Lay Summary: We used fine-scale measurements of resting metabolism to define seasonal patterns of baseline energy demands for spotted, ringed, and bearded seals. These data reveal energetic consequences of different molting strategies and provide source data for population and bioenergetics models aimed at assessing the sensitivity of Arctic seals to environmental warming.
Keywords: Bearded seals, climate change, resting metabolic rate, ringed seals, sea ice loss, spotted seals
Abstract
Arctic seals, including spotted (Phoca largha), ringed (Pusa hispida) and bearded (Erignathus barbatus) seals, are directly affected by sea ice loss. These species use sea ice as a haul-out substrate for various critical functions, including their annual molt. Continued environmental warming will inevitably alter the routine behavior and overall energy budgets of Arctic seals, but it is difficult to quantify these impacts as their metabolic requirements are not well known—due in part to the difficulty of studying wild individuals. Thus, data pertaining to species-specific energy demands are urgently needed to better understand the physiological consequences of rapid environmental change. We used open-flow respirometry over a four-year period to track fine-scale, longitudinal changes in the resting metabolic rate (RMR) of four spotted seals, three ringed seals and one bearded seal trained to participate in research. Simultaneously, we collected complementary physiological and environmental data. Species-specific metabolic demands followed expected patterns based on body size, with the largest species, the bearded seal, exhibiting the highest absolute RMR (0.48 ± 0.04 L O2 min−1) and the lowest mass-specific RMR (4.10 ± 0.47 ml O2 min−1 kg−1), followed by spotted (absolute: 0.33 ± 0.07 L O2 min−1; mass-specific: 6.13 ± 0.73 ml O2 min−1 kg−1) and ringed (absolute: 0.20 ± 0.04 L O2 min−1; mass-specific: 7.01 ± 1.38 ml O2 min−1 kg−1) seals. Further, we observed clear and consistent annual patterns in RMR that related to the distinct molting strategies of each species. For species that molted over relatively short intervals—spotted (33 ± 4 days) and ringed (28 ± 6 days) seals—metabolic demands increased markedly in association with molt. In contrast, the bearded seal exhibited a prolonged molting strategy (119 ± 2 days), which appeared to limit the overall cost of molting as indicated by a relatively stable annual RMR. These findings highlight energetic trade-offs associated with different molting strategies and provide quantitative data that can be used to assess species-specific vulnerabilities to changing conditions.
Introduction
Rapid human-induced climate change is having disproportionate effects in the Arctic, where intense shifts in environmental conditions threaten the overall health and stability of Arctic and sub-Arctic ecosystems (Hinzman et al., 2005; Post et al., 2009; Serreze and Barry, 2011; Comiso and Hall, 2014). The present rate of change may be too rapid for long-lived and slowly reproducing species, such as marine mammals, to effectively adjust routine behavior or alter timing of life-history events. Associated threats to Arctic marine mammals include significant loss of sea ice habitats, changes in prey distribution and abundance, climate-induced stressors to body condition and health, and increased disturbance (Laidre et al., 2008; Moore and Huntington, 2008). Rapid warming and dramatic declines in sea ice are of particular concern for ice-dependent Arctic seals, as loss of haul-out substrate could prevent individuals from successfully completing key life-history events and/or alter individual energy budgets. For many of these species, we lack appropriate data to make robust and meaningful predictions about the physiological impacts of environmental change. Specifically, directed studies examining energetic requirements and physiological constraints are urgently needed.
Resting metabolic rate (RMR) is a standard measure of individual energy expenditure, devoid of the influences of digestion, thermoregulation and reproduction. RMR is commonly used to compare energy needs across taxa (Kleiber, 1947; Thompson and Nicoll, 1986; Williams and Maresh, 2016), has been shown to scale to other measures of energy expenditure in marine mammals (Kooyman, 1989; Castellini et al., 1992; Costa and Williams, 1999; Richmond et al., 2006) and is a key component of many predictive modeling efforts (Winship et al., 2002; Ophir et al., 2010; Rechsteiner et al., 2013; Villegas-Amtmann et al., 2015; Costa et al., 2016; Beltran et al., 2017). Among phocids (i.e. true seals), RMR and overall energy budgets fluctuate annually in concert with environmental cues and internal biological cycles (Renouf and Noseworthy, 1990; Boily and Lavigne, 1997; Rosen and Renouf, 1998; Beck et al., 2003; Sparling et al., 2006). As a result, species-specific metabolic data—encompassing time-periods both inside and outside critical life-history events—are essential to more accurately forecast the consequences of sea ice loss for Arctic seals.
Although there are some studies of metabolism in Arctic seals (Gallivan and Ronald, 1981; Ashwell-Erickson et al., 1986; Renouf and Gales, 1994; Ochoa-Acuña et al., 2009), the majority of physiological data comes from field sampling of harvested animals that is often conducted in cooperation with native subsistence communities (Lenfant et al., 1970; Ryg et al., 1990a, 1990b; Lydersen et al., 1992; Andersen et al., 1999; Chabot and Stenson, 2002; Tryland et al., 2006; Burns et al., 2007; Quakenbush and Citta, 2008; Quakenbush et al., 2009, 2011a, 2011b; Lestyk et al., 2009; Ferreira et al., 2011; Harwood et al., 2012; Routti et al., 2013). These sampling events generally take place in spring during annual breeding and molting periods when seals are most visible and accessible. Such efforts are useful for inter-annual comparisons of specific demographic groups but, due to the terminal nature of data collection, cannot provide fine-scale longitudinal data for individuals. Similarly, physiological data obtained during tagging expeditions are typically acquired once for each individual, as free-ranging Arctic seals are difficult to recapture for repeated measurements. Instrumentation secured to the pelage can be useful in collecting longitudinal behavioral data; however, the efficacy of such tags is reduced during the spring molt, as the probability of tag loss increases with molt progression. The overrepresentation of point-sampled data from individuals and data obtained from one period each year may bias our understanding of reference ranges for key parameters and limit our ability to understand the impact of specific life-history events on annual energetic profiles.
The molt is a significant physiological event that occurs following the breeding season each spring. During this time seals shed and re-grow their fur as well as several layers of epidermis. To facilitate this annual process, seals haul out for extended periods, increase blood flow to the skin and maintain elevated skin temperatures (Chang, 1926; Feltz and Fay, 1966; Boily, 1995). Hence, it has been proposed that the molt period should be characterized by increased metabolic rates; however, the cost of molt has been measured in a number of phocids with mixed results. Grey seals (Halichoerus grypus) (Boily, 1996; Boily and Lavigne, 1997), harp seals (Pagophilus groenlandicus) (Renouf and Gales, 1994; Hedd et al., 1997; Chabot and Stenson, 2002), Hawaiian monk seals (Neomonachus schauinslandi) (Williams et al., 2011) and southern elephant seals (Mirounga leonia) exhibit the predicted increase in metabolism coincident with molt. Northern elephant seals (Mirounga angustirostris) have been suggested to experience little to no energetic cost associated with molt (Worthy et al., 1992). Alternatively, studies of spotted (Phoca largha) and harbor (Phoca vitulina) seals have documented decreases in metabolism during the molt (Ashwell-Erickson et al., 1986; Rosen and Renouf, 1998). These conflicting findings suggest that the physiological consequences of molt are not fully resolved in phocids.
Given that the timing of molt is typically entrained to specific environmental cues like photoperiod (Ling, 1984; Mo et al., 2000), and has evolved to coincide with particular sea ice conditions for Arctic seals, the ongoing and rapid loss of adequate haul-out substrate may have significant energetic implications for ice seals during this time. If seals must increase time in cold polar water during the molt, lowered skin temperatures could inhibit hair growth, resulting in prolonged, disrupted, or failed molt cycles (Boily, 1995). More time in water during molt may also lead to increased heat loss and an overall increase in the metabolic cost of molting. Alternately, seals may modify their behavior if suitable sea ice becomes unavailable in preferred areas, choosing instead to molt on substrate farther away from foraging grounds (Von Duyke et al., 2020) and/or molt on land, which could raise both daily energy budgets (Hamilton et al., 2015) and predation risk (Smith, 1980). Such behavioral changes will likely influence the degree to which Arctic seals can maintain energy balance.
In this study we use a comparative framework to examine annual patterns in metabolic requirements for three Arctic phocids that differ greatly in their use of and dependence on sea ice. Spotted seals are an ice-associated species found in both sub-Arctic and Arctic regions that utilize seasonal pack ice edges for pupping and molting, but may also use coastal haul-outs during the summer and fall in some locations (Burns, 1970; Lowry et al., 1998). Ringed (Pusa hispida) and bearded (Erignathus barbatus) seals have circumpolar Arctic distributions and are strongly ice-dependent. Ringed seals maintain breathing holes and excavate subnivean lairs in order to remain in areas of extensive fast ice for much of the year, only hauling out on exposed sea ice for extended periods during the spring molt (Burns, 1970; Smith and Stirling, 1975). In contrast, bearded seals use broken and moving pack ice, particularly in regions over shallow foraging grounds, as a platform for pupping and molting (Burns, 1970; Breed et al., 2018; Cameron et al., 2018). Sea ice loss will impact the ability of all three species to successfully carry out important life-history events and alter individual energy budgets (Laidre et al., 2015; Hamilton et al., 2016); however, the extent and severity of effects will depend on the physiology, life-history characteristics and behavioral flexibility of each species.
Due to the remote habitats and cryptic behavior of these species, the only way to obtain year-round metabolic data is through the study of captive individuals. This approach enables the examination of fine-scale changes in parameters over time, under controlled conditions. Working with four spotted seals, three ringed seals and one bearded seal trained to participate in physiological sampling, we document fine-scale changes in the RMR of individuals over a four year-period, with particular focus on using within-subject repeated measures to determine the metabolic implications of molt. Here, we provide measures of RMR for each species, describe seasonal changes, examine potential drivers of observed patterns and discuss the relevance of our findings for each species in light of rapid sea ice loss and ongoing climate change.
Methods
Animals
Eight ice seals participated in this multi-year study at two facilities (Table 1). The spotted and ringed seals stranded at young ages in the wild and were brought to the Alaska SeaLife Center (ASLC) for rehabilitation. Individual seals spent an average of two to three months in rehabilitation and were deemed healthy by veterinary staff prior to entry into this study. The bearded seal was collected in the wild, brought to ASLC for one and a half months and then moved to Long Marine Laboratory (LML) at six months of age for participation in research. One ringed seal and one bearded seal were housed at LML, Santa Cruz, CA (36.9497° N, 122.0656° W). Four spotted seals and two ringed seals were housed at ASLC, Seward, AK (60.0999° N, 149.4410° W). Study animals included both males and females, as well as sub-adult and adult individuals. The seals were trained to voluntarily participate in data collection and routine husbandry care using positive reinforcement methods. Seals at both facilities were housed in natural seawater pools with water temperatures that ranged seasonally with local environmental conditions (LML Tw = 8.6 °C—19.3 °C; ASLC Tw = 4.0 °C—11.5 °C) and had access to haul-out areas at all times of day and during all seasons. TidBit v2 temperature data loggers (Onset Computer Corporation, Bourne, MA, USA) were used to measure and record ambient air and water temperatures at each facility at one-hour intervals. Mean daily air and water temperatures were determined by averaging hourly measurements for each 24-hour period. Seals were kept outdoors under local photoperiod at each facility.
Table 1. Subject information for study animals, with age, mass and data collection dates presented as ranges across the sampling period. Location of study animals include the ASLC in Seward, AK, and the LML in Santa Cruz, CA.
| Species | Individual | Location | Sex | Age (yrs) | Mass (kg) | Data collection |
|---|---|---|---|---|---|---|
| Phoca largha | Amak | ASLC | M | 5.8–9.2 | 49.2–85.0 | Feb 2016—Jun 2019 |
| Tunu | ASLC | M | 5.8–9.2 | 59.5–85.5 | Feb 2016—Jun 2019 | |
| Sura | ASLC | F | 2.8–5.2 | 36.0–59.0 | Feb 2017—Jun 2019 | |
| Kunik | ASLC | M | 1.8–4.1 | 37.0–59.0 | Feb 2017—Jun 2019 | |
| Pusa hispida | Nayak | LML | F | 5.5–8.1 | 24.9–32.5 | Nov 2016—Jun 2019 |
| Pimniq | ASLC | M | 3.5–5.2 | 25.3–31.7 | Oct 2017—Jun 2019 | |
| Dutch | ASLC | F | 2.8–3.2 | 27.3–28.6 | Jan 2019—May 2019 | |
| Erignathus barbatus | Noatak | LML | M | 2.0–4.3 | 102.4–144.6 | Mar 2017—Jun 2019 |
Seals were fed a daily diet composed of several prey types that included relatively high-fat clupeid fish (e.g. herring—Clupea spp.) and relatively high-protein osmerid fish (e.g. capelin—Mallotus villosus), supplemented with cephalopod or bivalve mollusks. Vita-Zu marine mammal tablets (Mazuri, PMI Nutrition International LLC) were included in the diet to ensure proper nutrition. Metrics of food motivation and appetite were scored daily by experienced staff and used to determine optimal diet each day, which allowed caloric intake and body mass to vary seasonally in a natural manner. A subsample of each prey batch was analyzed for proximate and energetic analyses (Michelson Labs Inc., Commerce, CA) and daily food consumption was recorded in both mass (kg) and energy (kcal). Animals were weighed weekly to the nearest tenth of a kilogram using a calibrated platform scale.
Research was conducted under United States National Marine Fisheries Permit 18 902 issued to C. Reichmuth with authorization from the Ice Seal Committee. Institutional Animal Care and Use Committees at the University of California Santa Cruz and Alaska SeaLife Center approved research protocols and provided oversight of animal welfare.
Metabolic measurements
In-water, RMRs were determined for all ice seals using an open-flow respirometry system designed for marine mammals. Individuals were trained to remain stationary beneath a custom-built metabolic dome attached to a PVC piping frame that floated at the top of the water surface (101 cm long x 90 cm wide x 46 cm high). Animals were able to move into and out of the dome freely, but were trained to voluntarily station and rest beneath the dome for extended intervals. Sessions were conducted in the morning following an overnight fast to ensure that seals were postprandial. To establish consistent and relaxed cooperative behavior, seals rehearsed this routine for 4–8 min each morning and were rewarded with 30 to 40% of their scheduled diet. Data collection trials were attempted weekly with average duration ranging between 10–13 min depending on the pre- and during-trial behavior of each animal on a given day. RMR measurements were considered useable only when animals were at continuous rest beneath the dome for a minimum of 5 min, and the metabolic trace contained an interval of resting equilibrium that exceeded 4 min.
Rate of oxygen consumption (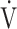 O2) during metabolic trials was determined by pulling ambient air through a metabolic dome at known rates between 150–200 L/min by a mass flow controller (Sable Systems International, North Las Vegas, NV, USA). Exact flow rates were dependent on individual mass and set to ensure that the oxygen content within the dome was maintained above 20.10% at all times. Subsamples of dome exhaust were dried (Drierite, W. A. Hammond Drierite, Xenia, OH, USA), scrubbed of carbon dioxide (Sodasorb, Smiths Medical Inc., Minneapolis, MN) and dried again, before entering an oxygen analyzer (Sable Systems International, North Las Vegas, NV, USA). Oxygen content of dome exhaust was recorded every 1.0 s on a laptop computer using EXPEDATA software (Sable Systems International, North Las Vegas, NV, USA). Ambient air baselines were collected before and after animals were under the dome to account for system drift and for use in
O2) during metabolic trials was determined by pulling ambient air through a metabolic dome at known rates between 150–200 L/min by a mass flow controller (Sable Systems International, North Las Vegas, NV, USA). Exact flow rates were dependent on individual mass and set to ensure that the oxygen content within the dome was maintained above 20.10% at all times. Subsamples of dome exhaust were dried (Drierite, W. A. Hammond Drierite, Xenia, OH, USA), scrubbed of carbon dioxide (Sodasorb, Smiths Medical Inc., Minneapolis, MN) and dried again, before entering an oxygen analyzer (Sable Systems International, North Las Vegas, NV, USA). Oxygen content of dome exhaust was recorded every 1.0 s on a laptop computer using EXPEDATA software (Sable Systems International, North Las Vegas, NV, USA). Ambient air baselines were collected before and after animals were under the dome to account for system drift and for use in 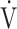 O2 calculations. Temperature and relative humidity of chamber air was determined during trials using a handheld temperature and humidity sensor (Vaisala HM40, Vantaa, Finland). Flow rates were corrected to standard temperature and pressure dry (STPD) and
O2 calculations. Temperature and relative humidity of chamber air was determined during trials using a handheld temperature and humidity sensor (Vaisala HM40, Vantaa, Finland). Flow rates were corrected to standard temperature and pressure dry (STPD) and 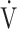 O2 determined using EXPEDATA software (Sable Systems International, North Las Vegas, NV, USA) and standard equations (Withers, 1977). Before each trial the oxygen analysis system was calibrated with dry, ambient air. Metabolic systems at both facilities were routinely checked for leaks and accuracy using 100% nitrogen gas (Fedak et al 1981; Davis et al. 1985).
O2 determined using EXPEDATA software (Sable Systems International, North Las Vegas, NV, USA) and standard equations (Withers, 1977). Before each trial the oxygen analysis system was calibrated with dry, ambient air. Metabolic systems at both facilities were routinely checked for leaks and accuracy using 100% nitrogen gas (Fedak et al 1981; Davis et al. 1985).
Molt status
We documented the timing, progression and overall duration of the visible molt for each animal annually. Data on molt were compiled from detailed photographic records, molt monitoring data sheets and husbandry records. The start date of each molt was defined as the first documentation of loose hair and/or active hair loss. The end of each molt was defined by the complete loss of the old coat and complete re-growth of a new coat. The 50% molt date was the day at which half of the new coat was determined to have grown in. For statistical analyses the molt status of an individual for a given metabolic data collection session was categorized in one of four ways: not molting, 1 month pre-molt, actively molting or 1 month post-molt.
Statistical analyses
We did not assume each species would respond similarly to changes in fixed parameters and therefore, evaluated each species independently.
To examine potential drivers of metabolism in ringed and spotted seals we used linear mixed-effects (LME) models. We included individual as a random effect within each LME model for each species, which allowed us to account for repeated measures and avoid issues associated with pseudo-replication (Harrison et al., 2018). Absolute 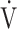 O2 data (rather than mass-specific) were used in statistical analyses to avoid any a priori bias based on scaling (Nagy, 1987; Glazier, 2005). We assessed potential fixed effects for each model—mass, age, sex, molt status, air temperature and water temperature—for collinearity using scatterplot matrices. Animal location (ASLC, LML) was not included as a fixed effect, as the inclusion of air and water temperature accounted for regional differences. Although photoperiod is known to affect the timing of molt in seals (Mo et al., 2000), we did not include it in our models as photoperiod is strongly correlated with molt status, and our aim was to determine the effect of molt status, not photoperiod, on metabolism.
O2 data (rather than mass-specific) were used in statistical analyses to avoid any a priori bias based on scaling (Nagy, 1987; Glazier, 2005). We assessed potential fixed effects for each model—mass, age, sex, molt status, air temperature and water temperature—for collinearity using scatterplot matrices. Animal location (ASLC, LML) was not included as a fixed effect, as the inclusion of air and water temperature accounted for regional differences. Although photoperiod is known to affect the timing of molt in seals (Mo et al., 2000), we did not include it in our models as photoperiod is strongly correlated with molt status, and our aim was to determine the effect of molt status, not photoperiod, on metabolism.
Prior to model building we assessed dependent variables for normality. Spotted seal absolute 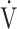 O2 data were log transformed to improve normality. Ringed seal absolute
O2 data were log transformed to improve normality. Ringed seal absolute 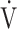 O2 data were normally distributed and no transformation was needed. For spotted seals and ringed seals, age and mass were highly collinear and age was subsequently dropped. In addition, we examined data for homogeneity of variance. Sex violated the homogeneity of variance assumption using Bartlett’s test for both spotted seals and ringed seals. Given the inclusion of only one female and one male in the study for spotted and ringed seals, respectively, we dropped sex as a fixed factor in both models. We used backwards elimination and corrected Akaike’s Information Criteria (AICc) for model selection. Finally, residuals of the final models were plotted to confirm homoscedasticity and normality, and thus proper model selection.
O2 data were normally distributed and no transformation was needed. For spotted seals and ringed seals, age and mass were highly collinear and age was subsequently dropped. In addition, we examined data for homogeneity of variance. Sex violated the homogeneity of variance assumption using Bartlett’s test for both spotted seals and ringed seals. Given the inclusion of only one female and one male in the study for spotted and ringed seals, respectively, we dropped sex as a fixed factor in both models. We used backwards elimination and corrected Akaike’s Information Criteria (AICc) for model selection. Finally, residuals of the final models were plotted to confirm homoscedasticity and normality, and thus proper model selection.
For bearded seal data analysis we began by using multiple linear regressions. Absolute 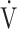 O2 data were log transformed to improve normality. We assessed potential fixed effects (age, mass, molt status, air temperature and water temperature) for collinearity using scatterplot matrices. Age and mass, as well as air and water temperature were highly correlated. Age and air temperature were subsequently dropped. Using backwards elimination and corrected Akaike’s Information Criteria (AICc) in model selection, we found there was no appropriate regression model to describe the bearded seal data. Therefore, we directly tested the effect of molt status on absolute
O2 data were log transformed to improve normality. We assessed potential fixed effects (age, mass, molt status, air temperature and water temperature) for collinearity using scatterplot matrices. Age and mass, as well as air and water temperature were highly correlated. Age and air temperature were subsequently dropped. Using backwards elimination and corrected Akaike’s Information Criteria (AICc) in model selection, we found there was no appropriate regression model to describe the bearded seal data. Therefore, we directly tested the effect of molt status on absolute 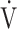 O2 using a one-way ANOVA with Tukey’s post hoc comparisons. Homogeneity of variance was confirmed using Bartlett’s test.
O2 using a one-way ANOVA with Tukey’s post hoc comparisons. Homogeneity of variance was confirmed using Bartlett’s test.
All statistical analyses were completed using JMP14 statistical software program (SAS Institute, Cary, NC). Metabolic data are presented as mean values ±SD. Results were considered significant if P < 0.05.
Results
Over a four-year period, we collected 812 metabolic data points from eight individuals across three species. Specifically, we collected 542 RMR measurements from four spotted seals, 175 measurements from three ringed seals and 95 measurements from one bearded seal (Table 1). On average, four data points were collected for each individual per month. To account for differences in sample sizes between individuals when determining species-average RMR values we calculated a grand species mean from individual seal means. Although there were intraspecific differences in metabolism, interspecific demands for these individuals followed expected metabolic relationships based on body size (Table 2). Ringed seals had the lowest absolute energy demands (0.20 ± 0.04 L O2 min−1), but the highest mass-specific demands (7.01 ± 1.38 ml O2 min−1 kg−1). Spotted seals exhibited a mean absolute RMR of 0.33 ± 0.07 L O2 min−1, with a mean mass-specific RMR of 6.13 ± 0.73 ml O2 min−1 kg−1. The bearded seal had the highest absolute RMR (0.48 ± 0.04 L O2 min−1) and lowest mass-specific RMR (4.10 ± 0.47 ml O2 min−1 kg−1).
Table 2. Summary of molt and metabolic data for all study animals. Data are presented as means ± standard deviations with sample sizes displayed in parentheses. Molt cycle sample sizes refer to the number of documented molt cycles, while metabolic sample sizes refer to number of data points.
| Species | Individual | Typical molt timing | Molt duration (d) | Absolute RMR (L O2 min−1) | Mass-specific RMR (ml O2 min−1 kg−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Annual mean | Non-molting | Molting | Annual mean | Non-molting | Molting | ||||
| Phoca largha | Amak | May–Jun | 38±13 (4) | 0.43±0.08 (154) | 0.42±0.06 (131) | 0.51±0.13 (23) | 6.98±1.54 (154) | 6.68±1.34 (131) | 8.69±1.48 (23) |
| Tunu | May–Jun | 31±6 (4) | 0.36±0.06 (172) | 0.35±0.06 (150) | 0.42±0.06 (22) | 5.47±1.00 (172) | 5.34±0.96 (150) | 6.41±0.75 (22) | |
| Sura | Apr–May | 36±21 (3) | 0.28±0.06 (106) | 0.27±0.04 (84) | 0.33±0.08 (22) | 6.49±1.07 (106) | 6.22±1.04 (84) | 7.53±1.08 (22) | |
| Kunik | Apr–May | 27±2 (3) | 0.25±0.07 (110) | 0.24±0.05 (92) | 0.31±0.11 (18) | 5.58±1.03 (110) | 5.35±0.78 (92) | 6.79±1.31 (18) | |
| Pusa hispida | Nayak | Mar–Apr | 36±8 (3) | 0.25±0.03 (122) | 0.24±0.03 (110) | 0.30±0.03 (12) | 8.93±1.29 (122) | 8.78±1.21 (110) | 10.31±1.21 (12) |
| Pimniq | May–Jun | 21±1 (2) | 0.18±0.05 (42) | 0.17±0.04 (35) | 0.25±0.06 (7) | 6.37±1.66 (42) | 5.90±1.29 (35) | 8.72±1.33 (7) | |
| Dutch | May | 26 (1) | 0.16±0.05 (11) | 0.13±0.03 (7) | 0.21±0.03 (4) | 5.73±1.73 (11) | 4.68±0.95 (7) | 7.57±1.04 (4) | |
| Erignathus barbatus | Noatak | Dec–Apr | 119±2 (3*) | 0.48±0.04 (95) | 0.47±0.04 (60) | 0.50±0.05 (35) | 4.10±0.47 (95) | 3.96±0.33 (60) | 4.34±0.56 (35) |
*Actual start date of molt could not be determined for this seal during its first year; thus, average molt duration for this animal was determined using two of three molt cycles during the study.
We documented apparent species-level differences in the timing and duration of the annual molt. Spotted and ringed seals molted over relatively short time frames, 33 ± 4 days and 28 ± 6 days, respectively. In contrast, the bearded seal exhibited prolonged molting intervals that lasted an average of 119 ± 2 days. Within individuals, the timing and duration of the annual molt was consistent across years (Table 2), but there was some variation between individuals. Spotted seals generally molted between April and June in Alaska, with the two immature spotted seals beginning their molt a month earlier than the two adults. Both ringed seals in Alaska molted over a similar time frame (May–June), while the ringed seal in California molted earlier; generally, between March and April. The bearded seal molt, which lasted approximately 4 months, consistently occurred between December and April in California.
We observed clear annual patterns in RMR for spotted (Fig. 1) and ringed (Fig. 2) seals that were highly consistent within individuals across years, with both absolute and mass-specific energy expenditure peaking coincident with the annual molt. The longitudinal metabolic patterns for spotted seals were strongly driven by molt status (F3,534 = 67.79, P < 0.001), air temperature (F1,533 = 24.71, P < 0.001) and body mass (F1,482 = 101.26, P < 0.001). RMR increased during molt, as well as with increasing body mass and air temperature (Table 3). Individual accounted for 46% of the observed variance in the spotted seal data. Annual patterns in RMR for ringed seals were driven most strongly by molt status (F3,168.1 = 43.91, P < 0.001) and water temperature (F1,170 = 5.35, P = 0.022). For ringed seals, RMR increased in association with the annual molt and decreased with increasing water temperature (Table 4). Individual accounted for 83% of the observed variance for ringed seals. For both species, molt status had a strong effect on metabolism. Mean molting RMR values—in both absolute and mass-specific terms—were consistently higher than non-molting values (Table 2; Supplementary Data), with maximum energetic costs occurring coincident with the 50% new coat date for both species (Figs 1, 2); for some individuals RMR more than doubled at this time relative to non-molting levels.
Figure 1. Longitudinal RMR data collected for four spotted seals (a–d) over a 4-year period. Data from each individual in successive years are stacked and displayed in two adjacent panels. Left panels display monthly mean mass-specific RMR (±SD) for each animal. On average, four data points (range: 1–11) were collected each month. Dashed vertical lines denote the typical molting period. Source data for the left panels, along with corresponding absolute RMR values and subject metadata, are provided as Supplementary Data. Right panels display individual metabolic data points collected during the 100 days preceding and 100 days following the 50% molt date each year (solid vertical line). Dashed vertical lines denote the mean start and end dates of molt referenced to the 50% molt date.
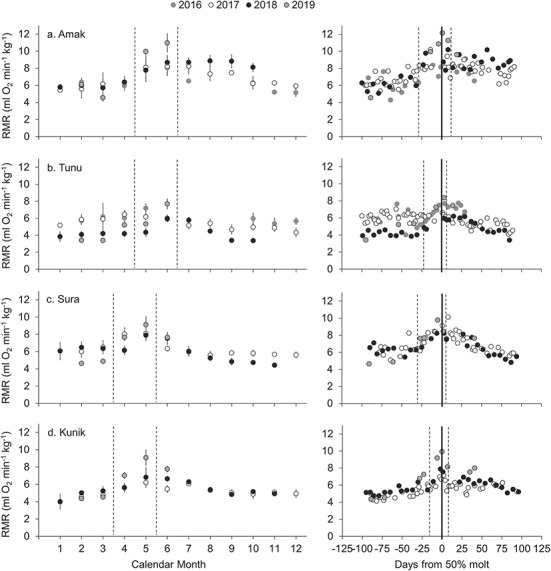
Figure 2. Longitudinal RMR data collected for three ringed seals (a-c) over a four-year period. Data from each individual are stacked and each individual’s data are displayed in two adjacent panels. Left panels display monthly mean mass-specific RMR (±SD) for each animal. On average, three data points (range: 1–10) were collected each month. Dashed vertical lines denote the typical molting period. Source data for the left panels, along with corresponding absolute RMR values and subject metadata, are provided as Supplementary Data. Right panels display individual metabolic data points collected during the 100 days preceding and 100 days following the 50% molt date each year (solid vertical line). Dashed vertical lines denote the mean start and end dates of molt referenced to the 50% molt date.
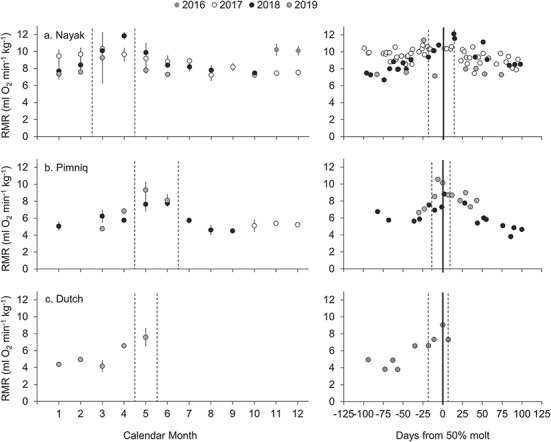
Table 3. LME model best fit results for spotted seals with molt status (F3,534 = 67.79, P < 0.001), air temperature (F1,533 = 24.71, P < 0.001) and body mass (F1,482 = 101.26, P < 0.001) as fixed effects against absolute  O2. Individual was included as a random effect and accounted for 46% of the variance in the model.
O2. Individual was included as a random effect and accounted for 46% of the variance in the model.
| Parameters | β | S.E. | DFDen | t-ratio | P |
|---|---|---|---|---|---|
| Intercept | −0.8161 | 0.044 | 11.08 | −18.44 | <0.001 |
| Molt – during | 0.0529 | 0.006 | 533 | 8.33 | <0.001 |
| Molt – no | −0.0566 | 0.005 | 534.1 | −12.10 | <0.001 |
| Molt – 1 mo. post | 0.0225 | 0.008 | 535 | 2.97 | 0.003 |
| Air Temp | 0.0043 | 0.001 | 533.3 | 4.97 | <0.001 |
| Mass | 0.0053 | 0.001 | 481.8 | 10.06 | <0.001 |
Table 4. LME model best fit results for ringed seals with molt status (F3,168 = 43.91, P < 0.001) and water temperature (F1,170 = 5.35, P = 0.022) as fixed effects against absolute  O2. Individual was included as a random effect and accounted for 83% of the variance in the model.
O2. Individual was included as a random effect and accounted for 83% of the variance in the model.
| Parameters | β | S.E. | DFDen | t-ratio | P |
|---|---|---|---|---|---|
| Intercept | 0.2399 | 0.039 | 2.42 | 6.18 | 0.016 |
| Molt – during | 0.0373 | 0.005 | 168.1 | 7.09 | <0.001 |
| Molt – no | −0.0349 | 0.004 | 168 | −9.80 | <0.001 |
| Molt – 1 mo. post | 0.0051 | 0.006 | 168 | 0.85 | 0.395 |
| Water Temp | −0.0035 | 0.001 | 170 | −2.31 | 0.022 |
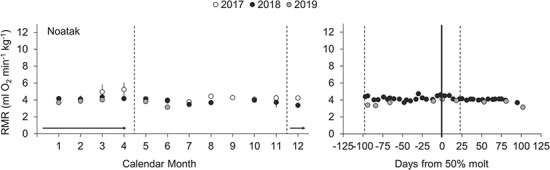
In contrast to spotted and ringed seals, the bearded seal did not exhibit notable seasonal patterns or discrete peaks in metabolism during the study period (Fig. 3). Despite comparably stable absolute and mass-specific RMR values (Table 2; Supplementary Data), we did discern an effect of molting on bearded seal metabolism (one-way ANOVA, F3,91 = 4.51, P = 0.005), with the only significant post-hoc comparison between molting and non-molting periods (Tukey’s HSD, P = 0.003). However, the percentage difference in mean mass-specific RMR between molting and non-molting periods was much smaller for the bearded seal (9%) than for the spotted seals (18–26%) and ringed seals (16–47%).
Figure 3. Longitudinal RMR data collected for one bearded seal over 3 years. Left panel displays monthly mean mass-specific RMR (±SD). On average, four data points (range: 1–6) were collected each month. Typical molt period denoted by a horizontal arrow between dashed vertical lines. Source data for the left panel, along with corresponding absolute RMR values and subject metadata, are provided as Supplementary Data. Right panel displays individual metabolic data points collected in the 100 days preceding and 100 days following the 50% molt date each year (solid vertical line). Dashed vertical lines denote the mean start and end dates of molt referenced to the 50% molt date.
Discussion
Baseline energy demands
Through the collection of fine-scale longitudinal data we were able to provide year-round, empirical RMR data for eight individuals comprising three Arctic seal species. Energy demands between species followed predicted trends based on body size with the bearded seal exhibiting the highest absolute and lowest mass-specific mean annual RMR, followed by the spotted seals and then the ringed seals. Most notably, our data revealed annual patterns in RMR that were strongly related to the distinct molting strategy of each species. Although we could not establish population-level values due to limited sample sizes for each species, by testing individual seals multiple times per month while in a consistent and calm state, we were able to establish high repeatability for our metabolic measurements both within individuals and across years. Thus, we are confident in the regularity of the annual patterns described here. Further, these types of fine-scale data are valuable for parameterizing predictive population and bioenergetics models.
Given that metabolic data are only available for a limited number of phocid seals, the values reported here improve our understanding of phocid energetics and provide species-specific information pertaining to the metabolic needs of ice-associated seals. Specifically, we contribute metabolic data for the smallest phocid species (i.e. ringed seal) as well as the largest Arctic phocid (i.e. bearded seal). Indeed, this is the first study to empirically measure metabolism in a bearded seal, which exhibited highly stable annual energy demands. The mean mass-specific RMR we determined for spotted seals was slightly lower than the value previously reported by Ashwell-Erickson et al. (1986); however, only immature individuals (≤2 yrs. old) were measured in that study, which would be expected to have elevated mass-specific demands relative to mature individuals (Kleiber, 1961). Further, as the seals in that study had not been conditioned to participate voluntarily in data collection, they may not have been in a true resting state during metabolic measurements. Thus, our slightly lower values likely reflect the increased age and larger body size of our spotted seals as well as our training protocol. For ringed seals, we found a higher mean RMR in-water than that reported by Ochoa-Acuña et al. (2009) in-air, who collected metabolic data for a single adult male in a haul-out chamber. Of note, the authors provided one in-water value for the same individual in that study, which was nearly twice that of his in-air RMR, but comparable to the mean in-water RMR values reported here.
As would be expected, there were some differences in mean RMR between individuals of the same species. Between-individual differences were minor in spotted seals and pronounced in ringed seals. Across our ringed seals, the greatest differences were observed between the ringed seal in California and those in Alaska. The female ringed seal in California had higher absolute and mass-specific energy demands than either ringed seal in Alaska, likely driven by differences in temperature regime and associated proportions of lean body mass and blubber. We do not believe the ringed seal in California was housed in conditions outside her thermoneutral zone (TNZ), as there was overlap between the ambient temperature ranges at both facilities; however, TNZ has not been established for this species and therefore this possibility cannot be ruled-out. Importantly, although RMR varied between individuals, we observed the same relative patterns across all three ringed seals with similar changes in RMR associated with molt.
Metabolic consequences of molt
We documented annual changes in RMR that were strongly related to species-specific molting phenology. Spotted and ringed seals molted over relatively short periods and exhibited markedly increased RMRs during this time, which peaked midway through the visible molt. This pattern was considerably different than that of the bearded seal, which took approximately four times longer to molt and exhibited a more subtle increase in RMR across the molting period. This prolonged molting strategy is unique among phocid seals. Although the bearded seal in this study molted earlier than free-ranging conspecifics, the duration and diffuse pattern of molt documented here agree with accounts of wild bearded seals (for review, see Cameron et al., 2010). Further, latitudinal gradients and ontogenetic differences in the timing of molt are common among Arctic seals; seals in lower latitudes tend to molt earlier than seals in higher latitudes likely driven by differences in photoperiod (Mo et al., 2000), and younger seals tend to molt before mature seals (for review, see Boveng et al., 2008; Boveng et al., 2009; Cameron et al., 2010; Kelly et al., 2010). Thus, given that our bearded seal was a young individual (1–3 years) housed in California under local photoperiod, his earlier molting period aligns with geographic and ontogenetic trends in molt timing observed for wild seals. Similarly, the ringed seal housed in California molted earlier than the two ringed seals housed in Alaska. All spotted seals housed in Alaska molted around the same time generally reported for their wild counterparts, with the two younger seals molting prior to the two sexually mature individuals.
The annual molt is generally considered an energetically expensive period for phocids (Boyd et al., 1993; Renouf and Gales, 1994; Boily, 1995, 1996; Boily and Lavigne, 1997; Hedd et al., 1997; Chabot and Stenson, 2002; Williams et al., 2011; Walcott et al., 2020); however, there are inconsistencies in the literature (Ashwell-Erickson et al., 1986; Worthy et al., 1992; Rosen and Renouf, 1998) that have led to the suggestion of a typical pattern of little to no cost of molt in phocids (Beltran et al., 2018). In those species for which we have strong evidence of increased metabolic costs during molt, it is unclear whether the main drivers of those additional costs relate to energetic requirements of generating new hair or to thermal losses associated with sending blood to the periphery to maintain the elevated skin temperatures necessary for cellular growth (Boily, 1995). Our data reveal significant, short-term increases in RMR during the molt for ringed and spotted seals, but also suggest that these costs may be mitigated in some species (i.e. bearded seals) by molting over a longer time frame.
The majority of studies examining phocid molting energetics have used methods similar to those described here. Of those studies, significant molt-associated metabolic costs have been reported for grey seals (Boily, 1996; Boily and Lavigne, 1997), harp seals (Hedd et al., 1997; Chabot and Stenson, 2002) and Hawaiian monk seals (Williams et al., 2011); however, metabolic depression during molt has been reported for spotted and harbor seals (Ashwell-Erickson et al., 1986; Rosen and Renouf, 1998). It is not apparent why there would be species-specific differences in the metabolic consequences of molt across phocids; however, resolution of this issue may be found through closer examination of the underlying data in the literature. For example, while Ashwell-Erickson et al. (1986) has long been cited as evidence of metabolic depression during molt, the authors actually documented increasing metabolic rates during the regenerative phase of molt. It appears that the interpretation of metabolic depression during molt was based on the early decline in metabolism observed during the 20–60 days preceding the visible molt. When considering only the visible molt, during which time hair is actively shed and regrown, their data largely agree with our findings—and those of other investigators—of additional costs associated with molting.
Increased levels of thyroid hormones have been linked with increased metabolism during the molt. In addition to their effect on metabolism, thyroid hormones stimulate the growth of new fur (Ramot et al., 2009). Elevated thyroid hormones during the visible molt have been documented in grey (Boily, 1996) and harp (John et al., 1987) seals, both species for which there are known metabolic costs associated with molt (Boily and Lavigne, 1997; Hedd et al., 1997; Chabot and Stenson, 2002). Similarly, in penguins, increased thyroid hormone levels are associated with new feather synthesis and increased metabolism (Groscolas and Cherel, 2014). Routti et al. (2013) proposed an energetic cost of molt for ringed seals given a documented rise in thyroid hormones during this period, which was confirmed by the direct measures of RMR presented here. In spotted seals, thyroid hormones decline to a minimum just before the molting period and then increase to their maximum values during the period of most rapid hair growth (Ashwell-Erickson et al., 1986). This peak in spotted seal thyroid hormones during the period of most rapid hair growth support the increase in RMR documented in the present study, with peak RMR coinciding with the 50% molt date. Interestingly, in harbor seals—the only other species besides spotted seals for which a metabolic depression during molt has been proposed (Ashwell-Erickson et al., 1986; Rosen and Renouf, 1998)—there are no documented changes in thyroid hormones in association with molting (Renouf and Brotea, 1991).
Although not well studied, it has long been believed that wild bearded seals undergo a protracted molt that differs from other phocids. Some have suggested that the diffuse molt of bearded seals involves year-round shedding of hair with a peak in molting activity during the spring when seals are regularly observed hauling out (for review, see Cameron et al., 2010). Data from the bearded seal in this study supports this notion and a similar molting pattern was observed in two additional captive individuals (C. Reichmuth, unpublished data). Our bearded seal was housed under local photoperiod in California, rather than an Arctic photoperiod, but we do not believe this was the cause of its prolonged molt. Although photoperiod is known to affect timing of molt in seals (Mo et al., 2000), its effect on duration is less clear. Indeed, the ringed seal housed in California consistently had an earlier onset of molt than the ringed seals in Alaska, but the progression and duration of molting was similar for all ringed seals. Thus, photoperiod was likely responsible for the early onset of the bearded seal’s molt, but not a direct cause of its prolonged duration.
The pronounced elevation in RMR we observed in spotted and ringed seals during molt was noticeably absent in the bearded seal, with no observable increase associated with the 50% molt date. By having a diffuse molt over an extended period, bearded seals may limit the overall energetic cost of this annual event and/or spread out the costs to reduce the metabolic impact at any one point in time. This energy-saving strategy may be facilitated by the much larger body size of bearded seals relative to other ice seals. This strategy may be physiologically impractical for ringed and spotted seals due their smaller body sizes, higher surface area to volume ratios and potential rates of heat loss, which may necessitate more rapid molt cycles. However, there are likely other trade-offs associated with this protracted molting strategy that should be considered, such as the impact on haul-out behavior. Overall, our results support the notion of an extended molt in bearded seals, enhance our understanding of individual molt dynamics and reveal the potential benefits of this unique strategy.
One conflicting finding from our study was the species-specific effects of air and water temperature on metabolism. RMR increased with increasing temperature in spotted seals, but decreased with increasing temperature in ringed seals. Further, we found no effect of temperature on the bearded seal’s RMR. While additional thermoregulatory costs can be accrued when animals are in environments outside their TNZ, this seems unlikely here. Based on studies of harbor seals, ambient temperatures experienced by our spotted seals were likely within their TNZ (Hansen, 1995; Hansen et al., 1995). The pattern we observed for ringed seals might be explained if individuals were tested below their lower critical temperature at points during the study. However, the lowest water temperature experienced by our ringed seals was 4.0 °C, a temperature well within the normal range of their wild counterparts (Kelly et al., 2010). Therefore, rather than temperature driving observed patterns in RMR, it is more likely that the timing of molt is associated with seasonal changes in temperature that do not themselves have independent effects on metabolism.
Conservation implications
Climate change is a pervasive threat to all ice-associated seals (Kovacs et al., 2011; Laidre et al., 2015), although the ability of these seals to adjust to changing conditions will depend on the unique characteristics of each species. Here, we provide some of the most comprehensive metabolic data for spotted, ringed and bearded seals with which to better predict the species-specific consequences of ongoing Arctic warming. These data fill important gaps in our understanding of the physiology of each species, and provide source data for predictive population and bioenergetic models that are important for conservation and management efforts. Further, this work highlights species-specific physiological attributes that are important when considering the impact of sea ice loss during key life-history events, such as molt.
In polar regions, the increased skin temperatures that are necessary to promote tissue regeneration during molt can only be achieved by ice seals when hauled out (Feltz and Fay, 1966; Boily, 1995; Walcott et al., 2020). If seals were unable to do so, increased skin perfusion in polar water for prolonged periods would result in untenably high rates of heat loss (Watts et al., 1993). Thus, adequate haul-out substrate is essential for polar seals during molt, raising specific concerns about the physiological consequences of declining sea ice. We found that spotted and ringed seals exhibit significant peaks in energy expenditure associated with their discrete molt and we observed them spending more time hauled out during this period than at any other time during the year. Despite the fact that our bearded seal exhibited minimal changes in metabolism associated with molt, we also observed his haul-out time to increase markedly during molt, particularly from around the 50% molt date through the end of molt. This suggests benefits of increased skin temperatures for molting bearded seals, despite their muted metabolic response.
Declining ice cover can also place energetic burdens on ice-dependent seals in other ways. To ensure adequate haul-out substrate during molt, there is evidence that ringed seals remain with preferred sea ice habitat as it retreats, even into marginal foraging areas (Hamilton et al., 2015; Von Duyke et al., 2020). Our data suggest that this behavior reflects the bioenergetic priorities of seals during molt. The advantages of hauling out to promote tissue regeneration may outweigh the caloric benefits of remaining in preferred foraging areas. This behavioral change occurs despite the additional energetic investment that individuals must make to move with or travel to preferred sea ice regions. Bearded seals tend to select areas of broken, drifting pack ice over shallow foraging grounds (Burns, 1967; Smith and Stirling, 1975; Bengtson et al., 2005). As this type of sea ice retreats from preferred foraging grounds, suitable haul-out substrate for bearded seals may become decoupled from important regions. This would force bearded seals to either move to areas of preferred ice cover, as has been observed for ringed seals, or to move coastal regions to haul out on land where risk of predation will be greater (Smith, 1980). Tagging data from juvenile bearded seals suggests they respond similarly to ringed seals, opting to move with preferred sea ice as it retreats (Breed et al., 2018; Cameron et al., 2018).
Although behavioral adjustments may currently allow seals to maintain contact with preferred sea ice habitat during molt, continued sea ice loss will likely require individuals to spend greater amounts of time in water. It remains unclear whether individuals can successfully molt without hauling out (Feltz and Fay, 1966; Boily, 1995). If seals curtail surface blood flow in response to spending more time in water, or cannot maintain elevated skin temperatures, this could slow, delay, or disrupt the overall process of tissue regeneration. Recent unusual mortality events (UMEs) for ice associated pinnipeds (2011–2016; 2018–present) may provide glimpses into the physiological consequences of reduced sea ice during molt (NOAA Fisheries, 2011a, 2011b, 2016, 2020). Although the cause of the 2011–2016 Alaska Pinniped UME is still unknown, seals and walruses presented with abnormal skin lesions and what appeared to be abnormal or disrupted molts (NOAA Fisheries, 2018). This UME coincided with many years of early sea ice breakup. A more recent UME, specific to ringed, bearded and spotted seals, began in 2018 and is ongoing (NOAA Fisheries, 2020). As part of this UME, seals are presenting with similar symptoms and appear in poor body condition, suggesting widespread energetic losses. These UMEs may be some of the first examples of the detrimental effects of the loss of sea ice for ice-associated seals.
The 2012 decision to list distinct population segments of bearded seals as threatened under the United States Endangered Species Act was driven by concern that sea ice loss would substantially reduce or eliminate adequate haul-out platforms for pupping and molting, leading to decreases in reproduction and survival (NMFS, 2012). Similar concerns exist for ringed seal populations, which are predicted to be at risk of major declines due to ongoing sea ice loss (Kelly et al., 2010; Kovacs et al., 2011; Reimer et al., 2019). Spotted seals may be less impacted by reductions in sea ice as they are already known to successfully utilize coastal haul outs for pupping and molting in lower latitudes (Wang, 1986; Nesterenko and Katin, 2009); however, the cascading consequences of climate change will inevitably affect all three species. Ultimately, this work highlights potential energetic trade-offs associated with different molting strategies in these three species and more broadly, provides valuable quantitative data regarding annual patterns in energy demands that can be used to assess species-specific vulnerabilities of Arctic seals to changing conditions.
Funding
This work was supported by the National Oceanic and Atmospheric Administration’s Alaska Pinnipeds Program [NA15NMF4390166, NA16NMF4390027, NA19NMF4390083].
Supplementary Material
Acknowledgements
We are grateful to the husbandry and veterinary teams at the Alaska SeaLife Center and Long Marine Laboratory for their dedicated work on this long-term project, especially Jenna Sullivan, Brandi Ruscher, Taylor Abraham, Mariah Tengler, Dave Casper, Jamie Mullins, Juliana Kim, Shelby Burman, Margaret Black, Brett Long, Derek Woodie, Kathy Woodie and Carrie Goertz. The authors thank John Goodwin, Alex Whiting, Andrew Rouse, Markus Horning, Lori Polasek, Lisa Hartman, Tara Reimer and Gary Griggs for their contributions. We also acknowledge the NOAA Polar Ecosystems Program and the Alaska Department of Fish and Game’s Marine Mammals Program for thoughtful discussions about this work.
References
- Andersen M, Hjelset AM, Gjertz I, Lydersen C, Gulliksen B (1999) Growth, age at sexual maturity and condition in bearded seals (Erignathus barbatus) from Svalbard, Norway. Polar Biol 21: 179–185. [Google Scholar]
- Ashwell-Erickson S, Fay FH, Elsner R, Wartzok D (1986) Metabolic and hormonal correlates of molting and regeneration of pelage in Alaskan harbor and spotted seals (Phoca vitulina and Phoca largha). Can J Zool 64: 1086–1094. [Google Scholar]
- Beck CA, Bowen DW, Iverson SJ (2003) Sex differences in the seasonal patterns of energy storage and expenditure in a phocid seal. J Anima 72: 280–291. [Google Scholar]
- Beltran RS, Burns JM, Breed GA (2018) Convergence of biannual moulting strategies across birds and mammals. Proc R Soc London B 285: 20180318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran RS, Testa JW, Burns JM (2017) An agent-based bioenergetics model for predicting impacts of environmental change on a top marine predator, the Weddell seal. Ecol Modell 351: 36–50. [Google Scholar]
- Bengtson JL, Hiruki-Raring LM, Simpkins MA, Boveng PL (2005) Ringed and bearded seal densities in the eastern Chukchi Sea, 1999–2000. Polar Biol 28: 833–845. [Google Scholar]
- Boily P (1995) Theoretical heat flux in water and habitat selection of phocid seals and beluga whales during the annual molt. J Theor Biol 172: 235–244. [Google Scholar]
- Boily P (1996) Metabolic an hormonal changes during the molt of captive gray seals (Halichoerus grypus). Am J Physiol 5: 1051–1058. [DOI] [PubMed] [Google Scholar]
- Boily P, Lavigne DM (1997) Developmental and seasonal changes in resting metabolic rates of captive female grey seals. Can J Zool 75: 1781–1789. [Google Scholar]
- Boveng PL, Bengtson JL, Buckley TW, Cameron MF, Dahle SP, Kelly BP, Megrey BA, Overland JE, Williamson NJ (2009) Status review of the spotted seal (Phoca largha). NOAA Tech Memo NMFS-AFSC 200i–xiii: 1–153. [Google Scholar]
- Boveng PL, Bengtson JL, Buckley TW, Cameron MF, Dahle SP, Megrey BA, Overland JE, Williamson NJ (2008) Status review of the ribbon seal (Histriophoca fasciata). NOAA Tech Memo NMFS-AFSC 191i–xiv: 1–115. [Google Scholar]
- Boyd I, Arnbom T, Fedak M (1993) Water flux, body composition, and metabolic rate during molt in female southern elephant seals (Mirounga leonina). Physiol Zool 66: 43–60. [Google Scholar]
- Breed GA, Cameron MF, Ver Hoef JM, Boveng PL, Whiting A, Frost KJ (2018) Seasonal sea ice dynamics drive movement and migration of juvenile bearded seals Erignathus barbatus. Mar Ecol Prog Ser 600: 223–237. [Google Scholar]
- Burns JJ (1967) The Pacific bearded seal. Alaska Department of Fish and Game, Pittman-Robertson Project Report W-6-R and W-14-R. 66 p.
- Burns JJ (1970) Remarks on the distribution and natural history of pagophilic pinnipeds in the Bering and Chukchi Seas. J Mammal 51: 445–454. [Google Scholar]
- Burns JM, Lestyk KC, Folkow LP, Hammill MO, Blix AS (2007) Size and distribution of oxygen stores in harp and hooded seals from birth to maturity. J Comp Physiol B 177: 687–700. [DOI] [PubMed] [Google Scholar]
- Cameron MF et al. (2010) Status review of the bearded seal (Erignathus barbatus). NOAA Tech Memo NMFS-AFSC 211: i. [Google Scholar]
- Cameron MF, Frost KJ, Ver Hoef JM, Breed GA, Whiting AV, Goodwin J, Boveng PL (2018) Habitat selection and seasonal movements of young bearded seals (Erignathus barbatus) in the Bering Sea. PLoS One 13: e0192743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellini MA, Kooyman GL, Ponganis PJ (1992) Metabolic rates of freely diving Weddell seals: correlations with oxygen stores, swim velocity and diving duration. J Exp Biol 165: 181–194. [DOI] [PubMed] [Google Scholar]
- Chabot D, Stenson GB (2002) Growth and seasonal fluctuations in size and condition of male Northwest Atlantic harp seals Phoca groenlandica: an analysis using sequential growth curves. Mar Ecol Prog Ser 227: 25–42. [Google Scholar]
- Chang HC (1926) Specific influence of the tyroid gland on hair growth. Am J Physiol 77: 562–567. [Google Scholar]
- Comiso J, Hall D (2014) Climate trends in the Arctic as observed from space. Wiley Interdiscip Rev Clim Chang 5: 389–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa DP, Schwarz L, Robinson P, Schick RS, Morris PA, Condit R, Crocker DE, Kilpatrick AM (2016) A bioenergetics approach to understanding the population consequences of disturbance: elephant seals as a model system In The Effects of Noise on Aquatic Life II. Springer, New York, pp. 161–169. [DOI] [PubMed] [Google Scholar]
- Costa DP, Williams TM (1999) Marine mammal energetics In JE Reynolds, S Rommel, eds, Biology of Marine Mammals. Smithsonian Institution Press, Washington, DC, pp. 176–217. [Google Scholar]
- Davis RW, Williams TM, Kooyman GL (1985) Swimming Metabolism of Yearling and Adult Harbor Seals Phoca vitulina. Physiol Zool 58: 590–596. [Google Scholar]
- Fedak MA, Rome L, Seeherman HJ (1981) One-step N2-dilution technique for calibrating open-circuit VO2 measuring systems. J Appl Physiol 51: 772–776. [DOI] [PubMed] [Google Scholar]
- Feltz ET, Fay FH (1966) Thermal requirements in vitro of epidermal cells from seals. Cryobiology 3: 261–264. [DOI] [PubMed] [Google Scholar]
- Ferreira EO, Loseto LL, Ferguson SH (2011) Assessment of claw growth-layer groups from ringed seals (Pusa hispida) as biomonitors of inter-and intra-annual Hg, d15N, and d13C variation. Can J Zool 89: 774–784. [Google Scholar]
- Gallivan GJ, Ronald K (1981) Apparent specific dynamic action in the harp seal (Phoca groenlandica). Comp Biochem Physiol Part A Physiol 69: 579–581. [Google Scholar]
- Glazier DS (2005) Beyond the ‘3/4-power law’: variation in the intra- and interspecific scaling of metabolic rate in animals. Biol Rev 80: 611–662. [DOI] [PubMed] [Google Scholar]
- Groscolas R, Cherel Y (2014) How to molt while fasting in the cold: the metabolic and hormonal adaptations of emperor and king penguins. Ornis Scand 23: 328–334. [Google Scholar]
- Hamilton C, Lydersen C, Ims R, Kovacs K (2016) Coastal habitat use by ringed seals Pusa hispida following a regional sea-ice collapse: importance of glacial refugia in a changing Arctic. Mar Ecol Prog Ser 545: 261–277. [Google Scholar]
- Hamilton CD, Lydersen C, Ims RA, Kovacs KM (2015) Predictions replaced by facts: a keystone species’ behavioural responses to declining arctic sea-ice. Biol Lett 11: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S (1995) Temperature Effects on Metabolic Rate, and the Thermal Limits of Harbour (Phoca vitulina) and Grey Seals (Halichoerus grypus). University of Guelph, Guelph Ont. [Google Scholar]
- Hansen S, Lavigne DM, Innes S (1995) Energy metabolism and thermoregulation in juvenile harbor seals (Phoca vitulina) in air. Physiol Zool 68: 290–315. [Google Scholar]
- Harrison XA, Donaldson L, Correa-Cano ME, Evans J, Fisher DN, Goodwin CED, Robinson BS, Hodgson DJ, Inger R (2018) A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 6: e4794. doi: 10.7717/peerj.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood LA, Smith TG, Melling H, Alikamik J, Kingsley MCS (2012) Ringed seals and sea ice in Canada’s Western Arctic: harvest-based monitoring 1992-2011. Arctic 65: 377–390. [Google Scholar]
- Hedd A, Gales R, Renouf D (1997) Inter-annual consistency in the fuctuating energy requirements of captive harp seals Phoca groenlandica. Polar Biol 18: 311–318. [Google Scholar]
- Hinzman LD et al. (2005) Evidence and implications of recent climate change in Northern Alaska and other Arctic regions. Clim Change 72: 251–298. [Google Scholar]
- John TM, Ronald K, George JC (1987) Blood levels of thyroid hormones and certain metabolites in relation to moult in the harp seal (Phoca groenlandica). Comp Biochem Physiol 88A: 655–657. [DOI] [PubMed] [Google Scholar]
- Kelly BP et al. (2010) Status review of the ringed seal (Phoca hispida). NOAA Tech Memo NMFS-AFSC 212i–xiv: 1–250. [Google Scholar]
- Kleiber M (1947) Body size and metabolic rate. Physiol Rev 27: 511–541. [DOI] [PubMed] [Google Scholar]
- Kleiber M (1961) The Fire of Life: An Introduction to Animal Energetics. John Wiley & Sons, New York. [Google Scholar]
- Kooyman GL (1989) Diverse Divers. Springer, New York. [Google Scholar]
- Kovacs KM, Lydersen C, Overland JE, Moore SE (2011) Impacts of changing sea-ice conditions on Arctic marine mammals. Mar Biodivers 41: 181–194. [Google Scholar]
- Laidre KL et al. (2015) Arctic marine mammal population status, sea ice habitat loss, and conservation recommendations for the 21st century. Conserv Biol 29: 724–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidre KL, Stirling I, Lowry LF, Wiig Ø, Heide-Jørgensen MP (2008) Quantifying the sensitivity of Arctic marine mammals to climate-induced habitat change. Ecol Appl 18: S97–S125. [DOI] [PubMed] [Google Scholar]
- Lenfant C, Johansen K, Torrance JD (1970) Gas transport and oxygen storage capacity in some pinnipeds and the sea otter. Respir Physiol 9: 277–286. [DOI] [PubMed] [Google Scholar]
- Lestyk KC, Folkow LP, Blix AS, Hammill MO, Burns JM (2009) Development of myoglobin concentration and acid buffering capacity in harp (Pagophilus groenlandicus) and hooded (Cystophora cristata) seals from birth to maturity. J Comp Physiol B 179: 985–996. [DOI] [PubMed] [Google Scholar]
- Ling JK (1984) Epidermal cycles and moulting in marine mammals. Acta Zool Fenn 171: 23–26. [Google Scholar]
- Lowry LF, Frost KJ, Davis R, DeMaster DP, Suydam RS (1998) Movements and behavior of satellite-tagged spotted seals (Phoca largha) in the Bering and Chukchi Seas. Polar Biol 19: 221–230. [Google Scholar]
- Lydersen C, Ryg MS, Hammill MO, O’Brien PJ (1992) Oxygen stores and aerobic dive limit of ringed seals (Phoca hispida). Can J Zool 70: 458–461. [Google Scholar]
- Mo G, Gili C, Ferrando P (2000) Do photoperiod and temperature influence the molt cycle of Phoca vitulina in captivity? Mar Mammal Sci 16: 570–577. [Google Scholar]
- Moore SE, Huntington HP (2008) Arctic marine mammals and climate change: impacts and resilience. Ecol Appl 18: S157–S165. [DOI] [PubMed] [Google Scholar]
- Nagy K (1987) Field metabolic rate and food requirement scaling in mammals and birds. Ecol Monogr 57: 111–128. [Google Scholar]
- Nesterenko VA, Katin IO (2009) Haulout: scope of the term and procedure for identification. Russ J Ecol 40: 48–54. [Google Scholar]
- NMFS (2012) Endangered and Threatened Species; Threatened Status for the Beringia and Okhotsk Distinct Population Segments of the Erignathus barbatus nauticus Subspecies of the Bearded Seal. 77: 76740–76768. [Google Scholar]
- NOAA Fisheries (2011a) 2011 Arctic Seal Disease Outbreak Fact Sheet. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, AK, USA. [Google Scholar]
- NOAA Fisheries (2011b) 2011 Arctic Seal Disease Outbreak Update on Investigation and Findings. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, AK, USA. [Google Scholar]
- NOAA Fisheries (2016) 2011 Arctic Pinniped Unusual Mortality Event (UME) Fact Sheet. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, AK, USA. [Google Scholar]
- NOAA Fisheries (2018) Special Notice: The UME Involving Ice Seals with Hair Loss and Skin Lesions Is Now Closed U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, AK, USA. [Google Scholar]
- NOAA Fisheries (2020) 2018–2020 Ice Seal Unusual Mortality Event in Alaska, https://www.fisheries.noaa.gov/alaska/marine-life-distress/2018-2020-ice-seal-unusual-mortality-event-alaska (last accessed 11 June 2020).
- Ochoa-Acuña HG, Mcnab BK, Miller EH (2009) Seasonal energetics of northern phocid seals. Comp Biochem Physiol Part A 152: 341–350. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Schrader SB, Gillooly JF (2010) Energetic cost of calling: general constraints and species-specific differences. J Evol Biol 23: 1564–1569. [DOI] [PubMed] [Google Scholar]
- Post E et al. (2009) Ecological dynamics across the Arctic associated with recent climate change. Science 325: 1355–1358. [DOI] [PubMed] [Google Scholar]
- Quakenbush L, Citta J (2008) Biology of the Ribbon Seal in Alaska, Final Rep to Natl Mar Fish Serv.
- Quakenbush L, Citta J, Crawford J (2009) Biology of the Spotted Seal (Phoca largha) in Alaska from 1962–2008, Final Rep to Natl Mar Fish Serv.
- Quakenbush L, Citta J, Crawford J (2011a) Biology of the Ringed Seal (Phoca hispida) in Alaska, 1960–2010, Final Rep to Natl Mar Fish Serv.
- Quakenbush L, Citta J, Crawford J (2011b) Biology of the Bearded Seal (Erignathus barbatus) in Alaska, 1961–2009, Final Rep to Natl Mar Fish Serv.
- Ramot Y, Paus R, Tiede S, Zlotogorski A (2009) Endocrine controls of keratin expression. BioEssays 31: 389–399. [DOI] [PubMed] [Google Scholar]
- Rechsteiner EU, Rosen DAS, Trites AW (2013) Energy requirements of Pacific white-sided dolphins (Lagenorhynchus obliquidens) as predicted by a bioenergetic model. J Mammal 94: 820–832. [Google Scholar]
- Reimer JR, Caswell H, Derocher AE, Lewis MA (2019) Ringed seal demography in a changing climate. Ecol Appl 0: 1–18. [DOI] [PubMed] [Google Scholar]
- Renouf D, Brotea G (1991) Thyroid hormone concentrations in harbour seals (Phoca vitulina): No evidence of involvement in the moult. Comp Biochem Physiol 99A: 185–194. [Google Scholar]
- Renouf D, Gales R (1994) Seasonal variation in the metabolic rate of harp seals: unexpected energetic economy in the cold ocean. Can J Zool 72: 1625–1632. [Google Scholar]
- Renouf D, Noseworthy E (1990) Feeding cycles in captive harbour seals (Phoca vitulina): weight gain in spite of reduced food intake and increased thermal demands. Mar Freshw Behav Physiol 17: 203–212. [Google Scholar]
- Richmond JP, Burns JM, Rea LD (2006) Ontogeny of total body oxygen stores and aerobic dive potential in Steller sea lions (Eumetopias jubatus). J Comp Physiol B 176: 535–545. [DOI] [PubMed] [Google Scholar]
- Rosen DAS, Renouf D (1998) Correlates of seasonal changes in metabolism in Atlantic harbour seals (Phoca vitulina concolor). Can J Zool 76: 1520–1528. [Google Scholar]
- Routti H, Munro B, Lydersen C, Bäckman C, Arukwe A (2013) Hormone, vitamin and contaminant status during moulting and fasting period in ringed seals (Phoca hispida) from Svalbard. Comp Biochem Physiol Part A 155: 70–76. [DOI] [PubMed] [Google Scholar]
- Ryg M, Lydersen C, Markussen NH, Smith TG, Øritsland NA (1990a) Estimating the blubber content of phocid seals. Can J Fish Aquat Sci 47: 1223–1227. [Google Scholar]
- Ryg M, Smith TG, Ortisland NA (1990b) Seasonal changes in body mass and body composition of ringed seals (Phoca hispida) on Svalbard. Can J Zool 68: 470–475. [Google Scholar]
- Serreze MC, Barry RG (2011) Processes and impacts of Arctic amplification: a research synthesis. Glob Planet Change 77: 85–96. [Google Scholar]
- Smith G (1980) Polar bear predation of ringed and bearded seals in the land-fast sea ice habitat. Can J Zool 58: 2201–2209. [Google Scholar]
- Smith TG, Stirling I (1975) The breeding habitat of the ringed seal (Phoca hispida). The birth lair and associated structures. Can J Zool 53: 1297–1305. [Google Scholar]
- Sparling CE, Speakman JR, Fedak MA (2006) Seasonal variation in the metabolic rate and body composition of female grey seals: fat conservation prior to high-cost reproduction in a capital breeder? J Comp Physiol B 176: 505–512. [DOI] [PubMed] [Google Scholar]
- Thompson SD, Nicoll ME (1986) Basal metabolic rate and energetics of reproduction in therian mammals. Nature 321: 690–693. [DOI] [PubMed] [Google Scholar]
- Tryland M, Krafft BA, Lydersen C, Kovacs KM, Thoresen SI (2006) Serum chemistry values for free-ranging ringed seals (Pusa hispida) in Svalbard. Vet Clin Pathol 35: 405–412. [DOI] [PubMed] [Google Scholar]
- Villegas-Amtmann S, Schwarz LK, Sumich JL, Costa DP (2015) A bioenergetics model to evaluate demographic consequences of disturbance in marine mammals applied to gray whales. Ecosphere 6: 1–19. [Google Scholar]
- Von Duyke AL, Douglas DC, Herreman JK, Crawford JA (2020) Ringed seal (Pusa hispida) seasonal movements, diving, and haul-out behavior in the Beaufort, Chukchi, and Bering Seas (2011–2017). Ecol Evol 00: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott SM, Kirkham AL, Burns JM (2020) Thermoregulatory costs in molting Antarctic Weddell seals: impacts of physiological and environmental conditions. Conserv Physiol 8: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P (1986) Distribution, ecology, and resource conservation of the spotted seal in the Huanghai and Bohai Seas. Acta Oceanol Sin 5: 126–133. [Google Scholar]
- Watts P, Hansen S, Lavigne DM (1993) Models of heat loss by marine mammals: thermoregulation below the zone of irrelevance. J Theor Biol 163: 505–525. [Google Scholar]
- Williams TM, Maresh JL (2016) Exercise energetics In MA Castellini, J Mellish, eds, Marine Mammal Physiology: Requisites for Ocean Living. CRC Press, Boca Raton, FL, pp. 47–68. [Google Scholar]
- Williams TM, Richter B, Kendall T, Dunkin R (2011) Metabolic demands of a tropical marine carnivore, the Hawaiian monk seal (Monachus schauinslandi): implications for fisheries competition. Aquat Mamm 37: 372–376. [Google Scholar]
- Winship AJ, Trites AW, Rosen DAS (2002) A bioenergetic model for estimating the food requirements of Steller sea lions Eumetopias jubatus in Alaska, USA. Mar Ecol Prog Ser 229: 291–312. [Google Scholar]
- Withers PC (1977) Measurment of VO2, VCO2, and evaporative water loss with a flow-through mask. J Appl Physiol 42: 120–123. [DOI] [PubMed] [Google Scholar]
- Worthy GAJ, Morrie PA, Costa DP, Le Boeuf BJ (1992) Moult energetics of the northern elephant seal. J Zool London 227: 257–265. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


