Abstract
The gut microbiota plays a role in a wide range of diseases and disorders, with low microbial diversity and richness emerging as notable risk factors. This longitudinal study addressed the impact of marital quality (assessed by the Couples Satisfaction Index) on changes in depressive symptoms, and gut diversity, richness, and permeability. On two occasions an average of 90 days apart, 162 people provided stool and blood samples, and completed questionnaires. Depressive symptoms, assessed by the Center for Epidemiological Studies Depression Scale (CES-D), increased from visit 1 to visit 2 in those with clinically significant relationship problems, in contrast to the lack of change among their more satisfied counterparts. These changes in depression were consequential: the gut microbiota’s diversity and richness decreased in tandem with the increase in depressive symptoms. Lower relationship satisfaction also foreshadowed increases in lipopolysaccharide binding protein from visit 1 to visit 2, reflecting greater translocation of bacterial endotoxin from the gut to blood circulation, a process that fuels inflammation. Lower diversity and richness provide a pathway from depressive symptoms and marital distress to subsequent health risk.
Keywords: Marriage, couples, depression, gut microbiota, intestinal permeability, lipopolysaccharide binding protein
Introduction
Growing evidence implicates the gut microbiota’s influence in a wide range of diseases and disorders including cardiovascular disease, obesity, neurologic and psychiatric disorders, metabolic dysregulation, chronic inflammatory diseases, allergies, and cancer (Rook et al., 2014; Wargo, 2020). Low microbial diversity and richness (fewer microbial species, and disproportionate species abundance) have emerged as notable risk factors across this broad spectrum. Maladaptive changes in the microbiota's diversity and composition can also provoke dysregulated immune responses including heightened inflammation, one pathway to accelerated aging (Shen and Wong, 2016). In fact, the microbiota's composition may alter the rate of aging, in tandem with age-linked changes in lifestyle, nutrition, frailty, and inflammation (Claesson et al., 2012; Vaiserman et al., 2017).
The autonomic nervous system (ANS) plays a key role in regulating gastrointestinal function (Sandhu et al., 2017). Stress and depression amplify sympathetic nervous system activity while lowering parasympathetic activation (Frasure-Smith et al., 2009; Kemp et al., 2010), providing a route to adverse gut changes. In support of these pathways, multiple cross-sectional studies have found gut microbiota differences between clinically depressed patients and non-depressed controls (Jiang et al., 2015; Kelly et al., 2016; Naseribafrouei et al., 2014; Valles-Colomer et al., 2019; Zheng et al., 2016); however, there is little consensus on the hallmark microbial features of depression. For instance, there is some evidence of lower diversity, but a number of studies have not found this (Jiang et al., 2015; Kelly et al., 2016; Naseribafrouei et al., 2014; Zheng et al., 2016). Longitudinal studies with repeated measures could shed light on these divergent findings by eliminating the noise of between-subject comparisons. Moreover, previous studies have addressed syndromal depression; capturing depressive symptoms and examining the corresponding components (negative affect, anhedonia, and somatic symptoms) may provide a more sensitive test and more nuanced insights into how different aspects of depression relate to the gut microbiota.
Marital distress provides a useful context for understanding routes to gut dysfunction because the stress of a troubled relationship can trigger depression. Indeed, marital discord carries a 10-fold increased risk for depressive symptomatology as well as greater risks for syndromal depression (Beach, 2014). Beyond its effects on depression, chronically abrasive relationships provoke recurring conflicts that worsen important health behaviors including sleep, diet, and physical activity (Kiecolt-Glaser et al., 2019), which can alter the gut microbiota. In a prior study we found that partners with more hostile marital interactions (a signature of marital distress) had higher levels of lipopolysaccharide-binding protein (LBP), i.e., greater translocation of bacterial endotoxin (lipopolysaccharide, LPS) from the gut microbiota to blood circulation (Kiecolt-Glaser et al., 2018)—a process that reflects greater gut barrier permeability. A “leaky gut” stimulates systemic inflammatory responses (Kelly et al., 2016; Stehle et al., 2012), and hostile partners’ higher LBP was associated with heightened inflammation (Kiecolt-Glaser et al., 2018).
In addition, other researchers have shown that cohabiting couples’ microbiotas are more similar to each other than to those from other households (Dill-McFarland et al., 2019). Couples’ microbiota similarities reflect the partners’ behavioral concordance in key health behaviors that influence the microbiome -- diet, exercise, sleep, smoking, and alcohol consumption (Kiecolt-Glaser and Wilson, 2017).
This longitudinal study assessed couples’ baseline relationship quality and changes in their depressive symptoms, gut microbiota diversity and richness, and leaky gut markers across two occasions, 2-7 months apart. In line with prior work, we expected that members of a couple would have more similar microbiotas than random or unrelated pairs (Dill-McFarland et al., 2019). Additionally, we anticipated that greater marital distress would predict worsening depressive symptoms and maladaptive decrements in gut diversity and richness, as well as greater gut permeability. In turn, we predicted that increased depressive symptoms would be linked to adverse changes in gut diversity, richness, and permeability. Finally, supplemental analyses explored whether the effects of depressive symptoms on the gut were driven by its individual features (negative affect, anhedonia, and somatic symptoms) or explained by parallel changes in health behaviors.
Methods
We recruited 143 couples, 116 of which completed both study visits. Of the 116 couples with two visits, seven same-sex couples were excluded in order to allow for estimation of within-couple and between-couple similarity. Microbiome data at both visits were available for 162 individuals. Of these, 140 were members of a couple; for the other 22 individuals their spouse had missing data at one or both visits and was thus excluded from the analysis sample. These 162 individuals ranged in age from 21 to 73 and had been with their partner at least 2 years (Mrelationship length = 14.5 years, SD = 11.1). Table 1 provides demographic data. The two assessments were 58-206 days apart (M = 90.35, SD = 33.01). Exclusions included recent antibiotic use, pregnancy, breastfeeding, malignancies, stroke, heart attack, immune disorders including lupus, multiple sclerosis, ulcerative colitis, inflammatory bowel disease, Crohn’s disease, and any ongoing nontrivial medical issues.
Table 1.
Baseline characteristics of participants (n=162).
| Characteristic | Mean ± SD (median, range) or N (%) |
|---|---|
| Demographics | |
| Age | 41. 6 ± 13.5 (38, 21-73) |
| BMI | 27.0 ± 5.6 (26.3, 18.2-51.7) |
| Race | |
| Asian | 10 (6.2%) |
| Black | 7 (4.3%) |
| White | 141 (87.0%) |
| Multi | 4 (2.5%) |
| Comorbidities | 0.26 ± 0.65 (0, 0-3) |
| Employment status | |
| Full time | 112 (69.1%) |
| Part time | 24 (14.8%) |
| Retired | 11 (6.8%) |
| Disabled | 2 (1.2%) |
| Unemployed | 13 (8.0%) |
| Income | |
| <$10,000 | 3 (1.9%) |
| $10,000-$24,999 | 9 (5.6%) |
| $25,000-$49,999 | 21 (13.0%) |
| $50,000-$74,999 | 32 (19.8%) |
| $75,000-$99,999 | 17 (10.5%) |
| >$100,000 | 71 (43.8%) |
| Refused to answer | 9 (5.6%) |
| Marital status | |
| Married | 146 (90.1%) |
| Not married | 10 (6.2%) |
| Common Law Marriage or Domestic Partnership | 6 (3.7%) |
| Bristol Stool Chart Value | 3.8 ± 1.2 (4, 1-6) |
| Behavioral | |
| Average drinks per week | 3.3 ± 3.9 (2, 0-24) |
| Number of alcoholic drinks in the last 48 hours at baseline visit | 1.08 ± 1.58 (0, 0-10) |
| Smoking status | |
| Current smoker | 2 (1.2%) |
| Former smoker | 23 (14.2%) |
| Never smoker | 137 (84.6%) |
| IPAQ met-adjusted minutes at baseline visit | 2704 ± 2631 (1980, 0-13518) |
| How rested did you feel upon rising today at baseline visit | 3.49 ± 0.91 (4, 1-5) |
| Psychological/Marital Quality | |
| CESD score at baseline visit | 7.9 ± 6.8 (6, 0-37) |
| Take any type of antidepressant | |
| No | 135 (83.3%) |
| Yes | 27 (16.7%) |
| Couple Satisfaction Index at baseline visit | 132.5 ± 22.3 (138.5, 65-161) |
| Microbiota Diversity and Richness | |
| Shannon diversity index at baseline visit | 2.3 ± 0.37 (2.3, 1.40-3.16) |
| Richness index at baseline visit | 64.1 ± 17.0 (64, 20-118) |
| Dietary | |
| Alternative Healthy Eating Index 2010 at baseline visit | 61.5 ± 16.0 (63.6, 19.8-96.2) |
| Total fat intake, gram per 1000 calories | 42.5 ± 10.6 (43.3, 15.7-66.2) |
| Total Dietary Fiber intake, gram per 1000 calories | 12.3 ± 4.9 (11.4, 3.3-29.3) |
| Oleic acid intake, gram per 1000 calories | 14.4 ± 5.0 (13.8, 4.4-31.3) |
| Total Saturated Fatty Acids, gram per 1000 calories | 14.1 ± 6.2 (13.2, 3.6-41.1) |
| Total Polyunsaturated Fatty Acids, gram per 1000 calories | 9.5 ± 3.9 (8.99, 3.1-34.1) |
| Total calories intake | 2206 ± 663 (2132, 960-3900) |
| Gut Permeability Markers | |
| LBP at baseline visit, ng/ml | 3785 ± 2394 (3568, 226-19185) |
| sCD14 at baseline visit, ng/ml | 1605 ± 313 (1570, 850-2660) |
| LBP to sCD14 ratio at baseline visit | 2.42 ± 1.53(2.23, 0.12-12.54) |
2.1. Questionnaires and Interviews
The 32-item Couples Satisfaction Index (CSI) assessed marital satisfaction at the first study visit (Funk and Rogge, 2007). Developed using item response theory, the CSI discriminates well between satisfied and dissatisfied couples with greater precision than the most common marital scales (Funk and Rogge, 2007).
The widely-used Center for Epidemiological Studies Depression Scale (CES-D) (Carleton et al., 2013) provided data on depressive symptoms at each of the two visits. The CES-D subscales (negative affect, anhedonia, somatic symptoms) provide a way to look at specific types of depressive symptoms (Carleton et al., 2013).
2.2. Health-Related Assessments
We assessed sleep, diet, exercise, and comorbidities based on their association with depression and the gut microbiota. The 5-item Women’s Health Initiative Insomnia Rating Scale assessed sleep over the last four weeks (Levine et al., 2003). The scale has well-established reliability and validity (Levine et al., 2003). The three 24-hour dietary recalls used the validated USDA Multiple Pass Approach method (Blanton et al., 2006). Two of the interviews took place on the days participants were seen for blood draws, and the third was administered via telephone. The International Physical Activity Questionnaire (IPAQ) was developed by an International Consensus Group and validated against accelerometer measurements. It has comparable or better measurement properties compared to other established self-reports (Craig et al., 2003). The Charlson Index provided data on comorbidities. This widely-used measure has good concurrent and predictive validity, and test-retest reliability (de Groot et al., 2003).
2.3. Stool Sample Protocol and Rating
After each study visit, participants received uBiome® kits that contained a cotton swab and a tube with a liquid preservative. Using the wipe collection method, participants swabbed soiled toilet paper, then swished the swab in a tube with preservative liquid for mailing. The uBiome® analysis (Almonacid et al., 2017) provided the study’s microbiota data.
The Bristol Stool Form Scale provided an assessment of transit time, which is significantly correlated with stool form and microbiota composition (Lewis and Heaton, 1997). Participants chose the picture and description that best corresponded to their sampled bowel movement.
2.4. Blood Samples and Assays
Non-fasting blood samples were collected during lab study visits between 7-11 AM to limit diurnal variation. Blood samples provided data on lipopolysaccharide (LPS)-binding protein (LBP) and soluble CD14 (sCD14), two biomarkers produced in response to microbial translocation (Amar et al., 2003; Stehle et al., 2012). LBP binds LPS and presents LPS to CD14, the receptor for LPS-LBP complexes (Stehle et al., 2012; Ulevitch and Tobias, 1995; Wright et al., 1990). Samples were measured using an electrochemilluminescence method with Meso Scale Discovery kits, following kit instructions. The stored serum samples for each subject were assayed for each marker in one run, thus using the same controls for both time points. The intra- and inter-assay coefficients of variation (CVs) were LBP were 5.61% and 9.37%, respectively, and the sensitivity was 0.038ng/mL. The intra- and inter-assay CVs for sCD14 were 5.47% and 6.30%, respectively, and the sensitivity was 125 pg/mL.
2.5. Microbiota Sequencing
The uBiome® analysis (Almonacid et al., 2017) provided the study’s microbiota data. DNA was extracted from the samples using bead beating followed by guanidine thiocyanate silica column-based purification. The V4 region of the 16S rRNA gene was PCR amplified using the 515F (GTGCCAGCMGCCGCGGTAA) and 806R (GGACTACHVGGGTWTCTAAT) primers. The primers also contained Illumina tags and barcodes to allow for amplicon identification (i.e., forward vs. reverse and sample identification) in multiplexed sequencing runs. PCR products were pooled prior to column-purification and size selection using microfluidic DNA fractionation. Libraries were quantified by quantitative real-time PCR using the Kapa Bio-Rad iCycler qPCR kit on a BioRad MyiQ, and 2x150 paired end sequencing was performed on the Illumina NextSeq 500 platform. Sequences were demultiplexed using the BCL2FASTQ algorithm (Illumina) and quality filtered to have a Q-score > 30. Primers and linker sequences were removed, and forward and reverse reads merged prior to clustering the merged reads using the Swarm algorithm. The most abundant sequence per cluster was considered the representative/correct sequence for the cluster. The representative sequences were checked for chimeras using the VSEARCH algorithm and representative sequences that remained were assigned a count based on the abundance of all sequences in the cluster. These sequences were then aligned, using 100% identity over 100% of the sequence length, to a hand-curated database of target 16S rRNA gene sequences and taxonomic annotations derived from version 123 of the SILVA database.
2.6. Statistical Methods
Shannon’s diversity index was used to quantify alpha diversity, and the simple count of observed operational taxonomic units (OTUs) was used to measure richness; for these measures higher values indicate higher diversity/higher richness. Significant associations with diversity and richness were probed using a short a priori list of taxa, chosen based on existing literature (Kurina et al., 2020; Naseribafrouei et al., 2014; Zheng et al., 2016). For these analyses, the percentage of each specific taxa was used as the summary measure.
Of primary interest were models for change, where change was defined as the visit 2 value minus the visit 1 value, so that a positive change score indicated an increase. In models with change scores as the outcome, the baseline level of the outcome was included as a covariate to account for regression to the mean. Additionally, all models controlled for age, visit 1 BMI, gender, and antidepressant use to guard against confounding. When a change score was used as a predictor (e.g., change in CES-D predicting change in diversity), the baseline level of the predictor was also included as a covariate (e.g., visit 1 CES-D). Since subjects were clustered into couples, linear mixed effects regression models with a random couple-level intercept were used to account for the within-couple clustering. The Kenward-Roger adjustment to the degrees of freedom was used to control type I error. The phyloseq package in R was used to calculate Shannon’s index; all other analyses were conducted in SAS version 9.4 (Cary, NC).
To quantify within-couple and within-subject similarity in microbiota, beta diversity was calculated using the Bray-Curtis distance based on presence/absence of OTUs. Three distances were calculated: 1) within-subject, between visits distance, 2) within-couple, and 3) between an individual and all other same-sex individuals who were not the spouse, averaged. Both the within-couple and between individuals and the non-spouse distances were calculated separately for each visit and averaged. Paired t-tests were used to test for differences between distance measures, separately for women and men.
With a sample size of 162 individuals, clustered into couples, the detectable effect size depends on how large the within-couple correlation (intraclass correlation, ICC) is. Thus, we estimated detectable effect sizes using a range of ICCs similar to those seen in our prior studies of cohabiting couples (Kiecolt-Glaser et al., 2015). We had 80% power to detect correlations ranging from r = 0.22 to r = 0.25 for ICCs ranging from 0 to 0.3.
Results
3.1. Description
Table 1 presents the baseline characteristics of the sample. The sample was on average 41.6 years old, predominately white (87%), and never smoked (85%). Ninety percent of the couples were married and the majority of participants were employed full-time (69%). Three-quarters of participants reported engaging in vigorous physical activity at least once a week, and the average BMI was 27.0. Twenty-seven participants (17%) reported current use of any type of antidepressant. The average CES-D score at baseline was 7.9 (SD = 6.8) with a wide range (0 to 37); 12% of participants had clinically elevated depressive symptoms.
On average there was not a significant change in mean alpha diversity between visits (mean change = +0.05, p=0.12) but there was a small increase in richness between visits (mean change = +3.2, p=0.049). There was large variability in the changes for both measures (Shannon diversity: range = −1.05 to +1.19; richness: range = −60 to +53), and 46% of subjects had decreased Shannon diversity at the second visit while 43% had decreased richness. On average there was a significant decrease in sCD14 between visits (mean change = −42.2, p=0.02), but no significant change in LBP (mean change = −154.1, p=0.14) or the LBP to sCD14 ratio (mean change = −0.03, p=0.60). There was not a significant change in CES-D between visits (mean change = 0.09, p = 0.85) but there was a wide range (−20 to +27). The majority of changes in CES-D were small; only eight individuals (4.9%) had large enough changes to cross the threshold of clinically significant depression (i.e., go from <16 to 16 or higher). Thus, all analyses used CES-D as a continuous score and not a binary one.
3.2. Gut Microbiota Similarity within Couples
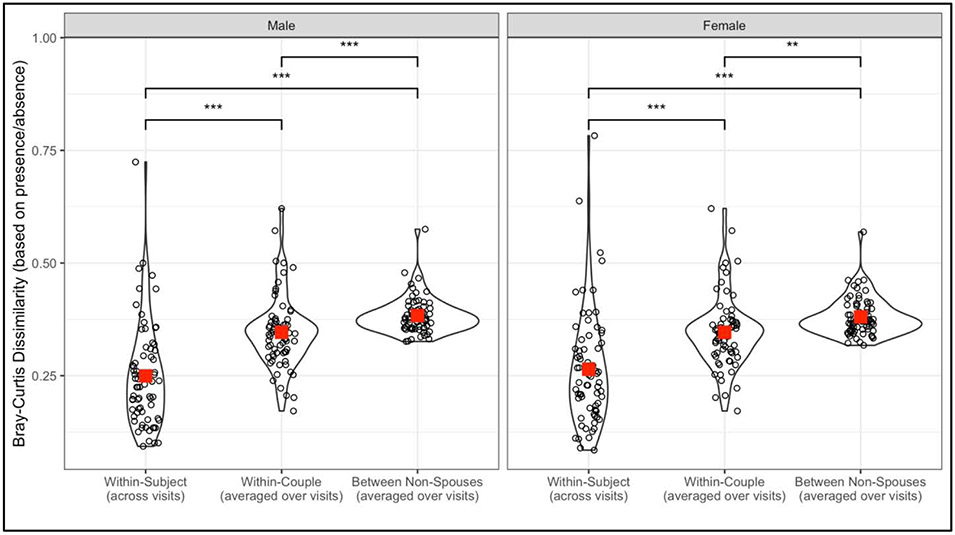
Beta diversity analyses revealed that individuals’ bacteriomes (bacterial communities in the gut) were more similar to their spouse than to same-sex individuals who were not the spouse (p < 0.001 for both women and men). Additionally, bacteriomes were more similar within-subject across visits than within-couple (p < 0.001 for both women and men) (Figure 1).
Figure 1:
Bray-Curtis distances using presence/absence data to quantify beta diversity within-subject/between-visit, within-couple (distance averaged over visits), and between individuals and all other same-sex individuals who were not the spouse (averaged over other individuals and over visit). Smaller Bray-Curtis distance corresponds to higher similarity. Large square is the group mean. ***p<0.001
3.3. Links between Marital Satisfaction and Changes in Depressive Symptoms and Gut Markers
Marital satisfaction (CSI), measured at visit 1, was significantly negatively associated with depression at each time point (p<0.0001 for both). Marital satisfaction was also associated with change in depression (p=0.01), with lower satisfaction at visit 1 associated with an increase in depression from visit 1 to visit 2 (B=−0.060, SE=0.023; Table 2; Figure 2). As shown in Table 2, marital satisfaction was also associated with decreased LBP from visit 1 to visit 2 (B=−8.8, SE=4.4, p = 0.049), but not with changes in sCD14 (p = 0.053) or the LBP to sCD14 ratio (p = 0.21). There were no significant associations between marital satisfaction and changes in either alpha diversity (p=0.87) or richness (p=0.60).
Table 2.
Associations of baseline marital satisfaction with changes in depression, alpha diversity and richness, and gut permeability markers.
| Outcome | Estimate (SE) | 95% CI | p-value |
|---|---|---|---|
| Depression change | −0.060 (0.023) | −0.11, −0.015 | 0.01 |
| Shannon diversity change | 0.00019 (0.0011) | −0.0021, 0.0025 | 0.87 |
| Richness change | −0.030 (0.056) | −0.14, 0.081 | 0.60 |
| LBP change | −8.8 (4.4) | −17.6, −0.023 | 0.049 |
| sCD14 change | −1.4 (0.74) | −2.9, 0.019 | 0.053 |
| LBP to sCD14 change | −0.0034 (0.0027) | −0.0087, 0.0020 | 0.21 |
Change defined as visit 2 minus visit 1
P-values from separate linear mixed effects models adjusting for age, gender, BMI, antidepressant usage, and baseline outcome level.
Sample sizes: Depression, Shannon diversity, Richness, n=162; LBP, sCD14, and LBP to sCD14 ratio, n=154.
Figure 2:
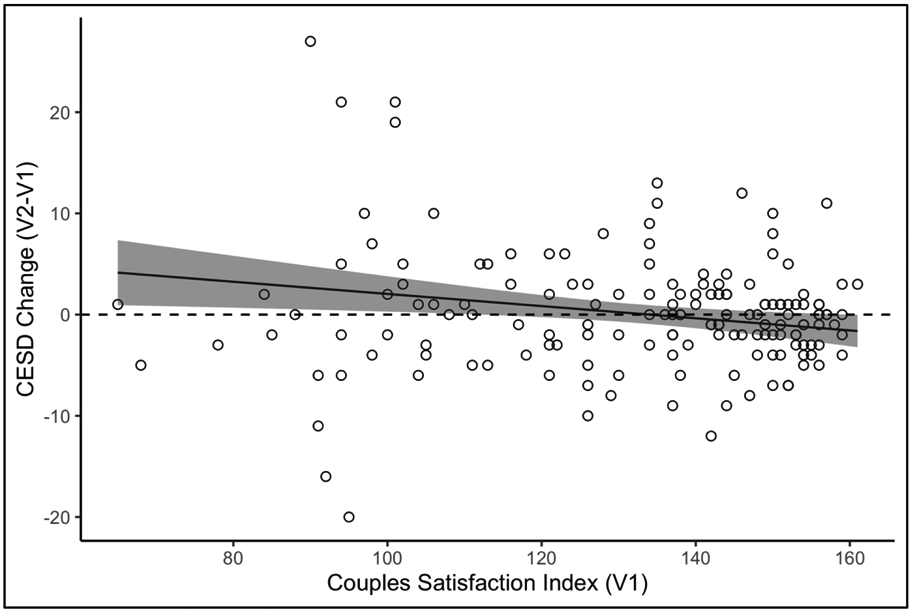
Predicted change in depression (CES-D) as a function of marital satisfaction (CSI). Results from mixed effects models controlling for age, gender, BMI, antidepressant use, and baseline depression. Shading indicates 95% confidence intervals.
3.4. Associations between Changes in Depressive Symptoms and Gut Markers
Results from models using change in depression to predict changes in alpha diversity and richness and gut permeability markers are shown in Table 3. Change in depression was negatively associated with both the change in Shannon diversity (B=−0.010, SE=0.0043, p=0.02) and the change in richness (B=−0.59, SE=0.21, p=0.005), with a decrease in depression associated with an increase in alpha diversity and richness (Figure 3). These significant effects were probed using individual taxa in place of alpha diversity and richness. There were no significant associations with the a priori selected phyla (Bacteroidetes, Actinobacteria, Firmicutes; p>0.4 for all) based on their association with depression in prior research (Naseribafrouei et al., 2014; Zheng et al., 2016) or for the prevalent and biomedically relevant human gut microbial genera (Kurina et al., 2020) (Alistipes, Coprococcus, Faecalibacterium, Akkermansia,Bacteroides, Bifidobacterium, Lactobacillus, Prevotella; p>0.09 for all).
Table 3.
Associations of change in depression with changes in alpha diversity and richness and gut permeability markers.
| Outcome | Estimate (SE) | 95% CI | p-value |
|---|---|---|---|
| Shannon diversity change | −0.010 (0.0043) | −0.019, −0.0016 | 0.02 |
| Richness change | −0.59 (0.21) | −1.00, −0.18 | 0.005 |
| LBP change | 19.8 (16.0) | −11.7, 51.3 | 0.22 |
| sCD14 change | 1.8 (2.8) | −3.8, 7.4 | 0.52 |
| LBP to sCD14 change | 0.011 (0.0097) | −0.0081, 0.030 | 0.26 |
Change defined as visit 2 minus visit 1
P-values from separate linear mixed effects models adjusting for age, gender, BMI, antidepressant usage, baseline outcome level, and baseline depression.
Sample sizes: Shannon diversity, Richness, n=162; LBP, sCD14, and LBP to sCD14 ratio, n=154.
Figure 3:
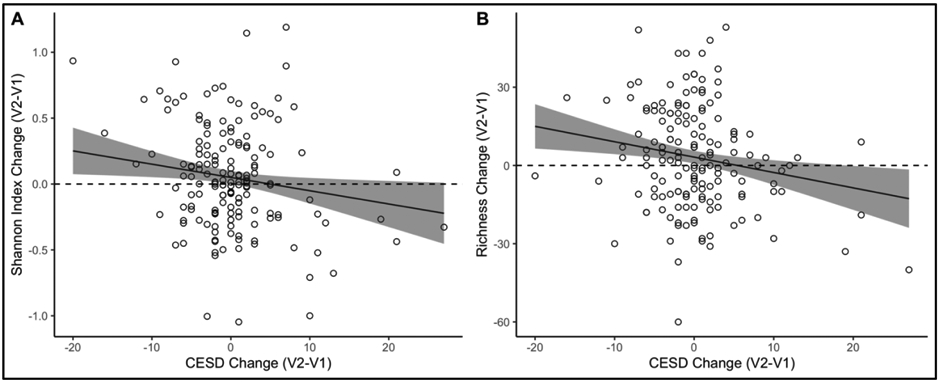
Changes in (A) alpha diversity (Shannon Index) and (B) richness as a function of change in depression (CES-D). Superimposed line is the regression line at the average level of covariates (age, gender, BMI, antidepressant use, baseline depression, and the baseline outcome level). Shading indicates 95% confidence intervals.
Change in depression was not associated with changes in LBP, sCD14, or their ratio (p > 0.2 for all). At visit 1, depression was not significantly associated with Shannon diversity, richness, LBP, sCD14, or the LBP/sCD14 ratio (p>0.13 for all).
3.5. Associations with Depressive Symptom Subscales
To probe the significant association between change in depression and change in diversity and richness further we repeated the analyses using each of the CES-D subscales (negative affect, anhedonia, somatic symptoms) (Carleton et al., 2013) in place of overall CES-D score. The change in negative affect was significantly associated with both change in Shannon index (B=−0.032, SE=0.014, p=0.02) and change in richness (B=−1.50, SE=0.69, p=0.03). Change in somatic symptoms was significantly associated with change in richness (B=−1.09, SE=0.54, p=0.04) but not with change in Shannon index (p=0.13). Change in anhedonia was not significantly associated with change in either Shannon index (p=0.42) or richness (p = 0.10).
3.6. Ancillary Analyses: Examining the Roles of Health Behavior Changes
We performed ancillary analyses to explore whether the link between changes in depression and changes in alpha diversity and richness might be explained by changes in health behaviors, including changes in diet. As shown in the supplementary online Table, increased depression from visit 1 to visit 2 was associated with decreased hours of restful sleep (p=0.002) but was not associated with changes in any other behavioral, physical, or dietary measures. When change in restful sleep was added to the models for changes in diversity and richness, the effects of depression change remained significant and similar in magnitude to the original models.
Discussion
Among a sample of cohabiting couples, depressive symptoms increased from visit 1 to visit 2 in those with lower marital satisfaction, in contrast to the nonsignificant changes in those with higher marital satisfaction. These changes in depression were consequential: fecal bacterial diversity and richness decreased in tandem with the increase in depressive symptoms. Further analysis of CES-D components showed that heightened negative affect drove changes in both diversity and richness; higher somatic symptoms were only linked with lower richness, and anhedonia was not associated with either dimension. These novel findings demonstrate the relevance of depressive symptoms, particularly negative affect, for changes in bacterial composition, extending cross-sectional work with clinical depression. We also found that gut microbiota diversity was more similar within couples than among other individuals, a successful replication of prior work (Dill-McFarland et al., 2019).
Depression’s health consequences have been well-documented. Depression co-occurs with many chronic diseases, and recent research suggests that it often temporally precedes disease onset. Indeed, depression is a central node in a multimorbid disease and poor heath behavior network (Birk et al., 2019). Across multiple meta-analyses, depression has predicted subsequent onset of heart disease and diabetes (Birk et al., 2019), two of the same diseases that track with low microbiota diversity, and both of these diseases have also been linked to marital discord (Gallo et al., 2003; Orth-Gomer et al., 2000; Whisman et al., 2014).
Extending previous cross-sectional findings (Kiecolt-Glaser et al., 2018), people in less satisfying relationships experienced greater elevations in LBP over time, reflecting increased translocation of bacterial endotoxin from the gut to blood circulation. In parallel, lower marital satisfaction also presaged increases in depressive symptoms between the two visits, consistent with prior theory and data (Beach et al., 2014). Notably, depressive symptoms were predicted to rise for partners who were near or exceeded the threshold for clinically significant relationship distress (CSI below 104.5, see Figure 2).
4.1. Depression and Gut Microbiota
Our data offer some of the first longitudinal evidence that increased depressive symptoms associated with marital distress can, in turn, provoke microbiota shifts. Indeed, depression and associated physiological processes, behaviors, and stress may remodel the gut environment, including shifting an otherwise stable gut microbial community. Depression promotes poor health behaviors that perturb the gut microbiota (Madison & Kiecolt-Glaser, 2019). Our analyses showed that worsening sleep accompanied increases in depressive symptoms. Nevertheless, links between depression and gut changes were robust to the inclusion of sleep in the model, suggesting that sleep changes were not the primary mechanism of this effect. Follow-up analyses of depression’s subcomponents revealed that negative affect most consistently predicted changes in gut bacterial diversity and richness, implicating a direct affective route through ANS dysregulation (Kemp et al., 2010; Sandhu et al., 2017). Increased depression may have also boosted stress (Hammen, 2006); among both animals (Bailey et al., 2011; Partrick et al., 2018) and humans (Knowles et al., 2008), the gut microbiota shifts with stress exposure.
However, there is an alternative pathway: it is plausible that higher levels of LBP and/or LBP/sCD14, indicative of a leaky gut, when combined with inflammation, may pave the way for depressive symptoms. In another sample, our lab found this to be the case (Madison et al., 2020). Thus, these blood markers may in fact mechanistically link the chronic stress of a troubled marriage with depression onset.
Finally, depression can usher in gut morphological and functional changes that render the gut environment less hospitable to pre-existing microbial communities. In one study, nearly all patients with syndromal depression reported gastrointestinal symptoms and a large majority met criteria for a functional gastrointestinal disorder (Koloski et al., 2012). In fact, a significant subset of depressed patients may experience chronic or frequent constipation (Koloski et al., 2012). Even in the absence of gastrointestinal symptoms, those with greater depressive symptoms had slower whole-gut transit times (Gorard et al., 1996) – an important determinant of which bacteria thrive (Vandeputte et al., 2016).
4.2. Consequences of Low Microbiota Diversity
In the gut, a greater number and depth of microbial species promotes resilience and stability, guards against infection, and minimizes the impact of any single species shift; thus, microbiota diversity and richness are informative metrics that provide a general indication of gut health (Sommer et al., 2017). Accordingly, lower bacterial diversity has been observed in many common chronic diseases, including diabetes and obesity (Sommer et al., 2017). Intriguingly, results from two cross-disease meta-analyses revealed a pattern in disease-associated bacterial shifts, such that there may be a signature, non-specific bacterial risk for or response to disease – especially lower diversity (Mancabelli et al., 2017).
Obesity and diabetes likely reflect, in part, the immunological consequences of low bacterial diversity, which is connected to elevated systemic inflammation, boosting risk for cardiovascular and other diseases (van den Munckhof et al., 2018). Also, the gut microbiota may drive a stress-induced inflammatory response (Bailey et al., 2011). Indeed, it has been argued that the decline in the diversity of core bacterial groups may be a stronger factor in age-related frailty than chronological age (Claesson et al., 2012; Vaiserman et al., 2017).
Along with these disease outcomes, low microbiota diversity predicts long-term weight gain: those with lower diversity had greater weight gain over a ten-year period than those with higher diversity (Menni et al., 2017). Gut bacterial richness has also been linked to metabolic markers (Le Chatelier et al., 2013). In one study those with low bacterial richness (23% of the study population) had greater overall adiposity, insulin resistance, dyslipidemia, and a "more pronounced inflammatory phenotype," which included differences in leptin, adiponectin, and CRP. The obese subjects in this subgroup also gained more weight over time (Le Chatelier et al., 2013).
Paralleling those data, our lab has shown that the confluence of marital distress and depression also alters metabolic responses. Men and women whose marital discussions were more hostile and who also had a mood disorder history had lower resting energy expenditure, higher insulin (which would heighten fat deposition), higher peak triglyceride responses, and higher inflammation than other participants following high-fat meals (Kiecolt-Glaser et al., 2015). Marital distress and depression can synergistically escalate risks for inflammation-related disorders.
Our sample had good variability in depression; overall, 12% had clinically significant depressive symptoms, which is well above the national prevalence rate of around 8% (Brody et al., 2018). Other studies have shown gut microbiota differences between individuals with and without clinical depression (Jiang et al., 2015; Kelly et al., 2016; Naseribafrouei et al., 2014; Valles-Colomer et al., 2019; Zheng et al., 2016). However, given the relatively small number of individuals meeting clinical criteria, we were unable to test differences in gut microbiome composition by CES-D clinical cutoff scores, one limitation. In addition, we only assessed marital satisfaction at the first visit, given its stability for most couples (Karney and Bradbury, 2020). Accordingly, we could not examine changes in marital satisfaction. Future work may consider addressing how changes in marital distress—particularly during turbulent relational phases—along with a current or past depressive disorder.may alter the gut microbiota and pose additional health risks.
The present study extends our understanding of how clinically significant social stress and depression may lead to gut dysbiosis, revealing key mind-body connections. Likewise, novel findings have traced reciprocal body-to-mind paths. In two studies, fecal matter from depressed and healthy humans was transferred to microbiota-deficient or germ-free rodents, and only the rodents given microbiota from depressed humans developed depressive-like behaviors (Kelly et al., 2016; Zheng et al., 2016). Taken together, these bidirectional processes may fuel a vicious cycle that compounds the health risks of marital discord and depression. Couples’ similarity in gut microbiota diversity—a reflection of their shared lives and overlapping risks—may further exacerbate the health consequences of discord, depression, and poor health behaviors.
In summary, these data suggest that increases in depressive symptoms can decrease microbiota diversity and richness in the context of marital discord. In turn, lower diversity and richness may link depressive symptoms and marital distress with subsequent health risk. Indeed, increased depressive symptoms accompanied by decreased microbiota diversity and richness in healthy adults may be an early premorbid harbinger of poor health.
Supplementary Material
Highlights.
81 couples provided stool and blood samples, and completed questionnaires twice
Depressive symptoms increased in those with significant relationship problems
Gut bacterial diversity and richness decreased when depressive symptoms increased
Lower gut bacterial diversity and richness may provide a path to later health threats
Acknowledgements
Work on this study was supported in part by NIH grants R01 AG057032, K05 CA172296, and UL1TRR001079, and uBiome® provided the kits and analysis for microbiota sampling free of charge. The sponsor and uBiome® had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors have no potential conflicts of interest. We are grateful to Michael Di Gregorio, M.A., for his role as a key organizer and experimenter, and Jennifer Hollyfield, M.S and Bryon Laskowski, B. S., for laboratory assays.
Footnotes
Conflict of interest: All authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almonacid DE, Kraal L, Ossandon FJ, Budovskaya YV, Cardenas JP, Bik EM, Goddard AD, Richman J, Apte ZS, 2017. 16s rrna gene sequencing and healthy reference ranges for 28 clinically relevant microbial taxa from the human gut microbiome. PLOS ONE 12, e0176555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amar J, Ruidavets JB, Bal Dit Sollier C, Bongard V, Boccalon H, Chamontin B, Drouet L, Ferrieres J, 2003. Soluble cd14 and aortic stiffness in a population-based study. J. Hypertens 21, 1869–1877. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M, 2011. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain. Behav. Immun 25, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach SRH, 2014. The couple and family discord model of depression: Updates and future directions, in: Agnew CR, South SC (Eds.), Interpersonal relationships and health: Social and clinical psychological mechanisms. Oxford University Press, New York, NY, US, pp. 133–155. [Google Scholar]
- Blanton CA, Moshfegh AJ, Baer DJ, Kretsch MJ, 2006. The usda automated multiple-pass method accurately estimates group total energy and nutrient intake. J. Nutr 136, 2594–2599. [DOI] [PubMed] [Google Scholar]
- Brody DJ, Pratt LA, Hughes JP, 2018. Prevalence of depression among adults aged 20 and over: United states, 2013–2016. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics, 1–8. [Google Scholar]
- Carleton RN, Thibodeau MA, Teale MJN, Welch PG, Abrams MP, Robinson T, Asmundson GJG, 2013. The center for epidemiologic studies depression scale: A review with a theoretical and empirical examination of item content and factor structure. PLOS ONE 8, e58067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Jeffery IB, Conde S, Power SE, O/'Connor EM, Cusack S, Harris HMB, Coakley M, Lakshminarayanan B, O/'Sullivan O, Fitzgerald GF, Deane J, O/'Connor M, Harnedy N, O/'Connor K, O/'Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O/'Toole PW, 2012. Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178–184. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P, 2003. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc 35, 1381–1395. [DOI] [PubMed] [Google Scholar]
- de Groot V, Beckerman H, Lankhorst GJ, Bouter LM, 2003. How to measure comorbidity. A critical review of available methods. J. Clin. Epidemiol 56, 221–229. [DOI] [PubMed] [Google Scholar]
- Dill-McFarland KA, Tang Z-Z, Kemis JH, Kerby RL, Chen G, Palloni A, Sorenson T, Rey FE, Herd P, 2019. Close social relationships correlate with human gut microbiota composition. Scientific Reports 9, 703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasure-Smith N, Lesperance F, Irwin MR, Talajic M, Pollock BG, 2009. The relationships among heart rate variability, inflammatory markers and depression in coronary heart disease patients. Brain. Behav. Immun [DOI] [PubMed] [Google Scholar]
- Funk JL, Rogge RD, 2007. Testing the ruler with item response theory: Increasing precision of measurement for relationship satisfaction with the couples satisfaction index. J. Fam. Psychol 21, 572–583. [DOI] [PubMed] [Google Scholar]
- Gallo LC, Troxel WM, Kuller LH, Sutton-Tyrrell K, Edmundowicz D, Matthews KA, 2003. Marital status, marital quality, and atherosclerotic burden in postmenopausal women. Psychosom. Med 65, 952–962. [DOI] [PubMed] [Google Scholar]
- Gorard DA, Gomborone JE, Libby GW, Farthing MJ, 1996. Intestinal transit in anxiety and depression. Gut 39, 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, 2006. Stress generation in depression: Reflections on origins, research, and future directions. J. Clin. Psychol 62, 1065–1082. [DOI] [PubMed] [Google Scholar]
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B, 2015. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behavior and Immunity 48, 186–194. [DOI] [PubMed] [Google Scholar]
- Karney BR, Bradbury TN, 2020. Research on marital satisfaction and stability in the 2010s: Challenging conventional wisdom. Journal of Marriage and Family 82, 100–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JR, Borre Y, C OB, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross P, Stanton C, Clarke G, Cryan JF, Dinan TG, 2016. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res 82, 109–118. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM, 2010. Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biol. Psychiatry 67, 1067–1074. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Jaremka L, Andridge R, Peng J, Habash D, Fagundes CP, Glaser R, Matarkey WB, Belury MA, 2015. Marital discord, past depression, and metabolic responses to high-fat meals: Interpersonal pathways to obesity. Psychoneuroendocrinology 52, 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Wilson SJ, 2017. Lovesick: How couples' relationships influence health. Annu Rev Clin Psychol 13, 421–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Wilson SJ, Bailey M, Andridge R, Peng J, Jaremka L, Fagundes CP, Malarkey WB, Laskowski B, Belury MA, 2018. Marital distress, depression, and a leaky gut: Translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinolgy 98, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Wilson SJ, Madison A, 2019. Marriage and gut (microbiome) feelings: Tracing novel dyadic pathways to accelerated aging. Psychosom. Med 81, 704–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles SR, Nelson EA, Palombo EA, 2008. Investigating the role of perceived stress on bacterial flora activity and salivary cortisol secretion: A possible mechanism underlying susceptibility to illness. Biol. Psychol 77, 132–137. [DOI] [PubMed] [Google Scholar]
- Koloski NA, Jones M, Kalantar J, Weltman M, Zaguirre J, Talley NJ, 2012. The brain--gut pathway in functional gastrointestinal disorders is bidirectional: A 12-year prospective population-based study. Gut 61, 1284–1290. [DOI] [PubMed] [Google Scholar]
- Kurina I, Popenko A, Klimenko N, Koshechkin S, Chuprikova L, Filipenko M, Tyakht A, Alexeev D, 2020. Development of qpcr platform with probes for quantifying prevalent and biomedically relevant human gut microbial taxa. Mol. Cell. Probes 52, 101570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jorgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clement K, Dore J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T, Bork P, Wang J, Ehrlich SD, Pedersen O, 2013. Richness of human gut microbiome correlates with metabolic markers. Nature 500, 541–546. [DOI] [PubMed] [Google Scholar]
- Levine DW, Kripke DF, Kaplan RM, Lewis MA, Naughton MJ, Bowen DJ, Shumaker SA, 2003. Reliability and validity of the women's health initiative insomnia rating scale. Psychol. Assess 15, 137–148. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Heaton KW, 1997. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol 32, 920–924. [DOI] [PubMed] [Google Scholar]
- Mancabelli L, Milani C, Lugli GA, Turroni F, Ferrario C, van Sinderen D, Ventura M, 2017. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ Microbiol 19, 1379–1390. [DOI] [PubMed] [Google Scholar]
- Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linlokken A, Wilson R, Rudi K, 2014. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil 26, 1155–1162. [DOI] [PubMed] [Google Scholar]
- Orth-Gomer K, Wamala SP, Horsten M, Schenck-Gustafsson K, Schneiderman N, Mittleman MA, 2000. Marital stress worsens prognosis in women with coronary heart disease - the stockholm female coronary risk study. JAMA 284, 3008–3014. [DOI] [PubMed] [Google Scholar]
- Partrick KA, Chassaing B, Beach LQ, McCann KE, Gewirtz AT, Huhman KL, 2018. Acute and repeated exposure to social stress reduces gut microbiota diversity in syrian hamsters. Behav. Brain Res 345, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GA, Raison CL, Lowry CA, 2014. Microbiota, immunoregulatory old friends and psychiatric disorders. Adv. Exp. Med. Biol 817, 319–356. [DOI] [PubMed] [Google Scholar]
- Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF, 2017. Feeding the microbiota-gut-brain axis: Diet, microbiome, and neuropsychiatry. Translational Research 179, 223–244. [DOI] [PubMed] [Google Scholar]
- Shen S, Wong CH, 2016. Bugging inflammation: Role of the gut microbiota. Clin Transl Immunology 5, e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P, 2017. The resilience of the intestinal microbiota influences health and disease. Nature Reviews Microbiology 15, 630–638. [DOI] [PubMed] [Google Scholar]
- Stehle JR Jr., Leng X, Kitzman DW, Nicklas BJ, Kritchevsky SB, High KP, 2012. Lipopolysaccharide-binding protein, a surrogate marker of microbial translocation, is associated with physical function in healthy older adults. J. Gerontol. A. Biol. Sci. Med. Sci 67, 1212–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch RJ, Tobias PS, 1995. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol 13, 437–457. [DOI] [PubMed] [Google Scholar]
- Vaiserman AM, Koliada AK, Marotta F, 2017. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res Rev 35, 36–45. [DOI] [PubMed] [Google Scholar]
- Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, Wijmenga C, Claes S, Van Oudenhove L, Zhernakova A, Vieira-Silva S, Raes J, 2019. The neuroactive potential of the human gut microbiota in quality of life and depression. Nature Microbiology 4, 623–632. [DOI] [PubMed] [Google Scholar]
- van den Munckhof ICL, Kurilshikov A, Ter Horst R, Riksen NP, Joosten LAB, Zhernakova A, Fu J, Keating ST, Netea MG, de Graaf J, Rutten JHW, 2018. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: A systematic review of human studies. Obes Rev 19, 1719–1734. [DOI] [PubMed] [Google Scholar]
- Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, Raes J, 2016. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 65, 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargo JA, 2020. Modulating gut microbes. Science 369, 1302–1303. [DOI] [PubMed] [Google Scholar]
- Whisman MA, Li A, Sbarra DA, Raison CL, 2014. Marital quality and diabetes: Results from the health and retirement study. Health Psychol. 33, 832–840. [DOI] [PubMed] [Google Scholar]
- Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC, 1990. Cd14, a receptor for complexes of lipopolysaccharide (lps) and lps binding protein. Science 249, 1431–1433. [DOI] [PubMed] [Google Scholar]
- Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P, 2016. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol. Psychiatry 21, 786–796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.