticoagulation (76%) during hospitalization. Not unexpectedly, as AFL/AFL was associated with comorbidities, markers of inflammation and disease severity, mortality was exceptionally high in this group (46%).
Previous studies have noted new-onset AF in patients with influenza (15). This had been attributed to higher rates of proinflammatory cytokines including IL-6, which is not specific to either influenza or COVID-19 infection (16). The observation that AF occurs in COVID-19 at a frequency similar to that in influenza argues against a unique effect of either virus on atrial rhythm. This similarity may be confounded by a higher rate of baseline comorbidities in the influenza group, more severe systemic illness in COVID-19, and longer duration of hospitalization during the COVID-19 pandemic. Importantly, as many patients with COVID-19 and influenza are managed as outpatients, our study only reflects those patients whose severity of illness warranted hospitalization.
The height of the COVID-19 pandemic imposed a unique stress on the medical system in New York hospitals as providers were called to perform unfamiliar roles. As a result, we believed it important to ascertain whether the automated data abstraction strategy applied to such a large dataset (using ICD 9/10 codes) accurately reflected the incidence of atrial arrhythmias. Manual chart review of a large subset of this population found that 23% of patients with AF/AFL were not identified by the automated analysis—accordingly, the true rate of AF/AFL increased from 10% to 13%. Furthermore, manual review identified substantially higher rates of common comorbidities that were not discovered by automated indexing of ICD codes. On the other hand, there were minimal differences in laboratory values, treatments, or outcomes between the 2 methods of data abstraction. This highlights the limitations and strengths of “big data” studies which have become commonplace during the COVID-19 pandemic.
Study limitations
This study was limited by several intrinsic challenges of the COVID-19 pandemic including limited access to telemetry monitoring in the nonintensive care unit setting, a high incidence of sedated and noncommunicative patients, and the potential for underdetection of ischemic stroke due to the difficulty of performing brain imaging tests in infected patients. From a methodological perspective, the comparison between the COVID-19 and influenza patients was performed with a similar automated strategy; however, it remains possible that unlike during the “normal” influenza season, the throes of a pandemic may have resulted in a differentially lower rate of detection of AF/AFL (or potential underreporting using ICD 9/10 codes) in the COVID-19Primary cohort. However, given the rapid ventricular response common with AF/AFL in the hospitalized COVID-19 pneumonia patient, it seems unlikely that there were many instances of clinically undetected AF/AFL. Additionally, the exact onset of AF/AFL cannot be accurately determined in part because of limitations in the available data and in part because of the potential delays in diagnosis of atrial arrhythmias during the COVID-19 pandemic. As such, it is difficult to discern the temporal relationship between the factors associated with the development of AF/AFL and their occurrence during the hospital course.
It also bears mentioning that these data only pertain to hospitalized patients: it is possible that nonhospitalized COVID-19 patients have different predictors of developing AF/AFL and different outcomes. Also, we cannot rule out the possibly that the decision-making for hospitalizing a COVID-19 patient during a pandemic may differ than for an influenza patient. However, the directionality of any such variance is unclear—perhaps there is a lower threshold for hospitalization with COVID-19 because of the apprehension surrounding a pandemic, or perhaps there is a higher threshold related to scarce resources or apprehension related to hospitalization. On the other hand, because AF/AFL was more likely to occur in the most critically ill patients regardless of the viral etiology, it is likely that the patients most likely to develop AF/AFL were hospitalized. Finally, because our follow-up only extended to hospital discharge, the impact of atrial arrhythmias on the patient’s clinical course post-hospitalization was not examined in this analysis.
Conclusions
In this study, we found that AF/AFL occurred in ∼13% of hospitalized patients with COVID-19. However, new-onset AF/AFL occurred in only a small minority (4%), a rate that was similar to that observed in hospitalized influenza patients. In both cohorts, new-onset AF/AFL correlated best with higher degrees of inflammation and disease severity, independent of patient baseline characteristics. These data suggest that these atrial arrhythmias are less likely specific to the COVID-19 viral infection but are rather a generalized response to the systemic inflammatory response of severe viral illness.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: This study addresses clinical competencies of medical knowledge regarding the incidence, risk factors, and outcomes associated with atrial arrhythmias of hospitalized patients during the ongoing COVID-19 pandemic in contrast with those hospitalized with influenza prior to the pandemic.
TRANSLATIONAL OUTLOOK: It is important to place the incidence and predictors of atrial arrhythmias during the COVID-19 pandemic in context with recent investigations into the importance of inflammation and myocardial injury in patients hospitalized with COVID-19.
Funding Support and Author Disclosures
Dr Koruth has received consulting fees from Abbott Laboratories, CardioFocus, Farapulse, and Vytron US, Inc. Dr Dukkipati has received grant support from Biosense Webster; and has equity with Farapulse and Manual Surgical Sciences, LLC. Dr Halperin has received consulting fees from Boehringer Ingelheim, Johnson & Johnson-Janssen Pharmaceuticals, and Medtronic. Dr Reddy is a consultant with Abbott, Ablacon, Acutus Medical, Affera, Apama Medical, Aquaheart, Atacor, Autonomix, Axon, Backbeat, BioSig, Biosense Webster, Biotronik, Boston Scientific, Cardiofocus, Cardionomic, CardioNXT/AFTx, Circa Scientific, Corvia Medical, Dinova-Hangzhou Nuomao Medtech Co., Ltd., East End Medical, EBR, EPD, Epix Therapeutics, EpiEP, Eximo, Fire1, Impulse Dynamics, Javelin, Kardium, Keystone Heart, LuxCath, Manual Surgical Sciences, Medlumics, Medtronic, Middlepeak, Newpace, Nuvera, Philips, Pulse Biosciences, Sirona Medical, Stimda, Surecor, Thermedical, and Valcare; and has equity in Ablacon, Acutus Medical, Affera, Apama, Aquaheart, Atacor, Autonomix, Backbeat, BioSig, Circa Scientific, Corvia Medical, Dinova-Hangzhou Nuomao Medtech Co., Ltd., East End Medical, EPD, Epix Therapeutics, EpiEP, Eximo, Fire 1, Javelin, Kardium, Keystone Heart, LuxCath, Manual Surgical Sciences, Medlumics, Middlepeak, Newpace, Nuvera, Sirona Medical, Surecor, Valcare, and Vizaramed. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Andrew Epstein, MD, served as Guest Editor for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental figure and tables, please see the online version of this paper.
Appendix
References
- 1.Hopkins J. Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html Available at:
- 2.Liu F., Li L., Xu M., et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian W., Jiang W., Yao J., et al. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2020;92:1875–1883. doi: 10.1002/jmv.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung M.K., Martin D.O., Sprecher D., et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 5.Wu N., Xu B., Xiang Y., et al. Association of inflammatory factors with occurrence and recurrence of atrial fibrillation: a meta-analysis. Int J Cardiol. 2013;169:62–72. doi: 10.1016/j.ijcard.2013.08.078. [DOI] [PubMed] [Google Scholar]
- 6.Hu L., Chen S., Fu Y., et al. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin Infect Dis. 2020;71:2089–2098. doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatla A., Mayer M.M., Adusumalli S., et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17:1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lala A., Johnson K.W., Januzzi J.L., et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klok F.A., Kruip M., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L., Yan X., Fan Q., et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colon C.M., Barrios J.G., Chiles J.W., et al. Atrial arrhythmias in COVID-19 patients. J Am Coll Cardiol EP. 2020;6:1189–1190. doi: 10.1016/j.jacep.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holt A., Gislason G.H., Schou M., et al. New-onset atrial fibrillation: incidence, characteristics, and related events following a national COVID-19 lockdown of 5.6 million people. Eur Heart J. 2020;41:3072–3079. doi: 10.1093/eurheartj/ehaa494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang T.Y., Chao T.F., Liu C.J., et al. The association between influenza infection, vaccination, and atrial fibrillation: a nationwide case-control study. Heart Rhythm. 2016;13:1189–1194. doi: 10.1016/j.hrthm.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 16.Julkunen I., Sareneva T., Pirhonen J., Ronni T., Melen K., Matikainen S. Molecular pathogenesis of influenza A virus infection and virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 2001;12:171–180. doi: 10.1016/s1359-6101(00)00026-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.