Abstract
Introduction
The dissemination of SARS-Cov2 may have delayed the diagnosis of new cancers. This study aimed at assessing the number of new cancers during and after the lockdown.
Methods
We prospectively collected the clinical data of the 11.4 million patients referred to the Assistance Publique Hôpitaux de Paris Teaching Hospital. We identified new cancer cases between 1st January 2018 and 31st September 2020 and compared indicators for 2018 and 2019 to 2020 with a focus on the French lockdown (17th March to 11th May 2020) across cancer types and patient age classes.
Results
Between January and September, 28,348, 27,272 and 23,734 new cancer cases were identified in 2018, 2019 and 2020, respectively. The monthly median number of new cases reached 3168 (interquartile range, IQR, 3027; 3282), 3054 (IQR 2945; 3127) and 2723 (IQR 2085; 2,863) in 2018, 2019 and 2020, respectively. From March 1st to May 31st, new cancer decreased by 30% in 2020 compared to the 2018–19 average; then by 9% from 1st June to 31st September. This evolution was consistent across all tumour types: −30% and −9% for colon, −27% and −6% for lung, −29% and −14% for breast, −33% and −12% for prostate cancers, respectively. For patients aged <70 years, the decrease of colorectal and breast new cancers in April between 2018 and 2019 average and 2020 reached 41% and 39%, respectively.
Conclusion
The SARS-Cov2 pandemic led to a substantial decrease in new cancer cases. Delays in cancer diagnoses may affect clinical outcomes in the coming years.
Keywords: Incidence, Early detection of cancer, Health policy, COVID-19
1. Introduction
The SARS-Cov2 pandemic led to specific health policies aiming at reducing both its dissemination and hospital crowding. Among them, lockdowns occurred worldwide, and from 17th March to 11th May 2020 in France. Health services adapted to the health situation,in order to avoid contaminations. For example, specialised health care centres adopted triage strategies for patients with acute organ dysfunctions [1]. As a result, in the United States, cardiac catheterisation for STEMI dropped by 38% during the outbreak, revealing a loss of opportunity for severe conditions due to pandemic-related health policy [2]. In the Paris area, the activity of interventional cardiology fell by 60% in April 2020 compared to April 2019 (unpublished data of Greater Paris University Hospital). Cancer screening was also affected. Cancer societies, such as the American Society of Clinical Oncology, asked to postpone any cancer screening procedures during the outbreak [3]. On 2nd April, the French National Institute of Cancer (INCa) decided to interrupt the national cancer screening programs [4]. Outpatient visits were also considerably reduced. In France, general practitioner’s, as well as specialist’s consultations, dropped by 40% and 50%, respectively, during the first month of lockdown [5]. The number of cancer diagnostic procedures dropped significantly during the lockdown in France, with the use of consumables such as iodinated contrasts for CT-scan, Gadolinium contrasts for RMN and laxative liquids for colonoscopy preparation decreasing by 500,000 (up to −72%), 280,000 (up to −72%) and 250,000 (up to −86%), respectively, between 16th March and 13th September 2020 [6]. Reduced patient encounters and diagnostic procedures, postponed screening procedures may explain a drop in new cancer cases during and after the outbreak. This would mean that patients with a new cancer are seen later and can, therefore, potentially suffer from a delay in the start of their treatment. Therefore, it is important to assess the importance of such drop, as treatment delays can dramatically impact the prognosis of patients with cancer [7,8].
This study has been aimed at assessing the impact of the pandemic and related French public health policies on the number of new cancer cases, across each type of cancer and main class ages, in Paris and suburban area, during and after the national lockdown.
2. Methods
The Greater Paris University Hospital (Assistance Publique Hôpitaux de Paris, AP-HP) is an organisation comprising 39 specialised health care centres in Paris and suburb area. Since 2015, AP-HP deployed a clinical data warehouse (CDW) based on the Informatics for Integrating Biology and the Bedside (i2b2) open-source software. This CDW prospectively collects from the hospital information system structured data (data directly reusable for further analysis, e.g. demographics, diagnosis codes, procedures, drug prescriptions, laboratory test results…) and unstructured data (i.e. data that need to be transformed before secondary use, including, but not limited to data stored in a free-text format such as radiology reports or discharge summaries). These data are refreshed daily [9], and the CDW presently contains medical data of 11.4 million patients.
In the present study, more specifically, we used the AP-HP administrative data used for the national hospital reporting system (PMSI – Programme de Medicalisation des Systemes d’Information), a system based on diagnosis-related groups (DRGs). A PMSI record contains, for each patient’s hospital stay, diagnoses (coded using International Classification of Diseases 10th revision (ICD-10)), procedures (coded using the Classification Commune des Actes Medicaux (CCAM), age, gender and place of residence. The hospital stay is described by the following variables: diagnostic-related group, length of stay, type of admission and discharge (home, referral, death), month and year of discharge and additional information about specific wards (e.g. intensive care unit) attended.
Patients with cancer were selected using a list of the ICD-10 codes related to a cancer diagnosis: C00 to D48, excluding benign tumours (D10 – D36) [10] (Supplementary Table 1). A patient was considered to have a newly diagnosed cancer if having a cancer-related ICD code and no cancer-related ICD code in the discharge summary recorded within the 2 previous years. We, therefore, counted cancer cases rather than patients, i.e. a patient with two primitive cancers during the study period was recorded twice, once for each cancer. We assessed the monthly number of new cancer cases for all types of cancer and for each cancer type, and for three patient age classes: <70 years, 70–84 years, and 85 years and older. We also assessed the number of in situ tumour cases: D00 to D09 [10] (Supplementary Table 1).
We compared the monthly number of cancers recorded in 2020 with those recorded in 2018 and in 2019 and with the average number recorded between 2018 and 2019. We performed aggregated comparisons between the 3-month periods corresponding to the French lockdown (1st March to 31st May 2020) and after the lockdown (1st June to 31st September 2020).
3. Results
3.1. Characteristics of the patient population
Between January and September, 28,348, 27,272 and 23,734 patients with a new cancer were referred to the AP-HP greater Paris University Hospital in 2018, 2019 and 2020, respectively. Table 1 summarises the characteristics of the patient population between 2018 and 2020.
Table 1.
Characteristics of the patient population, between January and September each year.
| Year | 2018 | 2019 | 2020 |
|---|---|---|---|
| Number of new cancer cases | 28,348 | 27,272 | 23,734 |
| Number of patients with at least one cancer diagnosis | 26,583 | 24,901 | 21,182 |
| Number of patients with at least two cancer diagnoses | 2847 | 1941 | 1159 |
| Age, median (IQR) (years) | 65 (54–75) |
66 (54–75) |
66 (54–75) |
| Female gender, n (%) | 13,098 (46%) | 12,593 (46%) | 10,980 (46%) |
IQR: interquartile range.
3.2. Number of new cases for all tumour types over time
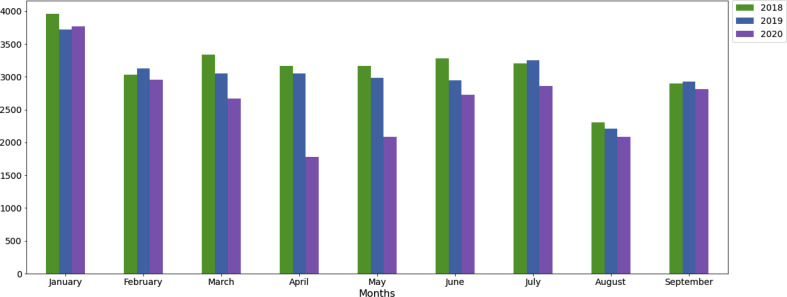
The evolution of the monthly number of overall new cancer cases over time is summarised in Fig. 1 . The related figures can be found in Table 2 . Between January and September, the monthly median number of new cancer cases reached 3168 (interquartile range, IQR, 3027; 3282), 3054 (IQR 2945; 3127) and 2723 (IQR 2085; 2863). During the French national lockdown period (i.e., from March to May 2020), compared to the two previous years, the median number of new cases dropped: by 30% in 2020 compared to the average of 2018–2019. In April 2020, the decrease reached 43% compared to April 2018 and April 2019. During the four months following the lockdown (i.e., from June to September 2020), the median number of new cases remained 9% lower than that recorded during the same period for the average of 2018–2019. In September 2020, the number of new cancer cases remained 3% and 4% lower than that recorded in September 2018 and September 2019, respectively.
Fig. 1.
Evolution of the monthly number of new cancer cases of any type over time in 2018, 2019 and 2020 in the Assistance Publique Hopitaux de Paris Teaching hospital Difference decrease (%).
Table 2.
Number of new cancer cases of any type in 2018, 2019 and 2020 in the AP-HP hospitals.
| Including in situ tumours | Number of new cancer cases |
Between-year difference (%) |
|||
|---|---|---|---|---|---|
| 2018 | 2019 | 2020 | 2020 vs. 2018 | 2020 vs. 2019 | |
| Median monthly number of new cases (IQR) | 3168 (3027–3282) |
3054 (2945–3127) |
2723 (2085–2863) |
||
| January | 3961 | 3723 | 3772 | −5 | 1 |
| February | 3027 | 3127 | 2952 | −2 | −6 |
| March | 3333 | 3054 | 2672 | −20 | −13 |
| April | 3168 | 3055 | 1776 | −44 | −42 |
| May | 3166 | 2984 | 2082 | −34 | −30 |
| June | 3282 | 2945 | 2723 | −17 | −8 |
| July | 3205 | 3248 | 2863 | −11 | −12 |
| August | 2307 | 2211 | 2085 | −10 | −6 |
| September | 2899 | 2925 | 2809 | −3 | −4 |
| In situ tumour cases solely | |||||
| Median monthly number of new cases (IQR) | 27 (26–29) |
41 (28–44) |
19 (17–23) |
||
| January | 29 | 45 | 23 | −21 | −49 |
| February | 26 | 45 | 26 | 0 | −42 |
| March | 30 | 44 | 17 | −43 | −61 |
| April | 29 | 28 | 19 | −34 | −32 |
| May | 42 | 33 | 17 | −60 | −48 |
| June | 27 | 41 | 18 | −33 | −56 |
| July | 27 | 42 | 22 | −19 | −48 |
| August | 16 | 17 | 16 | 0 | −6 |
| September | 25 | 19 | 24 | −4 | 26 |
iQR: interquartile range.
3.3. Number of new cases across cancer types
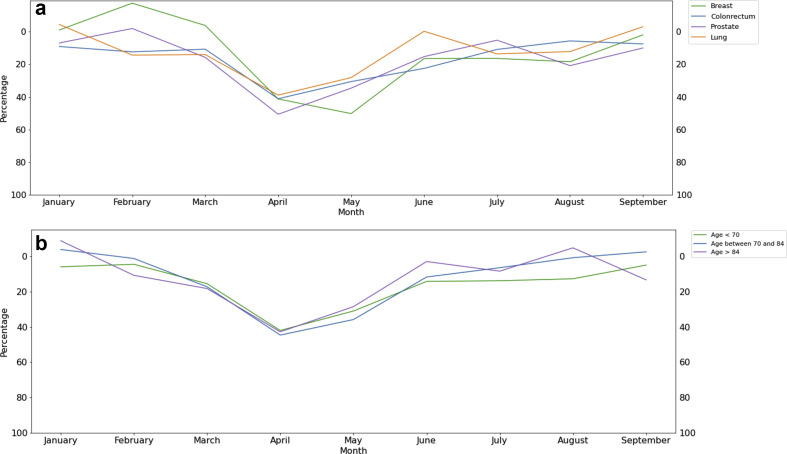
Fig. 2a summarises the evolution of the monthly number of new breast, colorectal, prostate and lung cancer cases between 2018 and 2020. Table 3 displays the figures across all tumour subtypes from March to May (during the lockdown) and from June to September (after the lockdown) for 2018, 2019 and 2020. Full monthly data for each cancer subtype are provided in Supplementary Table 2.
Fig. 2.
Difference (%) in the monthly number of new cancer cases between 2020 and the average of 2018–2019 in the Assistance Publique Hopitaux de Paris Teaching Hospital. a: according to main cancer type (breast, colorectal, prostate, lung cancer). b: according to the patient’s age class (<70 years; 70–84 years; 85 years and over).
Table 3.
Number of new cancer cases (any type) during and after the French lockdown period in 2018, 2019 and 2020 in the AP-HP hospitals.
| From 1st March to 31st May |
From 1st June to 31st September |
|||||||
|---|---|---|---|---|---|---|---|---|
| Number of new cancer cases |
Difference between 2020 and the 2018–2019 average (%) | Number of new cancer cases |
Decrease between 2020 and the 2018–2019 average (%) | |||||
| 2018 | 2019 | 2020 | 2018 | 2019 | 2020 | |||
| Overall | 9667 | 9093 | 6530 | −30 | 11,693 | 11,329 | 10,480 | −9 |
| Colon | 563 | 539 | 388 | −30 | 738 | 648 | 629 | −9 |
| Rectum | 210 | 209 | 167 | −20 | 317 | 294 | 249 | −18 |
| Oesophagus | 118 | 98 | 61 | −44 | 126 | 127 | 99 | −22 |
| Gastric | 185 | 168 | 111 | −37 | 192 | 208 | 175 | −13 |
| Pancreas | 375 | 340 | 235 | −34 | 517 | 464 | 410 | −16 |
| Biliary duct | 126 | 127 | 73 | −42 | 173 | 173 | 119 | −31 |
| Bowel | 74 | 77 | 38 | −50 | 72 | 88 | 62 | −23 |
| Anus | 45 | 31 | 35 | −8 | 49 | 48 | 51 | 5 |
| Liver | 450 | 398 | 303 | −29 | 513 | 522 | 469 | −9 |
| Digestive other | 135 | 115 | 53 | −58 | 124 | 183 | 116 | −24 |
| Endometrium | 126 | 111 | 97 | −18 | 143 | 140 | 143 | 1 |
| Cervix | 96 | 75 | 68 | −20 | 103 | 91 | 96 | −1 |
| Ovary | 119 | 130 | 117 | −6 | 164 | 169 | 157 | −6 |
| Breast | 792 | 638 | 507 | −29 | 896 | 844 | 752 | −14 |
| Gynaecology other | 35 | 29 | 25 | −22 | 45 | 48 | 39 | −16 |
| Lung | 828 | 829 | 602 | −27 | 1047 | 949 | 939 | −6 |
| Mesothelioma | 23 | 29 | 25 | −4 | 27 | 19 | 26 | 13 |
| Pneumology other | 46 | 63 | 36 | −34 | 57 | 65 | 59 | −3 |
| Head and neck | 353 | 257 | 221 | −28 | 409 | 385 | 321 | −19 |
| Eye | 6 | 3 | 9 | 100 | 8 | 9 | 7 | −18 |
| CNS | 500 | 430 | 317 | −32 | 598 | 538 | 531 | −7 |
| PNS | 4 | 15 | 3 | −68 | 26 | 12 | 13 | −32 |
| Soft tissue | 134 | 118 | 107 | −15 | 164 | 141 | 131 | −14 |
| Osteosarcoma | 132 | 111 | 94 | −23 | 158 | 135 | 157 | 7 |
| Myeloma | 230 | 231 | 201 | −13 | 286 | 315 | 318 | 6 |
| Hodgkin lymphoma | 110 | 95 | 94 | −8 | 118 | 122 | 113 | −6 |
| Non-Hodgkin lymphoma | 510 | 465 | 363 | −26 | 556 | 617 | 581 | −1 |
| Leukaemia | 339 | 338 | 258 | −24 | 425 | 409 | 426 | 2 |
| Other haematologic malignancies | 378 | 370 | 217 | −42 | 464 | 485 | 417 | −12 |
| Prostate | 503 | 498 | 335 | −33 | 664 | 576 | 544 | −12 |
| Testis | 44 | 35 | 29 | −27 | 62 | 48 | 45 | −18 |
| Kidney | 301 | 291 | 195 | −34 | 320 | 375 | 336 | −3 |
| Bladder | 399 | 388 | 275 | −30 | 493 | 487 | 407 | −17 |
| Other urothelial | 16 | 16 | 11 | −31 | 17 | 14 | 12 | −23 |
| Melanoma | 245 | 261 | 213 | −16 | 320 | 310 | 295 | −6 |
| Skin other | 568 | 645 | 339 | −44 | 715 | 693 | 646 | −8 |
| Thyroid | 322 | 305 | 187 | −40 | 287 | 297 | 332 | 14 |
| CUP | 74 | 54 | 51 | −20 | 86 | 89 | 77 | −12 |
| Other endocrine | 153 | 161 | 70 | −55 | 214 | 192 | 181 | −11 |
Abbreviations: CNS, central nervous system; CUP, carcinoma of unknown primitive; PNS, peripheral nervous system.
The decrease in new cancer cases was consistent across all tumour types and has continued after the lockdown: −30% and −9% for colon, −27% and −6% for lung, −29% and −14% for breast, −33% and −12% for prostate cancers, respectively, from March to May and from June to September 2020 compared to the 2018–2019 average, respectively. Similar decrease patterns were found for poor prognosis tumours: −34% and −16% for pancreatic, −30% and −17% for bladder, −32% and −7% for the central nervous system and −29% and −9% for liver cancers, respectively.
3.4. Number of new cases across age classes
Fig. 2b summarises the consistent evolution of the monthly number of new cancer cases between 2018 and 2020 across patient age classes: < 70 years; 70–84 years; 85 years and over. Full monthly data per age class for each cancer subtype are provided in Supplementary Table 2. In April 2020, the new cases fell by 42%, 45% and 43% in patients aged <70 years, 70–84 years, 85 years and older, respectively, compared to the April 2018–2019 average. Colorectal and breast cancer diagnoses are partially related to national screening programs that were interrupted during the lockdown. For patients aged less than 70 years, the decrease of colorectal and breast new cancer cases in April between 2018 and 2019 average and 2020 reached 41% and 39%, respectively (Supplementary Table 2).
4. Discussion
Public health policies applied for the SARS-Cov2 pandemic led to a substantial drop in new cancer cases in the Paris area. New cases were still lower than expected in September, showing that the situation had not returned to normal even after the lockdown was lifted. These results suggest a delay in new diagnoses and treatment of cancer cases.
Our results are in line with the published literature. In the Netherlands, the number of cancer diagnoses fell by 25% for cancers other than skin cancers and by 60% for skin cancers (excluding basal cell carcinomas) after ‘social distancing’ policies were implemented [11]. A cross-sectional American study showed that the weekly incidence of six main types of cancer fell by 46% during the pandemic crisis compared to baseline [12]. This decrease is consistent with other studies and reached 52% and 49% for breast and colorectal cancers, respectively [13]. An English survey showed a 76%-decrease in urgent cancer referrals for early diagnosis across eight tertiary care centres during the crisis [14]. An Italian study had reported a decrease in oral cancer screening during the first lockdown [15]. Similarly, an American multicentric observational study concluded that the incidence of new cancers had been divided into two between April 2019 and April 2020 across a 28-million patient population [16]. In this study, the drop of cancer incidence was higher for breast, prostate cancers, and melanoma. Screening for breast and colorectal cancers had decreased by 89% and 85%, respectively. An Italian survey concluded that the incidence of neuroendocrine neoplasia (NEN) decreased by 77% during the national lockdown [17].
This is the first study to assess the impact of the SARS-Cov2 outbreak on the number of new cancer cases of all tumour types across the main age classes during and after a national lockdown. Our results are based on administrative databases and should, therefore, be interpreted with caution, as this kind of epidemiologic studies carries a risk of internal biases [18]. However, since filling the medical record is mandatory for financing reasons, the probability of bias on the number of cases recorded is low. We will link our results with the national administrative database in order to neutralise false-positive cases. Further studies are planned to exploit the content of patients’ electronic health records and mitigate these risks of bias.
Our study results confirm the distraction effect induced by the SARS-Cov2 outbreak on the management of cancer patients [19]. The pandemic may induce subtle unintended consequences such as psychological stress and social isolation, decrease in care provision for life-threatening conditions, economic and logistic disruptions in drug administrations. In a French study performed on 125,000 healthcare professional practices, patients reported that fear of contamination (38%), reluctance to unduly disturb a physician in the middle of a crisis (28%) and closed medical practices (17%) were the main reason for not seeking medical attention [20]. In a context of reduced healthcare resources, efforts should be balanced between the acute threat of virus dissemination and the long-term clinical issues related to severe diseases, such as cancer. Delaying tumour diagnosis might prevent curable tumours from being treated effectively and impair significantly patient clinical outcomes [21,22]. Through a statistical model, Lai et al. estimated that this insufficient care could lead to an excess of 6270 and 33,890 deaths at one year in incident cancer English and American patients, respectively [23]. Another English model study evaluated that the 5-year additional cancer-related deaths due to the pandemic would reach 3500 for four main cancer types [24]. International cancer societies urge cancer patients to come back to clinics, while SARS-Cov2 keeps on disseminating through subsequent waves [25]. Anticipating a loss of opportunity for cancer patients, many scientific communities published guidelines related to cancer care during the pandemic [[26], [27], [28]]. General practitioners have advocated for a timely diagnosis of symptomatic cancer [29]. National cancer screening programs should go on while claiming patient safety regarding the risk of virus contamination in order to address the backlog within usual cancer screening programs [30]. Innovative, non-invasive, resilient to scarce resources screening procedures should be developed and deployed [31]. In an American survey of 3055 cancer patients and survivors, half of them reported some impact of the pandemic on their health care, and 27% of actively-treated patients expressed some delay to their treatment [32].
5. Conclusion
The SARS-Cov2 pandemic and related public health policies led to a significant decrease in the hospital visits of new patients with cancer in France. Stakeholders should pay attention to this issue during the next pandemic waves in order to avoid an excess in cancer-related deaths over the coming years. Further studies are warranted to analyse data from the second wave and the associated lockdown, as well as the assessment of the clinical prognostic impact of cancer diagnosis delays.
Author contributions
Emmanuelle Kempf: Conceptualisation, Methodology, Formal analysis, Data Curation, Writing - Original Draft, Project administration. Guillaume Lamé: Conceptualisation, Methodology, Formal analysis, Writing - Original Draft. Richard Layese: Conceptualisation, Methodology, Software, Formal analysis, Data Curation, Visualisation. Sonia Priou: Conceptualisation, Methodology, Software, Formal analysis, Data Curation, Visualisation. Gilles Chatellier: Conceptualisation, Methodology, Formal analysis, Writing - Original Draft, Supervision, Project administration. Hedi Chaieb: Conceptualisation, Methodology, Software, Formal analysis, Data Curation. Marc-Antoine Benderra: Conceptualisation, Methodology, Writing - Original Draft. Ali Bellamine: Conceptualisation, Methodology, Software, Writing - Original Draft. Romain Bey: Conceptualisation, Methodology, Resources. Stéphane Bréant: Conceptualisation, Methodology, Resources. Gilles Galula: Conceptualisation, Methodology, Writing - Original Draft, Supervision. Namik Taright: Conceptualisation, Methodology, Formal analysis, Writing - Original Draft, Supervision. Xavier Tannier: Conceptualisation, Methodology, Writing - Original Draft. Thomas Guyet: Conceptualisation, Writing - Original Draft. Elisa Salamanca: Conceptualisation, Methodology, Resources, Supervision. Etienne Audureau: Conceptualisation, Methodology, Supervision. Christel Daniel: Conceptualisation, Methodology, Formal analysis, Resources, Writing - Original Draft, Supervision, Project administration. Christophe Tournigand: Conceptualisation, Methodology, Formal analysis, Writing - Original Draft, Supervision.
Funding
This research was supported by a grant from the AP-HP Foundation and a grant from the ARC Foundation for cancer research.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Mrs. Patricia Serre and Dr. François Hemery for their help in the data access and analysis.
This research was supported by the teams in charge of the Clinical Data Warehouse of Greater Paris University Hospitals (AP-HP).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2021.02.015.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Elanko A., Khan J., Hamady Z.Z., Malik H. Cancer surgery sustainability in the light of COVID-19 pandemic. Eur J Surg Oncol. 2020 Jun 1;46(6):1174–1175. doi: 10.1016/j.ejso.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia S., Albaghdadi M.S., Meraj P.M., Schmidt C., Garberich R., Jaffer F.A., et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;vol. 75:2871–2872. doi: 10.1016/j.jacc.2020.04.011. Elsevier USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Society of Clinical Oncology . 2020. Cancer screening, diagnosis, staging, and surveillance.https://www.asco.org/asco-coronavirus-resources/care-individuals-cancer-during- covid-19/cancer-screening-diagnosis-staging 2 Updated June 22. [Internet]. Available from: [Google Scholar]
- 4.Ministère des Solidarités et de la Santé . Inst Natl du cancer; 2020. Continuité des activités des Centres régionaux de coordination des dépistages des cancers (CRCDC) [Google Scholar]
- 5.Nicolas Revel directeur général de la Caisse nationale de l’assurance maladie (CNAM) 2020. Hearing before the Commission des Affaires Sociales, Senat(15 April, 2020) [Google Scholar]
- 6.Weill A., Drouin J., Desplas D., Cuenot F., Dray-Spira R., Zureik M. EPI-PHARE GIS ANSM - CNAM; 2020. Usage des médicaments de ville en France durant l ’ épidémie de la Covid-19 – point de situation jusqu’au 13 septembre 2020.https://www.ansm.sante.fr/S-informer/Points-d-information-Points-d-information/Usage-des-medicaments-de-ville-en-France-durant-l-epidemie-de-Covid-19-point-de-situation-a-la-fin-du-confinement-Point-d-Information [Internet]. Available from: [Google Scholar]
- 7.Hanna T.P., King W.D., Thibodeau S., Jalink M., Paulin G.A., Harvey-Jones E., et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020 Nov;371:m4087. doi: 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartman H.E., Sun Y., Devasia T.P., Chase E.C., Jairath N.K., Dess R.T., et al. Integrated survival estimates for cancer treatment delay among adults with cancer during the COVID-19 pandemic. JAMA Oncol [Internet] 2020;48109:1–9. doi: 10.1001/jamaoncol.2020.5403. http://www.ncbi.nlm.nih.gov/pubmed/33119036 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniel C. La recherche clinique à partir d’entrepôts de données. L’expérience de l'Assistance Publique – Hôpitaux de Paris (AP–HP) à l’épreuve de la pandémie de Covid-19. Rev Med Interne [Internet] 2020 May 1;41(5):303–307. doi: 10.1016/j.revmed.2020.04.005. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7164890/ [cited 2020 Oct 20]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouvrage collectif édité par L’INCa . 2013. Algorithme de sélection des hospitalisations liées à la prise en charge du cancer dans les bases nationales d’activité hospitalière de court séjour «algorithme cancer»; pp. 1–192. [Google Scholar]
- 11.Dinmohamed A.G., Visser O., Verhoeven R.H.A., Louwman M.W.J., van Nederveen F.H., Willems S.M., et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands [Internet] Lancet Oncol. 2020;21:750–751. doi: 10.1016/S1470-2045(20)30265-5. https://pubmed.ncbi.nlm.nih.gov/32359403/ Lancet Publishing Group; [cited 2020 Nov 13]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufman H.W., Chen Z., Niles J., Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw open [Internet] 2020 Aug 3;3(8) doi: 10.1001/jamanetworkopen.2020.17267. https://jamanetwork.com/ [cited 2020 Oct 20]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Team TNUCI of S (NCIS) W A segregated-team model to maintain cancer care during the COVID-19 outbreak at an academic center in Singapore [Internet] Ann Oncol. 2020;31:840–843. doi: 10.1016/j.annonc.2020.03.306. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7174823/ Elsevier Ltd; [cited 2020 Nov 13]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai A.G., Pasea L., Banerjee A., Denaxas S., Katsoulis M., Chang W.H., et al. Estimating excess mortality in people with cancer and multimorbidity in the COVID-19 emergency. medRxiv [Internet] 2020;15(11) https://www.medrxiv.org/content/10.1101/2020.05.27.20083287v1 2020.05.27.20083287. Available from: [Google Scholar]
- 15.Arduino P.G., Conrotto D., Broccoletti R. The outbreak of Novel Coronavirus disease (COVID-19) caused a worrying delay in the diagnosis of oral cancer in north-west Italy: the Turin Metropolitan Area experience [Internet] Oral Dis. 2020 doi: 10.1111/odi.13362. https://pubmed.ncbi.nlm.nih.gov/32306459/ Blackwell Publishing Ltd; [cited 2020 Nov 13]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.London J.W., Fazio-Eynullayeva E., Palchuk M.B., Sankey P., McNair C. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin Cancer Informatics. 2020;(4):657–665. doi: 10.1200/CCI.20.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panzuto F., Maccauro M., Campana D., Faggiano A., Massironi S., Pusceddu S., et al. Impact of the SARS-CoV2 pandemic dissemination on the management of neuroendocrine neoplasia in Italy: a report from the Italian Association for Neuroendocrine Tumors (Itanet) J Endocrinol Invest [Internet] 2020 Aug 16 doi: 10.1007/s40618-020-01393-4. http://www.ncbi.nlm.nih.gov/pubmed/32803662 [cited 2020 Oct 20]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prada-Ramallal G., Takkouche B., Figueiras A. Bias in pharmacoepidemiologic studies using secondary health care databases: a scoping review [Internet] BMC Med Res Methodol. 2019;19:1–14. doi: 10.1186/s12874-019-0695-y. BioMed Central Ltd.; [cited 2020 Dec 11]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortiula F., Pettke A., Bartoletti M., Puglisi F., Helleday T. Managing COVID-19 in the oncology clinic and avoiding the distraction effect. Ann Oncol. 2020;31:553–555. doi: 10.1016/j.annonc.2020.03.286. Elsevier Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.COMMUNIQUE DE PRESSE Covid-19 : Doctolib alerte sur la chute de fréquentation des cabinets et s’engage pour permettre aux patients de retourner consulter [Internet]. [cited 2020 Nov 13]. Available from: www.community.doctolib.com.
- 21.Sud A., Torr B., Jones M.E., Broggio J., Scott S., Loveday C., et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Artic Lancet Oncol [Internet] 2020;21:1035–1079. doi: 10.1016/S1470-2045(20)30392-2. www.gov.uk/guidance/national- [cited 2020 Dec 11];Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sud A., Jones M.E., Broggio J., Loveday C., Torr B., Garrett A., et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol. 2020 Aug 1;31(8):1065–1074. doi: 10.1016/j.annonc.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai A.G., Pasea L., Banerjee A., Hall G., Denaxas S., Chang W.H., et al. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open [Internet] 2020;10 doi: 10.1136/bmjopen-2020-043828. http://bmjopen.bmj.com/ [cited 2020 Dec 11]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maringe C., Spicer J., Morris M., Purushotham A., Nolte E., Sullivan R., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020 Aug 1;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tempero M. COVID-19 and cancer: unintended consequences. J Natl Compr Canc Netw: JNCCN. 2020;18:1147. doi: 10.6004/jnccn.2020.0044. NLM (Medline) [DOI] [PubMed] [Google Scholar]
- 26.van de Haar J., Hoes L.R., Coles C.E., Seamon K., Fröhling S., Jäger D., et al. Caring for patients with cancer in the COVID-19 era [Internet] Nat Med. 2020;26:665–671. doi: 10.1038/s41591-020-0874-8. https://pubmed.ncbi.nlm.nih.gov/32405058/ Nature Research; [cited 2020 Nov 13]. Available from: [DOI] [PubMed] [Google Scholar]
- 27.Qadan M., Hong T.S., Tanabe K.K., Ryan D.P., Lillemoe K.D. A multidisciplinary team Approach for triage of elective cancer surgery at the Massachusetts general hospital during the novel coronavirus COVID-19 outbreak. Ann Surg [Internet] 2020 Jul 1;272(1) doi: 10.1097/SLA.0000000000003963. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7188033/ [cited 2020 Nov 13]:e20–1. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueda M., Martins R., Hendrie P.C., McDonnell T., Crews J.R., Wong T.L., et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward a common goal. JNCCN J Natl Compr Cancer Netw [Internet] 2020 Apr 1;18(4):366–369. doi: 10.6004/jnccn.2020.7560. https://pubmed.ncbi.nlm.nih.gov/32197238/ [cited 2020 Nov 13]. Available from: [DOI] [PubMed] [Google Scholar]
- 29.Jones D., Neal R.D., Duffy S.R.G., Scott S.E., Whitaker K.L., Brain K. Impact of the COVID-19 pandemic on the symptomatic diagnosis of cancer: the view from primary care [Internet] Lancet Oncol. 2020;21:748–750. doi: 10.1016/S1470-2045(20)30242-4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7251992/ Lancet Publishing Group; [cited 2020 Nov 13]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubois R.N. 2020. Cancer prevention research | commentary. [Google Scholar]
- 31.Dockter A.G., Angelos G.C. Molecular-based alternatives for colorectal cancer screening during the COVID-19 pandemic. Surg Technol Int. 2020 May 1;36:1–5. [PubMed] [Google Scholar]
- 32.American Cancer Society Cancer Action Network (ACS. COVID-19 pandemic impact on cancer patients and survivors [Internet]. Available from: https://www.fightcancer.org/sites/default/files/National Documents/SARS-COV2-Ongoing-Impact-Polling-Memo.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.