Abstract
The potent estrogen 17β-estradiol (E2) is known to enhance hippocampal memory and plasticity, however the molecular mechanisms underlying these effects remain unclear. Brain derived neurotrophic factor (BDNF) and its receptor tropomyosin receptor kinase B (TrkB) are regulated by E2, but the potential mechanistic roles of neurotrophic signaling in E2-induced enhancement of memory are not well understood. Here, we examined the effects of hippocampal TrkB signaling on E2-induced enhancement of memory consolidation in the object placement and recognition tasks. Bilateral infusion of the TrkB antagonist ANA-12 into the dorsal hippocampus of ovariectomized female mice blocked E2-induced enhancement of memory consolidation, supporting a role for TrkB-mediated signaling in estrogenic regulation of memory. Although dorsal hippocampal E2 infusion increased levels of phospho-TrkB and mature BDNF (mBDNF) in the dorsal hippocampus within 4–6 hours, E2-induced increases in hippocampal mBDNF expression were not required for hippocampal TrkB activation and were not inhibited by TrkB antagonism. Thus, E2 regulates TrkB signaling to facilitate memory consolidation in a manner independent of mBDNF expression. Together these results provide the first direct evidence that E2 modulation of hippocampal TrkB signaling is required for its beneficial effects on memory consolidation and provide additional characterization of the ways in which TrkB/BDNF signaling is regulated by E2 in the hippocampus.
Keywords: estrogen, hippocampus, neurotrophin, object recognition, spatial memory, mouse
Introduction
17β-estradiol (E2), a potent estrogen, powerfully modulates hippocampal plasticity and memory. E2 influences the structure and function of hippocampal pyramidal neurons, increasing dendritic spine density and synapse number, thereby leading to potentiated excitatory neurotransmission (Luine and Frankfurt, 2013; Woolley, 1998). These changes are associated with improved performance on hippocampal-dependent memory tasks, including object placement and object recognition tasks (Jacome et al., 2016; Kim et al., 2019; Phan et al., 2012). Although progress has been made in identifying molecular mechanisms that mediate effects of E2 in the hippocampus (Boulware et al., 2013; Fernandez et al., 2008; Fortress et al., 2013; Kramár et al., 2013; Phan et al., 2012), the field still lacks a comprehensive understanding of the pathways critical for E2-induced memory enhancement. In particular, accumulating research has suggested that BDNF/TrkB signaling may play a role, but few studies have directly tested this hypothesis.
BDNF is a member of the neurotrophin family of small, secreted peptides and is critically involved in hippocampal synaptic plasticity. Activity-dependent synthesis and release of BDNF drives long-term potentiation, protein synthesis, and structural remodeling in hippocampal synapses (Leal et al., 2015; Park and Poo, 2013) and is required for hippocampal memory (Bekinschtein et al., 2007; Heldt et al., 2007; Lee et al., 2004). BDNF exerts its trophic effects via the receptor TrkB, a tyrosine kinase receptor that activates multiple intracellular signaling pathways involved in neuroplasticity, including ERK, PI3K, and PLC (Park and Poo, 2013; Sasi et al., 2017). Interestingly, activation of these same pathways is also a hallmark of rapid, membrane-initiated E2 signaling. E2 treatment rapidly activates hippocampal ERK (Fernandez et al., 2008; Kuroki et al., 2000; Wu et al., 2005), PI3K/Akt (Fan et al., 2010; Hasegawa et al., 2015; Yang et al., 2010), and PLC signaling (Maruyama et al., 2013), and both ERK and PI3K signaling in the dorsal hippocampus are necessary for E2 to enhance memory in ovariectomized (OVX) mice (Fan et al., 2010; Fernandez et al., 2008; Fortress et al., 2013). The notable overlap in the mechanisms through which E2 and BDNF/TrkB influence hippocampal plasticity and memory has led many to question whether BDNF and TrkB are mediators of rapid E2 action in this region (Luine and Frankfurt, 2013; Scharfman and MacLusky, 2005; Srivastava et al., 2013).
E2 has been found to activate both BDNF and TrkB signaling in the rodent hippocampus, which lends considerable support to this hypothesis. E2 can regulate BDNF on multiple levels, including increasing gene expression through classical genomic activity at an ERE-like site on the Bdnf gene (Sohrabji et al., 1995) and epigenetic modification of the Bdnf gene (Fortress et al., 2014), and by stimulation of BDNF release (Briz et al., 2015; Sato et al., 2007). OVX of female rodents dramatically reduces BDNF levels in the hippocampus, particularly in the CA3 and dentate gyrus regions, and E2 replacement restores this expression (Berchtold et al., 2001; Liu et al., 2001; Pan et al., 2010; Scharfman et al., 2007; Singh et al., 1995). E2 also regulates TrkB activity, as measured by increases in TrkB phosphorylation. In gonadally intact female mice, levels of phospho-TrkB in the hippocampus are greatest during proestrus (Spencer et al., 2008; Spencer-Segal et al., 2011), the phase of the estrous cycle with the highest circulating levels of E2, and exogenous E2 treatment increases phospho-TrkB in both hippocampal slices (Kramár et al., 2013; Wang et al., 2016) and the hippocampus of OVX mice (Spencer-Segal et al., 2012). Whether E2-induced increases in hippocampal TrkB activation are a direct result of enhanced BDNF expression or activity is unclear, as some studies suggest that E2 can activate TrkB in a BDNF-independent manner (Spencer et al., 2008; Wang et al., 2016).
Despite clear connections between E2 and BDNF/TrkB in the hippocampus, few studies have directly examined whether BDNF or TrkB signaling play a mechanistic role in the effects of E2 in this region. In hippocampal culture, manipulation of BDNF or TrkB signaling inhibits E2-induced dendritic spine formation (Murphy et al., 1998; Sato et al., 2007), and in hippocampal slices from intact female rats, a TrkB inhibitor reduces CA3 hyperexcitability that occurs during proestrus (Scharfman et al., 2003). More recently, it has been shown that TrkB is required for E2 improvement of spatial memory. In OVX rats, systemic injection of the TrkB antagonist ANA-12 blocked the memory enhancing effects of long-term E2 replacement on an object placement task (Bohm-Levine et al., 2020). Previous work from our lab has found that a single infusion of E2 into the dorsal hippocampus (DH) given immediately after training improves spatial and object recognition memory consolidation through acute activation of cell-signaling cascades in the DH during the 1–3 hour consolidation period (Boulware et al., 2013; Fan et al., 2010; Fernandez et al., 2008; Fortress et al., 2014, 2013). Whether rapid local activation of TrkB in the DH underlies E2 enhancement of hippocampal memory consolidation remains unknown. Here, we used hippocampal-dependent object memory tasks to determine if TrkB in the DH is required for acute E2 infusion to enhance object recognition and spatial memory consolidation. OVX mice received bilateral DH infusions of ANA-12 and intracerebroventricular (ICV) infusion of E2 immediately following training on object placement or recognition tasks. The effects of E2 and ANA-12 on TrkB activation and BDNF expression in the DH were also examined. Our findings suggest that TrkB is a critical mediator of the enhancing effects of E2 on memory consolidation and that E2-induced TrkB activation is independent of changes to hippocampal BDNF expression.
Methods
Subjects
All subjects were female C57BL/6 mice obtained from Taconic Biosciences at 9 weeks of age. Mice were kept on a 12:12 light-dark cycle with food and water ad libitum and all procedures were conducted between 9:00 A.M. and 6:00 P.M. Mice were housed in groups of up to 5 prior to surgery, after which they were singly housed. All procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin-Milwaukee.
Surgeries
At 10 weeks of age, mice were bilaterally ovariectomized (OVX) and implanted with an indwelling guide cannula targeting the dorsal hippocampus (DH) as described previously (Boulware et al., 2013; Fortress et al., 2013). Mice were placed into a stereotaxic apparatus and anesthetized with 5% isoflurane in oxygen, then maintained at 2–3% isoflurane throughout the surgery. Bilateral OVX was performed first, and then guide cannulae (C232G; 22 gauge; Plastics One) were positioned at coordinates for the DH alone (−1.7 mm AP, +/− 1.5 mm ML, −2.3 mm DV) or the DH and dorsal third ventricle (intracerebroventricular (ICV); −0.9 mm AP, 0.0 mm ML, −.2.3 mm DV). Cannulae were implanted and fixed with dental cement, which also served to close the wound. Dummy cannulae (C232DC, Plastics One) were inserted to keep the guide cannulae clear throughout experiments. Cannula placements were verified visually during tissue dissection. Following surgery, mice were given a minimum of one week to recover before beginning behavioral testing.
Drugs and infusions
For intracranial infusions, mice were gently restrained, dummy cannulae were removed, and an infusion cannula (C313l; DH: 28 gauge, extending 0.8 mm beyond the 1.5 mm guide; ICV: 28 gauge, extending 1.0 mm beyond the 1.8 mm guide; Plastics One) attached to PE50 polyethylene tubing mounted on a 10 µl Hamilton syringe was inserted. Infusions were controlled by a microinfusion pump (KDS Legato 180, KD Scientific) and administered at a rate of 0.5 µl/min into the DH or 1 µl/2 min into the ICV as described previously (Boulware et al., 2013; Fortress et al., 2013). The infusion cannula was left in place for 1 min following infusion to allow for drug diffusion. In triple infusion experiments using both ANA-12 and E2 infusion, ANA-12 was first infused bilaterally into the DH, followed immediately by E2 infusion into the ICV. This triple infusion protocol was used to prevent tissue damage from multiple infusions into the DH in rapid succession (Boulware et al., 2013; Fernandez et al., 2008; Fortress et al., 2013; Zhao et al., 2010).
The TrkB inhibitor, ANA-12 (Tocris Bioscience), was dissolved in 100% DMSO for a stock solution of 4 µg/µl and stored at −20°C. On the day of the experiment, dilutions at concentrations of 1 and 2 µg/µl in 80% DMSO were prepared. ANA-12 was infused into the DH at doses of 0.5, 1, or 2 µg/hemisphere with 80% DMSO serving as the vehicle control. Cyclodextrin-encapsulated E2 (Sigma-Aldrich) was dissolved in 0.9% sterile saline at a concentration of 10 µg/µl and infused at a dose of 5 µg/hemisphere into the DH or 10 µg ICV. For the vehicle solution, 2-hydroxypropyl-β-cyclodextrin (HBC; Sigma-Aldrich) was dissolved in 0.9% sterile saline at the same concentration as the encapsulated E2 solution.
Memory testing
Object recognition (OR) and object placement (OP) tasks were used to measure object recognition and spatial memory as described previously (Boulware et al., 2013; Fernandez et al., 2008; Fortress et al., 2013; Kim et al., 2016). Because all mice completed both tasks, the order of training was counterbalanced within each group and a minimum of two weeks separated training on each task to minimize the potential influences of test order or previous testing on the results (Fig. 1A). Prior to the start of behavioral training, mice were briefly handled for three days and then habituated to the empty testing arena for 5 min on each of two consecutive days. To habituate mice to objects, a Lego Duplo block was placed in each home cage on the second day of handling and remained in the cage until behavioral training began. On the day of behavioral training, mice were briefly rehabituated to the empty arena for 1 min, removed, and then placed back into the arena with two identical objects placed near the upper right and left corners. Mice were allowed to explore the objects until they reached a criterion of 30 sec of exploration time or until 20 min had elapsed. Exploration was manually scored with ANY-maze tracking software (Stoelting) and was counted when the mouse was immediately adjacent to an object with its front paws and/or nose directed at or touching the object. Mice that successfully reached the 30 sec criterion were immediately given drug infusions to target the memory consolidation period and then returned to their home cages.
Figure 1.
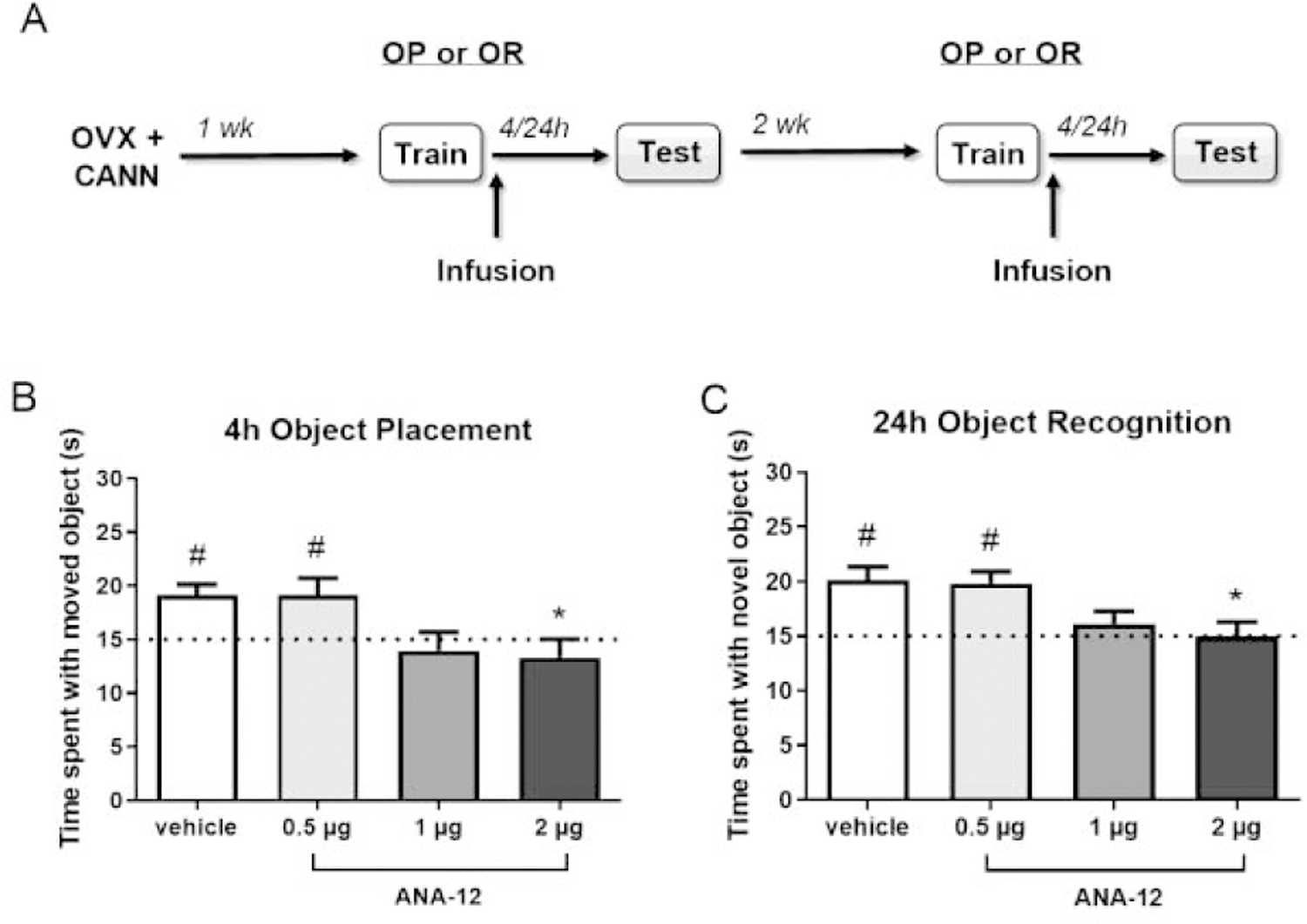
TrkB antagonism with 1 µg and 2 µg ANA-12, but not 0.5 µg ANA-12, blocks consolidation of object placement and object recognition memory. OVX female mice (n = 8–9/group) received bilateral DH infusion of vehicle or ANA-12 (0.5, 1, or 2 µg/hemisphere) immediately following object training (A). In an object placement test 4 hours later (B) or an object recognition test 24 hours later (C), mice receiving vehicle or 0.5 µg ANA-12 spent significantly more time with the moved or novel object than chance (dotted line at 15 s, #p < 0.05 relative to chance), whereas mice receiving 1 or 2 µg ANA-12 did not, suggesting impaired memory consolidation. Mice treated with 2 µg ANA-12 also spent significantly less time with the moved or novel object compared to vehicle treated animals (*p < 0.05). Error bars indicate mean ± SEM.
In the testing trial, one of the objects was either moved to a lower corner of the testing arena (OP) or replaced with a novel object (OR). Because mice inherently prefer novelty, those with intact memory for the training objects should spend more time than chance (15 sec) exploring the moved or novel objects. Memory was tested at timepoints previously established by our lab as ideal for observing either impairment or facilitation of memory consolidation (Boulware et al., 2013; Fortress et al., 2013; Kim et al., 2016). That is, to discern possible inhibitory effects of ANA-12 on memory, mice were tested using timepoints at which control mice demonstrate intact memory for the training objects (4 hr delay for OP, 24 hr delay for OR). To observe potential faciliatory effects of E2, mice were tested using timepoints at which control mice do not demonstrate intact memory (24 hr delay for OP, 48 hr delay for OR). As in training, mice were allowed to explore the objects during testing until they reached the 30-sec exploration criterion or until 20 min had elapsed. They were then returned to their home cages.
Tissue preparation and Western blot analysis
Because we previously showed that bilateral DH infusion of E2 increases pro-BDNF and mBDNF protein in the DH of middle-aged OVX mice 4 or 6 h later (Fortress et al., 2014), young behaviorally naïve OVX mice were first used to establish the time course of E2 effects on TrkB activation and mBDNF protein expression at this age. As in our previous study, tissue was collected 4 and 6 h after infusion. Next, to determine the influence of TrkB inhibition on E2-induced changes in TrkB and BDNF RNA and protein, the same mice that underwent behavioral testing for interactions between E2 and TrkB were used for PCR and Western blot analysis of the E2+ANA-12 interaction. These mice were euthanized two weeks following the completion of behavioral testing (Fig. 1A) to allow for any acute effects of previous infusion and behavioral training to dissipate prior to re-infusion and tissue collection. Tissue was collected 4 h, rather than 6 h, after infusion to capture potentially earlier changes in RNA expression that might precede alterations in protein levels. Mice received intracranial infusions as described above and were then cervically dislocated, decapitated, and brains were removed 4 or 6 hours later based on the time course of previously described E2/BDNF interactions (Fortress et al., 2014). The DH was rapidly dissected on ice and frozen at −80°C until homogenization. Cannula placements were visually verified during dissection and no missed placements were identified during the study. Western blotting was performed as described previously (Boulware et al., 2013; Fernandez et al., 2008; Fortress et al., 2014, 2013). Tissue was homogenized by sonication in a hypotonic lysis buffer (1:50 w/v) containing PMSF and EDTA-free protease inhibitor cocktail (ThermoFisher Scientific). Total protein concentrations were determined via Bradford assay and then proteins were separated on 4–15% TGX Stain-free precast gels (BioRad) and transferred to PVDF membranes. Membranes were blocked in 5% milk and then incubated with the following primary antibodies at 4°C overnight: phospho-TrkB (Tyr706, Signalway #11328, 1:1000), TrkB (Cell Signaling Technology #4603, 1:1000), BDNF (Abcam #108319; 1:1000), GAPDH (Cell Signaling Technology #5174, 1:10000). The next day, membranes were washed and incubated with an HRP conjugated secondary antibody (anti-rabbit IgG, Cell Signaling Technology, 1:5000) and developed using Clarity Max enhanced chemiluminescence substrate (Bio-Rad). Blots were imaged using a ChemiDoc MP gel imager (Bio-Rad) and densitometry analysis was performed using ImageLab software (Bio-Rad, Image Lab version 5.2). Lanes were manually defined and bands were detected automatically by the Image Lab software. The Lane Profile tool was used to make minor adjustments to bands and to subtract background. Phospho-TrkB blots were stripped and reprobed for total full-length TrkB (~120 kDa) for normalization. Mature BDNF (15 kDa) was normalized to GAPDH. All normalized proteins were expressed as a percentage relative to vehicle control.
RT-qPCR
Brain tissue was dissected as described above and stored in RNAlater (ThermoFisher Scientific) at −20°C until it was processed. RNA was prepared and quantified as described previously (Fortress et al., 2014; Zhao et al., 2010). Briefly, RNA was extracted using the Qiagen RNeasy Mini kit (Qiagen) following the manufacturer instructions, and cDNA samples were prepared from 1 µg of extracted RNA using the Bio-Rad iScript cDNA Synthesis kit (Bio-Rad). Real-time quantitative PCR (qPCR) was performed using SYBR Green master mix (Bio-Rad) on an Eppendorf Realplex 2 PCR System (Eppendorf). Predesigned and optimized RT2 qPCR Primer Assays (Qiagen) were used for analysis of total Bdnf (#PPM03006C), the TrkB gene Ntrk2 (#PPM04330A), and Gapdh (#PPM02946E; expression control). Results were analyzed using Realplex 2.2 software. Samples were run in triplicate and normalized to corresponding GAPDH values and the ΔΔCT method was used to calculate relative expression of each gene of interest.
Statistical Analysis
All statistical analyses were conducted with GraphPad Prism 7. All data were analyzed for outliers, defined by ±2 standard deviations from the mean, which were removed prior to additional analysis. To assess within-group learning effects, behavioral data were first analyzed with one sample t-tests to compare individual group performance to chance (15 sec). This analysis was used because time spent with the objects is not independent; time spent with one object necessarily reduces time spent with the other (Frick and Gresack, 2003). To assess between-group treatment effects, behavioral data were also analyzed with one- or two-way ANOVAs. For ANA-12 dose-response data, one-way ANOVAs were followed by post hoc Dunnett’s test comparing treatment doses to vehicle control. For E2/ANA-12 behavioral data, two-way ANOVAs (hormone × drug) were followed by planned post hoc comparisons using Fisher’s LSD test. Western blot data were analyzed using one- or two-way ANOVAs followed by post hoc Dunnett’s test or Tukey’s multiple comparison test, respectively. Significance was determined at p < 0.05.
Results
ANA-12 impairs hippocampal memory consolidation in a dose dependent manner
Because TrkB activity is involved in hippocampal memory consolidation (Bambah-Mukku et al., 2014; Blank et al., 2016; Minichiello et al., 1999), experiments testing the ability of the TrkB antagonist ANA-12 to inhibit E2-induced memory enhancement could be confounded by a general inhibitory effect of ANA-12 on memory consolidation. Therefore, we sought to determine a dose of ANA-12 that does not impair memory on its own for use in subsequent experiments with E2. Immediately after object training, OVX mice received bilateral DH infusion of vehicle or ANA-12 (0.5, 1, or 2 µg/hemisphere, Fig. 1A). To detect possible memory impairing effects of ANA-12, mice were tested 4 hrs later for OP and 24 hrs later for OR, which are time points at which vehicle-treated OVX mice show intact memory (Boulware et al., 2013; Kim et al., 2016). As expected, vehicle treated mice spent significantly more time than chance with the moved (t(7) = 4.01, p = 0.005, Fig. 1B) or novel (t(7) = 4.23, p = 0.004, Fig. 1C) objects during testing, indicating intact memory for the training objects. Both the 1 and 2 µg doses of ANA-12 blocked memory formation, as mice in these groups did not spend significantly more time than chance with the moved (1 µg: t(8) = 0.61, p = 0.56, 2 µg: t(8) = 0.98, p = 0.36) or novel objects (1 µg: t(8) = 1.0, p = 0.35, 2 µg: t(8) = 0.01, p = 0.99). In contrast, mice receiving 0.5 µg ANA-12 spent significantly more time with the moved (t(7) = 2.63, p = 0.030) and novel (t(7) = 4.41, p = 0.003) objects, indicating that this dose did not impair memory consolidation in either task. These within-group findings were generally supported by the results of between-group analyses. One-way ANOVAs revealed significant main effects of treatment for both the object placement (F(3, 31) = 4.15, p = 0.014) and object recognition tasks (F(3, 30) = 4.64, p = 0.009), with post hoc tests showing that mice treated with 2 µg ANA-12 spent significantly less time with the moved (p < 0.05) and novel (p < 0.05) objects than vehicle controls. Time to accumulate 30 seconds of object exploration during testing did not differ among treatment groups for either OP (vehicle: M = 482.9, SEM = 58.9; 0.5 µg: M = 523.1, SEM = 54.6; 1 µg: M = 536.4, SEM = 43.2; 2 µg: M = 567.4, SEM = 54.9) or OR (vehicle: M = 619.8, SEM = 81.9; 0.5 µg: M = 706.4, SEM = 77.9; 1 µg: M = 549.0, SEM = 47.5; 2 µg: M = 691.4, SEM = 79.8), as indicated by one-way ANOVA. Together, these data indicate that ANA-12 dose-dependently blocks memory consolidation, such that 2 µg impairs memory whereas 0.5 µg does not. These findings support an important role for TrkB signaling in mediating memory consolidation among OVX mice and establish a behaviorally sub-effective dose of ANA-12 for use in subsequent studies with E2.
ANA-12 blocks estradiol-induced enhancement of memory consolidation
To determine if TrkB signaling is necessary for E2 to enhance hippocampal memory consolidation, OVX mice received an ICV infusion of vehicle or E2 (10 µg) and a bilateral DH infusion of vehicle or 0.5 µg/hemisphere ANA-12 immediately following object training (Fig. 2A). To detect possible memory enhancing effects of E2, mice were tested 24 hrs later for OP and 48 hrs later for OR, which are time points at which vehicle-treated OVX mice exhibit impaired memory (Boulware et al., 2013; Kim et al., 2016). In both tasks, mice treated with vehicle+vehicle or vehicle+ANA-12 did not differ from chance in the time spent with the novel (vehicle+vehicle: t(14) = 0.47, p = 0.65, vehicle+ANA-12: t(9) = 1.81, p = 0.10) or moved objects (vehicle+vehicle: t(15) = 0.86, p = 0.41, vehicle+ANA-12: t(15) = 1.39, p = 0.18), whereas those treated with E2+vehicle demonstrated intact memory by spending significantly more time than chance with the moved (t(12) = 4.84, p = 0.0004, Fig. 2B) and novel objects (t(9) = 6.31, p = 0.0001, Fig. 2C). However, mice infused with both E2+ANA-12 did not spend more time than chance with the moved or novel objects in the object placement (t(9) = 1.82, p = 0.10) and object recognition tasks (t(15) = 1.39, p = 0.18), suggesting that ANA-12 blocked the memory-enhancing effects of E2. Further analysis by two-way (hormone × drug) ANOVA revealed a significant main effect of hormone for object placement (F(1,49) = 10.81, p = 0.002), and significant main effects of hormone (F(1, 52) = 17.44, p = 0.0001) and drug (F(1, 52) = 6.16, p = 0.016), as well as a marginal hormone × drug interaction (F(1, 52) = 3.90, p = 0.054), for object recognition. Planned post hoc comparisons showed that the E2+vehicle group spent significantly more time with the moved and novel object compared to the vehicle+vehicle group (OP: p = 0.0006, OR: p = 0.0002) and E2+ANA-12 group (OP: p = 0.042, OR: p = 0.004) in both tasks. Time to accumulate 30 seconds of object exploration did not differ among treatment groups in OR when compared by two-way ANOVA (vehicle+vehicle: M = 644.2, SEM = 58.0; E2+vehicle: M = 717.7, SEM = 68.1; vehicle+ANA-12: M = 626.2, SEM = 41.8; E2+ANA-12: M = 644.0, SEM = 58.9). For OP, E2-treated mice showed a small, but significant, increase in time to complete the task (vehicle+vehicle: M = 558.6, SEM = 44.2; E2+vehicle: M = 730.9, SEM = 45.8; vehicle+ANA-12: M = 580.7, SEM = 59.7; E2+ANA-12: M = 781.4, SEM = 66.7; main effect of hormone: F(1, 49) = 11.65, p = 0.001). However, this increase did not correlate with observed effects on memory consolidation, as the E2+vehicle and E2+ANA-12 groups did not differ in time to complete the task (p = 0.54). This, combined with the lack of effect on accumulation time for OR, suggests an incidental effect on this variable in OP that did not influence memory formation. Together, these results indicate that dorsal hippocampal TrkB activity is necessary for E2-induced enhancement of object recognition and spatial memory consolidation in OVX mice.
Figure 2.
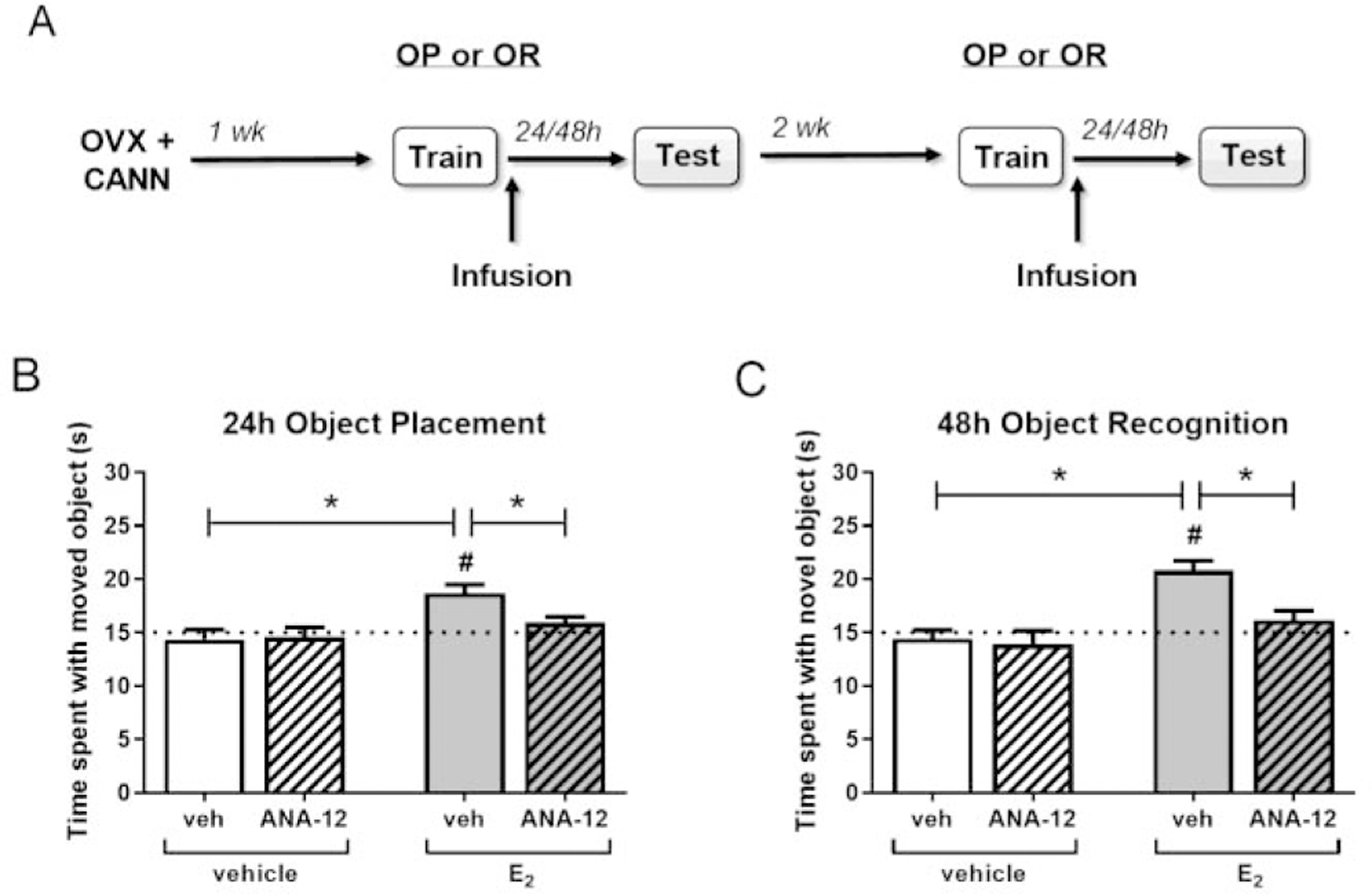
Estradiol enhancement of memory consolidation depends on hippocampal TrkB. OVX female mice (n = 10–16/group) received ICV infusion of vehicle or E2 (10 µg) and DH infusion of vehicle or ANA-12 (0.5 µg/hemisphere) immediately following object training (A). In an object placement test 24 hours later (B) or an object recognition test 48 hours later (C), mice infused with E2 + vehicle spent significantly more time with the moved or novel object than chance (dotted line at 15 s; #p < 0.05), and this effect was eliminated by ANA-12 treatment. Mice infused with E2 + vehicle also spent significantly more time with the moved or novel object compared to vehicle + vehicle or E2 + ANA-12 treated animals (*p < 0.05). Error bars indicate mean ± SEM.
Estradiol activates hippocampal TrkB signaling prior to increased expression of mBDNF protein
In female mice, E2 has been shown to increase hippocampal expression of both pro-BDNF and mature BDNF (Fortress et al., 2014; Gibbs, 1999; Scharfman et al., 2007; Singh et al., 1995; Sohrabji et al., 1995). E2 also increases levels of phosphorylated TrkB (Kramár et al., 2013; Spencer et al., 2008; Spencer-Segal et al., 2012, 2011; Wang et al., 2016), which is an indicator of TrkB activity. Changes in TrkB/BDNF expression and activity are often studied after long-term (>24 hrs) E2 exposure, however, evidence that E2 can increase both mature BDNF (mBDNF) protein (Fortress et al., 2014) and TrkB activation (Kramár et al., 2013; Sato et al., 2007) on more rapid timescales has also been reported. Whether TrkB activation influences this more rapid induction of mBDNF protein is unknown. Therefore, we next examined hippocampal TrkB phosphorylation and mBDNF protein expression following acute E2 treatment. Behaviorally naïve OVX mice were given bilateral DH infusion of vehicle or E2 (5 µg/hemisphere) and then the dorsal hippocampus was dissected 4 or 6 hours later (Fig. 3A), time points at which we have previously observed increased mBDNF expression following DH infusion of E2 (Fortress et al., 2014). Protein levels of full-length TrkB phosphorylated at Tyr706 (~120 kDa), total full-length TrkB (~120 kDa), and mBDNF (15 kDa) were assessed by Western blot (Fig. 3B). TrkB phosphorylation at Tyr706 positively correlates with tyrosine kinase activity of the receptor and phosphorylation at other sites, including Tyr515 and Tyr816 (Huang and McNamara, 2010; Segal et al., 1996), and can be modulated by OVX and E2 replacement (Hill et al., 2013, 2012). A significant main effect of E2 was found for phospho-TrkB expression (F(2, 31) = 3.90, p = 0.031, Fig. 3C), such that levels of phospho-TrkB were significantly higher than vehicle both 4 and 6 hrs after infusion (p < 0.05). A significant main effect of E2 for mBDNF expression was also observed (F(2, 32) = 11.49, p = 0.0002, Fig. 3D), but interestingly, mBDNF was increased only at 6 hrs post-treatment (p < 0.05). This difference in time courses suggests that E2-induced TrkB activation precedes increased levels of local mBDNF protein.
Figure 3.
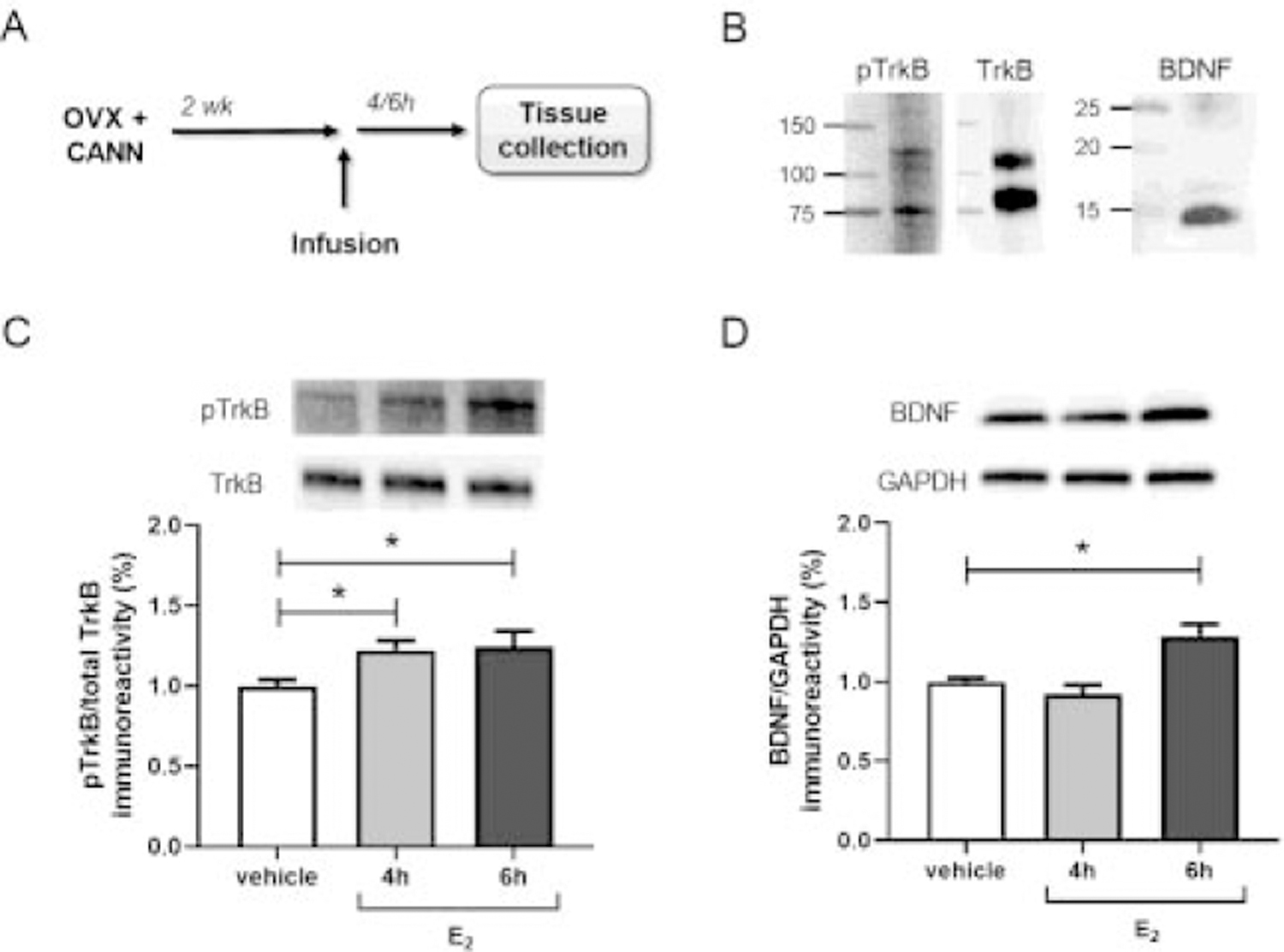
Estradiol activates hippocampal TrkB prior to increased mBDNF expression. TrkB phosphorylation and mBDNF expression were assessed 4 or 6 hours after bilateral DH infusion of vehicle or E2 (5 µg/hemisphere) in behaviorally naïve OVX female mice (A, n = 11–12/group). Full-length (~120 kDa) and truncated (~75 kDa) TrkB and monomeric mBDNF (15 kDa) were detected by Western blot (B). Levels of full-length phospho-TrkB (Tyr706) and full-length total TrkB were used for analysis. E2 infusion increased levels of phospho-TrkB both 4 and 6 hours later (C), but expression of mBDNF was only increased 6 hours following E2 treatment (D). *p < 0.05 compared to vehicle control. Error bars indicate mean ± SEM.
ANA-12 blocks estradiol-induced activation of TrkB and enhances BDNF expression
Finally, we assessed the interactive effect of E2 and ANA-12 on TrkB/BDNF signaling in the DH. OVX mice used two weeks prior in OR and OP behavioral tasks received ICV infusion of vehicle or E2 (10 µg) plus DH infusions of vehicle or ANA-12 (0.5 µg/hemisphere) and dorsal hippocampal tissue was collected 4 hours later (Fig. 4A). Analysis with two-way ANOVA revealed no effect of hormone (F(1, 23) = 0.17, p = 0.68), drug (F(1, 23) = 0.23, p = 0.64), or hormone × drug interaction (F(1, 23) = 0.33, p = 0.57) on Ntrk2 mRNA at this timepoint (Fig. 4B). However, E2 and ANA-12 interacted to influence phospho-TrkB levels (hormone × drug interaction: F(1, 29) = 10.05, p = 0.004). As seen in our previous experiment (Fortress et al., 2014), mice treated with E2+vehicle exhibited significantly increased levels of phospho-TrkB 4 hrs after treatment (p = 0.010), however, this effect was blocked by administration of ANA-12 (p = 0.020). For Bdnf mRNA, the main effect of hormone was significant (F(1,30) = 4.18, p = 0.049, Fig. 4B), such that transcript levels in E2-treated groups were increased relative to vehicle-treated groups, regardless of ANA-12 co-administration. For mBDNF protein, a significant main effect of E2 treatment (F(1,44) = 6.71, p = 0.013) and a marginal hormone × drug interaction (F(1,44) = 3.94, p = 0.053) were observed. Planned post hoc comparisons support that these effects were primarily driven by a combined effect of E2+ANA-12 treatment, with E2+ANA-12 treated mice expressing significantly higher levels of mBDNF compared to the vehicle+vehicle (p = 0.011), vehicle + ANA-12 (p = 0.017), and E2 + vehicle (p = 0.043) groups. Together, these results suggest that while ANA-12 treatment blocks E2-induced TrkB activation, it does not influence E2 regulation of Bdnf expression and may interact with E2 to accelerate increases in mBDNF protein.
Figure 4.
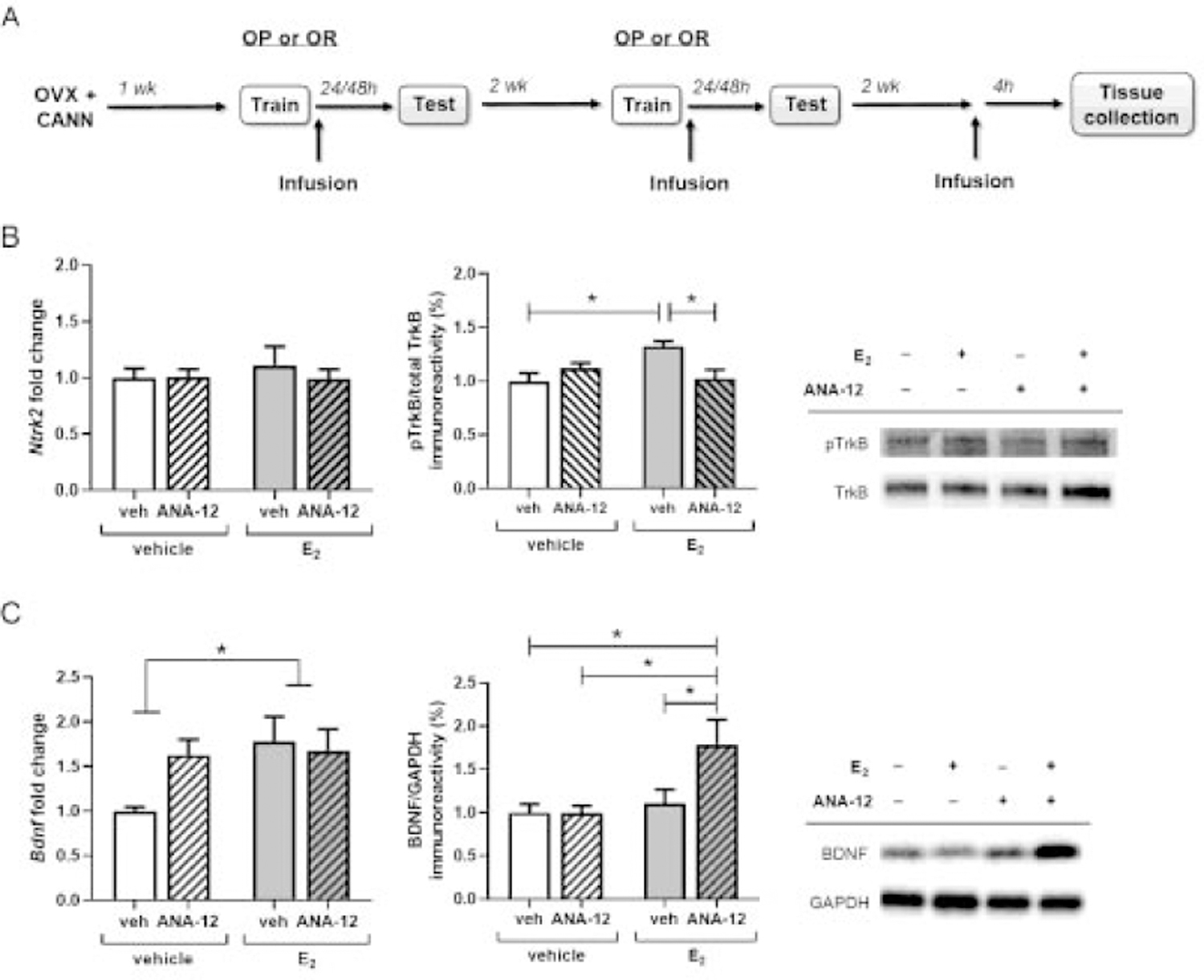
ANA-12 blocks estradiol-induced TrkB activation and accelerates increased mBDNF expression. TrkB and BDNF expression were assessed 4 hours after ICV infusion of vehicle or E2 (10 µg) and DH infusion of vehicle or ANA-12 (0.5 µg/hemisphere) in OVX female mice (A). Levels of TrkB mRNA were unaffected by E2 and ANA-12 treatment, but E2-induced TrkB phosphorylation was blocked by ANA-12 (B, n = 6–9/group). Levels of BDNF mRNA were significantly increased by E2 treatment and E2 and ANA-12 interacted to increase mBDNF protein at 4 hours post-infusion (C, n = 8–14/group). *p < 0.05 compared to vehicle control. Error bars indicate mean ± SEM.
Discussion
Previous literature supports a role for TrkB signaling in E2-induced enhancement of hippocampal memory, however, many of the details of this interaction remain unclear. Here, we examined the ability of E2 to facilitate hippocampal-dependent memory consolidation among OVX mice in the presence of the TrkB antagonist, ANA-12. We found that ANA-12 infusion into the DH blocked both the memory-enhancing effects of E2 in OVX mice and E2-induced phosphorylation of hippocampal TrkB, but had no influence on E2-induced expression of mBDNF. Further characterization of the relationship between E2, BDNF expression, and TrkB activation showed that TrkB phosphorylation precedes E2-induced increases in mBDNF protein. Together, these results demonstrate that activation of hippocampal TrkB signaling is a critical mediator of E2 enhancement of memory consolidation and provide insight into the complexity of E2 regulation of BDNF and TrkB.
TrkB activation is known to be important for hippocampal memory (Minichiello et al., 1999) and, in particular, appears to be critical for the process of memory consolidation (Bambah-Mukku et al., 2014; Bekinschtein et al., 2007; Lee et al., 2004). This putative role in consolidation has been studied primarily using inhibitory avoidance, a one-trial associative learning task. In this task, pharmacological blockade of TrkB signaling in the hippocampus during the consolidation period following training impairs memory during testing in male rodents (Bambah-Mukku et al., 2014; Blank et al., 2016; Kim et al., 2012). The present findings extend this work by showing similarly detrimental effects of ANA-12 on memory consolidation in OVX female mice during one-trial spatial and recognition memory tasks. Previous data from OVX rodents has shown that E2 facilitates consolidation in these tasks within 1–3 hours of testing (Fernandez et al., 2008; Frye et al., 2007; Walf et al., 2006). Here, immediate post-training infusion of ANA-12 into the dorsal hippocampus dose-dependently impaired object placement and object recognition memory in OVX mice, demonstrating a necessity for TrkB activation during the memory consolidation period. Together with previous studies, this finding highlights the importance of hippocampal TrkB in consolidation across multiple types of memory and irrespective of E2.
Next, we examined the interaction of hippocampal TrkB with E2 in promoting memory consolidation in these tasks. Immediate post-training infusion of E2 enhanced memory consolidation as previously observed (Boulware et al., 2013; Fernandez et al., 2008; Fortress et al., 2014; Kim et al., 2016). However, in mice that also had a post-training infusion of low dose ANA-12 into the hippocampus, this effect was abolished, demonstrating that E2 enhancement of memory consolidation on object placement and object recognition tasks depends on hippocampal TrkB activity. These results extend previous work correlating estrogenic modulation of hippocampal memory with BDNF/TrkB activity (Luine and Frankfurt, 2013; Pan et al., 2010; Scharfman et al., 2007) and the recent finding of TrkB’s involvement in long-term E2’s enhancement of spatial memory in OVX rats (Bohm-Levine et al., 2020) by demonstrating E2-induced enhancement of spatial and object recognition memory consolidation in OVX mice relies on dorsal hippocampal TrkB activation. The fact that TrkB is essential for the memory-enhancing effects of E2 in OVX rats and mice may indicate a generalizable role across species. Moreover, work focused on other brain regions and memory systems suggests that activation of TrkB signaling may be a common mechanism of E2-induced learning. Systemic ANA-12 treatment blocks E2-induced facilitation of extinction learning on a cocaine conditioned place preference test in OVX rats, and this behavior is correlated with a TrkB dependent potentiation of infralimbic medial prefrontal cortex neurons by E2 (Yousuf et al., 2019). Future work examining the mechanisms of E2 modulation of cognition should consider how E2 activation of TrkB signaling may work throughout the brain to modulate multiple types of learning and memory.
TrkB contributes to hippocampal plasticity and memory through its activation of multiple downstream signaling pathways, including MEK/ERK, PI3K/Akt, and PLC (Leal et al., 2015). These pathways are also critical for E2-induced plasticity and memory (Frick, 2015), which is a central reason that TrkB has been hypothesized to mediate the effects of E2 in the hippocampus. Our findings support a mechanistic role for TrkB activation in E2-induced enhancement of hippocampal memory consolidation; however, many questions remain about how E2 activation of TrkB facilitates memory and how TrkB activation can be integrated into our current understanding of the molecular mechanisms driving E2 effects on hippocampal plasticity and function. Previous studies examining the in vivo and ex vivo activation of kinase networks in male and female rodents by acute E2 treatment have shown that these pathways can be activated very rapidly, within 5–30 minutes of E2 exposure (Fan et al., 2010; Fernandez et al., 2008; Hasegawa et al., 2015; Kuroki et al., 2000). This rapid activation has also been shown to depend on other cell surface receptors and channels, such as mGluRs (Boulware et al., 2013, 2005) and L-type calcium channels (Wu et al., 2005). We anticipated that TrkB activation, however, might occur at later time points due to previous findings that hippocampal infusion of E2 does not increase mBDNF expression until 4–6 hours later (Fortress et al., 2014). Here, we observed phosphorylation of TrkB at Tyr706 at both 4 and 6 hours following E2 treatment, indicating an increase in TrkB kinase activity. Although we cannot rule out an earlier phosphorylation of TrkB at this or other sites, there is other evidence that TrkB activation is temporally distinct from other mechanisms of rapid E2 signaling in the hippocampus. Specifically, systemic E2 replacement in OVX mice induces early hippocampal Akt activation followed by a delayed increase in TrkB phosphorylation (Spencer-Segal et al., 2012), and in E2 treated hippocampal slices from male rats, β1-integrin activation that facilitates synaptic signaling precedes activation of TrkB (Wang et al., 2016). It is possible that E2’s influence on hippocampal plasticity and memory consolidation occurs in stages, with an initial rapid kinase activation mediated by one set of mechanisms followed by a TrkB-mediated wave of signaling that contributes to longer term plasticity and consolidation. Indeed, others have found evidence that neuroplasticity resulting from rapid E2 signaling requires further stimulation for changes to synaptic structure and transmission to persist (Srivastava, 2012). Previous work from our lab has found that hippocampal E2 infusions induce rapid epigenetic modifications within 30 minutes that impact BDNF expression 4–6 hours later (Fortress et al., 2014), lending further support to the idea that rapid E2 signaling is necessary for initiating longer term effects that contribute to memory consolidation. Similarly, work on the mechanisms of memory generally supports a role for BDNF signaling in later stages of memory consolidation and maintenance (Bekinschtein et al., 2007). In questioning how E2-induced TrkB activation contributes to hippocampal synaptic plasticity and memory, it is also worth considering the localization of E2-induced pTrkB. Among intact female mice, hippocampal pTrkB is expressed primarily in presynaptic terminals, and these levels become elevated during proestrus in stratum radiatum axons (Spencer-Segal et al., 2011). How this change in localization contributes to E2-induced neuroplasticity remains unclear. To further comprehend how E2 and TrkB interact to facilitate hippocampal memory consolidation, a more detailed understanding of when and how hippocampal TrkB is activated following E2 treatment and the specific molecular and cellular consequences of E2-induced TrkB signaling will be critical questions for future work.
In addition to testing the involvement of TrkB in E2 enhancement of hippocampal memory consolidation, we also examined the relationships among E2, hippocampal TrkB activation, and BDNF expression. E2 is known to activate hippocampal TrkB (Kramár et al., 2013; Spencer-Segal et al., 2012), but the mechanisms through which this occurs remain unclear. The ability of E2 to increase levels of hippocampal mBDNF offers one explanation (Murphy et al., 1998; Scharfman et al., 2003), but our finding that E2-induced increases in hippocampal pTrkB occur at least two hours prior to increased mBDNF suggests that E2 also regulates TrkB activity through mechanisms beyond a simple increase in ligand expression. Other research has found a similar uncoupling between E2 regulation of BDNF expression and TrkB activity that may be relevant to our findings. In a study of ER knockout mice, OVX ERα knockouts demonstrate a loss of E2-induced TrkB activation despite intact E2 induction of BDNF mRNA (Spencer-Segal et al., 2012). In hippocampal cultures, E2 increases PSD-95 in a TrkB-dependent manner, but without a correlating increase in mBDNF expression (Sato et al., 2007). Rather, E2 was found to increase BDNF release from dentate gyrus cells through a membrane-initiated rapid signaling mechanism (Sato et al., 2007). Similarly, TrkB-dependent E2 stimulation of mammalian target of rapamycin (mTOR) signaling in hippocampal culture is associated with a rapid E2-induced release of BDNF (Briz et al., 2015). Modulation of BDNF release is one possible route for E2-induced activation of TrkB in the absence of changes to BDNF levels, however, TrkB activation could also be independent of BDNF. BDNF-independent transactivation of TrkB is a well-established phenomenon that can occur through extracellular zinc release or through intracellular signaling downstream of GPCRs, such as adenosine and endocannabinoid receptors (Nagappan et al., 2008). An interesting point of convergence in both of these mechanisms is the activation of Src-family kinases to tyrosine phosphorylate, and ultimately activate, the TrkB receptor (Nagappan et al., 2008). Recently, evidence of TrkB transactivation downstream of E2 signaling has been found in the hippocampus. In hippocampal slices from male mice, E2 increases TrkB phosphorylation within 30 minutes of treatment and this effect remains intact in the presence of a BDNF scavenger (Wang et al., 2016), suggesting a BDNF-independent means of TrkB activation that appears to involve β1-integrins. Src kinases could also play a role, as E2 is known to activate Src signaling in the hippocampus (Bi et al., 2000; Wu et al., 2005). Determining which, if any, of these mechanisms contribute to E2 activation of hippocampal TrkB in vivo should be examined in future studies.
We also found that ICV administration of E2 had no effect of TrkB transcription 4 hours later and that E2+ANA-12 treatment blocked TrkB activation but did not block, and may have accelerated, induction of BDNF expression. Previous work on the effects of E2 on hippocampal TrkB expression indicate mixed results, showing both upregulation (Pan et al., 2010) and no change to TrkB expression (Solum and Handa, 2002; Spencer-Segal et al., 2012) following E2 treatment. This variability in findings is likely due to experimental differences, including length, dosage, and route of E2 replacement, as well as the animal models used. Further research will be needed to fully understand how E2 may regulate TrkB gene expression. The increase in hippocampal mBDNF expression following E2 treatment is in line with our previous findings demonstrating that DH infusion of E2 increases mBDNF protein in OVX mice within 6 hours (Fortress et al., 2014). That TrkB antagonism doesn’t inhibit this increase in expression is not necessarily surprising, as currently known mechanisms of E2 upregulation of BDNF expression, such as binding at an ERE-like site (Sohrabji et al., 1995) and epigenetic regulation (Fortress et al., 2014), are not thought to involve TrkB activation. The observed interaction between E2 and ANA-12 to increase mBDNF protein at 4 hours, prior to when increases from E2 alone occur, may be due to a compensatory mechanism that drives ligand upregulation as a result of TrkB antagonism.
In conclusion, this study provides important new insights about the role of TrkB in hippocampal memory consolidation and its interaction with E2 to mediate memory enhancement. We extend previous work on the function of TrkB in memory consolidation by demonstrating a necessity for TrkB signaling in spatial and object memory consolidation and provide the first evidence that hippocampal TrkB receptors are essential for E2 enhancement of hippocampal memory consolidation. Although further work must be conducted to elucidate the mechanisms through which E2 activates hippocampal TrkB, our data also provide evidence that E2 activation of TrkB occurs independently of E2-induced changes to mBDNF expression. Together, these findings shed new light on the importance of TrkB in E2-mediated regulation of hippocampal function and the complex interactions among E2, TrkB, and BDNF signaling in the hippocampus.
Highlights.
TrkB antagonist ANA-12 dose-dependently inhibits hippocampal memory consolidation
Hippocampal TrkB activity is required for estradiol to enhance memory consolidation
Estradiol increases hippocampal TrkB activity prior to increased BDNF expression
Estradiol-induced expression of hippocampal BDNF does not depend on TrkB activity
Acknowledgements:
This work was supported by the National Institutes of Health (R01MH107886, F32MH118782, 2R15GM118304-02), the Alzheimer’s Association (SAGA-17-419092), the University of Wisconsin-Milwaukee Office of Undergraduate Research, and the University of Wisconsin-Milwaukee College of Letters and Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
☐ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declarations of interest: Dr. Karyn M. Frick is a co-founder of, and shareholder in, Estrigenix Therapeutics, Inc., a company which aims to improve women’s health by developing safe, clinically proven treatments for the mental and physical effects of menopause. She also serves as the company’s Chief Scientific Officer. The other authors have no competing interests to declare.
References:
- Bambah-Mukku D, Travaglia A, Chen DY, Pollonini G, Alberini CM, 2014. A positive autoregulatory BDNF feedback loop via C/EBPβ mediates hippocampal memory consolidation. J. Neurosci 34, 12547–12559. 10.1523/JNEUROSCI.0324-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LRM, Izquierdo I, Medina JH, 2007. Persistence of long-term memory storage requires a late protein synthesis- and BDNF-dependent phase in the hippocampus. Neuron 53, 261–277. 10.1016/j.neuron.2006.11.025 [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW, 2001. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur. J. Neurosci 14, 1992–2002. 10.1046/j.0953-g816x.2001.01825.x [DOI] [PubMed] [Google Scholar]
- Bi R, Broutman G, Foy MR, Thompson RF, Baudry M, 2000. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc. Natl. Acad. Sci. USA 97, 3602–3607. 10.1073/pnas.97.7.3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank M, Petry FS, Lichtenfels M, Valiati FE, Dornelles AS, Roesler R, 2016. TrkB blockade in the hippocampus after training or retrieval impairs memory: protection from consolidation impairment by histone deacetylase inhibition. J. Neural. Transm 123, 159–165. 10.1007/s00702-015-1464-7 [DOI] [PubMed] [Google Scholar]
- Bohm-Levine N, Goldberg AR, Mariani M, Frankfurt M, Thornton J, 2020. Reducing luteinizing hormone levels after ovariectomy improves spatial memory: Possible role of brain-derived neurotrophic factor. Horm. Behav 118, 104590 10.1016/j.yhbeh.2019.104590 [DOI] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM, 2013. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J. Neurosci 33, 15184–15194. 10.1523/JNEUROSCI.1716-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG, 2005. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J. Neurosci 25, 5066–5078. 10.1523/JNEUROSCI.1427-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briz V, Liu Y, Zhu G, Bi X, Baudry M, 2015. A novel form of synaptic plasticity in field CA3 of hippocampus requires GPER1 activation and BDNF release. J. Cell. Biol 210, 1225–1237. 10.1083/jcb.201504092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM, 2010. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal ERK and PI3K activation. J. Neurosci 30, 4390–4400. 10.1523/JNEUROSCI.4333-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM, 2008. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J. Neurosci 28, 8660–8667. 10.1523/JNEUROSCI.1968-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM, 2013. Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn. Mem 20, 147–155. 10.1101/lm.026732.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Kim J, Poole RL, Gould TJ, Frick KM, 2014. 17β-Estradiol regulates histone alterations associated with memory consolidation and increases Bdnf promoter acetylation in middle-aged female mice. Learn. Mem 21, 457–467. 10.1101/lm.034033.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, 2015. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm Behav 74, 4–18. 10.1016/j.yhbeh.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Gresack JE, 2003. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav. Neurosci 117, 1283–1291. 10.1037/0735-7044.117.6.1283 [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA, 2007. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol. Learn. Mem 88, 208–216. 10.1016/j.nlm.2007.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, 1999. Treatment with estrogen and progesterone affects relative levels of brain-derived neurotrophic factor mRNA and protein in different regions of the adult rat brain. Brain Res 844, 20–27. 10.1016/S0006-8993(99)01880-6 [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Hojo Y, Kojima H, Ikeda M, Hotta K, Sato R, Ooishi Y, Yoshiya M, Chung B-C, Yamazaki T, Kawato S, 2015. Estradiol rapidly modulates synaptic plasticity of hippocampal neurons: Involvement of kinase networks. Brain Res 1621, 147–161. 10.1016/j.brainres.2014.12.056 [DOI] [PubMed] [Google Scholar]
- Heldt S, Stanek L, Chhatwal J, Ressler K, 2007. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol. Psychiatry 12, 656–670. 10.1038/sj.mp.4001957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Wu Y-WC, Gogos A, Buuse M van den, 2013. Sex-dependent alterations in BDNF-TrkB signaling in the hippocampus of reelin heterozygous mice: a role for sex steroid hormones. J. Neurochem 126, 389–399. 10.1111/jnc.12205 [DOI] [PubMed] [Google Scholar]
- Hill RA, Wu YWC, Kwek P, Buuse M. van den, 2012. Modulatory effects of sex steroid hormones on brain-derived neurotrophic factor-tyrosine kinase B expression during adolescent development in C57Bl/6 mice. J. Neuroendocrinol 24, 774–788. 10.1111/j.1365-2826.2012.02277.x [DOI] [PubMed] [Google Scholar]
- Huang YZ, McNamara JO, 2010. Mutual regulation of Src family kinases and the neurotrophin receptor TrkB. J. Biol. Chem 285, 8207–8217. 10.1074/jbc.M109.091041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome LF, Barateli K, Buitrago D, Lema F, Frankfurt M, Luine VN, 2016. Gonadal hormones rapidly enhance spatial memory and increase hippocampal spine density in male rats. Endocrinology 157, 1357–1362. 10.1210/en.2015-1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Kim JM, Park SJ, Cai M, Liu X, Lee S, Shin CY, Ryu JH, 2012. GABA A receptor blockade enhances memory consolidation by increasing hippocampal BDNF levels. Neuropsychopharmacol 37, 422–433. 10.1038/npp.2011.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Schalk JC, Koss WA, Gremminger RL, Taxier LR, Gross KS, Frick KM, 2019. Dorsal hippocampal actin polymerization is necessary for activation of G-protein-coupled estrogen receptor (GPER) to increase CA1 dendritic spine density and enhance memory consolidation. J. Neurosci 39, 9598–9610. 10.1523/JNEUROSCI.2687-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Szinte JS, Boulware MI, Frick KM, 2016. 17β-Estradiol and agonism of G-protein-coupled estrogen receptor enhance hippocampal memory via different cell-signaling mechanisms. J. Neurosci 36, 3309–3321. 10.1523/JNEUROSCI.0257-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramár EA, Babayan AH, Gall CM, Lynch G, 2013. Estrogen promotes learning-related plasticity by modifying the synaptic cytoskeleton. Neuroscience 239, 3–16. 10.1016/j.neuroscience.2012.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y, 2000. Putative membrane-bound estrogen receptors possibly stimulate mitogen-activated protein kinase in the rat hippocampus. Eur. J. Pharmacol 400, 205–209. 10.1016/S0014-2999(00)00425-8 [DOI] [PubMed] [Google Scholar]
- Leal G, Afonso PM, Salazar IL, Duarte CB, 2015. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res 1621, 82–101. 10.1016/j.brainres.2014.10.019 [DOI] [PubMed] [Google Scholar]
- Lee JLC, Everitt BJ, Thomas KL, 2004. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science 304, 839–843. 10.1126/science.1095760 [DOI] [PubMed] [Google Scholar]
- Liu Y, Fowler CD, Young LJ, Yan Q, Insel TR, Wang Z, 2001. Expression and estrogen regulation of brain-derived neurotrophic factor gene and protein in the forebrain of female prairie voles. J. Comp. Neuro 433, 499–514. 10.1002/cne.1156 [DOI] [PubMed] [Google Scholar]
- Luine V, Frankfurt M, 2013. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience 239, 34–45. 10.1016/j.neuroscience.2012.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama NO, Lucas TFG, Porto CS, Abdalla FMF, 2013. Estrogen receptor ESR1 regulates the phospholipase C-inositol phosphate signaling in the hippocampus from rats in proestrous and estrous phases. Steroids 78, 8–14. 10.1016/j.steroids.2012.10.005 [DOI] [PubMed] [Google Scholar]
- Minichiello L, Korte M, Wolfer D, Kühn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp H-P, Bonhoeffer T, Klein R, 1999. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron 24, 401–414. 10.1016/S0896-6273(00)80853-3 [DOI] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Segal M, 1998. Brain-derived neurotrophic factor mediates estradiol-induced dendritic spine formation in hippocampal neurons. Proc. Natl. Acad. Sci. USA 95, 11412–11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagappan G, Woo NH, Lu B, 2008. Ama “zinc” link between TrkB transactivation and synaptic plasticity. Neuron 57, 477–479. 10.1016/j.neuron.2008.02.004 [DOI] [PubMed] [Google Scholar]
- Pan M, Li Z, Yeung V, Xu R-J, 2010. Dietary supplementation of soy germ phytoestrogens or estradiol improves spatial memory performance and increases gene expression of BDNF, TrkB receptor and synaptic factors in ovariectomized rats. Nutr. Metab. (Lond) 7, 75 10.1186/1743-7075-7-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Poo M, 2013. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci 14, 7–23. 10.1038/nrn3379 [DOI] [PubMed] [Google Scholar]
- Phan A, Gabor CS, Favaro KJ, Kaschack S, Armstrong JN, MacLusky NJ, Choleris E, 2012. Low doses of 17 β -Estradiol rapidly improve learning and increase hippocampal dendritic spines. Neuropsychopharmacol 37, 2299–2309. 10.1038/npp.2012.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasi M, Vignoli B, Canossa M, Blum R, 2017. Neurobiology of local and intercellular BDNF signaling. Eur J Physiol 469, 593–610. 10.1007/s00424-017-1964-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K, 2007. β-Estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res 1150, 108–120. 10.1016/j.brainres.2007.02.093 [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Hintz TM, Gomez J, Stormes KA, Barouk S, Malthankar-Phatak GH, McCloskey DP, Luine VN, MacLusky NJ, 2007. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. Eur. J. Neurosci 26, 2595–2612. 10.1111/j.1460-9568.2007.05848.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ, 2005. Similarities between actions of estrogen and BDNF in the hippocampus: coincidence or clue? Trends Neurosci 28, 79–85. 10.1016/j.tins.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ, 2003. Hippocampal excitability increases during the estrous cycle in the rat: A potential role for brain-derived neurotrophic factor. J. Neurosci 23, 11641–11652. 10.1523/JNEUROSCI.23-37-11641.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal RA, Bhattacharyya A, Rua LA, Alberta JA, Stephens RM, Kaplan DR, Stiles CD, 1996. Differential utilization of Trk autophosphorylation sites. J. Biol. Chem 271, 20175–20181. 10.1074/jbc.271.33.20175 [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Simpkins JW, 1995. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology 136, 2320–2324. 10.1210/en.136.5.2320 [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD, 1995. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. USA 92, 11110–11114. 10.1073/pnas.92.24.11110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solum DT, Handa RJ, 2002. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J. Neurosci. 22, 2650–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, McEwen BS, 2008. Estrous cycle regulates activation of hippocampal Akt, LIMK, and neurotrophin receptors in C57BL6 mice. Neuroscience 155, 1106–1119. 10.1016/j.neuroscience.2008.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Segal JL, Tsuda MC, Mattei L, Waters EM, Romeo RD, Milner TA, McEwen BS, Ogawa S, 2012. Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience 202, 131–146. 10.1016/j.neuroscience.2011.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Segal JL, Waters EM, Bath KG, Chao MV, McEwen BS, Milner TA, 2011. Distribution of phosphorylated TrkB receptor in the mouse hippocampal formation depends on sex and estrous cycle stage. J. Neurosci 31, 6780–6790. 10.1523/JNEUROSCI.0910-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, 2012. Two-step wiring plasticity – A mechanism for estrogen-induced rewiring of cortical circuits. J. Steroid. Biochem 131, 17–23. 10.1016/j.jsbmb.2012.01.006 [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Evans PD, 2013. Mechanisms underlying the interactions between rapid estrogenic and BDNF control of synaptic connectivity. Neuroscience 239, 17–33. 10.1016/j.neuroscience.2012.12.004 [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA, 2006. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol. Learn. Mem 86, 35–46. 10.1016/j.nlm.2006.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Kantorovich S, Babayan AH, Hou B, Gall CM, Lynch G, 2016. Estrogen’s effects on excitatory synaptic transmission entail integrin and TrkB transactivation and depend upon β1-integrin function., estrogen’s effects on excitatory synaptic transmission entail integrin and TrkB transactivation and depend upon β1-integrin function. Neuropsychopharmacol. 41, 41, 2723, 2723–2732. 10.1038/npp.2016.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, 1998. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm. Behav 34, 140–148. 10.1006/hbeh.1998.1466 [DOI] [PubMed] [Google Scholar]
- Wu T-W, Wang JM, Chen S, Brinton RD, 2005. 17β-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: A potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience 135, 59–72. 10.1016/j.neuroscience.2004.12.027 [DOI] [PubMed] [Google Scholar]
- Yang L, Zhang Q-G, Zhou C, Yang F, Zhang Y, Wang R, Brann DW, 2010. Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus. PLoS One 5 10.1371/journal.pone.0009851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf H, Smies CW, Hafenbreidel M, Tuscher JJ, Fortress AM, Frick KM, Mueller D, 2019. Infralimbic estradiol enhances neuronal excitability and facilitates extinction of cocaine seeking in female rats via a BDNF/TrkB mechanism. Front. Behav. Neurosci 13 10.3389/fnbeh.2019.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Frick KM, 2010. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc. Natl. Acad. Sci. USA 107, 5605–5610. 10.1073/pnas.0910578107 [DOI] [PMC free article] [PubMed] [Google Scholar]


