Abstract
Provocative research has revealed both positive and negative effects of hormones on hearing as we age; with in some cases, mis-regulation of hormonal levels in instances of medical comorbidities linked to aging, lying at the heart of the problem. Animal model studies have discovered that hormonal fluctuations can sharpen hearing for improved communication and processing of mating calls during reproductive seasons. Sex hormones sometimes have positive effects on auditory processing, as is often the case with estrogen, whereas combinations of estrogen and progesterone, and testosterone, can have negative effects on hearing abilities, particularly in aging subjects. Too much or too little of some hormones can be detrimental, as is the case for aldosterone and thyroid hormones, which generally decline in older individuals. Too little insulin, as in Type 1 diabetics, or poor regulation of insulin, as in Type 2 diabetics, is also harmful to hearing in our aged population. In terms of clinical translational possibilities, hormone therapies can be problematic due to systemic side effects, as has happened for estrogen/progestin combination hormone replacement therapy (HRT) in older women, where the HRT induces a hearing loss. As hormone therapy approaches are further developed, it may be possible to lower needed doses of hormones by combining them with supplements, such as antioxidants. Another option will be to take advantage of emerging technologies for local drug delivery to the inner ear, including biodegradeable, sustained-release hydrogels and micropumps which can be implanted in the middle ear near the round window. In closing, exciting research completed to date, summarized in the present report bodes well for emerging biomedical therapies to prevent or treat age-related hearing loss utilizing hormonal strategies.
Keywords: Presbycusis, aldosterone, estrogen, testosterone, animal model, human
Introduction
Progress has been dramatic for advancing our knowledge of interactions between hormonal changes and the nature and progression of age-related hearing loss (ARHL). This field is rapidly reaching a point where basic science advances will be translated into clinical trials and new therapeutic approaches. Much of this progress has come from animal model and preclinical studies; as well as clinical research in humans (Frisina and Frisina 1997; 2016). Here we will present some of the most interesting and useful studies of animal models and humans regarding interplay between hormonal changes and ARHL/presbycusis, with an eye towards basic research approaches that may lead to therapies involving hormonal components to solve sensory aging problems.
Initially here, provocative results from some seminal animal model studies will be put forth, suggestive of translational approaches. Then studies of aging women and hormone replacement therapy (HRT) effects will be analyzed; followed by animal model studies that start to probe the biological bases for the interactions between HRT and ARHL. Building upon this, some additional preclinical and human investigations of sex hormones will be summarized, which have information useful for therapeutic strategies. Lastly, the potential of other hormones, in addition to the sex hormones, will be considered as pathways to future translational breakthroughs.
Animal Model Systems - Vertebrates
Sisneros and colleagues have contributed much to our knowledge of how sex hormone changes can modify auditory processing in neuroethologically relevant ways, paving the way for designing experiments in aging mammals and humans. Specifically, the vocal plainfin midshipman fish (Porichthys notatus) is an excellent model for characterizing neural mechanisms of auditory processing common to vertebrates (Sisneros 2009). Utilizing this animal model, evidence for steroid hormone-dependent modulation of hearing, leading to enhanced coupling of sender and receiver, has been revealed in this vocal-acoustic communication system. For example, non-reproductive females treated with either testosterone or17b-estradiol exhibit improved degrees of temporal coding by the auditory saccular afferents to the dominant frequency content of male vocalizations produced during reproductive behaviors. This hormonal augmented frequency sensitivity of females receiving hormone therapy mimics the reproductive female’s hearing phenotype, and appears to improve the detection and localization of conspecific mates who are calling during the summer breeding season. In sum, certain fish actively tune their hearing based upon hormonal levels related to their mating season and for identifying potential mates.
For the lower vertebrates, frogs have also been a very useful animal model system for investigating key relations between sex hormones and hearing plasticity (Arch and Narins 2009). Most anuran amphibians (frogs and toads) use acoustic communication to facilitate sexual and reproductive behaviors. For example, females find and select their mates using sound cues provided by males in the form of signature advertisement calls. Vocalization production and reception are closely tied to successful reproduction. Research with anurans has demonstrated that auditory communication is modulated by reproductive hormones, including gonadal steroids and peptide neuromodulators. Most of these reports have focused on the ways in which hormonal systems influence vocal signal production; however, hormonal modulation of hearing and call reception also occurs, revealing that reproductive hormones assist in the coordination of reproductive behaviors between signaler and receiver by changing sensitivity and spectral filtering of anuran hearing. Apparently, the hormonal systems that help guide reproductive behaviors are well-conserved among vertebrates; so studying the endocrine and neuromodulatory bases of acoustic communication in frogs and toads can lead to novel findings with broader applicability to hormonal modulation of vertebrate sensory physiology, behavior and changes with age.
Animal Model Systems – Mammalian Investigations of Sex Hormones and Auditory Processing
McFadden’s team carried out a series of elegant studies, using different mammals and humans, demonstrating that testosterone levels can alter auditory sensitivity and cochlear processing (McFadden, 2009). McFadden built upon knowledge that otoacoustic emissions (OAEs) differ between the sexes in humans, rhesus and marmoset monkeys, and sheep. Otoacoustic emissions give a non-invasive, physiological readout of the health and well-being of the cochlear outer hair cell system. This system is important for regulating the sensitivity of the cochlea, i.e., hearing thresholds; and often is the first system to decline in ARHL. OAEs also differ in some special populations of humans. McFadden analyzed OAEs from different animal models and human groups, and in the context of possible prenatal androgen effects on the auditory system, and concluded that a parsimonious explanation for several outcomes is that prenatal exposure to high levels of androgens (e.g., testosterone hormone) can weaken the cochlear amplifiers and thereby decrease OAE magnitudes. Functionally, this means that testosterone is linked to higher OAE thresholds, and lower OAE amplitudes, i.e., worse hearing. He also surmised that prenatal androgen exposure can alter auditory evoked potentials (AEPs), even at supra-threshold sound levels.
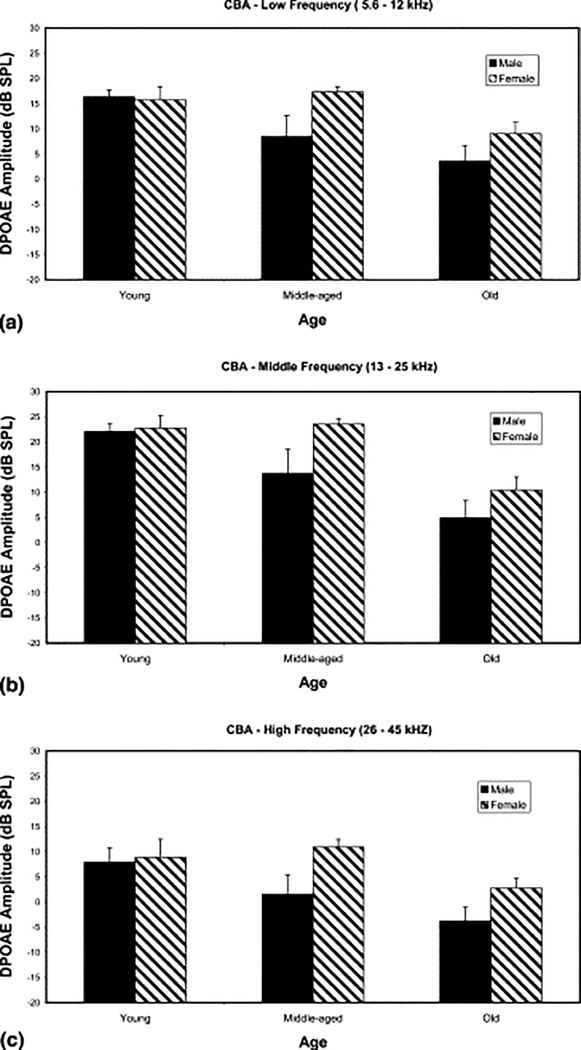
Female hormones have also been linked to general hearing differences between the sexes. Normally, on the average, women have better hearing than men, at least until they reach old age. Examination of available clinical evidence suggests this is due in part to more noise exposure and other ototoxic insults in men compared to women as they age across the lifespan. It has also been attributed to the fact that women have more circulating estrogen than men. Estrogen levels, aside from being an important component of the ovulatory cycle, and during pregnancy, is a growth factor hormone that supports cell proliferation for different systems of the body, including the inner ear. So, its elevated levels in females, for example in aging female mice, is partly responsible for better hearing in females relative to males, going into old age (e.g., Guimaraes et al. 2004). Guimaraes and colleagues measured auditory brainstem response (ABR) thresholds, and OAEs for 3 groups of aging CBA/CaJ mice: young adult, middle age and old. For the two younger groups, the females had better hearing, lower thresholds, than the males. However, this relative sex difference in hearing sensitivity was significantly smaller in the old age group, as shown here in Figure 1. Since these animals lived their entire life in a noise-free, non-ototoxic environment (unlike most humans), it is likely that the relatively better hearing of the females disappeared in old age, because the estrogen levels declined in the old females (post-menopausal mice), and the testosterone levels decreased in the old males; consistent with the general phenomenon of age-related declines in most hormones of the body. In addition, another study using CBA/J and CBA/CaJ mice as a model for age-related hearing loss (ARHL) found that males have higher ABR thresholds compared to female mice, coinciding with previous studies that have found this trend in humans (Henry 2004).
Figure 1.
Females show better hearing throughout life, but the difference declines in old age. (a) Low frequency range (5.6–12 kHz): the males presented with a progressive decline of DPOAE amplitude from young adult to middle-aged groups. The females maintained their DPOAE amplitudes through middle-aged and then showed a significant decline from middle to old age. The sex differences for the low frequencies using a two-way ANOVA proved to be significant (F=4.2, P<0.05). (b) Middle frequency range (13–25 kHz): the pattern in this graph was similar to that of the previous one. The females had high DPOAE amplitudes through middle age, with declines from middle age to old age. The males presented with a progressive decline throughout life. The two-way ANOVA confirmed this sex difference (F=4.57, P<0.05). (c) High frequency range (26–45 kHz): the age by sex findings were like those of the previous graphs (a,b). The two-way ANOVA was also significant (F=4.89, P<0.05). (From Guimaraes et al. 2004, Fig. 3; with permission).
A synthesis of these findings suggests that since estrogen is a growth factor, and supportive of cochlear cell health throughout life, losing this in old age hurts old females’ hearing relative to the males. Conversely, since testosterone may have a negative effect, see work of McFadden described above, diminishing levels of this hormone in the elderly males, could actually help their hearing somewhat, compared to the aged females.
Hormone Replacement Therapy - HRT in Aging Women
In recent decades, a consensus in the literature suggests that the sex hormone estrogen plays an important role in modulating hearing function. Some of the original studies focused on comparing thresholds and latency values from auditory brainstem responses (ABRs) and otoacoustic emissions (OAEs) between males and females using human and animal models. These studies found that females, regardless of species, had better thresholds and latency values compared to the men in the same age group (Jerger and Hall, 1980; Chung et al., 1983; Church and Cudahy, 1984; Jerger and Johnson, 1988; McFadden and Pasanen, 1998).
These studies led to a myriad of new reports investigating the effects of estrogen on hearing using female subjects still undergoing their menstrual cycle, postmenopausal females, male comparison subjects, and subjects using estrogen hormone-therapy (Coleman et al. 1994; Zhang et al. 2018; Kilicdag et al. 2004). For example, further supporting the important role of hearing-related estrogen effects, Da Silva Souza’s work with pre-menopausal women interestingly observed noticeable changes during the menstrual cycle. In periods of high serum estrogen levels such as the late-follicular phase, hearing thresholds were at their lowest. Conversely, the late-luteal phase and early follicular phase where serum estrogen levels were lowest, correlated with elevated hearing thresholds. (Da Silva Souza et al., 2017)
Guimaraes and colleagues performed the largest clinical research study of the effects of HRT on hearing in older women (Guimaraes et al. 2006). They did a comprehensive battery of hearing tests, including pure-tone audiograms, OAEs (DPOAEs), speech perception in quiet, and speech perception in background noise (HINT); and they obtained comprehensive health histories; allowing them to match the age of the subjects, and exclude subjects with confounding, ototoxic histories such as prolonged noise exposure, chemotherapy, diabetes, hypothyroidism and so on. They tested 3 groups of older women (post-menopausal): Controls who were otherwise healthy, and had never taken HRT; women who had taken the most common form of HRT: combination of estrogen and progestin (progesterone) (EP); and a group of old women who had undergone hysterectomies, and took estrogen HRT (E alone). Across the board, the EP subject group performed the worst on the hearing test battery; specifically, they had higher audiometric thresholds and poorer supra-threshold speech perception in background noise. The E group had similar hearing capabilities as the Controls; suggesting that the progesterone component of the combination HRT is ototoxic; or the combination/interaction of these two female sex hormones in HRT is harmful.
Building upon Guimaraes et al. clinical research study, Curhan et al. (2017), as part of the large Nurses’ Health Study II, investigated relations between hearing and HRT in aging women. They pointed out that menopause may be a risk factor for hearing loss, and that postmenopausal hormone therapy (HRT) was originally hypothesized to slow age-linked hearing declines. So, they quantitatively examined the independent relations between menopause, postmenopausal HRT and risk of self-reported hearing loss. Their large, prospective cohort study involved 80,972 women, baseline age 27–44 years, and they followed these women from 1991 to 2013. Baseline and updated data were obtained from validated biennial questionnaires. Cox proportional hazards regression models were used to examine independent associations between the variables. After 1,410,928 person-years of follow-up, 18,558 cases of hearing loss were reported. There was no significant overall association between menopausal status, natural or surgical, and risk of hearing loss; rather, older age at natural menopause was associated with higher risk. Among postmenopausal women, oral HRT [(estrogen therapy (E) or estrogen plus progestogen therapy (EP)] was associated with higher risk of hearing loss, and longer duration of use was associated with higher risk (p-trend <0.001). Compared with women who never used HRT, the risk of ARHL among women who used oral HRT for 5–9.9 years was 1.15 (95% CI 1.06, 1.24) and for 10+ years was 1.21 (95% CI 1.07, 1.37). They concluded that older age at menopause and longer duration of postmenopausal HRT are associated with higher risk of ARHL, similar to Guimaraes et al. (2006, reported above).
Biological Mechanisms and Neural Underpinnings of Sex Hormone Interactions with Hearing
To start determining the generality and neural bases of these clinical research findings, Price and coworkers performed an animal model (CBA/CaJ mice) experiment, modelled after the Guimaraes et al. (2006) clinical investigation just described (Price et al. 2009). They examined the effects of combination HRT (estrogen + progestin, EP) and estrogen alone (E) on hearing in peri-menopausal mice. Specifically, ABRs and OAEs (DPOAEs) were measured for middle age female CBA mice who received either a time-release, subcutaneous implanted EP, E, or placebo (Control) drug pellet. Longitudinal comparisons of the ABR threshold data obtained at 4 months of treatment revealed statistically significant declines, relative to Controls, in auditory sensitivity over time for the EP treatment group, with the E only group revealing milder changes at 3, 6 and 32 kHz. DPOAE measures revealed statistically significant differences (worse hearing) for the EP treatment group in the high and middle frequency ranges (15–29 and 30–45 kHz) after as early as 2 months of treatment. Statistically significant changes were also seen at 4 months of treatment across all frequencies for the EP HRT group. Consistent with the human research presented above (Guimaraes et al. 2006; Curhan et al. 2017) these data indicate that EP HRT therapy impairs outer hair cell functioning and overall auditory sensitivity. Taken together, these findings suggest that EP HRT, the most common form used clinically, may actually accelerate ARHL, relative to E monotherapy or no therapy.
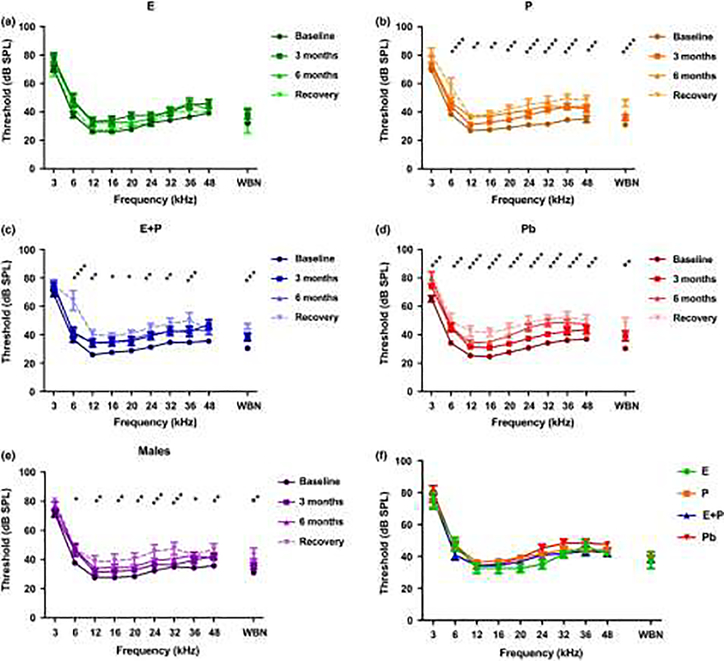
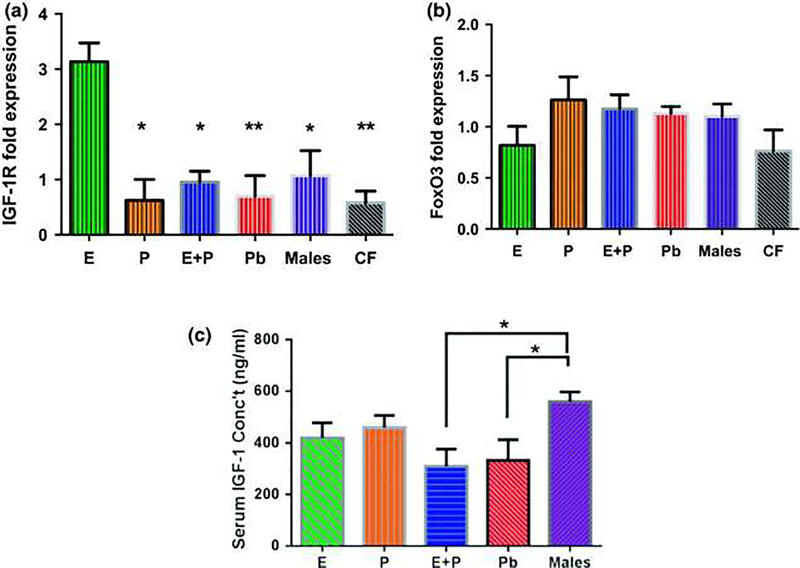
Williamson and colleagues (2019) expanded upon the work of Price et al. (2009), focusing upon the effects of progesterone alone (P) compared to EP and E, some of the biological mechanisms, and possible recovery of hearing after the termination of HRT. They pointed out that estradiol (estrogen - E) is a multitasking hormone that plays a prominent role in the reproductive system, and also contributes to physiological growth mechanisms in multiple body systems. Specifically, they evaluated hearing in aging female CBA/CaJ middle-aged mice during and after long-term hormone replacement therapy (HRT- like Price et al. 2009) via electrophysiological and molecular techniques. Their data revealed that the long-term effects of HRT are permanent for ABR thresholds and ABR gap-in-noise amplitude levels (GIN, a physiological measure of the auditory system’s temporal processing capabilities). Examples of key findings are shown in Figure 2: E-treated animals had lower thresholds and higher amplitude values compared to other hormone treatment subject groups. Interestingly, P-treated animals had ABR thresholds that increased but amplitude levels that remained relatively the same throughout treatment. These results were consistent with qPCR experiments that displayed high levels of IGF-1R in the stria vascularis (SV) of both E and P animal groups compared to combination treatment (EP) animals as displayed in Figure 3. IGF-1R plays a vital role in mediating anti-apoptotic responses via the PI3K/AKT pathway. Overall, their results gain insights into the neuro-protective properties of E hormone treatments as well as expand the scientific knowledge base to help women decide whether HRT is the right choice for them.
Figure 2.
Auditory brainstem response thresholds over the course of hormone treatments as well as during the recovery period. (a) Estrogen-treated (E) females show no significant signs of ARHL over the course of hormone therapy, thus indicating that E possesses protective properties for auditory function. (b) Progestin-treated (P) females show significantly poorer hearing at almost all of the tested frequencies. (c) The E + P-treated group of females displayed elevations in ABR thresholds as early as 3 months. Notable worsening of hearing could be seen in this group over time. (d) Placebo control females’ (Pb) thresholds changed drastically over the 6-month time period. Significant ARHL changes were observed for all frequencies. (e) Changes observed in the male group, more specifically during the recovery period (8 months), could be attributed to ARHL. (f) Recovery period group comparison shows that E-treated animals had lower thresholds at 12, 16, 20, 24, and 32 kHz compared to all the other HRT animals. Pb females had higher thresholds among the HRT groups at 24 and 32 kHz. These data suggest that the results of long-term HRT on ABR thresholds are permanent. No statistical differences were seen among the hormone groups during the recovery period. It should be noted that statistical differences for (a) through (e) are a comparison between the baseline and recovery. Statistical test: 2-way ANOVA followed by Bonferroni; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. From Williamson et al. 2019, Fig. 2; with permission.
Figure 3.
In vivo 1-month post-treatment IGF-1R and FoxO3 expression levels. (a) Estrogen-treated (E) females had the highest IGF-1R fold expression levels among the subject groups for stria vascularis (SV) tissue samples. Interestingly, placebo (Pb) and control female (CF) animals had the most significant differences among the groups, relative to E. This implies that lack of HRT during the aging process decreases IGF-1R levels. (b) FoxO3 gene expression was comparatively similar among the SV tissue sample groups. Congruous findings were observed for in vitro FoxO3 experiments. It can be noted that overall the CF group had the lowest expression levels for both genes. (c) Post-treatment IGF-1 concentration levels in the serum of HRT mice showed no significant differences among the female HRT groups. Only E + progestin (P) and Pb groups displayed statistical variances in comparison to the control male animals. Statistical test: 1-way ANOVA followed by Bonferroni; *p < 0.05, **p < 0.01 (E n = 3; P n = 3; E + P n = 3; Pb n = 3, Males n = 3; CF n = 3). Note : The CF group consists of age-matched females with their ovaries intact that did not undergo any type of HRT. From Williamson et al. 2019, Fig. 6; with permission.
In terms of further elucidation of biological mechanisms, in the auditory system, estrogen has been shown to exert its effects via specialized estrogen receptors (ER), namely ER-α and ER-β. These ERs have been found in tissues throughout the inner ear such as Reissner’s membrane, stria vascularis, spiral ligament, spiral ganglion, vestibular ganglion, and cochlear inner and outer hair cells. They are also expressed in various brain regions of the central auditory system: auditory cortex, cochlear nuclei, inferior colliculus, lateral superior olive, and ventral nucleus of the lateral lemniscus. (Charitidi and Canlon, 2010; Milon et al. 2018; Shuster et al. 2019). The localization of these receptors in the cochlea is important because they help determine auditory sound processing response parameters such as threshold, amplitude, and latency (Williamson et al. 2019).
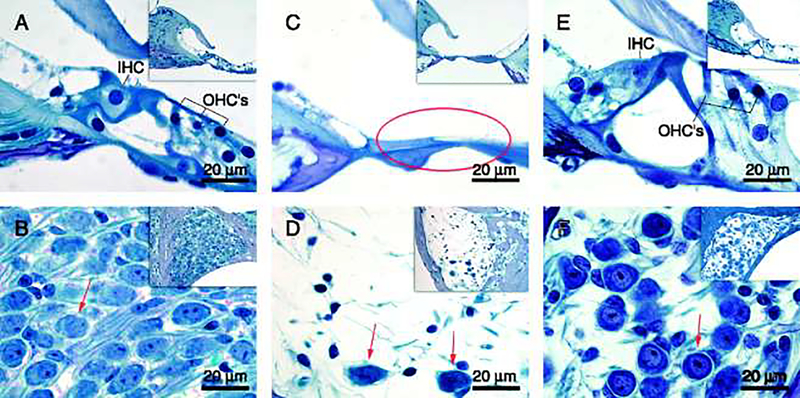
Simonoska et al. (2009) used ER-β knockout mice to compare the hearing and morphological characteristics of the inner ear to control C57BL/6J mice. Their results showed that ER-β knockout mice had severe damage in the of the organ of Corti of the cochlear basal turn, including an absence of hair cells, and missing spiral ganglion neurons as presented in Figure 4. Furthermore, when the ER-β knockout mice were 1 year old, they were already deaf. ER-α is also believed to play a role in sex differences for ARHL because its expression is higher in young CBA/CaJ female mice compared to male mice of the same age group (Motohashi et al. 2010). Another mechanism hypothesized to be involved in estrogen’s preservation of hearing is the insulin growth factor-1 (IGF-1) pathways. IGF-1 is known to have neuroprotective effects such as maintaining cellular metabolism and limiting cell death. It has also been shown that haplo-insufficiency of IGF-1 is related to ARHL (Rodríguez-de la Rosa et al. 2017). Recently, Williamson et al. (2019) showed that IGF-1 levels increased in postmenopausal mice after 6 months of estrogen hormone treatment. In addition, the ABR thresholds and gap-in-noise (GIN) amplitudes were maintained with this treatment compared to control animals who continued to suffer further hearing impairments due to ARHL. Moreover, it was found that estrogen interaction with ERs increased with the upregulation of IGF-1 (Rudzinski 2002). Lastly, glutamate toxicity has been connected to ARHL as glutamate accumulation with age can damage central auditory tissue (Tadros et al. 2007). It has been found that estrogen provides neuroprotective effects against glutamate toxicity (Tadros et al. 2007; Singer et al. 1996). Specifically, release of cellular lactate dehydrogenase (LDH) is a marker for cytotoxicity, specifically glutamate toxicity, when it is released from primary cortical neurons. In the presence of estrogen, cells show significant decrease in LDH release, thus playing a role in reducing glutamate excitotoxicity.
Figure 4.
Morphological photomicrographs from: the basal turn of the cochlea in a 3-month-old ER-β−/− mouse (A and B); 12-month-old ER-β−/− mouse (C and D), and 12-month-old WT mouse (E and F). (A) Shows a normal organ of Corti with intact inner hair cell (IHC) and three outer hair cells (OHCs) and in (B) the spiral ganglion is filled with spiral ganglion cells (red arrow). (C) Shows the organ of Corti in a 12-month-old ER-β−/− mouse that has degenerated, resulting in a flat epithelium (red circle) and in (D), there is an extensive loss of ganglion cells in the spiral ganglion, with a red arrow indicating the few cells left. (E) Shows fairly normal organ of Corti in 12-month-old WT mouse and (F) shows the spiral ganglion with loss of spiral ganglion cells (arrow), but in comparison with 12-month-old ER-β−/− mice (D) the loss is not as extensive. From Simonoska et al. 2009, Fig. 2; with permission.
For the peripheral auditory system, estrogen clearly plays key roles in the homeostasis and protection of inner ear cells. (Hultcranz et al. 2006) However, estrogen is also vital in the maintenance and processing of auditory signals in the central auditory system. Estrogen, or its main form estradiol, controls signal transmission through two unique mechanisms, non-genomic and genomic. Non-genomic control occurs on a fast timescale on the order of seconds allowing for rapid gain adjustment while classical genomic effects occur within minutes to hours in order to enhance and sustain neural coding over longer time periods (Pinaud and Tremer, 2012).
In normal hearing individuals, non-genomic effects are shown to be relatively instantaneous as seen in songbirds (Maney and Pinaud, 2011). Local estradiol production by estrogen synthase, within neurons, can send signals as both paracrine and autocrine ligands. Estradiol then exhibits control over presynaptic activity through modulation of GABA neurotransmission, thus controlling the subsequent release of miniature inhibitory postsynaptic currents (miPSCs). Interestingly, only frequency and not amplitude is attenuated for these miPSCs, leading to increased gain and improved sound discrimination. Estradiol effectively widens the bandwidth of the neural transmission while reducing neural background noise (Jeong et al. 2009). The slower timescale effects, coordinated by classical genomic effects, are mainly regulated by the mitogen-activated protein kinase (MAPK) cascade (Pinaud 2012). Estradiol binding to both the estrogen receptors, ER-α and ER-β, activates the MAPK pathway to regulate cellular proliferation, cell and neuronal survival via anti-apoptotic pathways, and mitochondrial viability through calcium signaling (Zhao et al., 2016); all of which assist in maintaining the auditory neurophysiological adaptations needed for sound processing. (Pinaud 2012)
Having now seen the importance of estradiol as a key modulator in auditory processing, perturbations of estrogen-related neural circuitry can lead to clinical pathologies both hearing-related and otherwise. Many of these pathologies are age-related disorders where estrogen production or receptor expression is inhibited. But, genetic disorders such as Turner’s syndrome can also lead to hearing loss. Syndromic, age-related disorders such as Alzheimer’s disease and Parkinson’s disease have also been linked to reduced levels of systemic estrogen and ERs, as well as noticeable loss in brain-derived estrogen (Cui et al. 2013). In these populations, estrogen replacement therapy (ERT) has been shown to mitigate some of the hallmarks associated with these genetic medical conditions, even promoting neurogenesis in certain brain regions such as the dentate gyrus of the hippocampus, which is important for auditory and other memories. Specifically, amyloid beta production was reduced for Alzheimer’s, while Parkinson’s patients experienced motor improvement (Cui et al.2013). As presented above, post-menopausal women are particularly at risk for of age-related disorders as their estrogen production ceases, dropping serum levels significantly. Concomitantly, hearing sensitivity, in this population, according to ABR measures is also reduced. Evidence of ERT as an effective treatment modality is also seen in post-menopausal women where ABR peak latencies are shortened, ABR peak amplitudes are increased, and pure-tone audiometric thresholds are reduced (Shuster et al. 2019; Caras 2013).
It should be noted that these hearing declines may not be the exclusive result of decreased estrogen production, but may in fact be due to a confluence of multiple age-related declines and perturbations. In studies involving animal models, expression levels of ERs were differentially distributed according to tissue and cell type in the brain (Mott and Pak, 2013). Additionally, ERs of both types were shown to be reduced with age, and the extent of downregulation for each individual receptor was location-dependent; lending credence to estrogen’s impact on ER autoregulation as local as system estrogen production is reduced with age. In one study involving the CBA/CaJ mouse model, Charitidi and colleagues probed for age-related changes of ER-α and ER-β that occur in the central auditory system. They found that significant declines in ER-α occurred in the auditory cortex, BIC, ECIC, DCIC, and some portions of the SOC when comparing young sexually mature mice to aged mice (26–28 months). ER-β was relatively unchanged but with an increase in the VCA. Interestingly, no sex differences were observed in the aged mice contrary to many other findings showing sexual dimorphism (Charitidi et al. 2010; Mott and Pak, 2013). Taken together, these results indicate that decreased estrogen production along with reduced ability to signal estrogen-related pathways may contribute to hearing perceptual processing deficits of ARHL. This also gives exciting frontiers for novel treatment as new knowledge continues to emerge from animal model studies and clinical research on relations between sex hormones and ARHL.
Aldosterone Physiological Regulation of Key Ions Important for Hearing
Trune’s team pioneered animal model investigations of the effects of steroid hormones, in particular aldosterone, on treating hearing loss. They pointed out that the standard treatment for some hearing disorders, such as sudden hearing loss, is glucocorticoid therapy but the cochlear mechanisms involved in steroid-responsive hearing loss are not well understood (Trune et al. 2006). To explore this area, they were able to demonstrate that autoimmune mice with significant degrees of hearing loss, could reduce their hearing loss by receiving treatments with the mineralocorticoid aldosterone, as effectively as with the glucocorticoid prednisolone (Trune et al. 2000). They reasoned that because aldosterone regulates sodium, potassium, and other electrolyte homeostasis in physiological systems of the body, this restoration of hearing with the mineralocorticoid was due to its facilitative impact on cochlear ion transport, particularly in the stria vascularis to bolster an impaired endocochlear potential, the inner ear biological battery that enables cochlear sound transduction via hair cells. In addition, they observed improved morphology of the cochlear stria vascularis with the aldosterone therapy. Continuing this line of investigation, Trune and colleagues (2001) examined hearing changes in in autoimmune MRL/MpJ-Faslpr mice. They gave these mice different doses of prednisolone or aldosterone in their drinking water for 2 months. Untreated, control mice showed elevation/worsening of ABR thresholds due to their ongoing autoimmune disease and aging. In contrast, the steroid groups had significantly more mice with improved or unchanged ABR thresholds. Both prednisolone and aldosterone improved stria vascularis morphology, although aldosterone was more effective. In conclusion, prednisolone and aldosterone restored auditory function in aging mice, suggesting that steroid reversal of autoimmune hearing loss in mice is due to increasing stria vascularis sodium transport, rather than suppression of systemic autoimmune responses.
Clinical Relations between Degree of ARHL and Serum Aldosterone Levels
Tadros et al. (2005) explored relations between hearing sensitivity and age-declining serum aldosterone levels; to discover if there were possible protective indications. They measured pure-tone audiograms for a group of aged human subjects and also obtained blood samples. The audiograms allowed them to classify the degree of hearing loss, and compare it quantitatively with the amount of aldosterone in the blood (circulating hormone levels). They found that the old subjects with the best hearing, i.e., lowest tone thresholds had the highest serum aldosterone concentrations. Conversely, those with the worst hearing (highest thresholds) had the lowest aldosterone levels. They also measured otoacoustic emissions (TEOAEs), gap detection, and speech perception abilities in background noise (hearing-in-noise test, HINT). They observed that HINT scores versus serum aldosterone levels were significantly correlated: higher aldosterone was related to better hearing-in-noise. On the contrary, no significant correlations were seen in the case of TEOAEs and gap detection. They concluded that aldosterone may have a protective effect on hearing in old age, where this effect is more peripheral than central, and appears to affect inner hair cell sound processing more than outer hair cells.
Prospective Studies in Aging Animal Models for Aldosterone Hormone Therapy Intervention
Walton, Frisina and colleagues performed a prospective animal-model experiment to test the hypothesis that aldosterone supplementation can provide hearing protection with age (Frisina et al. 2016; Halonen et al. 2016). This hypothesis builds upon their previous findings that aldosterone (1 mM) increased NKCC1 protein expression in vitro and that this up-regulation of NKCC1 was not dose-dependent (dosing range from 1 nM to 100 mM). Na-Kþ-Cl co-transporter 1 or NKCC1, which is involved in homeostatic maintenance of the endocochlear potential (Ding et al. 2014). So, they parsed middle age, CBA/CaJ mice, who normally lose their hearing slowly with age, like most humans; into an experimental group that received subcutaneous, time-release aldosterone hormone supplementation pellets and a control group that received saline. They measured their hearing physiologically with ABR audiogram thresholds, and behaviorally with pre-pulse inhibition of the acoustic startle response. After 4 months of treatment, so 4 months of aging, the experimental group had hearing sensitivity equivalent or better than at baseline (just prior to the start of the treatments); whereas the control group had worse hearing, i.e., their age-related hearing loss progression continued as expected.
In terms of cellular and molecular mechanisms underlying this therapeutic effect, additional experiments revealed that spiral ganglion cell survival was significantly improved, mineralocorticoid receptors were upregulated via post-translational protein modifications, and age-related intrinsic and extrinsic apoptotic pathways were blocked by the aldosterone therapy (Frisina et al. 2016). They concluded that taken together, these novel findings pave the way for translational drug development towards the first medication to prevent the progression of ARHL.
Other Hormones can Affect Age-Linked Hearing Declines- Thyroid
Thyroid hormone is essential for cochlear development and fluctuating levels of this hormone can affect age-related declines in hearing, so significant effects across the lifespan (Forrest et al. 1996; Rüsch et al. 2001; Song et al. 2008; Ng et al. 2015; Sundaresan et al. 2016; Sharlin et al. 2018). Thyroid hormones also play critical roles in the development of the middle ear, including the ossicular chain (Cordas et al. 2012). Song et al. (2008) reported that hypothyroid, Tshr mutant mice have abnormal threshold-frequency responses (tuning curves) and two-tone suppression at the postnatal three-week stage compared to controls (euthyroid) mice that possess regular cochlear development characteristics: sharp tuning curves and normal two-tone suppression responses. This study demonstrates the importance of thyroid hormones in murine cochlear development. Similarly, (Sharlin et al. 2018) showed that knockout of two thyroid hormone transporters (Slc16a2 – Mct8, and Slc16a10 – Mct10) together lead to retardation in postnatal development of mouse cochlear structures; inner hair cells, outer hair cells, support cells and loss of the endocochlear potential. Treatment with thyroid hormone T3 at P7 stage helped preserve cochlear morphology in knockout mice and limited some of the negative effects on auditory function, such as ABR responses.
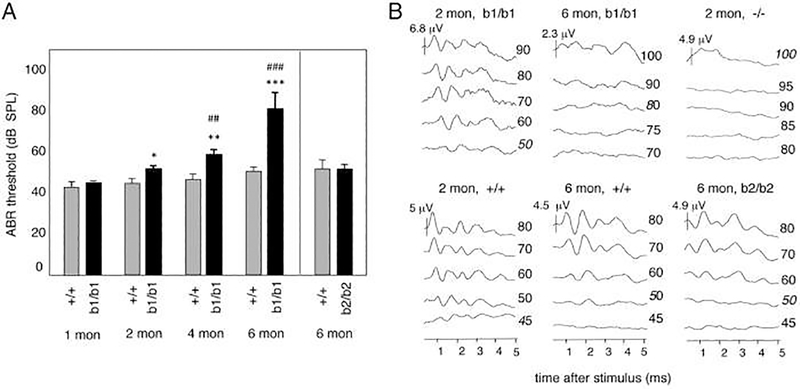
Along with effects on the development of auditory function, there are reports that thyroid hormones play an important role in age-linked hearing declines. Ng et al. (2015) investigated the function of thyroid hormone receptor β in the development as well as the maintenance of the cochlea with age. They investigated the role of two isoforms of thyroid receptors: β1 and β2. C57BL/6J mice were used as the background strain for the study. ABR thresholds were elevated for the β1 deficient mice with age, as compared to the controls, whereas β2 deficient mice had same thresholds as the controls, as shown in Figure 5. Cochlear morphological damage in the β1 knockouts are displayed in Figure 6. These findings indicate that thyroid hormone receptor β1 isoform plays an important role in maintenance of auditory function and cochlear hair cell survival in aging mice.
Figure 5.
Age-dependent loss of auditory function in b1/b1 mice; which is a knockout mouse for thyroid receptor β1. A, ABR for +/+ and b1/b1 mice at 1, 2, 4, and 6 months of age, and for b2/b2 mice (b2 is a knockout mouse for thyroid receptor β2) with their own +/+ group at 6 months of age. Responses to click stimulus are shown. Thresholds increased progressively in b1/b1 compared with +/+ mice, whereas b2/b2 mice had no increase compared with their +/+ group at 6 months. For comparison of b1/b1 vs +/+ groups at a given age: *, P < .05; **, P < .01; ***, P < .001; for each b1/b1 group vs its preceding younger group: ##, P < .01; ###, P < .001. B, Representative ABR waveforms for a click stimulus at different intensities (numbers on right, dB SPL) showing loss of specific waveforms by 6 months of age in b1/b1 mice, whereas +/+ mice show only modest shifts in thresholds. In contrast, −/− mice lacked distinct waveforms at younger ages. Waveforms were similar in b2/b2 and +/+ mice at 6 months of age. From Ng et al. 2015, Fig. 4; with permission.
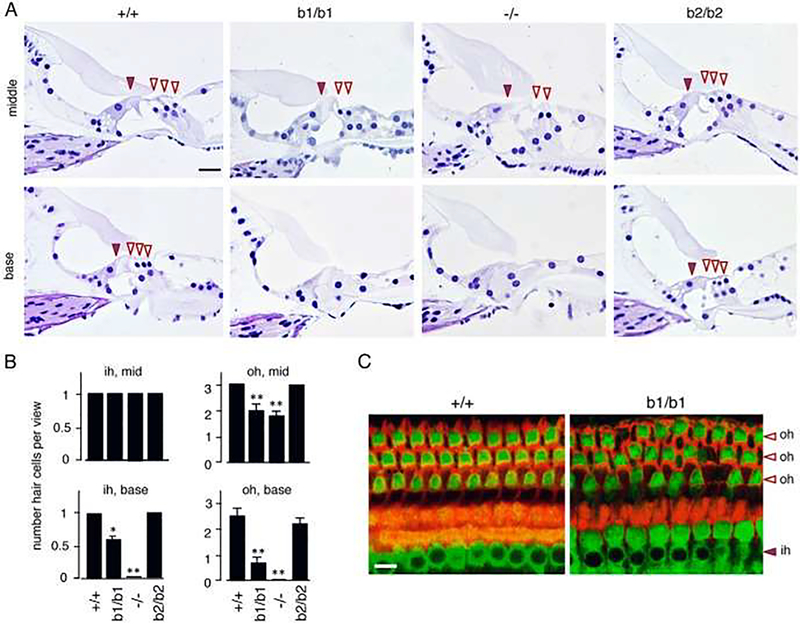
Figure 6.
Loss of hair cells in adult mice lacking thyroid receptor (TR)β1: b1. −/− mice lack both TRβ1 and TRβ2, Histological sections of mid and basal turns of the cochlea at 6 months of age. Brown arrowhead, inner hair cell; white arrowheads, outer hair cells. Loss of hair cells is obvious in b1/b1 and −/− mice but not in +/+ or b2/b2 mice. In b1/b1 and −/− mice, the sensory epithelium has also lost support cells and is flattened, especially in basal regions. Scale bar, 20 μm. B, Counts of hair cells showing loss of outer (oh) and inner hair cells (ih), determined on sections of middle (mid) and basal (base) regions. For a given genotype vs +/+ group: *, P < .005; **, P < .001. C, Immunofluorescent analysis of surface views of the organ of Corti stained for myosin VI (detects inner and outer hair cells, green) and phalloidin (detects filamentous actin, red). Gaps appear in the hair cell array resulting from loss of outer hair cells in this mid-region view of the cochlea in b1/b1 mice at 6 months of age. Scale bar, 10 μm. From Ng et al. 2015, Fig. 6; with permission.
There are human clinical studies which have revealed associations between hypothyroidism and hearing disorders. Santosh and Rao (2016) found a correlation between Meniere’s disease and hypothyroidism. Likewise, Hussein et al. (2017) investigated whether L-thyroxine hormone therapy can help hypothyroid patients, having mild to severe hearing loss. 30 subjects were recruited for the study, and nearly half of the patients reported improvement in hearing after 6 months of therapy, with 15% restored to normal hearing.
Growth Factors and Age-Related Auditory Declines
There are multiple reports suggesting that growth hormone; such as insulin like growth factor, can play a role in hearing disorders including ARHL (Iwai et al. 2006; Yamahara et al. 2015; Yoshida et al. 2015; Muus et al. 2017). Insulin like growth factor 1 (IGF–1), a neuroprotective agent which helps to maintain different cellular functions like growth activation, metabolism, proliferation and differentiation, is critical for development of the inner ear and central auditory system, and its receptor level expressions change with age, and its circulating levels decrease in older adults (Zou et al. 2009; Tafra et al. 2014; Vestergaard et al. 2014; Rodríguez-de La Rosa et al. 2017). For instance, Lassale et al. (2017) found a co-relation between IGF-1 and presbycusis among older people in a European cohort. Animal model studies have probed these relations further, showing that deficiency/knockout of IGF hormones increases the degeneration of cochlear structures. For instance, Riquelme et al. (2010) reported that IGF-1 knockout mice had a moderately severe hearing loss at a young age (3 months) while the control animals lost their hearing much more steadily, as typically occurs in ARHL. Both groups had a severe hearing loss at 1 year of age, although the waveform morphology of the knockouts was more abnormal. IGF knockout mice also show accelerated damage to inner ear neural structures as well as the “cochlear battery”, the stria vascularis. This study demonstrates the importance of IGF1 in cochlear development and aging.
Furthermore, IGF-1 has been linked to various neurodegenerative disorders which have some mechanistic similarities to ARHL. Given that IGF-1 declines with age, and is essential for maintenance of cochlear function, it is a potential therapeutic target for presbycusis. In fact, there are studies with animal models demonstrating IGF-1 protective effects for cochlear structures pertaining to various hearing pathologies and ototoxicities (Yamahara et al. 2015), noise exposure (Iwai et al. 2006) and neomycin ototoxicity (Yoshida et al. 2015). For humans, a clinical study reports on the effect of IGF-1 hormone therapy for 11 human subjects with Laron syndrome (Nakagawa et al. 2014; Attias et al. 2012). Specifically, young children who started early hormone treatment showed no signs of hearing loss, and no auditory hypersensitivity - hyperacusis. Also, there is a recent case report of growth hormone treatment for a 3.5-month-old newborn, having cerebral palsy and bilateral sensorineural hearing loss due to fetal distress (Guerra et al. 2019). The child was treated with growth hormones (0.4 mg/kg/day), Melatonin (5 mg/day and 10 mg/day after 6 months). The child’s hearing loss recovered fully. These researchers speculated that hearing recovery was due to the effects of growth hormones on the production of inner ear hair cells from stem cells that may be present in newborns.
So, hormone therapies may be a way forward for prevention/treatment of presbycusis. In general, there will need to be a dose optimization, since higher doses can lead to side-effects. One potential way to lower the effective dose of a hormone is to use it in combination with supplements to get an effective drug cocktail for presbycusis. There are different reports with results about the use of antioxidants to prevent/treat ARHL. For example, Seidman (2000) found only limited effects of different antioxidants (vitamin E – 2.475 mg/day, vitamin C – 0.44 mg/day, melatonin – 0.1 mg/day, lazaroid – 1.25 mg/day), at certain ABR frequencies only, in Fisher 344 rats. Seidman also found preservation of ABR thresholds with lecithin - a structural element of cell membranes; a treatment involving the upregulation of aging mitochondrial DNA deletion (mtDNA (4834)) (Seidman et al. 2002). However, Kashio et al. (2009) found no effects of vitamin C on ABRs. Sha et al. (2012) observed no effects of a combined diet of vitamin A, C and E, and L-cartinite on the progression of ARHL in CBA/CaJ mice. Someya et al. (2009) tested 17 different antioxidants (acetyl-L-carnitine, α-lipoic acid, β-carotene, carnosine, coenzyme Q10, curcumin, d-α-tocopherol, EGCG, gallic acid, lutein, lycopene, melatonin, N-acetyl-L-cysteine, proanthocyanidin, quercetin, tannic acid, and resveratrol) and found that α-lipoic acid almost fully preserved hearing of C57BL/6. He found evidence that this effect was mediated by the reduction of apoptotic spiral ganglion neuron cell death, due to the deletion of a BAK gene, a mitochondrial pro-apoptotic gene. All the other antioxidant treatments were not effective in reducing ARHL.
There are human studies as well with antioxidants. Like animal studies, they have also produced mixed results; some showing positive effects, and some show no benefits. For example, Takumida and Anniko (2009) carried out human clinical studies with rebamipide (300 mg/day), vitamin C (600 mg/day) and α-lipoic acid (60 mg/day). Elderly people, age 70 years or above, were recruited, and a significant improvement in hearing was observed. Polanski et al. (2013) conducted a human study with 120 patients (age 60 or older), divided into four groups. Each group received one of these treatments: ginkgo biloba dry extract, α-lipoic acid combined with vitamin C, papverine chlorhydrate combined with vitamin E, and placebo. There was no significant difference between any of these groups.
Insulin Mis-regulation and Age-Related Hearing Loss
Insulin is a hormone made in the pancreas, allowing the body to effectively utilize glucose for energy. Abnormal regulation of insulin has effects on intra- and extra-cellular biochemical signaling pathways in multiple physiological systems of the body and can lead to diabetes. Type II diabetes mellitus (T2DM) is a prevalent age-related metabolic disorder affecting about 7% of the population worldwide (Frisina et al., 2006). Accumulating evidence suggests that hearing loss is yet another disabling complication of diabetes, particularly in the aged (Bainbridge et al., 2008; Mitchell et al., 2009), with potential adverse effects on quality of life, the ability to understand speech, social-emotional well-being (Strawbridge et al., 2000), neuropathy, and mortality (Feeny et al., 2012). Although studies on diabetes and hearing loss often demonstrate that an association exists, interpretation of causation is often difficult (Konrad-Martin, 2015), and many of the differences between the hearing abilities of aged diabetics and their age-matched controls include measures of inner ear function. For example, Frisina et al. (2006) investigated the hearing impairment among older type II diabetes patients, whose insulin system was mis-regulated due to the diabetes: 30 diabetics, age 59 to 92 (mean age = 73 years) and 30 non-diabetics, age 59 to 88 years (mean age = 73 years). They discovered noticeable deficits in pure-tone audiograms, wideband noise and speech reception thresholds, and otoacoustic emissions. In addition, there was a strong tendency for diabetes to affect the right ear more than the left. One possible interpretation is that as one develops presbycusis, the right ear advantage is lost, and this decline is accelerated by diabetes.
In a prospective animal model study using middle age CBA/CaJ mice with Type 1 diabetes mellitus (T1DM) (STZ injection) or Type 2 diabetes mellitus (T2DM) (high fat diet, for a period of 6 months), ABR threshold elevations were found for both types of diabetes, but were most pronounced in the T2DM, starting as early as 2 months after induction of diabetes (Vasilyeva et al., 2009). A decline of mean DPOAE amplitudes was observed in both diabetic groups at high frequencies, and for the T2DM at low frequencies. In contrast to ABR thresholds, tone and noise thresholds in the inferior colliculus were lower for both diabetic groups, indicating either loss of inhibition, increase in central gain, or both, as a result of the peripheral hearing loss due to the diabetes. In conclusion, induction of diabetes in middle-aged CBA/CaJ mice promotes amplification of age-related peripheral hearing loss which makes it a suitable model for studying the interactions of ARHL and diabetes.
Pålbrink and coworkers found that insulin induced phosphorylation of protein kinase B (PKB/Akt) at Ser473, in a PI3-kinase-dependent manner (Pålbrink et al., 2020). The phosphorylation of PKB was inhibited by isoproterenol and IBMX, a general phosphodiesterase (PDE) inhibitor. PDE1B, PDE4D and the insulin-sensitive PDE3B were found to be expressed and catalytically active in HEI-OC1 cells, a cell line for cochlear hair cells. Insulin decreased and AICAR, an activator of AMP-activated protein kinase, increased the phosphorylation for Ser79 of acetyl-CoA carboxylase, the rate-limiting enzyme in de novo lipogenesis. Furthermore, the activity of hormone-sensitive lipase, the rate-limiting enzyme in lipolysis, was observed in HEI-OC1 cells. Based on this study, the organ of Corti could be a direct target tissue for insulin action, and inner ear insulin resistance might contribute to the links between diabetes and inner ear deficits. It will be important to further investigate the biological mechanisms of how diabetes can influence age-related auditory processing deficits to move closer to new interventional therapies.
Summary and Conclusions
The present article summarizes some of the noteworthy effects that hormones and hormonal level changes can have on auditory processing and sound perception. Animal models, including fish, amphibians and mammals have revealed changes in cellular processing and hearing due to natural changes in hormone levels as well as modifications that occur as a result of proactive hormone therapies. In some cases, for example estrogen, the prevailing evidence is that elevated or restorative levels of estrogen are beneficial to hearing and cochlear morphology and cellular pathways. In contrast, so-called combination hormone replacement therapy, estrogen + progestin, is detrimental to hearing in both aging animal models and older women. It may be that combining hormone therapies with antioxidant supplements may be beneficial to hearing in aging animals and humans, because theoretically, a lower dose of the hormone could be used to reduce or eliminate side effects of the hormone therapy. In addition, as local drug delivery to the cochlea moves closer to the clinic to treat age-related and other acquired hearing losses, the problem of hormone and drug systemic side effects will disappear (hydrogels, micropumps; e.g., Frisina et al. 2018; Forouzandeh et al. 2019). In closing, animal model studies and clinical research are moving us closer to translational successes using hormones and other compounds to prevent or treat the progression of ARHL.
Highlights.
Sex Hormones Modify Auditory Processing to Facilitate Mating
Hormonal Level Changes with Age Can Influence Age-Related Hearing Loss
Prospective Studies Reveal that Hormone Therapies Can Alter Hearing Abilities
Combining Hormones with Other Supplements May be a Key for Future Therapies
Co-Morbid Medical Conditions Can Have a Negative Impact on Hearing in the Elderly
Acknowledgements:
Work supported by NIH grants from the National Institute on Aging, P01 AG009524, and the National institute on Deafness and Communication Disorders, R21 DC017039. We thank Dr. Shannon Salvog for project support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arch VS and Narins PM (2009) Sexual hearing: The influence of sex hormones on acoustic communication in frogs. Hearing Res, 252(1):15–20. 10.1016/j.heares.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attias J, Zarchi O, Nageris BI and Laron Z (2012) Cochlear hearing loss in patients with Laron syndrome. Euro Arch Otorhinolaryngol, 269(2):461–466. 10.1007/s00405-011-1668-x. [DOI] [PubMed] [Google Scholar]
- Bainbridge KE, Hoffman HJ, Cowie CC (2008) Diabetes and hearing impairment in the United States: audiometric evidence from the National Health and Nutrition Examination Survey, 1999 to 2004. Ann Intern Med 149(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caras ML (2013) Estrogenic modulation of auditory processing: a vertebrate comparison. Frontiers in Neuroendocrinology, 34(4):285–299. 10.1016/j.yfrne.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charitidi K, & Canlon B (2010) Estrogen receptors in the central auditory system of male and female mice. Neurosci., 165(3):923–933. 10.1016/j.neuroscience.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Chung DY, Mason K, Gannon RP, & Willson GN (1983). The ear effect as a function of age and hearing loss. J Acoustical Soc, 73(4):1277–1282. 10.1121/1.389276. [DOI] [PubMed] [Google Scholar]
- Church GT, & Cudahy EA (1984) The time course of the acoustic reflex. Ear and Hearing, 5(4):235–242. 10.1097/00003446-198407000-00008. [DOI] [PubMed] [Google Scholar]
- Coleman JR, Campbell D, Cooper WA, Welsh MG, & Moyer J (1994) Auditory brainstem responses after ovariectomy and estrogen replacement in rat. Hearing Res, 80(2):209–215. 10.1016/0378-5955(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Cordas EA, Ng L, Hernandez A, et al. (2012) Thyroid hormone receptors control developmental maturation of the middle ear and the size of the ossicular bones. Endocrinology, 153(3):1548–1560. 10.1210/en.2011-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Shen Y, Li R (2013) Estrogen Synthesis and Signaling Pathways during Aging: From Periphery to Brain. Trends in Molecular Medicine, 19(3):197–209. 10.1016/j.molmed.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva Souza D, Luckwu B, Teobaldo Lopes de Andrade W, et al. (2017) Variation in the Hearing Threshold in Women during the Menstrual Cycle. International Archives of Otorhinolaryngology, 21(4):323–328. 10.1055/s-0037-1598601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B, Frisina RD, Zhu X, et al. (2014) Direct control of Na+-K+−2Cl- co-transport protein (NKCC1) expression with aldosterone. Am. J. Physiol.- Cell Physiol. Endocrinol. Metabolism, 306(1):C66–75. 10.1152/ajpcell.00096.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeny D, Huguet N, McFarland BH, Kaplan MS Orpana H, Eckstrom E (2012) Hearing, mobility, and pain predict mortality: a longitudinal population-based study. J Clin Epidemiol 65(7):764–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forouzandeh F, Zhu X, Alfadhel A, et al. (2019) A nanoliter resolution implantable micropump for murine inner ear drug delivery. J. Controlled Release, 298(1):27–37. 10.1016/j.jconrel.2019.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest D and Ng l. (2016) Thyroid hormone and the mammalian auditory system In: Bass AH, Sisneros JA, Popper AN, Fay RR (Eds.), Hearing and Hormones. Springer Handbook of Auditory Research, Vol. 57 Springer Science+Business Media, LLC, New York: Chapter 7, Pg. 163–189. ISBN-13: 978–3319265957. [Google Scholar]
- Frisina RD, Budzevich M, Zhu X, et al. (2018) Animal Model Studies Yield Translational Solutions for Cochlear Drug Delivery. Hearing Res, 368(1):67–74, 10.1016/j.heares.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisina RD, Ding B, Zhu X, Walton JP. (2016, Online) Age-Related Hearing Loss: Prevention of Threshold Declines, Cell Loss and Apoptosis in Spiral Ganglion Neurons. Aging, 8(1):1–19. ^equal 1st authors. 10.18632/aging.101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisina DR & Frisina RD (1997) Speech recognition in noise and presbycusis: Relations to possible neural sites. Hearing Res, 106(1):95–104. 10.1016/s0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Frisina RD and Frisina DR (2016) Hormone Replacement Therapy and its Effects on Human Hearing In: Bass A, Sisneros J, Popper A & Fay RR Eds.; Hormones and Hearing. Springer Handbook of Auditory Research, Vol. 57 Springer Science+Business Media, LLC, New York: Ch. 8, Pg. 191–209. ISBN-13: 978–3319265957. [Google Scholar]
- Frisina ST, Mapes F, Kim S, Frisina DR and Frisina RD (2006) Characterization of hearing loss in aged type II diabetics. Hearing Res, 211(1–2):103–113. 10.1016/j.heares.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra J, Devesa A, Llorente D, Mouro R, et al. (2019) Early Treatment with Growth Hormone (GH) and Rehabilitation Recovers Hearing in a Child with Cerebral Palsy. Reports, 2(1): 4 10.3390/reports2010004. [DOI] [Google Scholar]
- Guimaraes P, Frisina ST, Mapes F, et al. (2006) Progestin Negatively Affects Hearing in Aged Women. Proc. National Academy Sciences - PNAS, 103(38):14246–9. 10.1073/pnas.0606891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes P, Zhu X, Cannon T, Kim S-H, Frisina RD (2004) Sex Differences in Distortion Product Otoacoustic Emissions as a Function of Age in CBA Mice. Hearing Res, 192(1–2),83–89. 10.1016/j.heares.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Halonen J, Hinton A, Frisina RD, et al. (2016) Long-term Treatment with the Mineralocorticoid Aldosterone Slows Down the Progression of Age-Related Hearing Loss Measured Behaviorally. Hearing Res, 336(1):63–71. 10.1016/j.heares.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KR (2004) Males lose hearing earlier in mouse models of late-onset age-related hearing loss; females lose hearing earlier in mouse models of early-onset hearing loss. Hearing Res, 190(1):141–148. 10.1016/S0378-5955(03)00401-5. [DOI] [PubMed] [Google Scholar]
- Hultcrantz M, Simonoska R, Stenberg AE (2006) Estrogen and Hearing: A Summary of Recent Investigations. Acta Oto-Laryngologica, 126(1):10–14. 10.1080/00016480510038617. [DOI] [PubMed] [Google Scholar]
- Hussein MM, Asal SI, Salem TM & Mohammed (2017) The effect of L-thyroxine hormone therapy on hearing loss in hypothyroid patients. Egyptian J Otolar, 33(4):637 10.4103/ejo.ejo_25_17. [DOI] [Google Scholar]
- Iwai K, Nakagawa T, Endo T, et al. (2006) Cochlear Protection by Local Insulin-Like Growth Factor-1 Application Using Biodegradable Hydrogel. Laryngoscope, 116(4):529–533. 10.1097/01.mlg.0000200791.77819.eb. [DOI] [PubMed] [Google Scholar]
- Jeong JK, Tremere LA, Ryave MJ, Vuong VC, Pinaud R (2009) Anatomical and Functional Organization of Inhibitory Circuits in the Songbird Auditory Forebrain. J Exp Neurosci, 2:43–53. [PMC free article] [PubMed] [Google Scholar]
- Jerger J, & Hall J (1980) Effects of age and sex on auditory brainstem response. Archives of Otolaryngology—Head and Neck Surgery, 106(7):387–391. 10.1001/archotol.1980.00790.31001.1003. [DOI] [PubMed] [Google Scholar]
- Jerger J, & Johnson K (1988) Interactions of Age, Gender, and Sensorineural Hearing Loss on ABR Latency. Ear and Hearing, 9(4):168–176. 10.1097/00003446-198808000-00002. [DOI] [PubMed] [Google Scholar]
- Kashio A, Amano A, Kondo Y, Sakamoto T, Iwamura H, Suzuki M, Ishigami A, Yamasoba T (2009) Effect of Vitamin C depletion on age-related hearing loss in SMP30/GNL knockout mice. Biochem Biophys Res Commun 390(3):394–398. [DOI] [PubMed] [Google Scholar]
- Kilicdag EB, Yavuz H, Bagis T, et al. (2004) Effects of estrogen therapy on hearing in postmenopausal women. American Journal of Obstetrics & Gynecology, 190(1):77–82. 10.1016/j.ajog.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Konrad-Martin D, Reavis KM, Austin D, Reed N, Gordon J, McDermott D, Dille MF (2015) Hearing impairment in relation to severity of diabetes in a veteran cohort. Ear Hear. Jul-Aug; 36(4): 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassale C, Batty GD, Steptoe A and Zaninotto P (2017) Insulin-like growth factor 1 in relation to future hearing impairment: findings from the English Longitudinal Study of Ageing. Sci Rep, 7(1):1–9. 10.1038/s41598-017-04526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney D, & Pinaud R (2011) Estradiol-dependent modulation of auditory processing and selectivity in songbirds. Frontiers in Neuroendocrinology, 32(3):287–302. 10.1016/j.yfrne.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D (2009) Masculinization of the Mammalian Cochlea. Hearing Res, 252(1–2):37–48. 10.1016/j.heares.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, & Pasanen EG (1998) Comparison of the auditory systems of heterosexuals and homosexuals: click-evoked otoacoustic emissions. Proceedings of the National Academy of Sciences of the United States of America, 95(5):2709–2713. 10.1073/pnas.95.5.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milon B, Mitra S, Song Y, et al. (2018) The impact of biological sex on the response to noise and otoprotective therapies against acoustic injury in mice. Biology of Sex Differences, 9(1):12 10.1186/s13293-018-0171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P, Gopinath B, McMahon CM, Rochtchina E, Wang JJ, Boyages SC, Leeder SR (2009) Relationship of Type 2 diabetes to the prevalence, incidence and progression of age-related hearing loss. Diabet Med 26(5):483–8. [DOI] [PubMed] [Google Scholar]
- Motohashi R, Takumida M, Shimizu A, et al. (2010) Effects of age and sex on the expression of estrogen receptor alpha and beta in the mouse inner ear. Acta Oto-Laryngologica, 130(2):204–214. 10.3109/00016480903016570. [DOI] [PubMed] [Google Scholar]
- Mott NN, & Pak TR (2013) Estrogen Signaling and the Aging Brain: Context-Dependent Considerations for Postmenopausal Hormone Therapy. ISRN Endocrinology, 2013:814690:1–16. 10.1155/2013/814690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muus JS, Weir FW, Kreicher KL, Bowlby DA, et al. (2017) Hearing loss in children with growth hormone deficiency. Int J Pediatr Otorhinolaryngol, 100(1):107–113. 10.1016/j.ijporl.2017.06.037. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kumakawa K, Usami SI, Hato N, et al. (2014) A randomized controlled clinical trial of topical insulin-like growth factor-1 therapy for sudden deafness refractory to systemic corticosteroid treatment. BMC Med, 12(1):219 10.1186/s12916-014-0219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Cordas E, Wu X et al. (2015) Age-related hearing loss and degeneration of cochlear hair cells in mice lacking thyroid hormone receptor β1. Endocrinology, 156(10): 3853–3865. 10.1210/en.2015-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pålbrink A, Kopietz F, Morén B, In ‘t Zandt R, Kalinec F, Stenkula K, Göransson O, Holm C, Magnusson M, Degerman E (2020) Inner ear is a target for insulin signaling and insulin resistance: evidence from mice and auditory HEI-OC1 cells. BMJ Open Diabetes Res Care. 8(1):e000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R & Tremere LA (2012) Control of Central Auditory Processing by a Brain-Generated Oestrogen.” Nature Reviews Neuroscience, 13(8):521–527. 10.1038/nrn3291. [DOI] [PubMed] [Google Scholar]
- Polanski J & Cruz OL (2013) Evaluation of antioxidant treatment in presbyacusis: prospective, placebo-controlled, double-blind, randomised trial. J Laryngol Otol, 127(2):134–141. 10.1017/S0022215112003118. [DOI] [PubMed] [Google Scholar]
- Price K, Zhu X, Guimaraes P, Vasilyeva ON, Frisina RD (2009) Hormone replacement therapy diminishes hearing in peri-menopausal mice. Hearing Res, 252(1–2):29–36. 10.1016/j.heares.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme R, Cediel R, Contreras J, et al. (2010) A comparative study of age-related hearing loss in wild type and insulin-like growth factor I deficient mice. Front Neuroanat, 4(1): 27 10.3389/fnana.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-de la Rosa L, Lassaletta L, Calvino M, et al. (2017) The role of insulin-like growth factor 1 in the progression of age-related hearing loss. Frontiers in Aging Neuroscience, 9(1):411 10.3389/fnagi.2017.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzinski W, & Krejza J (2002) Effects of estrogens on the brain and implications for neuro-protection. Neurol Neurochir Pol, 36(1):143–156. PMID: 12053605. [PubMed] [Google Scholar]
- Rüsch A, Ng L, Goodyear R, et al. (2001) Retardation of cochlear maturation and impaired hair cell function caused by deletion of all known thyroid hormone receptors. J Neuroscience, 21(24):9792–9800. 10.1523/JNEUROSCI.21-24-09792.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman MD (2000) Effects of dietary restriction and antioxidants on presbyacusis. Laryngoscope, 110:727–738. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Khan MJ, Tang WX, Quirk WS (2002) Influence of lecithin on mitochondrial DNA and age-related hearing loss. Otolaryngology–Head Neck Surg, 127(3):138–144. [DOI] [PubMed] [Google Scholar]
- Sha SH, Kanicki A, Halsey K, et al. (2012) Antioxidant-enriched diet does not delay the progression of age-related hearing loss. Neurobiol Aging, 33(5):1010. e1015–1016. 10.1016/j.neurobiolaging.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharlin DS, Ng L, Verrey F, et al. (2018) Deafness and loss of cochlear hair cells in the absence of thyroid hormone transporters Slc16a2 (Mct8) and Slc16a10 (Mct10). Sci Rep, 8(1):4403 10.1038/s41598-018-22553-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster BZ, Depireux DA, Mong JA, & Hertzano R (2019) Sex differences in hearing: Probing the role of estrogen signaling. J Acoustical Society, 145(6):3656–3663. 10.1121/1.5111870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoska R, Stenberg AE, Duan M, et al. (2009) Inner ear pathology and loss of hearing in estrogen receptor-beta deficient mice. J Endocrinology, 201(3):397–406. 10.1677/joe-09-0060. [DOI] [PubMed] [Google Scholar]
- Singer CA, Rogers KL, Strickland TM, & Dorsa DM (1996) Estrogen protects primary cortical neurons from glutamate toxicity. Neuroscience Letters, 212(1):13–16. 10.1016/0304-3940(96)12760-9. [DOI] [PubMed] [Google Scholar]
- Sisneros JA (2009). Steroid-dependent auditory plasticity for the enhancement of acoustic communication: Recent insights from a vocal teleost fish. Hearing Res, 252(1–2):9–14. 10.1016/j.heares.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Xu J, Kondo K, Ding D, et al. (2009) Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc Natl Acad Sci USA, 106(46): 19432–19437. 10.1073/pnas.0908786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, McGee J and Walsh EJ (2008) The influence of thyroid hormone deficiency on the development of cochlear nonlinearities. J Assoc Res Otolaryngol, 9(4):464–476. 10.1007/s10162-008-0140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge WJ, Wallhagen MI, Shema SJ, Kaplan GA (2000) Negative consequences of hearing impairment in old age: a longitudinal analysis.Gerontologist 40(3):320–6. [DOI] [PubMed] [Google Scholar]
- Sundaresan S, Kong JH, Fang Q, et al. (2016) Thyroid hormone is required for pruning, functioning and long-term maintenance of afferent inner hair cell synapses. Euro J Neurosci, 43(2):148–161. 10.1111/ejn.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros SF, Frisina ST, Mapes F, et al. (2005) High Serum Aldosterone Levels Correlate with Lower Hearing Thresholds in Aged Humans: A Possible Protective Hormone against Presbycusis. Hearing Res, 209(1):10–18. 10.1016/j.heares.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Tadros SF, D’Souza M, Zettel ML, et al. (2007) Glutamate-related gene expression changes with age in the mouse auditory midbrain. Brain Research, 1127(1):1–9. 10.1016/j.brainres.2006.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafra R, Brakus SM, Vukojevic K, Kablar B, et al. (2014) Interplay of proliferation and proapoptotic and antiapoptotic factors is revealed in the early human inner ear development. Otol Neurotol, 35(4):695–703. 10.1097/MAO.0000000000000210. [DOI] [PubMed] [Google Scholar]
- Takumida M, Anniko M (2009) Radical scavengers for elderly patients with age-related hearing loss. Acta Otolaryngol 129(1):36–44. doi: 10.1080/00016480802008215. [DOI] [PubMed] [Google Scholar]
- Trune DR, Kempton JB, and Kessi M (2000) Aldosterone (mineralocorticoid) equivalent to prednisolone (glucocorticoid) in reversing hearing loss in MRL/MpJ-Fas1pr autoimmune mice. Laryngoscope, 110(11):1902–1906. 10.1097/00005537-200011000-00025. [DOI] [PubMed] [Google Scholar]
- Trune DR and Kempton JB (2001) Aldosterone and prednisolone control of cochlear function in MRL/MpJ-Fas(lpr) autoimmune mice. Hearing Res, 155(1–2):9–20. 10.1016/s0378-5955(01)00240-4. [DOI] [PubMed] [Google Scholar]
- Trune DR, Kempton JB, and Gross ND (2006) Mineralocorticoid receptor mediates glucocorticoid treatment effects in the autoimmune mouse ear. Hearing Res, 212(1–2):22–32. 10.1016/j.heares.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Vasilyeva ON, Frisina ST, Zhu X, Walton JP, Frisina RD (2009) Interactions of hearing loss and Diabetes Mellitus in the CBA/CaJ mouse model of presbycusis. Hearing Res 249: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard PF, Hansen M, Frystyk J, Espelund U, et al. (2014) Serum levels of bioactive IGF1 and physiological markers of ageing in healthy adults. Eur J Endocrinol, 170(2):229–236. 10.1530/EJE-13-0661. [DOI] [PubMed] [Google Scholar]
- Williamson TT, Ding B, Zhu X, Frisina RD (2019) Hormone Replacement Therapy Attenuates Hearing Loss: Mechanisms Involving Estrogen and the IGF-1 Pathway. Aging Cell, 18(3):e12939:1–15. 10.1111/acel.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamahara K, Yamamoto N, Nakagawa T and Ito J (2015) Insulin-like growth factor 1: a novel treatment for the protection or regeneration of cochlear hair cells. Hearing Res, 330:2–9. 10.1016/j.heares.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sugahara K, Hashimoto M, Hirose Y, et al. (2015) The minimum peptides of IGF-1 and substance P protect vestibular hair cells against neomycin ototoxicity. Acta Otolaryngol, 135(5):411–415. 10.3109/00016489.2014.979438. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang T, Yu L, et al. (2018). Effects of ovarian reserve and hormone therapy on hearing in premenopausal and postmenopausal women: A cross-sectional study. Maturitas, 111(1):77–81. 10.1016/j.maturitas.2018.01.019. [DOI] [PubMed] [Google Scholar]
- Zou S, Kamei H, Modi Z and Duan, (2009) Zebrafish IGF genes: gene duplication, conservation and divergence, and novel roles in midline and notochord development. PLoS One, 4(9):e7026 10.1371/journal.pone.0007026. [DOI] [PMC free article] [PubMed] [Google Scholar]