Abstract
Background:
People with PD who exhibit freezing of gait (FOG) also exhibit poor balance compared to those who do not freeze. However, balance is a broad construct that can be subdivided into subdomains that include dynamic balance (gait), anticipatory postural adjustments (APAs) & gait initiation, postural sway in stance, and automatic postural responses (e.g., reactive stepping). Few studies have provided a robust investigation on how each of these domains is impacted by FOG, and no studies have compared balance across groups while rigorously controlling for disease severity.
Methods:
Structural equation modeling was used to evaluate the relationships between FOG and balance domains constructed as latent variables and controlling for disease severity. Domains included: dynamic balance (gait), APAs, postural sway, and reactive stepping. Models were run relating domains to both the presence and severity of FOG.
Results:
Latent variables reflecting domains of Gait and APAs, but not postural sway or reactive stepping, were significantly related to the severity of FOG. Models for presence of FOG showed the same results, as Gait and APAs, but not postural sway or reactive stepping, were related to presence of FOG.
Conclusion:
These results are consistent with hypotheses that balance deficits in people with PD who freeze are most pronounced in gait and anticipatory postural adjustments. Reactive stepping and postural control domains are less effected. These findings suggest that rehabilitative strategies focused on gait and APAs may be most effective for people with PD who freeze.
Keywords: Posture, Gait, Freezing of Gait, Parkinson’s disease, Postural Instability
INTRODUCTION
Freezing of gait (FOG) has substantial negative impact on quality of life in people with Parkinson’s disease (PD) and is inadequately controlled by pharmacological, rehabilitative, or surgical treatments[1]. FOG is closely linked to falls [2] and, like falls, is a transient symptom that occurs more frequently under certain circumstances (e.g., while turning, stressed, etc.[1]). Determining which specific balance and/or gait characteristics are impaired in people with FOG may provide a deeper understanding of potential mechanisms of FOG and may facilitate identification of rehabilitative targets for FOG.
Previous studies have identified a robust relationship between the postural instability and gait dysfunction (PIGD) phenotype and FOG (e.g. [3]). However, postural instability and gait represents a broad and complex suite of abilities. Mancini, Nutt and Horak (2019) proposed four domains of balance, each of which are affected in PD, including balance during stance (i.e., postural sway), automatic postural responses, anticipatory postural adjustments (APAs), and dynamic balance during walking (i.e., gait)[4]. Recent studies have measured individual domains of balance in PD participants who do and do not freeze to facilitate a better understanding of the relationship between FOG and these specific signs (e.g.[5]). In 2018, Bekkers & colleagues conducted a narrative review to consolidate results of these studies. While results were markedly variable, weight shifts (APAs) and dynamic balance (i.e., gait), were consistently worse in people with PD and FOG compared to whose without FOG, while reactive postural adjustments and static postural control (i.e., quiet stance) were not typically different across these groups[6].
However, this literature has several limitations. First, each study typically measured balance outcomes in a single outcome or domain, making across-domain comparisons difficult. Second, studies were of relatively small samples (typically 10 and 20 participants per group), limiting generalizability of findings. Third, outcomes were assessed in freezing and non-freezing groups based on the presence of self-identified FOG symptoms. While this is a standard and commonly used approach, categorizing individuals into those who do and do not freeze can be challenging, especially considering that patients with PD often present with cognitive disturbances and may not be aware of FOG symptoms. Relating mobility outcomes to a continuous, objective measure of FOG may provide more sensitive and reliable relationships to balance domains. Fourth, FOG becomes more common later in the course of PD. Therefore, controlling for disease severity is critical to reduce the chance of parkinsonism severity confounding the relationship between FOG and posture and gait outcomes. Indeed, Bekkers et al. indicated that only 3 of the 30 studies included in the review controlled for PD severity[6].
The purpose of the current study is to determine the relationship between both the presence and severity of FOG and specific, objectively defined domains of balance, accounting for disease severity. This topic is relevant for at least two reasons. First, it is plausible that there may be a direct relationship between balance and FOG episodes, such that poor balance may contribute to precipitation of a FOG event. If so, interventions aimed at improving relevant aspects of balance and/or gait may reduce FOG frequency. Second, characterizing which aspects of balance are related to FOG severity can provide a deeper understanding of the progression and occurrence of FOG. Based on previous work[6], we hypothesized that outcomes related to gait (measured primarily as pace and variability) and anticipatory postural adjustments (weight-shifting) would be most closely linked to presence and severity of FOG.
METHOD
Participants
Participant characteristics are shown in Table 1. 144 participants were recruited through physician referral at OHSU, local patient support groups, and fliers placed throughout the community. Inclusion criteria were: aged 50–90 years, ability to stand and walk unassisted, meet Brain Bank Criteria for idiopathic PD[7] and six weeks of stable medications. Exclusion criteria were: major musculoskeletal or peripheral disorders that could impact balance or gait and any non-PD neurological disorders and inability to follow instructions. The present work is a secondary analysis of baseline data collected as part of a clinical trial (ClinicalTrials.gov NCT02231073 and NCT02236286), as well as additional, cross-sectionally collected data. Portions of these data have been examined previously without a focus on FOG [8, 9]. Also, a freezing/non-freezing comparison has been conducted with a portion (n=56, FOG-26, non-FOG-30) of the current dataset [10]. However, in addition to the smaller sample, the analyses and focus of this previous manuscript were distinct from the current report.
Table 1:
Participant characteristics for people with PD who did (FOG+) and did not (FOG−) experience freezing of gait.
| FOG+ (n=64) | FOG− (n=80) | ||||
|---|---|---|---|---|---|
| Mean | STD | Mean | STD | p | |
| Male Gender (%) | 44 | 68.80 | 49 | 61.3 | 0.384 |
| Age (y) | 68.06 | 8.04 | 68.75 | 8.04 | 0.611 |
| Disease Duration (y) | 7.8 | 5.4 | 5.0 | 4.2 | 0.001 |
| LEDD | 868.6 | 1355.0 | 609.3 | 416.8 | 0.176 |
| ABC (%) | 73.75 | 17.57 | 85.79 | 13.00 | 0.000 |
| MDS-UPDRS | 77.58 | 20.64 | 60.05 | 17.26 | 0.000 |
| MiniBESTest | 17.19 | 5.40 | 19.68 | 4.08 | 0.003 |
| MoCA | 25.49 | 3.78 | 25.99 | 3.06 | 0.400 |
| NFOGQ | 12.10 | 7.09 | -- | -- | -- |
| FOG ratio * | 2.64 | 6.03 | 0.68 | 0.76 | 0.001* |
LEDD: Levodopa Equivalent Daily Dose, ABC: Activities of Balance Confidence; MDS-UPDRS: Movement Disorders Society Unified Parkinson’s Disease Rating Scale, MoCA: Montreal Cognitive Assessment; NFOGQ: New FOG Questionnaire;
Mann-Whitney U Test
Procedures
Clinical scales were administered while subjects were in their practical Off state (at least 12 hours after their last dose of Levodopa), and included: Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS)[11], the Activities-specific Balance Confidence scale (ABC-scale)[12], and the Montreal Cognitive Assessment (MoCA)[13].
Motor tasks were then performed to characterize the following domains: Gait, APAs, Reactive Stepping, and Postural Sway. Data were collected via eight wearable, inertial sensors (Opals, APDM). The sensors were placed on both feet, shins, wrists, sternum and the lumbar region. Most motor tasks were collected while undergoing the Mini-Balance Evaluation System Test (Mini-BESTest)[8]. The data used to quantify performance in each domain are described below and shown in Figure 1a. Details on the algorithms used to calculate each outcome from wearable sensors can be found here[8, 14].
Figure 1.
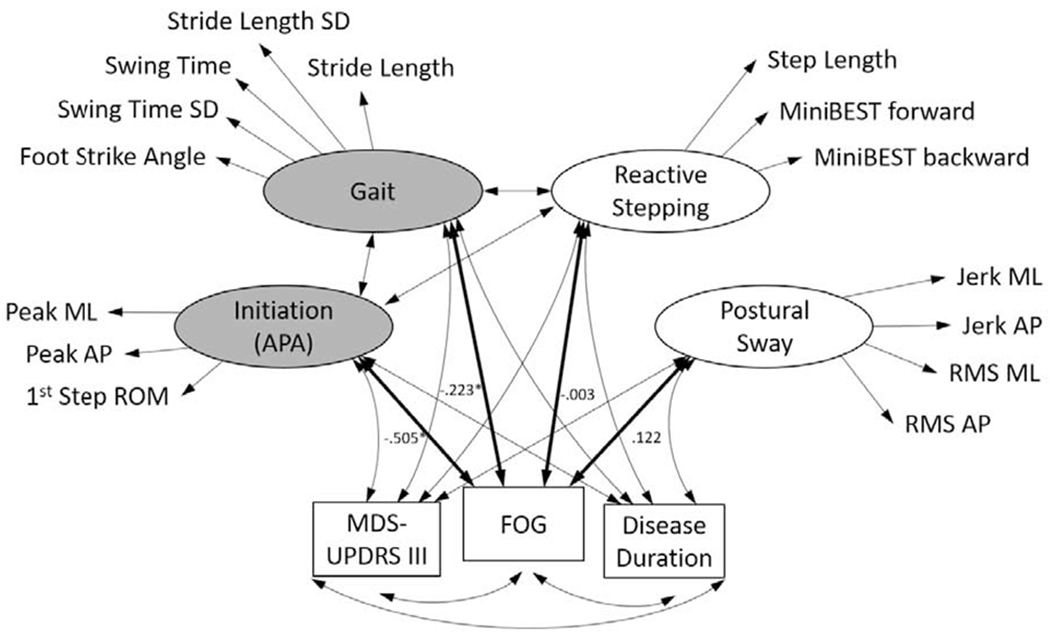
Schematic of the model showing the four latent variable mobility domains (and their respective objective measures selected to constitute each domain) and their potential relationship to FOG. Shaded domains represent those which were significantly related to FOG accounting for disease severity and duration. Covariances for the relationships between the latent variables and log-transformed Freezing severity are included. *indicates significant relationship for FOG-APA and FOG-Gait.
Dynamic balance (Gait):
Subjects walked at a comfortable pace continuously between two lines 25 feet (7.62m) apart for 2 min. From this task, stride length, stride length variability (quantified as standard deviation), swing time, swing time variability, step duration asymmetry (calculated as the natural log of the ratio of left to right step duration, with the smaller of the two values as the numerator), and foot strike angle (in sagittal plane) were calculated[14]. Although by no means comprehensive in capturing all components of gait, these outcomes were chosen to represent aspects of gait previously suggested to be related to FOG[1, 15, 16] (see also “Limitations”, point two).
APAs & Gait initiation:
APA outcomes were derived from the step initiation phase (prior to gait) of the Instrumented Stand and Walk test[17]. As such, these outcomes were derived from a single trial per participant. After 30s of quiet standing, participants began walking at their comfortable speed[8]. A template was used to achieve consistent foot placement (10cm between heels and 30° outward rotation of feet). Specific outcomes were: peak medio-lateral APA, peak anterior-posterior APA, angle of foot at first foot strike, first step latency[8].
Reactive stepping:
Postural responses to external perturbations were quantified with the backward Push and Release test within the MiniBESTest[18]. Standing subjects leaned against the tester’s hands just beyond their backward base of support. They were instructed to do whatever was necessary to regain balance, including taking steps, when the tester quickly removed support. As with APAs, only one trial was included for analysis. We chose only to include data from one reactive stepping trial because performance on reactive stepping can change considerably with repeated exposures (especially early in the exposures[19]). The first exposure is most likely to reflect participants’ most natural response. Outcomes to quantify reactive stepping were: first step latency, first step length, and MiniBESTest score (0, 1, or 2) on the backward reactive step item. MiniBESTest score on the forward reactive stepping item was also included. Instrumented outcomes (step latency, length, etc.) for forward Push and Release were not calculated as the algorithms for this movement have not yet been validated.
Postural Sway:
Quiet stance data (eyes open, firm surface) were collected for 30sec. During this period, jerk and root mean square of acceleration (medio-lateral and anterior-posterior axes) were calculated[20].
To provide an objective measure of FOG severity, acceleration data were collected from the left and right shins during a turning in place test. Participants were required to turn in place, alternating 360° turn to the right and left for 60 seconds as quickly as safely possible [21]. FOG ratio was calculated as the square of the total power in the frequency band corresponding with FOG (3.5–8 Hz) divided by the square of the total power in the locomotion band (0.5–3 Hz). This calculation was conducted separately for the left and right shins, and then averaged across legs [21]. Higher FOG ratio scores indicate greater FOG severity. FOG ratio has been validated against gold standard FOG severity video observation by trained movement disorders neurologists[21]. Finally, we assessed presence of FOG, indicated as scoring a 1 on question 1 of the New FOG Questionnaire (NFOGQ) “Did you experience ‘freezing episodes’ over the past month”[22].
Statistical approach:
Analyses were performed using Stata 15.1/IC. Structural equation modeling was used to evaluate the relationships between FOG (measured continuously with the FOG Ratio and dichotomously [absent-0; present-1] from the NFOGQ) and the balance and gait domains constructed as latent variables, controlling for disease duration and severity (MDS-UPDRS Part III). In rare cases (n=8), the participant noted 0 on the NFOGQ, but freezing episodes were observed during testing, and confirmed by an expert neurologist reviewing video. In these cases, this individual was placed in the FOG group. First, the latent variable measurement models were evaluated with a general confirmatory factoring approach. Issues with convergence were evaluated to inform remediation. The only instance of nonconvergence resulted from a Heywood case for the measurement model of Postural Sway. Maximum likelihood estimates produced a negative residual variance for the “sway area” indicator within the Postural Sway latent variable. Therefore, this indicator was removed from the measurement model to abet convergence.
Estimation of measurement models
We initially examined the measurement models to determine whether the observed variables measured their respective latent constructs. Models were performed using full information maximum likelihood to handling missing data. The fit indices reported beyond the χ2 test of model fit include Comparative Fit Index (CFI), Root Mean Squared Error of Approximation (RMSEA), CD (Coefficient of Determination), and Square Root Mean Residual (SRMR; reported where possible when no missing values exist.) The results from the measurement models are reported in Supplemental Tables 2–5. The models for APA and Reactive Stepping had good initial fit, but the step latency metrics in these models (first step, and reactive stepping, respectively) had weaker loadings and conceptually could be considered distinct in these domains. Thus, they were removed from the model. This meant that the final models for APA and Reactive Stepping were just identified and could not be evaluated with fit statistics based on the saturated model. However, the loadings for these models were in the expected directions and statistically significant. The models for Gait and Postural Sway did not fit well statistically or descriptively. To prevent suspect inferences from the full structural model, these models were modified to improve fit[23]. For Gait, step asymmetry did not load significantly, p = .364, was removed. We also added an error covariance between the measures of variability of Gait. After these modifications, the model did not fit well statistically, but descriptive measures of fit indicated acceptable-to-good fit (CFI = .965, SRMR = .051). For Postural Sway the RMS and Jerk values were natural log transformed and an error covariance was added between the RMS indicators. After this modification, the model did not fit well statistically, but most descriptive measures of fit indicated acceptable-to-good fit (CFI = .944, SRMR = .035).
Second, after establishing the fit of each measurement model, the full structural model was specified—once with FOG measured continuously across all participants and once with FOG measured dichotomously as freezers or non-freezers. To further measure the robustness of these findings, bootstrapping was performed using 500 random resamples with replacement. Bootstrapped standard errors (SEs) and bias-corrected (BC) confidence intervals (CIs) were computed to make inferential decisions within the context of bootstrapping for comparison to the observed information matrix (OIM) SEs and normal-theory-based CIs. The continuous measure of FOG severity (FOG ratio), was right skewed, and some participants were shown to be potential outliers. To help control for these potential effects, FOG-ratio data were log-transformed prior to running our primary analyses. However, because transformations can reduce interpretability of data, and to further investigate the robustness of findings, models were also run on original FOG ratio data. Finally, to provide a secondary assessment of the impact of FOG status on gait and balance outcomes, independent sample t-tests were run on each outcome.
RESULTS
Participant Characteristics
Participant characteristics are shown in table 1. PD who experienced FOG had longer disease, p = 0.001, and more severe PD, p < 0.001, and performed worse on both the ABC, p < 0.001, and MiniBESTest, p = 0.003. Age, levodopa equivalent daily dose, and MoCA were similar across groups, ps > 0.05. Notably, assessment of objective measures of Reactive Stepping was not possible from 13 people with FOG and 7 people without FOG as they experienced a fall without stepping, and measures of Postural Sway from 4 FOG and 1 non-FOG participant were not included as they could not stand for 30 seconds.
Across-group differences in gait outcomes
Means and standard deviations of all outcomes in PD with and without FOG, as well as simple uncorrected across group assessments are provided in Supplemental Table 1, indicating people with FOG performed worse in the Gait and APA, but not Sway and Reactive stepping aspects of balance compared to people without FOG.
FOG severity and balance domains
Our primary analysis utilizing structural equation modeling showed similar results. Using OIM for SEs and normal-theory CIs, Gait, 95% CI[−.4377, −.0083], and APA, 95% CI[−.7601, −.2498], were significantly and negatively related to the natural log of FOG severity (Table 2). That is, poorer natural log FOG ratio scores corresponded to worse gait and APA outcomes. Neither Postural Sway, 95% CI[−.1174, .3608], nor Reactive Stepping, 95% CI[−.2600, .2537], was significantly related to FOG severity. Bootstrapped analyses confirmed the significance of the Gait and APA domain findings. Using 500 resamples to compute bootstrapped SEs and BC CIs, both Gait, 95% CI[−.5939, −.0012] and APA 95% CI[−27.2058, −.0045] remained significantly and negatively related to the natural log of FOG severity.
Table 2:
Structural equation model outputs relating each posture or gait domain to FOG. For each domain, both fOg severity (measured as FOG ratio with natural log transformation), and dichotomous (presence of fOg) models are presented.
| Latent Variable (domain) | 95% BC CI | |||
|---|---|---|---|---|
| Model | Cov | LB | UB | |
| Gait | FOG Severity | −.223* | −.594 | −.001 |
| Dichotomous | −.206* | −.276 | −.004 | |
|
| ||||
| Anticipatory postural response (APA) | FOG Severity | −.505* | −27.206 | −.005 |
| Dichotomous | −.259* | −.614 | −.004 | |
|
| ||||
| Reactive Step | FOG Severity | −.003 | −.401 | .174 |
| Dichotomous | −.093 | −.220 | .002 | |
|
| ||||
| Posture | FOG Severity | .122 | −.062 | .462 |
| Dichotomous | −.019 | −.140 | .037 | |
Note: BC CI = Bias-Correct Confidence Interval computed using bootstrapped standard errors from 500 bootstrap resamples.
Significant at α = .05 as 95% BC CI does not contain 0.
FOG status and balance domains
Using OIM SEs and normal-theory CIs, Gait, 95% CI[−0.2867, −0.1259] , and APA, 95% CI[−0.3727, −0.1461], but not Postural Sway, 95% CI[−0.1059, 0.0673], or Reactive Stepping, 95% CI[−0.1922, 0.0068], were significantly related to FOG status. Using bootstrapped SEs and BC CIs, both Gait, 95% CI −0.2755, −0.0038], and APA, 95% CI[−0.6135, −0.0044], remained significantly and negatively related to FOG status.
For additional information regarding relationships between covariates and latent constructs for OIM and bootstrapped analyses, see Supplemental Tables 6 (for relationship to FOG severity) & 7 (for relationship to FOG status). Further, relationships between covariates and latent constructs with untransformed data were generally consistent with transformed results, and can be found in Supplemental Tables 8 and 9.
Finally, although not a primary outcome of the study, NFOGQ total score and FOG ratio in the FOG group were shown to be significantly correlated (Spearman’s Rho = 0.285, p=0.024; See supplemental Figure 1).
DISCUSSION
Our findings suggest that, when controlling for disease severity, dynamic balance (i.e., gait) and gait initiation (i.e., APAs & first step), were associated with the severity and presence of FOG, whereas automatic postural stepping responses and postural sway were not (see schematic in Figure 1b). Notably, the analysis used in this study had 4 important features: 1) data were included to capture four established and theoretically grounded balance domains[24], each containing 4 to 6 objectively measured outcomes, 2) a relatively large sample of participants (n=144 in their Off state) was included, 3) models were included for both dichotomous (presence or absence of FOG) and continuous (FOG severity) outcomes, and 4) the models were corrected for PD disease severity and duration. The relationship between FOG and each of the four balance domains are discussed in turn.
The finding that gait deficits are related to the presence and severity of FOG is consistent with previous work. Several aspects of gait are altered in freezers compared to non-freezers, even when excluding actual freezing events[15]. The underlying mechanism linking deficits in these continuous gait outcomes and transient FOG outcomes is not fully understood. Recent work suggests that gait may be more attentionally demanding in people who freeze compared to non-freezers, thus increasing variability of gait [1]. Indeed, dynamic balance activities including walking indicate that PD who freeze exhibit more activity of the frontal cortex than those who do not freeze[25]. Further, these increased demands on the cortico-basal ganglia system may place the individual closer to a freezing event, which could be triggered by a cognitive, affective, or motor conflict[26], underpinned by a de-coupling of the cortico-thalamic system [27].
APAs have been related to freezing prior to gait initiation or “start hesitation”. Although failure of gait initiation is a complex problem, it may be precipitated by abnormal APA production. More specifically, start hesitation, and the leg trembling that sometimes accompanies it, could reflect an uncoupling of the weight shift prior to the step (APA) and the step-related leg movement[28]. Consistent with the current report, some[29], although not all[30], recent work has shown people who freeze to exhibit smaller APAs than their non-freezing counterparts. The smaller APAs in PD who freeze may be related to brainstem and supplementary motor cortex dysfunction[16], as brainstem regions including the pontomedullary reticular formation are critical for APA production as well as the subsequent step[31]. The current study suggests that in addition to smaller APAs, people with PD who freeze also exhibit worse first voluntary steps, underscoring the functional significance of altered APAs. Notably, altered APA size has also been suggested to be a compensatory strategy for those who freeze. Schlenstedt and colleagues demonstrated that while APAs were smaller in people who freeze, they were unlikely to have been caused by poor APA production. Instead, the smaller APAs may have been caused by increased hip abductor co-contraction, possibly a compensatory strategy in those who freeze [29].
Reactive postural control was not related to the presence or severity of FOG. These results are consistent with a growing body of work that suggests postural responses to external perturbations are not significantly different in people who do and do not freeze[5, 32, 33]. Further, although both postural instability and FOG symptoms become more pronounced as PD progresses, a substantial proportion of people with PD who freeze have similar postural control performance to people who do not freeze[3]. Together, this work suggests that reactive postural control may be at least partially a distinct phenomenon to FOG and perhaps more linked to the progression of PD. Indeed, as shown in supplemental tables 7 and 8, disease severity (measured as MDS-UPDRS III) was a significant covariate for reactive postural control in both the “presence” and “severity” of FOG models. However, additional studies, with carefully selected participants, matched across different aspects of FOG and postural instability (See for example [32]) will be needed to fully clarify the relationship between these complex and multifaceted symptoms.
Postural sway was also not related to FOG in our cohort. Although data on this topic is mixed, previous work suggests that people with FOG do not consistently exhibit altered static postural control compared to non-freezers[6]. Interestingly however, a few studies have indicated that under complex conditions, such as dual-tasking or when sensory integration is challenged, freezers may exhibit altered sway characteristics (e.g.[29]). In the current study, sway was evaluated only with in the eyes-open, firm surface condition, limiting our ability to clarify this potential relationship.
In the current analysis, we evaluated the relationship among four balance domains and both the presence of FOG and the severity of FOG. Given the transient nature of FOG and challenges in dichotomizing PD patients into those who do and do not freeze, we hypothesized that continuous outcomes may be more able to capture relationships between FOG and outcomes. However, we observed that FOG presence and FOG severity (measured as FOG ratio) were similarly related to our balance domains. This may be a reflection of the relatively large dataset used in this study, as more continuous outcomes may become more important for prediction as the sample becomes smaller. Regardless, this finding provides circumstantial evidence of the relevance of the FOG ratio to quantify severity of FOG. Establishing quantitative outcomes of FOG severity is critical for tracking progression of FOG and evaluating the effect of interventions on this outcome.
LIMITATIONS
Several limitations should be noted. First, we acknowledge that while several objective outcomes were included for each domain, some outcomes (e.g., turning, sensory re-weighting, etc.) were not evaluated. Second, we included only one latent variable for each balance domain, despite the fact that each may be broken into several sub-domains. For example, “gait” is quite broad, and indicators chosen here were not comprehensive in capturing all aspects gait. In fact, it partially is because of this variability across gait outcomes (and also in other domains) that we chose to be conservative with the number of indicators per domain, focusing specifically on those that have been suggested to be related to FOG (e.g. stride length, variability, and asymmetry). Therefore, some potentially interesting outcomes were excluded. An investigation into the relationship between FOG and subdivisions of each domain (with expanded number of outcomes) is warranted; however such an analysis was outside the scope of the current manuscript. Third, for the reactive stepping domain, data from 13 people with and 7 without FOG were excluded because of falls. For the sway domain 4 people with and 1 without FOG were excluded due to an inability to stand for 30 seconds. Therefore, our analysis did not account for a small subset of severe participants. Fourth, data were collected in the practical Off state medication. Given that levodopa may have variable effects on posture and gait [34], addition of medication could also impact these outcomes as well as the relationship between FOG and such outcomes. Fifth, we acknowledge that while inertial sensors are commonly used for gait and balance assessments, the reliability of these outcomes is, in some cases, variable. Specifically, while reliability of stride length and time, and their respective variability have been shown to be good to excellent with the use of inertial sensors (ICC>0.75), some of the tested outcomes (e.g. swing time), exhibit poor ICCs. Therefore, although we have no reason to believe that these measures would have been biased asymmetrically across groups, data should be interpreted with caution. Further, other devices, such as an instrumented walking mat, may have been able to provide more detailed or accurate spatial and asymmetry outcomes. Sixth, the FOG ratio is calculated during stepping in place, and therefore could biased it toward a relationship with stepping or gait outcomes. Two points somewhat lessen this concern: 1) FOG ratio has been shown to be related to FOG severity assessed via video-review[21], and 2) the presence of FOG model also showed a relationship to Gait. Nevertheless, this limitation is notable as it may have implications for development of rehabilitative approaches for different sub-types of FOG such as doorways and dual-tasking triggers. Seventh, as noted in the methods section, while models for APA and Reactive Stepping fit well statistically and descriptively, models for Gait and Postural Sway did not fit well statistically. After adjusting the models, Gait and Postural sway did fit well descriptively, but not statistically. This lack of statistical fit could impact inferences. Lastly, it is possible that freezing events during initiation (start hesitation) or gait may have occurred, contributing to the relationship between FOG status and severity and gait/gait initiation. However, anecdotally, none of the participants exhibited freezing during gait or start hesitation during the tasks in question, reducing this concern.
CONCLUSIONS
We observed that presence and severity of freezing of gait was related to impairments of dynamic balance (gait) and gait initiation (APAs), but not reactive or static balance in people with PD when Off medication. These findings provide further support for the idea that dynamic balance and weight shifting are often impacted in people with PD who experience FOG. These domains may be especially important rehabilitative targets to improve balance in PD with FOG.
Supplementary Material
Highlights.
The gait & balance domains that are impacted in PD who freeze are poorly understood
We related FOG and domains of balance and gait via structural equation modeling
Gait & anticipatory postural adjustments (APAs) were related to FOG
Neither postural sway nor reactive stepping were related to FOG
Rehabilitation focused on gait and APAs may be particularly effective for PD & FOG
ACKNOWLEDGMENT
The authors thank participants, as well as Natassja Pal, Graham Harker and Michael Fleming for assisting in participant recruitment, screening and data collection.
FUNDING
The project was supported by grants from the US Department of Veteran’s Affairs Rehabilitation Research and Development Service (Career Development Award-1: #I01BX007080; PI: DSP) and VA Merit Award (I01 RX001075-01; PI: FBH) , the National Institutes of Health (R01 AG006457 29 PI: FH), a NIH Career Development Award K99 HD078492 0IAI (PI: MM) and NIH/NCATS (KL2TR000152; PI: BWF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS
Dr. Horak has a significant financial interest in ADPM, a company that may have a commercial interest in the results of this research and technology. This potential conflict has been reviewed and managed by OHSU and the Portland VA Health Care System.
LITERATURE CITED
- [1].Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A, Freezing of gait: moving forward on a mysterious clinical phenomenon, Lancet neurology 10(8) (2011) 734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bloem BR, van Vugt JP, Beckley DJ, Postural instability and falls in Parkinson’s disease, Adv Neurol 87 (2001) 209–23. [PubMed] [Google Scholar]
- [3].Amboni M, Stocchi F, Abbruzzese G, Morgante L, Onofrj M, Ruggieri S, Tinazzi M, Zappia M, Attar M, Colombo D, Simoni L, Ori A, Barone P, Antonini A, Group DS, Prevalence and associated features of self-reported freezing of gait in Parkinson disease: The DEEP FOG study, Parkinsonism Relat Disord 21(6) (2015) 644–9. [DOI] [PubMed] [Google Scholar]
- [4].Mancini M, Nutt J, Horak F, Balance Dysfunction in Parkinson’s Disease: Basic Mechanisms to Clinical Management, Academic Press; 2019. [Google Scholar]
- [5].Bekkers EMJ, Van Rossom S, Heremans E, Dockx K, Devan S, Verschueren SMP, Nieuwboer A, Adaptations to Postural Perturbations in Patients With Freezing of Gait, Frontiers in neurology 9 (2018) 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bekkers EMJ, Dijkstra BW, Heremans E, Verschueren SMP, Bloem BR, Nieuwboer A, Balancing between the two: Are freezing of gait and postural instability in Parkinson’s disease connected?, Neurosci Biobehav Rev 94 (2018) 113–125. [DOI] [PubMed] [Google Scholar]
- [7].Hughes AJ, Daniel SE, Kilford L, Lees AJ, Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases, J Neurol Neurosurg Psychiatry 55(3) (1992) 181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hasegawa N, Shah VV, Carlson-Kuhta P, Nutt JG, Horak FB, Mancini M, How to Select Balance Measures Sensitive to Parkinson’s Disease from Body-Worn Inertial Sensors-Separating the Trees from the Forest, Sensors 19(15) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].King LA, Peterson DS, Mancini M, Carlson-Kuhta P, Fling BW, Smulders K, Nutt JG, Dale M, Carter J, Winters-Stone KM, Horak FB, Do cognitive measures and brain circuitry predict outcomes of exercise in Parkinson Disease: a randomized clinical trial, BMC neurology 15 (2015) 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].de Souza Fortaleza AC, Mancini M, Carlson-Kuhta P, King LA, Nutt JG, Chagas EF, Freitas IFJ, Horak FB, Dual task interference on postural sway, postural transitions and gait in people with Parkinson’s disease and freezing of gait, Gait Posture 56 (2017) 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N, U.R.T.F. Movement Disorder Society, Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results, Mov Disord 23(15) (2008) 2129–70. [DOI] [PubMed] [Google Scholar]
- [12].Powell LE, Myers AM, The Activities-specific Balance Confidence (ABC) Scale, J Gerontol A Biol Sci Med Sci 50A(1) (1995) M28–34. [DOI] [PubMed] [Google Scholar]
- [13].Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H, The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment, J Am Geriatr Soc 53(4) (2005) 695–9. [DOI] [PubMed] [Google Scholar]
- [14].Morris R, Stuart S, McBarron G, Fino PC, Mancini M, Curtze C, Validity of Mobility Lab (version 2) for gait assessment in young adults, older adults and Parkinson’s disease, Physiological measurement 40(9) (2019) 095003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hausdorff JM, Schaafsma JD, Balash Y, Bartels AL, Gurevich T, Giladi N, Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait, Exp Brain Res 149(2) (2003) 187–94. [DOI] [PubMed] [Google Scholar]
- [16].Snijders AH, Takakusaki K, Debu B, Lozano AM, Krishna V, Fasano A, Aziz TZ, Papa SM, Factor SA, Hallett M, Physiology of freezing of gait, Ann Neurol 80(5) (2016) 644–659. [DOI] [PubMed] [Google Scholar]
- [17].Horak FB, Mancini M, Carlson-Kuhta P, Nutt JG, Salarian A, Balance and Gait Represent Independent Domains of Mobility in Parkinson Disease, Phys Ther 96(9) (2016) 1364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].El-Gohary M, Peterson DS, Gera G, Horak F, Huisinga J, Validity of the instrumented push and release test (IPUSH) to quantify postural responses in persons with multiple sclerosis, Arch Phys Med Rehabil Accepted and in Press (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nanhoe-Mahabier W, Allum JH, Overeem S, Borm GF, Oude Nijhuis LB, Bloem BR, First trial reactions and habituation rates over successive balance perturbations in Parkinson’s disease, Neuroscience 217 (2012) 123–9. [DOI] [PubMed] [Google Scholar]
- [20].Mancini M, Salarian A, Carlson-Kuhta P, Zampieri C, King L, Chiari L, Horak FB, ISway: a sensitive, valid and reliable measure of postural control, Journal of neuroengineering and rehabilitation 9 (2012) 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mancini M, Smulders K, Cohen RG, Horak FB, Giladi N, Nutt JG, The clinical significance of freezing while turning in Parkinson’s disease, Neuroscience 343 (2017) 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T, Giladi N, Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers, Gait Posture 30(4) (2009) 459–63. [DOI] [PubMed] [Google Scholar]
- [23].Maydeu-Olivares A, Assessing the Size of Model Misfit in Structural Equation Models, Psychometrika (2017). [DOI] [PubMed] [Google Scholar]
- [24].Horak FB, Wrisley DM, Frank J, The Balance Evaluation Systems Test (BESTest) to differentiate balance deficits, Phys Ther 89(5) (2009) 484–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Belluscio V, Stuart S, Bergamini E, Vannozzi G, Mancini M, The Association between Prefrontal Cortex Activity and Turning Behavior in People with and without Freezing of Gait, Neuroscience 416 (2019) 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ehgoetz Martens KA, Shine JM, The interactions between non-motor symptoms of Parkinson’s disease, Expert review of neurotherapeutics 18(6) (2018) 457–460. [DOI] [PubMed] [Google Scholar]
- [27].Georgiades MJ, Shine JM, Gilat M, McMaster J, Owler B, Mahant N, Lewis SJG, Hitting the brakes: pathological subthalamic nucleus activity in Parkinson’s disease gait freezing, Brain 142(12) (2019) 3906–3916. [DOI] [PubMed] [Google Scholar]
- [28].Jacobs JV, Nutt JG, Carlson-Kuhta P, Stephens M, Horak FB, Knee trembling during freezing of gait represents multiple anticipatory postural adjustments, Exp Neurol 215(2) (2009) 334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Schlenstedt C, Mancini M, Nutt J, Hiller AP, Maetzler W, Deuschl G, Horak F, Are Hypometric Anticipatory Postural Adjustments Contributing to Freezing of Gait in Parkinson’s Disease?, Front. Aging Neurosci 10(36) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lu C, Amundsen Huffmaster SL, Tuite PJ, Vachon JM, MacKinnon CD, Effect of Cue Timing and Modality on Gait Initiation in Parkinson Disease With Freezing of Gait, Arch Phys Med Rehabil 98(7) (2017) 1291–1299 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schepens B, Stapley P, Drew T, Neurons in the pontomedullary reticular formation signal posture and movement both as an integrated behavior and independently, J Neurophysiol 100(4) (2008) 2235–53. [DOI] [PubMed] [Google Scholar]
- [32].Nonnekes J, de Kam D, Oude Nijhuis LB, van Geel K, Bloem BR, Geurts A, Weerdesteyn V, StartReact effects support different pathophysiological mechanisms underlying freezing of gait and postural instability in Parkinson’s disease, PLoS One 10(3) (2015) e0122064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Peterson DS, Horak FB, Effects of freezing of gait on postural motor learning in people with Parkinson’s disease, Neuroscience 334 (2016) 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Curtze C, Nutt JG, Carlson-Kuhta P, Mancini M, Horak FB, Levodopa Is a Double-Edged Sword for Balance and Gait in People With Parkinson’s Disease, Mov Disord (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


