Abstract
Background:
We assessed the cross-sectional association of physical function measures with cognition in the Kaiser Healthy Aging and Diverse Life Experiences (KHANDLE) Cohort.
Methods:
Analyses included 1,369 participants (24% Asian, 26% Black, 18% Latino, 32% White). Grip strength was measured using a hand-held dynamometer (kilograms) and gait speed was measured over a 4-meter walk (seconds/meter). The Spanish and English Neuropsychological Assessment Scales (SENAS) was used to evaluate cognitive domains of executive function, semantic memory, and verbal episodic memory. Physical function measures (per standard deviation) were associated with cognitive test z-scores in linear regression models adjusted for demographic, behavioral, and clinical factors. Racial/ethnic differences were tested using interaction terms and stratification.
Results:
Stronger grip was associated with better executive function [β=0.10(0.05, 0.15)], semantic memory [β=0.13(0.09, 0.18)] and verbal episodic memory [β=0.07(0.02, 0.13)] with no racial/ethnic differences. Faster gait was associated with better executive function [β=0.29(0.22, 0.36)], semantic memory [β=0.23(0.16, 0.30)], and verbal episodic memory [β=0.20(0.13, 0.27)]; however, the association between gait speed and executive function varied by race/ethnicity with the strongest associations in Asians and Whites.
Conclusion:
Across race/ethnicity, grip strength and gait speed were associated with cognition with racial/ethnic differences in the association of gait speed and executive function.
Keywords: Disparities, Grip Strength, Gait Speed, Cognition
Introduction:
Among older adults, racial/ethnic minorities in the United States are more likely to experience declines in physical function associated with increased risk of disability and mortality compared to non-Latino Whites.1,2 Several studies have shown that there is often a decline in physical function prior to the onset of cognitive impairment.3 This is particularly salient to racial/ethnic minorities such as Black and Latino Americans who experience high rates of dementia compared to other racial/ethnic groups.4 As the U.S. population rapidly ages and becomes more diverse, understanding the relationship between measures of physical function and cognition has become more important for understanding how to maintain quality of life and possibly reduce the risk of cognitive decline and dementia among diverse populations of older adults.
Grip strength and gait speed are components of physical function that have been independently associated with cognitive decline, mild cognitive impairment (MCI), and dementia.5–14 Additionally, studies have found that among older adults, White Americans had stronger grip strength and faster gait speed than Latinos and Asians, while Black Americans had stronger grip strength, but slower gait speed than Whites.15–20 Despite evidence of racial/ethnic differences in these measures, to our knowledge, no studies have evaluated racial/ethnic differences in the associations of grip strength and gait speed with cognitive function.
Using the Kaiser Healthy Aging and Diverse Life Experiences (KHANDLE) Study, we aimed to evaluate racial/ethnic differences in the cross-sectional associations of grip strength and gait speed with cognition. We hypothesized that across racial/ethnic groups, greater grip strength or faster gait speed would be positively associated with cognitive function.
Methods:
The KHANDLE cohort includes community-dwelling older adults residing in the San Francisco Bay and Sacramento areas of California. KHANDLE aims to evaluate how race/ethnicity and life course health and sociocultural factors influence late-life brain health and cognitive decline. Individuals eligible for KHANDLE were long-term members of Kaiser Permanente Northern California, an integrated healthcare delivery system, were age 65 years or older on January 1, 2017, spoke English or Spanish, and had previously participated in Kaiser Permanente multiphasic health checkup (MHC) exams between 1964-1973 or 1977-1985. Stratified random sampling by race/ethnicity and educational attainment was used with the goal of recruiting approximately equal proportions of Asian, Black, Latino, and White participants as well as diversity in educational attainment. Exclusion criteria included electronic medical record diagnosis of dementia or other neurodegenerative disease (frontotemporal dementia, Lewy body disease, Pick’s disease, Parkinson’s disease with dementia, Huntington’s disease) and presence of health conditions that would impede participation in study interviews (defined by hospice activity in the past 12 months, history of severe chronic obstructive pulmonary disease in the past 6 months, congestive heart failure hospitalizations in the past 6 months, and history of end stage renal disease or dialysis in the past 12 months). At wave 1 (2017 – 2018), 1,712 individuals were enrolled.
Grip strength and gait speed were assessed by trained interviewers. Grip strength was measured in kilograms (kg) using a Jamar Hydraulic Hand Dynamometer calibrated by B & L Engineering (Santa Ana, CA) and adjusted for each participant’s hand size. Three measures were taken from the participant’s dominant hand and the average used for analysis. Grip strength was analyzed continuously per gender-specific standard deviation (SD women = 5.7 kg; SD men = 8.6 kg). Gender-specific SD was used due to known differences in measures of physical performance.2,19 Gait speed was measured over a 4-meter walk. Participants were able to use a walking aid (e.g. cane, walker) if needed. The walk was performed twice at a pace similar to if the participant was “walking down the street to the store.” The average of the two walking times was used for analysis and assessed continuously per gender-specific standard deviation (SD women = 0.7 s/m; SD men = 0.5 s/m).2,19
Three cognitive domains (verbal episodic memory, semantic memory, and executive function) were derived from the Spanish and English Neuropsychological Assessment Scales (SENAS). The SENAS is a battery of cognitive tests that has undergone extensive development using item response theory (IRT) methodology for valid comparisons of cognition and cognitive change across racial/ethnic and linguistically diverse groups. The SENAS was administered during wave 1 interviews in either English or Spanish, with language of administration determined by an algorithm that considered preferred language and everyday language usage in a variety of settings. IRT methods were used to derive a verbal episodic memory score from a multi-trial word-list-learning test. A semantic memory composite score was calculated from IRT based verbal (object-naming) and nonverbal (picture association) scores. An executive function composite score was calculated from IRT based component measures of category fluency, phonemic (letter) fluency, and working memory (digit-span backward, visual-span backward, list sorting). Details of the administration procedures, development, and psychometric characteristics have been described in detail elsewhere.21,22 Each domain was z-standardized using the mean and standard deviation from the full baseline sample.
Covariates obtained during the wave 1 interview were grouped into three sets. Our basic covariate set included age at interview in years (from the medical record and modeled linearly), self-reported gender (male/female), and race/ethnicity (Asian/Black/Latino/White). Our second covariate set included socioeconomic factors of education (less than high school/high school/GED, some college/trade school/certificate, Bachelor’s degree, or graduate school) and family income (<$55,000/≥$55,000). The third covariate set included cardiovascular risk factors including physical activity, alcohol consumption (current drinker/non-drinker), lifetime smoking (ever/never), and waist circumference (modeled linearly in centimeters). The physical activity questionnaire evaluated frequency of light and vigorous leisure and sport activity in the past year, and responses were assigned scores on a Likert scale ranging from 0 (no physical activity) to 4 (daily or almost daily activity).23 Question scores were summed for a total range of 0-16 with a higher score indicating higher levels of physical activity and modeled linearly. Waist circumference was measured in centimeters (cm) with tape positioned in a horizontal plane across the narrowest part of the torso and taken while the participant stood, abdomen relaxed, with their feet together and arms at their sides.
Statistical Analysis:
We described means and prevalences of participant characteristics stratified by race/ethnicity. Linear regression was used to assess cross-sectional associations of continuous measures of grip strength and gait speed with domain-specific cognition in separate models with multiple covariate adjustment.
First, we explored whether grip strength and gait speed were linearly associated with cognition. We plotted predicted domain-specific cognitive test z-scores based on grip strength and gait speed separately using spline models (with knots at the 5th, 35th, 65th, and 95th percentiles) adjusted for age, gender, and race/ethnicity using Stata version 14.0 (StataCorp, College Station, TX). We also tested quadratic terms for grip strength and gait speed. Due to a non-linear association between gait speed and cognition, we mean-centered gait speed and included a quadratic term in subsequent analyses.
Our primary analyses evaluated racial/ethnic differences in the associations of grip strength and gait speed (separately) with domain-specific cognition. These differences were assessed through a series of linear regression models in the overall sample, by testing race/ethnicity*grip strength and race/ethnicity*gait speed interaction terms, and in race/ethnicity stratified models. To understand whether grip strength and gait speed related to one another, we ran regression models with both physical function measures in the model (including a quadratic term for gait speed) as well as tested a grip strength*gait speed interaction.
Linear regression Model 1 adjusted for age, gender, and race/ethnicity (not included in race/ethnicity-stratified models). Model 2 adjusted for model 1 covariates plus education and income. Model 3 adjusted for model 2 covariates plus waist circumference, physical activity, alcohol consumption, and ever smoking. These analyses were completed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results:
Participants were excluded from analyses if they were missing information on race/ethnicity (n=4), gender (n=0), grip strength (n=96), gait speed (n=236) or cognitive measures (n=7) for a final analytic sample of 1,369 individuals. The primary reason for missing grip strength was due to being physically unable, and the primary reason for missing gait speed was due to lack of space to complete the measurement. Of the 1,369 participants, 24% were Asian, 26% were Black, 18% were Latino, and 32% were White (Table 1). Grip strength was greatest in Black participants (mean = 26.6 kg) followed by Whites (25.5 kg), Asians (24.3 kg), and Latinos (23.9 kg) (p < 0.0001 with adjustment for age). Black participants also had the slowest gait speed (1.4 s/m), followed by Asians (1.3 s/m), Latinos (1.3 s/m), and Whites (1.2 s/m) (p = 0.03 with adjustment for age).
Table 1.
Participant baseline characteristics by race/ethnicity, KHANDLE
| Variables | Overall | Asian | Black | Latino | White | P-value for Trend |
|---|---|---|---|---|---|---|
| n = 1369 | n = 323 | n = 365 | n = 247 | n = 434 | ||
| Age, years | 75.6 ± 6.7 | 75.1 ± 6.3 | 74.8 ± 6.4 | 75.8 ± 6.4 | 76.4 ± 7.1 | 0.0008 |
| Male, n (%) | 572 (41.8) | 156 (48.3) | 121 (33.2) | 106 (42.9) | 189 (43.6) | 0.0006 |
| Waist Circumference, cm | 95.7 ± 14.2 | 88.3 ± 12.4 | 98.8 ± 14.2 | 98.9 ± 13.6 | 96.9 ± 13.9 | <0.0001 |
| Education | ||||||
| ≤ High School Grad, n (%) | 215 (15.7) | 36 (11.2) | 59 (16.2) | 57 (23.2) | 63 (14.5) | <0.0001 |
| Trade or some college, n (%) | 486 (35.6) | 74 (23.0) | 182 (49.9) | 103 (41.9) | 127 (29.3) | |
| College Grad, n (%) | 352 (25.8) | 128 (39.8) | 59 (16.2) | 46 (18.7) | 119 (27.4) | |
| Graduate School, n (%) | 314 (23.0) | 84 (26.1) | 65 (17.8) | 40 (16.3) | 125 (28.8) | |
| Income ≥ $55,000, n (%) | 870 (67.0) | 238 (76.8) | 200 (58.5) | 139 (59.2) | 293 (71.1) | <0.0001 |
| Physical Activity, units | 9.0 ± 3.1 | 8.9 ± 3.0 | 9.0 ± 3.1 | 8.9 ± 3.3 | 9.2 ± 3.0 | 0.16 |
| Current Alcohol Drinker, n (%) | 976 (71.9) | 216 (66.9) | 220 (61.1) | 191 (78.3) | 349 (81.0) | <0.0001 |
| Ever Tobacco Smoker, n (%) | 617 (45.3) | 107 (33.1) | 160 (44.2) | 120 (49.2) | 230 (53.2) | <0.0001 |
| Grip Strength, kg | 25.2 ± 9.5 | 24.3 ± 9.0 | 26.6 ± 9.9 | 23.9 ± 9.5 | 25.5 ± 9.3 | <0.0001 |
| Gait Speed, s/m | 1.3 ± 0.7 | 1.3 ± 0.7 | 1.4 ± 0.9 | 1.3 ± 0.5 | 1.2 ± 0.5 | 0.04 |
Mean ± Standard Deviation
Testing Non-Linear Associations:
We examined non-linear associations of grip strength and gait speed with cognition using restricted cubic splines with 4 knots (Figure 1). We found that the association between grip strength and cognition was positive and linear. The association between gait speed and cognition was negative and resembled a U-shaped curve. Due to this non-linear association, subsequent models included a quadratic term for gait speed and results for gait speed were interpreted incorporating the quadratic term.
Figure 1.
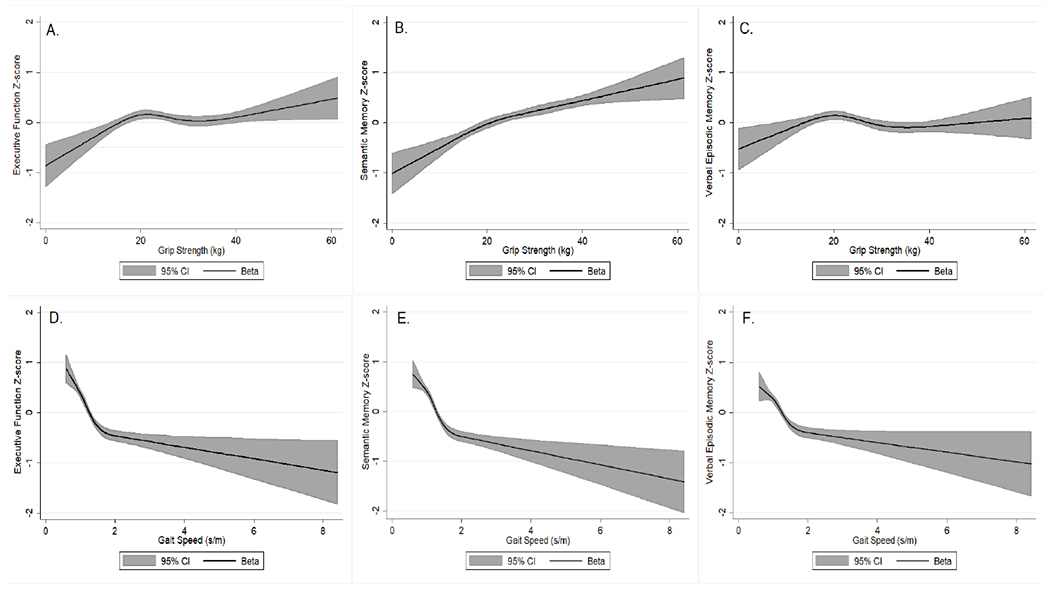
Restricted cubic spline regression† for the association between grip strength and gait speed with cognition, KHANDLE
†Analyzed using 4 knots at the 5th, 35th, 65th, and 95th percentiles of grip strength and gait speed and adjusted for age, gender, and race/ethnicity
A. Association between grip strength and executive function z-score; B. Association between grip strength and semantic memory z-score; C. Association between grip strength and verbal episodic memory z-score; D. Association between gait speed and executive function z-score; E. Association between gait speed and semantic memory z-score; F. Association between gait speed and verbal episodic memory z-score
Grip Strength:
A one SD greater grip strength was associated with a 0.10 (95% CI: 0.05, 0.15) higher z-score in executive function (Table 2; Model 1). A one SD greater grip strength was also associated with better semantic memory [β: 0.13 (0.09, 0.18)] and verbal episodic memory [β: 0.07 (0.02, 0.13)]. Race/ethnicity*grip strength interaction terms were tested, and, although there were small differences in point estimates across racial/ethnic group, there were no statistically significant interactions for any of the three cognitive domains (Table 2).
Table 2.
Association between grip strength (per standard deviation) and domain-specific cognition by race/ethnicity, KHANDLE
| Models | Overall | Race Interaction P-value | Asian | Black | Latino | White |
|---|---|---|---|---|---|---|
| n = 1369 | n = 323 | n = 365 | n = 247 | n = 434 | ||
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | ||
| Executive Function | ||||||
| 1 | 0.10 | 0.27 | 0.12 | 0.03 | 0.18 | 0.12 |
| (0.05, 0.15) | (0.01, 0.22) | (−0.05, 0.11) | (0.07, 0.29) | (0.008, 0.23) | ||
| 2 | 0.09 | 0.06 | 0.09 | −0.005 | 0.21 | 0.11 |
| (0.04, 0.13) | (−0.008, 0.19) | (−0.08, 0.07) | (0.10, 0.32) | (0.003, 0.21) | ||
| 3 | 0.08 | 0.07 | 0.07 | −0.001 | 0.19 | 0.09 |
| (0.03, 0.13) | (−0.03, 0.17) | (−0.08, 0.07) | (0.08, 0.30) | (−0.02, 0.20) | ||
| Semantic Memory | ||||||
| 1 | 0.13 | 0.54 | 0.23 | 0.10 | 0.15 | 0.10 |
| (0.09, 0.18) | (0.10, 0.35) | (0.02, 0.17) | (0.04, 0.25) | (0.02, 0.17) | ||
| 2 | 0.12 | 0.21 | 0.22 | 0.07 | 0.18 | 0.09 |
| (0.08, 0.17) | (0.10, 0.34) | (−0.003, 0.15) | (0.08, 0.28) | (0.01, 0.17) | ||
| 3 | 0.12 | 0.29 | 0.21 | 0.08 | 0.17 | 0.09 |
| (0.07, 0.17) | (0.08, 0.33) | (0.003, 0.16) | (0.07, 0.27) | (0.02, 0.17) | ||
| Verbal Episodic Memory | ||||||
| 1 | 0.07 | 0.53 | 0.07 | 0.05 | 0.06 | 0.12 |
| (0.02, 0.13) | (−0.05, 0.19) | (−0.03, 0.13) | (−0.06, 0.18) | (0.02, 0.22) | ||
| 2 | 0.07 | 0.41 | 0.05 | 0.03 | 0.08 | 0.12 |
| (0.02, 0.12) | (−0.06, 0.17) | (−0.05, 0.11) | (−0.04, 0.20) | (0.02, 0.22) | ||
| 3 | 0.06 | 0.34 | 0.08 | 0.01 | 0.09 | 0.09 |
| (0.01, 0.11) | (−0.05, 0.20) | (−0.07, 0.10) | (−0.03, 0.21) | (−0.01, 0.19) | ||
SD grip strength women = 5.7 kg; SD grip strength men = 8.6 kg
Model 1 adjusted for age, gender, and race (in Overall and Interaction models)
Model 2 adjusted for Model 1 + education and income
Model 3 adjusted for Model 2 + waist circumference (cm), physical activity, alcohol consumption, and ever smoking
Gait Speed:
Gait speed was significantly and curvilinearly associated with cognition across levels of adjustment for covariates (Table 3). In order to estimate the mean difference in cognition per standard deviation in gait speed, we mean-centered gait speed and incorporated the quadratic term into our beta estimates. Subsequent results differ slightly from those presented in Table 3 due to the incorporation of this quadratic term. A gait speed one standard deviation greater than the mean was associated with a 0.29 (0.22, 0.36) higher z-score in executive function and a gait speed two standard deviations greater than the mean was associated with a 0.54 (0.42, 0.67) higher executive function z-score after Model 1 adjustments. Increased gait speed was also associated with better semantic memory [+1 SD β: 0.23 (0.16, 0.30); +2 SD β: 0.43 (0.31, 0.56)] and verbal episodic memory [+1 SD β: 0.20 (0.13, 0.27); +2 SD β: 0.38 (0.25, 0.51)]. We found significant interactions between race/ethnicity and gait speed in predicting executive function (Model 1 interaction p = 0.10; Model 2 p = 0.02; Model 3 p = 0.02). In race/ethnicity stratified results, a gait speed one SD above the mean was associated with significantly better executive function across racial/ethnic groups after Model 1 adjustments. By Model 3 adjustment, associations were attenuated for Black participants and non-significant for Latino participants while remaining significantly associated in Asians and Whites after taking into account the quadratic term.
Table 3.
Association between gait speed (per standard deviation) and domain-specific cognition by race/ethnicity, KHANDLE
| Models | Overall | Race Interaction P-value | Asian | Black | Latino | White | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 1369 | n = 323 | n = 365 | n = 247 | n = 434 | |||||||
| Gait Speed β (95% CI) | Gait Speed2 β (95% CI) | Gait Speed β (95% CI) | Gait Speed2 β (95% CI) | Gait Speed β (95% CI) | Gait Speed2 β (95% CI) | Gait Speed β (95% CI) | Gait Speed2 β (95% CI) | Gait Speed β (95% CI) | Gait Speed2 β (95% CI) | ||
| Executive Function | |||||||||||
| 1 | 0.31 | −0.02 | 0.10 | 0.35 | −0.02 | 0.27 | −0.02 | 0.35 | −0.04 | 0.45 | −0.08 |
| (0.23, 0.38) | (−0.03, −0.01) | (0.20, 0.49) | (−0.03, −0.01) | (0.14, 0.39) | (−0.03, −0.01) | (0.13, 0.57) | (−0.10, 0.02) | (0.26, 0.65) | (−0.15, −0.02) | ||
| 2 | 0.27 | −0.02 | 0.02 | 0.40 | −0.02 | 0.20 | −0.02 | 0.26 | −0.02 | 0.37 | −0.06 |
| (0.19, 0.34) | (−0.02, −0.01) | (0.25, 0.55) | (−0.03, −0.01) | (0.07, 0.33) | (−0.03, −0.01) | (0.04, 0.49) | (−0.08, 0.04) | (0.17, 0.56) | (−0.13, 0.00) | ||
| 3 | 0.28 | −0.02 | 0.02 | 0.38 | −0.02 | 0.22 | −0.02 | 0.30 | −0.02 | 0.37 | −0.06 |
| (0.20, 0.37) | (−0.02, −0.01) | (0.22, 0.54) | (−0.03, −0.01) | (0.09, 0.36) | (−0.03, −0.01) | (0.07, 0.52) | (−0.08, 0.03) | (0.16, 0.57) | (−0.13, 0.02) | ||
| Semantic Memory | |||||||||||
| 1 | 0.24 | −0.01 | 0.81 | 0.26 | −0.01 | 0.22 | −0.01 | 0.37 | −0.04 | 0.25 | −0.03 |
| (0.17, 0.31) | (−0.02, −0.01) | (0.08, 0.44) | (−0.03, 0.00) | (0.10, 0.35) | (−0.03, −0.00) | (0.16, 0.57) | (−0.09, 0.02) | (0.11, 0.39) | (−0.08, 0.01) | ||
| 2 | 0.23 | −0.01 | 0.21 | 0.34 | −0.02 | 0.20 | −0.01 | 0.35 | −0.04 | 0.20 | −0.02 |
| (0.16, 0.30) | (−0.02, −0.01) | (0.15, 0.53) | (−0.03, −0.00) | (0.07, 0.33) | (−0.02, −0.00) | (0.14, 0.55) | (−0.09, 0.02) | (0.06, 0.34) | (−0.07, 0.02) | ||
| 3 | 0.25 | −0.01 | 0.25 | 0.36 | −0.02 | 0.20 | −0.01 | 0.29 | −0.03 | 0.21 | −0.00 |
| (0.17, 0.33) | (−0.02, −0.01) | (0.16, 0.57) | (−0.03, −0.00) | (0.06, 0.34) | (−0.02, −0.00) | (0.08, 0.50) | (−0.08, 0.02) | (0.07, 0.36) | (−0.06, 0.05) | ||
| Verbal Episodic Memory | |||||||||||
| 1 | 0.21 | −0.01 | 0.56 | 0.20 | −0.01 | 0.17 | −0.01 | 0.20 | −0.01 | 0.40 | −0.08 |
| (0.14, 0.29) | (−0.02, −0.01) | (0.04, 0.36) | (−0.02, 0.00) | (0.04, 0.31) | (−0.02, 0.00) | (−0.04, 0.43) | (−0.07, 0.06) | (0.22, 0.58) | (−0.14, −0.02) | ||
| 2 | 0.19 | −0.01 | 0.40 | 0.24 | −0.01 | 0.13 | −0.01 | 0.14 | 0.01 | 0.32 | −0.06 |
| (0.11, 0.27) | (−0.02, −0.00) | (0.06, 0.42) | (−0.03, −0.00) | (−0.01, 0.28) | (−0.02, 0.01) | (−0.10 0.38) | (−0.06, 0.07) | (0.14, 0.51) | (−0.12, 0.00) | ||
| 3 | 0.19 | −0.01 | 0.23 | 0.30 | −0.02 | 0.11 | −0.00 | 0.08 | 0.02 | 0.27 | −0.02 |
| (0.10, 0.27) | (−0.02, −0.00) | (0.11, 0.50) | (−0.03, −0.00) | (−0.05, 0.26) | (−0.02, 0.01) | (−0.17, 0.33) | (−0.05, 0.08) | (0.07, 0.46) | (−0.09, 0.05) | ||
SD gait speed women = 0.7 s/m; SD gait speed men = 0.5 s/m
Gait speed was mean-centered
Bolded results indicate statistical significance (p<0.05)
Model 1 adjusted for age, gender, and race (in Overall and Interaction models)
Model 2 adjusted for Model 1 + education and income
Model 3 adjusted for Model 2 + waist circumference (cm), physical activity, alcohol consumption, and ever smoking
Grip Strength and Gait Speed:
We tested grip strength and gait speed with cognition in the same model and found that the association between grip strength and cognition was attenuated and non-significant for executive function and verbal episodic memory, but it remained positively associated with semantic memory (Supplemental Table 1). The association between quadratic gait speed and cognition persisted with faster gait speed associated with better cognition in all three domains (Supplemental Table 1). To determine whether measures of physical function interacted with each other on cognition, we tested a grip strength*gait speed interaction term and found no statistically significant interactions after adjustment for age, gender, and race/ethnicity (executive function interaction p = 0.49; semantic memory interaction p = 0.42; verbal episodic memory interaction p = 0.60).
Discussion:
In this assessment of physical function and cognition in a diverse, community-dwelling cohort, we found stronger grip strength and faster gait speed were significantly associated with better cognition in domains of executive function, semantic memory, and verbal episodic memory. The level of difference in cognitive function associated with a SD greater grip strength was approximately equal to what would be expected in participants 2 years younger in age [(executive function β: 0.11 (0.10, 0.13), semantic memory β: 0.11 (0.09, 0.12); verbal episodic memory β: 0.11 (0.10, 0.13)] after adjusting for gender and race/ethnicity. For gait speed, the level of difference associated with a SD faster gait speed than the mean was closer to what would be expected in participants 4-6 years younger in age. There were some variations in the association of grip strength and cognition by race/ethnicity, but interaction terms were non-significant. We did find statistically significant racial/ethnic differences in the association of gait speed with executive function after accounting for the non-linearity of gait speed. Associations were significant among Asian and White participants across multiple levels of covariate adjustment while associations among Black participants were attenuated and associations among Latinos become non-significant. Overall, results suggest that grip strength and gait speed are correlated with late-life cognition; the relationship of gait speed with cognition was non-linear and differed by race/ethnicity for executive function.
Our finding that Black participants had the strongest mean grip strength, followed by Whites, Asians, and Latinos is consistent with previous work.15–20 There is evidence that Black individuals maintain greater muscle mass and muscle quality than Whites with advancing age; though, this association is complicated by fat composition and prevalence of age-related comorbidities.24 Low socioeconomic status or being foreign-born has been associated with worse physical function among older adults in prior studies; this may explain the finding of weaker grip strength among Asians and Latinos in KHANDLE who were most likely to be among the 22% (n=305) of foreign born participants.16,20,24 However, differences in baseline grip strength were not indicative of disparities in the association of grip strength with cognition. Despite these racial/ethnic differences, increased grip strength was associated with better cognition across all racial/ethnic groups and all cognitive domains. This finding is consistent with the literature and indicates that despite racial/ethnic differences in grip strength, this measure of physical function is independently associated with late-life cognition among diverse populations of older adults.7,8,11,12
White participants in KHANDLE had the fastest gait speed followed by Latino, Asian, and Black participants. Previous studies have primarily found that Whites have faster mean gait speed than other racial/ethnic groups15,16,18 These differences are thought to be related to higher prevalence of co-morbidities such as obesity, diabetes, and cardiovascular disease in racial/ethnic minorities as well as influences of socioeconomic status.2,19,25 As with previous literature, we found that increased gait speed was positively and non-linearly associated with cognition.6,7,10,26,27 However, we noted that the association of gait speed with executive function significantly differed by racial/ethnic group. To our knowledge, no previous work has assessed racial/ethnic differences in the association of gait speed and cognition in a diverse cohort. Our findings are in agreement with existing literature that gait speed is an important indicator of physical function and associated with multiple cognitive domains.28 The racial/ethnic differences in the association of gait speed and executive function are particularly notable given the growing body of evidence suggesting gait changes are related to executive function deficits.28,29 Further research is needed to understand why there may be racial/ethnic differences in the association of gait speed with executive function and whether these differences persist in longitudinal assessments.
Our findings underscore the important relationship between physical function and cognitive function among older adults. Our analyses have several strengths including a large racially/ethnically diverse cohort of community-dwelling older adults whose physical function and cognition were systematically measured to be able to assess racial/ethnic differences. Nevertheless, there were limitations. We were limited to a cross-sectional examination of the association of physical function and cognition. There is some evidence that the relationship between physical function and cognition may be bi-directional, and we are unable to determine temporality.3,29 Further, several of the covariates we included in our models relied on self-report, which may lead to issues of recall bias and measurement error. Another limitation was that we could not assess cognitive decline, only cognitive function at wave 1. While we found positive associations between greater grip strength and faster gait speed with cognition, we cannot determine whether those with weaker grip or slower gait are predisposed to pathologic cognitive decline or dementia, just that their cognition was worse compared to those with stronger grip and faster gait. However, evidence from the literature suggests these physical function measures are risk factors for subsequent MCI and dementia, though examination of potential racial/ethnic differences are still needed.7,11,26
There is clear evidence that grip strength and gait speed are related to late-life cognitive function, but our findings suggest there may be racial/ethnic differences in these associations, specifically gait speed and executive function, that require further examination. Efforts to retain physical function with advancing age, particularly among racial/ethnic minorities, may contribute to maintenance of cognitive function and reduction of health disparties.3 Future research is needed to examine whether there are racial/ethnic differences when assessing change in physical function over time as well as whether there are racial/ethnic differences in the relationship of physical measures with cognitive decline and dementia.
Supplementary Material
Acknowledgements:
This work was funded by the National Institutes of Health, National Institute on Aging under grant number RF1AG052132, R01AG066132 (PI: Gilsanz), and R00AG053410 (PI: Mayeda).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References:
- 1.van der Flier WM, Scheltens P. Epidemiology and risk factors of dementia. J Neurol Neurosurg Psychiatry. 2005;76 Suppl 5:v2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in Older Adults: A Nationally Representative Profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70(11):1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borges MK, Canevelli M, Cesari M, Aprahamian I. Frailty as a Predictor of Cognitive Disorders: A Systematic Review and Meta-Analysis. Front Med (Lausanne). 2019;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doi T, Tsutsumimoto K, Nakakubo S, et al. Physical Performance Predictors for Incident Dementia Among Japanese Community-Dwelling Older Adults. Phys Ther. 2019;99(9):1132–1140. [DOI] [PubMed] [Google Scholar]
- 6.Quan M, Xun P, Chen C, et al. Walking Pace and the Risk of Cognitive Decline and Dementia in Elderly Populations: A Meta-analysis of Prospective Cohort Studies. J Gerontol A Biol Sci Med Sci. 2017;72(2):266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hooghiemstra AM, Ramakers I, Sistermans N, et al. Gait Speed and Grip Strength Reflect Cognitive Impairment and Are Modestly Related to Incident Cognitive Decline in Memory Clinic Patients With Subjective Cognitive Decline and Mild Cognitive Impairment: Findings From the 4C Study. J Gerontol A Biol Sci Med Sci. 2017;72(6):846–854. [DOI] [PubMed] [Google Scholar]
- 8.Vancampfort D, Stubbs B, Firth J, Smith L, Swinnen N, Koyanagi A. Associations between handgrip strength and mild cognitive impairment in middle-aged and older adults in six low- and middle-income countries. Int J Geriatr Psychiatry. 2019;34(4):609–616. [DOI] [PubMed] [Google Scholar]
- 9.Sibbett RA, Russ TC, Allerhand M, Deary IJ, Starr JM. Physical fitness and dementia risk in the very old: a study of the Lothian Birth Cohort 1921. BMC Psychiatry. 2018;18(1):285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hackett RA, Davies-Kershaw H, Cadar D, Orrell M, Steptoe A. Walking Speed, Cognitive Function, and Dementia Risk in the English Longitudinal Study of Ageing. J Am Geriatr Soc. 2018;66(9):1670–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Grip strength and the risk of incident Alzheimer’s disease. Neuroepidemiology. 2007;29(1-2):66–73. [DOI] [PubMed] [Google Scholar]
- 12.Shin HY, Kim SW, Kim JM, Shin IS, Yoon JS. Association of grip strength with dementia in a Korean older population. Int J Geriatr Psychiatry. 2012;27(5):500–505. [DOI] [PubMed] [Google Scholar]
- 13.Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386(9990):266–273. [DOI] [PubMed] [Google Scholar]
- 14.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol. 2009;66(11):1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espinoza SE, Hazuda HP. Frailty in older Mexican-American and European-American adults: is there an ethnic disparity? J Am Geriatr Soc. 2008;56(9):1744–1749. [DOI] [PubMed] [Google Scholar]
- 16.Haas SA, Krueger PM, Rohlfsen L. Race/ethnic and nativity disparities in later life physical performance: the role of health and socioeconomic status over the life course. J Gerontol B Psychol Sci Soc Sci. 2012;67(2):238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Looker AC, Wang CY. Prevalence of reduced muscle strength in older U.S. adults: United States, 2011-2012. NCHS Data Brief. 2015(179):1–8. [PubMed] [Google Scholar]
- 18.Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55(8):1216–1223. [DOI] [PubMed] [Google Scholar]
- 19.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 20.Thorpe RJ, Simonsick E, Zonderman A, Evans MK. Association between Race, Household Income and Grip Strength in Middle- and Older-Aged Adults. Ethn Dis. 2016;26(4):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mungas D, Reed BR, Crane PK, Haan MN, Gonzalez H. Spanish and English Neuropsychological Assessment Scales (SENAS): further development and psychometric characteristics. Psychol Assess. 2004;16(4):347–359. [DOI] [PubMed] [Google Scholar]
- 22.Mungas D, Reed BR, Haan MN, Gonzalez H. Spanish and English neuropsychological assessment scales: relationship to demographics, language, cognition, and independent function. Neuropsychology. 2005;19(4):466–475. [DOI] [PubMed] [Google Scholar]
- 23.Brewster PW, Melrose RJ, Marquine MJ, et al. Life experience and demographic influences on cognitive function in older adults. Neuropsychology. 2014;28(6):846–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51(3):323–330. [DOI] [PubMed] [Google Scholar]
- 25.Blanco I, Verghese J, Lipton RB, Putterman C, Derby CA. Racial differences in gait velocity in an urban elderly cohort. J Am Geriatr Soc. 2012;60(5):922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dumurgier J, Artaud F, Touraine C, et al. Gait Speed and Decline in Gait Speed as Predictors of Incident Dementia. J Gerontol A Biol Sci Med Sci. 2017;72(5):655–661. [DOI] [PubMed] [Google Scholar]
- 27.Taylor ME, Lasschuit DA, Lord SR, et al. Slow gait speed is associated with executive function decline in older people with mild to moderate dementia: A one year longitudinal study. Arch Gerontol Geriatr. 2017;73:148–153. [DOI] [PubMed] [Google Scholar]
- 28.Demnitz N, Esser P, Dawes H, et al. A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait Posture. 2016;50:164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Best JR, Liu-Ambrose T, Boudreau RM, et al. An Evaluation of the Longitudinal, Bidirectional Associations Between Gait Speed and Cognition in Older Women and Men. J Gerontol A Biol Sci Med Sci. 2016;71(12):1616–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


