Abstract
Chimpanzees are the species most closely related to humans, yet age-related changes in brain and cognition remain poorly understood. The lack of studies on age-related changes in cognition in chimpanzees is particularly unfortunate in light of the recent evidence demonstrating that this species naturally develops Alzheimer’s disease (AD) neuropathology. Here, we tested 213 young, middle-aged, and elderly captive chimpanzees on the Primate Cognitive Test Battery (PCTB), a set of 13 tasks that assess physical and social cognition in nonhuman primates. A subset of these chimpanzees (n=146) were tested a second time on a portion of the PCTB tasks as a means of evaluating longitudinal changes in cognition. Cross-sectional analyses revealed a significant quadratic association between age and cognition with younger and older chimpanzees performing more poorly than middle-aged individuals. Longitudinal analyses showed that the oldest chimpanzees at the time of the first test showed the greatest decline in cognition, albeit the effect was mild. The collective data show that chimpanzees, like other nonhuman primates, show age-related decline in cognition. Further investigations into whether the observed cognitive decline is associated with AD pathologies in chimpanzees would be invaluable in understanding the comparative biology of aging and neuropathology in primates.
Keywords: Aging, Cognition, Chimpanzee, Alzheimer’s pathology, Age-related decline
Introduction
Decades of research in humans have documented age-related changes in cognition, brain, and behavior (Abe et al., 2008; Bertrand et al., 2018; Peters, 2006). Some loss in cognitive functions and integrity of cortical organization are considered a normal process of aging, but other decrements are attributable to specific neuropathological conditions, such as Alzheimer’s disease (AD) and frontotemporal dementia (FTD). Animal models, including primate models, have been developed as a means of understanding the mechanisms that underlie age-related changes in brain and cognition, as well as facilitating the development of effective therapeutic interventions (Didier et al., 2016; Lacreuse & Herndon, 2008; McQuail & Nicolle, 2012; Phillips et al., 2014; Rosen et al., 2016; Rosen, Walker, & LeVine, 2011; Verdier et al., 2015; Walker & Jucker, 2017).
One species that is often overlooked in studies on age-related changes in cognition and the brain is the chimpanzee (Pan troglodytes) (and other great apes). This is unfortunate given their genetic and neuroanatomical similarities to humans (Mikkelsen et al., 2005; Sherwood, Baurernfeind, Bianchi, Raghanti, & Hof, 2012) and more sophisticated cognitive and motor abilities (Tomasello & Call, 1997) when compared to more distantly related primates. Neuroimaging studies have reported small to moderate reductions in cortical surface area, gray matter thickness, gray matter volume, and white matter connectivity in aged chimpanzees (Autrey et al., 2014; Chen et al., 2013; Raz, Herndon, Hopkins, Schmidtke, & Lacreuse, in press; Sherwood et al., 2011). Moreover, recent studies have documented the co-occurrence of both neurofibrillary tangles (NFTs) and β amyloid plaques (Aβ) in elderly chimpanzee postmortem brains (Edler et al., in press; Edler et al., 2017; Gearing, Rebeck, Hyman, Tigges, & Mirra, 1994; Rosen et al., 2008). The presence of both Aβ plaques and NFTs is the key neuropathological feature used to definitively confirm a diagnosis of AD in humans; thus, chimpanzees appear to be the only known species to naturally develop AD-like pathology. Additional studies involving chimpanzees could lead to important findings on the comparative biology of aging, and may hold the key to understanding our own susceptibility to this neuropathological process (Edler et al., in press).
To date, there are only a handful of studies that have explicitly examined age-related changes in cognition in chimpanzees, and all but one used cross-sectional, rather than longitudinal, designs, which many have suggested are critically important in studies on aging (Raz et al., in press). In early work, Bernstein (1961) tested eight young and eight old chimpanzees and found no age differences in a series of object discriminations and on a wheel-rotating task. Riopelle and Rogers (1965) tested 19 chimpanzees (7 to 41 years of age divided into a young and old group) and reported no age-related deficits in discrimination tasks but did identify impairments in older chimpanzees on the shortest retention delays (0 and 5 s) of a delayed response task, and on an oddity task. In one recent study, Lacreuse, Parr, Chennareddi, and Herndon (2018) examined cognitive set switching in a modified version of the Wisconsin Card Sorting task in a sample of 30 female chimpanzees, and found that older individuals made more perseveration errors and required more trials to reach criterion in the set switching dimensions than younger ones. Manrique and Call (2015) tested 25 apes (including chimpanzees, bonobos, gorillas and orangutans) on a measure of cognitive flexibility, and reported that younger and older individuals made significantly more perseveration errors compared to middle-aged apes.
In the lone longitudinal study in chimpanzees, Lacreuse, Russell, Hopkins, and Herndon (2014) tested 38 females on the Primate Cognition Test Battery (PCTB), a 13-item test designed to assess social and physical cognition in nonhuman primates (Hermann, Call, Hernández-Lloreda, Hare, & Tomasello, 2007; E. Herrmann, Hare, Call, & Tomasello, 2010; E. Herrmann, Hernandez-Lloreda, Call, Hare, & Tomasello, 2010; Joly et al., 2017; Russell, Lyn, Schaeffer, & Hopkins, 2011; Schmitt, Pankau, & Fischer, 2011). Lacreuse et al. (2014) found very little evidence of age-related decline in overall and physical cognition performance. In contrast, performance on measures of social cognition, as well as motor function, declined with age in this sample. Lacreuse et al. (2014) noted that the four oldest chimpanzees in the sample performed significantly worse over the three-year study period than the remaining subjects on a measure of spatial memory.
In the current study, we tested for cross-sectional and longitudinal changes in cognition in a considerably larger sample of chimpanzees using the PCTB. From the cross-sectional data, we initially examined the linear and quadratic association between age and overall cognitive performance based on performance on the PCTB. We also tested a sub-sample of the chimpanzees three years later (on average) on a portion of the same PCTB tasks as a means of evaluating any longitudinal changes in performance with increasing age. We hypothesized that if chimpanzees exhibit age-related decline in cognitive functions, then both the cross-sectional and longitudinal data would reveal poorer performance in elderly individuals compared to middle-aged and young apes.
Methods
Ethics Statement
This study was conducted in accordance with the ethical standards and according to the Declaration of Helsinki and according to national and international guidelines and has been approved by the authors’ institutional review board. All procedures involving the chimpanzees were approved by the local Institutional Animal Care and Use Committees, and adhered to the legal requirements of the United States and to the American Society of Primatologists’ Principles for the Ethical Treatment of Primates.
Subjects
For the cross-sectional analysis, there were 218 chimpanzees (140 females, 78 males) ranging in age from 13 to 54 years (mean age = 28.47 years ± SD 10.92) housed at two facilities, including the National Center for Chimpanzee Care (NCCC, n = 123), which is part of the MD Anderson Cancer Center, and the Yerkes National Primate Research Center (YNPRC, n = 95) of Emory University. All chimpanzees were housed in indoor/outdoor corrals or Primadomes™, with 24-hour access to both areas, except during cleaning. The enclosures contained climbing structures, bedding, and daily environmental enrichment. Care staff fed the chimpanzees a diet of commercially produced primate chow, and fresh fruits and vegetables twice per day. The chimpanzees also had multiple foraging opportunities every day, and ad libitum access to water.
We classified the chimpanzees into three age groups, including young (< 25 years, n = 98), middle-aged (25 to 36 years, n = 72), and elderly (> 37 years, n = 48). We selected these age cut-points based on life span and mortality data reported in Finch (2010) that indicate a sharp increase in chimpanzee mortality between 35 and 40 years of age. For the longitudinal analysis, the sample was comprised of 146 chimpanzees (99 females, 47 males), including 76 young, 46 middle-aged, and 24 elderly individuals. The range in time between the administration of the first and second PCTB tests varied from one to seven years (mean = 3.41 ± SD 1.73).
Procedures
Subjects were tested on a modified version of the primate cognition test battery (PCTB) originally described by Hermann et al. (2007) and Hermann, Hare et al. (2010), and details have been described elsewhere (Russell et al., 2011). The PCTB attempts to assess subjects’ abilities in various areas of physical and social cognition. For our study, some aspects of the original PCTB were eliminated due to time and housing constraints. The previously published procedures were followed as similarly as possible, but some tasks were modified to better address the questions at hand given the past experiences and environmental constraints of our subjects. Each of the nine physical cognition tasks and the three social cognition tasks are described briefly below, with notes made when procedures were altered from those described by Herrmann et al. (2007). Subjects were generally tested in the order that the tasks are presented below and testing was completed over one to five testing sessions, depending on the motivation and attention of the subject. All chimpanzees were given the opportunity to participate in the social and physical cognitive testing. However, a small proportion of chimpanzees (< 7%) were not motivated to perform the PCTB tasks. Since subjects had to complete all tasks within the PCTB, these subjects were not included in analyses.
Physical Cognition Tasks
Nine tasks were utilized in the “Physical Cognition” portion of our test battery, including tasks exploring the apes’ spatial memory and understanding of spatial relationships, ability to differentiate between quantities, understanding of causality in the visual and auditory domains and their understanding of tools. Our test differed from the original PCTB in several ways. We excluded the Addition task, as well as certain components of the Tool Properties tasks. Spatial Memory (3 trials): This test assessed subjects’ ability to remember the locations of food rewards. In this task, the subject watched as food was hidden in two of three possible locations. Each subject received all three possible combinations of baited locations. The subject was then allowed to search the locations. The subject was scored as successful if he/she located both food items without searching in the unbaited location. Object Permanence (9 trials): Here we tested an individuals’ ability to follow a food reward after invisible displacement given three different possible displacements. During single displacement trials, only one of three possible locations was manipulated and thus, potentially baited. In the double displacement trials, two of three possible locations were manipulated, meaning that two of the three locations could potentially be baited. Double displacement trials were further divided by whether or not the baited locations were adjacent to one another. In order to be considered successful, the subject had to locate the hidden food item without searching in the location that was not manipulated. Rotation (9 trials): In the third task, we examined subjects’ ability to track a food reward as it is spatially rotated either 180 or 360 degrees. In this task, subjects watched as one of three possible locations was baited and then as the three locations were rotated as a unit on a horizontal plane. Three different manipulations were employed. In 180 degree middle trials, the middle location was baited and the platform was turned 180 degrees. In 360 degree side and 180 degree side trials, either the left or right location was baited, and the platform was then rotated 360 or 180 degrees, respectively. Subjects successfully completed a Rotation trial by tracking and identifying the correct location. Transposition (9 trials): In this task, subjects watch as a food reward is hidden in one of three possible locations and then as the baited location is changed in one of three ways. In one condition, the baited location is switched with one of the unbaited locations. In the second condition, the baited location is switched with one of the unbaited locations and then the two unbaited locations are switched. In the last condition, the baited location is switched with one of the unbaited locations and then with the other unbaited location. To be considered successful on this task, the subject had to track the reward and choose the baited location. Relative Numbers (13 trials): In the fifth task, subjects were tested for their ability to discriminate between different quantities when being presented with two plates containing different amounts of equally sized pieces of food. Each subject received the same set of 13 different quantity pairings, as used in the original PCTB (1:0, 5:1, 6:3, 6:2, 6:4, 4:3, 3:2, 2:1, 4:1, 4:2, 5:2, 3:1, and 5:3). During each trial, the subject was allowed to choose only one plate and received whatever reward was on the chosen plate. A correct response was recorded when the subject chose the plate containing the larger quantity of food. We did not include the task by Herrmann et al. (2007) referred to as Addition Numbers. Causality Noise (6 trials): In the sixth task, we assessed subjects’ understanding of causal relationships based on sound. In this task, the experimenter placed a hard food reward (i.e., peanut) in one of two metal containers such that the container with the food reward made a sound when shaken while the unbaited container did not. In “Full” trials, the metal container containing the food reward was lifted and shaken and then the unbaited container was lifted. In the “Empty” trials, the empty container was lifted and shaken and then the baited container was lifted. Subjects were then allowed to choose one of the two containers. A correct choice was recorded when the subject chose the baited container. Causality Visual (6 trials): In the seventh task, subjects were tested for their causal understanding of the physical world in the visual domain. Specifically, in one trial type, a food reward was placed underneath one of two boards laying flat on the testing table. The food caused the baited board to be tilted away from the subject (such that the chimpanzee could not see the food underneath the board), while the unbaited board remained flat. In the second trial type, a food reward was placed underneath one of two pieces of cloth laying flat on the testing table. The reward created a visible bump in the baited cloth while the unbaited cloth remained flat. In both trial types, the subject had to choose the baited item to be considered successful. Tool Properties (6 trials): This Physical Cognition task explored the apes’ understanding of the physical properties of tools and how those relate to achieving a goal. In each task, the subject was presented with a choice between two similar tools. However, one tool could be used to obtain a food reward while the other tool was ineffective. For the first task, subjects were presented with two identical pieces of paper. One piece of paper had a food reward sitting on top of the far end while the second piece of paper had a food reward sitting beside it. The subject could pull either piece of paper into their cage, but only by pulling the paper with the food sitting on top of it would they be able to retrieve the food reward. In the second task, one tool was identical to the effective tool in the first task. The second tool consisted of two smaller pieces of paper with a small gap between them, visually emphasizing that they were not connected. The food reward was placed on the out-of-reach piece of the two disconnected pieces of paper. The subject could pull in the reward using the effective tool, but pulling the piece of the disconnected paper was ineffective in obtaining the reward. Note that we did not include three tool properties tasks from the original PCTB including “Bridge”, “Broken Wool”, and “Tray Circle” (Hermann et al., 2007). Tool Use (1 trial): The last Physical Cognition task measured each ape’s ability to solve a simple problem using a tool. A food reward was placed on a table approximately 10 cm out of reach of the subject. A lollipop stick measuring 12 cm long was then placed on the table with one end inserted into the enclosure. To be successful, the subject had to use the stick to manipulate the reward within his or her reach within two minutes.
For all physical cognition tasks, experimenters received specific instructions for recording chimpanzee responses. With the exception of tool use, these tasks required that the chimpanzee make a choice among two or more options. The experimenters recorded the first choice of the chimpanzee, which was then classified as correct or incorrect. Before an experimenter could test chimpanzees, he/she first had to pass trial sessions in which a human acted as a subject until the experimenter appropriately recorded correct and incorrect responses according to instructions. During actual chimpanzee testing, any ambiguous responses or choices (e.g., a chimpanzee used both hands to point to more than one option) were not counted and the trial was repeated. Additionally, experimenters had worked with the chimpanzees for at least three years prior to conducting any testing for the current experiments, and therefore were very familiar with chimpanzee subjects and their behavior.
Social Cognition Tasks
There were three tasks within the “Social Cognition” dimension of the PCTB and they are designed to assess subjects’ initiation in joint attention (IJA) abilities, their response to joint attention (RJA) cues, and their ability to use appropriate communicative modalities based on the attentional status of a human experimenter (Attentional State). Initiation of Joint Attention (IJA): These data were taken from previously reported findings from Leavens et al. (2015). Briefly, a food item was placed outside one end of the subject’s home cage by an experimenter, who then left the area to start the trial. A second experimenter then entered and positioned themselves at the opposite end of the subject’s home cage. A correct response was recorded if the ape gestured toward the food item or the experimenter while alternating their gaze between the referent and experimenter. Trials lasted 60 seconds and all subjects received four trials separated by at least two minutes. Attentional State: Four trials were administered to test a subject’s sensitivity to an experimenter’s attentional state. An experimenter placed a food reward on the ground outside the subject’s enclosure, after which a second experimenter approached the cage and altered their attentional state in one of two ways. During the facing-toward trial, the experimenter’s face and body were oriented toward the reward and the subject. During the away body-facing trial, the experimenter’s body faced the subject, but his/her face was turned away. Subjects were scored as successful if they used communicative signals in the modality appropriate to the experimenter’s attentional state (i.e., the chimpanzee used a manual gesture in facing-toward trials, or first used an attention-getting signal followed by a manual gesture in away trials). Gaze Following/Response to Joint Attention (RJA): Four trials were administered to test a subject’s ability to follow gaze (Hopkins, Keebaugh, et al., 2014). Sitting approximately one meter from the subject’s enclosure, the experimenter initially captured the subject’s attention and then shifted their head and eyes to gaze at a point directly behind the focal subject for a period of 15 seconds. Subjects were scored as successful if they followed the gaze of the experimenter by looking behind them. For all the measures, the percentage of correct responses computed across trials was the initial dependent measure of interest.
Data Analysis
For the cross-sectional analysis, for each task, the percentage of correct responses was calculated for each subject. Within each chimpanzee colony, we converted the performance scores to standardized z-scores. We then averaged the z-scores across all physical and social cognition tasks to create separate unit weighted average (UWA) performance scores (called UWA-physical and UWA-social score) (Woodley, Fernandes, & Hopkins, 2015). For the longitudinal analysis, because of the retirement and subsequent relocation of some of the chimpanzees to the NIH funded sanctuary, the testing was limited to the tasks that assessed physical cognition only. For this set of data, we included only those chimpanzees that were tested twice. As a means of assessing change in performance, for each of the nine tasks, we computed difference scores by subtracting the average performance on test 1 from the average performance on test 2. Negative difference scores indicated a reduction in performance and positive values indicated improved performance over time. To compute overall change in performance, we averaged the difference scores across all nine tasks (Mean_Difference). In addition, based on the constructs originally proposed by Hermann et al. (2007), we also computed average changes in performance on the four tasks that assessed spatial cognition (Spatial_Difference), causality (Causal_Difference), understanding of tools (Tool_Difference) and quantity discrimination (Number_Difference). To evaluate longitudinal changes in performance, we used partial regression analysis with age at the onset of testing as the predictor variable, and the Mean_Difference, Spatial_Difference, Causal_Difference, Tool_Difference and Number_Different as the outcome measures. Colony, genetic relatedness, sex, and time between test 1 and 2 (in years and months) served as covariates. We note here that relatedness was included as a variable because we have previously found that (1) PCTB performance is significantly heritable in chimpanzees (Hopkins, Russell, & Schaeffer, 2014), and (2) some of the chimpanzees in our sample were related to each other. Relatedness coefficients were created for the chimpanzee population using available pedigree information, going back as early as the founder animals within each population (Hopkins, Reamer, Mareno, & Schapiro, 2015). All data were analyzed in SPSS Statistics 26 (IBM Corporation, Chicago, IL, USA). Data are available from the corresponding author upon reasonable request.
Results
Cross-Sectional Findings
For this analysis, we performed stepwise multiple regression on each of the cognition scores entering the following variables: sex, linear age, and quadratic age in that order. In addition to the overall model results, the stepwise regression also reported significant changes in R-squared with the inclusion of each predictor variable. For UWA-physical (F= 2.605; df = 3, 214; P = 0.050; R2 = 0.035) and UWA-social (F= 5.103; df = 3, 214; P = 0.002; R2 = 0.067), the full model regression analyses were significant. For the UWA-social measure, significant changes in R-squared were found when the variables linear age (F=9.871; df = 1, 215; P = 0.002) and quadratic age were entered into the model (F= 5.209; df = 1, 214; P = 0.023). For the UWA-physical score, significant changes in R-squared were only found when quadratic age was entered into the model (F = 5.417; df = 1, 214; P = 0.0218). Sex was not found to be a significant predictor of UWA-physical or UWA-social scores (P < 0.10). Scatterplots of the quadratic association between age and each cognition measure are shown in Figure 1a and 1b. As can be seen, younger and older apes performed more poorly than middle-aged apes.
Figure 1.
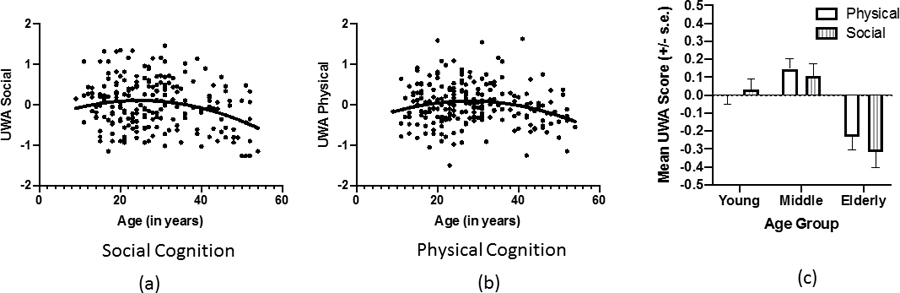
Scatterplots of quadratic association between age and (a) UWA-social and (b) UWA-physical cognition in chimpanzees. (c) Mean UWA scores (± SE) scores for young, middle-aged and elderly chimpanzees
In addition to multiple regression analyses, we also tested for age-related differences in the UWA-physical and UWA-social scores using a mixed-model analysis of covariance on age groups. Performance test (UWA-physical, UWA-social) was the repeated measure while sex (male, female) and age group (young, middle, elderly) were the between-group factors. Genetic relatedness was found to be a significant covariate (F= 4.66; df = 1, 211; p = 0.03). A significant main effect for age group was found (F=14.31; df = 2, 211; p = .000). Post-hoc analyses indicated that elderly chimpanzees performed worse than middle-aged and young chimpanzees, but there was no difference between these two latter groups (see Figure 1c). There was no effect of sex nor an interaction between sex and age group (P > 0.10).
We note above that genetic relatedness was a significant covariate. To further explore the potential heritability in PCTB performance, as we have done in the past (Hopkins, Russell, et al., 2014), we used the software program SOLAR to estimate heritability in overall PCTB performance (UWA). We confirmed that overall performance on the PCTB tasks was significantly heritable (h2 = .209 ± SE 0.139, P = 0.045) when controlling for sex, colony and age at the onset of PCTB testing.
Longitudinal Findings
For this analysis, we regressed age at the onset of PCTB testing on each cognition outcome measure, while statistically controlling for colony, sex, relatedness and difference in age between test 1 and 2. Significant negative associations were found between age at the onset of testing and the overall change in performance (Mean_Difference) (partial r = −.195, df = 136, P = 0.022), as well as the tasks that comprised the spatial cognition component of the PCTB (Spatial_Difference) (partial r = −.242, df = 136, P = 0.004). Thus, older chimpanzees at the onset of cognition testing showed greater loss (or lower performance values) in cognitive function. No significant associations were found between age at the onset of testing and performance on the Tool_Difference (partial r = −.094, df = 136, P = 0.271), Cause_Difference (partial r = .047, df = 136, P =0.583), and Number_Difference (partial r = −.060, df = 136, P = 0.482) components. Scatterplots showing the association between age at onset of PCTB testing and the difference in performance between tests 1 and 2 are shown in Figures 2a to 2e.
Figure 2.
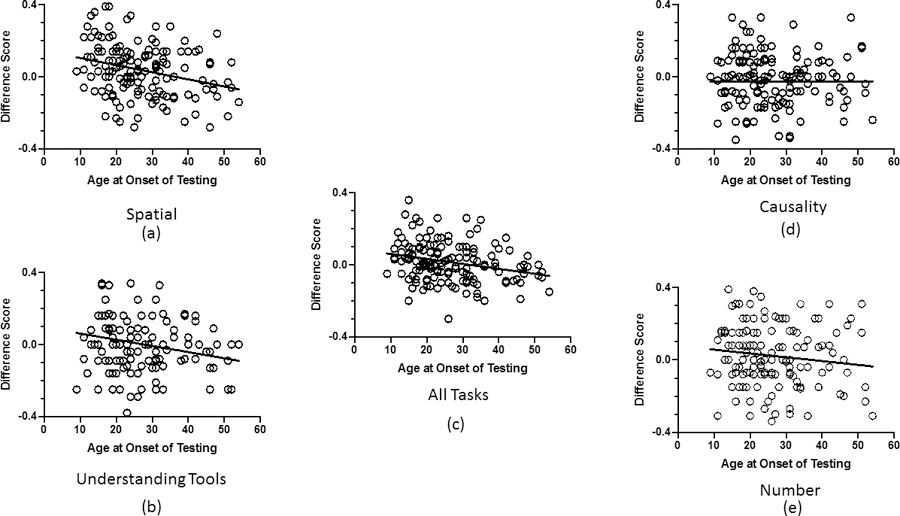
Average changes in performance, expressed as difference scores between test 1 and test 2, as a function of age at the onset of testing in the (a) spatial tasks, (b) understanding tools tasks, (c) across all PCTB tasks, (d) causality tasks, and (e) number task.
Discussion
As has been reported in other nonhuman primate species using cross-sectional methods (Hara, Rapp, & Morrison, 2012; Herndon, Moss, Rosene, & Killiany, 1997; Joly, Ammersdorfer, Schmidtke, & Zimmermann, 2014; King & Michels, 1989; LaClair & Lacreuse, 2016; Lacreuse, Herndon, Killiany, Rosene, & Moss, 1999; Lyons, Yang, Eliez, Reiss, & Schatzberg, 2004; Moss, Rosene, & Peters, 1988; Munger, Takemoto, Raghanti, & Nakamura, 2017; Nagahara, Bernot, & Tuszynski, 2010; Picq, 2007; Rapp & Amaral, 1989, 1992; Workman, Healey, Carlotto, & Lacreuse, 2019), we found an inverted U-shaped pattern of lifespan change in cognition, where middle-aged individuals performed best on both physical and social cognition tasks compared to younger and elderly chimpanzees. The longitudinal analysis also showed age-related decline in cognition in chimpanzees, particularly those classified as elderly during the initial test. Longitudinal studies of age-related changes in cognition are rare in nonhuman primates (Workman et al., 2019), and in the only other longitudinal study in chimpanzees, Lacreuse et al. (2014) reported a small but non-significant loss in cognition in a sample of 43 female chimpanzees, a finding that differs somewhat from the results reported here. The differences in findings between Lacreuse et al. (2014) and this report may be attributed to the larger sample size and the inclusion of male chimpanzees in the current study. Nonetheless, Lacreuse et al. (2014) did report that the four oldest individuals in their sample showed reductions in performance on the PCTB tasks linked to spatial cognition, and we found a similar result in the current, larger sample of apes. The decline in cognition as a function of older age in our sample mirrors the effect found in human subjects. In healthy adults, certain cognitive domains begin to show decline as early as 30–40 years of age, with substantial decline occurring after age 60 (Salthouse, 2009). Generally, this age-related cognitive decline in humans is linear, but some evidence suggests that there may be non-linear trends in age-related decline in certain neuropsychological cognitive functions (Albert, Duffy, & Naeser, 1987).
There are at least two limitations to this study. First, though the collective tasks within the PCTB did detect cross-sectional and longitudinal changes in cognition, it is possible that other kinds of tasks, such as those assessing memory and executive functions, may capture even more robust changes in cognition associated with age in chimpanzees. Second, the length of time between assessments was (1) relatively short, (2) limited to the physical cognition tasks, and (3) not well controlled or systematically manipulated in this sample. Though we used the duration in time that elapsed between the two tests as a covariate in the statistical analysis, ideally this should be better controlled from an experimental standpoint. This could have removed some potential sources of error variance and enhanced the rigor of the design. Additionally, given the longer lifespan of chimpanzees compared to other nonhuman primates, the elapsed time between tests should, perhaps, be longer, which may reveal more robust effects of age on cognition. Lastly, we no made attempt to minimize or limit intervening social events between tests 1 and 2 nor did we attempt to control for the physical housing of the apes between tests. Similarly, many of the chimpanzees in this study were involved in multiple studies of motor skill, cognition and social learning during the intervening time between the administration of the two PCTB tests. Arguably, the lack of control of these extraneous variables may account for the observed age-related changes in performance, particularly for the cross-sectional results. However, we believe that the longitudinal changes we report are less likely attributable to these variables because each ape would have carried those experiences forward and we know of no appreciable differences in the treatment or experiences of the apes between tests 1 and 2.
There is one fundamental question that arises from these findings when viewed in the context of the neurobiology of aging in chimpanzees. Notably, chimpanzees show age-related changes in gray volume and white matter integrity in certain regions, though not to the widespread extent that leads to the same degree of pronounced brain structure deterioration observed in human aging or AD (Autrey et al., 2014; Chen et al., 2013). Furthermore, a proportion of elderly individuals develop AD-like pathology (Edler et al., in press) or exhibit other neurological pathologies or infarcts (Hopkins & Latzman, 2017). Whether the loss in cognitive functions reported here in elderly chimpanzees is attributable to any of these conditions, but specifically those pathological features associated with AD, remains entirely unknown, yet warrants further investigation. If loss in cognition in aged chimpanzees is associated with AD pathology, this would have significant implications for our understanding of the uniqueness of neurodegenerative disorders in humans compared to other nonhuman primates. Additionally, such findings would have significant implications for the long-term management and care of the captive chimpanzee populations in the US and other countries. For instance, the National Institutes of Health owns more than 400 chimpanzees, most of whom are retired from biomedical research and housed in the federal sanctuary (i.e., Chimp Haven). Many of these chimpanzees are adults, will continue to age, and may potentially develop motor and cognitive impairments that present management challenges or compromise their wellbeing. In our view, this is a remarkable circumstance and opportunity in which continued documentation of longitudinal changes in cognition and behavior could be coupled with a rigorous, sustained brain collection (www.chimpanzeebrain.org) and a neuropathology program to produce data that benefit both our understanding of AD pathology in humans and the opportunity to advance wellbeing in captive chimpanzees.
One intriguing alternative possibility is that the cognitive loss we observe in chimpanzees may be relatively mild and due to subtle neuroanatomical changes in dendritic and synaptic function, but not linked with overt human-like AD pathology, despite a large number of chimpanzees exhibiting AD-like pathology on postmortem examination (Edler et al., 2017). Indeed, the loss in cognitive performance across age classes reported here is small, accounting for ~ 4% of the variance in performance. This is consistent with the fact that chimpanzees with AD pathology do not exhibit significant neuron loss (Edler et al., in press), a characteristic that is a hallmark of human AD. Some studies indicate a divergence between humans and chimpanzees in terms of the inflammatory responses associated with NFTs and amyloid plaques (Edler et al., 2018; Munger et al., 2018), which may contribute to neuron loss in humans and neuron preservation in chimpanzees.
In summary, this study allowed for the cross-sectional and longitudinal assessment of age-related changes in chimpanzee cognition using the PCTB test. The results reported here demonstrate that aging chimpanzees show moderate to small losses in cognition. Though there are limitations to this study, this is, by far, the single largest cross-sectional and longitudinal study on age-related changes in cognition in chimpanzees (and, indeed all nonhuman primates), the species most closely related to humans, and the only known one to naturally develop AD-like pathology similar to humans. Taken together, these lines of evidence highlight the importance of including chimpanzees in future research on cognition and aging that may provide important clues that will allow us to identify therapeutic targets for human AD.
Acknowledgements
This research was supported in part by NIH grants NS-42867, NS-73134, and HD-60563. The NCCC chimpanzees are supported by Cooperative Agreement U42-OD011197. The Yerkes Center and NCCC are fully accredited by AAALAC International. American Psychological Association guidelines for the ethical treatment of animals were adhered to during all aspects of this study.
Footnotes
The authors have no conflict of interest to declare.
References
- Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, … Ohtomo K (2008). Aging in the CNS: Comparison of gray/white matter volume and diffusion tensor data. Neurobiology of Aging, 29, 102–116. [DOI] [PubMed] [Google Scholar]
- Albert M, Duffy FH, & Naeser M (1987). Nonlinear changes in cognition with age and their neurophysiologic correlates. Canadian Journal of Experimental Psychology, 41, 141. [PubMed] [Google Scholar]
- Autrey MM, Reame LA, Mareno MC, Sherwood CC, Herndon JG, Preuss TM, … Hopkins WD (2014). Age-related effects in the neocortical organization of chimpanzees: gray and white matter volume, cortical thickness, and gyrification. NeuroImage, 101, 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein IS (1961). Response variability and rigidity in the adult chimpanzee. Journal of Gerontology, 16(4), 381–386. [DOI] [PubMed] [Google Scholar]
- Bertrand E, Azar M, Rizvi B, Brickman AM, Huey ED, Habeck C, … Cosentino S (2018). Cortical thickness and metacognition in cognitively diverse older adults. Neuropsychology, 32(6), 700–710. doi: 10.1037/neu0000458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XE, B., Li L, Glasser MF, Westyle LT, Fjell AM, Walhovd KB, … Rilling JK (2013). Brain aging in humans, chimpanzees (Pan troglodytes) and rhesus macaques (Macaca mulatta): magnetic resonance images of macro- and microstructural changes. Neurobiology of Aging, 34, 2248–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier ES, MacLean AG, Mohan M, Didier PJ, Lackner AA, & Kuroda MJ (2016). Contributions of nonhuman primates to research on aging. Veterinary Pathology, 53(2), 277–290. doi: 10.1177/0300985815622974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edler MK, Munger EL, Meindl RS, Hopkins WD, Ely JJ, Erwin JM, … Raghanti MA (in press). Age-related neuron density changes and Alzheimer’s-like pathology in the chimpanzee brain. Philosophical Transactions of the Royal Society: B Biological Sciences. [DOI] [PMC free article] [PubMed]
- Edler MK, Sherwood CC, Meindl RS, Hopkins WD, Ely JJ, Erwin JM, … Raghanti MA (2017). Aged chimpanzees exhibit pathologic hallmarks of Alzheimer’s disease. Neurobiology of Aging, 59, 107–120. doi: 10.1016/j.neurobiolaging.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edler MK, Sherwood CC, Meindl RS, Munger EL, Hopkins WD, Ely JJ, … Raghanti MA (2018). Microglia changes associated to Alzheimer’s disease pathology in aged chimpanzees. Journal of Comparative Neurology, 526(18), 2921–2936. doi: 10.1002/cne.24484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE (2010). Evolution in health and medicine Sackler colloquium: Evolution of the human lifespan and diseases of aging: Roles of infection, inflammation, and nutrition. Proceedings of the National Academy of Science, 107(S1), 1718–1724. doi: 10.1073/pnas.0909606106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing M, Rebeck GW, Hyman BT, Tigges J, & Mirra SS (1994). Neuropathology and apolipoportein E profile of aged chimpanzees: Implications of Alzheimer’s disease. Proceedings of the National Academy of Sciences, 91(20), 9382–9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Rapp PR, & Morrison JH (2012). Neuronal and morphological bases of cognitive decline in aged rhesus monkeys. Age, 34(5), 1051–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann E, Call J, Hernández-Lloreda MV, Hare B, & Tomasello M (2007). Humans have evolved specialized skills of social cognition: The cultural intelligence hypothesis. Science, 317(5843), 1360–1366. doi: doi: 10.1126/science.1146282 [DOI] [PubMed] [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, & Killiany RJ (1997). Patterns of cognitive decline in aged rhesus monkeys. Behavioural Brain Research, 87, 25–34. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Hare B, Call J, & Tomasello M (2010). Differences in the cognitive skills of bonobos and chimpanzees. PLOS ONE, 5(8), e12438. doi:e1243810.1371/journal.pone.0012438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann E, Hernandez-Lloreda MV, Call J, Hare B, & Tomasello M (2010). The structure of individual differences in the cognitive abilities of children and chimpanzees. Psychological Science, 21(1), 102–110. doi:095679760935651110.1177/0956797609356511 [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Keebaugh AC, Reamer LA, Schaeffer J, Schapiro SJ, & Young LJ (2014). Genetic influences on receptive joint attention in chimpanzees (Pan troglodytes). Scientific Reports 4(3774), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, & Latzman RD (2017). Future research with captive chimpanzees in the USA: Integrating scientific program with behavioral management In Schapiro SJ (Ed.), Handbook of Primate Behavioral Management (pp. 445–470). Boca Raton, FL: Taylor and Francis Group. [Google Scholar]
- Hopkins WD, Reamer L, Mareno MC, & Schapiro SJ (2015). Genetic basis for motor skill and hand preference for tool use in chimpanzees (Pan troglodytes). Proceedings of the Royal Society: B Biological Sciences, 282(1800). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell JL, & Schaeffer J (2014). Chimpanzee intelligence is heritable. Current Biology, 24(14), 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly M, Ammersdorfer S, Schmidtke D, & Zimmermann E (2014). Touchscreen-based cognitive tasks reveal age-related impairment in a primate aging model, the grey mouse lemur (Microcebus murinus). PLOS ONE, 9(10), e109393. doi: 10.1371/journal.pone.0109393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly M, Micheletta J, De Marco A, Langermans JA, Sterck EHM, & Waller BM (2017). Comparing physical and social cognitive skills in macaque species with different degrees of social tolerance. Proceedings of the Royal Society: B Biological Sciences, 284(1862). doi: 10.1098/rspb.2016.2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JE, & Michels RR (1989). Error analysis of delayed response in aged squirrel monkeys Animal Learning and Behavior, 17(2), 157–162. [Google Scholar]
- LaClair M, & Lacreuse A (2016). Reversal learning in gonadectomized marmosets with and without hormone replacement: are males more sensitive to punishment? Animal Cognition, 19(3), 619–630. doi: 10.1007/s10071-016-0966-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, & Herndon JG (2008). Nonhuman primate models of cognitive aging In Bizon JL & Woods AG (Eds.), Animal Models of Human Cognittive Aging (pp. 29–58). New York: Springer. [Google Scholar]
- Lacreuse A, Herndon JG, Killiany RJ, Rosene DL, & Moss MB (1999). Spatial cognition in rhesus monkeys: male superiority declines with age. Hormones and Behavior, 36(1), 70–76. doi: 10.1006/hbeh.1999.1532 [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Parr L, Chennareddi L, & Herndon JG (2018). Age-related decline in cognitive flexibility in female chimpanzees. Neurobiology of Aging, 72, 83–88. doi: 10.1016/j.neurobiolaging.2018.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Russell JL, Hopkins WD, & Herndon JG (2014). Cognitive and motor aging in female chimpanzees. Neurobiology of Aging, 35(3), 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Reamer LA, Mareno MC, Russell JL, Wilson DC, Schapiro SJ, & Hopkins WD (2015). Distal communication by chimpanzees (Pan troglodytes): Evidence for common ground? Child Development, 86(5), 1623–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DM, Yang C, Eliez S, Reiss AL, & Schatzberg AF (2004). Cognitive correlates of white matter growth and stress hormones in female squirrel monkey adults. Journal of Neuroscience, 24(14), 3655–3662. doi: 10.1523/JNEUROSCI.0324-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique HM, & Call J (2015). Age-dependent cognitive inflexibility in great apes. Animal Behaviour, 102, 1–6. [Google Scholar]
- McQuail JA, & Nicolle MM (2012). Animal models of aging and cognition. Current Translational Geriatrics and Experimental Gerontology Reports, 1, 21–28. [Google Scholar]
- Mikkelsen T, Hillier L, Eichler E, Zody M, Jaffe D, Yang S-P, … Altheide T (2005). Initial sequence of the chimpanzee genome and comparison with the human genome. Nature, 437(7055), 69–87. [DOI] [PubMed] [Google Scholar]
- Moss MB, Rosene DL, & Peters A (1988). Effects of aging on visual recognition memory in the rhesus monkey. Neurobiology of Aging, 9, 495–502. [DOI] [PubMed] [Google Scholar]
- Munger EL, Edler MK, Hopkins WD, Ely JJ, Erwin JM, Perl DP, … Raghanti MA (2018). Astrocytic changes with aging and Alzheimer’s disease-type pathology in chimpanzees. Journal of Comparative Neurology. doi: 10.1002/cne.24610 [DOI] [PMC free article] [PubMed]
- Munger EL, Takemoto A, Raghanti MA, & Nakamura K (2017). Visual discrimination and reversal learning in aged common marmosets (Callithrix jacchus). Neuroscience Research, 124, 57–62. doi: 10.1016/j.neures.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Bernot T, & Tuszynski MH (2010). Age-related cognitive deficits in rhesus monkeys mirror human deficits on an automated test battery. Neurobiology of Aging, 31(6), 1020–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R (2006). Ageing and the brain. Postgraduate Medical Journal, 82(964), 84–88. doi: 10.1136/pgmj.2005.036665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Caitanio JP, Conley A, Czoty PW, t Hart BA, … Voytko ML (2014). Why primate models matter. American Journal of Primatology, 76(9), 801–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picq JL (2007). Aging affects executive functions and memory in mouse lemur primates. Experimental Gerontology, 42(3), 223–232. doi: 10.1016/j.exger.2006.09.013 [DOI] [PubMed] [Google Scholar]
- Rapp PR, & Amaral DG (1989). Evidence for task-dependent memory dysfunction in the aged monkey. The Journal of Neuroscience, 9, 3568–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, & Amaral DG (1992). Individual differences in the cognitive and neurobiological consequences of normal aging. Trends in Neurosciences, 15(9), 340–345. [DOI] [PubMed] [Google Scholar]
- Raz N, Herndon JG, Hopkins WD, Schmidtke D, & Lacreuse A (in press). Age-related decline in executive function as a hallmark of cognitive aging across primates: A view from the laboratory. Philosophical Transactions of the Royal Society: B Biological Sciences. [DOI] [PMC free article] [PubMed]
- Riopelle AJ, & Rogers CM (1965). Age changes in chimpanzees In Schrier AM, Harlow HF, & Stollnitz F (Eds.), Behavior in Nonhuman Primates (pp. 499–462). New York/London: Academic Press. [Google Scholar]
- Rosen RF, Farberg AS, Gearing M, Dooyema J, Long PM, Anderson DC, … Walker LC (2008). Tauopathy with paired helical filaments in an aged chimpanzee Journal of Comparative Neurology, 509, 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RF, Tomidokoro Y, Farberg AS, Dooyema J, Ciliax B, Preuss TM, … Walker LC (2016). Comparative pathobiology of beta-amyloid and the unique susceptibility of humans to Alzheimer’s disease. Neurobiology of Aging, 44, 185–196. doi: 10.1016/j.neurobiolaging.2016.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RF, Walker LC, & LeVine H (2011). PIB binding in aged primate brains: Enrichment of high-affinity sites in humans with Alzheimer’s disease. Neurobiology of Aging, 32(3), 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JL, Lyn H, Schaeffer JA, & Hopkins WD (2011). The role of socio‐communicative rearing environments in the development of social and physical cognition in apes. Developmental Science, 14(6), 1459–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2009). When does age-related cognitive decline begin? Neurobiology of Aging, 30(4), 507–514. doi: 10.1016/j.neurobiolaging.2008.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt V, Pankau B, & Fischer J (2011). Old World monkeys compare to apes in the primate cognition test battery. PLOS ONE, 7(4), e32024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood CC, Baurernfeind AL, Bianchi S, Raghanti MA, & Hof PR (2012). Human brain evolution writ large and small In Hofman MA & Falk D (Eds.), Progress in Brain Research: Elsevier. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Gordon AD, Allen JS, Phillips KA, Erwin JM, Hof PR, & Hopkins WD (2011). Aging of the cerebral cortex differs between humans and chimpanzees. Proceedings of the National Academy of Sciences, 108, 13029–13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M, & Call J (1997). Primate Cognition. New York: Oxford University Press. [Google Scholar]
- Verdier JM, Acquatella I, Lautier C, Devau G, Trouche S, Lasbleiz C, & Mestre-Frances N (2015). Lessons from the analysis of nonhuman primates for understanding human aging and neurodegenerative diseases. Frontiers in Neuroscience, 9, 64. doi: 10.3389/fnins.2015.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, & Jucker M (2017). The exceptional vulnerability of humans to Alzheimer’s disease. Trends in Molecular Medicine, 23(6), 534–545. doi: 10.1016/j.molmed.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley MA, Fernandes HBF, & Hopkins WD (2015). The more g-loaded, the more heritable, evolvable, and phenotypically variable: Homology with humans in chimpanzee cognitive abilities. Intelligence, 50, 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman KP, Healey B, Carlotto A, & Lacreuse A (2019). One-year change in cognitive flexibility and fine motor function in middle-aged male and female marmosets (Callithrix jacchus). American Journal of Primatology, 81(2), e22924. doi: 10.1002/ajp.22924 [DOI] [PMC free article] [PubMed] [Google Scholar]


