Abstract
Background:
Prostate-specific membrane antigen (PSMA)-targeted radionuclide therapy (TRT) has demonstrated efficacy and tolerability with a dose-response effect in phase I/II trials in men with metastatic castration-resistant prostate cancer (mCRPC). The need for positive PSMA imaging prior to PSMA-TRT to select patients is largely practiced, but its utility is not proven. Given target heterogeneity, developing a biomarker to identify the optimal patient population remains an unmet need. The aim of this study was to assess PSMA uptake by imaging and response to PSMA-TRT.
Methods:
We performed an analysis of men with mCRPC enrolled in sequential prospective phase I/II trials of PSMA-TRT. Each patient had baseline PSMA imaging by planar 111In and/or 177Lu SPECT (N=171) or 68Ga-PSMA-11 PET/CT (N=44), but results were not used to include/exclude treatment. Semi-quantitative imaging scores (IS) on a 0–4 scale were assigned based on PSMA uptake in tumors compared to liver uptake. We compared the ≥50% PSA decline response proportions between low (0–1) and high (2–4) PSMA IS using the chi-square test. We used multivariable logistic regression analysis to understand the relationship between independent and dependent variables, including IS, radionuclide activity (dose) administered, CALGB (Halabi) prognostic risk score, prior taxane use.
Results:
215 men with progressive mCRPC received PSMA-TRT as follows: 177Lu-J591 (n = 137), 177Lu-PSMA-617 (n = 44), 90Y-J591 (n = 28), 177Lu-J591 + 177Lu-PSMA-617 (n = 6). High PSMA expression (IS 2–4) was found in 160 (74.4%) patients and was significantly associated with more frequent ≥50% PSA reduction (26.2 versus 7.3%, p = 0.006). On multivariate logistic regression analysis, higher IS was associated with ≥50% decrease in PSA, even after accounting for CALGB (Halabi) prognostic score, dose administered, and previous taxane use (OR 4.72, 95% CI: 1.71 −16.85, p = 0.006). Patients with low PSMA expression (N=55, 24.7%) were less likely to respond. 13 of 26 (50%) with no PSMA uptake (IS=0) had post-PSMA-TRT PSA decline with 2 (7.7%) having ≥50% PSA declines.
Conclusion:
Collectively, the data provide evidence in favor of the hypothesis that patients with high PSMA uptake and high administered radionuclide dose correlate with a higher chance of response.
1. INTRODUCTION
Prostate-specific membrane antigen (PSMA) is a cell surface marker present and enriched in 75–95% of metastatic castration-resistant prostate cancer (mCRPC). PSMA-targeted radionuclide therapy (TRT) is a promising therapeutic approach for mCRPC. We and others have performed a series of phase I and II studies utilizing antibodies or small molecule ligands conjugated with beta-emitting radionuclides including lutetium 177 (177Lu) and ytrium 90 (90Y).1–8 These sequential studies have demonstrated the safety and activity of PSMA-TRT1–8 as well as a dose-response relationship.3,4,6
Theranostics is an approach involving molecular imaging of an antigen followed by treatment via radionuclides targeting the same antigen. While there may be some heterogeneity, PSMA is expressed by nearly all CRPCs. Nevertheless, the requirement for positive PSMA imaging to respond to PSMA-TRT has not been proven. PSMA uptake on imaging with whole body gamma camera single-photon emission computed tomography (SPECT) scans or PSMA-positron emission tomography (PET) may be performed and has been suggested as a non-invasive approach for patient selection for PSMA-TRT.9–12 As PSMA was initially described as universally expressed and there was no available evidence that PSMA imaging positivitiy is a prerequisite for benefit from PSMA-TRT, several prospective studies have been performed including baseline PSMA imaging, but without selection based upon results. However, we hypothesized that the amount of PSMA-uptake may have a relationship with response to PSMA-TRT. To test this hypothesis, we prospectively included an analysis of PSMA imaging and outcome into each of our studies. Our data with individual studies has provided early evidence that those with poorer imaging have a lower chance of response to PSMA-TRT.3,4,6 However, each of the individual trials were small, and hence we pooled data across trials with similar entry criteria.
2. MATERIALS AND METHODS
2.1. Patient population
We performed a post-hoc analysis of all subjects who were included in our phase I/II beta radiation emitting PSMA-TRT studies with enrollment during the period between July 2002 and October 2018.1–4, 6,7 Key eligibility criteria included prospective mCRPC by Prostate Cancer Working Group criteria with no available standard therapies or refusal of standard therapies and intact performance status and organ function as previously described. In these prospective clinical trials, all patients undergoing PSMA-TRT remained on their pre-trial continuous androgen- deprivation therapy. No other concomitant anti-neoplastic therapies, including androgen-receptor signaling pathway inhibitors, chemotherapy, radium-223 or sipuleucel-T, were allowed during the assessment period. All subjects had gamma imaging (111In SPECT and/or 177Lu SPECT, N=171) and/or 68Ga-PSMA-11 PET/CT (N=44) but were not selected for treatment based upon imaging results (Table 1). Planar and SPECT gamma camera images were obtained 5 to 7 days after infusion with 177Lu-J591 (N=122) or 177Lu-PSMA-617 (N=6). Patients who were treated with 90Y-J591 (N=28)1 and expansion cohorts of the 177Lu-J591 phase II study (N=15]3 also underwent pretreatment imaging 3 to 4 days after 111In-J591 infusion. 68Ga-PSMA11 PET/CT was performed in patients who received 177Lu-PSMA-617 with or without 177Lu-J591 (N=44). With respect to radionuclide activity for pre-treatment imaging, 5mCi of 111In were used. As 177Lu is a therapeutic radionuclide with both beta and gamma emission, other than those that were imaged with 111In or 68Ga, there was no pre-treatment imaging specifically using 177Lu; rather images used for initial treatment served as baseline imaging.1–4, 6, 7
TABLE 1.
Abbreviations of radionuclides, ligands and imaging modalities used in this study.
| Abbreviation | Explanation |
|---|---|
| PET Nuclides | |
| 68Ga | Gallium-68, t1/2 : 68 minutes, B+ decay |
| SPECT Nuclides | |
| 111In | Indium-111, t1/2 : 67.2 hours, gamma |
| Therapeutic and SPECT Nuclides | |
| 177Lu | Lutetium-177, t1/2: 6.65 days, B- decay |
| PSMA targeting agents | |
| J591 | Monoclonal antibody to PSMA extracellular domain |
| Ligands | Binding the extracellular domain of PSMA |
| PSMA-11 | Small molecule PSMA ligand |
| PSMA-617 | Small molecule PSMA ligand |
| Imaging modalities | |
| Planar | Acquisition of 2D nuclear images, similar to plain films in x-ray imaging. |
| PET | Positron emission tomography - form of tomographic nuclear imaging that relies on the near simultaneous detection of the pair of gamma photons that are released from an annihilation of a positron and an electron. |
| SPECT | Single-photon emission computed tomography - method of obtaining cross-sectional nuclear images using single gamma photon detection that are produced by gamma photon decay. |
2.2. PSMA imaging score calculation
To measure PSMA uptake we used a semi-quantitative imaging with a five point imaging score as previously described.3,4,6,7 Imaging scores (IS) were assigned based upon PSMA uptake in tumors compared to liver uptake and scored by two independent radiologists on a 0–4 scale (Table S1). For planar imaging, the three lesions with the highest uptake were scored. PET images were scored by averaging SUVmax of the five lesions with highest uptake and then comparing that value with liver SUVmean.
2.3. Statistical analysis
To assess the association between imaging and response, we compared the ≥50% PSA decline response proportions between low (0–1) and high (2–4) PSMA IS using the chi-square test. We used multivariable logistic regression analysis to understand the relationship between independent and dependent variables, including IS, CALGB (Halabi) prognostic risk score (which includes sites of metastatic disease - bone, lymph node or/and visceral, opioid analgesic use, ECOG performance status, lactate dehydrogenase, albumin, hemoglobin, alkaline phosphatase, and PSA13), and prior taxane use. In addition, as several of our studies were dose-escalation studies and have previously reported a dose-response relationship, radionuclide activity (dose) administered was another variable utilized (lower dose vs recommended phase II dose = RP2D). Statistical significance was set at the 0.05 alpha level. Analyses were performed using STATA version 15.0 (StataCorp).
3. RESULTS
3.1. Patient characteristics
Two hundred fifteen men with progressive mCRPC received PSMA-TRT as follows: 177Lu-J591 (N = 137), 177Lu-PSMA-617 (N = 44), 90Y-J591 (N = 28), 177Lu-J591 + 177Lu-PSMA-617 (N = 6) (Table 2).1–4, 6,7 The majority of patients who were imaged with SPECT (N = 171) and were treated with 177Lu-J591 (N = 137). Additionally, all of the patients who were assessed with 68Ga-PSMA PET/CT for IS were treated with 177Lu-PSMA-617 alone (N = 38) or in combination with 177Lu-J591 (N = 6). As part of phase I/II dose-escalation studies, patients received low dose (less than recommended phase 2 dose, RP2D) or RP2D, as previously defined in the individual studies.1–4, 6,7
TABLE 2.
Clinical characteristics of patients with metastatic CRPC treated with beta-emitting PSMA-TRT
| Characteristics | Value (n=215) |
|---|---|
| Age (yrs), median (range) | 70.8 (44.6–93.4) |
| ECOG PS, n(%) | |
| 0 | 19 (8.8) |
| 1 | 177 (82.3) |
| 2 | 18 (8.4) |
| 3 | 1 (0.5) |
| Gleason sum, n(%) | |
| ≤6 | 35 (16.3) |
| 7 | 60 (27.9) |
| 8–10 | 117 (54.4) |
| PSA baseline (ng/ml), median (range) | 73.55 (0.49 – 2746) |
| Sites of metastases, n(%) | |
| Bone | 193 (89.8) |
| Lymph node | 117 (54.4) |
| Lung | 35 (16.3) |
| Liver | 19 (8.8) |
| Other | 7 (3.2) |
| CALGB prognostic group, n(%) | |
| High | 139 (64.6) |
| Intermediate | 67 (31.2) |
| Low | 9 (4.2) |
| Previous therapies, n(%) | |
| Taxanes | 129 (60.0) |
| Abiraterone/Enzalutamide | 65 (30.2) |
| Radium-223 | 15 (6.9) |
| Sipuleucel-T | 22 (10.2) |
| Beta-emitting PSMA-TRT types, n(%) | |
| 177Lu-J591 | 137 (63.7) |
| 177Lu-PSMA-617 | 44 (20.5) |
| 90Y-J591 | 28 (13.0) |
| 177Lu-J591 + 177Lu-PSMA-617 | 6 (2.8) |
| Dose level, n(%) | |
| Low | 111 (51.6) |
| Full | 104 (48.4) |
| PSMA imaging | |
| SPECT | 171 (79.5) |
| PET | 44 (20.5) |
3.2. PSMA imaging score and response
Fifty five (25.6%) patients had low PSMA expression by imaging (IS 0–1) whereas 160 (74.4%) had strong PSMA (IS 2–4) (Table 2, Figure 1). More intense PSMA imaging was associated more ≥50% PSA declines compared with less intense imaging (26.2 versus 7.3%; p = 0.006) (Table 3). Patients with high PSMA uptake who received RP2Ds were more likely to have ≥50% PSA decline (36.1%; p = 0.036) (Table 3). This analysis was similar in those imaged with SPECT versus PET. However, because antibody and small-molecule PSMA-TRT demonstrate different bio-distribution, toxicity profiles and efficacy across early phase studies,15 we performed separate analyses in patients who received J591-based PSMA-TRT (N = 165) and those who received PSMA-617 (N = 38) after excluding 6 patients who received the combination. We confirmed an association between higher doses and PSA decline in both groups, which was most prominent in patients receiving J591 (≥50% PSA decline: 15/77 or 19.5%; p = 0.002 and ≥30% PSA decline: 33/77 or 42.8%; p < 0.001).
FIGURE 1.
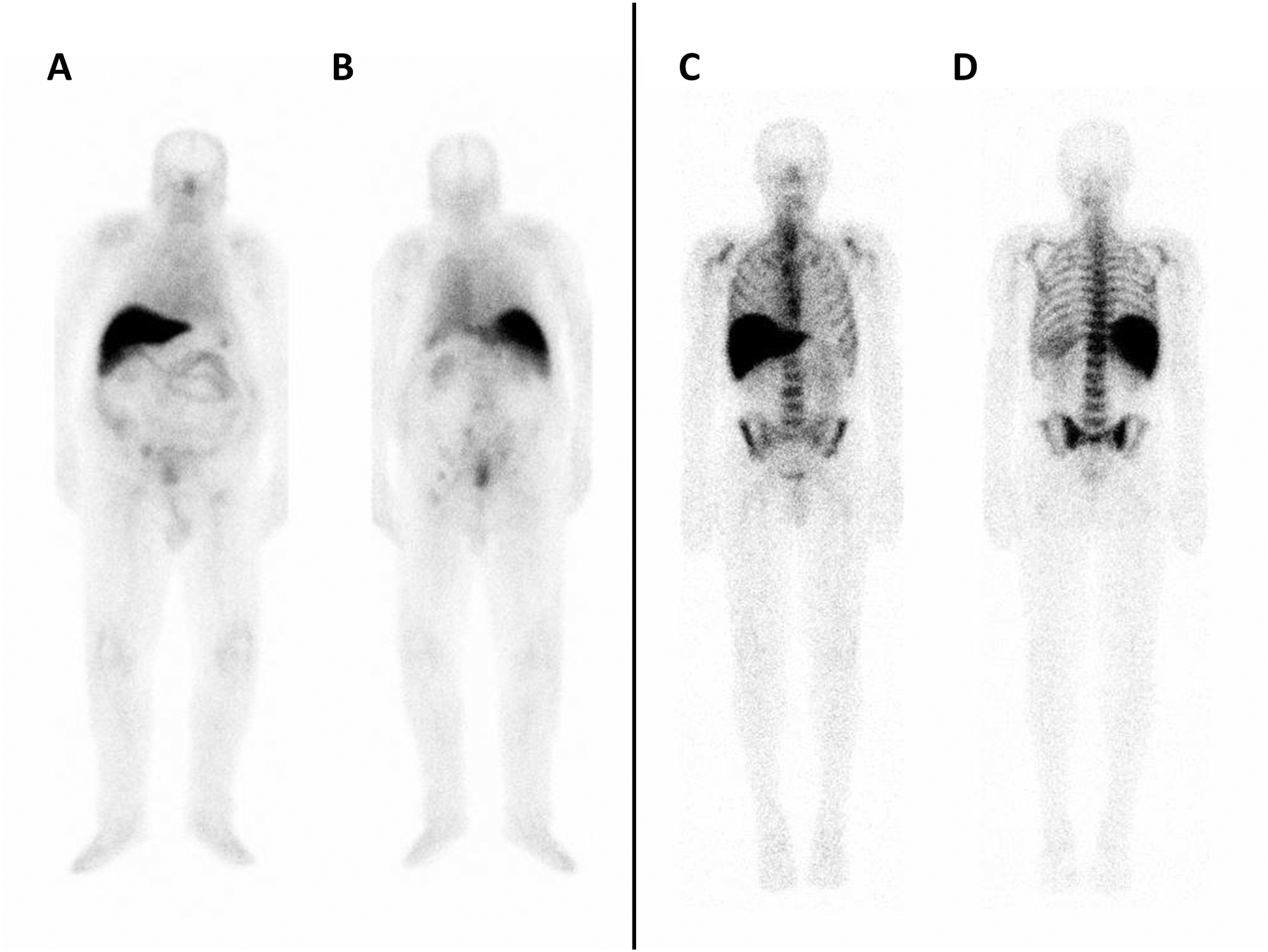
Planar imaging in two patients with different PSMA uptake. Left panel: Patient 1 (IS 1) following IV administration of 177Lu-J591. From left to right: (A) anterior, and (B) posterior view showing uptake in right external iliac node. Right panel: Patient 2 (IS 4) following IV administration of 177Lu-J591. From left to right: (C) anterior, and (D) posterior view showing uptake in the thoracolumbar spine, bilateral ribs, sacrum, and bilateral iliac bones.
TABLE 3.
PSA response by imaging score and dose
| Any dose (N=215) | N of patients with PSA decline | P value | ||
|---|---|---|---|---|
| <50% | ≥50% | |||
| Imaging score | 0–1 | 51 (92.7%) | 4 (7.27%) | 0.006 |
| 2–4 | 118 (73.8%) | 42 (26.2%) | ||
| Low dose (N=111) | N of patients with PSA decline | P value | ||
| <50% | ≥50% | |||
| Imaging score | 0–1 | 32 (94.1%) | 2 (5.88%) | 0.220 |
| 2–4 | 65 (84.4%) | 12 (15.6%) | ||
| Full dose (N=104) | N of patients with PSA decline | P value | ||
| <50% | ≥50% | |||
| Imaging score | 0–1 | 19 (90.5%) | 2 (9.52%) | 0.036 |
| 2–4 | 53 (63.9%) | 30 (36.1%) | ||
Two out of 26 (7.7%) patients with no PSMA uptake (IS=0), representing 0.9% of the total cohort, had ≥50% PSA declines. All patients with IS 0 (N = 26) underwent SPECT imaging. Of those, five patients had 111In and the rest had 177Lu SPECT.The majority of patients (N = 17) had lymph node or/and bone metastases (N = 15). Eight patients had visceral disease, with presence of lung (N = 6) or/and liver (N = 3) metastases.
To better understand the implications of complete absence of PSMA expression (IS=0) and response, we compared ≥30% PSA decline and any % PSA decline in three subgroups of patients: IS 0, IS 1, and IS 2–4. In addition to confirming an association between higher PSMA expression and response, we identified 3 patients out of 26 with complete lack of PSMA uptake (IS=0) who experienced ≥30% PSA decline and 13 (50% of those with IS=0) who had any PSA decline (Table S2).
To assess for an independent effect of IS on predicting PSA responses, we performed a multivariable logistic regression analysis including CALGB (Halabi) prognostic score, previous taxane use, and radionuclide dose administered. Higher imaging score was associated with ≥50% decrease in PSA, even after accounting for CALGB (Halabi) prognostic score, dose administered, and previous taxane-use (OR 4.72, 95%CI: 1.71 – 16.85, p = 0.006) (Table 4).
TABLE 4.
Multivariable Logistic Regression Associated with >50% PSA Response
| Odds Ratio | 95% CI | P value | |
|---|---|---|---|
| Imaging score (2–4 vs 0–1) |
4.72 | 1.71 – 16.85 | 0.006 |
| CALGB (Halabi) score (High vs. Low/Intermediate) |
0.66 | 0.32 – 1.39 | 0.272 |
| Dose (RP2D vs Lower) |
2.81 | 1.39 – 5.91 | 0.005 |
| Previous taxane (Yes vs No) |
0.69 | 0.34 – 1.39 | 0.296 |
4. DISCUSSION
The field of theranostics revolves around a “see what you treat” philosphophy, but relies on imaging as a predictive biomarker. In general, to fully assess a biomarker, one needs to treat both those positive and negative for that biomarker to assess its impact. Our study is the first to formally analyze response to beta-emitting PSMA-TRT by PSMA imaging expression in unselected patients with metastatic CRPC. According to our semi-quantitative assessment of PSMA uptake on PSMA imaging (SPECT or PET) and in line with our hypothesis, patients that have a higher PSMA uptake are more likely to respond to PSMA-TRT, as are those with higher (RP2D) doses administered. However, a small subset of patients without detectable PSMA uptake on imaging did demonstrate response. This may suggest the presence of micrometastatic disease that, although not detectable on imaging, may still be effectively targeted by the PSMA-TRT. In an ex vivo system of water-density spheres irradiated with various beta-emitters, the shorter-range emitter 177Lu outperformed 90Y in treating micrometastases (<100μM) and single cells, but required much higher dosing due to an inverse relationship between tumor size and dose delivered.14 In line with this observation, the presence of lesions below the level of detectability by planar/SPECT (<500–1000μm) and the fact that the majority (18/26, 69%) of our patients with no PSMA uptake (IS=0) received high (RP2D) doses, can both explain responses could still be achieved in such patients by administering higher doses that could “overcome” low PSMA expression. Notably, our analysis included patients from older prospective studies in which only planar single-photon imaging was available for assessment of PSMA uptake (i.e. before the development of PSMA PET). Planar imaging has an overall lower sensitivity for lesion detection compared to 68Ga/18F-PSMA PET/CT and may fail to detect small volume disease.11 Hence, it is likely that a proportion of our patients with IS of 0–1 (54/171, 31%) who were all assessed by SPECT imaging only, might have been re-classified as having detectable, PSMA-avid disease if they had been assessed with 68Ga-PSMA PET/CT. Irrespectively, membranous PSMA immunohistochemical expression in mCRPC patients may be characterized by marked intra-tumor and intra-patient heterogeneity between metastases.16 Thus, not all metastases may be detectable by 68Ga-PSMA PET/CT at baseline. Likewise post-PSMA-TRT, it was suggested by retrospective data from 48 patients with mCRPC treated with 177Lu-PSMA-617 that 68Ga-PSMA PET/CT has a sensitivity of only about 85% and a specificity of between 55% and 65% for predicting PSA responses, with negative and positive predictive values ranging between 70% and 78%.17
Other prospective studies as well as compassionate use treatment have excluded patients with “low”, often undefined, PSMA expression on imaging using variable cutoffs, sometimes in comparison with other imaging modalities such as fluorodeoxyglucose (FDG)-PET.18 While other studies have excluded those with no or discordant PSMA/FDG imaging, 68Ga-PSMA-11 SUV at screening has been associated with PSA reduction in some studies.19 Using a detection threshold of SUV>3, the mean intensity of PSMA-avid tumor uptake was reported to be predictive of overall survival (OS) in men with mCRPC treated with 177Lu-PSMA-617.20 The median OS of patients in this phase II trial of 177Lu-PSMA-617 was 13.3 months.21 These studies are in accordance with our results supporting a role of PSMA imaging in predicting higher probability of improved outcomes after beta-emitting PSMA-TRT in patients with detectable PSMA uptake. In their phase II study of 177Lu-PSMA-617, Hofman et al. reported a ≥50% PSA decline in 17/30 (57%) of their patients.5 Our lower rate of ≥50% PSA declines in this combined cohort (36.1%) is likely best explained by lower dose of radionuclide delivered to tumors in most of these studies, as several of the studies were dose-escalation by nature and J591 was used as the carrier in most, Specifically, most of the patients in the present report were treated with 177Lu-J591 (N = 137), of whom 44% (N = 60) received less than RP2Ds. A prospective head-to-head comparison between 177Lu-J591 and 177Lu-PSMA-617 would be required to evaluate potential differences in responses between these two therapies. While our findings support the use of PSMA imaging as a selection tool for single-agent PSMA-TRT, the data suggest that negative PSMA imaging might not be able to fully exclude all patients that could benefit. However, in further support of PSMA imaging selection for PSMA-TRT, is the randomized TheraP trial which compares PSA responses from 177Lu-PSMA-617 versus cabazitaxel chemotherapy in men with progressive mCRPC following docetaxel.22 In this study, using a higher than usual PSA entry criteria plus strict determination of PSMA avidity by 68 Ga-PSMA-11 and FDG-PET/CT to exclude discordant FDG-avid PSMA-negative sites of disease, PSA responses were superior with 177Lu-PSMA-617 compared to cabazitaxel.23
We sought out to assess whether PSMA imaging is associated with response to PSMA-TRT over a decade ago and built in this secondary endpoint into each of our prospective trials. To fully assess a biomarker, one must treat those with and without biomarker positivity. However, though all data was collected prospectively as part of registered clinical trials, limitations of this post-hoc analysis combining data across trials include uncertainty for cut-offs that may potentially define different levels of PSMA expression, the use of two different imaging modalities with different detection sensitivities, which may be differentially affected by tumor thickness,24 and use of different doses of therapy. A more homogeneous prospective study will be required to confirm our hypothesis-generating findings. Additionally, our study population was heterogeneous with respect to treatment schedules but homogenous in terms of the target (PSMA) and types of radionuclides used (beta-emitters only).
5. CONCLUSIONS
In conclusion, non-invasive PSMA imaging may become a useful tool to select the population of patients more likely to respond to single-agent PSMA-TRT. However, imaging might not completely exclude all patients that benefit and higher administered doses lead to more responses independent of imaging.
Supplementary Material
ACKNOWLEDGMENT
Thanks to Sharon Singh for providing administrative support.
Funding Statement
US Department of Defense grants W81XWH-13-PCRP-CCA, W81XWH-09-1-0596, W81XWH-04-1-0267, W81XWH-14-2-0159 and W81XWH-17-PCRP-IA; the Prostate Cancer Foundation; National Institutes of Health grants ULI RR024996, 1-KL2-RR024997-01, 7R01CA207645 and PTBF5405; the David H. Koch Foundation; the Robert Dow Foundation; and the Lawrence and Carol Zicklin Charitable Trust.
Footnotes
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Disclosure / Conflict of interest Statement
PJV certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: NHB is an inventor of patents assigned to the Cornell Center for Technology Licensing for the J591 antibody described in this article. He is also a paid consultant for and holds equity in BZL Biologics, LLC, the company to which these patents were licensed for further research and development. STT has served as a paid consultant to Endocyte/AAA/Novartis, POINT Biopharma and Blue Earth Diagnostics, as an unpaid consultant to Atlab and Telix, and Weill Cornell Medicine has received research funding from Endocyte/AAA/Novartis and Atlab/Telix. The other authors have no relevant disclosures.
Ethics Approval Statement
The study was approved by the Institutional Review Board of Weill Cornell Medicine – New York Presbyterian Hospital, New York, NY. The study was conducted in accordance with Helsinki Declaration as revised in 2013.
Patient Consent Statement
Written informed consent was obtained from the participants of all clinical trials used for this post-hoc analysis.
Clinical trial registration
All patients included in this study participated in the following clinical trials registered on ClinicalTrials.gov: NCT00003391, NCT00195039, NCT00538668, NCT03545165, NCT03042468, NCT03276572.
REFERENCES
- 1.Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ, Bander NH. Phase I trial of yttrium-90-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer. J Clin Oncol. 2004;22:2522–2531. [DOI] [PubMed] [Google Scholar]
- 2.Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol 2005;23:4591–4601. [DOI] [PubMed] [Google Scholar]
- 3.Tagawa ST, Milowsky MI, Morris M, et al. Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res. 2013;19:5182–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tagawa ST, Vallabhajosula S, Christos PJ, et al. Phase 1/2 study of fractionated dose lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 (177 Lu-J591) for metastatic castration-resistant prostate cancer. Cancer. 2019;125:2561–2569. [DOI] [PubMed] [Google Scholar]
- 5.Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33. [DOI] [PubMed] [Google Scholar]
- 6.Tagawa ST, Osborne JR, Hackett A, et al. Preliminary results of a phase I/II dose-escalation study of fractionated dose 177Lu-PSMA-617 for progressive metastatic castration resistant prostate cancer (mCRPC). Ann Oncol. 2019;30 (suppl_5):mdz248.006. doi: 10.1093/annonc/mdz248.006. [DOI] [Google Scholar]
- 7.Niaz MJ, Batra JS, Walsh RD, et al. Pilot study of hyperfractionated dosing of lutetium-177–labeled anti–prostate-specific membrane antigen monoclonal antibody J591 (177Lu-J591) for metastatic castration-resistant prostate cancer. Oncologist. 2020;25(6):477–e895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calais J, Fendler WP, Eiber M, et al. RESIST-PC phase 2 trial: 177Lu-PSMA-617 radionuclide therapy for metastatic castrate-resistant prostate cancer. J Clin Oncol. 2019;37(no. 15_suppl):5028–5028. [Google Scholar]
- 9.Pandit-Taskar N, O’Donoghue JA, Divgi CR, et al. Indium 111-labeled J591 anti-PSMA antibody for vascular targeted imaging in progressive solid tumors. EJNMMI Res. 2015;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damjanovic J, Janssen JC, Prasad V, Diederichs G, Walter T, Brenner W, Makowski MR. 68Ga-PSMA-PET/CT for the evaluation of liver metastases in patients with prostate cancer. Cancer Imaging. 2019;19:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Violet J, Jackson P, Ferdinandus J, et al. Dosimetry of 177Lu-PSMA-617 in Metastatic Castration-Resistant Prostate Cancer: Correlations Between Pretherapeutic Imaging and Whole-Body Tumor Dosimetry with Treatment Outcomes. J Nucl Med. 2019;60:517–523. [DOI] [PubMed] [Google Scholar]
- 12.Rathke H, Holland-Letz T2, Mier W, et al. Response Prediction of 177Lu-PSMA-617 Radioligand Therapy Using Prostate-Specific Antigen, Chromogranin A, and Lactate Dehydrogenase. J Nucl Med. 2020;61:689–695. [DOI] [PubMed] [Google Scholar]
- 13.Halabi S, Lin CY, Kelly WK, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32:671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hindié E, Zanotti-Fregonara P, Quinto MA, Morgat C, Champion C. Dose Deposits from 90Y, 177Lu, 111In, and 161Tb in Micrometastases of Various Sizes: Implications for Radiopharmaceutical Therapy. J Nucl Med. 2016;57:759–764. [DOI] [PubMed] [Google Scholar]
- 15.Wester HJ, Schottelius M. PSMA-Targeted Radiopharmaceuticals for Imaging and Therapy. Semin Nucl Med. 2019;49:302–312. [DOI] [PubMed] [Google Scholar]
- 16.Paschalis A, Sheehan B, Riisnaes R, et al. Prostate-specific Membrane Antigen Heterogeneity and DNA Repair Defects in Prostate Cancer. Eur Urol. 2019;76(4):469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinzel A, Boghos D, Mottaghy FM, Gaertner F, Essler M, von Mallek D, Ahmadzadehfar H. 68Ga-PSMA PET/CT for monitoring response to 177Lu-PSMA-617 radioligand therapy in patients with metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46:1054–1062. [DOI] [PubMed] [Google Scholar]
- 18.Thang SP, Violet J, Sandhu S, et al. Poor Outcomes for Patients with Metastatic Castration-resistant Prostate Cancer with Low Prostate-specific Membrane Antigen (PSMA) Expression Deemed Ineligible for 177Lu-labelled PSMA Radioligand Therapy. Eur Urol Oncol. 2019; 2(6):670–676. [DOI] [PubMed] [Google Scholar]
- 19.Emmett L, Crumbaker M, Ho B, et al. Results of a Prospective Phase 2 Pilot Trial of 177Lu-PSMA-617 Therapy for Metastatic Castration-Resistant Prostate Cancer Including Imaging Predictors of Treatment Response and Patterns of Progression. Clin Genitourin Cancer. 2019;17:15–22. [DOI] [PubMed] [Google Scholar]
- 20.Ferdinandus J, Violet J, Sandhu S, et al. Prognostic biomarkers in men with metastatic castration-resistant prostate cancer receiving [177Lu]-PSMA-617. Eur J Nucl Med Mol Imaging. 2020;47(10):2322–2327. [DOI] [PubMed] [Google Scholar]
- 21.Violet J, Sandhu S, Iravani A, et al. Long term follow-up and outcomes of re-treatment in an expanded 50 patient single-center phase II prospective trial of Lutetium-177 (177Lu) PSMA-617 theranostics in metastatic castrate-resistant prostate cancer. J Nucl Med. 2020; 61(6):857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofman MS, Emmett L, Violet J, et al. TheraP: a randomized phase 2 trial of 177 Lu-PSMA-617 theranostic treatment vs cabazitaxel in progressive metastatic castration-resistant prostate cancer (Clinical Trial Protocol ANZUP 1603). BJU Int. 2019;124 Suppl 1:5–13. [DOI] [PubMed] [Google Scholar]
- 23.Hofman MS, Emmett L, Sandhu SK, et al. TheraP: A randomised phase II trial of 177Lu-PSMA-617 (LuPSMA) theranostic versus cabazitaxel in metastatic castration resistant prostate cancer (mCRPC) progressing after docetaxel: Initial results (ANZUP protocol 1603). J Clin Oncol. 2020;38(suppl):5500–5500. doi: 10.1200/JCO.2020.38.15_suppl.5500 [DOI] [Google Scholar]
- 24.Hope TA, Calais J, Zhang L, Dieckmann W, Millo C. 111 In-Pentetreotide Scintigraphy Versus 68 Ga-DOTATATE PET: Impact on Krenning Scores and Effect of Tumor Burden. J Nucl Med. 2019;60:1266–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


