Abstract
The development of successful vaccines has been increasingly reliant on the use of immunoadjuvants - additives, which can enhance and modulate immune responses to vaccine antigens. Immunoadjuvants of the polyphosphazene family encompass synthetic biodegradable macromolecules, which attain in vivo activity via antigen delivery and immunostimulation mechanisms. Over the last decades, the technology has witnessed evolvement of next generation members, expansion to include various antigens and routes of administration, and progression to clinical phase. This was accompanied by gaining important insights into the mechanism of action and the development of a novel class of virus-mimicking nano-assemblies for antigen delivery. The present review evaluates in vitro and in vivo data generated to date in the context of latest advances in understanding the primary function and biophysical behavior of these macromolecules. It also provides an overview of relevant synthetic and characterization methods, macromolecular biodegradation pathways, and polyphosphazene-based multi-component, nanoparticulate, and microfabricated formulations.
Keywords: Immunoadjuvants, Vaccine delivery, Polyphosphazenes, TLR agonists, Resiquimod, Antigens, Proteins, Nanocomplexes, Biodegradable polymers
1. Introduction
Generation of robust primary immune responses by successful vaccines has become increasingly dependent on the aid of advanced immunoadjuvants. These additives are specifically designed to enhance, prolong, and shape antigen specific immune responses, as well as to enable antigen dose sparing [1–6]. The term immunoadjuvant broadly includes “true” immunostimulating compounds, such as Toll-Like Receptor (TLR) agonists (Monophosphoryl Lipid A - MPL and CpG), as well as vaccine delivery carriers, which are typically represented by aluminum-based hydrogels (Alhydrogel, Alum), emulsions, and nanoparticulate formulations, including liposomes [5]. Although Alhydrogel remains by far the most widely employed immunoadjuvant, a number of advanced systems, such as MF59, AS01, AS03, AS04, and AF03, were introduced in licensed vaccines recently [5, 6]. The majority of these newer adjuvants are multi-component, bi-phasic formulations, which include natural or semi-synthetic products, and may require sophisticated formulation and production efforts [5]. Despite these advances, the search for potent and safe immunoadjuvants continues.
Over the last two and a half decades, polyphosphazene technology has evolved as an independent immunoadjuvant platform with a number of distinct features, which differentiate it from other systems developed to date. The unique position of polyphosphazene adjuvants appears to stem from an exceptional antigen-binding feature of these water-soluble macromolecules, which also display molecular dimensions mimicking those of viruses - generally between 30 and 140 nm [7–10]. In fact, the ability of polyphosphazene macromolecules to spontaneously self-assemble with antigenic proteins in aqueous solutions resulting in multimeric supramolecular assemblies is gaining an increasing experimental support in a number of recent studies [7, 9–17]. Despite their virus-mimicking size and protein load, these assemblies retain excellent water-solubility at near physiological conditions. Biodegradability of polyphosphazene adjuvants - a mandatory requirement for using them as injectables, results from their hybrid nature - an inorganic phosphorus-nitrogen backbone in these macromolecules is “decorated” with organic side groups (Fig. 1A). These organic moieties are intentionally selected to be physiologically benign as they constitute the main degradation product along with small amounts of ammonium phosphate resulting from the backbone [18]. From a chemical standpoint, lead polyphosphazene adjuvants - poly[di(carboxylatophenoxy)phosphazene], PCPP and poly[di(carboxylatoethylphenoxy)phosphazene], PCEP, along with recently synthesized poly[di(carboxylatomethylphenoxy)phosphazene], PCMP, are members of homologous family of polyphosphazene polyelectrolytes (Fig. 1B).
Fig. 1.
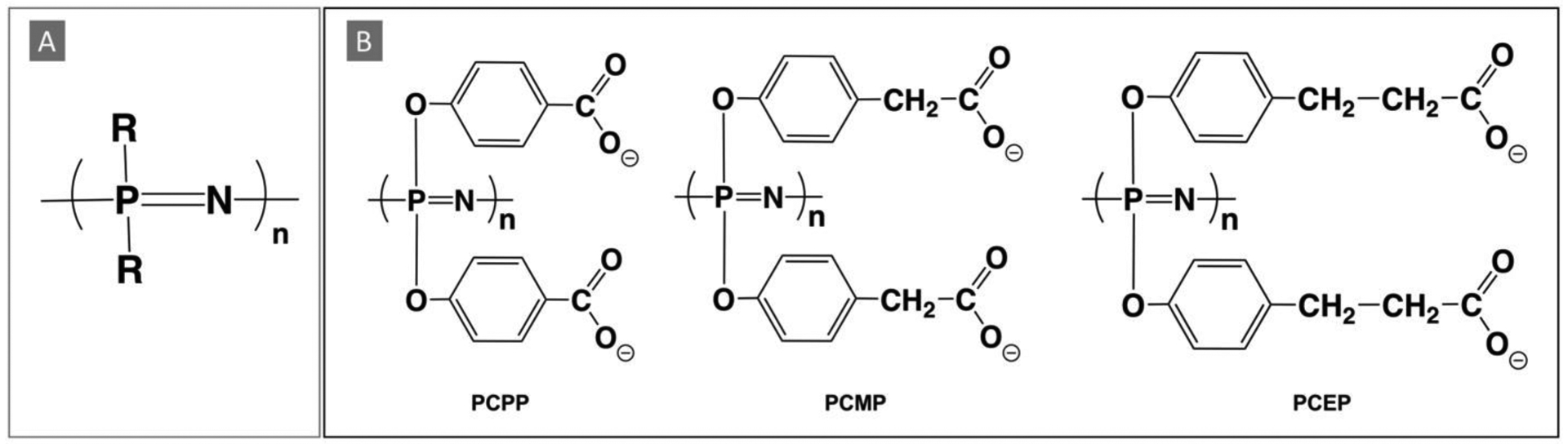
Chemical structures of (A) generic polyphosphazene and (B) lead polyphosphazene immunoadjuvants: PCPP, PCMP, and PCEP.
As reviewed below, polyphosphazene immunoadjuvants have been investigated in numerous animal studies, in which they displayed the ability to enhance and modulate the quality of immune responses, induce mucosal immunity, enable antigen dose sparing, and facilitate protection in challenge studies. PCPP has also been studied in several clinical trials, in which its safety and dose-dependent immunoadjuvant potency was validated in humans. The present review intends to analyze research and clinical data accumulated over almost three decades of technology development, in the context of recent advances in understanding biophysical behavior of polyphosphazenes, principles of their self-assembly, and their potential mechanism of action. The review also provides a summary of various polyphosphazene formulations, including microneedle and nanoparticulate systems, polyphosphazene degradation pathways, and a brief survey of synthetic and characterization methods relevant to these macromolecules.
2. Immunoadjuvant activity of polyphosphazenes
2.1. PCPP
The discovery of the immunoadjuvant effect of PCPP in the early-nineties [19] pioneered the field of polyphosphazene adjuvants and, despite the evolvement of new derivatives, this polymer remains the most investigated macromolecule of its class with approximately fifty published studies dedicated only to its in vivo activity (Table 1) and clinical trials (chapter 3). This remarkable synthetic polyelectrolyte (Fig. 1) was originally designed for the preparation of ionically cross-linked hydrogels [20–24], but showed impressive immunoadjuvant activity in vivo in both soluble and cross-linked forms [25–28]. Presently, in vivo potency of PCPP is documented for over thirty-five bacterial, viral, and model antigens in seven animal models, including non-human primates (Table 1). Even at the early stages of its development, it was noted that PCPP not only enhanced the immune response, but also shifted its onset to as little as two-three weeks after immunization (Figure 2A) [27–30], while sustaining antibody titers up to 41 weeks - the length of the experiment [27]. Addition of PCPP also allowed achievement of significant antigen dose sparing. PCPP adjuvanted influenza and HBsAg formulations were more potent than their non-adjuvanted counterparts containing up to 25 times higher doses of the antigen [13, 29, 31].
Table 1.
In vivo immunoadjuvant potency of PCPP
| Pathogen | Antigen | Animal Model | ROA | Comparison Adjuvants | REF |
|---|---|---|---|---|---|
| Viral Antigens | |||||
| Influenza virus | A/Texas/36/91 (H1N1); A/Shangdong/9/93 (H3N2); B/Panama/45/90; X-31 |
mice | SC | [27] | |
| A/Texas/36/91 (H1N1); A/Shangdong/9/93 (H3N2); B/Panama/45/90; X-31 |
mice | SC | [28] | ||
| X-31 | mice | SC | CFA, PCPP copolymers | [35 , 36] | |
| X-31, A/Aichi/68 (H3:N2) | mice | SC | Alum | [29] | |
| IN+SC | PCEP | [37] | |||
| A/Johannesburg (H3N2); A/Texas (H1N1); B/Harbin |
mice | PAREN | [38] | ||
| rgA/Vietnam/1203/2004 (H5N1) | ferrets | IM | [13] | ||
| PR8, inactivated | mice | IN | CpG | [33] | |
| HBV | HBsAg, plasma and recombinant | mice | SC | Alum | [28] |
| HBsAg, recombinant protein | mice | SC | PCEP | [39] | |
| pigs | ID or IM | [31, 40] | |||
| HBsAg, recombinant protein | mice | SC | PCEP, CpG | [30] | |
| HCV | E2, envelope glycoprotein | mice | IP | Alum, R848, PCPP-R | [9] |
| E2, envelope glycoprotein | mice | IP | Alum, Addavax, PCEP | [10] | |
| E2, multiple variants | mice | IP | [41] | ||
| RSV | RSV sF, postfusion glycoprotein | mice | IM | Alum | [7] |
| FI-BRSV, RSV ΔF | mice | IN, SC | CpG | [42, 43] | |
| Rotavirus | EDIM, inactivated | mice | IM | QS-21, QS-7, Ribi (MPL) | [34] |
| VP6 (EDIM) | mice | IN | CpG, QS-21, CTA1-DD, LT | [44] | |
| VLPs(Rf 8* −2/6/7) | mice | IM | [32] | ||
| SC or ORAL | [45] | ||||
| VLPs (various) | pigs | IN, IN+IM | [46] | ||
| inactivated | mice | IM | [47] | ||
| HSV | D2 glycoprotein | mice | SC | [28] | |
| BoHV-1 | tgD glycoprotein | sheep | SC | Emulsigen | [48] |
| BRSV | inactivated | mice | IN, SC | CpG | [49] |
| HIV | HIV-1, whole inactivated | macaques | IV | [50] | |
| HIV-1, Tat toxoid and protein | macaques | IM, ID (3- 5x) |
IFA | [51, 52] | |
| HIV-1 DNA and recombinant protein | mice | IM, IN | [53] | ||
| SIV | Gag protein | macaques | IM | [54] | |
| Bacterial antigens | |||||
| Clostridium tetani | TT | mice | SC | CFA | [35] |
| IN | [35] | ||||
| H. influenzae | Hib PRP conj. TT | mice | SC | Alum | [28] |
| Vibrio cholerae | TCP | mice | SC | CRL-1005 | [55] |
| Streptococcus pneumoniae | PspA | mice | IN | CpG | [33] |
| Bordetella pertussis | PTd | mice | IN | CpG | [33] |
| SC | PCEP, CpG, IDR, combinations | [56] | |||
| Bartonella henselae | inactivated | cats | SC | Carbopol | [57] |
| Model antigens | |||||
| BSA | mice | SC | Alum, PCEP | [11, 29] | |
| PSA | sheep | SC | [48] | ||
| Ovalbumin | mice | SC | PCPP, CpG, indol, combinations, Ca-PCPP | [58–60] | |
| mice | IN | PCEP, poly I:C, c-di-AMP, IDR | [61] | ||
| HEL | calves | SC | CpG, indol | [62] | |
(ROA-route of administration; REF-references; SC-subcutaneous; IM-intramuscular; PAREN-parenteral; ID-intradermal; IN-intranasal; IP-intraperitoneal; IV-intravenous; HBV-hepatitis B virus; HBsAg-hepatitis B Surface antigen; HCV-hepatitis C virus; RSV-respiratory syncytial virus; EDIM-epizootic diarrhea of infant mice; HSV-herpes Simplex Virus; BoHV-1-bovine herpes virus-1; BRSV-Bovine respiratory syncytial virus; HIV-human immunodeficiency virus; SIV-simian immunodeficiency virus; Hib-haemophilus influenza type B; PRP-capsular polysaccharide; TT-tetanus toxoid; TCP-toxin-coregulated pilin synthetic peptide; PspA-pneumococcal surface protein A; Ptd-pertussis toxoid; BSA-bovine serum albumin; PSA-porcine serum albumin; HEL-hen egg lysozyme; VLPs-virus-like particles; CpG-CpG oligodeoxynucleotide, c-di-AMP-bis-(3′,5′)-cyclic dimeric adenosine monophosphate; IDR-cationic innate defense regulator peptides; FI-BRSV-formalin-inactivated bovine RSV; CFA-complete Freund adjuvant; IFA-incomplete Freund adjuvant; CTA1-DD-chimeric A1 subunit of cholera toxin; LT-attenuated E. coli heat-labile toxin; CRL-1005-poly(ethylene oxide-co-propylene oxide); poly I:C-polyinosinic:polycytidylic acid; Indol-host defense peptide indolicidin)
Fig. 2.
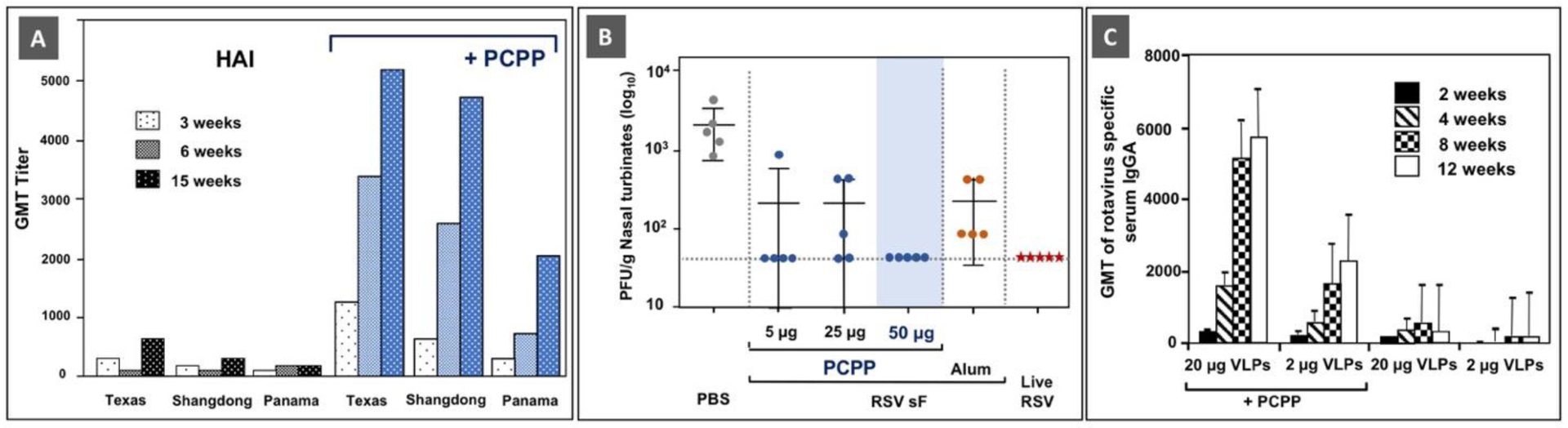
Immunoadjuvant activity of PCPP: (A) The effect of PCPP on hemagglutination-inhibition (HAI) antibody titers to trivalent influenza vaccine (A/Texas/36/91 (H1N1), A/Shangdong/9/93 (H3N2), B/Panama/45/90) in BALB/C mice. Reprinted from [27], Copyright 1997, with permission from Elsevier. (B) Nasal turbinates viral loads 4 days post-challenge with RSV A2 in mice. Infectious virus was detected by Plaque Assay on HEp-2 cells. Adapted with permission from [7]. Copyright 2017, American Chemical Society. (C) Rotavirus-specific serum IgA antibodies after intramuscular immunization with rotavirus VLPs with or without PCPP. Significantly higher IgA titers were observed in mice that received PCPP adjuvanted formulations (p < 0.002). (Reprinted from [32], Copyright 2008, with permission from Elsevier).
Benchmarking of PCPP adjuvanted formulations against those containing Alum implied superior performance of PCPP, both in terms of induction of antibody responses and protection in animal challenge studies (Figure 2B) and other studies [28, 29]. In comparison with another well-investigated adjuvant, CpG ODN, PCPP showed superior performance of PCPP in generating IgG and IgA antibody-forming cells in the nasal passage, lung, and sub-mandibular glands when administered intranasally with several vaccine antigens [33]. It was also noted that both adjuvants displayed synergy when used in combination in formulations with HBsAg [30]. PCPP was also compared with QS-21 and MPL-containing Ribi adjuvant systems, outperforming them in the induction of IgG, IgA, and neutralization antibody responses to inactivated rotavirus [34].
PCPP adjuvanted formulations enabled protection against disease in multiple challenge studies. In lethal challenge studies with the H5N1 influenza vaccine in ferrets - a well-established preclinical animal model, the PCPP formulated vaccine afforded 100% protection from mortality, whereas the non-adjuvanted formulation was not protective at a dose of at least 10 fold higher [13]. Similar results were later obtained in mice [63]. Protective immunity afforded by PCPP adjuvanted vaccines was also established against respiratory pathogens, cholera, and rotavirus [7, 32–34, 55]. The immunoadjuvant activity of PCPP was proven with recombinant and plasma proteins, peptides, nucleic acids, and inactivated virus particles among others, demonstrating the high level of antigen compatibility and versatility of this adjuvant (Table 1). It is noteworthy, that PCPP appears to work well with gentle antigenic constructs, such as virus-like particles (VLPs) [32]. A single intramuscular dose of PCPP formulated rotavirus VLPs induced rotavirus-specific serum IgG and IgA, fecal IgG titers that were enhanced 5–90-fold by the adjuvant (Fig. 2C).
Another interesting feature of PCPP is its apparent potential in the development of vaccines for neonates or elderly adults. There is an unmet medical need for vaccine formulations aimed towards generating immunity in the elderly population, newborns and young infants, who are more susceptible to infections and/or less likely to develop protective immunity [64, 65]. To that end, the ability of PCPP to facilitate induction of high antibody titers to the influenza vaccine in aged 22-month mice was reported [27]. These results are noteworthy in context of established PCPP potency in clinical trials in elderly subjects (Chapter 3). In vitro studies also demonstrated that PCPP formulations are effective in promoting maturation, activation and antigen presentation by both human adult and newborn dendritic cells (DCs) [14].
Taken together, the immunoadjuvant effect of PCPP can be characterized by modulations in the onset, magnitude, and duration of immune responses, protection against viral challenge, and antigen sparing effect. The ability of PCPP to modulate quality of immune responses remains under discussion, with publications suggesting either biased Th2 [29] or mixed Th1/Th2 responses [7, 33] ,and appear to be antigen-dependent. This subject is reviewed in more detail in Chapter 4.
2.2. PCPP-R
The innovative design of PCPP-R (Fig. 3A) may be viewed as a combination of PCPP and R848 (resiquimod) adjuvants. However, from a polyelectrolyte chemistry standpoint, cationic R848 merely serves as a counterion for a negatively charged PCPP and constitutes an integral part of its macromolecular structure (Fig. 3A). What is remarkable is that this ion-paired system, which is stabilized by hydrophobic interactions, resists dissociation under physiological conditions and apparently performs as a single entity in vivo [9]. The design of this adjuvant was inspired by some of the challenges faced by the potent small molecule TLR 7/8 agonist in its development as a vaccine adjuvant. In particular, inconsistent and somewhat disappointing performance of R848 as an immunoadjuvant is associated with its rapid clearance in vivo and quick dissociation from the antigen upon injection [66, 67]. It was also noted that the reduction of potential systemic toxicity of this cationic compound can be desirable [68].
Fig. 3.
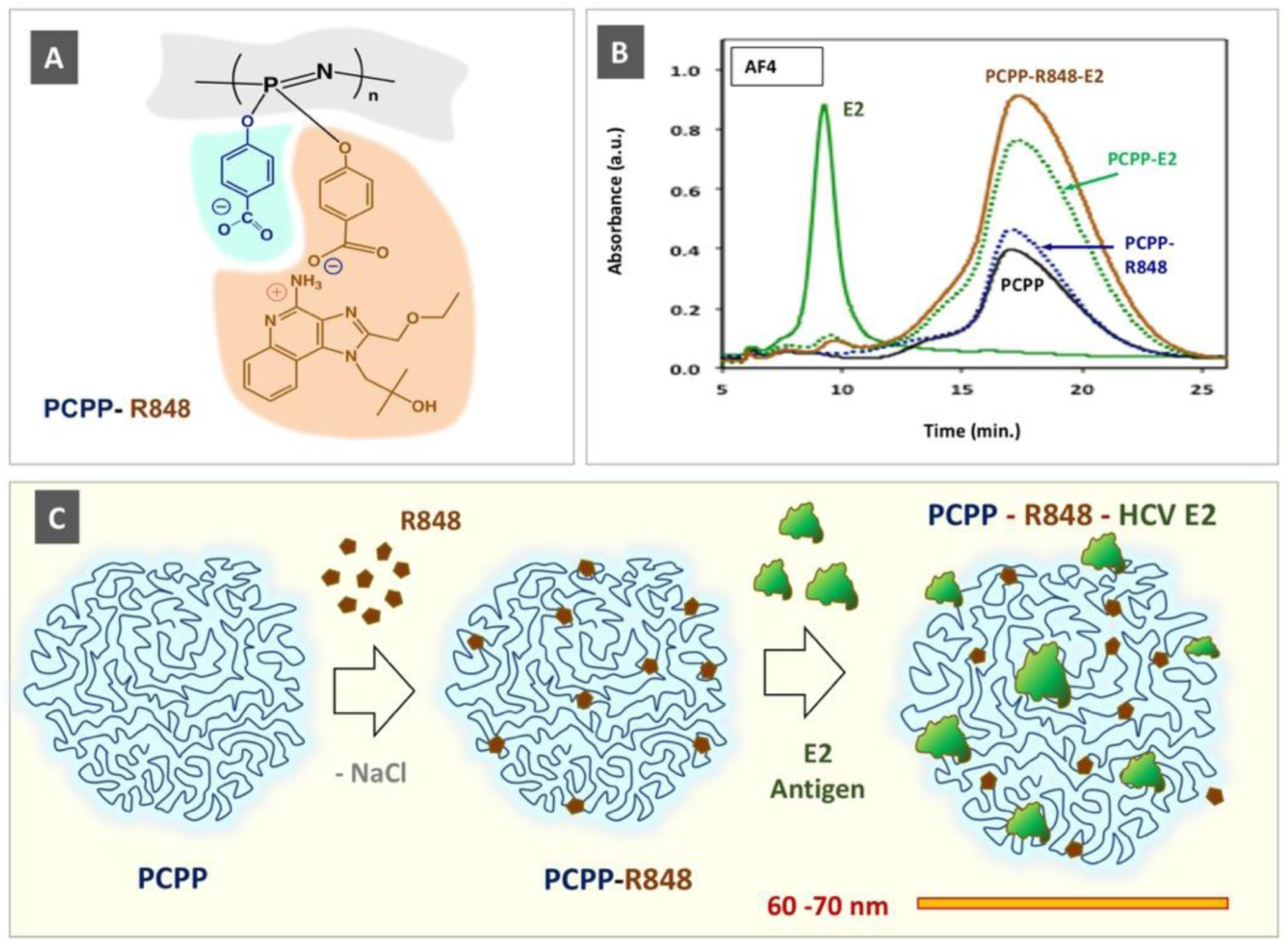
Supramolecular assembly of PCPP-R848-HCV E2 antigen ternary complex: (A) chemical structure of PCPP-R848; (B) AF4 fractograms of a complex and its components (Adapted with permission from [9]. Copyright 2020 American Chemical Society). (C) Schematic presentation of spontaneous self-assembly and resulting virus-size mimicking complex displaying antigen and TLR 7/8 agonist “danger signals” in their multimeric forms.
The molecular design of PCPP-R, which was extensively investigated using an array of physico-chemical methods, carries over one thousand R848 moieties per a single PCPP chain and retains about 250 of them even after in vitro release experiments at near physiological conditions [9]. This PCPP-assembled multimeric form of the TLR7/8 agonist displays higher immunostimulatory activity in vitro, as assessed in the engineered RAW Blue cell line, and reduced toxicity in a hemolysis test [9]. When co-formulated with a vaccine antigen (HCV E2 envelope glycoprotein), a non-covalent ternary complex was spontaneously formed in aqueous solution, which was clearly demonstrated by asymmetric flow field flow fractionation (AF4) analysis (Fig. 3B). This virus-mimicking polymer assembly (Fig. 3C), in terms of size (60–70 nm) and multiple copies of the associated antigen and TLR7/8 agonist (“danger signals”), was efficient both in antigen display (assessed by antibody binding) and immune stimulation in cellular assays. In vivo studies demonstrated that the PCPP-R adjuvanted HCV formulation induced higher serum neutralization titers in BALB/c mice and shifted the response towards desirable cellular immunity, as evaluated by antibody isotype ratio (IgG2a/IgG1) and ex vivo analysis of cytokine secreting splenocytes (higher levels of interferon gamma (IFN-γ) single and tumor necrosis factor alpha (TNF-α)/IFN-γ double producing cells).
PCPP-enabled non-covalent multimerization of R848 stands in contrast to previously suggested delivery methods for this compound, which involve covalent conjugation or encapsulation [69–81]. The ion-pairing concept can be extended to different members of the polyphosphazene homologous series, such as PCMP and PCEP, as well as other representatives of the imidazoquinoline family, such as gardiquimod and newer derivatives [82], or TLR 7/8 agonists of the oxoadenine series [83] among others. It can be further suggested that the stability and persistence of these assemblies can be modulated through careful selection of side groups on the polyphosphazene backbone, most likely by their hydrophobic modification.
2.3. PCEP
Molecular design of a new generation polyphosphazene immunoadjuvant - PCEP [39, 84], was contemplated with the following objectives. First, to amplify antigen binding to polyphosphazene - a key feature of the PCPP delivery modality [11], through increased contribution of hydrophobic interactions and hydrogen bonds. Second, to alleviate the observed high sensitivity of PCPP to sodium ions [85], which was deemed to be responsible for insufficient stability of its complexes with some antigens [11]. It was anticipated, that introduction of two methylene groups between the benzene ring and the carboxylic acid moiety (Fig. 1) will increase hydrophobicity of a new macromolecule, reduce the dissociation constant, and distort the molecular configuration of PCPP, which shows unique affinity to sodium, but not lithium or potassium ions [85]. In vitro studies revealed higher stability of antigen - PCEP complexes at near physiological conditions when compared to those formed by PCPP [10]. Interestingly, this relatively minor alteration in polyphosphazene structure resulted in pH dependent membrane destabilizing activity of PCEP within a pH range of early endosomes, which PCPP does not display [15]. The potential importance of these findings will be more thoroughly reviewed in Chapter 4.
Immunoadjuvant potency of PCEP in vivo generally shows superiority over PCPP in the induction of antibody titers, which is exemplified for HBsAg and influenza antigens in Fig. 4A and Fig. 4B, correspondingly. Remarkably, minor structural alterations that differentiate PCEP from PCPP appear to be sufficient to shift the immune response, which, as mentioned above, is mainly Th2 for PCPP, to a balanced Th1/Th2 immunity. This was demonstrated in multiple studies [29, 39, 86, 87] and is illustrated in Fig. 4C and Fig. 4D, which show IgG2a titers and antigen specific cytokine secreting cells in mice splenocytes for X:31 influenza antigen. It needs to be noted that a recent study appeared to indicate that the ability of PCEP to promote balanced response is not universal for all antigens [88]. Table 2 summarizes currently available publications, in which PCEP was investigated as an immunoadjuvant in vivo. It is important to note however, that this Table only includes studies, which investigated PCEP as an individual adjuvant, whereas a significant part of research conducted on this macromolecule included it as part of a combination with other adjuvants, which will be discussed in Chapter 7.
Fig. 4.
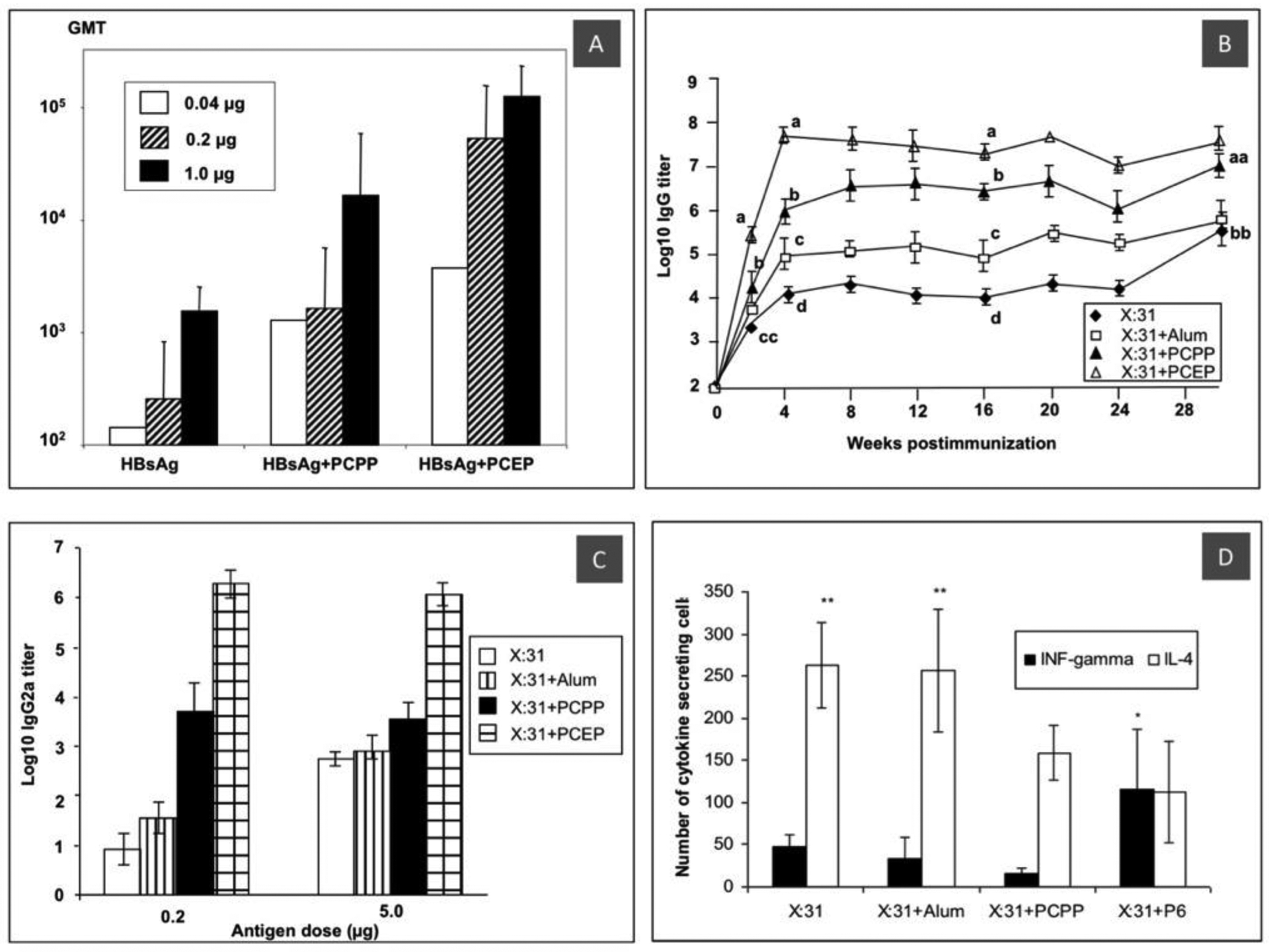
In vivo activity of PCEP: (A) serum IgG titers after immunization of mice with PCPP and PCEP adjuvanted and non-adjuvanted HBsAg at different antigen doses (IM, 4-week data. Reprinted with permission from [39]. Copyright 2006, American Chemical Society; (B) serum IgG titers after immunization of mice with 0.2 μg X31 influenza antigen; (C) IgG2a titers; and (D) frequency of X:31-specific INFγ and IL-4 secreting cells in mice splenocytes as determined by ELISPOT assay in the same experiment (SC, 16 weeks. All reprinted from [29]. Copyright 2006, with permission from Elsevier).
Table 2.
In vivo immunoadjuvant potency of PCEP
| Pathogen | Antigen | Animal Model | ROA | Comparison adjuvants | REF |
|---|---|---|---|---|---|
| Viral antigens | |||||
| Influenza | X-31, A/Aichi/68 (H3:N2) | mice | IN, SC, ORAL | PCPP | [29, 37, 39, 89] |
| HBV | HBsAg, recombinant | mice | SC | PCPP, CpG | [30, 39] |
| HCV | E2, envelope glycoprotein | mice | IP | Alum, Addavax, PCPP | [10] |
| IBHV | Recombinant or native hexon protein, inactivate virus | chicken | IM, in ovo | avian beta defensin 2, combination | [90, 91] |
| SwIV | H1N1, Inactivated | pigs | ID, IM | Emulsigen | [92, 93] |
| Bacterial antigens | |||||
| Bordetella pertussis | PTd | mice | SC | CpG, IDR, PCPP | [56, 94] |
| Actinobacillus pleuropneum oniae | OmlA | pigs | SC | CpG, Emulsigen | [86] |
| Model antigens | |||||
| BSA | mice | SC | Alum, PCPP | [29] | |
| Ovalbumin | mice | SC, IN | PCPP, CpG, Ca-PCPP, poly I:C, c-di-AMP, IDR, combinations | [58, 61] | |
(ROA-route of administration; REF-references; SC-subcutaneous; IM-intramuscular; ID-intradermal; IN-intranasal; IP-intraperitoneal; HBV-hepatitis B virus; HBsAg-hepatitis B Surface antigen; HCV-hepatitis C virus; IBHV-inclusion body hepatitis virus; SwIV-swine influenza virus; Ptd-pertussis toxoid; OmIA-outer membrane antigen; BSA-bovine serum albumin; CpG-CpG oligodeoxynucleotide; c-di-AMP-bis-(3′,5′)-cyclic dimeric adenosine monophosphate; IDR-cationic innate defense regulator peptides, Ca-PCPP-calcium cross-linked PCPP, poly I:C-polyinosinic:polycytidylic acid)
2.4. PCMP
PCMP is the most recently synthesized member of the polyphosphazene homologous series (Fig. 1). The side group of PCMP - 4-hydroxyphenylacetic acid is a constituent of numerous foods, such as olives, cocoa beans, oats, and beer, and is well-known for its antioxidant properties [95–100]. It can be also found throughout all human tissues and biofluids [97, 98, 101, 102]. The synthesis of this polymer extends the lineup of structurally similar macromolecules, which intends to facilitate the establishment of structure-activity relationship for this adjuvant class [84]. In vitro, PCMP displays pH-dependent membrane disruptive activity and antigen-complexation profiles resembling PCEP, rather than PCPP. In vivo evaluation of PCMP potency was conducted with Human Papillomavirus (HPV) VLPs based vaccine. Immunization experiments in mice demonstrated that the PCMP adjuvanted RG1-VLPs vaccine induced potent humoral immune responses and, in particular, on the level of highly desirable protective cross-neutralizing antibodies. It also outperformed PCPP and Alum adjuvanted formulations.
2.5. Other polyphosphazene derivatives
In an attempt to establish the structure - activity relationship for polyphosphazene adjuvants, a series of copolymers containing ionic carboxylatophenoxy groups along with electrostatically neutral oligo(ethylene glycol) side groups, such as methoxyethoxy (PCPP-MEP) and methoxyethoxyethoxy side groups (PCPP-MEEP), were synthesized [36]. The immunoadjuvant effects of these PCPP derivatives was investigated with the influenza antigen in mice and compared both with those of PCPP and of a water-soluble homopolymer containing only neutral methoxyethoxyethoxy side groups - MEEP (Fig. 5). MEEP alone did not display any immunostimulating capacity confirming that ionic moieties are needed to enable this feature in the polymer. Somewhat surprisingly, introduction of 20 % (mol) of “inactive” MEEP side groups in PCPP structure (PCPP-MEEP) resulted in a significant boost of immunoadjuvant activity, which was somewhat reduced when the content of neutral side groups was increased to 40 % (Fig. 5). Furthermore, a copolymer containing 20% (mol) of shorter methoxyethoxy side groups (PCPP-MEP) showed even higher immunostimulating activity than PCPP-MEEP. The above results suggest that the activity of PCPP and other polyphosphazene immunoadjuvants can be further enhanced by optimizing environment of “active” ionic moieties and polymer microstructure. In particular, introduction of non-polar, but hydrogen-bond forming side groups can be beneficial. The composition and structural dependences above indicate that optimal hydrophilicity-hydrophobicity balance, along with polymer solvation with water molecules is essential. These parameters are most likely to affect the way polyphosphazenes interact with vaccine antigens and may be critical for overall stability of the resulting complexes. Molecular interactions aspects are more thoroughly discussed in Chapter 4.
Fig. 5.
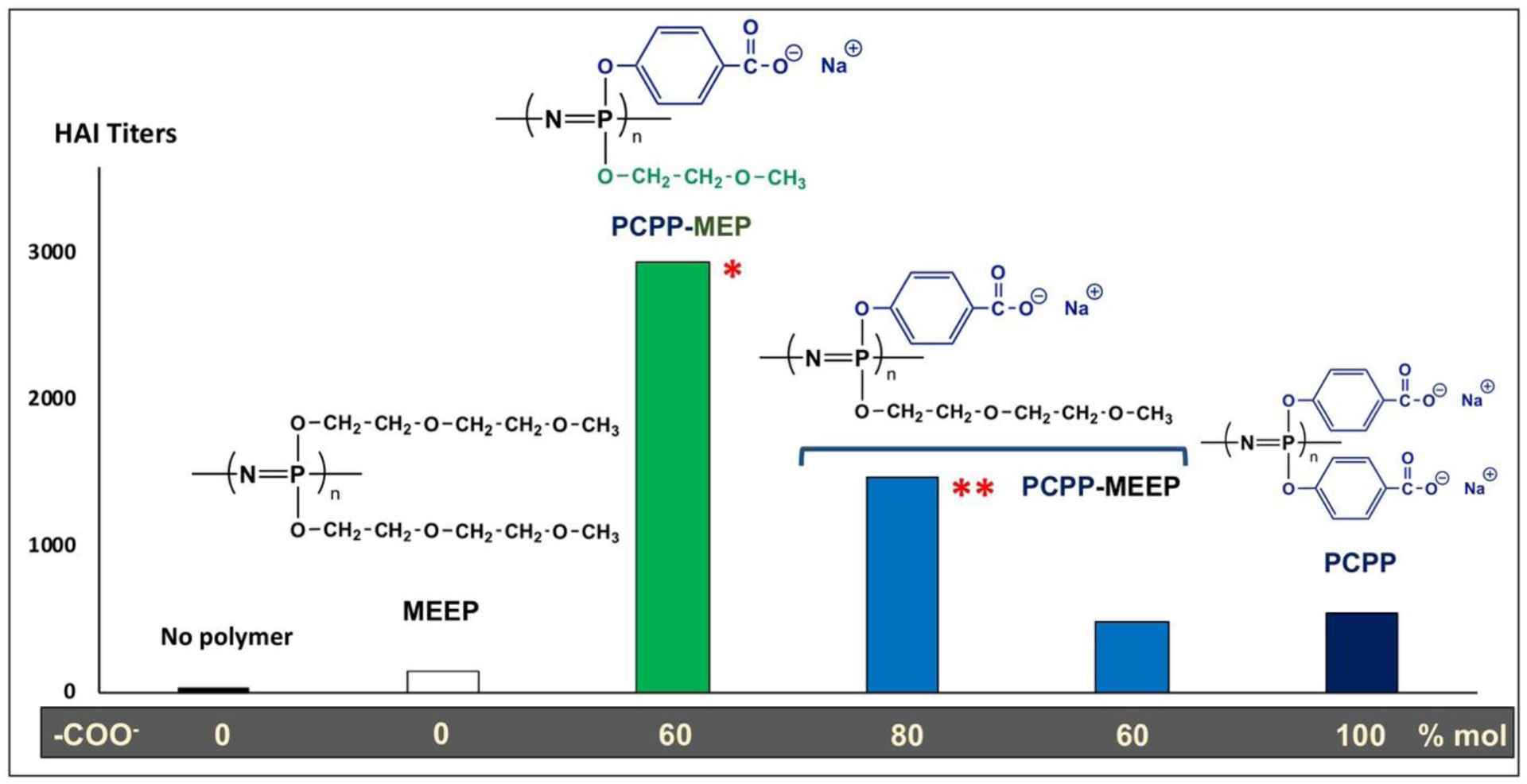
Serum hemagglutination (HAI) titers after immunization of mice with split X-31 influenza adjuvanted with PCPP derivatives (5 μg antigen, 30 μg polymer doses; geometric mean titers of 5 BALB/C mice; *statistically significantly different from PCPP, P = 0.0121; **statistically significantly different from PCPP, P = 0.0733; compiled from data published in [36]).
PCPP copolymers with various content of ethylpyrrolidone side groups [103] and analog of PCPP containing sulfonic acid groups instead of carboxylic [104] were also synthesized, however their immunoadjuvant activity has yet to be investigated.
A series of PCPP polymers, containing various amounts of unsubstituted hydroxyl groups, were synthesized with the objective of investigating how these structural irregularities affect the mechanism of its hydrolytic degradation [85]. These polymers were also evaluated in animal studies, but no comparison with completely substituted PCPP was attempted [43, 49, 60, 62]. Since the molecular structure of these polymers is no different than that of PCPP with the exception of minute quantities of hydroxyl groups, the references to these studies are included in Table 1.
Along with other derivatives, it is worth mentioning an amphiphilic polyphosphazene containing N,N-diisopropylethylenediamine and poly(ethylene glycol) side groups, which was used for the preparation of ovalbumin (OVA) containing polymersomes [105]. The resulting formulation induced enhanced IgG titers and isotypes compared to protein alone, however the polymer itself or other comparative adjuvants were not evaluated.
3. PCPP in clinical trials
Published results on clinical trials involving PCPP remain somewhat limited. A Phase I trial was conducted with an influenza vaccine and tested three PCPP doses (100, 200, and 500 μg) in comparison with a non-adjuvanted formulation [38]. Ninety-six subjects were enrolled including forty-one participants over 60 years old and they were followed for twelve months. Overall, authors state that the adjuvanted vaccine showed improved results both in young and elderly subjects. In particular, the immunogenicity of the A/H3N2 influenza strain adjuvanted with PCPP was greatly improved in an adjuvant dose-dependent manner in respect to all serological parameters - geometric mean titers, seroconversion, and seroprotection (Fig. 6). No serious adverse events related to the adjuvanted vaccine were observed [38].
Fig. 6.
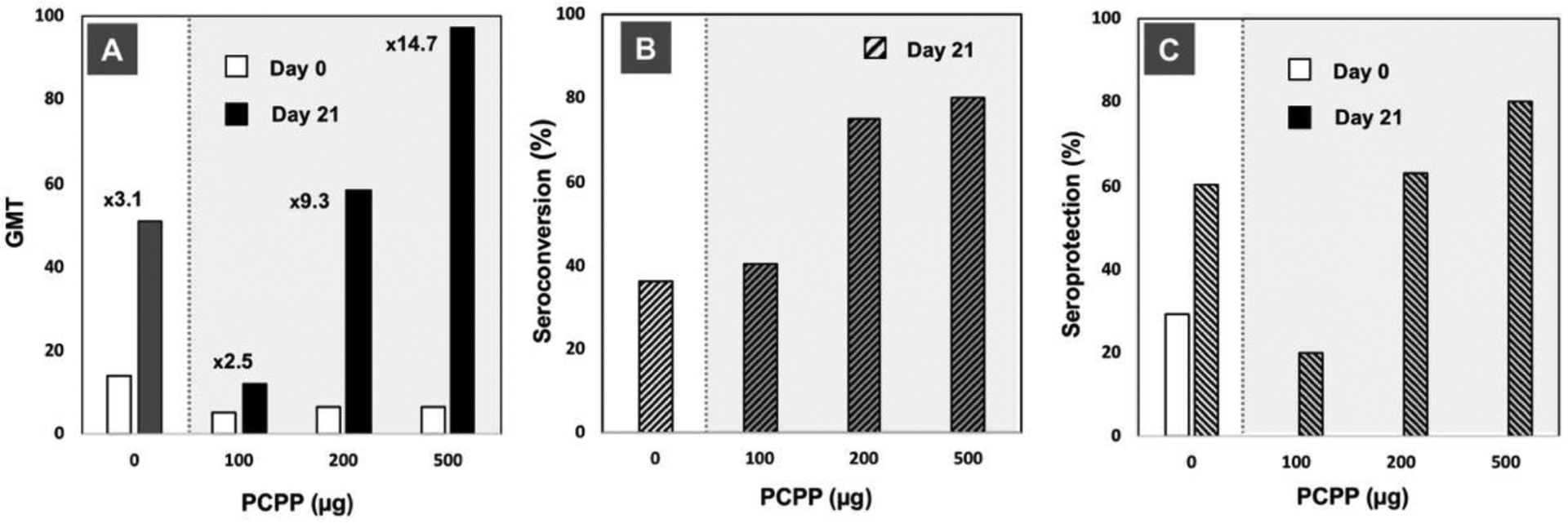
Immunoadjuvant activity of PCPP in clinical trials with influenza vaccine: (A) geometric mean titers (GMT) of A/H3N2 strain specific immune response (numbers indicate fold-increase in GMTs compared to day 0); (B) seroconversion, and (C) seroprotection data versus PCPP dose (strain-specific immunogenicity for A/Johannesburg/33/94 (H3N2) influenza, days 0 and 21 results are shown, 96 subjects). Figures plotted based on the data published in [38].
A phase I study with PCPP adjuvanted RSV A vaccine was conducted in healthy young adults, who received either Alum adjuvanted vaccine (10 participants) or RSV-PCPP vaccine (30 participants) [106]. One month after immunization, a 4-fold or greater increase in neutralizing antibodies was detected against RSV A or RSV B in greater than 77% of the participants. Both PCPP and Alum formulations were noted to have comparable safety and immunogenicity profiles [106].
PCPP was also tested in Phase 1/2 trials of ALVAC-HIV (vCP1521) recombinant canarypox vector vaccine, in which it was used in boost immunization with oligomeric envelope glycoprotein, ogp160 [107]. The authors concluded that the prime-boost regimens tested in this study appear to be safe and well tolerated displaying cell-mediated immune responses consistent with those in other trials of canarypox vectors [107].
A randomized phase 1 clinical trial to assess the safety and immunogenicity of ogp160 MN/LAI-2 HIV vaccine adjuvanted with either PCPP or alum following priming with live recombinant canarypox vector, ALVAC-HIV (vCP205) was conducted [108]. The vaccines were safe and well-tolerated, with no vaccine-related serious adverse events reported. The responses in the PCPP adjuvanted prime-boost groups were higher than those in protein-only and Alum-adjuvanted prime-boost groups. PCPP adjuvanted groups induced significantly higher end-of study responses than Alum, and durability improved with a higher-dose PCPP adjuvanted boost. Furthermore, among ogp160 recipients, those who received PCPP adjuvanted formulations were more likely than those who received Alum to have serum that neutralized resistant tier two viruses (12% vs 0%) [108].
Taken together, these findings demonstrate the immunoadjuvant potential of PCPP in humans with several different vaccine antigens and suggest that PCPP containing vaccine formulations are safe and well-tolerated.
4. Mechanistic insights
Although the mechanism of action for polyphosphazene adjuvants is yet to be fully elucidated, there is sufficient experimental evidence that these macromolecules spontaneously self-assemble with antigens in the formulation, thereby providing vaccine delivery modality. They also display intrinsic immunostimulatory activity in vitro and appear to create local immunocompetent environment at the injection site. This dual functionality of polyphosphazenes is discussed below.
4.1. Vaccine delivery modality - virus-mimicking supramolecular assemblies
The ability of PCPP to present the antigen to the immune system over an extended period of time was noted in the early stages of PCPP development. Maintenance of high antibody levels, antigen “dose sparing” effect, and long-lived IgM response were construed as indirect lines of evidence to support this hypothesis [27]. Furthermore, it was found that the excision of the injection site had no effect on the immune response, indicating that PCPP moves out of the site of injection within 24 hours [27]. These results constituted a strong basis for considering PCPP as a water-soluble vaccine delivery vehicle, which differs drastically from other bi-phasic antigen carriers, such as Alum, emulsions, or liposomes. It was also noted that conventional polyelectrolyte adjuvants, such as polyacrylic acid, show immunoadjuvant activity, but only when covalently conjugated with the antigen [109]. High activity of PCPP in a simple physical mixture with the antigen suggested presence of non-covalent interactions in the polyphosphazene system. However, it was not until recently that direct evidence of antigen binding by polyphosphazenes through non-covalent mechanisms was established [11, 14, 15].
Analysis of antigen-containing polyphosphazene formulations by dynamic light scattering (DLS), AF4, and HPLC with diode array detection provides unambiguous proof that, upon mixing, vaccine antigens spontaneously self-assemble with the polyphosphazene immunoadjuvant into supramolecular structures. Although these complexes are predominantly formed through electrostatic forces established between negatively charged polyphosphazene and positively charged patches on the protein surface, it was also demonstrated that hydrogen bonds and hydrophobic interactions play an important role in stabilizing these structures [15]. To date, the formation of protein-loaded polyphosphazene assemblies has been demonstrated for at least twenty vaccine antigens or model proteins [7, 9–16]. Depending on the type, dose, and whether multiple antigens are required, there could be several scenarios of self-assembly (Fig. 7). Small water-soluble antigens, such as proteins, can be assembled on PCPP or PCEP in multiple copies (multimeric assemblies) with resulting dimensions (z-average hydrodynamic diameter by DLS) generally corresponding to the size of unloaded polymers - 60–70 nm (Fig. 7A). When more than one antigen is used in the formulation, antigens can be added simultaneously or sequentially to form multivalent assemblies, which can also have multiple copies of each (Fig. 7B). Particulate antigens, such as VLPs, which are comparable in size to polyphosphazenes, undergo surface modification with a flexible polymer, essentially resulting in the formation of a polyphosphazene corona around the antigen with the size of up to 150 nm (Fig. 7C). In all cases, polyphosphazene-based assemblies display virus-mimicking features in terms of their dimensions and multimericity. Importantly, the flexibility of the polyphosphazene chain, which resembles a “cloud” due to segmental motions of the polymer, allows efficient display of antigens as confirmed by ELISA and AF4 analysis [7, 9, 10]. The dimensions of polyphosphazene assemblies appear to be optimal for antigen presentation. It has been reported that the size of delivery vehicles can affect internalization of antigen by DCs, trafficking to the draining lymph nodes, and induction of antibody and cytotoxic T cell responses, with smaller particles (up to 200 nm) generally performing more favorably than the larger ones [110].
Fig. 7.
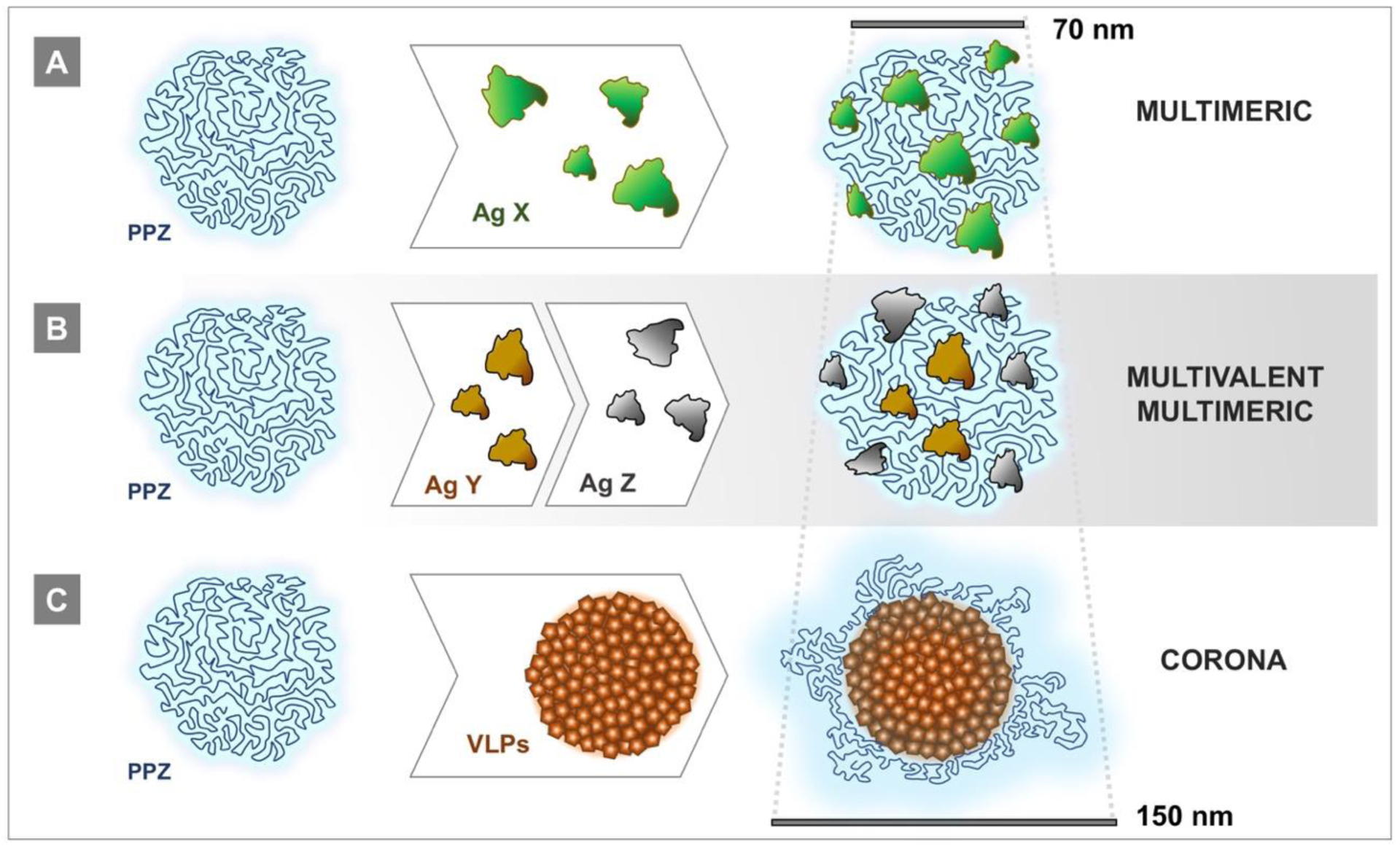
Schematic presentation of supramolecular assemblies of polyphosphazene adjuvants with antigens: (A) multimeric complexes containing multiple copies of the same adjuvant, (B) multivalent (containing more than one antigen)/multimeric complexes, and (C) corona (surface modified particulate antigen) assemblies formed with VLPs (bars represent approximate dimensions).
Generally, PCPP adjuvanted vaccine formulations are extremely easy to prepare, as no biphasic systems are formed, display excellent stability, and can be lyophilized and redissolved. Their water-solubility also allows for the efficient use of advanced physico-chemical characterization and quality control methods. However, in relatively rare cases of strong antigen-polymer interactions, which can potentially lead to some aggregation or complex compaction resulting in reduced activity, consideration should be given to optimizing formulation conditions or use of a microfluidics mixing technique while performing mandatory monitoring of the system by DLS and AF4 [7, 10, 11, 16].
4.2. Intrinsic immunostimulatory activity in cellular assays
Intrinsic immunostimulatory activity of polyphosphazene adjuvants were established in vitro, utilizing human newborn and adult dendritic cells (DCs) [14]. In these studies, PCPP was benchmarked to Alum, the most commonly used vaccine adjuvant. PCPP induced dose dependent cytokine production (release of danger signals) from both newborn and adult DCs. Up to a 1000-fold increase compared to unstimulated cells was detected, which was in contrast with Alum, which only induced a marginal increase - less than 10-fold (Fig. 8A). Both PCPP and its supramolecular complex with model antigen -HIV-Gag induced release of mixed Th1/Th2 cytokine responses, promoting both cellular and potentially humoral responses to PCPP assembled antigen. PCPP and its complex containing 10 molecules of HIV-Gag, but not the antigen alone, also induced maturation of DCs as assessed by surface expression levels of co-stimulatory markers - CD83 and CD86 (Fig. 8B). Importantly, interactions between PCPP and the antigen were observed to be gentle enough not to interfere with effective antigen internalization and presentation (Fig. 8C). It is particularly noteworthy that PCPP induced comparable levels of maturation and activation in both newborn and adult DCs, apparently bypassing the well-documented distinct early life impairment in Th1-polarizing cytokine production observed in response to multiple immune stimuli, which may have additional applicability for early life immunization [14].
Fig. 8.
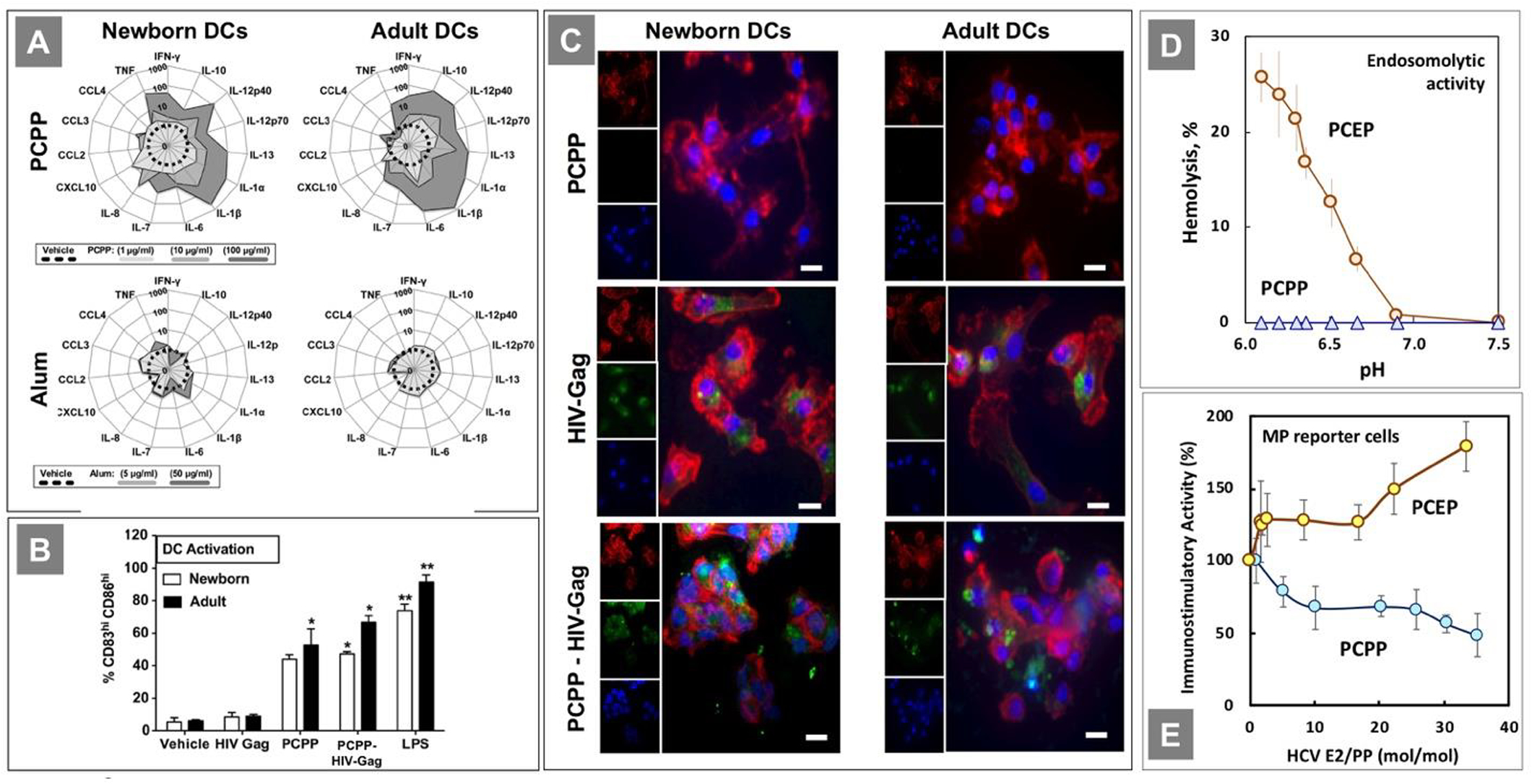
(A) PCPP-induced newborn and adult DC maturation profile is superior to Alum’s; (B) DC maturation profile as percentage of the total population of gated DCs per condition; (C) HIV-Gag - PCPP assemblies are internalized by human newborn and adult DCs. (All reprinted from [14], Copyright 2014, with permission from Elsevier). (D) Endosomolytic activity of PCEP as determined by hemolysis assay. (Reprinted with permission from [15]. Copyright 2016 American Chemical Society). (E) The effect of E2 antigen loading on the immunostimulatory activity of complexes as evaluated in macrophage reporter cell lines. (Reprinted with permission from [10]. Copyright 2020 American Chemical Society)
In a separate study, PCPP and PCEP were shown to directly stimulate production of cytokines in mouse spleen cells in the absence of antigen [30]. IL-4, IL-12 and IFNy responses were assessed and PCEP showed superior activity to PCPP. It should be noted, that PCEP induced direct activation of naïve mouse splenic B-cells in a dose dependent manner [111].
PCPP and PCEP were investigated for their ability to induce TLR activation using engineered HEK293 reporter cells with overexpressed human TLRs, specifically TLR2, 3, 4, 5, 7, 8, and 9 [15]. Although it is clear that both PCPP and PCEP were able to stimulate similar cellular responses, they do not appear specific as negative control cell lines also showed some activation. This underlines non-specific ionic type of interactions of polyphosphazene adjuvants with receptors on cell surfaces. AF4 experiments, which analyzed interactions of these polymers with soluble TLRs at near physiological conditions, revealed that both PCPP and PCEP effectively formed supramolecular complexes with all tested receptors [15].
The above results suggest that the differences observed for structurally similar PCPP and PCEP in terms of their in vivo performance (Chapter 2) are less likely to be derived from the distinctions in their interactions with specific receptors and that some physico-chemical features are probably responsible. One notable difference between the two polymers lies in their behavior at the pH range corresponding to the pH environment of early endosomes. PCEP, but not PCPP, displays pH-dependent membrane disruptive activity at pH below 6.8, as determined using red blood cells as endosomal membrane models (Fig. 8D). This can potentially result in different abilities of thetwo adjuvants to facilitate cross-presentation of the antigen. In fact, it has been previously noted that some vaccine delivery carriers are capable of increasing the amount of antigen that escapes from endosomes into the cytoplasm resulting in its more efficient direction into the major histocompatibility complex (MHC) class I antigen presentation pathway [112–114]. The other potential line of investigation to explain superior in vivo activity of PCEP may require more comprehensive investigation of the physiological stability of antigen loaded polyphosphazene assemblies. PCPP, but not PCEP, is well-known to show some sensitivity to sodium ions, which can potentially result in ion-induced phase separation [24, 85]. This phenomenon can be significantly amplified when some charges, which usually facilitate water-solubility of polyelectrolytes, are consumed in ionic interactions with protein antigens. It was reported [10] that immunostimulatory activity of HCV E2 antigen-loaded PCPP complexes in an engineered mouse macrophage reporter cell line assay decreases with increased antigen load, whereas PCEP complexes follow the opposite direction (Fig. 8E). The observed trend for PCPP complexes was correlated with increasing aggregation of PCPP complexes at higher antigen load [10]. Further combined physico-chemical, in vitro, and in vivo studies are required to better understand substantial differences observed for the two lead polyphosphazene adjuvants.
4.3. In vivo studies on the mechanism and structure-activity relationship
Early studies on the structure-activity relationship of ionic polyphosphazene macromolecules had a main objective of establishing to what extent their charge or molecular size had any influence on in vivo activity of these macromolecules. Studies, in which carboxylic acid groups of PCPP were partially replaced by non-ionic ester functionalities, reveal that the immune response was directly proportional to the content of ionic groups (charge) in the polymer (Fig. 9A). Since the ability of PCPP to form complexes with proteins is based primarily on electrostatic interactions, these results may be interpreted in favor of the importance of PCPP as a delivery vehicle. Similarly, immunoadjuvant activity of PCPP is directly dependent on the size of the complex with maximum activity observed with macromolecules of 500 kDa and above (Fig. 9B), which for PCPP typically corresponds to hydrodynamic diameter in excess of 60 nm. This may suggest that similarly to nanoparticles, the size of the polymer and corresponding complexes is important in targeting immune cells and the efficient induction of the immune response [110].
Fig. 9.
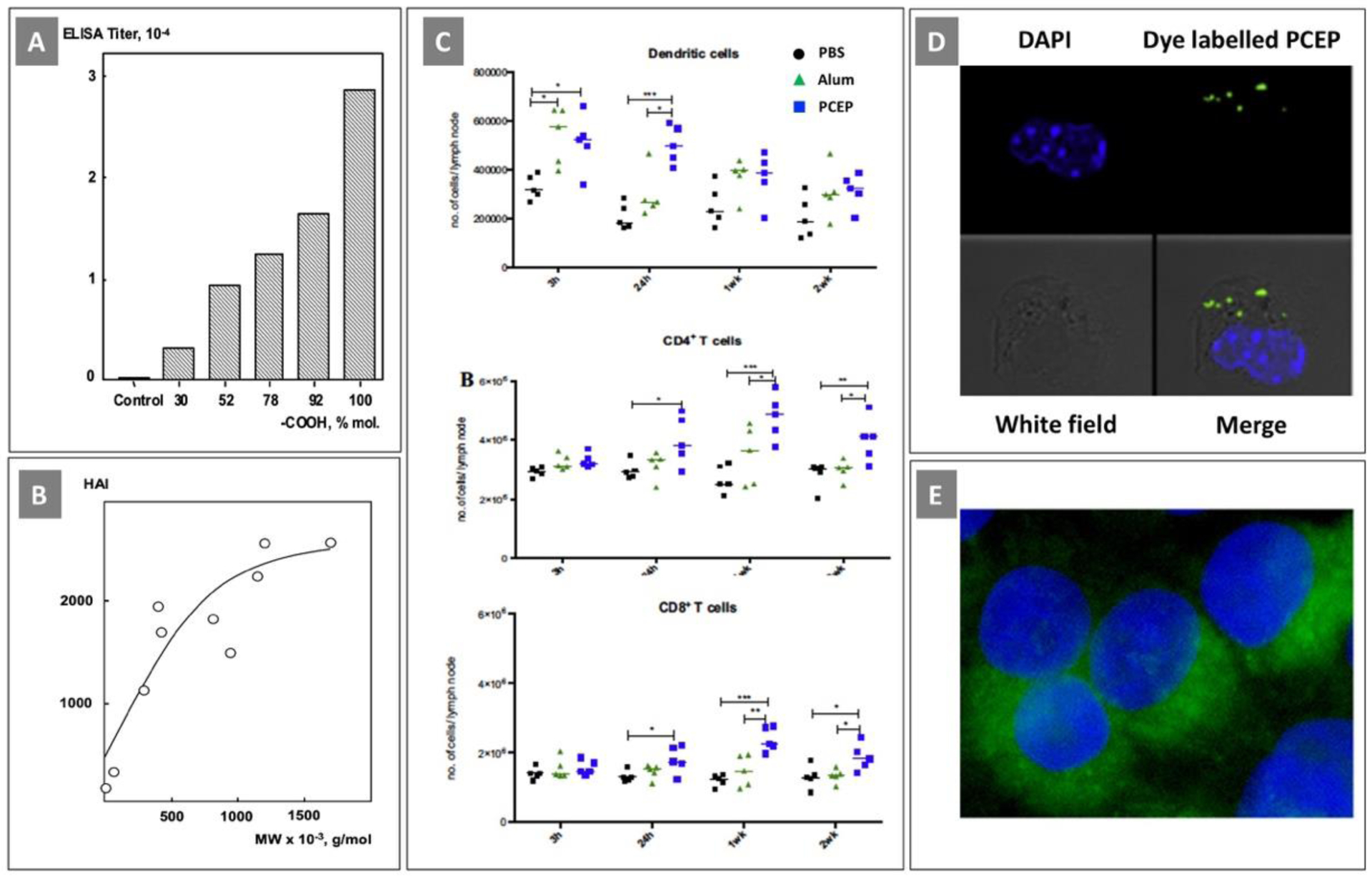
(A) Serum IgG titers after immunization of mice with influenza antigen adjuvanted with PCPP with variable content of carboxylic acid group. (Reprinted with permission from [85]. Copyright 2004, American Chemical Society); (B) HAI titers in mice immunized with influenza antigen adjuvanted with PCPP having various molecular weight. (Reprinted from [27], Copyright 1997, with permission from Elsevier). (C) PCEP stimulates increased immune cell numbers in the draining lymph nodes in mice; (D) Intracellular uptake of PCEP at the injection site in mice as analyzed using a confocal laser scanning microscope. (All reprinted from [115], Copyright 2014, with permission from Elsevier); (E) Fluorescence microscopy visualization of PCEP copolymer enhanced cytosolic delivery of a model protein cargo (Reprinted with permission from [116]. Copyright 2017 American Chemical Society)
The majority of recent studies on the mechanism of action in vivo were conducted using PCEP. PCEP was shown to be a strong modulator of innate immune responses at the site of injection [117]. It induced time-dependent changes in the gene expression of multiple adjuvant core response genes and triggered strong local production of cytokines and chemokines. It was noted that polyphosphazene adjuvant induced genes were distinct from those triggered by CpG, suggesting their different mechanisms of action. It was also established that PCEP up-regulated the gene expression of the inflammasome receptor and induced the production of pro-inflammatory cytokines IL-1β, and IL-18, which suggests activation of the inflammasome as a possible part of the mechanism of action [117]. In a somewhat contrast with these investigations, studies in pigs revealed that PCEP induced the expression of chemokine CCL2 and proinflammatory cytokine IL-6 - an inducer of B cell activity and differentiation, indicating the ability of PCEP to promote a Th2 type immune response [88].
Further studies in mice showed that PCEP caused recruitment of immune cells to the site of injection with subsequent trafficking to lymph nodes [115]. Similar results were obtained when PCEP was administered intradermally in pigs [118]. In mice, flow cytometry analysis revealed significantly higher numbers of myeloid and lymphoid cells (results for DCs, CD4+ and CD8+ T cells are shown in Fig. 9C) detected in the draining lymph nodes after injection of PCEP, when compared with results obtained for mice injected with Alum or PBS. The authors also reported that fluorescently labeled PCEP was detected in over 28 % of the total cells recruited to the site of injection [115]. It should be noted that no data was presented on the efficiency of fluorescent dye labeling, the stability of the conjugate or the effect of labeling on the biophysical properties of the polymer. Nonradiative labeling is notorious for causing significant alterations in biological behavior of water-soluble polymers [119]. Nevertheless, confocal microscopy results showed that fluorescently labeled PCEP localized within a defined region in the cytosol of various recruited cell populations (Fig. 9D). It is noteworthy, that in vitro investigations conducted on PCEP copolymers by another research group demonstrated that the polymer is capable of enhancing intracellular protein uptake of over 40-fold (Fig. 9E) [116]. Taken together, these findings emphasize the importance of further studies of the delivery capabilities of polyphosphazene adjuvants and their contribution in the mechanism of action.
5. Particulate and microfabricated formulations
5.1. Nano- and microparticulate systems
PCPP was originally introduced as a synthetic alternative to the well-known biocompatible polymer, alginic acid [120]. Similarly, to this natural polymer, PCPP is capable of forming ionotropic hydrogels when exposed to aqueous solutions of calcium salts [20, 21]. The resulting cross-linked materials, which are characterized by superb mechanical characteristics, have been studied for encapsulation of cells [21, 121], biomedical imaging [122–125], oral delivery of bioactives [35, 126, 127], sustained release [22, 23], and bone replacement applications [128, 129]. The concept of conforming water-soluble macromolecular adjuvant into nanoparticulates also appears attractive as a pathway to provide concurrent sustained release of antigen and immunoadjuvant [130]. Contrary to many other encapsulation methods, the technology does not require use of organic solvents, elevated temperatures, or sophisticated manufacturing processes.
Polyphosphazene-based micro and nanoparticulates can be produced by several methods, which result in various size distribution parameters (Fig. 10A). Initially, the technology relied on spraying polymer solutions into aqueous calcium chloride, which generated microspheres in a micrometer-millimeter size range [21–23, 25]. It also had severe limitations due to non-linear scale-up, issues related to frequent nozzle clogging, and challenges in containment of biologics. The evolvement of nozzle-free methods followed a discovery of atypical sensitivity of PCPP to sodium ions [85, 131]. This resulted in a development of a two-step method, in which PCPP solution undergoes sodium-induced coacervation followed by cross-linking with calcium chloride [24]. Nanoparticulates can be produced through complexation of polyphosphazenes with either physiologically benign multivalent amines, such as spermine and spermidine [130], or poly(oxyethylene) [132]. These techniques are not restricted to PCPP and can be applied to other derivatives as well [39, 103, 133]. The addition of PEGylated polyphosphazenes, such as PEGylated derivative of PCPP, not only stabilizes the system in the presence of salts, but also allows achievement of a more narrow size distribution [16]. The use of microfluidics mixing techniques has also demonstrated improved uniformity and reproducibility of the results [16, 124, 125]. A noticeable feature of all of the above methods is high encapsulation efficiency. Proteins first self-assemble with polyphosphazenes into non-covalent complexes, which are then conformed into micro- and nanoparticulates [15, 16, 130].
Fig. 10.
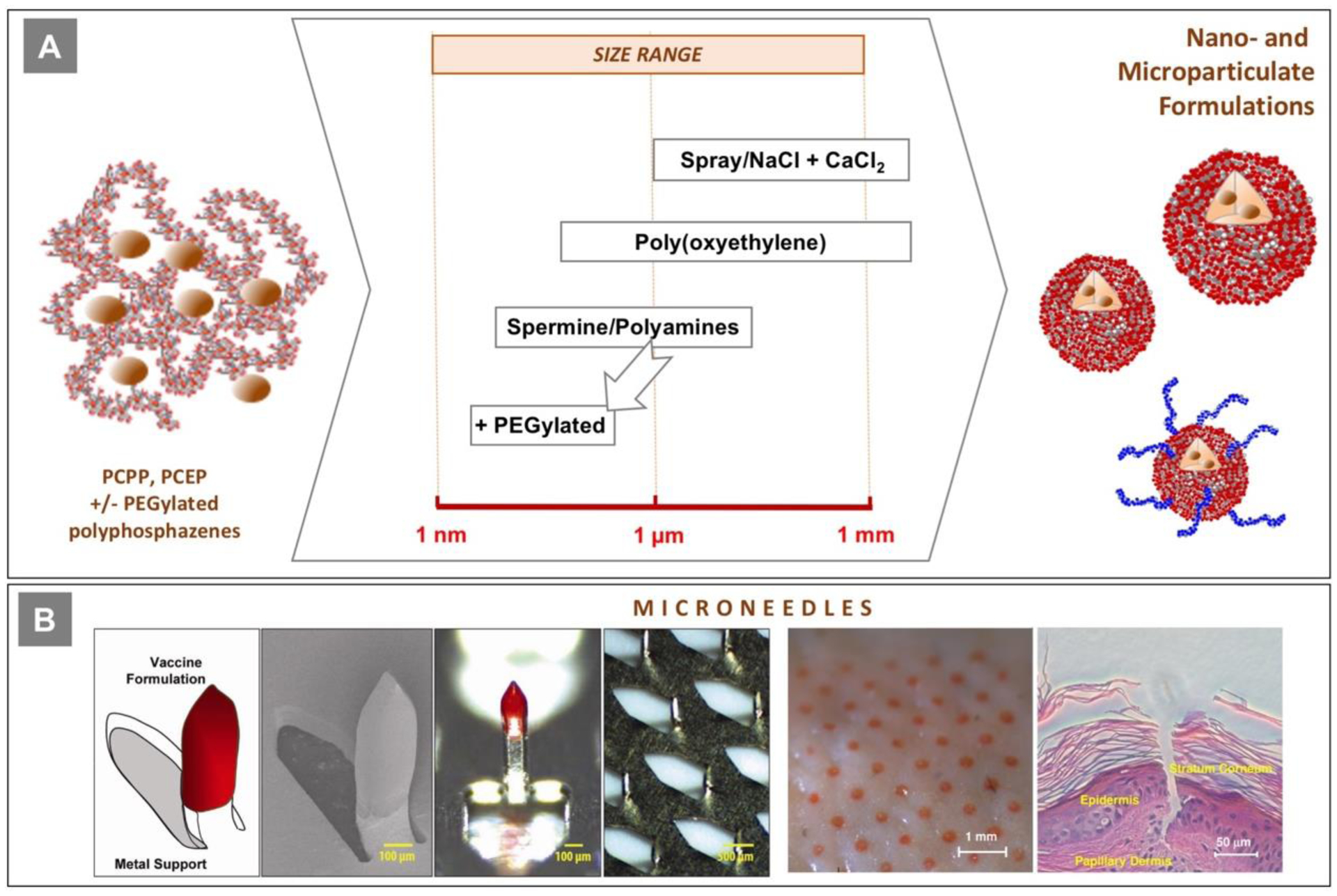
(A) The relationship between various methods for preparation of polyphosphazene nano- and microparticulates and their dimensions; (B) polyphosphazene coated microneedles - schematics, scanning electron and optical microscopy images of microneedles and their array; images of porcine skin surface after application of microneedle patch and histological analysis of pierced skin (reproduced with permission from [31]).
Despite a number of publications which investigated in vivo activity of ionically cross-linked PCPP and PCEP, the advantages of these systems over conventional water-soluble polyphosphazenes have yet to be demonstrated. An earlier study compared in vivo activity of intranasally administered tetanus toxoid, which was encapsulated in microspheres formulated on the basis of PCPP, alginic acid, and PCPP-alginic acid blend [25]. Although an adjuvant effect of PCPP microspheres was greatly superior compared to those composed of alginic acid, the maximum activity was achieved when both polymers were blended [35]. This effect may be explained by insufficient stability of unmodified polyphosphazene microspheres, which tend to aggregate under physiological conditions and can benefit from surface modification with PEGylated polyphosphazenes or polycations [16, 124, 125]. Investigation of the in vivo adjuvant effect of PCPP and PCEP particulates has been greatly extended to include various antigens and routes of vaccine administration [58, 61, 94, 134]. However, the lack of direct comparison with water-soluble formulations and absence of physico-chemical characterization and microsphere stability data in these studies dictate the need for further research in this area.
5.2. Microneedles and Intradermal Delivery
Delivery of vaccines to the skin - an anatomic space which contains a high density of dermal dendritic cells and epidermal Langerhans cells, is a promising approach both in terms of induction of superior immune responses and addressing the need for antigen sparing [135]. However, it also requires either special medical personnel training or development of technologies which do not rely on the use of conventional hypodermic needles. The use of microneedles - microfabricated structures that can pierce the skin and deliver vaccine to epidermis and dermis compartments is an especially attractive option [135, 136]. To that end, PCPP and other polyphosphazenes offer unprecedented opportunity as they can be used both as an immunoadjuvant and, due to their excellent mechanical properties, microfabrication material, thus minimizing the size of microneedle patches needed for effective vaccination [31, 40, 137, 138]. Arrays of 600-μm long microneedles were fabricated, which contained solid PCPP-HBsAg vaccine formulation as a coating on the titanium core (Fig. 10B). Once applied to pig skin as part of the band-aid type patch, these microneedles effectively cut the stratum corneum and the formulation quickly dissolves into the epidermis/dermis compartments (Fig. 10B). Immunization studies in pigs demonstrated that HBsAg containing PCPP microneedles induced antibody titers which were three orders of magnitude higher than those induced by microneedles with antigen-carboxymethylcellulose coatings and at least 10-fold higher than those resulting from intramuscular injection with the polyphosphazene adjuvanted HBsAg formulation [31]. In this study, PCPP microneedles also showed significant antigen dose sparing compared to the formulation delivered by intramuscular injection. Intradermal immunization with PCEP formulations was also investigated, but these studies utilized conventional injection methods [88, 92].
6. Polyphosphazene-based combination adjuvants
Due to the excellent water-solubility, ease of formulation, and delivery capabilities, polyphosphazenes provide an attractive platform for the development of combination adjuvants. The ability of PCPP to enable a synergistic response with the TLR4 agonist (MPL) was first demonstrated with influenza antigen in mice [139]. Similar effects were observed with combinations of PCPP or PCEP with CpG ODN in mice and pigs [30, 86, 140, 141]. Further studies were undertaken to combine PCEP or PCEP-GpG formulation with cationic host-defense peptides (HDPs) or their synthetic analogs - Innate Defense Regulators (IDRs) [56, 59, 90, 91, 142, 143]. By far the most investigated polyphosphazene-based combination adjuvant is a ternary system, which includes IDR-1002 peptide, poly(I:C) and PCEP - “TriAdj”. Immunoadjuvant potency of TriAdj was validated in mice, rats, cotton rats, hamsters, rabbits, lambs, calves, and pigs with a variety of antigens using different administration routes, such as intramuscular, intranasal, and intrapulmonary [63, 134, 144–159]. Triadj was also investigated as part of the liposomal delivery system [160]. A recent review outlines some of the developments in this field in more details [161]. It may be also noted that the use of anionic PCEP and cationic IDR peptides should inadvertently lead to the formation of supramolecular polymer-peptide complexes formed via ionic interactions. It can be expected that research into intermolecular interactions and optimization of such complexes in terms of their binding to the antigen can lead to further improvements in the performance of polyphosphazene-based combination adjuvants.
7. Biodegradation and shelf-life
Applications of water-soluble polyphosphazenes as injectables became possible due to the ability of these synthetic macromolecules to undergo hydrolytic degradation at near physiological conditions. The hydrolytic pathway for these macromolecules is well established and results in the cleavage of side groups and release of phosphate and ammonium ions [18, 29, 39, 85, 103, 116, 133, 162–165]. In particular, the degradation profile of PCPP is characterized by a relatively long half-life at near physiological conditions (150 days) and the release of the benign by-product - hydroxybenzoic acid, which is also a well-known metabolite of propyl paraben - an excipient generally regarded as safe (GRAS) by the FDA [18, 162]. Similarly, PCMP and PCEP degrade into 4-hydroxyphenylacetic acid and 3–4-hydroxyphenyl)propionic acid (desaminotyrosine), correspondingly. These compounds are constituents of numerous foods, such as olives, cocoa beans, oats, and beer, and are well-known human metabolites [95–100]. The rate of degradation can be modulated through addition of certain pendant groups, which introduce hydrolytically labile bonds in the polymer structure. For example, mixed substituent copolymers of PCPP containing N-ethylpyrrolidone [103] or PEG [165] side groups degrade faster than PCPP and the rate of hydrolysis increases proportionally to their content in the polymer. The degradation rate can be also amplified by the presence of residual chlorine atoms and hydroxyl groups - irregularities contained in incompletely substituted polyphosphazenes [85]. Therefore, careful control of the residual groups (“structural defects”) or “weak links” in polyphosphazene structures is needed to ensure reproducibility of results. It is also important to note that the degradation rate is dependent on the temperature and is dramatically reduced below 4°C, therefore allowing for adequate shelf-life in solution in a refrigerated or frozen state [18].
8. Synthesis and manufacturing of polyphosphazene adjuvants
All polyphosphazene immunoadjuvants reported to date were produced using the macromolecular substitution route - ring-opening polymerization of hexachlorocyclotriphosphazene (trimer) yielding polydichlorophosphazene (PDCP) - followed by replacement of chlorine atoms of PDCP with organic nucleophiles [20, 39, 85, 166]. Although this is a traditional route to polyphosphazene synthesis, several challenges had to be addressed before reproducible synthesis and the cGMP production process for PCPP had been established [85]. Conversion of highly hydrolytically sensitive PDCP into a water-soluble polyphosphazene adjuvant may result in partial polymer breakdown, cross-linking, or generation of undesirable residual groups, which can adversely affect its performance or shelf-life. A series of innovations have been made, which included discovery of the stabilizing effect of diglyme on PDCP, development of aqueous based deprotection methods and a single solvent - single pot approach, controlling the degree of conversion in the polymerization process and forcing substitution conditions [85, 131, 167–170]. This allowed the development of a cGMP process, which demonstrated its simplicity, scalability, and robustness in over two hundred production cycles, including manufacturing of cGMP consistency lots and millions of human doses of clinical grade material [85]. Synthesis of new generation PCEP and PCMP adjuvants, as well as their diverse copolymers, are essentially based on the same key elements of the process and share a greater part of their production processes with PCPP [39, 103, 116, 133, 165].
Several alternative synthetic routes to PDCP, which are based on condensation processes, have also been developed [171–174]. Among them, a method based on room temperature, “living” polymerization is introduced, which allows for the preparation of polyphosphazene block copolymers with controlled architecture [175, 176]. However, potential utility of these methods for the synthesis of polyphosphazene adjuvants is yet to be investigated.
9. Characterization of polyphosphazene adjuvants and their formulations with antigens
Physico-chemical characterization methods for polyphosphazene polyelectrolytes are well-established and are common for the analysis of either polyphosphazenes, or polyelectrolytes. Molecular structure and absence of structural irregularities, such as hydroxyl groups, ester groups, or chlorine atoms, are typically confirmed using 1H, 31P, and 13C nuclear magnetic resonance (NMR) spectroscopy and elemental analysis. Focus studies on the determination of absolute molecular weights of PCPP and its molecular weight distributions, preparation of narrow molecular weight standards, and the dependence of molecular weight parameters on conditions of PDCP to PCPP conversion process were undertaken [85, 166]. This included determination of the second virial coefficient, refractive index increment, and Mark-Houwink constants (for viscometry measurements) for PCPP - all at near physiological conditions (PBS, pH 7.4). The multi-angle laser light scattering (MALS) method, both as stand-alone and in combination with gel-permeation chromatography (GPC), was used for the determination of absolute molecular weight of PCPP [177]. Applicability of conventional GPC methods, using universal calibration curves, ionic, and neutral standards, were also evaluated [177].
Analysis of polyphosphazene formulations with antigenic proteins is no less important, however is somewhat more challenging. Knowledge of intermolecular interactions in the formulation, biophysical and biochemical properties of antigen-polyphosphazene complexes, and avoidance of potential undesirable aggregation in the system, are all critical for achieving reproducible and predictable behavior of formulations in vivo. Two interconnected aspects require specific attention here - compositional/conformational parameters of the complex and antigenicity of the protein. Isotherms of antigen - polyphosphazene binding were initially established for some systems using GPC equipped with a diode array detector [11, 14]. However, this technique proved to be of limited applicability due to existence of non-specific interactions between many complexes and resins which are commonly employed for analytical separation. Significant progress in the analysis of intermolecular complexes was achieved following introduction of AF4 method [7, 9, 10, 15, 16, 116, 165]. Similarly to GPC, this technique offers size-dependent separation of the analyte, yet allowes analysis of supramolecular assemblies and particulates up to micrometer size and minimizes interactions with the surface of a stationary phase [178].
Perhaps the most critical parameter that can affect in vivo performance of the formulation is the conformation of antigen-polyphosphazene assemblies. It was noted that compaction and, in extreme cases, immediate or slow aggregation occurring in the system appear to adversely affect antigen presentation and result in inferior in vivo activity [10, 11]. To that end, dynamic light scattering (DLS) or MALS analysis of the formulation to determine dimensions of the assembly and its short-term stability is mandatory. In case of extremely strong antigen-polyphosphazene interactions, component mixing facilitated by a microfluidics technique or careful selection of mixing volumes may be useful [7, 16]. It is always a good approach to verify that the protein assembled in a supramolecular complex maintains its antigenicity using conventional methods, such as ELISA [7] or SRID [13]. Finally, if a co-adjuvant is used, such as resiquimod, the formation of ternary assemblies can be confirmed by AF4, fluorescent, and NMR spectroscopy methods [9]. In summary, biophysical characterization of formulations and antigen-polyphosphazene supramolecular assemblies appear to be compulsory for generation of consistent and reproducible in vivo results.
10. Conclusion
Polyphosphazenes are uniquely positioned among other immunoadjuvants. These macromolecules do not resemble the majority of systems investigated as adjuvants and vaccine delivery vehicles to date. Unlike conventional antigen delivery carriers, which are typically represented by emulsions, liposomes, nanoparticles and aluminum-based hydrogels, these macromolecules are fully water-soluble. Contrary to “true immunostimulating compounds”, such as TLR agonists, they do not carry functionalities responsible for specific interactions with cell receptors. In contrast with conventional polyelectrolytes, which only show significant immunoadjuvant activity when chemically conjugated to the protein, polyphosphazenes do not require covalent attachment to the antigen.
As is the case with many other important immunoadjuvants, the mechanism of action, as well as structure-activity relationship for polyphosphazene family of adjuvants, are yet to be established in detail. Nevertheless, the hypothesis of their dual functionality - antigen delivery and immunostimulation - gains growing experimental support. Perhaps one of the most intriguing and yet to be answered questions is why two structurally similar members of polyphosphazene homologue series, PCPP and PCEP, behave so differently in vivo. Generally, PCEP, which only differs from PCPP by two methylene groups, not only promotes higher antibody levels to vaccine antigens, but shifts the response towards a desirable balanced Th1/Th2 immunity. The mechanistic cause of this is still poorly understood. Although the differences between two polymers are negligible from the structural chemistry prospective, properties of their complexes with antigens and, especially their stability, are shown to be dissimilar in a number of cases. As mentioned above, an overview of recent studies suggests that these distinctions may be antigen-dependent. Some instances of PCPP promoted balanced immunity and Th2 biased responses induced by PCEP formulations are now noted. Therefore, when two adjuvants are compared, the cause of varying performance must be sought not only in the properties of polyphosphazenes, but also in the behavior of their complexes with antigens. This, once again, mandates careful physico-chemical and biophysical characterization of the formulation prior to in vivo evaluation, which is not yet standard in the field.
As outlined in present review, in vivo potency of polyphosphazene adjuvants is proven with multiple viral and bacterial antigens in almost one hundred published studies. Moreover, PCPP also showed safety and immunoadjuvant activity in clinical trials. Contrary to many biphasic vaccine delivery systems, polyphosphazenes are well-defined synthetic macromolecules which have excellent solubility under physiological conditions. They are extremely simple to formulate and combine with other adjuvants. Polyphosphazenes also offer unprecedented opportunities for preparation of solid formulations, such as microneedles for intradermal immunization and nanoparticulates for improved targeting or concurrent antigen and adjuvant release. However, despite their proven potency, versatility, safety, and clinical history, polyphosphazenes remain one of the most overlooked classes of immunoadjuvants and vaccine delivery systems.
Highlights.
Polyphosphazenes are potent immunostimulants and vaccine delivery vehicles
Polyphosphazenes assemble antigens in virus-mimicking supramolecular constructs
Mechanism of action reviewed in context of molecular antigen-adjuvant interactions
Compendium of clinical and in vivo data presented for PCPP and PCEP adjuvants
Synthetic, formulation, and biophysical characterization methodologies are reviewed
Acknowledgements
This work was supported in part by the National Institutes of Health Grant R01AI132213 (A.A.) and the National Science Foundation under Award DMR-1808531 (A.A.). Authors are grateful to Zachary Tropeano-Lovatt and Elizaveta A. Andrianova for expert assistance with proof-reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Conflict of Interest
A.K.A. declares no conflict of interest. For a list of entities with which R.L. is involved, compensated or uncompensated, see www.dropbox.com/s/yc3xqb5s8s94v7x/Rev%20Langer%20COI.pdf?dl=0.
References
- [1].Rappuoli R, Pizza M, Del Giudice G, De Gregorio E, Vaccines, new opportunities for a new society, Proc. Natl. Acad. Sci. U. S. A, 111 (2014) 12288–12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].De Gregorio E, Rappuoli R, From empiricism to rational design: a personal perspective of the evolution of vaccine development, Nat. Rev. Immunol, 14 (2014) 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Reed SG, Orr MT, Fox CB, Key roles of adjuvants in modern vaccines, Nat. Med, 19 (2013) 1597–1608. [DOI] [PubMed] [Google Scholar]
- [4].Nanishi E, Dowling DJ, Levy O, Toward precision adjuvants: optimizing science and safety, Curr. Opin. Pediatr, 32 (2020) 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Del Giudice G, Rappuoli R, Didierlaurent AM, Correlates of adjuvanticity: A review on adjuvants in licensed vaccines, Semin. Immunol, 39 (2018) 14–21. [DOI] [PubMed] [Google Scholar]
- [6].Brito LA, Malyala P, O’Hagan DT, Vaccine adjuvant formulations: A pharmaceutical perspective, Semin. Immunol, 25 (2013) 130–145. [DOI] [PubMed] [Google Scholar]
- [7].Cayatte C, Marin A, Rajani GM, Schneider-Ohrum K, Snell Bennett A, Marshall JD, Andrianov AK, PCPP-Adjuvanted Respiratory Syncytial Virus (RSV) sF Subunit Vaccine: Self-Assembled Supramolecular Complexes Enable Enhanced Immunogenicity and Protection, Mol. Pharmaceutics, 14 (2017) 2285–2293. [DOI] [PubMed] [Google Scholar]
- [8].Martinez AP, Qamar B, Marin A, Fuerst TR, Muro S, Andrianov AK, Biodegradable “Scaffold” Polyphosphazenes for Non-Covalent PEGylation of Proteins, in: Andrianov A, Allcock HR (Eds.) Polyphosphazenes in Biomedicine, Engineering, and Pioneering Synthesis, American Chemical Society, 2018, pp. 121–141. [Google Scholar]
- [9].Andrianov AK, Marin A, Wang R, Karauzum H, Chowdhury A, Agnihotri P, Yunus, Abdul, R.A. Mariuzza, T.R. Fuerst, Supramolecular assembly of Toll-like receptor 7/8 agonist into multimeric water-soluble constructs enables superior immune stimulation in vitro and in vivo, ACS Appl. Bio Mater, 3 (2020) 3187–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Andrianov AK, Marin A, Wang R, Chowdhury A, Agnihotri P, Yunus AS, Pierce BG, Mariuzza RA, Fuerst TR, In Vivo and In Vitro Potency of Polyphosphazene Immunoadjuvants with Hepatitis C Virus Antigen and the Role of Their Supramolecular Assembly, Mol. Pharm, (2020) 10.1021/acs.molpharmaceut.1020c00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Andrianov AK, Marin A, Roberts BE, Polyphosphazene polyelectrolytes: A link between the formation of noncovalent complexes with antigenic proteins and immunostimulating activity, Biomacromolecules, 6 (2005) 1375–1379. [DOI] [PubMed] [Google Scholar]
- [12].Marin A, DeCollibus DP, Andrianov AK, Protein Stabilization in Aqueous Solutions of Polyphosphazene Polyelectrolyte and Non-Ionic Surfactants, Biomacromolecules, 11 (2010) 2268–2273. [DOI] [PubMed] [Google Scholar]
- [13].Andrianov AK, Decollibus DP, Marin A, Webb A, Griffin Y, Webby RJ, PCPP-formulated H5N1 influenza vaccine displays improved stability and dose-sparing effect in lethal challenge studies, J. Pharm. Sci, 100 (2011) 1436–1443. [DOI] [PubMed] [Google Scholar]
- [14].Palmer CD, Ninković J, Prokopowicz ZM, Mancuso CJ, Marin A, Andrianov AK, Dowling DJ, Levy O, The effect of stable macromolecular complexes of ionic polyphosphazene on HIV Gag antigen and on activation of human dendritic cells and presentation to T-cells, Biomaterials, 35 (2014) 8876–8886. [DOI] [PubMed] [Google Scholar]
- [15].Andrianov AK, Marin A, Fuerst TR, Molecular-Level Interactions of Polyphosphazene Immunoadjuvants and Their Potential Role in Antigen Presentation and Cell Stimulation, Biomacromolecules, 17 (2016) 3732–3742. [DOI] [PubMed] [Google Scholar]
- [16].Andrianov AK, Marin A, Deng J, Fuerst TR, Protein-loaded soluble and nanoparticulate formulations of ionic polyphosphazenes and their interactions on molecular and cellular levels, Mater. Sci. Eng., C, 106 (2020) 110179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Andrianov AK, Self-Assembling Ionic Polyphosphazenes and Their Biomedical Applications, in: Polyphosphazenes in Biomedicine, Engineering, and Pioneering Synthesis, American Chemical Society, 2018, pp. 27–49. [Google Scholar]
- [18].DeCollibus DP, Marin A, Andrianov AK, Effect of Environmental Factors on Hydrolytic Degradation of Water-Soluble Polyphosphazene Polyelectrolyte in Aqueous Solutions, Biomacromolecules, 11 (2010) 2033–2038. [DOI] [PubMed] [Google Scholar]
- [19].Andrianov AK, Jenkins SA, Payne LG, Roberts BE, Phosphazene polyelectrolytes as immunoadjuvants, U.S., 5,494,673, 1996, February 27. [Google Scholar]
- [20].Allcock HR, Kwon S, An ionically cross-linkable polyphosphazene: Poly[bis(carboxylatophenoxy)phosphazene] and its hydrogels and membranes, Macromolecules, 22 (1989) 75–79. [Google Scholar]
- [21].Cohen S, Bano MC, Visscher KB, Chow M, Allcock HR, Langer R, Ionically crosslinkable polyphosphazene: a novel polymer for microencapsulation, J. Am. Chem. Soc, 112 (1990) 7832–7833. [Google Scholar]
- [22].Andrianov AK, Cohen S, Visscher KB, Payne LG, Allcock HR, Langer R, Controlled release using ionotropic polyphosphazene hydrogels, J. Controlled Release, 27 (1993) 69–77. [Google Scholar]
- [23].Andrianov AK, Payne LG, Visscher KB, Allcock HR, Langer R, Hydrolytic degradation of ionically cross-linked polyphosphazene microspheres, J. Appl. Polym. Sci, 53 (1994) 1573–1578. [Google Scholar]
- [24].Andrianov AK, Chen J, Payne LG, Preparation of hydrogel microspheres by coacervation of aqueous polyphosphazene solutions, Biomaterials, 19 (1998) 109–115. [DOI] [PubMed] [Google Scholar]
- [25].Payne LG, Jenkins SA, Andrianov A, Roberts BE, Water-soluble phosphazene polymers for parenteral and mucosal vaccine delivery, Pharm. Biotechnol, 6 (1995) 473–493. [DOI] [PubMed] [Google Scholar]
- [26].Payne LG, Jenkins SA, Andrianov A, Langer R, Roberts BE, Xenobiotic polymers as vaccine vehicles, Adv. Exp. Med. Biol, 371 (1995) 1475–1480. [PubMed] [Google Scholar]
- [27].Payne LG, Jenkins SA, Woods AL, Grund EM, Geribo WE, Loebelenz JR, Andrianov AK, Roberts BE, Poly[di(carboxylatophenoxy)phosphazene] (PCPP) is a potent immunoadjuvant for an influenza vaccine, Vaccine, 16 (1998) 92–98. [DOI] [PubMed] [Google Scholar]
- [28].Payne L, Van Nest G, Barchfeld G, Siber G, Gupta R, Jenkins S, PCPP as a parenteral adjuvant for diverse antigens, Dev. Biol. Stand, 92 (1998) 79–88. [PubMed] [Google Scholar]
- [29].Mutwiri G, Benjamin P, Soita H, Townsend H, Yost R, Roberts B, Andrianov AK, Babiuk LA, Poly[di(sodium carboxylatoethylphenoxy)phosphazene] (PCEP) is a potent enhancer of mixed Th1/Th2 immune responses in mice immunized with influenza virus antigens, Vaccine, 25 (2007) 1204–1213. [DOI] [PubMed] [Google Scholar]
- [30].Mutwiri G, Benjamin P, Soita H, Babiuk LA, Co-administration of polyphosphazenes with CpG oligodeoxynucleotides strongly enhances immune responses in mice immunized with Hepatitis B virus surface antigen, Vaccine, 26 (2008) 2680–2688. [DOI] [PubMed] [Google Scholar]
- [31].Andrianov AK, DeCollibus DP, Gillis HA, Kha HH, Marin A, Prausnitz MR, Babiuk LA, Townsend H, Mutwiri G, Poly[di(carboxylatophenoxy)phosphazene] is a potent adjuvant for intradermal immunization, Proc. Natl. Acad. Sci. U.S.A, 106 (2009) 18936–18941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Istrate C, Hinkula J, Charpilienne A, Poncet D, Cohen J, Svensson L, Johansen K, Parenteral administration of RF 8–2/6/7 rotavirus-like particles in a one-dose regimen induce protective immunity in mice, Vaccine, 26 (2008) 4594–4601. [DOI] [PubMed] [Google Scholar]
- [33].Shim D-H, Ko H-J, Volker G, Potter AA, Mutwiri G, Babiuk LA, Kweon M-N, Efficacy of poly[di(sodium carboxylatophenoxy)phosphazene] (PCPP) as mucosal adjuvant to induce protective immunity against respiratory pathogens, Vaccine, 28 (2010) 2311–2317. [DOI] [PubMed] [Google Scholar]
- [34].McNeal MM, Rae MN, Ward RL, Effects of different adjuvants on rotavirus antibody responses and protection in mice following intramuscular immunization with inactivated rotavirus, Vaccine, 17 (1999) 1573–1580. [DOI] [PubMed] [Google Scholar]
- [35].Payne LG, Jenkins SA, Andrianov AK, Roberts BE Water-soluble phosphazene polymers for parenteral and mucosal vaccine delivery, in: Powell MF, Newman MJ (Eds.) Vaccine Design, New York, NY, 1995, pp. 473–493. [DOI] [PubMed] [Google Scholar]
- [36].Andrianov AK, Sargent JR, Sule SS, Le Golvan MP, Woods AL, Jenkins SA, Payne LG, Synthesis, physico-chemical properties and immunoadjuvant activity of water-soluble phosphazene polyacids, J. Bioact. Compat. Polym, 13 (1998) 243–256. [Google Scholar]
- [37].F Eng N, Garlapati S, Lai K, Gerdts V, Mutwiri GK, Polyphosphazenes enhance mucosal and systemic immune responses in mice immunized intranasally with influenza antigens, The Open Vaccine Journal, 2 (2009). [Google Scholar]
- [38].Bouveret Le Cam NN, Ronco J, Francon A, Blondeau C, Fanget B, Adjuvants for influenza vaccine, Res. Immunol, 149 (1998) 19–23. [Google Scholar]
- [39].Andrianov AK, Marin A, Chen J, Synthesis, properties, and biological activity of Poly[di(sodium carboxylatoethylphenoxy)phosphazene], Biomacromolecules, 7 (2006) 394–399. [DOI] [PubMed] [Google Scholar]
- [40].Andrianov AK, Mutwiri G, Intradermal immunization using coated microneedles containing an immunoadjuvant, Vaccine, 30 (2012) 4355–4360. [DOI] [PubMed] [Google Scholar]
- [41].Pierce BG, Keck Z-Y, Wang R, Lau P, Garagusi K, Elkholy K, Toth EA, Urbanowicz RA, Guest JD, Agnihotri P, Kerzic MC, Marin A, Andrianov AK, Ball JK, Mariuzza RA, Fuerst TR, Foung SKH, Structure-based design of hepatitis C virus E2 glycoprotein improves serum binding and cross-neutralization, J. Virol, 94 (2020) DOI: 10.1128/JVI.00704-00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mapletoft JW, Oumouna M, Kovacs-Nolan J, Latimer L, Mutwiri G, Babiuk LA, van S Drunen Littel-van den Hurk, Intranasal immunization of mice with a formalin-inactivated bovine respiratory syncytial virus vaccine co-formulated with CpG oligodeoxynucleotides and polyphosphazenes results in enhanced protection, J. Gen. Virol, 89 (2008) 250–260. [DOI] [PubMed] [Google Scholar]
- [43].Kovacs-Nolan J, Mapletoft J, Lawman Z, Babiuk L, Formulation of bovine respiratory syncytial virus fusion protein with CpG oligodeoxynucleotide, cationic host defence peptide and polyphosphazene enhances humoral and cellular responses and induces a protective type 1 immune response in mice, J. Gen. Virol, 90 (2009) 1892–1905. [DOI] [PubMed] [Google Scholar]
- [44].Choi AH, McNeal MM, Flint JA, Basu M, Lycke NY, Clements JD, Bean JA, Davis HL, McCluskie MJ, VanCott JL, Ward RL, The level of protection against rotavirus shedding in mice following immunization with a chimeric VP6 protein is dependent on the route and the coadministered adjuvant, Vaccine, 20 (2002) 1733–1740. [DOI] [PubMed] [Google Scholar]
- [45].Johansson E, Istrate C, Charpilienne A, Cohen J, Hinkula J, Poncet D, Svensson L, Johansen K, Amount of maternal rotavirus-specific antibodies influence the outcome of rotavirus vaccination of newborn mice with virus-like particles, Vaccine, 26 (2008) 778–785. [DOI] [PubMed] [Google Scholar]
- [46].El-Attar L, Oliver SL, Mackie A, Charpilienne A, Poncet D, Cohen J, Bridger JC, Comparison of the efficacy of rotavirus VLP vaccines to a live homologous rotavirus vaccine in a pig model of rotavirus disease, Vaccine, 27 (2009) 3201–3208. [DOI] [PubMed] [Google Scholar]
- [47].Johansen K, Hinkula J, Istrate C, Johansson E, Poncet D, Svensson L, Polyphosphazenes as Adjuvants for Inactivated and Subunit Rotavirus Vaccines in Adult and Infant Mice, in: Andrianov AK (Ed.) Polyphosphazenes for Biomedical Applications, John Wiley & Sons, Hoboken, New Jersey, 2009, pp. 85–99. [Google Scholar]
- [48].Mutwiri G, Babiuk LA, Potential of Polyphosphazenes in Modulating Vaccine-Induced Immune Responses, in: Andrianov AK (Ed.) Polyphosphazenes for Biomedical applications, John Wiley & Sons, Hoboken, New Jersey, 2009, pp. 77–84. [Google Scholar]
- [49].Mapletoft JW, Latimer L, Babiuk LA, Intranasal immunization of mice with a bovine respiratory syncytial virus vaccine induces superior immunity and protection compared to those by subcutaneous delivery or combinations of intranasal and subcutaneous prime-boost strategies, Clin. Vaccine Immunol, 17 (2010) 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lu Y, Salvato MS, Pauza CD, Li J, Sodroski J, Manson K, Wyand M, Letvin N, Jenkins S, Touzjian N, Chutkowski C, Kushner N, LeFaile M, Payne LG, Roberts B, Utility of SHIV for testing HIV-1 vaccine candidates in macaques, J Acquir Immune Defic Syndr Hum Retrovirol, 12 (1996) 99–106. [DOI] [PubMed] [Google Scholar]
- [51].Pauza CD, Trivedi P, Wallace M, Ruckwardt TJ, Le Buanec H, Lu W, Bizzini B, Burny A, Zagury D, Gallo RC, Vaccination with Tat toxoid attenuates disease in simian/HIV-challenged macaques, Proc. Natl. Acad. Sci. U. S. A, 97 (2000) 3515–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tikhonov I, Ruckwardt TJ, Hatfield GS, Pauza CD, Tat-Neutralizing Antibodies in Vaccinated Macaques, J. Virol, 77 (2003) 3157–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bråve A, Johansen K, Palma P, Benthin R, Hinkula J, Maternal immune status influences HIV-specific immune responses in pups after DNA prime protein boost using mucosal adjuvant, Vaccine, 26 (2008) 5957–5966. [DOI] [PubMed] [Google Scholar]
- [54].Steger KK, Waterman PM, Pauza CD, Acute Effects of Pathogenic Simian-Human Immunodeficiency Virus Challenge on Vaccine-Induced Cellular and Humoral Immune Responses to Gag in Rhesus Macaques, J. Virol, 73 (1999) 1853–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wu JY, Wade WF, Taylor RK, Evaluation of cholera vaccines formulated with toxin-coregulated pilin peptide plus polymer adjuvant in mice, Infect. Immun, 69 (2001) 7695–7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gracia A, Polewicz M, Halperin SA, Hancock REW, Potter AA, Babiuk LA, Gerdts V, Antibody responses in adult and neonatal BALB/c mice to immunization with novel Bordetella pertussis vaccine formulations, Vaccine, 29 (2011) 1595–1604. [DOI] [PubMed] [Google Scholar]
- [57].Regnery RL, Rooney JA, Jenkins SA, Composition to protect a mammal against Bartonella henselae infection, U.S., 5,958,414, 1999, September 28. [Google Scholar]
- [58].Garlapati S, Eng NF, Wilson HL, Buchanan R, Mutwiri GK, Babiuk LA, Gerdts V, PCPP (poly[di(carboxylatophenoxy)-phosphazene]) microparticles co-encapsulating ovalbumin and CpG oligo-deoxynucleotides are potent enhancers of antigen specific Th1 immune responses in mice, Vaccine, 28 (2010) 8306–8314. [DOI] [PubMed] [Google Scholar]
- [59].Wilson HL, Kovacs-Nolan J, Latimer L, Buchanan R, Gomis S, Babiuk L, van S Drunen Littel-van den Hurk, A novel triple adjuvant formulation promotes strong, Th1-biased immune responses and significant antigen retention at the site of injection, Vaccine, 28 (2010) 8288–8299. [DOI] [PubMed] [Google Scholar]
- [60].Kovacs-Nolan J, Latimer L, Landi A, Jenssen H, Hancock REW, Babiuk LA, van S Drunen Littel-van den Hurk, The novel adjuvant combination of CpG ODN, indolicidin and polyphosphazene induces potent antibody- and cell-mediated immune responses in mice, Vaccine, 27 (2009) 2055–2064. [DOI] [PubMed] [Google Scholar]
- [61].Schulze K, Ebensen T, Babiuk LA, Gerdts V, Guzman CA, Intranasal vaccination with an adjuvanted polyphosphazenes nanoparticle-based vaccine formulation stimulates protective immune responses in mice, Nanomedicine, 13 (2017) 2169–2178. [DOI] [PubMed] [Google Scholar]
- [62].Kovacs-Nolan J, Mapletoft JW, Latimer L, Babiuk LA, Hurk S.v.D.L.-v.d., CpG oligonucleotide, host defense peptide and polyphosphazene act synergistically, inducing long-lasting, balanced immune responses in cattle, Vaccine, 27 (2009) 2048–2054. [DOI] [PubMed] [Google Scholar]
- [63].Lu Y, Landreth S, Liu G, Brownlie R, Gaba A, Hurk S.v.D. Littel-van den, Gerdts V, Zhou Y, Innate immunemodulator containing adjuvant formulated HA based vaccine protects mice from lethal infection of highly pathogenic avian influenza H5N1 virus, Vaccine, 38 (2020) 2387–2395. [DOI] [PubMed] [Google Scholar]
- [64].Osterholm MT, Kelley NS, Sommer A, Belongia EA, Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis, Lancet Infect. Dis, 12 (2012) 36–44. [DOI] [PubMed] [Google Scholar]
- [65].Sanchez-Schmitz G, Levy O, Development of Newborn and Infant Vaccines, Sci. Transl. Med, 3 (2011) 90ps27–90ps27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tomai MA, Vasilakos JP, TLR-7 and −8 agonists as vaccine adjuvants, Expert Rev. Vaccines, 10 (2011) 405–407. [DOI] [PubMed] [Google Scholar]
- [67].Vasilakos JP, Tomai MA, The use of Toll-like receptor 7/8 agonists as vaccine adjuvants, Expert Rev. Vaccines, 12 (2013) 809–819. [DOI] [PubMed] [Google Scholar]
- [68].Tomai MA, Miller RL, Lipson KE, Kieper WC, Zarraga IE, Vasilakos JP, Resiquimod and other immune response modifiers as vaccine adjuvants, Expert Rev. of Vaccines, 6 (2007) 835–847. [DOI] [PubMed] [Google Scholar]
- [69].Peine KJ, Gupta G, Brackman DJ, Papenfuss TL, Ainslie KM, Satoskar AR, Bachelder EM, Liposomal resiquimod for the treatment of Leishmania donovani infection, J. Antimicrob. Chemother, 69 (2014) 168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sevimli S, Knight FC, Gilchuk P, Joyce S, Wilson JT, Fatty Acid-Mimetic Micelles for Dual Delivery of Antigens and Imidazoquinoline Adjuvants, ACS Biomater. Sci. Eng, 3 (2017) 179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Duong AD, Sharma S, Peine KJ, Gupta G, Satoskar AR, Bachelder EM, Wyslouzil BE, Ainslie KM, Electrospray Encapsulation of Toll-Like Receptor Agonist Resiquimod in Polymer Microparticles for the Treatment of Visceral Leishmaniasis, Mol. Pharm, 10 (2013) 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Thomsen LL, Topley P, Daly MG, Brett SJ, Tite JP, Imiquimod and resiquimod in a mouse model: adjuvants for DNA vaccination by particle-mediated immunotherapeutic delivery, Vaccine, 22 (2004) 1799–1809. [DOI] [PubMed] [Google Scholar]
- [73].Ilyinskii PO, Roy CJ, O’Neil CP, Browning EA, Pittet LA, Altreuter DH, Alexis F, Tonti E, Shi J, Basto PA, Iannacone M, Radovic-Moreno AF, Langer RS, Farokhzad OC, von Andrian UH, Johnston LPM, Kishimoto TK, Adjuvant-carrying synthetic vaccine particles augment the immune response to encapsulated antigen and exhibit strong local immune activation without inducing systemic cytokine release, Vaccine, 32 (2014) 2882–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Widmer J, Thauvin C, Mottas I, Nguyen VN, Delie F, Allémann E, Bourquin C, Polymer-based nanoparticles loaded with a TLR7 ligand to target the lymph node for immunostimulation, Int. J. Pharm, 535 (2018) 444–451. [DOI] [PubMed] [Google Scholar]
- [75].Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, García-Sastre A, Compans R, Pulendran B, Programming the magnitude and persistence of antibody responses with innate immunity, Nature, 470 (2011) 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zhou C-X, Li D, Chen Y-L, Lu Z-J, Sun P, Cao Y-M, Bao H-F, Fu Y-F, Li P-H, Bai X-W, Xie B-X, Liu Z-X, Resiquimod and polyinosinic-polycytidylic acid formulation with aluminum hydroxide as an adjuvant for foot-and-mouth disease vaccine, BM C Veterinary Research, 10 (2014) 2–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Weeratna RD, Makinen SR, McCluskie MJ, Davis HL, TLR agonists as vaccine adjuvants: comparison of CpG ODN and Resiquimod (R-848), Vaccine, 23 (2005) 5263–5270. [DOI] [PubMed] [Google Scholar]
- [78].Ding Y, Liu J, Lu S, Igweze J, Xu W, Kuang D, Zealey C, Liu D, Gregor A, Bozorgzad A, Zhang L, Yue E, Mujib S, Ostrowski M, Chen P, Self-assembling peptide for co-delivery of HIV-1 CD8+ T cells epitope and Toll-like receptor 7/8 agonists R848 to induce maturation of monocyte derived dendritic cell and augment polyfunctional cytotoxic T lymphocyte (CTL) response, J. Controlled Release, 236 (2016) 22–30. [DOI] [PubMed] [Google Scholar]
- [79].Shukla NM, Salunke DB, Balakrishna R, Mutz CA, Malladi SS, David SA, Potent Adjuvanticity of a Pure TLR7-Agonistic Imidazoquinoline Dendrimer, PLOS ONE, 7 (2012) e43612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lynn GM, Laga R, Darrah PA, Ishizuka AS, Balaci AJ, Dulcey AE, Pechar M, Pola R, Gerner MY, Yamamoto A, Buechler CR, Quinn KM, Smelkinson MG, Vanek O, Cawood R, Hills T, Vasalatiy O, Kastenmüller K, Francica JR, Stutts L, Tom JK, Ryu KA, Esser-Kahn AP, Etrych T, Fisher KD, Seymour LW, Seder RA, In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity, Nat. Biotechnol, 33 (2015) 1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Aichhorn S, Linhardt A, Halfmann A, Nadlinger M, Kirchberger S, Stadler M, Dillinger B, Distel M, Dohnal A, Teasdale I, Schöfberger W, A pH-sensitive Macromolecular Prodrug as TLR7/8 Targeting Immune Response Modifier, Chem. Eur. J, 23 (2017) 17721–17726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Miller SM, Cybulski V, Whitacre M, Bess LS, Livesay MT, Walsh L, Burkhart D, Bazin HG, Evans JT, Novel Lipidated Imidazoquinoline TLR7/8 Adjuvants Elicit Influenza-Specific Th1 Immune Responses and Protect Against Heterologous H3N2 Influenza Challenge in Mice, Fronti. Immunol, 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Evans JT, Bess LS, Mwakwari SC, Livesay MT, Li Y, Cybulski V, Johnson DA, Bazin HG, Synthetic Toll-like Receptors 7 and 8 Agonists: Structure-Activity Relationship in the Oxoadenine Series, ACS Omega, 4 (2019) 15665–15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Andrianov A, Marin A, Immunostimulating polyphosphazene compounds, US 9,687,546, 2017, June 27. [Google Scholar]
- [85].Andrianov AK, Svirkin YY, LeGolvan MP, Synthesis and biologically relevant properties of polyphosphazene polyacids, Biomacromolecules, 5 (2004) 1999–2006. [DOI] [PubMed] [Google Scholar]
- [86].Dar A, Lai K, Dent D, Potter A, Gerdts V, Babiuk LA, Mutwiri GK, Administration of poly[di(sodium carboxylatoethylphenoxy)]phosphazene (PCEP) as adjuvant activated mixed Th1/Th2 immune responses in pigs, Vet. Immunol. Immunopathol, 146 (2012) 289–295. [DOI] [PubMed] [Google Scholar]
- [87].Magiri R, Mutwiri G, Wilson HL, Recent advances in experimental polyphosphazene adjuvants and their mechanisms of action, Cell Tissue Res, 374 (2018) 465–471. [DOI] [PubMed] [Google Scholar]
- [88].Magiri RB, Lai K, Chaffey AM, Wilson HL, Berry WE, Szafron ML, Mutwiri GK, Response of immune response genes to adjuvants poly [di(sodium carboxylatoethylphenoxy)phosphazene] (PCEP), CpG oligodeoxynucleotide and emulsigen at intradermal injection site in pigs, Vet. Immunol. Immunopathol, 175 (2016) 57–63. [DOI] [PubMed] [Google Scholar]
- [89].Eng NF, Garlapati S, Gerdts V, Babiuk LA, Mutwiri GK, PCEP enhances IgA mucosal immune responses in mice following different immunization routes with influenza virus antigens, J. Immune Based Ther. Vaccines, 8 (2010) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Dar A, Tipu M, Townsend H, Potter A, Gerdts V, Tikoo S, Administration of Poly[di(sodium carboxylatoethylphenoxy)phosphazene] (PCEP) and Avian Beta Defensin as Adjuvants in Inactivated Inclusion Body Hepatitis Virus and its Hexon Protein-Based Experimental Vaccine Formulations in Chickens, Avian Dis, 59 (2015) 518–524, 517. [DOI] [PubMed] [Google Scholar]
- [91].Sarfraz M, Suleman M, Tikoo SK, Wheler C, Potter AA, Gerdts V, Dar A, Immune responses to in ovo vaccine formulations containing inactivated fowl adenovirus 8b with poly[di(sodium carboxylatoethylphenoxy)]phosphazene (PCEP) and avian beta defensin as adjuvants in chickens, Vaccine, 35 (2017) 981–986. [DOI] [PubMed] [Google Scholar]
- [92].Magiri R, Lai K, Chaffey A, Zhou Y, Pyo H-M, Gerdts V, Wilson HL, Mutwiri G, Intradermal immunization with inactivated swine influenza virus and adjuvant polydi(sodium carboxylatoethylphenoxy)phosphazene (PCEP) induced humoral and cell-mediated immunity and reduced lung viral titres in pigs, Vaccine, 36 (2018) 1606–1613. [DOI] [PubMed] [Google Scholar]
- [93].Magiri RB, Lai KJ, Mutwiri GK, Wilson HL, Experimental PCEP-Adjuvanted Swine Influenza H1N1 Vaccine Induced Strong Immune Responses but Did Not Protect Piglets against Heterologous H3N2 Virus Challenge, Vaccines, 8 (2020) 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Garlapati S, Eng NF, Kiros TG, Kindrachuk J, Mutwiri GK, Hancock REW, Halperin SA, Potter AA, Babiuk LA, Gerdts V, Immunization with PCEP microparticles containing pertussis toxoid, CpG ODN and a synthetic innate defense regulator peptide induces protective immunity against pertussis, Vaccine, 29 (2011) 6540–6548. [DOI] [PubMed] [Google Scholar]
- [95].Papadopoulos G, Boskou D, Antioxidant effect of natural phenols on olive oil, J. Am. Oil Chem. Soc, 68 (1991) 669–671. [Google Scholar]
- [96].Nardini M, Ghiselli A, Determination of free and bound phenolic acids in beer, Food Chem, 84 (2004) 137–143. [Google Scholar]
- [97].Nardini M, Natella F, Scaccini C, Ghiselli A, Phenolic acids from beer are absorbed and extensively metabolized in humans, J. Nutr. Biochem, 17 (2006) 14–22. [DOI] [PubMed] [Google Scholar]
- [98].Villaño D, Fernández-Pachón MS, Troncoso AM, García-Parrilla MC, Comparison of antioxidant activity of wine phenolic compounds and metabolites in vitro, Anal. Chim. Acta, 538 (2005) 391–398. [Google Scholar]
- [99].Sroka Z, Cisowski W, Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids, Food Chem. Toxicol, 41 (2003) 753–758. [DOI] [PubMed] [Google Scholar]
- [100].Vissiennon C, Nieber K, Kelber O, Butterweck V, Route of administration determines the anxiolytic activity of the flavonols kaempferol, quercetin and myricetin — are they prodrugs?, J. Nutr. Biochem, 23 (2012) 733–740. [DOI] [PubMed] [Google Scholar]
- [101].Bouatra S, Aziat F, Mandal R, Guo AC, Wilson MR, Knox C, Bjorndahl TC, Krishnamurthy R, Saleem F, Liu P, Dame ZT, Poelzer J, Huynh J, Yallou FS, Psychogios N, Dong E, Bogumil R, Roehring C, Wishart DS, The Human Urine Metabolome, PLOS ONE, 8 (2013) e73076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zhang K, Zuo Y, GC-MS Determination of Flavonoids and Phenolic and Benzoic Acids in Human Plasma after Consumption of Cranberry Juice, J. Agric. Food Chem, 52 (2004) 222–227. [DOI] [PubMed] [Google Scholar]
- [103].Andrianov AK, Marin A, Peterson P, Water-soluble biodegradable polyphosphazenes containing N-ethylpyrrolidone groups, Macromolecules, 38 (2005) 7972–7976. [Google Scholar]
- [104].Andrianov AK, Marin A, Chen J, Sargent J, Corbett N, Novel route to sulfonated polyphosphazenes: Single-step synthesis using “noncovalent protection” of sulfonic acid functionality, Macromolecules, 37 (2004) 4075–4080. [Google Scholar]
- [105].Gao M, Peng Y, Jiang L, Qiu L, Effective intracellular delivery and Th1 immune response induced by ovalbumin loaded in pH-responsive polyphosphazene polymersomes, Nanomedicine, 14 (2018) 1609–1618. [DOI] [PubMed] [Google Scholar]
- [106].Ison MG, Mills J, Openshaw P, Zambon M, Osterhaus A, Hayden F, Current research on respiratory viral infections: Fourth International Symposium, Antiviral Res, 55 (2002) 227–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Thongcharoen P, Suriyanon V, Paris RM, Khamboonruang C, de Souza MS, Ratto-Kim S, Karnasuta C, Polonis VR, Baglyos L, El Habib R, A Phase 1/2 Comparative Vaccine Trial of the Safety and Immunogenicity of a CRF01_AE (Subtype E) Candidate Vaccine: ALVAC-HIV (vCP1521) Prime With Oligomeric gp160 (92TH023/LAI-DID) or Bivalent gp120 (CM235/SF2) Boost, J. Acquired Immune Defic. Syndr, 46 (2007) 48. [DOI] [PubMed] [Google Scholar]
- [108].O’Connell RJ, Excler J-L, Polonis VR, Ratto-Kim S, Cox J, Jagodzinski LL, Liu M, Wieczorek L, McNeil JG, El-Habib R, Safety and Immunogenicity of a randomized Phase I prime-boost trial with ALVAC-HIV (vCP205) and Oligomeric gp160 MN/LAI-2 Adjuvanted in Alum or Polyphosphazene, J. Infect. Dis, 213 (2016) 1946–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kabanov VA, From synthetic polyelectrolytes to polymer-subunit vaccines, Pure Appl. Chem, 76 (2004) 1659–1677. [Google Scholar]
- [110].Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF, Nanoparticles target distinct dendritic cell populations according to their size, Eur. J. Immunol, 38 (2008) 1404–1413. [DOI] [PubMed] [Google Scholar]
- [111].Awate S, Eng N, Gerdts V, Babiuk L, Mutwiri G, Caspase-1 Dependent IL-1b Secretion and Antigen-Specific T-Cell Activation by the Novel Adjuvant, PCEP, Vaccines, 2 (2014) 500–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Shen H, Ackerman AL, Cody V, Giodini A, Hinson ER, Cresswell P, Edelson RL, Saltzman WM, Hanlon DJ, Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles, Immunology, 117 (2006) 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Stier EM, Mandal M, Lee K-D, Differential cytosolic delivery and presentation of antigen by listeriolysin O-liposomes to macrophages and dendritic cells, Mol. Pharmaceutics, 2 (2005) 74–82. [DOI] [PubMed] [Google Scholar]
- [114].Lin M-L, Zhan Y, Villadangos JA, Lew AM, The cell biology of cross-presentation and the role of dendritic cell subsets, Immunol. Cell Biol, 86 (2008) 353–362. [DOI] [PubMed] [Google Scholar]
- [115].Awate S, Wilson HL, Singh B, Babiuk LA, Mutwiri G, The adjuvant PCEP induces recruitment of myeloid and lymphoid cells at the injection site and draining lymph node, Vaccine, 32 (2014) 2420–2427. [DOI] [PubMed] [Google Scholar]
- [116].Martinez AP, Qamar B, Fuerst TR, Muro S, Andrianov AK, Biodegradable “Smart” Polyphosphazenes with Intrinsic Multifunctionality as Intracellular Protein Delivery Vehicles, Biomacromolecules, 18 (2017) 2000–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Awate S, Wilson HL, Lai K, Babiuk LA, Mutwiri G, Activation of adjuvant core response genes by the novel adjuvant PCEP, Mol. Immunol, 51 (2012) 292–303. [DOI] [PubMed] [Google Scholar]
- [118].Magiri R, Lai K, Huang Y, Mutwiri G, Wilson HL, Innate immune response profiles in pigs injected with vaccine adjuvants polydi(sodium carboxylatoethylphenoxy)phosphazene (PCEP) and Emulsigen, Vet. Immunol. Immunopathol, 209 (2019) 7–16. [DOI] [PubMed] [Google Scholar]
- [119].Morawetz H, Studies of Synthetic Polymers by Nonradiative Energy Transfer, Science, 240 (1988) 172–176. [DOI] [PubMed] [Google Scholar]
- [120].Lee KY, Mooney DJ, Alginate: Properties and biomedical applications, Prog. Polym. Sci, 37 (2012) 106–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Bano MC, Cohen S, Visscher KB, Allcock HR, Langer R, A Novel Synthetic Method for Hybridoma Cell Encapsulation, Nat Biotech, 9 (1991) 468–471. [DOI] [PubMed] [Google Scholar]
- [122].Cohen S, Andrianov AK, Wheatley M, Allcock HR, Langer RS, Gas-filled polymeric microbubbles for ultrasound imaging, U.S. Patent 5,487,390, 1996, [Google Scholar]
- [123].Cohen S, Andrianov AK, Wheatley M, Allcock HR, Langer RS, Polymeric microparticles containing agents for imaging, U.S. Patent 5,562,099, 1996, [Google Scholar]
- [124].Cheheltani R, Ezzibdeh RM, Chhour P, Pulaparthi K, Kim J, Jurcova M, Hsu JC, Blundell C, Litt HI, Ferrari VA, Allcock HR, Sehgal CM, Cormode DP, Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging, Biomaterials, 102 (2016) 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Chhour P, Gallo N, Cheheltani R, Williams D, Al-Zaki A, Paik T, Nichol JL, Tian Z, Naha PC, Witschey WR, Allcock HR, Murray CB, Tsourkas A, Cormode DP, Nanodisco Balls: Control over Surface versus Core Loading of Diagnostically Active Nanocrystals into Polymer Nanoparticles, ACS nano, 8 (2014) 9143–9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Andrianov AK, Payne LG, Polymeric carriers for oral uptake of microparticulates, Adv. Drug Delivery Rev, 34 (1998) 155–170. [DOI] [PubMed] [Google Scholar]
- [127].Andrianov AK, Jenkins SA, Payne LG, Roberts BE, Hydrogel microencapsulated vaccines, U.S. Patent 5,529,777, 1996, June 26. [Google Scholar]
- [128].Greish YE, Bender JD, Lakshmi S, Brown PW, Allcock HR, Laurencin CT, Formation of hydroxyapatite-polyphosphazene polymer composites at physiologic temperature, J. Biomed. Mater. Res., Part A, 77A (2006) 416–425. [DOI] [PubMed] [Google Scholar]
- [129].Greish Y, Bender J, Lakshmi S, Brown P, Allcock H, Laurencin C, Composite formation from hydroxyapatite with sodium and potassium salts of polyphosphazene, J. Mater. Sci.: Mater. Med, 16 (2005) 613–620. [DOI] [PubMed] [Google Scholar]
- [130].Andrianov AK, Chen J, Polyphosphazene microspheres: Preparation by ionic complexation of phosphazene polyacids with spermine, J. Appl. Polym. Sci, 101 (2006) 414–419. [Google Scholar]
- [131].Andrianov AK, LeGolvan MP, Svirkin Y, Sule SS, Purification of polyphosphazene polyacids, US, 5,842,471, 1998, December 1. [Google Scholar]
- [132].Andrianov AK, Marin A, Fuerst TR, Self-assembly of polyphosphazene immunoadjuvant with poly(ethylene oxide) enables advanced nanoscale delivery modalities and regulated pH-dependent cellular membrane activity, Heliyon, 2 (2016) Article e00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Andrianov AK, Marin A, Peterson P, Chen J, Fluorinated polyphosphazene polyelectrolytes, J. Appl. Polym. Sci, 103 (2007) 53–58. [Google Scholar]
- [134].Garlapati S, Garg R, Brownlie R, Latimer L, Simko E, Hancock R, Babiuk L, Gerdts V, Potter A, Enhanced immune responses and protection by vaccination with respiratory syncytial virus fusion protein formulated with CpG oligodeoxynucleotide and innate defense regulator peptide in polyphosphazene microparticles, Vaccine, 30 (2012) 5206–5214. [DOI] [PubMed] [Google Scholar]
- [135].Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK, Microneedle-Based Vaccines, in: Compans RW, Orenstein WA (Eds.) Curr. Top. Microbiol. Immunol. Vol 333: Vaccines for Pandemic Influenza Springer, 2009, pp. 369–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Kim Y-C, Park J-H, Prausnitz MR, Microneedles for drug and vaccine delivery, Adv. Drug Deliv. Rev, 64 (2012) 1547–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Andrianov A, Marin A, DeCollibus D, Microneedles with Intrinsic Immunoadjuvant Properties: Microfabrication, Protein Stability, and Modulated Release, Pharm. Res, 28 (2011) 58–65. [DOI] [PubMed] [Google Scholar]
- [138].Andrianov AK, DeCollibus DP, Gillis HA, Kha HH, Marin A, Polyphosphazene Immunoadjuvants for Intradermal Vaccine Delivery, in: Andrianov AK (Ed.) Polyphosphazene for Biomedical Applications, John Wiley & Sons, Hoboken, New Jersey, 2009, pp. 101–116. [Google Scholar]
- [139].Loebelenz JR, Roberts BE, Andrainov AK, Jenkins SA, Immunoadjuvants, US Patent 6,261,573, 2001, July 17. [Google Scholar]
- [140].Pasternak JA, Ng SH, Buchanan RM, Mertins S, Mutwiri GK, Gerdts V, Wilson HL, Oral antigen exposure in newborn piglets circumvents induction of oral tolerance in response to intraperitoneal vaccination in later life, BMC Vet. Res, 11 (2015) 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Pasternak JA, Ng SH, Wilson HL, A single, low dose oral antigen exposure in newborn piglets primes mucosal immunity if administered with CpG oligodeoxynucleotides and polyphosphazene adjuvants, Vet. Immunol. Immunopathol, 161 (2014) 211–221. [DOI] [PubMed] [Google Scholar]
- [142].Polewicz M, Gracia A, Garlapati S, van Kessel J, Strom S, Halperin SA, Hancock REW, Potter AA, Babiuk LA, Gerdts V, Novel vaccine formulations against pertussis offer earlier onset of immunity and provide protection in the presence of maternal antibodies, Vaccine, 31 (2013) 3148–3155. [DOI] [PubMed] [Google Scholar]
- [143].Khan SA, Waugh C, Rawlinson G, Brumm J, Nilsson K, Gerdts V, Potter A, Polkinghorne A, Beagley K, Timms P, Vaccination of koalas (Phascolarctos cinereus) with a recombinant chlamydial major outer membrane protein adjuvanted with poly I:C, a host defense peptide and polyphosphazine, elicits strong and long lasting cellular and humoral immune responses, Vaccine, 32 (2014) 5781–5786. [DOI] [PubMed] [Google Scholar]
- [144].Snider M, Garg R, Brownlie R, van den Hurk JV, Hurk S.v.D.L.-v.d., The bovine viral diarrhea virus E2 protein formulated with a novel adjuvant induces strong, balanced immune responses and provides protection from viral challenge in cattle, Vaccine, 32 (2014) 6758–6764. [DOI] [PubMed] [Google Scholar]
- [145].Sadat SMA, Snider M, Garg R, Brownlie R, van S Drunen Littel-van den Hurk, Local innate responses and protective immunity after intradermal immunization with bovine viral diarrhea virus E2 protein formulated with a combination adjuvant in cattle, Vaccine, 35 (2017) 3466–3473. [DOI] [PubMed] [Google Scholar]
- [146].Garg R, Latimer L, Gerdts V, Potter A, van S Drunen Littel-van den Hurk, Vaccination with the RSV fusion protein formulated with a combination adjuvant induces long-lasting protective immunity, J. Gen. Virol, 95 (2014) 1043–1054. [DOI] [PubMed] [Google Scholar]
- [147].Garg R, Latimer L, Gerdts V, Potter A, van S Drunen Littel-van den Hurk, Intranasal immunization with a single dose of the fusion protein formulated with a combination adjuvant induces long-term protective immunity against respiratory syncytial virus, Hum. Vaccines Immunother, 13 (2017) 2894–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Garg R, Latimer L, Gerdts V, Potter A, The respiratory syncytial virus fusion protein formulated with a novel combination adjuvant induces balanced immune responses in lambs with maternal antibodies, Vaccine, 33 (2015) 1338–1344. [DOI] [PubMed] [Google Scholar]
- [149].Garg R, Latimer L, Wang Y, Simko E, Gerdts V, Potter A, van S Drunen Littel-van den Hurk, Maternal immunization with respiratory syncytial virus fusion protein formulated with a novel combination adjuvant provides protection from RSV in newborn lambs, Vaccine, 34 (2016) 261–269. [DOI] [PubMed] [Google Scholar]
- [150].Sarkar I, Garg R, van S Drunen Littel-van den Hurk, Formulation of the respiratory syncytial virus fusion protein with a polymer-based combination adjuvant promotes transient and local innate immune responses and leads to improved adaptive immunity, Vaccine, 34 (2016) 5114–5124. [DOI] [PubMed] [Google Scholar]
- [151].Garg R, Theaker M, Martinez EC, van S Hurk Drunen Littel-van den, A single intranasal immunization with a subunit vaccine formulation induces higher mucosal IgA production than live respiratory syncytial virus, Virology, 499 (2016) 288–297. [DOI] [PubMed] [Google Scholar]
- [152].Sarkar I, Zardini Buzatto A, Garg R, Li L, van S Drunen Littel-van den Hurk, Metabolomic and Immunological Profiling of Respiratory Syncytial Virus Infection after Intranasal Immunization with a Subunit Vaccine Candidate, J. Proteome Res, 18 (2019) 1145–1161. [DOI] [PubMed] [Google Scholar]
- [153].Garg R, Brownlie R, Latimer L, Gerdts V, Potter A, van S Drunen Littel-van den Hurk, A chimeric glycoprotein formulated with a combination adjuvant induces protective immunity against both human respiratory syncytial virus and parainfluenza virus type 3, Antiviral Res, 158 (2018) 78–87. [DOI] [PubMed] [Google Scholar]
- [154].Martinez EC, Garg R, Shrivastava P, Gomis S, van S Drunen Littel-van den Hurk, Intranasal treatment with a novel immunomodulator mediates innate immune protection against lethal pneumonia virus of mice, Antiviral Res, 135 (2016) 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Pasternak JA, Hamonic G, Forsberg NM, Wheler CL, Dyck MK, Wilson HL, Intrauterine delivery of subunit vaccines induces a systemic and mucosal immune response in rabbits, Am. J. Reprod. Immunol, 78 (2017) e12732. [DOI] [PubMed] [Google Scholar]
- [156].Pasternak JA, Hamonic G, Van Kessel J, Wheler CL, Dyck MK, Wilson HL, Intrauterine vaccination induces a dose-sensitive primary humoral response with limited evidence of recall potential, Am. J. Reprod. Immunol, 80 (2018) e12855. [DOI] [PubMed] [Google Scholar]
- [157].Garg R, Brownlie R, Latimer L, Gerdts V, Potter A, van S Drunen Littel-van den Hurk, Vaccination with a human parainfluenza virus type 3 chimeric FHN glycoprotein formulated with a combination adjuvant induces protective immunity, Vaccine, 35 (2017) 7139–7146. [DOI] [PubMed] [Google Scholar]
- [158].Garg R, Latimer L, Gomis S, Gerdts V, Potter A, van S Drunen Littel-van den Hurk, Maternal vaccination with a novel chimeric glycoprotein formulated with a polymer-based adjuvant provides protection from human parainfluenza virus type 3 in newborn lambs, Antiviral Res, 162 (2019) 54–60. [DOI] [PubMed] [Google Scholar]
- [159].Hamonic G, Pasternak JA, Ng SH, Fourie KR, Simko OM, Deluco B, Wilson HL, Assessment of Immunological Response and Impacts on Fertility Following Intrauterine Vaccination Delivered to Swine in an Artificial Insemination Dose, Front. Immunol, 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Wasan EK, Syeda J, Strom S, Cawthray J, Hancock RE, Wasan KM, Gerdts V, A lipidic delivery system of a triple vaccine adjuvant enhances mucosal immunity following nasal administration in mice, Vaccine, 37 (2019) 1503–1515. [DOI] [PubMed] [Google Scholar]
- [161].Garg R, Babiuk L, van S Hurk Drunen Littel-van den, Gerdts V, A novel combination adjuvant platform for human and animal vaccines, Vaccine, 35 (2017) 4486–4489. [DOI] [PubMed] [Google Scholar]
- [162].Andrianov AK, LeGolvan MP, Sule SS, Payne LG, Degradation of poly[di(carboxylatophenoxy)phosphazene] in aqueous solution, PMSE Preprints 76 (1997) 369–370. [Google Scholar]
- [163].Andrianov AK, Marin A, Degradation of polyaminophosphazenes: Effects of hydrolytic environment and polymer processing, Biomacromolecules, 7 (2006) 1581–1586. [DOI] [PubMed] [Google Scholar]
- [164].Linhardt A, König M, Schöfberger W, Brüggemann O, Andrianov AK, Teasdale I, Biodegradable Polyphosphazene Based Peptide-Polymer Hybrids, Polymers, 8 (2016) 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Andrianov AK, Marin A, Martinez AP, Weidman JL, Fuerst TR, Hydrolytically Degradable PEGylated Polyelectrolyte Nanocomplexes for Protein Delivery, Biomacromolecules, 19 (2018) 3467–3478. [DOI] [PubMed] [Google Scholar]
- [166].Andrianov AK, Chen J, LeGolvan MP, Poly(dichlorophosphazene) as a precursor for biologically active polyphosphazenes: Synthesis, characterization, and stabilization, Macromolecules, 37 (2004) 414–420. [Google Scholar]
- [167].Andrianov AK, Sargent JR, Production of polyorganophosphazenes, US 5,760,271, 1998, June 2. [Google Scholar]
- [168].Andrianov AK, Sargent JR, Sule SS, Recovery of polyphosphazene polyacids or acids salts thereof, US, 5,814,704 1998, September 29. [Google Scholar]
- [169].Andrianov AK, Sargent JR, Sule SS, LeGolvan M, Polyhalophosphazene solutions stable against gelation, US, 5,707,597 1998, January 13. [Google Scholar]
- [170].Andrianov AK, LeGolvan MP, Svirkin Y, Sule SS, Production of polyorganophosphazenes having desired molecular weights, US, 5,869,016, 1999, February 9. [Google Scholar]
- [171].Allcock HR, Synthesis, structures, and emerging uses for poly (organophosphazenes), in: Polyphosphazenes in Biomedicine, Engineering, and Pioneering Synthesis, ACS Publications, 2018, pp. 3–26. [Google Scholar]
- [172].Strasser P, Teasdale I, Main-Chain Phosphorus-Containing Polymers for Therapeutic Applications, Molecules, 25 (2020) 1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Allcock HR, Chemistry and Applications of Polyphosphazenes, Wiley, Hoboken, NJ, 2002. [Google Scholar]
- [174].Rothemund S, Teasdale I, Preparation of polyphosphazenes: a tutorial review, Chem. Soc. Rev, 45 (2016) 5200–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Honeyman CH, Manners I, Morrissey CT, Allcock HR, Ambient Temperature Synthesis of Poly(dichlorophosphazene) with Molecular Weight Control, J. Am. Chem. Soc, 117 (1995) 7035–7036. [Google Scholar]
- [176].Allcock HR, Reeves SD, Nelson JM, Crane CA, Manners I, Polyphosphazene Block Copolymers via the Controlled Cationic, Ambient Temperature Polymerization of Phosphoranimines, Macromolecules, 30 (1997) 2213–2215. [Google Scholar]
- [177].Andrianov AK, Le Golvan MP, Characterization of poly[di(carboxylatophenoxy)-phosphazene] by an aqueous gel permeation chromatography, J. Appl. Polym. Sci, 60 (1996) 2289–2295. [Google Scholar]
- [178].Messaud FA, Sanderson RD, Runyon JR, Otte T, Pasch H, Williams SKR, An overview on field-flow fractionation techniques and their applications in the separation and characterization of polymers, Prog. Polym. Sci, 34 (2009) 351–368. [Google Scholar]


