Abstract
Respiratory tract infections caused by multidrug-resistant (MDR) Gram-negative bacteria such as Pseudomonas aeruginosa are serious burdens to public health, especially in cystic fibrosis patients. The combination of colistin, a cationic polypeptide antibiotic, and ivacaftor, a cystic fibrosis transmembrane regulator (CFTR) protein modulator, displays a synergistic antibacterial effect against P. aeruginosa. The primary aim of the present study is to investigate the transport, accumulation and toxicity in lung epithelial Calu-3 cells of a novel nanoparticle formulation containing colistin and ivacaftor. The cell viability results demonstrated that ivacaftor alone or in combination with colistin in the physical mixture showed significant toxicity at an ivacaftor concentration of 10 μg/mL or higher. However, the cellular toxicity was significantly reduced in the nanoparticle formulation. Ivacaftor transport into the cells reached a plateau rapidly as compared to colistin. Colistin transport across the Calu-3 cell monolayer was less than ivacaftor. A substantial amount (46–83%) of ivacaftor, independent of dose, was accumulated in the cell monolayer following transport from the apical into the basal chamber, whereas the intracellular accumulation of colistin was relatively low (2–15%). The nanoparticle formulation significantly reduced the toxicity of colistin and ivacaftor to Calu-3 cells by reducing the accumulation of both drugs in the cell and potential protective effects by bovine serum albumin (BSA), which could be a promising safer option for the treatment of respiratory infections caused by MDR P. aeruginosa.
Keywords: pulmonary drug delivery, cytotoxicity, drug transport, human lung epithelial cell, lung infection
Graphical Abstract
Nanoparticle formulation of colistin and ivacaftor was added to the Calu-3 cell monolayer. Cellular distribution of colistin, ivacaftor and the BSA tagged nanoparticles were investigated at different time points (1,2 and 4 h) using confocal microscopy. Both colistin and ivacaftor accumulated in the Calu-3 cell monolayer in a time-dependent manner.
1. Introduction
Cystic fibrosis (CF) is an autosomal recessive disorder caused by mutations in the CFTR gene, which encodes the cystic fibrosis transmembrane conductance regulator (CFTR) protein, a chloride anion channel in the lung cells (Kuk and Taylor-Cousar, 2015). Pseudomonas aeruginosa is a communal pathogen commonly associated with respiratory tract infections in CF patients (Sadikot et al., 2005). The presence of thick and viscous mucous often obstructs the airways and makes it difficult for the lung to clear the bacteria (Bos et al., 2017). Colonized P. aeruginosa in CF lung infections is very difficult to eradicate and is a leading cause of morbidity in CF patients (Schneider-Futschik et al., 2018).
The emergence of MDR Gram-negative pathogens combined with a decline in the development of novel antibiotics is a serious concern for CF patients who develop chronic MDR P. aeruginosa induced lung infections (Lodato and Kaplan, 2013; Schneider-Futschik et al., 2018). Polymyxins (i.e. colistin and polymyxin B) are outer membrane active lipopeptide antibiotics commonly formulated for inhalation therapy either as a dry powder or nebulizer solution in the treatment of this dangerous pathogen (Bos et al., 2017). Unfortunately, P. aeruginosa is acquiring resistance to polymyxins both communally and nosocomially (Orsi et al., 2019). Therefore, novel combination therapies are urgently needed (Schneider-Futschik et al., 2018; Schneider et al., 2016a).
Ivacaftor (Brand name Kalydeco) is the first marketed drug to potentiate CFTR protein activity in CF, treating the diseased state itself (Drugs.com, 2020). Ivacaftor demonstrates clinical efficacy as a CFTR corrector primarily in patients with the G551D mutation, who account for 4 – 5% cases of CF; demonstrating an increase in chloride transport, improvement in lung function (increase in FEV1) and a lower frequency of pulmonary exacerbation (Rosenfeld et al., 2018; Sermet-Gaudelus, 2013). Ivacaftor in combination with polymyxin has shown significant synergistic killing of polymyxin-resistant P. aeruginosa at clinically relevant concentrations (Schneider et al., 2016a). The disposition of inhaled antibiotics in lungs and antibacterial activity depends on several important factors including the solubility of drugs in epithelial lining fluids and mucus, the interaction of the antibiotic with mucus, penetration of the antibiotic through mucus, and in special cases, penetration through an alginate barrier produced by P. aeruginosa (Bos et al., 2017). It is therefore possible to imagine that ivacaftor’s CFTR potentiator activity could increase the penetration of polymyxins through mucus to the infection site in the lungs of CF patients (Schneider et al., 2016a).
Over the last decade, inhaled antibiotics have drawn significant attention for the treatment of respiratory infections, including those in patients with cystic fibrosis and ventilator-associated pneumonia (Quon et al., 2014). Inhaled antibiotics are delivered directly into infection sites in the lungs (Hickey et al., 2013; Mangal et al., 2019; Mehta et al., 2019; Xu et al., 2019), thereby achieving greater antibacterial efficacy with significant drug deposition in the epithelial lining fluid and lower systemic drug exposure (Lin et al., 2017a; Lin et al., 2017b; Yapa et al., 2014; Yu et al., 2020; Zhou et al., 2015). Dry powder inhaler formulations (DPIs) represent a significant technical advancement in pulmonary drug delivery and possess enhanced drug stability and greater delivery efficiencies as compared to the corresponding liquid formulations (Brunaugh and Smyth, 2018; Shetty et al., 2020; Zhou et al., 2014). In a recent study, we developed a nanoparticle formulation containing ivacaftor and colistin for inhalation therapy (Zhu et al., 2020). This formulation encapsulated ivacaftor in bovine serum albumin (BSA) nanoparticles (Iva-BSA-NPs) and used colistin as the matrix material, which exhibited improved dissolution for ivacaftor and superior aerosolization behavior. The aim of our current study is to examine the in vitro toxicity, transport, and accumulation of co-delivered colistin and ivacaftor nanoparticles in human lung epithelial cells.
2. Methods
2.1. Materials
Ivacaftor and colistin were procured from AOK Chem (Shanghai, China) and Betapharma Co. Ltd. (Jiangsu, China), respectively. Bovine serum albumin (BSA, purity > 98%, Mw 66.5 kDa), glutaraldehyde, L-leucine, Neutral Red and Alamar Blue dye were obtained from Sigma-Aldrich Inc. (St. Louis, MO, USA). MTT assay kits and DMSO were obtained from Thermo Fisher scientific Inc. (Waltham, MA, USA). Hoechst 33342 was provided by Becton Dickinson (Franklin Lakes, NJ, USA). The fetal bovine serum (FBS), Dulbecco’s Modified Eagle Medium (DMEM) and Hanks’ Balanced Salt Solution (HBSS) were purchased from Gibco Life Technologies Corporation (Eugene, OR, USA). BCA protein assay kit, TritonX-100 and RIPA buffer were obtained from Thermo Scientific (Rockford, IL, USA). Transwell permeable membrane cell culture inserts (pore diameter 0.4 μm, insert diameter 6.5 mm) were purchased from Corning Incorporated (Kennebunk, ME, USA). Paraformaldehyde (PFA) was purchased from Biotium, Inc. (Fremont, CA, USA). Mouse anti-polymyxin B IgM, Goat anti-mouse IgM antibody conjugated with Alexa Fluor™ 647, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Invitrogen (Life Technologies Corporation, Eugene, OR, USA).
2.2. Preparation of colistin and Iva-BSA-NPs DPIs for co-delivery
Iva-BSA-NPs were prepared using the established anti-solvent method (Zhu et al., 2020). First, BSA was dissolved in double-distilled water and ivacaftor was dissolved in dimethyl sulfoxide (DMSO), at a mass ratio of 50:4. Then the nanoparticles were obtained by continuously dropping the ivacaftor solution into the BSA solution at a rate of about 1.0 mL/min under sonication using an ultrasonic bath (Thermo Fisher Scientific Inc.). The volume ratio of ivacaftor solution into BSA solution was 1:3. After that, a small amount of glutaraldehyde solution (8.0%, v/v) was added into the above suspension and was kept stirring at 400 rpm overnight to maximize crosslinking with BSA. Finally, the ivacaftor nanoparticles (Iva-BSA-NPs) were collected by high-speed centrifugation (16,000 × g for 15 min) and washed three times with water to remove DMSO. Then colistin solution was added into the Iva-BSA-NPs suspension (mass ratio of Col:Iva=1:1) so that colistin would interact with the nanoparticles due to its positive charge. Subsequently, L-Leucine (Leu, mass ratio of Leu:Col=16.7:1) was added as the aerosolization enhancer. The suspension was sprayed using an ultrasonic atomizer (3.5 W) into liquid nitrogen at a rate of 2.0 mL/min. The frozen samples were then dried using a freeze dryer (Labconco, Kansas City, MO, USA). The lyophilization temperature was maintained at −54 °C and a vacuum level of 0.5 mbar was used for complete sublimation of ice. The dry powder was collected and stored in sealed tubes and desiccated containers for further studies.
2.3. Calu-3 cell culture
The Calu-3 bronchial epithelial cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The Calu-3 is a lung adenocarcinoma cell line, and has been widely used to evaluate pulmonary drug cytotoxicity and trans-epithelial drug transport assessments (Ong et al., 2011; Ong et al., 2013). Normal human bronchial primary cells (NHBE) are difficult to propagate and several subculture may cause impaired mucociliary differentiation (Min et al., 2016). For the drug transport and permeability measurement, we have used air liquid interface (ALI) culture conditions; however, NHBE cells form multiple layers of variable thickness and differentiated cellular composition in ALI conditions, which can complicate interpretation of drug transport data (Min et al., 2016). The culture medium was DMEM/F-12 and was supplemented with 10% (V/V) fetal bovine serum (FBS), 1% (v/v) nonessential amino acid and 1% (v/v) penicillin and streptomycin. The Calu-3 cells were cultured in the 75 cm2 flask and maintained at 37 °C and 95% relative humidity with 5% CO2. The medium was changed every two days and passaged weekly.
2.4. Cytotoxicity measurement
Calu-3 cells were seeded in the 96-well plates at a density of 5×104 cells/well. After the 24 h culture, the cells were exposed to different drug solutions (diluted with culture medium), including colistin solution, ivacaftor solution, colistin/ivacaftor combined solution, a physical mixture of both compounds, and a dry powder of nanoparticle formulation. For each drug solution, concentrations of 3, 6, and 10 μg/mL were used, and cellular viability and metabolic activity were examined at 24 h (n=6).
2.4.1. Cell mitochondrial activity assay with MTT
For viable cells, mitochondrial enzyme could transform MTT into formazan, whose UV intensity could serve as an index of intrinsic cytotoxicity. After the drug treatment, the drug solution was aspirated out and MTT solution (5 mg/mL diluted in serum free medium) was added into the 96-well plates and incubated for 4 h. The reagent was carefully removed and DMSO was added to dissolve the formazan previously formed. Absorbance was measured at 570 nm using a fluorescent microplate reader (SYNERGY 4, BioTek, Winooski, VT, USA).
2.4.2. Cell metabolic activity assay with Alamar Blue
Alamar Blue is commonly used as an indicator of cell metabolic ability because it will shift from blue to pink in the presence of reductases such as NADPH, in viable cells. After being exposed to drug solution, 200 μL of Alamar Blue solution (10 μg/mL, diluted with culture medium) was added and incubated for 6 h. Absorbance was measured (excitation wavelength 535 nm and emission wavelength 600 nm) using fluorescent microplate reader (SYNERGY 4, BioTek, Winooski, VT, USA).
2.4.3. Cell membrane integrity assay with Neutral Red
Neutral Red can accumulate in living cells with an intact lysosomal membrane and can be correlated with cell viability. For a cell membrane integrity study, 200 μL Neutral red solution (50 μg/mL, diluted with culture medium) was added after drug exposure and incubated for 3 h. Cells were washed with PBS and were treated with acetic acid-ethanol solution to solubilize incorporated dye. After agitation, fluorescence was measured using fluorescent microplate reader (SYNERGY 4, BioTek, Winooski, VT, USA) with excitation wavelength of 535 nm and emission wavelength of 600 nm.
2.5. In vitro trans-epithelial drug diffusion study
Calu-3 epithelial cells were cultured at ALI condition at a density of 5×105 cells/cm2 in 200 μL medium onto the apical side of the 24 well Transwell® polyester insert membrane (Chai et al., 2019). Medium was aspirated from the apical and basal chamber, and cells were incubated for 12–14 days with medium only in the basolateral chamber for differentiation (Haghi et al., 2018; Panduga et al., 2017). ALI culture shares a high similarity with human lung cells in terms of apical morphology and glycoprotein secretion (Haghi et al., 2010). Trans-epithelial electrical resistance (TEER) before and after the transport study was used to evaluate the integrity of the cell monolayer.
For the transport study, drug concentrations were selected based on the toxicity data. The drug was added into either the apical or basolateral side of the Transwell cell culture inserts for 4 h. For drug transport from the apical side (A) to basolateral side (B), a volume of 200 μL drug solution (colistin, ivacaftor, particle mixture or nanoparticle formulation in HBSS) was placed in the apical chamber of the insert as the donor solution, and 600 μL pre-warmed HBSS (37 °C) in the basolateral chamber as the recipient solution. For transport studies from B-A, 600 μL of different drug solutions were added into the basolateral chamber and 200 μL HBSS was put into the apical chamber. For the transport study, all samples were taken at 1, 2, 3 and 4 h for LC-MS/MS analysis. All experiments were replicated four times. The capacity of drug transport across the Calu-3 cell monolayer was evaluated by calculating the apparent permeability coefficient (Papp, cm·s−1) (Chai et al., 2019).
2.6. Drug accumulation in Calu-3 cell monolayer
To detect the amount of drug accumulated in the cells, the Calu-3 cell monolayer was harvested after drug exposure from both the apical and basolateral sides (Chai et al., 2019; Panduga et al., 2017). Cell monolayers were excised from the Transwell® and collected into 24 well culture plates. Cells were lysed using 400 μL of RIPA buffer with 30 min incubation at room temperature. An aliquot of 200 μL cell lysate was briefly mixed with 400 μL acetonitrile (containing 0.1% FA) to precipitate protein, and then highly centrifuged to produce supernatant for LC-MS/MS analysis. The Calu-3 cell uptake of ivacaftor/colistin was then normalized with the cellular protein content. Cellular protein content was measured using 200 μL of the BCA protein assay kit mixed with 25 μL of the cell lysate, with absorbance measured at 562 nm.
2.7. Determination of colistin and ivacaftor by LC-MS/MS
Drug concentrations were determined by LC-MS/MS using a previously published method with slight modifications(Chai et al., 2019). The LC-MS/MS was equipped with a C18 column (2.6 μm, 50 mm×3 mm, Phenomenex, Torrance, CA, USA), a liquid chromatography system (Agilent 1200) and a mass spectrometer (Agilent 6460 QQQ) with an electrospray ionization source (Santa Clara, CA, USA). The mobile phase was 0.1% formic acid solution in water (A) and 0.1% formic acid solution in acetonitrile (B) with a flow rate of 0.6 mL/min. The gradient was set as: 0–0.5 min, 90% A; 1.5 min, 30% A; 2.5 min, 10% A; 3.0 min, 10% A; 3.5 min, 90% A; 5.5 min, stop. Multiple reaction monitoring (MRM) was used to identify each drug from the area under the curve ratio for the compound and the internal standard. The main colistin ions were m/z 585.7 and m/z 578.8, which was consistent with previous research. Electrospray ionization (ESI) was the ionization source. For polymyxin B (as the internal standard), the dominant ions were m/z 602.3 and m/z 595.3. For ivacaftor, the dominant ion was m/z 172.1.
2.8. Evaluation of drug distribution by Confocal Laser Scanning Microscopy (CLSM)
Calu-3 cells were cultured at the ALI condition on the Transwell insert membrane and grown for 14 days to form a differentiated monolayer (Chai et al., 2019). The colistin and ivacaftor nanoparticle formulation was added to the apical chamber of cell monolayers and incubated for up to 4 h. Drug solution was aspirated at 1, 2 and 4 h and the cell monolayer was washed three times with PBS. The insert membrane with cell monolayer was then fixed with 4% PFA followed by permeabilization (0.1 % Triton X-100 for 1 h). Non-specific sites were blocked with 1% goat serum for 3 h (room temperature). For the detection of colistin, cells were incubated overnight in a dark chamber at 4 °C with a mouse anti-polymyxin B antibody (1:500) after blocking. Polymyxin B antibody has been proved as the best option and successfully used to visualize colistin in lung epithelial cells and primary neurons cells (Ahmed et al., 2017; Ahmed et al., 2018; Dai et al., 2018; Velkov et al., 2016). Following overnight incubation, cells were incubated with the goat anti-mouse IgM conjugated with Alexa Fluor™ 647 (1:500) at room temperature for 2 h. The nucleus was stained with propidium iodide (100 ng/mL) for 20 min (Ahmed et al., 2017; Ahmed et al., 2018).
The distribution of ivacaftor, colistin and nanoparticles in the Calu-3 cell monolayer were imaged by confocal microscopy (Nikon A1R, Nikon America Inc., Melville, NY, USA). Fluorescence detection of ivacaftor was very sensitive at 317 nm excitation and 407 nm emission (Schneider et al., 2016b). Settings for camera aperture, image capture and gain parameters, and laser poser were adjusted with untreated control and maintained throughout the study. Three-dimensional views of drug transport through the Calu-3 cell monolayer were made by collecting images in every 0.3 μm along the z-axis.
2.9. Statistical analysis
All the data are presented as mean ± standard deviation (SD). Analysis of variance (ANOVA) for statistical significance was analyzed using GraphPad Prism 8.0 (GraphPad Software, San Diego, USA), and the statistical significance was assigned at p<0.05.
3. Results and discussions
Physico-chemical properties, aerosolization performance and dissolution profiles of the DPI formulation with Iva-BSA-NPs and colistin (as the matrix material) have been published somewhere else (Zhu et al., 2020). The Iva-BSA-NPs are amorphous with particle sizes of 173.2 ± 4.1 nm and encapsulation efficiency of 78.7 ± 2.6 %. The spray freeze dried DPI formulation exhibited irregular-shaped morphology with FPF values of 73.8 ± 5.2% for Col and 80.9 ± 4.1% for Iva. The formulation demonstrated much faster dissolution rate than the physical mixture of jet-milled pure drug particles, with almost completely drug release within 3 hours (Zhu et al., 2020). In the present study, we examined the in vitro toxicity, transport and accumulation of co-delivered ivacaftor nanoparticles and colistin in Calu-3 human lung epithelial cells.
3.1. Cell viability
There were no significant differences in cell viability for the nanoparticle formulation group across all tested concentrations (p > 0.05, Fig 1). Colistin alone was not cytotoxic for Calu-3 cells at 100 μg/mL or lower, which was in agreement with previously published results (Chai et al., 2019). However, ivacaftor alone, both in the solution mixture and the physical mixture, showed concentration-dependent cell cytotoxicity at 15 μg/mL and 20 μg/mL (p < 0.05). Cell viability reduced to 56.4 ± 9.0 % and 17.4 ± 0.6 % respectively with 15 μg/mL and 20 μg/mL ivacaftor, which substantially increased to 98.8 ± 13.5 % (p < 0.001) and 96.1 ± 9.3 % (p < 0.0001) while using in combination with colistin in the nanoparticle formulation. Alamar Blue and Neutral Red assays showed that both the solution mixture and the physical mixture of ivacaftor alone disrupted the membrane integrity of Calu-3 cells in a concentration-dependent manner.
Figure 1.
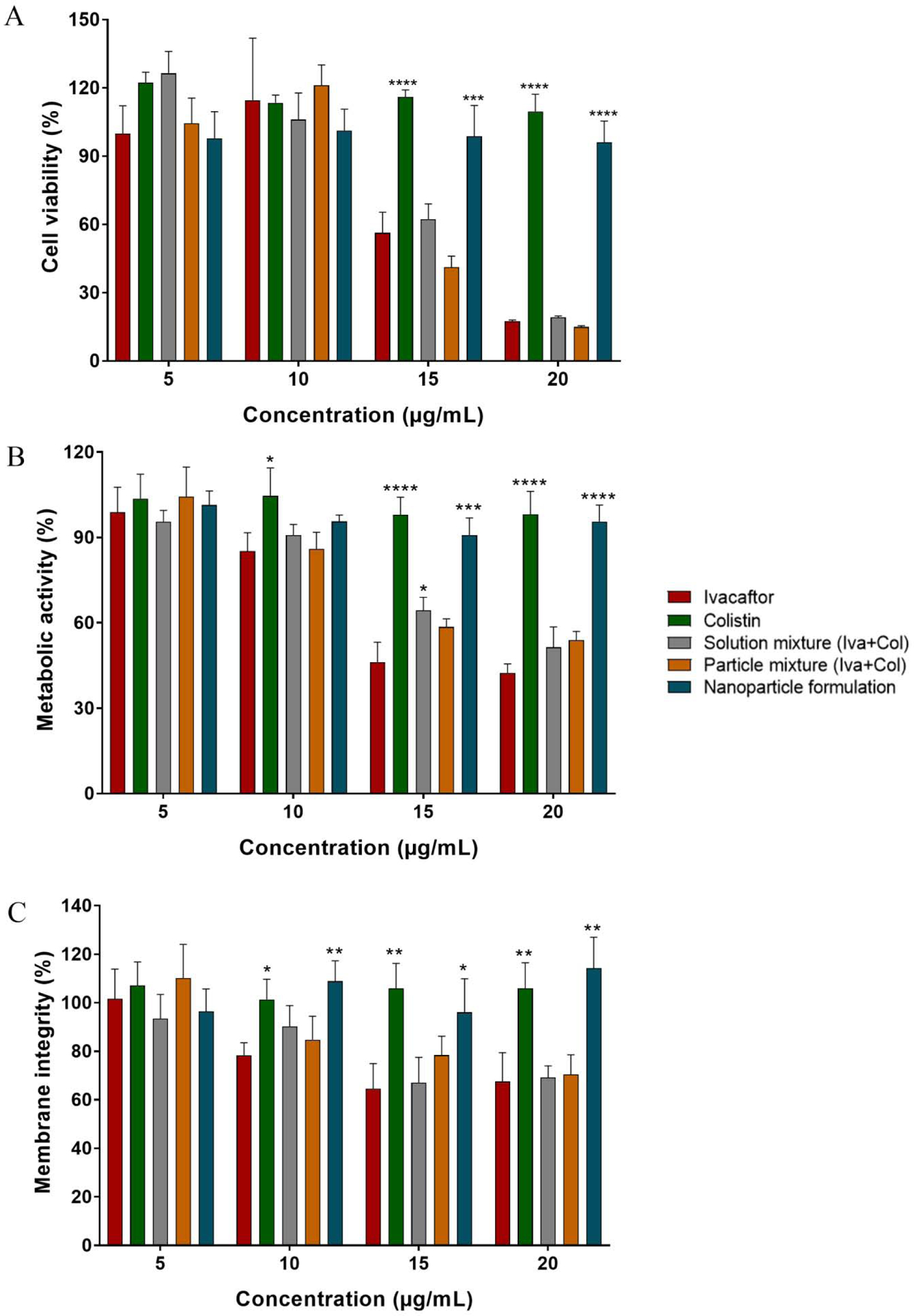
Cytotoxicity data in Calu-3 cells from: A. cell viability (MTT) assay, B. cellular metabolic activity assay (Alamar Blue) and C. Cellular membrane integrity assay (Neutral Red). All treatments were normalized compared to control group and presented as relative absorbance (mean ± SD; n=6). Statistical significance was calculated compared to the ivacaftor group to show significant reduction in toxicity (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).
3.2. In vitro drug transport across Calu-3 cell layer
Calu-3 cells cultured at the ALI culture condition were used for the drug transport study. The relevant transport parameters, including Papp, remaining drugs (ivacaftor and colistin), and the drug accumulation in cells from apical side to basolateral side (A-B) and basolateral side to apical side (B-A), were all measured. For both A-B and B-A transport studies, the TEER values were more than 500 Ω·cm2 with no dramatic change before and after testing, which indicates the integrity of the cell layer during the transport studies (Chai et al., 2019).
3.2.1. Ivacaftor transport across Calu-3 cell layer
Ivacaftor had a relatively fast transport rate based on the Papp value (the order of magnitude ranged from 10−6 cm·s−1 to 10−5 cm·s−1) and the Papp values from A-B were larger than those from B-A with the same drug concentration (Fig. 2). A dose-dependent decrease in the ivacaftor transport from A-B was observed. Ivacaftor accumulation in the cells was independent of dose throughout different formulations, which means a significant amount of drug remained in the donor chamber with the increase in concentration (Fig. 2). Ivacaftor penetration from A-B through the cell monolayer was significantly higher at 3 μg/mL and 6 μg/mL concentration for the nanoparticle formulation, compared to the ivacaftor alone and the particle mixture group (Fig. 2). As for 10 μg/mL, the penetration of nanoparticle formulation is also higher that solutions for Iva but without significance due to a bit higher SD of solution. But the penetration of nanoparticle formulation is significantly higher than that of physical mixtures (p<0.05). For B-A transport study, substantial amount of ivacaftor remained (57–71%) in the donor chamber resulting in poor penetration (3–12%) and lower intracellular accumulation (18–34%) across different concentration and formulations. Ivacaftor had very low solubility in aqueous medium and drug precipitation was observed when the DMSO solution was diluted with HBSS. For this reason, the precipitated ivacaftor crystal, although very small, tended to settle on the bottom and was difficult to transport from basal to apical chamber (B-A).
Figure 2.
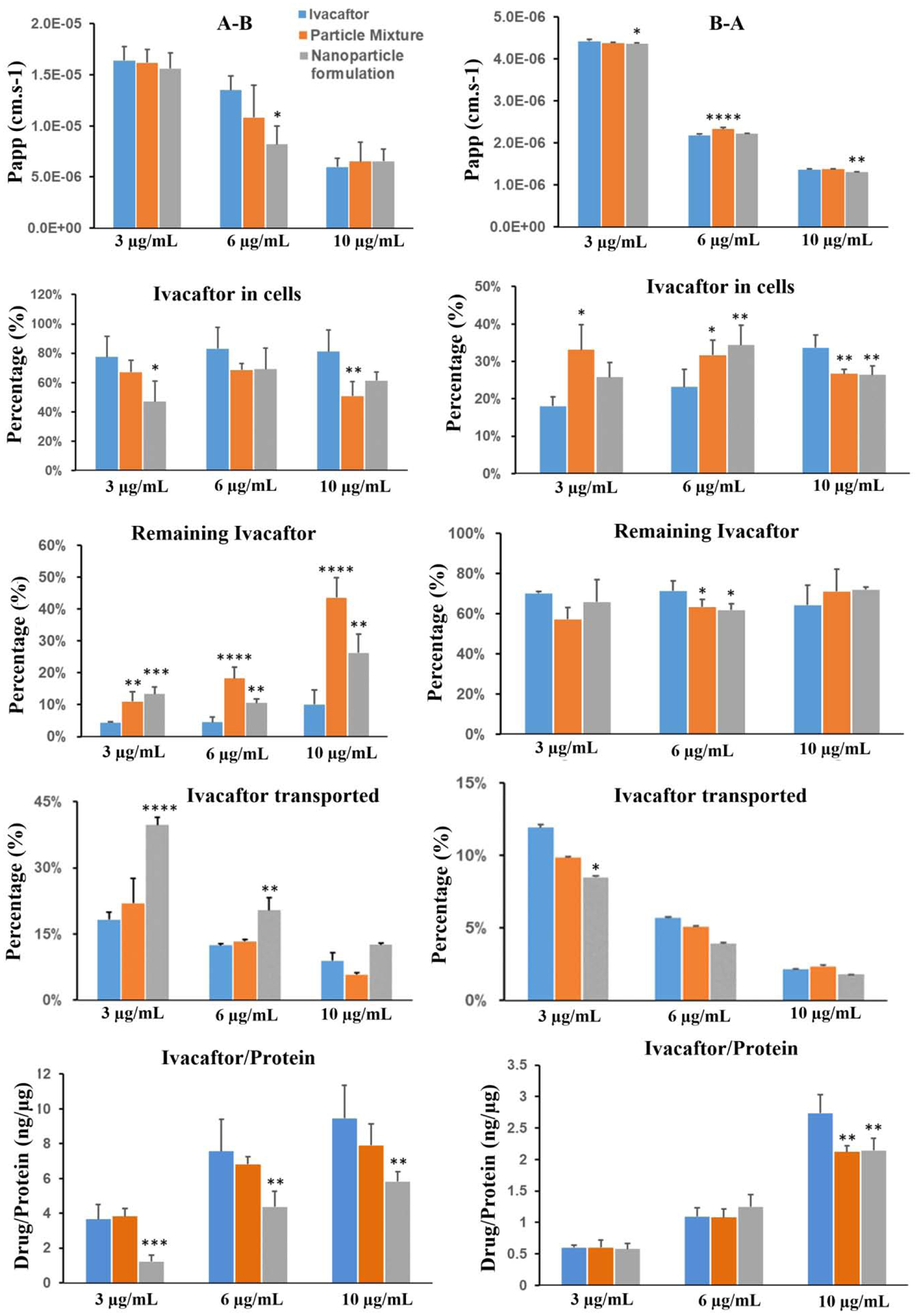
Transport results of ivacaftor across the Calu-3 cell layer. A-B: from apical side to basolateral side (left panel); B-A: from basolateral side to apical side (right panel). All data are presented as mean ± SD (n=4) and compared to the ivacaftor group: *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001.
3.2.2. Colistin transport across Calu-3 cell layer
The permeability coefficient (Papp) for colistin transport was less than that of ivacaftor in A-B. Colistin transport from both A-B and B-A decreased in a concentration-dependent manner. Colistin accumulation in Calu-3 cells was significantly higher (p<0.05) in the colistin solution group as compared to other treatment groups (Fig. 3) except at 6 μg/mL from A-B, which could be due to BSA in the physical mixture and nanoparticle formulation. BSA usually processes as a negative charge in the pH neutral solution, which could adsorb cationic colistin (Shouren et al., 1998) by electrostatic interaction and hinder drug release and diffusion. For the remaining drug content, the colistin solution group had similar levels as compared to the other groups (p > 0.05). Colistin penetration from A-B through the cell monolayer was unaffected by both the particle mixture and the nanoparticle formulation at low concentration (3 and 6 μg/mL). However, at 10 μg/mL colistin penetration was significantly decreased by the nanoparticle formulation (Fig. 3). For the B-A transport study, colistin penetration was significantly decreased by both the particle mixture and the nanoparticle formulation throughout all concentrations used in the study (Fig. 3).
Figure 3.
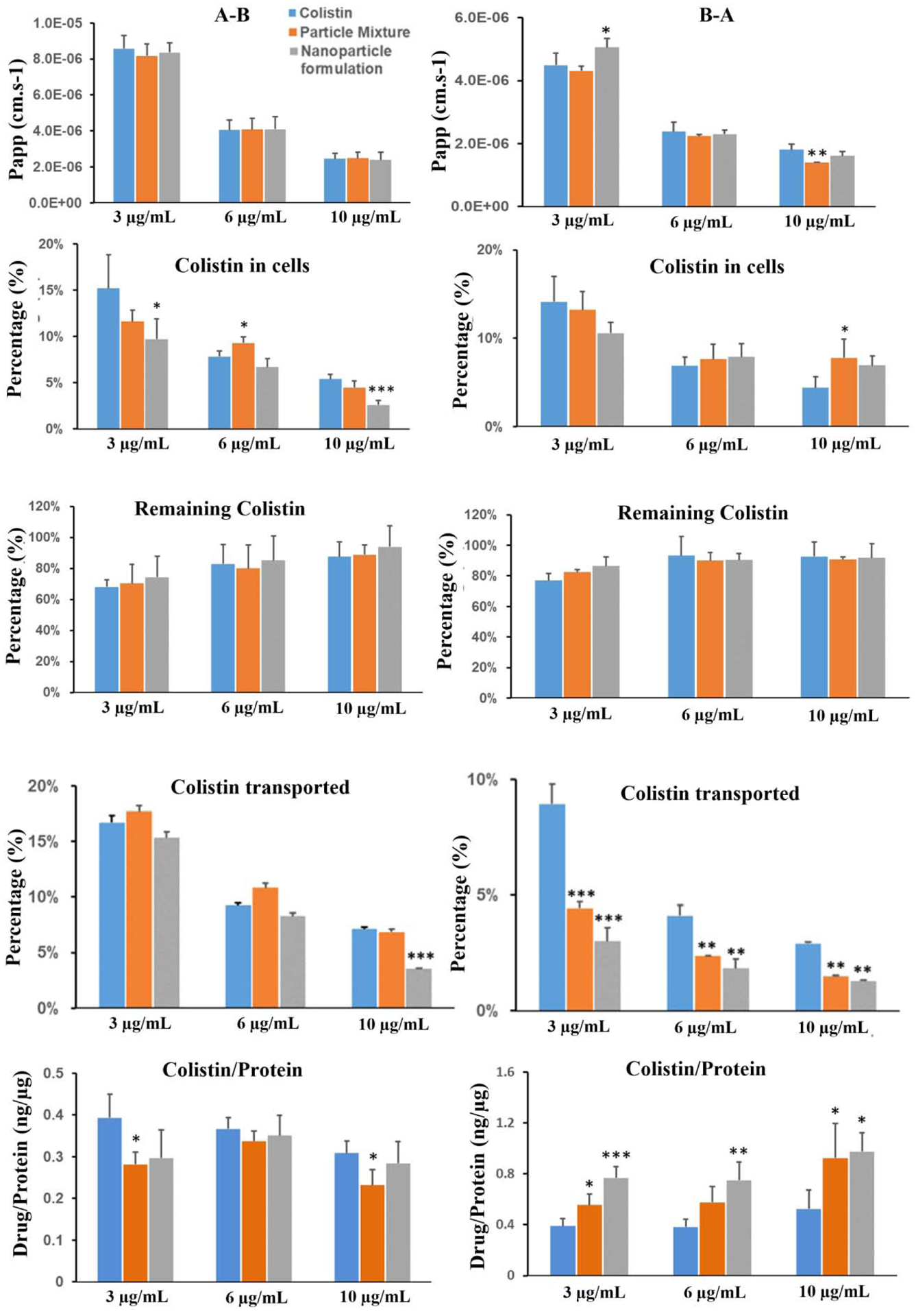
Transport results of colistin across the Calu-3 cell layer. A-B: from apical side to basolateral side (left panel); B-A: from basolateral side to apical side (right panel). All data are presented as mean ± SD (n=4) and compared to the colistin group: *p<0.05, **p<0.01 and ***p<0.001.
3.3. Imaging of ivacaftor and colistin distribution in Calu-3 cell monolayer
For the imaging study, the insert membrane was placed on top of a slide, covered with a coverslip, and inversely placed on top of the objecting for imaging. As shown in Fig. 4, ivacaftor and colistin were accumulated in the cell monolayers in a time-dependent manner (up to 4 h).
Figure 4.
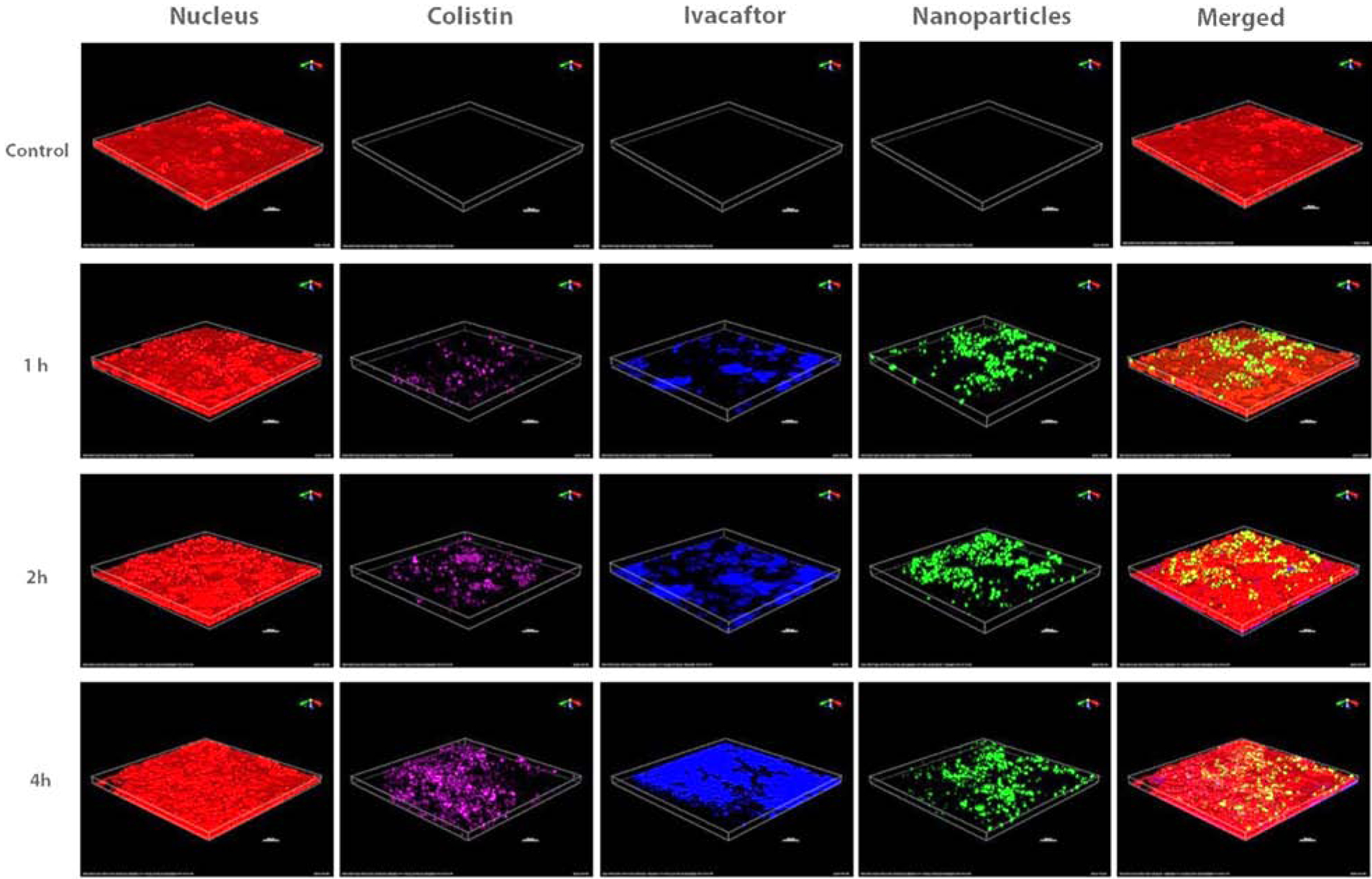
Distribution of colistin (purple), ivacaftor (blue) and nanoparticles (green) in the Calu-3 cell monolayer. Nucleus is in red and scale bar is 100 μm.
Discussion
In this study we investigated the transport, accumulation and toxicity in lung epithelial Calu-3 cells of a novel ivacaftor nanoparticle formulation with the potential addition of colistin. We observed how ivacaftor displayed a dose-dependent cytotoxic effect on Calu-3 cells. Ivacaftor was dissolved in dimethyl sulfoxide (DMSO) and then diluted with culture medium due to the low solubility of ivacaftor in the culture medium. The percentage of DMSO was no more than 0.5% (v/v) and did not show significant toxicity as compared to the control group. The excipients (BSA and Leu) were also safe for cells at the tested range. Thus, the cytotoxic effect of the solution mixture and the physical mixture was attributed to ivacaftor. A previous study also showed that ivacaftor is toxic to A549 cells at 8 μg/mL or higher (Zhu et al., 2020). The present study hypothesized that the cytotoxicity of ivacaftor was associated with its high hydrophobicity and cellular accumulation, which we observed in our transport studies (Fig. 2).
Interestingly, the nanoparticle formulation was relatively safer than the physical mixture (Rashidipour et al., 2019). For the colistin transport data, a significant amount of colistin remained in the donor chamber for both A-B and B-A by the end of the transport study (Fig. 3). BSA, a major drug carrier used in several controlled release formulations to affect drug transport and absorption through biological membranes, might affect the transport behavior of ivacaftor, although the mechanism is not clear (Kim et al., 2019; Zaman et al., 2018). Cross-linked BSA may bind with ivacaftor and affect the transport behavior in Calu-3 cells in both the particle mixture and nanoparticle formulation group (Fonseca et al., 2017; Sripriyalakshmi et al., 2014). We believe BSA played a significant role in affecting the colistin transport because BSA can bind with colistin and they share a common transport protein, megalin, in the cellular uptake by endocytosis (Ahmed et al., 2017; Buchackert et al., 2012; Dai et al., 2014; Suzuki et al., 2013). The accumulation of ivacaftor and colistin in the Calu-3 cell monolayer increased in a time-dependent manner (Fig. 4). Higher hydrophobicity and lower molecular weight might be the reason for the higher accumulation of ivacaftor in Calu-3 cells as compare to colistin. Ivacaftor-induced cytotoxicity (Fig. 1) could be due to its substantial accumulation in Calu-3 cells.
A recent study demonstrated that ivacaftor amorphous nanoparticle formulation with colistin showed superior aerosol performance by using BSA as a carrier and L-Leucin as an aerosolization enhancer (Zhu et al., 2020). Since drug dissolution is the first step for drug efficacy and it is well known that drugs transport strictly depends on drug dissolution (Siepmann and Peppas, 2011), effects of dissolution on drug transport behavior were examined in this study. Earlier study showed that the amorphous nanoparticle formulation achieved enhanced dissolution rate for ivacaftor as compared with the physical mixture, due to its larger specific surface area and amorphous form. Such enhanced dissolution was reflected by less ivacaftor found in the donor chamber from A-B for the high ivacaftor concentrations of 6 and 10 μg/mL. It is not surprising that the ivacaftor solution group showed a faster ivacaftor transport rate, as well as greater retention in cells, as compared to the nanoparticle formulation group as the solution group do not have the dissolution step for ivacaftor, a poorly soluble drug. Interestingly, ivacaftor penetration across the cell monolayer from A-B significantly increased for the nanoparticle formulation group, which explained overall less ivacaftor accumulation in the cells than the solution group.
Data from our current study shows the potential of the ivacaftor-colistin nanoparticle formulation for pulmonary drug delivery, with faster-release of drugs on the lung epithelial surface as compared to the physical mixtures. Synergistic killing activity of polymyxin B and ivacaftor combination against polymyxin-resistant P. aeruginosa isolates has been reported in a previous study (Schneider et al., 2016a). Since significant accumulation of ivacaftor inside lung cells caused toxicity, the nanoparticle formulation showed a safer profile in the human lung epithelial cell study, due to reduced cell accumulation of both drugs and potential protective effects from BSA. It would offer a safer and promising therapy for treatment against MDR Gram-negative bacteria associated lung infections. In addition, in vivo pharmacokinetic/pharmacodynamic (PK/PD) study are warranted to evaluate the toxicity and efficacy further in the established mouse lung infection model (Lin et al., 2018).
Conclusion
Pulmonary delivery of colistin in combination with the synergistic drug ivacaftor using dry powder inhalers directly into the lungs could be effective in treating lung infections caused by MDR P. aeruginosa. In the present study, we examined cytotoxicity, drug transport, and cell accumulation of ivacaftor and colistin as well as their mixtures and nanoparticle formulation in Calu-3 human lung epithelial cells. Ivacaftor alone, in both solution mixtures and particle mixtures, showed significant dose-dependent cytotoxicity in the lung epithelial Calu-3 cells. Surprisingly, nanoparticle formulation of ivacaftor combined with colistin showed much less cytotoxicity than the solution mixture and physical mixture. Such reduced toxicity is attributed to the decrease in the cellular accumulation of ivacaftor in the lung cells and potential protective effects by BSA. This study clearly demonstrate that our nanoparticle formulation may lead to a safer therapy for the treatment of lung infections associated with MDR Gram-negative bacteria.
Acknowledgement
This study was supported by the National Institutes of Health / National Institute of Allergy and Infectious Diseases (R01AI132681 and R01AI146160). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. J.C. is supported by a scholarship from China Scholarship Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahmed MU, Velkov T, Lin YW, Yun B, Nowell CJ, Zhou F, Zhou QT, Chan K, Azad MAK, Li J, 2017. Potential toxicity of polymyxins in human lung epithelial cells. Antimicrobial agents and chemotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed MU, Velkov T, Zhou QT, Fulcher AJ, Callaghan J, Zhou F, Chan K, Azad MAK, Li J, 2018. Intracellular localization of polymyxins in human alveolar epithelial cells. Journal of Antimicrobial Chemotherapy, dky409-dky409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos AC, Passé KM, Mouton JW, Janssens HM, Tiddens HAWM, 2017. The fate of inhaled antibiotics after deposition in cystic fibrosis: How to get drug to the bug? Journal of Cystic Fibrosis 16, 13–23. [DOI] [PubMed] [Google Scholar]
- Brunaugh AD, Smyth HDJIJo.P., 2018. Formulation techniques for high dose dry powders. 547, 489–498. [DOI] [PubMed] [Google Scholar]
- Buchackert Y, Rummel S, Vohwinkel CU, Gabrielli NM, Grzesik BA, Mayer K, Herold S, Morty RE, Seeger W, Vadasz I, 2012. Megalin mediates transepithelial albumin clearance from the alveolar space of intact rabbit lungs. The Journal of physiology 590, 5167–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai G, Park H, Yu S, Zhou F, Li J, Xu Q, Zhou QT, 2019. Evaluation of co-delivery of colistin and ciprofloxacin in liposomes using an in vitro human lung epithelial cell model. Int J Pharm 569, 118616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Ciccotosto GD, Cappai R, Tang S, Li D, Xie S, Xiao X, Velkov T, 2018. Curcumin Attenuates Colistin-Induced Neurotoxicity in N2a Cells via Anti-inflammatory Activity, Suppression of Oxidative Stress, and Apoptosis. Molecular Neurobiology 55, 421–434. [DOI] [PubMed] [Google Scholar]
- Dai C, Li J, Tang S, Li J, Xiao X, 2014. Colistin-induced nephrotoxicity in mice involves the mitochondrial, death receptor, and endoplasmic reticulum pathways. Antimicrobial agents and chemotherapy 58, 4075–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugs.com, 2020. Kalydeco Approval History: Development History and FDA Approval Process for Kalydeco. [Google Scholar]
- Fonseca DP, Khalil NM, Mainardes RM, 2017. Bovine serum albumin-based nanoparticles containing resveratrol: Characterization and antioxidant activity. Journal of Drug Delivery Science and Technology 39, 147–155. [Google Scholar]
- Haghi M, Young PM, Traini D, 2018. A Simple and Rapid Method for Deposition and Measurement of Drug Transport Across Air Interface Respiratory Epithelia. AAPS PharmSciTech 19, 3272–3276. [DOI] [PubMed] [Google Scholar]
- Haghi M, Young PM, Traini D, Jaiswal R, Gong J, Bebawy M, 2010. Time-and passage-dependent characteristics of a Calu-3 respiratory epithelial cell model. Drug development and industrial pharmacy 36, 1207–1214. [DOI] [PubMed] [Google Scholar]
- Hickey AJ, Misra A, Fourie P.B.J.J.o.p.s., 2013. Dry powder antibiotic aerosol product development: inhaled therapy for tuberculosis. 102, 3900–3907. [DOI] [PubMed] [Google Scholar]
- Kim D, Maharjan P, Jin M, Park T, Maharjan A, Amatya R, Yang J, Min KA, Shin MC, 2019. Potential Albumin-Based Antioxidant Nanoformulations for Ocular Protection against Oxidative Stress. Pharmaceutics 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuk K, Taylor-Cousar JL, 2015. Lumacaftor and ivacaftor in the management of patients with cystic fibrosis: current evidence and future prospects. Therapeutic advances in respiratory disease 9, 313–326. [DOI] [PubMed] [Google Scholar]
- Lin Y-W, Zhou QT, Han M-L, Chen K, Onufrak NJ, Wang J, Turnidge JD, Howden BP, Forrest A, Chan H.-K.J.A.a., chemotherapy, 2018. Elucidating the Pharmacokinetics/Pharmacodynamics of Aerosolized Colistin against Multidrug-Resistant Acinetobacter baumannii and Klebsiella pneumoniae in a Mouse Lung Infection Model. 62, e01790–01717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YW, Zhou Q, Onufrak NJ, Wirth V, Chen K, Wang J, Forrest A, Chan HK, Li J, 2017a. Aerosolized Polymyxin B for Treatment of Respiratory Tract Infections: Determination of Pharmacokinetic-Pharmacodynamic Indices for Aerosolized Polymyxin B against Pseudomonas aeruginosa in a Mouse Lung Infection Model. Antimicrobial agents and chemotherapy 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YW, Zhou QT, Cheah SE, Zhao J, Chen K, Wang J, Chan HK, Li J, 2017b. Pharmacokinetics/Pharmacodynamics of Pulmonary Delivery of Colistin against Pseudomonas aeruginosa in a Mouse Lung Infection Model. Antimicrobial agents and chemotherapy 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodato EM, Kaplan W, 2013. Background Paper 6.1 Antimicrobial resistance. World Health Organization, Geneva. [Google Scholar]
- Mangal S, Huang J, Shetty N, Park H, Lin YW, Yu HH, Zemlyanov D, Velkov T, Li J, Zhou QT, 2019. Effects of the antibiotic component on in-vitro bacterial killing, physico-chemical properties, aerosolization and dissolution of a ternary-combinational inhalation powder formulation of antibiotics for pan-drug resistant Gram-negative lung infections. Int J Pharm 561, 102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P, Kadam S, Pawar A, Bothiraja C, 2019. Dendrimers for pulmonary delivery: current perspectives and future challenges. New Journal of Chemistry 43, 8396–8409. [Google Scholar]
- Min KA, Rosania GR, Kim CK, Shin MC, 2016. Functional and cytometric examination of different human lung epithelial cell types as drug transport barriers. Archives of pharmacal research 39, 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HX, Traini D, Bebawy M, Young PM, 2011. Epithelial profiling of antibiotic controlled release respiratory formulations. Pharmaceutical research 28, 2327–2338. [DOI] [PubMed] [Google Scholar]
- Ong HX, Traini D, Young PM, 2013. Pharmaceutical applications of the Calu-3 lung epithelia cell line. Expert opinion on drug delivery 10, 1287–1302. [DOI] [PubMed] [Google Scholar]
- Orsi TD, Perdigão Neto LV, Martins RCR, Levin AS, Costa SF, 2019. Polymyxin-resistant Pseudomonas aeruginosa assigned as ST245: First report in an intensive care unit in São Paulo, Brazil. Journal of global antimicrobial resistance 16, 147–149. [DOI] [PubMed] [Google Scholar]
- Panduga V, Stocks MJ, Bosquillon C, 2017. Ipratropium is ‘luminally recycled’ by an inter-play between apical uptake and efflux transporters in Calu-3 bronchial epithelial cell layers. Int J Pharm 532, 328–336. [DOI] [PubMed] [Google Scholar]
- Quon BS, Goss CH, Ramsey BW, 2014. Inhaled Antibiotics for Lower Airway Infections. Annals of the American Thoracic Society 11, 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidipour M, Maleki A, Kordi S, Birjandi M, Pajouhi N, Mohammadi E, Heydari R, Rezaee R, Rasoulian B, Davari B, 2019. Pectin/Chitosan/Tripolyphosphate Nanoparticles: Efficient Carriers for Reducing Soil Sorption, Cytotoxicity, and Mutagenicity of Paraquat and Enhancing Its Herbicide Activity. Journal of Agricultural and Food Chemistry 67, 5736–5745. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M, Wainwright CE, Higgins M, Wang LT, McKee C, Campbell D, Tian S, Schneider J, Cunningham S, Davies JC, Rosenfeld M, Wainwright CE, Higgins M, Wang LT, McKee C, Campbell D, Tian S, Schneider J, Cunningham S, Davies JC, Harris W, Mogayzel P, McCoy K, Milla C, Rubenstein R, Walker S, Black P, Montgomery G, McColley S, Hiatt P, Sawicki G, Rock M, Aurora P, Ratjen F, Maitra A, Ives A, Gaillard E, McNalley P, Selvadurai H, Robinson P, 2018. Ivacaftor treatment of cystic fibrosis in children aged 12 to 24 months and with a CFTR gating mutation (ARRIVAL): a phase 3 single-arm study. The Lancet Respiratory Medicine 6, 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadikot RT, Blackwell TS, Christman JW, Prince AS, 2005. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. American journal of respiratory and critical care medicine 171, 1209–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Futschik EK, Paulin OKA, Hoyer D, Roberts KD, Ziogas J, Baker MA, Karas J, Li J, Velkov T, 2018. Sputum Active Polymyxin Lipopeptides: Activity against Cystic Fibrosis Pseudomonas aeruginosa Isolates and Their Interactions with Sputum Biomolecules. ACS infectious diseases 4, 646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider EK, Azad MAK, Han M-L, Tony Zhou Q, Wang J, Huang JX, Cooper MA, Doi Y, Baker MA, Bergen PJ, Muller MT, Li J, Velkov T, 2016a. An “Unlikely” Pair: The Antimicrobial Synergy of Polymyxin B in Combination with the Cystic Fibrosis Transmembrane Conductance Regulator Drugs KALYDECO and ORKAMBI. ACS infectious diseases 2, 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider EK, Reyes-Ortega F, Wilson JW, Kotsimbos T, Keating D, Li J, Velkov T, 2016b. Development of HPLC and LC-MS/MS methods for the analysis of ivacaftor, its major metabolites and lumacaftor in plasma and sputum of cystic fibrosis patients treated with ORKAMBI or KALYDECO. J Chromatogr B Analyt Technol Biomed Life Sci 1038, 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sermet-Gaudelus I, 2013. Ivacaftor treatment in patients with cystic fibrosis and the G551D-CFTR mutation. European Respiratory Review 22, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty N, Cipolla D, Park H, Zhou QT, 2020. Physical stability of dry powder inhaler formulations. Expert Opinion on Drug Delivery 17, 77–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouren G, Kojio K, Takahara A, Kajiyama T, 1998. Bovine serum albumin adsorption onto immobilized organotrichlorosilane surface: Influence of the phase separation on protein adsorption patterns. Journal of Biomaterials Science, Polymer Edition 9, 131–150. [DOI] [PubMed] [Google Scholar]
- Siepmann J, Peppas NA, 2011. Higuchi equation: derivation, applications, use and misuse. Int J Pharm 418, 6–12. [DOI] [PubMed] [Google Scholar]
- Sripriyalakshmi S, Anjali C, George Priya Doss C, Rajith B, Ravindran A, 2014. BSA Nanoparticle Loaded Atorvastatin Calcium - A New Facet for an Old Drug. PloS one 9, e86317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Yamaguchi H, Ogura J, Kobayashi M, Yamada T, Iseki K, 2013. Megalin contributes to kidney accumulation and nephrotoxicity of colistin. Antimicrobial agents and chemotherapy 57, 6319–6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velkov T, Yun B, Schneider EK, Azad MAK, Dolezal O, Morris FC, Nation RL, Wang J, Chen K, Yu HH, Wang L, Thompson PE, Roberts KD, Li J, 2016. A Novel Chemical Biology Approach for Mapping of Polymyxin Lipopeptide Antibody Binding Epitopes. ACS infectious diseases 2, 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Wang Y, Guo Z, Chen J, Lin L, Wu J, Tian H, Chen X, 2019. Pulmonary delivery by exploiting doxorubicin and cisplatin co-loaded nanoparticles for metastatic lung cancer therapy. J Control Release 295, 153–163. [DOI] [PubMed] [Google Scholar]
- Yapa WS, Li J, Patel K, Wilson JW, Dooley MJ, George J, Clark D, Poole S, Williams E, Porter CJ, Nation RL, McIntosh MP, 2014. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: targeting advantage of inhalational administration. Antimicrobial agents and chemotherapy 58, 2570–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Wang S, Zou P, Chai G, Lin Y-W, Velkov T, Li J, Pan W, Zhou QT, 2020. Inhalable liposomal powder formulations for co-delivery of synergistic ciprofloxacin and colistin against multi-drug resistant gram-negative lung infections. Int J Pharm 575, 118915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman RU, Mulla NS, Braz Gomes K, D’Souza C, Murnane KS, D’Souza MJ, 2018. Nanoparticle formulations that allow for sustained delivery and brain targeting of the neuropeptide oxytocin. Int J Pharm 548, 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Leung SSY, Tang P, Parumasivam T, Loh ZH, Chan H-K, 2015. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Advanced drug delivery reviews 85, 83–99. [DOI] [PubMed] [Google Scholar]
- Zhou QT, Tang P, Leung SSY, Chan JGY, Chan H-K, 2014. Emerging inhalation aerosol devices and strategies: where are we headed? Advanced drug delivery reviews 75, 3–17. [DOI] [PubMed] [Google Scholar]
- Zhu C, Chen J, Yu S, Que C, Taylor LS, Tan W, Wu C, Zhou QT, 2020. Inhalable Nanocomposite Microparticles with Enhanced Dissolution and Superior Aerosol Performance. DOI: 10.1021/acs.molpharmaceut.0c00390. Molecular Pharmaceutics 17, 3270–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]


