Abstract
Glioblastoma – the most aggressive form of brain cancer, comprises a complex mixture of tumor cells and nonmalignant stromal cells, including neurons, astrocytes, microglia, infiltrating monocytes/macrophages, lymphocytes, and other cell types. All non-malignant cells within and surrounding the tumor are affected by the presence of glioblastoma. Astrocytes use multiple modes of communication to interact with neighboring cells. Extracellular vesicle-directed intercellular communication has been found to be an important component of signaling between astrocytes and glioblastoma in tumor progression. In this review we focus on recent findings on extracellular vesicle-mediated bilateral crosstalk between glioblastoma cells and astrocytes, highlighting the pro- and anti-tumor roles of astrocytes in glioblastoma development.
Keywords: Astrocytes, Glioma, Exosomes, Tumor microenvironment, Crosstalk
ASTROCYTES WITHIN THE TUMOR MICROENVIRONMENT
The focus of neuro-oncological research has expanded to include the cellular microenvironment of the tumor [1-3]. Hence, it is now acknowledged that tumor cells are part of a dynamic cellular network rather than being completely independent, with this network contributing to the aggressive nature of the tumor. Glioblastoma – the most malignant form of brain cancer is characterized by its diffuse growth, heterogeneous genetic and phenotypic composition, and complex mixture of tumor cells and nonmalignant cells [3]. In this review, we will refer to different grades of glioma (see Glossary) as glioblastoma in vivo, since in most studies, the level of malignancy was not addressed, and use the term glioma for most culture cell studies according to the authors’ designation. The heterogeneity of glioblastoma occurs at multiple levels which makes this brain tumor challenging to study and currently incurable [4]. First, inter-tumor heterogeneity is reflected by different subtypes of tumors, which are typically classified into proneural, classical and mesenchymal based on transcriptional expression profiles [5, 6]. These subtypes dynamically change over time, alter their induced expression profile upon therapy, and can co-exist in different regions of the same tumor [7]. A single-cell sequencing study characterized this heterogeneity of the cell states of a tumor in detail showing that these are influenced by the tumor microenvironment [8].
The second layer of heterogeneity is the unique tumor microenvironment of the tumor. The cell population within the tumor as well as the tumor surroundings consists of a variety of glia subtypes, including astrocytes, oligodendrocytes, ependymal cells and microglia, as well as infiltrating adaptive and innate immune cells, such as lymphocytes and myeloid-derived monocytes/macrophages [3]. Among these cell types, astrocytes are the most abundant. Overall, astrocytes make up about half of the total brain cell population and have many roles in both healthy and diseased brain [9]. Their functions are broad, spanning many aspects of brain (patho)physiology, and while addressing all of them in detail is beyond the scope of this review, they have been summarized in numerous reports [10-12]. There is a range of astrocyte phenotypes, as they are highly variable in morphology and function, but they can broadly be defined as “homeostatic cells” because one of their main functions is the preservation of homeostasis in the healthy brain [12].
Astrocytes have an enormous impact on healthy and diseased brain functioning, and their critical role in glioblastoma progression has been increasingly recognized [11]. Therefore, it is not surprising that they maintain complex interactions with these tumor cells. Indeed, astrocytes actively interact with tumor cells and utilize a variety of communication strategies including direct cell-cell contact through gap junctions, ion channels, microtubules and tunneling nanotubes [13, 14], and indirectly through the secretion of molecules, the release of gliotransmitters, and extracellular vesicles (EVs) [13]. These interactions influence the growth, migration, survival and treatment resistance of brain tumors [13-16]. Here we will focus on recent insights into EV-directed communication between astrocytes and tumor cells, highlighting its contribution to glioblastoma development.
ASTROCYTE ACTIVATION
To understand the role of astrocytes in glioblastoma development it is important to discuss the process of astrocyte activation (i.e. astrogliosis). Astrogliosis is a defense mechanism of the central nervous system (CNS) to minimize and repair initial damage from brain injuries, with the main purpose to isolate non-injured tissue from damaged brain areas; it is characterized by specific molecular, cellular and functional alterations in glial cells, resulting in so called reactive astrocytes [17]. This transformation (Fig 1), in which astrocytes are activated in response to threats, such as trauma, infections, inflammation and tumors, is characterized by increased expression of glial fibrillary acidic protein (GFAP), nestin and vimentin [18], as well as markedly enhanced expression of the gap junction protein Connexin 43 (Cx43) [19-21]. On top of increased GFAP levels, severe brain injuries can also lead to phenotypic changes within astrocytes characterized by cell body hypertrophy associated with the diffuse extension of distant processes [22]. These phenotypic changes have been described in mouse models extensively [22], but can also be found when studying the interactions between astrocytes and glioma cells using co-cultures (Box 1). Astrocytes are triggered into an activated state induced during glioblastoma development and are transformed into reactive astrocytes as the tumor progresses. This was shown using transgenic mice expressing luciferase regulated by the GFAP promotor. Here, astrogliosis has been found to peak three days after intracranial injection of glioblastoma cells in xenograft mouse models [23]. In the latter case, astrocytes migrate directly towards the tumor border where they transform into reactive astrocytes, facilitating tumor progression, proliferation and migration through multiple relatively well-understood signaling pathways, such as the nuclear factor kappa B (NF-κB) and transforming growth factor-β (TGF-β) pathways [24, 25]. Although this process is normally intended to protect healthy brain tissue, these signaling pathways appear to be co-opted by glioblastoma cells to change the astrocytes towards a more tumor-supportive phenotype [26]. Examples of their tumor-supportive role can be found in in vitro studies, where injury results in an astrocyte reactive phenotype together with subsequent transcriptional and secretome changes associated with increased tumor cell proliferation and migration. Astrocytes activated by glioblastoma produce tumor-promoting factors, such as TGF-β, thereby enhancing glioblastoma cell invasion [27]. In addition, signal transducer and activator of transcription 3 (STAT-3) is expressed in reactive astrocytes surrounding metastatic brain lesions [28]. Expression of this gene is also elevated in a subpopulation of reactive astrocytes associated with metastases of peripheral tumors to the brain and correlates with reduced patient survival time [28]. Most importantly, blocking STAT-3 had an effective anti-tumor effect, indicating that targeting reactive astrocytes could offer a possible therapeutic strategy against CNS tumors [28]. Activation of astrocytes can also be caused by treatment of tumors. Upon tumor resection, as demonstrated in a murine model, the brain injury causes reactive astrocytes to promote growth and invasion of the recurrent glioma [29]. This demonstrates that defining the stimuli and mechanisms of astrocyte activation can yield insights into potential therapeutic opportunities targeting this tumor-supportive process.
Figure 1. Astrocyte activation upon tumor development.
Anti-tumor effects of astrocytes (left): In the early phase of tumor progression, astrocytes (orange) act to protect healthy cells from glioma cells (pink). Astrocytes express IL1-β and uptake neurotransmitters, such as glutamate, which protects neurons and leads to reduced tumor migration and growth as well as increased tumor cell apoptosis. Pro-tumor effects of astrocytes (right): As the tumor develops and tumor cells move into a more advanced stage, astrocytes lose the ability to re-uptake neurotransmitters, and the expression of the voltage-gated K+ channel, Kv1.3 which regulates glutamate buffering, increases. These changes disturb the homeostatic properties of astrocytes and cause elevated levels of extracellular glutamate. Over time, astrocytes transform into reactive astrocytes, which are characterized by increased levels of GFAP and the gap-junction protein Cx43, and changes in morphology including a hypertrophic cell body and extended processes. The signaling pathways TGF-β, NF-κB and STAT3 are highly active in reactive astrocytes. Reactive astrocytes typically migrate to the tumor border, and the secretion of cytokines, growth factors such as TGF-β and MMPs are enhanced. Together, this results in a breakdown of the extracellular matrix (ECM), and increased invasion, migration and proliferation of the tumor cells.
Box 1: Co-culturing astrocytes with glioma cells to study cell-cell interactions.
It has been challenging to study the communication between brain tumors and its microenvironment due to the paucity of adequate models. One of the first co-culture models to study glioblastoma and astrocyte interactions was reported in 1996 [79]. In co-cultures, cells were grown with some degree of contact between them, allowing multiple cell types, such as astrocytes and/or microglia, to be cultured together with tumor cells to examine the effect of one cell type on the other [80]. Co-culturing approaches may more closely represent the complex network of CNS and tumor cells rather than single cell cultures, thus allowing the study of physiological release-uptake dynamics and enabling the assessment of multiple mediators between the different cell types [60]. Studying the tumor microenvironment interactions is largely hampered by the difficulty of co-culturing glioma cells with primary glia, as they have distinct culture conditions. Yet, multiple co-culture assays are currently used to address cell-to-cell communication including: Transwell plates [81]; microfluidic platforms [82] or solid support, such as three-dimensional scaffolds [83]; hydrogels [84]; and microarrays [85]. These methods are used in studying aspects of intracellular cross-talk such as metabolic interactions, vessel formation, the role of glioma stem cell-like cells and the impact of astrocytes on the response of glioma cells to different therapeutic agents [60, 86]. Of note, co-culturing may shed light on non-direct cellular interactions, including EV transfer, which do not require adjacent colocalization in the tumor microenvironment. Intriguingly, patient-derived glioblastoma organoids might provide an even better model to study intercellular communication, since organoids closer recapitulate the parental tumor heterogeneity[87].
The pro-tumor versus anti-tumor capacity of reactive astrocytes is a dynamic process. Interestingly, immunohistochemistry analyses of seven post-mortem glioblastoma cases indicated that reactive astrocytes at the tumor edge do not express IL-6, important for tumor growth, but do express interleukin (IL)1-β, an immune suppressive cytokine. This may indicate that astrocytes at the tumor border are conflicted in their response. Early in tumor formation they may tend towards protecting healthy cells from the damage caused by the tumor [30]. The protective role of astrocytes in the context of glioma has been shown in studies focusing on the ability of astrocytes to re-uptake neurotransmitters, such as glutamate. Glutamate is an excitatory transmitter that can cause cell toxicity when overexpressed [31]. In a healthy brain, glutamate is released from neurons and predominantly taken up by astrocytes to maintain balanced levels of glutamate [31]. In early-stage glioma development, a border of astrocytes is created around the tumor. When mixing glioma cells with astrocytes in culture it was found that large numbers of astrocytes are able to completely eliminate excessive glutamate levels, thus preventing neuronal cell death, while at the same time limiting glioma expansion, through reduction of the stimulatory role of glutamate on glioma growth. However, when glutamate is deaminated by glutaminase, ammonia is released, partly by glioma cells, which induces astrocyte swelling and dysfunction of glutamate uptake. This means, in advanced stages of glioma, astrocytes are no longer able to maintain glutamate homeostasis, as high levels of ammonia damaged the astrocytic glutamate uptake, resulting in neuronal injury providing space for tumor growth which is beneficial for glioma progression [32]. Interestingly, the voltage-gated K+ channel, Kv1.3 regulates glutamate buffering within astrocytes. In the context of glioma, the Kv1.3 channel has increased activity which disturbs the homeostatic properties of astrocytes. The inhibition of Kv1.3 through a specific inhibitor 5-(4-phenoxybutoxy) psoralen (PAP-1), increased glutamate uptake by astrocytes, thus reducing glioma-induced neurotoxicity [33]. These findings suggest that astrocytes have a role in neuronal protection and anti-tumorigenic properties at an early stage in glioma development, however over time under the influence of glioma cells, astrocytes lose the ability to buffer glutamate and their protective effect on neurons. We hypothesize that, as the tumor develops, astrocytes lose their protective role and change towards a pro-tumorigenic phenotype (Fig 1). Together, recent studies highlight how astrocytes respond to brain tumor development, which is achieved through multiple modes of communication. Here, we will focus on the EV-mediated crosstalk between astrocytes and glioblastoma (summarized in Fig 2, Key Figure).
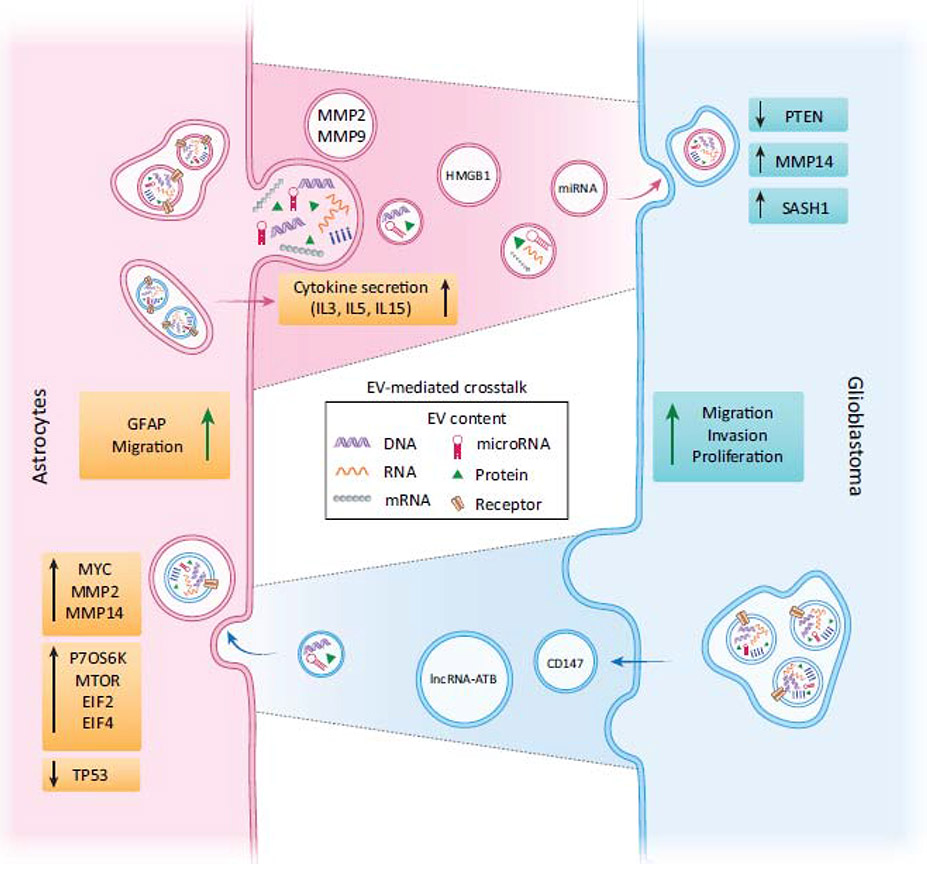
Figure 2 (Key Figure). Schematic illustration of bi-directional EV-mediated communication between astrocytes and glioblastoma cells.
EVs can transport different types of cargo, including DNA, RNA, receptors and other proteins. Both astrocytes and glioblastoma cells shed EVs, carrying various cargoes as illustrated in the schematic. Moreover, EVs that originate from glioblastoma cells contain CD147, which increases MMP expression in astrocytes and in turn contributes to the breakdown of the ECM, leading to enhanced invasion of glioblastoma cells. Gfap mRNA is upregulated in astrocytes, indicating a conversion to reactive astrocytes in the context of glioblastoma. Moreover, the MYC signaling pathway, an oncogene, is activated in astrocytes, whereas TP53, a tumor suppressor gene is downregulated upon glioblastoma-derived EV uptake, suggesting that astrocytes transform to tumor-like cells. Both mTOR and p70S6K signaling pathways, critical for translational regulation, and initiation and control of protein synthesis, are upregulated in astrocytes as a result of exposure to glioblastoma-derived EVs. Concurrently, EVs shed by astrocytes contain multiple PTEN targeting miRNAs, including miR-19b and miR-20a, leading to a decrease in PTEN expression in the tumor cells, which enhances tumor cell proliferation. In addition, astrocytes induce an upregulation of MMP14 in glioblastoma cells which promotes ECM degradation and tumor cell migration. Taken together, glioblastoma cells are able to alter astrocytes by the release of EVs, which change the phenotype of astrocytes to become supportive of tumor progression. The “transformed” astrocytes shed EVs supporting glioblastoma cells instead of defending the CNS.
EV-MEDIATED COMMUNICATION BETWEEN ASTROCYTES AND GLIOBLASTOMA
Several studies have shown that EVs, signaling vehicles with multifaceted functions in neuronal and glial crosstalk, have a crucial role in the communication among cell types within glioblastoma and its microenvironment [34, 35]. EVs are membrane-enclosed nanospheres that contain proteins, lipids and nucleic acids, such as DNA, mRNA and non-coding RNAs, and are released by most cells [36, 37]. EV-mediated communication is unique compared to other communication pathways, since it allows the delivery of combinatorial vesicle-enclosed cargo, which is not soluble, and is protected from degradation, with access not only to the neighboring tumor cells, but also to distant sites [38-40]. A number of studies have demonstrated that the tumor microenvironment, particularly the stromal cells in the tumor, contributes to the malignant behavior of tumor cells [3, 41, 42]. Almost all normal cells within and surrounding the tumor are affected by the glioblastoma, including astrocytes [1].
Effects of glioblastoma-derived EVs on astrocytes
Importantly, glioblastoma-derived EVs can be taken up by different cells in the tumor environment, such as endothelial cells, microglia, monocytes [43-45] and astrocytes [46]. EVs represent one of the mechanisms by which cancer cells modulate the tumor microenvironment to stimulate their growth, invasion, neo-angiogenesis, oncogenic transformation and suppression of the immune response [47, 48]. Evidence suggests that the content of glioblastoma-derived EVs, at least to a certain extent, mirrors the phenotypic and genotypic signature of the respective glioblastoma cells. This supports the use of EV-associated biomarkers, such as CD44, a multifunctional plasma membrane protein with an important predictive value for glioblastoma subtyping [49, 50], and detection of tumor driver mutations, such as EGFRvIII [51, 52].
What is the functional effect of the uptake of glioblastoma-derived EV by astrocytes? One study found that uptake of EVs derived from surgically resected primary glioblastoma tumors drove primary human astrocytes towards a tumor-enhancing phenotype in culture [46]. Glioblastoma-derived EVs taken up by primary human astrocytes in vitro increased their migratory capabilities, activated oncogenic signaling pathways, including epidermal growth factor (EGF), receptor-type tyrosine-protein kinase (FLT), interleukin 22 (IL-22) and mitogen activated protein kinase (MAPK), and converted them into reactive astrocytes defined by increased Gfap levels.
From a neurodevelopmental perspective, the increase in GFAP levels raises the question whether these astrocytes are part of the tumor stem cell evolution. Interestingly, abnormal development of glial progenitors, including astrocyte precursor cells appear to contribute to tumorigenesis [53], and gliomas consist of a substantial proportion of glial progenitor-like cells [54]. In addition, it was revealed that distinct glial progenitors present in brain development are similar to the progenitors present during tumorigenesis [55, 56].
Another observation showing the dynamics between astrocyte phenotype and tumors is found in the notion that astrocytes undergo changes based on their location in the healthy and pathological brain [57-59]. This all indicates that astrocyte progenitors can potentially transform into glioma cells, which can also be driven by glioma-derived EVs. To start, it was confirmed that these two cells types communicate by showing that extracellular RNA was transferred from glioma stem cells to astrocytes through EVs [60]. Interestingly, glioblastoma-derived EVs facilitated neoplastic growth of astrocytes but not normal human or mouse astrocytes by reprogramming glycolysis and oxidative phosphorylation through EV-mediated transfer of miRNAs, inducing proliferation, self-renewal, and colony formation of activated astrocytes and enhanced malignant growth of astrocytes in a mouse allograft model [61]. In vitro studies have also shown that transformed astrocytes have an increased ability to take up EVs. In detail, the spontaneous cellular transformation of human astrocytes during serial culturing resulted in a higher level of EV uptake [62]. The implications of this observation and whether this also occurs during the activation of pro-tumorigenic astrogliosis is still to be examined. However, EVs released by patient-derived glioblastoma stem cells do stimulate astrocytes to acquire a transformed-like phenotype through inhibition of TP53, an important tumor suppressor gene [63], in combination with activation of MYC, a proto-oncogenic signaling molecule important in the regulation of cell proliferation [64]. Thus, changes in these signaling pathways in normal astrocytes exposed to glioblastoma-derived EVs reveals mechanisms by which glioblastomas manipulate astrocytes to create a tumorigenic microenvironment [65].
In addition, EVs isolated from genetically unstable, oncogene-driven glioma cells can trigger the formation of micronuclei in neighboring cells which are associated with genotoxicity and chromosomal instability. Micronuclei were found in both endothelial cells and hTERT-immortalized human astrocytes, exposed to glioma-derived EVs demonstrating their impact on chromosomal stability in recipient cells [66]. Extensive proteomic analyses have been performed on astrocytes treated with EVs derived from glioblastoma stem-like cells compared to untreated astrocytes. Here, EIF2, eIF4, p70S6K, and mTOR signaling pathways, critical for translational regulation, and initiation and control of protein synthesis, were shown to be significantly enhanced stimulating proteome changes and mediating a transformed phenotype in in astrocytes treated with glioblastoma-derived EVs [65]. Taken together, glioblastoma-derived EVs can morph astrocytes into tumor-like cells.
More indications of the functional consequences of EVs were found when astrocytes were incubated with glioblastoma-derived EVs and the medium collected from these cells, with medium collected from astrocytes not exposed to glioblastoma-derived EVs serving as a control. Interestingly, glioblastoma cells incubated, in turn, with the ‘EV-treated astrocyte growth medium’ proliferated at a significantly faster rate than the same cells treated with ‘control astrocyte growth medium’, as measured by a NAD(P)H-dependent dehydrogenase- based assay evaluating the metabolic activity of cells [46]. In parallel, by using a rat glioma cell line, treatment of astrocytes with tumor cell-derived EVs increased the rate of astrocyte proliferation and elevated the mRNA expression levels of Gfap [67]. Several pieces of evidence showed that this is consistent with the ability of glioblastoma cells to convert astrocytes into reactive astrocytes – as measured by increased GFAP expression, mediated at least in part via EVs [68]. One of the mechanisms through which astrocytes can be activated is through the EV-mediated transfer of the glioma-derived lncRNA activated by TGFβ - lncRNA-ATB [68]. Upon uptake, this glioma-derived lncRNA downregulates miR-204-3p in astrocytes leading to an increase in miR-204-3p mRNA target Gfap. This lncRNA-ATB related to the activation of astrocytes is also associated with increased glioma cell invasion [68]. Importantly, the involvement of a specific lncRNA in increased glioma cell invasion provides a promising opportunity to target this lncRNA for therapeutic purposes.
Why would the activation of astrocytes be impactful in glioblastoma development? One possible explanation is because it is accompanied by the increased ability to breakdown the extracellular matrix (ECM) through elevated matrix metalloproteinase (MMP) levels, which is important for tumor growth and invasion [68]. The secretion and overexpression of MMPs, including MMP2, MMP9 and MMP14, by astrocytes exposed to glioblastoma-derived EVs, contributes to protease-based destruction of the ECM to facilitate glioblastoma cell invasion [46, 67]. As discussed above, treatment of tumors can have an adverse effect through astrocytes, a process which can be mediated by EVs [28]. In response to ionizing radiation, glioblastoma cells increase the levels of CD147 shed in EVs. This protein, involved in regulation of MMPs enhances MMP9 release from astrocytes exposed to these EVs by increasing JNK signaling, a process that was blocked by knocking out CD147 in glioma cells [69]. Since MMPs break down ECM, this result supports a tumor microenvironment-mediated role of CD147 in glioblastoma invasiveness and reveals a prominent role for ionizing radiation, used in treatment for glioblastoma, in enhancing this effect [69]. In addition, astrocytes exposed to glioblastoma-derived EVs were tested in an ‘invadopodia’ assay to determine the effects on astrocyte migration and ECM degradation [65]. Invadopodia are specialized actin-based dynamic cell membrane protrusions that degrade the ECM to facilitate cell migration. Interestingly, the gelatin degradation ability of astrocytes was significantly enhanced after treatment with glioblastoma-derived EVs relative to untreated cells, supporting breakdown of the ECM in assisting glioblastoma cell invasion [65]. These data suggest that through the secretion of different MMPs, astrocytes facilitate in the remodeling of the tumor surroundings.
To further support growth and invasion of the tumor, astrocytes can also create an immune favorable environment for glioma cells. Human astrocytes treated with glioblastoma-derived EVs showed increased expression of interferon gamma (IFN-γ), IL12, IL1A, IL8, and IL1B, chemokine CXCL10, and factor C5 compared to astrocytes exposed to EVs derived from normal epithelial cells [46]. Several of these cytokines, such as CSF2 and 3, and IL4, 10 and 13, secreted by astrocytes upon treatment with glioblastoma-derived EVs are thought to regulate T cells and promoting immune suppression [46, 63]. In addition, uptake of glioblastoma-derived EVs upregulated the release of certain cytokines from astrocytes, such as the pro-inflammatory cytokine IFNγ and various interleukins (IL1A, IL1B, IL8 and IL12), implicating an autocrine stimulation of astrocytes to maintain the inflammatory milieu in a brain tumor setting [46]. Together, the pro-inflammatory cytokine environment of astrocytes treated with glioblastoma-derived EVs could potentially promote immune suppression, which is highly prevalent in glioblastoma patients.
While numerous studies have examined the EV-mediated communication in culture, the first indication (to our knowledge) of the communication between glioma cells and astrocytes in vivo was achieved using a transgenic mouse model [70]. In this model, astrocytes express the fluorescent marker tdTomato under a tamoxifen-inducible Cre-regulated GFAP promoter, and GFP-expressing glioma cells were transplanted into the brains of these mice. The interaction between astrocytes and glioma was monitored over 10 days post injection using intravital imaging. Here, the first indication of glioma-derived EV transfer was detection of GFP-positive astrocytes. Combining the Cre/LoxP system with longitudinal time-lapse live imaging, GFP/Cre-expressing glioma cells were found to transfer Cre to stromal cells as measured by tdTomato expression resulting from Cre recombinase activity. The majority of the tdTomato-positive cells were identified as astrocytes by GFAP staining. Interestingly, after EV purification by ultracentrifugation, both full length Cre and GFP mRNAs were present in EVs derived from glioma cells, supporting their ability to transport Cre and GFP mRNA and proteins derived from the tumor to astrocytes. To confirm that glioma-derived EV could transfer Cre to astrocytes, isolated EVs from GFP/Cre expressing glioma cells were injected into one hemisphere of C57BL/6J mouse brains. EV-mediated Cre transfer and subsequent recombination was detected by the presence of tdTomato-positive, GFAP positive astrocytes eight days post injection.
There are several outstanding questions related to effects of therapy on EV-mediated communication between astrocytes and glioma and whether therapeutic directions should be tethered towards this interaction. The first indication that EV-mediated communication between astrocytes and glioma cells could be changed under the influence of therapy was found in the observation that chemotherapy (i.e. temozolomide) alters the composition of EVs shed by glioma cells [71]. Whether this results in a pro-tumor or anti-tumorigenic switch of astrocytes after EV uptake needs to be studied.
Taken together, these results support the profound effect of glioblastoma-derived EVs on neighboring astrocytes, which contributes to alterations in the tumor microenvironment in ways which are beneficial to tumor progression. Future studies should focus on the effects of standard therapy on the uptake of EVs and subsequent phenotypical changes of the astrocytes.
Effects of astrocyte-derived EVs on glioblastoma
What effect do EVs secreted by astrocytes have on glioblastoma development? Recently, a growing body of evidence indicates that astrocytes, like many other cell types, use EVs as a mode to transfer information. The shedding of EVs from astrocytes was shown to be partly dependent on the activation of acidic sphingomyelinase [72]. Astrocytes are able to secrete both small and large EVs (ranging from 350 nm to 6.5 μm as determined by electron microscopy) from the cell-surface, with the latter containing lipid droplets, ATP and mitochondria, which possibly could be defined as apoptotic bodies [73]. Like most different cell types, astrocytes shed EVs with a heterogeneous composition in size and cargo, and that mediate both physiological and pathological functions. EVs are able to transfer pathogenic proteins, suggesting that EVs may contribute to particular pathologies [74].
It is thought that astrocytes initially act as a defensive mechanism against glioblastoma cells, since the presence of the tumor activates them, presumably as a protective shield for the surrounding neurons [75]. As discussed above, in response to glioblastoma infiltration, astrocytes undergo a series of structural and functional changes, first changing into reactive astrocytes associated with repair of the affected tissue. However, in the case of glioblastoma development, this process seems to be quickly diverted to support tumorigenesis and further contributes to tumor growth and invasion, including resistance to treatment (Fig 1) [26]. In co-culture models of astrocytes and brain metastatic tumor cells, EVs shed by astrocytes were found to decrease levels of tumor suppressor gene, PTEN, in tumor cells. Here, PTEN-targeting miRNAs, including miR-17, miR-19a, miR-19b, miR-20a and miR-92, which are transported via EVs from astrocytes to brain metastatic tumor cells, are involved in PTEN downregulation. This study showed that both human and mouse tumor cells have decreased PTEN expression, after dissemination to the brain, but not to other organs, indicating that PTEN is a key factor in priming the brain tumor microenvironment and critical for tumor growth [76]. Although recent studies have reported both pro- and anti-tumor roles for astrocytes in glioblastoma progression, the balance of evidence seems to point toward a pro-tumorigenic role for astrogliosis as the tumor progresses, with the release of growth factors and matrix remodeling enzymes. Although EV-mediated communication may play an important role in this transformation, further studies regarding astrocyte-derived EVs are necessary to establish a more complete picture of the complex tumor cell-astrocyte networks [77].
CONCLUDING REMARKS
Recently, the crosstalk between glioblastoma cells and astrocytes through the transfer of EV cargo has become an area of interest. Here, the balance of evidence seems to point toward a pro-tumorigenic role for astrocytes in later stages of glioma development, promoting tumor growth, migration and invasiveness. While the EV community has sought to provide consistent guidelines for how to isolate and setup EV-oriented experiments [78], the different findings discussed in this review on the role of EVs in astrocyte-glioblastomas communication used a variety of techniques (Table 1). Of note, the EV incubation times show a clear distinction between acute and chronic exposure, potentially affecting the reprogramming of cells present in the tumor microenvironment differently. For future research it will be important to adhere to the aforementioned guidelines to be able to qualitatively compare different studies. That said, one can conclude that glioblastoma-derived EVs participate in astrocyte activation in support of the migration and proliferation of glioma cells in vitro [46, 65] and glioblastomas in vivo [70]. Unraveling the biology of EV exchange between astrocytes and tumor cells may provide important therapeutic options to impede the transfer of genetic material and other cargo, and wrest control of the tumor microenvironment from glioblastoma cells. However, there are many unresolved questions regarding communication between glioblastoma cells and astrocytes via EVs (see Outstanding Questions). Now that more sophisticated animal models are available [70], the field can begin to clarify the complex role of astrocytes in the tumor microenvironment in vivo. Functional imaging techniques, such as methods visualizing calcium signaling will be important in understanding the homeostatic properties of astrocytes, and other novel imaging approaches will allow a better understanding of how astrocytes communicate and respond within the tumor microenvironment. Not only do these techniques potentially allow visualization and quantification of EV-mediated communication, but they may yield mechanistic insights that could potentially lead to novel therapeutic approaches. Taken together, further investigation of the intercellular communication between glioblastoma cells and astrocytes within their surroundings will bring researchers closer to understanding glioblastoma biology.
Table 1.
Extracellular vesicle procedures used in different studies
| Ref | Method | Cell lines | EV handling | |||
|---|---|---|---|---|---|---|
| Assay | EV incubation |
Glioblastoma | Astrocytes | EV isolation | EV characterization | |
| [69] |
in vitro in vivo |
12 and 24 hours | T98G U-87 MG U-118 MG Human glioma |
Human fetal glial cells SVG (ATCC) | Ultracentrifugation | Nanoparticle tracking analysis (NanoSight) |
| [66] | in vitro | 7 days | U373P U373VIII Human glioma |
Normal human astrocytes | Ultracentrifugation | Transmission electron microscopy |
| [70] |
in vitro in vivo |
Not done | 73 C mice glioma | Mouse primary astrocytes | Ultracentrifugation | • Transmission electron microscopy • Dynamic laser scattering technique |
| [65] | in vitro | 24 hours | U-87 MG human glioblastoma | Human primary astrocytes | Ultracentrifugation Iodixanol density gradient |
• Nanoparticle tracking analysis (NanoSight) • Invadopodia assay Transmission Electron Microscopy |
| [46] | in vitro | 4, 8 and 24 hours | Surgically resected primary tumors | Normal human astrocytes | Ultracentrifugation | • Nanoparticle tracking analysis (NanoSight) • Exo-Check arrays (System Biosciences) |
| [67] | in vitro | 1, 3 and 5 days | C6 Rat glioma | Normal human astrocytes | Ultracentrifugation | • Dynamic light scattering • Transmission electron microscopy |
| [61] |
in vitro in vivo |
2 and 5 days | GBM8 GBM4 Human glioma |
Normal human astrocytes | Ultracentrifugation | • Transmission electron microscopy |
| [62] | in vitro | 7 days | U373p U87 Human glioma |
Normal human astrocytes | Ultracentrifugation | • Transmission electron microscopy |
OUTSTANDING QUESTION BOX.
To what extent do the different types of EVs secreted by glioblastoma cells influence astrocytes? Does the transition to pro-tumorigenic astrocytes lead to increased EV uptake by astrocytes? Are there ways to suppress specific EV-mediated communication?
Astrocytes collectively form a border around the tumor edge. Is this migration due to signals transferred through EVs?
The glial scar is claimed to function as a defense mechanism of the brain to protect non-tumor cells from being invaded by glioblastoma cells. Many studies, however, have shown that these tumor-associated astrocytes become tumor supportive. This raises the question, do all astrocytes become pro-tumorigenic over time, or are there different subtypes of astrocytes that are “lured” into the tumor microenvironment? To what extent is this dependent on the glioma subtype?
Now that more sophisticated animal models are available, scientists can start to investigate the complex interactions between astrocytes and the tumor microenvironment. How can these results be translated to humans?
Astrocytes are influenced by the uptake of glioma-derived EVs. Can EVs loaded ex vivo or in vivo with specific compounds be used as therapeutic agents?
HIGHLIGHTS.
The ability of glioblastoma cells to recruit healthy brain cells into pro-tumor cells has gained interest. This affects all cell types, including astrocytes, the homeostatic cells of the brain, which are designed to protect the brain, but may be coerced into supporting gliomas.
Astrocytes act to isolate non-injured tissue from damaged brain areas, and accordingly, migrate towards the tumor border as a possible defense mechanism. However, there is evidence that glioblastoma can transform astrocytes into an active state, defined by increased levels of glial fibrillary acidic protein (GFAP) and pro-tumor factors.
Astrocytes maintain close interactions with tumor cells, creating complex communication networks. Extracellular vesicle-mediated crosstalk has an important role in tumor progression. Further understanding of these mechanisms may open perspectives for future therapy.
ACKNOWLEDGEMENT
We thank Mrs. Suzanne McDavitt for her skilled editorial assistance. Xandra Breakefield acknowledges NIH NCI CA179563, CA069246 and CA232103 grants for supporting this work. U19 CA179563 is supported by the NIH Common Fund, through the Office of Strategic Coordination/Office of the NIH Director.
GLOSSARY
- Extracellular vesicles
Are nanosized, membrane bound particles with a lipid bilayer able to transport functional cargo. Most if not all cell-types release extracellular vesicles, which carry a heterogeneous cargo consisting of proteins, DNA, RNA, lipids, metabolites and even organelles derived from the parent cell and are known to have an important role in intercellular communication. Extracellular vesicles hold promise as diagnostic biomarkers, therapeutic targets and therapeutic vehicles.
- Glioma
A general term to describe the malignant tumors originated from the glia and stem cells in the brain. Glioma defines a broad category of brain and spinal cord tumors which are named based on the specific type of brain cells affected. Gliomas are categorized in four grades according to the level of aggressiveness and each has different types of cells present and different treatment strategies. Glioblastoma, the most common form of primary brain tumors, is a grade IV glioma, which is the most aggressive form and has three major genetic subtypes.
- Gliotransmitters
Chemicals released by all glial cell-types. Glia-derived transmitters released by astrocytes include glutamate, D-serine and adenosine triphosphate (ATP) which act on neurons to regulate synaptic neurotransmission and neuronal plasticity. It is still unknown whether there is a difference in gliotransmitters between different types of astrocytes, or how they affect glioma growth.
- Reactive astrocytes
Astrocytes which have undergone astrogliosis – a process defined by proliferation and morphological alterations, such as hypertrophy, enhanced synthesis of neurotrophins and inflammatory mediators, and increased levels of GFAP. The transformation of reactive astrocytes is a widely observed phenomena and functions as a defense mechanisms to minimize destruction of brain tissue after CNS injuries, including tumor development.
- Tumor microenvironment
The area within and around the tumor, including the inclusive and surrounding blood vessels, extracellular matrix and a mixture of stromal cell types, secreted factors, signaling molecules and EVs. The tumor and its inclusive environment are in close contact and dynamically interact, which affects tumor development and progression, and even influences the therapeutic responses and resistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1.Broekman ML et al. (2018) Multidimensional communication in the microenvirons of glioblastoma. Nature reviews. Neurology 14 (8), 482–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charles NA et al. (2012) The brain tumor microenvironment. Glia 60 (3), 502–14. [DOI] [PubMed] [Google Scholar]
- 3.Quail DF and Joyce JA (2017) The Microenvironmental Landscape of Brain Tumors. Cancer cell 31 (3), 326–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen PY et al. (2020) Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol 22 (8), 1073–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verhaak RG et al. (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17 (1), 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q et al. (2017) Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer cell 32 (1), 42–56.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel AP et al. (2014) Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344 (6190), 1396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neftel C et al. (2019) An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 178 (4), 835–849.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dossi E et al. (2018) Human astrocytes in the diseased brain. Brain Res Bull 136, 139–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimelberg HK and Nedergaard M (2010) Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics 7 (4), 338–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandao M et al. (2019) Astrocytes, the rising stars of the glioblastoma microenvironment. Glia 67 (5), 779–790. [DOI] [PubMed] [Google Scholar]
- 12.Parpura V and Verkhratsky A (2012) The astrocyte excitability brief: from receptors to gliotransmission. Neurochem Int 61 (4), 610–21. [DOI] [PubMed] [Google Scholar]
- 13.Guan X et al. (2018) Reactive Astrocytes in Glioblastoma Multiforme. Mol Neurobiol 55 (8), 6927–6938. [DOI] [PubMed] [Google Scholar]
- 14.Gurke S et al. (2008) The art of cellular communication: tunneling nanotubes bridge the divide. Histochem Cell Biol 129 (5), 539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osswald M et al. (2016) A malignant cellular network in gliomas: potential clinical implications. Neuro Oncol 18 (4), 479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi SS et al. (2014) Human astrocytes: secretome profiles of cytokines and chemokines. PLoS One 9 (4), e92325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sofroniew MV (2014) Astrogliosis. Cold Spring Harb Perspect Biol 7 (2), a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Middeldorp J and Hol EM (2011) GFAP in health and disease. Prog Neurobiol 93 (3), 421–43. [DOI] [PubMed] [Google Scholar]
- 19.Theodoric N et al. (2012) Role of gap junction protein connexin43 in astrogliosis induced by brain injury. PLoS One 7 (10), e47311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liddelow SA and Barres BA (2017) Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 46 (6), 957–967. [DOI] [PubMed] [Google Scholar]
- 21.Sin WC et al. (2016) Astrocytes promote glioma invasion via the gap junction protein connexin43. Oncogene 35 (12), 1504–1516. [DOI] [PubMed] [Google Scholar]
- 22.Schiweck J et al. (2018) Important Shapeshifter: Mechanisms Allowing Astrocytes to Respond to the Changing Nervous System During Development, Injury and Disease. Front Cell Neurosci 12, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J et al. (2011) Non-invasive quantification of brain tumor-induced astrogliosis. BMC Neurosci 12, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H et al. (2020) Novel insights into astrocyte-mediated signaling of proliferation, invasion and tumor immune microenvironment in glioblastoma. Biomed Pharmacother 126, 110086. [DOI] [PubMed] [Google Scholar]
- 25.Matias D et al. (2018) Microglia/Astrocytes-Glioblastoma Crosstalk: Crucial Molecular Mechanisms and Microenvironmental Factors. Front Cell Neurosci 12, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien ER et al. (2013) The role of astrocytes in CNS tumors: pre-clinical models and novel imaging approaches. Front Cell Neurosci 7, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JK et al. (2014) Tumoral RANKL activates astrocytes that promote glioma cell invasion through cytokine signaling. Cancer Lett 353 (2), 194–200. [DOI] [PubMed] [Google Scholar]
- 28.Priego N et al. (2018) STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med 24 (7), 1024–1035. [DOI] [PubMed] [Google Scholar]
- 29.Okolie O et al. (2016) Reactive astrocytes potentiate tumor aggressiveness in a murine glioma resection and recurrence model. Neuro-oncology 18 (12), 1622–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagashima G et al. (2002) Immunohistochemical analysis of reactive astrocytes around glioblastoma: an immunohistochemical study of postmortem glioblastoma cases. Clin Neurol Neurosurg 104 (2), 125–31. [DOI] [PubMed] [Google Scholar]
- 31.Bak LK et al. (2006) The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem 98 (3), 641–53. [DOI] [PubMed] [Google Scholar]
- 32.Yao PS et al. (2014) Glutamate/glutamine metabolism coupling between astrocytes and glioma cells: neuroprotection and inhibition of glioma growth. Biochem Biophys Res Commun 450 (1), 295–9. [DOI] [PubMed] [Google Scholar]
- 33.Grimaldi A et al. (2018) Kv1.3 activity perturbs the homeostatic properties of astrocytes in glioma. Sci Rep 8 (1), 7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciccocioppo F et al. (2020) Extracellular Vesicles Involvement in the Modulation of the Glioblastoma Environment. J Oncol 2020, 3961735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matarredona ER and Pastor AM (2019) Extracellular Vesicle-Mediated Communication between the Glioblastoma and Its Microenvironment. Cells 9 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abels ER and Breakefield XO (2016) Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cellular and Molecular Neurobiology 36 (3), 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tkach M and Théry C (2016) Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 164 (6), 1226–1232. [DOI] [PubMed] [Google Scholar]
- 38.Mathivanan S et al. (2010) Exosomes: extracellular organelles important in intercellular communication. J Proteomics 73 (10), 1907–20. [DOI] [PubMed] [Google Scholar]
- 39.van der Pol E et al. (2012) Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 64 (3), 676–705. [DOI] [PubMed] [Google Scholar]
- 40.Maas SLN et al. (2017) Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends in cell biology 27 (3), 172–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whiteside TL (2008) The tumor microenvironment and its role in promoting tumor growth. Oncogene 27 (45), 5904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abels ER et al. (2019) Glioblastoma-Associated Microglia Reprogramming Is Mediated by Functional Transfer of Extracellular miR-21. Cell reports 28 (12), 3105–3119.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maas SLN et al. (2020) Glioblastoma hijacks microglial gene expression to support tumor growth. J Neuroinflammation 17 (1), 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abels ER et al. (2019) Glioma EVs Contribute to Immune Privilege in the Brain. Trends in Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lucero R et al. (2020) Glioma-Derived miRNA-Containing Extracellular Vesicles Induce Angiogenesis by Reprogramming Brain Endothelial Cells. Cell Rep 30 (7), 2065–2074.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oushy S et al. (2018) Glioblastoma multiforme-derived extracellular vesicles drive normal astrocytes towards a tumour-enhancing phenotype. Philos Trans R Soc Lond B Biol Sci 373 (1737). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dubois LG et al. (2014) Gliomas and the vascular fragility of the blood brain barrier. Front Cell Neurosci 8, 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D'Asti E et al. (2016) Extracellular Vesicles in Brain Tumor Progression. Cell Mol Neurobiol 36 (3), 383–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lane R et al. (2019) Cell-derived extracellular vesicles can be used as a biomarker reservoir for glioblastoma tumor subtyping. Commun Biol 2, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rak J (2013) Extracellular vesicles - biomarkers and effectors of the cellular interactome in cancer. Front Pharmacol 4, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skog J et al. (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nature cell biology 10 (12), 1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yekula A et al. (2020) Large and small extracellular vesicles released by glioma cells in vitro and in vivo. J Extracell Vesicles 9 (1), 1689784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gallo V and Deneen B (2014) Glial development: the crossroads of regeneration and repair in the CNS. Neuron 83 (2), 283–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tirosh I et al. (2016) Single-cell RNA-seq supports a developmental hierarchy in human oligodendroglioma. Nature 539 (7628), 309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weng Q et al. (2019) Single-Cell Transcriptomics Uncovers Glial Progenitor Diversity and Cell Fate Determinants during Development and Gliomagenesis. Cell Stem Cell 24 (5), 707–723.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhaduri A et al. (2020) Outer Radial Glia-like Cancer Stem Cells Contribute to Heterogeneity of Glioblastoma. Cell Stem Cell 26 (1), 48–63.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Batiuk MY et al. (2020) Identification of region-specific astrocyte subtypes at single cell resolution. Nat Commun 11 (1), 1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sosunov AA et al. (2014) Phenotypic heterogeneity and plasticity of isocortical and hippocampal astrocytes in the human brain. J Neurosci 34 (6), 2285–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Dalahmah O et al. (2020) Single-nucleus RNA-seq identifies Huntington disease astrocyte states. Acta Neuropathol Commun 8 (1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei Z et al. (2019) Co-cultures of Glioma Stem Cells and Primary Neurons, Astrocytes, Microglia, and Endothelial Cells for Investigation of Intercellular Communication in the Brain. Front Neurosci 13, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeng A et al. (2020) Glioblastoma-Derived Extracellular Vesicles Facilitate Transformation of Astrocytes via Reprogramming Oncogenic Metabolism. iScience 23 (8), 101420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee TH et al. (2016) Barriers to horizontal cell transformation by extracellular vesicles containing oncogenic H-ras. Oncotarget 7 (32), 51991–52002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jackson C et al. (2011) Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin Dev Immunol 2011, 732413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dang CV (2012) MYC on the path to cancer. Cell 149 (1), 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hallal S et al. (2019) Extracellular Vesicles Released by Glioblastoma Cells Stimulate Normal Astrocytes to Acquire a Tumor-Supportive Phenotype Via p53 and MYC Signaling Pathways. Mol Neurobiol 56 (6), 4566–4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chennakrishnaiah S et al. (2020) Extracellular vesicles from genetically unstable, oncogene-driven cancer cells trigger micronuclei formation in endothelial cells. Sci Rep 10 (1), 8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taheri B et al. (2018) C6 glioma-derived microvesicles stimulate the proliferative and metastatic gene expression of normal astrocytes. Neurosci Lett 685, 173–178. [DOI] [PubMed] [Google Scholar]
- 68.Bian EB et al. (2019) Exosomal lncRNA-ATB activates astrocytes that promote glioma cell invasion. Int J Oncol 54 (2), 713–721. [DOI] [PubMed] [Google Scholar]
- 69.Colangelo NW and Azzam EI (2020) Extracellular vesicles originating from glioblastoma cells increase metalloproteinase release by astrocytes: the role of CD147 (EMMPRIN) and ionizing radiation. Cell Commun Signal 18 (1), 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao X et al. (2020) Gliomas Interact with Non-glioma Brain Cells via Extracellular Vesicles. Cell Rep 30 (8), 2489–2500.e5. [DOI] [PubMed] [Google Scholar]
- 71.Garnier D et al. (2018) Divergent evolution of temozolomide resistance in glioblastoma stem cells is reflected in extracellular vesicles and coupled with radiosensitization. Neuro Oncol 20 (2), 236–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bianco F et al. (2009) Acid sphingomyelinase activity triggers microparticle release from glial cells. Embo j 28 (8), 1043–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Falchi AM et al. (2013) Astrocytes shed large membrane vesicles that contain mitochondria, lipid droplets and ATP. Histochem Cell Biol 139 (2), 221–31. [DOI] [PubMed] [Google Scholar]
- 74.Fruhbeis C et al. (2013) Extracellular vesicles as mediators of neuron-glia communication. Front Cell Neurosci 7, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burda JE et al. (2016) Astrocyte roles in traumatic brain injury. Exp Neurol 275 Pt 3 (0 3), 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang L et al. (2015) Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 527 (7576), 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Graner MW (2019) Roles of Extracellular Vesicles in High-Grade Gliomas: Tiny Particles with Outsized Influence. Annu Rev Genomics Hum Genet 20, 331–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Théry C et al. (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of extracellular vesicles 7 (1), 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lal PG et al. (1996) Astrocyte-astrocytoma cell line interactions in culture. J Neurosci Res 44 (3), 216–22. [DOI] [PubMed] [Google Scholar]
- 80.Goers L et al. (2014) Co-culture systems and technologies: taking synthetic biology to the next level. J R Soc Interface 11 (96). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hatherell K et al. (2011) Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J Neurosci Methods 199 (2), 223–9. [DOI] [PubMed] [Google Scholar]
- 82.Wu MH et al. (2010) Microfluidic cell culture systems for drug research. Lab Chip 10 (8), 939–56. [DOI] [PubMed] [Google Scholar]
- 83.Campbell JJ et al. (2011) A multifunctional 3D co-culture system for studies of mammary tissue morphogenesis and stem cell biology. PLoS One 6 (9), e25661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Albrecht DR et al. (2006) Probing the role of multicellular organization in three-dimensional microenvironments. Nat Methods 3 (5), 369–75. [DOI] [PubMed] [Google Scholar]
- 85.Felton EJ et al. (2012) Heterotypic cell pair co-culturing on patterned microarrays. Lab Chip 12 (17), 3117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chekhonin IV et al. (2018) Glioma Cell and Astrocyte Co-cultures As a Model to Study Tumor-Tissue Interactions: A Review of Methods. Cell Mol Neurobiol 38 (6), 1179–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jacob F et al. (2020) A Patient-Derived Glioblastoma Organoid Model and Biobank Recapitulates Inter- and Intra-tumoral Heterogeneity. Cell 180 (1), 188–204.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]