Abstract
The National Forensic Laboratory Information System (NFLIS) is a program of the U.S. Drug Enforcement Administration, Diversion Control Division. The NFLIS-Drug component collects drug identification results and associated information from drug cases submitted to and analyzed by federal, state, and local forensic laboratories. This paper presents national annual estimates and national and regional yearly trend differences for clonazepam, diazepam, flubromazolam, clonazolam, and etizolam using annual report rates per 100,000 persons aged 15 or older between 2015 and 2018. An estimated 263,538 benzodiazepine reports were identified by state and local laboratories between 2015 and 2018. Methamphetamine, cocaine, and heroin accounted for 32% of the drugs reported in the same item as alprazolam. Depressants and tranquilizers and narcotic analgesics were the drug classes most frequently identified in the same item as etizolam. A timeline of some benzodiazepines’ emergence in NFLIS-Drug is shown, as well as state- and county-level data for selected benzodiazepines.
Keywords: U.S. drug enforcement administration, National forensic laboratory information system, Drug trends, Benzodiazepines
1. Introduction
Benzodiazepines are a class of drugs prescribed to treat a variety of conditions that include insomnia and anxiety [1,2]. They contain the core structure shown in Fig. 1. Controlled as Schedule IV in the Controlled Substances Act, benzodiazepines have a low potential of abuse and a low risk of dependence [3]. They replaced barbiturates as the most widely prescribed central nervous system depressants because they are safer in the event of an overdose and have fewer side effects. Chlordiazepoxide was the first benzodiazepine to be used therapeutically as the brand name Librium® [1,4].
Fig. 1.
Benzodiazepine core structure.
Benzodiazepines are typically described as being approved for use by the U.S. Food and Drug Administration (FDA), which means they are prescribed drugs in the United States. The most common benzodiazepine prescription drugs approved for therapeutic use in the United States are alprazolam (Xanax®), clonazepam (Klonopin®), lorazepam (Ativan®), and diazepam (Valium®) [2]. Sometimes, benzodiazepines are not FDA approved as prescription drugs in the United States but are prescribed for therapeutic use in other countries. Phenazepam and etizolam are examples of benzodiazepines prescribed for therapeutic use in other countries but are typically used for recreational purposes in the United States [5,6]. Other benzodiazepines not approved by the FDA (e.g., clonazolam, flubromazepam, flubromazolam, and pyrazolam) are called “designer benzodiazepines” [[7], [8], [9]] and have no acceptable medical use in any country. Although benzodiazepines that have no current medical use in the United States are not federally scheduled, some are scheduled in other countries and by individual states, such as Virginia, Florida, and Louisiana [[10], [11], [12], [13]].
Although benzodiazepines are considered a safer alternative to barbiturates, they can have adverse effects under certain conditions: (a) if they are not taken as prescribed; (b) if they are taken in combination with other substances, such as alcohol or narcotic analgesics; or (c) if they are benzodiazepines that have not been approved by the FDA for therapeutic use. The FDA has warned about concurrent use of benzodiazepines and narcotic analgesics, and the Centers for Disease Control and Prevention (CDC) released guidelines that cautioned clinicians to avoid co-prescribing these substances [14,15]. In 2017, benzodiazepines were present in 33% and 17% of prescription opioid overdose deaths and synthetic opioid overdose deaths, respectively [16]. Prevalence data show that in 2018, an estimated 5.4 million people aged 12 or older were past year misusers of prescription benzodiazepines, which corresponded to 2% of the U.S. population at that time [17].
Blood concentrations of designer benzodiazepines have been documented in drug impairment cases [18,19]. In Norway, diclazepam and phenazepam were the most prevalent designer benzodiazepines in forensic cases between June 1, 2016, and September 30, 2019 [19], while flubromazolam and flubromazepam were commonly identified between July 1, 2013, and May 31, 2016 [18]. Amphetamine, tetrahydrocannabinol, clonazepam, and methamphetamine were often identified along with these designer benzodiazepines [18,19]. Etizolam and fentanyl were identified in samples from individuals who ingested counterfeit alprazolam tablets [20]. The National Poison Data System showed the most common exposures from etizolam and clonazolam [21]. The United Nations Office on Drugs and Crime (UNODC) Early Warning Advisory monitors the emergence of novel psychoactive substances (NPS) from a global perspective. A UNODC report showed the recent uptick in benzodiazepine-type NPS, with flubromazolam, flualprazolam, and etizolam being the most prevalent in cases involving driving under the influence of drugs [22].
One well-established surveillance program in the United States that has been tracking benzodiazepines for the past two decades is the U.S. Drug Enforcement Administration’s (DEA’s) National Forensic Laboratory Information System (NFLIS). Its NFLIS-Drug component systematically collects drug identification results and associated information from drug cases submitted to and analyzed by federal, state, and local forensic laboratories. These laboratories analyze controlled and noncontrolled substances secured in law enforcement operations across the United States, making NFLIS-Drug an important resource in monitoring illicit drug use and trafficking, including the diversion of legally manufactured pharmaceuticals into illegal markets. NFLIS-Drug data are used to support drug scheduling efforts and to inform drug policy and drug enforcement initiatives nationally and in local communities around the United States. The data provide information on diversion of prescription drugs, emerging drugs of abuse, and national and regional drug trends. NFLIS-Drug also serves as a complementary resource to other drug data collections, such as overdose data from the CDC and the UNODC Early Warning Advisory.
Although the NFLIS program has generally published NFLIS-Drug annual and midyear reports, these higher-level reports do not always showcase emerging drugs of abuse if they are not in the top 25 most identified drugs in NFLIS-Drug or highly reported in their drug class. To provide more visibility and usability of the NFLIS-Drug data, the NFLIS program recently began reporting NFLIS-Drug data in different formats, such as its “Snapshot” series of reports and data tables that are publicly available on the NFLIS website. In addition, NFLIS-Drug special reports are released to highlight timely topics on drugs that have emerged in the community. For example, the NFLIS Special Report: Benzodiazepines Reported in NFLIS, 2009–2014 [23] provided a review of the state of benzodiazepines and their initial emergence.
The purpose of this paper is to provide an updated view of prescription benzodiazepines and nonprescription benzodiazepines observed in NFLIS-Drug and a comparison of these benzodiazepines between 2015 and 2018. NFLIS-Drug data are shown for national annual estimates of selected benzodiazepines and regional trends for diazepam, clonazepam, flubromazolam, clonazolam, and etizolam. Data are shown for selected benzodiazepines of interest that have emerged in NFLIS-Drug that are not prescribed for therapeutic use in the United States. Combinations of alprazolam and etizolam reported with other drugs are examined. State- and county-level data are shown for etizolam, clonazolam, and flubromazolam. Lastly, total prescription benzodiazepines dispensed during three time periods (i.e., 2001 to 2005, 2009 to 2014, and 2015 to 2018) are compared along with a seizure-to-prescription drug ratio based on drug reports identified in NFLIS-Drug per 10,000 prescriptions dispensed.
2. Methods
In the United States, approximately 275 individual laboratories voluntarily participate in NFLIS-Drug, representing 50 state laboratory systems and 103 local or municipal laboratories/laboratory systems. The DEA and U.S. Customs and Border Protection laboratories represent the federal data submitted to NFLIS-Drug.
NFLIS produces annual and midyear estimates of the total numbers of national and regional drug reports, as well as rates of drug reports per 100,000 persons aged 15 or older. These estimates are based on drug reports submitted to the state and local laboratories participating in NFLIS-Drug from January 1st through December 31st for each reported year and analyzed by March 31st of the following year. The estimates are generated using the National Estimates Based on All Reports method, which incorporates data from the originally sampled laboratories and from additional laboratories later recruited and submitting their data to NFLIS [24]. Imputation methods are in place to account for nonresponse at the monthly reporting level and drug level within each laboratory or laboratory system, and the estimates are weighted to account for the state and local laboratories that are in the known NFLIS-Drug universe but are not currently participating in NFLIS-Drug. This paper presents national annual estimates for 18 selected benzodiazepines, as well as national and regional yearly trend differences for clonazepam, diazepam, flubromazolam, clonazolam, and etizolam using annual report rates per 100,000 persons aged 15 or older. Clonazepam and diazepam were selected as compounds of interest because they are two of the three most identified prescription benzodiazepines within NFLIS during the referenced time period. Flubromazolam, clonazolam, and etizolam were selected as compounds of interest because they are the most identified nonprescription benzodiazepines in NFLIS and have increasing reports during the referenced time period. Yearly differences nationally and within regions were tested for statistical significance (p < 0.05) using pairwise t tests.
Beyond the national and regional estimates, raw counts of drug reports from the NFLIS-Drug data are also presented. Raw counts are those directly received by NFLIS-Drug and have not undergone any weighting or imputation adjustments to account for laboratory nonresponse; they are simply the number of reports of drugs recorded and submitted by participating NFLIS-Drug laboratories.
First, data from federal laboratories were combined with submissions from state and local laboratories to show the first instances of selected benzodiazepines reported in NFLIS-Drug, the cumulative number of drug reports through December 31, 2018, and a highlight of selected emerging benzodiazepines in 2019. An additional analysis focuses on alprazolam and etizolam, which were the two most prevalent prescription and nonprescription benzodiazepines in 2018 in the United States. NFLIS-Drug data from state and local laboratories are shown for reported items that contained alprazolam or etizolam and at least one other drug submitted for analysis between January 1, 2018, and December 31, 2018, and analyzed by March 31, 2019. These items are not necessarily true drug combinations but may be reports of separate drugs reported together in the same item.
Finally, raw counts of reports of etizolam, clonazolam, and flubromazolam stemming from state and local laboratories to NFLIS-Drug are presented at the state level for 2015 and 2018. County-level data for etizolam are also presented for 2018. It is important to note that a small number of laboratories within a few states were not reporting data to NFLIS-Drug, and their absence may affect the relative distribution of drugs seized and analyzed.
The IQVIA National Prescription Audit™ database provides the number of prescription drugs dispensed in the United States. It was queried for selected benzodiazepine prescriptions between 2015 and 2018 and compared with NFLIS-Drug data from 2001 to 2005 [25] and from 2009 to 2014 [23]. During these three time periods, a diversion factor was generated for alprazolam, diazepam, and clonazepam. This is a seizure-to-prescription ratio based on drug reports identified in NFLIS-Drug per 10,000 prescriptions dispensed.
3. Results
3.1. National estimates
From 2015 to 2018, an estimated 263,538 benzodiazepine reports were identified by state and local laboratories. Estimated benzodiazepine reports decreased by 14% between 2015 and 2018. Of the benzodiazepines listed in Table 1, alprazolam, clonazepam, diazepam, and lorazepam accounted for 97% of benzodiazepine reports between 2015 and 2018. Etizolam, clonazolam, flubromazolam, clorazepate, diclazepam, flualprazolam, flubromazepam, oxazepam, and bromazepam increased between 2015 and 2018, with flubromazolam, clonazolam, and etizolam having the greatest percent increases. Alprazolam, clonazepam, diazepam, lorazepam, temazepam, chlordiazepoxide, phenazepam, triazolam, and midazolam decreased, with phenazepam having the greatest percent decrease (-75%).
Table 1.
National Annual Estimates of Selected Benzodiazepines in NFLIS-Drug, 2015–2018. Includes total drug reports submitted to laboratories from January 1st through December 31st for each reported year and analyzed by March 31st of the following year.
| Selected Benzodiazepine | 2015 | 2016 | 2017 | 2018 | Total |
|---|---|---|---|---|---|
| Alprazolam | 45,584 | 51,271 | 47,160 | 40,195 | 184,211 |
| Clonazepam | 12,269 | 12,274 | 10,869 | 9551 | 44,963 |
| Diazepam | 5306 | 4702 | 4249 | 3345 | 17,602 |
| Lorazepam | 2635 | 2563 | 2229 | 1855 | 9282 |
| Etizolam | 504 | 573 | 844 | 1506 | 3427 |
| Temazepam | 331 | 295 | 219 | 200 | 1044 |
| Clonazolam | 34 | 99 | 177 | 531 | 841 |
| Flubromazolam | 22 | 17 | 233 | 354 | 626 |
| Other benzodiazepine | 166 | 58 | 101 | 58 | 384 |
| Chlordiazepoxide | 94 | 68 | 70 | 65 | 298 |
| Phenazepam | 106 | 19 | 20 | 26 | 172 |
| Clorazepate | 23 | 60 | 32 | 26 | 141 |
| Triazolam | 27 | 24 | 29 | 21 | 101 |
| Diclazepam | 18 | 15 | 32 | 36 | 100 |
| Midazolam | 23 | 24 | 29 | 20 | 96 |
| Flualprazolam | 0 | 0 | 0 | 83 | 83 |
| Flubromazepam | 6 | 5 | 57 | 13 | 80 |
| Oxazepam | 12 | 18 | 14 | 15 | 59 |
| Bromazepam | 6 | 12 | 2 | 9 | 29 |
| Total | 67,166 | 72,097 | 66,366 | 57,909 | 263,539 |
3.2. National and regional trends for diazepam, clonazepam, flubromazolam, clonazolam, and etizolam
Nationally and by each region, diazepam reports significantly (p < 0.05) decreased between 2015 and 2018 (Fig. 2). For all regions, diazepam reports decreased each year from 2015 to 2018, with the exception of a slight increase in the Midwest from 2015 to 2016. The West and Northeast had lower diazepam rates compared with the Midwest and South.
Fig. 2.
NFLIS-drug national and regional trends in diazepam reported per 100,000 persons aged 15 or older, January 2015–December 2018.
Nationally and by each region, clonazepam reports significantly (p < 0.05) decreased between 2015 and 2018. The estimated number of clonazepam reports in the Midwest peaked in 2016 at a rate of 5.15 (2840 reports) per 100,000 persons, then gradually decreased to a rate of 4.38 (2430 reports) per 100,000 persons in 2018 (Fig. 3). Peak rates of clonazepam in the South and West were observed in 2015 at 6.36 (6205 reports) and 1.95 (1187 reports) per 100,000 persons, respectively. These rates gradually decreased in both regions through 2018. The estimated number of clonazepam reports in the Northeast peaked in 2016 at a rate of 5.10 (2381 reports) per 100,000 persons, then gradually decreased to a rate of 3.64 (17,09 reports) per 100,000 persons in 2018. Unlike the results for diazepam reports, only the West had the lowest rates overall for clonazepam compared with all other regions, ranging from a rate of 1.95 to 0.98 reports per 100,000 persons from 2015 to 2018.
Fig. 3.
NFLIS-drug national and regional trends in clonazepam reported per 100,000 persons aged 15 or older, January 2015–December 2018.
Nationally, flubromazolam significantly (p < 0.05) increased between 2015 and 2018 (Fig. 4). The Midwest had the highest rate of flubromazolam reports at 0.22 (123 reports) per 100,000 persons. Flubromazolam significantly (p < 0.05) increased in the Midwest between 2015 and 2018.
Fig. 4.
NFLIS-drug national and regional trends in flubromazolam reported per 100,000 persons aged 15 or older, January 2015–December 2018.
Nationally, reports of clonazolam significantly (p < 0.05) increased between 2015 and 2018 (Fig. 5). The highest reports of clonazolam were in the South at a peak rate of 0.28 (282 reports) per 100,000 persons in 2018. The West and Northeast regions slightly decreased in reports of clonazolam in 2017, but both regions peaked at a rate of 0.11 (69 reports) and 0.10 (46 reports) per 100,000 persons in 2018, respectively. Reports of clonazolam significantly (p < 0.05) increased in the Midwest between 2015 and 2018.
Fig. 5.
NFLIS-drug national and regional trends in clonazolam reported per 100,000 persons aged 15 or older, January 2015–December 2018.
Nationally, reports of etizolam significantly (p < 0.05) increased between 2015 and 2018 and significantly (p < 0.05) increased in the Midwest during the same period (Fig. 6). Etizolam reports were stable in the Northeast between 2015 and 2017, then decreased in 2018 to a rate of 0.15 (70 reports) per 100,000 persons. The opposite was observed in the West, with fairly steady rates of etizolam from 2015 to 2017, but a more drastic increase in 2018 to a rate of 0.32 (202 reports) per 100,000 persons. The Midwest was relatively stable between 2015 and 2016 and increased to a rate of 0.54 (301 reports) per 100,000 persons in 2018, while the rates in the West and South regions for etizolam reports increased in 2018.
Fig. 6.
NFLIS-drug national and regional trends in etizolam reported per 100,000 persons aged 15 or older, January 2015–December 2018.
3.3. Selected benzodiazepines of interest
Selected benzodiazepines of interest were chosen based on a review of the literature [21,22,26,27]. Many of the selected benzodiazepines of interest were reported as exposures in the National Poison Data System [21]. From 2014 to 2017, etizolam and clonazolam had the most exposures reported in the National Poison Data System, and the instances of designer benzodiazepine exposures increased from 2014 to 2017 [21]. As noted in Table 2, phenazepam was reported earliest, with reports as far back as 2005, followed by etizolam in 2012. Reports of phenazepam peaked in 2015. Etizolam continued to increase between 2015 and 2018 (Fig. 7). Five other prevalent benzodiazepines of interest are also presented in Fig. 7, which shows their increasing and decreasing patterns over the 4-year time period.
Table 2.
Selected Benzodiazepines of Interest, by Year First Reported and Total Number of Reports in NFLIS-Drug through December 31, 2018. Cumulative data were derived from NFLIS-Drug’s Data Query System as of August 3, 2020, at https://www.nflis.deadiversion.usdoj.gov/.
| Year first reported in NFLIS-Drug | Selected benzodiazepine | Cumulative number of reports from first year in NFLIS-Drug to 2018 |
|---|---|---|
| 2005 | Phenazepam | 594 |
| 2012 | Etizolam | 4033 |
| 2014 | Diclazepam | 141 |
| Flubromazepam | 99 | |
| 2015 | Clonazolam | 963 |
| Flubromazolam | 748 | |
| 2016 | 3-Hydroxyphenazepam | 1 |
| Bromazolam | 5 | |
| 2017 | Meclonazepam | 1 |
| Flualprazolam | 114 | |
| Norflunitrazepam | 3 | |
| 2018 | 4-Chlorodiazepam | 1 |
| Deschloroetizolam | 1 | |
| Flunitrazolam | 6 | |
| Metizolam | 1 | |
| Nitrazolam | 1 |
Fig. 7.
Seven benzodiazepines of interest in NFLIS-Drug total reports, by year, 2015–2018.
3.4. Emerging benzodiazepines in 2019
Data in this section were retrieved from NFLIS-Drug’s Data Query System as of August 3, 2020. This section provides a snapshot in time of the raw data in NFLIS-Drug in 2018 and 2019. As noted in Table 3, in 2019, two new benzodiazepines appeared in NFLIS-Drug—pyrazolam and adinazolam. Etizolam has been one of the most prominent emerging benzodiazepines over the years; it increased 99% between 2018 and 2019. An emerging benzodiazepine, flualprazolam, increased 1565% between 2018 and 2019.
Table 3.
Emerging Benzodiazepines of Interest, by Total Number of Reports by year in NFLIS-Drug from January 1, 2018 through December 31, 2019. Data were retrieved from NFLIS-Drug’s Data Query System as of August 3, 2020, at https://www.nflis.deadiversion.usdoj.gov/.
| Selected benzodiazepine | Number of reports in 2018 | Number of reports in 2019 |
|---|---|---|
| Etizolam | 1680 | 3338 |
| Clonazolam | 623 | 695 |
| Flubromazolam | 440 | 359 |
| Flualprazolam | 113 | 1881 |
| Diclazepam | 63 | 67 |
| Phenazepam | 22 | 4 |
| Flubromazepam | 17 | 24 |
| Bromazolam | 2 | 20 |
| Adinazolam | 0 | 69 |
| Pyrazolam | 0 | 2 |
3.5. Alprazolam and etizolam reported with other drugs in the same item
Understanding true drug combinations (e.g., powders mixed together) identified by laboratories across the United States is important to law enforcement agencies, public health departments, and other state, local, and federal entities. NFLIS-Drug data can provide some insight into potential combinations but can provide limited knowledge on true combinations. Fig. 8, Fig. 9 show combinations of alprazolam and etizolam, respectively, identified in the same item as another drug. The data shown are not mutually exclusive.
Fig. 8.
NFLIS-drug reports of alprazolam identified with other drugs within the same item, 2018.
Fig. 9.
NFLIS-drug reports of etizolam identified with other drugs within the same item, 2018.
In 2018, there were 35,322 reports of alprazolam, and of those, 916 were reported with at least one other drug in the same item for a total of 1053 drugs reported in combination with alprazolam. Methamphetamine (168 reports), cocaine (103 reports), and heroin (69 reports) accounted for 32% of the drugs reported in the same item as alprazolam (Fig. 8). Of the narcotic analgesics reported in the same item as alprazolam (16%), fentanyl (43 reports), fentanyl-related compounds (42 reports), and oxycodone (31 reports) accounted for the majority of substances. Depressants and tranquilizers accounted for 26% of drugs reported in the same item as alprazolam, with etizolam accounting for more than half of these. Interestingly, FUB-AMB was the most prevalent synthetic cannabinoid identified in the same item as alprazolam, and it was the second most commonly identified synthetic cannabinoid reported by participating laboratories in 2018 [28]. The major difference observed between 2014 and 2018 in the number of alprazolam reports with other drugs in the same item was the emergence of fentanyl-related compounds and other emerging benzodiazepines, such as clonazolam, in 2018. Examples of other substances include caffeine, lidocaine, acetaminophen.
In 2018, there were 1325 reports of etizolam, and of these 267 were reported with at least one other drug in the same item for a total of 328 drugs reported in combination with etizolam. Depressants and tranquilizers (52%) and narcotic analgesics (18%) were the drug classes most frequently identified in the same item as etizolam (Fig. 9). Alprazolam (49%) accounted for most of the depressants and tranquilizers, while emerging benzodiazepines accounted for 3% of drugs identified in the same item as etizolam. These emerging benzodiazepines include diclazepam, flualprazolam, flubromazepam, and flubromazolam. Of narcotic analgesics, fentanyl (8%) and U-47700 (6%) were most commonly identified in the same item as etizolam. Examples of other substances include lidocaine, caffeine, and cocaine.
3.6. Rates of NFLIS reports of etizolam, clonazolam, and flubromazolam, by state and county
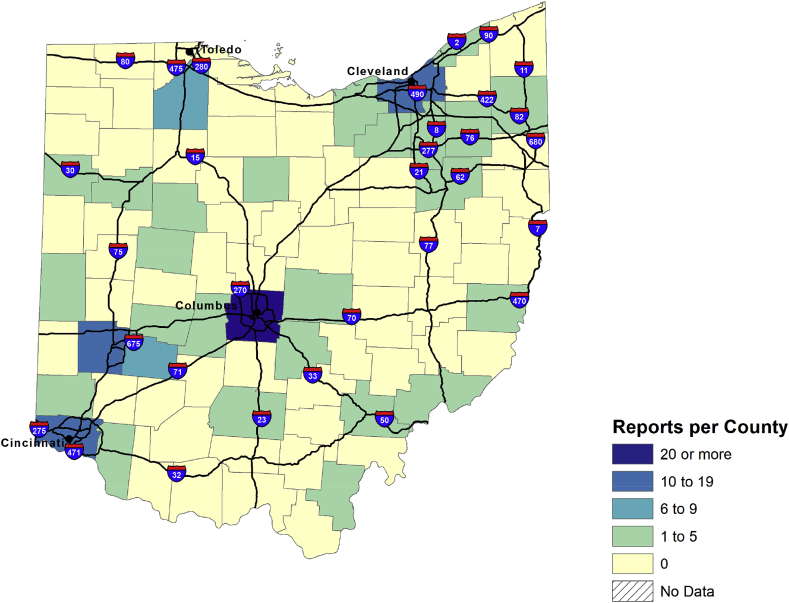
Fig. 10, Fig. 11, Fig. 13, Fig. 14, Fig. 15, Fig. 16 present rates of drug reports per 100,000 persons aged 15 or older by state. Population sizes are based on U.S. census annual estimates of the resident populations as of July 1, 2019. A total of 28 states had rates of etizolam reports that were 0.01 or more during 2015 (Fig. 10). Only four states had rates of etizolam reports that were 0.60 or more per 100,000 persons during 2015, including one state that had a rate of 1.00 or more during that time. By 2018, 38 states had rates of etizolam reports that were 0.01 or more, and the number of states that had rates of 1.00 or more increased to 8 (Fig. 11). Most states with the highest rates of etizolam reports during 2018 were in the South, and most states in the South had rates of 0.30 or more. In comparison, only seven states in the Midwest and only two each in the West and Northeast had rates of etizolam reports of 0.30 or more during 2018. South Carolina and Arkansas had the highest rates of etizolam reports during 2018. Generally, the highest numbers of etizolam reports were found in urban areas. For example, during 2018, etizolam reports in Ohio were concentrated in the Columbus, Cleveland, Cincinnati, and Dayton areas (Fig. 12).
Fig. 10.
Rate of etizolam reports per 100,000 persons aged 15 or older in NFLIS-Drug, by state, 2015.
Fig. 11.
Rate of etizolam reports per 100,000 persons aged 15 or older in NFLIS-Drug, by state, 2018.
Fig. 13.
Rate of clonazolam reports per 100,000 persons aged 15 or older in NFLIS-Drug, by state, 2015.
Fig. 14.
Rate of clonazolam reports per 100,000 persons aged 15 or older in NFLIS-Drug, by state, 2018.
Fig. 15.
Rate of flubromazolam reports per 100,000 persons aged 15 or older in NFLIS-Drug, by state, 2015.
Fig. 16.
Rate of flubromazolam reports per 100,000 persons aged 15 or older in NFLIS-Drug, by state, 2018.
Fig. 12.
Etizolam reports in NFLIS-Drug in Ohio, by county, 2018.
As shown in Fig. 13, only 10 states had rates of clonazolam reports that were 0.01 or more during 2015, with only 1 state having a rate of 0.10 or more. By 2018, 31 states had rates of clonazolam reports that were 0.01 or more, including 23 states with rates that were 0.10 or more, with 5 of those states having rates of 0.50 or more (Fig. 14). States with the highest rates of clonazolam reports during 2018 were in the Midwest and South. Although most states in the West and Northeast had clonazolam report rates of less than 0.01 during 2018, California, Oregon, Washington, New Jersey, and Pennsylvania had clonazolam report rates of 0.10 or more during that time. Louisiana and Georgia had substantially higher clonazolam report rates compared with other states during 2018, with the highest numbers of reports clustered in areas just outside of Atlanta in Cobb, Fulton, and Cherokee Counties (data not shown).
Only seven states had flubromazolam report rates of 0.01 or more during 2015, and no states had rates of 0.10 or more (Fig. 15). By 2018, 27 states had flubromazolam report rates of 0.01 or more, including 8 with rates of 0.30 or more (Fig. 16). Of the eight states with flubromazolam report rates of 0.30 or more in 2018, three were in the South, three were in the Midwest, two were in the Northeast, and none were in the West. Only 4 of the 13 states in the West had flubromazolam report rates of 0.01 or more compared with more than half of the states in each of the South and the Midwest and four out of nine states in the Northeast. Kansas had the highest rate of flubromazolam reports during 2018, with nearly all reports from the two most populous counties in the state, Sedgwick and Johnson (data not shown).
3.7. Selected benzodiazepine prescriptions dispensed
The IQVIA Launch (formerly IMS Health) database provides data on the number of legitimately dispensed prescriptions in the United States. The number of benzodiazepine prescriptions decreased each year from 2015 (131.7 million) to 2018 (108.3 million), amounting to an overall decline of nearly 18% (Table 4). It is possible that some of the decline in benzodiazepine prescriptions may be due to the 2016 FDA boxed warning of co-prescribing opioids and benzodiazepines [14]. In 2020, an FDA Drug Safety Communication was issued requiring boxed warning updates about benzodiazepines [36]. Although the number of dispensed prescriptions for midazolam—the least commonly prescribed benzodiazepine—increased by 25% from 2015 to 2018, the numbers of prescriptions dispensed for all of the other selected benzodiazepines decreased between 2015 and 2018, ranging from a 13% decrease for clonazepam to a 49% decrease for flurazepam. Alprazolam was the most commonly prescribed benzodiazepine across all years, accounting for over one third (37%) of all the dispensed benzodiazepines in 2018. The number of alprazolam prescriptions decreased by 18% from 2015 to 2018. Lorazepam decreased by 16%, with 28.5 million prescriptions in 2015 and 23.8 million prescriptions in 2018.
Table 4.
IQVIA total prescriptions dispensed, by selected benzodiazepines, 2015–2018.
| Selected benzo-diazepine | 2015 | 2016 | 2017 | 2018 | % change (2015–2018) | % of total (2018) |
|---|---|---|---|---|---|---|
| Alprazolam | 48,648,574 | 47,709,548 | 43,586,794 | 39,916,469 | -17.9 | 36.9 |
| Clonazepam | 29,712,779 | 29,525,024 | 27,360,959 | 25,840,153 | -13.0 | 23.9 |
| Lorazepam | 28,513,707 | 27,901,421 | 25,683,689 | 23,833,390 | -16.4 | 22.0 |
| Diazepam | 14,410,484 | 13,833,258 | 12,287,771 | 10,928,546 | -24.2 | 10.1 |
| Temazepam | 8,043,438 | 7,673,689 | 6,784,393 | 6,024,801 | -25.1 | 5.6 |
| Triazolam | 1,104,740 | 1,068,923 | 968,964 | 912,828 | -17.4 | 0.8 |
| Clorazepate | 689,284 | 624,664 | 521,408 | 451,336 | -34.5 | 0.4 |
| Oxazepam | 258,496 | 225,251 | 185,989 | 160,173 | -38.0 | 0.1 |
| Flurazepam | 214,892 | 182,492 | 136,152 | 109,859 | -48.9 | 0.1 |
| Estazolam | 107,950 | 96,453 | 84,709 | 74,471 | -31.0 | 0.1 |
| Midazolam | 29,372 | 33,371 | 32,176 | 36,824 | 25.4 | 0.0 |
| Total | 131,733,716 | 128,874,094 | 117,633,004 | 108,288,850 | -17.8 | 100.0 |
3.8. Diversion of selected benzodiazepines
By comparing national prescription data with forensic laboratory data on drugs seized by law enforcement agencies, NFLIS-Drug has been able to approximate the extent to which prescription drugs are diverted in the United States. It is probable that some of the drugs seized are clandestinely manufactured, which may lead these seizure-to-prescription ratios to overestimate the extent of diversion. The DEA’s 2006 special report on controlled substance prescription drugs reported in NFLIS-Drug between 2001 and 2005 revealed drugs with the highest seizure-to-prescription ratios [25]. The list of drugs with high seizure-to-prescription ratios included the following benzodiazepines: diazepam, alprazolam, clonazepam, and lorazepam.
When an updated DEA report was published in 2016 with the results from 2009 to 2014 using NFLIS-Drug and IMS Health’s prescription data, alprazolam had the largest seizure-to-prescription drug ratio in that time period (9.39 drug reports identified in NFLIS per 10,000 prescriptions dispensed) [23]. Moreover, alprazolam had the largest ratio change (58%) between 2001–2005 and 2009–2014. Diazepam was the only drug with a percent decrease in the seizure-to-prescription drug ratio between these two periods.
In the current analysis (Table 5), the 2001–2005 and 2009–2014 seizure-to-prescription drug ratios from previous report time periods are compared with the results from 2015 to 2018 using NFLIS-Drug and IQVIA Launch data. Alprazolam had the largest seizure-to-prescription drug ratio in 2015–2018 (10.24 drug reports identified in NFLIS-Drug per 10,000 prescriptions dispensed), which points to the highest level of diversion given the availability of the drug. Moreover, alprazolam had the largest ratio change between 2001–2005 and 2009–2014. Clonazepam and lorazepam both showed modest decreases for their respective seizure-to-prescription drug ratios from 2009 to 2014 compared with 2015–2018.
Table 5.
Drug Reports Identified in NFLIS-Drug per 10,000 Prescriptions Dispensed, 2001–2005, 2009–2014, and 2015–2018. Source for 2001–2005 NFLIS-Drug data is U.S. Drug Enforcement Administration, Office of Diversion Control [25]. Source for 2009–2014 NFLIS data is U.S. Drug Enforcement Administration, Office of Diversion Control [23]. Prescription data are from the IQVIA Launch database (2001–2005, 2009–2014, 2015–2018).
| Selected benzo-diazepine | 2001–2005 |
2009–2014 |
2015–2018 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Average annual number of drug reports | Average annual number of prescriptions dispensed | Drug reports per 10,000 prescriptions dispensed | Average annual number of annual drug reports | Average annual number of prescriptions dispensed | Drug reports per 10,000 prescriptions dispensed | Average annual number of annual drug reports | Average annual number of prescriptions dispensed | Drug reports per 10,000 prescriptions dispensed | |
| Alprazolam | 20,227 | 33,938,200 | 5.96 | 40,523 | 43,157,333 | 9.39 | 46,053 | 44,965,250 | 10.24 |
| Clonazepam | 5868 | 16,506,800 | 3.55 | 11,203 | 24,667,167 | 4.54 | 11,241 | 28,109,750 | 4.00 |
| Lorazepam | 1608 | 22,463,600 | 0.72 | 2442 | 24,846,333 | 0.98 | 2321 | 26,483,000 | 0.88 |
| Diazepam | 7913 | 13,059,800 | 6.06 | 6625 | 12,964,833 | 5.11 | 4401 | 12,865,000 | 3.42 |
4. Discussion
This study analyzed results of drug seizure data submitted to NFLIS-Drug by participating laboratories. The analysis focused on benzodiazepines, with an emphasis on designer benzodiazepines reported in NFLIS-Drug. Analyzing seized drug data can provide insight into the illicit drug markets and alert law enforcement agencies, public health departments, and other state and federal entities of state, regional, and emerging drug trends. Seized drug data have been shown as a complementary resource to other data resources, such as overdose deaths [[29], [30], [31]]. NFLIS-Drug can serve as a warning system for new drugs of abuse while other data collection systems provide complementary information.
For example, bromazolam was first reported by NFLIS-Drug in 2016, while a toxicology laboratory and research center reported it in May 2020 [32]. Sometimes, drugs of abuse are reported in other countries first, and NFLIS-Drug can monitor when they start to appear in the United States. Nifoxipam has not been reported in NFLIS-Drug but has been identified in other countries [26]. NFLIS-Drug data show that the estimated total benzodiazepine reports decreased by 14% between 2015 and 2018, and the number of benzodiazepine prescriptions decreased by 18% between 2015 and 2018. A decrease in benzodiazepines can be seen around this time through nonfatal drug overdoses that were treated in emergency departments [33]. Although NFLIS-Drug data and IQIVIA Launch data showed a decrease in benzodiazepines, there are still issues of concern due to misuse and abuse, co-prescribing or concurrent use with other substances, and the emergence of designer benzodiazepines [34].
NFLIS-Drug data showed significant decreases both nationally and regionally of prescription benzodiazepines, clonazepam, and diazepam between 2015 and 2018 but increases in nonprescribed benzodiazepines, flubromazolam, clonazolam, and etizolam. Regional differences were observed between these nonprescribed benzodiazepines. In 2018, the highest rates of flubromazolam were in the Midwest, and the highest rates of clonazolam were in the South. For etizolam, the Northeast showed a decreased rate in 2018.
Alprazolam accounted for almost half (49%) of the drug reports identified in the same item as etizolam. In the first quarter of 2020, etizolam toxicology and seized drug combinations were reported through a toxicology laboratory and research center [35]. Etizolam, methamphetamine, and narcotic analgesics each accounted for 15% of the drug reports identified in the same item as alprazolam.
5. Conclusions
As the only nationally representative database of law enforcement drug seizures submitted to and analyzed by laboratories, NFLIS-Drug provides the DEA, law enforcement agencies, public health agencies, the treatment community, and other entities with the ability to track drug trends nationally, regionally, and locally. NFLIS-Drug provides the community with information on new and evolving drugs, such as designer benzodiazepines, and helps identify changing trends of drugs, such as prescription benzodiazepines. It serves as a complementary dataset to other local or state drug seizure datasets and overdose data.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge the assistance of Neelima Kunta and Jeffrey Ancheta for providing NFLIS-Drug data and for their review of the manuscript. This work was supported by the DEA through the NFLIS program. This publication was reviewed and approved by the DEA Publication Review Board (DEA PRB 08-21-20-27a).
References
- 1.Levine B., editor. Principles of Forensic Toxicology. AACC Press; Washington, DC: 2017. [Google Scholar]
- 2.U.S. Drug Enforcement Administration Diversion Control division, benzodiazepines (street names: benzos, downers, nerve pills, tranks) December 2019. https://www.deadiversion.usdoj.gov/drug_chem_info/benzo.pdf
- 3.U.S. Department of Justice Drug enforcement administration, diversion Control division, title 21 United States code (USC) controlled substance Act, schedules of controlled substances. 2016. https://www.deadiversion.usdoj.gov/21cfr/21usc/812.htm
- 4.Wick J.Y. The history of benzodiazepines. Consult. Pharm. 2013;28:538–548. doi: 10.4140/tcp.n.2013.538. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Drug Enforcement Administration Diversion Control division, etizolam (trade names: etilaam, etizest, depas, etizola, sedekopan, pasaden) March 2020. https://www.deadiversion.usdoj.gov/drug_chem_info/etizolam.pdf
- 6.U.S. Drug Enforcement Administration Diversion Control division, phenazepam (street names: bonsai, soviet benzo, fenaz, panda) March 2020. https://www.deadiversion.usdoj.gov/drug_chem_info/phenazepam.pdf
- 7.Marin M.J., van Wijk X.M.R. The evolution of designer benzodiazepines: challenges for detection and monitoring. https://www.aacc.org/publications/cln/articles/2019/november/the-evolution-of-designer-benzodiazepines AACC Clinical Lab. News.
- 8.van Wijk X.M.R., Yun C., Hooshfar S., Arens A.M., Lung D., Wu A.H.B., Lynch K.L. A liquid-chromatography high-resolution mass spectrometry method for non-FDA approved benzodiazepines. J. Anal. Toxicol. 2019;43:316–320. doi: 10.1093/jat/bky092. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Drug Enforcement Administration Diversion Control division, flubromazolam (street name: liquid xanax) August 2019. https://www.deadiversion.usdoj.gov/drug_chem_info/flubromazolam.pdf
- 10.State of Virginia Virginia general assembly, Schedule I, 54.1, code of Virginia, sect. 3446. 2020. https://law.lis.virginia.gov/vacode/title54.1/chapter34/section54.1-3446/
- 11.State of Florida Florida legislature, 2019 statutes, title XLVI, ch. 893. 2019. http://www.leg.state.fl.us/Statutes/index.cfm?App_mode=Display_Statute&URL=0800-0899/0893/Sections/0893.03.html
- 12.State of Louisiana Louisiana state legislature. Statutes. 2020 http://legis.la.gov/Legis/Law.aspx?d=98877 RS 40, section 964. [Google Scholar]
- 13.World Health Organization Expert committee on drug dependence, critical review report: etizolam, 42nd meeting. 2019. https://www.who.int/medicines/access/controlled-substances/Final_Etizolam.pdf?ua=1 21–25 October 2019, Geneva, Switzerland.
- 14.U.S. Food and Drug Administration FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. https://www.fda.gov/Drugs/DrugSafety/ucm518473.htm 20 September 2017.
- 15.Dowell D., Haegerich T.M., Chou R. CDC guideline for prescribing opioids for chronic pain—United States. J. Am. Med. Assoc. 2016;315:1624–1645. doi: 10.1001/jama.2016.1464. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tori M.E., Larochelle M.R., Naimi T.S. Alcohol or benzodiazepine co-involvement with opioid overdose deaths in the United States, 1999-2017. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Substance Abuse and Mental Health Services Administration Key substance use and mental health indicators in the United States: results from the 2018 national survey on drug use and health (HHS publication No. PEP19-5068, NSDUH series H-54) August 2019. https://www.samhsa.gov/data/report/2018-nsduh-annual-national-report
- 18.Høiseth G., Tuv S.S., Karinen R. Blood concentrations of new designer benzodiazepines in forensic cases. Forensic Sci. Int. 2016;268:35–38. doi: 10.1016/j.forsciint.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Heide G., Høiseth G., Middelkoop G., Øiestad A.M.L. Blood concentrations of designer benzodiazepines: relation to impairment and findings in forensic cases. J. Anal. Toxicol. 2020 doi: 10.1093/jat/bkaa043. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arens A.M., van Wijk X.M.R., Vo K.T., Lynch K.L., Wu A.H.B., Smollin C.G. Adverse effects from counterfeit alprazolam tablets. JAMA Intern. Med. 2016;176:1554–1555. doi: 10.1001/jamainternmed.2016.4306. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter J.E., Murray B.P., Dunkley C., Kazzi Z.N., Gittinger M.H. Designer benzodiazepines: a report of exposures recorded in the national Poison data system, 2014-2017. Clin. Toxicol. 2019;57:282–286. doi: 10.1080/15563650.2018.1510502. [DOI] [PubMed] [Google Scholar]
- 22.United Nations Office on Drugs and Crime UNODC . II. January 2020. https://www.unodc.org/documents/scientific/Current_NPS_Threats_Volume_II_Web.pdf (Current NPS Threats). [Google Scholar]
- 23.U.S. Drug Enforcement Administration . 2016. Office of Diversion Control, National Forensic Laboratory Information System Special Report: benzodiazepines Reported in NFLIS, 2009–2014.https://www.nflis.deadiversion.usdoj.gov/Reports.aspx [Google Scholar]
- 24.U.S. Drug Enforcement Administration . September 2017. Diversion Control Division, National Forensic Laboratory Information System: statistical Methodology.https://www.nflis.deadiversion.usdoj.gov/Reports.aspx [Google Scholar]
- 25.U.S. Drug Enforcement Administration . 2006. Office of Diversion Control, NFLIS Special report: controlled Substance Prescription Drugs, 2001–2005.https://www.nflis.deadiversion.usdoj.gov/Reports.aspx [Google Scholar]
- 26.Helander A., Bäckber M., Beck O. Drug trends and harm related to new psychoactive substances (NPS) in Sweden from 2010 to 2016: experiences from the STRIDA project. PloS One. 2020;15 doi: 10.1371/journal.pone.0232038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mei V., Concheiro M., Pardi J., Cooper G. Validation of an LC-MS/MS method for the quantification of 13 designer benzodiazepines in blood. J. Anal. Toxicol. 2019;43:688–695. doi: 10.1093/jat/bkz063. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Drug Enforcement Administration Diversion Control division, national forensic laboratory information system: NFLIS-drug 2018 annual report. 2019. https://www.nflis.deadiversion.usdoj.gov/Reports.aspx
- 29.Rosenblum D., Unick J., Ciccarone D. The rapidly changing US illicit drug market and the potential for an improved early warning system: evidence from Ohio drug crime labs. Drug Alcohol Depend. 2020;208:10779. doi: 10.1016/j.drugalcdep.2019.107779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pardo B., Davis L.M., Melinda M. Characterization of the synthetic opioid threat profile to inform inspection and detection solutions (RAND Homeland Security Operational Analysis Center) 2019. https://www.rand.org/content/dam/rand/pubs/research_reports/RR2900/RR2969/RAND_RR2969.pdf
- 31.O’Donnell J.K., Gladden R.M., Seth P. Trends in deaths involving heroin and synthetic opioids excluding methadone, and law enforcement drug product reports, by census region—United States, 2006–2015, Morbidity Mortal. Wkly. Rep. 2017;66:897–903. doi: 10.15585/mmwr.mm6634a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krotulski A.J., Fogarty M.F., Papsun D.M., Logan B.K. Bromazolam: seized material & biological fluid. https://www.npsdiscovery.org/wp-content/uploads/2020/05/Bromazolam_050120_NMSLabs_Report.pdf 1 May 2020.
- 33.Vivolo-Kantor A.M., Hoots B.E., Scholl L., Pickens C., Roehler D.R., Board A., Mustaquim D., Smith H., Snodgrass S., Liu S. Nonfatal drug overdoses treated in emergency departments—United States, 2016–2017, Morbidity Mortal. Wkly. Rep. 2020;69:371–376. doi: 10.15585/mmwr.mm6913a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitz A. Benzodiazepines: the time for systematic change is now. Addiction. 2020 doi: 10.1111/add.15095. [DOI] [PubMed] [Google Scholar]
- 35.Krotulski A.J., Mohr A.L.A., Logan B.K. NPS benzodiazepines in the United States (trend report: Q1 2020) https://www.npsdiscovery.org/wp-content/uploads/2020/04/2020-Q1_NPS-Benzodiazepines_Trend-Report.pdf 8 April 2020.
- 36.U.S. Food and Drug Administration FDA Drug Safety Communication: FDA requiring Boxed Warning updated to improve safe use of benzodiazepine drug class. 2020. https://www.fda.gov/media/142368/download