Abstract
Abnormalities in the spectral power of offenders’ neural oscillations have been noted within select Resting-State Networks (RSNs); however, no study has yet evaluated the influence of cocaine dependence, drug use severity, and psychopathic traits on these abnormalities. To this end, the present study compared rest-related power spectral characteristics between two groups of offenders (with and without a DSM-IV-TR cocaine-dependence diagnosis) and a non-offender control group. Results indicated that both offender groups presented with lower low frequency power ratio (LFPR) scores (i.e. across all RSNs) than non-offenders. These differences in LFPR scores were found to be due to both higher high-frequency power (0.15-0.25 Hz; within seven of eight investigated networks) and decreased low-frequency power (0.01-0.10 Hz; within six of eight investigated networks) in both offender groups compared to non-offenders. Thus, both cocaine-dependent and non-dependent offenders displayed consistently abnormal neural oscillations, suggesting that these oscillatory abnormalities could serve as a broad neurobiological marker of offender status. Offenders’ LFPR levels correlated with lifetime years of cocaine use, but not with the level of psychopathic traits. These findings supplement our knowledge of the influence of substance use on resting-state activity in offenders; moreover, they provide further indication of the importance of evaluating shared/unique variance associated with drug use and antisocial personality traits.
Keywords: ICA, psychopathy, cocaine use, offenders, resting-state, fMRI
1. Introduction
The spectral dynamics of resting-state networks (RSNs), which can be assessed through the evaluation of frequency oscillations across the power spectrum, have been hypothesized to underlie the synchronicity of rest-related activity in RSNs (Baria et al., 2011; Thompson & Fransson, 2015; Yaesoubi et al., 2017), and to provide generalized insights into the development and maintenance of healthy brain function (Pizoli et al., 2011). A typical normalized resting-state spectral profile consists of peak activity within low-frequencies (< 0.10 Hz), with sharply decreased activity occurring within mid- (0.10-0.149 Hz), and particularly within high-frequencies (0.15-0.25 Hz; Allen et al., 2011; Kalcher et al., 2014). A higher proportion of low-frequency activity at rest is believed indicative of a more stable, functional network (Biswal et al., 1996), whereas a higher proportion of high-frequency activity at rest has been linked to instability of network activity (Buzsáki & Draguhn, 2004; Salvador et al., 2008; but see DeRamus et al., 2020). Consistent with this view, considerable work has associated decreased low-frequency activity (Cauda et al., 2009; Garrity et al., 2007; Han et al., 2011; Malinen et al., 2010; Wang et al., 2016; Xu et al., 2014; Yu et al., 2014) and/or increased high-frequency activity (Cauda et al., 2009; Malinen et al., 2010; Otti et al., 2013; Sambataro et al., 2017) with numerous neurological and psychiatric disorders, including schizophrenia, chronic pain, bipolar disorder, major depressive disorder and amnestic mild cognitive impairment. The consistency and breadth of this body of work suggests that aberrant spectral patterns may serve as a common underlying feature of a wide variety of psychiatric disorders.
To date, only a handful of studies have reported on spectral dynamics within offender populations, and work has almost exclusively been conducted within adolescent samples (Liu et al., 2014; Thijssen et al., 2017; Zhou et al., 2015). Zhou and colleagues (2015) reported decreased fractional amplitude of low frequency fluctuation (fALFF) within adolescent offenders’ bilateral amygdala/hypothalamus, right lingual gyrus, left cuneus and right insula. More recently, Thijssen and colleagues (2017) demonstrated that more prolonged cannabis use related to decreased low-frequency power within the Default-Mode Network (DMN), Executive Control Network (ECN) and sensory networks of adolescent offenders (more extended history of alcohol use also related to decreased low-frequency power within the right frontoparietal, salience, dorsal attention and sensory networks). Finally, the only study to have investigated spectral abnormalities within an adult offender sample to date (though the mean age of the sample was still only 20; Liu et al., 2014) reported reduced low-frequency fluctuations within the right orbitofrontal cortex, left temporal pole, right inferior temporal gyrus and left cerebellum of 32 offenders with antisocial personality disorder compared to 35 healthy controls. The consistency of these findings does provide preliminary support for the existence of spectral abnormalities in offending populations. However, the relative sparseness of the field, the small sample sizes employed, and the predominant focus on adolescent offenders does significantly limit the ability to form firm conclusions.
One distinct possibility is that offenders’ osscilatory abnormalities are due to their generally higher levels of substance use disorders (SUD), which can reach prevalence rates as high as 74% (Peters et al., 1998) or even 95% (Kouri et al., 1997), and which are well-established to impart long-lasting changes to neural integrity (e.g. Barrós-Loscertales et al., 2011; Cisler et al., 2013; Ma et al., 2015; Beard et al., 2019). Indeed, a growing body of work has begun reporting spectral disturbances within substance-dependent populations (Ide et al., 2016; Jiang et al., 2011; Wang et al., 2013), including evidence of increased thalamic fALFF (Ide et al., 2014) and decreased power spectrum scale invariance (i.e. weaker autocorrelation and signal persistence) in various frontoparietal regions (Ide et al., 2016) in cocaine dependent individuals. Similar results have been reported in heroin-dependent individuals within a variety of frontal, temporal, occipital and parietal regions (Jiang et al., 2011; Wang et al., 2013) in heroin dependent individuals. It should be noted, however, that the vast majority of this work has not been conducted within an offender population. Thus, important gaps remain regarding the extent to which dependence status underpins spectral abnormalities in offender populations.
To this end, the present study sought to evaluate the integrity of resting-state power spectral dynamics within two groups of offenders (those who do and do not meet DSM-IV diagnosis for cocaine-dependence) and within a control group without offending behavior. Resting-state networks were identified using Independent Component Analysis (ICA), and power spectral dynamics were evaluated within and between the three study groups. Based on the handful of studies conducted within offender samples, and on the work conducted to date within substance-using populations, we predicted decreased low-frequency activity and increased high-frequency activity (resulting in decreased low frequency power ratio (LFPR; the ratio of the integral of low-frequency activity to the integral of high-frequency activity, see Allen et al., 2011) in both offender groups compared to the non-offender controls. Identifying such power spectra disruptions in offenders could indicate incoherence in baseline neural activity in this population. Additionally, we sought to explore the extent to which network-related disruptions in offenders were characteristic of all RSNs or were instead specific to only certain resting-state networks. To this end, exploratory analyses compared/contrasted oscillatory patterns within each identified RSN, to evaluate for the existence of global versus network-specific effects.
Another possibility is that offenders’ abnormal spectral patterns are due to their generally higher levels of antisocial traits, which have also been linked to substantive neural abnormalities, including decreased amygdalar and frontal activity (Blair, 2010; Poeppl et al., 2019; Umbach et al., 2015), as well as increased fronto-insular cortex activity (Poeppl et al., 2019). As a complicating factor, antisociality and substance abuse are themselves highly comorbidity (as high as 90% comorbidity by some estimates; Smith & Newman, 1990), making dissection of SUD/antisociality distinctions extremely difficult to parse. As this parcellation is a particular focus of work in our laboratory, we also conducted subsequent exploratory regression analyses to evaluate the extent to which SUD participants’ spectral patterns could be explained as a function of either their drug-use severity or their psychopathic personality. To our knowledge, no work has yet investigated these issues as they pertain to spectral power in adult offenders; thus, while exploratory, results may provide preliminary insights that can help guide future progress into this underrepresented area.
2. Methods
2.1. Participants
One hundred and twelve right-handed male participants were recruited as part of a larger project focused on identifying various biomarkers of cocaine use disorders. Offenders were recruited through active recruitment at New Mexico probation/parole offices, temporary employment agencies, alcohol/drug treatment centers, and through print/online ads. Non-offenders were recruited solely through print/online ads. Offenders met initial screening criteria if they were on probation/parole, were 18-55 years of age, had a felony-level conviction history, did not self-report psychotic disorders or use of antipsychotic medications, and matched fMRI acquisition criteria (e.g. no metal in the body, no history of major head trauma, no pregnancy, right-handedness). Subsequent screening excluded for lifetime history of a psychotic disorder (one exclusion), major depressive disorder within the last six months, current use of antipsychotic medications (9 exclusions), and full IQ estimates < 70. Non-offenders were matched on important demographic variables to the extent possible, but self-reported having no criminal record. Following all screening mechanisms, 102 participants were included in final analyses (37 Cocaine-Dependent, 47 Non-Dependent and 18 non-offenders; see Table 1 for full demographics). This study was approved by the Research Ethics Board of the University of New Mexico. Participants provided written informed consent in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Table 1.
Participant demographics
| O (N = 84) |
DO (N = 37) |
NDO (N = 47) |
NO (N = 18) |
Group differences (t scores) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Mean Range SD |
N |
Mean Range SD |
N |
Mean Range SD |
Mean Range SD |
O vs NO | DO vs NO | NDO vs NO | DO vs NDO | |
| Age | 33.44 | 35.11 | 32.13 | 30.87 | 2.63 * | 3.32 * | 1.90 | −1.52 | ||
| 20-59 | 22-56 | 20-59 | 18-50 | |||||||
| 8.97 | 8.36 | 9.30 | 9.27 | |||||||
| IQ | 105.76 | 106.03 | 105.55 | 112.60 | −1.99 * | −1.77 | −1.93 | −0.18 | ||
| 77-137 | 80-137 | 77-131 | 89-131 | |||||||
| 11.84 | 11.74 | 12.04 | 12.50 | |||||||
| ASI-X | ||||||||||
| LCU | 3.35 | 7 | 0.47 | 0.33 | 4.48 ** | 5.89 ** | 0.31 | −5.82 ** | ||
| 0-24 | 0-24 | 0-10 | 0-5 | |||||||
| 5.61 | 6.66 | 1.67 | 1.29 | |||||||
| Major Drugs | 4.51 | 6.38 | 3.04 | 1.58 | ||||||
| 0-31 | 0-31 | 0-29 | ||||||||
| 6.87 | 7.24 | 6.26 | ||||||||
| Minor Drugs | 21.55 | 27.05 | 17.21 | 1.05 | ||||||
| 0-74 | 1-61 | 0-74 | ||||||||
| 16.25 | 14.82 | 16.15 | ||||||||
| PCL-R | ||||||||||
| Total | 20.79 | 23.19 | 18.90 | −2.76 * | ||||||
| 4-34 | 12-34 | 4-34 | ||||||||
| 7.34 | 6.57 | 7.43 | ||||||||
| Factor 1 | 7.32 | 8.09 | 6.71 | −1.81 | ||||||
| 2-15 | 2-14 | 2-15 | ||||||||
| 3.51 | 3.21 | 3.64 | ||||||||
| Factor 2 | 12.09 | 13.38 | 11.08 | −2.66 * | ||||||
| 2-20 | 3-20 | 2-19 | ||||||||
| 4.09 | 3.83 | 4.05 | ||||||||
| SCID | ||||||||||
| GAD | 1 | 1 | ||||||||
| MDD | 7 | 4 | ||||||||
Demographic data on age, IQ, Lifetime Cocaine Use (LCU), Major Drug use (combining years of use of methamphetamines, opiates/analgesics, heroin), Minor Drug use (combining year of use of alcohol, cannabis, nicotine, hallucinogens and inhalants), PCL-R and SCID diagnosis (i.e. General Anxiety Disorder (GAD) and Major Depression Disorder (MDD)) reported separately for Dependent Offenders (DO), Non-Dependent Offenders (NDO) and Non-Offenders (NO). Group differences between Offenders (O), DO, NDO and NO were evaluated via between-group t-tests.
p < 0.05
p < 0.001
2.2. Clinical/Forensic Assessments
2.2.1. SCID-I/P
The Structured Clinical Interview for DSM-IV-TR (SCID-I/P; First, Spitzer, Gibbon, & Williams, 2002) was used to evaluate participants for Axis I and II disorders. Interviews were videotaped and conducted by highly trained Master’s level research personnel, under the guidance of a senior SCID trainer (R.C.; see acknowledgements).
2.2.2. Psychopathic traits
Offenders were assessed for psychopathic traits via a semi-structured Psychopathy Checklist-Revised (PCL-R) interview (Hare, 2003), videotaped and conducted by highly-trained research personnel (trained by M.S.). Subsequent file reviews were not possible; thus, participants were scored 0 to 2 on each of the 20 PCL-R items based on the clinical interview alone (see Denomme et al., 2018, 2020; Forth et al., 1996; Kosson et al., 1997 for evidence of the validity of this approach).
2.2.3. Cocaine use
The number of years of regular drug use was evaluated using a modified Addiction Severity Index - Expanded (ASI-X; McLellan et al., 1992), administered orally by a trained examiner. As offenders were recruited based on cocaine abuse/dependency diagnoses, total years of Lifetime Cocaine Use (LCU) was included in relevant analyses throughout this study.
2.2.4. Drug use
Following administration of the ASI-X, composite scores of total drug use (other than cocaine) were calculated by summing the total years of use of drugs that corresponded to two categories: Major drugs (i.e. methamphetamines, opiates/analgesics, heroin) and Minor drugs (i.e. alcohol, cannabis, nicotine, hallucinogens and inhalants). Thus, for example, if a participant used cannabis for 3 years, hallucinogens for 5 years and nicotine for 5 years, their Minor drug use score was calculated as 13 years. In addition, a ‘Total drug use” score was calculated as the sum of major + minor drug use. These drug use scores were included as covariates in supplementary regression analyses, to examine whether reported results were specific to cocaine use, or instead generalized to other drugs of abuse.
2.2.5. WAIS-III
Full-scale intelligence quotient was approximated using the vocabulary and matrix reasoning scales of the Wechsler Adult Intelligence Scale 3rd edition (WAIS-III).
2.3. Data acquisition
Resting-state data was acquired using a 3T Magnetom Trio Tim Siemens scanner at the Mind Research Network (MRN). Participants kept their eyes open and directed at a fixation cross. The 5.5 minutes acquisition was performed using a fast gradient-echo EPI sequence. Imaging parameters were as follows: TR = 2000ms, TE = 29ms, FA = 75°, matrix = 63 x 63, slices = 33 acquired in an ascending interleaved fashion, slice thickness = 3.5 mm, FOV = 240mm x 240mm and voxel size = 3.8 x 3.8 x 3.5mm.
2.4. Preprocessing
Resting-state data was preprocessed using SPM12. Data was motion-corrected using INRIAlign (Freire et al., 2002). Realignment was handled using a distance cut-off of 2.5, with data coregistered with a full-width-as-half-maximum (FWHM) of 8mm. Slice timing correction was applied using the 16th slice as reference. Data was then normalized using the SPM5 EPI template; the mean image was used for parameter estimation. Finally, data was smoothed using a 10mm Gaussian kernel. No participant presented movement above 5mm. Hence, after preprocessing, all participants were included in analyses.
2.5. Independent Component Analysis (ICA)
Following preprocessing, ICA was performed on all 102 participants using the GIFT toolbox (GroupICAT v4.0b, https://trendscenter.org/software/) following well-established methodology (Allen et al., 2011). The spatial group ICA (GICA) was set to identify a high number of components (75) using the Infomax algorithm. To ensure the stability of the estimation, the Infomax ICA algorithm was repeated in Icasso 20 times. Two GICA back-reconstruction steps were performed to produce subject-specific spatial maps (SMs) and time courses (TCs); results were subsequently scaled to z-scores.
The ICA analysis yielded 75 components, constructed by grouping individual voxels that were synchronously activated during rest. Components were manually classified into two groups: resting state and noise. Components were classified into resting-state based on the methodology recommended by Allen et al. (2011): component t-maps showed activity in grey matter areas, activity peaks between 0.01-0.08 Hz followed by flat activity in high frequencies, a low to high-frequency ratio higher than 4, and a cluster stability index (Iq) of at least 0.80. Components displaying spectral activity non-typical of resting-state networks (i.e. activity outside of grey matter, a flat power spectrum, or power spectrum with an activity peak in high-frequency) were classified as noise. One-sample t-tests were performed using SPMstats in GIFT to create SPM12 compatible t-maps. Activity peaks in each component were visually inspected, identified using PickAtlas 3.0.5 (Maldjian et al., 2003), matched with key regions of known resting-state networks using the 90 fROIs Atlas (Shirer et al., 2012), and classified into the corresponding RSN category.
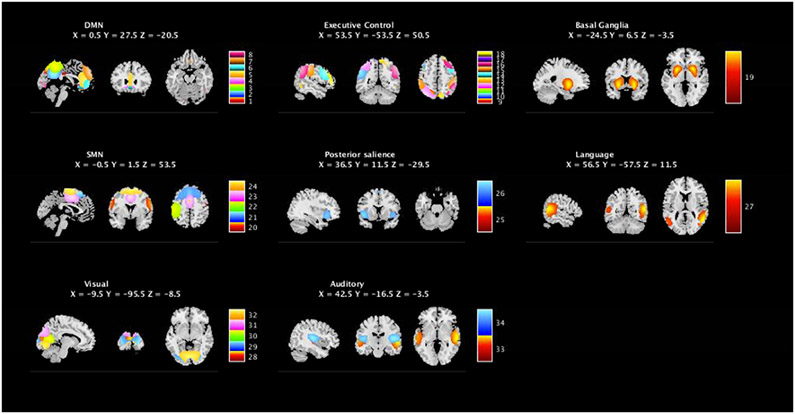
Following component selection (Allen et al., 2011), 34 components (from the original 75) were selected as representing valid BOLD activity. Following network categorization based on the 90 fROIs atlas (Shirer et al., 2012), these 34 components were categorized into one of DMN, ECN, Posterior Salience (PS), Visual, Basal Ganglia (BG), SMN, Language (LG) and Auditory (AU) networks (see Figure 1).
Figure 1. Network classification of valid resting-state components.
Resting-state component classification yielded 34 components of interest. RSN classification, using the peak of activity of the components, allowed us to categorize those components in 8 RSNs: Default mode (DMN), Executive Control (ECN), Basal Ganglia, Sensorimotor (SMN), Posterior Salience, Languahe, Visual and Auditory networks.
p(FWE) < 0.05
2.6. Spectral Analyses
Power spectra were computed for each participant using spectral group comparison analyses in GIFT. Following the widely used process for categorizing frequency bands (Allen et al., 2011; Bryant et al. , 2016), power spectra data were pooled into three bins representing low- (< 0.10 Hz), mid- (0.10 to 0.149 Hz) and high-frequency (0.15 to 0.25 Hz) activity. Analyses focused exclusively on the low and high frequency bands, as both the function and dynamics of mid-frequency activity are significantly less characterized in the broader clinical literature. Spectral data for each participant was then extracted for further analysis in SPSS 22.
2.7. Data Analytic Strategy
A 3 (Group) x 2 (Bins) x 8 (Network) omnibus ANOVA was used to evaluate all higher-order effects, followed by targeted comparisons used to evaluate specific hypothesized effects. This analytic strategy provides the greatest granularity with regard to the nature of spectral distributions in offenders, and thus served as our primary analytic pipeline. However, because the literature tends to focus predominantly on low-frequency centered metrics (e.g. ALFF, fALFF, LFPR) as a single predictive metric, we also report differences in LFPR scores, which have previously been used as a predictor in a clinical setting (see Yu et al., 2013).
In addition, hierarchical regression models were employed, using interview-based PCL-R scores, LCU, and their interaction as regressors, to explore the potential influence of psychopathic traits and LCU on power spectra integrity. These models were run with and without lifetime use of other substances as covariates in the model, to ensure that reported effects were specific to lifetime cocaine burden rather than the burden of other comorbid substances. Results of these analyses can be found in the supplementary material Sections 7 to 12.
3. Results
3.1. Demographics
As can be seen in Table 1, rates of comorbid psychiatric disorders were generally low, with no prevalence differences between offender groups, χs = 1.97, 0.03). Interview-based PCL-R scores (M = 20.79) were closely in line with previously published norms collected through interview + file review methods in offender populations (Hare, 2003).
One-way ANOVA identified group differences in LCU, (F = 28.79, p < 0.001), with the Dependent group (M = 7.00, SD = 6.66) showing higher LCU than either the Non-Dependent (M = 0.47, SD = 1.67; t = −5.82, p < 0.001) or Non-Offender (M = 0.33, SD = 1.29; t = 5.89, p < 0.001) groups. As expected, the Dependent group (M = 23.19, SD = 6.57) also manifested higher PCL-R scores than the Non-Dependent group (M = 18.90, SD = 7.43; t = −2.76, p < 0.05; Non-Offenders did not receive the PCL-R interview). No main effect of IQ was identified; however, the Dependent group (M = 35.11, SD = 8.36) was somewhat older than the Non-Dependent (M = 32.13, SD = 9.3; t = −1.52, p > 0.05), and Non-Offender (M = 30.87, SD = 9.27; t = 3.32, p < 0.05) groups. Analyses reported within were reported without controlling for age, as the well-established collinearity between age and LCU (Wagner & Anthony, 2002) may influence validity/interpretability of age-controlled models. However, we also provide age-controlled results in Section 1 of Supplementary Materials.
Correlational analyses indicated that PCL-R scores were positively correlated with negatively correlated with IQ (r = −0.26, p = 0.02), and uncorrelated with age (r = −0.01, p = 0.95), whereas LCU was positively correlated with age (r 0.45, p < 0.001), but uncorrelated with IQ (r = −0.11, p = 0.27). LCU and PCL-R scores showed a modest positive relationship (r = 0.26, p = 0.02).
3.2. Spectra analyses
The omnibus ANOVA identified a main effect of Group, F(2, 99) = 9.92, p < 0.001, with pairwise comparisons indicating that Dependent (M = 0.83; SD = 0.05) and Non-Dependent (M = 0.83; SD = 0.05) groups showed similar overall power spectra, p = 1.00, that were both higher than Non-Offenders (M = 0.79; SD = 0.07; both ps < .05). Main effects of Network, F(7, 99) = 8.13, p < 0.001, and Bin F(1, 99) = 1116.57, p < 0.001, were also observed, with the low frequency bin (M = 1.15; SD= 0.08) showing higher overall power than the high-frequency bin (M = 0.56; SD = 0.10). These main effects were influenced by significant Group x Bin, F(2, 99) = 6.19, p = 0.003, and Network x Bin, F(7, 99) =13.20, p < 0.001, interactions. The Group x Network, F(14, 99) = 1.39, p = 0.15, and Group x Bin x Network, F(14, 99) = 0.94, p = 0.63, interactions did not reach significance.
As no interactions with Network were found, we collapsed mean spectral values across all networks and evaluated for group differences in low-/high-frequency bins via Bonferroni-corrected pairwise comparisons. As can be seen in Figure 2 (see also Section 2 of Supplementary materials), while the two offender groups did not present with differences across either low or high frequency bins, several differences were observed between these groups and the Non-Offender group. Specifically, both offender groups showed significantly decreased low-frequency power spectra compared to Non-Offenders (Dependent Offenders: t = −3.29, p(Family Wise Error (FWE)) = 0.002 ; Non-Dependent Offenders: t =−2.26, p(FWE) = 0.03), and significantly increased high-frequency power compared to Non-Offenders (Dependent Offenders: t = 4.25, p(FWE) < 0.001; Non-dependent Offenders: t = 3.24, p(FWE) = 0.002). As may be expected, given the pattern of low/high frequency activity reported, both Dependent Offenders (M = 5.83, SD = 2.94; t = −2.29, p(FWE) = 0.03) and Non-Dependent Offenders (M = 5.64, SD = 3.13; t = −2.38, p(FWE) = 0.03) presented with significantly decreased LFPR in comparison to Non-Offenders (M = 10.76, SD = 8.91; see Figure 3).
Figure 2. Mean power spectra differences between groups during the overall resting-state activity.
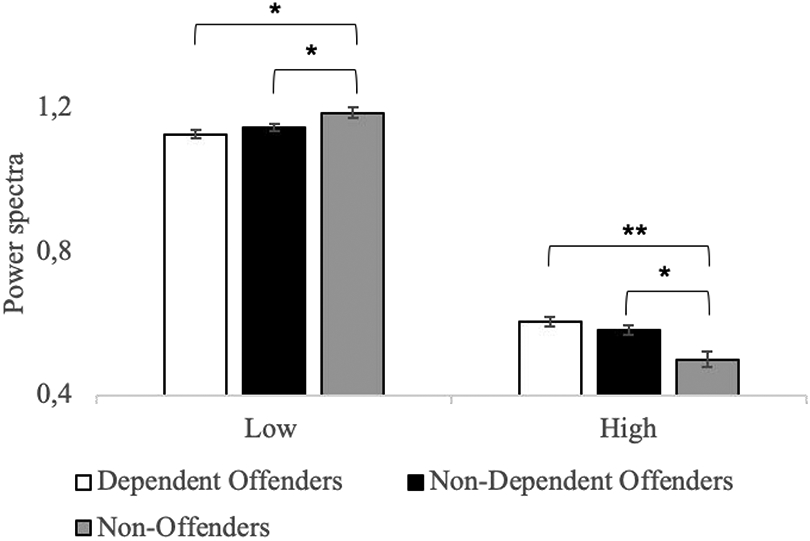
Mean power spectra differences in the low-, mid- and high-frequency bins between Dependent Offenders (white), Non-Dependent Offenders (black), and Non-Offenders (grey) during overall resting-state activity. Dependent and Non-Dependent Offenders exhibited an overall lower activity amplitude during low-frequency activity and a higher activity amplitude during high-frequency activity in comparison to Non-Offenders. However, no significant differences were observed between the two Offender sub-groups in any of the frequency bins.
* p(FWE) < 0.05 ** p(FWE) < 0.001, error bars = SEM
Figure 3. LFPR differences between groups across all networks.
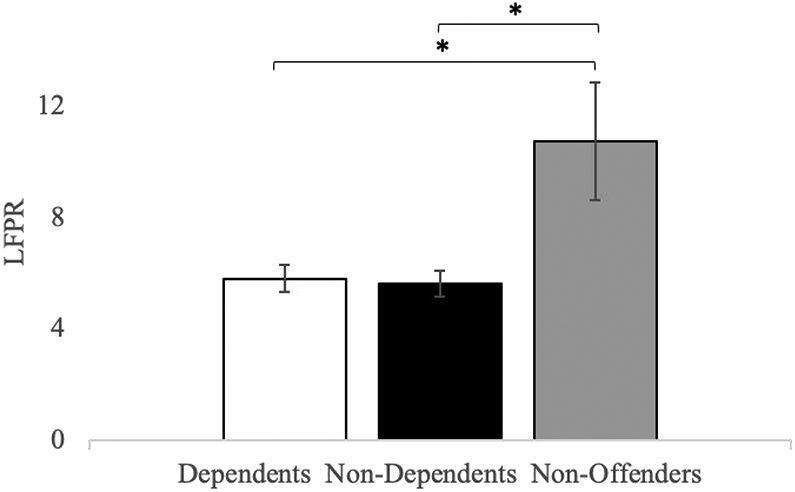
Mean differences in LFPR between Dependent Offenders, Non-Dependent Offenders and Non-Offenders during overall resting-state activity (i.e. across all networks). Both Offender sub-groups displayed significantly lower LFPR in comparison to Non-Offenders; however, no differences were observed between Dependent and Non-Dependent Offenders.
* p(FWE) < 0.05, error bars = SEM
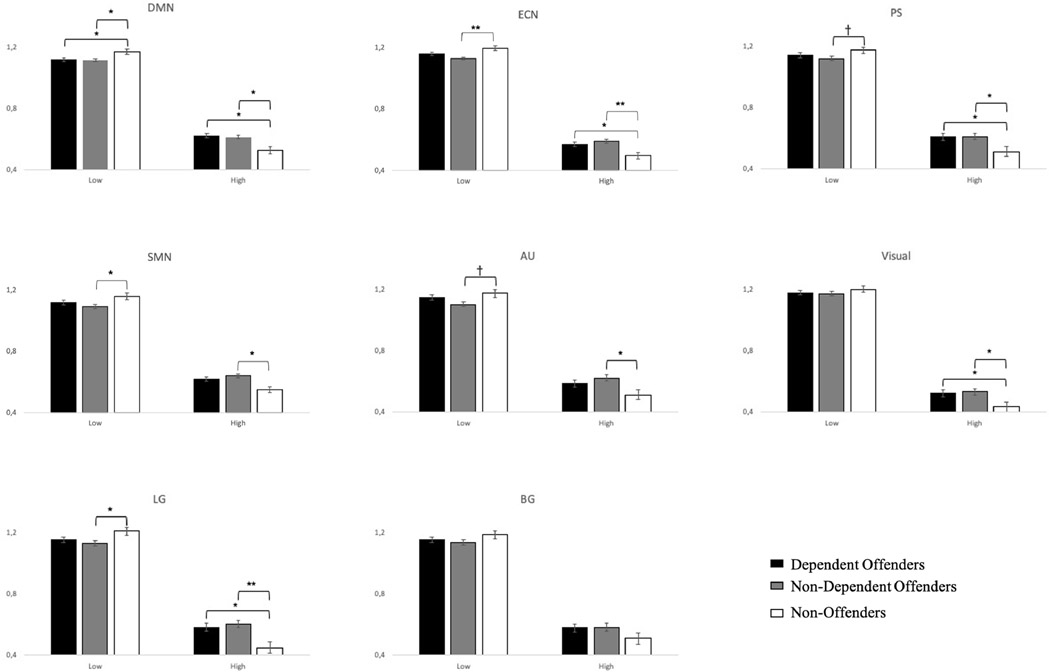
3.3. Exploratory network analyses
The non-significant interactions with Network suggested that observed differences in spectral power occurred similarly across all RSNs. To more formally evaluate the extent to which this was true, we compared LFPR between our three participant groups within each of the eight RSNs. As displayed in Figure 4, the two offender groups presented with highly similar spectral profiles within each RSN (all ps > 0.05), which differed from the Non-Offender group’s frequency profiles within several RSNs. Most consistent was an increase in high-frequency power, which was characteristic of five of the Dependent groups’ RSNs, and seven of the Non-Dependent groups’ RSNs. On the other hand, reductions in low-frequency power were identified within six of the Non-Dependent group’s RSNs, but only one of the Dependent group's RSNs (the DMN; see Section 3 of Supplementary materials for results of higher order effects). Together, this led to significantly reduced network-specific LFPR scores compared to Non-Offenders in a highly overlapping set of four of the Dependent group’s RSNs (DMN, ECN, LG and AU), and four of the Non-Dependent group’s RSNs (DMN, ECN, LG and BG; see Section 4 of Supplementary materials).
Figure 4. Pairwise comparisons in power spectra between groups in each RSN.
Mean power spectra differences between Dependent Offenders, Non-Dependent Offenders and Non-Offenders in the low- and high-frequency bins in each RSN during rest.
† p(FWE) < 0.10 * p(FWE) < 0.05 ** p(FWE) < 0.001, error bars = SEM
3.4. Exploratory contributions of LCU and psychopathy
Exploratory regression models evaluating the influence of LCU and/or interview-based PCL-R scores on LFPR were conducted separately for Dependent and Non-Dependent groups, as LCU levels showed a quite restricted range within the Non-Dependent group. In the Dependent group, only LCU negatively predicted Total LFPR, while PCL-R and the LCU x PCL-R interaction were unpredictive (see Section 5 of Supplementary materials for detailed results). Importantly, this negative relationship with LCU was identified within seven of the eight investigated RSNs (see section 6 of Supplementary materials); moreover, these findings were only minimally affected by the inclusion of ‘major’, ‘minor’ or ‘total’ substance use metrics as model covariates (see section 2.2.4 for definitions of major/minor/total substance use). Thus, LCU appeared to serve as a broad-spectrum marker of spectral imbalance in the cocaine-dependent offenders that could not be attributed to the use of comorbid substances.
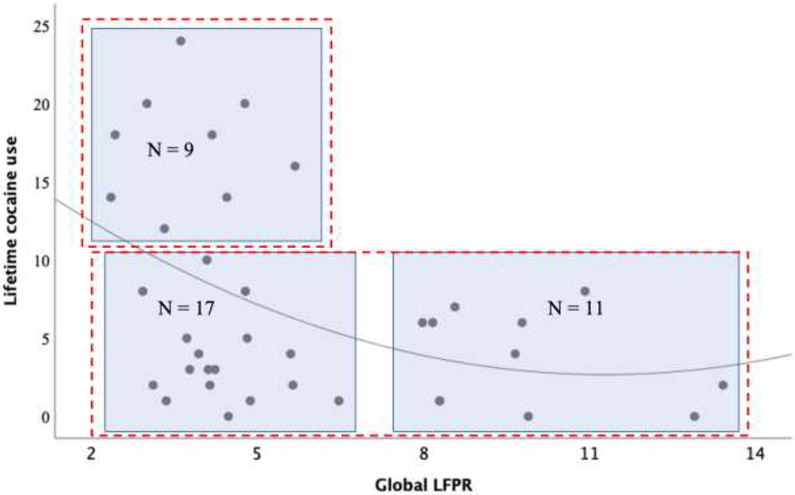
Figure 5 displays a plot of LCU and overall LFPR within the Dependent offenders. As can be seen, this relationship was curvilinear and negatively-skewed, r = −0.39, p = 0.016; see the best-fit line in Figure 5), with data populating only three of four quadrants. Of potential importance, visual inspection of the data suggests that all offenders who presented with LCU levels > 10 years showed lower LFPR scores (i.e. less than 6), while all offenders with LFPR > 8 reported LCU levels < 8 years. To formalize these observations, we used k-means clustering to evaluate the efficacy of both 2-cluster and 3-cluster models. Results indicated that the 2-cluster model (Silhouette coefficient = 0.62, Calinski-Harabasz index = 78.76, Davies-Boulding index = 0.50) performed better than the 3-cluster model (Silhouette coefficient = 0.45, Calinski-Harabasz index = 66.58, Davies-Boulding index = 0.82). Given the modest sample size, the precision of this solution should be considered preliminary; however, previous guidance on the use of clustering techniques suggests that a minimum of 30-40 participants per variable (in line with the present study) is sufficient to provide adequate reliability (Clatworthy et al., 2007; Dalmaijer et al., 2020; Dolnicar et al., 2014).
Figure 5. Relationship between lifetime cocaine use and global LFPR in the Dependent Offender group.
The negative curvilinear relationship between lifetime cocaine use and LFPR during overall resting-state activity in Dependent Offenders. The red dotted-lined rectangles represent a 2-cluster representation of the relationship between LCU and LFPR with 9 Dependent Offenders presenting LCU > 10 and LFPR <7, as well as 28 with LCU <10 and ranging LFPR. The blue rectangles represent a 3-cluster representation between LCU and LFPR with 9 Dependent Offenders presented LCU > 10 years with a LFPR < 7, 17 with LCU < 10 years and LFPR < 7, 11 with LCU < 10 years and LFPR > 7, while no Dependent Offenders presented a LCU > 10 years and LFPR > 7.
Similar regression models undertaken within the Non-Dependent group showed no significant relationships. Nor were any relationships revealed when regression models were conducted across the entire offender sample. However, two caveats: First, it should be reiterated that the range of LCU scores in the Non-Dependent group were significantly restricted, making interpretation of these null results with cocaine difficult. Second, supplementary analyses indicated that ‘minor’ and ‘total’ drug use metrics (see section 2.2.4 for definitions) did correlate negatively with LFPR in the Non-Dependent participants (see Sections 7 to 12 of Supplementary materials).
4. Discussion
The primary aim of our study was to investigate the integrity of rest-related spectral power in antisocial offenders with and without DSM-IV diagnoses of cocaine dependence. Our results indicated broadly disrupted neural oscillations within both offender groups, compared to a group of non-offender controls. Indeed, both offender groups showed reduced low-frequency power, and increased high-frequency power, compared to the Non-Offender group. In total, these spectral disruptions led to lower global LFPR levels in both offender groups within seven of the eight investigated RSNs. Work of this nature is currently sparse; however, the current results are consistent with a handful of studies that have reported spectral abnormalities in a variety of offender populations, including adult offenders with antisocial personality disorder (Liu et al., 2014), and adolescent offenders with antisocial tendencies (Thijssen et al., 2017; Zhou et al., 2015). These results expand on this previous work by suggesting that the observed abnormalities may a) represent a pervasive disturbance characteristic of a majority of RSNs, and b) be largely independent of cocaine dependence status.
4.1. Interpretations of spectral abnormalities in offenders
An understanding of the role of low- and high-frequency activity during rest remains in its infancy; however, several interpretations have been offered. The traditional view posits low-frequency activity during rest as a marker of neural efficiency, and high-frequency activity during rest as indicative of increased ‘neural noise’ (Biswal et al., 1995). In line with this view, the decreased LFPR levels in both offender groups may be interpreted as indicative of neural inefficiencies. Importantly, our results extend previous findings by suggesting that disruptions in offenders are not network-specific, but rather extend broadly to a majority of rest-related neural activity disruptions.
Other research, relying more on task-related activity (i.e. non-rest), suggests that high-frequency activity may play an active, functional role in network dynamics (see Baria et al., 2011; Craig et al., 2017; Gohel & Biswal, 2015). For instance, Baria and colleagues (2011) reported that high-frequency activity often related to increased BOLD response within cortical regions involved in higher-level cognitive processes (i.e. OFC, temporal cortex). In contrast, the low-frequency activity often related to BOLD response within subcortical regions involved in lower-level processes (i.e. posterior precuneus, posterior cingulate). Moreover, Craig and colleagues (2017) observed that high-frequency activity in the DMN was strongly engaged during cognitively demanding tasks. These results suggest that in the DMN, at least, high-frequency activity may play an as-yet-undetermined functional role (Craig et al., 2017). Though speculative, through this lens, our results could suggest the increased engagement of resource-demanding activity during rest in offenders.
Interestingly, some research has linked variation in spectral activity to variation in the synchrony/coherence across disparate neural regions (Anderson et al., 2014; Biswal et al., 1995; Di et al., 2013). For instance, Anderson and colleagues (2014) found a positive relationship between low-frequency amplitudes and local connectivity across the brain. Similarly, low-frequency fluctuations have been associated with connectivity within the motor network during rest (Biswal et al., 1995) and ALFF values (calculated between 0.01 and 0.08 Hz) were strongly related to connectivity strength within and between various RSNs/ROIs (Di et al., 2013). One possibility then is that our result of altered power dynamics in offenders could underpin disruptions in functional connectivity. This interpretation is consistent with numerous reports of connectivity disruptions in offenders (Contreras-Rodríguez et al., 2015; Hoppenbrouwers et al., 2014; Jiang et al., 2017; Leutgeb et al., 2016) and cocaine users (Geng et al., 2017; Ide et al., 2016; Martins et al., 2018; McCarthy et al., 2017; Prichep et al., 2002). Hence, our results demonstrating that both offender groups present decreased LFPR in the DMN, ECN, and language networks in comparison to non-offenders could be indicative of functional connectivity disruptions in these networks.
4.2. Implications for Dependence
Interestingly, both offender samples showed similar oscillatory differences compared to the non-offender controls; moreover, no between-group differences were observed between the Cocaine-Dependent and Non-Dependent Offenders. Thus, all offenders appeared to show common atypical neural oscillations that were largely independent of their drug dependence status. While these results could be seen as running somewhat counter to reports of spectral disruptions in cocaine-dependent (Ide et al., 2016, 2014) and heroin-dependent (Jiang et al., 2011; Wang et al., 2013) individuals, it should be noted that these previous studies were conducted within non-offender samples (and showed a combination of increased and decreased fALFF levels). This distinction may be important, should an aspect of the offender lifestyle itself underpin the observed spectral abnormalities. For instance, we note a handful of studies that have connected neural/cognitive abnormalities to environmental factors such as early life adversity (Dismukes et al., 2015; Kolla et al., 2014), and incarceration itself (Gendreau et al., 1972; Johnson et al., 2015; Umbach et al., 2018). It would be interesting for future research to evaluate the effects of these environmental factors on spectral integrity and/or other neural features.
The results from the present findings will require additional confirmation, optimally with larger samples of participants. However, if the results hold, it would be interesting to consider the potential for targeting abnormal oscillatory patterns in offender populations as treatment/rehabilitative targets. To our knowledge, no fMRI-based power spectra treatment protocols have been tested within offending populations, however, an EEG-based protocol has shown preliminary efficacy reducing aggression and impulsivity in violent offenders through regulation of frequency dynamics (Konicar et al., 2015). Clearly only so much should be made of this at present; however, the finding does serve as an early demonstration that oscillatory disruptions can be remedied, and that this change can have an impact on downstream behavioral/cognitive constructs in offenders.
4.3. Influence of LCU and/or PCL-R scores on spectral abnormalities in offenders
While we identified no differences in spectral dysfunction between the Dependent and Non-Dependent offender groups, the length of lifetime cocaine use (but not interview-based PCL-R scores) was negatively predictive of spectral dysfunction within the Dependent group. One possibility then is that it is not dependence status, but rather the lifetime drug burden, that is most relevant to the degree of spectral dysfunction in offenders. These findings partially replicate a recent report regarding the detrimental influences of drug use on spectral integrity in antisocial adolescents (Thijssen et al., 2017), but serve as the first demonstration of this effect within adult offenders. In contrast, neither LCU nor PCL-R scores predicted the magnitude of spectral dysfunction in the Non-Dependent group. Given the restricted range of LCU in the Non-Dependent group, it would be premature to read too much into these group differences. However, one possibility deserving of future consideration is that drug use severity may serve as an important predictor of dysfunction only after it reaches a certain threshold, whereas other (as of yet unidentified) factors (e.g. personality traits, early life adversity, length of incarceration) could serve more explanatory roles in individuals with less substance use/abuse problems. Consistent with this interpretation, supplementary analyses indicated that some metrics of noncocaine drug use (ie. ‘minor’ and ‘total’ drug use), which the Non-Dependent group did show measurable levels of, did correlate with LFPR. At the least, these results further highlight the need for increased work that treats the offender population as a heterogenous group, with unique, if overlapping, risk factors.
5. Limitations
Several limitations are important to note. First, as the highest identifiable frequency in a power spectrum is equal to ½ the sampling rate (Weik, 2001), the upper-bound limit for the observable frequencies of the time series was limited to < 0.25 Hz given the 2 sec TR. However, high-frequency activity has been shown to contribute to network activity up to 1.4 Hz when using a shorter TR of less than 500 ms (Boubela et al., 2013). A faster TR should be used in future work to investigate the contribution of all ranges of high-frequency activity to resting-state activity in offenders. Second, while our reliance on male-only data will provide the greatest comparison to the extant literature, generalization to female offenders should be undertaken with caution. Particularly given reports of significant differences between male/female offenders (Colins et al., 2017; Rogstad & Rogers, 2008), a future evaluation within a sample of offending women will be crucial. Third, in light of the non-significant effects with PCL-R scores, we should note that our assessment of psychopathy entailed an interview-only process that did not include subsequent file review. While this assessment process has been previously accepted (e.g. Forth et al., 1996; Kosson et al., 1997), and has elicited valid, significant findings in several previous imaging studies (Denomme et al., 2018, 2020; Arbuckle & Shane, 2016; Shane & Groat, 2018), it does not follow official PCL-R scoring guidelines (Hare, 1991). Thus, the possibility that differences in scoring methodologies are responsible for the null findings should not be completely overlooked. Finally, we note that participants’ LCU scores correlated moderately-to-highly with their age in our sample. This is hardly unexpected, however, the inherent collinearity between substance use burden and age does complicate the ability to dissect their influence on rest-related neural activity (see supplementary analyses for results that control for age, but these should be interpreted with caution given due to this collinearity). That said, the fact that LCU only correlated with LFPR in the Dependent group (where LCU variance was sufficient to reliably interrogate the effect) suggests that LCU, and not age, wielded the important influence in our dataset.
Supplementary Material
Highlights.
Offenders present lower whole-brain rest-related low-frequency power ratio (LFPR) compared to non-offenders
Offenders present increased high- and decreased low-frequency power in various RSNs
LFPR in cocaine-dependent offenders correlates with lifetime years of cocaine use
Dependence status does not influence power spectra profiles in offenders
Oscillatory abnormalities could serve as a neurobiological marker of offender status
Acknowledgements
This work was supported by National Institute on Drug Abuse grant (R01DA026932) to M.S. and a CGS-SSHRC doctoral award to I.S. Funding agencies did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. We would like to acknowledge Mr. Ben Wasserott, as well as Ms. Roberta Chavez and all of the staff at the Program Evaluation Services at the Center on Alcoholism, Substance Abuse, and Addictions (CASAA) at the University of New Mexico, for recruitment of participants and collection of study data.
Footnotes
Conflict of interest statement
None of the material included in our research article has been published previously, and it is not under consideration in another journal. All the coauthors have contributed significantly to this study and agree on the content and authorship of the manuscript. Isabelle Simard conceptualized and designed the study, analyzed and interpreted the data, as well as drafted the article and gave final approval of the version hereby submitted. William J. Denomme and Matthew S. Shane both contributed to the analysis and interpretation of data, critically revised the manuscript and gave final approval of the version submitted, additionally Dr. Shane acquired the data. None of us have conflicts of interest related to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, & Silva RF (2011). A baseline for the multivariate comparison of resting-state networks, 5, 2 10.3389/fnsys.2011.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle NL, & Shane MS (2017). Up-regulation of neural indicators of empathic concern in an offender population. Social neuroscience, 12(4), 386–390. 10.1080/17470919.2016.1179669 [DOI] [PubMed] [Google Scholar]
- Baria AT, Baliki MN, Parrish T, & Apkarian AV (2011). Anatomical and functional assemblies of brain BOLD oscillations. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 31(21), 7910–7919. 10.1523/JNEUROSCI.1296-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrós-Loscertales A, Garavan H, Bustamante JC, Ventura-Campos N, Llopis JJ, Belloch V, … Ávila C (2011). Reduced striatal volume in cocaine-dependent patients. Neuroimage, 56(3), 1021–1026. 10.1016/j.neuroimage.2011.02.035 [DOI] [PubMed] [Google Scholar]
- Beard CL, Schmitz JM, Soder HE, Suchting R, Yoon JH, Hasan KM, … Lane SD (2019). Regional differences in white matter integrity in stimulant use disorders: A meta-analysis of diffusion tensor imaging studies. Drug and Alcohol Dependence, 201, 29–31. 10.1016/j.drugalcdep.2019.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, DeYoe EA, & Hyde JS (1996). Reduction of physiological fluctuations in fMRI using digital filters. Magnetic Resonance in Medicine, 35(1), 107–113. 10.1002/mrm.1910350114 [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, & Hyde JS (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541. 10.1002/mrm.1910340409 [DOI] [PubMed] [Google Scholar]
- Blair RJR (2010). Neuroimaging of psychopathy and antisocial behavior: a targeted review. Current Psychiatry Reports, 12(1), 76–82. 10.1007/s11920-009-0086-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boubela RN, Kalcher K, Huf W, Kronnerwetter C, Filzmoser P, & Moser E (2013). Beyond Noise: Using Temporal ICA to Extract Meaningful Information from High-Frequency fMRI Signal Fluctuations during Rest. Frontiers in Human Neuroscience, 7, 168 10.3389/fnhum.2013.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, Felmingham KL, Liddell B, Das P, & Malhi GS (2016). Association of FKBP5 polymorphisms and resting-state activity in a frontotemporal-parietal network. Translational Psychiatry, 6(10), e925 10.1038/tp.2016.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, & Draguhn A (2004). Neuronal oscillations in cortical networks. Science, 304(5679), 1926–1929. 10.1126/science.1099745 [DOI] [PubMed] [Google Scholar]
- Cauda F, Sacco K, Duca S, Cocito D, D’Agata F, Geminiani GC, & Canavero S (2009). Altered resting state in diabetic neuropathic pain. PLoS ONE, 4(2), e4542 10.1371/journal.pone.0004542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Elton A, Kennedy AP, Young J, Smitherman S, James GA, & Kilts CD (2013). Altered functional connectivity of the insular cortex across prefrontal networks in cocaine addiction. Psychiatry Research: Neuroimaging, 213(1), 39–46. 10.1016/j.pscychresns.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatworthy J, Hankins M, Buick D, Weinman J, & Horne R (2007). Cluster analysis in illness perception research: A Monte Carlo study to identify the most appropriate method. Psychology and Health, 22(2), 123–142. 10.1080/14768320600774496 [DOI] [Google Scholar]
- Colins OF, Fanti KA, Salekin RT, & Andershed H (2017). Psychopathic Personality in the General Population: Differences and Similarities Across Gender. Journal of Personality Disorders, 31(1), 49–74. 10.1521/pedi_2016_30_237 [DOI] [PubMed] [Google Scholar]
- Craig MM, Manktelow AE, Sahakian BJ, Menon DK, & Stamatakis EA (2017). Spectral Diversity in Default Mode Network Connectivity Reflects Behavioral State. Journal of Cognitive Neuroscience, 30(4), 526–539. 10.1162/jocn_a_01213 [DOI] [PubMed] [Google Scholar]
- Dalmaijer ES, Nord CL, & Astle DE (2020). Statistical power for cluster analysis. arXiv preprint arXiv:2003.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denomme WJ, Simard I, & Shane MS (2018). Neuroimaging Metrics of Drug and Food Processing in Cocaine-Dependence, as a Function of Psychopathic Traits and Substance Use Severity. Frontiers in Human Neuroscience, 12, 350 10.3389/fnhum.2018.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denomme WJ, & Shane MS (2020). History of withdrawal modulates drug-and food-cue reactivity in cocaine dependent participants. Drug and Alcohol Dependence, 208, 107815 10.1016/j.drugalcdep.2019.107815 [DOI] [PubMed] [Google Scholar]
- DeRamus TP, Faghiri A, Iraji A, Agcaoglu O, Vergara VM, Fu Z, … & Wang YP (2020). Modular and state-relevant connectivity in high-frequency resting-state BOLD fMRI data: An independent component analysis. bioRxiv. 10.1101/2020.07.22.212720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dismukes AR, Johnson MM, Vitacco MJ, Iturri F, & Shirtcliff EA (2015). Coupling of the HPA and HPG axes in the context of early life adversity in incarcerated male adolescents. Developmental Psychobiology, 57(6), 705–718. 10.1002/dev.21231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolnicar S, Grün B, Leisch F, & Schmidt K (2014). Required sample sizes for data-driven market segmentation analyses in tourism. Journal of Travel Research, 53(3), 296–306. 10.1177/0047287513496475 [DOI] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JB (2002). Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (pp. 94–1). New York, NY, USA:: SCID-I/P. [Google Scholar]
- Forth AE, Brown SL, Hart SD & Hare RD (1996). The assessment of psychopathy in male and female noncriminals: Reliability and validity. Personality and Individual Differences, 20(5): 531–543. 10.1016/0191-8869(95)00221-9 [DOI] [Google Scholar]
- Freire L, Roche A, & Mangin JF (2002). What is the best similarity measure for motion correction in fMRI time series?. IEEE Transactions on Medical Imaging, 21(5), 470–484. 10.1109/TMI.2002.1009383 [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, & Calhoun VD (2007). Aberrant “default mode” functional connectivity in schizophrenia. American Journal of Psychiatry, 164(3), 450–457. 10.1176/ajp.2007.164.3.450 [DOI] [PubMed] [Google Scholar]
- Gendreau P, Freedman NL, Wilde GJ, & Scott GD (1972). Changes in EEG alpha frequency and evoked response latency during solitary confinement. Journal of Abnormal Psychology, 79(1), 54 10.1037/h0032339 [DOI] [PubMed] [Google Scholar]
- Gohel SR, & Biswal BB (2015). Functional Integration Between Brain Regions at Rest Occurs in Multiple-Frequency Bands. Brain Connectivity, 5(1), 23–34. 10.1089/brain.2013.0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Wang J, Zhao Z, Min B, Lu J, Li K, … Jia J (2011). Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: A resting-state fMRI study. NeuroImage, 55(1), 287–295. 10.1016/j.neuroimage.2010.11.059 [DOI] [PubMed] [Google Scholar]
- Hare RD (2003). The psychopathy checklist–Revised. Toronto, ON, 2003. [Google Scholar]
- Ide JS, Hu S, Zhang S, Mujica-Parodi LR, & Chiang-shan RL (2016). Power spectrum scale invariance as a neural marker of cocaine misuse and altered cognitive control. NeuroImage: Clinical, 11, 349–356. 10.1016/j.nicl.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Zhang S, Hu S, Sinha R, Mazure CM, & Chiang-shan RL (2014). Cerebral gray matter volumes and low-frequency fluctuation of BOLD signals in cocaine dependence: duration of use and gender difference. Drug and Alcohol Dependence, 134, 51–62. 10.1016/j.drugalcdep.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Qiu Y, Zhang X, Han L-J, Lv X-F, Li L, … Tian J (2011). Amplitude low-frequency oscillation abnormalities in the heroin users: a resting state fMRI study. Neuroimage, 57(1), 149–154. 10.1016/j.neuroimage.2011.04.004 [DOI] [PubMed] [Google Scholar]
- Johnson MM, Mikolajewski A, Shirtcliff EA, Eckel LA, & Taylor J (2015). The association between affective psychopathic traits, time incarcerated, and cortisol response to psychosocial stress. Hormones and Behavior, 72, 20–27. 10.1016/j.yhbeh.2015.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalcher K, Boubela RN, Huf W, Bartova L, Kronnerwetter C, Derntl B, … Moser E (2014). The spectral diversity of resting-state fluctuations in the human brain. PloS One, 9(4), e93375 10.1371/journal.pone.0093375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla NJ, Gregory S, Attard S, Blackwood N, & Hodgins S (2014). Disentangling possible effects of childhood physical abuse on gray matter changes in violent offenders with psychopathy. Psychiatry Research: Neuroimaging, 221(2), 123–126. 10.1016/j.pscychresns.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konicar L, Veit R, Eisenbarth H, Barth B, Tonin P, Strehl U, & Birbaumer N (2015). Brain self-regulation in criminal psychopaths. Scientific Reports, 5, 9426 10.1038/srep09426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosson DS, Steuerwald BL, Forth AE, & Kirkhart KJ (1997). A new method for assessing the interpersonal behavior of psychopathic individuals: Preliminary validation studies. Psychological Assessment, 9(2): 89 10.1037/1040-3590.9.2.89 [DOI] [Google Scholar]
- Kouri EM, Pope HG, Powell KF, Oliva PS, & Campbell C (1997). Drug use history and criminal behavior among 133 incarcerated men. The American Journal of Drug and Alcohol Abuse, 23(3), 413–419. 10.3109/00952999709016886 [DOI] [PubMed] [Google Scholar]
- Liu H, Liao J, Jiang W, & Wang W (2014). Changes in Low-Frequency Fluctuations in Patients with Antisocial Personality Disorder Revealed by Resting-State Functional MRI. PLOS ONE, 9(3), e89790 10.1371/journal.pone.0089790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Steinberg JL, Moeller FG, Johns SE, & Narayana PA (2015). Effect of cocaine dependence on brain connections: clinical implications. Expert Review of Neurotherapeutics, 15(11), 1307–1319. 10.1586/14737175.2015.1103183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, & Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–1239. 10.1016/S1053-8119(03)00169-1 [DOI] [PubMed] [Google Scholar]
- Malinen S, Vartiainen N, Hlushchuk Y, Koskinen M, Ramkumar P, Forss N, … Hari R (2010). Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proceedings of the National Academy of Sciences, 107(14), 6493–6497. 10.1073/pnas.1001504107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, … Argeriou M (1992). The Fifth Edition of the Addiction Severity Index. Journal of Substance Abuse Treatment, 9(3), 199–213. 10.1016/0740-5472(92)90062-S [DOI] [PubMed] [Google Scholar]
- Otti A, Guendel H, Wohlschlager A, Zimmer C, & Noll-Hussong M (2013). Frequency shifts in the anterior default mode network and the salience network in chronic pain disorder. BMC Psychiatry, 13(1), 84 10.1186/1471-244X-13-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters RH, Greenbaum PE, Edens JF, Carter CR, & Ortiz MM (1998). Prevalence of DSM-IV substance abuse and dependence disorders among prison inmates. The American Journal of Drug and Alcohol Abuse, 24(4), 573–587. 10.3109/00952999809019608 [DOI] [PubMed] [Google Scholar]
- Pizoli CE, Shah MN, Snyder AZ, Shimony JS, Limbrick DD, Raichle ME, … Smyth MD (2011). Resting-state activity in development and maintenance of normal brain function. Proceedings of the National Academy of Sciences, 108(28), 11638–11643. 10.1073/pnas.1109144108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppl TB, Donges MR, Mokros A, Rupprecht R, Fox PT, Laird AR, … & Eickhoff SB (2019). A view behind the mask of sanity: meta-analysis of aberrant brain activity in psychopaths. Molecular psychiatry, 24(3), 463–470. 10.1038/s41380-018-0122-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogstad JE, & Rogers R (2008). Gender differences in contributions of emotion to psychopathy and antisocial personality disorder. Clinical Psychology Review, 28(8), 1472–1484. 10.1016/j.cpr.2008.09.004 [DOI] [PubMed] [Google Scholar]
- Salvador R, Martinez A, Pomarol-Clotet E, Gomar J, Vila F, Sarro S, … Bullmore E (2008). A simple view of the brain through a frequency-specific functional connectivity measure. NeuroImage, 39(1), 279–289. 10.1016/j.neuroimage.2007.08.018 [DOI] [PubMed] [Google Scholar]
- Sambataro F, Visintin E, Doerig N, Brakowski J, Holtforth MG, Seifritz E, & Spinelli S (2017). Altered dynamics of brain connectivity in major depressive disorder at-rest and during task performance. Psychiatry Research: Neuroimaging, 259, 1–9. 10.1016/j.pscychresns.2016.11.001 [DOI] [PubMed] [Google Scholar]
- Shane MS, & Groat LL (2018). Capacity for upregulation of emotional processing in psychopathy: all you have to do is ask. Social cognitive and affective neuroscience, 13(11), 1163–1176. 10.1093/scan/nsy088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, & Greicius MD (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex, 22(1), 158–165. 10.1093/cercor/bhr099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, & Newman JP (1990). Alcohol and drug abuse-dependence disorders in psychopathic and nonpsychopathic criminal offenders. Journal of Abnormal Psychology, 99(4), 430 10.1037/0021-843X.99.4.430 [DOI] [PubMed] [Google Scholar]
- Thijssen S, Rashid B, Gopal S, Nyalakanti P, Calhoun VD, & Kiehl KA (2017). Regular cannabis and alcohol use is associated with resting-state time course power spectra in incarcerated adolescents. Drug and Alcohol Dependence, 178, 492–500. 10.1016/j.drugalcdep.2017.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WH, & Fransson P (2015). The frequency dimension of fMRI dynamic connectivity: Network connectivity, functional hubs and integration in the resting brain. NeuroImage, 121, 227–242. 10.1016/j.neuroimage.2015.07.022 [DOI] [PubMed] [Google Scholar]
- Umbach R, Berryessa C, & Raine A (2015). Brain Imaging Research on Psychopathy: Implications for Punishment, Prediction, and Treatment in Youth and Adults Brain Imaging Research on Psychopathy: Implications for Punishment. Journal of Criminal Justice, 43(4), 295–306. [Google Scholar]
- Umbach R, Raine A, & Leonard NR (2018). Cognitive decline as a result of incarceration and the effects of a CBT/MT intervention: A cluster-randomized controlled trial. Criminal Justice and Behavior, 45(1), 31–55. 10.1177/0093854817736345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FA, & Anthony JC (2002). From first drug use to drug dependence: Developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology, 26(4), 479–488. 10.1016/S0893-133X(01)00367-0 [DOI] [PubMed] [Google Scholar]
- Wang L, Kong Q, Li K, Su Y, Zeng Y, Zhang Q, … Yu X (2016). Frequency-dependent changes in amplitude of low-frequency oscillations in depression: A resting-state fMRI study. Neuroscience Letters, 614, 105–111. 10.1016/j.neulet.2016.01.012 [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhu J, Li Q, Li W, Wu N, Zheng Y, … Wang W (2013). Altered fronto-striatal and fronto-cerebellar circuits in heroin-dependent individuals: a resting-state FMRI study. PLoS One, 8(3), e58098 10.1371/journal.pone.0058098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weik MH (2001). Nyquist sampling rate In Computer Science and Communications Dictionary (p. 1127). Boston, MA: Springer US; 10.1007/1-4020-0613-6_12653 [DOI] [Google Scholar]
- Xu K, Liu H, Li H, Tang Y, Womer F, Jiang X, … Fan G (2014). Amplitude of low-frequency fluctuations in bipolar disorder: A resting state fMRI study. Journal of Affective Disorders, 152, 237–242. 10.1016/j.jad.2013.09.017 [DOI] [PubMed] [Google Scholar]
- Yaesoubi M, Miller RL, & Calhoun VD (2017). Time-varying spectral power of resting-state fMRI networks reveal cross-frequency dependence in dynamic connectivity. PloS One, 12(2), e0171647 10.1371/journal.pone.0171647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Sui J, Liu J, Plis SM, Kiehl KA, Pearlson G, & Calhoun VD (2013). Disrupted correlation between low frequency power and connectivity strength of resting state brain networks in schizophrenia. Schizophrenia research, 143(1), 165–171. 10.1016/j.schres.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Chien YL, Wang HLS, Liu CM, Liu CC, Hwang TJ, … Tseng WYI (2014). Frequency-specific alternations in the amplitude of low-frequency fluctuations in schizophrenia. Human Brain Mapping, 35(2), 627–637. 10.1002/hbm.22203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Yao N, Fairchild G, Zhang Y, & Wang X (2015). Altered Hemodynamic Activity in Conduct Disorder: A Resting-State fMRI Investigation. PLOS ONE, 10(3), e0122750 10.1371/journal.pone.0122750 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.