Abstract
Endocytic pathways provide the primary route for therapeutic and diagnostic nanoparticles (NPs) to enter cells and subcellular compartments. A better understanding of these cell entry processes will not only aid in nanomaterial applications but also broaden our knowledge of cell biology. Among the endocytic routes, macropinocytosis has unique characteristics for engulfing NPs and other large cargo, yet its molecular machinery and involvement in NP uptake are far less characterized relative to other pathways. In this review, we summarize the current knowledge on the macropinocytic machinery, and its involvement in NP internalization. Particularly, we differentiate ligand (specifically peptide)-functionalized and unfunctionalized NPs (bystander NPs). While most of previous research focused on ligand-functionalized NPs, we showcase here a synergistic effect between these two NP types during their cell entry through receptor-mediated macropinocytosis. The regulation of NP uptake efficiency by extracellular amino acids is also highlighted in the context of interconnections between macropinocytosis and metabolic signaling. These discussions may fuel future research interests in improving NP internalization through this pathway, and open a new avenue to study the interplay among endocytosis, metabolism and nanomedicine.
Introduction
Nanoparticles (NPs) are typically defined as entities with a diameter between 1 and 100 nm. Due to their tunable physicochemical characteristics (e.g. size, shape and surface chemistry), NPs have proven useful as therapeutic and imaging agents [1]. Since the liposomal doxorubicin, Doxil, was first approved in 1995, nanomedicine has attracted great interest in both academia and pharmaceutical industry [2]. One common prerequisite for NP-formulated drugs to achieve desirable clinical outcomes is efficient entry into cells or even certain subcellular compartments [3]. Therefore, it is important to understand cellular pathways for NP entry into cells, and explore ways to increase their efficiency.
The plasma membrane of mammalian cells is made of a lipid bilayer that separates the intracellular region from the outside environment, and regulates the movement of substances in and out of cells. It allows small molecules, such as ions and amino acids, to pass through it freely or via specific transmembrane transporters and ion channels [4]. Macromolecules, such as proteins and nanosized particles, however, generally cannot permeate the cell membrane by diffusion. Moreover, hydrophilic polymers are often coated on NPs to increase solubility in the circulation, but they form a barrier for NPs to pass through lipophilic structures (e.g. the plasma membrane). Therefore, NP internalization is mainly mediated by an active cellular process, endocytosis [5]. One type of endocytosis is phagocytosis (cell eating). This occurs in phagocytic cells, such as dendritic cells, neutrophils and macrophages, and is specialized in engulfing large solid particles (~ μm in diameter) [6]. The other type is pinocytosis (cell drinking). Generally, relatively small particles are internalized by this process, which occurs in almost all mammalian cells. Pinocytosis is further subdivided into macropinocytosis, clathrin-mediated endocytosis, caveolin-mediated endocytosis [6].
In this review, we will first provide a brief overview of endocytic pathways. Then we will focus on macropinocytosis, which has unique characteristics to mediate NP internalization. While the majority of previous studies were centered around ligand-functionalized NPs [7], we will also discuss bystander NPs, which are not equipped with cell-penetrating ligands and thus cannot enter the cells by themselves or very inefficiently. However, an important observation is that a synergistic transport exists between peptide-functionalized and bystander NPs [8]. Finally, we will review the regulation of amino acid concentration on NP internalization, and its connection to metabolic signaling and macropinocytosis machinery.
Endocytic pathways and their roles in nanoparticle uptake
The main physiological function of phagocytosis is to eliminate exogeneous pathogens as well as endogenous senescent cells [9]. When NPs are systemically administered, immunoglobins, complement components and other serum proteins absorb onto the NP surface and form a protein shell, which facilitates adherence to phagocytic cells (opsonization) [10]. These opsonized NPs then bind to cell surface receptors, including Fc receptors, complement receptors, mannose receptors and scavenger receptors. The receptor-ligand interaction triggers a signaling cascade mediated by Rho-family GTPases, which involves activation of Cdc42, Rac and downstream kinases [11]. As a result, actin rearrangements induce cup-like membrane protrusion around the particle in preparation for engulfment [11]. With complete encirclement, the resulting phagosome carries the cargo throughout the cytoplasm to the lysosome. The lysosome fuses with the phagosome to form a phagolysosome for the purpose of degrading the NPs. With destruction, the NP payload is released. Relatively large particles are usually engulfed by phagocytosis, as phagosomes are sufficiently large (> 250 nm) [12]. Champion et al. described the striking effect of the particle shape on phagocytosis by fabricating six different shapes of polystyrene NPs in the range of 1 – 10 μm. They found that NPs with a high aspect ratio underwent minimal phagocytosis [13]. Due to the presence of phagocytic cells in the reticulum endothelium system (RES) organs, such as liver and spleen, considerable effort has been devoted to avoiding phagocytosis and thus increasing the accumulation of NPs at the diseased sites [14]. Notable examples include coating NP surface with poly (ethylene glycol) (PEG) or “marker of self” CD47 peptide [15, 16].
Clathrin-mediated endocytosis (CME) is the major pathway for receptor-mediated endocytosis, which has been estimated to contribute to over 95% of endocytic flux [17]. The major structural protein of its endocytic vesicles is clathrin, which has led to the vesicle name as clathrin-coated pits. These vesicles are 60 – 120 nm in diameter [18]. The sequential steps of the CME process has been reviewed elsewhere in greater detail [18]. The endocytosed vesicles are merged into early endosomes, which are further sorted into either recycling endosomes and returned to the plasma membrane or degradative lysosomes resulting in drug and/or content release [5]. CME appears to be the most prominent mechanism for cells to engulf a wide range of NPs functionalized with ligands, such as transferrin, low-density lipoprotein and epidermal growth factor [19]. CME also provides a pathway, albeit minor, for unfunctionalized NPs provided that they possess the required physicochemical properties. Vasir et al. reported that CME-mediated uptake of poly (D, L-lactide-co-glycolide) NPs was increased with poly-L-lysine functionalization compared to those unmodified [20]. Furthermore, pH-sensitive and enzyme-cleavable modifications of NPs can be exploited as two strategies to enhance endosomal escape [5]. For example, Benyettou et al. found that by using the acid-mediated release of payloads, the NP delivery system showed significantly improved anticancer outcomes [21].
Caveolin-mediated endocytosis (CavME) is driven by flask-shaped membrane invaginations called caveolae [22]. These endocytic vesicles are 60 – 80 nm in diameter and rich in cholesterol and sphingolipids [6]. Upon specific receptor-ligand interactions, cargo, such as NPs, is concentrated in caveolae invaginations [23]. Subsequent activation of signaling molecules, including G proteins, kinases and endothelial nitric-oxide synthase, trigger caveolae endocytosis. Similar to CME, GTPase dynamin oligomerizes around the “flask” neck to pinch off membrane vesicles. The resulting cytosolic caveosomes are ultimately delivered to the Golgi apparatus and endothelium reticulum, and thereby the degradative lysosomes are avoided. Therefore, NPs can take use of this internalization mechanism to target specific organelles and avoid the hydrolytic sensitivity. For example, Xin et al. reported rod-shaped drug particles enabled efficient delivery of miRNA, which bypassed the lysosomal route in cytosol [24]. The role of caveolae is also associated with transcytosis in endothelial cells, allowing NPs to overcome the endothelial barrier in the body and increase the exposure to diseased tissues. Work by Liu et al. demonstrated that polyamidoamine dendrimer NPs modified with a rabies virus glycoprotein had a higher ability to cross the blood-brain barrier through CavME [25]. It has been reported that anionic particles and specific surface ligands, such as folic acid, albumin and cholesterol, undergo uptake by this mechanism [26, 27].
Molecular machinery of macropinocytosis
Macropinocytosis is characterized by actin-dependent formation of large-sized (200 nm - 5 μm in diameter) membrane protrusions [28]. It occurs in almost all cell types and has been implicated a diverse array of physiological functions [29]. It is commonly regarded as a nonselective pathway for cells to engulf extracellular fluid and the solutes contained therein. Its non-selective nature is suggested by the fact that the cargo does not rely on binding to specific cellular receptors to initiate the internalization process. Macropinocytosis is distinct from other endocytic pathways mainly in the following aspects. First, there is no known structural protein to define macropinosomes (like clathrin for CME or caveolin for CavME). Second, the size of macropinosomes is heterogeneous and much larger than clathrin-coated pits and caveolae. Although the sizes of endocytic vacuoles of phagocytosis and macropinocytosis overlap to some extent, phagocytosis almost solely occurs in phagocytic cells (e.g. macrophages and dendritic cells) initiated by interactions between cellular receptors and pathogen components [9]. In contrast, macropinocytosis can be receptor-independent, accounts for fluid uptake instead of large solid particles, and occurs in almost all cell types.
Innate immune cells, such as macrophages and dendritic cells, undergo a high rate of macropinocystosis constitutively to engulf sample antigens from the nearby environment [30]. Many viruses and pathogenic bacteria were reported to promote membrane ruffling and macropinosome formation via this pathway, including adenovirus, Ebola virus and Legionella pneumophila [31, 32]. The activity of this process depends on extracellular calcium [33]. Depletion of calcium in the medium leads to the loss of membrane ruffles. A G protein-coupled calcium sensing receptor (CaSR) on the cell surface is responsible for calcium sensation and activation of phosphatidylinositol 3-kinase (PI3K) and phospholipase C (PLC), which are involved in actin filaments nucleation [34]. Malignant cells can also adapt macropinocytosis to acquire extracellular nutrients (e.g. proteins) for survival and proliferation, which serves as a hallmark of cancer metabolism [35]. The macropinocytotic internalized proteins undergo degradation into intracellular amino acids that can fuel metabolism and cell growth [35]. Growth factors, including epidermal growth factor, platelet-derived growth factor and colony-stimulating factor-1 or tumor-promoting factor, can stimulate the activity of macropinocytosis [10]. Depletion of nutrients can drive activation of growth factor receptors in a variety of cancer cells, notable examples of which are those bearing Ras oncogenic mutations [29, 36, 37]. As the central regulator, mechanistic target-of-rapamycin complex-1 (mTORC1) coordinates these external signaling and controls the subsequent metabolic responses [28]. To be specific, growth factors activate the PI3K-Akt pathway, which then activates Rheb. Moreover, amino acids can activate two GTPases Rag and Rheb on the lysosomal surface. After being recruited by Rag, mTORC1 is directly activated by Rheb, which functions to increase protein synthesis and inhibit autophagy (Figure 1) [28].
Figure 1. Endocytic pathways and macropinocytosis-mediated activation of mTOR signaling.
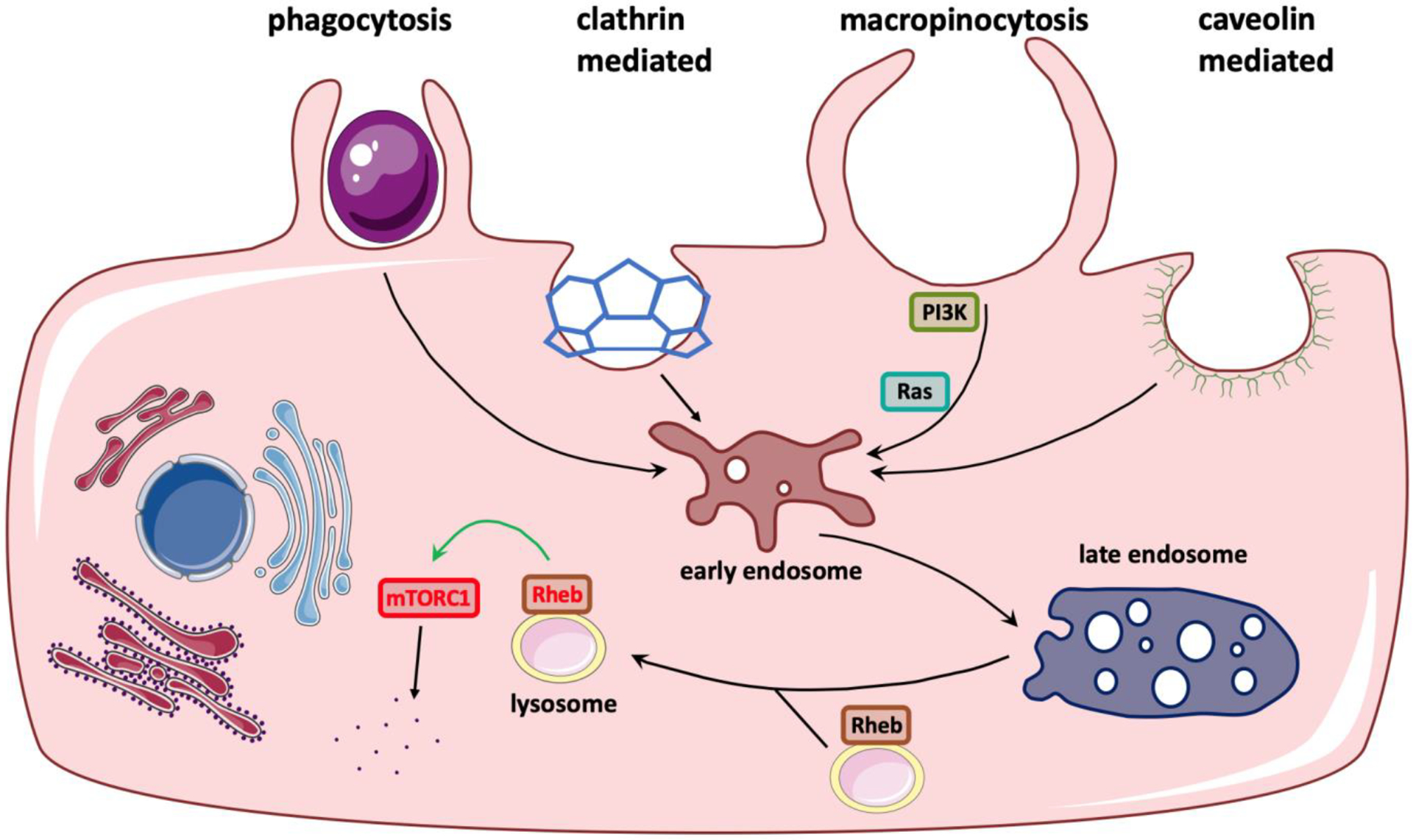
NP enter cells via multiple mechanisms of endocytosis, including phagocytosis, clathrin-mediated, caveolin-mediated, or macropinocytosis. PI3K is recruited to enhance PI (4,5) P2 conversion to PIP3 which accumulate in macropinocytic cups. Ruffles and early macropinosomes are active sites for Akt signaling, resulting in direct Rheb activation on the lysosomal surface. Formation of complete macropinosomes relies on Ras. As the central regulator of cell growth, mTORC1 is then activated by Rheb.
The irregular movements of membrane ruffles initially transform into cup-like structures and then close fully to form mature macropinosomes [38]. During the initial macropinocytic cup formation, PI3K transiently converts phosphatidylinositol 4,5-bisphosphate [PI (4,5) P2] on the cytosolic side of the membrane into phosphatidylinositol 3,4,5-trisphosphate (PIP3), which can be further hydrolyzed into diacylglycerol (DAG) by PLC [39]. The resulting PIP3 spike is associated with recruitment of several guanine nucleotide exchange factors (GEFs), which can activate Rho-family GTPases, including Rac1/2 and Cdc42. These in turn promote the Arp2/3-dependent branched actin polymerization. During the cup closure into the macropinosome, Ras is considered to be crucial. It can not only regulate PI3K via its Ras-binding domain but also directly activate the formin ForG, a cytoskeletal protein involved in actin assembly [40]. An early study showed that Hela cells expressing a mutated Ras gene exhibited elevated levels of membrane ruffles and macropinocytosis [41]. Vacuolar ATPase (V-ATPase) trafficking to the plasma membrane is a prerequisite for ruffles in oncogenic Ras-transformed cells [42]. However, MEF cells with triple knockout of Ras still retained the capability of performing macropinocytosis [43]. It remains largely undefined whether the role of Ras can be performed by other proteins, or PIP3 alone is sufficient to form complete macropinosomes.
After macropinosomes are fully formed, PIP3 is sequentially dephosphorylated, while DAG is converted to phosphatidic acid (PtdOH), which continues to bolster the activation of actin nucleation [40]. Macropinosomes then move along the microtubule network and fuse with lysosomes, although recycling to the cell surface does rarely occur [28]. During cup closure, the Rab GTPase family begins to accumulate on macropinosomes and continues throughout their maturation [39]. Specifically, localization of Rab5 marks newly internalized vesicles, whereas Rab7 is associated with later phases [44]. In the early stage, macropinosomes show the ability of tubulation and shrinkage to concentrate the vesicle cargo that would require the removal of membrane and water [45]. After fusion with lysosomes in the late stage, V-ATPase accumulates on the membrane to acidify quickly the macropinosomal lumen, and lysosomal hydrolases are activated to degrade their substrates [34]. Amino acids either delivered directly into the lysosomes or generated from protein degradation can activate Rheb, which activates mTORC1 subsequently [46]. For macrophages and dendritic cells, the intracellular destinations are different from those reported in other cell types [39]. Macropinosomes in these cells often fuse with both early and late endosomes from other endocytic pathways, such as CME and CavME [32]. This may facilitate the digested peptides to bind to the Major Histocompatibility Complex II-loaded vesicles for antigen presentation.
Directing NPs to macropinocytosis as the pathway for internalization would provide several advantages over other endocytic pathways. First, its relatively large and heterogenous endocytic vesicles are more suitable to accommodate a greater capacity of NPs of various sizes and shapes than that of CME or CavME pathway [32]. Second, compared to phagocytosis, macropinocytosis provides a universal route into a much broader range of cell types. Third, a high rate of macropinocytosis occurs constitutively in antigen-presenting immune cells and many cancer cells, which can be advantageous for certain NP applications. One such example is NP-based vaccination [86]. The strong macropinocytotic activity in immature dendritic cells allows efficient engulfment of peptide antigens conjugated to NPs, which boosts vaccination efficacy. Macropinocytic uptake of nab-paclitaxel, an albumin-formulated NP carrying paclitaxel, was also shown to induce the polarization of tumor-associated macrophages towards an immunostimulatory phenotype [47]. This result is significant as it indicates that immunity can be modulated through this pathway. As the functional relevance of macropinocytosis is linked with nutrient acquisition, its metabolic role is highlighted under pathological conditions. NP associated with proteins, such as albumin-bound fatty acids, are more likely to enter cancer cells via macropinocytosis [87]. A lipoprotein-based NP system carrying miRNA in the core achieved efficient accumulation and therapeutic effect in gliomas through CXCR4 receptor-stimulated macropinocytosis [48]. Although the large size of macropinosomes is considered to favor uptake of nano- and micro-sized particles, many NPs have been reported to utilize more than one endocytic route [6]. For instance, a cationic and hydrophobic glycol chitosan NP with the mean diameter of 359 nm could be internalized by multiple distinct pathways, including CME, CavME and macropinocytosis [49]. To trace macropinocytosis, 70kDa Dextran (Dextran thereafter if not indicated otherwise) is a commonly-used fluid-phase marker as its large hydrodynamic size excludes the possibility of being engulfed by CME and CavME [50].
Peptide-functionalized nanoparticles and macropinocytosis
In order to enter the cells, NPs are often functionalized with cell-penetrating ligands. Notable examples are cell-penetrating peptides (CPPs), a family of short peptides (typically 5 – 30 amino acids) capable in crossing cellular membranes [51]. Coupling cargo with CPPs by either covalent bonds or noncovalently electrostatic/hydrophobic interactions enables the conjugates to access their intracellular targets, thereby increasing the therapeutic effect [52]. Based on the physical-chemical properties, CPPs can be divided into three classes: cationic, amphipathic and hydrophobic peptides. Cationic CPPs, mainly rich in arginine and lysine, were discovered first and consist of the majority of known CPPs [51]. They have been widely used in carrying diverse cargo types into cell, such as proteins, nucleic acids, small molecule drugs and NPs [53].
Not surprisingly, the cell entry mechanisms of cationic CPPs are also relatively well-characterized [54, 55]. One such example is CendR peptides. These peptides contain a positively charged R/KXXR/K motif at their C-terminus (thus C-end Rule, CendR) [56]. They were discovered using in vivo phage display, a powerful technology to identify tissue-homing peptides under physiological conditions [57]. The prototypic tumor-homing CendR peptide, iRGD, exhibits two unique properties. First, iRGD can selectively recognize tumor vasculatures and penetrate across the vessel wall and deep into the extravascular tumor tissues [58]. This tumor-penetrating property applies to the peptide itself and its coupled cargo, which can range from small molecules to NPs. Second, iRGD can also enhance the tumor penetration and accumulation of co-administered but not covalently linked cargo [59]. This property is termed as the “bystander effect”. The implication of this discovery is highly significant, because the delivery and antitumor efficacy of clinically approved drugs can be enhanced by simple co-administration instead of chemical modifications. The tumor penetration and bystander effect were found with other CendR peptides as well [79, 80, 81, 82].
CendR peptides were demonstrated to invoke endocytosis upon binding to the cell surface receptor, neuropilin-1 (NRP1) [60]. Neuropilins are non-tyrosine kinases of 120 – 130 kDa and are widely expressed in various tissue types, including endothelium and solid tumors [61]. Their physiological functions are implicated in angiogenesis and neuron development. NRP1, and NRP2 to a lesser extent, serve as receptors or co-receptors for multiple growth factors, for example, vascular endothelial growth factor (VEGF)-A isoforms [62]. Particularly, VEGF-A165 contains an active CendR motif [63]. A prototypic CendR peptide, RPARPAR, was shown to enhance vascular permeability upon subcutaneous injection similarly as VEGF. This led to the initial but incorrect speculation that the bystander effect mechanism was achieved by increasing the vascular permeability locally in tumors. However, later studies demonstrated CendR peptides use an active transport process for vascular and tissue penetration, rather than passive diffusion through intercellular gaps in highly permeable vessels, [56, 58, 64]. Liu et al. directly visualized iRGD-mediated vascular penetration in tumors by transmission electron microscopy (TEM) and further proved that iRGD-NP complexes crossed the endothelium via transcytosis (Figure 2A) [65].
Figure 2. Receptor-mediated macropinocytosis and the uptake of CPP-functionalized nanoparticles.
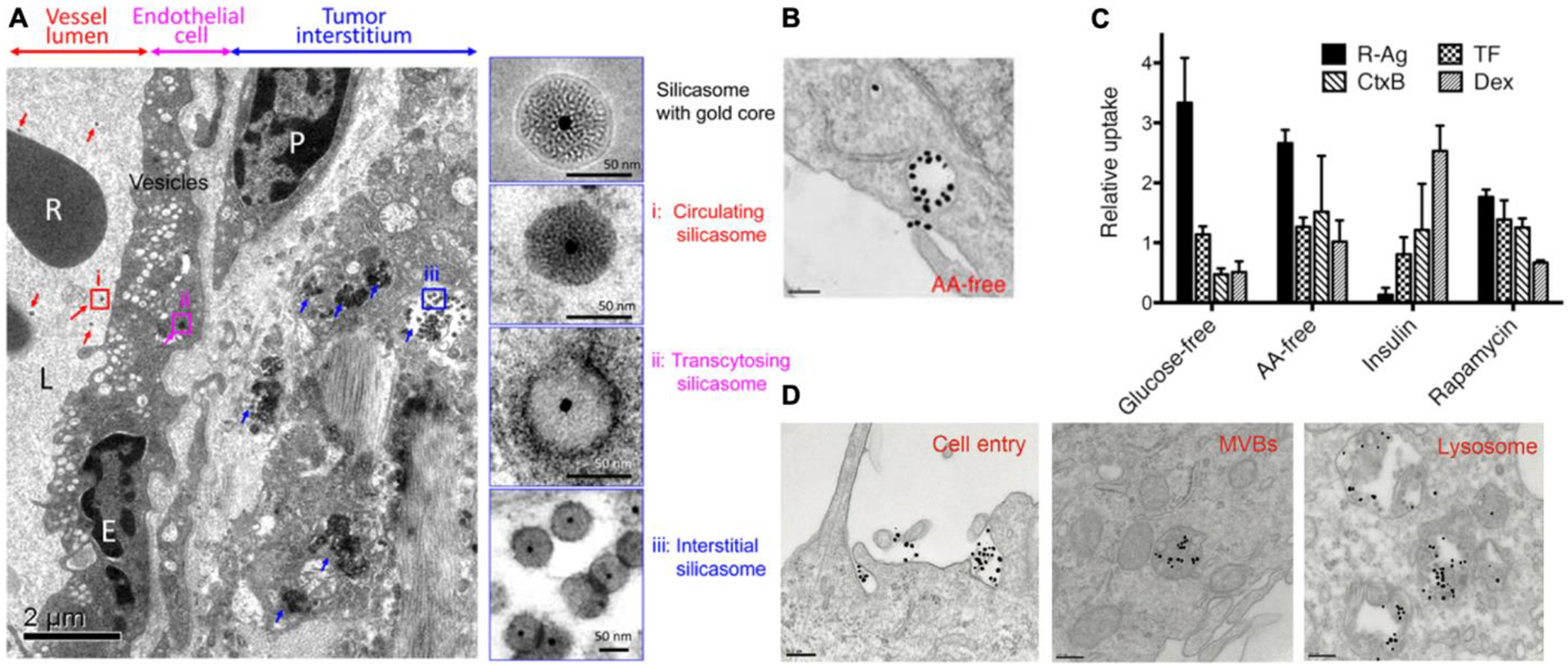
(A) TEM images shows that CendR peptide induced a transcytosis for NP vascular penetration. Silicasome NPs co-administered with iRGD appeared in (i) the blood vessel lumen (red arrows), (ii) transport vesicles in endothelial cells (pink arrows) and (iii) the tumor matrix (blue arrows). Right panels were magnified regions (i) through (iii). R, red blood cell; E, endothelial cell; P, pericyte. Scale bar = 2 μm (left panel) or 50 nm (right panel). The image is adapted from [65].
(B) RPARPAR coated gold NPs (dense dark dots) were observed to be internalized into macropinocytosis-like vacuoles, which were evident in the medium depleted of amino acids. Scale bar = 200 nm. The TEM image is adapted from [66].
(C) The cellular uptake of endocytic tracers (indicated on the upper left corner of the figure) responds differently to environmental cues. The y-axis shows the cellular uptake of each tracer relative to that in the complete cell culture medium (glucose- or AA-free) or treated by control solvents (insulin or rapamycin). The image is adapted from [66].
(D) Gold NPs coated with TAT (dense dark dots) were observed to be engulfed by macropinocytosis-like vesicles, and sequentially transported through the indicated compartments (from left to right). Scale bar = 200 nm. The image is adapted from [70].
Pang et al. performed a genome-wide RNA interfering (RNAi) screen to identify genes and signaling pathways that regulated the cell entry of CendR cargo [66]. RPARPAR was conjugated to silver NPs (R-AgNPs) as the model CendR cargo [66]. AgNPs were used to take advantage of etching technology that can be used to dissolve extracellular AgNPs rapidly, while internalized NPs remain intact [67]. The screen identified macropinocytosis genes, such as Rac1, Cdc42, Rab5, some members of PI3Ks and PLCs, but not clathrins or caveolins [66]. The uptake of R-AgNPs was sensitive to macropinocytosis inhibitors and also depended on CendR-NRP1 ligation. Ultrastructurally, the endocytic vesicles resembled macropinosomes, which were over 250 nm in diameter and showed irregular shapes (Figure 2B). When co-administered with Dextran, R-AgNPs exhibited limited colocalization, highlighting a difference between CendR-NRP1 initiated and conventional receptor-independent macropinocytosis. Together, these results provide strong evidence that CendR-mediated cell entry occurred through receptor (NRP1)-mediated macropinocytosis.
Another finding was that CendR-mediated endocytosis was governed by nutrient sensing and translation regulatory pathways, especially mTOR signaling [66]. Genome screen hits included members of mTOR complex 1 and 2, up- and down-stream targets, such as PI3K/Akt-related genes and translational initiating factors (EIF3s and EIF4s). Specifically, mTOR inhibition or deprivation of extracellular nutrients (e.g. glucose and amino acids) enhanced the cell entry efficiency of CendR cargo, including NPs and proteins (Figure 2C) [66]. The response of CendR endocytosis to mTOR inhibition and nutrient deprivation was different from that of CME, CavME as well as conventional macropinocytosis traced by Dextran (Figure 2C). This further supports the possibility of receptor (NRP1)-mediated macropinocytosis being a novel endocytic pathway [66]. Despite previous studies on the activation of mTOR pathway by amino acid influx mediated by macropincytosis, this was among the first reports showing the regulation of macropinocytosis by mTOR or nutrient availability. The upregulated expression of NRP1 on the cell surface partly accounts for this phenomenon, but other possible mechanisms remain to be investigated [66].
In terms of physiological relevance, NRP1-mediated bulk transport pathway may be activated for essential material acquisition in solid tumors, where oxygen and nutrients are often limited [68]. CendR peptides can therefore commandeer this pathway for delivery therapeutic cargo. The nutrient regulation is also consistent with previous reports on the connection between macropinocytosis and mTOR signaling as discussed above. The underlying mechanism and physiological relevance are potentially of significance to both cell biology and nanomedicine research.
Besides CendR peptides, other cationic CPPs also use receptor-mediated macropinocytosis to transport NPs into cells. Transactivator of transcription (TAT), derived from human immunodeficiency virus-1 (HIV-1), was first found in 1988 and later considered as the prototype cationic CPP [69]. Pang et al. coated NPs with TAT made of L- and D-amino acids and found that TAT-NPs were taken up in the macropinosome-like structures (> 250 nm) (Figure 2D) [70]. This engulfment relied on TAT interaction with its receptors, heparan sulfate proteoglycans (HSPGs). Consistently, TAT-fusion proteins were shown to transduce into cells via macropinocytosis and exhibit endosomal escape [71]. This HSPG-mediated macropinocytosis was also reported to mediate internalization of albumin conjugated with a TAT-like peptide, polyarginines [72]. Multiple endocytic pathways and even direct translocation across the plasma membrane have been proposed as the internalization mechanism for TAT peptide [73, 74, 75]. In contrast, when TAT peptides are multiplexed on proteins or NPs, macropinocytosis was the main mechanism [70, 76]. The difference between monomer peptide and its multiplexed form on NP may contribute to distinct receptor interactions and eventually determines the endocytic route.
NRPs and HSPGs are present in immune cells and may involve in phagocytosis as well [88]. However, the processes observed above appear distinct from receptor-mediated phagocytosis. Phagocytosis normally involves membrane wrapping of a single solid particle [85]. The cell membrane is in close proximity to the particle surface. However, the size of peptide-functionalized NPs in these studies was much smaller than the diameter of endocytic vesicles, and multiple NPs were simultaneously engulfed within a single endocytic vacuole (Figure 2B and 2D) [66, 70]. Additionally, tumor cell lines were primarily used to perform these studies. As such, this process is more related to receptor-mediated macropinocytosis rather than phagocytosis. On the other hand, the main features setting this process apart from traditional macropinocytosis, include receptor dependence and distinctive responses to mTOR signaling and nutrient availability, as discussed above. One key factor contributing to these differences may be the internalization dynamics. In these studies, the authors used a relatively short time (~1h) for internalization, while a much longer time (~24h) was shown to be required for the efficient uptake of unfunctionalized NPs [89]. Considering the blood flow rate and the time frame for circulatory cargo to encounter a certain tissue (e.g. tumor), it is unlikely for systemically administered NPs to have such a long time together with target cells for efficient internalization. Therefore, this receptor-initiated macropinocytosis will be of greater use to improve the efficacy of NPs in vivo.
Other than cell entry, an in vitro cellular assay was established to study the possibility of transcellular transport of CPP-NPs [66]. This study proved that both CendR- and TAT-functionalized NPs could be exported from one cell and be re-absorbed into another. This cell-to-cell transfer was demonstrated to be mediated by membrane-enclosed structures, as etching did not significantly lower the transcellular efficiency [66]. Here, this result provides a possible mechanism for CendR-mediated tumor penetration and raises an intriguing question regarding the final destination of engulfed cargo by this pathway. Future investigations on this process are needed to shed light on cellular transport machineries that are responsible for not only internalizing cargo into cells but also transporting across the tissues of multilayered cells.
Nanoparticle uptake in the bystander manner via receptor-mediated macropinocytosis
Receptor-mediated macropinocytosis also provides a possible mechanism for the bystander effect [56]. The bystander effect is the phenomenon when the cell entry of one molecule (an active ligand) enables the internalization of co-administered cargo (bystander cargo). This is most convincingly demonstrated when the bystander cargo has little or no ability to enter the cells themselves and has no physical or chemical interaction with the active ligand. Sugahara et al. first tested the bystander effect of iRGD by co-injection with a variety of anticancer drugs [59]. The tumor accumulation and antitumor efficacy were significantly enhanced for a small molecule drug (doxorubicin), nanoparticles (nab-paclitaxel and liposomal doxorubicin), and an antibody (trastuzumab) when used together with iRGD. It should be noted that this effect circumvents the limited availability of target receptors on the cell membrane, since bystander cargo are engulfed through a bulk transport system [59]. Since then, several preclinical studies have taken advantage of this effect to increase the specificity and therapeutic effects of various drugs [77, 78].
Besides iRGD, other CendR peptides have been shown to exhibit the bystander activity. LyP-1 is a tumor-homing peptide with a cryptic CendR motif and preferentially binds to tumor macrophages after extravasation [56]. Both LyP-1 and its linear truncated form tLyP-1 were reported to generate the bystander effect [79, 80, 81]. Timur et al. combined LyP-1 and doxorubicin hydrochloride in a self-microemulsifying drug delivery system that showed enhanced in vitro cytotoxicity and reduced tumor growth in vivo [79]. When tLyP-1 was co-administered with tumor-targeted NPs, deeper penetration into brain and greater therapeutic effects were observed in mice bearing glioma [80]. Another example is iNGR, a de novo designed tumor-penetrating peptide with the C-terminal consensus motif [82]. Co-administration of iNGR with doxorubicin enhanced the anticancer efficacy. A more thorough review of peptides with the bystander activity can be found elsewhere [56].
Considering potential therapeutic value, it is of great importance to decode the cellular mechanism for this bystander activity. Despite intensive characterization in vivo, the bystander effect of CendR peptides has rarely been studied in vitro. A closest phenomenon came from cell entry of TAT peptide. Kaplan et al. showed that co-administration with TAT peptide could increase the cellular uptake of Dextran (Figure 3A) [73]. TAT and Dextran were not linked chemically or physically so Dextran is likely a bystander payload. Fluid-phase macropinocytosis is believed to account for this improved transport, because the formation of macropinosomes may sweep nearby cargo in bulk into the large endocytic vesicles (> 200 nm in diameter) [56]. Table I provides the list of the peptides that have served as active ligands. Table II summarizes the studies that have shown an elevated intracellular delivery in the bystander manner.
Figure 3. Receptor-mediated macropinocytosis and bystander uptake.
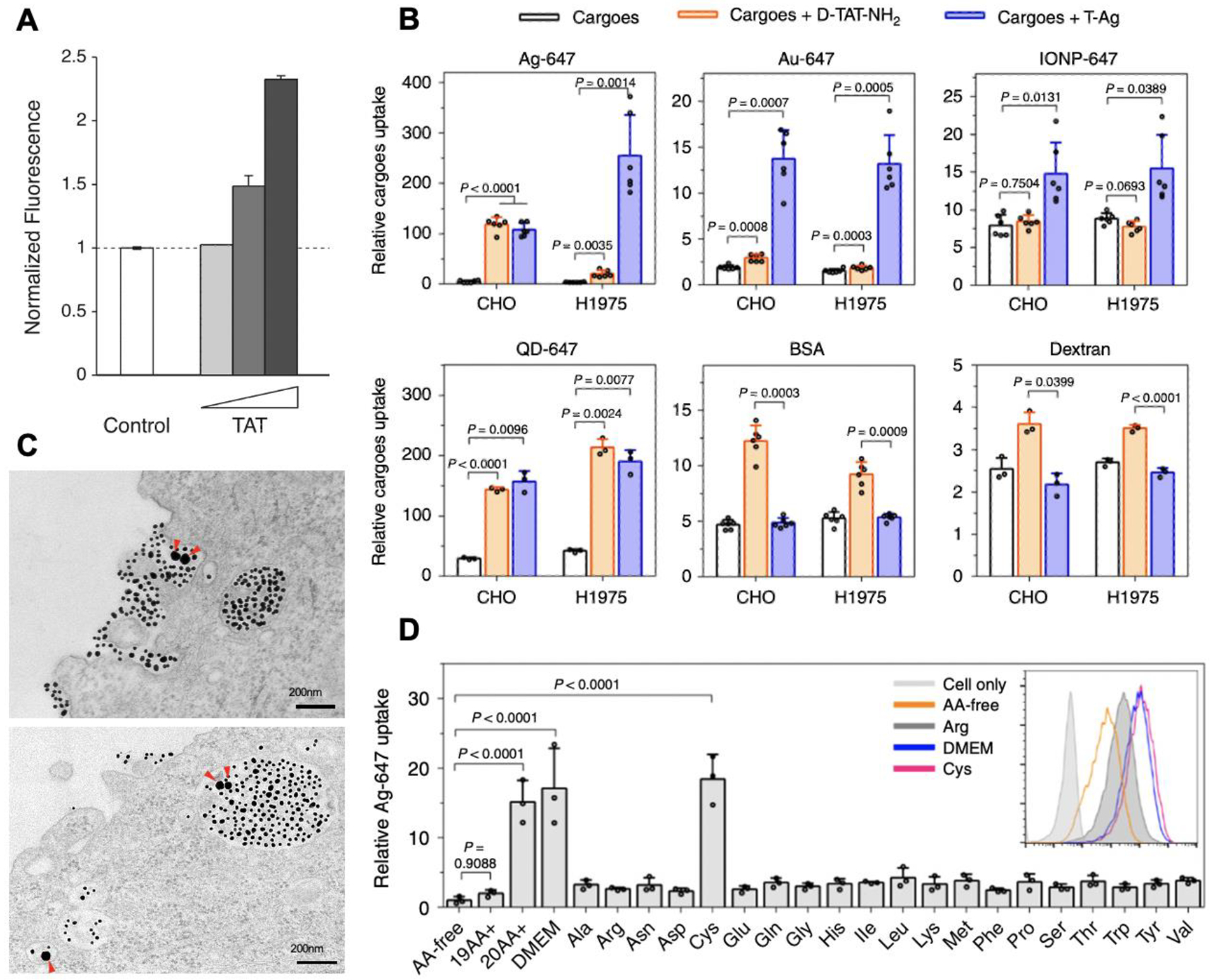
(A) TAT peptide stimulated the bystander uptake of Dextran. The image is adapted from [73].
(B) The bystander uptake of the indicated bystander cargo by TAT peptide (orange bars) and TAT-NPs (blue bars). The image is adapted from [8].
(C) TEM images shows that TAT-coated gold NPs (smaller dark dots) and bystander gold NPs (larger dark dots, pointed by red arrows) are engulfed together by macropinocytosis-like vesicles. Scale bar = 200 nm. The image is adapted from [8].
(D) Extracellular cysteine stimulated TAT-NP-induced bystander uptake. Cells were incubated with TAT-NPs and bystander AgNPs in the culture media containing various amino acid compositions: no amino acid (AA-free), the indicated amino acid alone (e.g. Cys, Arg), 19 amino acids without cysteine (19AA+) and all the amino acids (20AA+). TAT-NP-mediated bystander uptake is much higher in the media containing all 20 AAs or only cysteine, than that in the media without Cysteine. The image is adapted from [8].
Table I.
Examples of “bystander effect” peptides, their sequences and origins
Table II.
Examples of bystander uptake under preclinical development
| Bystander Inducer | Description | Result | Reference |
|---|---|---|---|
| TAT | TAT co-incubation could provoke the uptake of the macropinosytosis marker 70kDa Dextran. | in vitro | [73] |
| TAT-NPs | NPs conjugated with TAT could induce bystander uptake of all NP-type cargo but not common fluid markers, the activity of which was regulated by extracellular cysteine presence both in vitro and in vivo. | in vitro & in vivo | [8] |
| Polyarginines | TAT-fused albumin induced macropinocytosis through interactions with HSPG and CXCR4 to activate PKC/PI3K/JNK/mTOR signaling pathways. | in vitro | [72] |
| iRGD | iRGD could facilitate the delivery of small molecule, nanoparticle and antibody into tumors when administered as a combination therapy. | in vivo | [59] |
| Despite little enhancement of cytotoxicity in vitro, co-administered iRGD promoted the delivery of PLGA nanoparticles selectively into tumors in vivo. | in vitro & in vivo | [78] | |
| LyP-1 | The LyP-1-containing self-microemulsifying formulation not only increased in vitro cytotoxic effect of co-administered doxorubicin hydrochloride, but also achieved preferential accumulation in tumors via intraperitoneal injection. | in vitro & in vivo | [79] |
| tLyP-1 | Following tLyP-1 co-administration, tumor-targeted NPs displayed improved cytotoxicity of the loaded paclitaxel and deeper penetration into the glioma parenchyma. | in vitro & in vivo | [80] |
| iNGR | iNGR enhanced accumulation and efficacy of co-administered doxorubicin by triggering the tumor-specific penetration. | in vivo | [82] |
Although it is challenging to stimulate the bystander effect with free CPPs, a recent breakthrough has been made with CPP-conjugated NPs. Using TAT-functionalized NPs, Wei et al. investigated the bystander activity on a variety of cargo, including NPs, albumin and Dextran [8]. The tested NP types included AgNPs, gold nanoparticles (AuNPs), iron oxide nanoparticles (IONPs) and quantum dots (QDs). The authors found that TAT-NP co-administration significantly enhanced the cellular uptake of all four NP types in the bystander manner (Figure 3B) as this effect was not due to TAT exchange between NPs or any interactions between the two NPs. This phenomenon was observed when TAT was conjugated to different NP types, in multiple cell line, as well as ex vivo tissues and in vivo settings. Interestingly, the bystander effect induced by TAT-NPs did not apply to common fluid markers, albumin and Dextran. In comparison, TAT peptide exhibited bystander activity to fluid markers and some types of bystander NPs but not all (Figure 3B). This cargo selectivity highlighted the difference between bystander uptake processes invoked by free peptide and its multivalent form on NP. Moreover, the level of bystander activity showed dependence on the composition of TAT-NPs and cell types, and the concentration of either TAT-NPs or bystander NPs.
The authors further showed that the bystander uptake induced by TAT-NPs used receptor-mediated macropinocytosis [8]. Initiation of this process relied on the interaction between TAT-NPs and cellular receptors, HSPGs. Inhibitor treatment demonstrated that TAT-NP-induced bystander uptake was primarily sensitive to macropinocytosis inhibitors. TEM studies provided the visual evidence that bystander cargo are located in the same cup-like macropinosomes (> 200 nm in diameter) with TAT-NPs (Figure 3C). Inside the cells, bystander NPs co-localized almost exclusively with TAT-NPs. Together, the results support that TAT-NPs invoked receptor (HSPG)-mediated macropinocytosis to engulf bystander NPs.
When studying the cell entry of NPs, especially the synergy between different NP types, an important consideration is to avoid the contribution of NP aggregates. The aggregation will not only render the above conclusion invalid and invoke different internalization routes but also induce unwanted cytotoxicity. In vivo, NP aggregation may influence biodistribution and cause severe adverse effects, such as capillary blockage [90]. Uptake of solid NP aggregates is primarily reported in phagocytosis, which involves internalization of two-phase (water-solid) materials [91]. One study investigated the clustering state of NPs which entered the cells via multiple fluid-phase endocytic pathways, including macropinocytosis [92]. They found that NPs appeared separately in the early stages of endocytosis but only clustered during vesicular maturation (e.g. late endosome and lysosomes). In the above studies, various procedures have been taken to ensure no aggregation. Hydrophilic polymers (e.g. PEG) were coated on NPs to promote dispersion. Multiple methods were used to ensure that there was no physical interaction between CPP-NPs and bystander NPs [8]. Based on TEM images, NPs are well dispersed at both early and late stages of endocytosis, further supporting that this phenomenon does not arise from NP aggregates (Figure 2B, 2D and 3C) [8, 66, 70].
Inspired by previous studies, the authors investigated the impact of extracellular amino acids (AAs) on the bystander effect [8]. They found that among all 20 natural AAs, there was only one AA, cysteine, whose concentration affected bystander activity (Figure 3D). TAT-NP-mediated bystander uptake was much higher in the cysteine-containing medium than that without cysteine. This cysteine regulation occurred in all tested NP types (as active ligands or bystander cargo), a variety of cell lines, and under ex vivo and in vivo conditions. Cysteine variants, cystine and glutathione (GSH), had similar effects. Blockage of cysteine transporter with an inhibitor significantly reduced bystander activity, indicating that this effect depended on cysteine uptake into cells and the intracellular redox status. Interestingly, the authors observed little or modest cysteine effect on TAT-NP uptake itself [8]. Although these results show that the uptake of functionalized and bystander NPs was not merely coincidental engulfment, further investigation is needed to dissect the interconnection between individual amino acids and endocytic machinery.
Regarding bystander uptake and amino acid regulation, there are more questions than answers. First, the physiochemical properties of NPs that determine their bystander activity are far from being understood. The particle size may be an important factor since QDs, with the smallest size, exhibited the lowest activity as bystander cargo [8]. This may also explain the above cited result of albumin and Dextran. Previous research that focused on NP-cell interactions reported that 50-nm spherical AuNPs showed the highest uptake compared to any rod-like shapes in the range of 30 – 90 nm diameter [83]. However, it remains a question whether this optimal size and shape apply to NPs as bystander cargo. Other parameters of NPs, such as rigidity and surface charge, may contribute to bystander efficiency as well. Second, the nature and molecular components for macropinosomes specifically responsible for bystander uptake remain to be investigated. This belongs to the even larger question in cell biology, as the structural marker of macropinosomes is still unknown [29]. Exploring of this aspect may reveal how bystander cargo are engulfed into the same vesicles as TAT-NPs and how this endocytic process drives cargo selectivity. Third, to our best knowledge, Wei et al is the first study to show the regulation of endocytosis and NP uptake by an individual amino acid. Cysteine and its derivative, GSH, are well known for protecting cells from oxidative stress and reactive oxygen species (ROS) [84]. Although the exact mechanism connecting cysteine with bystander uptake remains to be studied, ROS and oxidative signaling are speculated to interfere with macropinocytosis activity through interactions with PI3K-Akt and mTOR pathways. The cysteine concentration did not influence TAT-NP internalization, signifying a delicate distinction between functionalized and bystander NPs, even though they both enter the cells via receptor-mediated macropinocytosis. Decoding this disparity will aid in a better understanding of macropinocytosis, its cargo selectivity and metabolic regulation.
Concluding Remarks
The molecular machinery involved in macropinocytosis has been described with an emphasis on receptor-mediated macropinocytosis process. This pathway is used by ligand-functionalized and bystander NPs for cell entry, which has important therapeutic consequences. Bystander uptake presents a useful way to synergize internalization of two NP types and circumvent the limitation of receptor availability on the cell surface [56]. These investigations also unveil several novel properties, including cargo selectivity and amino acid regulation. Addressing the details of this transport mechanism will advance our knowledge on macropinocytosis in general, help identify molecular signatures for macropinosomes, and clarify the factors determining bystander uptake efficiency and cysteine regulation. Such efforts will further improve the cell entry and hold the promise of advancing therapeutic efficacy of nanomedicine.
Highlights.
This review summarizes the macropinocytosis machinery and its internalization of nanoparticles, with special focus on receptor-mediated macropinocytosis.
This review describes a synergistic entry of peptide-functionalized and bystander nanoparticles into cells through receptor-mediated macropinocytosis.
This review highlights the regulation of nanoparticle uptake efficiency by extracellular amino acids, which opens up a new avenue to study the interplay among drug delivery, endocytosis, nutrient availability and metabolic signaling.
Acknowledgements
The authors acknowledge the funding support from the National Institute of Health (R01CA214550, R01GM133885 and R21EB022652) and the State of Minnesota (MNP #19.08).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- [1].Li Bin, and Lane Lucas A.. “Probing the biological obstacles of nanomedicine with gold nanoparticles.” Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 11.3 (2019): e1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Barenholz Yechezkel Chezy. “Doxil®—the first FDA-approved nano-drug: lessons learned.” Journal of controlled release 160.2 (2012): 117–134. [DOI] [PubMed] [Google Scholar]
- [3].Mattias Björnmalm, et al. “Bridging bio-nano science and cancer nanomedicine.” ACS nano 11.10 (2017): 9594–9613. [DOI] [PubMed] [Google Scholar]
- [4].Conner Sean D., and Schmid Sandra L.. “Regulated portals of entry into the cell.” Nature 422.6927 (2003): 37–44. [DOI] [PubMed] [Google Scholar]
- [5].Donahue Nathan D., Acar Handan, and Wilhelm Stefan. “Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine.” Advanced drug delivery reviews 143 (2019): 68–96. [DOI] [PubMed] [Google Scholar]
- [6].Yameen Basit, et al. “Insight into nanoparticle cellular uptake and intracellular targeting.” Journal of Controlled Release 190 (2014): 485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sahay Gaurav, Alakhova Daria Y., and Kabanov Alexander V.. “Endocytosis of nanomedicines.” Journal of controlled release 145.3 (2010): 182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wei Yushuang, Tang Tang, and Pang Hong-Bo. “Cellular internalization of bystander nanomaterial induced by TAT-nanoparticles and regulated by extracellular cysteine.” Nature communications 10.1 (2019): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hillaireau Hervé, and Patrick Couvreur. “Nanocarriers’ entry into the cell: relevance to drug delivery.” Cellular and molecular life sciences 66.17 (2009): 2873–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bahrami Behdokht, et al. “Nanoparticles and targeted drug delivery in cancer therapy.” Immunology letters 190 (2017): 64–83. [DOI] [PubMed] [Google Scholar]
- [11].Mao Yingyu, and Finnemann Silvia C.. “Regulation of phagocytosis by Rho GTPases.” Small GTPases 6.2 (2015): 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rabinovitch Michel. “Professional and non-professional phagocytes: an introduction.” Trends in cell biology 5.3 (1995): 85–87. [DOI] [PubMed] [Google Scholar]
- [13].Champion Julie A., and Mitragotri Samir. “Shape induced inhibition of phagocytosis of polymer particles.” Pharmaceutical research 26.1 (2009): 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ajdari Niloofar, et al. “Gold nanoparticle interactions in human blood: a model evaluation.” Nanomedicine: Nanotechnology, Biology and Medicine 13.4 (2017): 1531–1542. [DOI] [PubMed] [Google Scholar]
- [15].Dai Qin, Walkey Carl, and Chan Warren CW. “Polyethylene glycol backfilling mitigates the negative impact of the protein corona on nanoparticle cell targeting.” Angewandte Chemie International Edition 53.20 (2014): 5093–5096. [DOI] [PubMed] [Google Scholar]
- [16].Sosale Nisha G. et al. ““Marker of Self” CD47 on lentiviral vectors decreases macrophage-mediated clearance and increases delivery to SIRPA-expressing lung carcinoma tumors.” Molecular Therapy-Methods & Clinical Development 3 (2016): 16080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bitsikas Vassilis, Corrêa Ivan R. Jr, and Nichols Benjamin J.. “Clathrin-independent pathways do not contribute significantly to endocytic flux.” Elife 3 (2014): e03970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kaksonen Marko, and Roux Aurélien. “Mechanisms of clathrin-mediated endocytosis.” Nature Reviews Molecular Cell Biology 19.5 (2018): 313. [DOI] [PubMed] [Google Scholar]
- [19].McMahon Harvey T., and Boucrot Emmanuel. “Molecular mechanism and physiological functions of clathrin-mediated endocytosis.” Nature reviews Molecular cell biology 12.8 (2011): 517. [DOI] [PubMed] [Google Scholar]
- [20].Vasir Jaspreet K., and Labhasetwar Vinod. “Quantification of the force of nanoparticle-cell membrane interactions and its influence on intracellular trafficking of nanoparticles.” Biomaterials 29.31 (2008): 4244–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Benyettou F, et al. “Synthesis of silver nanoparticles for the dual delivery of doxorubicin and alendronate to cancer cells.” Journal of Materials Chemistry B 3.36 (2015): 7237–7245. [DOI] [PubMed] [Google Scholar]
- [22].Carver Lucy A., and Schnitzer Jan E.. “Caveolae: mining little caves for new cancer targets.” Nature Reviews Cancer 3.8 (2003): 571–581. [DOI] [PubMed] [Google Scholar]
- [23].Bareford Lisa M., and Swaan Peter W.. “Endocytic mechanisms for targeted drug delivery.” Advanced drug delivery reviews 59.8 (2007): 748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Xin Xiaofei, et al. “Rod-shaped active drug particles enable efficient and safe gene delivery.” Advanced Science 4.11 (2017): 1700324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu Yang, et al. “Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles.” Biomaterials 30.25 (2009): 4195–4202. [DOI] [PubMed] [Google Scholar]
- [26].Perumal Omathanu P., et al. “The effect of surface functionality on cellular trafficking of dendrimers.” Biomaterials 29.24–25 (2008): 3469–3476. [DOI] [PubMed] [Google Scholar]
- [27].Blanco Elvin, Shen Haifa, and Ferrari Mauro. “Principles of nanoparticle design for overcoming biological barriers to drug delivery.” Nature biotechnology 33.9 (2015): 941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yoshida Sei, et al. “Macropinocytosis, mTORC1 and cellular growth control.” Cellular and Molecular Life Sciences 75.7 (2018): 1227–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stow Jennifer L., Hung Yu, and Wall Adam A.. “Macropinocytosis: Insights from immunology and cancer.” Current Opinion in Cell Biology 65 (2020): 131–140. [DOI] [PubMed] [Google Scholar]
- [30].Steinman Ralph M., Brodie Scott E., and Cohn Zanvil A.. “Membrane flow during pinocytosis. A stereologic analysis.” The Journal of cell biology 68.3 (1976): 665–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Saeed Mohammad F., et al. “Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes.” PLoS pathogens 6.9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Swanson Joel A., and King Jason S.. “The breadth of macropinocytosis research.” (2019): 20180146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Canton Johnathan, et al. “Calcium-sensing receptors signal constitutive macropinocytosis and facilitate the uptake of NOD2 ligands in macrophages.” Nature communications 7.1 (2016): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Canton Johnathan. “Macropinocytosis: New insights into its underappreciated role in innate immune cell surveillance.” Frontiers in immunology 9 (2018): 2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Commisso Cosimo, et al. “Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells.” Nature 497.7451 (2013): 633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bar-Sagi Dafna, and Feramisco James R.. “Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins.” Science 233.4768 (1986): 1061–1068. [DOI] [PubMed] [Google Scholar]
- [37].Cullis Jane, Das Shipra, and Dafna Bar-Sagi. “Kras and tumor immunity: friend or foe?.” Cold Spring Harbor Perspectives in Medicine 8.9 (2018): a031849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yoshida Sei, et al. “Sequential signaling in plasma-membrane domains during macropinosome formation in macrophages.” Journal of cell science 122.18 (2009): 3250–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].King Jason S., and Kay Robert R.. “The origins and evolution of macropinocytosis.” Philosophical Transactions of the Royal Society B 374.1765 (2019): 20180158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Junemann Alexander, et al. “A Diaphanous-related formin links Ras signaling directly to actin assembly in macropinocytosis and phagocytosis.” Proceedings of the National Academy of Sciences 113.47 (2016): E7464–E7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Porat-Shliom Natalie, Kloog Yoel, and Donaldson Julie G.. “A unique platform for H-Ras signaling involving clathrin-independent endocytosis.” Molecular biology of the cell 19.3 (2008): 765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ramirez Craig, et al. “Plasma membrane V-ATPase controls oncogenic RAS-induced macropinocytosis.” Nature 576.7787 (2019): 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Palm Wilhelm, et al. “Critical role for PI3-kinase in regulating the use of proteins as an amino acid source.” Proceedings of the National Academy of Sciences 114.41 (2017): E8628–E8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Egami Youhei, et al. “Small GTPases and phosphoinositides in the regulatory mechanisms of macropinosome formation and maturation.” Frontiers in physiology 5 (2014): 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Krishna Shefali, et al. “PIKfyve regulates vacuole maturation and nutrient recovery following engulfment.” Developmental cell 38.5 (2016): 536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yoshida Sei, et al. “Growth factor signaling to mTORC1 by amino acid-laden macropinosomes.” Journal of Cell Biology 211.1 (2015): 159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cullis Jane, et al. “Macropinocytosis of nab-paclitaxel drives macrophage activation in pancreatic cancer.” Cancer immunology research 5.3 (2017): 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jiang Gan, et al. “Tailored Lipoprotein-Like miRNA Delivery Nanostructure Suppresses Glioma Stemness and Drug Resistance through Receptor-Stimulated Macropinocytosis.” Advanced Science 7.5 (2020): 1903290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nam Hae Yun, et al. “Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles.” Journal of Controlled Release 135.3 (2009): 259–267. [DOI] [PubMed] [Google Scholar]
- [50].Li Lei, et al. “The effect of the size of fluorescent dextran on its endocytic pathway.” Cell biology international 39.5 (2015): 531–539. [DOI] [PubMed] [Google Scholar]
- [51].Guidotti Giulia, Brambilla Liliana, and Rossi Daniela. “Cell-penetrating peptides: from basic research to clinics.” Trends in pharmacological sciences 38.4 (2017): 406–424. [DOI] [PubMed] [Google Scholar]
- [52].Copolovici Dana Maria, et al. “Cell-penetrating peptides: design, synthesis, and applications.” ACS nano 8.3 (2014): 1972–1994. [DOI] [PubMed] [Google Scholar]
- [53].Bechara Chérine, and Sagan Sandrine. “Cell-penetrating peptides: 20 years later, where do we stand?.” FEBS letters 587.12 (2013): 1693–1702. [DOI] [PubMed] [Google Scholar]
- [54].Guo Zhengrong, et al. “Cell-penetrating peptides: Possible transduction mechanisms and therapeutic applications.” Biomedical reports 4.5 (2016): 528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Borrelli Antonella, et al. “Cell penetrating peptides as molecular carriers for anti-cancer agents.” Molecules 23.2 (2018): 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ruoslahti Erkki. “Tumor penetrating peptides for improved drug delivery.” Advanced drug delivery reviews 110 (2017): 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ruoslahti Erkki. “Peptides as targeting elements and tissue penetration devices for nanoparticles.” Advanced materials 24.28 (2012): 3747–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sugahara Kazuki N., et al. “Tissue-penetrating delivery of compounds and nanoparticles into tumors.” Cancer cell 16.6 (2009): 510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sugahara Kazuki N., et al. “Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs.” science 328.5981 (2010): 1031–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Teesalu Tambet, Sugahara Kazuki NMD, and Ruoslahti Erkki. “Tumor-penetrating peptides.” Frontiers in oncology 3 (2013): 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ellis Lee M. “The role of neuropilins in cancer.” Molecular cancer therapeutics 5.5 (2006): 1099–1107. [DOI] [PubMed] [Google Scholar]
- [62].Makinen Taija, et al. “Differential binding of vascular endothelial growth factor B splice and proteolytic isoforms to neuropilin-1.” Journal of Biological Chemistry 274.30 (1999): 21217–21222. [DOI] [PubMed] [Google Scholar]
- [63].Teesalu Tambet, et al. “C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration.” Proceedings of the National Academy of Sciences 106.38 (2009): 16157–16162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Nel Andre, Erkki Ruoslahti and Huan Meng. “New insights into “permeability” as in the enhanced permeability and retention effect of cancer nanotherapeutics.” (2017): 9567–9569. [DOI] [PubMed] [Google Scholar]
- [65].Liu Xiangsheng, et al. “Tumor-penetrating peptide enhances transcytosis of silicasome-based chemotherapy for pancreatic cancer.” The Journal of clinical investigation 127.5 (2017): 2007–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Pang Hong-Bo, et al. “An endocytosis pathway initiated through neuropilin-1 and regulated by nutrient availability.” Nature communications 5.1 (2014): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Braun Gary B., et al. “Etchable plasmonic nanoparticle probes to image and quantify cellular internalization.” Nature materials 13.9 (2014): 904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sagnella Sharon M., McCarroll Joshua A., and Maria Kavallaris. “Drug delivery: beyond active tumour targeting.” Nanomedicine: Nanotechnology, Biology and Medicine 10.6 (2014): 1131–1137. [DOI] [PubMed] [Google Scholar]
- [69].Frankel Alan D., and Pabo Carl O.. “Cellular uptake of the tat protein from human immunodeficiency virus.” Cell 55.6 (1988): 1189–1193. [DOI] [PubMed] [Google Scholar]
- [70].Pang Hong-Bo, Braun Gary B., and Ruoslahti Erkki. “Neuropilin-1 and heparan sulfate proteoglycans cooperate in cellular uptake of nanoparticles functionalized by cationic cell-penetrating peptides.” Science advances 1.10 (2015): e1500821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wadia Jehangir S., Stan Radu V., and Dowdy Steven F.. “Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis.” Nature medicine 10.3 (2004): 310–315. [DOI] [PubMed] [Google Scholar]
- [72].Ichimizu Shota, et al. “Cell-penetrating mechanism of intracellular targeting albumin: contribution of macropinocytosis induction and endosomal escape.” Journal of Controlled Release 304 (2019): 156–163. [DOI] [PubMed] [Google Scholar]
- [73].Kaplan Ian M., Wadia Jehangir S., and Dowdy Steven F.. “Cationic TAT peptide transduction domain enters cells by macropinocytosis.” Journal of Controlled Release 102.1 (2005): 247–253. [DOI] [PubMed] [Google Scholar]
- [74].Ben-Dov Nadav, and Korenstein Rafi. “The uptake of HIV Tat peptide proceeds via two pathways which differ from macropinocytosis.” Biochimica et Biophysica Acta (BBA)-Biomembranes 1848.3 (2015): 869–877. [DOI] [PubMed] [Google Scholar]
- [75].Jiao Chen-Yu, et al. “Translocation and endocytosis for cell-penetrating peptide internalization.” Journal of Biological Chemistry 284.49 (2009): 33957–33965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Tyagi Mudit, et al. “Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans.” Journal of Biological Chemistry 276.5 (2001): 3254–3261. [DOI] [PubMed] [Google Scholar]
- [77].Akashi Y, et al. “Anticancer effects of gemcitabine are enhanced by co-administered iRGD peptide in murine pancreatic cancer models that overexpressed neuropilin-1.” British journal of cancer 110.6 (2014): 1481–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zhong Yi, et al. “Co-Administration Of iRGD Enhances Tumor-Targeted Delivery and Anti-Tumor Effects of Paclitaxel-Loaded PLGA Nanoparticles for Colorectal Cancer Treatment.” International Journal of Nanomedicine 14 (2019): 8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Timur Selin S., et al. “Efficacy of a novel LyP-1-containing self-microemulsifying drug delivery system (SMEDDS) for active targeting to breast cancer.” European Journal of Pharmaceutics and Biopharmaceutics 136 (2019): 138–146. [DOI] [PubMed] [Google Scholar]
- [80].Miao Deyu, et al. “Co-administration of dual-targeting nanoparticles with penetration enhancement peptide for antiglioblastoma therapy.” Molecular pharmaceutics 11.1 (2014): 90–101. [DOI] [PubMed] [Google Scholar]
- [81].Hu Quanyin, et al. “F3 peptide-functionalized PEG-PLA nanoparticles co-administrated with tLyp-1 peptide for anti-glioma drug delivery.” Biomaterials 34.4 (2013): 1135–1145. [DOI] [PubMed] [Google Scholar]
- [82].Alberici Luca, et al. “De novo design of a tumor-penetrating peptide.” Cancer research 73.2 (2013): 804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Malugin Alexander, and Ghandehari Hamidreza. “Cellular uptake and toxicity of gold nanoparticles in prostate cancer cells: a comparative study of rods and spheres.” Journal of Applied Toxicology: An International Journal 30.3 (2010): 212–217. [DOI] [PubMed] [Google Scholar]
- [84].Townsend Danyelle M., Tew Kenneth D., and Tapiero Haim. “The importance of glutathione in human disease.” Biomedicine & pharmacotherapy 57.3–4 (2003): 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Bloomfield Gareth, and Kay Robert R.. “Uses and abuses of macropinocytosis.” Journal of cell science 129.14 (2016): 2697–2705. [DOI] [PubMed] [Google Scholar]
- [86].Hirosue Sachiko, et al. “Antigen delivery to dendritic cells by poly (propylene sulfide) nanoparticles with disulfide conjugated peptides: Cross-presentation and T cell activation.” Vaccine 28.50 (2010): 7897–7906. [DOI] [PubMed] [Google Scholar]
- [87].Kamphorst Jurre J., et al. “Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids.” Proceedings of the National Academy of Sciences 110.22 (2013): 8882–8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Roy Sohini, et al. “Multifaceted role of neuropilins in the immune system: potential targets for immunotherapy.” Frontiers in immunology 8 (2017): 1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wu Meiyu, et al. “Size-dependent cellular uptake and localization profiles of silver nanoparticles.” International Journal of Nanomedicine 14 (2019): 4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Moore Thomas L., et al. “Nanoparticle colloidal stability in cell culture media and impact on cellular interactions.” Chemical Society Reviews 44.17 (2015): 6287–6305. [DOI] [PubMed] [Google Scholar]
- [91].Geiser Marianne, et al. “Nanoparticle uptake by airway phagocytes after fungal spore challenge in murine allergic asthma and chronic bronchitis.” BMC pulmonary medicine 14.1 (2014): 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Liu Mengmeng, et al. “Real-time visualization of clustering and intracellular transport of gold nanoparticles by correlative imaging.” Nature communications 8.1 (2017): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


