Abstract
Background
Zika virus (ZIKV) is a mosquito-borne virus that is also transmitted sexually; however, the epidemiological relevance of ZIKV sexual transmission in endemic regions is unclear.
Methods
We performed a household-based serosurvey in Northeast Brazil to evaluate the differential exposure to ZIKV and chikungunya virus (CHIKV) among households. Individuals who participated in our previous arboviral disease cohort (indexes) were recontacted and enrolled, and their household members were newly enrolled.
Results
The relative risk of sexual partners being ZIKV-seropositive when living with a ZIKV-seropositive index participant was significantly higher, whereas this was not observed among nonsexual partners of the index. For CHIKV, both sexual and nonsexual partner household members living with a CHIKV-seropositive index had a significantly higher risk of being seropositive. In the nonindex-based dyadic and generalized linear mixed model analyses, the odds of sexual dyads having a concordant ZIKV plaque reduction neutralization test result was significantly higher. We have also analyzed retrospective clinical data according to the participants’ exposure to ZIKV and CHIKV.
Conclusions
Our data suggest that ZIKV sexual transmission may be a key factor for the high ZIKV seroprevalence among households in endemic areas and raises important questions about differential disease from the 2 modes of transmission.
Keywords: chikungunya, epidemiology, mosquito, sexual transmission, Zika
Our household-based serological survey for Zika and chikungunya viruses in Northeast Brazil suggests that sexual contact may be a strong driver of ZIKV exposure in endemic regions, where the virus is transmitted by both mosquitoes and sexual contact.
Endemic transmission of arthropod-borne viruses (arboviruses) is established when the compatible vectors, viruses, and hosts occur in sympatry and under sustained suitable environmental and ecological conditions. The 4 dengue virus serotypes (DENV1–4), Zika virus (ZIKV), and chikungunya virus (CHIKV) circulate and are coendemic in many periurban and urban regions of the tropics, where they are transmitted by Aedes spp, most notably Aedes aegypti. Several studies show that clustered transmission of arboviruses by A aegypti occurs at a household level [1–5]. This is because once this endophilic and anthropophagic mosquito is settled within a microenvironment (eg, household), it does not need to fly long distances (>100 meters) to blood feed or lay eggs [2]. However, each of these arboviruses has unique features that impact transmission dynamics and disease epidemiology in unclear ways. Zika virus stands out because it is transmitted by mosquitoes as well as sexually and vertically (from mother to fetus) [6, 7]. These different modes of transmission may dictate the course of infection and disease presentation in humans. For instance, vertical transmission can lead to serious neurological sequelae in fetuses, termed congenital Zika syndrome, which includes microcephaly [8]. However, the impact of sexual transmission in disease presentation is unclear, as is its relevance in endemic transmission dynamics.
In 2007, ZIKV spread out of Africa and Asia, where it had been circulating for decades, to the Pacific region [9, 10]. In 2013–2014, the virus was introduced in the Americas, where it rapidly disseminated, causing a pandemic [11, 12]. High ZIKV seroprevalence (up to 73%) has been observed in places affected by the pandemic ZIKV strain [13–16]. The fast spread of the virus during the pandemic and the high ZIKV seroprevalence contradicts data showing the relatively poor efficiency of ZIKV transmission by A aegypti from the Americas. First, ZIKV viremia in natural human infections is low relative to CHIKV and DENV [17–20], and titer is a key variable in mosquito infection efficiency [21–24]. Second, gold-standard experiments using sympatric mosquito and virus strains show variable but mostly low vector competence for ZIKV [21, 22, 25–27]. A possible contributing factor to the virus’ rapid and efficient dissemination is sexual transmission; however, assessing the relative importance of this mode of transmission in places where mosquito transmission also occurs is challenging because of the difficulty in identifying the source of infection (mosquito vs sexual) in people. Several human cohort projects were designed to conduct a more thorough assessment on the role of ZIKV sexual transmission in endemic areas [28, 29], but data from many of these studies have been limited due to the very low ZIKV transmission that followed the pandemic [30]. Most human data so far have come from case reports and observational studies from travelers arriving from endemic countries to nonendemic areas where mosquito-borne transmission is implausible [6, 7]. Nevertheless, recent data from a prospective household-based Zika cohort in Puerto Rico showed that sexual partners living with ZIKV RNA-positive index participants had higher odds of also being acutely infected by ZIKV than nonsexual partner residents [31], indicating an important epidemiological role of ZIKV sexual transmission in endemic regions.
Northeast (NE) Brazil is endemic for DENV, ZIKV, and CHIKV [32, 33]. In Pernambuco State (PE), our group characterized the end of a ZIKV outbreak immediately followed by a CHIKV outbreak in a municipality within the Recife Metropolitan Region [34, 35]. Among the 263 participants presenting with arboviral disease symptoms, 60% were acutely infected with ZIKV or CHIKV. In 2017, 2 years after the outbreaks, we rerecruited many of the same participants of our previous cohort and newly enrolled their household members to assess ZIKV and CHIKV seroprevalence at the household level. We then compared the seropositivity to ZIKV and CHIKV among sex partner and nonsex partner household members to evaluate the differential risk of being seropositive for the viruses. The inclusion of the chikungunya group was an important control in our study because CHIKV is transmitted by the same household-biting, urban Aedes spp mosquitoes, but it is not transmitted through sex. We also assessed retrospective clinical data from all participants. Our data raise critical questions about ZIKV transmission in endemic regions and about differential clinical disease from mosquito versus sexual transmission.
METHODS
Cohort Recruitment
Participants from our previous arboviral disease cohort in Paulista, PE, NE Brazil, who had presented with symptoms and a suspected infection with DENV, ZIKV, or CHIKV in 2015–2016 [34], were recontacted and recruited in the current study and were considered the index participants (regardless of their arbovirus infection status in the previous study). In addition, the household members of the index were newly enrolled, including up to 2 nonsexual partner household members (NSP-HM) and a sexual partner household member (SP-HM), when these were available. Enrollment occurred from April to November 2017 and all participants were ≥5 and ≤80 years of age. Retrospective clinical data and blood samples were obtained by trained nurses through residential visits. Informed consent and assent (individuals 12–17 years) to participate was obtained from the participants or their legal representatives. This research protocol was approved by the Aggeu Magalhaes Institute (no. 63441516.6.0000.5190) and Colorado State University (no. 16-6579HH) Institutional Board Reviews.
Zika Virus and Chikungunya Virus Serological Assays and Analyses
Total anti-ZIKV or anti-CHIKV IgG were measured with the EUROIMMUN ELISA kits (catalog no. EI 2668-9601 G for ZIKV and EI 293a-9601 G for CHIKV), following the manufacturer’s instructions. Zika virus neutralizing antibodies (nAbs) were titrated by plaque reduction neutralization tests (PRNTs) as previously described, using Vero cells and ZIKV strain BR-PE243/2015 [34, 36]. Antibody titers resulting in a 50% plaque reduction (PRNT50) were estimated using a 4-parameter nonlinear regression. The PRNT50 titers ≥100 were considered positive. The proportion of seropositive samples with the respective 95% confidence interval was calculated for each assay through a modified Wald method. These results were also stratified by sex and age. Retrospective clinical data were analyzed with the participants’ combined ZIKV (PRNT50) and CHIKV (immunoglobulin IgG) serology. The ZIKV/CHIKV serological groups were as follows: ZIKV+/CHIKV+, ZIKV+/CHIKV−, ZIKV−/CHIKV+, and ZIKV−/CHIKV−.
The statistical tests described in this section were performed in GraphPad Prism 8.3.1. All serological assays were performed blinded by the responsible researcher.
Spatial Distribution of Households With the Relative Percentage of Seropositivity for Zika Virus and Chikungunya Virus
The addresses of participants were georeferenced and superimposed in the Paulista-PE map, which was acquired from the Brazilian Institute of Geography and Statistics. The relative percentage of people within each household who were positive for each virus was calculated and represented by proportional symbols associated with the addresses, by following previously reported methodologies [37, 38].
Relative Risk and Dyadic Analyses With Zika Virus and Chikungunya Virus Serological Data
The relative risk (RR) of nonindex household members being seropositive for ZIKV or CHIKV based on the index’s serological status for the respective virus was calculated by inputting the serological results in contingency tables on GraphPad Prism 8.3.1. Living with an index participant who was seropositive for ZIKV or CHIKV was considered the “exposure” and being seropositive for ZIKV or CHIKV was considered the “outcome.” Significance was calculated through Fisher’s exact test and P ≤ .05 were considered significant. For both ZIKV and CHIKV serological data, contingency tables were constructed with the results from the overall study population or from the NSP-HM and SP-HM subgroups. A subanalysis was also done with participants ≥18 years old. Data from ZIKV PRNTs and enzyme-linked immunosorbent assays (ELISAs) were analyzed independently.
Dyadic analyses were performed with the serological data from each test, using the nonsexual or sexual relationship between any participant pair within a household, where each pair was determined as a dyad (thus, this type of analysis was nonindex based). First, the number of concordant serological results, whether positive or negative (+/+ or −/−), and discordant serological results (+/− or −/+) among nonsexual and sexual dyads were plotted in contingency tables to calculate the RR of having a concordant serological result and being part of a sexual dyad. A second analysis was then performed with the number of nonsexual and sexual dyads with both members having a positive serological result (+/+) and those having any other serological results (−/−, +/−, or −/+) among the households to calculate the RR of having a positive result and being part of a sexual dyad. In both cases, a subanalysis was performed with participants ≥18 years old. Because the number of participants per group was low in several cases, limiting the power of statistical analyses, P ≤ .1 were considered marginally significant, whereas P ≤ .05 were considered significant. Both the RR and dyadic analyses were performed in SAS 9.4. All analyses in the current study were performed with new serological assays from 2017 blood collections, even in the case of the index participants.
Generalized Linear Mixed Model With Zika Virus and Chikungunya Virus Serological Data
To account for the correlations between members of a household, dyadic outcomes were examined in generalized linear mixed models (GLMMs) with a nested covariance structure (dyads nested within households). Two separate models for each serological test were conducted to assess the association between sexual relationship and serological status to ZIKV or CHIKV. The first model assessed the association between sexual relationship and dyad serological concordance, whether positive or negative (+/+ or −/−), compared with discordant dyad serological status (+/− or −/+). The second model assessed the association between sexual relationship and both dyad members having a positive serological result (+/+) compared with all other serological outcomes (−/−, +/−, or −/+) between the dyad members. A subanalysis was performed with participants ≥18 years old. Odds ratios were calculated using the GLIMMIX procedure in SAS 9.4.
Retrospective Clinical Questionnaire
A clinical questionnaire was administered to participants through an interview to gather information on demographic data, arboviral disease history, occurrence of atypical symptoms, and pregnancy history. Data were first collected in the residences by the nurses, who filled the questionnaires by hand, and then entered into a REDCap electronic data capture tool hosted at the Heidelberg University Hospital [39]. Assessment of arboviral disease history was initiated with a question of whether the individual had been ill with dengue, Zika, or chikungunya in the previous 2 years, followed by questions on the symptoms he/she presented with when the response was positive (regardless of whether the participant went to a health clinic). The purpose was to determine whether ZIKV- or CHIKV-seropositive individuals recalled having had disease. Arboviral disease history was only assessed among nonindex participants because the index attended a local health unit in 2015–2016 when having symptoms suggestive of an arboviral infection. The sexual relationship of any participant pair living in a household was assessed by asking the individuals what type of relationship they had with the other household members (parent, daughter/son, sibling, husband/wife, boyfriend/girlfriend, other) and whether there was sexual contact between them.
Clinical data comparisons were performed among the 4 ZIKV/CHIKV serological groups described above. The differences between proportions was analyzed by Fisher’s exact test on GraphPad Prism 8.3.1. P ≤ .1 were considered marginally significant, and P ≤ .05 were considered significant.
RESULTS
Cohort Recruitment
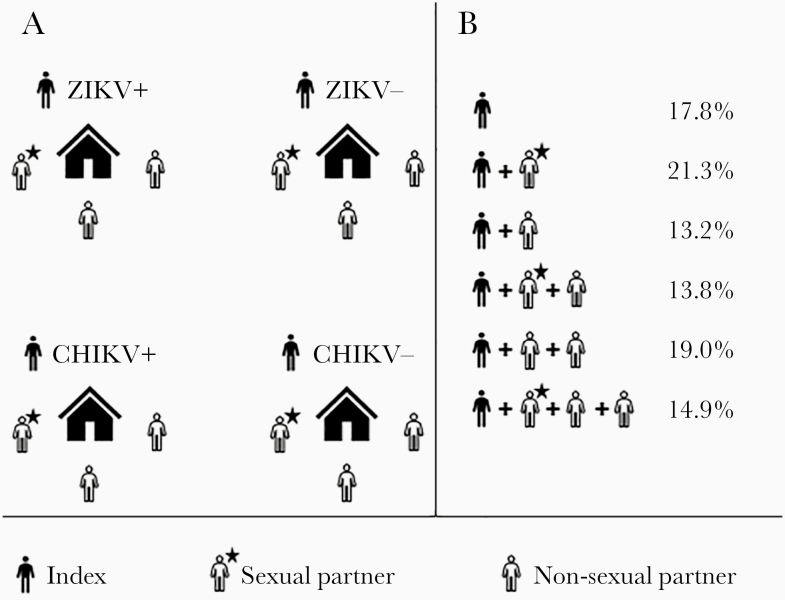
From April to December 2017, 425 participants were enrolled in the current study, including 174 index (individuals who participated in our previous cohort), 165 index-associated NSP-HM and 86 index-associated SP-HM. The numbers of nonsexual and sexual dyads within households were 286 and 100, respectively. The percentages of houses having 1–4 enrolled participants are presented in Figure 1. The proportion of enrolled females and males and within age groups can be seen in Table 1.
Figure 1.
Study design (A) and enrollment data (B). CHIKV, chikungunya virus; ZIKV, Zika virus.
Table 1.
Demographics of Enrolled Participants and Positive Rates of Zika Virus and Chikungunya Virus Serology
| Category | Total Participants | Positive for ZIKV IgG (ELISA) | Positive for ZIKV nAbs (PRNT50) | Positive for CHIKV IgG (ELISA) |
|---|---|---|---|---|
| % | % [95% CI] | % [95% CI] | % [95% CI] | |
| N/Total | N/Total | N/Total | N/Total | |
| Gender | ||||
| Female | 55.1 | 81.2 [76.2–86.4] | 65.2 [58.8–71.1] | 47.9 [41.6–54.3]↓ |
| 234/425 | 180/220 | 150/230 | 112/234 | |
| Male | 45.9 | 79.0 [72.5–84.3] | 66.3 [59.2–72.8] | 57.2 [50.1–64.1]↑ |
| 191/425 | 143/181 | 122/184 | 107/187 | |
| Total | 100.0 | 80.6 [76.4–84.1] ▲ | 65.7 [61.0–70.1]▲ | 52.0 [47.3–56.8]▼ |
| 425/425 | 323/401 | 272/414 | 219/421 | |
| Age Group | ||||
| 5–11 | 8.5 | 58.3 [42.2–72.9] | 60.0 [43.5–74.5] | 45.7 [30.5–61.8] |
| 36/425 | 21/36 | 21/35 | 16/35 | |
| 12–17 | 8.2 | 78.1 [61.0–89.3] | 66.7 [49.5–80.3] | 64.7 [47.9–78.6] |
| 35/425 | 25/32 | 22/33 | 22/34 | |
| 18–24 | 9.4 | 79.0 [63.4–89.2] ▲ | 73.7 [57.8–85.2] ▲ | 46.2 [31.6–61.4] ▼ |
| 40/425 | 30/38 | 28/38 | 18/39 | |
| 25–34 | 22.6 | 81.1 [71.7–88.0] ▲ | 65.3 [55.3–74.1] ▲ | 45.8 [36.2–55.8] ▼ |
| 96/425 | 73/90 | 62/95 | 44/96 | |
| 35–44 | 22.3 | 82.4 [73.2–89.0] ▲ | 59.3 [49.1–68.9] | 54.7 [44.7–64.4] ▼ |
| 95/425 | 75/91 | 54/91 | 52/95 | |
| 45–54 | 14.1 | 81.5 [69.0–89.8] ▲ | 66.1 [53.3–76.9] | 57.6 [44.9–69.4] ▼ |
| 60/425 | 44/54 | 39/59 | 34/59 | |
| 55–64 | 10.4 | 88.1 [74.5–95.3] ▲ | 68.2 [53.4–80.1] ▲ | 43.2 [29.7–57.8]▼ |
| 44/425 | 37/42 | 30/44 | 19/44 | |
| 65–74 | 3.1 | 100.0 [71.8–100.0]△ | 76.9 [49.1–92.5] | 69.2 [42.0–87.7] ▽ |
| 13/425 | 12/12 | 10/13 | 9/13 | |
| 75–80 | 1.4 | 100.0 [55.7–100.0] | 100.0 [55.7–100.0] | 83.3 [41.8–98.9] |
| 6/425 | 6/6 | 6/6 | 5/6 |
Values significantly or marginally different are indicated in bold.
Abbreviations: CI, confidence interval; CHIKV, chikungunya virus; ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; nAbs, neutralizing antibodies; PRNT50, plaque reduction neutralization test resulting in 50% plaque reduction; ZIKV, Zika virus.
aPercent (%) and CI were calculated from the total number of samples resulting in a positive or negative ZIKV or CHIKV serological result (inconclusive results were excluded from the analysis) within the respective group (eg, females, males or total; individual age groups).
▲ vs ▼ Significantly or △ vs ▽ marginally different between serology groups (row data). ↑ vs ↓ Marginally different between females vs males (column data). Significantly different: P ≤ .05; marginally different P ≤ .1. The proportions and the respective 95% CIs were calculated through a modified Wald method.
Zika Virus and Chikungunya Virus Seropositivity Rates
The percentage of positive samples for ZIKV and CHIKV IgG in the overall study population was 80% and 52%, respectively. Twenty-one samples (5%) gave an inconclusive result in the ZIKV IgG ELISA, whereas only 1 sample (0.24%) tested inconclusive in the CHIKV IgG ELISA. In the ZIKV PRNT50, 66% of the study population tested positive. The percentage of females and males positive for ZIKV IgG and ZIKV PRNT50 was similar, whereas a marginally higher number of males were positive for CHIKV IgG when compared with females. Overall, most adult age groupings had significantly higher seropositivity for ZIKV (through ELISA or PRNT50) than for CHIKV (Table 1). The demographics of the 4 ZIKV/CHIKV serological groups is shown in Supplementary Table S1.
The agreement between ZIKV IgG and PRNT50 results was 75.6% (298 of 394). Most of the discordant results consisted of samples that were positive for ZIKV IgG and negative for ZIKV PRNT50 (Supplementary Table S2). Because the ZIKV IgG assay data are likely to be inflated by cross-reactive anti-DENV IgG in our study population, the ZIKV/CHIKV serological groups used to evaluate the retrospective clinical data were determined with ZIKV PRNT50 and CHIKV IgG data only.
Relative Percentage of Zika Virus and Chikungunya Virus Seropositivity in Households
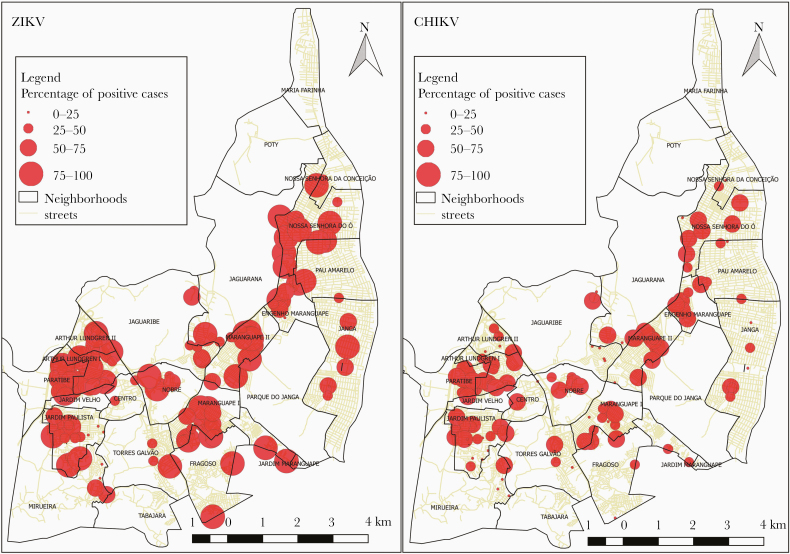
The relative proportion of people positive for ZIKV in households was higher than for CHIKV (P ≤ .005) (Figure 2). Less than 10% of the houses were negative for ZIKV, whereas 23% of houses were negative for CHIKV.
Figure 2.
Relative percentage of people positive in each house for Zika virus ([ZIKV] left) and chikungunya virus ([CHIKV] right). Addresses outside of Paulista-PE were removed in this analysis.
Relative Risk for Zika Virus and Chikungunya Virus Seropositivity
Zika virus PRNT and CHIKV IgG data showed that the RR of nonindex household members being seropositive for the respective virus was significantly higher among those living in a house with ZIKV-positive or CHIKV-positive index participants. When stratified by NSP-HM and SP-HM subgroups, only the SP-HM had a significantly higher risk of being ZIKV-positive when living with a ZIKV-positive index, whereas both NSP-HM and SP-HM had a significantly higher risk of being CHIKV-positive when living with a CHIKV-positive index (Table 2). Similar results to the ZIKV PRNT RR were obtained when data from ZIKV IgG were analyzed (Table 2), and the results were consistent or slightly stronger when limited to only analyzing participants ≥18 years old (who are more likely to be sexually active).
Table 2.
Relative Risk of Household Members Being Seropositive for Zika Virus (ZIKV) or Chikungunya Virus (CHIKV) When Exposed to ZIKV or CHIKV Index Cases, Respectively
| Serological Data | Relative Risk (95% CI) | P Value | ||
|---|---|---|---|---|
| ZIKV PRNT50 | ||||
| Positive HM | Negative HM | |||
| Positive Index | 112 | 68 | 1.49 [1.11–2.11] | .0066 |
| Negative Index | 25 | 35 | ||
| Positive NSP-HM | Negative NSP-HM | |||
| Positive Index | 71 | 46 | 1.16 [0.86–1.65] | .3667 |
| Negative Index | 22 | 20 | ||
| Positive SP-HM | Negative SP-HM | |||
| Positive Index | 41 | 22 | 3.91 [1.62–11.29] | .0004 |
| Negative Index | 3 | 15 | ||
| Positive HM ≥18 yo | Negative HM ≥18 yo | |||
| Positive Index ≥18 yo | 77 | 45 | 1.85 [1.24–2.98] | .0018 |
| Negative Index ≥18 yo | 14 | 27 | ||
| Positive NSP-HM ≥18 yo | Negative NSP-HM ≥ 18 yo | |||
| Positive Index ≥18 yo | 36 | 24 | 1.26 [0.82–2.12] | .3345 |
| Negative Index ≥18 yo | 11 | 12 | ||
| Positive SP-HM ≥18 yo | Negative SP-HM ≥ 18 yo | |||
| Positive Index ≥18 yo | 41 | 21 | 3.97 [1.64–11.47] | .0003 |
| Negative Index ≥18 yo | 3 | 15 | ||
| ZIKV IgG | ||||
| Positive HM | Negative HM | |||
| Positive Index | 150 | 29 | 1.27 (1.06–1.63] | .0125 |
| Negative Index | 31 | 16 | ||
| Positive NSP-HM | Negative NSP-HM | |||
| Positive Index | 94 | 21 | 1.17 [0.96–1.57] | .1493 |
| Negative Index | 23 | 10 | ||
| Positive SP-HM | Negative SP-HM | |||
| Positive Index | 56 | 8 | 1.53 [1.09–2.70] | .0153 |
| Negative Index | 8 | 6 | ||
| Positive HM ≥18 yo | Negative HM ≥18 yo | |||
| Positive Index ≥18 yo | 106 | 14 | 1.35 [1.09–1.84] | .0054 |
| Negative Index ≥18 yo | 21 | 11 | ||
| Positive NSP-HM ≥18 yo | Negative NSP-HM ≥18 yo | |||
| Positive Index ≥18 yo | 50 | 7 | 1.22 [0.96–1.80] | .1454 |
| Negative Index ≥18 yo | 13 | 5 | ||
| Positive SP-HM ≥18 yo | Negative SP-HM ≥18 yo | |||
| Positive Index ≥18 yo | 56 | 7 | 1.56 (1.11–2.74] | .0104 |
| Negative Index ≥18 yo | 8 | 6 | ||
| CHIKV IgG | ||||
| Positive HM | Negative HM | |||
| Positive Index | 86 | 73 | 2.27 [1.55–3.42] | <.0001 |
| Negative Index | 21 | 67 | ||
| Positive NSP-HM | Negative NSP-HM | |||
| Positive Index | 56 | 50 | 2.47 [1.50–4.27] | .0001 |
| Negative Index | 12 | 44 | ||
| Positive SP-HM | Negative SP-HM | |||
| Positive Index | 30 | 23 | 2.01 [1.16–3.78] | .0138 |
| Negative Index | 9 | 23 | ||
| Positive HM ≥18 yo | Negative HM ≥18 yo | |||
| Positive Index ≥18 yo | 62 | 43 | 2.19 [1.45–3.44] | <.0001 |
| Negative Index ≥18 yo | 17 | 46 | ||
| Positive NSP-HM ≥18 yo | Negative NSP-HM ≥18 yo | |||
| Positive Index ≥18 yo | 32 | 21 | 2.34 [1.32–4.54] | .0031 |
| Negative Index ≥18 yo | 8 | 23 | ||
| Positive SP-HM ≥18 yo | Negative SP-HM ≥18 yo | |||
| Positive Index ≥18 yo | 30 | 22 | 2.05 [1.18–3.85] | .0128 |
| Negative Index ≥18 yo | 9 | 23 |
Values significantly or marginally different are indicated in bold.
Abbreviations: CI, confidence interval; CHIKV, chikungunya virus; IgG, immunoglobulin G; NSP-HM, nonsexual partner household members; PRNT50, plaque reduction neutralization test resulting in 50% plaque reduction; SP-HM, sexual partner household members; yo, years old; ZIKV, Zika virus.
aFor each serological assay, data is first shown from the whole study population (all household members and when subdivided into NSP-HM and SP-HM), and from participants ≥18 yo (all household members and when subdivided into NSP-HM and SP-HM).
Dyadic Analyses with Concordant and Discordant Zika Virus and Chikungunya Virus Serological Data
The RR of dyads having a concordant (+/+ or −/−) serological result was significantly higher among sexual partners when ZIKV PRNT50 data were used (Table 3). For ZIKV IgG and CHIKV IgG data, the risk of having a concordant result was not higher among sexual dyads (Table 3). The RR of having a positive (as opposed to a concordant) serological result for ZIKV (PRNT50 or IgG) or CHIKV was not higher in sexual dyads (Supplementary Table S3).
Table 3.
Relative Risk of Household Members Having Concordant (+/+ or −/−) Serology for Zika Virus or Chikungunya Virus Within Sexual and Nonsexual Dyads
| Serological Data | Relative Risk (95% CI) | P Value | ||
|---|---|---|---|---|
| ZIKV PRNT50 | ||||
| Concordant serological result (+/+ or −/−) | Discordant serological result (+/− or −/+) | |||
| Member of sexual dyad | 63 | 31 | 1.22 [1.01–1.45] | .0407 |
| Member of nonsexual dyad | 150 | 123 | ||
| Concordant serological result (+/+ or −/−) | Discordant serological result (+/− or −/+) | |||
| Member of sexual dyad ≥18 yo | 63 | 30 | 1.20 [0.98–1.48] | .0817* |
| Member of nonsexual dyad ≥18 yo | 76 | 59 | ||
| ZIKV IgG | ||||
| Concordant serological result (+/+ or −/−) | Discordant serological result (+/− or −/+) | |||
| Member of sexual dyad | 70 | 21 | 1.07 [0.92–1.22] | .3442 |
| Member of nonsexual dyad | 186 | 73 | ||
| Concordant serological result (+/+ or −/−) | Discordant serological result (+/− or −/+) | |||
| Member of sexual dyad ≥18 yo | 64 | 20 | 1.03 [0.87–1.20] | .7442 |
| Member of nonsexual dyad ≥18 yo | 92 | 32 | ||
| CHIKV IgG | ||||
| Concordant serological result (+/+ or −/−) | Discordant serological result (+/− or −/+) | |||
| Member of sexual dyad ≥18 yo | 63 | 35 | 1.02 [0.84–1.19] | .8496 |
| Member of nonsexual dyad ≥18 yo | 177 | 103 | ||
| Concordant serological result (+/+ or −/−) | Discordant serological result (+/− or −/+) | |||
| Member of sexual dyad ≥18 yo | 63 | 34 | 0.96 [0.79–1.15] | .6390 |
| Member of nonsexual dyad ≥18 yo | 93 | 44 |
Values significantly or marginally different are indicated in bold.
Abbreviations: CI, confidence interval; CHIKV, chikungunya virus; IgG, immunoglobulin G; PRNT50, plaque reduction neutralization test resulting in 50% plaque reduction; yo, years old; ZIKV, Zika virus.
*Marginally different (P ≤ .1). Relative risk significance was calculated through Fisher’s exact test.
The results of the GLMM models based on dyadic data were similar to those from the dyadic frequency distribution analyses. The odds of having a concordant (+/+ or −/−) serological result was marginally higher among sexual dyads when data from ZIKV PRNT50 was used (Table 4). The odds of having a positive serological result compared with any other serological result was not higher among sexual dyads (Supplementary Table S4).
Table 4.
Generalized Linear Mixed Model With Zika Virus and Chikungunya Virus Serology Using a Similar Approach as Table 3 (+/+ or −/− Dyads vs +/− or −/+ Dyads)
| Category | Serological Data | Odds Ratio [95% CI] | P Value for Type 3 Effects | Covariance Parameter Estimates (SE) |
|---|---|---|---|---|
| All participants | ZIKV PRNT50 | 1.65 [0.97–2.78] | .06* | 0.33 (0.25) |
| ZIKV IgG | 1.19 [0.62–2.27] | .60 | 0.87 (0.37) | |
| CHIKV IgG | 0.98 [0.58–1.66] | .93 | 0.41 (0.26) | |
| Participants ≥18 yo | ZIKV PRNT50 | 1.72 [0.91–3.24] | .09* | 0.74 (0.40) |
| ZIKV IgG | 1.11 [0.54–2.29] | .78 | 0.72 (0.48) | |
| CHIKV IgG | 0.84 [0.47–1.51] | .55 | 0.28 (0.35) |
Values significantly or marginally different are indicated in bold.
Abbreviations: CI, confidence interval; CHIKV, chikungunya virus; IgG, immunoglobulin G; PRNT50, plaque reduction neutralization test resulting in 50% plaque reduction; SE, standard error; yo, years old; ZIKV, Zika virus.
*Marginally different (P ≤ .1). Calculations were performed using the GLIMMIX procedure.
Retrospective Clinical Data
The percentage of adults reporting having had arboviral disease among the serological, sexual, and age groups is shown in Table 5. In brief, more people who were positive for CHIKV reported having had arboviral disease than people who were positive for ZIKV-only or who were negative for both viruses. More people who were positive for ZIKV-only reported having had arboviral disease than those negative for both viruses. A marginally higher percentage of adults who were positive for ZIKV-only reported having had arboviral disease in nonsexual dyads than in sexual dyads, and no women reported having had arboviral disease in the sexual dyads. Arboviral disease history among children and teenagers is shown in Supplementary Table S5. Among individuals who went to a health clinic, chikungunya was the most clinically diagnosed disease in the 2 CHIKV-positive groups (61.5%–77.8%); however, chikungunya was diagnosed in a high percentage (up to 75%) of participants who were CHIKV-negative and ZIKV-positive. Zika was clinically diagnosed in 6.7%–25% of people within the 2 ZIKV-positive groups but was not diagnosed in any participants of the 2 ZIKV-negative groups. Dengue diagnosis was variable among all groups, ranging from 0% to 100% (Table 5 and Supplementary Table S5). Atypical symptoms were reported similarly among serological groups and between sexual and nonsexual dyads (Supplementary Tables S6 and S7). Most pregnancy history data and risk factors for adverse pregnancy outcomes among the groups with different serological statuses were relatively similar (Supplementary Table S8).
Table 5.
Arboviral Disease History in Nonindex Participants ≥18 Years Old Relative to Their Serological Statusa
| Arboviral Disease History | ZIKV+/CHIKV+ % (N/Total) | ZIKV+/CHIKV− % (N/Total) | ZIKV−/CHIKV+ % (N/total) | ZIKV−/CHIKV− % (N/Total) |
|---|---|---|---|---|
| Member of a Nonsexual Dyad | ||||
| Reported Having Had Arboviral Disease | 84.2 (16/19) ▲ | 41.9 (13/31) ▼,△,^ | 87.5 (14/16) ▲ | 15.8 (3/19) ▼,▽ |
| Female | 90.0 (9/10) | 40.0 (10/25) | 81.8 (9/11) | 14.3 (2/14) |
| Male | 77.8 (7/9) | 50.0 (3/6) | 100.0 (5/5) | 20.0 (1/5) |
| 18–50 years | 87.5 (7/8) | 56.3 (9/16) | 88.9 (8/9) | 13.3 (2/15) |
| >50 years | 81.8 (9/11) | 26.7 (4/15) | 85.7 (6/7) | 25.0 (1/4) |
| Symptoms During Disease | ||||
| Fever | 81.2 (13/16) | 76.9 (10/13) | 100.0 (14/14) | 100.0 (3/3) |
| Female | 88.9 (8/9) | 80.0 (8/10) | 100.0 (9/9) | 100.0 (2/2) |
| Male | 71.4 (5/7) | 66.7 (2/3) | 100.0 (5/5) | 100.0 (1/1) |
| 18–50 years | 71.4 (5/7) | 77.8 (7/9) | 100.0 (8/8) | 100.0 (2/2) |
| >50 years | 88.9 (8/9) | 75.0 (3/4) | 100.0 (6/6) | 100.0 (1/1) |
| Rash | 62.5 (10/16) | 84.6 (11/13) ▲ | 42.8 (6/14) ▼ | 100.0 (3/3) |
| Female | 55.5 (5/9) | 90.0 (9/10) | 55.5 (5/9) | 100.0 (2/2) |
| Male | 71.4 (5/7) | 66.7 (2/3) | 20.0 (1/5) | 100.0 (1/1) |
| 18–50 years | 71.4 (5/7) | 88.9 (8/9) | 25.0 (2/8) | 100.0 (2/2) |
| >50 years | 55.6 (5/9) | 75.0 (3/4) | 66.7 (4/6) | 100.0 (1/1) |
| Myalgia | 87.5 (14/16) | 84.6 (11/13) | 92.8 (13/14) | 66.7 (2/3) |
| Female | 100.0 (9/9) | 100.0 (10/10)↑ | 100.0 (9/9) | 50.0 (1/2) |
| Male | 71.4 (5/7) | 33.3 (1/3)↓ | 80.0 (4/5) | 100.0 (1/1) |
| 18–50 years | 100.0 (7/7) | 77.8 (7/9) | 87.5 (7/8) | 100.0 (2/2) |
| >50 years | 77.8 (7/9) | 100.0 (4/4) | 100.0 (6/6) | 0.0 (0/1) |
| Arthralgia | 80.0 (12/15) | 83.3 (10/12) | 85.7 (12/14) | 66.7 (2/3) |
| Female | 100.0 (9/9) | 100.0 (9/9)↑ | 88.9 (8/9) | 50.0 (1/2) |
| Male | 50.0 (3/6) | 33.3 (1/3)↓ | 80.0 (4/5) | 100.0 (1/1) |
| 18–50 years | 85.7 (6/7) | 77.8 (7/9) | 87.5 (7/8) | 100.0 (2/2) |
| >50 years | 75.0 (6/8) | 100.0 (3/3) | 83.3 (5/6) | 0.0 (0/1) |
| Redness in the Eye or Retroorbital Pain | 43.7 (7/16) | 61.5 (8/13) | 42.8 (6/14) | 33.3 (1/3) |
| Female | 44.4 (4/9) | 70.0 (7/10) | 44.4 (4/9) | 0.0 (0/2) |
| Male | 42.8 (3/7) | 33.3 (1/3) | 40.0 (2/5) | 100.0 (1/1) |
| 18–50 years | 57.1 (4/7) | 55.6 (5/9) | 25.0 (2/8) | 50.0 (1/2) |
| >50 years | 33.3 (3/9) | 75.0 (3/4) | 66.7 (4/6) | 0.0 (0/1) |
| Photophobia | 62.5 (10/16) ^ | 61.5 (8/13) | 42.8 (6/14) | 33.3 (1/3) |
| Female | 77.8 (7/9) | 60.0 (6/10) | 55.5 (5/9) | 0.0 (0/2) |
| Male | 42.8 (3/7) | 66.7 (2/3) | 20.0 (1/5) | 100.0 (1/1) |
| 18–50 years | 71.4 (5/7) | 66.7 (6/9) | 50.0 (4/8) | 50.0 (1/2) |
| >50 years | 55.6 (5/9) | 50.0 (2/4) | 33.3 (2/6) | 0.0 (0/1) |
| Went to the Doctor When Having the Disease | 81.3 (13/16) | 69.2 (9/13) | 64.3 (9/14) | 66.7 (2/3) |
| Female | 77.8 (7/9) | 70.0 (7/10) | 77.8 (7/9) | 100.0 (2/2) |
| Male | 85.7 (6/7) | 66.7 (2/3) | 40.0 (2/5) | 0.0 (0/1) |
| 18–50 years | 71.4 (5/7) | 66.7 (6/9) | 37.5 (3/8)↓ | 50.0 (1/2) |
| >50 years | 88.9 (8/9) | 75.0 (3/4) | 100.0 (6/6)↑ | 100.0 (1/1) |
| Hospitalized | 12.5 (2/16) | 0.0 (0/9) | 0.0 (0/9) | 0.0 (0/2) |
| Female | 22.2 (2/9) | - | - | - |
| Male | 0.0 (0/7) | - | - | - |
| 18–50 years | 14.3 (1/7) | - | - | - |
| >50 years | 11.1 (1/9) | - | - | - |
| Clinical Diagnosis | 100.0 (13/13) | 100.0 (9/9) | 88.9 (8/9) | 100.0 (2/2) |
| Dengue | 30.8 (4/13) | 44.4 (4/9) | 11.1 (1/9) | 100.0 (2/2) |
| Zika | 7.7 (1/13) | 11.1 (1/9) | 0.0 (0/9) | 0.0 (0/2) |
| Chikungunya | 61.5 (8/13) | 44.4 (4/9) | 77.8 (7/9) | 0.0 (0/2) |
| Member of a Sexual Dyad | ||||
| Reported Having Had Arboviral Disease | 71.4 (20/28) ▲ | 19.3 (6/31) ▼ | 85.0 (17/20) ▲ | 14.3 (4/28) ▼ |
| Female | 76.5 (13/17) | 0.0 (0/14)↓ | 88.9 (8/9) | 23.1 (3/13) |
| Male | 63.6 (7/11) | 35.3 (6/17)↑ | 81.8 (9/11) | 6.7 (1/15) |
| 18–50 years | 75.0 (18/24) | 16.7 (4/24) | 81.3 (13/16) | 14.3 (3/21) |
| >50 years | 50.0 (2/4) | 28.6 (2/7) | 100.0 (4/4) | 14.3 (1/7) |
| Symptoms During Disease | ||||
| Fever | 80.0 (16/20) | 83.3 (5/6) | 88.2 (15/17) | 75.0 (3/4) |
| Female | 76.9 (10/13) | - | 100.0 (8/8) | 100.0 (3/3) |
| Male | 85.7 (6/7) | 83.3 (5/6) | 77.8 (7/9) | 0.0 (0/1) |
| 18–50 years | 77.8 (14/18) | 100.0 (4/4) | 84.6 (11/13) | 100.0 (3/3) |
| >50 years | 100.0 (2/2) | 50.0 (1/2) | 100.0 (4/4) | 0.0 (0/1) |
| Rash | 70.0 (14/20) | 66.7 (4/6) | 52.9 (9/17) | 25.0 (1/4) |
| Female | 76.9 (10/13) | - | 75.0 (6/8) | 33.3 (1/3) |
| Male | 57.1 (4/7) | 66.7 (4/6) | 33.3 (3/9) | 0.0 (0/1) |
| 18–50 years | 66.7 (12/18) | 75.0 (3/4) | 53.8 (7/13) | 33.3 (1/3) |
| >50 years | 100.0 (2/2) | 50.0 (1/2) | 50.0 (2/4) | 0.0 (0/1) |
| Myalgia | 100.0 (19/19) | 100.0 (6/6) | 94.1 (16/17) | 100.0 (4/4) |
| Female | 100.0 (12/12) | - | 87.5 (7/8) | 100.0 (3/3) |
| Male | 100.0 (7/7) | 100.0 (6/6) | 100.0 (9/9) | 100.0 (1/1) |
| 18–50 years | 100.0 (17/17) | 100.0 (4/4) | 100.0 (13/13) | 100.0 (3/3) |
| >50 years | 100.0 (2/2) | 100.0 (2/2) | 75.0 (3/4) | 100.0 (1/1) |
| Arthralgia | 95.0 (19/20) | 100.0 (6/6) | 94.1 (16/17) | 100.0 (4/4) |
| Female | 92.3 (12/13) | - | 100.0 (8/8) | 100.0 (3/3) |
| Male | 100.0 (7/7) | 100.0 (6/6) | 88.9 (8/9) | 100.0 (1/1) |
| 18–50 years | 94.4 (17/18) | 100.0 (4/4) | 92.3 (12/13) | 100.0 (3/3) |
| >50 years | 100.0 (2/2) | 100.0 (2/2) | 100.0 (4/4) | 100.0 (1/1) |
| Redness in the Eye or Retroorbital Pain | 35.0 (7/20) | 50.0 (3/6) | 29.1 (5/17) | 0.0 (0/4) |
| Female | 38.5 (5/13) | - | 25.0 (2/8) | - |
| Male | 28.6 (2/7) | 50.0 (3/6) | 33.3 (3/9) | - |
| 18–50 years | 38.9 (7/18) | 75.0 (3/4) | 23.1 (3/13) | 0.0 (0/3) |
| >50 years | 0.0 (0/2) | 0.0 (0/2) | 50.0 (2/4) | 0.0 (0/1) |
| Photophobia | 30.0 (6/20) | 80.0 (4/6) | 47.1 (8/17) | 0.0 (0/4) |
| Female | 38.5 (5/13) | - | 50.0 (4/8) | - |
| Male | 14.3 (1/7) | 66.7 (4/6) | 44.4 (4/9) | - |
| 18–50 years | 33.3 (6/18) | 75.0 (3/4) | 46.2 (6/13) | 0.0 (0/3) |
| >50 years | 0.0 (0/2) | 5.0 (1/2) | 50.0 (2/4) | 0.0 (0/1) |
| Went to the Doctor When Having the Disease | 75.0 (15/20) | 66.7 (4/6) | 64.7 (11/17) | 50.0 (2/4) |
| Female | 69.2 (9/13) | - | 50.0 (4/8) | 66.7 (2/3) |
| Male | 85.7 (6/7) | 66.7 (4/6) | 77.8 (7/9) | 0.0 (0/1) |
| 18–50 years | 77.8 (14/18) | 75.0 (3/4) | 61.5 (8/13) | 66.7 (2/3) |
| >50 years | 50.0 (1/2) | 50.0 (1/2) | 75.0 (3/4) | 0.0 (0/1) |
| Hospitalized | 0.0 (0/15) | 0.0 (0/4) | 0.0 (0/11) | 0.0 (0/2) |
| Female | - | - | - | - |
| Male | - | - | - | - |
| 18–50 years | - | - | - | - |
| >50 years | - | - | - | - |
| Clinical Diagnosis | 86.7 (13/15) | 100.0 (4/4) | 100.0 (11/11) | 100.0 (2/2) |
| Dengue | 13.3 (2/15) | 0.0 (0/4) | 36.4 (4/11) | 50.0 (1/2) |
| Zika | 6.7 (1/15) | 25.0 (1/4) | 0.0 (0/11) | 0.0 (0/2) |
| Chikungunya | 66.7 (10/15) | 75.0 (3/4) | 63.6 (7/11) | 50.0 (1/2) |
Values significantly or marginally different are indicated in bold.
Abbreviations: CHIKV, chikungunya virus; ZIKV, Zika virus.
aDetermination of seropositivity was from ZIKV plaque reduction neutralization test resulting in a 50% plaque reduction (PRNT50) and CHIKV IgG enzyme-linked immunosorbent assay (ELISA).
▲ vs ▼ Significantly or △ vs ▽ marginally different between serology groups (row data). ↑ vs ↓ Significantly different between females vs males or 18–50 vs >50 years old (column data). ^ vs Marginally different between nonsexual and sexual dyad (column data). Significantly different, P ≤ .05; marginally different, P ≤ .1. Differences between proportions was analyzed by Fisher’s exact test.
DISCUSSION
Our study shows intense arbovirus transmission within the index-based households, in accordance with the clustered, household-level arbovirus transmission by human-biting A aegypti seen with DENV [1–5]. However, seroprevalence for ZIKV was significantly higher than CHIKV, from both PRNT and IgG data. We believe that seroprevalence from PRNT (66%) more reliably reflects the proportion of the study population exposed to ZIKV because the ZIKV IgG ELISA exhibits suboptimal specificity and sensitivity with samples from dengue-endemic areas [40, 41]. Several factors might explain the high ZIKV seroprevalence found here. The most obvious is the possibility that our data incorporate an inflated measure of cross-reactive anti-flavivirus antibodies in serological tests due to previous infections of participants with DENV. This is likely to be true with the less specific ZIKV IgG ELISA assay, but much less so with the PRNTs because of the high PRNT cutoff used here and because the level of DENV/ZIKV cross-reactive nAbs decreases significantly over time [42]. In fact, differential ZIKV seropositivity among household members were observed, in some cases, only with PRNT data and not with IgG data. Others have proposed that non-aedine mosquitoes might facilitate transmission of ZIKV in tropical urban cities, which may be a factor that inflates ZIKV transmission and thus seroprevalence; however, these data have not been replicated in numerous other studies and so have been called into question [43–47]. The factor most parsimonious with our data is that sexual exposure of ZIKV is driving inflated seroprevalence to this arbovirus, which is modestly transmitted by A aegypti [21, 25–27, 48].
The RR analyses treating index participants as the exposure demonstrated that people living in a house with a ZIKV- or CHIKV-seropositive index have a higher risk of also being seropositive for the respective virus. However, when the household members were subgrouped into sexual and nonsexual partners of the index, there was a substantially higher risk of sexual partners being ZIKV-positive when living with a ZIKV-seropositive index, but not of nonsexual partners, indicating that people engaged in a sexual relationship with the index had significantly higher risk of being exposed to ZIKV. Nonsexual household members of ZIKV indexes had a modestly higher but nonsignificant risk of being ZIKV-seropositive, providing epidemiological support for the numerous studies suggesting that A aegypti vector competence for ZIKV is modest [21, 25–27, 48]. In stark contrast, both sexual and nonsexual household members had significantly higher risk of being seropositive when living with a CHIKV-positive index, strengthening our general understanding of efficient A aegypti transmission of some arboviruses at the household level. The dyadic and GLMM analyses on concordant ZIKV PRNT results corroborated the data from the index-based RR analysis. However, in dyadic and GLMM analyses using ZIKV IgG data, sexual dyads had no higher risk of having a concordant result, which, again, might be explained by the fact that IgG data incorporates cross-reactive DENV IgG, making the differential serology between nonsexual and sexual dyads undetectable. A possible limitation of our study is that it was index-biased, and thus our results may not reflect ZIKV and CHIKV household transmission dynamics in the overall Paulista-PE population. We also had few participants in certain subgroups, which constrained the statistical power of some analyses. Future studies should assess similar factors of household transmission of these viruses at a population level in this area and others, which would also increase the number of participants. In a related study to ours conducted in Puerto Rico, Rosenberg et al [31] studied household ZIKV infection by reverse-transcription polymerase chain reaction (RT-PCR). They showed that while sexual partners of index case patients were not at significantly higher risk to also be RT-PCR-positive, their network analysis of sexual partner dyads demonstrated a higher probability of both members being ZIKV-positive than other dyads. These important data are consistent with ours, and both studies suggest that sexual contact may be a stronger driver of ZIKV exposure in places where the virus is transmitted by both mosquitoes and sexual contact.
In the retrospective clinical data, a greater proportion of CHIKV-exposed participants recalling having had an arboviral disease suggest a higher ratio of apparent or symptomatic disease from CHIKV infections. Nonetheless, a similar proportion of participants reported having had symptoms such as fever, rash, myalgia, and arthralgia among the different ZIKV/CHIKV serological groups, highlighting the challenges of distinguishing these arboviral diseases based on common symptoms [49, 50]. It is notable that fewer participants and no women who were seropositive for ZIKV-only within sexual dyads reported having had arboviral disease compared with those from nonsexual dyads. If participants from the sexual dyads were more likely to be infected with ZIKV through sex, this raises the important question of whether the 2 routes of transmission (sex vs mosquito) leads to different symptomatology. Although the frequency of recall of atypical symptoms (eg, dysuria) did not differ significantly among the serological groups, future prospective studies designed to capture atypical symptoms during acute infection with ZIKV are warranted.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Table S1. Demographics of participants according to combined Zika virus (ZIKV) and chikungunya virus (CHIKV) serology.
Supplementary Table S2. Agreement between Zika virus (ZIKV) serology from plaque reduction neutralization test (PRNT) and IgG ELISA.
Supplementary Table S3. Relative risk of household members being seropositive for Zika virus (ZIKV) or chikungunya virus (CHIKV) within sexual and nonsexual dyads.
Supplementary Table S4. Generalized linear mixed model (GLMM) with Zika virus (ZIKV) and chikungunya virus (CHIKV) serology using a similar approach as Supplementary Table S3 (+/+ dyads vs +/−, −/+ or −/− dyads).
Supplementary Table S5. Arboviral disease history in nonindex participants 5–17 years old relative to their serological status.
Supplementary Table S6. Arboviral disease-related symptoms in nonindex participants ≥ 18 years old relative to their serological status in non-sexual and sexual dyads.
Supplementary Table S7. Arboviral disease-related symptoms in nonindex participants from 5–17 years old relative to their serological status.
Supplementary Table S8. Pregnancy history and abnormal gynecological exam results in participants ≥12 years old relative to their serological status.
Notes
Acknowledgments. We are grateful to the participants for their willingness in being part of this study and to the nurses who helped collect the clinical data and blood samples. We are also thankful to Frank Tobian from the Heidelberg University Hospital for having developed the study’s REDCap database.
Financial support. This work was funded by the National Institutes of Health (Grant Number R21AI129464), the European Union’s Horizon 2020 Research and Innovation Program (ZIKAlliance Grant Agreement Number 734548), and the European Union’s 7th Framework Programme (IDAMS Grant Agreement Number 281803).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Anders KL, Nga LH, Thuy NT, et al. Households as foci for dengue transmission in highly urban Vietnam. PLoS Negl Trop Dis 2015; 9:e0003528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harrington LC, Scott TW, Lerdthusnee K, et al. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg 2005; 72:209–20. [PubMed] [Google Scholar]
- 3. Mammen MP, Pimgate C, Koenraadt CJ, et al. Spatial and temporal clustering of dengue virus transmission in Thai villages. PLoS Med 2008; 5:e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stoddard ST, Forshey BM, Morrison AC, et al. House-to-house human movement drives dengue virus transmission. Proc Natl Acad Sci U S A 2013; 110:994–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoon IK, Getis A, Aldstadt J, et al. Fine scale spatiotemporal clustering of dengue virus transmission in children and Aedes aegypti in rural Thai villages. PLoS Negl Trop Dis 2012; 6:e1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Magalhaes T, Foy BD, Marques ETA, Ebel GD, Weger-Lucarelli J. Mosquito-borne and sexual transmission of Zika virus: recent developments and future directions. Virus Res 2018; 254:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Runge-Ranzinger S, Morrison AC, Manrique-Saide P, Horstick O. Zika transmission patterns: a meta-review. Trop Med Int Health 2019; 24:523–9. [DOI] [PubMed] [Google Scholar]
- 8. Moore CA, Staples JE, Dobyns WB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr 2017; 171:288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gutiérrez-Bugallo G, Piedra LA, Rodriguez M, et al. Vector-borne transmission and evolution of Zika virus. Nat Ecol Evol 2019; 3:561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wikan N, Smith DR. Zika virus: history of a newly emerging arbovirus. Lancet Infect Dis 2016; 16:e119–26. [DOI] [PubMed] [Google Scholar]
- 11. Faria NR, Quick J, Claro IM, et al. Establishment and cryptic transmission of Zika virus in Brazil and the Americas. Nature 2017; 546:406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Q, Sun K, Chinazzi M, et al. Spread of Zika virus in the Americas. Proc Natl Acad Sci U S A 2017; 114:E4334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–43. [DOI] [PubMed] [Google Scholar]
- 14. Netto EM, Moreira-Soto A, Pedroso C, et al. High Zika virus seroprevalence in Salvador, Northeastern Brazil limits the potential for further outbreaks. mBio 2017; 8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rodriguez-Barraquer I, Costa F, Nascimento EJM, et al. Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. Science 2019; 363:607–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zambrana JV, Bustos Carrillo F, Burger-Calderon R, et al. Seroprevalence, risk factor, and spatial analyses of Zika virus infection after the 2016 epidemic in Managua, Nicaragua. Proc Natl Acad Sci U S A 2018; 115:9294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mansuy JM, Mengelle C, Pasquier C, et al. Zika virus infection and prolonged viremia in whole-blood specimens. Emerg Infect Dis 2017; 23:863–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Musso D, Rouault E, Teissier A, et al. Molecular detection of Zika virus in blood and RNA load determination during the French Polynesian outbreak. J Med Virol 2017; 89:1505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simmons G, Bres V, Lu K, et al. High incidence of Chikungunya virus and frequency of viremic blood donations during epidemic, Puerto Rico, USA, 2014. Emerg Infect Dis 2016; 22:1221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Waggoner JJ, Gresh L, Vargas MJ, et al. Viremia and clinical presentation in nicaraguan patients infected with Zika virus, chikungunya virus, and dengue virus. Clin Infect Dis 2016; 63:1584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roundy CM, Azar SR, Rossi SL, et al. Variation in Aedes aegypti mosquito competence for Zika virus transmission. Emerg Infect Dis 2017; 23:625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tesla B, Demakovsky LR, Packiam HS, et al. Estimating the effects of variation in viremia on mosquito susceptibility, infectiousness, and R0 of Zika in Aedes aegypti. PLoS Negl Trop Dis 2018; 12:e0006733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vazeille M, Madec Y, Mousson L, et al. Zika virus threshold determines transmission by European Aedes albopictus mosquitoes. Emerg Microbes Infect 2019; 8:1668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nguyet MN, Duong TH, Trung VT, et al. Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A 2013; 110:9072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garcia-Luna SM, Weger-Lucarelli J, Rückert C, et al. Variation in competence for ZIKV transmission by Aedes aegypti and Aedes albopictus in Mexico. PLoS Negl Trop Dis 2018; 12:e0006599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chouin-Carneiro T, Vega-Rua A, Vazeille M, et al. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl Trop Dis 2016; 10:e0004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lozano-Fuentes S, Kenney JL, Varnado W, Byrd BD, Burkhalter KL, Savage HM. Susceptibility and vectorial capacity of American Aedes albopictus and Aedes aegypti (Diptera: Culicidae) to American Zika virus strains. J Med Entomol 2019; 56:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim CR, Counotte M, Bernstein K, et al. ; Sexual Transmission of Zika virus Expert Meeting participants Investigating the sexual transmission of Zika virus. Lancet Glob Health 2018; 6:e24–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calvet GA, Kara EO, Giozza SP, et al. ; ZIKABRA Study Team Study on the persistence of Zika virus (ZIKV) in body fluids of patients with ZIKV infection in Brazil. BMC Infect Dis 2018; 18:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization (WHO): Pan American Health Organization / World Health Organization. Zika Epidemiological Update, 25 August 2017. Washington, DC: PAHO/WHO; 2017. Available at: https://www.paho.org/hq/dmdocuments/2017/2017-aug-25-phe-epi-update-zika-virus.pdf. Accessed 30 July 2020. [Google Scholar]
- 31. Rosenberg ES, Doyle K, Munoz-Jordan JL, et al. Prevalence and incidence of Zika virus infection among household contacts of patients with Zika virus disease, Puerto Rico, 2016–2017. J Infect Dis 2019; 220:932–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pessôa R, Patriota JV, Lourdes de Souza Md, Felix AC, Mamede N, Sanabani SS. Investigation into an outbreak of dengue-like illness in Pernambuco, Brazil, revealed a cocirculation of Zika, chikungunya, and dengue virus type 1. Medicine (Baltimore) 2016; 95:e3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ministério da Saúde [Monitoramento dos casos de arboviroses urbanas transmitidas pelo Aedes (dengue, chikungunya e Zika), Semanas Epidemiológicas 01 a 52]. In: Secretaria de Vigilância em Saúde, ed. Vol. 51 Brasília: Ministério da Saúde, 2020: pp 6. [Google Scholar]
- 34. Magalhaes T, Braga C, Cordeiro MT, et al. Zika virus displacement by a chikungunya outbreak in Recife, Brazil. PLoS Negl Trop Dis 2017; 11:e0006055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jaenisch T, Sakuntabhai A, Wilder-Smith A; IDAMS ; DENFREE; DengueTools. Dengue research funded by the European Commission-scientific strategies of three European dengue research consortia. PLoS Negl Trop Dis 2013; 7:e2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Castanha PM, Cordeiro MT, Martelli CM, Souza WV, Marques ET Jr, Braga C. Force of infection of dengue serotypes in a population-based study in the northeast of Brazil. Epidemiol Infect 2013; 141:1080–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cabello S, Haverkort H, van Kreveld M, Speckmann B. Algorithmic aspects of proportional symbol maps. Algorithmica 2010; 58:543–65. [Google Scholar]
- 38. Kunigami G, Rezende PJ, Souza CC, Yunes T. Generating optimal drawings of physically realizable symbol maps with integer programming. The Visual Computer 2012; 28:1015–26. [Google Scholar]
- 39. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. L’Huillier AG, Hamid-Allie A, Kristjanson E, et al. Evaluation of Euroimmun anti-Zika virus IgM and IgG enzyme-linked immunosorbent assays for Zika virus serologic testing. J Clin Microbiol 2017; 55: 2462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matheus S, Talla C, Labeau B, et al. Performance of 2 commercial serologic tests for diagnosing Zika virus infection. Emerg Infect Dis 2019; 25:1153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Montoya M, Collins M, Dejnirattisai W, et al. Longitudinal analysis of antibody cross-neutralization following Zika virus and dengue virus infection in Asia and the Americas. J Infect Dis 2018; 218:536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lourenco-de-Oliveira R, Marques JT, Sreenu VB, et al. Culex quinquefasciatus mosquitoes do not support replication of Zika virus. J Gen Virol 2018; 99:258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fernandes RS, Campos SS, Ferreira-de-Brito A, et al. Culex quinquefasciatus from Rio de Janeiro is not competent to transmit the local Zika virus. PLoS Negl Trop Dis 2016; 10:e0004993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van den Hurk AF, Hall-Mendelin S, Jansen CC, Higgs S. Zika virus and Culex quinquefasciatus mosquitoes: a tenuous link. Lancet Infect Dis 2017; 17:1014–6. [DOI] [PubMed] [Google Scholar]
- 46. Amraoui F, Atyame-Nten C, Vega-Rua A, Lourenco-de-Oliveira R, Vazeille M, Failloux AB. Culex mosquitoes are experimentally unable to transmit Zika virus. Euro Surveill 2016; 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kenney JL, Romo H, Duggal NK, et al. Transmission incompetence of Culex quinquefasciatus and Culex pipiens pipiens from North America for Zika virus. Am J Trop Med Hyg 2017; 96:1235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Souza-Neto JA, Powell JR, Bonizzoni M. Aedes aegypti vector competence studies: a review. Infect Genet Evol 2019; 67:191–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Braga JU, Bressan C, Dalvi APR, et al. Accuracy of Zika virus disease case definition during simultaneous dengue and chikungunya epidemics. PLoS One 2017; 12:e0179725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brito CA, Cordeiro MT. One year after the Zika virus outbreak in Brazil: from hypotheses to evidence. Rev Soc Bras Med Trop 2016; 49:537–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.