Abstract
Viral immunotherapy has shown clinical efficacy in treating cancers (e.g., melanoma). Given that viral immunotherapy commonly uses intratumoral injection, prolonging the duration of therapeutic virus at the tumor site can further enhance the antitumor efficacy and reduce potential off-target effects. In this work, we describe a “double-punch” strategy by combining dendrimer platform and injectable hydrogel encapsulation for delivery of an adenovirus encoding Flagrp170 (Adv-Flagrp170), which has been shown to effectively mount a cytotoxic T lymphocyte response through enhanced tumor immunogenicity and optimized antigen cross-presentation. We first complexed PAMAM generation 4 (G4) with Adv (G4/Adv) to strengthen its transfection efficiency and then loaded G4/Adv into a biocompatible and injectable supramolecular hydrogel (SH) made of α-cyclodextrin and 4-arm polyethylene glycol via host-guest interaction. When tested in a murine melanoma model, the G4/Adv complex was shown to have improved retention at the tumor site. The presence of SH facilitated the targeted gene expression in tumor-infiltrating leukocytes, including antigen-presenting dendritic cells. Delivery of Adv-Flagrp170 by both G4 coating and SH encapsulation significantly enhanced its therapeutic efficacy in controlling mouse melanoma (8-fold reduction in tumor volume), which is associated with increased immune activation in the tumor microenvironment as well as decreased adenovirus-reactive antibodies. Taken together, this new formulation may be used to improve the treatment outcome of adenovirus-based cancer immunotherapy.
Keywords: Adenovirus, Cancer immunotherapy, Dendrimer, Hydrogel, Melanoma
1. Introduction
Cancer immunotherapy using replication defective or competent virus to deliver immunostimulatory molecules represents a promising approach to the management of advanced malignancies [1,2]. For example, administration of oncolytic virus is able to induce tumor cell lysis while inducing antitumor immunity [1,3]. Additionally, the combination of viral immunotherapy with other agents, e.g., immune checkpoint inhibitors, immunomodulating factors, or chemotherapeutics, has shown improved anticancer efficacy [3,4]. We recently created a highly immunostimulatory molecule by strategically engineering a pathogen (i.e., flagellin)-derived ‘danger’ signal into chaperone-based antigen-carrying cargo [5–8]. This chimeric molecule, termed Flagrp170, retains a superior antigen-presenting capability and is equipped with additional capacity of effectively augmenting the function of antigen-presenting cells (APCs) [9]. Intratumoral delivery of Flagrp170 using an adenovirus (Adv-Flagrp170) induces multiple immunostimulatory cytokines (e.g., IL-12, IFN-γ) in the tumor microenvironment (TME), hence re-establishing systemic host antitumor immune surveillance.
Despite the excellent capacities of Adv in gene therapy of cancers, efficient delivery of Adv to targeted cells can be limited by the scarcity of coxsackievirus and adenovirus receptor (CAR) on the tumor cell surfaces [10]. Efforts have been made to bypass CAR-mediated endocytosis of Adv by engineering the surface of Adv using various strategies, including coating with cationic polymers and lipids [11–14]. The layer of cationic polymer on the surface of Adv can realize increased cell internalization by overcoming the repulsion of negatively surface charged Adv and targeted cells [15,16]. Poly(ethylenimine) (PEI) is a highly branched cationic polymer and has been extensively utilized as the coating of Adv [17,18]. PEI with higher molecular weight forms stronger complexation with Adv, resulting in improved transfection efficiency but in association with high cytotoxicity [19,20]. PEI with lower molecular weight shows less toxicity but displays lower complexation with Adv and minimal transfection efficiency [21]. Polyamidoamine (PAMAM) dendrimers with primary amine termini are an ideal substitute for PEI to lower cytotoxicity and maintain transfection efficiency [22]. PAMAM alone and modified PAMAM have been broadly explored as a non-viral gene vector for the delivery of DNA and small interfering RNA (siRNA) [23–25].
Conventional tumor-targeted viral gene therapy exhibits direct cytotoxic effects on cancer cells and often requires large volume of viral transduction. However, given the distinct mechanism underlying the induction of antitumor immunity, in situ viral immunotherapy that mainly targets the host immune system does not require extensive infection of cancer cells. Tumor antigens released from cancer cells, with assistance from immunostimulatory molecules (e.g., Flagrp170) delivered via a viral vector, can be efficiently directed to the specialized antigen-presenting cells, resulting in activation of tumor-reactive T lymphocytes. Nonetheless, prolonging the duration of therapeutic virus such as Adv at the tumor site and limiting its leakiness can further enhance its immunostimulatory efficacy while reducing potential off-target effects. Injectable hydrogels are excellent delivery carriers for gene or viral vectors [26,27]. Surface-engineered virus can be in-situ loaded to hydrogel components and retained at the injection site following gel solidification. The hydrogel also enables sustained localized release of Adv at the tumor site, which could significantly increase the therapeutic index of viral immunotherapy.
To improve effective delivery and retention of Adv-Flagrp170 for cancer immunotherapy, we developed a “double-punch” strategy by combining dendrimer gene delivery and injectable hydrogel encapsulation into a combinatorial regimen. We first complexed PAMAM generation 4 (G4) with Adv (G4/Adv) and then formulated it into a biocompatible and injectable supramolecular hydrogel (SH). Delivery of Adv-Flagrp170 using our newly developed platform significantly enhanced its therapeutic efficacy in the treatment of murine melanoma, which was associated with elevated immune activation against tumor as well as a decreased humoral response to adenoviral vector.
2. Materials and methods
2.1. Materials, cell lines, and cell culture
EDA-core PAMAM dendrimer generation 4 (G4) was purchased from Dendritech (Midland, MI, USA). α-Cyclodextrin (α-CD, ≥98%), and cell proliferation reagent WST-1 were purchased from Sigma-Aldrich. 4-arm polyethylene glycol (4-PEG, Mn = 10,000 g/mol) was purchased from Xiamen SINOPEG Biotech (Fujian, China). HN12 head and neck squamous carcinomas cells were cultured in DMEM supplemented with 10% Cosmic calf serum, 100 units/mL of penicillin, and 100 μg/mL of streptomycin at 37 °C in 95% air/5% CO2. B16 -gp100 murine melanoma cells (gift from Dr. A Rakhmilevich, University of Wisconsin-Madison) [28] were cultured in DMEM supplemented with 10% fetal bovine serum, 100 units/mL of penicillin, 100 μg/mL of streptomycin, and 1 M of HEPES at 37 °C in 95% air/5% CO2.
2.2. Generation of replication-defective recombinant adenoviruses
Replication-defective recombinant adenoviruses carrying Flagrp170 (Adv-Flagrp170) was prepared as we previously described [9]. Briefly, N-terminal (amino acid 1–176) and C-terminal (amino acid 402–505) fragments of flagellin (GenBank accession #AAL20871.1) from Salmonella enterica serovar Typhimurium LT2 was fused to the ATP-binding domain-truncated Grp170 (GenBank accession #AF228709, amino acid 431–994). A 34-aa signal peptide for Grp170 was added to the N-terminus of the fusion construct, and the “KNDEL” ER retention sequence was removed to generate a secretable form of Flagrp170. Adenovirus carrying GFP (Adv-GFP) was also prepared as a control to study the transduction efficiency in vitro and in vivo in the presence or absence of dendrimers. Recombinant adenoviruses were purified using AdenoPACK Maxi spin columns (Sartorius Stedim Biotech).
2.3. Coating of Adv with G4
To prepare the G4/Adv complex, various concentrations of G4 were mixed with Adv-GFP particles (1.28 × 109 vp/mL), resulting in molar ratios of 0.13, 1.3, 6.5, 13, 65, 130 (×106) G4 molecule/Adv-GFP particle in PBS buffer (1 × PBS, pH 7.4). The complexes were then incubated for 30 min at R.T. under mild shaking prior to further use.
2.4. Size and ζ potential of naked Adv and G4/Adv complex
Size and ζ potential of naked Adv-GFP and G4/Adv-GFP complexes were measured by dynamic laser scattering (DLS) on a Malvern Zetasizer Nano ZS90 (Malvern Instruments, Malvern, Worcestershire, U.K.). All the measurements were performed in triplicate. The morphologies of both naked Adv-GFP and G4/Adv-GFP complexes were observed by TEM (JEM-1400 Plus). Adv-GFP or G4/Adv-GFP complexes was fixed in PBS containing 4% glutaraldehyde for 30 min. Fixed sample was then applied on carbon-supported grids and incubated for 30 min at R.T. The grids were washed by floating on a drop of filtered DI H2O for 3 times on parafilm. The samples were then stained by 1% UA solution, washed and air-dried before imaging.
2.5. Transduction efficiency study
HN12 cells (2×104 cells/well) were seeded in 48-well plates and cultured overnight for cell attachment. The first group of cells were incubated with Adv-GFP, G4/Adv-GFP, Adv-GFP SH, or G4/Adv-GFP SH (5 vp/cell) for 3 h, rinsed with PBS and cultured for another 21 h at 37 °C. The second group of cells were incubated with Adv-GFP, G4/Adv-GFP, Adv-GFP SH, or G4/Adv-GFP SH (5 vp/cell) for 24 h. The two groups of cells were imaged under a fluorescence microscope (Nikon Instruments Inc., Melville, NY). Another set of cells treated as described above were subjected to flow cytometry (BD LSRFortessa-X20, BD Biosciences, San Jose, CA).
2.6. In vivo therapeutic efficacy assessment of G4/Adv-Flagrp170
C57BL/6 mice with B16 tumors were established by injecting 2 × 105 B16-gp100 tumor cells s.c. into the right dorsal flank. Mice were treated for three doses of Adv-Flagrp170 or G4/Adv-Flagrp170 intratumorally when tumor reached 4–5 mm in diameter every other day (2 × 109 vp of Adv-Flagrp170 in 50 μL of PBS for every dose). Tumor growth was monitored by measuring the perpendicular diameters of tumors. T cell infiltration into the tumor was assayed by flow cytometry. Ten days after last treatment, spleens were collected and splenocytes were cultured in the presence of gp10025–33 peptide for 72 h. IFN-γ in the culture media was determined by ELISA.
2.7. G4/Adv-GFP loaded supramolecular hydrogel (G4/Adv-GFP SH) characterization
G4/Adv-GFP was loaded to a supramolecular hydrogel (SH) following a two-step procedure: 1) G4 solution (10 μL, 1.28 mg/mL) and Adv-GFP (32 μL, 1.28 × 109 vp/mL) were mixed with 2.1 mg of 4-PEG, vortexed and kept shaking at 100 rpm for 30 min, 2) α-CD (10 mg) in 58 μL PBS was added to the above mixture and vortexed.
Rheological measurements of G4/Adv-GFP SH at 25 °C were carried out on Discovery Hybrid Rheometer HR-3 (TA Instruments) and a 20 mm parallel plate geometry was used. An amplitude sweep was first performed at a constant angular frequency of 1 rad/s in the strain range of 0.1%–100%. Within the linear viscoelastic region (LVR), oscillatory frequency sweeps were then carried out under a constant strain of 0.5% in the frequency range of 0.1–100 rad/s. A flow sweep cycle (viscosity vs. shear rate) was carried out from 0.1 s−1 to 100 s−1.
Lyophilized G4/Adv-GFP SH sample was coated with platinum for 90 s using an ion sputter. SEM images were taken under SEM (Hitachi FE-SEM Su-70) with an acceleration voltage of 5 kV. SEM images were analyzed using ImageJ2 software to determine the pore size of the dehydrated hydrogel. The analysis was performed on at least 20 measurements.
To determine the cytotoxicity of the G4/Adv-GFP SH, B16 cells or HN12 cells were seeded at 1 × 104 cells/well in a 96-well culture plate overnight at 37 °C. Spent media was replaced with fresh media containing G4/Adv-GFP SH at different doses (1.2, 2.4, 6 mg/mL). After 48 h of continuous incubation, cell viability was measured via WST-1 colorimetric assay (absorbance wavelengths measured at 450 nm and 650 nm).
2.8. Transduction efficiency of G4/Adv-GFP SH in tumor-associated myeloid cells
C57BL/6 mice with B16 tumors were established following the same protocol as described in 2.6. One dose of Adv-GFP, G4/Adv-GFP, SH, or G4/Adv-GFP SH was intratumorally injected into tumors. At 72 h post-injection, tumors were collected for evaluation of GFP expression in myeloid and non-myeloid cells. For analysis of leukocyte infiltration, tumor tissues were digested with collagenase D (1 mg/mL) and DNase I (100 μg/mL), and cell suspensions were filtered through a 70 mm cell strainer. Viability of tumor cells (gated on CD45− cells) was evaluated by flow cytometry after Zombie dye staining.
2.9. In vivo therapeutic efficacy assessment of G4/Adv-GFP SH immunotherapy
C57BL/6 mice bearing B16 tumors were established following the same protocol as describe in 2.6. When tumors reached the size of 4–5 mm in diameter, mice received intratumorally Adv-Flagrp170 (3 or 5 doses, 2 × 109 vp of Adv-Flagrp170 in 50 μL of PBS for each dose), SH (three doses, 50 μL for each dose), or G4/Adv-Flagrp170 SH (three doses, 50 μL for each dose) were intratumorally injected every other day. Tumor growth was monitored by measuring the perpendicular diameters of tumors. Transcription of il12p40 was assayed by real-time PCR upon the termination of the experiment. A separate set of experiments was conducted following the same dosing regimen. Ten days after the last treatment, the titer of antibodies that recognize adenoviral vector was assayed.
2.10. Evaluation of adenovirus-reactive humoral response
Humoral response to adenoviral vector was assayed using serum samples by ELISA. 96-well ELISA plates were coated with 108 vp of adenovirus in 100 μL of PBS at 4 °C overnight, washed three times in PBS/0.05% Tween and blocked in PBS/3% BSA for 1 h at room temperature. Serially diluted serum samples were added and incubated for 2 h at room temperature. Plates were washed followed by incubation with peroxidase conjugated anti-mouse IgG2a (BD Biosciences, San Jose, CA) for 30 min at room temperature. Plates were washed as described above and TMB substrate (Biolegend, San Diego, CA) was added. Optical density was recorded at 450 nm.
3. Results
3.1. Physicochemical properties of G4/Adv
The complexation of G4/Adv was formed via electrostatic interaction between the positively charged PAMAM dendrimer and negatively charged Adv (Fig. 1a). A series of G4/Adv complexes were prepared at various ratios of G4/Adv. The size and morphologies of Adv and G4/Adv were characterized by TEM. The images of Adv display a characteristic hexon structure and the icosahedral shape of the adenoviral particle (Fig. 1b). The size of dry Adv is ~69 nm according to TEM image analysis. Adv particles appear to aggregate in G4/Adv complex and the surface is rough (Fig. 1c), suggesting successful coating of dendrimers into the Adv particles. The hydrodynamic diameter and ζ potential of all the complexes and naked Adv are shown in Fig. 1d. The hydrodynamic size of naked Adv is 126 nm. The hydrodynamic size increases to ~1 μm when the ratio of G4/Adv is 6.5 × 106. Naked Adv possesses a negatively charged surface with the ζ potential value of −20 mV. ζ potential values of G4/Adv complexes increase with the increasing ratio of G4/Adv. When the ratio of G4/Adv is raised to 6.5 × 106, ζ potential turns from negative to positive. Based on the characterization, we chose G4/Adv prepared at the ratio of 13 × 106 in our later studies.
Fig. 1.
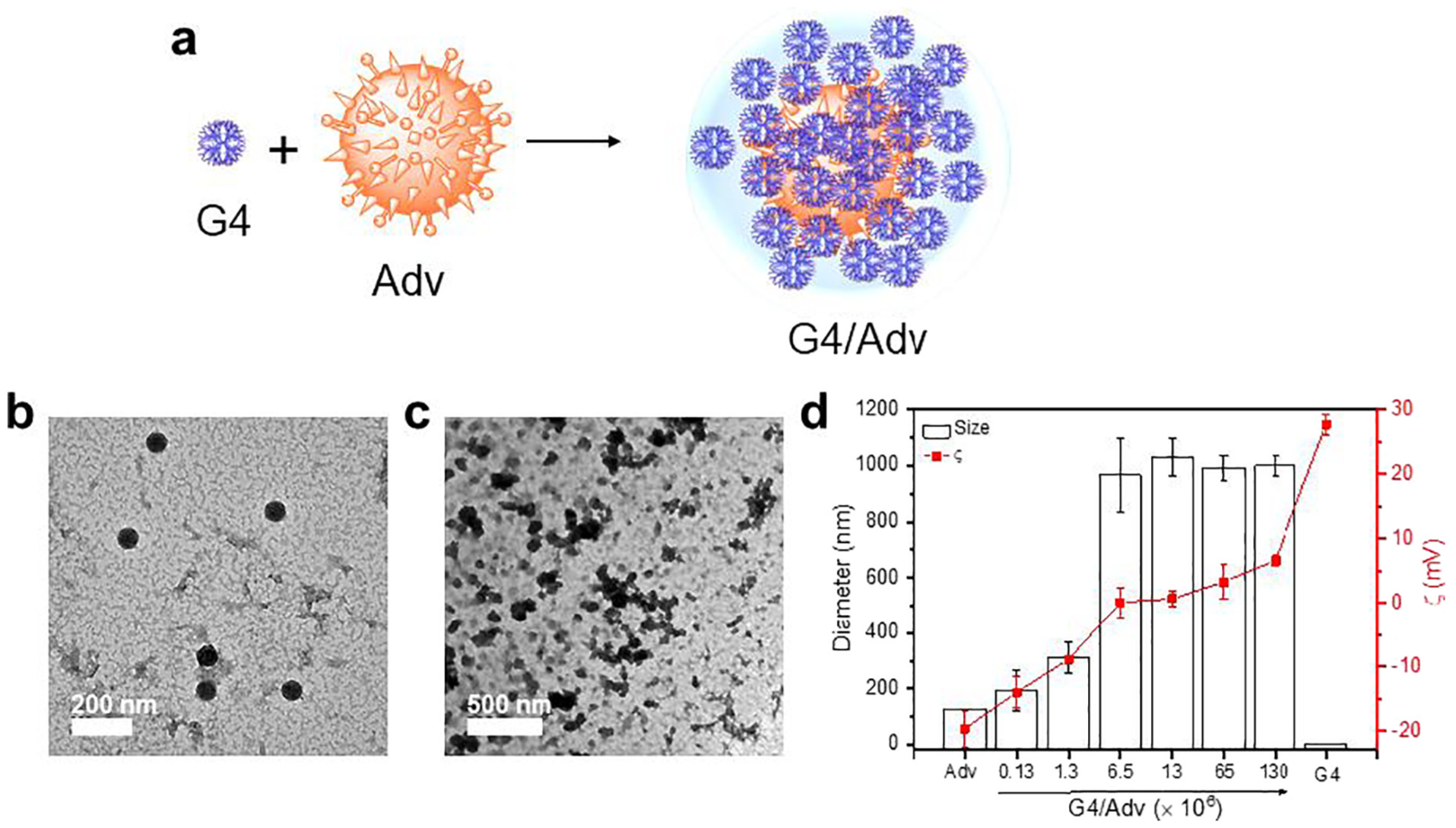
Preparation and characterization of G4/Adv complexes. a) Schematic illustration of forming G4/Adv complexes via electrostatic interactions. b, c) Representative TEM image of Adv and G4/Adv. d) Size and zeta potential of G4/Adv complexes prepared at various molar ratios.
3.2. Enhanced transduction efficiency of G4/Adv-GFP
We tested the gene transduction efficiency of both naked Adv-GFP and G4/Adv-GFP on HN12 cell lines. Cells were transduced with naked Adv-GFP or G4/Adv-GFP at Adv/cell ratio 5/1 for 3 h or 24 h, respectively. The GFP intensity was visualized using fluorescence microscopy and quantified using FACS analysis (Fig. 2). At the same dosage of Adv-GFP, G4/Adv-GFP transfected more cells than naked Adv-GFP at 3 h (Fig. 2a). Quantitative FACS analysis (Fig. 2c) showed that the G4/Adv-GFP transduced HN12 cells at a higher level (46.85 ± 18.03%) than naked Adv-GFP (5.75 ± 1.63%) after 3 h incubation. Continuous culturing of HN12 cells with Adv-GFP or G4/Adv-GFP for up to 24 h resulted in increased transfection, 91.5 ± 1.62% of cells transfected by Adv-GFP, while 83.8 ± 0.99% transfected by G4/Adv-GFP. Under the in vitro cell culture conditions, there is no detectable leak. At the same dose of adenovirus, G4/Adv-GFP and Adv-GFP showed a comparable transduction rate during a long period (24 h). There was no significant transduction observed after culturing cells with hydrogel formulations (Adv-GFP SH, and G4/Adv-GFP SH) for 3 h. Even after 24 h-culture, the gel formulations of adenovirus transfected less cells than those without gels (Fig. 2b, c). There were 42.9 ± 0.99% of cells expressing GFP after culture with Adv-GFP SH for 24 h, whereas only 8.10 ± 0.50% of cells incubated with G4/Adv-GFP SH were GFP positive.
Fig. 2.
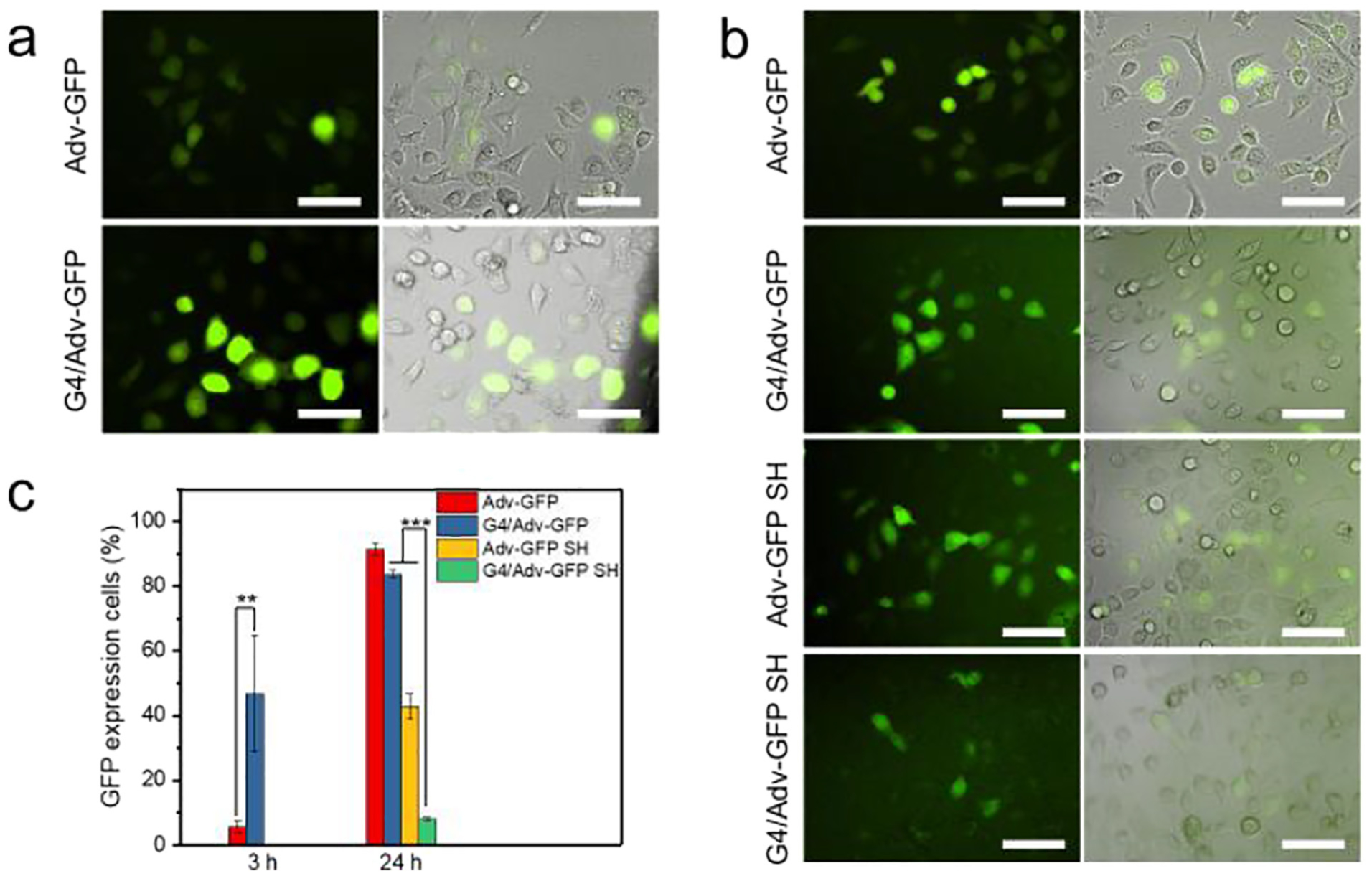
In vitro transfection efficiency of Adv-GFP. a) Fluorescence images of cells incubated with Adv-GFP or G4/Adv-GFP for 3 h, scale bar = 100 μm. b) Fluorescence images of cells incubated with Adv-GFP, G4/Adv-GFP, Adv-GFP SH, or G4/Adv-GFP SH for 24 h, scale bar = 100 μm. c) Quantitative analysis of GFP-expressing cells incubated with Adv-GFP, G4/Adv-GFP, Adv-GFP SH, or G4/Adv-GFP SH by FACS. **, p <0.01. ***, p <0.001.
Evaluation of in vivo therapeutic efficacy revealed that G4/Adv-Flagrp170 had a similar tumor inhibitory effect compared to Adv-Flagrp170 (Fig. 3a). However, G4/Adv-Flagrp170 appeared to cause a modest increase in recruitment of CD8+ and CD4+ T cells (Fig. 3b, c) as well as significantly elevated IFN-γ production from splenocytes in response to gp10025–33 peptide stimulation, an indicator of enhanced immune response to melanoma antigen gp100 (Fig. 3d).
Fig. 3.
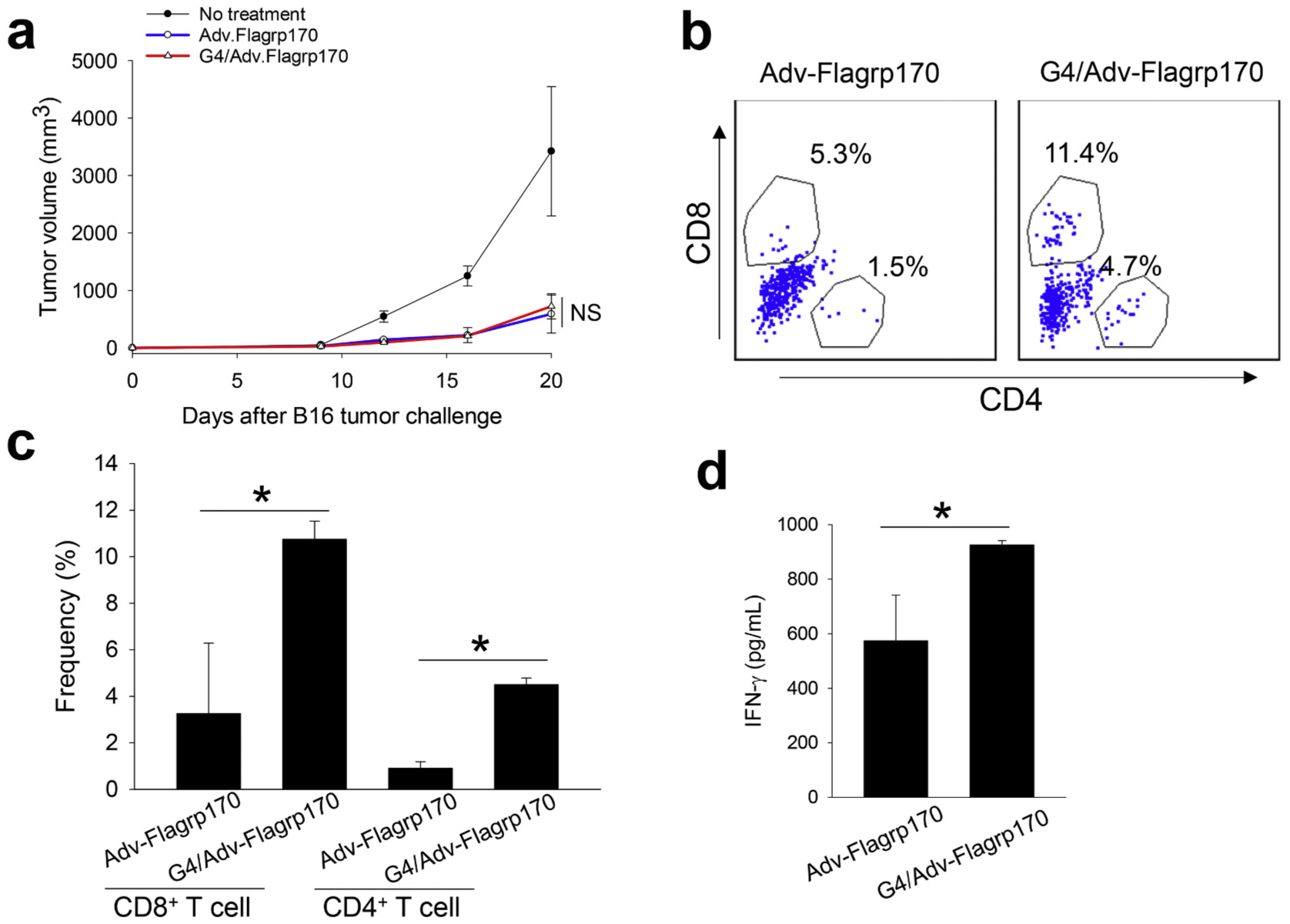
In vivo therapeutic efficacy assessment of G4/Adv-Flagrp170. When tumor reached 4–5 mm in diameter, C57BL/6 mice bearing B16 tumors were injected with Adv-Flagrp170 or G4/Adv-Flagrp170 every other day for a total of three doses. a) Tumor growth was monitored during the treatment. b, c) T cell infiltration into the TME was assayed by flow cytometry. d) Ten days after the last treatment, spleens were collected. Splenocytes were cultured in the presence of gp10025–33 peptide for 72 h. IFN-γ in the culture media was determined by ELISA. NS, not significant. *, p <0.05.
3.3. G4/Adv-GFP SH facilitates the transduction efficiency of adenovirus in tumor-associated myeloid cells
We speculated that no significant difference in in vivo therapeutic efficacy between G4/Adv-Flagrp170 and naked Adv/Flagrp170 might have been caused by quick in vivo clearance of therapeutic viruses. To address this issue, we used an injectable α-CD/4-PEG SH as the carrier of dendrimer/adenovirus to enhance viral retention at the tumor site. The G4/Adv-GFP loaded SH hydrogel (G4/Adv-GFP SH) was prepared on the basis of the host-guest interaction between α-CD and 4-PEG (Fig. 4a). Our previous studies indicated that the SH is biocompatible, injectable and easily prepared [29]. By tuning the host/guest ratio (α-CD to 4-PEG ratio), we could manipulate the network pore size, solidification time, and mechanical properties of the SH. The SH at a host/guest ratio of 50/1 shows a solidification time of 2 min, a pore size ranges at 2–5 μm, and a relative high storage modulus. All the properties of SH at a host/guest ratio of 50/1 meets the criteria for an injectable in-situ forming hydrogel that could help increase the retention of adenovirus. Following the preparation of the gel and in-situ loaded Adv, i.e., G4/Adv SH, we tested whether the performances of the gel could maintain after G4/Adv loading, especially for the pore size, and the mechanical properties. According to the amplitude sweep test (Fig. 4b), a linear viscoelastic range (LVR) was determined to be 0.1%–10%. A frequency sweep test was then conducted within the LVR, particularly at a fixed strain of 0.5%. The G4/Adv-GFP SH displayed typical hydrogel viscoelastic behavior as its storage modulus (G’) is higher than its loss modulus (G”) with frequency-independence from 1 rad/s to 100 rad/s (Fig. 4c). The flow sweep (Fig. 4d) showed that the viscosity of this supramolecular hydrogel dropped quickly with an increasing shear rate but recovered to its initial status when the shear rate was reduced to zero. This shear-thinning thixotropic property also endows the SH injectability. G4/Adv-GFP SH has a porous gel structure with micro-sized pores (Fig. 4e). Statistics of the pore size showed that there are two kinds of pore sizes: the larger ones distribute around 46 μm, while most of the pores ranging at 3–10 μm (Fig. 4f). Given the size of G4/Adv-GFP around 1 μm, this SH is suitable for release of G4/Adv-GFP through a diffusion process. We next tested the cytotoxicity of G4/Adv-GFP SH on both B16 cells and HN12 cells. G4/Adv-GFP SH at the selected doses of 1.2, 2.4, 6 mg/mL did not show toxicity on either B16 cells or HN12 cells (Fig. 4g).
Fig. 4.
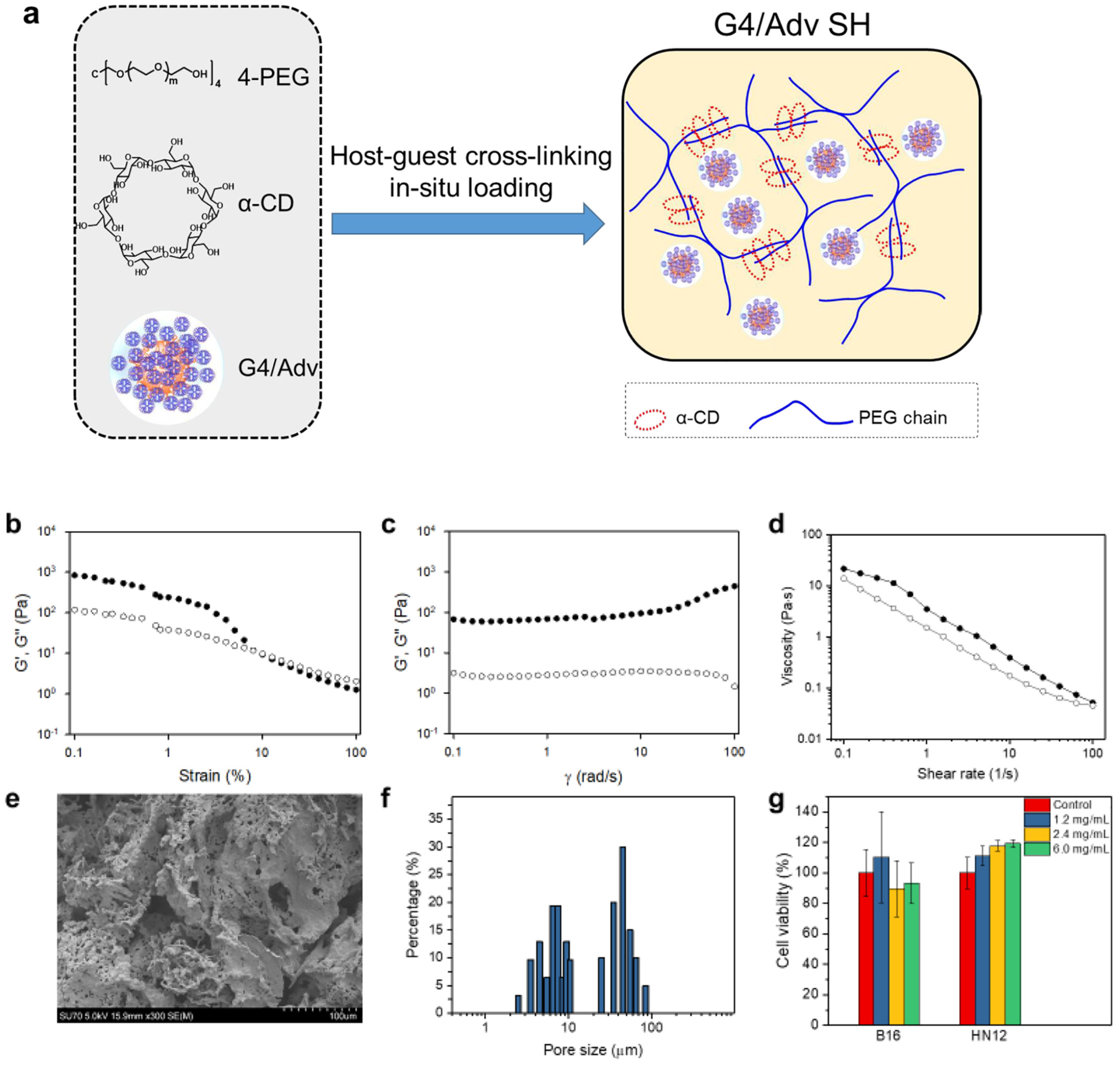
Preparation and characterization of G4/Adv supramolecular hydrogel, i.e., G4/Adv SH. a) Schematic illustration of a general approach to preparing G4/Adv SH by mixing G4/Adv complexes with 4-armed PEG (4-PEG) and α-cyclodextrin (α-CD) on the basis of host-guest interaction. b) Oscillatory amplitude sweep test on G4/Adv SH. c) Oscillatory frequency sweep test on G4/Adv SH. d) Oscillatory flow sweep test on G4/Adv SH. e) SEM image of dehydrated G4/Adv SH. f) Pore size distribution of the dehydrated G4/Adv SH. g) Assessment of cytocompatibility of G4/Adv SH at different doses (n =6).
We also assessed the effect of hydrogel matrix on in vitro transfection efficiency of the viral complex. There was no significant transduction observed after culturing cells with hydrogel formulations for 3 h. This was probably caused by the fact that it takes long time for adenovirus to diffuse out of the network of the gel. It is not surprising to observe a significant low transfection by G4/Adv-GFP SH when compared with Adv-GFP SH in in vitro transfection assay, which we believe were caused by the slow diffusion of large size adenoviruses from the hydrogel network. These results suggest that the gel formulation can retain the adenovirus for a potential slow release, especially when delivered to the targeted tissues in vivo.
Since our previous study suggested that in situ Flagrp170 immunotherapy involves infection of both cancer cells and immune cells [9], we examined whether G4/Adv-GFP SH formulation can facilitate the transduction of these two cell populations in the tumor microenvironment. We injected Adv-GFP, G4/Adv-GFP, G4/Adv-GFP SH or SH to established B16 melanomas, followed by analysis of the frequency of GFP positive cells using flow cytometry. G4/Adv-GFP did not show any advantages in facilitating adenoviral infection when compared to naked virus. However, G4/Adv-GFP SH significantly enhanced transduction efficiency, indicated by elevation of GFP signals in immune cells including CD11b+ myeloid cells and CD11c+ dendritic cells. (Fig. 5a). While the G4/Adv-GFP SH formulation showed its superiority in enhancing GFP transduction of both CD45+ leukocytes as well as CD45− cells (e.g., tumor cells), this formulation appeared to target adenovirus preferentially to leukocytes (Fig. 5b). Cell viability in the tumor microenvironment was also evaluated by Zombie dye staining. No evident cytotoxicity associated with all formulations was detected as compared with injection with naked Adv-GFP (Fig. 5c). These results indicate that G4/Adv-GFP SH formulation can enhance the transfer of immunostimulatory gene into immune cells and cancer cells in the setting of adenovirus-based immunotherapies.
Fig. 5.
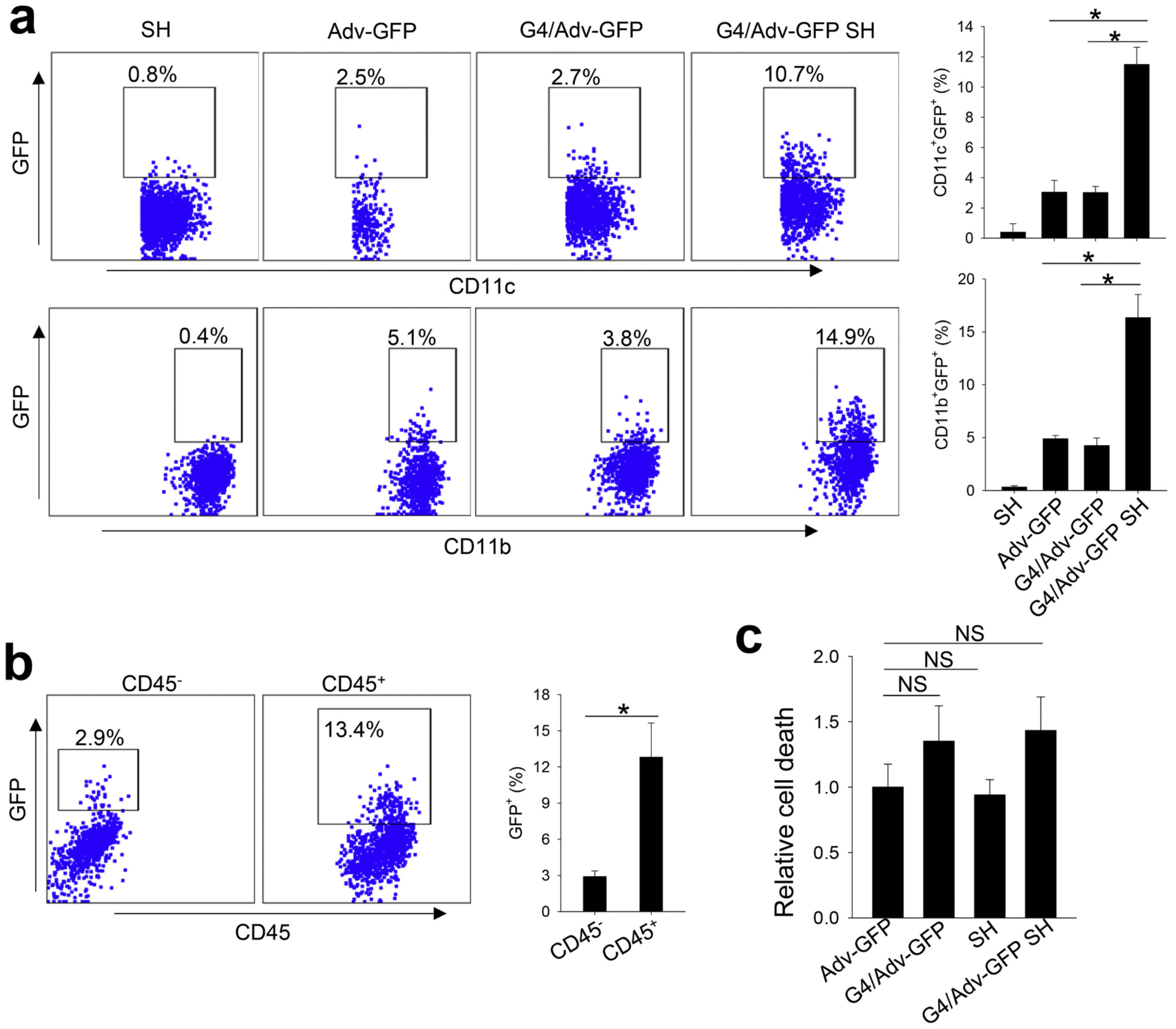
Supramolecular hydrogel formulation enhances the transfection efficiency of adenovirus in tumor associated myeloid cells. One dose of Adv-GFP, G4/Adv-GFP, SH, or G4/Adv-GFP SH was intratumorally injected into B16 tumors. At 72 h post injection, tumors were collected for evaluation of GFP expression in myeloid cells (a) and leukocytes (b) by flow cytometry. Tumor cell (gated on CD45− cells) viability (%) was evaluated by flow cytometry after Zombie dye staining (c). NS, not significant. *, p <0.05.
3.4. SH enhances the therapeutic potency of G4/Adv-Flagrp170
Lastly, we examined whether this hydrogel formation could improve therapeutic potency of Adv-Flagrp170 (Fig. 6a). SH alone did not show any effect on tumor growth. Tumor growth was significantly inhibited in mice receiving three doses of intratumorally injected naked Adv-Flagrp170. Compared to the control (untreated) group, the tumor volume at day 23 was reduced by 2-fold following three doses of Adv-Flagrp170 treatment. Treatment with five doses of Adv-Flagrp170 modestly enhance growth inhibition. Notably, G4/Adv-Flagrp170 SH was shown to be most effective in suppressing tumor growth. Three doses of G4/Adv-Flagrp170 SH resulted in reduction of tumor volume by nearly 8-fold. In addition, G4/Adv-Flagrp170 SH induced the highest upregulation of il12p40 in the tumor tissues, a Th1 cytokine that is critically involved in the Adv-Flagrp170 therapy-augmented antitumor immunity (Fig. 6b). More strikingly, there was a sharp decrease in the titers of antibodies reactive with adenoviral vector in mice receiving G4/Adv-Flagrp170 SH compared with those treated with all other formulations (Fig. 6c), indicating that retention of G4/Adv-Flagrp170 by SH at the tumor site also substantially decreased a humoral response to the adenoviral vector.
Fig. 6.
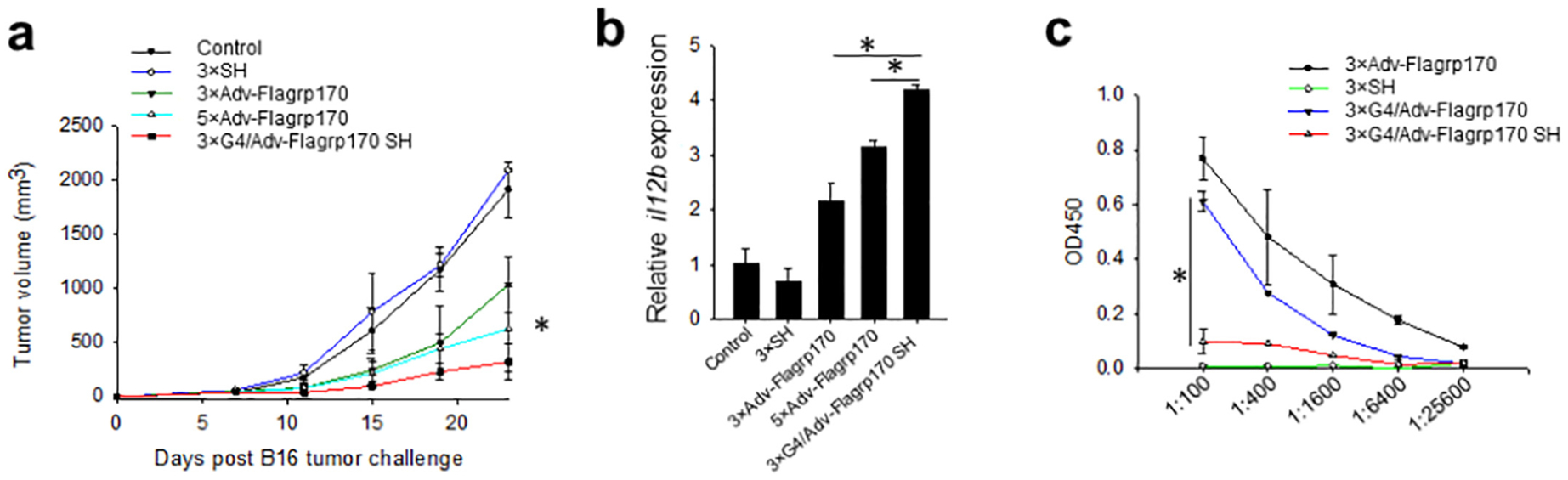
Supramolecular hydrogel formulation enhances the therapeutic efficacy of Flagrp170 immunotherapy. B16 tumors were established in C57BL/6 mice. When tumors reached 4–5 mm in diameter, Adv-Flagrp170, SH, or G4/Adv-Flagrp170 SH were intratumorally injected for a total of three or five doses. (a) Tumor growth was followed. *, p <0.05, 3 × Adv-Flagrp170 vs 3 × G4/Adv-Flagrp170 SH. (b)Transcription of il12p40 was assayed by real-time PCR upon the termination of experiment (c) At ten days after the last treatment, the titer of anti-adenovirus antibodies in the serum was assayed. *, p <0.05.
4. Discussion
In this work, we have prepared a dendrimer/adenovirus complex based on the electrostatic interaction between the negatively surface-charged adenovirus and positively surface-charged PAMAM den-drimer. The size of the dendrimer/adenovirus complex increases with increasing the ratio of dendrimer/adenovirus. The zeta potential of the dendrimer/adenovirus complex turns from negative to positive when the ratio of dendrimer/adenovirus reaches to 6.5 × 106, which confirms the electrostatic interaction. Compared to the negatively surface-charged naked adenovirus, dendrimer/adenovirus complex can enhance cellular uptake of adenovirus, as validated by enhanced transduction efficiency in HN12 cancer cells after 3 h-culture. Despite the increased transduction efficiency of dendrimer/adenovirus complex in vitro, it fails to improve the therapeutic index of Adv-Flagrp170 in inhibiting tumor progression in vivo as compared to naked Adv-Flagrp170.
To achieve prolonged retention of therapeutic adenovirus such as Adv-Flagrp170 at the tumor site we have also evaluated an injectable α-CD/4-PEG SH as the carrier of dendrimer/adenovirus. The SH is a biocompatible hydrogel with microscopic microporous structures formed by dynamic host-guest supramolecular complexation between α-CD and 4-PEG. The typical viscoelasticity and shear-thinning property of SH measured by oscillatory rheological tests validate its gelation and injectability. The injectability makes SH an ideal formulation for intratumoral injection. The relatively high storage modulus and high viscosity at static state of SH indicates its potential for prolonged retention in vivo after injection. The micro-sized porous structures of this SH can retain therapeutic adenovirus locally while allowing its slow release. In vitro transfection results show that there is a delayed transfection using the gel formulations, which may be explained by the time necessary for adenovirus to diffuse out from the network of the gel. These features of this hydrogel formulation will retain virus in the tumor microenvironment for a sustained period of time, which not only enhances therapeutic potency of viral immunotherapy, but also potentially reduces treatment doses for achieving therapeutic responses. Indeed, this hydrogel formulated Flagrp170 is highly potent than all other treatment in controlling tumor growth in vivo, which is further supported by the observation that the three doses of G4/Adv-Flagrp170 SH is more therapeutically effective than five doses of Adv-Flagrp170.
Although the research tools currently available limit our ability to dissect the relative importance of adenoviral infection of cancer cells vs immune cells, our data generated during the study of this in situ Flagrp170 immunotherapy suggest that infection of both tumor cells and immune cells (e.g., dendritic cells specifically) are important for the therapeutic efficacy of Flagrp170. Genetically modifying cancer cells to produce Flagrp170 significantly increases tumor immunogenicity for immune recognition. [9] Additionally, infection of dendritic cells markedly enhances their activation and functions in antigen-presentation and T cell priming [9]. Studies are currently under way to engineer tumor- or host cell (e.g., dendritic cell)-specific construct encoding Flagrp170, which will help delineate the effects of infection of cancer cells or dendritic cells on overall immunotherapeutic outcome.
Considering that the host response to adenoviral vector may limit the activity of therapeutic viral therapy [30,31], we have assessed the impact of this hydrogel formulation on the titers of antibodies that can recognize the adenovirus after the Flagrp170 immunotherapy. We demonstrate that use of the G4/Adv-Flagrp170 SH formulation profoundly reduces the humoral response to the adenoviral vector. Therefore, this formulation can potentiate the therapeutic outcome of the Flagrp170 immunotherapy while minimizing the potential neutralizing effect of the host response to adenoviral vector, since repeated treatments are often required for cancer immunotherapy.
5. Conclusions
We have developed a “double-punch” strategy by combining den-drimer gene delivery and injectable hydrogel encapsulation for delivery of immunostimulatory Flagrp170-encoding adenovirus (Adv-Flagrp170). PAMAM dendrimer helps strengthen Adv’s in vitro transfection efficiency. The injectable α-CD/4-PEG supramolecular hydrogel retains Adv and facilitates targeted gene expression at the tumor site. Adv-Flagrp170 delivered by the combined use of PAMAM dendrimer and SH potently inhibit growth of mouse melanoma by enhancing immune activation in the tumor microenvironment while limiting development of host response to adenoviral vector. Thus, this novel hydrogel formulation may be broadly used to improve both the therapeutic benefits and the safety profile of virus-based cancer immunotherapy.
Acknowledgements
This work was supported in part by Department of Defense Grant W81XWH-11-0481, the VCU Massey Cancer Center Novel Agent Initiatives, National Cancer Institute Grants CA099326, CA154708, and CA175033. Services in support of the research project were provided by the VCU Massey Cancer Center Flow Cytometry Core, supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059.
References
- [1].Cerullo V, Vähä-Koskela M, Hemminki A, Oncolytic adenoviruses: a potent form of tumor immunovirotherapy, Oncoimmunology 1 (6) (2012) 979–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rosewell Shaw A, Suzuki M, Recent advances in oncolytic adenovirus therapies for cancer, Curr. Opin. Virol 21 (2016) 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Goradel NH, Mohajel N, Malekshahi ZV, Jahangiri S, Najafi M, Farhood B, Mortezaee K, Negahdari B, Arashkia A, Oncolytic adenovirus: a tool for cancer therapy in combination with other therapeutic approaches, J. Cell. Physiol 234 (6) (2019) 8636–8646. [DOI] [PubMed] [Google Scholar]
- [4].Jiang H, Rivera-Molina Y, Gomez-Manzano C, Clise-Dwyer K, Bover L, Vence LM, Yuan Y, Lang FF, Toniatti C, Hossain MB, Fueyo J, Oncolytic adenovirus and tumor-targeting immune modulatory therapy improve autologous cancer vaccination, Cancer Res. 77 (14) (2017) 3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang XY, Kazim L, Repasky EA, Subjeck JR, Characterization of heat shock protein 110 and glucose-regulated protein 170 as cancer vaccines and the effect of fever-range hyperthermia on vaccine activity, J. Immunol 166 (1) (2001) 490–497. [DOI] [PubMed] [Google Scholar]
- [6].Park JE, Facciponte J, Chen X, MacDonald I, Repasky EA, Manjili MH, Wang XY, Subjeck JR, Chaperoning function of stress protein grp170, a member of the hsp70 superfamily, is responsible for its immunoadjuvant activity, Cancer Res. 66 (2) (2006) 1161–1168. [DOI] [PubMed] [Google Scholar]
- [7].Wang XY, Sun X, Chen X, Facciponte J, Repasky EA, Kane J, Subjeck JR, Superior antitumor response induced by large stress protein chaperoned protein antigen compared with peptide antigen, J. Immunol 184 (11) (2010) 6309–6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang XY, Arnouk H, Chen X, Kazim L, Repasky EA, Subjeck JR, Extracellular targeting of endoplasmic reticulum chaperone glucose-regulated protein 170 enhances tumor immunity to a poorly immunogenic melanoma, J. Immunol 177 (3) (2006) 1543–1551. [DOI] [PubMed] [Google Scholar]
- [9].Yu X, Guo C, Yi H, Qian J, Fisher PB, Subjeck JR, Wang XY,A multifunctional chimeric chaperone serves as a novel immune modulator inducing therapeutic antitumor immunity, Cancer Res. 73 (7) (2013) 2093–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Beatty MS, Curiel DT, Adenovirus strategies for tissue-specific targeting, Adv. Cancer Res 115 (2012) 39–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Howard F, Muthana M, Designer nanocarriers for navigating the systemic delivery of oncolytic viruses, Nanomedicine 15 (1) (2020) 93–110. [DOI] [PubMed] [Google Scholar]
- [12].Lv P, Liu X, Chen X, Liu C, Zhang Y, Chu C, Wang J, Wang X, Chen X, Liu G, Genetically engineered cell membrane Nanovesicles for oncolytic adenovirus delivery: a versatile platform for cancer virotherapy, Nano Lett. 19 (5) (2019) 2993–3001. [DOI] [PubMed] [Google Scholar]
- [13].Na Y, Nam J-P, Hong J, Oh E, Shin HC, Kim HS, Kim SW, Yun C-O, Systemic administration of human mesenchymal stromal cells infected with polymer-coated oncolytic adenovirus induces efficient pancreatic tumor homing and infiltration, J. Control. Release 305 (2019) 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kasala D, Lee S-H, Hong JW, Choi J-W, Nam K, Chung YH, Kim SW, Yun C-O, Synergistic antitumor effect mediated by a paclitaxel-conjugated polymeric micelle-coated oncolytic adenovirus, Biomaterials 145 (2017) 207–222. [DOI] [PubMed] [Google Scholar]
- [15].Choi J-W, Lee YS, Yun C-O, Kim SW, Polymeric oncolytic adenovirus for cancer gene therapy, J. Control. Release 219 (2015) 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kasala D, Yun C-O, Polymer-anchored adenovirus as a therapeutic agent for Cancer gene therapy, in: Curiel DT (Ed.), Adenoviral Vectors for Gene Therapy (Second Edition), Academic Press, San Diego, 2016, pp. 707–737. [Google Scholar]
- [17].Choi J-W, Nam J-P, Nam K, Lee YS, Yun C-O, Kim SW, Oncolytic adenovirus coated with multidegradable bioreducible core-cross-linked polyethylenimine for cancer gene therapy, Biomacromolecules 16 (7) (2015) 2132–2143. [DOI] [PubMed] [Google Scholar]
- [18].Lee C-H, Kasala D, Na Y, Lee MS, Kim SW, Jeong JH, Yun C-O, Enhanced therapeutic efficacy of an adenovirus-PEI-bile-acid complex in tumors with low coxsackie and adenovirus receptor expression, Biomaterials 35 (21) (2014) 5505–5516. [DOI] [PubMed] [Google Scholar]
- [19].Putnam D, Gentry CA, Pack DW, Langer R, Polymer-based gene delivery with low cytotoxicity by a unique balance of side-chain termini, Proc. Natl. Acad. Sci 98 (3) (2001) 1200–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yang C, Cheng W, Teo PY, Engler AC, Coady DJ, Hedrick JL, Yang YY, Mitigated cytotoxicity and tremendously enhanced gene transfection efficiency of PEI through facile one-step carbamate modification, Adv. Healthc. Mater 2 (10) (2013) 1304–1308. [DOI] [PubMed] [Google Scholar]
- [21].Peng Q, Zhong Z, Zhuo R, Disulfide cross-linked Polyethylenimines (PEI) prepared via Thiolation of low molecular weight PEI as highly efficient gene vectors, Bioconjug. Chem 19 (2) (2008) 499–506. [DOI] [PubMed] [Google Scholar]
- [22].Choi YJ, Kang SJ, Kim YJ, Lim Y.-b., Chung HW, Comparative studies on the genotoxicity and cytotoxicity of polymeric gene carriers polyethylenimine (PEI) and polyamidoamine (PAMAM) dendrimer in Jurkat T-cells, Drug Chem. Toxicol 33 (4) (2010) 357–366. [DOI] [PubMed] [Google Scholar]
- [23].Dufès C, Uchegbu IF, Schätzlein AG, Dendrimers in gene delivery, Adv. Drug Deliv. Rev 57 (15) (2005) 2177–2202. [DOI] [PubMed] [Google Scholar]
- [24].Yang J, Zhang Q, Chang H, Cheng Y, Surface-engineered dendrimers in gene delivery, Chem. Rev 115 (11) (2015) 5274–5300. [DOI] [PubMed] [Google Scholar]
- [25].Chen J, Lu Y, Cheng Y, Ma R, Zou J, Zheng H, Wang R, Zhu Z, Li F, Novel strategy of gene delivery system based on dendrimer loaded recombinant Hirudine plasmid for Thrombus targeting therapy, Mol. Pharm 16 (4) (2019) 1648–1657. [DOI] [PubMed] [Google Scholar]
- [26].Caronia JM, Sorensen DW, Leslie HM, van Berlo JH, Azarin SM, Adhesive thermosensitive gels for local delivery of viral vectors, Biotechnol. Bioeng 116 (9) (2019) 2353–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Paul A, Hasan A, Kindi HA, Gaharwar AK, Rao VTS, Nikkhah M, Shin SR, Krafft D, Dokmeci MR, Shum-Tim D, Khademhosseini A, Injectable graphene oxide/hydrogel-based Angiogenic gene delivery system for Vasculogenesis and cardiac repair, ACS Nano 8 (8) (2014) 8050–8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rakhmilevich AL, Imboden M, Hao Z, Macklin MD, Roberts T, Wright KM, Albertini MR, Yang NS, Sondel PM, Effective particle-mediated vaccination against mouse melanoma by coadministration of plasmid DNA encoding Gp100 and granulocyte-macrophage colony-stimulating factor, Clin. Cancer Res 7 (4) (2001) 952–961. [PubMed] [Google Scholar]
- [29].Wang J, Williamson GS, Yang H, Branched polyrotaxane hydrogels consisting of alpha-cyclodextrin and low-molecular-weight four-arm polyethylene glycol and the utility of their thixotropic property for controlled drug release, Colloids Surf. B: Biointerfaces 165 (2018) 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gabitzsch ES, Xu Y, Balint JP Jr., Z.C. Hartman, H.K. Lyerly, F.R. Jones, Antitumor immunotherapy despite immunity to adenovirus using a novel adenoviral vector Ad5 [E1-, E2b-]-CEA, Cancer Immunol. Immunother 59 (7) (2010) 1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sun C, Feng L, Zhang Y, Xiao L, Pan W, Li C, Zhang L, Chen L, Circumventing Antivector immunity by using adenovirus-infected blood cells for repeated application of adenovirus-vectored vaccines: proof of concept in rhesus macaques, J. Virol 86 (20) (2012) 11031–11042. [DOI] [PMC free article] [PubMed] [Google Scholar]


