Abstract
Foxp3+ regulatory T cells (Treg) maintain the integrity of the organism by preventing excessive immune responses. These cells protect against autoimmune diseases but are also important regulators of other immune responses including inflammation, allergy, infection, and tumors. Furthermore, they exert non-immune functions such as tissue repair and regeneration. In the periphery, Foxp3+ Treg have emerged as a highly heterogeneous cell population with distinct molecular and functional properties. Foxp3+ Treg mainly develop within the thymus where they receive instructive signals for their differentiation. Recent studies have revealed that thymic Treg are also heterogeneous with two distinct precursors that give rise to mature Foxp3+ Treg exhibiting non-overlapping regulatory activities characterized by a differential ability to control different types of autoimmune reactions. Furthermore, the thymic Treg cell pool is not only composed of newly developing Treg, but also contain a large fraction of recirculating peripheral cells. Here, we review the two pathways of thymic Treg cell differentiation and their potential impact on Treg activity in the periphery. We also summarize our current knowledge on recirculating peripheral Treg in the thymus.
Keywords: immune tolerance, regulatory T cells, thymus, thymic recirculation, regulatory T cell heterogeneity, autoimmune disorders
Introduction
CD4+Foxp3+ regulatory T cells (Treg) crucially maintain immune tolerance. These cells are endowed with the unique ability to protect from life-threatening autoimmune reactions and dampen inflammatory responses. The absence of Foxp3+ Treg in IPEX (Immune dysregulation, Polyendocrinopathy, Enteropathy, X-linked syndrome) patients, and scurfy mice results in a severe lymphoproliferation and multi-organ autoimmunity (1–4). Treg cells are also involved in numerous physio-pathological processes such as foeto-maternal tolerance, allergy, anti-tumoral immunity, tissue homeostasis, and repair (5, 6). This large array of functions implies a division of labor by several Treg subsets. This heterogeneity is illustrated by the central Treg (cTreg)/effector Treg (eTreg) dichotomy (7). cTreg are found in lymphoid organs and depend on IL-2, whereas eTreg populate non-lymphoid tissues and depend on TCR signals for their proliferation. Similarly to conventional CD4+ T helper (Th) cells, the cytokine microenvironment induces the polarization of Treg into Th1-, Th2-, Th17-, and follicular Treg (Tfr)-like cells, showing distinct chemokine receptor expression patterns (8). This polarization allows Treg to specifically antagonize their conventional CD4+ Th counterparts.
The majority of Foxp3+ Treg develop in the thymus (tTreg) although conventional CD4+ T cells can also convert in the periphery into Foxp3+ Treg under certain conditions (pTreg) (9). Several precursors including CD25+Foxp3− cells, recent thymic emigrants (RTE) and FR4hiCD73hi cells have been proposed to differentiate into pTreg, which have both overlapping and unique TCR repertoire and functions compared to tTreg (9–17). Thymic development of Treg cells was initially suggested by seminal work during the 1960's describing generalized autoimmune manifestations in neonatal thymectomies, before day 3 but not later (18, 19). Further works demonstrated that suppressive T cells exerting a dominant tolerance develop during this neonatal period in the thymus and were called “regulatory T cells” (20–24). Thymic Treg cells represent only ~2–3% of developing CD4+ single positive (SP) thymocytes. Their development is driven by strong TCR stimulation coupled to the CD28 co-stimulatory signal. A third signal mediated by γ-chain cytokines promotes their survival and progression into a mature CD25+Foxp3+ Treg stage (25).
Recent advances have revealed that thymic Treg constitute a more diverse and dynamic population than previously thought. Emerging evidences indicate that the phenotypically and functional diversity of Treg could be imprinted during their ontogeny in the thymus. In this review, we discuss our current knowledge on the two identified precursors leading to the development of mature Treg, showing distinct regulatory activities. We also highlight a new population of mature Treg recirculating from the periphery into the thymus.
Distinct Thymic Foxp3+ Treg Subsets Generated by Two Developmental Pathways
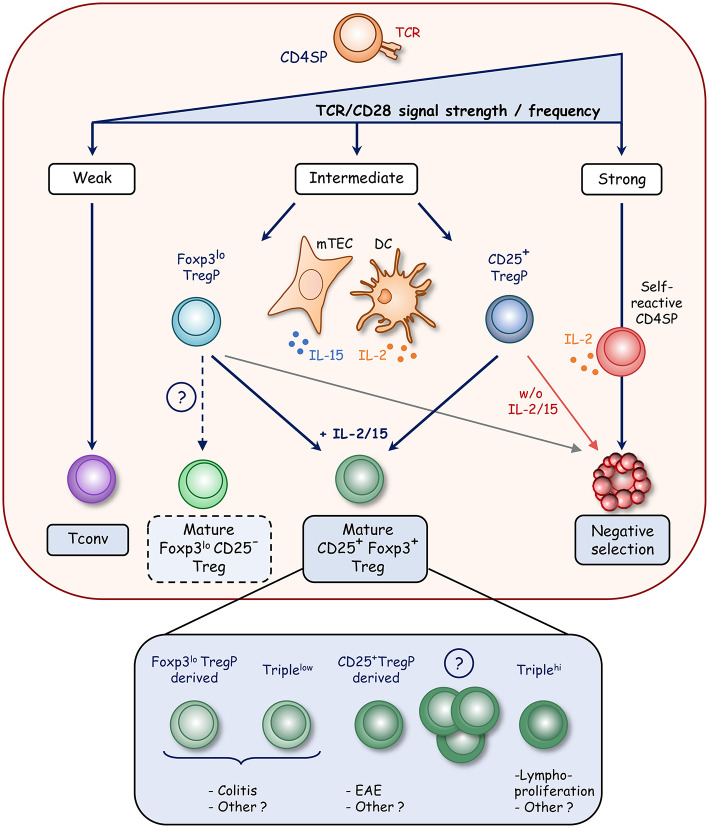
T-cell fate decision toward clonal deletion (also called negative selection) or the diversion into the Treg or T conventional cell lineage is dictated by TCR affinity and/or avidity for self-peptides presented by thymic antigen presenting cells [APC (26)]. The relative contributions of the different APC subsets such as medullary thymic epithelial cells (mTEC), cortical TEC (cTEC), B cells, conventional, and plasmacytoid dendritic cells (DC) in T-cell fate decision remain poorly understood although bone marrow-derived APC and mTEC seem to play non-overlapping roles in shaping the TCR repertoire (27). The introduction of a cognate antigen in TCR transgenic mice leads to both clonal T-cell deletion and Treg induction, indicating that the diversion into the Treg cell lineage is also induced by the recognition of agonist self-peptide (28). Treg thus show a highly self-reactive TCR repertoire, distinct from conventional T cells, which contributes to their functional diversity (29–32). The prevailing paradigm of Treg development is based on the integration of signals mediated by TCR/CD28 stimulation and cytokine signaling. It relies on a “two-step model” in which strong agonist TCR signals in CD25−Foxp3− CD4+ SP thymocytes results in the induction of CD25 (also known as IL-2Rα) and thus in the development of CD25+Foxp3− Treg precursors (CD25+ TregP) (step one) (Figure 1). However, the TCR signal strength required to select Treg was described to be lower than that leading to clonal deletion of CD4+ SP thymocytes (30, 33). This could be due to a lower intrinsic TCR affinity or to the limited expression of the cognate antigen by APC, thus reducing multiple antigen encounters (34, 35). The CD28-CD80/86 costimulatory axis is also essential for Treg development from their precursor stage (36–38). This is well illustrated in Cd28−/− and Cd80−/− × Cd86−/− mice showing impaired generation of CD25+ TregP and a severe defect in mature Treg. Furthermore, the TCR signaling pathway in association with the CD28 co-stimulation signal drives the upregulation of tumor-necrosis factor receptor superfamily (TNFRSF) members such as GITR, OX40, and TNFR2 in CD25+ TregP (39). The CD28 co-stimulation in developing thymocytes has also been reported to induce Foxp3 expression (40).
Figure 1.
Model of Foxp3+ Treg development in the thymus. During thymic development, strong TCR signals lead to clonal deletion of highly self-reactive CD4+ SP thymocytes whereas weak signals result in the generation of conventional T cells (Tconv). In contrast, intermediate TCR signals result in the diversion of CD4+ SP thymocytes into the Treg cell lineage with the development of two distinct precursors. CD25+ Treg precursors (CD25+ TregP) develop upon strong TCR signals that induce CD25 expression whereas Foxp3lo Treg precursors (Foxp3lo TregP) develop upon weaker TCR signals, which drive the upregulation of Foxp3. Further development of these two precursors requires survival signals mediated by γc cytokine signaling. IL-2 produced by DC and self-reactive CD4+ SP thymocytes and IL-15 produced by mTEC rescue Treg precursors from cell death and lead to mature CD25+Foxp3+ Treg development. Interestingly, mature CD25+Foxp3+ Treg appear to have distinct functional properties according to the precursors from which they are derived. CD25−Foxp3lo cells also likely contain a subset of mature Treg that egress to the periphery (dotted line). Mature Treg also encompass Triplehi (GITRhiPD1hiCD25hi) and Triplelo (GITRloPD1loCD25lo) Treg subsets, which show distinct functional activities.
The second step of Treg development that drives the maturation of CD25+ TregP into mature CD25+Foxp3+ Treg depends on γ-chain cytokines, in particular IL-2 and/or IL-15 signaling (Figure 1) (41–43). Mice deficient for the IL-2rγ chain, which is shared by IL-2,-4,-7,-9,-15, and -21 receptors, lack this cell type (42, 44). However, mice deficient for IL-2 or IL-2rα display only half of Foxp3+ Treg, indicating that IL-2 plays a major role in Treg development (44). Furthermore, mice lacking the IL-2rβ chain, shared by IL-2 and IL-15 receptors, were also found to almost lack mature Treg (42, 44, 45). Accordingly, the generation of IL-2 × IL-15 double-deficient mice has revealed a similar Treg defect as that observed in Il2rβ−/− mice, indicating that IL-15 is also implicated in Treg cell development (42, 46, 47). These two cytokines promote survival and thus rescue Treg from the pro-apoptotic effects of Foxp3 (48). In addition, the stimulation of GITR, OX40, or TNFR2 participates in this second step by enhancing the conversion of CD25+ TregP into mature Treg through an increase responsiveness to IL-2 (39).
Recent advances have shed new light on the cellular source of IL-2 and IL-15 cytokines. DC have been initially proposed to constitute a privileged developmental niche for Treg cell generation by providing both cognate self-antigens and IL-2 (49). However, this concept has been recently challenged. Although IL-2 can be produced by thymic DC, the selective ablation of the Il2 gene in DC and T cells in the absence of IL-15 revealed that only IL-2 provided by T cells severely impairs Treg development (50). Accordingly, IL-2 production was more precisely assigned to self-reactive CD4+ thymocytes, including Treg precursors (51). These observations suggest that the size of the Treg cell population is regulated by self-reactive thymocytes through IL-2 production. In contrast to IL-2, IL-15 is provided by stromal cells, mainly TEC including cTEC and mTEC expressing both low and high levels of MHC class II molecules (46, 52, 53).
A second developmental pathway has recently emerged based on the identification of another Treg precursor subset expressing low level of Foxp3 and lacking CD25 cell surface expression (Foxp3lo TregP) (46, 54). Similarly to CD25+ TregP, a substantial proportion of Foxp3lo TregP differentiates into mature Treg upon intrathymic transfer. These observations indicate that both CD25+ and Foxp3lo TregP contribute to the generation of the mature Treg pool. Foxp3lo TregP seems to depend on IL-15 for their survival, and then on IL-2 to fully differentiate into mature Treg cells whereas CD25+ TregP likely depend on IL-2 for their development. Treg cells are thus generated via two distinct developmental programs involving CD25+ and Foxp3lo TregP (54) (Figure 1). CD25+ TregP show a higher affinity for self-antigens than Foxp3lo TregP. Accordingly, these two precursors exhibit a distinct TCR repertoire and thus make unique contribution to the mature Treg TCR repertoire. Furthermore, they have distinct transcriptomes, regulated by different signaling pathways and enhancers. The NFκB signaling pathway and the Foxp3 regulatory element Cns3 were found to be required for the development of Foxp3lo TregP but dispensable for CD25+ TregP. Interestingly, although these two precursors can give rise to mature Treg, they have distinct functional capacities. Treg derived from CD25+ TregP protect from experimental auto-immune encephalomyelitis, whereas those derived from Foxp3lo TregP protect from T-cell induced colitis. These observations open new perspectives to treat autoimmune disorders by Treg-cell based therapy, which is currently extremely promising in clinical trials (55). Moreover, single-cell transcriptomic analysis suggests the presence of CD25+ and Foxp3lo TregP in the human thymus (56), indicating conserved developmental pathways, although further investigations are required.
The discovery of these two Treg cell precursors raises the question about the existence of a common or a distinct pre-precursor. It has been recently proposed that CD4+GITR+CD122+CD25−Foxp3− cells represent a Treg pre-precursor population, which remains to be clearly established (57, 58). Furthermore, CD25−Foxp3lo cells seem to be at a more mature stage than CD25+Foxp3− cells based on their CD24loQa2hi phenotype or their “thymic age” measured by their green fluorescent protein (GFP) expression level using transgenic mice expressing GFP under the recombination-activating gene 2 promoter (Rag2-GFP model) (59). These observations could be due to a later emergence of these cells during T-cell development or by the presence of mature developing Treg among this population as suggested by other experiments (60). In accordance with this latter hypothesis, a substantial fraction of RTE and functional peripheral Treg are CD25−Foxp3+ cells (7, 46, 61). Thus, further investigations are required to better define the cellular composition of the CD25−Foxp3lo compartment.
Two mature Treg cell subsets were also identified based on the expression level of CD25, GITR, and PD-1 and were defined as GITRloPD-1loCD25lo (Triplelo) and GITRhiPD-1hiCD25hi (Triplehi) cells (61). These cells develop within the thymus and show a distinct TCR repertoire with different affinities for self-antigens with Triplehi Treg being more self-reactive than Triplelo Treg. Interestingly, Triplehi Treg control the lympho-proliferation in peripheral lymph nodes, suggesting an ability to protect against auto-immune disorders, whereas Triplelo Treg limit the development of colitis by promoting the conversion of conventional CD4+ T cells into pTreg. Nevertheless, their respective relationship with CD25+ and Foxp3lo TregP remains to be established. Therefore, these recent advances on Treg development have revealed a developmentally regulated heterogeneity in the thymic Treg population (Figure 1). These findings thus indicate that the thymus generates multiple Treg subsets with non-overlapping regulatory activities.
Heterogeneity and Function of Recirculating Thymic Treg Cells
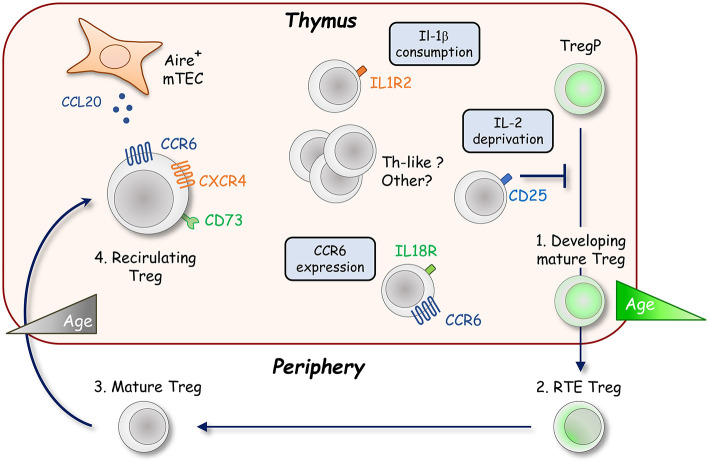
Although thymic Treg were thought to represent a homogenous population of developing cells for a long time, several recent studies have described the presence of mature cells derived from the periphery or in retention upon their development in the thymus. The homing of mature peripheral Treg into the thymus was initially reported in the context of parabiosis experiments and lymphopenic mice (62, 63). The generation of Rag2-GFP mice was particularly useful to distinguish developing Rag2-GFP+ Treg and older Rag2-GFP− Treg (59). After positive selection, the expression of the Rag2 gene stops and GFP undergoes exponential decay with a half-life of ~56 h (64). Using this model, a consequent fraction of thymic Treg were found to be Rag2-GFP− cells (65). It was later demonstrated that these cells contain peripheral Treg migrating back to their organ of origin (Figure 2) (66, 67). Some Rag2-GFP− Treg can also correspond to resident cells that have never left the thymus, making more complex the heterogeneity of this mature Treg population (68). Interestingly, mature Rag2-GFP− Treg represent around half of the thymic Treg pool in 8 week-old B6 mice, and almost their totality in 6 month-old B6 mice, unraveling an age-dependent accumulation of these cells within the thymus (66, 68). These cells display an activated and effector phenotype, which is required for their migration into the thymus (69). Their trafficking is likely directed by CXCR4/CXCL12 and CCR6/CCL20 chemokine axes (66, 70). CCL20 is mainly produced by mTEC under the control of the transcription factor Aire (Autoimmune regulator), which is crucial for the establishment of T-cell tolerance (71). The CCR6/CCL20 axis could thus be responsible of the almost exclusive medullary localization of recirculating Treg (69, 70). It was recently described that the IL-18 stimulation of peripheral IL-18R-expressing Treg allows the expression of CCR6 that favors their recirculation into the thymus (72). In addition to CCR6, CD73 was found to discriminate mature Rag2-GFP− Treg from newly produced Rag2-GFP+ Treg (54, 69). Recirculating mature Treg can thus be identified as CCR6+CD73+ in the absence of Rag2-GFP transgenic reporter mice (Figure 2).
Figure 2.
Recirculation of peripheral Foxp3+ Treg into the thymus. The thymic Foxp3+ Treg population is not only composed of developing Rag2-GFP+ Treg (green cell) but also contains a large fraction of recirculating mature Rag2-GFP− Treg from the periphery showing an activated phenotype (gray cells). The recirculation of peripheral Treg likely depends on CCR6 and CXCR4 chemokine receptors. These cells mainly localize into the thymic medulla in response to the production of the CCR6 ligand, CCL20, by Aire+ mTEC. Once in the thymus, recirculating CCR6+CD73+ Treg inhibit the development of de novo CD25+Foxp3+ Treg in an age dependent manner by IL-2 deprivation. Distinct subsets of recirculating Treg were recently described such as IL-1R2+ Treg that maintain Treg development by quenching IL-1β under inflammatory conditions. An IL-18R+ subset, endowed with a higher ability to recirculate into the thymus than their IL-18R− Treg counterparts, was also described. In this subset, IL-18 signaling likely promotes the thymic entry of peripheral Treg by upregulating CCR6 expression.
However, is this recirculation an epiphenomenon or do these cells have a function in the thymus? The medullary localization, the effector phenotype and the accumulation of mature Rag2-GFP− Treg during aging that occurs concomitantly with the reduced development of Rag2-GFP+ Treg suggests a suppressive function of these cells. In line with this hypothesis, the addition of mature Treg either to fetal thymic organ cultures (FTOC) or adult thymic tissue slices inhibits de novo Treg production by consuming locally available IL-2 (49, 66). Accordingly, an increase in Treg development is observed if the amount of recirculating Treg is naturally (different mouse strains) or artificially (peripheral CD4+ T-cell depletion) diminished (69). Thus, the thymic Treg niche seems to be stable with age, but dynamic in its composition and regulated by the amount of mature Treg that repopulate the thymus. This feedback loop regulation of Treg production by recirculating cells has been recently challenged by observations made in Aire−/−, Ccr6−/−, and Il18r1−/− mice, which all show a decrease in Treg recirculation with no obvious impact on de novo generated Treg cells (70, 72). These observations could question the dynamic balance between developing and recirculating Treg. However, Treg recirculation is only partially abrogated in these mice and it cannot be excluded that the remaining recirculating mature Treg cells are able to inhibit Treg production. Furthermore, since numbers of Aire+ mTEC were reported to influence antigen-mediated Treg selection, the expected stronger Treg development in Aire−/− mice could be hidden by impaired basal Treg induction, which was described in neonatal mice lacking recirculating Treg (73). Conversely, mice deficient for OPG (osteoprotegerin; encoded by Tnfrsf11b), the soluble decoy receptor for RANKL (receptor activator of nuclear factor-κB ligand; encoded by Tnfsf11), which controls the development of Aire+ mTEC, show an increase Treg recirculation with no defect in de novo Treg production (74). Nevertheless, the strong inhibition by recirculating Treg in these mice could be counterbalanced by an increased development of de novo Treg mediated by exacerbated Aire+ mTEC numbers (27, 75–77). Further studies are definitely required to clarify the impact of peripheral Treg on de novo Treg production. The generation of transgenic mice either fully lacking recirculating Treg, or with adjustable amount of recirculating Treg into the thymus, is expected to clarify this issue.
Since Treg promote the homeostasis of several other tissues such as skeletal muscle, visceral adipose tissue, lung, central nervous system, and skin (5, 6), recirculating Treg could also participate in thymus homeostasis. In particular, the crosstalk with CD4+ SP thymocytes controls the development of Aire+ mTEC that are responsible for peripheral Treg re-entry into the thymus (78, 79). Since recirculating Treg express RANKL and that the development of Aire+ mTEC is mediated by the RANK-RANKL signaling pathway (75, 79–81), it would be of special interest to define whether recirculating Treg are in turn implicated in the regulation of Aire+ mTEC cellularity or other stromal cells throughout lifespan. In line with this hypothesis, excessive RANK signaling driven by activated CD4+ T cells has been reported to enhance Aire+ mTEC development and in fine promote the exhaustion of epithelial progenitors (82). Further investigations are required to evaluate the function of mature recirculating Treg in the thymus. One important step before is undoubtedly to better characterize the cell subsets that reside within the recirculating mature Treg compartment. Similarly to developing Treg, a heterogeneity is emerging among recirculating mature Treg. Two subsets, IL-18R−, and IL-18R+ cells, the latter showing molecular similarities with tissue-resident effector Treg, have been described (72). Based on the differential expression of the decoy receptor for the inflammatory cytokine IL-1, IL-1R2−, and IL-1R2+ recirculating Treg have been also reported with IL-1R2+ Treg protecting Treg development under inflammatory conditions by quenching IL-1 (83). Furthermore, the Triplehi and Triplelo subsets described in the developing Treg compartment were also observed among recirculating Treg (61). However, the relationship between IL-18R−, IL-18R+, IL-1R2−, IL-1R2+, Triplehi, and Triplelo subsets in recirculating Treg remain to be determined. Interestingly, high-throughput RNA sequencing on the bulk of Rag2-GFP− Treg revealed a high expression for many genes specific of Th1-, Th2-, Th17-, Tfr-like Treg and markers found in adipose- and muscle-tissue Treg (Figure 2) (66). These observations suggest a greater heterogeneity in the Rag2-GFP− Treg compartment than currently described. Single cell transcriptomic analysis of recirculating Treg is expected to shed new light on the composition of distinct effector cells that resides in this compartment. Interestingly, peripheral Treg also likely migrate back to the thymus in humans, indicative of a conserved mechanism. These cells, identified as CD31−, a marker expressed by developing thymocytes and RTE and downregulated during peripheral maturation, display an activated and differentiated phenotype (66). Further investigations are now required to better understand human mature Treg heterogeneity and functionality.
Concluding Remarks
Recent advances have highlighted an unsuspected heterogeneity in the thymic Treg cell population, including in developing and recirculating Treg. Given that at least two precursors give rise to several subsets of mature Foxp3+ Treg, with distinct abilities to suppress autoimmunity, it would be not surprising that a functional imprinting is acquired from Treg cell development in the thymus. Beyond the TCR repertoire, this imprinting could be driven in specific niches providing different molecular and cellular cues such as cytokines, TNFRSF signals, and APC subsets. A heterogeneity in the recirculating Treg compartment is also currently emerging, opening new interesting questions on their tissue origin and their respective roles in the thymic function. Furthermore, it remains to be determined whether different recirculating subsets could interact with distinct stromal cells and regulate the thymic selection, not only of Treg, but also of conventional and unconventional T cells. Moreover, recirculating Treg could participate to the homeostasis of the thymus similarly to their peripheral counterparts. Finally, future investigations aimed at deciphering the developmental pathways of specific functional Treg subsets are expected to elaborate personalized Treg therapies in the context of auto-immunity, inflammation, and regenerative medicine.
Author Contributions
JS and MI conceived and wrote the manuscript. AB critically read and discussed the content prior to submission. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work received funding from Excellence Initiative of Aix-Marseille University-A*Midex, a French Investissements d'avenir program (LTalpha-Treg to MI) and the Agence Nationale de la Recherche (grant ANR-19-CE18-0021-01, RANKLthym to MI) and was supported by institutional grants from Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique and Aix-Marseille Université. AB was supported by a Ph.D. fellowship from the Ministère de l'Enseignement Supérieur et de la Recherche.
References
- 1.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, et al. JM2, encoding a fork head–related protein, is mutated in X-linked autoimmunity–allergic disregulation syndrome. J Clin Invest. (2000) 106:R75–81. 10.1172/JCI11679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. (2001) 27:20–1. 10.1038/83713 [DOI] [PubMed] [Google Scholar]
- 3.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. (2001) 27:18–20. 10.1038/83707 [DOI] [PubMed] [Google Scholar]
- 4.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko S, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nature. (2001) 27:68–73. 10.1038/83784 [DOI] [PubMed] [Google Scholar]
- 5.Panduro M, Benoist C, Mathis D. Tissue tregs. Annu Rev Immunol. (2016) 34:609–33. 10.1146/annurev-immunol-032712-095948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma A, Rudra D. Emerging functions of regulatory T cells in tissue homeostasis. Front Immunol. (2018) 9:1–26. 10.3389/fimmu.2018.00883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smigiel KS, Richards E, Srivastava S, Thomas KR, Dudda JC, Klonowski KD, et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J Exp Med. (2014) 211:121–36. 10.1084/jem.20131142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cretney E, Kallies A, Nutt SL. Differentiation and function of Foxp3+ effector regulatory T cells. Trends Immunol. (2013) 34:74–80. 10.1016/j.it.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 9.Savage PA, Klawon DEJ, Miller CH. Regulatory T cell development. Annu Rev Immunol. (2020) 38:421–53. 10.1146/annurev-immunol-100219-020937 [DOI] [PubMed] [Google Scholar]
- 10.Paiva RS, Lino AC, Bergman M-L, Caramalho Í, Sousa AE, Zelenay S, et al. Recent thymic emigrants are the preferential precursors of regulatory T cells differentiated in the periphery. Proc Natl Acad Sci USA. (2013) 110:6494–9. 10.1073/pnas.1221955110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schallenberg S, Tsai P-Y, Riewaldt J, Kretschmer K. Identification of an immediate Foxp3- precursor to Foxp3+ regulatory T cells in peripheral lymphoid organs of nonmanipulated mice. J Exp Med. (2010) 207:1393–407. 10.1084/jem.20100045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalekar LA, Schmiel SE, Nandiwada SL, Lam WY, Barsness LO, Zhang N, et al. CD4+ T cell anergy prevents autoimmunity and generates regulatory T cell precursors. Nat Immunol. (2016) 17:304–14. 10.1038/ni.3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlenner SM, Weigmann B, Ruan Q, Chen Y, von Boehmer H. Smad3 binding to the Foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut. J Exp Med. (2012) 209:1529–35. 10.1084/jem.20112646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Alexander R. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflic. Cell. (2012) 150:29–38. 10.1016/j.cell.2012.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. (2012) 482:395–9. 10.1038/nature10772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohnmacht C, Park J-H, Cording S, Wing JB, Atarashi K, Obata Y, et al. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORγt+ T cells. Science. (2015) 349:989–93. 10.1126/science.aac4263 [DOI] [PubMed] [Google Scholar]
- 17.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio C-W, Santacruz N, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. (2011) 478:250–4. 10.1038/nature10434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller J. Effect of neonatal thymectomy on the immunological responsiveness of the mouse. Proc R Soc Lond B Biol Sci. (1962) 156:415–28. 10.1098/rspb.1962.0048 [DOI] [Google Scholar]
- 19.Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. (1969) 166:753–5. 10.1126/science.166.3906.753 [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune disease induced in mice by elimination of Y cell subset. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp. (1985) 161:72–87. 10.1084/jem.161.1.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. (1995) 155:1151–64. 10.4049/jimmunol.0903301 [DOI] [PubMed] [Google Scholar]
- 22.Ohki H, Martin C, Corbel C, Coltey M, Le Douarin NN. Tolerance induced by thymic epithelial grafts in birds. Science. (1987) 237:1032–5. 10.1126/science.3616623 [DOI] [PubMed] [Google Scholar]
- 23.Modigliani Y, Thomas-vaslintt V, Bandeira A, Colteyt M, Douarint NMLE, Coutinho A, et al. Lymphocytes selected in allogeneic thymic epithelium mediate dominant tolerance toward tissue grafts of the thymic epithelium haplotype. Proc Natl Acad Sci USA. (1995) 92:7555–9. 10.1073/pnas.92.16.7555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in postthymectomy autoimmune oophoritis in mice II. Requirement of Lyt-1 cells in normal female mice for the prevention of oophoritis. J Exp Med. (1982) 156:1577–86. 10.1084/jem.156.6.1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owen DL, Sjaastad LE, Farrar MA. Regulatory T cell development in the thymus. J Immunol. (2019) 203:2031–41. 10.4049/jimmunol.1900662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein L, Robey EA, Hsieh CS. Central CD4+T cell tolerance: deletion versus regulatory T cell differentiation. Nat Rev Immunol. (2019) 19:7–18. 10.1038/s41577-018-0083-6 [DOI] [PubMed] [Google Scholar]
- 27.Perry JSA, Lio CWJ, Kau AL, Nutsch K, Yang Z, Gordon JI, et al. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity. (2014) 41:414–26. 10.1016/j.immuni.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. (2001) 2:301–6. 10.1038/86302 [DOI] [PubMed] [Google Scholar]
- 29.Romagnoli P, Hudrisier D, van Meerwijk JPM. Preferential recognition of self antigens despite normal thymic deletion of CD4+CD25+ regulatory T cells. J Immunol. (2002) 168:1644–8. 10.4049/jimmunol.168.4.1644 [DOI] [PubMed] [Google Scholar]
- 30.Hsieh C, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. (2006) 7:401–10. 10.1038/ni1318 [DOI] [PubMed] [Google Scholar]
- 31.Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity. (2006) 25:249–59. 10.1016/j.immuni.2006.05.016 [DOI] [PubMed] [Google Scholar]
- 32.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. (2004) 21:267–77. 10.1016/j.immuni.2004.07.009 [DOI] [PubMed] [Google Scholar]
- 33.Lee H, Bautista JL, Scott-browne J, Mohan JF, Hsieh C. A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self. Immunity. (2012) 37:475–86. 10.1016/j.immuni.2012.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malhotra D, Linehan JL, Dileepan T, Lee YJ, Purtha WE, Lu JV, et al. Tolerance is established in polyclonal CD4+T cells by distinct mechanisms, according to self-peptide expression patterns. Nat Immunol. (2016) 17:187–95. 10.1038/ni.3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Legoux FP, Lim JB, Cauley AW, Dikiy S, Ertelt J, Mariani TJ, et al. CD4+T cell tolerance to tissue-restricted self antigens is mediated by antigen-specific regulatory T cells rather than deletion. Immunity. (2015) 43:896–908. 10.1016/j.immuni.2015.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, et al. B7/CD28 Costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. (2000) 12:431–40. 10.1016/S1074-7613(00)80195-8 [DOI] [PubMed] [Google Scholar]
- 37.Vang KB, Yang J, Pagán AJ, Li L-X, Wang J, Green JM, et al. Cutting edge: CD28 and c-Rel–dependent pathways initiate regulatory T cell development. J Immunol. (2010) 184:4074–7. 10.4049/jimmunol.0903933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lio C-WJ, Dodson LF, Deppong CM, Hsieh C-S, Green JM. CD28 facilitates the generation of Foxp3− cytokine responsive regulatory T cell precursors. J Immunol. (2010) 184:6007–13. 10.4049/jimmunol.1000019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahmud SA, Manlove LS, Schmitz HM, Xing Y, Wang Y, Owen DL, et al. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat Immunol. (2014) 15:473–81. 10.1038/ni.2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tai X, Michelle C, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. (2005) 6:152–62. 10.1038/ni1160 [DOI] [PubMed] [Google Scholar]
- 41.Lio CWJ, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. (2008) 28:100–11. 10.1016/j.immuni.2007.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. (2007) 178:280–90. 10.4049/jimmunol.178.1.280 [DOI] [PubMed] [Google Scholar]
- 43.Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. (2008) 28:112–21. 10.1016/j.immuni.2007.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. (2005) 6:1142–51. 10.1038/ni1263 [DOI] [PubMed] [Google Scholar]
- 45.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rb-deficient mice: implications for the nonredundant function of IL-2. Immunity. (2002) 17:167–78. 10.1016/S1074-7613(02)00367-9 [DOI] [PubMed] [Google Scholar]
- 46.Marshall D, Sinclair C, Tung S, Marshall D, Sinclair C, Tung S, et al. Differential requirement for IL-2 and IL-15 during bifurcated development of thymic regulatory T cells. J Immunol. (2014) 193:5525–33. 10.4049/jimmunol.1402144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Michael A, et al. IL-2,−7, and−15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+regulatory T cell development. J Immunol. (2008) 181:3285–90. 10.4049/jimmunol.181.5.3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tai X, Erman B, Alag A, Mu J, Kimura M, Katz G, et al. Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity. (2013) 38:1116–28. 10.1016/j.immuni.2013.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weist BM, Kurd N, Boussier J, Chan SW, Robey EA. Thymic regulatory T cell niche size is dictated by limiting IL-2 from antigen-bearing dendritic cells and feedback competition. Nat Immunol. (2015) 16:635–41. 10.1038/ni.3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Owen DL, Mahmud SA, Vang KB, Kelly RM, Blazar BR, Smith KA, et al. Identification of cellular sources of IL-2 needed for regulatory T cell development and homeostasis. J Immunol. (2018) 200:3926–33. 10.4049/jimmunol.1800097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hemmers S, Schizas M, Azizi E, Dikiy S, Zhong Y, Feng Y, et al. IL-2 production by self-reactive CD4 thymocytes scales regulatory T cell generation in the thymus. J Exp Med. (2019) 216:2466–78. 10.1084/jem.20190993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cui G, Hara T, Simmons S, Wagatsuma K, Abe A, Miyachi H, et al. Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo. Proc Natl Acad Sci USA. (2013) 111:1915–20. 10.1073/pnas.1318281111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White AJ, Jenkinson WE, Cowan JE, Parnell SM, Bacon A, Jones ND, et al. An essential role for medullary thymic epithelial cells during the intrathymic development of invariant NKT cells. J Immunol. (2014) 192:2659–66. 10.4049/jimmunol.1303057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Owen DL, Mahmud SA, Sjaastad LE, Williams JB, Spanier JA, Simeonov DR, et al. Thymic regulatory T cells arise via two distinct developmental programs. Nat Immunol. (2019) 20:195–205. 10.1038/s41590-018-0289-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferreira LMR, Muller YD, Bluestone JA, Tang Q. Next-generation regulatory T cell therapy. Nat Rev Drug Discov. (2019) 18:749–69. 10.1038/s41573-019-0041-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park J-E, Botting RA, Conde CD, Popescu D-M, Lavaert M, Kunz DJ, et al. A cell atlas of human thymic development defines T cell repertoire formation. Science. (2020) 367:eaay3224. 10.1101/2020.01.28.911115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuster M, Plaza-sirvent C, Matthies A, Heise U, Jeron A, Bruder D, et al. c-REL and IκBNS govern common and independent steps of regulatory T cell development from novel CD122-expressing pre-precursors. J Immunol. (2017) 199:920–30. 10.4049/jimmunol.1600877 [DOI] [PubMed] [Google Scholar]
- 58.Schuster M, Plaza-sirvent C, Visekruna A, Huehn J, Schmitz I. Generation of Foxp3+CD25– regulatory T-cell precursors requires c-rel and IκBNS. Front Immunol. (2019) 10:1–6. 10.3389/fimmu.2019.01583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu W, Nagaoka H, Jankovic M, Misulovin Z, Suh H, Rolink A, et al. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. (1999) 400:682–7. 10.1038/23287 [DOI] [PubMed] [Google Scholar]
- 60.Bending D, Martín PP, Paduraru A, Ducker C, Marzaganov E, Laviron M, et al. A timer for analyzing temporally dynamic changes in transcription during differentiation in vivo. J Cell Biol. (2018) 217:2931–50. 10.1083/jcb.201711048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wyss L, Stadinski BD, King CG, Schallenberg S, McCarthy NI, Lee JY, et al. Affinity for self antigen selects Treg cells with distinct functional properties. Nat Immunol. (2016) 17:1093–101. 10.1038/ni.3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bosco N, Agenes F, Rolink AG, Ceredig R. Peripheral T cell lymphopenia and concomitant enrichment in naturally arising regulatory T cells: the case of the pre-Talpha gene-deleted mouse. J Immunol. (2006) 177:5014–23. 10.4049/jimmunol.177.8.5014 [DOI] [PubMed] [Google Scholar]
- 63.Zhan Y, Bourges D, Dromey JA, Harrison LC, Lew AM. The origin of thymic CD4+CD25+regulatory T cells and their co-stimulatory requirements are determined after elimination of recirculating peripheral CD4+cells. Int Immunol. (2007) 19:455–63. 10.1093/intimm/dxm010 [DOI] [PubMed] [Google Scholar]
- 64.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. (2004) 5:418–25. 10.1038/ni1049 [DOI] [PubMed] [Google Scholar]
- 65.McCaughtry TM, Wilken MS, Hogquist KA. Thymic emigration revisited. J Exp Med. (2007) 204:2513–20. 10.1084/jem.20070601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thiault N, Darrigues J, Adoue V, Gros M, Binet B, Perals C, et al. Peripheral regulatory T lymphocytes recirculating to the thymus suppress the development of their precursors. Nat Immunol. (2015) 16:628–34. 10.1038/ni.3150 [DOI] [PubMed] [Google Scholar]
- 67.Cowan JE, McCarthy NI, Anderson G. CCR7 controls thymus recirculation, but not production and emigration, of Foxp3+T cells. Cell Rep. (2016) 14:1041–8. 10.1016/j.celrep.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang EJ, Zou T, Leichner TM, Zhang SL, Kambayashi T. Both retention and recirculation contribute to long-lived regulatory T-cell accumulation in the thymus. Eur J Immunol. (2014) 44:2712–20. 10.1002/eji.201444529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Darrigues J, Santamaria JC, Galindo-Albarrán A, Robey EA, Joffre OP, Meerwijk JPM, et al. Robust intrathymic development of regulatory T cells in young NOD mice is rapidly restrained by recirculating cells. Eur J Immunol. (2020). 10.1002/eji.202048743. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 70.Cowan JE, Baik S, McCarthy NI, Parnell SM, White AJ, Jenkinson WE, et al. Aire controls the recirculation of murine Foxp3+regulatory T-cells back to the thymus. Eur J Immunol. (2018) 48:844–54. 10.1002/eji.201747375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perniola R. Twenty years of AIRE. Front Immunol. (2018) 9:98 10.3389/fimmu.2018.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peligero-Cruz C, Givony T, Sebé-Pedrós A, Dobeš J, Kadouri N, Nevo S, et al. IL18 signaling promotes homing of mature Tregs into the thymus. Elife. (2020) 9:e58213. 10.7554/eLife.58213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. (2015) 348:589–94. 10.1126/science.aaa7017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCarthy NI, Cowan JE, Nakamura K, Bacon A, Baik S, White AJ, et al. Osteoprotegerin-mediated homeostasis of rank+thymic epithelial cells does not limit Foxp3+regulatory T cell development. J Immunol. (2015) 195:2675–82. 10.4049/jimmunol.1501226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, et al. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. (2008) 29:438–50. 10.1016/j.immuni.2008.06.018 [DOI] [PubMed] [Google Scholar]
- 76.Malchow S, Leventhal DS, Lee V, Nishi S, Socci ND, Savage PA. Aire enforces immune tolerance by directing autoreactive T cells into the regulatory T cell lineage. Immunity. (2016) 44:1102–13. 10.1016/j.immuni.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khan IS, Mouchess ML, Zhu ML, Conley B, Fasano KJ, Hou Y. Enhancement of an anti-tumor immune response by transient blockade of central T cell tolerance. J Exp Med. (2014) 211:761–8. 10.1084/jem.20131889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, Williams IR, et al. Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity. (2008) 29:451–63. 10.1016/j.immuni.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 79.Lopes N, Sergé A, Ferrier P, Irla M. Thymic crosstalk coordinates medulla organization and T-cell. Front Immunol. (2015) 6:365. 10.3389/fimmu.2015.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Desanti GE, Cowan JE, Baik S, Parnell SM, White AJ, Penninger JM, et al. Developmentally regulated availability of RANKL and CD40 ligand reveals distinct mechanisms of fetal and adult cross-talk in the thymus medulla. J Immunol. (2012) 189:5519–26. 10.4049/jimmunol.1201815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Irla M. RANK signaling in the differenciation and regeneration of thymic epithelial cells. Front Immunol. (2021) 11:3592. 10.3389/fimmu.2020.623265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yin C, Pei XY, Shen H, Gao YN, Sun XY, Wang W, et al. Thymic homing of activated CD4+T cells induces degeneration of the thymic epithelium through excessive RANK signaling. Sci Rep. (2017) 7:1–12. 10.1038/s41598-017-02653-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nikolouli E, Elfaki Y, Herppich S, Schelmbauer C, Delacher M, Falk C, et al. Recirculating IL-1R2+mtecs fi ne-tune intrathymic Treg development under inflammatory conditions. Cell Mol Immunol. (2020) 18:182–93. 10.1038/s41423-019-0352-8 [DOI] [PMC free article] [PubMed] [Google Scholar]