Abstract
Congenital anomalies of the kidney and urinary tract (CAKUT) occur in 0.5–1/100 newborns and as a group they represent the most frequent cause for chronic kidney failure in children. CAKUT comprise clinically heterogeneous conditions, ranging from mild vesicoureteral reflux to kidney aplasia. Most forms of CAKUT share the pathophysiology of an impaired developmental interaction of the ureteric bud (UB) and the metanephric mesenchyme (MM). In most cases, CAKUT present as an isolated condition. They also may occur as a component in rare multi-organ syndromes. Many CAKUT probably have a multifactorial etiology. However, up to 20% of human patients and > 200 transgenic mouse models have a monogenic form of CAKUT, which has fueled our efforts to unravel molecular kidney (mal-)development. To date, genetic variants in more than 50 genes have been associated with (isolated) CAKUT in humans. In this short review, we will summarize typical imaging findings in patients with CAKUT and highlight recent mechanistic insight in the molecular pathogenesis of monogenic forms of CAKUT.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40348-021-00112-0.
Congenital anomalies of the kidney and urinary tract (CAKUT) are a major challenge in pediatric nephrology
The term “CAKUT” (congenital anomalies of the kidney and urinary tract) encompasses a clinically broad spectrum of malformations, including kidney hypoplasia and dysplasia, ureteropelvic junction obstruction (UPJO), primary megaureter, vesicoureteral reflux (VUR), ureterovesical junction obstruction (UVJO), or posterior urethral valves (PUV) (Fig. 1). Together with heart defects, CAKUT are the most common group of malformations in humans, affecting 0.5-1% of newborns according to the EUROCAT database. In most of the cases, CAKUT present as an isolated condition. However, there are numerous rare syndromes that have CAKUT as one component among other malformations (e.g., Fraser syndrome; “cryptophthalmos, syndactyly, and CAKUT;” OMIM #219000). About 40% of children on kidney replacement therapy are suffering from a CAKUT diagnosis, making the CAKUT spectrum the most frequent single cause of chronic kidney failure in children [1].
Fig. 1.
Imaging studies in patients with different CAKUT. (a) Mild dilatation of the kidney pelvis (11 mm); (b and c) severe dilatation of the kidney pelvis (290 mm); and calyxes due to ureteropelvic junction obstruction (UPJO). (d) Small and hyperechogenic right kidney without corticomedullary differentiation suggesting kidney dysplasia; (e) Dilatation of the right ureter retrovesically; (f) voiding cysturetrography (VCUG) demonstrating bilateral VUR V° in the filling phase in the same patient as in e (International Reflux Study Committee (1981)). Contrast agent was administered using a transurethral catheter. (g) Contrast-enhanced uro-sonography in a patient with VUR III°. Contrast agent is detectable in the right kidney pelvis
CAKUT usually are detected by ultrasonography, either prenatally, or due to a complication later in life (Fig. 1), or as an incidental finding. Symptomatic individuals may present with recurrent urinary tract infections, with chronic kidney disease of any stage and at any age, with perinatal kidney failure being the most severe form. Medical care for children with symptomatic CAKUT may be complex and require patient-tailored multidisciplinary approaches. Therapeutic options are restricted to symptomatic treatments that focus on preserving kidney function, preventing urinary tract infections, optimizing quality of life, mitigating symptoms of chronic kidney disease, and, if necessary, providing kidney replacement therapy.
A minority of patients has monogenic CAKUT
The etiologies of CAKUT often remain elusive, as etiology likely is “multifactorial” in many cases [2]. Recent advances in “disease gene” identification have confirmed that in human patients with CAKUT variants can be found in many genes that are implicated in early kidney development and that are also affected in mouse models with CAKUT [3]. Today, up to 20% of cases may be explained by one of > 50 rare monogenic forms of CAKUT (Supplemental Table 1) [4, 5]. An additional 5-10% of CAKUT cases seem to be caused by larger genetic variations, i.e., copy number variations (CNV) [6, 7]. From a clinical point of view, monogenic CAKUT may be undistinguishable from multifactorial CAKUT. A careful evaluation of family members and thorough screening for involvement of other organs may indicate a monogenic form of CAKUT that could be confirmed by next generation sequencing (NGS)-based genetic testing. Physicians caring for families with CAKUT need to be aware that different individuals from the same family may have different CAKUT phenotypes (“variable expressivity”) and that variant carriers may not be affected (“reduced or incomplete penetrance”), which further complicates the identification of familial cases and counseling of families.
More than 50 “CAKUT-genes” have been described so far (Supplemental Table 1), each of them being responsible for less than 1% of cases in mixed CAKUT cohorts [4, 5]. Variants in almost all of these genes may present as multi-organ syndrome, the most common ones being HNF1B (OMIM #13792 Renal cysts and diabetes syndrome) and PAX2 (OMIM #120330 papillorenal syndrome). However, pathogenic variants in the same genes may also lead to either truly isolated CAKUT, or to CAKUT with only subtle syndromic features that easily can be missed, or to CAKUT with syndromic features presenting later in life (such as cognitive impairment). Consequently, genetic diagnostics in individuals with isolated CAKUT should be rather inclusive. Genetic variants in individuals with CAKUT (and in general) have to be interpreted with caution as recommended by the American College of Medical Genetics (ACMG criteria) [8]. Genetic variants in “CAKUT-genes” should be interpreted by experienced geneticists because multiple variants published as “pathogenic” have turned out to be suspiciously frequent in healthy individuals and thus may merely represent genetic risk factors or even be neutral. For many “CAKUT genes,” functional data supporting causality is lacking (Supplemental Table 1).
CAKUT arise from a disturbed differentiation/interaction of the ureteric bud and the metanephric mesenchyme
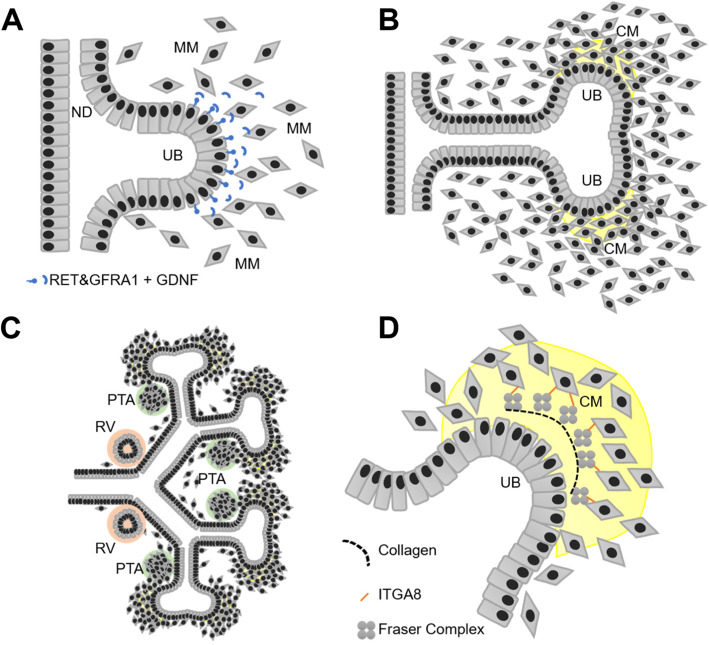
Despite their clinical divergence, most CAKUT have in common an early mal-development of nephro-uro-genic tissues, i.e., the ureteric bud (UB) and the metanephric mesenchyme (MM). CAKUT of the lower urinary tract (e.g., posterior urethral valves, PUV) likely have a different pathogenesis, which is yet not well understood and will not be the focus of this review. In humans, (meta-)nephrogenesis begins at around gestational week four when the UBs form from the nephric ducts (ND) under the influence of temporospatial molecular cues, e.g., GDNF-RET/GFRA1 signaling (Fig. 2a) [9, 10]. The UBs invade the MM, undergo multiple generations of dichotomous divisions, thereby forming a “UB-tree” that is fully embedded in MM (Fig. 2b and c). Keeping with this analogy, the “UB-trunk” will turn into the ureter, the “UB-branches” will give rise to the upper urinary collecting system, whereas the MM contributes by adjoining the “leaves,” namely, the nephrons (and the kidney/ureter stroma). The centrifugally growing UB-tips are capped by a subpopulation of MM cells, i.e., the cap mesenchyme (Fig. 2b), which harbors a pool of SIX1+/CITED1+ nephron progenitor cells (NPC) [9]. Repeatedly, groups of NPC leave the cap-mesenchyme and become committed to the formation of nephrons by initially forming pretubular aggregates (PTA) (Fig. 2c) [10]. PTA cells undergo a mesenchymal-epithelial transition becoming renal vesicles (RV) (Fig. 2c). RV elongate to form “comma-shaped” and then bend to “S-shaped” bodies, which eventually differentiate into mature, segmented nephrons.
Fig. 2.
Illustration of ureteric budding, ureteric branching, initiation of nephron formation, and the extra cellular matrix (ECM) interface between UB and MM. a Ureteric budding from the nephric duct (“ND”) requires active GDNF/RET/GFRA1 signaling between the ureteric bud (“UB”) and the metanephric mesenchyme (“MM”). b Branching of the ureteric bud (“UB”) tip covered by cap mesenchyme (“CM,” yellow) containing nephron progenitor cells (“NPC”). c Subpopulations of NPC leave the cap mesenchyme niche, form peritubular aggregates (“PTA,” green), and undergo mesenchymal-epithelial transition to form renal vesicles (“RV,” orange) that will develop further into nephrons (not shown). d Illustration of the UB-cap mesenchyme (“CM”)-interface with the “Fraser Complex”-associated extra cellular matrix (ECM). FRAS1 and FREM2 (secreted from the UB) and FREM1 and nephronectin (expressed in the MM/CM) assemble in the extracellular space and bind to the Integrin a8/ß1 receptor located in cells derived from the MM
Numerous mouse models with CAKUT phenotypes and identification of monogenic CAKUT in humans have provided mechanistic insight into the molecular pathogenesis of CAKUT. Most of the “CAKUT-genes” can be assigned to one of the following three groups: (I) transcription factors (e.g., PAX2, HNF1B), (II) signaling molecules (e.g., ROBO2), and (III) extracellular matrix (ECM) components of the developing kidney (e.g., FRAS1, FREM2, ITGA8).
Transcription factors are implicated in CAKUT in humans
The spatiotemporal expression of distinctive sets of transcription factors is essential for proper programming of multi-potent cells, such as SIX1+/CITED1+ positive nephron progenitor cells (NPC), as well as for their descendants, e.g., LHX1+/EMX2+/HNF1B+ cells in pretubular aggregates and renal vesicles (Fig. 2) [9]. Deleterious variants in many of the genes encoding for these kidney developmental transcription factors lead to CAKUT in mice and humans (Supplemental Table 1).
Probably the most recognized example for monogenic CAKUT in human patients among clinicians is HNF1B nephro-uro-pathy. Deleterious heterozygous variants in HNF1B, such as the frequently reported whole gene deletion (about 50% of cases), lead to heterogeneous malformations of the kidney and/or the urinary tract and/or diabetes mellitus. Commonly, affected patients exhibit ultrasonographic small and bright kidneys with multiple small cysts and different degrees of kidney function impairment. HNF1B is expressed in epithelial cells in the liver, pancreas, and in the kidney, more specifically in UB cells, nephron precursor cells, and in kidney tubules. In developing kidney tubules, HNF1B promotes SOCS3 expression, which plays an important role in tubulogenesis [11]. Tubular dysgenesis in patients with deleterious variants in HNF1B is appreciated by the clinical term “HNF1B associated Autosomal dominant Tubular Kidney Disease” (HNF1B-ADTKD), which is characterized by chronic kidney disease including hyperuricemia with or without gout, hypokalemia, hypomagnesemia, and polyuria [12]. In a German multicenter childhood registry study for HNF1B nephropathy, 87% of probands (54/62) had bilateral kidney dysplasia, whereas the other mentioned symptoms were less frequently observed [13]. The broad expression of HNF1B in UB, liver, and pancreas explains additional extrarenal features, such as “Maturity Onset Diabetes of the Young Type 5” (MODY5) and defects of the urinary collecting system.
Variants in PAX2 are another well-established example of monogenic CAKUT. Mutations in PAX2 may cause syndromic CAKUT with ocular anomalies, such as optic nerve coloboma (OMIM # 120330) [14]. PAX2 is expressed in multiple embryonic tissues including the optical disk and the cap mesenchyme (see expression data on www.gudmap.org). In mesenchymal-epithelial transition, PAX2 promotes expression of the podocyte transcription factor WT1. Failure to promote WT1 expression seems to result in kidney dysplasia through impairment of nephron differentiation. Interestingly, patients carrying a deleterious variant in PAX2 may also present with steroid resistant nephrotic syndrome (SRNS) and focal segmental glomerulosclerosis (FSGS), similar to patients with a deleterious variant in WT1 [15]. To this notion, patients with PAX2-CAKUT may have early albuminuria, exceeding the level of albuminuria that is expected solely on the basis of chronic kidney disease. Hence, physician should carefully search for eye involvement and consider genetic testing for PAX2 mutations in patients with CAKUT and early albuminuria.
Other recent examples for transcription factors implicated in CAKUT are TBX18 and NRIP1. TBX18 is essential for differentiation of MM cells into ureter smooth muscle cells. Heterozygous deleterious mutations in TBX18 prevent smooth muscle differentiation in the ureteral wall. The absence of peristaltic contraction ability causes ureteropelvic junction obstruction (UPJO) and congenital hydronephrosis. This has been discovered and studied in a Tbx18+/− mouse model and was later found to also be a rare cause of UPJO and hydronephrosis in human patients [16, 17].
NRIP1 is a co-transcription factor of the retinoic acid receptor RARα. A heterozygous truncating variant in NRIP1 recently has been identified in a large kindred with different forms of CAKUT (kidney cysts, kidney dysplasia, dilatation of the ureter, and VUR) [18]. A causative role of this variant is supported by knock-down of Nrip1 in X. laevis larvae that causes a similar CAKUT phenotype (hydroureter, hydronephrosis, and ureterocele). Interestingly, this discovery is in line with the historic observation that alternations in maternal vitamin A supply during pregnancy increase the risk for CAKUT and thereby provides insight into a possible interplay of genetic and environmental factors [19].
Signaling molecules are implicated in CAKUT in humans
The presence of specific transcription factors in nephrogenic cells goes hand in hand with expression of specific sets of cell-signaling proteins. Metanephric kidney formation is initiated when mesenchymal cells at the site of ureteric budding secrete the ligand GDNF which finds its receptor RET and co-receptor GFRA1 on epithelial cell of the nephric duct (ND) that will start proliferating to form a bud. Failure in budding leads to kidney agenesis, failure to restrict budding to a single site leads to duplex kidneys. These landmark findings have been studied extensively in mouse models and embryonic kidney organ cultures [20]. Heterozygous variants in RET and biallelic variants in GFRA1 in human patients with (bilateral) kidney agenesis have been described as very rare causes of CAKUT (Supplemental Table 1). Other ligands involved in ureter induction are BMP4 and GREM1, which seem to restrict GDNF signaling to the actual site of budding. Involvement of rare genetic variants in BMP4 and GREM1 in CAKUT in humans has been suggested [3, 21].
Another signaling pathway that is involved in nephrogenesis and CAKUT is SLIT2-ROBO2 signaling. The ligand SLIT2 is expressed in MM and cap mesenchyme. The SLIT2 signal is interpreted by epithelial cells of the nephric duct and the UB through the trans-membranous receptor ROBO2 [22]. Variants in ROBO2, and likely also variants in SLIT2 and the downstream small GTPase activator SRGAP1, lead to CAKUT (mostly multicystic dysplastic kidneys, MCDK) in humans [23]. SLIT2/ROBO2/SRGAP1 signaling is also present during nephrongenesis in podocytes [24]. Possibly, genetic disturbances in SLIT2/ROBO2 signaling during initial budding lead to ureteral defects, whereas disturbances during nephron formation may lead to kidney hypoplasia or dysplasia.
Extracellular matrix components are implicated in CAKUT in humans
The cellular compartments of the developing kidney are scaffolded by an extracellular matrix that seems to include a set of proteins which are essential for kidney development, as supported by human and mouse genetic data: The UB epithelial cells express three “Fraser Syndrome” genes FRAS1, FREM2, and GRIP1. Biallelic pathogenic variants in any of these genes lead to ECM defects, e.g., in the skin and developing kidney. Affected human individuals and animal models exhibit a syndromic spectrum of malformations ranging from the most severe Fraser Syndrome (OMIM #219000), to the “mildest” manifestation in form of isolated CAKUT [3]. GRIP1 is essential for secretion of FRAS1 and FREM2 into the extracellular space where they interact with FREM1 and nephronectin (NPNT) in a multi-protein complex (Fig. 2d). This “Fraser Complex” interacts with the Integrin a8/ß1 heterodimer located in the membrane of cells derived from the MM (Fig. 2d). Binding of the “Fraser complex” to Integrin a8/ß1 enables a signaling event that likely acts upstream of GDNF expression in MM, which may explain the kidney agenesis phenotype in mice and humans with deleterious variants in the integrin encoding gene ITGA8 [25]. In this context, variants in the “CAKUT gene” HPSE2, that encodes for a peptidase that cleaves the heparan sulfate side chains to permit the remodeling of the extracellular matrix for cell movement (Supplemental Table 1), are an additional line of evidence for the importance of the nephrogenic ECM in CAKUT.
In summary, CAKUT impose a high burden on affected families and health care. Establishing a molecular genetic diagnosis in patients with monogenic CAKUT helps affected families to understand the etiology of their child’s medical condition. It enables physicians to classify it as accurately as possible and it might open the possibility for a more personalized care. In our pediatric nephrology center, we consider genetic testing in CAKUT in patients with severe CAKUT (i.e., relevant chronic kidney disease), syndromic CAKUT, and/or familial CAKUT. Since CAKUT are a developmental group of conditions with genetic-environmental etiology, there is no causative therapy after birth. Therapeutic strategies focus on prevention and symptomatic treatment, including surgical correction/improvement, avoiding complications, and providing kidney replacement therapy. Studying molecular mechanisms of genes implicated in monogenic CAKUT and animal models has provided insight in nephrogenesis on a cellular and molecular level that may serve as basic research on our journey to growing an artificial, personalized kidney in the future.
Supplementary Information
Additional file 1: Supplemental Table 1. 50 genes that represent monogenic causes/candidate genes of “isolated” CAKUT in humans.
Acknowledgements
None
Abbreviations
- Array-CGH
Array-based comparative genomic hybridization
- CAKUT
Congenital anomalies of the kidney and urinary tract
- ECM
Extracellular matrix
- MCDK
Multicystic dysplastic kidneys
- MM
Metanephric mesenchyme
- ND
Nephric duct
- UB
Ureteric bud
- UPJO
Ureteropelvic junction obstruction
- UVJO
Ureterovesical junction obstruction
- VUR
Vesicoureteral reflux
- WES
Whole exome sequencing
Authors’ contributions
S.K. conceptualized and wrote the manuscript, designed the figures, and Supplemental Table 1. S.H. and L.T.W. participated in writing the manuscript. M.C.L. conceptualized this review and participated in writing the manuscript. The authors read and approved the final manuscript.
Funding
S.K. is funded by a young investigator’s stipend of the Cologne Fortune research program. S.H., L.T.W., and M.C.L. have nothing to declare. Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
N/A
Ethics approval and consent to participate
Written consent was obtained from all patients who anonymously donated clinical imaging data for this educational review.
Consent for publication
We consent the terms for publication in Molecular and Cellular Pediatrics.
Competing interests
No competing interests declared.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Harambat J, Bonthuis M, Groothoff JW, et al. Lessons learned from the ESPN/ERA-EDTA Registry. Pediatr Nephrol. 2016;31:2055–2064. doi: 10.1007/s00467-015-3238-8. [DOI] [PubMed] [Google Scholar]
- 2.Nicolaou N, Renkema KY, Bongers EMHF, et al. Genetic, environmental, and epigenetic factors involved in CAKUT. Nat Rev Nephrol. 2015;11:720–731. doi: 10.1038/nrneph.2015.140. [DOI] [PubMed] [Google Scholar]
- 3.Kohl S, Hwang D-Y, Dworschak GC, et al. Mild recessive mutations in six Fraser syndrome-related genes cause isolated congenital anomalies of the kidney and urinary tract. J Am Soc Nephrol. 2014;25:1917–1922. doi: 10.1681/ASN.2013101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang D-Y, Dworschak GC, Kohl S, et al. Mutations in 12 known dominant disease-causing genes clarify many congenital anomalies of the kidney and urinary tract. Kidney Int. 2014;85:1429–1433. doi: 10.1038/ki.2013.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heidet L, Morinière V, Henry C, et al. Targeted exome sequencing identifies PBX1 as involved in monogenic congenital anomalies of the kidney and urinary tract. J Am Soc Nephrol. 2017;28:2901–2914. doi: 10.1681/ASN.2017010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanna-Cherchi S, Kiryluk K, Burgess KE, et al. Copy-number disorders are a common cause of congenital kidney malformations. Am J Hum Genet. 2012;91:987–997. doi: 10.1016/j.ajhg.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verbitsky M, Westland R, Perez A, et al. The copy number variation landscape of congenital anomalies of the kidney and urinary tract. Nat Genet. 2019;51:117–127. doi: 10.1038/s41588-018-0281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindström NO, McMahon JA, Guo J, et al. Conserved and divergent features of human and mouse kidney organogenesis. J Am Soc Nephrol. 2018;29:785–805. doi: 10.1681/ASN.2017080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Combes AN, Davies JA, Little MH. Cell-cell interactions driving kidney morphogenesis. Curr Top Dev Biol. 2015;112:467–508. doi: 10.1016/bs.ctdb.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Ma Z, Gong Y, Patel V, et al. Mutations of HNF-1beta inhibit epithelial morphogenesis through dysregulation of SOCS-3. Proc Natl Acad Sci U S A. 2007;104:20386–20391. doi: 10.1073/pnas.0705957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrè S, Igarashi P. New insights into the role of HNF-1β in kidney (patho)physiology. Pediatr Nephrol. 2019;34:1325–1335. doi: 10.1007/s00467-018-3990-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okorn C, Goertz A, Vester U, et al. HNF1B nephropathy has a slow-progressive phenotype in childhood-with the exception of very early onset cases: results of the German Multicenter HNF1B Childhood Registry. Pediatr Nephrol. 2019;34:1065–1075. doi: 10.1007/s00467-018-4188-8. [DOI] [PubMed] [Google Scholar]
- 14.Weber S, Moriniere V, Knüppel T, et al. Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: results of the ESCAPE study. J Am Soc Nephrol. 2006;17:2864–2870. doi: 10.1681/ASN.2006030277. [DOI] [PubMed] [Google Scholar]
- 15.Vivante A, Chacham OS, Shril S, et al. Dominant PAX2 mutations may cause steroid-resistant nephrotic syndrome and FSGS in children. Pediatr Nephrol. 2019;34:1607–1613. doi: 10.1007/s00467-019-04256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Airik R, Bussen M, Singh MK, et al. Tbx18 regulates the development of the ureteral mesenchyme. J Clin Invest. 2006;116:663–674. doi: 10.1172/JCI26027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vivante A, Kleppa M-J, Schulz J, et al. Mutations in TBX18 cause dominant urinary tract malformations via transcriptional dysregulation of ureter development. Am J Hum Genet. 2015;97:291–301. doi: 10.1016/j.ajhg.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vivante A, Mann N, Yonath H, et al. A dominant mutation in nuclear receptor interacting protein 1 causes urinary tract malformations via dysregulation of retinoic acid signaling. J Am Soc Nephrol. 2017;28:2364–2376. doi: 10.1681/ASN.2016060694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lelièvre-Pégorier M, Vilar J, Ferrier ML, et al. Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int. 1998;54:1455–1462. doi: 10.1046/j.1523-1755.1998.00151.x. [DOI] [PubMed] [Google Scholar]
- 20.Pepicelli CV, Kispert A, Rowitch DH, McMahon AP. GDNF induces branching and increased cell proliferation in the ureter of the mouse. Dev Biol. 1997;192:193–198. doi: 10.1006/dbio.1997.8745. [DOI] [PubMed] [Google Scholar]
- 21.Weber S, Taylor JC, Winyard P, et al. SIX2 and BMP4 mutations associate with anomalous kidney development. J Am Soc Nephrol. 2008;19:891–903. doi: 10.1681/ASN.2006111282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu W, van Eerde AM, Fan X, et al. Disruption of ROBO2 is associated with urinary tract anomalies and confers risk of vesicoureteral reflux. Am J Hum Genet. 2007;80:616–632. doi: 10.1086/512735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang D-Y, Kohl S, Fan X, et al. Mutations of the SLIT2-ROBO2 pathway genes SLIT2 and SRGAP1 confer risk for congenital anomalies of the kidney and urinary tract. Hum Genet. 2015;134:905–916. doi: 10.1007/s00439-015-1570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan X, Yang H, Kumar S, et al. SLIT2/ROBO2 signaling pathway inhibits nonmuscle myosin IIA activity and destabilizes kidney podocyte adhesion. JCI Insight. 2016;1:e86934. doi: 10.1172/jci.insight.86934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humbert C, Silbermann F, Morar B, et al. Integrin alpha 8 recessive mutations are responsible for bilateral renal agenesis in humans. Am J Hum Genet. 2014;94:288–294. doi: 10.1016/j.ajhg.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Table 1. 50 genes that represent monogenic causes/candidate genes of “isolated” CAKUT in humans.
Data Availability Statement
N/A