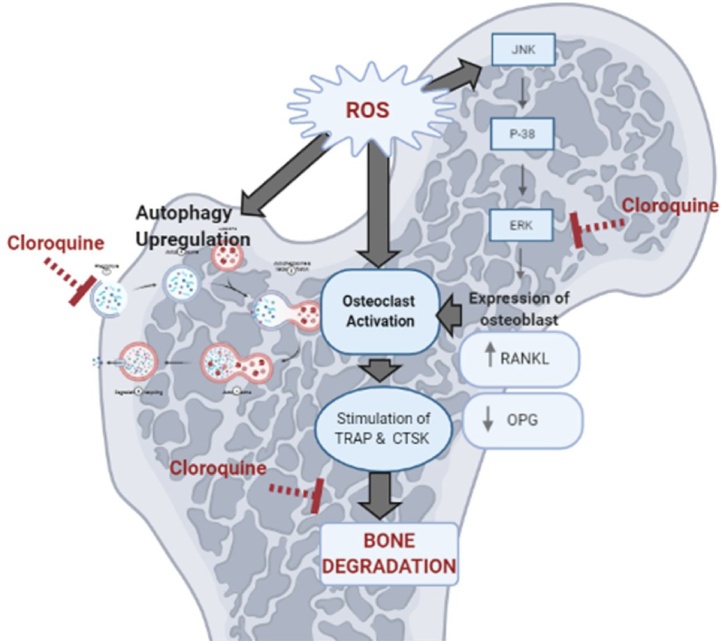
Graphical abstract
Keywords: Chloroquine, Osteoporosis, ERK, RANKL, OPG, Rats
Highlights
-
•
Cloroquine (CQ) has reduced the adverse bone changes caused by d-galactose.
-
•
It improved bone health, switched off nuclear factor kappa-B ligand (RANKL) receptor activator activation and decreased ERK bone expression.
-
•
CQ treatment inhibited osteoclastogenesis and consequently restored the RANKL/OPG ratio.
-
•
CQ demonstrated an antioxidant effect in bone where it increased both catalase (CAT) and superoxide dismutase (SOD).
-
•
CQ is a possible anti-osteoporotic agent through the suppression of osteoclastogenesis associated with ERK.
Abstract
Chloroquine (CQ); a lysosomotropic agent used for decade ago as anti-malarial, was tested against aging induced osteoporosis. Osteoporosis in male rats was induced using d-galactose (D-gal) as a reducing sugar at a dose of 200 mg/kg/day; i.p. Osteoporotic rats were orally treated with CQ (10 mg/kg/day) for four successive weeks. Bone densitometry of tibia and femur were evaluated. Bone formation biomarkers; osteoprotegrin (OPG), bone specific alkaline phosphatse (BALP), and osteocalcin (OCN), and bone resorption biomarker; receptor activator of nuclear factor kappa-B ligand (RANKL), cathepsin-k (CTSK), tartrate-resistant acid phosphatase (TRAP) were estimated. Moreover, the expression of extracellular regulated kinase (ERK) in bone was determined. CQ ameliorated the bone detrimental changes induced by d-galactose. It enhanced bone health as revealed by measurement of bone densitometry, halted the activation of receptor activator of nuclear factor kappa-B ligand (RANKL) and reduced bone manifestation of ERK. Furthermore, CQ treatment abated serum cathepsin-k (CTSK) and serum tartrate-resistant acid phosphatase (TRAP) thus inhibited osteoclastogenesis and consequently restored the RANKL/OPG ratio. CQ demonstrated an antioxidant effect in bone where it increased both Catalase (CAT) and Superoxide dismutase (SOD). These CQ preserving effect in rats treated with d-galactose were confirmed by the histopathological examination. The present study points to the potential therapeutic effect of CQ as anti-osteoporotic agent possibly through its antioxidant effects and suppression of ERK associated osteoclastogenesis.
1. Introduction
Osteoporosis is signalized by decrease in bone mass ensuing from the disproportion among bone resorption and bone formation rate which enhances bone fragility and subsequently increases fracture risk [1]. When the bone resorption cells predominate, bone turnover takes place in both genders at the tissue levels which impairs osteoblastic bone formation and causes bone loss particularly during aging [2].
Free radical and oxidative stress subsequently produced from the excessive formation of reactive oxygen species (ROS) are the most promising reasons for the process of aging, inflammatory arthritis, and age-related bone loss [3]. Mitochondrial ROS as hydrogen peroxide and superoxide are metabolic by-products produced by complex I in electron transport chain [4,5]. NADPH oxidase (NOX) contributes to the production of ROS through oxidation of the intracellular NADPH to NADP+ and donating electrons through membranes which reduced the molecular oxygen and engendered superoxide anions [6]. Failure to maintain healthy mitochondria could result in overproduction of ROS and successively leads to chronic diseases such as osteoporosis [7]. Moreover, a former study [8] showed that, SOD regulates the osteoblast activity and hence osteoclastogenesis through RANKL signaling.
Antioxidants act as a defense mechanism against the reactive species derived from oxygen and nitrogen by reducing the oxidative damage to the tissues [9]. Superoxide dismutase (SOD) and catalase (CAT) both have significant roles as antioxidants. SOD reduces the superoxide radicle to hydrogen peroxide and molecular oxygen while CAT converts the hydrogen peroxide into water [10]. However, accumulation of ROS decreases the activity of the antioxidant enzymes which subsequently lead to oxidative stress [11,12] by disturbing the balance between the oxidant and the antioxidants [13]. It also induces mitochondrial malfunction and lipid peroxidation which result in further increase of ROS production and osteoclasts differentiation through upregulation of the receptor activator of nuclear factor-κβ ligand RANKL [14,15]. Activation of RANKL/RANK signaling has an important role in bone turnover which could be prevented by the expression of the decoy receptor osteoprotegrin (OPG) on osteoblastic cells which protects against bone turnover [16]. ROS modulated the mitogen-activated protein kinase; extracellular regulated kinase (ERK) pathway to induce expression of RANKL in osteoblasts [[17], [18], [19]] which in-turn altered the ratio of receptor activator of nuclear factor kappa-B ligand and osteoprotegrin (RANKL/OPG) and subsequently enhanced the conversion to bone-catabolizing osteoclastic cells [20]. Activation of osteoclasts (OCs) by ROS also causes the expression of tartrate-resistant acid phosphatase (TRAP) and cathepsin-k (CTSK) that are important to osteoclastogenesis. They both have an essential role in bone remodeling as they are found abundantly in the ruffled borders of osteoclastic cells and cause degradation and demineralization of bone matrix especially type-1 collagen which contributes to bone loss [21]. Moreover, excessive formation of ROS leads to the production of autophagy which is a highly conserved intracellular catabolic process that maintains cellular homeostasis through which the damaged cellular components are degraded for the production of energy [22]. However, a previous study by Shi et al. [23] revealed a significant upregulation in autophagic activity followed by OCs differentiation during oxidative stress.
Chloroquine (CQ) is approved for the treatment of uncomplicated malaria due to susceptible plasmodium strains [24]. It may be used for musculoskeletal inflammatory disease rheumatoid arthritis as well as for treating discoid and systemic lupus erythematosus [25]. Due to its alkaline properties, CQ is highly lipid soluble and can neutralize the acidic compartments of the cell as endosomes and lysosomes [26]. Several reports showed that CQ and its derivative hydroxychloroquine prevent vascular damage by inhibiting angiogenesis in discoid lupus erythematosus [27]. Moreover, CQ has been used to control influenza virus type A and type B as well as inhibiting the multiplication of H1N1 and avian viruses [28]. A former study showed that it also prevents expression of inflammatory mediators by blocking autophagic flux through inhibition of autolysosomes formation [29]. Moreover, a previous study demonstrated CQ interference with ERK activation in-vitro is related to its inflammatory effect [30].
The present study was carried out to investigate the possible repurposing of CQ to bone health. CQ was investigated in d-galactose (D-gal) induced osteoporosis in male rats through its impact on the activation of ERK/RANKL pathway. Furthermore, CQ effect on antioxidant enzymes was examined together with its subsequent influence on the inhibition of TRAP and CTSK during osteoclastogenesis. Osteoporosis was monitored through dual energy X-ray absorptiometry and histopathological examination.
2. Material and methods
2.1. Experimental animal
Thirty-six adult male Wistar albino rats (three months old) gauging 150−180 g and were supplied from the animal house colony of the National Research Center (Giza, Egypt). They were kept and housed in proper environmental conditions all throughout the time of examination; surrounding temperature (25 ± 2 °C), moisture (60 ± 10 %), and light-dark cycles every 12 h. Animals were fed on a standard rat pellet diet (United Co. for poultry production, El-Obour city, Cairo, Egypt) and indorsed free admittance to water. The investigational procedure of this study was reliable with the National Institute of Health Guidelines (NIH Publications No. 80-23; 1996). Furthermore, moral endorsement was accommodated for the present study by the research Ethical Committee, Faculty of Pharmacy, Cairo University, Cairo, Egypt (ethical approval No. PT 1395; 2015).
2.2. Study design
After a 2 weeks acclimatization period, all animals were divided into three groups each of 12 rats; Group 1: normal rats received equivalent volume of saline act as a control group, Group 2: rats received d-gal dosage of 200 mg/kg/day, i.p. for 8 consecutive weeks and served as osteoporotic rats, Group 3: rats received d-gal dosage of 200 mg/kg/day, i.p. for 8 consecutive weeks followed by CQ at a dose of 10 mg/kg/day for 4 successive weeks. d-gal dosage of 200 mg/kg/day, i.p. for 8 consecutive weeks was based on a previous study [12,31]. Dosage of CQ was selected based on the previous studies [32,33]. By the end of the twelfth week, blood samples were gathered and kept frozen at −70 °C. Femoral and tibia bone samples were then detached and washed off soft tissues to be scanned for bone densitometry as illustrated in Fig. 1.
Fig. 1.
Schematic diagram showing the experimental design of the present study.
2.3. Determination of femoral and tibia bone densitometry using central dual energy x-ray absorptiometry (DEXA)
Rats were forfeited; bone femur and tibia were removed, eviscerated, and then placed under the scanner arm of a Norland XR-46 to perform a DEXA scan. The device estimates bone mineral content (BMC) in grams and area in cm2 through directing a flimsy imperceptible x-ray beam through the bone. After scanning; the information is kept by the computer unit and used to analyze bone mineral density (BMD) in g/cm2 by dividing BMC over area of bone measured. Fig. 2 shows the scanning of bone tibia and femur.
Fig. 2.
Represents bone densitometry of tibia and femur scanned by NORLND XR-46. The scanning was performed with a speed of 60 mm/s and 1.0 × 1.0 mm resolution with the same duration of time for each group. Control (A), d-galactose (B), and CQ (C).
2.4. Determination of serum calcium and phosphorus
The serum levels of calcium and phosphorus in blood were evaluated colorimetrically (Bio-diagnostic, Cairo, Egypt) at 585 nm conferring to the technique designated by [34] and at 640 nm relying on the process defined by [35] respectively.
2.5. Determination of bone turnover biomarkers
The serum concentration of bone-specific alkaline phosphatase (BALP), osteocalcin (OCN), and osteoprotegrin (OPG), receptor activator of nuclear factor kappa beta ligand (RANKL), tartrate-resistant acid phosphatase (TRAP), Cathepsin k (CTSK) were evaluated by sandwich ELISA kit (Biotang Inc., Waltham, Massachusetts, USA).
2.6. Measurement of anti-oxidant enzymes
The oxidoreductases catalase (CAT) in U/L and superoxide dismutase (SOD) in U/mL serum activity were valued using colorimetric kit (Bio-diagnostic, Cairo, Egypt) at 510 nm agreeing to the procedure pronounced by [36] and at 560 nm conferring to the method pronounced by [37].
2.7. Quantitative western blot of bone ERK Protein
The prepared samples of the scraped bone tissues were treated by the ReadyPrep™ protein extraction kit provided by Bio-Rad Inc. (Catalog #163-2086) according to the manufacturer constructions. Quantitative measurements for protein assay were done by Bradford Protein Assay Kit (Cat # SK3041, Bio Basic Inc., Ontario, Canada). Parting of the protein samples due to the difference in their molecular weight was done on a polyacrylamide gel in a regular running buffer after 20 μg of total protein were encumbered into each mini-gel well. The gel was then collected in a transferal sandwich with PVDV membrane, located in an allocation container (Bio-Rad Trans-Blot Turbo) with 1x transfer buffer to permit protein bands movement from gel to membrane. In order to completely block the membrane for 1 h, Tris-buffered saline which comprises Tween 20 (TBST) and 3% bovine serum albumin (BSA) is used at lukewarm that diluted the Primary ERK antibodies concentration referring to the constructor's guidelines. Incubation at 4 °C was done during night in the primary antibody solution alongside the marked aimed protein. HRP-conjugated secondary antibody solution (goat anti-rabbit IgG- HRP-1 mg goat mAb -Novus Biologicals) was added to the membrane and incubation takes place against the blotted target protein for 1 h at ambient temperature. The chemiluminescent substrate (Clarity™ Western ECL substrate Bio-Rad cat#170-5060) was applicable to the blot conferring to the constructor’s endorsement. Computer program used to evaluate the images by interpreting the band intensity of the marked proteins opposing the control tester beta actin; housekeeping protein on the ChemiDoc MP imager. The primary antibodies against ERK (Cat # sc-1647), were purchased from Santa Cruz Biotechnology, Inc. (Dallas, Texas, USA), and the secondary antibody (goat anti-rabbit IgG; HRP-1 mg goat mAb) were purchased from Novus Biologicals (Littleton, Colorado, USA).
2.8. Histopathological examination
The animals were sacrificed via pentobarbital overdose (100 mg/kg). Then, tibia and femur bone were detached and preserved in 10 % neutral buffered formalin for 48 h, and then were fixed in 10 % ethylene diamine tetra-acetic acid (EDTA) for decalcification. Tissue handling was done through graded ethanol, xylol, and paraffin for dehydration, clearing, impregnation, and embedding. Histologic sections with a thickness of 6 μm were arranged and afterword they were regularly tainted with hematoxylin and eosin. A scoring scale was used to assess osteoporosis according to [38]. The scoring was 1: <25 % bone showing osteopenia, 1+: 25−50 % bone showing osteopenia, 2+: 50−75 % bone showing osteopenia 3+: >75 % bone showing osteopenia.
2.9. Statistical analysis
The statistical outcomes are obtainable as mean ± S.E.M. and were factually investigated using one-way ANOVA followed by Tukey’s multiple comparisons test. GraphPad Prism software version 6 (La Jolla, CA, USA) was engaged for the statistical inquiry. Statistical significance was set up as *p < 0.05 vs Normal control group, @ p < 0.05 vs D-gal treated group.
3. Results
3.1. Effect of CQ on bone mineral density in d-gal induced osteoporotic rats
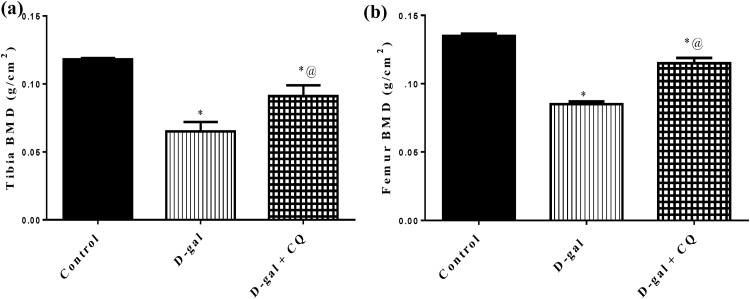
The perusing and examination of bone engendered by DEXA showed that d-gal produced reductions in tibia and femur BMD by around 45 % and 37 % respectively regarded to the control group represented in Fig. 3. The d-gal induced reductions in BMD of tibia and femur were alleviated by oral CQ treatment and showed a marked elevation in tibia and femur bone by about 40 % and 35 % respectively as regard to d-gal treated group.
Fig. 3.
Effect of CQ on BMD in d-gal induced osteoporotic rats.
Osteoporosis was induced in male rats via injecting d-gal of 200 mg/kg/day, i.p. for 8 consecutive weeks. Afterwards, osteoporotic rats were treated orally with CQ (10 mg/kg) for 4 successive weeks. 24 h after the last dose of CQ, Tibia bone and Femur bone were scanned using a regional high-resolution scan of the right and left femurs. Results are expressed as mean ± S.E.M. (n = 10–12). Data were statistically estimated using one-way ANOVA followed by Tukey’s multiple comparisons test. Variances were deemed statistically significant when *p < 0.05 in contrast to the control group and @ p < 0.05 in contrast to D-gal treated group.
3.2. Effect of CQ on serum calcium and phosphate in d-gal induced osteoporotic rats
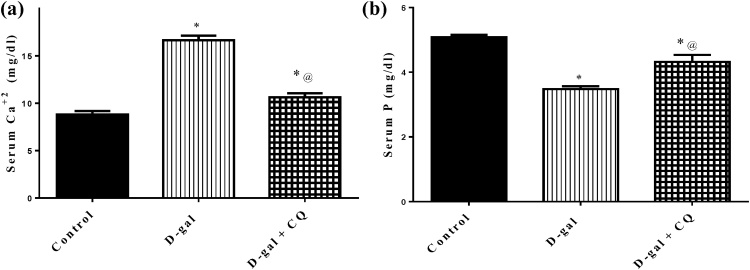
D-gal treatment produced a significant rise in serum calcium and a decline in serum phosphorus in d-gal treated group by 89 % and 32 %, in respect to the control group. Treatment with CQ decreased serum calcium by 36 % while elevated serum phosphorus by 24 %, as compared to d-gal treated group. Results are displayed in Fig. 4.
Fig. 4.
Effect of CQ on bone minerals in d-gal induced osteoporotic rats.
Osteoporosis was induced in male rats via injecting d-gal of 200 mg/kg/day, i.p. for 8 consecutive weeks. Afterwards, osteoporotic rats were treated orally with CQ (10 mg/kg) for 4 successive weeks. 24 h after the last dose of CQ, serum Ca+2 and serum P were determined using UV–vis spectrophotometer. Results are expressed as mean ± S.E.M. (n = 10–12). Data were statistically estimated using one-way ANOVA followed by Tukey’s multiple comparisons test. Variances were deemed statistically significant when *p < 0.05 in contrast to the control group and @ p < 0.05 in contrast to D-gal treated group.
3.3. Effect of CQ on anti-oxidant enzymes in d-gal induced osteoporotic rats
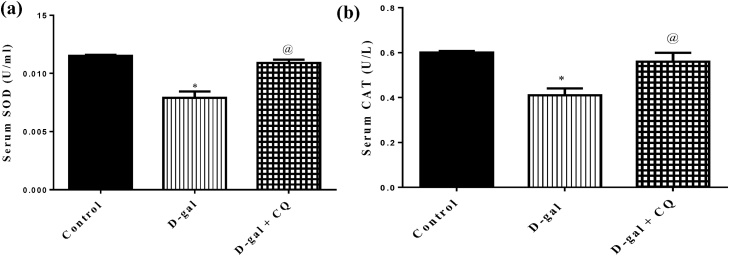
Both serum SOD and CAT showed a prominent decrease by about 31 % and 32 % respectively after being treated with d-gal for 8 successive weeks regarding the control group as depicted in Fig. 5. A marked elevation in serum SOD and CAT was observed after treatment with CQ that succeeded to restore the anti-oxidant enzyme levels nonsignificant from normal values.
Fig. 5.
Effect of CQ on antioxidants biomarkers in d-gal induced osteoporotic rats. Osteoporosis was induced in male rats via injecting d-gal of 200 mg/kg/day, i.p. for 8 consecutive weeks. Afterwards, osteoporotic rats were treated orally with CQ (10 mg/kg) for 4 successive weeks. 24 h after the last dose of CQ, serum superoxide dismutase (a) and serum catalase (b) were established using UV–vis spectrophotometer. Results are expressed as mean ± S.E.M. (n = 10-12). Data were statistically estimated using one-way ANOVA followed by Tukey’s multiple comparisons test. Variances were deemed statistically significant when *p < 0.05 in contrast to the control group and @ p < 0.05 in contrast to D-gal treated group.
3.4. Effect of CQ on bone formation biomarkers in d-gal induced osteoporotic rats
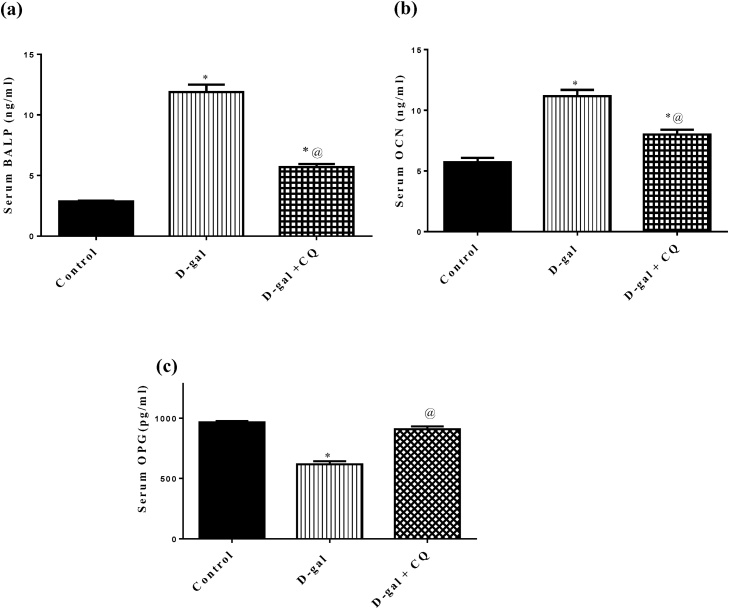
Results presented in Fig. 6 showed that d-galactose induced osteoporotic rats caused a marked upsurge in serum levels of (A) BALP and (B) OCN by about 3 and 1folds respectively while hampered serum (C) OPG by 36 % in respect to the control group. Treatment by CQ blunted the elevation in serum levels of BALP and OCN by about 52 % and 28 % respectively and replenished serum OPG in relation to d-gal treated group.
Fig. 6.
Effect of CQ on bone formation biomarkers in d-gal induced osteoporotic rats. Osteoporosis was induced in male rats via injecting d-gal of 200 mg/kg/day, i.p. for 8 consecutive weeks. Afterwards, osteoporotic rats were treated orally with CQ (10 mg/kg) for 4 successive weeks. 24 h after the last dose of CQ, serum BALP (a), serum OCN (b), serum OPG (c), was established via the sandwich ELISA technique. Results are expressed as mean ± S.E.M. (n = 10-12). Data were statistically estimated using one-way ANOVA followed by Tukey’s multiple comparisons test. Variances were deemed statistically significant when *p < 0.05 in contrast to the control group and @ p < 0.05 in contrast to D-gal treated group.
3.5. Effect of CQ on bone resorption biomarkers in d-gal induced osteoporotic rats
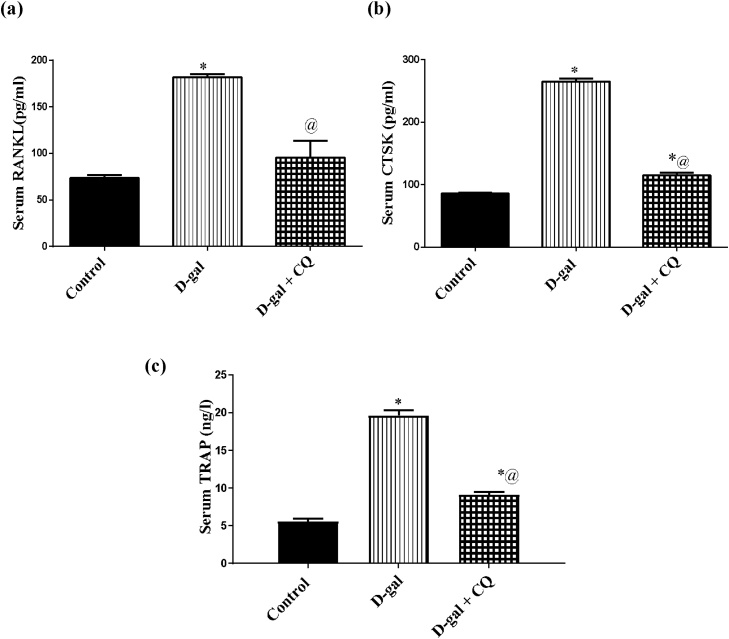
d-gal induced osteoporotic rats augmented serum levels of (A) RANKL, (B) CTSK, and (C) TRAP by 1.5, 2, and 2.5 folds after 8 consecutive weeks respectively in respect to the control group. Conversely, treatment with CQ abrogated the elevated aforementioned parameters in the order of the control group as demonstrated in Fig. 7
Fig. 7.
Effect of CQ on bone resorption biomarkers in d-gal induced osteoporotic rats. Osteoporosis was induced in male rats via injecting d-gal of 200 mg/kg/day, i.p. for 8 consecutive weeks. Afterwards, osteoporotic rats were treated orally with CQ (10 mg/kg) for 4 successive weeks. 24 h after the last dose of CQ, serum RANKL (a), serum CTSK (b), and serum TRAP (c) were established via the sandwich ELISA technique. Results are expressed as mean ± S.E.M. (n = 10-12). Data were statistically estimated using one-way ANOVA followed by Tukey’s multiple comparisons test. Variances were deemed statistically significant when *p < 0.05 in contrast to the control group and @ p < 0.05 in contrast to D-gal treated group.
3.6. Effect of CQ on bone ERK protein kinase in d-gal induced osteoporotic rats
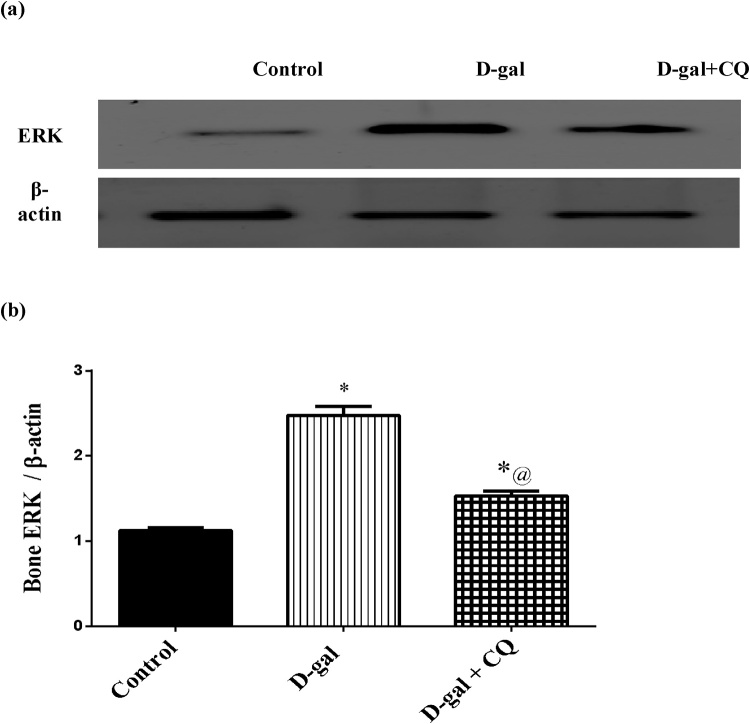
ERK was measured in bone using western blot method to determine its effect on the osteoclastogenesis in bone matrix as demonstrated in Fig. 8. Osteoporotic group exhibited an elevation in ERK by about 1.2 folds as compared to the control group. Once more, treatment with CQ turned off the increment and significantly mitigated ERK by 38 % in respect to the d-gal treated group.
Fig. 8.
Effect of CQ on femoral bone head expression of ERK protein kinase in d-gal induced osteoporotic rats.
Osteoporosis was induced in male rats via injecting d-gal of 200 mg/kg/day, i.p. for 8 consecutive weeks. Afterwards, osteoporotic rats were treated orally with CQ (10 mg/kg) for 4 successive weeks. 24 h after the last dose of CQ, It was determined using Western blot technique. Results are expressed as mean ± S.E.M. (n = 10–12). Data were statistically estimated using one-way ANOVA followed by Tukey’s multiple comparisons test. Variances were deemed statistically significant when *p < 0.05 in contrast to the control group and @ p < 0.05 in contrast to D-gal treated group.
3.7. Effect of CQ on histopathology in d-gal induced osteoporotic rats
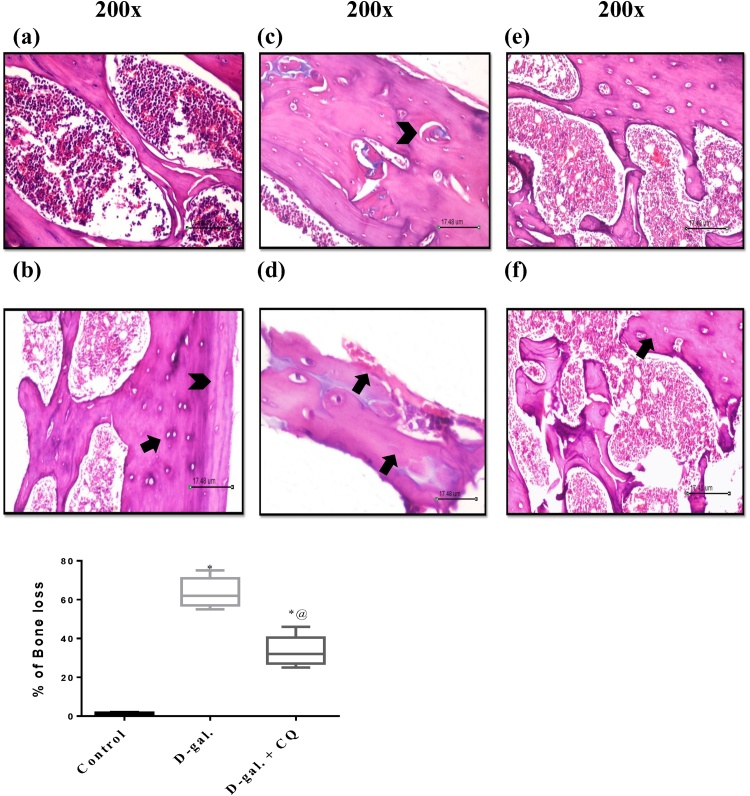
Fig. 9 represents the photomicrograph of a longitudinal segment of cancellous bone and represents the histological scoring report of the osteoporotic bone. Bone tissue sequestered from d-gal treated group exhibited irregularly sporadically disintegrated endosteal surface and resorption cavities. Multinucleated osteoclasts with their acidic compartments were also detected kept in the eroded bone surface. Moreover, damage of the normal architecture of bone trabeculae with obvious surge in the intertrabecular distance, which led to noticeable widening of the interconnected bone marrow spaces, and apparent increment in the adipocytes compared to (A) and (B) control group that has normal bone structure. CQ treatment showed mild changes of microarchitectural of bone, appearance of osteocytes in trabecula bone along with improvement of trabecular arrangement. However, signs of resorption with micro-fissures were still obvious. The histopathological scoring was according to [38] as shown in Table 1.
Fig. 9.
Effect of CQ on Histopathological examination in d-gal induced osteoporotic rats. Section of (C) and (D) d-gal induced osteoporosis group shows revealed irregularly sporadically disintegrated endosteal surface and resorption cavities, irregular thickness of the remaining trabeculae, osteoclasts with their acidic compartments and multiple nuclei were detected kept in the eroded bone surface as well as an increment in bone marrow adipocytes compared to the (A) and (B) control groups that showed regular histological structure. Section of (E) and (F) CQ treated group shows mild changes of microarchitectural of bone, trabecular continuity shows some resorption micro-fissures and bony trabeculae enclosing osteocytes in their lacunae. The percentage of osteoporosis were valued by mean of 5 and represented as mean ± S.E.M. Data were statistically estimated using one-way ANOVA followed by Tukey’s multiple comparisons test. Variances were deemed statistically significant when *p < 0.05 in contrast to the control group and @ p < 0.05 in contrast to D-gal treated group.
Table 1.
Histopathological scoring of osteoporotic rats treated with CQ according to [38].
| Parameters | Groups |
||
|---|---|---|---|
| Control | D-galactose | CQ | |
| % of Bone loss | 0 (< 5%) | 2+ (50−75 %)* | 1+ (25−50 %)*@ |
The histopathological sections were examined for osteoporosis in 2 different fields. Data are expressed as median (min-max) and analyzed Kruskel-Wallis followed by Dunn's Multiple Comparison test. Variances were deemed statistically significant when *p < 0.05 in contrast to the control group and @ p < 0.05 in contrast to D-gal treated group.
4. Discussion
Aged rat model has been established using d-gal which is a well-known reducing sugar that deteriorates the biological conditions of the cell as it imitates aging. It accumulates inside the cell at higher levels and is reduced to galactitol that causes osmosis stress, swelling, and subsequently ROS formation [39,40]. In the current study, d-galactose induced bone loss was confirmed by marked decline in BMD of femur and tibia. This was consistent with the findings of Djordjevic [41] and El-Baz et al. [42] that showed a negative correlation between increase in oxidative stress and BMD. The alterations in BMD assured osteoporosis in d-galactose treated group compared to the control rats.
Marked increase in serum calcium levels together with the decline in serum phosphate in the present study also established bone loss. This could be attributed to the activation of OCs that leads to the infusion of the extracellular fluid with calcium thus lowering the synthesis of 125 (OH)2D3 [43]. The further decline in serum 1,25 (OH)2D3 leads to the reduction in the intestinal calcium and phosphate absorption [44,45]. Moreover, augmentation in serum OCN and BALP was observed which could also contribute for the inhibition of calcium mineralization and to the osteoclast break down of bone matrix according to [46,47].
Daily administration of osteoporotic male rats with CQ improved BMD. It also mitigated serum calcium and subsequently raised serum phosphate. Moreover, it showed a prominent reduction in serum OCN and BALP. The mechanism could be accredited to the inhibition of OCs which in turn elevated bone formation biomarkers; BALP and OCN that resulted in calcium mineralization in bone matrix [48]. Also, the histopathological examination revealed that, CQ had lessened the altered bone structure compared to the osteoporotic control group. Notably, CQ had no direct effect on bone formation biomarkers in bone marrow stromal cells proposing that the main effect was on OCs [49].
Regular treatment of male rats with d-galactose increased the production of ROS that was indicated by the blunted antioxidant enzymes SOD and CAT serum activity. These results are in harmony with previous study of Muthusami et al. [50] in ovariectomized rats. On the opposite side, CQ in the present study normalized the oxidoreductases serum levels. Likewise, Iyawe et al. [51] reported the increase in antioxidant enzyme activity in plasmodium berghei infested mice treated with CQ and ascorbic acid. The mechanism by which CQ restored the antioxidant enzymes is still unclear however, it could be attributed to the ability of CQ as a lysosomotropic agent to prevent osteoclastogenesis [52].
Accelerated bone resorption via osteoclastic cells in osteoporotic rats in the present work was illuminated by the increased serum level of TRAP and CTSK; lysosomal proteases enzymes that are expressed in osteoclast. The ruffled borders of osteoclast start degrading bone matrix through the fusion of lysosomes with TRAP and CTSK [53]. It has been reported that, deletion of in-vivo CTSK in osteoclastic cells resulted in the activation of osteoblastic cells which in turn enhanced bone formation [54]. CQ as a lysosomotropic agent prohibited the acidification of lysosome by neutralizing its pH thereby preventing TRAP and CTSK activation and subsequently osteoclastogenesis [55].
Increased production of ROS following d-galactose treatment in the current study resulted in a noticeable increase in bone ERK. Bhatt et al. [56] reported that production of ROS stimulated ERK signaling which is a very important pathway as it increases RANKL expression on osteoblastic cells hence activating osteoclasts which in turn re-stimulate ROS formation [57]. Likewise, in our study, sharp elevation in serum RANKL together with a significant reduction in serum OPG thereby altered RANKL/OPG ratio which indicated osteoclastogenesis [58]. Also, upregulation of autophagy during oxidative stress contributes to differentiation of osteoclasts [59].
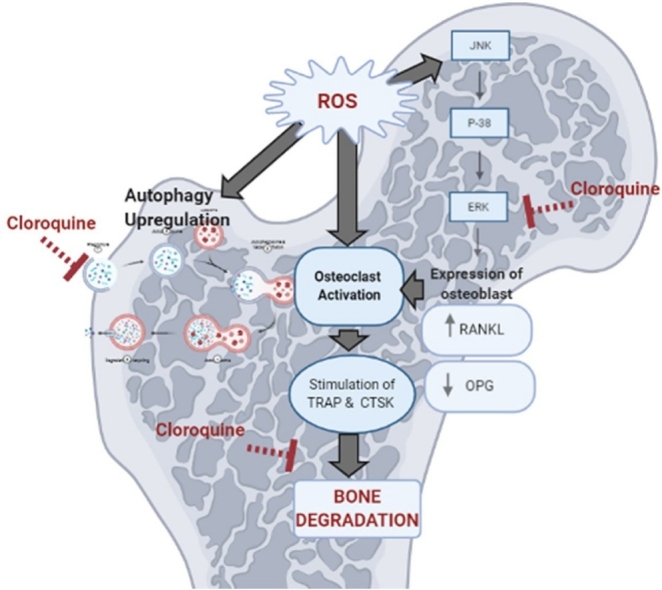
The osteo-protective effect of the CQ informed in the present study was associated by lowering bone ERK. It has been reported that, CQ hinder the activation of early members of the ERK signaling pathway thus inhibiting RANKL expression and blocked the activation of different inflammatory mediators [30] as illustrated in Fig. 10. CQ as autophagy-lysosome inhibitor abrogated the autophagic flux thus prohibited osteoclast activation and subsequently the activation of ROS [60]. Besides, CQ restored serum OPG which in turn increased expression of OPG on osteoblastic cells and promotes bone formation. Moreover, CTSK expression was found to be inhibited by normal levels of OPG [61].
Fig. 10.
Diagram summarizing the different bases upon which CQ inhibits osteoclastogenesis.
ROS: reactive oxygen species, ERK: extracellular regulated kinase, OCs: osteoclasts, RANKL: receptor activator of nuclear factor-κβ ligand, OPG: osteoprotegrin, TRAP: tartrate resistant acid phosphatase, CTSK: cathepsin K.
5. Conclusion
The results of the present study revealed that in d-gal induced osteoporosis; CQ showed valuable anti-osteoporotic effects which can be attributed to its inhibitory effects on autophagy-lysosomal pathway thus inhibiting osteoclast differentiation. Furthermore; CQ inhibited ERK protein kinases activation with subsequent decrease in RANKL expression on osteoblastic cells. This decline in RANKL results in an increase in OPG which decreases TRAP and CTSK in the ruffled border of the OCs thus preventing bone degradation.
Authorship statement
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Furthermore, each author certifies that this material or similar material has not been and will not be submitted to or published in any other publication before its appearance in the Journal.
Authorship contributions
Category 1
Conception and design of study: Dalia O. Saleh, Marwa M. Safar, Azza M. Agha, Mahmoud, M. Khattab
Acquisition of data: Mohamed A. Aziz Mahmoud, Dalia O. Saleh; Analysis and/or interpretation of data: Mohamed A. Aziz Mahmoud.
Category 2
Drafting the manuscript: Mohamed A. Aziz Mahmoud, Dalia O. Saleh
Revising the manuscript critically for important intellectual content: Marwa M. Safar, Azza M. Agha, Mahmoud, M. Khattab
Category 3
Approval of the version of the manuscript to be published (the names of all authors must be listed): Marwa M. Safar, Azza M. Agha, Mahmoud, M. Khattab: Signature: Mohamed Aziz Mahmoud: Dalia Saleh: Marwa Safar: Azza Agha: Mahmou Khattab
Declaration of Competing Interest
The authors declare no conflict of interest.
Edited by Dr. A.M. Tsatsaka
References
- 1.Woo J., Kwok T., Leung J., Tang N. Dietary intake, blood pressure and osteoporosis. J. Hum. Hypertens. 2009;23(7):451–455. doi: 10.1038/jhh.2008.156. [DOI] [PubMed] [Google Scholar]
- 2.Khosla S., Riggs B.L. Pathophysiology of age-related bone loss and osteoporosis. Endocrinol. Metab. Clin. North Am. 2005;34(4):1015–1030. doi: 10.1016/j.ecl.2005.07.009. xi. [DOI] [PubMed] [Google Scholar]
- 3.Kouka P., Priftis A., Stagos D., Angelis A., Stathopoulos P., Xinos N. Assessment of the antioxidant activity of an olive oil total polyphenolic fraction and hydroxytyrosol from a Greek Olea europea variety in endothelial cells and myoblasts. Int. J. Mol. Med. 2017;40(3):703–712. doi: 10.3892/ijmm.2017.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dirks A.J., Leeuwenburgh C. The role of apoptosis in age-related skeletal muscle atrophy. Sport. Med. 2005;35(6):473–483. doi: 10.2165/00007256-200535060-00002. [DOI] [PubMed] [Google Scholar]
- 5.Sabharwal S.S., Schumacker P.T. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nature reviews. Cancer. 2014;14(11):709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta K.J., Kumari A., Florez-Sarasa I., Fernie A.R., Igamberdiev A.U. Interaction of nitric oxide with the components of the plant mitochondrial electron transport chain. J. Exp. Bot. 2018;69(14):3413–3424. doi: 10.1093/jxb/ery119. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell T., Chacko B., Ballinger S.W., Bailey S.M., Zhang J., Darley-Usmar V. Convergent mechanisms for dysregulation of mitochondrial quality control in metabolic disease: implications for mitochondrial therapeutics. Biochem. Soc. Trans. 2013;41(1):127–133. doi: 10.1042/BST20120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nojiri H., Saita Y., Morikawa D., Kobayashi K., Tsuda C., Miyazaki T. Cytoplasmic superoxide causes bone fragility owing to low-turnover osteoporosis and impaired collagen cross-linking. J. Bone Miner. Res. 2011;26(11):2682–2694. doi: 10.1002/jbmr.489. [DOI] [PubMed] [Google Scholar]
- 9.do Nascimento Cavalcante A., Lima L.K.F., Araujo C.M., da Silva Santos F.P., do Nascimento M.O., de Castro E.S.J.M. Toxicity, cytotoxicity, mutagenicity and in vitro antioxidant models of 2-oleyl-1,3-dipalmitoyl-glycerol isolated from the hexane extract of Platonia insignis MART seeds. Toxicol. Rep. 2020;7:209–216. doi: 10.1016/j.toxrep.2020.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikumawoyi V.O., Awodele O., Agbaje E.O., Alimba C.G., Bakare A.A., Akinloye O. Bioactivity and modulatory functions of Napoleona vogelii on oxidative stress-induced micronuclei and apoptotic biomarkers in mice. Toxicol. Rep. 2019;6:963–974. doi: 10.1016/j.toxrep.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juranek I., Nikitovic D., Kouretas D., Hayes A.W., Tsatsakis A.M. Biological importance of reactive oxygen species in relation to difficulties of treating pathologies involving oxidative stress by exogenous antioxidants. Food Chem. Toxicol. 2013;61:240–247. doi: 10.1016/j.fct.2013.08.074. [DOI] [PubMed] [Google Scholar]
- 12.Hung Y.T., Tikhonova M.A., Ding S.J., Kao P.F., Lan H.H., Liao J.M. Effects of chronic treatment with diosgenin on bone loss in a D-galactose-induced aging rat model. Chin. J. Physiol. 2014;57(3):121–127. doi: 10.4077/CJP.2014.BAC199. [DOI] [PubMed] [Google Scholar]
- 13.Sharifi-Rad M., Anil Kumar N.V., Zucca P., Varoni E.M., Dini L., Panzarini E. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020;11:694. doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long J., Wang X., Gao H., Liu Z., Liu C., Miao M. D-galactose toxicity in mice is associated with mitochondrial dysfunction: protecting effects of mitochondrial nutrient R-alpha-lipoic acid. Biogerontology. 2007;8(3):373–381. doi: 10.1007/s10522-007-9081-y. [DOI] [PubMed] [Google Scholar]
- 15.Kim M.S., Yang Y.M., Son A., Tian Y.S., Lee S.I., Kang S.W. RANKL-mediated reactive oxygen species pathway that induces long lasting Ca2+ oscillations essential for osteoclastogenesis. J. Biol. Chem. 2010;285(10):6913–6921. doi: 10.1074/jbc.M109.051557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W., Cong X.L., Qin Y.H., He Z.W., He D.Y., Dai S.M. IL-18 upregulates the production of key regulators of osteoclastogenesis from fibroblast-like synoviocytes in rheumatoid arthritis. Inflammation. 2013;36(1):103–109. doi: 10.1007/s10753-012-9524-8. [DOI] [PubMed] [Google Scholar]
- 17.Mine Y., Makihira S., Yamaguchi Y., Tanaka H., Nikawa H. Involvement of ERK and p38 MAPK pathways on Interleukin-33-induced RANKL expression in osteoblastic cells. Cell Biol. Int. 2014;38(5):655–662. doi: 10.1002/cbin.10249. [DOI] [PubMed] [Google Scholar]
- 18.Lee K., Seo I., Choi M.H., Jeong D. Roles of mitogen-activated protein kinases in osteoclast biology. Int. J. Mol. Sci. 2018;19(10) doi: 10.3390/ijms19103004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salehi B., Rescigno A. Avocado-soybean unsaponifiables: a panoply of potentialities to be exploited. Biomolecules. 2020;10(1) doi: 10.3390/biom10010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y., Zhang Y., Kou J., Wang C., Wang K. Role of reactive oxygen species in angiotensin II: induced receptor activator of nuclear factor-kappaB ligand expression in mouse osteoblastic cells. Mol. Cell. Biochem. 2014;396(1-2):249–255. doi: 10.1007/s11010-014-2160-x. [DOI] [PubMed] [Google Scholar]
- 21.Leung P., Pickarski M., Zhuo Y., Masarachia P.J., Duong L.T. The effects of the cathepsin K inhibitor odanacatib on osteoclastic bone resorption and vesicular trafficking. Bone. 2011;49(4):623–635. doi: 10.1016/j.bone.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Yin X., Zhou C., Li J., Liu R., Shi B., Yuan Q. Autophagy in bone homeostasis and the onset of osteoporosis. Bone Res. 2019;7:28. doi: 10.1038/s41413-019-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi J., Wang L., Zhang H., Jie Q., Li X., Shi Q. Glucocorticoids: dose-related effects on osteoclast formation and function via reactive oxygen species and autophagy. Bone. 2015;79:222–232. doi: 10.1016/j.bone.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Brenda C.-T., Rivera N., Rojas-Lemus M., López-Valdez N., Fortoul T. Evaluation of the genotoxicity, cytotoxicity and antimalarial effect of sodium metavanadate po in a Plasmodium yoelii yoelii infected murine model. Toxicol. Rep. 2020;7 doi: 10.1016/j.toxrep.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rainsford K.D., Parke A.L., Clifford-Rashotte M., Kean W.F. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23(5):231–269. doi: 10.1007/s10787-015-0239-y. [DOI] [PubMed] [Google Scholar]
- 26.Thome R., Lopes S.C., Costa F.T., Verinaud L. Chloroquine: modes of action of an undervalued drug. Immunol. Lett. 2013;153(1-2):50–57. doi: 10.1016/j.imlet.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Lesiak A., Narbutt J., Kobos J., Kordek R., Sysa-Jedrzejowska A., Norval M. Systematic administration of chloroquine in discoid lupus erythematosus reduces skin lesions via inhibition of angiogenesis. Clin. Exp. Dermatol. 2009;34(5):570–575. doi: 10.1111/j.1365-2230.2008.03006.x. [DOI] [PubMed] [Google Scholar]
- 28.Yan Y., Zou Z., Sun Y., Li X., Xu K.F., Wei Y. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;23(2):300–302. doi: 10.1038/cr.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mauthe M., Orhon I., Rocchi C., Zhou X., Luhr M., Hijlkema K.J. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 2018;14(8):1435–1455. doi: 10.1080/15548627.2018.1474314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber S.M., Chen J.M., Levitz S.M. Inhibition of mitogen-activated protein kinase signaling by chloroquine. J. Immunol. 2002;168(10):5303–5309. doi: 10.4049/jimmunol.168.10.5303. [DOI] [PubMed] [Google Scholar]
- 31.Seif A., Fawzy N., Abdelrahman S., Emara M., Zaki H., Khattab M. Stimulation of ACE2/ANG(1–7)/Mas Axis by diminazene ameliorates alzheimer’s disease in the D-Galactose-Ovariectomized rat model: role of PI3K/Akt pathway. Mol. Neurobiol. 2018;55 doi: 10.1007/s12035-018-0966-3. [DOI] [PubMed] [Google Scholar]
- 32.Adeeko A.O., Dada O.A. Chloroquine reduces fertilizing capacity of epididyma sperm in rats. Afr. J. Med. Med. Sci. 1998;27(1-2):63–64. [PubMed] [Google Scholar]
- 33.Somsak V., Damkaew A., Onrak P. Antimalarial activity of Kaempferol and its combination with chloroquine in Plasmodium berghei infection in mice. J. Pathog. 2018 doi: 10.1155/2018/3912090. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gindler E.M., King J.D. Rapid colorimetric determination of calcium in biologic fluids with methylthymol blue. Am. J. Clin. Pathol. 1972;58(4):376–382. doi: 10.1093/ajcp/58.5.376. [DOI] [PubMed] [Google Scholar]
- 35.El-Merzabani M.M., Anwer-El-Aaser A., Zakhary N.I. A new method for determination of inorganic phosphorus in serum without deproteinization. J. Clin. Chem. Clin. Biochem. 1977;15(12):715–718. doi: 10.1515/cclm.1977.15.1-12.715. [DOI] [PubMed] [Google Scholar]
- 36.Aebi H. Catalase in vitro. Meth. Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 37.Rao N.A., Nishikimi M., Yagi K. Reactivity of D-amino acid oxidase with artificial electron acceptors. Biochimica et biophysica acta. 1972;276(2):350–362. doi: 10.1016/0005-2744(72)90995-3. [DOI] [PubMed] [Google Scholar]
- 38.Aswar U.M., Mohan V., Bodhankar S.L. Antiosteoporotic activity of phytoestrogen-rich fraction separated from ethanol extract of aerial parts of Cissus quadrangularis in ovariectomized rats. Indian J. Pharmacol. 2012;44(3):345–350. doi: 10.4103/0253-7613.96310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadzi-Petrushev N., Stojkovski V., Mitrov D., Mladenov M. D-galactose induced changes in enzymatic antioxidant status in rats of different ages. Physiol. Res. 2015;64(1):61–70. doi: 10.33549/physiolres.932786. [DOI] [PubMed] [Google Scholar]
- 40.Jeong H., Liu Y., Kim H.S. Dried plum and chokeberry ameliorate d-galactose-induced aging in mice by regulation of Pl3k/Akt-mediated Nrf2 and Nf-kB pathways. Exp. Gerontol. 2017;95:16–25. doi: 10.1016/j.exger.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Djordjevic V.B. Free radicals in cell biology. Int. Rev. Cytol. 2004;237:57–89. doi: 10.1016/S0074-7696(04)37002-6. [DOI] [PubMed] [Google Scholar]
- 42.El-Baz F.K., Saleh D.O., Abdel Jaleel G.A., Hussein R.A., Hassan A. Heamatococcus pluvialis ameliorates bone loss in experimentally-induced osteoporosis in rats via the regulation of OPG/RANKL pathway. Biomed. Pharmacother. 2019;116 doi: 10.1016/j.biopha.2019.109017. [DOI] [PubMed] [Google Scholar]
- 43.Ma Y.L., Cain R.L., Halladay D.L., Yang X., Zeng Q., Miles R.R. Catabolic effects of continuous human PTH (1--38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology. 2001;142(9):4047–4054. doi: 10.1210/endo.142.9.8356. [DOI] [PubMed] [Google Scholar]
- 44.Danilovich N., Babu P.S., Xing W., Gerdes M., Krishnamurthy H., Sairam M.R. Estrogen deficiency, obesity, and skeletal abnormalities in follicle-stimulating hormone receptor knockout (FORKO) female mice. Endocrinology. 2000;141(11):4295–4308. doi: 10.1210/endo.141.11.7765. [DOI] [PubMed] [Google Scholar]
- 45.Heaney R.P. Toward a physiological referent for the vitamin D requirement. J. Endocrinol. Invest. 2014;37(11):1127–1130. doi: 10.1007/s40618-014-0190-6. [DOI] [PubMed] [Google Scholar]
- 46.Ivaska K.K., Hentunen T.A., Vaaraniemi J., Ylipahkala H., Pettersson K., Vaananen H.K. Release of intact and fragmented osteocalcin molecules from bone matrix during bone resorption in vitro. J. Biol. Chem. 2004;279(18):18361–18369. doi: 10.1074/jbc.M314324200. [DOI] [PubMed] [Google Scholar]
- 47.Boulbaroud S., Mesfioui A., Arfaoui A., Ouichou A., el-Hessni A. Preventive effects of flaxseed and sesame oil on bone loss in ovariectomized rats. Pak. J. Biol. Sci.: PJBS. 2008;11(13):1696–1701. doi: 10.3923/pjbs.2008.1696.1701. [DOI] [PubMed] [Google Scholar]
- 48.Zoch M.L., Clemens T.L., Riddle R.C. New insights into the biology of osteocalcin. Bone. 2016;82:42–49. doi: 10.1016/j.bone.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiu Y., Xu H., Zhao C., Li J., Morita Y., Yao Z. Chloroquine reduces osteoclastogenesis in murine osteoporosis by preventing TRAF3 degradation. J. Clin. Invest. 2014;124(1):297–310. doi: 10.1172/JCI66947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muthusami S., Ramachandran I., Muthusamy B., Vasudevan G., Prabhu V., Subramaniam V. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin. Chim. Acta. 2005;360(1-2):81–86. doi: 10.1016/j.cccn.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 51.Iyawe H., A.O O, O.O A Effect of chloroquine and ascorbic acid interaction on the oxidative stress status of Plasmodium berghei infested mice. Int. J. Pharmacol. 2006;2 [Google Scholar]
- 52.Klionsky D.J., Abdalla F.C., Abeliovich H., Abraham R.T., Acevedo-Arozena A., Adeli K. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8(4):445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Antika L., Kim Y.-H., Kang M.-K., Park S.-H., Lee E.-J., Choi Y.-J. Dietary compound gossypetin inhibits bone resorption through down-regulating lysosomal cathepsin K activity and autophagy-related protein induction in actin ring-bearing osteoclasts. J. Funct. Foods. 2016;24:390–402. [Google Scholar]
- 54.Lotinun S., Kiviranta R., Matsubara T., Alzate J.A., Neff L., Luth A. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J. Clin. Invest. 2013;123(2):666–681. doi: 10.1172/JCI64840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rieman D.J., McClung H.A., Dodds R.A., Hwang S.M., Holmes M.W., James I.E. Biosynthesis and processing of cathepsin K in cultured human osteoclasts. Bone. 2001;28(3):282–289. doi: 10.1016/s8756-3282(00)00445-2. [DOI] [PubMed] [Google Scholar]
- 56.Bhatt N.Y., Kelley T.W., Khramtsov V.V., Wang Y., Lam G.K., Clanton T.L. Macrophage-colony-stimulating factor-induced activation of extracellular-regulated kinase involves phosphatidylinositol 3-kinase and reactive oxygen species in human monocytes. J. Immunol. 2002;169(11):6427–6434. doi: 10.4049/jimmunol.169.11.6427. [DOI] [PubMed] [Google Scholar]
- 57.Agidigbi T.S., Kim C. Reactive oxygen species in osteoclast differentiation and possible pharmaceutical targets of ROS-Mediated osteoclast diseases. Int. J. Mol. Sci. 2019;20(14) doi: 10.3390/ijms20143576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lerner U.H. New molecules in the tumor necrosis factor ligand and receptor superfamilies with importance for physiological and pathological bone resorption. Crit. Rev. Oral Biol. Med. 2004;15(2):64–81. doi: 10.1177/154411130401500202. [DOI] [PubMed] [Google Scholar]
- 59.Liu S., Zhu L., Zhang J., Yu J., Cheng X., Peng B. Anti-osteoclastogenic activity of isoliquiritigenin via inhibition of NF-kappaB-dependent autophagic pathway. Biochem. Pharmacol. 2016;106:82–93. doi: 10.1016/j.bcp.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Redmann M., Benavides G.A., Berryhill T.F., Wani W.Y., Ouyang X., Johnson M.S. Inhibition of autophagy with bafilomycin and chloroquine decreases mitochondrial quality and bioenergetic function in primary neurons. Redox Biol. 2017;11:73–81. doi: 10.1016/j.redox.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skoumal M., Haberhauer G., Kolarz G., Hawa G., Woloszczuk W., Klingler A. Serum cathepsin K levels of patients with longstanding rheumatoid arthritis: correlation with radiological destruction. Arthritis Res. Ther. 2005;7(1):R65–70. doi: 10.1186/ar1461. [DOI] [PMC free article] [PubMed] [Google Scholar]