Abstract
The role of autophagy and lysosomal degradation pathway in the regulation of skeletal muscle metabolism was previously studied. However, underlying molecular mechanisms are poorly understood. L-lactate which is utilized as an energetic substrate by skeletal muscle can also augment genes expression related to metabolism and up-regulate those being responsive to reactive oxygen species (ROS). Since ROS is the most important regulator of autophagy in skeletal muscle, we tested if there is a link between cellular lactate metabolism and autophagy in differentiated C2C12 myotubes and the gastrocnemius muscle of male wistar rats. C2C12 mouse skeletal muscle was exposed to 2, 6, 10, and 20 mM lactate and evaluated for lactate autophagic effects. Lactate dose-dependently increased autophagy and augmented ROS generation in differentiated C2C12 myotubes. The autophagic effect of lactate deterred in N-acetylcysteine presence (NAC, a ROS scavenger) indicated lactate regulates autophagy with ROS participation. Lactate-induced up-regulation of extracellular signal-regulated kinase 1/2 (ERK1/2) through ROS was required to regulate the autophagy by lactate. Further analysis about ERK1/2 up- and downstream indicated that lactate regulates autophagy through ROS-mediated the activation of ERK1/2/mTOR/p70S6K pathway in skeletal muscle. The in vitro effects of lactate on autophagy also occurred in the gastrocnemius muscle of male Wistar rats. In conclusion, we provided the lactate-associated regulation evidence of autophagy in skeletal muscle by activating ROS-mediated ERK1/2/mTOR/p70S6K pathway. Since the increase in cellular lactate concentration is a hallmark of energy deficiency, the results provide insight into a skeletal muscle mechanism to fulfill its enhanced energy requirement.
Keywords: Autophagy, Differentiated C2C12 myotubes, Extracellular signal-regulated kinase ½, Lactate, Skeletal muscle
Introduction
Autophagy, lysosomal degradation pathway, is a natural mechanism of cell which functions during basal conditions in organelle and protein quality control (Glick et al. 2010). The autophagic/lysosome system allows cytosolic renovation through degradation and recycling of unnecessary or dysfunctional cellular components (Yu et al. 2017) and permits a cell to degrade a part of its materials to fulfill energetic needs through the starvation and stress s (Young et al. 2006). In general, the autophagy is categorized by three main types: microautophagy, chaperone-mediated autophagy (CMA), and macroautophagy. They mainly differ in their mode of substrate delivery to lysosome (Yu et al. 2017). Macroautophagy, hereafter referred to as autophagy, delivers cytoplasmic cargo to lysosome through the intermediary of a double-membrane structure containing sequestered cytoplasmic material; autophagosome which ultimately fuses with lysosome in which the cargo degrades into amino acids takes place (Yu et al. 2017).
Several factors and signaling pathways were shown to contribute to a basal- and stress-induced autophagy regulation in skeletal muscle. Phosphatidylinositol 3-phosphate kinase/Akt/mammalian target of rapamycin (mTOR) is the most potent autophagy inhibitor in skeletal muscles which exerts its inhibitory effects by blocking autophagosome formation (Risson et al. 2009). On the other hand, the activation of forkhead box O3 (FoxO3a) by AMP-activated protein kinase (AMPK) augments the expression of several autophagy-specific genes (Atgs) including LC3-phosphatidylethanolamine conjugate (LC3-II) and GABAA receptor-associated protein-like 1 (Gabarapl1) which acts as the promoters of autophagosome fabrication (Sanchez et al. 2012). Moreover, p38 αβ MAPK pathway was described to regulate Atgs expression independent of FoxO3. (McClung et al. 2009).
A growing body of work suggests that reactive oxygen species (ROS) are important cellular signal transducers controlling autophagy in skeletal muscle (Dobrowolny et al. 2008; McClung et al. 2009; Rahman et al. 2014; Scherz-Shouval et al. 2007). Endogenous ROS, particularly mitochondrial-derived ROS, was shown to promote basal- and nutrient starvation-induced autophagy in differentiated C2C12 myotubes, the effect which is mediated through AMPK activation and AKT inhibition (Rahman et al. 2014). Nutrient starvation-induced autophagy requires hydrogen peroxide (H2O2) production, which directly inhibits Atg4, an enzyme involved in Atg8 protein maturation and delipidation, thereby increasing the formation of LC3-associated autophagosomes (Scherz-Shouval et al. 2007). Also, H2O2 induces early transcriptional activation of genes involved in autophagy lysosome-mediated proteolysis in cultured C2C12 myotubes through the activation of p38 αβ MAPK (McClung et al. 2009). Moreover, in MLC/SOD1G93A transgenic mice expressing a mutant form of superoxide dismutase 1 (SOD1), ROS accumulation serves as a signal to initiate autophagy and muscle atrophy (Dobrowolny et al. 2008). These results indicate that alterations in redox balance and subsequent oxidative stress are major factors which regulate muscle autophagy. Therefore, any factor changing muscle redox balance could be involved in skeletal muscle autophagy.
For several years, L-lactate was largely considered to be a dead-end product of anaerobic glycolysis. However, recent evidence proposed a lactate role in regulating genes related to its metabolism (Aveseh et al. 2014; Gabriel-Costa et al. 2015; Hashimoto et al. 2007; Latham et al. 2012) and redox state in cells (Echigoya et al. 2012; Galardo et al. 2014; Hashimoto et al. 2007; Hunt et al. 2007). Indeed, the conversion of lactate to pyruvate catalyzed by lactate dehydrogenase (LDH) is accompanied by NADH production, and consequently, by a change in redox balance which could be associated to alterations in ROS levels. To support this idea, lactate was shown to increase ROS production and up-regulate various genes in L6 cells, many known to be responsive to ROS (Hashimoto et al. 2007). Meanwhile, the oxidation of lactate to pyruvate within germ cells results in NADH accumulation, a substrate for NAD(P)H oxidase (NOX), which causes ROS production and, consequently, up-regulation of Akt- and p38-MAPK signaling pathways (Galardo et al. 2014). Also, Lactic acid induces increased cellular H2O2 levels in K562 cells and promotes erythroid differentiation in a ROS-dependent manner (Luo et al. 2017). Also, there is some evidence of enhanced mitochondrial activity following lactate treatment in neuroblastoma cells, associated to a mild elevation in ROS levels (Tauffenberger et al. 2019). There is a possibility that NADH, produced during the conversion of lactate into pyruvate, could enter the mitochondria, thereby boosting the electron transport chain and ROS levels. These findings raise the possibility that there is also a link between cellular lactate metabolism and autophagy-lysosome system-mediated protein degradation through ROS production and changes in cellular redox state in skeletal muscle. However, there is still no substantial evidence.
This study’s primary goals were to test the central hypothesis which lactate affects autophagy in skeletal muscle. To evaluate this hypothesis, we investigated: 1) whether lactate is able to regulate autophagy-related gene expression in differentiated C2C12 myotubes, if ROS participates in this regulation; 2) possible mechanisms which may be involved in the lactate stimulation of autophagy regulation in differentiated C2C12 myotubes, 3) the in vivo effects of lactate on autophagy regulation in skeletal muscle of male Wistar rats.
Materials and methods
Cell culture
C2C12 mouse skeletal muscle cells were obtained from Pasteur Institute. C2C12 myoblast cells were cultured in Dulbecco’s Modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin (Invitrogen) at 37 °C and 5% CO2. Myogenic differentiation was induced at 80% confluence by changing growth medium to differentiation medium (DM) supplemented with 2% horse serum instead of FBS for 6 days. DM used to generate myotubes was daily replaced with a fresh medium. Then, differentiated C2C12 myotubes were treated with L-lactate (Sigma-Aldrich) at different final dilutions (2, 6, 10, and 20 mM) in the absence or presence of 5 mM N-acetylcysteine (NAC), 10 mM oxamate, 10 µM SB203580, 2 mM 3-hydroxy-butyrate (3-OBA), 10 µM BI-D1870, 10 nM rapamycin, or 2.5 µM U0126, and dependent variables were measured as described below.
Lactate treatment
Differentiated C2C12 myotubes were exposed to 2, 6, 10, and 20 mM of lactate for 8 h for protein and RNA measurements. Autophagy-related gene expression (Beclin 1, Atg7, Atg9, Lc3b, Gabarapl1, and P62) and autophagic flux (protein levels of free (LC3-I), lipidated (LC3-II) forms, and LC3-I/LC3-II ratio) were measured following lactate treatment. Differentiated C2C12 myotubes incubated with 2, 6, 10, and 20 mM of lactate for 15, 30, or 60 min were used for thiobarbituric acid reactive substances (TBARS) or reactive oxygen species (ROS) assay. Positive control was included H2O2 500 mM for 15 min.
Reactive oxygen species (ROS) assay
The intracellular levels of ROS in C2C12 myocytes were measured using a dichlorofluorescein assay, as previously described. (Wang and Joseph 2005). In brief, differentiated C2C12 myocytes in 96-well plates were incubated with 10 µM 2´,7´-dichlorodihydrofluorescein diacetate (H2DCFDA; Sigma-Aldrich) in Krebs–Ringer buffer solution for 30 min at 37 C. After washing with PBS, the cells were treated for 15, 30, or 60 min with 2, 6, 10, and 20 mM lactate or H2O2 500 mM in phenol red-free Minimum Essential Media. Then, intracellular intensity ROS was monitored at excitation and emission wavelengths of 480 and 520 nm, respectively, or fluorescence images were acquired. The fluorescent images were captured using a Nikon Eclipse TE2000-U fluorescence inverted microscope (Nikon, Tokyo, Japan). Images were acquired using an Olympus C-5060 digital camera attached to Nikon TE2000U inverted microscope with a 4x objective. Fluorescence intensity was measured using excitation and emission wavelengths at 495 and 529 nm, respectively. Fluorescence intensity was quantified using ImageJ software.
Thiobarbituric acid reactive substances (TBARS) assay
Lipid peroxidation in C2C12 myotubes was measured using a TBARS assay as previously described (Keles et al. 2001). The methodology measures malondialdehyde (MDA) and other aldehydes which were produced by lipid peroxidation and induced by hydroxyl free radicals. The cells were resuspended in PBS at 1 × 106 cells/ml. The cellular suspension (400 mL) or 400 mL of PBS for blank were mixed with 500 mL of 35% TCA and 500 mL of Trishydrochlorid (Tris–HCl; 200 mM, pH 7.4) which incubated for 10 min at room temperature. Then, 1 ml solution involving 2 M Na2SO4 and 55 mM thiobarbituric acid was added. Then, the samples were incubated at 95 °C for 45 min. In the next step, the samples were cooled on ice for 5 min, mixed with 1 ml of 70% TCA, centrifuged at 15,000 × g for 3 min, and supernatant absorbance was read at 530 nm. A baseline absorbance was taken by running a blank along with all samples during the measure-ment. The total protein concentration in cell suspension was assayed using Bradford method. Calculating TBARS concentration was based on the molar extinction coefficient of malondialdehyde.
Autophagic flux in vitro
Differentiated C2C12 myotubes were preincubated in DM in the absence or presence of 5 mM NAC, 10 mM oxamate, 10 µM SB203580, 2 mM 3-OBA, 10 µM BI-D1870, rapamycin, 10 nM rapamycin, or 2.5 µM U0126 for 2 h and then exposed to lactate (2, 6, 10, and 20 mM) for 8 h. Protein levels of free (LC3-I), lipidated (LC3-II) forms, and LC3-I/LC3-II ratio) were measured to reflect autophagic flux.
Western blotting
Differentiated C2C12 myotubes were resuspended in 200 µl lysis buffer (50 mM Tris pH 7.6; 250 mM NaCl; 5 mM EDTA; 0.1% NP-40; protease inhibitor) which passed through a syringe. The cell extracts were centrifuged at 5000 g for 5 min to pellet cell debris, and the supernatant was extracted. For skeletal muscle sample, approximately 100-150 mg of whole gastrocnemius muscle was homogenized in ice-cold RIPA buffer (50 mM TriseHCl, 1 mM EDTA,150mMNaCl, NP40 1%, Na deoxycholate 1%, SDS 1%, Protease and phosphatase inhibitor 0.01 M, pH 7.4), centrifuged at 14,000 RPM for 15 min at 4 °C, and supernatant was recovered. Total protein was determined by Bradford protein assay using bovine serum albumin (BSA) as a standard and western blotting was performed (Mansouri et al. 2014). In brief, 20 μg total protein of each sample was loaded and separated on 12.5% SDS- PAGE and transferred by electroblotting onto polyvinylidenedifluoride membranes (Amersham). Thereafter, PVDF membranes were stained in 0.1% Ponceau S in 1% acetic acid for 3 min by shaking. Membranes were destained by rinsing in water for 2 min. Ponceau S staining total protein loading was recorded to monitor transfer efficiency and quantification of whole protein loading. Then, after washing in TBST buffer (150 mM NaCl, 5% skimmed milk, 0.1% Tween 20, and 50 mM Tris, pH 7.5) for 5 min, the membranes were incubated for 1.5 h at room temperature on an orbital shaker in + 5% skimmed milk in TBST and incubated in primary antibody overnight at 4 °C in blocking buffer. Membranes were washed and incubated for 90 min at room temperature with a secondary antibody in TBST. Membranes were washed, and protein expression was detected by enhanced chemiluminescence according to manufacture instructions. Autoradiographic film were exposed to membranes and developed. Molecular weight standards (Prestained Protein Ladder, Abcam Cat# ab116027) were used to identify appropriate antibody binding. Band densities were determined with ImageJ densitometer software. Applied primary antibodies were LC3-II/LC3-I (Cell Signaling Technology Cat# 2775, RRID:AB_915950), P62 (Cell Signaling Technology Cat# 5114, RRID:AB_10624872), ERK 1/2 (Cell Signaling Technology Cat# 9102, RRID:AB_330744), Phospho-mTOR (Cell Signaling Technology Cat# 2971, RRID:AB_330970), Phospho-Erk1/2 (Cell Signaling Technology Cat# 4370, RRID:AB_2315112) p38-MAPK (Abcam Cat# 197348), Phospho-p38-MAPK (Abcam Cat# ab45381, RRID:AB_881838). Goat Anti-Rabbit IgG Antibody, Peroxidase Conjugated (Millipore Cat# AP132P, RRID:AB_90264) was used as the secondary antibody.
Real Time-PCR
Differentiated C2C12 myotubes or powdered muscle samples (~ 50 mg) was added to 1 ml ice-cold Isol RNA-Lysis reagent (5 prime, Cat# 2302700) and homogenized. Homogenates were centrifuged at 12,000 × g for 10 min at 4 °C to remove the pellet. Chloroform (200 μl) was added to supernatant fraction and vigorously shaken for 15 s. The organic and aqueous phases were separated by centrifugation at 12,000 × g for 15 min. The aqueous phase was removed, 600 μl isopropanol was added, and RNA was isolated according to the manufacturer’s instructions (5 prime, Cat# 2302700). RNA concentration and purity were estimated by OD 260/280. cDNA synthesis was performed with 1 μg RNA in a total reaction volume of 20 μl using random hexamer oligonucleotides. Reverse transcriptase reactions were performed according to the manufacturer’s instructions (Thermo Scientific, Cat# K1622). Quantitative real-time PCR was performed using a 7300 Real-Time PCR System (Applied Biosystems, Step One, Germany). PCR reaction was carried out using SYBR Green II, and ROX was used as a reference dye. The thermocycling conditions were: 10 min at 95 °C, followed by 40 cycles of 95 °C for 15 s and 60 °C for 45 s. The primer sequences used are listed in Table 1. Gene expressions were expressed relative to the expression of 18S housekeeping gene. To avoid detecting nonspecific PCR products, each amplified product’s purity was confirmed using a melting curve analysis. Data quantification was carried out using the 2−ΔΔCT method.
Table 1.
Primer sequences used for Real-time PCR
| Gene | Species | Forward primer (5ʹ to 3ʹ) | Reverse primer (5ʹ to 3ʹ) |
|---|---|---|---|
| Atg7 | Mouse | CCTGCACAACACCAACACAC | CACCTGACTTTATGGCTTCCC |
| Rat | TGTCAGCCTGGCATTTGATAA | TCACTCATGTCCCAGATCTCA | |
| Beclin 1 | Mouse | GGCCAATAAGATGGGTCTGA | CACTGCCTCCAGTGTCTTCA |
| Rat | TTGGCCAATAAGATGGGTCTGAA | TGTCAGGGACTCCAGATACGAGTG | |
| Gabarapl1 | Mouse | CATCGTGGAGAAGGCTCCTA | ATACAGCTGGCCCATGGTAG |
| Rat | AGAAGGCTCCTAAAGCCAGG | GTCCTCAGGTCTCAGGTGGA | |
| p62 | Mouse | CACAGGCACAGAAGACAA | CCGACTCCAAGGCTATCT |
| Rat | TCCCTGTCAAGCAGTATCC | TCCTCCTTGGCTTTGTCTC | |
| Lc3B | Mouse | CGTCCTGGACAAGACCAAGT | ATTGCTGTCCCGAATGTCTC |
| Rat | CAAGCCTTCTTCCTCCTGGTGA | CGCTCTCGTACACTTCAGAGA | |
| 18S | Mouse | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
| Rat | GTTGGTTTTCGGAACTGA GGC | GTCGGCATCGTTTATGGTCG | |
| GPR81 | Rat | GGCTGAGAAAAGCGGTATGA | TCGTTAACTCTCTCCGAGCTAGA |
In vivo study of lactate effect
Male Wistar rats (8-weeks- old) were purchased from the Neuroscience Research Center of Kerman, housed in standard conditions, and randomley divided into two groups: control (C; n = 18) and experimental (EX; n = 18). The animals from EX group were intramuscularly injected with 0.27 ml/kg of a 0.25 M sodium lactate solution (pH 7.2). This lactate value was selected to elevate muscle lactate levels in the gastrocnemius muscle to an average value of 10 mM, as previousely described (Nikooie and Samaneh 2016). C group was administered with PBS at a volume which approximated the average volume administered to EX group. The animals were killed at intervals of 3, 10, and 24 h (six animals for each time) following lactate injection, and gastrocnemius was harvested, frozen in liquid nitrogen, and stored at 80 C for subsequent. The Ethical Committees approved all procedures in the present study on Animal Care at the Neuroscience Research Centre of Kerman, University of Medical Sciences, Kerman, Iran. This study was conducted due to the principles of Declaration of Helsinki (version October 2013).
Measurment of autophagic flux in vivo
Autophagic flux in skeletal muscle was measured using Colchicine method, as previousely described (Mofarrahi et al. 2013). Male Wistar rats (8-weeks-old) were randomly divided into two groups: control (C; n = 12) and experimental (EX; n = 12). The animals received an i.p. injection of PBS (C group) or colchicine (0.4 mg/kg/day, EX group). Twenty-four hours later, the animals were subjected to i.p. injections of PBS (C group) or colchicine combined with an intramuscular injection of lactate in gastrocnemius muscle (EX group). This design provided us four different cohorts of rats: untreated which received PBS for two continuous days (n = 6); colchicine treated which received colchicine for two continuous days (n = 6); lactate treated which received PBS at the fisrt day, followed by a second injection of lactate on the second day (n = 6); lactate plus colchicine treated which received a colchicine on the first day, followed by a second injection of lactate and colchicine on the second day (n = 6). Gastrocnemius muscles were harvested 24 h following the second injection, frozen in liquid nitrogen, and stored at 80 C for subsequent. The whole muscle homogenate was used for all protein and RNA analysis to eliminate inconsistency in the sampling.
Statistical analysis
Data are represented as mean ± SD. One- or Two-way analysis of variance followed by post hoc Bonferroni evaluation was used for multiple groups to determine significant differences. In Two-way ANOVA, a significant interaction was interpreted by a subsequent simple-effects analysis with Bonferroni correction. Before the final analysis, the assumptions for ANOVA, homogeneity of variance, and normal distribution were tested. In all comparisons, the significant level was set at α = 0.05.
Results
Lactate regulates autophagy in differentiated C2C12 myotubes
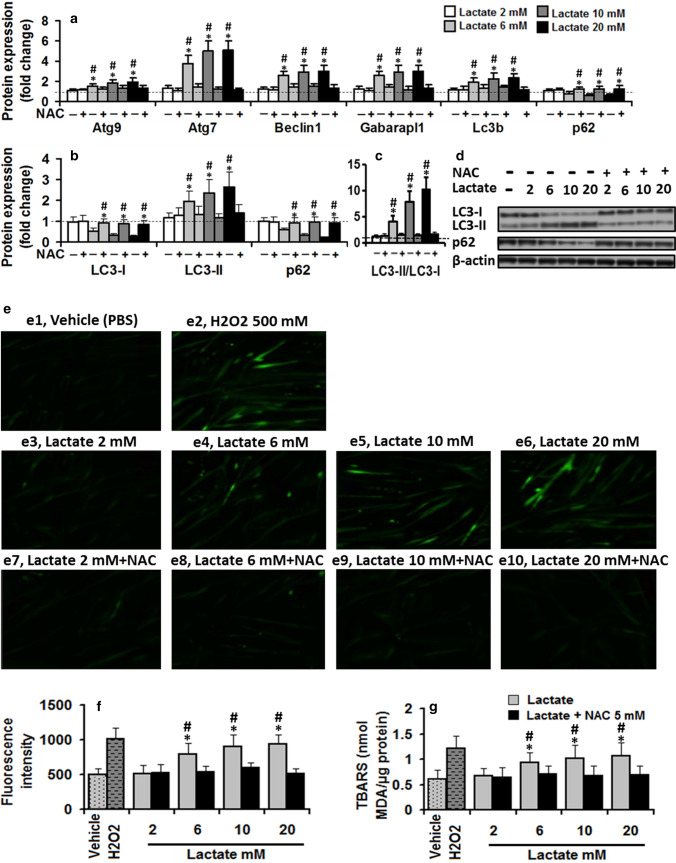
To explore possible autophagy regulation by lactate in vitro, autophagy-related gene expression (Atg9 which is the sole multi-spanning membrane protein essential for the autophagosome formation; Beclin 1 which plays a vital role to regulate early stages of autophagosome formation; Atg7 which is involved in LC3-I conjugation process to PE during autophagosome formation; Gabarapl1 which is crucial for autophagosome formation and the sequestration of cytosolic cargo into double-membrane vesicles; LC3B which is involved in autophagosome membrane expansion and fusion events; and p62, a cargo receptor for ubiquitinated substrates which is itself degraded during autophagy) in differentiated C2C12 myotubes treated with vehicle or lactate (2, 6, 10, and 20 mM) for 8 h were analyzed. The results are reported in Fig. 1a–d. Autophagy-related genes expression data displayed significant dose effect (all p < 0.01), treatment effect (all p < 0.01), and treatment × dose interaction (all p < 0.01). Lactate treatment increased the transcription of autophagy-related genes expression in a dose-dependent manner while the vehicle did not induce any change in analyzed genes. All genes were no more expressed upon treatment with 2 mM lactate compared to control values. However, significant effects started to be observed at 6 mM, increased up to 10 mM, which were maximally expressed upon treatment with 20 mM lactate.
Fig. 1.
Lactate increased ROS generation, thereby augmenting autophagy in differentiated C2C12 myotubes. a Lactate treatment increased transcription of autophagy-related gene expression (two-way ANOVA: main effect of dose, Atg9, F = 6; Atg7, F = 22.9; Beclin 1, F = 12.3; Gabarapl1, F = 12.3; LC3B, F = 8.9; p62, F = 6.1; all p < 0.01; main effect of treatment, Atg9, F = 51; Atg7, F = 228; Beclin 1, F = 146; Gabarapl1, F = 135; LC3B, F = 89; p62, F = 44; treatment × dose interaction, Atg9, F = 5.7; Atg7, F = 22.7; Beclin 1, F = 12.2; Gabarapl1, F = 12.2; LC3B, F = 8.7; p62, F = 6.4; all p < 0.01) and b, c autophagic flux (two-way ANOVA: increase in LC3-II, main effect of dose, F = 7.2, p < 0.01; main effect of treatment, F = 52, p < 0.01; treatment × dose interaction, F = 5.1, p < 0.01; increase in LC3II/LC3I, main effect of dose, F = 38, p < 0.01; main effect of treatment, F = 209, p < 0.01: treatment × dose interaction, F = 35, p < 0.01; decrease in p62, main effect of dose, F = 4.8, p < 0.01; main effect of treatment, F = 74, p < 0.01; treatment × dose interaction, F = 10.1, p < 0.01) in a dose-dependent manner in C2C12 myotubes maintained in vehicle or lactate (2, 6, 10, and 20 mM, 8 h) compared to the control values (dashed lines). d In-gel profile for densitometric scanning analysis of LC3-I, LC3-II, and p62. e Representative images of H2DCFDA fluorescence. Lactate dose-dependently increased ROS generation and TBARS in differentiated C2C12 myotubes maintained in 2, 6, 10, and 20 mM lactate for 60 min e3-6 compared to the baseline values (vehicle), but exerted no effect in C2C12 myotubes co-incubated with 5 mM N-acetylcysteine (NAC) and lactate e7-10 (two-way ANOVA: ROS, main effect of dose, F = 12.3, p < 0.01; main effect of treatment, F = 42.1, p < 0.01; dose × treatment interaction, F = 8.4, p < 0.01; TBARS, main effect of dose, F = 4.5, p < 0.01, main effect of treatment, F = 4.5, p < 0.01; dose × treatment interaction, F = 2.2, p = 0.08). 500 mM H2O2 was used as a positive control. (f, g) Quantification of H2DCFDA fluorescence. Pre-treatment with NAC (5 mM, 2 h) deterred stimulatory effect of lactate on autophagy-related gene expression a (two-way ANOVA: main effect of dose, Atg9, F = 7.4; Atg7, F = 23.1; Beclin 1, F = 12.3; Gabarapl1, F = 11.6; LC3B, F = 7.7; p62, F = 6.9; all p < 0.01; main effect of treatment, Atg9, F = 13.9; Atg7, F = 187; Beclin 1, F = 71.7; Gabarapl1, F = 78.2; LC3B, F = 37.4; p62, F = 41; two-way ANOVA: treatment × dose interaction, Atg9, F = 2.0, p = 0.9; Atg7, F = 19.7; Beclin 1, F = 7.2; Gabarapl1, F = 7.5; LC3B, F = 5.6; p62, F = 3.1; all p < 0.01) and autophagic flux (b, c) (two-way ANOVA: main effect of dose, LC3B-I, F = 1.2; LC3B-II, F = 6.6; p62, F = 8.7; LC3B-II/LC3B-I, F = 38.7; all p < 001; Fig. 1b; main effect of treatment, LC3B-I, F = 46.9; LC3B-II, F = 31.2; p62, F = 50.4; LC3B-II/LC3B-I, F = 38.7; all P < 001; treatment × dose interaction, LC3B-I, F = 5; LC3B-II, F = 5.7; p62, F = 7.9; LC3B-II/LC3B-I, F = 34; all p < 001), suggesting lactate regulates autophagy in C2C12 myotubes with ROS participation. The values are mean ± SD. For autophagy-related gene expression, values expressed as mean ± SD of fold change from basal values (vehicle). For autophagy flux markers, the fold changes in protein levels were normalized for β-actin and relative to their expression in basal condition (vehicle). # Significant difference with basal values (P < 0.01), * Significant difference with lactate + NAC group (P < 0.01). N = 6 per group
Autophagic flux was monitored by measuring the protein levels of P62, LC3-I, LC3-II and LC3-I conversion into LC3-II, assessed by LC3-II/LC3-I ratio (Fig. 1b–d). LC3-II expression and the ratio of LC3II/LC3I displayed significant dose effect (all p < 0.01), treatment effect (all p < 0.01), and treatment × dose interaction (all p < 0.01). Lactate dose-dependently increased LC3-II expression and LC3II/LC3I ratio. LC3-II and LC3II/LC3I levels were significantly larger at 6, 10, and 20 mM lactate compared to control values. To support increasing LC3B-II protein, p62, an autophagic adaptor protein, which can be degraded through increased autophagy was dramatically reduced in differentiated C2C12 myotubes treated with lactate which could be interpreted as an increase in autophagy flux. These results suggest that lactate significantly induces autophagy in differentiated C2C12 myotubes.
Lactate regulates autophagy in C2C12 myotubes with the participation of ROS
To establish the role of intracellular ROS in lactate-induced autophagy regulation, differentiated C2C12 myotubes were treated with vehicle or lacate (2, 6, 10, and 20 mM) for variable periods (15, 30, or 60 min) or 500 mM H2O2 (positive control), and ROS levels were indirectly estimated by TBARS assay. As shown in Table 2, TBARS indicated a significant dose effect (p < 0.01) and treatment effect (all p < 0.05). Higher doses of lactate (6, 10, and 20 mM) induced a significant increase in TBARS generation compared to control values. TBARS values were significantly larger at 30- and 60-min time points in C2C12 myotubes treated with lactate compared to control values. To ensure accurate quantification of ROS generation, H2DCFDA, a common ROS detection dye, was used to monitor intracellular ROS levels in cells treated with lactate (2, 6, 10, and 20 mM) for 60 min. Figures 1e1, 2, 3, 4, 5, 6 shows that ROS generation significantly increased following treatment with 6, 10, and 20 mM lactate (one-way ANOVA: F = 14.5, p < 0.01), not for lactate 2 mM compared to value found in untreated myotubes.
Table 2.
TBARS (nmol MDA/µg protein) values in C2C12 myotubes incubated with different doses of lactate. H2O2 was used as a positive control
| Time | |||
|---|---|---|---|
| 15 min | 30 min | 60 min | |
| Vehicle | 0.60 ± 0.16 | 0.63 ± 0.16 | 0.61 ± 0.18 |
| H2O2 | 1.02 ± 0.28* | 1.21 ± 0.23* | 1.21 ± 0.25* |
| Lactate 2 mM | 0.69 ± 0.16 | 0.62 ± 0.17 | 0.67 ± 0.14 |
| Lactate 6 mM | 0.74 ± 0.18 | 0.88 ± 0.16* | 0.93 ± 0.19* |
| Lactate 10 mM | 0.74 ± 0.19 | 0.95 ± 0.17* | 1.02 ± 0.26* |
| Lactate 20 mM | 0.76 ± 0.21 | 0.95 ± 0.17 | 1.07 ± 0.25 |
Values are means ± SD, each value is the average for six samples, * significant difference with C2C12 myotube treated with vehicle, P < 0.01; two-way ANOVA: main effect of dose, F = 10.4, p < 0.01; main effect of time, F = 4.8, p < 0.05; dose × time interaction, F = 1.1, p = 0.39)
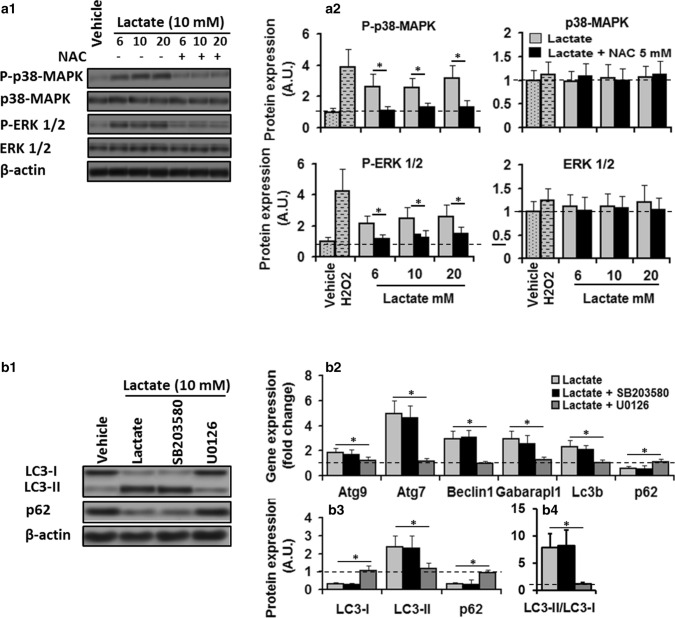
Fig. 2.
Activation of ERK1/2 was required for lactate-induced regulation of autophagy through ROS. a1 In-gel profile for densitometric scanning analysis of p38-MAPK and ERK l/2 and their phosphorylated level in C2C12 myotubes maintained in vehicle or H2O2, or lactate 10 mM for 8 h in the absence or presence of NAC. a2 Lactate increased phosphorylataion of p38-MAPK (two-way ANOVA: main effect of treatment, F = 90.1 p < 0.01; dose × treatment interaction, F = 1.5, p = 0.23) and ERK l/2 (two-way ANOVA: main effect of treatment, F = 113 p < 0.01; dose × treatment interaction, F = 0.86, p = 0.43) compared to control values (dashed lines), but its effects were deterred in the presence of NAC (two-way ANOVA: main effect of treatment, p38-MAPK, F = 56.8, p < 0.01; dose × treatment interaction, F = 0.53, p = 0.59; main effect of treatment, P-Erk1/2, F = 64.2, p < 0.01; dose × treatment interaction, F = 0.44, p = 0.64). b1 In-gel profile and b2–4 bar plots for autophagy-related gene expression and autophagic flux in C2C12 cells maintained in vehicle or lactate (6, 10, and 20 mM, 8 h) in the absence or presence of ERK1/2 inhibitor U0126 (2.5 µM) and p38-MAPK inhibitor SB203580 (10 µM) for 8 h. ERKl/2 inhibition by U0126 abolished the stimulatory effect of lactate on autophagy-related gene expression (one-way ANOVA, all p < 0.01) Atg9, F = 4.7; Atg7, F = 32.8; Beclin 1, F = 31.5; Gabarapl1, F = 13.9; LC3B, F = 18.7; p62, F = 10; all p < 0.01) and autophagic flux (one-way ANOVA: LC3B-I, F = 52; LC3B-II, 12.9; p62, F = 21.7, LC3B-II/LC3B-I, F = 19.3; all p < 0.01), but p38-MAPK inhibition by SB203580 had no effect, suggesting lactate-induced autophagy regulation in C2C12 myotubes is mediated through activation of ERK1/2. * Significant difference between groups (P < 0.01). N = 6 per group
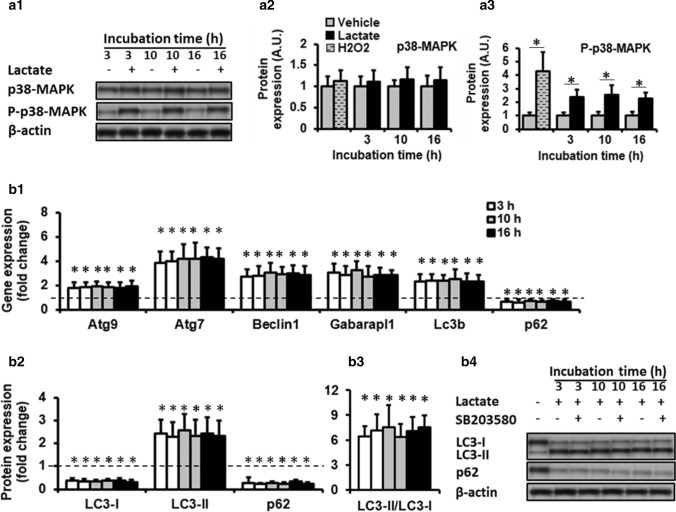
Fig. 3.
P38-MAPK pathway was not involved in regulating the autophagy by lactate. (a1 and b4) In-gel profile for densitometric scanning analysis of P-p38-MAPK, LC3-I, LC3-II, and p62. Three different incubation times (3 h, 10 h and 16 h) with lactate 10 mM significantly increased P-p38-MAPK (a3; two-way ANOVA: main effect of treatment, F = 90.3 p < 0.01) compared to control values, and upregulated autophagy-related gene expression (b1; two-way ANOVA: main effect of time, Atg9, F = 11.3; Atg7, F = 31.7; Beclin 1, F = 22.7; Gabarapl1, F = 26.2; LC3B, F = 16.7; p62, F = 14.5; all p < 0.01; and autophagic flux (b2, 3; two-way ANOVA: main effect of time, LC3B-I, F = 83.9; LC3B-II, F = 27.7; p62, F = 89.5; LC3B-II/LC3B-I, F = 13.2; all p < 001) compared to baseline values (dashed line). For autophagy-related gene expression, values expressed as mean ± SD of fold change from basal values (vehicle). For all protein expression the fold changes in protein levels were normalized for β-actin and relative to their expression in basal condition (vehicle). * Significant difference with basal values (P < 0.01). N = 6 per group
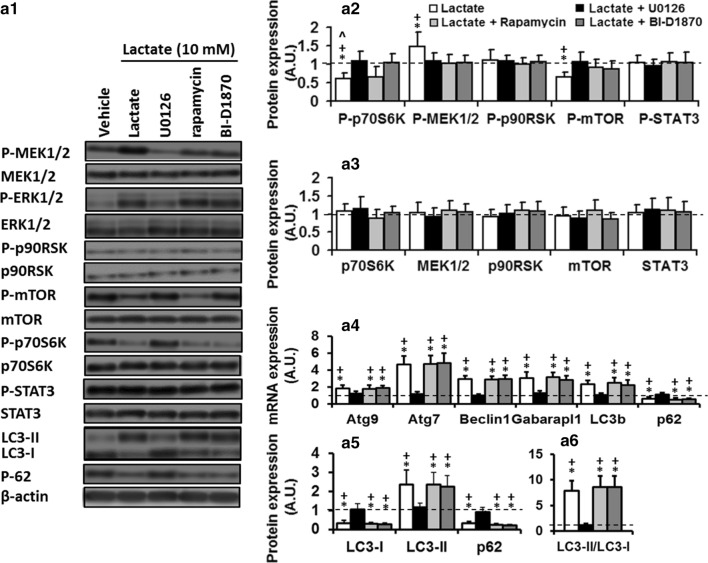
Fig. 4.
ERK1/2/mTOR/p70S6K pathway contributes to lactate-induced autophagy activation in differentiated C2C12 myotubes. a1–a3 Effects of U0126 (2.5 µM), Rapamycin (10 nM), or BI-D1870 (10 µM) on protein levels of ERK1/2/mTOR/p70S6K signaling pathway components in C2C12 cells maintained in the vehicle or lactate 10 mM for 8 h. Erk1/2 inhibition by U0126 deterred lactate effects on values of P-mTOR and p-p70S6K expression which restored them to their control values (dashed line). mTOR inhibition by SB203580 decreased p-p70S6K levels, p70S6K inhibition had no effect on P-ERK 1/2 and P-mTOR levels. a4 Autophagy-related gene expression and a5, 6 autophagic flux in C2C12 cells maintained in vehicle or lactate (6, 10, and 20 mM, 8 h) in the absence or presence of U0126, Rapamycin, or BI-D1870. For all protein measurements, fold changes in protein levels were normalized for β-actin and relative to their expression in basal condition (vehicle). * Significant difference with basal values (P < 0.01), + Significant difference with lactate + U0126 group (P < 0.01), ^ Significant difference with lactate + Rapamycin group (P < 0.01). N = 6 per group
Fig. 5.
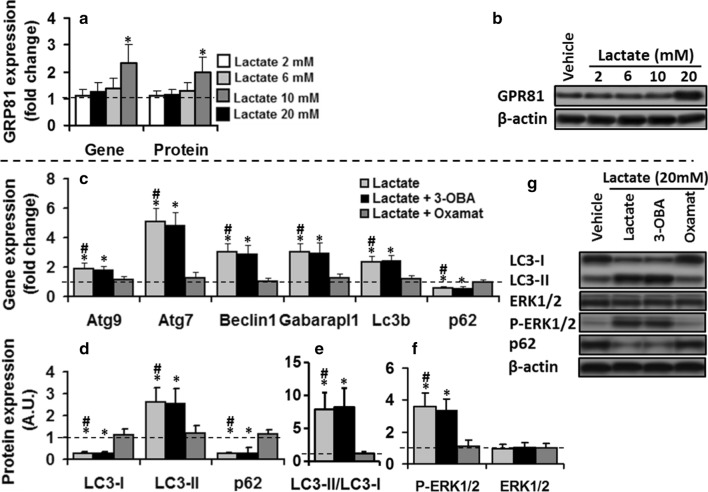
Changing the cellular redox associated to lactate oxidation is vital for ERK1/2-mediated autophagic effect of lactate on C2C12 myotubes. C2C12 cells maintained in the vehicle or lactate (2, 6, 10, and 20 mM, 8 h) in the absence or presence of 3-OBA (2 mM) or Oxamate (10 mM) for 8 h. a and b Only 20 mM lactate increased GPR81 gene and protein expression compared to the control values (dashed line). lactate oxidation inhibition by Oxamate abolished lactate stimulatory effect on autophagy-related gene expression c; one-way ANOVA, Atg9, F = 13.5; Atg7, F = 537; Beclin1, F = 34.3, Gabarapl1, F = 23.6; LC3B, F = 29.8, p62, F = 19.4; all p < 0.01), autophagic flux markers (d, e; one-way ANOVA: LC3B-II, F = 23.3; LC3B-II/LC3B-I, F = 142.1; LC3B-I, 32.5; p62, F = 19.4, all p < 0.01), and P-ERK 1/2 (f), inhibition of GPR81 by 3-OBA had no effect. The values expressed as mean ± SD of fold change from basal values (dashed line). For all protein measurement, fold changes in protein levels were normalized for β-actin and relative to their expression in basal condition (dashed line). * Significant difference with basal values (P < 0.01), # Significant difference with lactate + oxamate group (P < 0.01), N = 6 per group
Fig. 6.
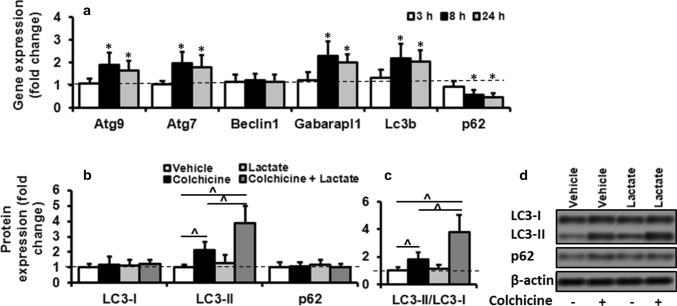
Lactate regulated autophagy in skeletal muscle in vivo. a The autophagy-related gene expression results in gastrocnemius muscles of rats injected with the vehicle or lactate. Animals euthanized at 3, 8, or 24 h following vehicle or lactate injection. All genes significantly increased following lactate treatment (two-way ANOVA: main effect of time, Atg9, F = 7; Atg7, F = 7.5; Gabarapl1, F = 8.6; LC3B, F = 4.3; p62, F = 6.6; all p < 0.01; main effect of the treatment, Atg9, F = 32.7; Atg7, F = 32; Gabarapl1, F = 58.5; LC3B, F = 46.6; p62, F = 42; treatment × dose interaction, Atg9, F = 7.2; Atg7, F = 7.7; Gabarapl1, F = 8.8; LC3B, F = 5.5; p62, F = 5.9; all p < 0.01). The values expressed as mean ± SD of fold change from basal values (PBS). # Significant difference with basal values (dashed line, P < 0.01), N = 6 per group. b–d In-gel profile for densitometric scanning analysis of LC3-I, LC3-II, and p62 in rats injected with PBS or colchicine (0.4 mg/kg/day), followed by a second injection of PBS or lactate. Animals euthanized at 24 h following second injection. The fold changes in protein levels were normalized for β-actin and relative to their expression in basal condition (PBS). Values expressed as mean ± SD. ^ Significant difference among groups (P < 0.01), N = 6 per group
Then, we sought to test whether the autophagy regulation induced by lactate is due to intracellular ROS generation. Differentiated C2C12 myotubes were pre-treated with NAC (a ROS scavenger) and incubated with 2, 6, 10, and 20 mM lactate for 60 min (Fig. 1e–g). ROS indicated significant dose effect (all p < 0.01), treatment effect (all p < 0.01), and treatment × dose interaction (all p < 0.01). ROS generation were increased following lactate treatment but had no changes following co-incubation with lactate and NAC (Fig. 1e, f). TBARS data indicated a significant dose effect (p < 0.01) and treatment effect (p < 0.01). TBARS levels were increased following lactate treatment but had no changes following co-incubation with lactate and NAC (Fig. 1g). TBARS levels were significantly lower in cells co-incubated with lactate and NAC compared to those values found in cells treated solely with lactate.
Furthermore, autophagy-related gene expression and autophagy flux in cells solely treated with lactate indicated significant dose effect (all p < 0.01), treatment effet (all p < 0.01), and treatment × dose interaction (all p < 0.01), while co-incubation with lactate and NAC did not induce any change in analyzed genes (Fig. 1a). Autophagy-related gene expression was significantly lower in cells co-incubated with lactate and NAC compared to those values found in cells treated solely with lactate (Fig. 1a). Moreover, in NAC pre-incubated myotubes, lactate exerted no effect on autophagy flux markers but enhanced LC3B-II protein intensity and LC3B-II/LC3B-I ratio and attenuated p62 and LC3-I in cells treated solely with lactate in a dose dependent manner (Fig. 1b, c). LC3B-II and LC3B-II/LC3B-I values were significantly lower, while LC3B-I and p62 were significantly higher in NAC pre-incubated myotubes compared to the cells treated solely with lactate. These results suggest that ROS may act as mediators of lactate actions on autophagy regulation in C2C12 myotubes.
ERK1/2 activation was required for lactate-induced regulation of autophagy through ROS
To understand the signaling pathway by which lactate regulates autophagy through ROS in C2C12 myotubes, this study evaluated whether mitogen-activated protein kinase signal transduction pathway (p-38-MAPK and Erk1/2) mediates lactate-induced up-regulation of autophagy in C2C12 myotubes. P-38-MAPK and Erk1/2 were shown implicated in mediating oxidative stress-induced regulation of autophagy in C2C12 myotubes (Ding et al. 2017; McClung et al. 2009). To this purpose, differentiated C2C12 myotubes were treated with 6, 10, and 20 mM lacate or vehicle for 8 h in the absence or presence of NAC or SB203580, and the level of P-p38-MAPK and P-Erk1/2 were measured. The values of P-p38-MAPK and P-Erk1/2 only revealed a significant treatment effect (all p < 0.01, Fig. 2a2). P-p38-MAPK and P-Erk1/2 values were significantly higher in cells treated with lactate compared to control values (Fig. 2a2).
These results suggest possible roles of p38-MAPK and Erk1/2 in ROS-mediated regulation of autophagy by lactate. Therefore, P-p38-MAPK and P-Erk1/2 levels in myotubes co-incubated with lactate (6, 10, 20 mM) and 5 mM NAC for 8 h were first measured to check whether elevated levels of ROS and lactate regulation of P-p38-MAPK and P-Erk1/2 are related. P-p38-MAPK and P-Erk1/2 values only indicated a significant treatment effect (all p < 0.01, (Fig. 2a2). P-p38-MAPK and P-Erk1/2 values were significantly lower in cells co-incubated with lactate and NAC compared to those values found in cells treated solely with lactate (Fig. 2a2). These results suggest that ROS mediate the lactate regulation of P-p38-MAPK and P-Erk1/2 in C2C12 myotubes. Then, to confirm the roles of P-p38-MAPK and P-Erk1/2 in regulating the autophagy by lactate, differentiated myotubes were pre-incubated with SB203580 (p38-MAPK inhibitor) or U0126 (ERK1/2 inhibitor) for 2 h and then treated with lactate 10 mM for 8 h. Pre-incubation of myotubes with SB203580 did not affect the stimulatory effect of lactate on autophagy-related gene expression (Fig. 2b2) and autophagy flux indexes (Fig. 2b3, 4), suggesting p38-MAPK is not involved in the lactate-induced regulation of autophagy. To further evaluate this key regulatory pathway’s involvement, the effect of 3 different incubation times (3 h, 10 h and 16 h) with lactate 10 mM were also tested. P-p38-MAPK values only indicated a significant treatment effect (all p < 0.01, Fig. 3a3). In any incubation time, lactate treatment significantly increased P-p38-MAPK compared to baseline values, while the vehicle did not induce any change (Fig. 3a3). Incubation time had no significant effect on the expression levels of P-p38-MAPK. Autophagy-related gene expression and autophagy flux in cells solely treated with lactate indicated significant time effect (all p < 0.01). Autophagy-related gene expression and autophagy flux were significantly higher compared to baseline values (time 0) (Fig. 3b1). However, there was no significant difference regarding the expression levels of autophagy-related gene expression and autophagy flux among 3 different incubation times (3 h, 10 h, and 16 h) (Figs. 3b1–4). Altogether these results suggest that the p38-MAPK pathway is not involved in regulating autophagy by lactate. On the other hand, co-incubation with U0126 prevented the lactate-induced changes in autophagy-related gene expression (Fig. 2b3). Autophagy-related gene expression was significantly lower in cells co-incubated with lactate and NAC compared to those values found in cells solely treated with lactate (one-way ANOVA, all p < 0.01) (Fig. 2b2). Moreover, in NAC pre-incubated myotubes, LC3B-II and LC3B-II/LC3B-I ratio were significantly lower, and p62 and LC3B-II had higher values compared to the cells solely treated with lactate (one-way ANOVA, all p < 0.01) (Fig. 2b3). These results suggest that lactate regulates autophagy in C2C12 myotubes with ROS participation, and P-Erk1/2 activation is required for this regulation.
To understand signaling pathways by which Erk1/2 mediates lactate’s autophagic effect, the expression levels of P-p70S6K, P-p90RSK, P-MEK1/2, P-mTOR, and P-STAT3 were analyzed in myotubes incubated with lactate or lactate and U0126 for 8 h. The results are reported in Fig. 4. P-MEK1/2 (50%) and Erk1/2 (112%) were increased in C2C12 myotubes treated with lactate, while p-mTOR (34%) and p-p70S6K (40%) expression were downregulated compared to control values (one-way ANOVA, all p < 0.01, Fig. 4a2). These results suggest ERK1/2/mTOR/p70S6K may be involved in the autophagic effect of lactate. To confirm this, myotubes were incubated in U0126 for 2 h and then treated with lactate 10 mM for 8 h. Erk1/2 inhibition decreased P-Erk1/2 and restored p-mTOR and p-p70S6K expression to their control values (Fig. 4a2), suggesting mTOR and p70S6K are indeed downstream targets of ERK1/2. Therefore, we hypothesized that ERK1/2/mTOR/p70S6K pathway positively regulates autophagy in C2C12 myotubes following lactate treatment. To confirm this, myotubes were incubated with rapamycin (mTOR inhibitor) or BI-D1870 (p90RSK inhibitor) for 2 h and then treated with lactate 10 mM for 8. Inhibition of mTOR by rapamycin did not change P-ERK1/2 expression, but significantly inhibited P-mTOR and P-p90RSK expression following lactate treatment, suggesting p90RSK may be a downstream target of ERK1/2/mTOR (Fig. 4a2). On the other hand, myotubes’ co-incubation with lactate and BI-D1870, a specific inhibitor of p90RSK, did not affect P-ERK1/2 P-mTOR expression (Fig. 4a2). All proteins’ expression did not differ from those values found in the myotubes treated solely with lactate. We measured autophagy level in C2C12 myotubes through inhibiting mTOR and p70S6K (Fig. 4a4, 5, 6). The autophagic effect of lactate occurred in the presence of mTOR and p70S6K inhibitors, while inhibition of ERK1/2 reversed their effect on autophagy. These data suggest that lactate regulates autophagy through ROS-mediated activation of the ERK1/2/mTOR/p70S6K pathway in skeletal muscle.
Lactate-induced regulation of autophagy is independent of GPR81
We were interested in understanding the molecular mechanism in which lactate activates P-ERK1/2 signaling pathway and regulates autophagy in C2C12 myotubes. Lactate was shown to activate signaling pathways either by binding to its receptor, G protein-coupled receptor 81 (GPR81), (Liu et al. 2009; Ohno et al. 2018; Rooney and Trayhurn 2011), or changing the cell’s redox balance resulting from its conversion to pyruvate (Galardo et al. 2014). First, GPR81 contribution was checked. GPR81, a selective receptor for lactate, is expressed in adipocytes as well as skeletal muscle cells in mice (Liu et al. 2009), and some biological action of lactate in skeletal muscle is mediated by GPR81 activation (Ohno et al. 2018; Rooney and Trayhurn 2011; Sun et al. 2016a). To address GPR81 contribution in the lactate regulation of P-ERK1/2 and autophagy, protein and mRNA of GPR81 were examined in C2C12 myotubes following treatment with 2, 6, 10, and 20 mM lactate for 8 h. Figure 5a shows that only treatment with 20 mM lactate induced a significant increase in GPR81 mRNA (one-way ANOVA, F = 43.3, p < 0.01), and protein expression (one-way ANOVA, F = 10.3, p < 0.01) compared to control values. These results suggest, at least at high doses, lactate induced GPR81 expression in C2C12 cells and raised the possibility of GPR81 contribution in P-ERK1/2-mediated autophagic effects of lactate in C2C12 myotubes. Therefore, to investigate whether the lactate-induced increase in ROS and autophagy-related gene expression is induced GPR81 activation, differentiated C2C12 myotubes were pre-treated to 3-OBA (a specific antagonist for GPR81) for 2 h, and incubated with 20 mM lactate for 8 h. Neither autophagy markers (Fig. 5c–e) nor P-ERK1/2 (Fig. 5f) was influenced GPR81 receptor inhibition, suggesting that P-ERK1/2-mediated autophagic effects of lactate in C2C12 myotubes may not be associated to its binding to GPR81.
Then, we checked the role of changing cellular redox balance associated to lactate conversion to pyruvate in autophagy regulation by lactate. Differentiated C2C12 myotubes were pre-treated with oxamate for 2 h which inhibits lactate oxidation to pyruvate utilizing LDH inhibition, and then incubated with 20 mM lactate for 8 h. Co-incubation significantly decreased P-ERK1/2 levels (one-way ANOVA, F = 19, p < 0.01)(Fig. 5f) which inhibited the stimulatory effect of lactate on autophagy-related gene expression (one-way ANOVA, all p < 0.01) compared to those values found in cells solely treated with lactate (Fig. 5c–e). Moreover, in myotubes incubated with lactate and oxamate, LC3B-II and LC3B-II/LC3B-I ratio were significantly lower, and p62 and LC3B-I had higher values compared to the cells solely treated with lactate (one-way ANOVA, all p < 0.01) (Fig. 5d, e). These results suggest that P-ERK1/2-mediated autophagic effect of lactate on C2C12 myotubes is mediated by changing cellular redox balance associated to lactate conversion to pyruvate rather than to its binding to GPR81. It is worth mentioning that in cell culture experiments with lactate and other additions, the possible effect of changing the medium’s osmolarity was not checked, which is a limitation of the current study.
In vivo study of autophagic effects of lactate
To understand whether in vitro effect of lactate on autophagy regulation in C2C12 myotube could occur in in vivo skeletal muscle, male wistar rats were intramuscularly injected with PBS or lactate, autopsied at 3, 8, or 24 h following injection, and autophagy-related gene expression was measured. Autophagy-related gene expression displayed significant time effect (all p < 0.01), treatment effet (all p < 0.01), and treatment × time interaction (all p < 0.01) (Fig. 6). Lactate treatment increased the transcription of autophagy-related gene expression in a time-dependent manner (Fig. 6a). While, PBS did not induce any change in analyzed genes. All genes remained unchanged 3 h following lactate injection compared to control values. However, in the majority of genes, mRNA abundances were significantly increased at 8 h after lactate injection which significantly remained higher than baseline values after 24 h (Fig. 6a).
Colchicine treatment significantly increased LC3-II level compared to those values found in the animals solely treated with PBS (one-way ANOVA, F = 42.1, p < 0.01) (Fig. 6b). Lactate treatment alone did not significantly increase LC3-II levels, however; lactate plus colchicine increased LC3-II levels which seen with colchicine alone (one-way ANOVA, F = 42.1, p < 0.01, Fig. 6b, c). Neither LC3-I nor p62 levels had a significant difference among the four groups.
Discussion
The current study examined the possible role of lactate in autophagy regulation in differentiated C2C12 myotubes. Four novel results were ascertained and discussed in more detail as follows: (1) lactate dose-dependently regulates autophagy in differentiated C2C12 myotubes with ROS participation; (2) lactate-induced regulation of autophagy through ROS is dependent on Erk1/2 activation, which might not be associated to its binding to GPR81; (3) changes in redox state associated to the conversion of lactate to pyruvate is vital to regulate the autophagy by lactate in skeletal muscle; (4) The in vitro effects of lactate on autophagy also occur in in vivo skeletal muscle.
The primary novel and most important finding of current study is a high concentration of lactate (6, 10, 20 mM) regulates autophagy, quantified by LC3-I lipidation, LC3-II flux, and measurements of autophagy-related gene expression in skeletal muscle. Our choice for using these lactate values was designed to reflect lactate values through rest (2 mM) and exercise in blood circulation (6 and 10 mM) (Hasanli et al. 2015) and skeletal muscle (20 mM) (Nikooie and Samaneh 2016). High lactate values caused LC3-II accumulation, an early autophagy biomarker and attenuated p62 expression. Upon activating the autophagic pathway, LC3-I is lipidated to form LC3-II which is specifically recruited to phagophore membrane. LC3-II levels therefore correlate well with autophagosome number (Glick et al. 2010). However, LC3-II could be found in some intracellular structures which could not develop into autophagic vacuoles (Xie and Klionsky 2007). Also, it can be targeted to phagosomes to promote maturation in a process called LC3-associated phagocytosis (Klionsky et al. 2016). Thus, the increase in LC3-II should be considered by caution when it is used as an index of autophagy. To avoid the problem of using LC3B-II intensity alone, expression level of p62, an autophagic adaptor protein which can be degraded during increased autophagy, expression was also checked in differentiated C2C12 myotubes treated with lacate. Increased LC3B-II was accompanied by reducing in P62 expression following lactate treatment, suggesting that lactate induces autophagy in differentiated C2C12 myotubes (Klionsky et al. 2016). The ability of lactate to regulate autophagy was previously indicated in SiHa (Brisson et al. 2016) and B16 melanoma cells (Matsuo and Sadzuka 2018). For the first time, we showed regulatory effect in C2C12 cells. The lack of lower lactate concentration effect (2 mM) on autophagy regulation could suggest that lactate cannot be involved in basal autophagy regulation which occurs under basal conditions when low levels of lactate are available (1 mM to 2.5 mM). In vivo blockage of LC3-II degradation with colchicine enabled us to quantitate autophagy induction following lactate treatment in mature skeletal muscle. We selected colchicine because rats well tolerate it, unlike other blockers (i.e., chloroquine and bafilomycinA1), and it rapidly increases LC3-II protein in mature skeletal muscle within two days of treatment (Ju et al. 2010). Significant increases in LC3-II amount in muscle from the colchicine-injected rats compared to vehicle-treated animals, indicated the success of method. Colchicine inhibits microtubule polymerization and impairs autophagosomes’ ability to travel to the lysosome for fusion and degradation (Ju et al. 2010). Consistence to our in vitro experiments, lactate was capable to regulate autophagy in vivo skeletal muscle, and lactate plus colchicine significantly increased LC3-II levels compared to colchicine treatment. Interpreting LC3-II levels using colchicine in vivo should consider compensatory response possibility against colchicine-induced blocking of autophagosome-lysosome fusion. However, prolonged use of colchicine (more than five days) is required to induce such a response, and it does not seem to be apparent at two days (Ju et al. 2010). Consistent to previous studies, no significant change in LC3-I and p62 protein levels following colchicine treatment in skeletal muscle (Ju et al. 2016; Ju et al. 2010), we found no significant difference between colchicine-injected rats and vehicle-treated animals regarding LC3-I and p62 protein levels. Notably, p62 levels were unchanged following colchicine treatment despite significant increase in LC3-II levels. These two substrates may have different degradation kinetics in mature skeletal muscle as compared to cell culture. Being associated to myofibrillar proteins such as titin, p62 may turnover more slowly than LC3-II (Lange et al. 2005).
To understand intracellular ROS role in lactate-induced autophagy regulation, we investigated whether ROS production undergoes any change due to lactate injection and how it may be involved in the autophagic effect of lactate. ROS levels were up-regulated, similar to those previously reported (Rahman et al. 2014; Willkomm et al. 2014), following lactate injection. This regulatory effect of lactate on ROS could be attributed either to its cellular metabolism catalyzed with LDH enzyme or to NAD (P)H oxidase activation. NADH generated during lactate oxidation to pyruvate by LDHB is associated to the changes in a cell’s redox state (NAD+/NADH) which can act as stimuli for ROS production. Also, lactate would be able to augment ROS production by activating NAD (P)H oxidase, membrane-bound epithelial superoxide (O2●−), and hydrogen peroxide (H2O2) producers, which is dependent on LDH activity (Mohazzab-H et al. 1997). The current findings showed that LDH inhibition by oxamate significantly abolished the stimulatory effect of lactate on ROS production which provided evidence for increased ROS through lactate oxidation. Nevertheless, ROS production through lactate-dependent NADH activity cannot be excluded. Also, it could be speculated that mitochondrial membranes are possible target places for lactate to enhance ROS formation (Willkomm et al. 2014). NAC deterred a lactate-induced increase in ROS content could support this notion because NAC’s antioxidant effects are mainly done in the mitochondrion (Moreira et al. 2007).
To determine whether the lactate-induced up-regulation of autophagy and ROS are related, the differentiated C2C12 myotubes were co-incubated with lactate and NAC. The results showed that ROS might act as mediators of lactate effect on autophagy. These results agree with previous studies in which ROS mediated the lactate up-regulation of PGC1-α in C2C12 (Nalbandian et al. 2019), p38-MAPK signaling pathways in germ (Galardo et al. 2014), and IL-8 expression in HUVEC cells (Végran et al. 2011). ROS’s nature mediating the autophagic effect of lactate in skeletal muscle cannot be determined from the present study. However, lactate was shown to up-regulate O2●− and H2O2 production in different tissue including skeletal muscles (Hashimoto et al. 2007), the liver (de Bari et al. 2010), and the heart (Mohazzab-H et al. 1997). O2●− and H2O2 can act as second messengers, regulating the transcription of several genes such as those involved in lactate oxidation in skeletal muscle cells. This response is associated to increased H2O2 levels (Hashimoto et al. 2007). Therefore, they could be candidates to mediate the autophagic effect of lactate in skeletal muscle. For more evaluation, the consequences of incubating C2C12 myotubes with pyrrolidine dithiocarbamate (PDTC), selective scavengers of O2●− (Shi et al. 2000), were studied, and the results were compared to those obtained from incubating C2C12 myotubes with NAC which mainly scavenge H2O2 (not shown). PDTC incubation did not completely deter lactate-induced increase in ROS production, as was found for NAC incubation suggest that H2O2 may be a more important regulator of lactate-induced autophagy regulation in C2C12 myotubes than other ROS. Nevertheless, the exact role of different ROS in regulating the autophagic effect of lactate in skeletal muscle should be elucidated in future studies.
To understand signaling pathways mediating the lactate-induced autophagy regulation through ROS in C2C12 myotubes, p-38-MAPK, and ERK1/2 changes were evaluated following lactate treatment. These pathways were selected because they are heavily involved in ROS-induced regulation of autophagy in C2C12 myotubes (Ding et al. 2017; McClung et al. 2009; Rossetti et al. 2018). The results showed that ERK1/2 activation, not p-38-MAPK, is vital for the autophagic effect of lactate in skeletal muscle. The lack of p-38-MAPK effect on the autophagic effect of lactate is hard to interpret because it is involved in autophagy regulation in skeletal muscle (Ding et al. 2017; McClung et al. 2009; Rossetti et al. 2018). The autophagic response mediated by p-38-MAPK may depend upon the nature of stimulus and strength and duration of activated MAPK pathways. The lack of p-38-MAPK effect on the autophagic effect of lactate, therefore, might be related to the time window of lactate incubation which was probably insufficient to induce enough transcription of p-38-MAPK which consequently affected the autophagy. To analyze this possibility, the effect of 3 different incubation times (3 h, 10 h, and 16 h) with lactate 10 mM were tested. 3 h incubation was used to check a possible early upregulation of P-p38-MAPK by lactate. Also, we test 10 h and 16 h incubation times to increase lactate stimulus strength. Neither a long time nor a short time further increased P-p38-MAPK expression and autophagy. No further up-regulation of P-p38-MAPK following longer incubation times indicates P-p38-MAPK was sufficiently expressed following lactate treatment which indicates P-p38-MAPK is not involved in lactate-induced regulation of autophagy in skeletal muscle. The stimulatory effect of 3 h and 16 h incubation with lactate on autophagy regulation was identical indicates even a short-time increase in muscle lactate concentration results in extensive regulation of autophagy in skeletal muscle. This result is so important from a physiological perspective since skeletal muscle lactate clearance usually occurs within 1 to 2 h. It means that every muscle lactate increase, at least in high concentration, plays a major regulatory role in muscle autophagy.
Considering previous researches which lactate activates ERK1/2 in skeletal muscle (Cerda-Kohler et al. 2018; Willkomm et al. 2014), current data indicated that lactate-induced up-regulation of ERK1/2 is necessary for the autophagic effect of lactate in C2C12 myotubes. ERK1/2, as a positive regulator of autophagy flux, negatively regulates mTOR, thereby promoting autophagy (Xu et al. 2018; Zhang et al. 2015). To check ERK1/2 role in autophagy regulation by lactate, the expression levels of ERK1/2 up- and downstream targets were analyzed following ERK1/2 inhibition. Lactate-induced augmentations of autophagy and reduction in mTOR and p70S6K were deterred following ERK1/2 inhibition. The fact that p90RSK, an ERK1/2 canonical downstream effector, was not changed following lactate treatment suggested ERK1/2 may cross-talk with other pathways or use non-canonical downstream effectors to regulate lactate-induced autophagy regulation in skeletal muscle. Because the inhibition of ERK1/2 blocked mTOR/p70S6K up-regulation, in which mTOR inhibition did not affect ERK1/2, it is proposed that ERK1/2/mTOR/p70S6K is a signaling pathway in which lactate regulates autophagy in skeletal muscle. Also, this notion could be indirectly supported by current finding that the autophagic effects of lactate in C2C12 myotubes were independent of GPR81 because lactate binding to GPR81 activates ERK1/2 canonical pathway in skeletal muscle (Li et al. 2014; Ohno et al. 2018).
Changing cellular redox associated to lactate oxidation was vital for ERK1/2-mediated autophagic effect of lactate, while binding to GPR81 was not required for lactate effects. However, lactate at high doses induced GPR81 expression at mRNA and protein levels in C2C12 myotubes. EC50 value for L-lactate to activate GPR81 is extremely high (~ 5 mM)(100), and 1.5–3 mM lactate is required to induction GPR81 in adipocytes where expression of GPR81 is very high (Liu et al. 2009). In skeletal muscle expressing lower values of GPR81, higher values of lactate (more than 16 mM) are required to induce GPR81 (Sun et al. 2016b). Since GRP81 induction was not involved in the autophagic effect of lactate in the current study, the GPR81 response probably acts as a positive feedback regulation of other lactate activities in muscle. It seems to be up-regulation of genes related to lactate metabolism, including proton-linked monocarboxylate transporters 1/4 (MCT1/4) which move lactate in and out of cells. This lactate function is mediated by GPR81-induced inhibition of the cyclic AMP-protein kinase A (cAMP-PKA) pathway (Sun et al. 2016b). There is also some evidence which lactate may induce intramuscular triglyceride (TG) storage and mitochondrial maintenance in myotubes (Sun et al. 2016b) and suppress stimulated, not basal lipolysis in adipocytes (De Pergola et al. 1989). All these lactate activities are mediated by GPR81 activation which require high levels of lactate (more than 16 mM). This condition is very similar to our results which showed increase in GRP81 expression only at lactate 20 mM.
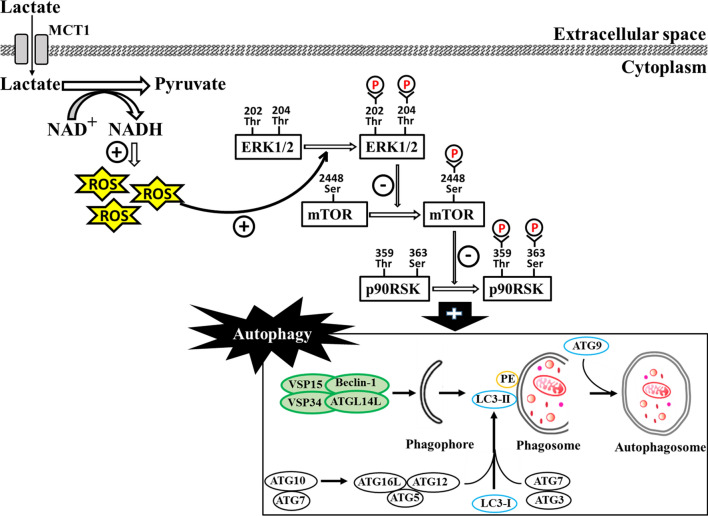
In conclusion, we provided the evidence of the lactate-associated regulation of autophagy in differentiated C2C12 myotubes through the activation of ROS-mediated ERK1/2/mTOR/p70S6K pathway (Fig. 7.). Lactate metabolism might have a significant contribution to skeletal muscle metabolism.
Fig. 7.
Changing cell’s redox balance (NAD +/NADH) resulting from lactate conversion to pyruvate augments reactive oxygen species (ROS) levels in differentiated C2C12 myotubes and mature skeletal muscle. The enhanced levels of ROS activate ERK1/2/mTOR/p70S6K signaling pathway, thereby increasing autophagy in skeletal muscle
Acknowledgements
We gratefully acknowledge the support of all our collaborators.
Authors’ contributions
Conception and design, drafting and editing the manuscript: Nikooie R; Writing the manuscript: Darioush moflehi; Data collection, analysis, and interpretation: Samira Zand.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not for-profit sectors.
Availability of data and material
Data are not shared.
Compliance wih ethical standards
Conflict of interest
No conflict of interest, financial or otherwise, is declared by the authors.
Ethics approval
All procedures in the present study were approved by the Ethical Committees on Animal Care at the Neuroscience Research Centre of Kerman, University of Medical Sciences, Kerman, Iran (No. 1398036IR.KMU.REC).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rohollah Nikooie, Email: r_nikooie@uk.ac.ir.
Daruosh Moflehi, Email: d_moflehi@uk.ac.ir.
Samira Zand, Email: zand.s.a.71@gmail.com.
References
- Aveseh M, Nikooie R, Sheibani V, Esmaeili-Mahani S. Endurance training increases brain lactate uptake during hypoglycemia by up regulation of brain lactate transporters. Mol Cell Endocrinol. 2014;394:29–36. doi: 10.1016/j.mce.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Brisson L, et al. Lactate dehydrogenase B controls lysosome activity and autophagy in cancer. Cancer Cell. 2016;30:418–431. doi: 10.1016/j.ccell.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Cerda-Kohler H, Henríquez-Olguín C, Casas M, Jensen TE, Llanos P, Jaimovich E. Lactate administration activates the ERK 1/2, mTORC 1, and AMPK pathways differentially according to skeletal muscle type in mouse. Physiol Rep. 2018;6:e13800. doi: 10.14814/phy2.13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bari L, Valenti D, Atlante A, Passarella S. l-Lactate generates hydrogen peroxide in purified rat liver mitochondria due to the putative l-lactate oxidase localized in the intermembrane space. FEBS Lett. 2010;584:2285–2290. doi: 10.1016/j.febslet.2010.03.038. [DOI] [PubMed] [Google Scholar]
- De Pergola G, et al. Influence of lactate on isoproterenol-induced lipolysis and b-adrenoceptors distribution in human fat cells. Horm Metab Res. 1989;21:210–213. doi: 10.1055/s-2007-1009193. [DOI] [PubMed] [Google Scholar]
- Ding H, Zhang G, Sin KWT, Liu Z, Lin RK, Li M, Li YP. Activin A induces skeletal muscle catabolism via p38β mitogen-activated protein kinase. J Cachexia Sarcopenia Muscle. 2017;8:202–212. doi: 10.1002/jcsm.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolny G, et al. Skeletal muscle is a primary target of SOD1 G93A-mediated toxicity. Cell Metab. 2008;8:425–436. doi: 10.1016/j.cmet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Echigoya Y, Morita S, Itou T, Sakai T. Effects of extracellular lactate on production of reactive oxygen species by equine polymorphonuclear leukocytes in vitro. Am J Vet Res. 2012;73:1290–1298. doi: 10.2460/ajvr.73.8.1290. [DOI] [PubMed] [Google Scholar]
- Gabriel-Costa D, et al. Lactate up-regulates the expression of lactate oxidation complex-related genes in left ventricular cardiac tissue of rats. PLoS ONE. 2015;10:e0127843. doi: 10.1371/journal.pone.0127843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galardo MN, Regueira M, Riera MF, Pellizzari EH, Cigorraga SB, Meroni SB. Lactate regulates rat male germ cell function through reactive oxygen species. PLoS ONE. 2014;9:e88024. doi: 10.1371/journal.pone.0088024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanli M, Nikooie R, Aveseh M, Mohammad F. Prediction of aerobic and anaerobic capacities of elite cyclists from changes in lactate during isocapnic buffering phase. J Strength Cond Res. 2015;29:321–329. doi: 10.1519/JSC.0000000000000640. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J. 2007;21:2602–2612. doi: 10.1096/fj.07-8174com. [DOI] [PubMed] [Google Scholar]
- Hunt TK, et al. Aerobically derived lactate stimulates revascularization and tissue repair via redox mechanisms. Antioxid Redox Signal. 2007;9:1115–1124. doi: 10.1089/ars.2007.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju J-S, Varadhachary AS, Miller SE, Weihl CC. Quantitation of” autophagic flux” in mature skeletal muscle. Autophagy. 2010;6:929–935. doi: 10.4161/auto.6.7.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju J-s, Jeon S-i, Park J-y, Lee J-y, Lee S-c, Cho K-j, Jeong J-m. Autophagy plays a role in skeletal muscle mitochondrial biogenesis in an endurance exercise-trained condition. J Physiol Sci. 2016;66:417–430. doi: 10.1007/s12576-016-0440-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keles M, Taysi S, Sen N, Aksoy H, Akcay F. Effect of corticosteroid therapy on serum and CSF malondialdehyde and antioxidant proteins in multiple sclerosis. Can J Neurol Sci. 2001;28:141–143. doi: 10.1017/s0317167100052823. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S, et al. The kinase domain of titin controls muscle gene expression and protein turnover. Science. 2005;308:1599–1603. doi: 10.1126/science.1110463. [DOI] [PubMed] [Google Scholar]
- Latham T, et al. Lactate, a product of glycolytic metabolism, inhibits histone deacetylase activity and promotes changes in gene expression. Nucleic Acids Res. 2012;40:4794–4803. doi: 10.1093/nar/gks066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Wang H-q, Wang L-h, Chen R-p, Liu J-p. Distinct pathways of ERK1/2 activation by hydroxy-carboxylic acid receptor-1. PLoS ONE. 2014;9:e93041. doi: 10.1371/journal.pone.0093041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, et al. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J Biol Chem. 2009;284:2811–2822. doi: 10.1074/jbc.M806409200. [DOI] [PubMed] [Google Scholar]
- Luo S-T, et al. The promotion of erythropoiesis via the regulation of reactive oxygen species by lactic acid. Sci Rep. 2017;7:38105. doi: 10.1038/srep38105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri M, Nikooie R, Keshtkar A, Larijani B, Omidfar K. Effect of endurance training on retinol-binding protein 4 gene expression and its protein level in adipose tissue and the liver in diabetic rats induced by a high-fat diet and streptozotocin. J Diabetes Investig. 2014;5:484–491. doi: 10.1111/jdi.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T, Sadzuka Y. Extracellular acidification by lactic acid suppresses glucose deprivation-induced cell death and autophagy in B16 melanoma cells. Biochem Biophys Res Commun. 2018;496:1357–1361. doi: 10.1016/j.bbrc.2018.02.022. [DOI] [PubMed] [Google Scholar]
- McClung JM, Judge AR, Powers SK, Yan Z. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol. 2009;298:C542–C549. doi: 10.1152/ajpcell.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mofarrahi M, et al. Autophagic flux and oxidative capacity of skeletal muscles during acute starvation. Autophagy. 2013;9:1604–1620. doi: 10.4161/auto.25955. [DOI] [PubMed] [Google Scholar]
- Mohazzab-H KM, Kaminski PM, Wolin MS. Lactate and PO2 modulate superoxide anion production in bovine cardiac myocytes: potential role of NADH oxidase. Circulation. 1997;96:614–620. doi: 10.1161/01.cir.96.2.614. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Harris PL, Zhu X, Santos MS, Oliveira CR, Smith MA, Perry G. Lipoic acid and N-acetyl cysteine decrease mitochondrial-related oxidative stress in Alzheimer disease patient fibroblasts. J Alzheimers Dis. 2007;12:195–206. doi: 10.3233/jad-2007-12210. [DOI] [PubMed] [Google Scholar]
- Nalbandian M, Radak Z, Takeda M. N-acetyl-l-cysteine prevents Lactate-Mediated PGC1-alpha expression in C2C12 Myotubes. Biology. 2019;8:44. doi: 10.3390/biology8020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikooie R, Samaneh S. Exercise-induced lactate accumulation regulates intramuscular triglyceride metabolism via transforming growth factor-β1 mediated pathways. Mol Cell Endocrinol. 2016;419:244–251. doi: 10.1016/j.mce.2015.10.024. [DOI] [PubMed] [Google Scholar]
- Ohno Y, et al. Lactate increases myotube diameter via activation of MEK/ERK pathway in C2C12 cells. Acta Physiol. 2018;223:e13042. doi: 10.1111/apha.13042. [DOI] [PubMed] [Google Scholar]
- Rahman M, Mofarrahi M, Kristof AS, Nkengfac B, Harel S, Hussain SN. Reactive oxygen species regulation of autophagy in skeletal muscles. Antioxid Redox Signal. 2014;20:443–459. doi: 10.1089/ars.2013.5410. [DOI] [PubMed] [Google Scholar]
- Risson V, et al. Muscle inactivation of mTOR causes metabolic and dystrophin defects leading to severe myopathy. J Cell Biol. 2009;187:859–874. doi: 10.1083/jcb.200903131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney K, Trayhurn P. Lactate and the GPR81 receptor in metabolic regulation: implications for adipose tissue function and fatty acid utilisation by muscle during exercise. Br J Nutr. 2011;106:1310–1316. doi: 10.1017/S0007114511004673. [DOI] [PubMed] [Google Scholar]
- Rossetti ML, Steiner JL, Gordon BS. Increased mitochondrial turnover in the skeletal muscle of fasted, castrated mice is related to the magnitude of autophagy activation and muscle atrophy. Mol Cell Endocrinol. 2018;473:178–185. doi: 10.1016/j.mce.2018.01.017. [DOI] [PubMed] [Google Scholar]
- Sanchez AM, Csibi A, Raibon A, Cornille K, Gay S, Bernardi H, Candau R. AMPK promotes skeletal muscle autophagy through activation of forkhead FoxO3a and interaction with Ulk1. J Cell Biochem. 2012;113:695–710. doi: 10.1002/jcb.23399. [DOI] [PubMed] [Google Scholar]
- Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Leonard SS, Wang S, Ding M. Antioxidant properties of pyrrolidine dithiocarbamate and its protection against Cr(VI)-induced DNA strand breakage. Ann Clin Lab Sci. 2000;30:209–216. [PubMed] [Google Scholar]
- Sun J, Ye X, Xie M, Ye J. Induction of triglyceride accumulation and mitochondrial maintenance in muscle cells by lactate. Sci Rep. 2016;6:33732. doi: 10.1038/srep33732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Ye X, Xie M, Ye J. Induction of triglyceride accumulation and mitochondrial maintenance in muscle cells by lactate. Sci Rep. 2016;6:1–10. doi: 10.1038/srep33732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauffenberger A, Fiumelli H, Almustafa S, Magistretti PJ. Lactate and pyruvate promote oxidative stress resistance through hormetic ROS signaling. Cell Death Dis. 2019;10:1–16. doi: 10.1038/s41419-019-1877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Végran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71:2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- Wang H, Joseph JA. Quantifying Cellular Oxidative Stress by Dichlorofluorescein Assay Using Microplate Reader. Free Radic Biol Med. 2005;39:1290. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- Willkomm L, et al. Lactate regulates myogenesis in C2C12 myoblasts in vitro. Stem Cell Res. 2014;12:742–753. doi: 10.1016/j.scr.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- Xu X, et al. ERK1/2/mTOR/Stat3 pathway-mediated autophagy alleviates traumatic brain injury-induced acute lung injury. Biochim Biophys Acta. 2018;1864:1663–1674. doi: 10.1016/j.bbadis.2018.02.011. [DOI] [PubMed] [Google Scholar]
- Young AR, et al. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J Cell Sci. 2006;119:3888–3900. doi: 10.1242/jcs.03172. [DOI] [PubMed] [Google Scholar]
- Yu L, Chen Y, Tooze SA. Autophagy pathway: cellular and molecular mechanisms. Autophagy. 2017;14(2):207–215. doi: 10.1080/15548627.2017.1378838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, et al. Autophagy protects against ischemia/reperfusion-induced lung injury through alleviating blood–air barrier damage. J Heart Lung Transplant. 2015;34:746–755. doi: 10.1016/j.healun.2014.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are not shared.