Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive- fibrosing disease characterized by extensive deposition of extracellular matrix (ECM), scarring of the lung parenchyma. Despite increased awareness of IPF, etiology and physiological mechanism of IPF are unclear. Therefore, preclinical model will require relevant and recapitulative features of IPF. Recently, pluripotent stem cells (PSC)-based organoid studies are emerging as an alternative approach able to recapitulate tissue architecture with remarkable fidelity. Moreover, these biomimetic tissue models can be served to investigate the mechanisms of diverse disease progression. In this review, we will overview the current organoids technology for human disease modeling including lung organoids for IPF.
Keywords: Idiopathic pulmonary fibrosis (IPF), Organoid, Disease modeling, Lung, Pluripotent stem cells
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic disorder characterized by the progressive decline in pulmonary function and fibrosis (1). Recently, both IPF prevalence and incidence have been consistently higher; hence, it is considered that severe exposure to particulate matter, smoking, and viral or bacterial respiratory infections can be potentially exacerbating risk factors for IPF (2). Despite emerging therapeutic attempts for IPF, its pathophysiologic mechanisms are poorly understood. One reason is the lack of a general IPF experimental model to determine pathologic mechanisms/drug screening, except by relying on the bleomycin-induced IPF animal model (3). However, this animal model has a limitation in recapitulating the human IPF progression and response to therapy. Therefore, in this respect, a novel process for developing a 3-D culture system allows us to establish a model that is similar to human disease in vitro. Human-induced pluripotent stem cells (hiPSCs) have been developed into organoids that mimic in vivo tissues and organs at a 3-D level (4). Recent bioengineering approaches have attempted to establish 3-D models of human diseases that applies gene editing, chemical injury, and exogenous physical stimuli.
Current Experimental IPF Modeling
Animal models
The IPF animal model is complicated due to its unknown etiology, although animal models have proven to effectively determine clinical, radiographical and histopathologic features in chronic pulmonary diseases (5). Representatively, IPF animal models have generally been established through bleomycin, and it was based on the fact that fibrosis is a major side effect in bleomycin-related human cancer therapy (3). Various chemicals such as silica and asbestos, have been used to derive IPF in rodents; however, bleomycin-induced animal models are widely accepted animal models available for preclinical investigation (6-8). Several studies have demonstrated that bleomycin induces inflammatory response at the earlier timepoints (within a week), and then it causes collagen deposition, extracellular matrix (ECM) accumulation, fibroblast proliferation/differentiation, and epithelial injury. Typically, mice are intratracheally injected with bleomycin for 2∼3 weeks. Several reports suggested that fibrotic lung injury resolves at longer timepoints after bleomycin injection as a self-limiting response in rodents. It seems that bleomycin-induced animal models are well-characterized and relatively easily established; however, this model has a limitation in recapitulating physiologic IPF findings or histopathologic patterns of interstitial pneu-monia. Furthermore, animal models that are used for experimental studies may raise animal welfare issues, which require researchers to switch to the most advanced technology ex vivo.
Two-dimensional (2-D) cell culture
Chronic alveolar epithelial injury and progressive proliferation of myofibroblasts are a major hallmark of higher IPF occurrence (9); therefore, most studies have established in vitro IPF models by inducing myofibroblast differentiation. Fibroblasts, primarily considered as myofibroblast precursors, have been used as key effector cells in fibrotic disease models (10). Routinely, in a 2-D culture system, human or rodent cells (both immortalized cell lines and primary cells) are used, and they are exposed to exogenous stimuli. For example, transforming growth factor beta (TGF-β), one of the most studied pro-fibrotic factors, is a multi-functional cytokine that regulates a wide range of biological processes including in several cell types (11). Besides TGF-β treatment, recent studies have attempted to mimic structural stress conditions. They have shown that mechanical stresses, including mechanical tension, compression, and shear stress also cause progressive lung fibrosis by ECM remodeling and fibroblast contraction associated with tissue tension or stiffness (12). In IPF pathogenesis, alveolar epithelial cells undergo phenotypic and functional reprogramming as a response to injury triggering multistep processes that involve fibroblast activation and ECM remodeling (12). Primary human lung epithelial cells are theoretically ideal for maintaining physiologic function compared with immortalized cell lines. Furthermore, primary cells have limited life spans and after several phases of cell division, these cells become senescent. A549 is an adenocarcinomic human alveolar basal epithelial cell line, that is widely used as a model of alveolar epithelial-like behavior in several studies. A549 cell monolayers subjected to TGF-β1 treatment regarding EMT, induced changes in claudin expression and permeability alteration similar to respiratory epithelial cells (13). Immortalized bronchial epithelial cells using the AD12- SV40 virus, BEAS-2B cells, are used to represent an early phase of IPF-related epithelial abnormalities. BEAS-2B cells highly express jag1 and p63 levels during TGF-β1 induced squamous differentiation (13). Continuous alveolar epithelial cell lines are easier to handle than primary cells, and they have reliable variability when derived from a single donor, even if it is simply grown in inexpensive culture media. However, these cells are difficult to control in terms of dynamic differentiation into ciliated, goblet, and club cells. Furthermore, viral onco-proteins that are inserted by immortalized cell lines may differ depending on cellular processes, including cell cycle regulation, and differentiation. Since lung tissues have several response mechanisms for inhalation, the single 2-D layer model has a drawback in conducting airways for gas exchange and clearance of carbon dioxide.
hPSC-based 3-D organoid culture
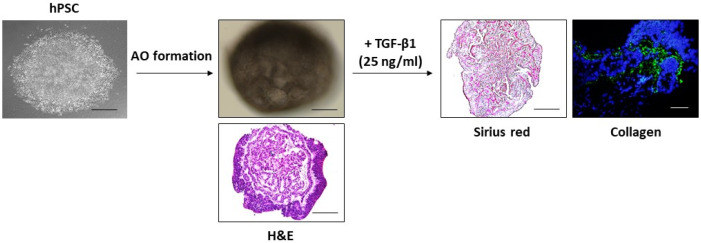
Recently, emerging respiratory diseases due to particulate matters or viral/bacterial infection seriously present a major threat to public health worldwide. Accordingly, respiratory disease modeling has been rapidly directed to public attention in the field of human medical research. Chen et al. (14) showed that lung bud organoids (LBOs) generated from hPSCs contained mesoderm and pulmonary endoderm that developed into branching airway and early alveolar structures, and they suggested that these 3-D LBOs may be a useful tool for human lung diseases, such as infection with respiratory syncytial virus (RSV) and fibrosis. At the second day after RSV infection, the organoids expanded, and infected cells were increased in the lumen of the branching structures. Furthermore, they also induced fibrosis-like organoids through HPS1 deletion, in the EPCAM-positive population only (14). Additionally, another research of organoids infected with a virus was indicated by Porotto et al. They investigated HPIV3 infection in PSC-derived organoids, a prevalent cause of lower respiratory tract disease in children. Consequently, they showed that clinical viruses acquire genome-wide changes, leading to alterations in the viral surface glycoproteins, which is conductive for viral entry (15). Heo et al. (16) have shown that hPSC-derived alveolar epithelial cells alleviated apoptotic and inflammatory responses against cadmium exposure. Recently, the established hPSC-derived epithelial cells are generated with alveolar epithelial organoids (AOs), and AOs treated with TGF-β1 exhibited collagen accumulation and fibrotic changes in the interstitial area of AOs (Fig. 1, unpublished). These aforementioned research data demonstrated that PSC-derived organoids are powerful models to use studying respiratory infection. Besides viral infection modeling, studies that use cystic fibrosis and IPF modeling from PSC-derived organoids are being established (17).
Fig. 1.
Generation of AOs from hPSCs and fibrosis induction. AOs exhibi-ted an alveolar sac-like structure with multiple alveoli and layers of epithelial cells. For in vitro model for studying IPF, AOs were treated with TGF-β1 (25 ng/ml) for 48 hrs. Histochemical and immunofluorescence staining displayed the fibrotic area (Sirius red stai-ning) and expression of collagen in AOs. Scale bars, 100 μm.
Development of Lung Organoids
Lung development
The respiratory systemcomprises a complex branched system of progressively smaller airways where gas exchange occurs. Apparently, lung development undergoes highly coordinated and multi-staged processes governed by mechanical and anatomical axes. Initially, the lungs arise from the anterior foregut endoderm, in which multiple organs, such as the salivary glands, esophagus, thyroid, and liver are generated and continue to develop through the branching process, which is surrounded by mesoderm and a vascular network extending into proximal airways and distal alveoli (18, 19). Human lung development is broadly divided into five stages, namely, embryonic, pseudoglandular, canalicular, saccular and alveolar (20). In the human embryonic stage at day 26, the anlage of the right and left lungs forms two buds of the ventral wall of the primitive foregut, and the buds stretch out vilaterally from the trachea. At E32, the anlages of the two lungs give rise to the two future main bronchi. With continuous branching, the lobar bronchi are formed by the end of week 5, day 37 (20). During the pseudoglandular stage (weeks 5∼17), formation of secondary bronchi, conducting airways, and the primitive capillary plexus and the differentiation of mesenchyme occur, and smooth muscle and mucous glands become visible. At this stage, the gland-like structures separated by the abundant mesenchyme can be observed (21). During the canalicular stage at 16∼26 post- conception weeks (pcw), the alveolar regions are generated, and further epithelial branching occurs, and the existing airway increases in size. For alveoli generation, the distal epithelial tubes widen into air spaces. In the saccular stage, 24∼38 pcw, epithelial and air space appear as thin-walled terminal saccules, and they more expand and become completely wrapped in a capillary bilayer (20).
Lung organoid generation from hPSCs
Since Yamanaka’s fundamental discovery in 2006, the research field using pluripotent stem cells rapidly evolved, developing highly efficient and effective sources for the generation of 3-D organized tissue or organoids (22). In this review, lung organoids refer to self-assembling structures that are generated from PSC-derived epithelial progenitor cells with/without mesenchymal support cells. Lung organoids have been considered as the most indispensable tools, although they are not enough to recapitu-late highly vascularized and delicate alveoli. During early development, the respiratory system is firstly marked by the NKX2.1, a transcription factor required for lung fate, and it is activated by tightly regulated signaling, such as activin A, FGF, BMP, Hedgehog, and WNT (23). At this stage, the dual inhibition of TGF-β/BMP signaling using their inhibitors, including Noggin and SB141524, led to the anterior foregut endoderm lineage from definitive endoderm (23). Indeed, retinoic acid (RA) is an important factor during lung bud development; however, RA concentration is higher in the proximal region than in the distal region. As such, multiple signaling activation successfully led to NKX2.1 expressing lung progenitors. To develop organoids from NKX2.1-positive lung progenitors, these cells need to be seeded in ECM-functioned gels, such as Matrigel or collagen, so they can further differentiate into conducting airway or alveolar epithelial cells (24). In the lung, the alveolar epithelium is composed of two distinct epithelial cells: alveolar epithelial type I (AT1) cells comprise the major gas exchange cells in the alveoli and homeostasis maintenance, whereas alveolar epithelial type II (ATII) cells are progenitors of AT1 cells and responsible for surfactants synthesis and homeostasis after injury (25). In respiratory diseases, ATII cells are considered as major cell targets because of their high regenerative capacity. Nikolić et al. (26) demonstrated that human embryonic lung tip cells express both SOX2 and SOX9, and this is a distinguishing feature of mouse models. Indeed, highly expressed SOX2 and SOX9 tip cells differentiate into both airway and alveolar cells and could be used to model pediatric lung diseases that are difficult to derive in normal lung development. Recently, pulmonary epithelial bud tip progenitor organoids have been developed to differentiate into a specific functional epithelial cell type with a supportive growth using FGF7 (27). These findings suggest that these bud tip progenitor organoids are useful for epithelial–mesenchymal network during lung development and hold great potential for further applications in pharmaceutical safety and efficacy testing.
hPSC-Based Organoids for Human Disease Modeling
The past decade has seen tremendous developments in disease modeling and generating suitable models that displayed quite an identical phenotype with human diseases, from co-culturing techniques to 3-D printed scaffolds to organization on dish (28). Organoids are cultured structures that consist of multiple organ-specific types, and their components are derived from tissue progenitors or pluripotent stem cells. The remarkable ability of PSCs to differentiate into all cell types offers an opportunity to model human diseases in a highly comprehensive way. In studying human diseases, several types of organoids, such as lung, intestinal, brain, liver, and pancreatic organoids have been established (Table 1).
Table 1.
The studies of disease modeling in iPSCs-derived organoids
| Organ | Disease | Induced cell types | Modeling | Reference |
|---|---|---|---|---|
| Lung | Infection with respiratory syncytial virus | Lung bud organoids (LBOs) | For RSV infection of day 170 LBOs, 107 plaque-forming units (PFU) of RSV in 1 l was directly added onto each organoid and incubated for 3 h at 37℃. | (14) |
| Fibrosis | LBOs | Mutation of HPS1 | (14) | |
| Infection with human para-influenza virus 3 (HPIV3) | LBOs | Recombinant HPIV3 for 32 days | (15) | |
| IPF | Mesenchymal organoids | 10 ng/ml TGF-β1 for 2 days | (42) | |
| Intestine | Salmonellae (bacterial) infection | intestinal organoids (iHO)-gut | Add of 1×106 bacteria/well in 24 well | (31) |
| Intestinal fibrosis | iHO -mid/hindgut | 2 ng/ml TGFβ for 96 hours | (32) | |
| Brain | Zika virus infection | Cerebral organoids | ZIKV for 7 days | (33) |
| Zika virus infection | Neural organoids | 3×105 PFU/ml 2 h at 37℃ | (34) | |
| Parkinson’s disease (PD) | Midbrain-like organoids | PD patient-derived organoids | (35) | |
| Alzheimer’s disease (AD) | Neural organoids | AD patient-derived organoids | (36) | |
| Liver | Steatohepatitis (fibrosis with inflammation) | Liver organoids | 800 mM oleic acid (OA) for 7 days | (37) |
| Infection with hepatitis B virus | Hepatic organoids | HBV (500, 5000 GEQ/cell) for 10 days | (39) |
Intestine
At the beginning of intestinal tissue-derived organoid culture, the tissue pieces can be maintained for several days only and were not self-renewing. Ever since Barker et al. (29) reported intestinal stem cells, known as Lgr5- positive stem cells, the field of intestinal organoid proliferation has been rapidly developing. In agreement with Lgr5 stem cells, Sato et al. (30) demonstrated that Lgr5 stem cells autonomously grow into crypt-like structures in a Matrigel culture system containing EGF, and Paneth cells provide essential support to Lgr5 stem cells. This suggests that Paneth cells and Lgr5 stem cells can be therapeutically applied for ileal Crohn’s disease, as well as necrotizing enterocolitis. Forbester et al. (31) have shown the between the enteric pathogen Salmonella typhimurium and human intestinal epithelium in an iPSC-induced intes-tinal organoid system. When S. Typhimurium was injected in intestinal organoids, the organoids retained their inoculum for 3 h incubation, exhibiting close to microvilli, and residing in structures that resemble Salmonella-containing vacuoles. Recently, hESC-derived intestinal organoids have been implicated in intestinal fibrosis. Intestinal organoids treated with TGF-β increased αSMA expressing cells, ECM components COL1A1 and FN1, and the myofibroblast marker and component of actin stress fibers ACTA2. Additionally, the effect of anti-fibrotic drug spironolactone and aldosterone receptor antagonists used in these organoids have been evaluated in TGF-β mediated fibrotic injury implicating a viable fibrosis model related to Crohn’s disease (32).
Brain
Compared with the 2-D culture of neural cells, the 3-D methods successfully mimicked key features of the developing brain. The brain parenchyma is a complex composite biological tissue, comprising various interacting constituents in a multi-layered network. In this view, brain organoids were developed to resemble particular brain regions and were even combined into “brain assembloids” for studying neural interactions and diseases formation. Indeed, brain organoids are useful in investigating cancer, Alzheimer’s disease (AD) and Parkinson’s diseases (PD), and unusual microcephaly seen in some bodies infected with Zika virus (ZIKV) before birth. Janssens et al demonstrated that ZIKV caused congenital microcephaly by alter DNA methylation of neural genes in an ESC-derived organoid. In the cerebral organoid-derived neural progenitor cells, ZIKV-induced differentially methylated regions were linked to conditions, such as mental retardation, as well as intellectual and developmental disorders, using PsyGeNET analysis (33). Additionally, ZIKV-infected brain organoids have shown a pronounced reduction of ZIKA production following sofosbuvir treatment, an anti-hepatitis C drug (34). These findings suggest that the brain organoids can be tested for potential secondary utilization of antiviral drugs against ZIKV in a broad range of neurologic disorders. On another note, mid-brain specific organoid modeling for PD has been successfully developed from PD patients carrying the LRRK2-G2019S mutation. In PD-patient derived midbrain organoids, FOXA2-positive progenitor cells were increased, and specification of midbrain dopaminergic neurons promoted by the LRRK2- G2019S mutation in the PD-patient-specific organoids was impaired (35). Additionally, AD-patient-derived iPSC generated 3-D human neural organoids for AD modeling (36). These modeling methods enable not only the screening of disease patterns, but also efficient validation of potential pharmaceutical agents for neurodegenerative disorders.
Liver
Three-dimensional liver organoids allow us to establish disease modeling for various hepatic disorders, including monogenic liver disease, cancer, fibrosis, and infection. Representatively, cystic fibrosis (CF) from the mutation of the CFTR gene was first described in human monogenic disease modeled with organoids. CFTR gene mutation impairs secretion and alkalization of bil,e leading to increased susceptibility to infectious agents and other toxic components secreted with the bile. Ouchi et al. (37) have shown that free fatty acid exposure induces the steatohepatitis phenotype in iPSC-derived liver organoids. When the liver organoids were exposed to oleic acid, they ovserved the severe lipid accumulation leading to inflammation and fibrosis. Furthermore, they applied organoid modeling to Wolman disease patient-derived iPSC lines to investigate the clinical relevance of steatohepatitis models. Patients with Wolman disease showed fibrotic phenotypes that initiated lipid accumulation in hepato-cytes. In their disease organoids, FGF9 treatment exhibited therapeutic effects by reducing lipid accumulation and reactive oxygen species production (38). Liver organoids are powerful tools for modeling viral infections. Nie et al. (39) utilized hiPSC-derived liver organoids as an infection model against the hepatitis-B virus. As mentioned, the CF organoid model has been applied in clinical studies that aim to restore the function of CFTR protein mutants to analyze the drug response of individual subjects (40). It suggests that developing of new compounds and screening drug efficacy at an individual level may contribute to the improvement of drug efficacy by selecting drug-responsive subgroups.
Lung
Organoid technology is rapidly developing, because organoids allow the same basic intrinsic pattern of events similar to human organs and they are a useful tool for studying development, repair, and homeostasis in several human diseases (41). The lung, however, is one of the most complex organs, and IPF involves complex interactions between different cell types. Hence, the lung is more challenging than other organ system in terms of pathologic research. Wilknson et al. (42) developed the human fetal-derived mesenchymal organoids for IPF modeling. When they treated the organoids with TGF-β for 2-days organoid contraction and high α-SMA and collagen I expression were observed, which are related to fibrosis. Furthermore, Strikoudis et al. (17) modeled fibrotic lung organoids using CRISPR/Cas9 gene editing system. They previously demonstrated that Hermansky–Pudlak syndrome (HPS)-associated interstitial pneumonia (HPSIP) shared similarities with IPF, and these similarities were determined using HPS1−/− ESC-derived 3-D lung organoids. The HSP1−/− organoids showed fibrotic changes, and additionally, the introduction of HPS1, 2, and 4 mutations promoted fibrosis in the lung organoids (17). ESC switches an HPS mutant generated structurally abnormal organoids, which were observed to have increased accumulation of mesenchymal cells and fibrosis-associated genes, such as Col1a1, fibronectin, and vimentin. These models may contribute to understanding unknown pathologic mechanisms or etiologies behind IPF, and they are worth developing in the field of fibrotic diseases research.
Future Application
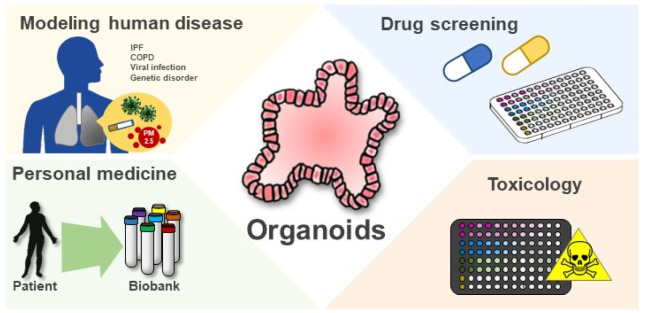
Although organoid technology is consistently and rapidly being developed, its limitations still remain. A general feature of PSC-derived cells, tissues, and organoids is that they similarly maintain the transcriptional state of human fetal tissues. This similarity in functional state becomes a hurdle, because established organoids fail to “mature” in the laboratory, thereby requiring better understanding of the associated cellular mechanisms. On another note, the inherently immature state allows efficient models to recapitulate the genetic structure or disease progression of immature human organs. In the respiratory system, the organoid technology using PSC will provide an opportunity for patient-specific organ modeling, which can also be used for manufacturing medicine. Furthermore, 2-D culture systems or animal models may not easily recapitulate infectious diseases because of the difference in pathway among cell types or host species (Fig. 1). In this view, 3-D organoids are an excellent novel system to model basic clinical scenarios, including infectious diseases, drug safety, toxicity, or efficiency, and applied lung research.
Fig. 2.
Applications of organoids. Organoids have proven to be particular information for modeling human diseases. The ability to generate 3D organoids in large quantities with high consistency is expected the organoid-based platforms for drug screening and toxicity validation. Using organoid as a platform, drug response associated with genetic profiles derived from patients can be employed to predict treatment response and guide personalized therapies for individual patient.
Acknowledgments
This study was supported by grants from the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) 2017M3A9B3061838, NRF-2020R1A2C201 0712 and the Research grant of Kangwon National University in 2019.
Footnotes
Potential Conflict of Interest
The authors have no conflicting financial interest.
References
- 1.Meltzer EB, Noble PW. Idiopathic pulmonary fibrosis. Orphanet J Rare Dis. 2008;3:8. doi: 10.1186/1750-1172-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sauleda J, Núñez B, Sala E, Soriano JB. Idiopathic pulmonary fibrosis: epidemiology, natural history, phenotypes. Med Sci (Basel) 2018;6:110. doi: 10.3390/medsci6040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.B Moore B, Lawson WE, Oury TD, Sisson TH, Raghavendran K, Hogaboam CM. Animal models of fibrotic lung disease. Am J Respir Cell Mol Biol. 2013;49:167–179. doi: 10.1165/rcmb.2013-0094TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JY, Hong SH. Hematopoietic stem cells and their roles in tissue regeneration. Int J Stem Cells. 2020;13:1–12. doi: 10.15283/ijsc19127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu T, De Los Santos FG, Phan SH. The bleomycin model of pulmonary fibrosis. Methods Mol Biol. 2017;1627:27–42. doi: 10.1007/978-1-4939-7113-8_2. [DOI] [PubMed] [Google Scholar]
- 6.Tashiro J, Rubio GA, Limper AH, Williams K, Elliot SJ, Ninou I, Aidinis V, Tzouvelekis A, Glassberg MK. Exploring animal models that resemble idiopathic pulmonary fibrosis. Front Med (Lausanne) 2017;4:118. doi: 10.3389/fmed.2017.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugimoto N, Suzukawa M, Nagase H, Koizumi Y, Ro S, Kobayashi K, Yoshihara H, Kojima Y, Kamiyama-Hara A, Hebisawa A, Ohta K. IL-9 blockade suppresses silica-induced lung inflammation and fibrosis in mice. Am J Respir Cell Mol Biol. 2019;60:232–243. doi: 10.1165/rcmb.2017-0287OC. [DOI] [PubMed] [Google Scholar]
- 8.Cheresh P, Morales-Nebreda L, Kim SJ, Yeldandi A, Williams DB, Cheng Y, Mutlu GM, Budinger GR, Ridge K, Schumacker PT, Bohr VA, Kamp DW. Asbestos-induced pulmonary fibrosis is augmented in 8-oxoguanine DNA glycosylase knockout mice. Am J Respir Cell Mol Biol. 2015;52:25–36. doi: 10.1165/rcmb.2014-0038OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Upagupta C, Shimbori C, Alsilmi R, Kolb M. Matrix abnormalities in pulmonary fibrosis. Eur Respir Rev. 2018;27:180033. doi: 10.1183/16000617.0033-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito A, Horie M, Nagase T. TGF-β signaling in lung health and disease. Int J Mol Sci. 2018;19:2460. doi: 10.3390/ijms19082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H, Yu Y, Huang H, Hu Y, Fu S, Wang Z, Shi M, Zhao X, Yuan J, Li J, Yang X, Bin E, Wei D, Zhang H, Zhang J, Yang C, Cai T, Dai H, Chen J, Tang N. Progressive pulmonary fibrosis is caused by elevated mechanical tension on alveolar stem cells. Cell. 2020;180:107–121.e17. doi: 10.1016/j.cell.2019.11.027. [DOI] [PubMed] [Google Scholar]
- 13.Togami K, Yamaguchi K, Chono S, Tada H. Evaluation of permeability alteration and epithelial-mesenchymal transition induced by transforming growth factor-β1 in A549, NCI-H441, and Calu-3 cells: development of an in vitro model of respiratory epithelial cells in idiopathic pulmonary fibrosis. J Pharmacol Toxicol Methods. 2017;86:19–27. doi: 10.1016/j.vascn.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 14.Chen YW, Huang SX, de Carvalho ALRT, Ho SH, Islam MN, Volpi S, Notarangelo LD, Ciancanelli M, Casanova JL, Bhattacharya J, Liang AF, Palermo LM, Porotto M, Moscona A, Snoeck HW. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat Cell Biol. 2017;19:542–549. doi: 10.1038/ncb3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porotto M, Ferren M, Chen YW, Siu Y, Makhsous N, Rima B, Briese T, Greninger AL, Snoeck HW, Moscona A. Authentic modeling of human respiratory virus infection in human pluripotent stem cell-derived lung organoids. mBio. 2019;10:e00723–19. doi: 10.1128/mBio.00723-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heo HR, Kim J, Kim WJ, Yang SR, Han SS, Lee SJ, Hong Y, Hong SH. Human pluripotent stem cell-derived alveolar epithelial cells are alternatives for in vitro pulmotoxicity assessment. Sci Rep. 2019;9:505. doi: 10.1038/s41598-018-37193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strikoudis A, Cieślak A, Loffredo L, Chen YW, Patel N, Saqi A, Lederer DJ, Snoeck HW. Modeling of fibrotic lung disease using 3D organoids derived from human pluripotent stem cells. Cell Rep. 2019;27:3709–3723.e5. doi: 10.1016/j.celrep.2019.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kearns NA, Genga RM, Ziller M, Kapinas K, Peters H, Brehm MA, Meissner A, Maehr R. Generation of organized anterior foregut epithelia from pluripotent stem cells using small molecules. Stem Cell Res. 2013;11:1003–1012. doi: 10.1016/j.scr.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Snoeck HW. Modeling human lung development and disease using pluripotent stem cells. Development. 2015;142:13–16. doi: 10.1242/dev.115469. [DOI] [PubMed] [Google Scholar]
- 20.Nikolić MZ, Sun D, Rawlins EL. Human lung development: recent progress and new challenges. Development. 2018;145:dev163485. doi: 10.1242/dev.163485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ornitz DM, Yin Y. Signaling networks regulating development of the lower respiratory tract. Cold Spring Harb Perspect Biol. 2012;4:a008318. doi: 10.1101/cshperspect.a008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCauley HA, Wells JM. Pluripotent stem cell-derived organoids: using principles of developmental biology to grow human tissues in a dish. Development. 2017;144:958–962. doi: 10.1242/dev.140731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green MD, Chen A, Nostro MC, d'Souza SL, Schaniel C, Lemischka IR, Gouon-Evans V, Keller G, Snoeck HW. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol. 2011;29:267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gkatzis K, Taghizadeh S, Huh D, Stainier DYR, Bellusci S. Use of three-dimensional organoids and lung-on-a-chip methods to study lung development, regeneration and disease. Eur Respir J. 2018;52:1800876. doi: 10.1183/13993003.00876-2018. [DOI] [PubMed] [Google Scholar]
- 25.Barkauskas CE, Chung MI, Fioret B, Gao X, Katsura H, Hogan BL. Lung organoids: current uses and future promise. Development. 2017;144:986–997. doi: 10.1242/dev.140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikolić MZ, Caritg O, Jeng Q, Johnson JA, Sun D, Howell KJ, Brady JL, Laresgoiti U, Allen G, Butler R, Zilbauer M, Giangreco A, Rawlins EL. Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. Elife. 2017;6:e26575. doi: 10.7554/eLife.26575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller AJ, Dye BR, Ferrer-Torres D, Hill DR, Overeem AW, Shea LD, Spence JR. Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc. 2019;14:518–540. doi: 10.1038/s41596-018-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaushik G, Ponnusamy MP, Batra SK. Concise review: current status of three-dimensional organoids as preclinical models. Stem Cells. 2018;36:1329–1340. doi: 10.1002/stem.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 30.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forbester JL, Goulding D, Vallier L, Hannan N, Hale C, Pickard D, Mukhopadhyay S, Dougan G. Interaction of salmonella enterica serovar Typhimurium with intestinal organoids derived from human induced pluripotent stem cells. Infect Immun. 2015;83:2926–2934. doi: 10.1128/IAI.00161-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodansky ES, Johnson LA, Huang S, Spence JR, Higgins PD. Intestinal organoids: a model of intestinal fibrosis for evaluating anti-fibrotic drugs. Exp Mol Pathol. 2015;98:346–351. doi: 10.1016/j.yexmp.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janssens S, Schotsaert M, Karnik R, Balasubramaniam V, Dejosez M, Meissner A, García-Sastre A, Zwaka TP. Zika virus alters DNA methylation of neural genes in an organoid model of the developing human brain. mSystems. 2018;3:e00219–17. doi: 10.1128/mSystems.00219-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sacramento CQ, de Melo GR, de Freitas CS, Rocha N, Hoelz LV, Miranda M, Fintelman-Rodrigues N, Marttorelli A, Ferreira AC, Barbosa-Lima G, Abrantes JL, Vieira YR, Bastos MM, de Mello Volotão E, Nunes EP, Tschoeke DA, Leomil L, Loiola EC, Trindade P, Rehen SK, Bozza FA, Bozza PT, Boechat N, Thompson FL, de Filippis AM, Brüning K, Souza TM. The clinically approved antiviral drug sofosbuvir inhibits Zika virus replication. Sci Rep. 2017;7:40920. doi: 10.1038/srep40920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smits LM, Reinhardt L, Reinhardt P, Glatza M, Monzel AS, Stanslowsky N, Rosato-Siri MD, Zanon A, Antony PM, Bellmann J, Nicklas SM, Hemmer K, Qing X, Berger E, Kalmbach N, Ehrlich M, Bolognin S, Hicks AA, Wegner F, Sterneckert JL, Schwamborn JC. Modeling Parkinson's disease in midbrain-like organoids. NPJ Parkinsons Dis. 2019;5:5. doi: 10.1038/s41531-019-0078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raja WK, Mungenast AE, Lin YT, Ko T, Abdurrob F, Seo J, Tsai LH. Self-organizing 3D human neural tissue derived from induced pluripotent stem cells recapitulate Alzheimer's disease phenotypes. PLoS One. 2016;11:e0161969. doi: 10.1371/journal.pone.0161969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouchi R, Togo S, Kimura M, Shinozawa T, Koido M, Koike H, Thompson W, Karns RA, Mayhew CN, McGrath PS, McCauley HA, Zhang RR, Lewis K, Hakozaki S, Ferguson A, Saiki N, Yoneyama Y, Takeuchi I, Mabuchi Y, Akazawa C, Yoshikawa HY, Wells JM, Takebe T. Modeling steatohepatitis in humans with pluripotent stem cell-derived orga-noids. Cell Metab. 2019;30:374–384.e6. doi: 10.1016/j.cmet.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aguisanda F, Yeh CD, Chen CZ, Li R, Beers J, Zou J, Thorne N, Zheng W. Neural stem cells for disease modeling of Wolman disease and evaluation of therapeutics. Orphanet J Rare Dis. 2017;12:120. doi: 10.1186/s13023-017-0670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nie YZ, Zheng YW, Miyakawa K, Murata S, Zhang RR, Sekine K, Ueno Y, Takebe T, Wakita T, Ryo A, Taniguchi H. Recapitulation of hepatitis B virus-host interactions in liver organoids from human induced pluripotent stem cells. EBioMedicine. 2018;35:114–123. doi: 10.1016/j.ebiom.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NW, Bijvelds MJ, Scholte BJ, Nieuwenhuis EE, van den Brink S, Clevers H, van der Ent CK, Middendorp S, Beekman JM. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19:939–945. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 41.Lancaster MA, Huch M. Disease modelling in human organoids. Dis Model Mech. 2019;12:dmm039347. doi: 10.1242/dmm.039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkinson DC, Alva-Ornelas JA, Sucre JM, Vijayaraj P, Durra A, Richardson W, Jonas SJ, Paul MK, Karumbayaram S, Dunn B, Gomperts BN. Development of a three-dimensional bioengineering technology to generate lung tissue for personalized disease modeling. Stem Cells Transl Med. 2017;6:622–633. doi: 10.5966/sctm.2016-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]