Abstract
Introduction.
The Trier Social Stress Test (TSST) is the most widely used protocol for activating a stress response of the hypothalamic-pituitary-adrenocortical (HPA) axis and other stress-mediating systems. A number of variants of the TSST exist, including ones for children, groups, and virtual reality. All of these versions, though, require in-person assessment. The COVID-19 pandemic has made in-person assessment impossible or extremely difficult and potentially dangerous. The purpose of this study was to validate a completely remote, online, version of the TSST for children.
Method.
A sample of 68 (27 female) 15- and 16-year old participants were administered the TSST-Online (TSST-OL) during the late afternoon hours (3pm-6pm start time). The participants, judges (one male, one female), and experimenter (female) all joined the assessment from their own homes via the online platform, ZOOM™. Two sessions were conducted, one to obtain consent, explain procedures, work with the family to arrange the computer and room set-up for the TSST-OL and one within two weeks to conduct the procedure. The participants were trained to take their own saliva samples and a saliva sampling kit was mailed to the home in between the first and second session. The samples were then mailed to the researchers within a day of collection. The participant was observed during saliva collection to determine correct procedures were followed. Salivary cortisol, salivary α-amylase and self-reports of stress were measured multiple times over the second session.
Results.
rmANOVAs yielded a significant effect of trials, for cortisol, F(1.37,90.46)=15.13, p=.001, sAA, F(2.75,146.68)=6.91, p=.001, and self-rated stress, F(3.43,222.69)=118.73, p=.001. There were no significant sex by trials interactions for any measure, although females reported more stress than males, F(1,65)=9.14, p=.004. For cortisol, from baseline to expected peak (30 min after the onset of speech preparation), the Cohen’s effect size was dz =0.57. Using 1.5 nmol/l (or 0.54 μg/dl) as the criterion for a response (Miller, Plessow, Kirschaum, & Stalder, 2013), 63% of the participants produced a significant increase in cortisol.
Conclusions.
The responses to the TSST-OL are consistent with in-person responses among children and adolescents (see recent meta-analysis (Seddon et al., 2020 ). The protocol is a viable way of assessing reactivity of the HPA axis and other stress systems without needing to bring the participant into the research laboratory. This method will be useful during periods of widespread infection. It should also work to study populations who all live too far from the research laboratory to be assessed in person.
Keywords: Stress, Trier Social Stress Test, Adolescents, Cortisol, α−Amylase, Online
1.0. Introduction
Life confronts all of us with stressors and challenges. We differ in the frequency and severity of the stressors we encounter, their timing in our development, and, critically, how intensely we react (Ebner & Singewald, 2017; Lupien, McEwen, Gunnar, & Heim, 2009). Because individual differences in stress reactivity play a role in the etiology of physical and mental health disorders, measuring stress reactivity is common in studies of adults and children (Turner et al., 2020 ). Stress mediating systems, including the hypothalamic-pituitary-adrenocortical (HPA) system and the sympathetic nervous system (SNS), respond to endogenous and exogenous threats to our safety and well-being (Herman, McKlveen, Solomon, Carvalho-Netto, & Myers, 2012; McEwen, 2013). In addition to threats to our physical selves, threats to our social selves produce behavioral and physiological stress responses (Dickerson & Kemeny, 2004). Adjudicated performances are forms of social evaluation that are ethical to use in the laboratory to elicit stress. Thus, performing in front of judges who evaluate a participant’s performance forms the core of several stressor protocols (Kirschbaum, Pirke, & Hellhammer, 1993; Smith, Leitzke, & Pollak, 2020; Sumter, Bokhorst, Miers, Van Pelt, & Westenberg, 2010).
Developed in Trier, Germany by Kirschbaum and colleagues (Kirschbaum et al., 1993), the Trier Social Stress Test (TSST) is the most frequently used social evaluative stress tasks in adults (Goodman, Janson, & Wolf, 2017), children, and adolescents (Seddon et al., 2020 ). The TSST consists of five stages: (1) adaptation, (2) speech preparation, (3) speech delivery, (4) mental arithmetic, and (5) recovery. The performance time (speech and math) lasts 10 minutes. Initially developed to assess individuals in person, adaptations of the TSST include group assessment (Hostinar, McQuillan, Mirous, Grant, & Adam, 2014; von Dawans, Kirschbaum, & Heinrichs, 2011) and several that use virtual reality (VR) (Montero-López et al., 2016). The TSST versions for children and adolescents use different speech prompts than the versions for adults as well as modifications to the subtraction task (TSST for children, TSST-C;(Buske-Kirschbaum et al., 1997), TSST-Modified, (Yim, Quas, Cahill, & Hayakawa, 2010)). What they all have in common is the need to bring the participant into the laboratory. Thus, many research groups who use the TSST have had to stop their research during the COVID-19 pandemic because in-person testing has not been feasible.
However, given the development of various online video conference platforms, giving a live talk in front of an audience online is now perfectly feasible. Therefore, we devised a version of the TSST for children and adolescents conducted entirely remotely with the participants, experimenter, and judges joining from their own homes. If this new TSST-online (TSST-OL) produced activation of the HPA axis comparable to traditional, in-person TSST protocols, it would allow continued use of the TSST during periods when infectious disease prevents in-person testing. It would also allow the study of populations challenging to reach for in-person stress assessments, such as populations with rare conditions or widely dispersed populations.
There are several reasons to be concerned that an online version of the TSST might not effectively elevate cortisol. First, uncertainty or unpredictability is a critical element of protocols that activate the HPA axis. Familiarity with one’s surroundings might reduce uncertainty. Consistent with this concern, one meta-analysis of the TSST performed with adults reported that longer periods of adaptation to the laboratory were associated with smaller cortisol responses (Goodman et al., 2017). Likewise, a recent study that administered the TSST for children (TSST-C) in the adolescents’ homes yielded no evidence of elevated cortisol but did find an increase in salivary alpha-amylase (sAA; (DeJoseph, Finegood, Raver, & Blair, 2019). Furthermore, VR versions of the TSST that use screens have been shown to produce smaller effects than in-person, traditional TSST protocols (Helminen, Morton, Wang, & Felver, 2019). Thus, while a significant cortisol response may be less likely when participants join a video call from their familiar home environments, a sympathetic nervous system (SNS) response seemed likely.
We hypothesized that this new TSST-online (TSST-OL) protocol would produce increases in participant self-reported stress and activation of the HPA axis, measured with salivary cortisol response. To determine whether it was as effective as in person versions of the TSST for children, we compared the effect size from baseline to expected peak to that found in the recent meta-analysis of community samples of children and adolescents which revealed a meta-analytic effect size of .48 with a standard error of .17 (Seddon et al., 2020 ). We also hypothesized that an online TSST would produce a significant sympathetic nervous system (SNS) response as indexed by salivary alpha-amylase. We chose to study adolescents because social evaluation is a particularly appropriate stressor for them because of their heightened sensitivity to social acceptance and exclusion (Pfeifer et al., 2013; van den Bos, de Rooij, Miers, Bokhorst, & Westenberg, 2014).
1. Methods
1.1. Participants
Sixty-eight (27 female) 15- and 16- years old were assessed. Participants were solicited from a department-maintained participant pool of families interested in being contacted for research. This pool was recruited through letters sent to all families giving birth each month in the greater metropolitan area augmented through recruitment annually at the state’s fair and through sources such as Craig’s List. Exclusion criteria were: major congenital abnormality, a psychiatric condition requiring medication, steroid or beta-adrenergic medication within two weeks of testing, any symptoms of COVID19, and being currently quarantined for COVID19. In addition, we required families to have a stable internet connection that could sustain a 2-hr session, computers/devices with at least a 13-inch screen, and a separate room for the TSST assessment where the participant could be alone for 2 hours. No families were excluded due to a lack of functional requirements for the study. Two families took part in only the consent call but then declined the TSST-OL session. They are not included in the sample size listed above. The sample size noted above also reflects all other exclusions. Descriptive data on the sample is shown in Table 1. This study was conducted in accordance with university Institutional Review Board (IRB) approval standards.
Table 1:
Descriptive and Demographic Data
| Variable | Unit | Values |
|---|---|---|
| Sex | ||
| Female | N | 27 |
| Males | N | 41 |
| Participant Age | Mean (SD) | 15.84 (0.47) |
| Tanner Stage | ||
| Three | % | 13.2 |
| Four | % | 77.9 |
| Five | % | 8.8 |
| Menstrual Phase1 | ||
| Follicular | N | 17 |
| Luteal | N | 10 |
| Birth Control (female) | N | 1 |
| Child Race | ||
| Black | % | 1 |
| White | % | 92 |
| Mixed | % | 7 |
| Parent 1 Education: ≥ Four year degree |
% | 92.6 |
| Parent 2 Education: ≥ Four year degree |
% | 83.3 |
| Median Family Income (pre-COVID19) | Median | $100–125,000 |
| Effect of COVID19 on Family Income | ||
| Increase Income | % | 2.9 |
| Decrease Income | % | 35.3 |
| No Change | % | 61.8 |
Luteal phase defined as < 10 days from next menstruation
2.2. Procedures
Following recruitment, two online sessions were completed using the Zoom™ platform: (1) the consent and set up session and (2) the TSST-OL protocol session. Note that images that show the face and body are considered personal health information (PHI) in the United States. Thus, to protect the participants’ PHI, a secured and HIPAA (health insurance portability and accountability act) compliant version Zoom™ Health Care Component (Zoom HCC) was used. The Zoom HCC structure is designed to reduce the risk of possible non-compliance with Protected Health Information (PHI). Zoom HCC is a HIPAA-compliant tool, but it depends on responsible use to remain in compliance. All researchers were required to use the HIPAA compliant Zoom and completed additional training prior to use. Most importantly for this study, cloud recording was disabled, and recordings (containing images of faces and body from the waist up) are only stored on encrypted servers. We used REDCap (Harris et al., 2009) for all forms, including questionnaires and experimenter notes. REDCap uses a MySQLdatabase via a secure web interface that is HIPAA compliant. Access is restricted by username and duo-authentication.
Session 1 consisted of a video call that lasted approximately 30-min and involved both parent and adolescent. At this session, the consent/assent process was completed with parent and youth electronically signing forms. The parent and youth also completed questionnaires (see measures, below). This session’s primary concern was to help the family work out the Session 2 logistics and set up in preparation for conducting the TSST-OL. First, participants showed experimenters around the space that participants thought would work for the TSST session and experimenters verified that traffic flow through that room could cease for the 2 hr duration of Session 2. Next, the experimenter and participant determined the computer set up so that the participant’s video camera was eye-level when the adolescent was seated. The experimenter then had the adolescent stand up and back away from the computer until they were visible from the waist up. The experimenter trained the adolescent to set the Zoom™ settings to “gallery view,” which ensured that the adolescent would see both judges and themselves in the video chat window during the TSST. The adolescent used their phone to show the experimenter the set-up. The experimenter also demonstrated to the adolescent how to collect saliva. A saliva collection kit was sent to the family as soon as Session 1 was completed to allow adequate time for it to arrive before the start of Session 2. Throughout Session 1, experimenters were able to determine the strength of the internet connection. If unstable, alternatives were explored to stabilize the connection. If the alternative internet solutions were still unstable, the family was thanked for their interest, paid for Session 1, and excluded from Session 2. It was not necessary to exclude any of the participants for internet connectivity issues.
Session 2 was scheduled approximately two weeks after Session 1 and began between 1500 and 1800 hours to control for diurnal rhythms. Figure 1 provides a diagram of the timeline and set up for Session 2. The experimenter who worked with the family during Session 1 served as the experimenter for Session 2. After the adolescent, parent, and experimenter were online together, the experimenter changed the adolescent’s screen name to “participant” and informed the participant that the starting to record the session. The experimenter then checked on whether the adolescent had recently eaten or drunk anything, whether all the saliva collection supplies were readily available, and had the adolescent take the first saliva sample. The experimenter then sent a link through the chat function to have the adolescent complete the health check for COVID-19 symptoms (see measures). None of the participants reported symptoms of illness. The experimenter then went over the TSST Zoom™ set-up from Session 1, had the adolescent close the chat function, and display Zoom™ full-screen set in gallery mode. When all of this was completed, the parent who had been present until this point left the room. The experimenter then played a calming PG-rated children’s movie until 30 minutes had elapsed from the onset of the Session 2 Zoom™ call.
Figure 1:
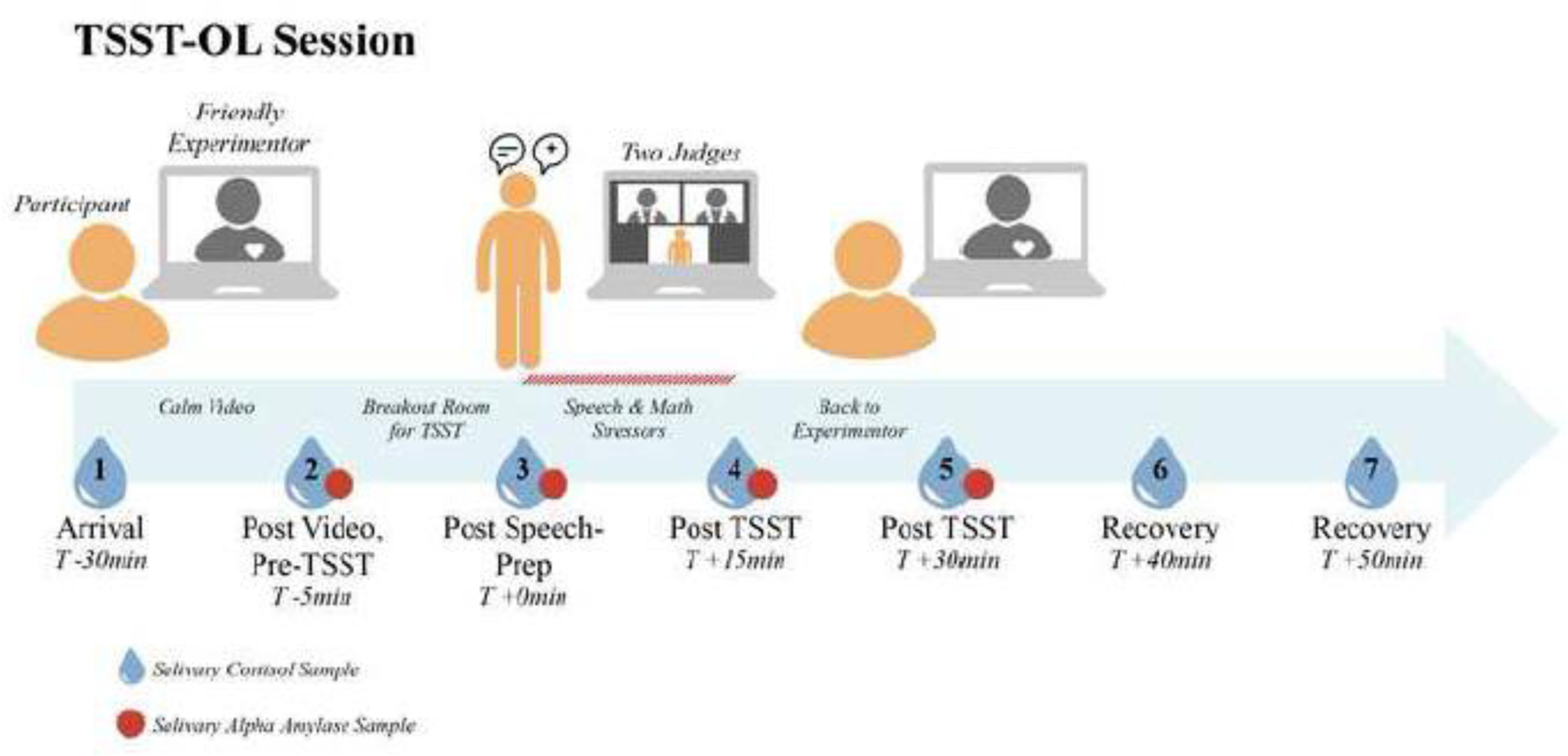
TSST-OL Session
Layout of the TSST-OL session with sampling times shown.
At this point, the experimenter informed the adolescent that they would move on to the test period with the judges. The adolescent produced a second saliva sample and the experimenter moved the adolescent into a breakout room where the judges were waiting. The judges were identified on their screens as “Judge A” and “Judge B.” Figure 1 provides a diagram of the typical screen for the TSST speech/math period of the test. There was always a male and female judge wearing white lab coats and maintaining neutral demeanors. The male judge always introduced the requirements. The Yim and colleagues (Yim et al., 2010) stem for the speech was used. This stem has the participant imagine they are introducing themselves to a class of their peers and to note several good and several not so good things about themselves. The judges then turned off their videos for the 5-min speech preparation. After four minutes, the female judge announced, “you have one minute until your speech.”
At the end of speech preparation, the judges turned their videos back on. The male judge had the participant take another saliva sample. Once done, the female judge said, “Make sure you are in gallery mode so that you can see both of us. Next, stand up and use your phone to show us what you see on your screen. Now turn your phone off so that we are not interrupted, and you may step back to the spot where you will give your speech. You should know that you are being recorded so that we can judge how well you did on your speech compared to others.” The male judge then said, “Your speech should be five minutes long, and if you stop early, we will tell you to continue. You may give your speech…(pause)…now.” If the participants stopped speaking for a period of 20 seconds, the female judge told them to keep going as the time was not up. This continued for 5 minutes.
When five minutes of speech were up, the male judge interrupted (if necessary) to say, “time’s up,” and then moved on to the 5-min math segment of the TSST. The male judge introduced the math task and provided an example of subtracting by 2’s from 200. The judge told the participant that it was “important to work both quickly and accurately,” and that if the participant made an error, they would be asked to start over. Each participant began with 938 and subtracted by 13’s. If they made an error, the female judge interrupted and had them start over. If the participant went exceptionally slowly, the male judge interrupted to remind the participant to work “both quickly and accurately,” and had them start over. If the participant made multiple errors in a row, the female judge interrupted and moved them to subtraction by a smaller number, first 7s and then 3s. If the participant performed exceptionally well while subtracting by 13s, the female judge interrupted and moved them to subtraction by 17s. At the end of the math segment, the recording was turned off, the participant was returned to the main Zoom™ room to interact with the experimenter.
The experimenter helped the adolescent take the remaining saliva samples (see figure 1) and uploaded the links for the questionnaires into chat. After the last sample, the adolescent was debriefed and were told that we were not actually rating their speech or math performance, but were interested in how their body was responding to the challenge.
All protocol materials, including detailed step-by-step manual, is provided online for researchers to access (https://osf.io/aqg9u/).
2.3. Measures
2.3.4. Salivary Cortisol.
Whole unstimulated samples of saliva for cortisol determination were obtained. Adolescents selected the appropriately pre-numbered Eppendorf Safe-Lock 1.5 ml vial, showed it to the experimenter or to the judges, opened it, and removed the straw. They spit through the straw into the vial. Once 50 ml was obtained (or 2 min had passed) the participant was told to stop collecting, tightly close the lid and placed the sample in a sealable plastic bag. The experimenter or judge noted the time of each sample. Once all the samples were collected, they were mailed to the researchers in a pre-labeled and pre-paid package. Seven samples were obtained for salivary cortisol (see Figure 1 for timing).
Once received, the samples were stored frozen at −20°C until being shipped to the endocrine laboratory in Trier, Germany where samples were assayed for cortisol concentration in duplicate using a time-resolved fluorescence immunoassay (DELFIA). The intra- and inter-assay coefficients of variation were less than 10%. Samples from each participant were included in the same assay batch. Samples were examined for values that were more than 4SDs from the mean. None were obtained; thus, all samples were analyzed. Cortisol in μg/dl was log10 transformed to resolve positive skew.
2.3.5. Salivary Alpha-Amylase (sAA).
sAA was assayed from the same saliva samples as those used to assay cortisol, with the exception that only samples 2 through 5 were used (shown in Figure 1). At assay,the saliva was diluted 1:200 with assay diluent. 8µl of the diluted saliva were pipetted in duplicates into a 96-well microtiter plate. After adding 320µl Substrate solution (CNP-G3), the plate was incubated for 60 seconds, 37°C, and 500rpm in a microtiter plate incubator. After exactly one minute, the plate was read kinetically in a plate reader at 405nm and incubated again for exactly two minutes at 37°C and 500rpm. After this incubation, the OD was read again at 405nm. The OD change was calculated by subtracting the OD from the first reading from the OD from the second reading. The activity of the α-Amylase was calculated and expressed in U/ml. The intra-assay coefficient of variation was between 2.8% and 6.3%, and the corresponding inter-assay coefficients of variation were between 5.5% - 7.6%. All samples for a given participant were assayed in the same batch. Fourteen samples from 9 participants that were implausible (i.e., < 2 U/mL ) were removed. The remaining sAA data were log10 transformed. One of the 9 participants with missing data were only missing sample 3. Because samples 2 and 3 were highly correlated, r=.65, p<.001, to preserve one more participant for the analysis, the values for sample 2 was assigned to sample 3 yielding 60 participants for repeated measure analysis.
2.3.6. Self Rating of Stress.
At the end of session two, the participants were asked to rate “how stressed did you feel” at seven points during the session. For this analysis, we examined the last five ratings: (1) the period when they watched the calming video, (2) preparing their speech, (3) giving the speech, (4) conducting the math portion, and (5) after the speech and math portions were completed. Each rating was on a 5-point scale, with 1 = “not at all stressed” and 5 = “highly stressed.”
2.3.6. Questionnaires.
Parents provided information about parental education, family income before COVID-19 (in 25 thousand dollar units), and whether the pandemic resulted in increased, decreased or no change in income. Both parents and adolescents completed the Petersen Pubertal Development Scale (Petersen, Crockett, Richards, & Boxer, 1988) to assess the adolescents pubertal development. Scores were converted to Tanner stages following the guidance of Shirtcliff and colleagues (Shirtcliff, Dahl, & Pollak, 2009). At each session, girls reported the date of their last menstrual period. Using Session 2 data were calculated whether they were in the follicular or luteal phase. At Session 2, adolescents provided information on when they awoke and when they last ate or drank. The health- screening questionnaire covered questions about symptoms of COVID19 on the day of testing. Following the TSST-OL, the adolescent also completed a number of stress and coping questionnaires and questionnaires about the impact of the COVID-19 pandemic on the adolescent and their family. These last data are not examined in this report as the sample size is not yet adequate for those analyses, but we are continuing to collect data for a future report.
2.4. Data Analysis
Cortisol, sAA and self-ratings of stress were each analyzed using sex by trials repeated measures analysis of variance (rmANOVA) with Greenhouse-Geisser corrections. In order to compare the cortisol effect size with the previous meta-analysis (Seddon et al., 2020 ), for cortisol only, Cohen’s dz for paired samples was computed by taking a difference score between baseline (sample 2) and expected peak (sample 5) and dividing the mean by the standard deviation of the difference score (Cohen, 1988), pg 48). GPower 3.1 was used to perform a statistical power analysis to determine the minimum sample size to detect the difference between baseline and expected peak for salivary cortisol response based on the effect size in (Seddon et al., 2020 ). With an effect size of d = .48 for, an alpha = .05 and power = 0.80, the projected sample size needed to detect a difference between dependent cortisol means (baseline and peak) is N = 37.
3.0. Results
3.1. Salivary Cortisol.
The 2 (sex) by 6 (trials) repeated measure ANOVA yielded no significant main effect of sex, F(1,66)=1.22, p=.27 or sex by trials interaction, F(1.37,90.47)=0.13, p=.80. The trials effect was highly significant, F(1.37,90.47)=15.13, p=.0001, with the expected increase from baseline to peak roughly 30 minutes from the onset of speech preparation and then return towards baseline (see Figure 2, Panel A). The Cohen’s effect size for baseline to expected peak response was dz =0.57. In addition, we analyzed the percentage of responders and non-responders based on a response of 0.054 μg/dl (Miller et al., 2013). There were 43 responders (63%) and 25 (37%) non-responders.
Figure 2.

Panel A. Salivary Cortisol Response in mg/dl with lg10 transformation with standard error bars. Panel B. Salivary a-Amylase response in U/ml with lg10 transformation. Panel C. Self-reported stress before (during film), during (speech preparation, speech and math) and after the TSST-OL
3.2. Salivary Alpha-Amylase.
Eight participants had one or more out of range sAA values; they were not included in the rmANOVA when all four sampling times were included. There was no significant effect of sex, F(1,58)=0.20, p=.66, nor did the sex and trials effect reach significance, F(2.57, 146.68)=2.29, p=.09. There was a significant main effect of trials, F(2.57,146.68)=6.9, p=.0001, with the expected increase to peak at the end of the math section and then decrease (see Figure 2, panel B).
3.3. Self Ratings of Stress.
There was a main effect of sex, with girls rating themselves as more stressed than boys, F(1,65)=9.14, p=.004, with girls reporting higher stress overall than boys, average Mgirls=2.91, N=26, SE=0.13; average Mboys =2.47, N=41, SE=0.8. There was no sex by trials interaction, F(3.43,222.69)=1.11, p=.35. There was a significant effect of trials, F(3.43,222.69)=118.73, p=.0001 (see Figure 2, panel c). The peak stress ratings corresponded to the speech and math period, followed by speech preparation, and the calming video segment and after completion of speech and math segment were rated similarly low.
4.0. Discussion
The results showed that this online TSST was successful in elevating cortisol, sAA, and self-rated stress. Notably, the cortisol results were consistent with the meta-analysis results of in-person TSST studies (Seddon et al., 2020 ). The meta-analysis yielded an effect size of .47 (SE-.17), and our effect size of .57 was within that range. The cortisol response in the meta-analysis and our online version of the TSST is smaller than seen among adults using the in-person, traditional TSST. One meta-analysis placed the effect size at .95 for adults (Goodman et al., 2017). It is unclear why the meta-analytic effect sizes for children and adolescents and our effect size are so much smaller than in adults. Studies that have directly compared children and adults often have not reported an age effect (Kudielka, Buske-Kirschbaum, Hellhammer, & Kirschbaum, 2004; Yim et al., 2010). It may be that when adolescents are studied, failure to account for menstrual cycle may be a factor, although until the cycle has become regular this can be difficult to accurately assess. It is also uncommon for studies of children and adolescents to give participants a sugar-loaded drink prior to testing in order to equate momentary blood sugar levels among participants. We had too few participants to account for menstrual cycle phase and we did not manipulate momentary blood sugar levels. Thus, while we did not obtain an effect size consistent with in-person adult TSST findings, it is evident that this protocol is successful and consistent with other studies of children and adolescents.
A limitation of the protocol was the need to conduct two sessions for each youth. This was necessary to confirm that the internet connection, participant computer set up, and room arrangements would accommodate the study. Only if we determined that the study could be conducted in the family’s home did we ship the saliva collection materials. This did make for a longer overall session than the in-person version. We also had to have the saliva mailed back to the laboratory. There were also several limitations to this report. Although we had a sufficient sample size to examine whether the protocol worked to activate the HPA axis, we did not have a sufficient sample size to examine the effects of subgroups (e.g., girls at different phases of the menstrual cycle, youth at different pubertal stages). These analyses will be important to report once the sample size is larger. We are continuing to collect participants and will report these analyses with associations with the impact of COVID-19 on the family and youth.
Nevertheless, there are several advantages to this online version of the TSST. Because we conducted the TSST online with adolescents in their homes, there was no need for the family to commute to the testing site. The time and hassle needed to commute to a university is frequently cited by participants who decline laboratory sessions. The TSST-OL offers advantages in that there were no space availability/conflicts within the university for this time-locked session. We were also able to run two TSST sessions offset by 50 minutes with four people: two experimenters and two judges. This presents numerous benefits to staffing: while experimenters worked with each participant for the full testing period, judges only needed to be with a participant for about 20 minutes. Thus, they could sign on and test one participant and sign off and shift to another participant. We routinely tested 2–4 participants on the same day while maintaining a three-hour late afternoon start window. Taken together, there is sufficient reason to conclude that this protocol can effectively increase salivary cortisol, salivary α-amylase, and self-reported stress in adolescents. Of course, it remains to be determined whether this protocol will be generalizable to populations that are more diverse in income, race, and ethnicity. Indeed, a limitation of the protocol is the need for the family to have a good internet connection and computers with at least a 13-inch screen. It also will be essential to determine whether it can be used in adults and in children in future work.
Highlights.
The Trier Social Stress Test (TSST) can be conducted successfully completely online with all parties joining remotely from their own homes.
This TSST-online (TSST-OL) produced the typical elevation in cortisol followed by a return towards baseline. The effect size of .57 was comparable to a recent meta-analysis of child and adolescent in-person TSST’s. Significant salivary α−Amylase and self-reported stress responses were also noted.
The TSST can be conducted online without any in person contact.
Acknowledgements
We express our gratitude to the families who make our research possible. Thanks are also due to Bao Moua for her assistance with REDCap™. This research was supported by a grant from the National Institutes of Health [R01 HD095904] to Drs Gunnar & Thomas, and in part by the National Institutes of Health’s National Center for Advancing Translational Sciences, grant UL1TR002494. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s National Center for Advancing Translational Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
5.0 References
- Buske-Kirschbaum A, Jobst S, Psych D, Wustmans A, Kirschbaum C, Rauh W, & Hellhammer D (1997). Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine, 59(4), 419–426. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical Power Analysis for the Behavioral Sciences. New York, NY: Routledge Academic. [Google Scholar]
- DeJoseph ML, Finegood E, Raver C, & Blair CB (2019). Measuring stress reactivity in the home: Preliminary findings from a version of the Trier Social Stress Test (TSST-H) appropriate for field-based research. [Google Scholar]
- Dickerson SS, & Kemeny ME (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. [DOI] [PubMed] [Google Scholar]
- Ebner K, & Singewald N (2017). Individual differences in stress susceptibility and stress inhibitory mechanisms. Current Opinion in Behavioral Sciences, 14, 54–64. [Google Scholar]
- Goodman WK, Janson J, & Wolf JM (2017). Meta-analytical assessment of the effects of protocol variations on cortisol responses to the Trier Social Stress Test. Psychoneuroendocrinology, 80, 26=35. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Information, 42, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helminen EC, Morton ML, Wang Q, & Felver JC (2019). A meta-analysis of cortisol reactivity to the Trier Social Stress Test in virtual environments. Psychoneuroendocrinology, 110, 104437. [DOI] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, & Myers B (2012). Neural regulation of the stress response: glucocorticoid feedback mechanisms. Brazilian Journal of Medical Biological Research, 45, 292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, McQuillan MT, Mirous HJ, Grant KE, & Adam EK (2014). Cortisol responses to a group public speaking task for adolescents: variations by age, gender, and race. Psychoneuroendocrinology, 50, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, & Hellhammer D (1993). The “Trier Social Stress Test”- A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28, 76–81. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, & Kirschbaum C (2004). HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuoendocrinology, 29, 83–98. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10, 434–445. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2013). The brain on stress: Toward an integrative approach to brain, body, and behavior. Perspectives in Psychological Science, 8, 673–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R, Plessow R, Kirschaum C, & Stalder T (2013). Classifcation criteria for distinguishing cortisol responders from nonresponders to psychosocial stress. Psychosomatic Medicine, 75, 832–840. [DOI] [PubMed] [Google Scholar]
- Montero-López E, Santos-Ruiz A, García-Ríos MC, Rodríguez-Blázquez R, Pérez-García M, & Peralta-Ramírez MI (2016). A virtual reality approach to the Trier Social Stress Test: Contrasting two distinct protocols. Behavioral Research Methods, 48, 223–232. [DOI] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17, 117–133. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Kahn LE, Merchant JS, Peake SJ, Veroude K, Masten CL, … Dapretto M (2013). Longitudinal change in the neural bases of adolescent social self-evaluations: effects of age and pubertal development. Journal of Neuroscience, 33, 7415–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon JA, Rodriguez VJ, Provencher Y, Raftery-Helmer J, Hersh J, Labelle PR, & Thomassin K (2020. ). Meta-analysis of the effectiveness of the Trier Social Stress Test in eliciting physiological stress responses in children and adolescents. Psychoneuroendocrinology, e-pub a head of press. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, & Pollak SD (2009). Pubertal development:correspondence between hormonal and physical development. Child Development, 80(2), 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Leitzke BT, & Pollak SD (2020). Youths’ processing of emotion information: Responses to chronic and video-based laboratory stress. Psychoneuroendocrinology, 122, 104873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumter SR, Bokhorst CL, Miers AC, Van Pelt J, & Westenberg PM (2010). Age and puberty differences in stress responses during a public speaking task: do adolescents grow more sensitive to social evaluation? Psychoneuroendocrinology, 35, 1510–1516. [DOI] [PubMed] [Google Scholar]
- Turner AI, Smyth N, Hall SJ, Torres SJ, Hussein M, Jayasinghe SU, … Clow AJ (2020. ). Psychological stress and future health and disease outcomes: A systematic review of prospective evidence. Psychoneuroendocrinology, e-pub ahead of press. doi: 10.1016/j.psyneuen [DOI] [PubMed] [Google Scholar]
- van den Bos E, de Rooij M, Miers AC, Bokhorst CL, & Westenberg PM (2014). Adolescents’ increasing stress response to social evaluation: pubertal effects on cortisol and alpha-amylase during public speaking. Child Development, 85, 220–236. [DOI] [PubMed] [Google Scholar]
- von Dawans B, Kirschbaum C, & Heinrichs M (2011). The Trier Social Stress Test for Groups (TSST-G): A new research tool for controlled simultaneous social stress exposure in a group format. Psychoneuroendocrinology, 36, 514–522. [DOI] [PubMed] [Google Scholar]
- Yim IS, Quas JA, Cahill L, & Hayakawa CM (2010). Children’s and adults’ salivary cortisol responses to an identical psychosocial laboratory stressor. Psychoneuroendocrinology, 35(2), 241–248. [DOI] [PubMed] [Google Scholar]


