Abstract
Introduction:
Nanocarrier-based delivery systems offer multiple benefits to overcome limitations of the traditional drug dosage forms, such as protection of the drug, enhanced bioavailability, targeted delivery to disease site, etc. Nanocarriers have exhibited tremendous successes in targeted delivery of therapeutics to the desired tissues and cells with improved bioavailability, high drug loading capacity, enhanced intracellular delivery, and better therapeutic effect. A specific design of stimuli-responsive nanocarriers allows for changing their structural and physicochemical properties in response to exogenous and endogenous stimuli. These nanocarriers show a promise in site specific controlled release of therapeutics under certain physiological conditions or external stimuli.
Areas covered:
This review highlights recent progresses on the multifunctional and stimuli-sensitive nanocarriers for targeted therapeutic drug delivery applications.
Expert opinion:
The progress from single functional to multifunctional nanocarriers has shown tremendous potential for targeted delivery of therapeutics. On our opinion, the future of targeted delivery of drugs, nucleic acids, and other substances belongs to the site-targeted multifunctional and stimuli-based nanoparticles with controlled release. Targeting of nanocarriers to the disease site enhance the efficacy of the treatment by delivering more therapeutics specifically to the affected cells and substantially limiting adverse side effects upon healthy organs, tissues, and cells.
Keywords: Nanotherapeutics for cancer, nanoscale-based delivery systems, endogenous and exogenous stimuli-responsive nanocarriers, liposomes, targeted delivery
1. Introduction
The primary objective in developing a drug delivery system is to carry the drug effectively at its target site with minimum side effects. In conventional drug delivery methods, it is difficult to achieve the desired therapeutic efficiency of many drugs because of various associated issues which include low bioavailability, sensitive toxicity, poor specificity, etc. [1,2,3]. Therefore, a suitable carrier is required to ensure site-specific delivery of therapeutics. Nanomedicine, which deals with nanosized biomaterials for applications in disease diagnosis and therapies, showed tremendous promise in target-specific delivery of therapeutics [4,5,6]. Over the decades, various nano-based materials and methods have been developed for disease diagnosis and treatment applications. For examples, nanocarriers such as nanoparticles [7–10], liposomes [11–13], nanostructures lipid carriers [14,15], dendrimers [16,17], Janus nanoparticles [18], etc. are the most widely exploited carriers for the delivery of drugs, bio-active agents, genes, imaging probes, etc. for several targeted diseases. Further, to achieve active targeted drug delivery, multifunctional nanocarrier system have been designed in recent years by conjugating various targeting ligands such as folic acid (FA), peptide, antibodies, etc. on the surface of the nanomaterials [19,20,29]. Such multifunctional nanocarriers were found to increase the accumulation of therapeutics in the specific tissues resulting in improved therapeutic efficacy, as well as decreased availability of the therapeutic into other organs thereby reducing adverse side effects observed in systemic delivery of nontargeted free drug. However, such active targeting nanomaterials also suffer from inadequate delivery of therapeutics at targeted sites. To overcome such shortcomings in targeted drug delivery, researchers paid significant attention in recent years to develop stimuli-responsive nanocarriers [30,31,32,33,34], which can undergo property change upon various stimuli, leading to controlled and adequate delivery of drugs and gene at the target site [35,36,37,41]. Stimuli-responsive nanocarrier is usually composed with various environmental sensitive functionalities within their structures and thus it can release the loaded therapeutics in response to various environmental factors such as temperature, pH, redox potential, enzymes, etc. endogenous stimuli as well as electromagnetic, light, radiation, ultrasound, etc. exogenous stimuli. A schematic representation displaying design of various stimuli-based multifunctional nanocarriers and its therapeutic release application has been outlined in Figure 1 [42]. In this review, we will be highlighting recent reports on the multifunction nanocarriers as well as various endogenous and exogenous stimuli-responsive nanocarriers and their corresponding therapeutic delivery applications.
Figure 1.
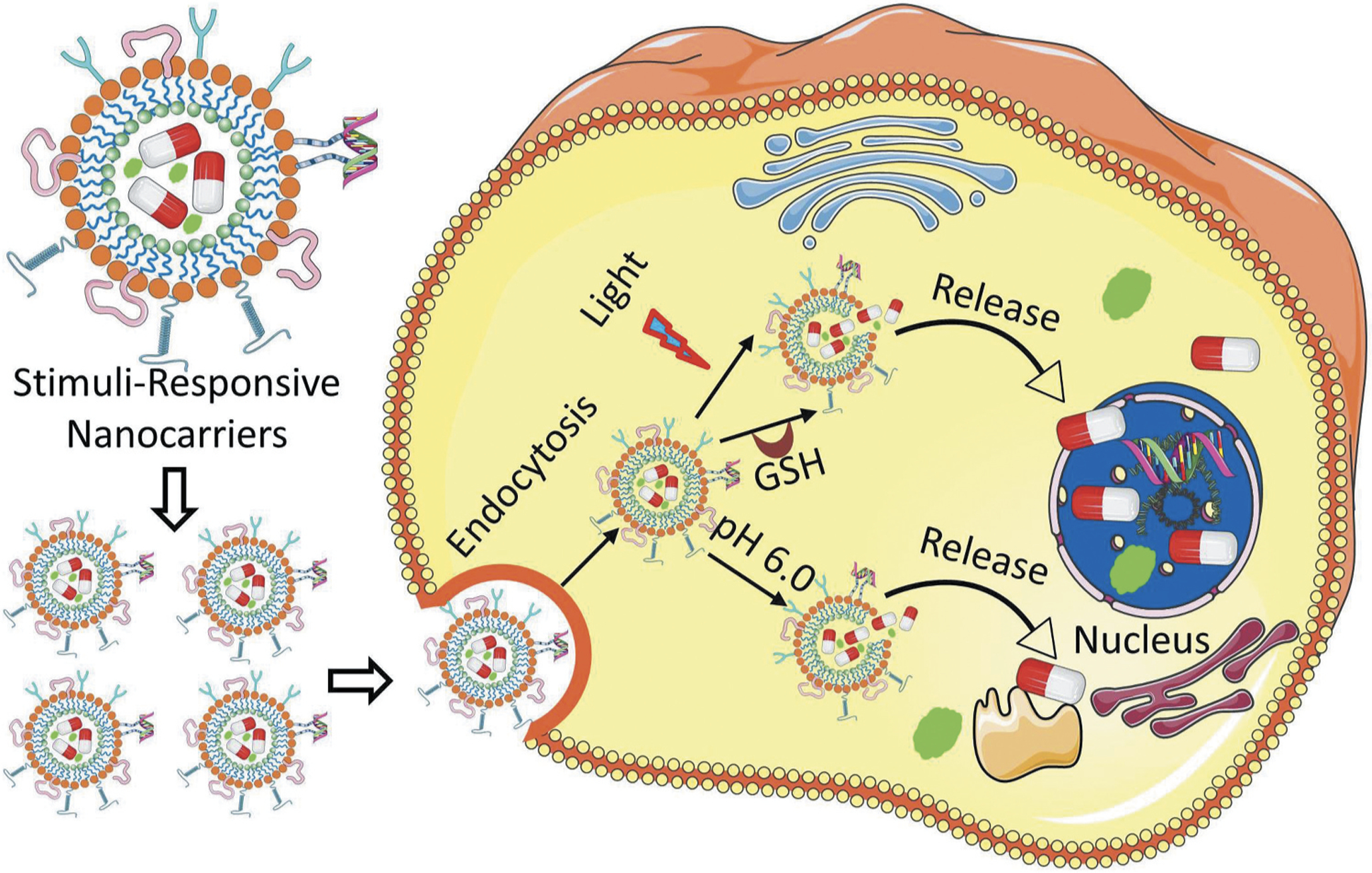
Schematic representation of stimuli-responsive delivery of therapeutics and contrast agents into a diseased cell. After entering a targeted cell through endocytosis, nanoparticles undergo disassembly through various internal (acidity, actions of intracellular enzymes, high temperature) as well as external (light, magnetic field, temperature, ultrasoud, etc.) stimuli. On dissociation of the nanostructure, the encapsulated drugs or imaging agents are released and delivered to their specific targets in nucleus and cytoplasm.
2. Multifunctional nanocarriers
Nanoscale carriers have been intensively explored in the past two decades for selective delivery of therapeutic agents to its targeted cells with minimum exposure to the healthy tissues [26,43,44,45,46]. Because of both small size and large surface area to volume ratio, nanocarriers possess unique properties which allow them to carry various drugs, therapeutics and imaging agents with high loading efficiency [47]. Over the decades, various nanocarriers such as liposomes, nanostructured lipids, dendrimers, micelles, nanoemulsions, polymeric nanoparticles, Janus nanoparticles, organic-based nanocarriers as well as carbon nanotubes, quantum dots, mesoporous silica nanoparticles, magnetic nanoparticles, inorganic-based nanocarriers, etc. have been studied for targeted therapeutic delivery applications. These nanocarriers were found not only to improve bioavailability of the drugs, but also protect its payloads from degradation – thereby making it a suitable device for effective drug delivery application [48]. Though such first generation of single-functional nanocarriers capable of carrying a drug addressed issues of bioavailability, stability, control release, etc. of many drugs; design of more complex nanocarriers came with additional functions such as more specific targeting as well as imaging of the tissue and cells simultaneously to address challenges associated with many diseases of different characteristics. These complex nanocarriers were named as ‘multifunctional nanocarriers’ which can simultaneously perform multiple functions including drug, nucleic acid delivery, and peptide delivery, optical imaging, etc. [26,49,50,49,51,52,53,54,55,56]. A common strategy to develop targeted multifunctional nanocarriers included surface modification of the parent nanocarriers via physical or covalent attachment of affinity ligands selective for certain receptors on the target cell, imaging agents, stimuli-sensitive components, cell-penetrating agents, etc. through a polymeric linker such as polyethylene glycol (PEG) as outlined in Figure 2. Many targeting ligands such as monoclonal antibodies, folate, aptamers, peptides, etc. have been explored for active targeting of nanocarriers to its disease cells as well as their receptor specific targeting features have been outlined in Figure 3 [21,22,57,58]. For instance, targeting of nanocarrier-based delivery systems by specific peptide to luteinizing hormone-releasing hormone (LHRH) receptors that are overexpressed in plasma membrane of different cancer cells leads to the preferential accumulation of nanocarrier and its payloads in the tumor and, as a result, enhancing efficiency of the treatment and limiting adverse side effects (Figure 4) [16,23,24,25,27,29,46,59,60]. As shown in Figure 4A, LHRH targeting peptides are attached to the surface of nanocarrier (dendrimer, nanostructured lipid carrier, neutral liposome, cationic liposome or mesoporous silica nanostructure) through linkers. Different types of such spacers (citric acid, PEG polymer, etc.) were conjugated to the carrier via nondegradable (e. g. amide) bond. Therapeutic components (drugs, nucleic acids, etc.) can be conjugated to the carrier via bio-degradable (e.g. ester) bond, coupled using electrostatic interaction between an active component (e.g. negatively charged nucleic acids) and charged carrier (e.g. cationic liposomes) or incorporated into the outer lipid membrane or lipid inner core (for lipophilic payload), dissolved in water-based inner core (for hydrophilic cargo) of nanoparticles or encapsulated into inner pores of transporters. Despite such differences in the mechanisms of drug loading into nanoparticles, one basic requirement should always be fulfilled, namely, drug or other active payload of nanoscale based carrier must be released inside targeted cells. Such release can be achieved by the disruption of spacers used for conjugation of cargo to vehicles (or stoppers that seal internal pores of nanoparticle) or complete degradation of entire nanoparticles in order to release a payload inside the targeted cell or its specific organelle(s). Targeting of drugs and nucleic acids to the site of action usually guarantees more favorable drug/nanoparticle distribution in the organism with a predominant accumulation of payload in targeted organs, cells or cellular organelles (Figure 4B). In addition, a complex multifunctional system can provide not only a desired therapeutic effect, but also suppress cellular defensive mechanisms that fight against the drug. For instance, adding siRNAs that suppress multidrug resistance in cancer cells and antiapoptotic cellular defense to the cancer targeted delivery system (LHRH PPI dendrimer) containing an anticancer drug (paclitaxel) significantly enhanced its antitumor activity to the level that cannot be achieved either by anticancer drug or siRNAs alone (Figure 4C).
Figure 2.
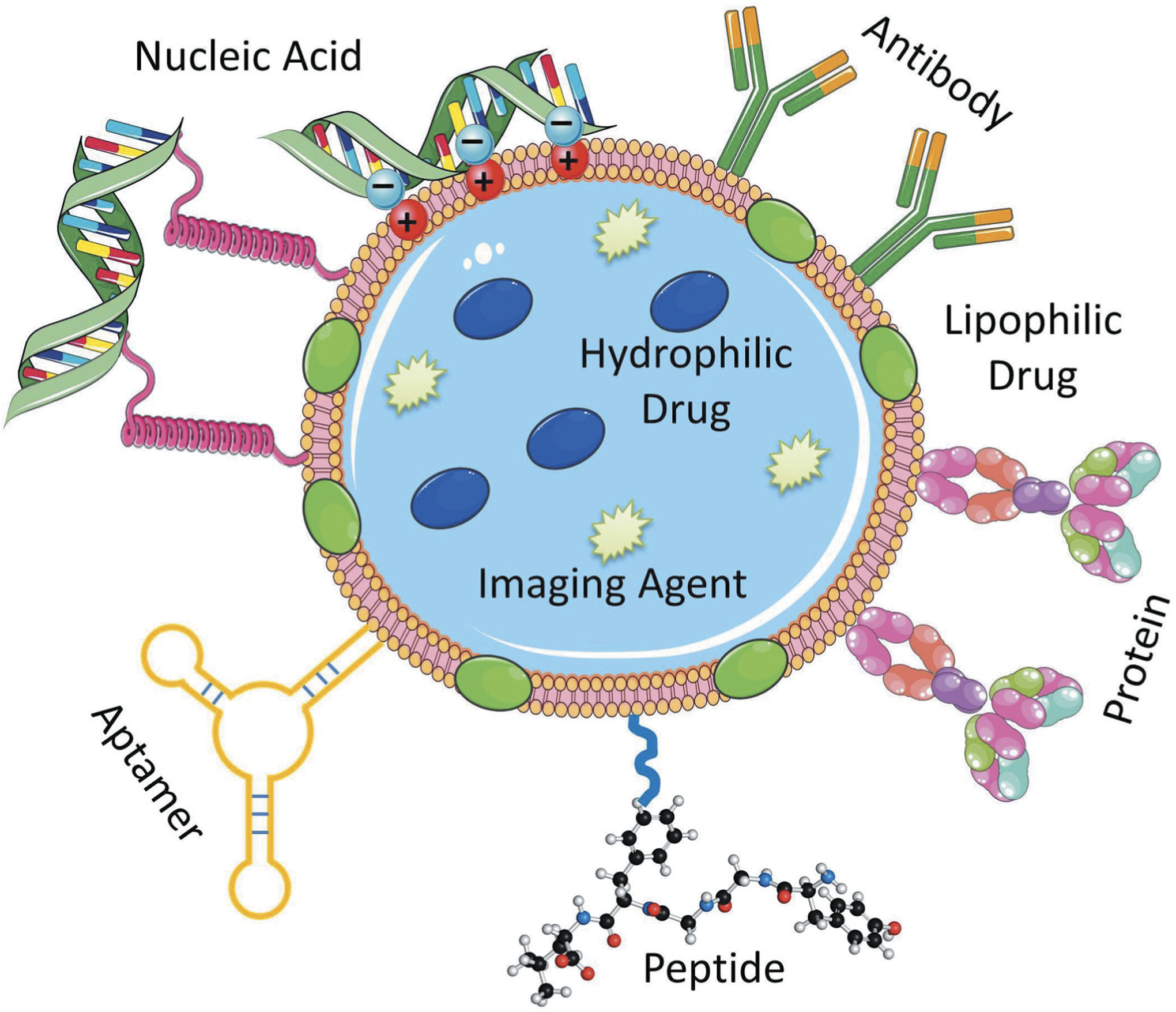
A multifunctional nanocarrier-based drug delivery system containing targeting moieties (antibody, peptide, aptamer, etc.) and active components (drug, nucleic acid, protein, contrast agent, etc.). Drugs and imaging agents are loaded in the core structure (or membrane) of the multifunctional nanocarrier, whereas the targeting moieties such as antibody, peptide, aptamer, etc. and nucleic acids in most cases are conjugated on the surface of the nanostructure through various linkers, electrostatic interactions as well as covalent or noncovalent bond formation.
Figure 3.
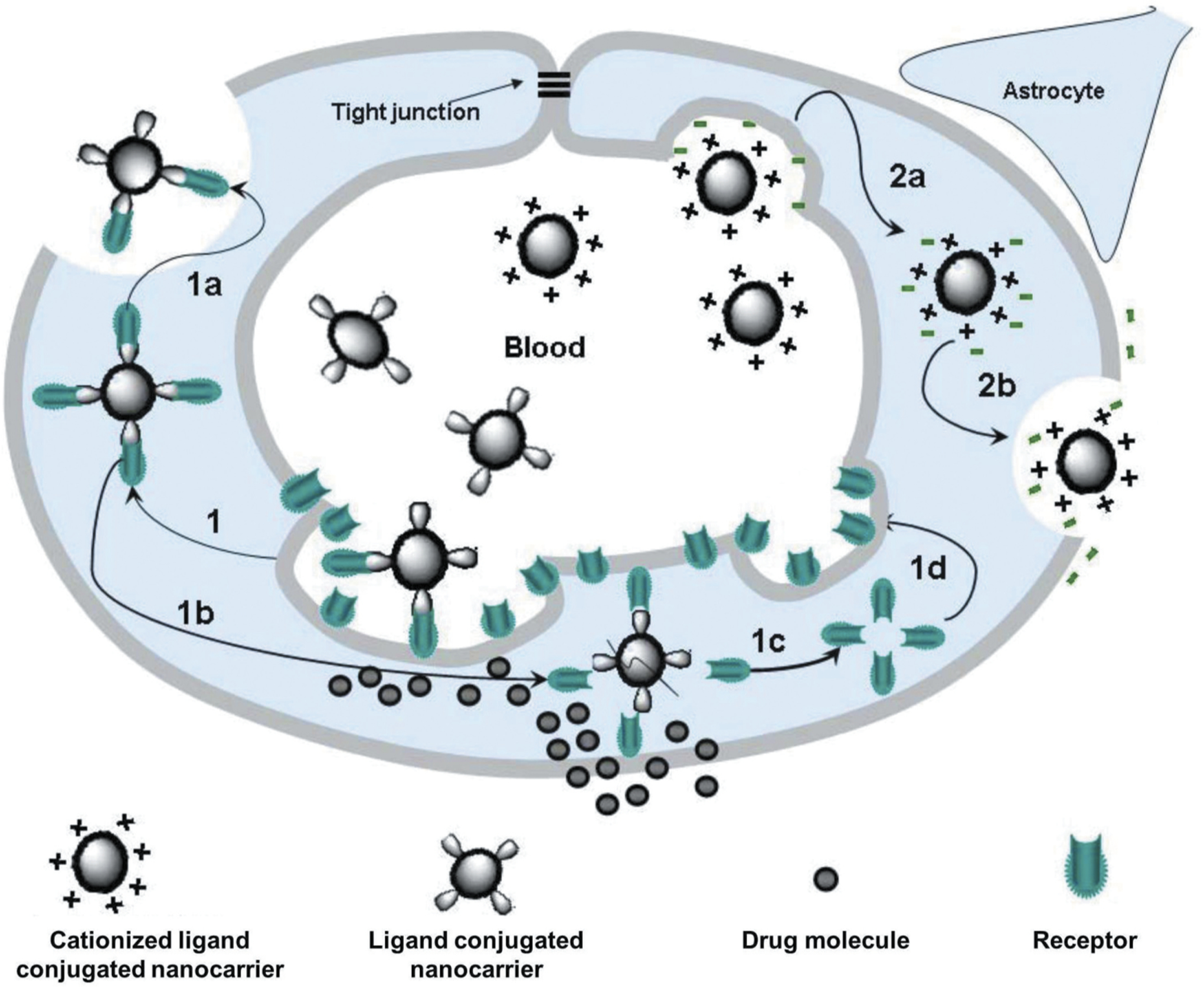
Mechanisms of drug transport through the blood-brain barrier using nanocarriers conjugated to receptor-specific ligands and cationized ligands. (1) Receptor-mediated endocytosis of the nanocarrier; (1a) Exocytosis of the nanocarrier; (1b) Dissociation of the receptor from the ligand-conjugated nanocarrier and acidification of the vesicle leading to the degradation of the nanocarrier and the release of the drug into the brain; (1 c and 1d) Recycling of receptors at the luminal cytoplasmic membrane; (2a) Adsorptive-mediated endocytosis of the nanocarrier conjugated to cationized ligands; (2b) Exocytosis of positively charged nanocarriers. Reproduced with permission [58].
Figure 4.
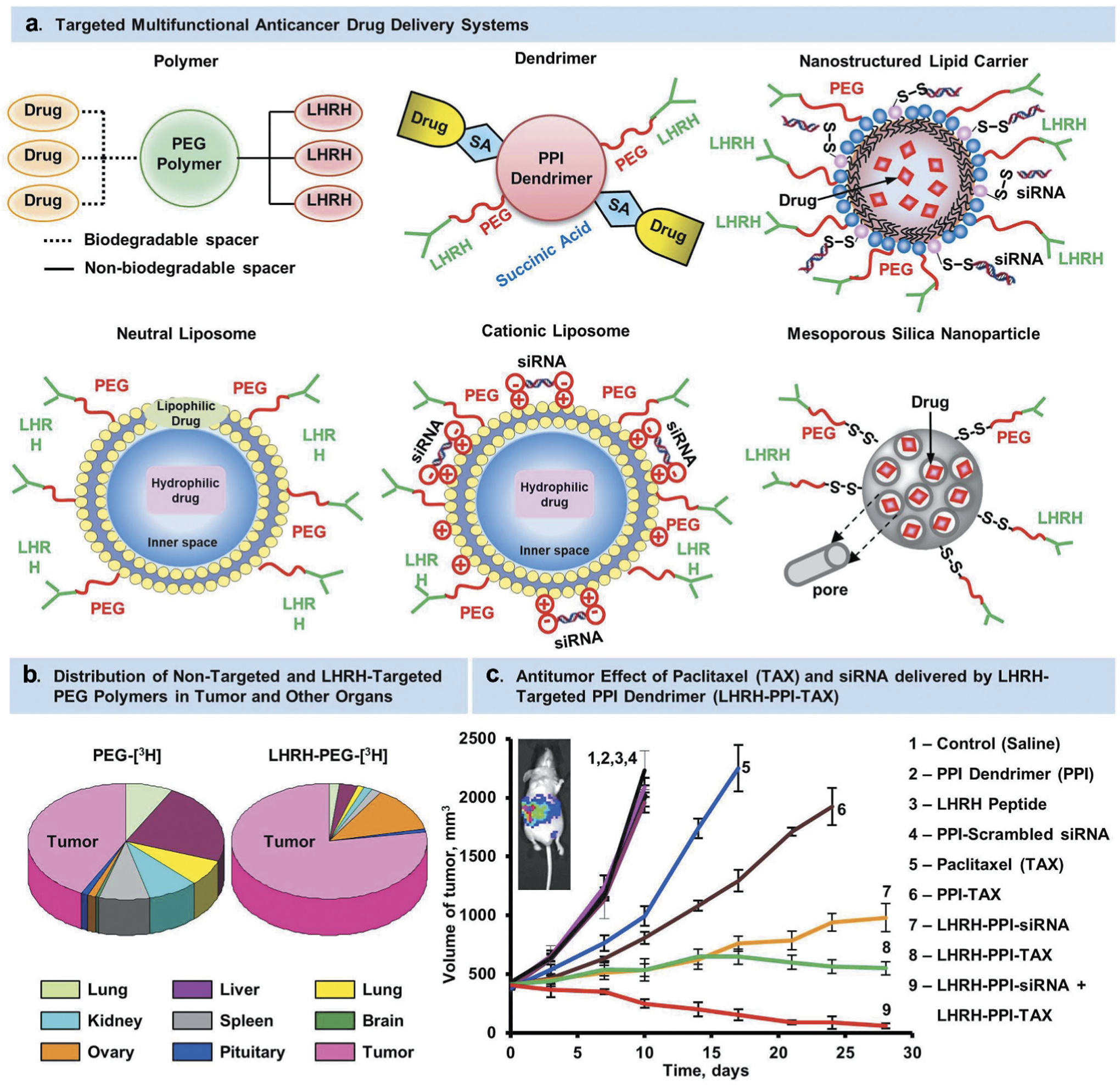
LHRH-targeted delivery systems. (A) Examples of different architecture of nanocarriers. (B) Distribution of tritium-labeled PEG and LHRH-PEG conjugates in tumor and different organs of mice bearing xenografts of human ovarian cancer. (C) Volume of tumor in mice bearing xenografts of human ovarian cancer treated with substances indicated. Means ± SD are shown. Modified from [8,9,16,17,25,27,46,61,82].
Various complex multifunctional nanocarriers have been developed in recent years for therapeutic and diagnostic purposes. Here, we will be discussing recent developments of both liposome and nanostructured lipid carrier (NLC)-based multifunctional nanocarriers and their various biomedical applications. To keep a distinctive perspective, here we have summarized widely used nanocarriers such as multifunctional liposome- and lipid-based nanocarriers for drug delivery applications. Contributions for drug delivery through others organic and inorganic nanocarriers such as polymeric nanoparticle [62], dendrimers [63], micelles [64], emulsions [65], and metal nanoparticle [66] have been already described in corresponding reviews.
2.1. Liposomes
Liposomes are lipid-based nanosized vesicles consisting of a lipid bilayer of biodegradable and biocompatible phospholipids, steroids and an aqueous core. The lipid bilayers and the aqueous core of liposomes can carry both hydrophilic and hydrophobic therapeutics [67]. Hydrophilic drugs are typically trapped inside the aqueous core of the liposome whereas hydrophobic drugs are found in the hydrocarbon chain of the liposome [60,68]. Liposomes are usually prepared by thin layer hydration followed by solvent evaporation and surfactant solubilization [69]. Surface of the liposomes is usually conjugated with polyethylene glycol (PEG) and its analog for maintaining steric equilibration. Various affinity ligands, antibodies, peptides etc. can be attached with the PEG linker for targeting specific disease cells [27,70]. Liposome is one of the widely explored nano-carrier-based systems for targeted therapeutic delivery [26,71,72]. Doxorubicin-based liposome ‘Doxil’ was the first FDA approved liposome-based drug delivery system for the treatment of AIDS-related Kaposi’s sarcoma, breast cancer, ovarian cancer, and other solid tumors. Many liposomes-based anticancer therapeutics such as Doxil, AmBisome, Abelcet, Amphotec, DaunoXome, VincaXome, DepoCyt are currently under investigation in clinical trials [73,74]. A list of FDA-approved liposome-based drug delivery systems have been summarized in Table 1 [75]. By incorporating various cationic lipids, a new series of cationic liposome-based systems have been also prepared for targeted delivery of anionic therapeutics such as small interfering RNA (siRNA), antisense oligonucleotides, aptamers etc. [17,76,77,77,78,79]. For examples, Peddada et al. designed a complex nanocarrier by decorating poly (propyl-acrylic acid) (PPAA) polymer backbone with a cationic DOTAP (1,2-dioleoyl-3-trimethylammonium-propane) liposome, an anionic copolymer and antisense oligonucleotide. This complex nanocarrier with grafted poly (alkylene oxides) (g-PAO) increased antisense gene silencing activity in human ovarian cancer A2780 cells. Authors also observed higher amount delivery of antisense oligonucleotide in ovarian tumor xenografts and demonstrated that this DOTAP/PPAA-g-PAO nanocarrier system can be used for delivery of antisense oligonucleotide for gene silencing [80].
Table 1.
List of FDA-approved liposome formulations combined with drugs or biologics. Modified from [75].
| Name | Material description | Nanoparticle advantage | Indication(s) | Year approved |
|---|---|---|---|---|
| Doxir®/Caelyx™ (Janssen) | Liposomal doxorubicin | Improved delivery to site of disease; decrease in systemic toxicity of free drug | Kaposi’s Sarcoma | 1995 |
| Abelcet® (Sigma-tau) | Liposomal Amphotericin B lipid complex | Reduced toxicity | Fungal infections | 1995 |
| DaunoXome® (Galen) | Liposomal Daunorubicin | Increased delivery to tumor site; lower systemic toxicity arising from side effects | Kaposi’s Sarcoma | 1996 |
| DepoCyt© (Sigma-Tau) | Liposomal Cytarabine | Increased delivery to tumor site; lower systemic toxicity arising from side effects | Lymphomatous meningitis | 1996 |
| AmBisome® (Gilead Sciences) | Liposomal Amphotericin B | Reduced nephrotoxicity | Fungal/protozoal infections | 1997 |
| Curosurf®/Poractant alpha (Chiesei farmaceutici) | Liposome-proteins SP-B and SP-C | Increased delivery for smaller volume; reduced toxicity | Pulmonary surfactant for Respiratory Distress Syndrome | 1999 |
| Visudyne® (Bausch and Lomb) | Liposomal Verteporfin | Increased delivery to site of diseased vessels; photosensitive release | Macular degeneration, wet age-related; myopia; ocular histoplasmosis | 2000 |
| Marqibo® (Onco TCS) | Liposomal Vincristine | Increased delivery to tumor site; lower systemic toxicity arising from side effects | Acute Lymphoblastic Leukemia | 2012 |
| Onivyde® (Merrimack) | Liposomal Irinotecan | Increased delivery to tumor site; lower systemic toxicity arising from side effects | Pancreatic Cancer | 2015 |
Kang et al. developed a dual targeted liposomal system using Pep-1 peptide as a cell penetrating peptide and folic acid as an affinity ligand for folate receptor (FR). The authors prepared this dual ligand (Pep-1 and folate)-modified liposome by separately attaching both the ligands on the surface of the liposomal using a short (PEG-2000) and long (PEG-3400) polymer linker. Cellular uptake of various fluorescent tagged liposomes was studied in FR-positive HeLa and FR-negative HaCaT cells. Higher cellular uptake was observed in FR positive cells as compared to that of the FR negative cells – representing suitability of this multifunctional liposomal system for FR-selective drug targeting [81]. Previously, we reported a liposome-based nanoscale delivery system prepared from the anticancer drug doxorubicin and MRP1 mRNA targeting antisense oligonucleotides as a suppressor of pump resistance. This nanoscale system was evaluated in vivo using an ortho-topic murine model of human lung cancer and observed increased antitumor activity as compared to the separate inhalation treatment of the individual components [82].
Another important factor of complexation of nucleic acids to the positively charged carriers is a dramatic decrease in the cyto- and genotoxicity of cationic carriers. We investigated a wide range of nanocarriers with different composition, architecture size and surface charge including poly(ethylene glycol) polymers, neutral and cationic liposomes, micelles, poly-(amidoamine) and poly(propyleneimine) dendrimers, quantum dots, mesoporous silica, and supermagnetic iron oxide (SPIO) nanoparticles [83]. It was found that even when being applied in noncytotoxic concentrations some of these nanocarriers demonstrated a significant genotoxicity. The magnitude of such genotoxicity positively correlates with zeta potential of nanoparticles. However, the complexation of nucleic acids with cationic carriers dramatically decreased their genotoxicity.
2.2. Nanostructured lipid carriers (NLCs)
Solid lipid nanoparticles consisting of both solid and liquid lipids matrix has been employed in recent years for therapeutic delivery applications. Such lipid-based nanocarriers are termed as nanostructured lipid carriers (NLCs). Most therapeutic drugs are usually more soluble in liquid lipids than in solid lipids [84], and NLCs are prepared with higher composition of liquid lipids. The lipid phase is first heated above its melting point, and the drug is dissolved in the melted lipid. The resulting drug containing melted lipid mixture is dispersed in the aqueous phase. The hot microemulsion is then homogenized using a homogenizer to get the NLC mixture. NLCs are generally prepared from biocompatible and biodegradable lipid materials and surfactants and capable of carrying both hydrophobic and hydrophilic therapeutics to its target tissues.
Because of ease of preparation, small size, enhanced drug loading capacity, and improved stability, NLCs have become a versatile platform for targeted therapeutic delivery applications [85]. As compared to other lipid-based nanocarriers, NLCs have demonstrated successful delivery of therapeutics through various routes such as ocular, topical, pulmonary, intranasal, and oral administration [86]. The use of siRNA as therapeutic is limited due to its poor stability and delivery to the target tissue. To enhance therapeutic efficacy of siRNA, a new strategy such as encapsulation of siRNA into NLCs was explored by many research groups. Over the years, researchers attempted to develop various lipid-based multifunctional NLCs for targeted drug and siRNA delivery. For examples, Minko and her group developed a multifunctional NLC-based system composed of anticancer drug (doxorubicin or paclitaxel), siRNA targeted to MRP1, another siRNA targeted to BCL2 mRNA, and a synthetic peptide derivative as a targeting moiety for luteinizing hormone-releasing hormone (LHRH). These lipid nanocarrier systems showed effective inhalation delivery of the loaded drugs in lungs as well as enhanced antitumor activity [17] and codelivery with high loading efficiency of two drugs Lumacaftor and Ivacaftor by inhalation route in treating lung manifestations of cystic fibrosis [87,88]. In a recent study, Emami et al. reported paclitaxel (PTX)-loaded and transferrin (Tf)-conjugated multifunctional nanostructured lipid carrier (Tf-PTX-NLC) for the treatment of glioblastoma. The authors first prepared the PTX loaded NLCs followed by conjugating transferrin as targeting ligands with the PTX-NLC. Average particle size and entrapment efficiency of these Tf-PTX-NLCs were 205.4 ± 11 nm and 91.8 ± 0.5% respectively. Cytotoxicity of the Tf-PTX-NLCs was tested against U87MG brain cancer cell line and the results revealed that Tf-PTX-NLC was more cytotoxic as compared to nontargeted NLCs and free drug treatments [89].
2.3. Iron-oxide nanoparticles
Iron oxide nanoparticles are the widely studied inorganic nanocarriers, which have received popularity in various biomedical and imaging application [66,90]. Because of magnetic nature, this nanoparticle can be observed by Magnetic Resonance Imaging (MRI) thereby making it suitable for MRI based applications [28]. Recently, various drug conjugated iron oxide nanoparticles have been extensively explored for targeted delivery applications. For example, Marcu et al. prepared iron oxide nanoparticle by laser pyrolysis with an average diameter of 8–10 nm for drug delivery of antracyclinic antibiotic Violamycine B1 to breast cancer cells [91]. The authors observed better internalization of these nanoparticles and the accumulation of the anticancer agent in the cytoplasm of human breast cancer MCF-7 cells when compared with other commercially available chemically prepared iron oxide nanoparticles with a larger size. Finally, these nanoparticles reduced the proliferation of the breast cancer cells. In recent years, iron oxide nanoparticles were explored for gene therapy of tumor cells. For this end, supermagnetic iron oxide nanoparticles (SPIONs) can be functionalized with cationic polymers (e.g. polyethylenimine) allowing for an attachment of nucleic acids on their surface [92]. In addition to the delivery of nucleic acid for cancer therapy, such theranostics SPIONs can be successfully used for imaging of cancer cells. In 2019, Jin et al. developed iron oxide Fe3O4 nanoparticles for targeted delivery of siRNAs to oral cancer cells [93]. These magnetic Fe3O4 nanoparticles were modified with polyethyleneimine (PEI) for attachment of the therapeutic siRNAs on the surface of the nanostructure. The nanoparticle system was capable of delivering siRNAs specifically to the B-cell lymphoma-2 (BCL2) targeted gene in Ca9–22 cells and inhibited in vitro proliferation of the oral cancer cells. Ferumoxytol, ferridex I.V., combidex etc. are a few commercial iron oxide nanoparticles, which are used for various therapeutic or imaging applications [94].
2.4. Mesoporous silica nanoparticles
Mesoporous silica particles with nanosized diameter have been also exploited in the past decades for targeted drug delivery and imaging applications. Mesoporous silica nanoparticles (MSNs) possess large and tunable pore size along with a high surface area and thermal stability. Their sealable pores allows for the incorporation of different payloads. Various functional groups, ligands, peptides etc. can be conjugated on the large surface area of the MSNs for targeted drug delivery applications. For examples, Cheng et al. designed and synthesized pH responsive multifunctional MSN system comprised of poly dopamine, poly (ethylene glycol) and folic acid for targeted delivery of doxorubicin [95]. The authors observed release of the encapsulated drug from this MSNPDA-PEG-FA nanosystems in acidic pH and high antitumor activity [95]. Researchers have also prepared multifunctional MSNs by decorating their surface with cationic polymers which facilitate electrostatic attachment and delivery of anionic therapeutics such as nucleic acids, siRNA, etc. [96]. In 2016, Yang et al. developed a disulfide-bridged ‘degradable dendritic mesoporous organosilica nanoparticles (DDMONs) for therapeutic protein delivery to cancer cells [97]. This DDMONs system showed a higher rate of glutathione (GSH)-responsive degradation and release of the therapeutic protein in B16F0 cancer cells, while the degradation of the nanoparticle was low in the normal HEK293t cells.
2.5. Carbon nanomaterials
Carbon based nanomaterials such as 1D carbon nanotubes (CNTs) [98], graphene oxide, diamond-like carbon etc. have received significant attention in recent years for various biomedical applications including drug delivery. Among the various carbon materials, single-wall CNTs possess unique structural, thermal, mechanical properties as well as good biocompatibility which made them one of the widely used carbon nanomaterials for targeted drug delivery. The surface of the CNTs is usually coated with functional polymer such as PEG and drugs are conjugated via non-ovalent or covalent bonding with the functional groups on the surface of CNTs [99,100,101]. CNTs have been explored as nanocarriers for the delivery of drugs [102] as well as biomolecules such as DNA, siRNA etc. [103,104]. In an early study, researchers developed a PEGylated CNT complex loaded with paclitaxel for breast cancer treatment [105]. This CNT-paclitaxel complex was tested in a 4T1 murine breast cancer model and showed better treatment efficacy when compared to free paclitaxel alone. Recently, Cheng et al. designed a poly (lactic-coglycolic) (PLGA) functionalized CNT system for delivery of proapoptotic protein caspase-3 (CP3) in bone cancer cells with reduced toxicity [106]. The authors successfully prepared CNT-PLGA-CP3 nanocomplex which showed efficient transfection of CP3 in cells and suppressed their proliferation. Such CNT-PLGA system showed good transfection rate with the transcription factors and the release profile of the payload can be controlled by changing the molecular weight and ratio of the PLGA polymer [106]. Mehra et al. developed a multi wall PEG-CTN complex loaded with doxorubicin (DOX) for the treatment of cancer [107]. Both folic acid (FA) and estrone (ES) were attached as targeting moieties on the surface of this DOX/ES-PEG-MWCNT system. The authors treated Balb/c mice bearing MCF-7 tumor with this DOX/ES-PEGMWCNT nanoformulation and observed a long survival of the mice.
2.6. Quantum dots
Quantum dots (QDs) are a type of nanosized semiconductor materials with good photoluminescence, optical and electronic properties, which made these materials suitable for image guided drug delivery applications [108]. QDs can be decorated with the targeting ligands for tissue specific therapeutic delivery application. Over the years, various such targeted QDs were studied for diagnosis and therapeutic delivery applications [109] For examples, Chen et al. developed a quantum dot-based FRET system for image guided drug delivery in the cellular nucleus [110]. The authors prepared graphene quantum dots (GQDs) and decorated its surface with the TAT peptide to facilitate the nuclear localization of the nano carriers and delivery of the GQDs in the nucleus. This quantum dot-based FRET system showed real-time monitoring of the therapeutic delivery as well as image-based tracing of the release process. A recent report showed that conjugation of QDs with lipid nanocarriers reduced their cytotoxicity specifically for metal based QDs and improved the safety profile [111]. Mahajan et al. developed a quantum dot system comprised of antiretroviral drug saquinavir and a targeting ligand transferrin (Tf), which was conjugated on its surface carboxyl groups through carbodiimide coupling reaction [112]. This quantum dot formulation was prepared for targeting transferrin receptors in the apical surface of the blood brain barrier. The authors investigated this system to enhance the transport of saquinavir through blood brain barrier for treating HIV-1 infected cells in brain. A pH responsive QD system for the treatment of ovarian cancer was designed and developed in our laboratory [113]. The surface of the developed QDs was decorated with a DNA aptamer for targeting of mutated MUC1 mucin overexpressed in ovarian cancer. Doxorubicin was linked on the surface of the QD through a pH labile hydrazine linker, which undergo hydro-lysis at acidic pH of the tumor microenvironment resulting in controlled release of the drug. Both in vitro and in vivo studies revealed better therapeutic outcome for this QD system when compared with free doxorubicin.
3. Stimuli-responsive nanocarriers
Nanocarriers which can change their certain property upon endogenous or exogenous stimuli, offered a wide range of biomedical applications including controlled release of therapeutics directly at the desired site thereby reducing side effects in the surrounding healthy tissue [114]. Such nanocarriers are referred to as stimuli-responsive nanocarriers, which have attracted enormous attention in recent years in the field of targeted drug delivery from various research groups throughout the world. Stimuli-responsive nanocarriers are generally made of a hydrophobic inner core and a hydrophilic or amphiphilic outer shell, which is usually an amphiphilic stimulus-responsive polymer sensitive to various endogenous or exogenous stimuli. The amphiphilic compound can be any of the natural lipids or lipid like materials or surfactants which are usually pH, redox, temperature, light, enzyme, or magnetic responsive polymers. For some nanocarriers, the hydrophobic part of amphiphilic polymers is modified with cationic groups for conjugating anionic agents such as nucleic acids in the hydrophobic core [115]. In addition, these nanocarriers sometime contain another component such as a targeting ligand or adhesion ligand which is specific to its receptor on target cells or tissues. Various stimuli-sensitive nanocarriers developed for drug and gene delivery application have been summarized in Table 2 [34,38,39,40,116–126]. We will be discussing various endogenous or exogenous stimuli-responsive nanocarriers for targeted therapeutic delivery applications.
Table 2.
Stimuli-sensitive various nanocarriers developed for drug and gene delivery application [34,38,116,117].
| Stimuli type | Nanocarrier type | Drug or gene | Therapeutic use | Reference |
|---|---|---|---|---|
| Temperature stimuli | Liposomes Polymeric nanoparticles |
Lucifer yellow iodoacetamide Doxorubicin |
Activity against CT-26 colon tumor Activity against Lewis lung carcinoma cells |
[118,119] |
| pH stimuli | Liposomes Micelles |
Doxorubicin Doxorubicin |
Activity against B-lymphoma cells Activity against MCF-7 breast cancer cells |
[120,121] |
| Redox stimuli | Gelatin nanoparticles Cationic lipoic acid |
Plasmid DNA Plasmid DNA |
Enhances gene transfection and DNA delivery via glutathione redox response | [122,123] |
| Increases transgene expression | ||||
| Enzyme stimuli | Silica-coated magnetic nanoparticles | Doxorubicin Polyhexanide |
Colon carcinoma Infection treatment |
[124,125,126] |
| Hyaluronic acid nanocapsule | Doxorubicin | Breast carcinoma | ||
| Liposomal nanocarrier |
3.1. Endogenous stimuli-sensitive nanocarriers
Here, we will discuss stimuli-responsive nanocarrier-based drug delivery systems, which are sensitive to various endogenous stimuli factors such as pH, redox potential, enzymes, etc.
3.1.1. pH-sensitive systems
pH-sensitive systems have been widely used for delivery of several therapeutics in specific organs where pH changes are found due to pathological conditions including cancer, inflammation etc. pH sensitive nanocarrier systems are usually designed to have a surface polymer with acid-sensitive bonds which undergo dissociation in response to environmental pH resulting in release of the loaded therapeutics specifically at the target disease tissues. pH responsive nanocarriers decorated with a targeting moiety or ligand can bind to its target cell resulting in its internalization. After entering in acidic intracellular environment, pH responsive nanocarriers can dissociate and deliver the loaded therapeutics. Thus, pH responsive nanocarriers can be exploited for selective delivery of therapeutics into target cells over nontarget cells [127]. Recently, Nikravan et al. developed a pH responsive cross-linked nanoparticle system derived from different molar ratios of poly (acrylic acid) (PAA) and ethylene glycol dimethacrylate for controlled release of doxorubicin. pH-responsive behavior of this nanocarrier was less effective with higher cross-linking degrees. The release of model drug doxorubicin was studied at pH values of 1.2, 5.3, and 7.4. Lower burst release of doxorubicin (change in hydrodynamic diameter) was observed with increase in the cross-linker content and pH of the release medium. pH-sensitive behavior of this PAA nanoparticle systems with higher and lower cross-linking ratios has been shown schematically in Figure 5 [128].
Figure 5.
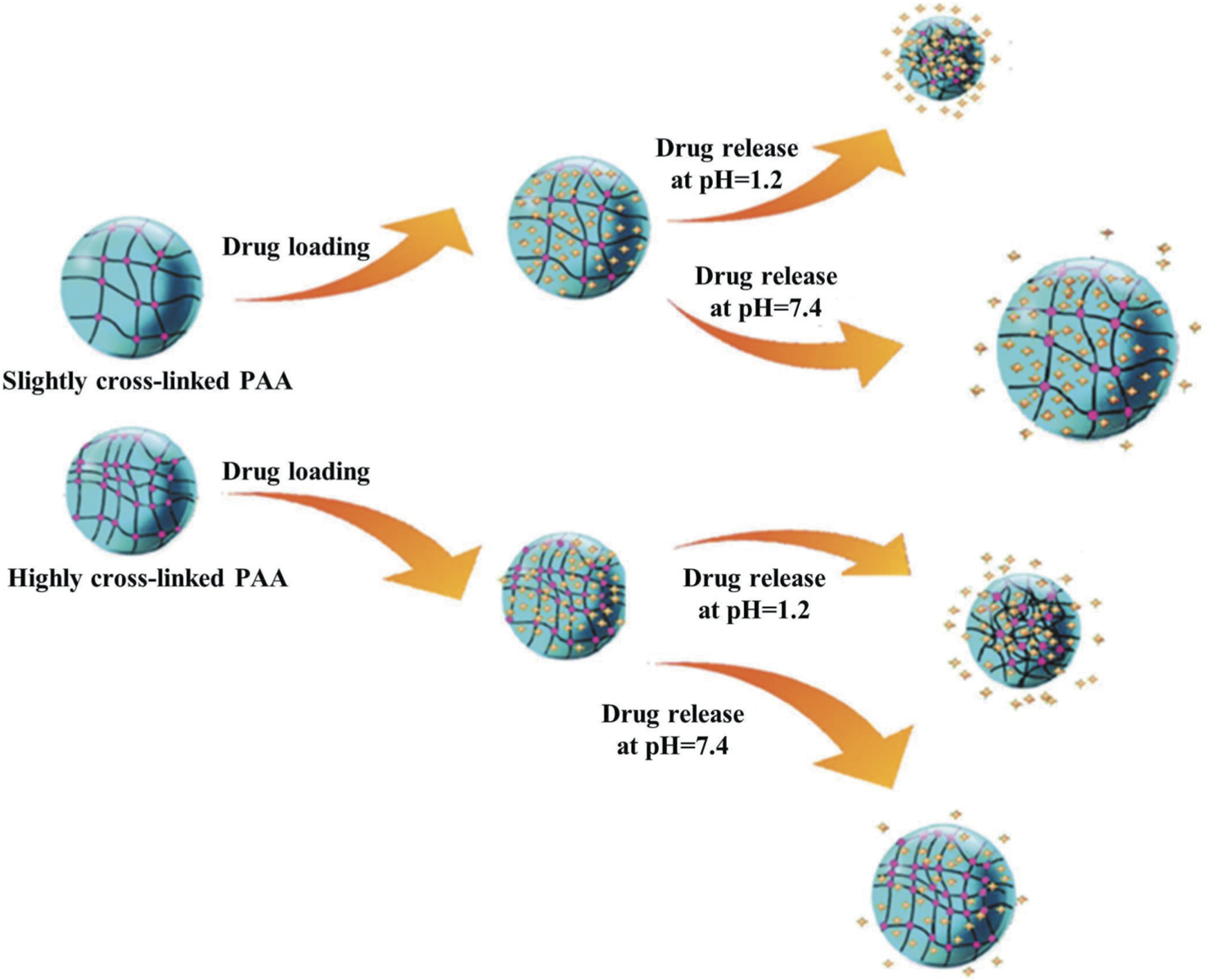
Responses of the poly(acrylic acid) (PAA) nanoparticles with different monomer to cross-linker ratios to pH and temperature. Reproduced with permission [128]. The change in hydrodynamic diameter and drug release from high and low cross-linked PAA has been displayed schematically.
3.1.2. Redox-sensitive systems
The release of therapeutics from nanocarriers can be triggered through a redox reaction by decorating the nanocarriers with redox sensitive bonds and/or linker such as di-sulfide bond. Nanocarriers decorated with di-sulfide-based bond and/or linker that is known to cleave by glutathione (GSH), can be used to deliver the therapeutics in response to redox reaction. Drug release can be triggered from di-sulfide linker-based nanocarriers by GSH, which is known to present in the intracellular compartments and tumor tissues compared with normal ones. A schematic illustration for GSH mediated intracellular drug release has been shown in Figure 6 [117,129]. As shown in this Figure, the disulfide bond of this nanocarrier is reduced to thiol groups after endocytosis inside the tumor cell with high level of GSH resulting in the dissociation of the nanostructure and release of the encapsulated drugs. Cho et. al, reported a redox-sensitive polymeric nanoparticle for tumor-targeted drug delivery. Authors used a redox-responsive biodegradable polymer to prepare the paclitaxel-incorporated nanoparticle, which was capable of delivery paclitaxel in response to reduction reaction [130]. Other examples include liposomes prepared from quinone-lipid conjugate [131] or di-sulfide crosslinked nanomaterials [132] showed promising results. Yang et al. designed a redox-responsive magnetic nanovectors (RMNs) system comprised of a redox-responsive polymer and Fe3O4 nanoparticles for effective delivery of therapeutic proteins [133]. The authors loaded fluorophore cya-nine 5.5 (Cy5.5) linked human serum albumin (HSA) and observed low cytotoxicity and high stability of this RMN structure. This RMNs-HSA-Cy5.5 system showed low release of the protein under physiological conditions, whereas the release rate was higher at the presence of glutathione. RMNs-HSA-Cy5.5 can be used for simultaneous fluorescence and magnetic resonance imaging as revealed in the in vivo study. Such multifunctional RMNs potentially can be used for effective protein delivery applications.
Figure 6.
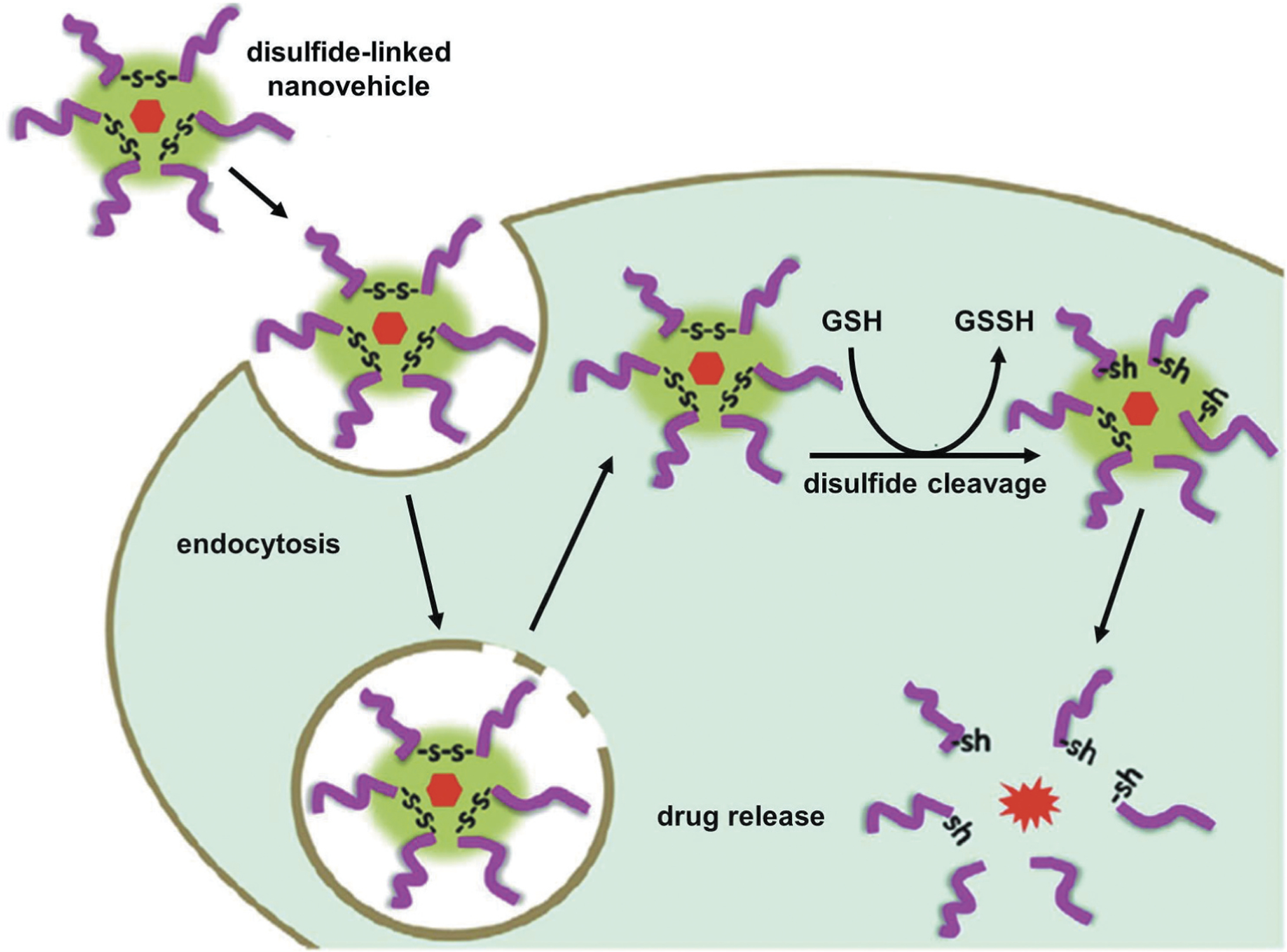
Internalization of a redox-sensitive nanocarrier, cleavage of disulfide linker and drug release inside cells. Disulfide-linked nanocarrier is stable outside the tumor microenvironment with low level of GSH. However, the disulfide bond of this nanocarrier is reduced to thiol groups after endocytosis inside the tumor cell with high level of GSH resulting in the dissociation of the nanostructure and release of the encapsulated drugs. Reproduced with permission [117].
3.1.3. Enzyme-sensitive systems
Many enzymes such as proteases, phospoholipases, glycosidases, etc. are known to be found at the site of tumors, and inflammatory tissues. Substrate of such enzymes can be used as the component of drug delivery system to achieve enzyme mediated drug release at the target tissue. In a recent study, researchers used a metalloproteinases cleavable short peptide as a linker between the surface PEG chains and TAT-functionalized liposomes as represented in Figure 7 [34,134,135]. After cleavage of this linker, loaded drugs and bioactive agents were exposed to the target site as compared to that of nanocarriers without such linker. Such nanocarriers systems were used for siRNA delivery showing almost 70% gene-silencing activity in tumor-bearing mice [136,137,138]. In another recent study, Zhang et al. designed a mesoporous silica nanoparticle by grafting hyaluronic acid (HA) as a targeting moiety on its surface via biotin–streptavidin interaction for enzyme triggered delivery of doxorubicin (MSN-HA/Dox). A schematic outline showing MSN-HA/Dox nanoparticle mediated delivery of doxorubicin to cancer cells has been displayed in Figure 8. In vitro results revealed that the release of doxorubicin (Dox) was significantly higher in the presence of enzyme hyaluronidase. MSN-HA/Dox system also displayed increased antitumor effects in vivo as compared to that of the free drug doxorubicin treatment only (Figure 9) [139].
Figure 7.
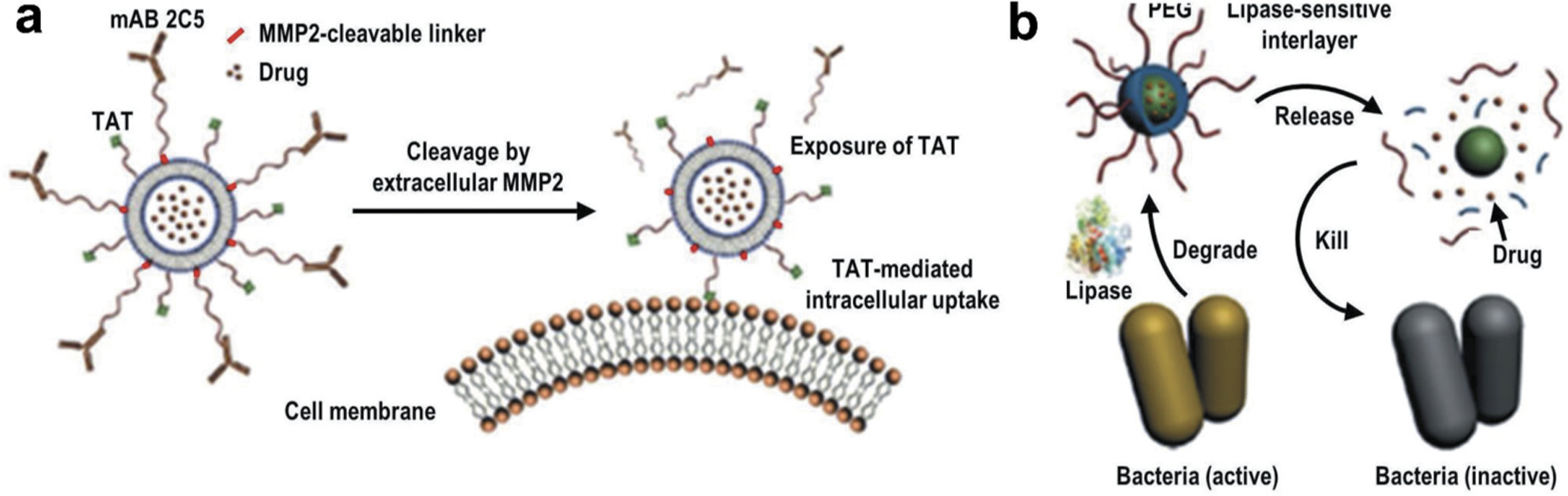
Enzyme-sensitive drug delivery. (A) Multifunctional liposomal nanocarrier responsive to matrix metalloproteinases (MMP2) for drug delivery via TAT-mediated internalization. mAB 2C5 – nucleosome-specific monoclonal antibody 2C5. (B) On-demand drug delivery triggered by bacterial lipase. Bacterial lipase causes cleavage of the internal PEG shell of the nanocarrier – resulting in release of the surface bioactive agents as well as encapsulated drugs. Reproduced with permission [34].
Figure 8.

Delivery and controlled release of doxorubicin (Dox) at cancer cells expressing hyaluronidase using mesoporous silica nanoparticles functionalized with biotin-modified hyaluronic acid (MSN-HA). (A) Drug loading steps to yield MSN-HA/Dox delivery system. Propylamine-functionalized silica (MSN-NH2) was first modified with desthiobiotin to obtain MSN-desthiobiotin, then by employing biotin (or desthiobiotin)–SA interaction, SA, and biotinylated HA were self-assembled on the external surface of MSN to yield MSN-HA. Optionally, therapeutic drug, doxorubicin, can be loaded into mesoporous silica nanoparticles to obtain MSN-HA/Dox. (B) Schematic illustration of the CD44 receptor-mediated endocytosis and triggering of drug release in tumor cells. MSN-HA/Dox is internalized by cancer cells via receptor-mediated endocytosis (HA–CD44 interaction), then loaded doxorubicin was released from the pore of MSN by the triggering of HAase and intracellular biotin. Reproduced with permission [139].
Figure 9.
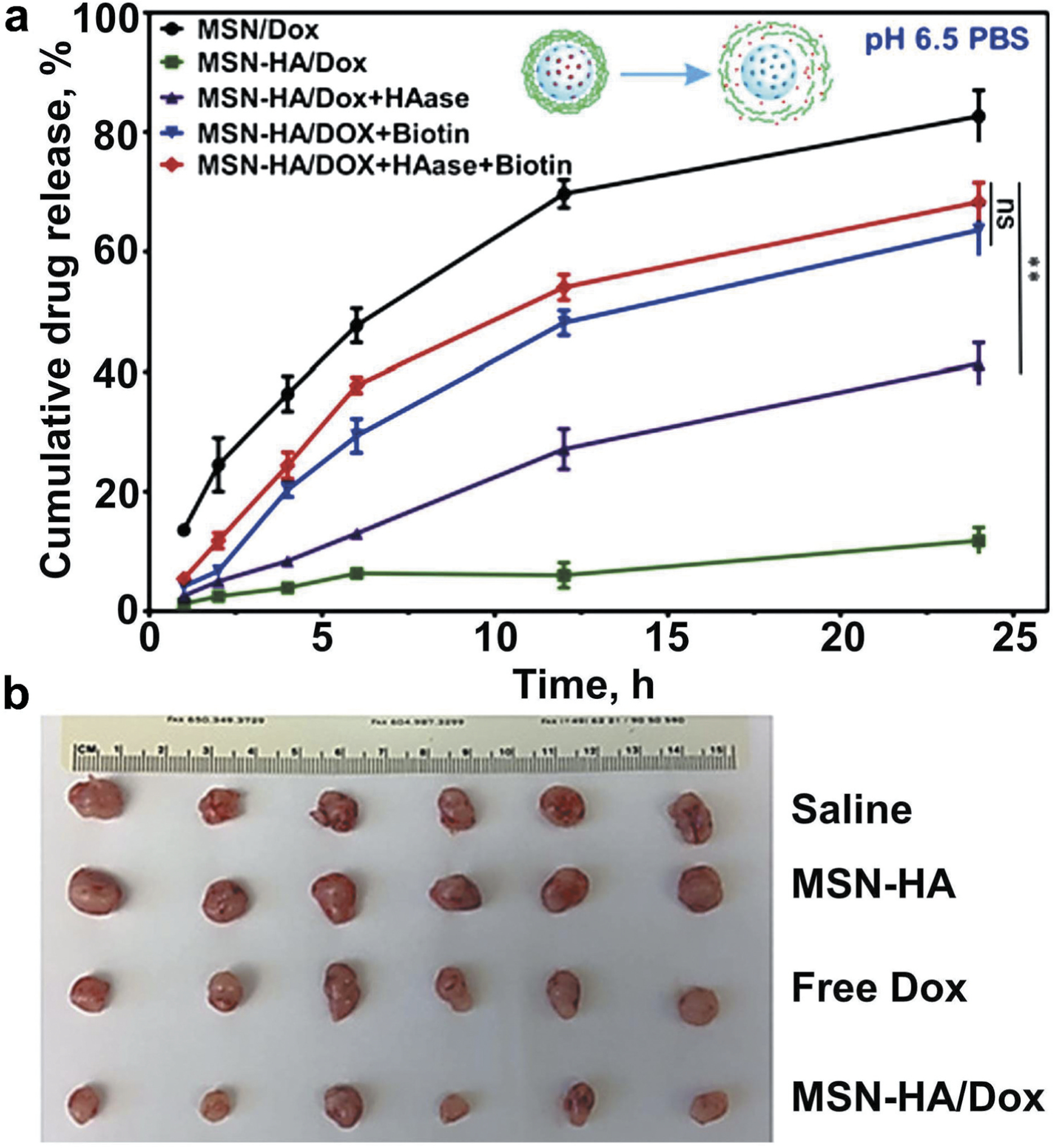
Doxorubicin (Dox) release from mesoporous silica nanoparticles functionalized with biotin-modified hyaluronic acid (MSN-HA) and antitumor effect of the formulation. (A) The biotin-and hyaluronidase-responsive release profiles of doxorubicin (Dox) under pH 6.5. The biotin- and HAase-responsive release profiles of doxorubicin (Dox) were evaluated. Drug release under pH 6.5 was conducted to mimic the condition of tumor microenvironment. Under different stimulus condition, biotin (2 mmol/L), HAase (150 U/mL), or both were added to MSN-HA/Dox solution; as a control, MSN-Dox was employed. At specified time points (1, 2, 4, 6, 12, and 24 hours), cumulative drug release was measured and compared. **P < 0.01. (B) Representative tumor images obtained from tumor-bearing mice treated for 18 days with saline (control), empty MSN-HA, free nonbound Dox, or MSN-HA/Dox. Modified with permission [139].
3.2. Exogenous stimuli-sensitive nanocarriers
Delivery of therapeutics from nanocarriers can be induced by various external stimuli such as light, temperature, or ultrasound application. Such external stimuli can be applied to a specific location or organ to trigger release of therapeutics from the nanocarrier while it passes the targeted location or organ. By applying heat or cold, a local temperature shift can be achieved in the target location or organ in the body to dissociate the nanocarriers at that location resulting in delivery of the therapeutics. Similarly, nanocarriers can be formed that are sensitive to various radiation (ionizing radiation, micro-waves, or radio-waves); light (ultraviolet, near infrared or far infrared, etc.) and sound waves (ultrasound). Few examples of such exogenous stimuli-responsive nanocarriers have been summarized below.
3.2.1. Thermoresponsive systems
Thermoresponsive system is a widely explored stimuli-responsive drug delivery approach for several diseases. Thermoresponsive nanocarrier is usually made up of one component with temperature sensitive material and it can release its loaded drug in response to its surrounding temperature. Such nanocarrier systems can usually be capable of holding their load at body temperature but deliver the loaded drug at sites of locally heated tissues which are usually observed at the sites of inflammation, injury, infection, and cancer [140]. Thermoresponsiveness is also associated with decrease in temperature phenomena. In this case, a locally cooling effect can increase the porosity of the nanocarrier system leading to free diffusion of the loaded therapeutics at the target site. For example, Pluronic F127–polyethyleneimine (PEI) nanocapsule was explored for effective delivery of siRNA into the target cell resulting in silencing of a target messenger RNA (mRNA) [141]. Poly (N-isopropylacrylamide) (PNIPAM) is a commonly used building block for the preparation of thermosensitive polymeric nanocarriers because of its temperature transition property [142,143]. Such thermosresponsive nanocarriers can be further decorated with receptor affinity ligands for target specific therapeutic delivery. For example, Chen et al. designed a bubble-generating thermoresponsive liposomal system by encapsulating doxorubicin and ammonium bicarbonate inside the nanocarriers. At an elevated temperature, ammonium bicarbonate undergoes decomposition generating carbon dioxide bubbles which creates permeable pores in the lipid bilayer of the liposomal nanocarriers resulting in rapid release of the loaded drug doxorubicin [144].
3.2.2. Ultrasound-triggered drug delivery
Ultrasound has received significant attention in recent years in the targeted-drug delivery research [145,146]. Ultrasound-triggered drug delivery systems offer spatiotemporal control of therapeutic delivery at the specific site thereby reducing side effects to the normal tissues. Major advantages of such system include its noninvasiveness nature, no requirement of ionization radiations and its easy exposure and tissue penetration depth, etc. Ultrasound waves trigger the release of the drug via thermal and/or physical forces induced by the radiation phenomena [147,148,149,150]. Jung et al. prepared a dual functional Gd(III)-DOTA-modified sonosensitive liposomes for targeted delivery of doxorubicin as well as to acquire magnetic resonance imaging. About 30–40% release of doxorubicin was observed after inducing the sonosensitive liposome with 20 kHz ultrasound. In addition, this sonosensitive liposome system displayed excellent contrast efficiency in magnetic resonance imaging [151].
3.2.3. Light-triggered drug delivery
Light-responsive nanocarriers have also received significant attention in targeted drug delivery research. Light-responsive drug delivery systems are generally noninvasive and can be remotely controlled in spatiotemporal manner. These systems can deliver therapeutics at the target site in response to application of specific wavelength of light such as UV, visible or near-infrared (NIR) etc. [152]. Nanocarriers-based systems have been developed in recent years. Usually, drug release is triggered by either reversible or irreversible photo-induced structural modifications of the nanocarriers. For examples, azobenzene and its derivative-based nanocarriers are widely used for control drug delivery at its target by site specific application of ultraviolet–visible light and/or light in the visible region that allow its structural modification resulting in drug release as outlined in Figure 10 [34,153,154,155,156]. One such light triggered drug delivery system has been prepared via azobenzene functionalization of the pore interior of mesoporous silica nanoparticles [157,158]. Li and co-others reported a near infrared laser-triggered nanocarrier system made of gold nanorod core surrounded with mesoporous silica shell [159]. In addition, single-stranded DNA valves were capped within the nanocarrier. This system displayed good stability, biocompatibility, resistance nuclease enzymes and controlled release of the cargo molecule with response to the laser on/off conditions. Upon laser radiation, the valves of this nanocarrier opened and the cargo molecules were released through the mesopores. Intracellular imaging experiments revealed that the controlled release was successful in living cells (Figure 11) [159].
Figure 10.
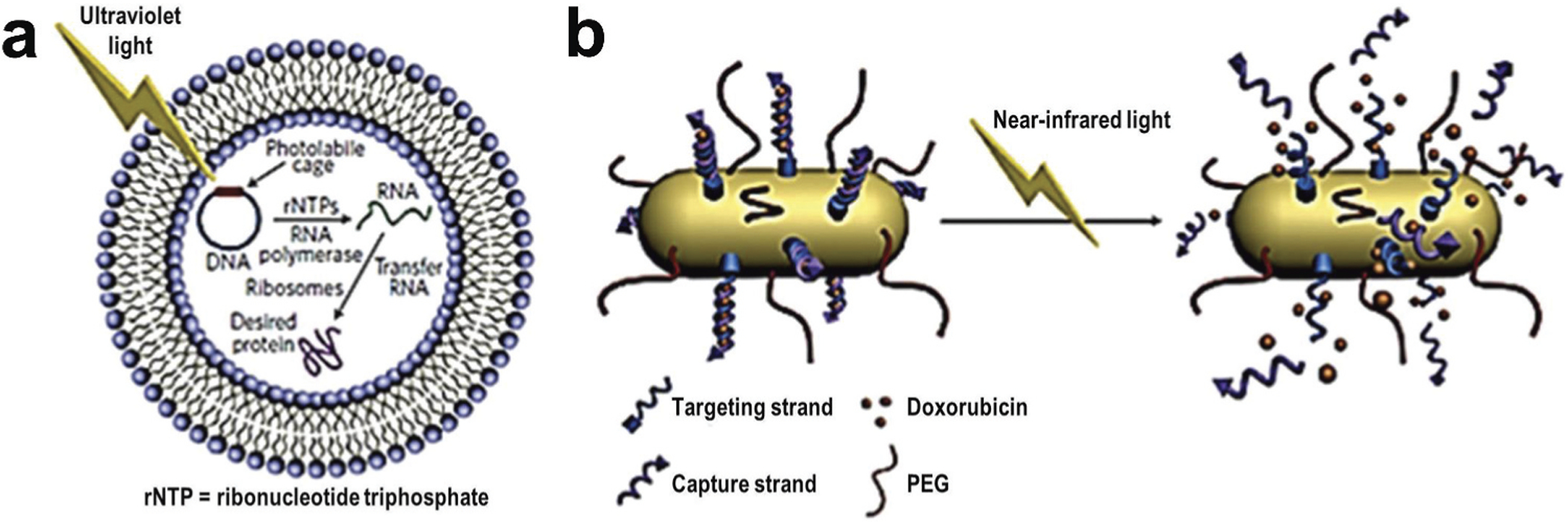
Examples of light triggered drug delivery. (A) Schematic representation of an encapsulated in vitro transcription–translation liposomal system triggered by irradiating caged DNA with light. (B) Delivery of doxorubicin through the near-infrared-triggered induction of dehybridization of the DNA conjugated at the surface of gold nanorods. Upon irradiation of the gold nanorods with NIR laser, the resulting light to heat transformation increases local temperature and leads to the detachment of the DNA helices from the gold surface – resulting in the release of the encapsulated doxorubicin. Reproduced with permission [34].
Figure 11.
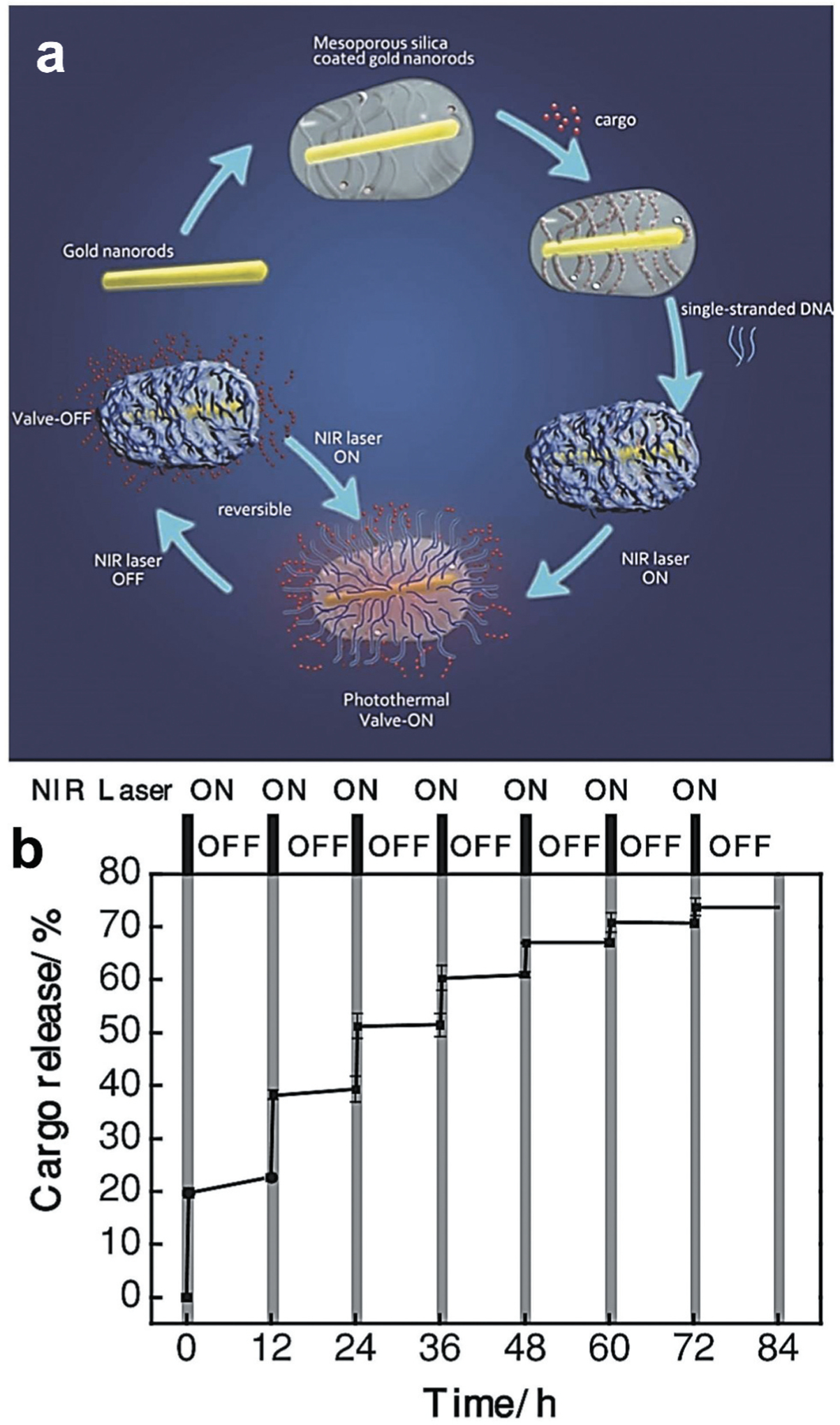
Near-infrared (NIR) light-triggered nanocarrier with reversible DNA valves for controlled release. (A) A schematic of light-controlled release of nucleic acid. Cargo loaded gold nanorods were coated with mesoporous silica shell and covered with reversible single stranded DNA valves. When such nanocarrier is irradiated with the NIR laser, the resulting photothermal effect disrupt the electrostatic bonding between silicon shell and DNA and activate the DNA valves to the ‘on’ state leading to the release of the encapsulated cargo molecules. Such release of the cargo is stopped when the NIR laser is turned off. (B) Controlled release profile of a cargo under NIR laser irradiation (808 nm, 2.7 W·cm−2) for different on/off cycles. Reproduced with permission [159].
3.3. Tumor microenvironment sensitive nanocarriers
Cancer is one of the prime causes of death globally for both men and women [160]. The physiochemical properties of tumor microenvironment are different from that of the normal tissues [161,162]. For examples, the extracellular matrix (ECM) pH in tumor tissues is more acidic (6.5–6.9) than that of the blood pH (7.4) at 37°C [163]. Besides, other physiochemical properties such as temperature is higher, oxygen concentration is less (hypoxia) and many chemical and cytokines are known to over express in tumor cell microenvironment as compared to normal cells [164,165,166,167,168,172]. Therefore, developing stimuli-responsive nanocarriers that can respond to tumor cell microenvironment will be a promising basis for targeted delivery of therapeutics to combat cancers. A schematic representation of pH activation of nanocarrier system by tumor microenvironment has been described in Figure 12. Nanocarriers, which are known to be sensitive to endogenous stimuli such as pH, reactive oxygen species, glutathione etc. and exogenous stimuli such as light, ultrasound, heat etc., can be used as stimuli-responsive anticancer nanomaterials for tumor tissue specific delivery of therapeutics. Over the years, researchers have developed such tumor microenvironment responsive nanocarriers for targeted drug delivery applications [173,174,175,176,177]. For examples, He et al. developed dual stimuli nanocarriers derived from polyethyleneimine-nitroimidazole micelles (PEI-NI) and Ce6-linked hyaluronic acid for delivery of anticancer drugs with response to both hypoxia and photo-induction at the tumor microenvironment (TME). A schematic illustration of hypoxia-responsive drug delivery has been shown in Figure 13. Hydrophobic nitroimidazole, a known hypoxia-responsive electron acceptor was used in the nanocarriers to induce hypoxia mediated condition. Hydrophobic nitroimidazole converts into hydrophilic 2-aminoimidazole under hypoxia condition resulting in delivery of the loaded doxorubicin from the nanocarrier system to the TME [116,169,170,171]. Wang et. al. reported multifunctional nanocapsules-based smart drug delivery system with both light and tumor microenvironment triggered release of anticancer drugs. The authors prepared the nanocapsules using a PLGA-polymer matrix along with Fe/FeO core-shell nanocrystals, chemotherapy drug and photothermal agent. The nanocapsules were able to respond in the TME to produce reactive oxygen species resulting in hypoxia condition for triggering drug release [178]. Recently, Yang et al. designed and synthesized a negatively charged hierarchical tumor acidity-responsive magnetic nanobomb (HTAMN) made of polypeptide ligand, mPEG, superparamagnetic iron oxide nanoparticles for tumor acidity responsive theranostics applications [179]. These negatively charged HTAMNs converted into positively charged nanoparticles in the acidic tumor environment resulting in accumulation in the tumor cells. The photosensitizers of the HTAMNs are activated inside the more acidic intracellular component of the cancer cells allowing the image-based diagnosis of tumor cells. Such HTAMNs based systems are highly promising for the development of cancer theranostics.
Figure 12.
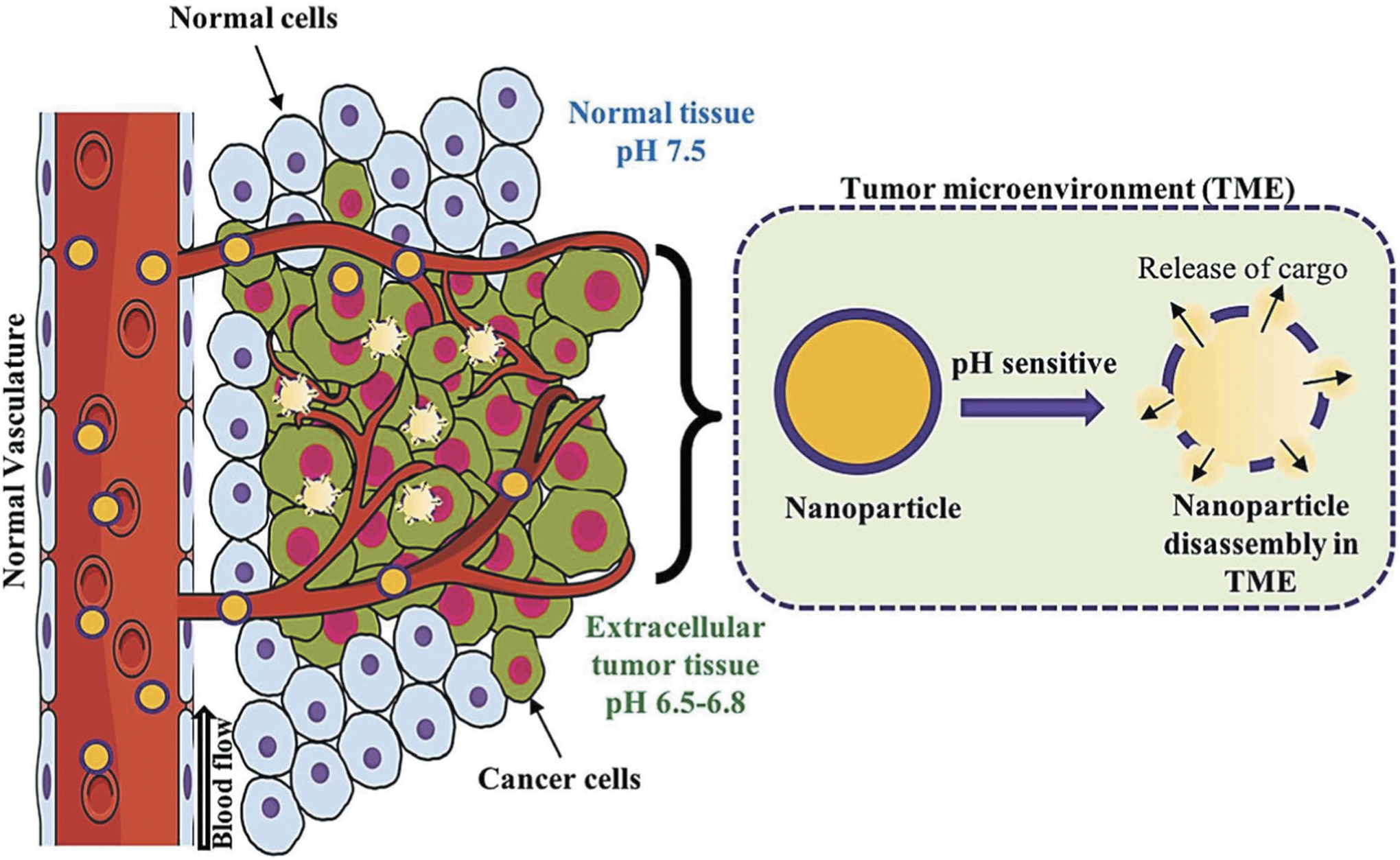
pH-triggered release of cargo inside the tumor microenvironment (TME). pH-sensitive nanocarriers are usually made off with acid responsive polymer or moieties. At the slightly acidic pH of tumor microenvironment, the acid sensitive moieties of such nanocarriers undergo protonation leading to the disruption of the nanostructure and the release of the encapsulated therapeutics. Reproduced with permission [116].
Figure 13.

Hypoxia-responsive drug delivery. (A) Dual-stimuli-sensitive nanoparticles prepared from polyethyleneimine-nitroimidazole micelles (PEI-NI) co-assembled with Ce6-linked hyaluronic acid (HC). Hypoxia-mediated activation was achieved by the incorporation of a hypoxia-responsive electron acceptor (nitroimidazole, NI) converted to hydrophilic 2-aminoimidazole under hypoxic conditions leading to the release of nanoparticle-loaded doxorubicin. (B) CD 44-mediated targeted delivery of doxorubicin in cancer stem cells and release of DOX in response to hypoxia generated by laser irradiation. CD44 is a cell surface receptor, which is overexpressed in most of the cancer stem cells. Hyaluronic acid (HA), a strong affinity ligand for CD 44, is usually decorated on the surface of nanoparticles for recognizing and binding to the cancer stem cell leading to the delivery of the drug to the specific tumor cell. Reproduced with permission [116].
3.4. Dual/multi-stimuli responsive nanocarriers
To make more specific and effective nanocarrier systems, recently researchers have designed multi-stimuli responsive nanocarriers by combining two or more stimuli-responsive components in a single nanocarrier system [180,181,182]. For example, Jia et al. designed a redox/enzyme responsive nanoparticle capable of releasing nitric oxide (NO) for anti-cancer therapy [183]. This nanoparticle was made of an organic-inorganic composite and encapsulated with a glutathione S-transferases π (GSTπ) – responsive NO releasing drug. In response to GSH at tumor site, these nanoparticles undergo biodegradation resulting in the release of the NO within the tumor [184]. In another case, Menon et al. reported a multi-stimuli responsive nanoparticles with both pH and temperature sensitive system, comprised of a copolymer of poly (N-isopropylacrylamide) and carboxymethylchitosan as the shell and PLGA as the core of the nanosystem. This nanoparticle was loaded with an image contrast agent such as superparamagnetic iron oxide (SPIO), which showed dual function of image contrast agent and inductor of temperature change by external magnetic field. This temperature alternation caused conformational change of the nanosystem resulting in drug release. The pH sensitive shell of the nanosystem also facilitated drug release at the acidic pH cancer environment [185]. Lee et al. prepared reactive oxygen species and light responsive nanoparticles encapsulated with an antioxidant agent bilirubin [186]. The structure of these bilirubin nanoparticles disrupted upon exposure to either reactive oxygen species or external laser light resulting in release of the encapsulated drugs. This bilirubin nanoparticle loaded with the anticancer drug doxorubicin inhibited tumor growth in a xenograft model upon irradiation with 650 nm laser light. Xiong et al. developed pH/redox sensitive micelles for the delivery of doxorubicin in cancer cells [187]. This nanosystem was made of an amphiphilic copolymer of poly(ε-caprolactone)-ss-poly(2-(dimethylamino) ethyl methacrylate) (PCL-SS-PDMAEMA). Doxorubicin was loaded in hydrophobic PCL region and gold nanoparticle was loaded in the hydrophilic PDMAEMA region of the micelle. At low pH in tumor site, the PDMAEMA part undergo protonation resulting in swelling of the outer shell leading to release of the drugs. Similarly, di-sulfide bond undergo cleavage in response to GHS, thereby degrading the micellular shell and releasing the drugs and imaging agent gold nanoparticles [12]. In a recent study, Yang et al. developed a redox/pH dual stimulus responsive nanosized polypeptide micelle system using a disulfide linker between methoxy poly(ethyleneglycol) and poly[2-(dibutylamino)ethylamine-L-glutamate] copolymers (mPEG-SS-PNLG) for delivery of anti-cancer drugs [188]. An anticancer drug doxorubicin (DOX) was loaded inside these mPEG-SS-PNLG micelles for the treatment of liver cancer. The authors observed a low rate of DOX release at pH 7.4, while the drug release rate was significantly higher at lower pH of 5.0 and a higher redox potential condition indicating a pH/redox dual responsive system. Drug loaded DOX-mPEG-SS-PNLG micelle system displayed improved in vivo activity against the HepG2 liver cancer model as compared to the free drug DOX only treatment. This micelle system showed a promising result for targeted delivery of anticancer drugs. Development of such multistimuli responsive nanocarriers systems showed promising results for more precision and targeted delivery of therapeutics at the disease site. A schematic representation of different stimuli-responsive multifunctional nanocarriers for targeted drug delivery applications has been shown in Figure 14.
Figure 14.
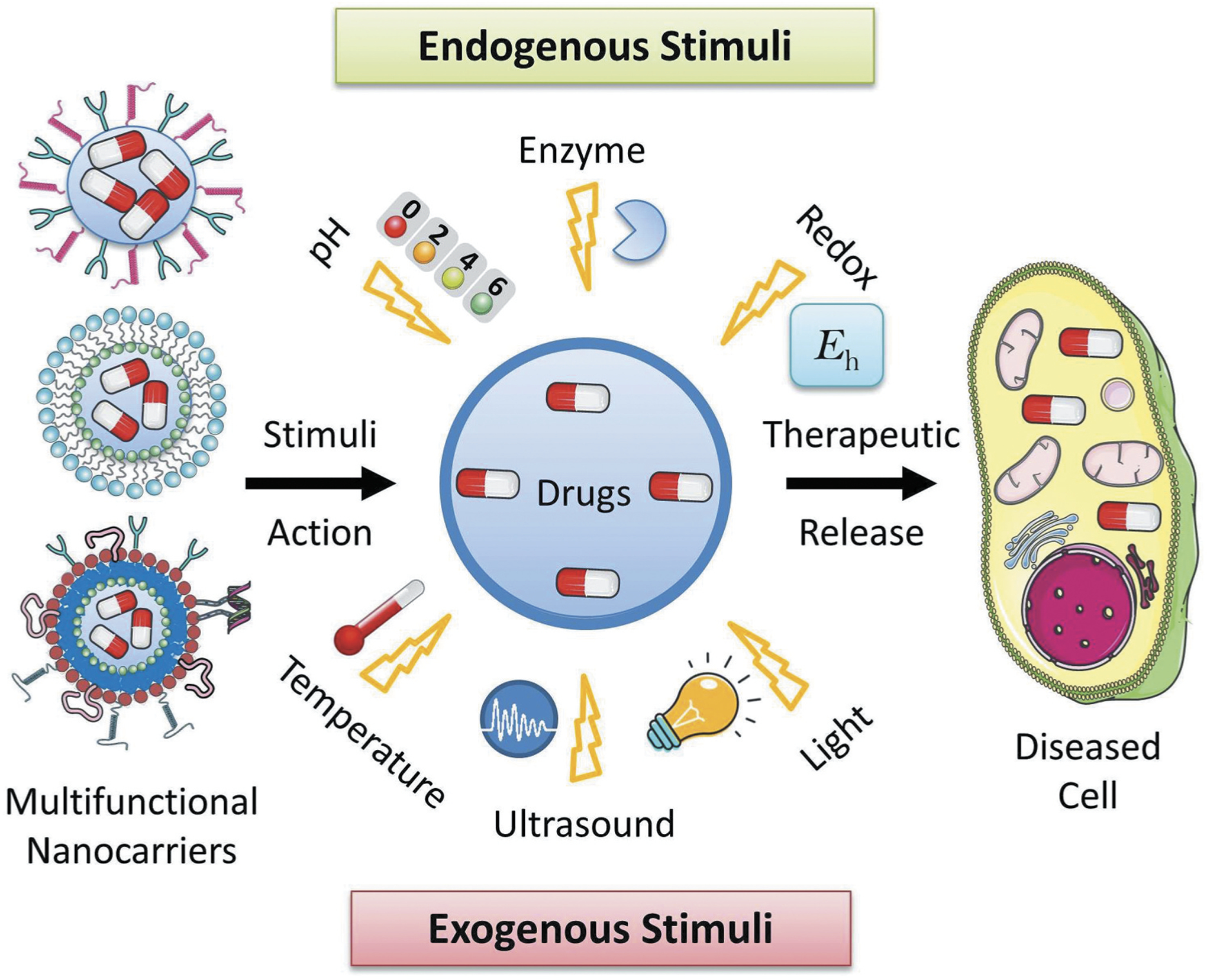
Schematic representation of different stimuli-responsive multifunctional nanocarriers for targeted drug delivery applications. Various stimuli-responsive multifunctional nanocarriers such as polymeric nanoparticles, liposomes, nanostructured lipid carriers, etc. can disassemble their nanostructure under various exogenous (temperature, ultrasound, light, etc.) and endogenous (pH, enzyme, redox potential, etc.) stimuli actions resulting in release of the encapsulated drugs at the targeted disease cells.
4. Clinical studies of nano-drug delivery systems
Because of their ability to protect the therapeutic agents from the physiological and enzymatic degradation as well as reduce the adverse drug side effects, nanocarrier-based systems have been evaluated in a number of clinical trials for the treatment of various diseases [189]. Most of the current nano-drug delivery systems in clinical trials included existing drugs which were encapsulated in the nanocarriers to improve their therapeutic benefit [75]. Liposome-based nanocarriers have been extensively studied in several clinical trials for the treatment of various cancers. For example, anticancer drug doxorubicin-based liposomal formulation namely Doxil® was one of the first tested nanoparticle-based drug in clinical trials for Karposi’s sarcoma, ovarian cancer and multiple myeloma [190]. ThermoDOX is another doxorubicin-based thermosensitive liposomal formulation which have been evaluated in clinical trial for the treatment of breast cancer (phase II) and hepatocellular carcinoma (phase III). ThermoDOX was stable at body temperature, but it dissociates and delivers the encapsulated drugs at the elevated temperature at the tumor microenvironment. Recently, ThermoDOX was also tested for other cancers by various research groups [191]. A list of nano-drug delivery systems in clinical trials is shown in Table 3 [192].
Table 3.
| Nanocarrier type | Name | Drug or gene | Therapeutic use |
|---|---|---|---|
| Liposomal nanodrug | Doxil Abelce Onivyde |
Doxorubicin Aamphotericin B Irinotecan |
Karposi’s sarcoma, ovarian cancer, multiple myeloma |
| ThermoDOX | Doxorubicin | Activity against Lewis lung carcinoma cells | |
| Pancreatic cancer | |||
| Liver cancer | |||
| Polymeric nanodrug | Adagen | Pegademase bovine | SCID |
| Eligard | Leuprolide acetate | Prostate cancer | |
| Peglntron | Pegylated IFN alpha-2b | Hepatitis C | |
| Micelle nanodrug | Estrasorb | Vasomotor symptoms in menopause |
As discussed in this review, the past two decades have witnessed significant progresses on the development of various nanosized carriers for targeted therapeutic delivery and disease diagnostic applications. However, the success rate of their translation into clinical application was very limited [193]. Detail toxicology studies, quality control assay and cost-effectiveness of the production are the key challenges for the successful translation developed nanomaterials into clinical application [193]. To achieve this goal, various studies such as bulk scale laboratory preparation of nanomedicines as per the industry standards, performing preclinical, conducting early clinical trials as well as cost-benefit analysis need to be carried out to expedite the translation of the nanotechnology based products into the clinical or commercial applications. Further, to improve the safety and efficacy of the product, there is a need to establish a standard preparation and characterization methods throughout the development of the nanomaterials. Over the years, researchers paid attention to establish strategies for improving efficacy and safety of the nanomaterials. Recently, researchers introduced ‘Safe-by-Design’ approach as a strategy for the development of safe and effective nanomaterials [194–197]. The purpose of ‘Safe-by-Design’ approach is to assess the safety of the nanomaterials from its early stage of innovation process, reduce adverse effects on human health by modifying the design of the nanomaterials as well as to maintain safety of the nanomaterials throughout their development stages. In traditional risk assessment method, safety is accessed when the product is fully developed, but in ‘Safe-by-Design’ concept, safety is considered throughout the all development stages of the product [198]. Designing safer nanomaterials has become a key focus in latest years. In a recent report, Borchard and coauthors have discussed ‘Safe-by-Design’ approach to enhance the safety and efficacy of chitosan-based protein drug delivery systems. Taking insulin as a model protein drug, the authors have discussed various challenges still need to be overcome for successful encapsulation and release of insulin from the chitosan nanoparticles [195,196]. In another recent report, researchers addressed various parameters that affect safety and efficacy of iron nanoparticles for the treatment of iron deficiency diseases. As an alternative to oral iron supplements, the authors have summarized the factors affecting intravenous iron-carbohydrate nanoparticles during their production, storage, and clinical studies. Such information is needed for the clinicians to establish the best clinical protocol for the product. Thus, successful implementation of ‘Safe-by-Design’ approach would be beneficial in developing safe nanomaterials from the beginning of the innovation.
5. Human studies of nanodrug delivery systems
Over the last two decades, various nanomedicines have been evaluated for therapeutic delivery application [199,200]. Because of small size, nanocarriers can easily cross various biological barriers in the human body [201] Lipid and polymer based nanocarriers (such as liposomes, dendrimers) have been extensively used for various medical applications and human studies [202]. The distribution of nanoparticles in the body after inhalation and oral delivery was examined in several animal and human studies [203,204] It was revealed that in contrast to low molecular weight drugs, physiology and anatomy of the organism, biodistribution, tissue penetration, phagocytosis, opsonization, and endocytosis of nanosized materials as well as specific properties have a major impact on their toxicity [205]. Also, larger size nanocarriers were found to be more toxic as compared to the smaller size nanocarrier of the same composition [206]. However, available safety and toxicity data of nanoparticles are highly inconsistent [207]. Therefore, more evaluation studies should be conducted and novel methods need to be developed for reducing the toxicity issues as well as safe use of the nano-sized medicines in human health.
6. Conclusions and outlook
In summary, here we report recent advances of multifunctional and stimuli-responsive nanocarriers in the development of site-specific delivery of therapeutics. Multifunctional and stimuli-responsive nanocarriers are able of increasing therapeutic efficacy and limiting the systemic adverse side effects usually associated with the conventional drug delivery methods. Such smart nanocarriers have shown a great promise in the improvement of disease diagnosis and therapy. Specially, stimuli-responsive nanocarriers showed possibility of controlled release of drugs at the target cells by acting as an active participant, rather than passive vehicle. However, there are still a few challenges associated with the multifunctional and stimuli-responsive nanocarriers. For examples, some endogenous stimuli are uncertain, such as variation of pH range in different diseased or normal organs and cells, presence of stimulus proteins in normal cells, etc. Though nanocarriers showed promising results against specific diseases, few drawbacks such as limited absorption, frequent injection of the nanomedicine for patients, etc. are associated with these nanocarrier-based delivery systems. Besides, such nanocarriers may undergo enzymatic degradation or physical entrapment on their way to the desired target site. Therefore, better understanding of the physiological environments of normal and disease cells as well as further development of nanocarrier-based drug systems are necessary for targeted therapeutic delivery applications. In summary, as discussed sequentially in this review, the progress from single functional to multifunctional nanocarriers has shown tremendous potential for targeted delivery of therapeutics. Further development of stimuli-based multifunctional feature would make an unprecedented control over the delivery and release of therapeutics at the disease site.
7. Expert opinion
Nanoscale-based delivery systems are intensively explored in the past few decades for the improvement of disease treatment and diagnosis. Initially developed for solving only few limitations of free non-bound drugs (e.g. low water solubility, poor pharmacokinetics and cellular availability, degradation in the gastrointestinal tract, blood stream or by hepatic metabolism, etc.), further expansion of the approach led to the extension of the diagnostic and/or therapeutic aims of nanotherapeutic drug delivery. As a result, many modern nanoparticle-based drugs substantially enhance a specific activity of parent drugs. Enhanced drug activity (especially of anticancer, antiviral, or other toxic drugs) in turn may cause severe adverse side effects on healthy organs, tissues, and cells. In order to limit such adverse side effects and further augment drug activity, different approaches for specific targeting of an entire delivery system to the site of action including a whole organ, definite parts of the organ, specific cell types or even intracellular organelles were developed. Many different targeting approaches have been exploited including passive or active targeting that employs specific properties of the nanocarriers or site of action (e.g. specific accumulation of nanoparticles with a certain size of molecular mass in deceased parts of the body, such as solid tumor), targeting to specific receptors or molecules expressed on the plasma membrane of cells under the attack (e.g. receptors, antigens, proteins, lipids, etc.) targeting to specific conditions in the site of action or neighboring tissues (e.g. pH, redox potential, specific enzymes, etc.) and many other relatively simple or complex approaches. Exploration of specific environment properties inside the targeted cells, tissues or entire organs led to the development of stimuli-responsive nanocarriers. Such nanocarriers mainly provide for a release of active components of a delivery system only under specific conditions (e.g. low pH in tumor or hypoxic microenvironment) or after the action of specific enzymes (e.g. oxidoreductases, transferases, hydrolases, etc.), which can destroy the bond between a carrier and active components or change a configuration of the nanoparticle triggering the drug release. In addition to endogenous conditions, various exogenous stimuli are used for targeting nanoparticles and release their components. These exogenous stimuli include but not limited to temperature, ultrasound, light, etc. In most cases one specific drug or simple nanocarrier-based system normally has one (or few) definite type(s) of action and solves only a limited number of tasks. By combining different active components in one complex, a simple pharmaceutical formulation with a limited number of functions can be converted to a complex multifunctional drug delivery system with ability to fulfill simultaneously several tasks. For instance, for the effective cancer treatment, a cell death should be induced in cancer cells alone with the suppression of cellular defensive mechanisms. Moreover, both these actions should be applied as an Ehrlich’s ‘magic bullet’ only to cancer cells leaving healthy cells intact. Based on such concept, we proposed several types of targeted multifunctional nanotechnology-based anticancer proapoptotic drug delivery systems which generally have four major components: a carrier (a nanoparticle which combines and holds all components together), targeting ligand (peptide, antibody or other class of agents that direct an entire system specifically to tumor, cancer cells or even intracellular organelles), cell death inducer (anticancer drug), suppressor(s) of multidrug resistance (antisense oligonucleotides, siRNAs, etc.) and suppressors of antiapoptotic cellular defense. In our opinion, this is a minimal configuration of anticancer delivery system that can provide an exceptionally high efficacy of cancer treatment, prevention and fighting over metastases and limiting severe adverse side effects upon healthy cells, tissues, and organs. Certainly, such system can be further enhanced to provide a stimuli-triggered release of the drugs, combine diagnostic features (such as imaging of tumor and metastases), improve pharmacokinetics and drug release, etc. However, the designing, testing, and especially production in large quality require tremendous efforts and financial expenses. These obstacles substantially limit interest to such complex multifunctional systems from industry and pharmaceutical companies which instead still prefer to develop small molecular weight anticancer drugs with relatively low efficacy and severe adverse side effects. Nevertheless, we hope that advances in research, development and formulation finally will result in the production of such complex multifunctional systems in large scale and implementation into clinical practice
Article highlights.
Various nano-based materials and methods have been developed for disease diagnosis and treatment applications.
The use of nanocarriers for the drug delivery helps to solve most limitations of traditional nonbound drugs including but not limited to poor water solubility, low cellular penetration, unfavorable pharmacokinetics and body distribution, uncontrolled drug release, degradation in the process of journey to the targeted site, adverse side effects, etc.
In general, the use of nanoparticles enhances the specific activity of drugs, diagnostic, and imaging agents.
In order to avoid severe adverse side effects upon healthy organs, tissue, and cells and further enhance the specific effects of active components, a targeting to the specific site(s) of action is employed.
With the intention of providing a controlled drug release and additional targeting to the specific conditions in the site of action, stimuli-responsive nanocarriers were developed.
Although in the early stage, nanocarrier-based drugs were developed to fulfill predominately one task, modern nanoparticle drugs are multifunctional and often contain several distinct components.
This box summarizes key points contained in the article.
Acknowledgments
Part of figures was created using Servier Medical Art images, which are licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com).
Funding
This work has been supported in part by National Institutes of Health (R01 CA238871, R01 CA209818, R01 HL118312).
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Mainardes RM, Silva LP. Drug delivery systems: past, present, and future. Curr Drug Targets. 2004. July;5(5):449–455. [DOI] [PubMed] [Google Scholar]
- 2.Robinson DH, Mauger JW. Drug delivery systems. Am J Health-Syst Pharm. 1991;48(10_suppl):S14–S23. [PubMed] [Google Scholar]
- 3.Zang X, Lee JB, Deshpande K, et al. Prevention of paclitaxel-induced neuropathy by formulation approach. J Control Release. 2019. June;10(303):109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majumder J, Taratula O, Minko T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv Drug Deliv Rev. 2019. April;144:57–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain V, Jain S, Mahajan SC. Nanomedicines based drug delivery systems for anti-cancer targeting and treatment. Curr Drug Deliv. 2015;12(2):177–191. [DOI] [PubMed] [Google Scholar]
- 6.Lombardo D, Kiselev MA, Caccamo MT. Smart nanoparticles for drug delivery application: development of versatile nanocarrier platforms in biotechnology and nanomedicine. J Nanomater. 2019;2019:1–26. [Google Scholar]
- 7.Tran S, DeGiovanni PJ, Piel B, et al. Cancer nanomedicine: a review of recent success in drug delivery. Clin Transl Med. 2017. December 11;6 (1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taratula O, Garbuzenko OB, Chen AM, et al. Innovative strategy for treatment of lung cancer: targeted nanotechnology-based inhalation co-delivery of anticancer drugs and siRNA. J Drug Target. 2011. December;19(10):900–914. [DOI] [PubMed] [Google Scholar]
- 9.Taratula O, Garbuzenko O, Savla R, et al. Multifunctional nanomedicine platform for cancer specific delivery of siRNA by superparamagnetic iron oxide nanoparticles-dendrimer complexes. Curr Drug Deliv. 2011. January;8(1):59–69. [DOI] [PubMed] [Google Scholar]
- 10.Chen AM, Taratula O, Wei D, et al. Labile catalytic packaging of DNA/siRNA: control of gold nanoparticles “out” of DNA/siRNA complexes. ACS Nano. 2010. July 27;4(7):3679–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zylberberg C, Matosevic S. Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016. November;23(9):3319–3329. [DOI] [PubMed] [Google Scholar]
- 12.Minko T, Pakunlu RI, Wang Y, et al. New generation of liposomal drugs for cancer. Anticancer Agents Med Chem. 2006. November; 6 (6):537–552.] [DOI] [PubMed] [Google Scholar]
- 13.Mendes LP, Sarisozen C, Luther E, et al. Surface-engineered polyethyleneimine-modified liposomes as novel carrier of siRNA and chemotherapeutics for combination treatment of drug-resistant cancers. Drug Deliv. 2019. December;26(1):443–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almousallam M, Moia C, Zhu H. Development of nanostructured lipid carrier for dacarbazine delivery. Int Nano Lett. 2015;5 (4):241–248. [Google Scholar]
- 15.Gomes MJ, Martins S, Ferreira D, et al. Lipid nanoparticles for topical and transdermal application for alopecia treatment: development, physicochemical characterization, and in vitro release and penetration studies. Int J Nanomedicine. 2014;9:1231–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taratula O, Garbuzenko OB, Kirkpatrick P, et al. Surface-engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery. J Control Release. 2009. December 16;140(3):284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taratula O, Kuzmov A, Shah M, et al. Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J Control Release. 2013. November 10;171(3):349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garbuzenko OB, Winkler J, Tomassone MS, et al. Biodegradable Janus nanoparticles for local pulmonary delivery of hydrophilic and hydrophobic molecules to the lungs. Langmuir. 2014. November 4;30(43):12941–12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X, Li H, Lee RJ. Targeted drug delivery via folate receptors. Expert Opin Drug Deliv. 2008. March;5(3):309–319. [DOI] [PubMed] [Google Scholar]
- 20.Alley SC, Okeley NM, Senter PD. Antibody-drug conjugates: targeted drug delivery for cancer. Curr Opin Chem Biol. 2010. August;14 (4):529–537. [DOI] [PubMed] [Google Scholar]
- 21.Alibakhshi A, Kahaki AF, Ahangarzadeh S, et al. Targeted cancer therapy through antibody fragments-decorated nanomedicines. J Control Release. 2017. December;28(268):323–334. [DOI] [PubMed] [Google Scholar]
- 22.Chu DT, Bac ND, Nguyen KH, et al. An update on Anti-CD137 antibodies in immunotherapies for cancer. Int J Mol Sci. 2019. April 12;20(8):1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandna P, Saad M, Wang Y, et al. Targeted proapoptotic anticancer drug delivery system. Mol Pharm. 2007. Sep-Oct;4(5):668–678. [DOI] [PubMed] [Google Scholar]
- 24.Dharap SS, Qiu B, Williams GC, et al. Molecular targeting of drug delivery systems to ovarian cancer by BH3 and LHRH peptides. J Control Release. 2003. August 28;91(1–2):61–73. [DOI] [PubMed] [Google Scholar]
- 25.Dharap SS, Wang Y, Chandna P, et al. Tumor-specific targeting of an anticancer drug delivery system by LHRH peptide. Proc Natl Acad Sci U S A. 2005. September 6;102(36):12962–12967. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An excellent paper that show advantages of LHRH-targeted delivery to cancer cells in order to enhance efficacy ot treatment and limit adverse side effects.
- 26.Garbuzenko OB, Kuzmov A, Taratula O, et al. , Strategy to enhance lung cancer treatment by five essential elements: inhalation delivery, nanotechnology, tumor-receptor targeting, chemo-and gene therapy. Theranostics. 2019;9(26): 8362–8376. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An extreamly important manuscript that demonstrates advantages of complex multifunctional targeted delivery systems for treatment of cancer.
- 27.Saad M, Garbuzenko OB, Ber E, et al. Receptor targeted polymers, dendrimers, liposomes: which nanocarrier is the most efficient for tumor-specific treatment and imaging? J Control Release. 2008. September 10;130(2):107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Very important investigation that demonstrates that “all nanocarriers are created equal“ if they are used with the same optimal efficient targteting agent; it provides a new avenue for design of nanocarriers for cancer treatment and imaging.
- 28.Savla R, Garbuzenko OB, Chen S, et al. Tumor-targeted responsive nanoparticle-based systems for magnetic resonance imaging and therapy. Pharm Res. 2014. December;31(12):3487–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Taratula O, Taratula O, et al. LHRH-targeted drug delivery systems for cancer therapy. Mini Rev Med Chem. 2017;17(3):258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onaca O, Enea R, Hughes DW, et al. Stimuli-responsive polymer-somes as nanocarriers for drug and gene delivery. Macromol Biosci. 2009. February 11;9(2):129–139. [DOI] [PubMed] [Google Scholar]
- 31.Rao NV, Ko H, Lee J, et al. Recent progress and advances in stimuli-responsive polymers for cancer therapy. Front Bioeng Biotechnol. 2018;6:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakayama M, Akimoto J, Okano T. Polymeric micelles with stimuli-triggering systems for advanced cancer drug targeting. J Drug Target. 2014. August;22(7):584–599. [DOI] [PubMed] [Google Scholar]
- 33.Hu YW, Du YZ, Liu N, et al. Selective redox-responsive drug release in tumor cells mediated by chitosan based glycolipid-like nanocarrier. J Control Release. 2015. May;28(206):91–100. [DOI] [PubMed] [Google Scholar]
- 34.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013. November;12(11):991–1003. [DOI] [PubMed] [Google Scholar]; • An excellent review that summarizes advances in the design of nanoscale stimuli-responsive systems.
- 35.Alvarez-Lorenzo C, Garcia-Gonzalez CA, Bucio E, et al. Stimuli-responsive polymers for antimicrobial therapy: drug targeting, contact-killing surfaces and competitive release. Expert Opin Drug Deliv. 2016. August;13(8):1109–1119. [DOI] [PubMed] [Google Scholar]; • Very important expert opinion that highlights macrophage-, bacteria-, and/or biofilm-targeted delivery depending on changes in enzymes, pH, and pO2 and recognize microorganisms for triggered (competitive/affinity-driven) drugrelease.
- 36.Do HD, Couillaud BM, Doan BT, et al. Advances on non-invasive physically triggered nucleic acid delivery from nanocarriers. Adv Drug Deliv Rev. 2019. 1;January(138):3–17. [DOI] [PubMed] [Google Scholar]
- 37.Fouladi F, Steffen KJ, Mallik S. Enzyme-responsive liposomes for the delivery of anticancer drugs. Bioconjug Chem. 2017. April 19;28 (4):857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganta S, Devalapally H, Shahiwala A, et al. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release. 2008. March 20;126(3):187–204. [DOI] [PubMed] [Google Scholar]
- 39.Hatakeyama H Recent advances in endogenous and exogenous stimuli-responsive nanocarriers for drug delivery and therapeutics. Chem Pharm Bull (Tokyo). 2017;65(7):612–617. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Xiao K, Zhu W, et al. Stimuli-responsive cross-linked micelles for on-demand drug delivery against cancers. Adv Drug Deliv Rev. 2014. February;66:58–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tayo LL. Stimuli-responsive nanocarriers for intracellular delivery. Biophys Rev. 2017. December;9(6):931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raza A, Rasheed T, Nabeel F, et al. Endogenous and exogenous stimuli-responsive drug delivery systems for programmed site-specific release. Molecules. 2019. March 21;24:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lokina S, Stephen A, Kaviyarasan V, et al. Cytotoxicity and antimicrobial activities of green synthesized silver nanoparticles. Eur J Med Chem. 2014. April;9(76):256–263. [DOI] [PubMed] [Google Scholar]
- 44.Wolfbeis OS. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem Soc Rev. 2015. July 21;44(14):4743–4768. [DOI] [PubMed] [Google Scholar]
- 45.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. [DOI] [PubMed] [Google Scholar]
- 46.Khandare JJ, Chandna P, Wang Y, et al. Novel polymeric prodrug with multivalent components for cancer therapy. J Pharmacol Exp Ther. 2006. June;317(3):929–937. [DOI] [PubMed] [Google Scholar]
- 47.Shen S, Wu Y, Liu Y, et al. High drug-loading nanomedicines: progress, current status, and prospects. Int J Nanomedicine. 2017;12:4085–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh R, Lillard JW Jr. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009. June;86(3):215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nie S, Xing Y, Kim GJ, et al. Nanotechnology applications in cancer. Annu Rev Biomed Eng. 2007;9:257–288. [DOI] [PubMed] [Google Scholar]
- 50.Sawant RR, Torchilin VP. Multifunctional nanocarriers and intracellular drug delivery. Curr Opin Solid State Mater Sci. 2012;16 (6):269–275. [Google Scholar]
- 51.Gao X, Cui Y, Levenson RM, et al. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004. August;22(8):969–976. [DOI] [PubMed] [Google Scholar]
- 52.Kim J, Lee JE, Lee SH, et al. Designed fabrication of a multifunctional polymer nanomedical platform for simultaneous cancer- targeted imaging and magnetically guided drug delivery. Adv Mater. 2008;20(3):478–483. [Google Scholar]
- 53.Yang J, Lee CH, Ko HJ, et al. Multifunctional magneto-polymeric nanohybrids for targeted detection and synergistic therapeutic effects on breast cancer. Angew Chem Int Ed Engl. 2007;46 (46):8836–8839. [DOI] [PubMed] [Google Scholar]
- 54.Liong M, Lu J, Kovochich M, et al. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008. May;2(5):889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo Y, Shi D, Cho H, et al. In vivo imaging and drug storage by quantum-dot-conjugated carbon nanotubes. Adv Funct Mater. 2008;18(17):2489–2497. [Google Scholar]
- 56.Li JL, Day D, Gu M. Ultra-low energy threshold for cancer photothermal therapy using transferrin-conjugated gold nanorods. Adv Mater. 2008;20(20):3866–3871. [Google Scholar]
- 57.Trapani G, Denora N, Trapani A, et al. Recent advances in ligand targeted therapy. J Drug Target. 2012. January;20(1):1–22. [DOI] [PubMed] [Google Scholar]
- 58.Bhaskar S, Tian F, Stoeger T, et al. Multifunctional Nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: perspectives on tracking and neuroimaging. Part Fibre Toxicol. 2010;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chandna P, Khandare JJ, Ber E, et al. Multifunctional tumor-targeted polymer-peptide-drug delivery system for treatment of primary and metastatic cancers. Pharm Res. 2010. November;27 (11):2296–2306. [DOI] [PubMed] [Google Scholar]
- 60.Zhang M, Garbuzenko OB, Reuhl KR, et al. Two-in-one: combined targeted chemo and gene therapy for tumor suppression and prevention of metastases. Nanomedicine (Lond). 2012. February;7(2):185–197. [DOI] [PubMed] [Google Scholar]
- 61.Chen AM, Zhang M, Wei D, et al. Co-delivery of doxorubicin and Bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small. 2009. December;5(23):2673–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jaimes-Aguirre L, Gibbens-Bandala BV, Morales-Avila E, et al. Polymer-based drug delivery systems, development and pre-clinical status. Curr Pharm Des. 2016;22(19):2886–2903. [DOI] [PubMed] [Google Scholar]
- 63.Mendes LP, Pan J, Torchilin VP. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules. 2017. August 23;22:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007. January;24(1):1–16. [DOI] [PubMed] [Google Scholar]
- 65.Sutradhar KB, Amin ML. Nanoemulsions: increasing possibilities in drug delivery. Eur J Nanomed. 2013;5(2):97–110. [Google Scholar]
- 66.Wang Z, Qiao R, Tang N, et al. Active targeting theranostic iron oxide nanoparticles for MRI and magnetic resonance-guided focused ultrasound ablation of lung cancer. Biomaterials. 2017. May;127:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mohan A, Narayanan S, Sethuraman S, et al. Novel resveratrol and 5-fluorouracil coencapsulated in PEGylated nanoliposomes improve chemotherapeutic efficacy of combination against head and neck squamous cell carcinoma. Biomed Res Int. 2014;2014:424239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kotla NG, Chandrasekar B, Rooney P, et al. Biomimetic lipid-based nanosystems for enhanced dermal delivery of drugs and bioactive agents. ACS Biomater Sci Eng. 2017;3(7):1262–1272. [DOI] [PubMed] [Google Scholar]
- 70.Sercombe L, Veerati T, Moheimani F, et al. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lamichhane N, Udayakumar TS, D’Souza WD, et al. Liposomes: clinical applications and potential for image-guided drug delivery. Molecules. 2018. January 30;23(2):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deshpande PP, Biswas S, Torchilin VP. Current trends in the use of liposomes for tumor targeting. Nanomedicine. 2013. September;8 (9):1509–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An excellent and important manuscript that highlights advances in liposome research in tumor targeting and pH-triggered drug delivery.
- 73.Chang HI, Yeh MK. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int J Nanomedicine. 2012;7:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bulbake U, Doppalapudi S, Kommineni N, et al. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017. March 27;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bobo D, Robinson KJ, Islam J, et al. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016. October;33(10):2373–2387. [DOI] [PubMed] [Google Scholar]
- 76.Li SD, Chono S, Huang L. Efficient gene silencing in metastatic tumor by siRNA formulated in surface-modified nanoparticles. J Control Release. 2008. February 18;126(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Betigeri S, Zhang M, Garbuzenko O, et al. Non-viral systemic delivery of siRNA or antisense oligonucleotides targeted to Jun N-terminal kinase 1 prevents cellular hypoxic damage. Drug Deliv Transl Res. 2011. February;1(1):13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pakunlu RI, Wang Y, Tsao W, et al. Enhancement of the efficacy of chemotherapy for lung cancer by simultaneous suppression of multidrug resistance and antiapoptotic cellular defense: novel multicomponent delivery system. Cancer Res. 2004. September 1;64 (17):6214–6224. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y, Saad M, Pakunlu RI, et al. Nonviral nanoscale-based delivery of antisense oligonucleotides targeted to hypoxia-inducible factor 1 alpha enhances the efficacy of chemotherapy in drug-resistant tumor. Clin Cancer Res. 2008. June 1;14 (11):3607–3616. [DOI] [PubMed] [Google Scholar]
- 80.Peddada LY, Garbuzenko OB, Devore DI, et al. Delivery of antisense oligonucleotides using poly(alkylene oxide)-poly(propylacrylic acid) graft copolymers in conjunction with cationic liposomes. J Control Release. 2014. November;28(194):103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kang MH, Yoo HJ, Kwon YH, et al. Design of multifunctional liposomal nanocarriers for folate receptor-specific intracellular drug delivery. Mol Pharm. 2015. December 7;12(12):4200–4213. [DOI] [PubMed] [Google Scholar]
- 82.Garbuzenko OB, Saad M, Pozharov VP, et al. Inhibition of lung tumor growth by complex pulmonary delivery of drugs with oligonucleotides as suppressors of cellular resistance. Proc Natl Acad Sci U S A. 2010. June 8;107(23):10737–10742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shah V, Taratula O, Garbuzenko OB, et al. Genotoxicity of different nanocarriers: possible modifications for the delivery of nucleic acids. Curr Drug Discov Technol. 2013. March;10(1):8–15. [PMC free article] [PubMed] [Google Scholar]
- 84.Uner M, Yener G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int J Nanomedicine. 2007;2(3):289–300. [PMC free article] [PubMed] [Google Scholar]
- 85.Khosa A, Reddi S, Saha RN. Nanostructured lipid carriers for site-specific drug delivery. Biomed Pharmacother. 2018. July;103:598–613. [DOI] [PubMed] [Google Scholar]
- 86.Battaglia L, Serpe L, Foglietta F, et al. Application of lipid nanoparticles to ocular drug delivery. Expert Opin Drug Deliv. 2016. December;13 (12):1743–1757. [DOI] [PubMed] [Google Scholar]
- 87.Garbuzenko OB, Kbah N, Kuzmov A, et al. Inhalation treatment of cystic fibrosis with lumacaftor and ivacaftor co-delivered by nanostructured lipid carriers. J Control Release. 2019. February;28 (296):225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuzmov A, Minko T. Nanotechnology approaches for inhalation treatment of lung diseases. J Control Release. 2015. December;10 (219):500–518. [DOI] [PubMed] [Google Scholar]
- 89.Emami J, Rezazadeh M, Sadeghi H, et al. Development and optimization of transferrin-conjugated nanostructured lipid carriers for brain delivery of paclitaxel using Box-Behnken design. Pharm Dev Technol. 2017. May;22(3):370–382. [DOI] [PubMed] [Google Scholar]
- 90.Jiang S, Eltoukhy AA, Love KT, et al. Lipidoid-coated iron oxide nanoparticles for efficient DNA and siRNA delivery. Nano Lett. 2013. March 13;13(3):1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marcu A, Pop S, Dumitrache F, et al. Magnetic iron oxide nanoparticles as drug delivery system in breast cancer. Appl Surf Sci. 2013;281:60–65. [Google Scholar]
- 92.Stephen ZR, Dayringer CJ, Lim JJ, et al. Approach to rapid synthesis and functionalization of iron oxide nanoparticles for high gene transfection. ACS Appl Mater Interfaces. 2016. March;8(10):6320–6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jin L, Wang Q, Chen J, et al. Efficient delivery of therapeutic siRNA by Fe3O4 magnetic nanoparticles into oral cancer cells. Pharmaceutics. 2019. November 17;11(11):615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang YX, Idee JM. A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant Imaging Med Surg. 2017. February;7(1):88–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng W, Nie J, Xu L, et al. pH-sensitive delivery vehicle based on folic acid-conjugated polydopamine-modified mesoporous silica nanoparticles for targeted cancer therapy. ACS Appl Mater Interfaces. 2017. June 7;9(22):18462–18473. [DOI] [PubMed] [Google Scholar]
- 96.Hom C, Lu J, Liong M, et al. Mesoporous silica nanoparticles facilitate delivery of siRNA to shutdown signaling pathways in mammalian cells. Small. 2010. 6;6;June(11):1185–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang Y, Wan J, Niu Y, et al. Structure-dependent and glutathione-responsive biodegradable dendritic mesoporous organosilica nanoparticles for safe protein delivery. Chem Mater. 2016;28(24):9008–9016. [Google Scholar]
- 98.Ray H, Baughman AAZ, de Heer WA. Carbon nanotubes—the route toward applications. Science. 2002;297:787–792. [DOI] [PubMed] [Google Scholar]
- 99.Wu W, Wieckowski S, Pastorin G, et al. Targeted delivery of amphotericin B to cells by using functionalized carbon nanotubes. Angew Chem Int Ed Engl. 2005. October 7;44(39):6358–6362. [DOI] [PubMed] [Google Scholar]
- 100.Feazell RP, N-R N, Dai H, et al. Soluble single-walled carbon nanotubes as longboat delivery systems for Platinum(IV) anticancer drug design. J Am Chem Soc. 2007;129:8438–8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dhar S, Liu Z, Thomale J, et al. Targeted single-wall carbon nanotube-mediated Pt(IV) prodrug delivery using folate as a homing device. J Am Chem Soc. 2008;130:11467–11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hilder TA, Hill JM. Probability of encapsulation of paclitaxel and doxorubicin into carbon nanotubes. Micro Nano Lett. 2008;3:2. [Google Scholar]
- 103.Pantarotto D, Singh R, McCarthy D, et al. Functionalized carbon nanotubes for plasmid DNA gene delivery. Angew Chem Int Ed Engl. 2004. October 4;43(39):5242–5246. [DOI] [PubMed] [Google Scholar]
- 104.Ravi Singh DP, McCarthy D, Chaloin O, et al. Binding and condensation of plasmid DNA onto functionalized carbon nanotubes: toward the construction of nanotube-based gene delivery vectors. J Am Chem Soc. 2005;127:4388–4396. [DOI] [PubMed] [Google Scholar]
- 105.Liu Z, Chen K, Davis C, et al. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008. August 15;68 (16):6652–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheng Q, Blais MO, Harris GM, et al. PLGA-carbon nanotube conjugates for intercellular delivery of caspase-3 into osteosarcoma cells. PLoS One. 2013;8(12):e81947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mehra NK, Jain NK. One platform comparison of estrone and folic acid anchored surface engineered MWCNTs for doxorubicin delivery. Mol Pharm. 2015. February 2;12(2):630–643. [DOI] [PubMed] [Google Scholar]
- 108.Valizadeh A, Mikaeili H, Samiei M, et al. Quantum dots: synthesis, bioapplications, and toxicity. Nanoscale Res Lett. 2012;7(480):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ozkan M Quantum dots and other nanoparticles: what can they offer to drug discovery? Drug Discov Today. 2004;9(24):1065–1071. [DOI] [PubMed] [Google Scholar]
- 110.Chen H, Wang Z, Zong S, et al. A graphene quantum dot-based FRET system for nuclear-targeted and real-time monitoring of drug delivery. Nanoscale. 2015. October 7;7(37):15477–15486. [DOI] [PubMed] [Google Scholar]
- 111.Olerile LD, Liu Y, Zhang B, et al. Near-infrared mediated quantum dots and paclitaxel co-loaded nanostructured lipid carriers for cancer theragnostic. Colloids Surf B Biointerfaces. 2017. February;1(150):121–130. [DOI] [PubMed] [Google Scholar]
- 112.Mahajan SD, Roy I, Xu G, et al. Enhancing the delivery of anti retroviral drug “Saquinavir” across the blood brain barrier using nanoparticles. Curr HIV Res. 2010;8(5):396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Savla R, Taratula O, Garbuzenko O, et al. Tumor targeted quantum dot-mucin 1 aptamer-doxorubicin conjugate for imaging and treatment of cancer. J Control Release. 2011. July 15;153(1):16–22. [DOI] [PubMed] [Google Scholar]
- 114.An X, Zhu A, Luo H, et al. Rational design of multi-stimuli-responsive nanoparticles for precise cancer therapy. ACS Nano. 2016. June 28;10(6):5947–5958. [DOI] [PubMed] [Google Scholar]
- 115.Castillo B, Bromberg L, Lopez X, et al. Intracellular delivery of siRNA by polycationic superparamagnetic nanoparticles. J Drug Deliv. 2012;2012:218940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Uthaman S, Huh KM, Park IK. Tumor microenvironment-responsive nanoparticles for cancer theragnostic applications. Biomater Res. 2018;22:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu M, Du H, Zhang W, et al. Internal stimuli-responsive nanocarriers for drug delivery: design strategies and applications. Mater Sci Eng C Mater Biol Appl. 2017. February;1(71):1267–1280. [DOI] [PubMed] [Google Scholar]
- 118.Wells J, Sen A, Hui SW. Localized delivery to CT-26 tumors in mice using thermosensitive liposomes. Int J Pharm. 2003;261(1–2):105–114. [DOI] [PubMed] [Google Scholar]
- 119.Na K, Lee KH, Lee DH, et al. Biodegradable thermo-sensitive nanoparticles from poly(L-lactic acid)/poly(ethylene glycol) alternating multi-block copolymer for potential anti-cancer drug carrier. Eur J Pharm Sci. 2006. February;27(2–3):115–122. [DOI] [PubMed] [Google Scholar]
- 120.Ishida T, Kirchmeier MJ, Moase EH, et al. Targeted delivery and triggered release of liposomal doxorubicin enhances cytotoxicity against human B lymphoma cells. Biochim Biophys Acta. 2001;1515 (2):144–158. [DOI] [PubMed] [Google Scholar]
- 121.Lee E Polymeric micelle for tumor pH and folate-mediated targeting. J Control Release. 2003;91(1–2):103–113. [DOI] [PubMed] [Google Scholar]
- 122.Kommareddy S, Amiji M. Poly(Ethylene Glycol)-modified thiolated gelatin nanoparticles for glutathione-responsive intracellular DNA delivery. Nanomedicine. 2007;3:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Balakirev M, Schoehn G, Chroboczek J. Lipoic acid-derived amphiphiles for redox-controlled DNA delivery. Chem Biol. 2000;7:813–819. [DOI] [PubMed] [Google Scholar]
- 124.Yang Y, Aw J, Chen K, et al. Enzyme-responsive multifunctional magnetic nanoparticles for tumor intracellular drug delivery and imaging. Chem Asian J. 2011. 6;6; June(6): 1381–1389. [DOI] [PubMed] [Google Scholar]
- 125.Baier G, Cavallaro A, Vasilev K, et al. Enzyme responsive hyaluronic acid nanocapsules containing polyhexanide and their exposure to bacteria to prevent infection. Biomacromolecules. 2013. April 8;14 (4):1103–1112. [DOI] [PubMed] [Google Scholar]
- 126.Zhu L, Kate P, Torchilin VP. Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS Nano. 2012. April 24;6(4):3491–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang X, Zhao M, Cao N, et al. Construction of a tumor microenvironment pH-responsive cleavable PEGylated hyaluronic acid nano-drug delivery system for colorectal cancer treatment. Biomater Sci. 2020. March 31;8(7):1885–1896. [DOI] [PubMed] [Google Scholar]
- 128.Nikravan G, Haddadi-Asl V, Salami-Kalajahi M. Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles. e-Polymers. 2019;19:203–214. [Google Scholar]
- 129.Cook JA, Pass HI, lype SN, et al. Cellular glutathione and thiol measurements from surgically resected human lung tumor and normal lung tissue. Cancer Res. 1991;51:4287–4294. [PubMed] [Google Scholar]
- 130.Cho H, Bae J, Garripelli VK, et al. Redox-sensitive polymeric nanoparticles for drug delivery. Chem Commun (Camb). 2012. June 18;48 (48):6043–6045. [DOI] [PubMed] [Google Scholar]
- 131.Ong W, Yang Y, Cruciano AC, et al. Redox-triggered contents release from liposomes. J Am Chem Soc. 2008;130:14739–14744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ryu J-H, Chacko RT, Jiwpanich S, et al. Self-cross-linked polymer nanogels: a versatile nanoscopic drug delivery platform. J Am Chem Soc. 2010;132:17227–17235. [DOI] [PubMed] [Google Scholar]
- 133.Yang HY, Jang MS, Li Y, et al. Multifunctional and redox-responsive self-assembled magnetic nanovectors for protein delivery and dual-modal imaging. ACS Appl Mater Interfaces. 2017. June 7;9 (22):19184–19192. [DOI] [PubMed] [Google Scholar]
- 134.Harris TJ, von Maltzahn G, Lord ME, et al. Protease-triggered unveiling of bioactive nanoparticles. Small. 2008. September;4(9):1307–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Terada T, Iwai M, Kawakami S, et al. Novel PEG-matrix metalloproteinase-2 cleavable peptide-lipid containing galactosylated liposomes for hepatocellular carcinoma-selective targeting. J Control Release. 2006. April 10;111(3):333–342. [DOI] [PubMed] [Google Scholar]
- 136.Banerjee J, Hanson AJ, Gadam B, et al. Release of liposomal contents by cell-secreted matrix metalloproteinase-9. Bioconjug Chem. 2009. July;20(7):1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Singh N, Karambelkar A, Gu L, et al. Bioresponsive mesoporous silica nanoparticles for triggered drug release. J Am Chem Soc. 2011. December 14;133(49):19582–19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hatakeyama H, Akita H, Ito E, et al. Systemic delivery of siRNA to tumors using a lipid nanoparticle containing a tumor-specific cleavable PEG-lipid. Biomaterials. 2011. June;32(18):4306–4316. [DOI] [PubMed] [Google Scholar]
- 139.Zhang M, Xu C, Wen L, et al. A hyaluronidase-responsive nanoparticle-based drug delivery system for targeting colon cancer cells. Cancer Res. 2016. December 15;76(24):7208–7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rodbard D, Moore MJ, Rodbard D. The role of regional body temperature in the pathogenesis of disease. N Engl J Med. 1981;305:808–814. [DOI] [PubMed] [Google Scholar]
- 141.Lee SH, Choi SH, Kim SH, et al. Thermally sensitive cationic polymer nanocapsules for specific cytosolic delivery and efficient gene silencing of siRNA: swelling induced physical disruption of endosome by cold shock. J Control Release. 2008. January 4;125 (1):25–32. [DOI] [PubMed] [Google Scholar]
- 142.Zhang J, Chen H, Xu L, et al. The targeted behavior of thermally responsive nanohydrogel evaluated by NIR system in mouse model. J Control Release. 2008;131(1):34–40. [DOI] [PubMed] [Google Scholar]
- 143.Cheng Y, Hao J, Lee LA, et al. Thermally controlled release of anticancer drug from self-assembled gamma-substituted amphiphilic poly(epsilon-caprolactone) micellar nanoparticles. Biomacromolecules. 2012. July 9;13(7):2163–2173. [DOI] [PubMed] [Google Scholar]
- 144.Chen K-J, Liang H-F, Chen H-L, et al. A thermoresponsive bubble-generating liposomal system for triggering localized extracellular drug delivery. ACS Nano. 2013;7(1):438–446. [DOI] [PubMed] [Google Scholar]
- 145.Rapoport NY, Kennedy AM, Shea JE, et al. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J Control Release. 2009. September 15;138(3):268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang CH, Kang ST, Lee YH, et al. Aptamer-conjugated and drug-loaded acoustic droplets for ultrasound theranosis. Biomaterials. 2012. February;33(6):1939–1947. [DOI] [PubMed] [Google Scholar]
- 147.Schroeder A, Honen R, Turjeman K, et al. Ultrasound triggered release of cisplatin from liposomes in murine tumors. J Control Release. 2009. July 1;137(1):63–68. [DOI] [PubMed] [Google Scholar]
- 148.Kheirolomoom A, Mahakian LM, Lai C-Y, et al. Copper-doxorubicin as a nanoparticle cargo retains efficacy with minimal toxicity. Mol Pharm. 2010;7:1948–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sirsi SR, Borden MA. State-of-the-art materials for ultrasound-triggered drug delivery. Adv Drug Deliv Rev. 2014. June;72:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gao ZG, Fain HD, Rapoport N. Controlled and targeted tumor chemotherapy by micellar-encapsulated drug and ultrasound. J Control Release. 2005. January 20;102(1):203–222. [DOI] [PubMed] [Google Scholar]
- 151.Jung SH, Na K, Lee SA, et al. Gd(III)-DOTA-modified sonosensitive liposomes for ultrasound-triggered release and MR imaging. Nanoscale Res Lett. 2012;7:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Juan L, Vivero-Escoto IIS, Wu C-W, et al. Photoinduced intracellular controlled release drug delivery in human cells by gold-capped mesoporous silica nanosphere. J Am Chem Soc. 2009;131:3462–3463. [DOI] [PubMed] [Google Scholar]
- 153.Liu YC, Le Ny AL, Schmidt J, et al. Photo-assisted gene delivery using light-responsive catanionic vesicles. Langmuir. 2009. May 19;25(10):5713–5724. [DOI] [PubMed] [Google Scholar]
- 154.Tong R, Hemmati HD, Langer R, et al. Photoswitchable nanoparticles for triggered tissue penetration and drug delivery. J Am Chem Soc. 2012. May 30;134(21):8848–8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.He D, He X, Wang K, et al. A light-responsive reversible molecule-gated system using thymine-modified mesoporous silica nanoparticles. Langmuir. 2012. February 28;28(8):4003–4008. [DOI] [PubMed] [Google Scholar]
- 156.Agasti SS, Chompoosor A, You CC, et al. Photoregulated release of caged anticancer drugs from gold nanoparticles. J Am Chem Soc. 2009. April 29;131(16):5728–5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lu J, Choi E, Tamanoi F, et al. Light-activated nanoimpeller-controlled drug release in cancer cells. Small. 2008. 4; April(4): 421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yuan Q, Zhang Y, Chen T, et al. Photon-manipulated drug release from a mesoporous nanocontainer controlled by azobenzene-modified nucleic acid. ACS Nano. 2012. July 24;6 (7):6337–6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li N, Yu Z, Pan W, et al. A near-infrared light-triggered nanocarrier with reversible DNA valves for intracellular controlled release. Adv Funct Mater. 2013;23(18):2255–2262. [Google Scholar]
- 160.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019. January;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 161.Wang YA, Li XL, Mo YZ, et al. Effects of tumor metabolic microenvironment on regulatory T cells. Mol Cancer. 2018. November 26;17 (1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Spill F, Reynolds DS, Kamm RD, et al. Impact of the physical microenvironment on tumor progression and metastasis. Curr Opin Biotechnol. 2016. August;40:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Alfarouk KO, Muddathir AK, Shayoub ME. Tumor acidity as evolutionary spite. Cancers (Basel). 2011. January 20;3(1):408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Korneev KV, Atretkhany KN, Drutskaya MS, et al. TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine. 2017. January;89:127–135. [DOI] [PubMed] [Google Scholar]
- 165.Hao Y, Zheng C, Wang L, et al. Tumor acidity-activatable manganese phosphate nanoplatform for amplification of photodynamic cancer therapy and magnetic resonance imaging. Acta Biomater. 2017. October;15(62):293–305. [DOI] [PubMed] [Google Scholar]
- 166.Shi X, Ma X, Hou M, et al. pH-Responsive unimolecular micelles based on amphiphilic star-like copolymers with high drug loading for effective drug delivery and cellular imaging. J Mater Chem B. 2017. September 7;5(33):6847–6859. [DOI] [PubMed] [Google Scholar]
- 167.Lin W, Yao N, Qian L, et al. pH-responsive unimolecular micelle-gold nanoparticles-drug nanohybrid system for cancer theranostics. Acta Biomater. 2017. August;58:455–465. [DOI] [PubMed] [Google Scholar]
- 168.Chen Q, Feng L, Liu J, et al. Intelligent Albumin-MnO2 nanoparticles as pH-/H2 O2 -responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy. Adv Mater. 2016. September;28(33):7129–7136. [DOI] [PubMed] [Google Scholar]
- 169.Yang G, Zhang R, Liang C, et al. Manganese dioxide coated WS2 @Fe3 O4/sSiO2 nanocomposites for pH-responsive MR imaging and oxygen-elevated synergetic therapy. Small. 2018;14:2. [DOI] [PubMed] [Google Scholar]
- 170.Li J, Liu F, Shao Q, et al. Enzyme-responsive cell-penetrating peptide conjugated mesoporous silica quantum dot nanocarriers for controlled release of nucleus-targeted drug molecules and real-time intracellular fluorescence imaging of tumor cells. Adv Healthc Mater. 2014. August;3(8):1230–1239. [DOI] [PubMed] [Google Scholar]
- 171.Hu Q, Katti PS, Gu Z. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale. 2014. November 7;6(21):12273–12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.de la Rica R, Aili D, Stevens MM. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv Drug Deliv Rev. 2012. August;64 (11):967–978. [DOI] [PubMed] [Google Scholar]
- 173.Kanapathipillai M, Brock A, Ingber DE. Nanoparticle targeting of anti-cancer drugs that alter intracellular signaling or influence the tumor microenvironment. Adv Drug Deliv Rev. 2014. December;15;79–80:107–118. [DOI] [PubMed] [Google Scholar]
- 174.Huang WC, Chen SH, Chiang WH, et al. Tumor microenvironment-responsive nanoparticle delivery of chemotherapy for enhanced selective cellular uptake and transportation within tumor. Biomacromolecules. 2016. December 12;17(12):3883–3892. [DOI] [PubMed] [Google Scholar]
- 175.Peng J, Yang Q, Xiao Y, et al. Tumor microenvironment responsive drug-dye-peptide nanoassembly for enhanced tumor-targeting, penetration, and photo-chemo-immunotherapy. Adv Funct Mater. 2019;29:19. [Google Scholar]
- 176.Du J, Lane LA, Nie S. Stimuli-responsive nanoparticles for targeting the tumor microenvironment. J Control Release. 2015. December;10 (219):205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fernandes C, Suares D, Yergeri MC. Tumor microenvironment targeted nanotherapy. Front Pharmacol. 2018;9:1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang Z, Ju Y, Ali Z, et al. Near-infrared light and tumor microenvironment dual responsive size-switchable nanocapsules for multi-modal tumor theranostics. Nat Commun. 2019. September 27;10(1):4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yang HY, Jang MS, Li Y, et al. Hierarchical tumor acidity-responsive self-assembled magnetic nanotheranostics for bimodal bioimaging and photodynamic therapy. J Control Release. 2019. May;10 (301):157–165. [DOI] [PubMed] [Google Scholar]
- 180.Cheng R, Meng F, Deng C, et al. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials. 2013. May;34(14):3647–3657. [DOI] [PubMed] [Google Scholar]
- 181.Curcio A, Marotta R, Riedinger A, et al. Magnetic pH-responsive nanogels as multifunctional delivery tools for small interfering RNA (siRNA) molecules and iron oxide nanoparticles (IONPs). Chem Commun. 2012;48:2400–2402. [DOI] [PubMed] [Google Scholar]
- 182.Hathout RM, Metwally AA, El-Ahmady SH, et al. Dual stimuli-responsive polypyrrole nanoparticles for anticancer therapy. J Drug Deliv Sci Tec. 2018;47:176–180. [Google Scholar]
- 183.Jia X, Zhang Y, Zou Y, et al. Dual intratumoral redox/enzyme-responsive NO-releasing nanomedicine for the specific, high-efficacy, and low-toxic cancer therapy. Adv Mater. 2018. July;30(30):e1704490. [DOI] [PubMed] [Google Scholar]
- 184.Gan Q, Lu X, Yuan Y, et al. A magnetic, reversible pH-responsive nanogated ensemble based on Fe3O4 nanoparticles-capped mesoporous silica. Biomaterials. 2011. March;32(7):1932–1942. [DOI] [PubMed] [Google Scholar]
- 185.Menon JU, Kuriakose A, Iyer R, et al. Dual-drug containing core-shell nanoparticles for lung cancer therapy. Sci Rep. 2017. October 16;7(1):13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lee Y, Lee S, Lee DY, et al. Multistimuli-responsive Bilirubin nanoparticles for anticancer therapy. Angew Chem Int Ed Engl. 2016. August 26;55(36):10676–10680. [DOI] [PubMed] [Google Scholar]
- 187.Xiong D, Zhang X, Peng S, et al. Smart pH-sensitive micelles based on redox degradable polymers as DOX/GNPs carriers for controlled drug release and CT imaging. Colloids Surf B Biointerfaces. 2018. March;1(163):29–40. [DOI] [PubMed] [Google Scholar]
- 188.Yang HY, Jang M-S, Gao GH, et al. Construction of redox/pH dual stimuli-responsive PEGylated polymeric micelles for intracellular doxorubicin delivery in liver cancer. Polym Chem. 2016;7 (9):1813–1825. [Google Scholar]
- 189.Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018. September 19;16(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A very interesting review that highlights FDA-approved nano-technology-based products and clinical trials.
- 190.Barenholz Y Doxil(R)–the first FDA-approved nano-drug: lessons learned. J Control Release. 2012. June 10;160(2):117–134. [DOI] [PubMed] [Google Scholar]
- 191.Ta T, Porter TM. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J Control Release. 2013. July 10;169(1–2):112–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ventola CL. Progress in nanomedicine: approved and investigational nanodrugs. P&T. 2017;42:742–755. [PMC free article] [PubMed] [Google Scholar]
- 193.Hua S, de Matos MBC, Metselaar JM, et al. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization [review]. Front Pharmacol. 2018. [2018 July 17];9(790). doi: 10.3389/fphar.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schmutz M, Borges O, Jesus S, et al. A methodological safe-by-design approach for the development of nanomedicines [perspective]. Front Bioeng Biotechnol. 2020. [2020 April 02];8(258). doi: 10.3389/fbioe.2020.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Marques C, Som C, Schmutz M, et al. How the lack of Chitosan characterization precludes implementation of the safe-by-design concept [review]. Front Bioeng Biotechnol. 2020. [2020 March 10];8 (165). doi: 10.3389/fbioe.2020.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mauri E, Perale G, Rossi F. Nanogel Functionalization: A versatile approach to meet the challenges of drug and gene delivery. ACS Appl Nano Mater. 2018. [2018 December 28];1(12):6525–6541. [Google Scholar]
- 197.Pinelli F, Sacchetti A, Perale G, et al. Is nanoparticle functionalization a versatile approach to meet the challenges of drug and gene delivery? Ther Deliv. 2020;11(7):401–404. [DOI] [PubMed] [Google Scholar]
- 198.Schwarz-Plaschg C, Kallhoff A, Eisenberger I. Making nanomaterials safer by design? NanoEthics. 2017. [2017 December 01];11 (3):277–281. [Google Scholar]
- 199.De Jong WH B PJA. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine. 2008;3:133–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008. January;4(1):26–49. [DOI] [PubMed] [Google Scholar]
- 201.Pourmand A, Abdollahi M. Current opinion on nanotoxicology. DARU J Pharma Sci. 2012;20(1):95–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dreher KL. Health and environmental impact of nanotechnology: toxicological assessment of manufactured nanoparticles. Toxicol Sci. 2004. January;77(1):3–5. [DOI] [PubMed] [Google Scholar]
- 203.Hagens WI, Oomen AG, de Jong WH, et al. What do we (need to) know about the kinetic properties of nanoparticles in the body? Regul Toxicol Pharmacol. 2007. December;49(3):217–229. [DOI] [PubMed] [Google Scholar]
- 204.Nemmar A, Hoet PHM, Vanquickenborne B, et al. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. [DOI] [PubMed] [Google Scholar]
- 205.Garnett MC, Kallinteri P. Nanomedicines and nanotoxicology: some physiological principles. Occup Med (Lond). 2006. August;56 (5):307–311. [DOI] [PubMed] [Google Scholar]; • An essential manuscript that shows the importance in studying biodistribution, phagocytosis, opsonization, and endocytosis of nanosized materials in predicting potential toxicity of nanoparticles.
- 206.Yang L, Watts DJ. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol Lett. 2005. August 14;158(2):122–132. [DOI] [PubMed] [Google Scholar]
- 207.Patel P, Shah J. Safety and toxicological considerations of nanomedicines: the future directions. Curr Clin Pharmacol. 2017;12 (2):73–82. [DOI] [PubMed] [Google Scholar]


