Abstract
After spinal cord injury (SCI), the majority of individuals develop spasticity, a debilitating condition involving involuntary movements, co-contraction of antagonistic muscles, and hyperreflexia. By acting on GABAergic and Ca2+-dependent signaling, current anti-spastic medications lead to serious side effects, including a drastic decrease in motoneuronal excitability which impairs motor function and rehabilitation efforts. Exercise, in contrast, decreases spastic symptoms without decreasing motoneuron excitability. These functional improvements coincide with an increase in expression of the chloride co-transporter KCC2 in lumbar motoneurons. Thus, we hypothesized that spastic symptoms can be alleviated directly through restoration of chloride homeostasis and endogenous inhibition by increasing KCC2 activity. Here, we used the recently developed KCC2 enhancer, CLP257, to evaluate the effects of acutely increasing KCC2 extrusion capability on spastic symptoms after chronic SCI. Sprague Dawley rats received a spinal cord transection at T12 and were either bike-trained or remained sedentary for 5 weeks. Increasing KCC2 activity in the lumbar enlargement improved the rate-dependent depression of the H-reflex and reduced both phasic and tonic EMG responses to muscle stretch in sedentary animals after chronic SCI. Furthermore, the improvements due to this pharmacological treatment mirror those of exercise. Together, our results suggest that pharmacologically increasing KCC2 activity is a promising approach to decrease spastic symptoms in individuals with SCI. By acting to directly restore endogenous inhibition, this strategy has potential to avoid severe side effects and improve the quality of life of affected individuals.
Keywords: spinal cord injury, rehabilitation, chloride homeostasis, neuroplasticity, KCC2, CLP257
Introduction
Disruption in synaptic inhibition is a common feature of an ever-growing list of diseases, ranging from neurodevelopmental conditions such as Down syndrome, epilepsy, and autism, to psychiatric disorders including schizophrenia and depression (De Koninck, 2007; Ben-Ari et al., 2012; Kaila et al., 2014; Ben-Ari, 2017). Targeting chloride equilibrium has therefore become recognized as a promising therapeutic strategy (De Koninck, 2007; Ben-Ari et al., 2012; Gagnon et al., 2013; Kahle et al., 2014; Puskarjov et al., 2014; Lorenzo et al., 2020). The importance of chloride homeostasis in the context of spinal cord injury (SCI) has been acknowledged with the identification of its involvement in both the development and maintenance of spasticity and neuropathic pain in animal models (reviewed in Côté, 2020, Coull et al., 2003; Boulenguez et al., 2010; Sanchez-Brualla et al., 2018; Mapplebeck et al., 2019) and more recently in humans (Klomjai et al., 2019).
After SCI, there is a progressive decrease in expression of the chloride extruder KCC2 (Boulenguez et al., 2010) that plateaus 4 weeks post-injury (Côté et al., 2014). The subsequent decrease in chloride extrusion drives the system toward a state resembling early development, in which GABAA and glycine mediated responses are depolarizing (Payne et al., 2003). Thus, after SCI there is reduced inhibition, decreased ability to suppress excitatory events, and facilitation of incoming excitatory inputs (Hubner et al., 2001; Jean-Xavier et al., 2006; Vinay and Jean-Xavier, 2008; Boulenguez et al., 2010; Bos et al., 2013). In adult lumbar motoneurons, the disruption in chloride homeostasis has been associated with the development of spasticity (Vinay and Jean-Xavier, 2008; Boulenguez et al., 2010; Viemari et al., 2011; Bos et al., 2013). Spasticity is characterized by a velocity-dependent increase in the stretch reflex which leads to symptoms such as increased muscle tone, hyperreflexia, painful muscle contractures, and co-contraction of antagonist muscles (Nielsen et al., 2007). This debilitating condition emerges in up to 75% of SCI individuals, and critically hampers functional recovery (Maynard et al., 1990; Skold et al., 1999; Biering-Sorensen et al., 2006; Holtz et al., 2017).
Most anti-spastic drugs currently available, such as baclofen and benzodiazepines, act via GABA-dependent mechanisms, leading to serious side effects. These include a deep, indiscriminate depression of CNS excitability, significant reductions in muscle activity, and muscle weakness, all of which further impede residual motor function and its recovery (Dario and Tomei, 2004; Taricco et al., 2006; Lapeyre et al., 2010; Simon and Yelnik, 2010; Angeli et al., 2012). There is a great need for strategies to combat spasticity that avoid depressing CNS excitability and muscle activity. While activity-based therapies are effective in a subset of SCI individuals (Petropoulou et al., 2007; Elbasiouny et al., 2010; Dietz and Sinkjaer, 2012), they are expensive and not widely available. In addition, SCI is accompanied by many co-morbidities that make exercise difficult or even impossible for some patients (Yelnik et al., 2009; Simon and Yelnik, 2010), especially early after injury. We have previously shown that exercise prevents the development of spasticity by increasing KCC2 expression (Côté et al., 2014; Beverungen et al., 2019). Thus, a possible alternative to current anti-spastic drugs and to activity-based therapies is to restore chloride homeostasis by increasing KCC2.
Recently, a family of selective KCC2 activators known as CLPs was developed, allowing us to directly target KCC2 activity (Gagnon et al., 2013; Kahle et al., 2014; Ferrini et al., 2017). CLPs have been shown to restore impaired KCC2-mediated Cl− extrusion in neurons in vitro and in vivo (Gagnon et al., 2013), and were later shown to increase KCC2 activity in various disease models in which KCC2 is pathologically decreased (Gagnon et al., 2013; Ostroumov et al., 2016; Ferrini et al., 2017; Chen et al., 2018; Thomas et al., 2018; Lizhnyak et al., 2019; Lorenzo et al., 2020).
Here, we show that pharmacologically increasing KCC2 activity in chronic SCI animals with CLP257 reduces hyperreflexia and spastic symptoms. Furthermore, we demonstrate that this pharmacological treatment mimics the beneficial effects of exercise. This work paves the way for future investigation of KCC2 enhancers as alternatives to exercise-based therapies for the treatment of spasticity after SCI.
Materials and Methods
Experimental Design.
In a rat model of complete thoracic SCI (T12), we investigated the effects of pharmacologically increasing KCC2 activity on spasticity and hyperreflexia and how this approach interacts with exercise. The KCC2 enhancer, CLP257, was used to increase KCC2 activity in chronic SCI rats, whereas control rats received saline. SCI animals were randomly assigned to one of the following groups: chronic spinal cord injured + CLP257 (SCI+CLP257), chronic spinal cord injured + saline (SCI), chronic spinal cord injured + bike-training + CLP257 (SCI+Ex+CLP257), and chronic spinal cord injured + bike-training + saline (SCI+Ex). Five weeks post injury, a terminal experiment was conducted in which either CLP257 or saline was administered, and signs of hyperreflexia were compared pre- and post-CLP257 or saline. Hyperreflexia and spasticity were assessed by examining: 1) the excitability of the plantar H-reflex and its modulation through rate-dependent depression (SCI+CLP257: n=10, SCI: n=8, SCI+Ex+CLP257: n=11, SCI+Ex: n=5), muscle forces and EMG activity in response to stretches to the triceps surae (SCI+CLP257: n=4, SCI: n=6, SCI+Ex+CLP257: n=3, SCI+Ex: n=4) and, where it was present, spontaneous motor unit activity in the lateral gastrocnemius (SCI+Ex+CLP257: n=5, SCI+Ex: n=4). A summary of group number for each outcome measure is provided in Table 1.
Table 1:
Number of animals included in analysis per group
| Number of Animals per Group | |||||||
|---|---|---|---|---|---|---|---|
| Experiment | SCI | SCI+CLP 257 |
SCI+Ex | SCI+Ex+CL P257 |
SCI+Ex+CLP257+VU 0240551 |
||
|
Figure 2 H-reflex Modulation |
H and M Properties | ||||||
| Frequency Dependent Depression |
11 | N/A | |||||
|
Figure 3 Mechanical Stretch Reflex |
Phasic EMG response | ||||||
| Tonic EMG response | N/A | N/A | |||||
|
Figure 4 Muscle Forces |
Peak Force |
Slow Stretch |
|||||
| Fast Stretch |
|||||||
| Plateau Force |
Slow Stretch |
||||||
| Fast Stretch* |
|||||||
| Reflex Mediate d Force |
Slow Stretch |
||||||
| Fast Stretch |
3 | N/A | |||||
|
Figure 5 Spont. Activity |
Spike Count over Time |
27 Motor units (5 rats) | 12 Motor Units (2 rats)** | ||||
|
Figure 6 Immunohistoch emistry |
Cytosol-Membrane ratio of KCC2 expression | 18 Motoneuron s (3 rats) |
N/A | ||||
Plateau forces resulting from fast stretches were separated and compared based off of presence of tonic EMG responses.
These rats derive from the SCI+Ex+CLP257 group. VU0240551 was added after CLP257
All procedures were performed in accordance with protocols approved by Drexel University College of Medicine Institutional Animal Care and Use Committee, followed National Institutes of Health guidelines for the care and use of laboratory animals, and complied with Animal Research: Reporting of In Vivo Experiments (ARRIVE).
Surgical procedures and postoperative care.
Adult female Sprague Dawley rats (240–300g, Charles River Laboratories) underwent a complete spinal transection at the low thoracic level (T12) as described previously (Côté et al., 2011; 2014; Beverungen et al., 2019). Briefly, rats were anesthetized with isoflurane (1–4%) in O2 and, under aseptic conditions, a laminectomy was performed at the T10–T11 vertebral level. The dura was carefully slit open, the spinal cord completely severed with small scissors, and the cavity filled with absorbable hemostats (Pfizer, New York, NY, USA) to promote hemostasis. The completeness of the lesion was ensured by the distinctive retraction of the rostral and caudal spinal tissue and by examining the ventral floor of the spinal canal during surgery and was later confirmed post-mortem. Paravertebral muscles were sutured, and the skin closed with wound clips. Upon completion of the surgery, animals received a single injection of slow-release buprenorphine (0.05 mg/kg, s.c.), then saline (5ml, s.c.) and Baytril (15mg/kg, s.c.) were given daily for 7 days to prevent dehydration and infection, respectively. Bladders were expressed manually at least twice daily until the voiding reflex returned.
Exercise regimen.
Beginning 4–5 days post-injury, exercised groups received 20 min of daily cycling (hereafter referred to as “exercise”), 5 days/week until completion of the study. No exercise was provided the day of the terminal experiment, so that the last exercise session took place >24h beforehand. Animals were secured in a support harness with the hindlimbs hanging and the feet fastened to pedals with surgical tape. The hindlimbs went through a complete range of motion during pedal rotation (45 rpm). Although the movement of the hindlimbs is passively generated by a custom-built motor-driven apparatus (Houle et al., 1999; Côté et al., 2011; 2014), this exercise protocol evokes rhythmic activity in both flexor and extensor muscles (Beverungen et al., 2019).
Drug Preparation.
The KCC2 enhancer CLP257 (generous gift from Dr. Y. de Koninck, Université Laval, Qc, Canada) was re-suspended in dimethyl sulfoxide (DMSO) as 100mM stock solution, then was freshly diluted to 100μM in saline immediately before administration. The KCC2 inhibitor VU0240551 (Cat# 3888, Tocris Bioscience, Minneapolis, MN) was prepared from a 50mM stock solution re-suspended in DMSO and diluted to 30μM in saline immediately before administration during the terminal experiment. Drugs were administered directly onto the exposed lumbar enlargement of the spinal cord, as described below.
Terminal experiment.
Five weeks post-injury, rats were anesthetized with isoflurane (1–4%) in O2 for the terminal experiment. Body temperature was maintained ~37°C through a DC heating pad via a rectal probe. The lumbar enlargement of the spinal cord was exposed, and the dura carefully removed. Using skin flaps of the back, an agar bath was created, and a small window was made in the solidified agar above the exposed lumbar enlargement (Gagnon et al., 2013) (Fig. 1C). The agar bath was filled with saline for baseline recordings and refilled with 0.5mL of the CLP257 solution for post-drug testing (or saline for control). As the half-life of CLP257 is ~15 minutes and is known to take effect at this time point (Gagnon et al., 2013), post-treatment recordings were collected 15 minutes after the application of CLP257/saline. The tibial nerve of the right hindlimb was dissected free and fitted with a cuff electrode for stimulation. The triceps surae (TS) muscles were isolated, the Achilles tendon severed distally, and tied to the lever of a servo-motor muscle puller (Fig. 1A) (Aurora Scientific, Aurora, ON, Canada). Bipolar wire electrodes (Cooner Wire, Chatsworth, CA) were inserted into the lateral gastrocnemius (LG) to record EMG activity in response to stretches of the TS (Fig. 1B), and into the interosseous muscle for H-reflex recordings (Fig. 1D). Muscle Force, muscle length and EMG activity obtained during the experiment were amplified (100–1000x; A–M Systems, Carlsborg, WA), band-pass filtered (10–5,000Hz) and the signal was digitized (10kHz) before being stored on a computer for analysis in Signal version 6 or Spike2 version 8 (Cambridge Electronic Design Limited, Cambridge, UK).
Figure 1: Experimental Setup.
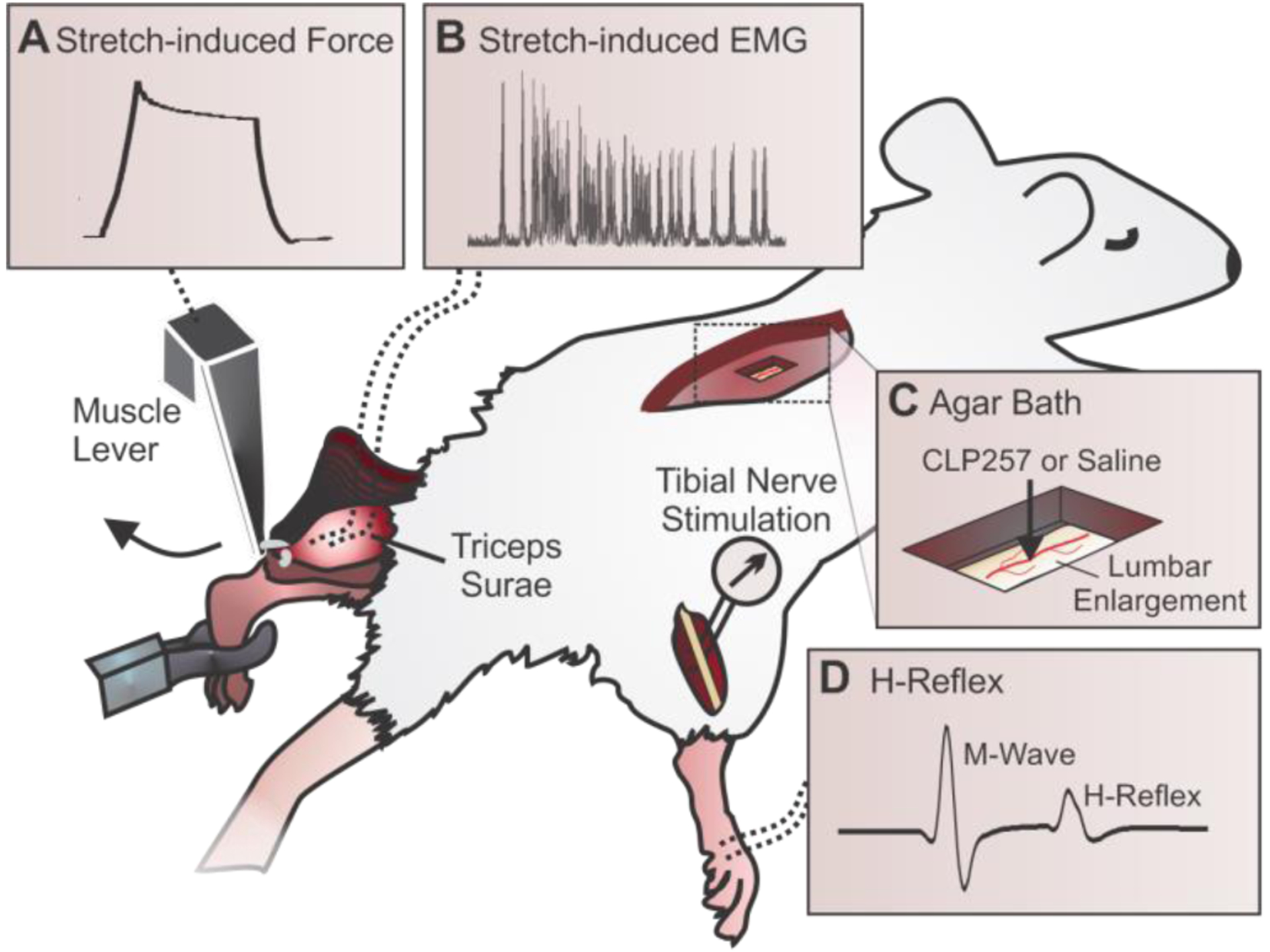
A) A dual mode muscle lever is attached to the left Achilles tendon to stretch the isolated triceps surae at varying lengths and speeds. The resultant force is recorded throughout the experiment. B) Bipolar electrodes are inserted into the lateral gastrocnemius (LG) muscle to measure EMG in response to physiological stretches. C) Window cut into an agar bath just above exposed lumbar enlargement for drug application. D) The H-reflex is evoked by a stimulation to the tibial nerve and recorded in the interosseous muscles of the right leg.
H-Reflex.
The H-reflex was evoked in the interosseus muscles by stimulating the tibial nerve with an isolated pulse stimulator delivering single biphasic pulses (100μs, A–M Systems). H-reflexes and M-waves were first recorded in response to a range of increasing stimulus intensities to determine the threshold for activation of the reflex (H-reflex threshold) and the motor response (MT). A recruitment curve was plotted by expressing the peak-to-peak amplitude of the H-reflex and M-wave responses as a function of stimulus intensity. The maximal amplitude of the H-reflex (Hmax) and the amplitude of the response of all motor units with supra-maximal stimulation of the tibial nerve axons (Mmax) were determined. Response latencies for the H-reflex and M-wave, the Hmax/Mmax and the Hmax/M ratios were measured before and 15 minutes after treatment.
The rate-dependent depression (RDD) of the H-reflex was estimated as described previously (Côté et al., 2014; Beverungen et al., 2019). Briefly, the stimulation intensity that evoked approximately 70% of the Hmax response was used for a series of 20 consecutive stimulations at 0.3, 5 and 10Hz. The 0.3Hz series was then repeated to verify that the M-wave amplitude was still within 95% of the initial trial. The first five responses in a stimulation series were discarded to allow for reflex stabilization, and the peak-to-peak amplitude of the remaining 15 responses was averaged for each animal and frequency. The change in H-reflex response at 5Hz and 10Hz was calculated as a percentage of the response measured at 0.3Hz. The properties of the H-reflex and M-wave and the RDD were assessed before, and 15 minutes after application of CLP257 or saline onto the exposed spinal cord. All data are presented as mean ± standard error of the mean (SEM).
Stretch-Induced Reflex and Muscle Force.
The ankle joint was held at 90° flexion and the muscle was positioned to achieve a baseline tension of 0.3 Newtons (N), which corresponds to the muscle length of the animal with an intact Achilles tendon in this joint position (Pingel et al. 2016). Passive and reflex-mediated forces in the triceps surae were assessed by using a protocol similar to those previously described in rodents (Pingel et al., 2016) and humans (Lorentzen et al., 2010; Willerslev-Olsen et al., 2013). Data were collected in length servo-mode, with computer driven TS muscle stretches evoked while monitoring TS muscle force and length, and EMG activity in LG. Two different ramp-hold-release stretches were applied to the TS muscle: a ‘slow’ stretch of 3mm with 150ms rising phase (20mm/s velocity) (Fig. 4Ai) and a ‘fast’ stretch of 3mm with 5ms rising phase (600mm/s) (Fig. 4Aii). The slow stretch had a hold phase of 500ms and a release phase of 150ms, while the fast stretch had a hold phase of 200ms and a release phase of 100ms. Force output from the TS muscle and EMG activity from the LG muscle were recorded in response to a series of 8–12 stretches repeated at 12s intervals. A second series of stretches was collected 15 minutes after application of CLP257, or saline for control animals. Response latencies were measured between the onset of stretch and onset of EMG response.
Figure 4: CLP257 reduces reflex-mediated forces.
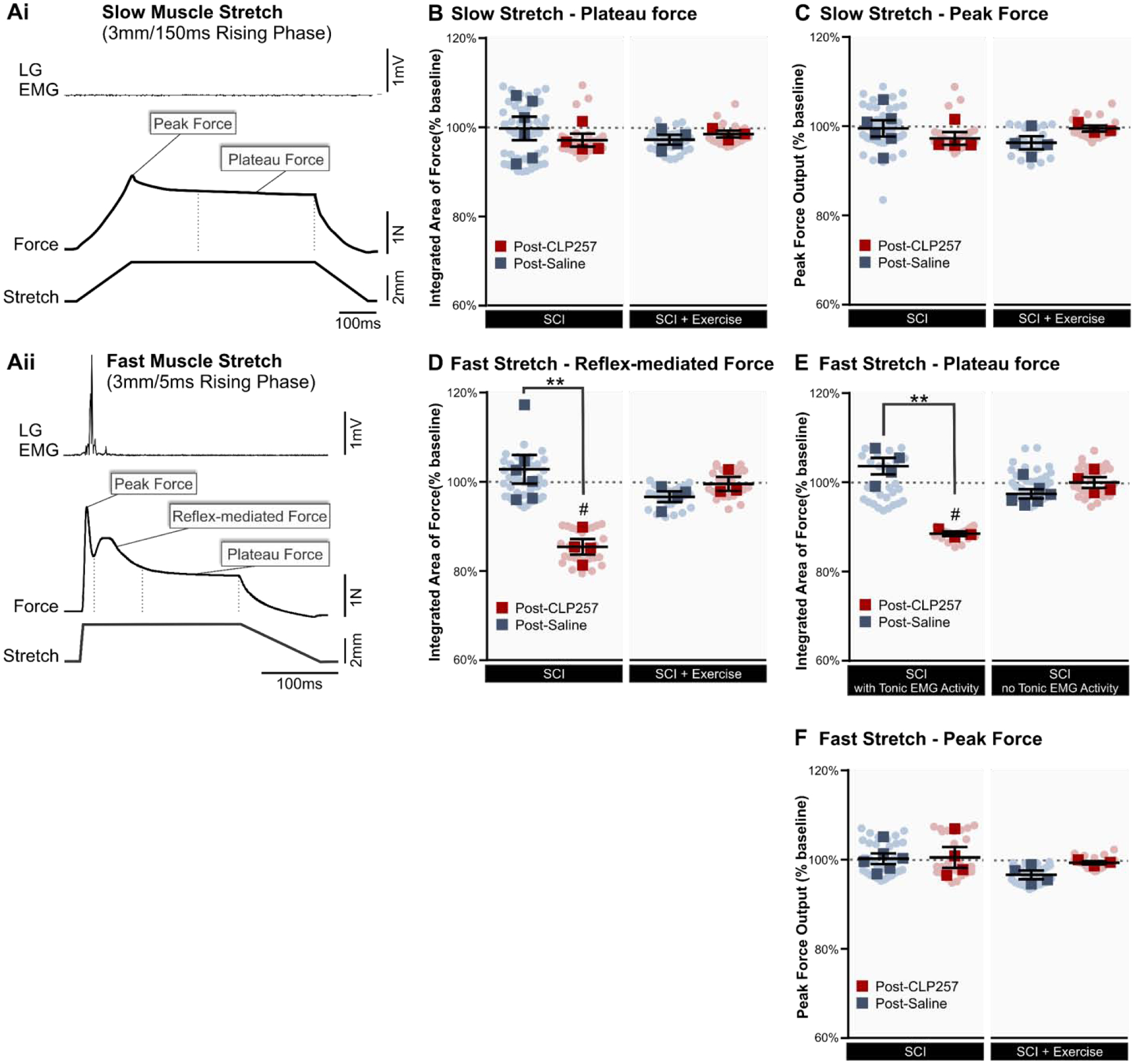
A) Example force output of triceps surae and EMG activity in LG muscle in response to stretches. When a slow stretch (Ai) was applied, no EMG activity was present and no reflex was elicited. With a fast stretch (Aii) a stretch reflex was reliably evident in the EMG, and a small increase in reflex-mediated force was present. Depicted also in each stretch are the peak force and the plateau force. B–C) With slow stretches that elicited no reflex activity, CLP257 had no significant effect on either the plateau force (B) or the peak force (C). D) With fast stretches that elicited reflex activity, the reflex-mediated force was significantly reduced by CLP257 (t(3) = 8.466, p =0.0035 n=4), but not by saline (t(5) = 0.8251, p =0.4469 n=6) in SCI animals. In exercised animals, the reflex-mediated force was not affected by either CLP257 (n=3) or by saline (n=4). E) In animals displaying tonic EMG activity, the plateau force was significantly reduced by CLP257 (t(2) = 21.24, p = 0.0022, n=3) but not by saline (t(2) = 1.998, p = 0.1396, n=4). In animals without tonic EMG activity however, CLP257 had no significant effect on the plateau force, indicating that CLP257 only affects forces mediated by reflex activity. F) Similarly, the peak force was unaffected by CLP257 in all groups. SCI n=6, SCI+CLP257 n=4. SCI+Ex n=4, SCI+Ex+CLP257 n=3. Analysis was conducted on responses averaged for each animal, represented by squares. Circles represent individual trials. All values are expressed as percentage of baseline values. **p<0.01 between groups. # p<0.05 compared to baseline.
EMG recordings were analyzed in response to the ‘fast’ stretch (3mm amplitude, 5ms rising time) as this parameter reliably yielded the most discernable reflex responses. The signal was rectified and band-pass filtered 70Hz–2500Hz offline for analysis. Muscle stretch evoked a phasic EMG response in LG that was measured as the integrated area of a window from 0–15ms following the onset of the stretch. An additional tonic component, defined as EMG activity that persisted >15ms after stretch onset, was also quantified when present, using the integrated area from 15ms to the end of the hold phase of the stretch. EMG responses were averaged across 8 trials before and after CLP257 or saline.
For both slow and fast stretches, the peak force and the plateau force were determined. The peak force was measured using peak-to-peak amplitude, and the plateau force was determined as the integrated area of the force over the final 60% of the hold phase of the stretch, before release. Each were averaged across 8 trials both before and after drug or saline. The reflex-mediated force was also measured to confirm results of phasic EMG analysis. Approximately 30ms following the onset of the stretch, a clear increase in force is observed whenever a stretch reflex is present in the EMG (Fig. 4Aii). This additional force response, elicited by a stretch reflex contraction of the muscle, represents the reflex-mediated force (Pingel et al., 2016). The reflex-mediated force was measured as the integrated area within a 60ms window beginning 20ms into the stretch (following the peak force) and preceding the plateau force (Fig. 4Aii). As with EMG recordings, this was solely analyzed for the stretch of 600mm/s velocity (3mm amplitude, 5ms rising time) as this stretch reliably yielded reflex responses.
Spontaneous spiking recordings and analysis.
In some animals, EMG activity in LG muscle was recorded continuously and the effect of CLP257 (n=5) or saline (n=4) on spontaneous spiking activity was assessed. Using a template matching function (Spike2, CED), individual motor units were discriminated based on spike shape, amplitude, and width, and confirmed with visual inspection. Spike count was averaged for each motor unit over five-minute intervals before (baseline), and up to 20 minutes after application of CLP257 or saline. In two animals (12 firing motor units), CLP257 was then washed off the exposed lumbar enlargement 20 minutes following its application and was replaced with the KCC2 inhibitor VU0240551. The average spike count following application of VU0240551 was then measured to determine if the effects of CLP257 on spiking could be attributed to changes in KCC2 function. In addition to spiking frequency, the number of recruited motor units was also estimated. A motor unit was termed as “recruited” when the template matching software in Spike2 plotted a unit exclusively after application of terminal treatment. Care was taken to validate that each recruited unit was in fact separate from those that fired during baseline recordings.
Immunofluorescent staining.
Rats were sacrificed using an overdose of Euthasol (390 mg/kg sodium pentobarbital and 50mg/kg phenytoin, i.p.) immediately following the terminal experiment, and perfused transcardially with cold saline followed by 4% paraformaldehyde in PBS. The lumbar spinal cord (L1–L6) was harvested, post-fixed overnight, and transferred to 30% sucrose in PBS for cryoprotection. Spinal cord tissue was then sectioned transversally (25μm) on a cryostat and mounted on slides for immunostaining. Sections were first pre-incubated in BSA (1%), donkey serum (10%) and 0.2% Triton x-100 OX in PBS for one hour at room temperature. Sections were then incubated with the following primary antibodies at 4°C overnight: rabbit anti-KCC2 (1:1000, Cat#07–432, Millipore, RRID: AB_310611) and goat anti-ChAT (1:100, Cat#AB144P, Millipore, RRID: AB_2079751). Species-specific secondary antibodies (1:1000, goat anti-rabbit conjugated to Alexafluor 488 for KCC2, and donkey anti-goat conjugated to rhodamine for ChAT; Jackson ImmunoResearch) were applied for 2h at room temperature, and the slides were cover-slipped before imaging. Spinal cord sections were imaged using a Leica DM550B fluorescent microscope and a Retiga-SRV digital color camera (QImaging) controlled by Slidebook imaging software version 6.0.11 (Olympus). Eighteen lumbar motoneurons were averaged per group (6 motoneurons for each animal, n=3 per group), and were identified with ChAT+ labeling, typical large size, and location within the ventral horn. The fluorescence intensity of KCC2 immunolabelling for the motoneuronal membrane was measured using ImageJ software and averaging the integrated area of the density curve obtained by drawing three lines across each motoneuron (yielding six data points per cell) (Boulenguez et al., 2010; Côté et al., 2014; Liabeuf et al., 2017). To examine the amount of KCC2 expressed in the membrane compared to the cytosol, mean pixel intensities for membranes were normalized to intensities in the cytoplasm for each motoneuron (Boulenguez et al., 2010).
Statistical Analysis.
Paired t-tests were used to compare differences in H-reflex and M-wave maximum amplitudes, latencies, and threshold for activation, the Hmax/Mmax ratio and the stimulation intensity required to evoke Hmax (expressed at x MT). These were compared for each animal before and after receiving either CLP257 or Saline. Where data was not normally distributed, a Wilcoxon test was used. For RDD measurements, three-way mixed ANOVAs followed by Holm-Sidak post-hoc tests were used to determine significant differences in H-reflex amplitude across stimulation frequencies (5Hz and 10Hz, normalized to 0.3Hz; within-subject factor), treatments (CLP257/Saline; between subject factor) and condition (pre and post treatment; within-subject factor). This was done separately for SCI and SCI+Ex groups. To compare effects of CLP257 and exercise on the RDD in SCI animals, a two-way mixed ANOVA was run followed by Holm-Sidak post-hoc test. Differences in H-reflex amplitude were compared across stimulation frequencies (5Hz and 10Hz, normalized to 0.3Hz; within-subject factor) and groups (un-exercised + CLP257 or exercised; between-subjects factor). Unless otherwise stated, values were normally distributed as assessed by Shapiro-Wilk’s test (p > 0.05) and Normal Q-Q plot. There was homogeneity of variances, as assessed by Levene’s test of homogeneity of variance (p > 0.05). Mauchly’s test of sphericity indicated that the assumption of sphericity was met (p > 0.05). Where it was violated, a Greenhouse-Geisser correction was applied.
Phasic and tonic EMG responses, as well as peak, plateau and reflex-mediated forces following CLP257 or saline were averaged across 8 trials and normalized to the average baseline response (also averaged over 8 baseline trials) in each animal. Unpaired t-tests were then used to compare normalized post-drug/saline responses between CLP257 and saline treated animals. This was done separately for the SCI and SCI + Ex groups. Where data was not normally distributed, a Mann-Whitney test was used. One sample t-tests were used to compare normalized post-treatment values in each group to the normalized baseline value with a theoretical mean of 100%. Where data was not normally distributed, a Wilcoxon test was used.
To analyze the effect of CLP257 on spike count, we used a generalized linear mixed model with a negative binomial distribution to account for the data not being normally distributed. Fixed effects for time, treatment and their interaction were included. Using a stacked random effect for each motor unit and animal, the model controlled for inter-individual differences. The glmmTMB package (Brooks et al., 2017) for R 3.2 was used to fit the model and account for zero-inflation and overdispersion. Backwards elimination was used starting with the full factorial model for the random effect, zero inflation and dispersion terms. Effects were removed from the model if the likelihood-ratio test was not significant. The intercepts for the random effect and the zero-inflation remained in the model, along with the dispersion terms (time and treatment). Q-Q plots and histograms of Pearson’s residuals were used to confirm negative binomial distribution of the residuals. Post-hoc comparisons using the Tukey’s test were used to assess spike count following VU0240551 application compared to CLP257 application.
Finally, unpaired t-tests were used to compare membrane-cytosol ratios of KCC2 immunolabelling between CLP257 and saline treated motoneurons. This was done separately for SCI and SCI + Ex groups. Where standard deviations were unequal, Welch’s correction was applied.
Results
We have previously shown that restoring KCC2 activity after SCI is critically involved in preventing the development of spasticity with activity-based therapies (Côté, 2020; Beverungen et al., 2019; Côté et al., 2014). Given that many patients present a significant number of co-morbidities that render them unable to participate in an exercise program, particularly in the acute phase of injury (Simon and Yelnik, 2010), our objective was to investigate if pharmacologically restoring chloride homeostasis is a viable alternative to reduce correlates of spasticity after chronic SCI.
CLP257 restores H-reflex modulation after chronic SCI
We first investigated the effect of restoring chloride homeostasis on hyperreflexia and spasticity after chronic SCI by measuring H-reflex excitability before and after application of the KCC2 enhancer, CLP257, to the exposed spinal lumbar enlargement five weeks post-SCI (Fig. 1C). Following a stimulation to the tibial nerve, two EMG responses are evoked in the plantar muscles: 1) the M-wave, which is the response to the direct activation of motor axons, and 2) the H-reflex, which results from the activation of Ia afferents that directly contact motoneurons (Fig. 1D). Table 2 displays the general properties of the M-wave and H-reflex such as maximal amplitude, latency and threshold for activation before and 15 minutes after CLP257 or saline application. There was no significant difference in any of the H-reflex or M-wave properties between groups before application of either CLP257 or saline (unpaired t-test, p > 0.05). CLP257 did not significantly alter H-reflex or M-wave latencies or thresholds after chronic SCI (paired t-test, p > 0.05), similar to saline (p > 0.05). To assess the proportion of motoneurons recruited through the monosynaptic reflex compared to the activation of the whole motor pool, the Hmax/Mmax ratio was also measured.
Table 2.
Properties of the M-Wave and H-Reflex: Latency, Amplitude, and Threshold
| Motor threshold | Mmax | Hmax | M latency | H latency | H-reflex threshold | Hmax | Hmax/Mmax | |||
|---|---|---|---|---|---|---|---|---|---|---|
| n = | (mA) | (mV) | (mV) | (msec) | (msec) | (x MT) | (x MT) | Ratio | ||
| SCI | Pre-CLP257 | 10 | 0.031 ± 0.015 | 6.97 ± 1.00 | 2.37 ± 0.40 | 2.63 ± 0.06 | 9.37 ± 0.08 | 1.08 ± 0.07 | 1.46 ± 0.09 | 0.37 ± 0.07 |
| Post-CLP257 | 10 | 0.036 ± 0.018 | 6.62 ± 1.22 | 2.18 ± 0.54 | 2.66 ± 0.08 | 9.45 ± 0.12 | 1.09 ± 0.07 | 1.39 ± 0.11 | 0.34 ± 0.05 | |
| Pre-Saline | 8 | 0.080 ± 0.025 | 5.75 ± 1.27 | 1.20 ± 0.25 | 2.54 ± 0.10 | 8.99 ± 0.22 | 1.09 ± 0.05 | 1.37 ± 0.09 | 0.24 ± 0.04 | |
| Post-Saline | 8 | 0.083 ± 0.026 | 5.71 ± 1.21 | 1.32 ± 0.27 | 2.47 ± 0.09 | 8.96 ± 0.24 | 1.10 ± 0.05 | 1.38 ± 0.07 | 0.27 ± 0.04 | |
| SCI+Ex | Pre-CLP257 | 11 | 0.032 ± 0.012 | 8.44 ± 1.34 | 3.51 ± 0.87 | 2.48 ± 0.09 | 9.33 ± 0.28 | 0.92 ± 0.09 | 1.20 ± 0.15 | 0.41 ± 0.06 |
| Post-CLP257 | 11 | 0.071 ± 0.023 | 7.77 ± 1.03 | 3.96 ± 0.90 | 2.45 ± 0.10 | 9.40 ± 0.25 | 0.99 ± 0.13 | 1.51 ± 0.21 | 0.49 ± 0.08 | |
| Pre-Saline | 5 | 0.060 ± 0.025 | 7.93 ± 2.58 | 2.50 ± 0.37 | 2.40 ± 0.14 | 9.25 ± 0.25 | 1.28 ± 0.19 | 1.49 ± 0.17 | 0.40 ± 0.09 | |
| Post-Saline | 5 | 0.067 ± 0.035 | 8.06 ± 1.96 | 3.14 ± 0.39 | 2.44 ± 0.13 | 9.25 ± 0.24 | 1.16 ± 0.12 | 1.44 ± 0.11 | 0.46 ± 0.08 |
Values are mean±SEM. MT, motor threshold
There was no significantly change in this ratio after either CLP257 or saline were applied (paired t-test, p > 0.05). Similarly, the Hmax (xMT), an estimate of the relative activation of the motor pool required to reach maximal reflex amplitude, also showed no significant difference after saline or CLP257 application (paired t-test, p > 0.05) (Table 2).
We then measured the effect of CLP257 after chronic SCI on the rate-dependent depression (RDD) of the plantar H-reflex. In the intact spinal cord, the amplitude of the H-reflex is decreased by the repetitive activation of Ia afferents (Hultborn et al., 1996; Thompson et al., 1992; Crone and Nielsen, 1989). SCI disrupts reflex modulation and leads to a decrease in the RDD in animals (Thompson et al., 1992) and in humans (Grey et al., 2008; Trimble et al., 2001; Calancie et al., 1993). Decreased RDD after chronic SCI has been associated with decreased KCC2 activity and subsequent depolarization in ECl− that contributes to hyperreflexia and spasticity (Bos et al., 2013; Boulenguez et al., 2010). To assess if CLP257 improves RDD after chronic SCI, H-reflexes were evoked at 0.3Hz, 5Hz and 10Hz before and after application of CLP257 or saline. Figure 2A shows representative traces of the H-reflex evoked by a 10Hz stimulation train to the tibial nerve in the same animal before and 15 minutes after application of CLP257 to the lumbar spinal cord. In this animal, CLP257 markedly decreased the amplitude of the H-reflex as compared to baseline. A three-way mixed ANOVA revealed a statistically significant interaction between treatment (CLP257/saline), and condition (pre/post-treatment) (F(1,16) = 11.11, p = 0.004). There was a significant difference in H-reflex amplitude across stimulation frequencies (F(1,16) = 23.32, p < 0.001), suggesting that some level of depression is still present after chronic SCI whether CLP257 was applied or not. However, within-subjects contrasts found a significant effect of condition (pre/post-treatment) on H-reflex depression in CLP257 treated animals (F(1,9) = 31.72, p < 0.001, n=10) but not in saline treated animals (F(1,7) = 0.04, p = 0.839, n=8) (Fig. 2B), suggesting that CLP257 affected the depth of depression. Overall, CLP257 decreased the H-reflex amplitude from 42 ± 6% to 21 ± 5% at 5Hz (t(9) = 6.50, p < 0.001) and from 36 ± 6% to 21 ± 5% at 10Hz (t(9) = 3.91, p = 0.004) as compared to 0.3Hz. Saline however, had no effect, with values similar to baseline at 5Hz (t(7) = 0.20, p = 0.844) and 10Hz (t(7) = −0.50, p = 0.633). These results suggest that CLP257 improved reflex modulation and improved the RDD after chronic SCI.
Figure 2: Enhancing KCC2 activity restores the rate-dependent depression of the H-reflex after chronic SCI.
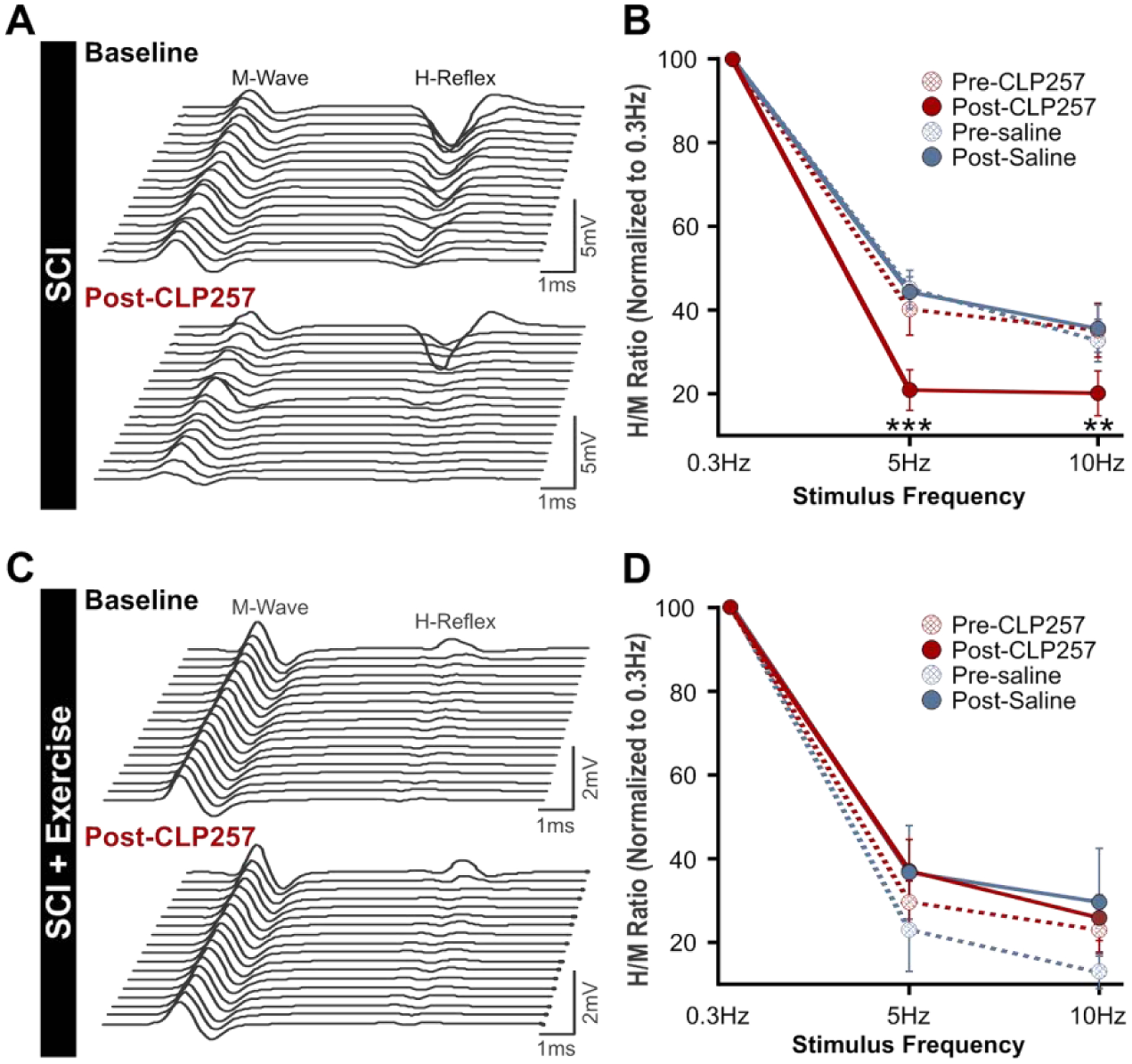
A) Representative H-reflex recordings over a series of 20 stimulations to the tibial nerve at 10Hz from a chronic SCI animal before (top) and after CLP257 administration (bottom). CLP257 dramatically decreased H-reflex amplitude and increased the rate-dependent depression of the H-reflex. B) There was a statistically significant interaction between treatment (CLP257/saline) and condition (pre/post treatment) in chronic SCI animals (F(1,16) = 11.11, p = 0.004). CLP257 decreased H-reflex amplitude at 5Hz (t(9) = 6.50, p < 0.001) and 10Hz (t(9) = 3.91, p = 0.004), whereas saline had no effect at either 5Hz (t(7) = 0.20, p = 0.844) or 10Hz (t(7) = −0.50, p = 0.633). SCI n=8, SCI+CLP257 n=10. C) Representative H-reflex recordings over a series of 20 stimulations to the tibial nerve at 10Hz from a chronic SCI animal that was exercised for 4 weeks, before (top) and after CLP257 administration (bottom). CLP257 showed no noticeable effect on the H-reflex modulation. D) There was no significant interaction between frequency, treatment, and condition (F(1, 14) = 2.273, p = 0.154), nor was there a significant interaction between condition and treatment (F(1, 14) = 1.251, p = 0.282). SCI+Ex n=5, SCI+Ex+CLP257 n = 11. **p<0.01 ***p<0.001.
Pharmacologically restoring chloride homeostasis parallels the effect of exercise on the H-reflex after chronic SCI
We have previously shown that the impairment in RDD is prevented by activity-based therapies (Côté et al., 2014) via a BDNF-dependent increase in KCC2 expression on lumbar motoneuronal membranes (Beverungen et al., 2019). To determine if CLP257 further ameliorates reflex excitability and modulation in animals undergoing an exercise program, a group of rats received bike-training for 4 weeks following SCI. The general features of the M-wave and H-reflex before and 15 minutes after CLP257 or saline application are displayed in Table 2. CLP257 did not significantly alter any of these parameters (p > 0.05, paired t-test), with both M-wave and H-reflex properties similar post-CLP257/saline as compared to their respective baseline values. We then assessed whether CLP257 improves H-reflex modulation in exercised animals following chronic SCI by quantifying the RDD. H-reflexes were evoked at 0.3Hz, 5Hz and 10Hz before and after CLP257 or saline application. Figure 2C shows representative traces of the M-wave and H-reflex in the same animal in response to a 10Hz stimulation train before and 15 minutes after application of CLP257 to the lumbar enlargement. CLP257 had no further effect on the amplitude of the H-reflex in this animal, as a decrease in amplitude was already present before drug application (Côté et al., 2014; Beverungen et al., 2019). A three-way mixed ANOVA was run to compare the effects of CLP257 (n=11) or saline (n=5) on H-reflex amplitude at increasing stimulation frequencies. There was a statistically significant main effect of stimulation frequencies (F(1,14) = 13.48, p = 0.003). However, there was no statistically significant interaction between stimulation frequency, treatment (CLP257/saline), and condition (pre/post-treatment) (F(1,14) = 2.27, p = 0.154). There was also no significant two-way interaction between condition and treatment (F(1,14) = 1.25, p = 0.282). Overall, CLP257 did not significantly affect the amplitude of the H-reflex in SCI+Ex animals (Fig. 2D), with similar values pre- and post-CLP257 at 5Hz (31 ± 5 % vs. 38 ± 7%) (t(10) = −1.58, p = 0.146) and 10Hz (25 ± 5% vs. 27 ± 5%) (t(10) = −0.54, p = 0.602).
As both CLP257 and exercise affect KCC2 activity, we further investigated if their effect in restoring the RDD after chronic SCI is comparable. A two-way mixed ANOVA found no significant main effect of treatment (CLP257 vs. Exercise) on H-reflex depression (F(1,20) = 1.02, p = 0.326), indicating that there was no significant difference in H-reflex depression in chronic SCI animals that received CLP257 compared to those that followed an exercise program. The amplitude of the H-reflex was similar between SCI + CLP257 and SCI + Ex at 5Hz (22 ± 5% vs. 31 ± 5 %) (t(40) = 1.36, p = 0.328) and at 10Hz (21 ± 5% vs. 25 ± 5%) (t(40) = 0.62, p = 0.539). This suggests that pharmacologically increasing KCC2 activity can reduce hyperreflexia to similar levels observed following an exercise program.
CLP257 reduces the phasic and tonic stretch-induced EMG responses after SCI
After chronic SCI, quick muscle stretches elicit a large phasic EMG response, with a latency corresponding to a monosynaptic reflex. This exaggerated monosynaptic reflex is characteristic of SCI and is thought to contribute to the production of clonus and hyperreflexia (Nielsen et al., 2007). In addition to exaggerated phasic responses, long-lasting tonic responses can also be present (Murray et al., 2011b; Li et al., 2004; Bennett et al., 2004; 1999) and contribute to triggering spasms in SCI patients (Norton et al., 2008). When slow stretches (60mm/s, 3mm, 50ms rising time) were applied to chronic SCI animals, little or no EMG activity was elicited (not shown). However, faster stretches with a velocity of 600mm/s (3mm, 5ms rising time) evoked large phasic responses (Fig. 3A) with a latency of ~4–5ms (see below). In addition to large phasic responses, a considerable number of chronic SCI animals (n= 7/10) displayed robust and long-lasting EMG activity with an onset ~1.5–15ms after the phasic response had terminated, and in some cases persisted for up to 3.5 seconds after the muscle stretch ended, suggesting both the contribution of polysynaptic pathways and sustained firing. We then evaluated the effect of CLP257 on LG muscle EMG activity evoked by these muscle stretches. Figure 3A shows example traces of chronic SCI animals displaying both the phasic and tonic EMG responses in LG muscle before and after application of either CLP257 or saline. CLP257 did not affect the latency of the response (Post-CLP257: 4.54 ± 0.7ms vs Pre-CLP257: 4.63 ± 0.5ms (paired t-test, t(3) = 0.2848, p = 0.7943)) similar to saline (Post-Saline = 4.13 ± 0.5ms vs Pre-Saline = 4.03 ± 0.5ms (paired t-test, t(5) = 0.7124, p = 0.5081)). However, CLP257 significantly reduced the phasic response amplitude to 57.0 ± 11.9% of baseline (one sample t-test, t(3) = 3.16, p = 0.036, n=4), whereas saline had no significant effect, with values remaining at 101.1 ± 5.0% of baseline (one sample t-test, t(5) = 0.220, p = 0.834, n=6). We further compared post-treatment values (normalized within each animal to baseline values) for CLP257 and saline groups. An unpaired t-test found that post-treatment values for CLP257 and saline groups were significantly different (t(8) = 3.89, p = 0.0046) (Fig. 3B, left).
Figure 3: CLP257 reduces stretch-induced EMG responses after chronic SCI.
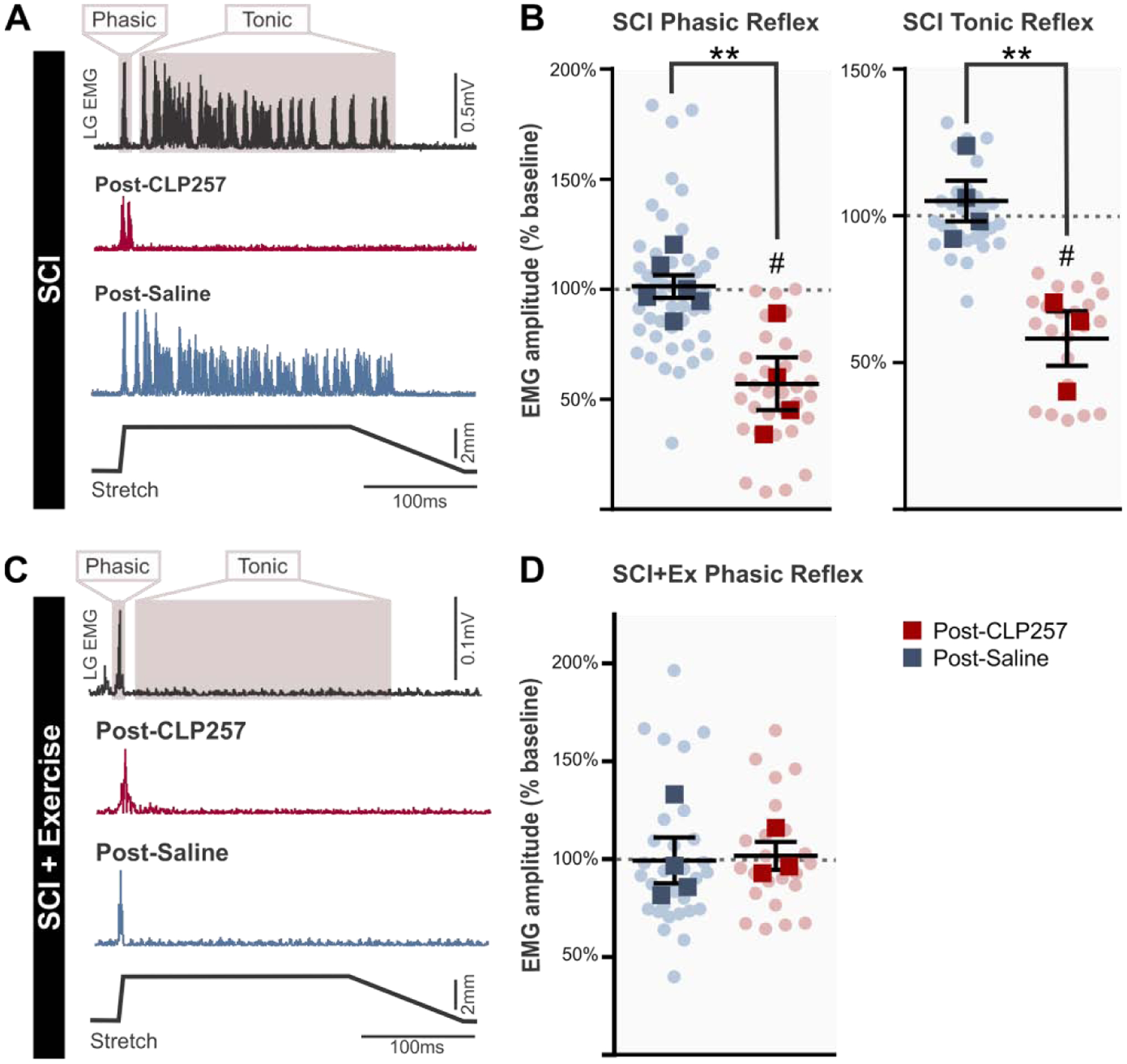
After chronic SCI, EMG responses to stretch have a phasic (early, short-lasting) and/or tonic (late, long-lasting) component. A) CLP257 decreases the amplitude of the phasic response after chronic SCI and prevents the development of a tonic response. B) Phasic responses following CLP257 treatment were significantly reduced compared to saline treatment (t(8) = 3.89, p = 0.0046) in chronic SCI animals. SCI n=6, SCI+CLP257 n=4. Similarly, the tonic response was also significantly reduced by CLP257 compared to saline treatment (t(5) = 4.169, p = 0.0087). SCI n=4, SCI+CLP257 n=3. C) Exercise prevented the development of tonic EMG activity in LG muscle in response to triceps surae muscle stretch, and CLP257 did not alter the phasic response. D) CLP257 had no significant effect on the amplitude of the phasic response in exercised animals, with values similar to saline values (t(5) = 0.159, p = 0.8798). SCI+Ex n=4, SCI+Ex+CLP257 n=3. Analysis was conducted on responses averaged for each animal, represented by squares. Circles represent individual trials. All values are expressed as percentage of baseline values. **p<0.01 between groups, # p<0.05 compared to baseline.
Tonic responses, when present, were also significantly reduced by CLP257 to 58.1 ± 9.25% of baseline values (t(2) = 4.531, p = 0.0454, n=3). Saline had no significant effect on the tonic response, which remained at 105.0 ± 6.8% of baseline (t(3) = 0.718, p = 0.524, n=4) (Fig. 3B, right). An unpaired t-test revealed that post-treatment values for the two groups were significantly different (t(5) = 4.169, p = 0.0087). This indicates that increasing KCC2 activity robustly attenuates long polysynaptic reflexes that initiate muscle spasms. Figure 3C shows a representative EMG response to stretch in a SCI+Ex animal after a chronic SCI. Interestingly, tonic EMG responses were not observed in any animal that followed an exercise program (n=7/7). In contrast to SCIs, the phasic response amplitude was not affected by either CLP257 (101.8 ± 7.2% of baseline, t(2) = 0.249, p = 0.8269, n=3) or saline in SCI+Ex (99.4 ± 11.7% of baseline, t(3) = 0.053, p = 0.9610, n=4), and values for the two groups were not significantly different (t(5) = 0.159, p = 0.8798) (Fig. 3D). This again suggests that CLP257 parallels the effects of exercise on hyperreflexia.
CLP257 reduces reflex-mediated force
To further assess how increasing KCC2 activity decreases hyperreflexia, we evaluated the force output in response to stretches of the triceps surae (TS). Figure 4A shows representative force in response to two different muscle stretches in the same animal. For slow stretches (Fig. 4Ai), the force increases linearly with the stretch until reaching a small peak of maximal force (hereafter referred to as the peak force), then plateauing during the hold phase of the stretch (hereafter referred to as the plateau force). Slow stretches did not evoke reflex activity in LG, as suggested by the lack of EMG response. For the plateau force following slow stretches, unpaired t-tests found that post-treatment values for CLP257 (n=4) and saline groups (n=6) were not significantly different from pre-treatment values in SCI animals (t(8) = 0.759, p = 0.4697). Similarly, there was no significant difference between the CLP257 (n=3) and saline groups (n=4) in SCI+Ex (t(5) = 0.855, p = 0.4314) (Fig. 4B). Peak forces resulting from slow stretches were also unaffected, with no significant difference between CLP257 and saline groups for either SCI animals (t(8) = 0.883, p = 0.4029) or SCI+Ex animals (t(5) = 1.784, p =0.1346) (Fig. 4C). In addition, no post-treatment group significantly differed from baseline values for plateau forces or peak forces resulting from slow stretches (one sample t-test, p >0.5). Together, this suggests that CLP257 (or saline) had no effect on passive properties of the muscle.
With faster stretches, the peak is more pronounced, and a stretch reflex was reliably evoked (Fig. 4Aii). This is evident from both the EMG response and the clear increase in force that peaks approximately 30ms following peak EMG activity (Pingel et al., 2016). By evaluating the force associated with the phasic EMG response (hereafter referred to as the reflex-mediated force), we could ensure that differences in EMG read-outs observed with CLP257 (Fig. 3) were obtained in similar experimental conditions, and not due to the deterioration of the preparation. In SCI animals, there was a significant difference between post-CLP257 and post-saline values (t(8) = 4.108, p = 0.0034), with CLP257 reducing the reflex-mediated force to 85.3 ± 1.7% of baseline values (t(3) = 8.466, p = 0.0035) and saline remaining at 102.6 ± 3.2% (t(5) = 0.8251, p = 0.4469) (Fig. 4D). In SCI+Ex animals, there was no significant change from baseline values post-CLP257 (99.4 ± 1.6% of baseline, t(2) = 0.3501, p = 0.7597) or post-saline (96.5 ± 1.2% of baseline, t(3) = 2.881, p = 0.0635), and no significant difference between post-CLP257 and post-saline (t(5) = 1.498, p = 0.1943). In agreement with EMG responses, these results indicate that CLP257 reduces the amplitude of the phasic reflex response in SCI animals, but not in animals that were exercised.
We further examined the plateau force, which follows immediately after the phasic reflex force, in response to fast stretches (Fig. 4Aii). We found that in animals displaying tonic EMG activity (7 SCI animals) in response to these stretches, there was a significant difference between groups (t(5) = 6.708, p = 0.0011), with the plateau force being significantly reduced by CLP257 (88.6 ± 0.5% of baseline, t(2) = 21.24, p = 0.0022, n=3) but not by saline (103.7 ± 1.9% of baseline, t(2) = 1.998, p = 0.1396, n=4). However, in animals that did not display tonic EMG activity (3 SCI and 7 SCI+Ex animals), the plateau force was unchanged by either CLP257 (100.1 ± 1.2% of baseline, t(3) = 0.059, p = 0.9565, n=1 SCI, 3 SCI+Ex) or saline (97.5.1 ± 1.0% of baseline, t(5) = 2.427, p = 0.0596, n=2 SCI, 4 SCI+Ex), and there was no significant difference between groups (t(8) = 1.601, p = 0.1480) (Fig. 4E). Similarly, the peak forces in response to fast stretches were also unaffected by CLP257. There was no significant difference in peak force between CLP257 and saline groups in either SCI animals (t(8) = 0.1311, p = 0.8990) or SCI+Ex animals (t(5) = 2.234, p = 0.0758), and one sample t-tests found that no group significantly differed from baseline values (p>0.5) (Fig. 4F).
CLP257 increases spontaneous motor-unit activity in hindlimb muscles in exercise animals
We also monitored spontaneous motor unit spiking in LG muscle before and after application of either CLP257 or saline after chronic SCI. Interestingly, exercise increased the occurrence of animals displaying spontaneous spiking activity (n=9/12) as compared to chronic SCI animals that did not follow an exercise program (n=3/17) (χ2(1, N = 29) = 7.321, p = 0.007, odds ratio = 0.07) (Fig. 5A). Figure 5B shows a representative recording from a SCI+Ex rat that displayed spontaneous spiking at baseline. CLP257 increased the spiking frequency, while the KCC2 inhibitor VU0240551 greatly reduced spiking back towards baseline levels (Fig. 5C). CLP257 had no effect on animals that did not display spontaneous spiking activity at baseline, with the EMG remaining silent after CLP257 application, whether animals were exercised or not (data not shown). Animals not displaying spontaneous activity were excluded from further analysis. A generalized linear mixed model found a significant interaction between treatment (CLP257/saline) and condition (pre/post-treatment) on the average spike count per motor unit (χ2(4) = 21.387, p = 0.0003). CLP257 increased average spike count at 5 minutes (t(217) = 3.254, p = 0.011), 10 minutes (t(217) = 2.895, p = 0.0337), 15 minutes (t(217) = 3.519, p = 0.0048) and 20 minutes (t(217) = 4.250, p = 0.0003) post-application. Saline treatment had no significant effect on average spike count at any time point (p > 0.05), suggesting that our preparation was stable, and that spontaneous spiking activity did not vary over time. There was also a significant difference in spike count between CLP257 and saline treated animals at 10 minutes (t(217) = 2.338, p = 0.0203), 15 minutes (t(217) = 2.744, p = 0.0066), and 20 minutes (t(217) = 2.890, p = 0.0042) post-treatment (Fig. 5D). To confirm that the observed effects could be attributed to an increase in KCC2 activity, we applied VU0240551 on the lumbar spinal cord 20 minutes after receiving CLP257 treatment in a subgroup of animals (n=2 rats, 12 motor units). Post-hoc comparisons using the Tukey’s test determined that blocking KCC2 with VU0240551 significantly reduced mean spike count from 96.5 counts per 5 min to 12.9 counts per 5 min (t(172) = 5.226, p < 0.0001). The mean spike count after VU0240551 application was significantly smaller than at any time point after CLP257 application (p < 0.005) and was not statistically significant from pre-CLP257 values (t(172) = −1.480, p = 0.6775) (Fig.5D). In addition to increasing spontaneous spiking of motor units that were already active, application of CLP257 induced the recruitment of additional motor units. After CLP257 treatment, an average of 5 additional motor units were recruited in each animal, whereas saline on average recruited no further motor units (Fig. 5E).
Figure 5: CLP257 increases spontaneous spiking activity in LG muscle after SCI.
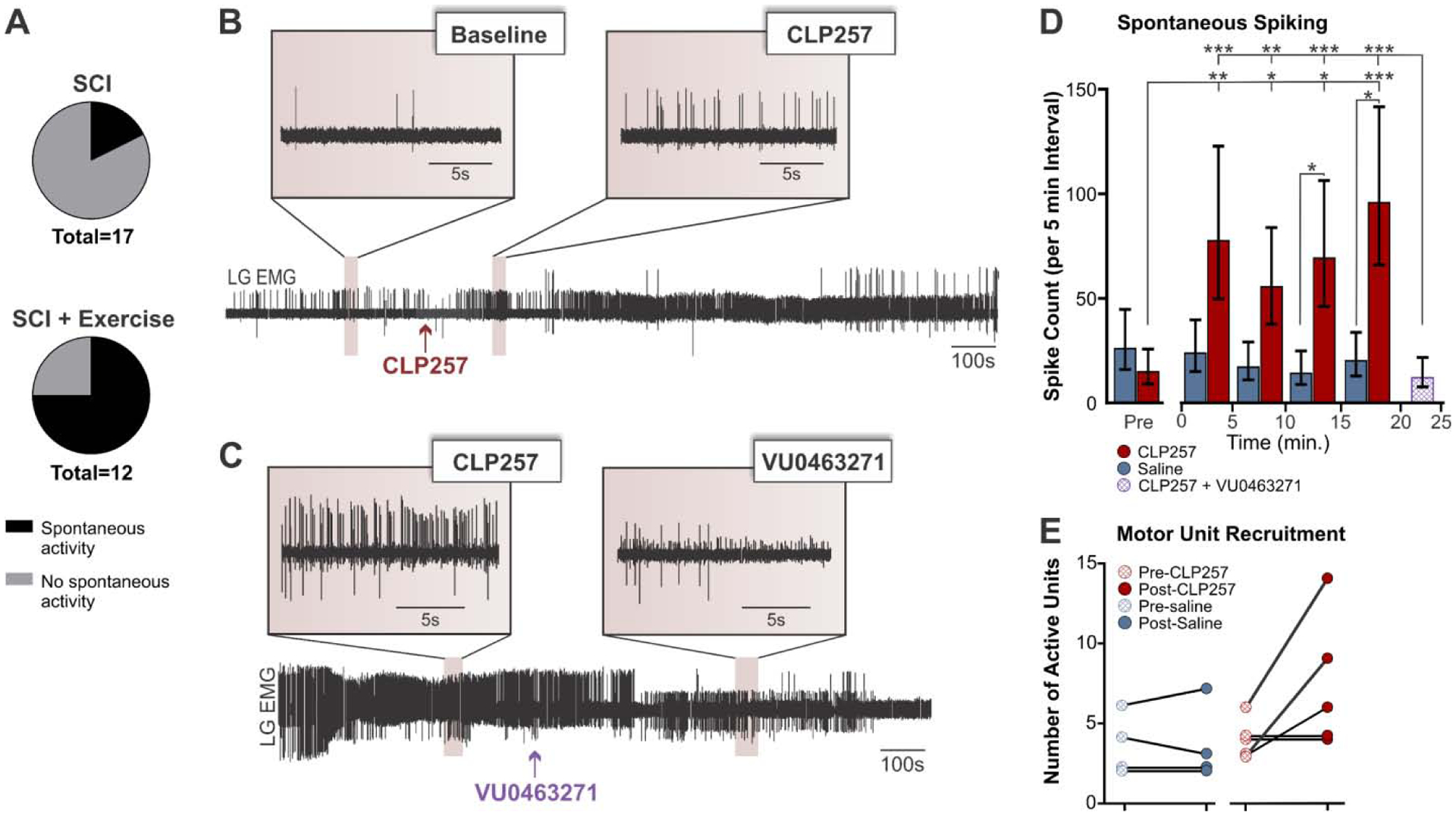
A) Exercise increased the occurrence of spontaneous spiking activity in the lateral gastrocnemius muscle as compared to un-exercised SCI (χ2(1, N = 29) = 7.321, p = 0.007). B–C) Representative EMG recording of an animal displaying spontaneous activity at rest in LG muscle. CLP257 increased spiking frequency (B) while VU0240551 returned spiking activity toward baseline levels (C). D) Application of CLP257 increased spontaneous spiking activity in exercised animals (n=5) as early as 5 minutes after drug application. Blocking KCC2 with VU0240551 post-CLP257 reversed this effect (n=2). E) CLP257 also increased the number of motor units recruited as compared to saline application. SCI+Ex n=4, SCI+Ex+CLP257 n=5. *p<0.05. **p<0.01. ***p<0.001
CLP257 restores KCC2 membrane expression in lumbar motoneurons after chronic SCI
Following chronic SCI, there is a ~10–20% decrease in motoneuronal KCC2 expression, and the insertion of KCC2 to the motoneuronal membrane is significantly reduced (Boulenguez et al., 2010; Bos et al., 2013). As KCC2 must be expressed on the membrane to actively extrude chloride, increased endocytosis leads to reduced chloride extrusion (Lee et al., 2007; 2010). Because CLP257 works post-translationally to increase KCC2 membrane expression (Ferrini et al., 2017; Gagnon et al., 2013), we compared the immunolabelling ratio for membrane and cytosolic expression of KCC2 between groups. Figure 6 shows KCC2 immunoreactivity in lumbar motoneurons after chronic SCI in animals that received saline (Fig. 6A) or CLP257 (Fig. 6B), and SCI+Ex animals that received saline (Fig. 6C) or CLP257 (Fig. 6D). Overall, the membrane-cytosol KCC2 ratio was significantly larger post-CLP257 than post-saline after chronic SCI (Fig. 6E; unpaired t-test, t(19.93) = 6.352, p < 0.0001). Similarly, CLP257 induced a significant increase in the membrane-cytosol ratio in exercised animals (Fig. 6E; t(28.73) = 3.531, p = 0.0014), albeit a more modest one. This demonstrates that CLP257 increases KCC2 membrane expression in lumbar motoneurons, both in SCI animals and in their exercised counterparts.
Figure 6: CLP257 increases KCC2 membrane expression in lumbar motoneurons.
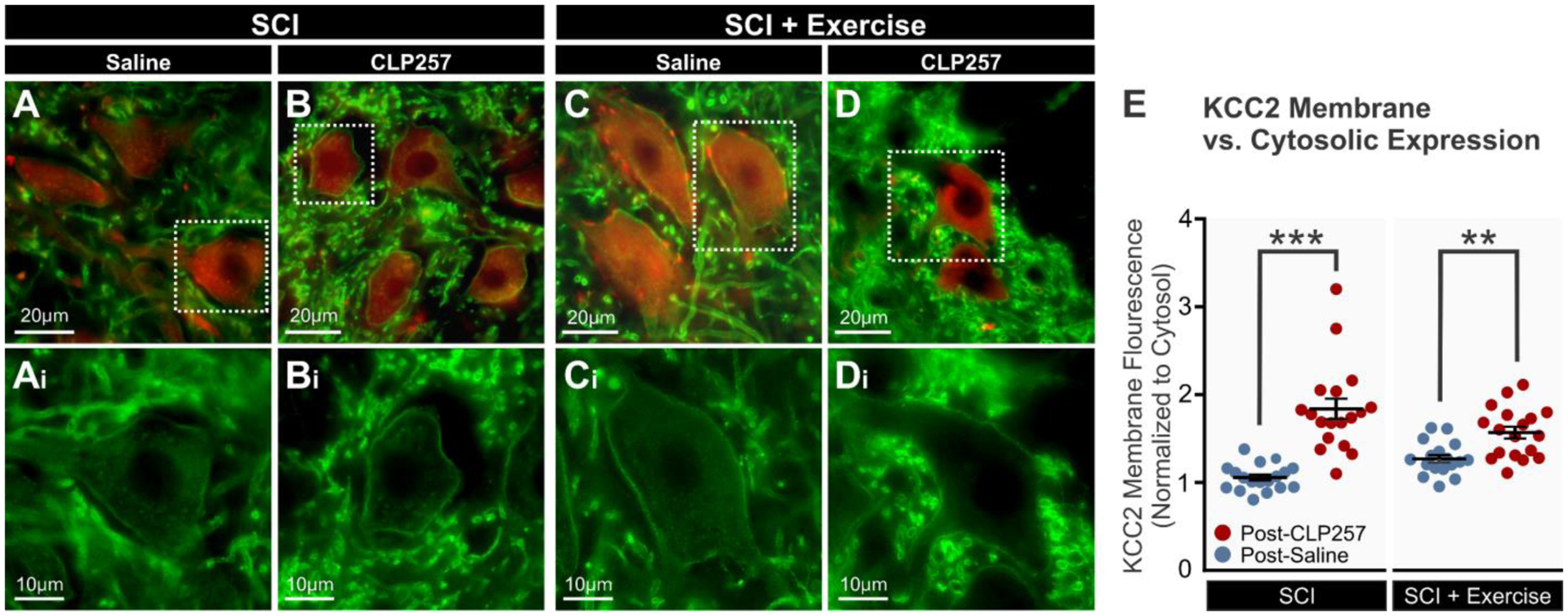
A–D) Representative images showing KCC2 (green) and ChAT (red) immunoreactivity in lumbar motoneurons of chronic SCI rats treated with saline (A) or CLP257 (B), and exercised rats treated with saline (C) or CLP257 (D). E) Mean pixel intensities of KCC2 motoneuronal membrane immunolabeling normalized to a determined area of the cytosol. Membrane-cytosol KCC2 ratio was significantly larger post-CLP257 than post-saline after chronic SCI whether the animals were unexercised (unpaired t-test, t(19.93) = 6.352, p < 0.0001) or exercised (t(28.73) = 3.531, p = 0.0014). Circles represent individual motoneurons. All groups contain 6 motoneurons per animal, 3 animals per group. **p<0.01, ***p<0.001.
Discussion
It is widely accepted that multiple factors play a role in the emergence and maintenance of spasticity and hyperreflexia. In particular, the role of increased motoneuronal intrinsic excitability and loss of descending inhibition have been thoroughly studied (Nielsen et al., 2007; Bennett et al., 2001). More recently, a disruption in chloride homeostasis was identified as a significant contributor to spasticity after SCI (Boulenguez et al., 2010). Under normal physiological conditions, the intracellular concentration of chloride is kept low by the chloride extruder KCC2, which favors neuronal hyperpolarization mediated by the chloride influx upon GABAAR activation (Kaila et al., 2014). After chronic SCI, there is a decrease in KCC2 expression in lumbar motoneurons (Boulenguez et al., 2010), likely due to increased calpain-mediated proteolysis (Plantier et al., 2019). This leads to a reversal of GABA-mediated responses from hyperpolarization to depolarization and increases the probability that a motoneuron will fire an action potential (Boulenguez et al., 2010; Vinay and Jean-Xavier, 2008). This shift in chloride homeostasis contributes to the development of spasticity, as indicated by the decrease in H-reflex RDD (Boulenguez et al., 2010; Vinay and Jean-Xavier, 2008). We and others have previously shown that exercise increases KCC2 expression and function after SCI, and that this correlates with improvements in H-reflex RDD (Tashiro et al., 2015; Chopek et al., 2015; Côté et al., 2014). In this study, we demonstrate that pharmacologically increasing KCC2 activity with CLP257 in the lumbar spinal cord reduces correlates of spasticity after chronic SCI.
CLP257 increases KCC2 membrane expression on lumbar motoneurons
After chronic SCI, KCC2 expression is decreased on lumbar motoneuronal membranes, while the presence of cytoplasmic clusters increases (Côté et al., 2014; Boulenguez et al., 2010), suggesting changes in post-translational membrane insertion and endocytosis of KCC2 (Lee et al., 2010; 2007). Previous studies have explored numerous methods to increase KCC2 expression in spinal cord neurons, albeit indirectly. Increasing BDNF (Boulenguez et al. 2010; Côté et al., 2014, Tashiro et al., 2015), activating 5-HT1A/2A receptors (Bos et al., 2013; Huang and Grau, 2018; Sanchez-Brualla et al., 2018) or administering the dopamine receptor (D2) antagonist, PCPZ (Liabeuf et al., 2017), all increase KCC2 activity and have proven effective in combatting hyperreflexia, central sensitization and neuropathic pain. However, these strategies were considered clinically suboptimal due to the severe side effects they can induce (reviewed in Côté 2020). Thus, the development of a small molecule that can directly act on KCC2 activity emerged as a critical need (Kaila et al., 2014).
There are many potential advantages to this approach, mainly that KCC2 is neuron-specific and expressed solely in the CNS (Rivera et al., 1999; Williams et al., 1999; Medina et al., 2014), and is therefore less likely to produce significant peripheral effects. Additionally, by restoring endogenous inhibition rather than indiscriminately depressing global excitability, reestablishing chloride homeostasis is likely to avoid detrimental effects on locomotor rehabilitation seen with current antispastic medications. Indeed, when using CLP to treat neuropathic pain in rats, Gagnon et al. (2013) found that CLP had no effect on performance in a rotarod assay, suggesting that CLP has no motor side effects. Although the precise mechanism of action remains unknown, CLPs increase chloride extrusion by rescuing plasmalemmal KCC2 protein turnover post-translationally (Ferrini et al., 2017; Gagnon et al., 2013). While the effectiveness of CLP257 has been challenged (Cardarelli et al., 2017), our results further support that CLP257 increases cell surface expression of KCC2 in spinal neurons (Chen et al., 2018; Gagnon et al., 2017).
CLP257 decreases hyperreflexia after chronic SCI
Using CLPs to increase KCC2 activity has rapidly gained popularity, having been used to treat several models of neuropathic pain (Gagnon et al., 2013; Ferrini et al., 2017; Lorenzo et al., 2020), restore hippocampal long-term potentiation after aging (Ferando et al., 2016), reduce alcohol self-administration (Ostroumov et al., 2016; Thomas et al., 2018), and improve functional recovery after traumatic brain injury (Lizhnyak et al., 2019). Our results show that by enhancing KCC2 function in lumbar motoneurons, CLP257 also ameliorates symptoms of spasticity.
A defining hallmark of spasticity is the hyperexcitability of the stretch reflex (Lance, 1980), which can be assessed either through electrical stimulation of the nerve (H-reflex), or through a mechanical stretch of the muscle. In our experiments, high velocity stretches evoked large phasic monosynaptic EMG responses as well as long-lasting tonic activity after chronic SCI, both of which were attenuated by CLP257. This was corroborated by examining the reflex-mediated forces: CLP257 reduced both the reflex-mediated force and also the plateau force for stretches in which tonic EMG activity was present. It is worth noting that while EMG recordings were performed only in the LG muscle, CLP257 affected reflex-mediated forces for the whole triceps surae muscle group. This suggests that CLP257 may have reduced reflex responses not only for the lateral gastrocnemius, but also the medial gastrocnemius and the soleus muscles (Nichols, 1999).
CLP257 also significantly improved the modulation of the H-reflex after chronic SCI, suggesting a change in the contribution of homosynaptic, presynaptic and/or postsynaptic inhibitory mechanisms (Hultborn et al. 1996; Zucker & Regehr, 2002; Caron et al., 2016). Interestingly, CLP257 reduced the phasic EMG response to stretch and restored H-reflex modulation but did not affect any basic properties of the H-reflex. This may be explained by differences in how H-reflexes and stretch responses are generated: electrical stimulation directly and synchronously activates the largest axons (group I afferents), while tendon stretch mechanically activates muscle spindles (group Ia and II), producing a more dispersed afferent volley and longer-lasting activation of the motor pool than electrical stimulation (Burke et al., 1984; Birnbaum and Ashby, 1982). As a result, responses to mechanical stretch are more likely affected by changes in polysynaptic pathways (Burke et al., 1984; Birnbaum and Ashby, 1982). Because KCC2 is not expressed in primary afferents (Kanaka et al., 2001; Mao et al., 2012), this suggests that the effects of CLP257 are likely due to increased postsynaptic inhibition, but this remains to be directly demonstrated.
It is unclear, however, whether this mainly involves motoneurons (Fig.6) or interneurons (Chen et al., 2018). CLP257 not only reduced the exaggerated monosynaptic reflexes that contribute to clonus and hyperreflexia (De Serres et al., 2002; Bennett et al., 1996), but also attenuated the long-lasting polysynaptic responses that contribute to hypertonia and the initiation of spasms (Murray et al., 2011b; Li et al., 2004; Bennett et al., 1999; 2004). This suggests an effect on multiple spinal pathways, monosynaptic and/or polysynaptic, that play a role in spasticity (Lucas-Osma et al., 2019; D’Amico et al., 2013a–b; Murray et al., 2010; 2011a). Motoneuronal excitability ultimately dictates the final motor output, and thus its role is critical to motor recovery. Indeed, restoring chloride homeostasis in lumbar motoneurons is not only of significant importance to improve hindlimb hyperreflexia (Boulenguez et al., 2010; Côté et al., 2014; Tashiro et al., 2015), but is also essential for it (Beverungen et al., 2019). Our results further support this finding. However, it is likely that restoring chloride homeostasis in spinal interneurons after SCI also contributes to functional recovery. Increasing KCC2 expression and chloride extrusion specifically in inhibitory relay interneurons was recently shown to improve locomotor recovery after an incomplete SCI (Chen et al., 2018), and spinal interneurons are known to play a role in the initiation of spasms (Lin et al., 2019; Bellardita et al., 2017). In particular, the interneurons in disynaptic reflex pathways from group II afferents have been highlighted as major contributors to the abnormally strong reflex responses observed in spastic individuals (Jankowska and Hammar, 2002). Therefore, it is likely that CLP257 reduced polysynaptic responses to stretch by affecting chloride homeostasis of interneurons in addition to motoneurons. Whether CLP257 specifically decreased group II excitation and/or increased group I inhibition remains to be determined.
CLP257 mimics the effect of exercise on hyperreflexia after chronic SCI
After chronic SCI, exercise prevents the downregulation of KCC2, which correlates with improvements in reflex modulation. It has also been shown that blockers of KCC2 activity impair the RDD in intact rats (Boulenguez et al., 2010), remove the beneficial effects of exercise in rats with SCI when delivered acutely (Côté et al., 2014) and prevent the beneficial effects of exercise when delivered chronically (Beverungen et al., 2019). Therefore, an increase KCC2 activity is required for exercise to induce beneficial effects on spasticity. In agreement with this, exercised animals in this study displayed high levels of KCC2 membrane expression in lumbar motoneurons and had a RDD of the H-reflex that resembled those of intact rats (Beverungen et al., 2019; Côté et al., 2014; Boulenguez et al., 2010; Thompson et al., 1992). In addition, exercised animals displayed no tonic EMG responses to muscle stretches, and smaller phasic responses. In sedentary animals, CLP257 affected both phasic and tonic EMG responses and RDD in a similar manner to exercise. This suggests that KCC2 enhancers mimic the effects of exercise on several correlates of spasticity.
Interestingly, CLP257 did not enhance the benefits of exercise on spastic symptoms. Perhaps because they act on the same pathway, but this remains to be thoroughly examined. More importantly, CLP257 did not interfere with the beneficial effects of exercise, and even increased spiking activity (see next section), suggesting that KCC2 enhancers may be used in tandem with exercise-based therapies without detrimental effects. These results indicate that KCC2 enhancers may be a promising alternative for SCI individuals and could reduce the need for exercise-based rehabilitation and the associated costs.
CLP257 increases spontaneous activity in exercised animals without hindering benefits on spasticity
There was a notable presence of spontaneous EMG activity in exercised animals. CLP257 further increased this activity, while blocking KCC2 with VU0240551 returned the activity back to baseline levels. This suggests that while increasing KCC2 activity reduces hyperreflexia, it does not decrease overall spinal excitability and increases activity in certain pathways, perhaps by restoring inhibition on inhibitory interneurons within the spinal cord (Chen et al., 2018). Importantly, the spontaneous activity we observe appears distinct from muscle spasms. It resembles activity often seen in SCI individuals, which involves weak contractions (usually unnoticed by subjects) that have no obvious trigger (Zijdewind and Thomas, 2001). Spasms, in contrast, involve strong contractions, vary in intensity and duration, and are easily initiated (Kawamura et al., 1989; Little et al., 1989). In fact, studies have reported such spontaneous activity either to have no correlation (Kirshblum et al., 2001) or to be negatively correlated (Campbell et al., 1991) with spasticity in patients. Accordingly, the heightened presence of spontaneous spiking in SCI + Ex animals in our study was not associated with increased measures of hyperreflexia or spasticity. Whether this contributes to functional recovery merits further investigation.
Conclusions
Current pharmacological treatments for spasticity result in severe side effects, including depression of motor function, seizures, hallucinations, liver toxicity, impaired memory, and possible addiction (Kita and Goodkin, 2000; Burchiel and Hsu, 2001; Elovic, 2001; Adams and Hicks, 2005). All of these severely hinder functional recovery and quality of life. This work has demonstrated the beneficial effects of pharmacologically increasing KCC2 with CLPs on hyperreflexia and spasticity after chronic SCI. By restoring endogenous inhibition rather than depressing overall excitability, restoring chloride homeostasis is likely to avoid the detrimental effects that current antispastic medications have on locomotor rehabilitation. What’s more, CLPs were recently found to improve spontaneous locomotor recovery in staggered hemi-transected mice (Chen et al., 2018). This, along with our results, implicates pharmacological manipulation of KCC2 as a promising new strategy to alleviate symptoms of spasticity in individuals with SCI (Klomaj et al., 2019).
Highlights.
Spasticity develops in a majority of SCI individuals and interferes with recovery
Exercise was shown to attenuate spasticity after SCI through an increase in KCC2 expression
However, early implementation of exercise programs in the clinic is often challenging
We show that spastic symptoms are reduced with CLP257 after chronic SCI
This suggests the potential to pharmacologically restore chloride homeostasis to treat spasticity
Acknowledgements:
We thank Drs. Yves de Koninck and Annie Castonguay from Université Laval for the generous gift of CLP257 and technical assistance. This work was supported by the National Institute of Health (grant number NS083666) and the Craig H. Neilsen Foundation (grant number 189758).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement:
The authors declare no competing financial interests.
References
- Adams MM, Hicks AL (2005) Spasticity after spinal cord injury. Spinal Cord 43:577–586. [DOI] [PubMed] [Google Scholar]
- Angeli C, Ochsner J, Harkema S (2012) Effects of chronic baclofen use on active movement in an individual with a spinal cord injury. Spinal Cord 50:925–927. [DOI] [PubMed] [Google Scholar]
- Bellardita C, Caggiano V, Leiras R, Caldeira V, Fuchs A, Bouvier J, Low P, Kiehn O (2017) Spatiotemporal correlation of spinal network dynamics underlying spasms in chronic spinalized mice. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y (2017) NKCC1 Chloride Importer Antagonists Attenuate Many Neurological and Psychiatric Disorders. Trends Neurosci 40:536–554. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E (2012) The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist 18:467–486. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, De Serres SJ, Stein RB (1996) Gain of the triceps surae stretch reflex in decerebrate and spinal cats during postural and locomotor activities. J Physiol 496 (Pt 3):837–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Harvey PJ, Gorassini M (2001) Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. Journal of Neurophysiology 86:1972–1982. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Sanelli L, Cooke CL, Harvey PJ, Gorassini MA (2004) Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. Journal of Neurophysiology 91:2247–2258. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Gorassini M, Fouad K, Sanelli L, Han Y, Cheng J (1999) Spasticity in rats with sacral spinal cord injury. JNeurotrauma 16:69–84. [DOI] [PubMed] [Google Scholar]
- Beverungen H, Klaszky SC, Klaszky M, Côte MP (2019) Rehabilitation Decreases Spasticity by Restoring Chloride Homeostasis through the Brain-Derived Neurotrophic Factor-KCC2 Pathway after Spinal Cord Injury. J Neurotrauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biering-Sorensen F, Nielsen JB, Klinge K (2006) Spasticity-assessment: a review. Spinal Cord 44:708–722. [DOI] [PubMed] [Google Scholar]
- Birnbaum A, Ashby P (1982) Postsynaptic potentials in individual soleus motoneurons in man produced by achilles tendon taps and electrical stimulation of tibial nerve. Electroencephalogr Clin Neurophysiol 54:469–471. [DOI] [PubMed] [Google Scholar]
- Bos R, Sadlaoud K, Boulenguez P, Buttigieg D, Liabeuf S, Brocard C, Haase G, Bras H, Vinay L (2013) Activation of 5-HT2A receptors upregulates the function of the neuronal K-Cl cotransporter KCC2. Proc Natl Acad Sci U S A 110:348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M, Vinay L (2010) Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. NatMed 16:302–307. [DOI] [PubMed] [Google Scholar]
- Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Machler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9:378–400. [Google Scholar]
- Burchiel KJ, Hsu FP (2001) Pain and spasticity after spinal cord injury: mechanisms and treatment. Spine (Phila Pa 1976) 26:S146–160. [DOI] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, McKeon B (1984) Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. J Neurophysiol 52:435–448. [DOI] [PubMed] [Google Scholar]
- Calancie B, Broton JG, Klose KJ, Traad M, Difini J, Ayyar DR (1993) Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal cord injury in man. ElectroencephalogrClinNeurophysiol 89:177–186. [DOI] [PubMed] [Google Scholar]
- Campbell JW, Herbison GJ, Chen YT, Jaweed MM, Gussner CG (1991) Spontaneous electromyographic potentials in chronic spinal cord injured patients: relation to spasticity and length of nerve. Arch Phys Med Rehabil 72:23–27. [PubMed] [Google Scholar]
- Cardarelli RA et al. (2017) The small molecule CLP257 does not modify activity of the K(+)-Cl(−) co-transporter KCC2 but does potentiate GABAA receptor activity. Nat Med 23:1394–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Li Y, Yu B, Zhang Z, Brommer B, Williams PR, Liu Y, Hegarty SV, Zhou S, Zhu J, Guo H, Lu Y, Zhang Y, Gu X, He Z (2018) Reactivation of Dormant Relay Pathways in Injured Spinal Cord by KCC2 Manipulations. Cell 174:521–535 e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopek JW, Sheppard PC, Gardiner K, Gardiner PF (2015) Serotonin receptor and KCC2 gene expression in lumbar flexor and extensor motoneurons posttransection with and without passive cycling. J Neurophysiol 113:1369–1376. [DOI] [PubMed] [Google Scholar]
- Côté M-P (2020) Role of chloride cotransporters in the development of spasticity and neuropathic pain after Spinal Cord Injury In: Neuronal Chloride Transporters in Health and Disease (Tang X, ed), p 650: Elsevier. [Google Scholar]
- Côté M-P, Gandhi S, Zambrotta M, Houle JD (2014) Exercise modulates chloride homeostasis after spinal cord injury. J Neurosci 34:8976–8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté M-P, Azzam GA, Lemay MA, Zhukareva V, Houle JD (2011) Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J Neurotrauma 28:299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De KP, De KY (2003) Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424:938–942. [DOI] [PubMed] [Google Scholar]
- Crone C, Nielsen J (1989) Methodological implications of the post activation depression of the soleus H-reflex in man. Exp Brain Res 78:28–32. [DOI] [PubMed] [Google Scholar]
- D’Amico JM, Li Y, Bennett DJ, Gorassini MA (2013a) Reduction of spinal sensory transmission by facilitation of 5-HT1B/D receptors in noninjured and spinal cord-injured humans. J Neurophysiol 109:1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico JM, Yavuz SU, Saracoglu A, Atis ES, Gorassini MA, Turker KS (2013b) Activation properties of trigeminal motoneurons in participants with and without bruxism. J Neurophysiol 110:2863–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dario A, Tomei G (2004) A benefit-risk assessment of baclofen in severe spinal spasticity. Drug Saf 27:799–818. [DOI] [PubMed] [Google Scholar]
- De Koninck Y (2007) Altered chloride homeostasis in neurological disorders: a new target. Curr Opin Pharmacol 7:93–99. [DOI] [PubMed] [Google Scholar]
- De Serres SJ, Bennett DJ, Stein RB (2002) Stretch reflex gain in cat triceps surae muscles with compliant loads. J Physiol 545:1027–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Sinkjaer T (2012) Spasticity. Handb Clin Neurol 109:197–211. [DOI] [PubMed] [Google Scholar]
- Elbasiouny SM, Moroz D, Bakr MM, Mushahwar VK (2010) Management of spasticity after spinal cord injury: current techniques and future directions. Neurorehabil Neural Repair 24:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovic E (2001) Principles of pharmaceutical management of spastic hypertonia. Phys Med Rehabil Clin N Am 12:793–816, vii. [PubMed] [Google Scholar]
- Ferando I, Faas GC, Mody I (2016) Diminished KCC2 confounds synapse specificity of LTP during senescence. Nature neuroscience 19: 1197–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrini F, Lorenzo LE, Godin AG, Quang ML, De Koninck Y (2017) Enhancing KCC2 function counteracts morphine-induced hyperalgesia. Sci Rep 7:3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon M, Bergeron MJ, Perez-Sanchez J, Plasencia-Fernandez I, Lorenzo LE, Godin AG, Castonguay A, Bonin RP, De Koninck Y (2017) Reply to The small molecule CLP257 does not modify activity of the K(+)-Cl(−) co-transporter KCC2 but does potentiate GABAA receptor activity. Nat Med 23:1396–1398. [DOI] [PubMed] [Google Scholar]
- Gagnon M, Bergeron MJ, Lavertu G, Castonguay A, Tripathy S, Bonin RP, Perez-Sanchez J, Boudreau D, Wang B, Dumas L, Valade I, Bachand K, Jacob-Wagner M, Tardif C, Kianicka I, Isenring P, Attardo G, Coull JA, De Koninck Y (2013) Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat Med 19:1524–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey MJ, Klinge K, Crone C, Lorentzen J, Biering-Sorensen F, Ravnborg M, Nielsen JB (2008) Post-activation depression of soleus stretch reflexes in healthy and spastic humans. Experimental Brain Research 185:189–197. [DOI] [PubMed] [Google Scholar]
- Holtz KA, Lipson R, Noonan VK, Kwon BK, Mills PB (2017) Prevalence and Effect of Problematic Spasticity After Traumatic Spinal Cord Injury. Arch Phys Med Rehabil 98:1132–1138. [DOI] [PubMed] [Google Scholar]
- Houle JD, Morris K, Skinner RD, Garcia-Rill E, Peterson CA (1999) Effects of fetal spinal cord tissue transplants and cycling exercise on the soleus muscle in spinalized rats. Muscle Nerve 22:846–856. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Grau JW (2018) Ionic plasticity and pain: the loss of descending serotoninerfic fibers after spinal cord injury transforms how GABA affects pain. Exp Neurol 206:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner CA, Stein V, Hermans-Borgmeyer I, Meyer T, Ballanyi K, Jentsch TJ (2001) Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron 30:515–524. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H (1996) On the mechanism of the post-activation depression of the H-reflex in human subjects. Experimental Brain Research 108:450–462. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I (2002) Spinal interneurons; how can studies in animals contribute to the understanding of spinal interneuronal system in man? Brain Res Brain Res Rev 40:19–28. [DOI] [PubMed] [Google Scholar]
- Jean-Xavier C, Pflieger JF, Liabeuf S, Vinay L (2006) Inhibitory postsynaptic potentials in lumbar motoneurons remain depolarizing after neonatal spinal cord transection in the rat. J Neurophysiol 96:2274–2281. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Khanna A, Clapham DE, Woolf CJ (2014) Therapeutic restoration of spinal inhibition via druggable enhancement of potassium-chloride cotransporter KCC2-mediated chloride extrusion in peripheral neuropathic pain. JAMA Neurol 71:640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J (2014) Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci 15:637–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura J, Ise M, Tagami M (1989) The clinical features of spasms in patients with a cervical cord injury. Paraplegia 27:222–226. [DOI] [PubMed] [Google Scholar]
- Kirshblum S, Lim S, Garstang S, Millis S (2001) Electrodiagnostic changes of the lower limbs in subjects with chronic complete cervical spinal cord injury. Arch Phys Med Rehabil 82:604–607. [DOI] [PubMed] [Google Scholar]
- Kita M, Goodkin DE (2000) Drugs used to treat spasticity. Drugs 59:487–495. [DOI] [PubMed] [Google Scholar]
- Klomjai W, Roche N, Lamy J-C, Bede P, Giron A, Bussel B, Bensmail D, Katz R, Lackmy-Vallée A (2019) Furosemide Unmasks Inhibitory Dysfunction after Spinal Cord Injury in Humans: Implications for Spasticity. J. Neurotrauma 36: 1469–1477. [DOI] [PubMed] [Google Scholar]
- Lance JW (1980) Symposium synopsis In: Spasticity: Disordered Motor Control (Feldman RG, Young RR, Koella WP, ed), pp 485–494. [Google Scholar]
- Lapeyre E, Kuks JB, Meijler WJ (2010) Spasticity: revisiting the role and the individual value of several pharmacological treatments. NeuroRehabilitation 27:193–200. [DOI] [PubMed] [Google Scholar]
- Lee HH, Jurd R, Moss SJ (2010) Tyrosine phosphorylation regulates the membrane trafficking of the potassium chloride co-transporter KCC2. Mol Cell Neurosci 45:173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Walker JA, Williams JR, Goodier RJ, Payne JA, Moss SJ (2007) Direct protein kinase C-dependent phosphorylation regulates the cell surface stability and activity of the potassium chloride cotransporter KCC2. J Biol Chem 282:29777–29784. [DOI] [PubMed] [Google Scholar]
- Li Y, Harvey PJ, Li X, Bennett DJ (2004) Spastic long-lasting reflexes of the chronic spinal rat studied in vitro. J Neurophysiol 91:2236–2246. [DOI] [PubMed] [Google Scholar]
- Liabeuf S, Stuhl-Gourmand L, Gackiere F, Mancuso R, Sanchez Brualla I, Marino P, Brocard F, Vinay L (2017) Prochlorperazine Increases KCC2 Function and Reduces Spasticity after Spinal Cord Injury. J Neurotrauma 34:3397–3406. [DOI] [PubMed] [Google Scholar]
- Lin S, Li Y, Lucas-Osma AM, Hari K, Stephens MJ, Singla R, Heckman CJ, Zhang Y, Fouad K, Fenrich KK, Bennett DJ (2019) Locomotor-related V3 interneurons initiate and coordinate muscles spasms after spinal cord injury. J Neurophysiol 121:1352–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little JW, Micklesen P, Umlauf R, Britell C (1989) Lower extremity manifestations of spasticity in chronic spinal cord injury. Am J Phys Med Rehabil 68:32–36. [DOI] [PubMed] [Google Scholar]
- Lizhnyak PN, Muldoon PP, Pilaka PP, Povlishock JT, Ottens AK (2019) Traumatic Brain Injury Temporal Proteome Guides KCC2-Targeted Therapy. J Neurotrauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorentzen J, Grey MJ, Crone C, Mazevet D, Biering-Sorensen F, Nielsen JB (2010) Distinguishing active from passive components of ankle plantar flexor stiffness in stroke, spinal cord injury and multiple sclerosis. Clin Neurophysiol 121:1939–1951. [DOI] [PubMed] [Google Scholar]
- Lorenzo L, Godin AG, Ferrini F et al. Enhancing neuronal chloride extrusion rescues α2/α3 GABAA-mediated analgesia in neuropathic pain. Nat Commun 11, 869 (2020). 10.1038/s41467-019-14154-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Osma AM, Li Y, Murray K, Lin S, Black S, Stephens MJ, Ahn AH, Heckman CJ, Fenrich KK, Fouad K, Bennett DJ (2019) 5-HT1D receptors inhibit the monosynaptic stretch reflex by modulating C-fiber activity. J Neurophysiol 121:1591–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao S, Garzon-Muvdi T, Di Fulvio M, Chen Y, Delpire E, Alvarez FJ, Alvarez-Leefmans (2012) Molecular and functional expression of cation-chloride cotransporters in dorsal root ganglion neurons during postnatal maturation. J Neurophysiol 108: 834–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapplebeck JCS, Lorenzo LE, Lee KY, Gauthier C, Muley MM, De Koninck Y, Prescott SA, Salter MW (2019) Chloride Dysregulation through Downregulation of KCC2 Mediates Neuropathic Pain in Both Sexes. Cell Rep 28:590–596 e594. [DOI] [PubMed] [Google Scholar]
- Maynard FM, Karunas RS, Waring WP 3rd (1990) Epidemiology of spasticity following traumatic spinal cord injury. Archives of physical medicine and rehabilitation 71:566–569. [PubMed] [Google Scholar]
- Medina I, Friedel P, Rivera C, Kahle KT, Kourdougli N, Uvarov P, Pellegrino C (2014) Current view on the functional regulation of the neuronal K(+)-Cl(−) cotransporter KCC2. Front Cell Neurosci 8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KC, Stephens MJ, Ballou EW, Heckman CJ, Bennett DJ (2011a) Motoneuron excitability and muscle spasms are regulated by 5-HT2B and 5-HT2C receptor activity. J Neurophysiol 105:731–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KC, Stephens MJ, Rank M, D’Amico J, Gorassini MA, Bennett DJ (2011b) Polysynaptic excitatory postsynaptic potentials that trigger spasms after spinal cord injury in rats are inhibited by 5-HT1B and 5-HT1F receptors. J Neurophysiol 106:925–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ, Rank M, D’Amico J, Harvey PJ, Li X, Harris RL, Ballou EW, Anelli R, Heckman CJ, Mashimo T, Vavrek R, Sanelli L, Gorassini MA, Bennett DJ, Fouad K (2010) Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. NatMed 16:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TR. Receptor mechanisms underlying heterogenic reflexes among the triceps surae muscles of the cat. J Neurophysiol. 1999. February;81(2):467–78. doi: 10.1152/jn.1999.81.2.467. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Crone C, Hultborn H (2007) The spinal pathophysiology of spasticity--from a basic science point of view. Acta Physiol (Oxf) 189:171–180. [DOI] [PubMed] [Google Scholar]
- Norton JA, Bennett DJ, Knash ME, Murray KC, Gorassini MA (2008) Changes in sensory-evoked synaptic activation of motoneurons after spinal cord injury in man. Brain : a journal of neurology 131:1478–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroumov A, Thomas AM, Kimmey BA, Karsch JS, Doyon WM, Dani JA (2016) Stress Increases Ethanol Self-Administration via a Shift toward Excitatory GABA Signaling in the Ventral Tegmental Area. Neuron 92:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipio J, Kaila K (2003) Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci 26:199–206. [DOI] [PubMed] [Google Scholar]
- Petropoulou KB, Panourias IG, Rapidi CA, Sakas DE (2007) The importance of neurorehabilitation to the outcome of neuromodulation in spasticity. Acta Neurochir Suppl 97:243–250. [DOI] [PubMed] [Google Scholar]
- Pingel J, Wienecke J, Lorentzen J, Nielsen JB (2016) Botulinum toxin injection causes hyperreflexia and increased muscle stiffness of the triceps surae muscle in the rat. J Neurophysiol 116:2615–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantier V, Sanchez-Brualla I, Dingu N, Brocard C, Liabeuf S, Gackiere F, Brocard F (2019) Calpain fosters the hyperexcitability of motoneurons after spinal cord injury and leads to spasticity. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puskarjov M, Kahle KT, Ruusuvuori E, Kaila K (2014) Pharmacotherapeutic targeting of cation-chloride cotransporters in neonatal seizures. Epilepsia 55:806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K (1999) The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397:251–255. [DOI] [PubMed] [Google Scholar]
- Sanchez-Brualla I, Boulenguez P, Brocard C, Liabeuf S, Viallat-Lieutaud A, Navarro X, Udina E, Brocard F (2018) Activation of 5-HT2A Receptors Restores KCC2 Function and Reduces Neuropathic Pain after Spinal Cord Injury. Neuroscience 387:48–57. [DOI] [PubMed] [Google Scholar]
- Simon O, Yelnik AP (2010) Managing spasticity with drugs. Eur J Phys Rehabil Med 46:401–410. [PubMed] [Google Scholar]
- Skold C, Levi R, Seiger A (1999) Spasticity after traumatic spinal cord injury: nature, severity, and location. Archives of physical medicine and rehabilitation 80:1548–1557. [DOI] [PubMed] [Google Scholar]
- Taricco M, Pagliacci MC, Telaro E, Adone R (2006) Pharmacological interventions for spasticity following spinal cord injury: results of a Cochrane systematic review. Eura Medicophys 42:5–15. [PubMed] [Google Scholar]
- Tashiro S, Shinozaki M, Mukaino M, Renault-Mihara F, Toyama Y, Liu M, Nakamura M, Okano H (2015) BDNF Induced by Treadmill Training Contributes to the Suppression of Spasticity and Allodynia After Spinal Cord Injury via Upregulation of KCC2. Neurorehabil Neural Repair 29:677–689. [DOI] [PubMed] [Google Scholar]
- Thomas AM, Ostroumov A, Kimmey BA, Taormina MB, Holden WM, Kim K, Brown-Mangum T, Dani JA (2018) Adolescent Nicotine Exposure Alters GABAA Receptor Signaling in the Ventral Tegmental Area and Increases Adult Ethanol Self-Administration. Cell Rep 23:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson FJ, Reier PJ, Lucas CC, Parmer R (1992) Altered patterns of reflex excitability subsequent to contusion injury of the rat spinal cord. Journal of Neurophysiology 68:1473–1486. [DOI] [PubMed] [Google Scholar]
- Trimble MH, Behrman AL, Flynn SM, Thigpen MT, Thompson FJ (2001) Acute effects of locomotor training on overground walking speed and H- reflex modulation in individuals with incomplete spinal cord injury. JSpinal CordMed 24:74–80. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Bos R, Boulenguez P, Brocard C, Brocard F, Bras H, Coulon P, Liabeuf S, Pearlstein E, Sadlaoud K, Stil A, Tazerart S, Vinay L (2011) Chapter 1--importance of chloride homeostasis in the operation of rhythmic motor networks. ProgBrain Res 188:3–14. [DOI] [PubMed] [Google Scholar]
- Vinay L, Jean-Xavier C (2008) Plasticity of spinal cord locomotor networks and contribution of cation-chloride cotransporters. Brain ResRev 57:103–110. [DOI] [PubMed] [Google Scholar]
- Willerslev-Olsen M, Lorentzen J, Sinkjaer T, Nielsen JB (2013) Passive muscle properties are altered in children with cerebral palsy before the age of 3 years and are difficult to distinguish clinically from spasticity. Dev Med Child Neurol 55:617–623. [DOI] [PubMed] [Google Scholar]
- Williams JR, Sharp JW, Kumari VG, Wilson M, Payne JA (1999). The neuron-specific K-Cl cotransporter, KCC2. Antibody development and initial characterization of the protein. J. Biol. Chem 274, 12656–12664. [DOI] [PubMed] [Google Scholar]
- Yelnik AP, Simon O, Bensmail D, Chaleat-Valayer E, Decq P, Dehail P, Quentin V, Marque P, Parratte B, Pellas F, Rousseaux M, Trocello JM, Uzzan M, Dumarcet N, Agence francaise de securite sanitaire des produits de s (2009) Drug treatments for spasticity. Ann Phys Rehabil Med 52:746–756. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Thomas CK (2001) Spontaneous motor unit behavior in human thenar muscles after spinal cord injury. Muscle Nerve 24:952–962. [DOI] [PubMed] [Google Scholar]


