Abstract
In recent decades, nano-scale zero valent iron is reported to have plant growth enhancement capacity under laboratory conditions, but till date, there is no report to highlight its effect on the growth and yield of field-grown plants. In this study, we have evaluated the potential of nZVI priming on rice yield. A two-year field study has been conducted with different concentrations (10, 20, 40, and 80 mg l−1) of nZVI for seed priming. The efficacy of nanopriming was compared with the hydroprimed control set. Seeds were treated for 72 h and sown in nursery beds and after 30 days seedlings were transplanted in the field. Root anatomy and morphology were studied in 7 days old seedlings where no changes were found. RAPD analysis also confirmed that low doses of nZVI were not genotoxic. Nanoprimed plants also had broader leaves, higher growth, biomass, and tiller number than control plants. Maximum yield was obtained from the 20 mg l−1 nZVI primed set (3.8 fold higher than untreated control) which is achieved primarily because of the increase in fertile tiller numbers (two fold higher than untreated control). Higher values of other agronomic parameters like growth rate, net assimilation rate proved that nZVI priming enhanced photosynthetic efficiency and helped in the proper storage of photo-assimilates. All these attributed to increased accumulation of phytochemicals like starch, soluble sugar, protein, lipid, phenol, riboflavin, thiamine, and ascorbic acid in the grains. The elemental analysis confirmed that nZVI priming also promoted higher accumulations of macro and micronutrients in grains. Thus, nanoprimed seeds showed better crop performance compared to the traditional hydropimed seeds. Hence, nZVI can be considered as ‘pro-fertilizer’ and can be used commercially as a seed treatment agent which is capable of boosting plant growth and yield along with minimum interference to the soil ecosystem.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00344-021-10335-0.
Keywords: Nano-scale zero valent iron, Rice, Seed priming, Agronomic traits, Yield, Grain nutrient
Introduction
According to UN (2013), the world population is projected to reach 9.6 billion by the year 2050. Hence, to meet food demands for the rapidly increasing world population, global grain production has to be increased by 70% by 2050 (FAO 2013). The recent global pandemic of novel coronavirus disease (COVID-19) may slow down food production as extensive lockdowns are making it harder for the farmers to obtain fertilizers, seeds, and also restricts the movement of seasonal field laborers (Poudel et al. 2020). Hence, we must embrace future agro-productive tools which can be implemented with minimal resources. The use of nanotechnology for increasing crop productivity can be a viable strategy for farmers.
Nanotechnology can be utilized to treat seeds as it can ensure rapid seed germination and enhancement of seedling vigor which are primarily essential for efficient crop production. According to Mahakham et al. (2017), seed quality declines gradually due to storage for a prolonged period which leads to the cumulative increase of ROS. Thus, the cells undergo a decline in their antioxidant potential, which mediates various biochemical changes in seeds, resulting in loss of seedling vigor. For rice cultivation, uniform seedling establishment is necessary before transplantation. According to Reddy (2004), although 30 days old seedlings are considered to be ideal for transplantation, farmers are often forced to transplant older seedlings that are 40–45 days old due to delayed germination, seedling emergence, slow growth rate of seedlings, and unfavorable climatic condition (mainly lack of precipitation). Transplantation of older seedlings reduces tillering capacity which is a major yield-limiting factor in the traditional rice cultivation system. Since improvement in seed quality can be achieved by seed treatment strategies, several researchers are working on various seed treatment protocols aiming to enhance plant vigor.
Seed priming is a pre-sowing seed treatment process that involves a regulated seed hydration step before radical emergence and this technique can induce rapid seed germination, stress tolerance, and synchronized seedling establishment (Bewley et al. 2013; Paparella et al 2015). Among different seed priming methods, nutri-priming with inorganic fertilizers is considered to be quite advantageous in stimulating plant growth (Farooq et al. 2007; Rehman et al. 2011; Mirshekari, 2015). However, inorganic fertilizers (such as, Fe and Cu sulphate fertilizers)often contain a very high amount of heavy metals (like Cd, Cr, Pb, and Ni) which can be severely toxic for the environment and human health, and repeated use of these inorganic salts at such early stage of seedling development must be avoided (Gimeno-Gracia et al. 1996; Otero et al. 2005). Thus, in the context of sustainable agriculture and food security, the application of nanoparticles (NPs) can be an alternative approach for nutrient delivery in plants.
Recently, several studies have been conducted on nanopriming which provided positive results. Macronutrient NP, like Mg(OH)2 is reported to be capable of promoting seed germination and enhance growth and photosynthetic efficiency of Zea mays seedlings (Shinde et al. 2020), priming with MgO NPs also stimulated seed germination and seedling vigor of Vigna radiata (Anand et al. 2020). Ca NP can also act as growth-promoting agents for plants (Yugandhar and Savithramma 2013). Existing literature is replete with several studies on nanopriming of seeds with micro-nutrient NPs like Zn, Cu, and Fe. Nadeem et al. (2019) and Itroutwar et al. (2019), conducted field studies with ZnO NP primed wheat and rice seeds respectively. According to their reports, Zn nutrition not only improved crop productivity but also enhanced grain nutrient qualities and aided in grain biofortification.
It can be said that the application of iron NPs can address the concerns regarding limited iron supply to plants. Foliar application of nano Fe (concentration 0.5 g l−1) in black-eyed pea plants has increased seedling vigor and plant Fe content as well (Delfani et al., 2014). According to Rui et al. (2016), Fe3O4 NPs can be utilized as an effective iron source for Arachis hypogaea as, Fe3O4 NP could boost plant growth, photosynthetic efficiency, antioxidant defense mechanism, and plant Fe levels. Das et al. (2018b), has made an interesting report regarding the effects of nano-pyrite rice seed treatment and they confirmed that this nanopriming technique can be an efficient alternative to the traditional NPK fertilization technique for rice cultivation. Recently there are few reports on the growth-promoting roles of nanoscale zero-valent iron. Li et al. (2015), first reported that very low concentrations of nZVI (40–80 μmol l−1) can promote peanut seed germination rate and seedling growth. Yoon et al. (2019), reported that treatment with 500 mg nZVI kg−1 soil, can significantly enhance biomass and photosynthetic efficiency in Arabidopsis thaliana. They also found nZVI not only increased plant Fe content but other macro-and micronutrients such as Mg, P, Mn, and Zn levels were increased significantly. However, till date, there is no report on field study which explores the yield enhancement potential of nZVI.
It can be said that the application of iron NPs can effectively address the global concern regarding iron deficiency which is scarcely available to the plants in aerobic and neutral pH environments despite being the fourth most abundant element in the lithosphere. Since iron plays an active role in plant antioxidant defense system, electron respiration, and photosynthesis, and embryogenesis, iron deficiency can hamper plant growth and productivity (Roschzttardtz et al. 2013; Grillet et al. 2013). Iron deficiency may further give rise to hidden hunger and other ailments like anemia. Researchers are already devoted to developing several biofortification techniques through conventional breeding or transgenic approaches to increase iron content in food grains. However, agronomic biofortification techniques that involve nutrient delivery through seed priming, soil, or foliar fertilizers are safer, cost-effective, and also user-friendly efficient short-term approaches to grain nutrient enhancement. Previously, we have found that seed priming with nZVI can improve seed germination, early seedling growth, water relations, photosynthetic efficiency, root metabolism, and antioxidant defense system in Oryza sativa L. cv. Gobindobhog seedlings under lab conditions in a hydroponic system (Guha et al. 2018). In addition to this, iron content was also increased in the nZVI treated seedlings; hence nZVI can be an alternative solution for providing Fe to plants.
In the present study, the yield performance of Oryza sativa L. cv. Gobindobhog seeds after priming with nZVI have been studied in detail. Since most of the researches regarding the effects of nZVI on plants are confined to short-term in-vitro studies in hydroponics or pot culture, field studies are necessary to evaluate the long-term effects of nZVI. Our study is the first report dealing with the application of nZVI in field conditions and its potential in increasing crop productivity. Initially, after priming the seeds with different concentrations of nZVI (0, 10, 20, 40, and 80 mg l−1), they were transferred to the nursery bed, and after 30 days seedlings were transplanted in the field. Agronomic traits have been evaluated at different growth stages to evaluate crop performance and lastly, kernel quality was checked by estimation of phytochemicals and mineral nutrient contents. Our results indicated that nZVI priming treatment can significantly increase rice productivity and enhance grain nutrient qualities as well. All these highlight the possibility of the application of nZVI as nano fertilizer for increasing crop productivity.
Materials and Methods
nZVI Synthesis and Characterization
nZVI particles were synthesized by sodium borohydride method using ferrous sulphate (FeSO4·7H2O) and EDTA (C10H14N2Na2O8·2H2O). The synthesis procedure was followed from a previous report (Ravikumar et al. 2016). nZVI was characterized using XRD (Bruker Advanced D8, Germany), UV–Visible Spectroscopy (Hitachi, U2910, Japan), Particle size analysis (NanoBrook 90Plus PALS Particle Size Analyzer, Brookhaven Instruments Corporation, USA), and TEM (Philips CM12, Netherlands). The characterization data are published in our previous report (Guha et al. 2018).
Site Description
Two-year field study was carried out in Agricultural Experimental Farm (88°26.164′E and 22°22.526′N) (Fig. S1), University of Calcutta, Baruipur, South 24 Parganas in Gangetic alluvial region of West Bengal, India during the Kharif season in the year 2018 and 2019 (June to October). Field soil had following properties: pH: 6.75, soil electrical conductivity: 0.011 dS m–1, organic carbon content: 7.4 g kg−1, Total Nitrogen: 0.73 g kg−1, Available Phosphorus: 23.35 kg ha−1, Available Potassium: 150 kg ha−1 (Mukherjee et al. 2019). Meteorological data during the experimental period is given in Table 1.
Table 1.
Weather data at the experimental station during course of experimentation
| Months | Rainfall (mm) | Relative humidity (%) | Temperature (°C) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Daily minimum | Daily Maximum | Daily mean | ||||||||
| 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | |
| June | 45.68 | 101.21 | 59 | 61 | 30 | 30 | 37 | 38 | 33 | 35 |
| July | 121.86 | 153.3 | 72 | 66 | 28 | 29 | 34 | 35 | 31 | 33 |
| August | 147.89 | 294.9 | 76 | 75 | 27 | 28 | 33 | 33 | 30 | 31 |
| September | 129.04 | 218.8 | 74 | 78 | 27 | 26 | 33 | 31 | 29 | 29 |
| October | 105.1 | 194.6 | 59 | 72 | 26 | 25 | 33 | 31 | 30 | 29 |
Source India Meteorological Department, Ministry of Earth Sciences, Government of India. (AWS ARG Networks)
Plant Material and Growth Conditions
Oryza sativa L. cv. Gobindobhog seeds were obtained from Rice Research Station, Chinsurah (Govt. of WB). For priming four concentrations (10, 20, 40, and 80 mg l−1) of nZVI were freshly prepared by dispersing the particles in deionized water using ultrasonic vibration (100 W, 40 kHz) for 30 min Mahakham et al. (2017). The control set seeds were hydroprimed using deionized water. Seeds were placed in Petri plates with moist filter papers containing 10 ml of priming solution (0, 10, 20, 40,80 mg l−1 nZVI) for 3 days in the dark, after surface sterilization with 0.2% Dithane M-45 (anti-fungal agent, Dow AgroSciences). The seeds were then transferred 7 days after germination in separate seedbeds on 20 June 2018 and 18 June 2019. 30 days old seedlings were transplanted from the nursery seedbeds to the main field. The experimental design was a randomized complete block design (RCBD) in three replications with a plot size of 1 square meter, row to row distance of 20 cm, and plant to plant spacing of 20 cm. The plants were grown in rain-fed irrigation conditions till maturity without any fertilizers and pesticides. Grains were harvested on 30th October 2018 and 31st October 2019.
Nursery Seedling Characteristics
After germination of the nanoprimed seeds, the mean emergence time (MET) and final emergence percentage (FEP) were calculated according to Ellis and Roberts (1981). The seedlings were tested for growth analysis 30 days after sowing (DAS). Shoot length (SL) and root length (RL) were recorded from 5 randomly selected seedlings.
Localization of Iron in nZVI Primed Seeds
Perl’s Prussian blue (PPB) staining technique was performed according to Wang and Cuschieri (2013) to investigate the localization of iron in rice seeds. Longitudinal sections of the seeds were incubated for 30 min in a solution containing 2.5% HCl (v/v) and 2.5% potassium ferrocyanide (w/v). The sections were examined with a simple light microscope after repeated washing.
Study of Anatomical Changes of Seedling Root
Embryonic root tips of the 7 days old seedlings were cut into 1 mm pieces from the tip and transverse sections of the root were made, followed by fixing the samples in 2% glutaraldehyde in 0.05 M phosphate buffer (pH 7.2) for 48 h at room temperature. After fixation, samples were dehydrated in ethanol gradient (30, 50, 70, 80, 90, and 100%), sputter-coated with platinum, and observed by SEM (ZEISS EVO-MA 10; Carl Zeiss Pvt. Ltd., Oberkochen). EDX was performed simultaneously for the detection of atomic Fe on the root surface.
DNA Extraction and RAPD Analysis
200 mg of embryonic root tissue was collected from 7 days old seedlings primed with 0, 20 and 80 mg l−1 nZVIfor genomic DNA extraction according to CTAB (cetyltrimethylammonium bromide)method (Doyle and Doyle 1987). The dose with the best yield and the highest treatment dose were elected for this assay. Quantity and purity of the extracted DNA were determined from Eppendorf BioSpectrometer® Basic with μCUVETTE® and DNA integrity was checked by gel electrophoresis on 0.8% Agarose gel (Fig. S2). RAPD analysis of isolated DNA was performed using 10 random primers among which 6 produced reproducible bands (OPA-2—5′-TGCCGAGCTG-3′, OPA-3—5′-AGTCAGCCAC-3′, OPA-4—5′-AATCGGGCTG-3′, OPA-5—5′-AGGGGTCTTG-3′, OPA-9—5′-GGGTAACGCC-3′, OPA-10—5′-GTGATCGCAG-3′). The total reaction mixture was 20 μL in volume having 50 ng DNA, 1× reaction buffer, 2.5 mM MgCl2, 100pmoles of decamer primer, 0.2 mM dNTPs, and 1 U Taq DNA polymerase (Promega, USA). After amplification by PCR, the RAPD products were analyzed electrophoretically in a 1.8% agarose gel, and for observing and photographing the gel, ultraviolet (UV) trans-illuminator, and gel documentation system was used.
The amplified bands were primarily scored based on the presence or absence of the band (binary scoring, 0 and 1) against the negative (untreated) control.
Genomic template stability (%) among nZVI primed seedlings was calculated from the formula used by Atienzar and Jha (2006):
where a is the average number of the polymorphic band (new bands appeared or disappeared compared to their non-treated control) and n is the total band number noted in the control.
Study of Agronomic and Yield Parameters
After maturity, rice plants from each treatment plot were manually harvested and observations regarding agronomic traits and yield components were recorded following the standard procedures. Leaf area was calculated using the length width method according to the formula: Leaf area = 0.75 × Leaf length × Leaf width proposed by Palaniswamy and Gomez (1974). Agronomic parameters like absolute growth rate (AGR), crop growth rate (CGR), and net assimilation rate (NAR) were calculated for three different harvest points (30, 60, and 130 DAS) according to Ghule et al. (2013) and Hunt (1979).
Plants were sun-dried to determine grain yield. Total panicles per plant and panicle length were recorded for each treatment. Filled and unfilled grains were separated and counted from each panicle to obtain grain filling percentage. 100-grain weight was recorded by weighing randomly sampled 100 filled grains.
Quantitative Analysis of Grain Phytochemicals
After maturity harvested rice grains from each treatment plot were collected for quantification of phytochemicals produced as a result of primary and secondary metabolism.
Assay of Soluble Sugar and Starch
1 g of powdered rice grain sample was at first boiled in 80% aqueous methanol for 15 min in water-bath. Alcoholic extract was taken for colorimetric assay of total sugar estimation with the phenol–sulphuric acid reagent. The residue obtained after extraction was dried and boiled with 3% HCl for 3 h in the water bath for acid hydrolysis of starch. The glucose released from starch hydrolysis was estimated again by phenol–sulphuric acid reagent and the value of glucose was converted to starch by multiplying with a factor of 0.9 (Das et al. 2018a).
Protein Content Determination
The total protein content from 1 g powdered grain was determined by using Bradford’s method. 100 µl of the sample extract was estimated according to Lowry et al 1951.
Total Lipid Estimation
Lipid estimation of rice grains was done by chloroform–methanol extraction procedure according to Bhattacharyya and Roy (2018).
Phenol Estimation
Phenol extraction was done by blending 1 g of powdered rice samples with 10 ml 80% ethanol. After extraction 200 µl of the sample extract, was mixed with 800 µl FolinCiocalteu reagent and 2 ml of 7.5% sodium carbonate added. After dark incubation for 30 min, absorbance was taken at 765 nm (Su et al. 2007).
Ascorbic Acid Estimation
0.1 g rice grain sample was homogenized with 1.5 ml 10% TCA at 4 °C and centrifuged at 3000×g for 5 min. The final assay mixture contained 0.5 ml supernatant, 0.5 ml DNPH reagent (2% DNPH, and 4% thiourea in 9 M sulphuric acid). After 3 h of incubation at room temperature, 2.5 ml of 85% H2SO4 was added and absorbance was recorded at 530 nm after 30 min(Okolie et al. 2014).
Riboflavin Content
For riboflavin estimation, 1g powdered rice grain was extracted with 50% ethanol and after filtration 2.5 ml 5% KMnO4 was added followed by the addition of 2.5 ml 30% H2O2 solution. The extract is then heated at 80 °C on a water-bath for 30 min. Riboflavin was quantified after adding 1 ml 40% Na2SO4 absorbance was recorded at 510 nm according to Nandagoapalan et al. (2016).
Thiamine Content
Thiamine extraction was done from 1 g of powered grain sample by mixing with 10 ml 20% ethanolic NaOH. After filtration of the extract 1 ml filtrate was mixed with 1 ml 2%K2Cr2O7 solution and absorbance was recorded at 360 nm (Bhattacharyya and Roy 2018).
Estimation of Macro and Micro-nutrient Contents by ICP-AES
After harvest, rice root, shoot and grains were dried in an oven at 80 °C for 48 h. The oven-dried plant parts of each were then ground into powder. About 0.1 g of the ground samples were weighed and digested with a tri-acid mixture of HNO3:HCl:HClO4 (4:2:1 v/v), and the resultant solutions were diluted to 25 ml with deionized water. The concentration of macronutrients (Ca, Mg, P, K, and S) and micro-nutrients (Fe, Cu, Mn, Zn) and cadmium were determined by using Inductively Coupled Plasma-Optical Emission Spectroscopy (ARCOS, Spectro, Germany).
Partition Quotient of Nutrients
Partitioning of minerals within rice plants was also checked. Changes of mineral content in each tissue were normalized to changes in each tissue’s weight, relative to the whole plant dry weight (DW). The DW of each organ was calculated as a percentage of total plant DW and the mineral content of each organ was calculated as a percentage of total plant mineral content. Finally, normalized partitioning of each mineral within the plant or the partition quotient (PQ) was calculated by dividing each organ’s percentage mineral content by its percentage DW and multiplying by 100 (Waters and Grusak 2008).
Statistical Analysis
Statistical analysis was conducted using IBM SPSS Statistics Version. 23. Significant differences between different nZVI treatments were determined using One-way ANOVA followed by the Tukey test. A significant difference in plant responses based on the year of study was determined by t test. The probability of p ≤ 0.05 was considered to be statistically significant. Pearson’s correlation analyses were carried out using two significance levels (p ≤ 0.05 and 0.01).
Results
Rainfall Variation During Growth Stages
Rainfall and temperature ranges during the growing seasons are presented in Table 1. The average temperature range during the growth session of 2018 and 2019 varied around 31 ± 2 °C. However, rainfall distribution differed significantly in the 2 years. During the vegetative growth phase, average rainfall was 134 mm and 224 mm, precipitation level during booting was 147 mm and 294 nm, and before maturity average rainfall was 105 nm and 194 nm respectively in the year 2018 and 2019. The average sunshine level during the heading and maturity stage was also significantly lower in 2019 compared to that of 2018.
Nursery Seedling Characteristics
Priming with nZVI showed significant effects on MET, FEP, and seedling growth (Table 2). Seed priming with different concentrations of nZVI (10–80 mg l−1) could significantly reduce the MET of seeds. Maximum reduction of MET was observed upon seed priming treatment with 20 mg l−1 nZVI solution while FEP increased significantly by 1.3 fold over the control set. At the same time, seedling growth also increased upon nanopriming. Compared to the control hydroprimed sets, shoot length increased by 1.17, 1.3, 1.23, and 1.12 fold, and root length increased by 1.12, 1.27, and 1.12 fold in plants treated with 10, 20, 40, and 80 mg l−1 nZVI, respectively. However, the root length of seeds primed with 80 mg l−1 nZVI was similar to that of the hydroprimed control set (Table 2).
Table 2.
Effect of seed priming on the nursery seedlings, flowering and maturity time
| Nzvi (mg l−1) | Mean emergence time (days) | Final emergence % | Seedling root length (cm) | Seedling shoot length (cm) | Flowering (DAS) | Maturity (DAS) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | |
| 0 | 6.4 ± 0.3a | 6.2 ± 0.3a | 63.8 ± 4a | 64.1 ± 4.1a | 8.1 ± 0.2a | 7.8 ± 0.3a | 34.2 ± 0.6a | 33.9 ± 0.6a | 94.4 ± 1.1a | 95.6 ± 1.5a | 130.8 ± 0.8a* | 133 ± 0.8a* |
| 10 | 4.2 ± 0.2b | 4.03 ± 0.2b | 76.3 ± 1.7b | 76.8 ± 1.5b | 9.1 ± 0.2b* | 8.6 ± 0.3b* | 40.1 ± 0.8b | 39.6 ± 0.9b | 90 ± 1.6b | 92.5 ± 2.4a | 124.8 ± 0.8* | 129.8 ± 3.4* |
| 20 | 3.3 ± 0.3c | 3.1 ± 0.3c | 85.6 ± 2.1c | 85.9 ± 2.02c | 10.34 ± 0.3c | 10.02 ± 0.4c | 44.7 ± 0.9c | 44.4 ± 1.01c | 82.8 ± 1.3c | 84.4 ± 1.29b | 120 ± 1.6c* | 123 ± 2.3b* |
| 40 | 4.3 ± 0.2b | 4.04 ± 0.3b | 81.24 ± 1.7c | 81.6 ± 1.7c | 9.1 ± 0.2b* | 8.7 ± 0.3b* | 42.3 ± 0.7d | 41.8 ± 0.8d | 85 ± 1.2c | 87.2 ± 1.9b | 122.6 ± 1.1d* | 127 ± 2bc* |
| 80 | 5.38 ± 0.3d | 5.17 ± 0.3d | 73.12 ± 2b | 73.5 ± 2.1b | 8.1 ± 0.2a* | 7.7 ± 0.3a* | 38.5 ± 0.8e | 38.1 ± 0.9e | 90.8 ± 1.3b | 92.9 ± 2.4a | 127.4 ± 0.9e* | 130.2 ± 1ac* |
Data represents means of 5 replicas and means sharing the same letters in a column do not differ significantly at p ≤ 0.5 according to Tukey’s HSD multiple comparison test. Significant differences between session 2018 and 2019 plants are indicated by symbol ‘*’ as obtained from unpaired t test analysis
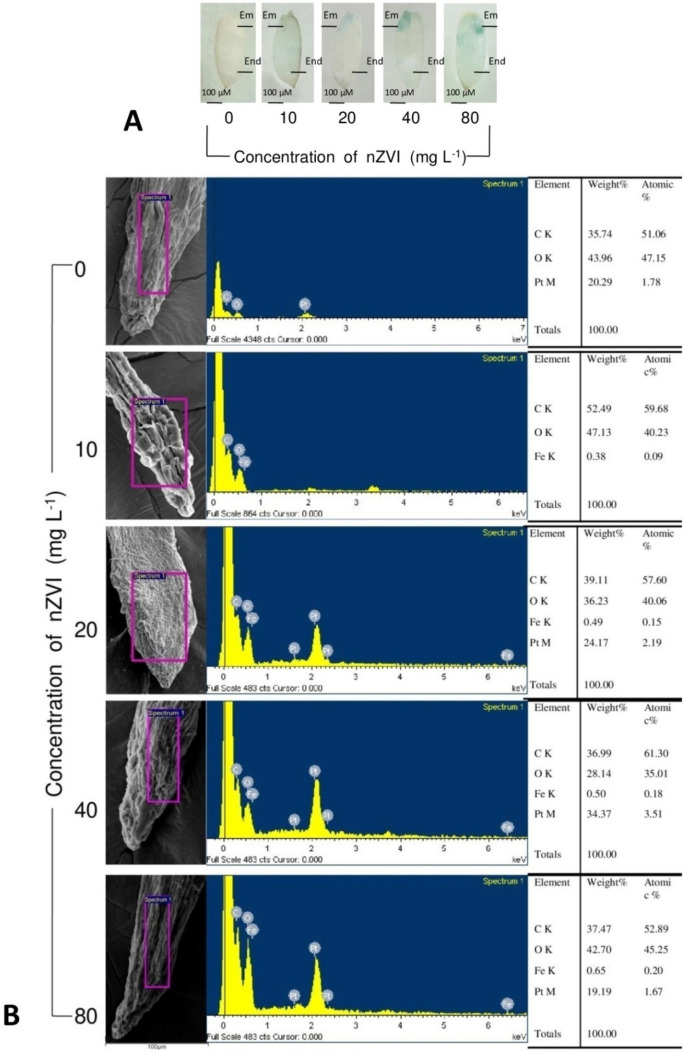
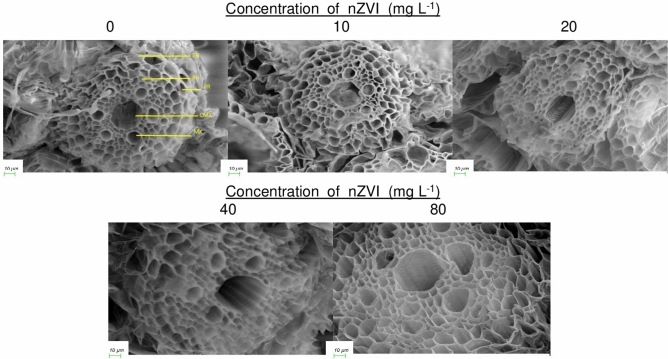
Longitudinal sections of rice seeds primed with nZVI for 72 h, showed bright blue color mainly near the embryonic region of the seeds, confirmed presence of iron, and the intensity of blue color increased in a dose-dependent manner (Fig. 1a). Scanning electron micrographs revealed the cellular details of root vascular bundles of nZVIprimed and unprimed rice seedlings. The cells were more or less intact with regular shape and size. In the nZVI primed seedlings, there were no morphological alterations near the root tip region. EDX analysis confirmed the depositions of atomic iron on the root surface. The amount of internalized iron was proportional to the concentration of nZVI and control seedlings had shown no iron depositions (Fig. 1b). The transverse section of the root also revealed no damage in internal root tissues (Fig. 2).
Fig. 1.
a Localization of iron in the embryonic region of nZVI primed seeds after Perl’s Prussian blue staining. (EM embryo, End endosperm). b EDX data of root tip surface of 7 days old seedlings treated with 0–80 mg l−1nZVI
Fig. 2.
Scanning electron micrograph of 7 days old rice seedlings A, B, C, D, and E represents control, 10, 20, 40, and 80 mg l−1nZVI primed seedling root vascular tissue after transverse sections (EN endodermis, PR pericycle, PH phloem, MX metaxylem, CMX central metaxylem)
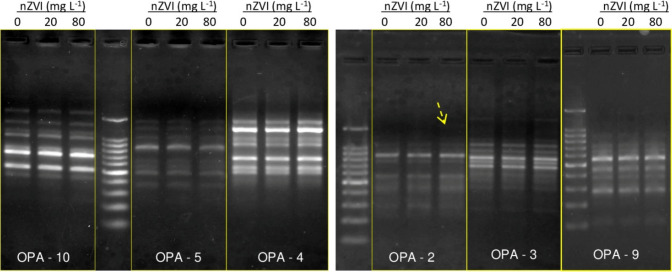
RAPD was performed to evaluate genotoxic impacts of nZVI in terms of induction of DNA damage in the embryonic root tissue of nZVI primed seedlings with respect to untreated seedlings. Data obtained from RAPD profiles showed that there was no significant difference in banding patterns in seedlings treated with 20 mg l−1 nZVI. Compared to 57 amplified bands, only 1 new band appeared with the use of the primer OPA-2 in the 80 mg l−1 nZVI primed seedlings and GTS% decreased to 98.25% (Fig. 3).
Fig. 3.
Influence of nZVI treatment on RAPD profiles of 7 days old rice root tissue (► indicates the disappearance of the band)
Agronomic Traits and Growth Analysis
Nanopriming induced significant changes in agronomic and yield-related parameters (Fig. 4a and b). Firstly, the duration of flowering and maturity was significantly less compared to that of the untreated control. Minimum days for flowering and maturity were recorded in seedlings primed with 20 mg l−1 nZVI (Table 2).
Fig. 4.
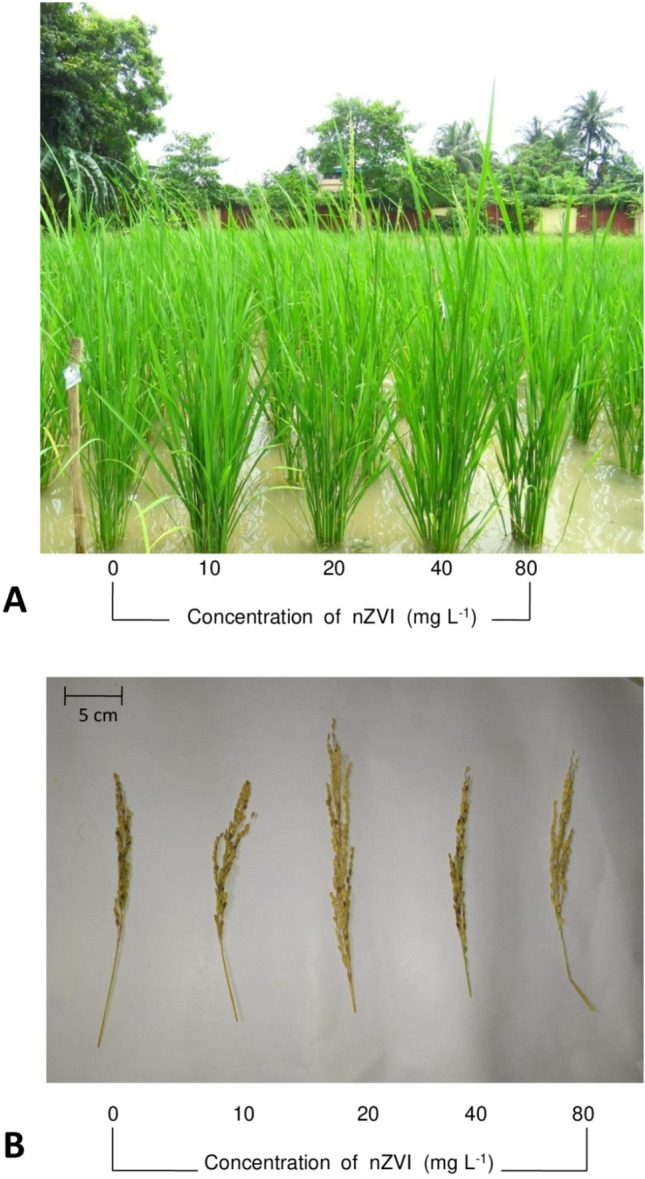
Photographic images of a 60 days old rice seedlings and b panicles of 0–80 mg l−1 nZVI primed sets
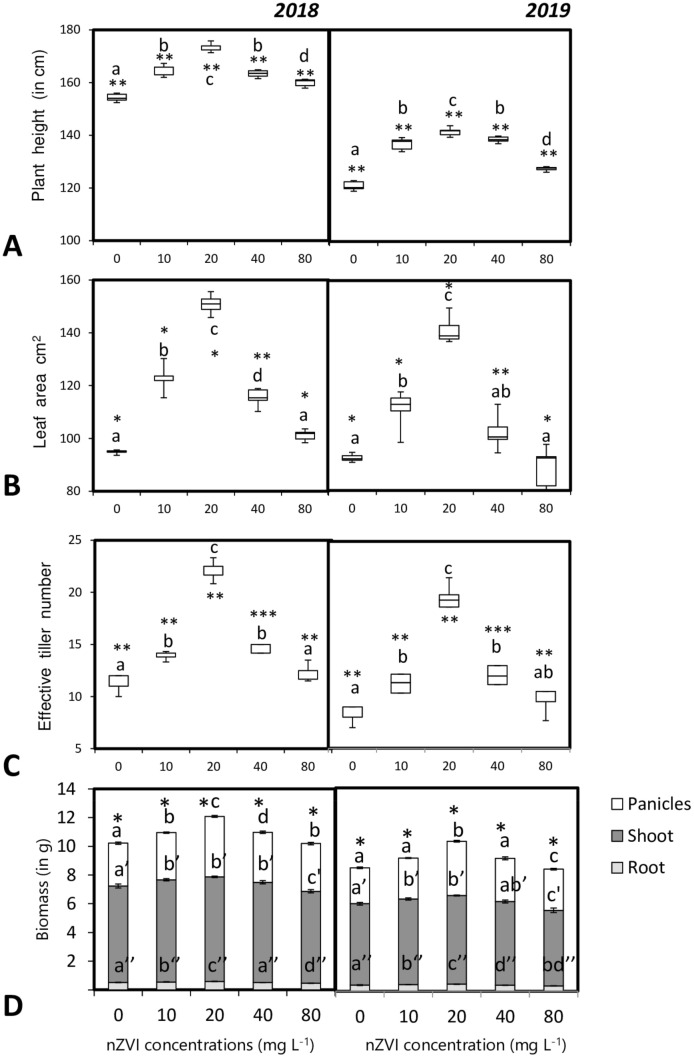
After analysis of plant morphological features like plant height, leaf area, it was revealed that there was a significant difference between the seedlings primed with nZVI compared to that of hydroprimed control sets. Maximum growth and leaf area increase were observed for the plants treated with 20 mg l−1 nZVI. However, in the 2nd year, overall plant growth and leaf area were reduced but in the nanoprimed sets, plant growth was still higher than the control hydroprimed sets (Fig. 5c and b).
Fig. 5.
Influence of seed priming with different concentrations of nZVI on a plant height, b leaf area, c effective tiller number, d biomass. of rice plants after maturity. Data represent the mean of five replicates and error bars represent standard error. Means with the same letters are not significantly different (Tukey’s HSD multiple comparisons at p ≤ 0.05). Significant differences between session 2018 and 2019 plants are indicated by symbol ‘*’ as obtained from unpaired t test analysis
Similar trends were also visible for plant biomass and panicle bearing effective tiller number. In both the year, seedlings primed with 20 mg l−1 nZVI, had a twofold increase in effective tiller numbers compared to that of the hydroprimed control set (Fig. 5c). Similarly, overall plant biomass was also significantly enhanced in the seedlings primed with 20 mg l−1 nZVI (Fig. 5d).
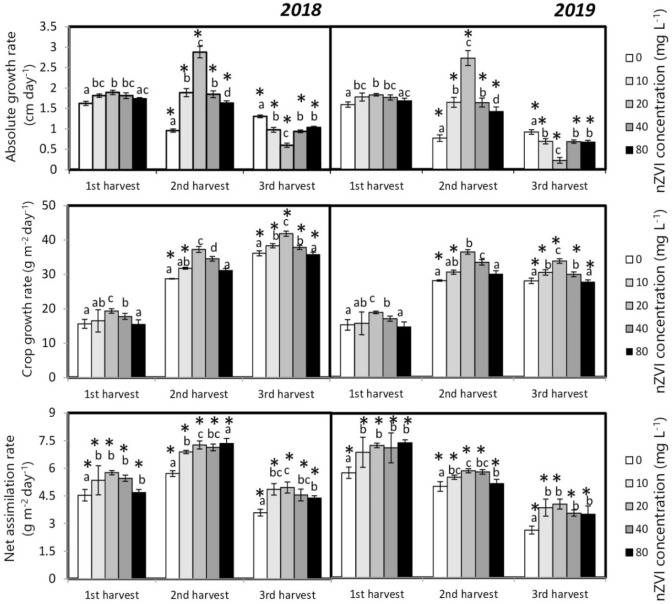
Agronomic parameters like AGR, CGR, and NAR were estimated based on plant height, biomass, and leaf area to evaluate the growth of seedlings in different growth stages. AGR values on 30 and 60 DAS were higher in nanoprimed seedlings compared to the control plants. Maximum AGR value was obtained from seedlings primed with 20 mg l−1 nZVI. However, the AGR value declined significantly in the nanoprimed sets during the 3rd harvest (Fig. 6a). On the other hand, CGR and NAR values in all the nanoprimed sets were higher than the control during all three harvest points (Fig. 6b and c).
Fig. 6.
Influence of seed priming with different concentrations of nZVI on a AGR, b CGR, c NAR of rice plants after maturity (1st harvest—on 30 DAS, 2nd harvest—on 60 DAS, 3rd harvest—at maturity). Data represent the means of five replicates and error bars represent standard error. Means with the same letters are not significantly different (Tukey’s HSD multiple comparisons at p ≤ 0.05). Significant differences between session 2018 and 2019 plants are indicated by symbol ‘*’ as obtained from unpaired t test analysis
Yield Components
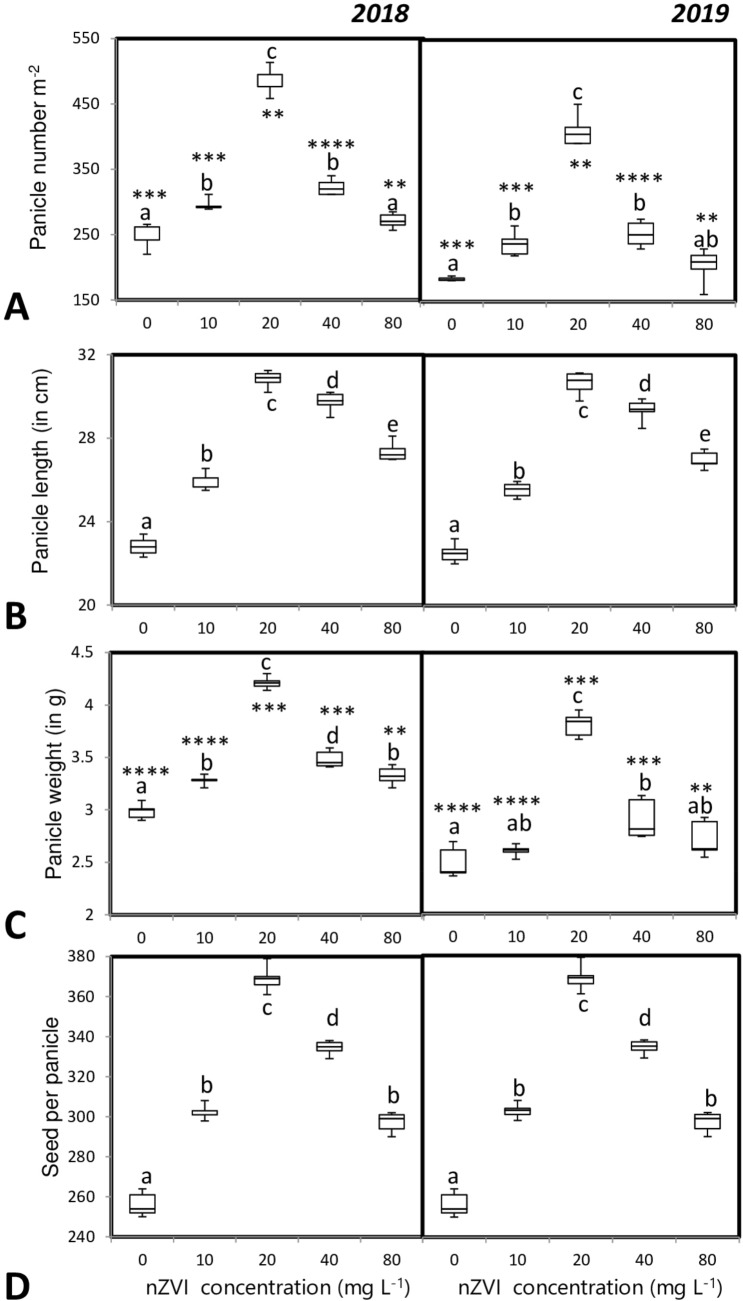
Due to the increase in effective tiller number in the nanoprimed sets the total panicle amount also increased significantly compared to that of the control set. Panicle size, panicle weight, and the number of grains per panicles were significantly higher than control. The highest panicle number and weight were noted in the seedlings primed with 20 mg l−1 nZVI (Fig. 7a–d).
Fig. 7.
Influence of seed priming with different concentrations of nZVI on panicle characters like a Panicle number per m2, b panicle length, c panicle weight, d total seed per panicle. Data represent the mean of five replicates and error bars represent standard error. Means with the same letters are not significantly different (Tukey’s HSD multiple comparisons at p ≤ 0.05). Significant differences between session 2018 and 2019 plants are indicated by symbol ‘*’ as obtained from unpaired t test analysis
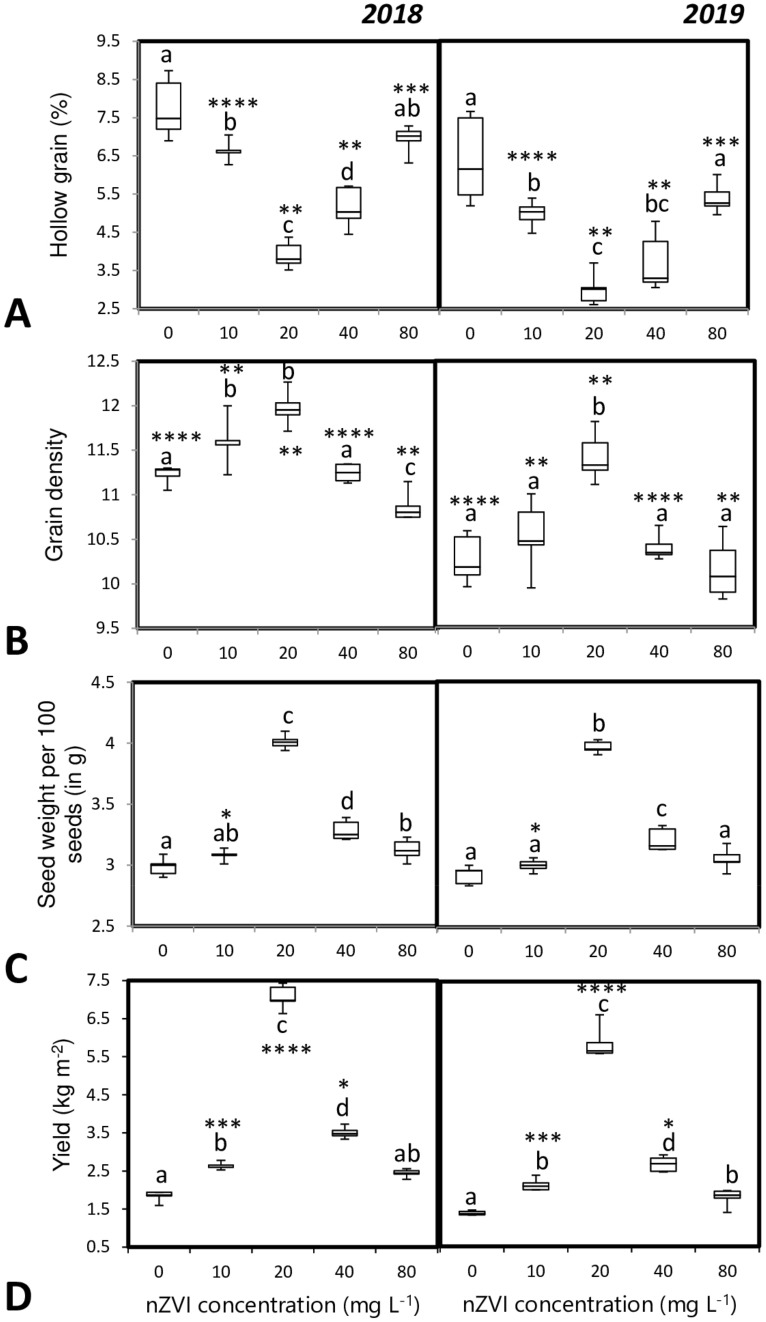
Additionally, hollow grain percentage was lower in the nanoprimed sets and panicles also had higher grain density compared to that of control (Fig. 8a, b). Seed weight and crop yield of nanoprimed seedlings were also improved with nZVI treatment. Around 1.34 fold and 3.8 fold increase in grain weight and grain yield respectively were observed in seedlings treated with 20 mg l−1 nZVI (Fig. 8c, d).
Fig. 8.
Influence of seed priming with different concentrations of nZVI on yield-related traits; a percentage of hollow grains, b grain density, c seed weight per 100 seeds, d.Yield (kg m−2). Data represent the mean of five replicates and error bars represent standard error. Means with the same letters are not significantly different (Tukey’s HSD multiple comparisons at p ≤ 0.05). Significant differences between session 2018 and 2019 plants are indicated by symbol ‘*’ as obtained from unpaired t test analysis
Kernel Qualities
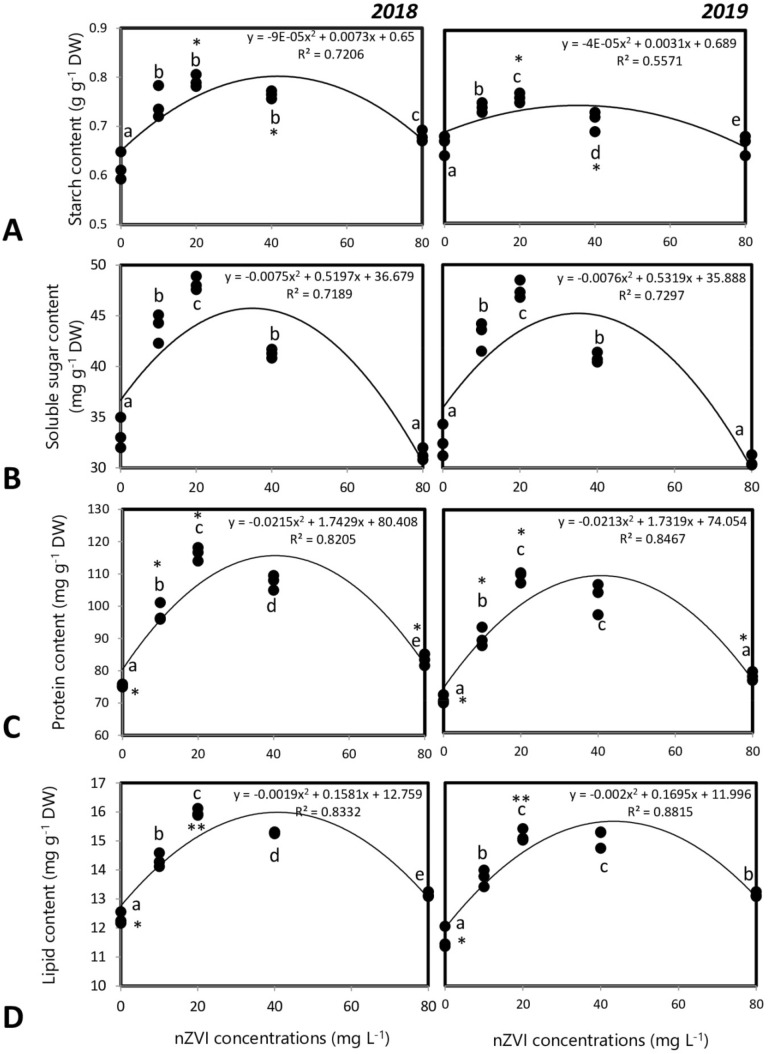
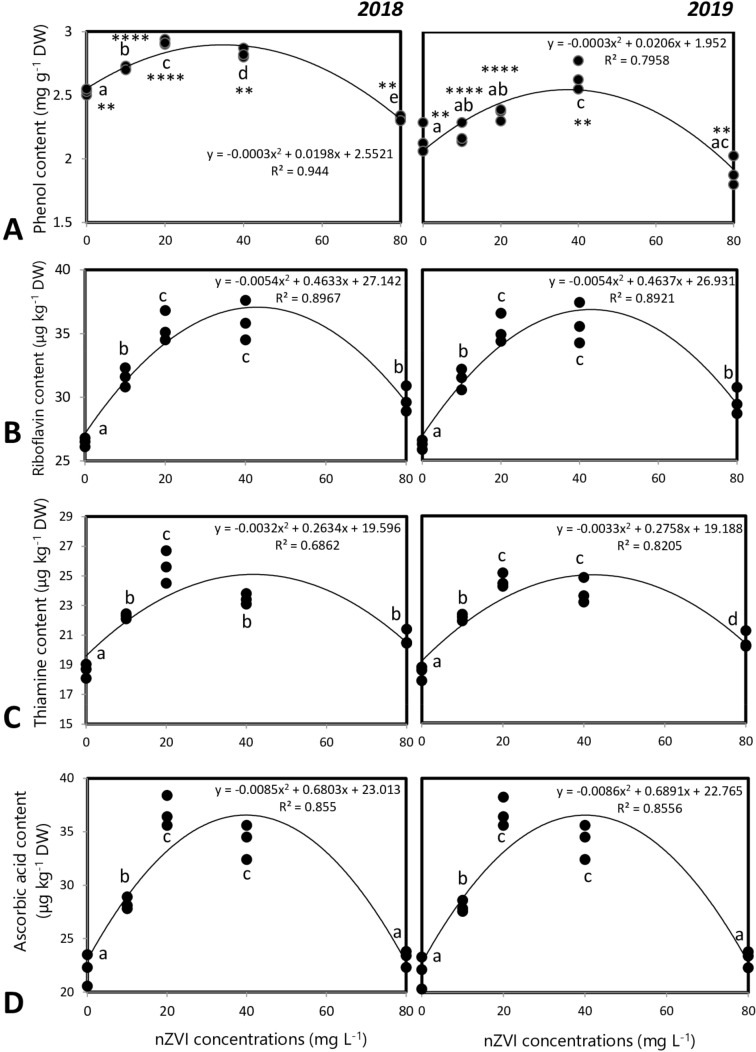
Concentration of phytochemicals in the rice grains obtained after seed priming varied significantly with respect to control sets in both the years. The total starch content varied with nZVI concentration (y2018 = − 0.00009x2 + 0.0073x + 0.65; y2019 = − 0.00004x2 + 0.0031x + 0.689). The maximum starch content was seen after 20 mg l−1 nZVI priming treatment and starch content of control set was similar with that of 80 mg l−1 nZVI treatment sets. The total soluble sugar content also varied quadratically with nZVI concentration (y2018 = − 0.0075x2 + 0.5197x + 36.679; y2019 = − 0.0076x2 + 0.5319x + 35.888) (Fig. 9a and b).
Fig. 9.
Influence of seed priming with different concentrations of nZVI on primary metabolite levels in rice grains a starch, b soluble sugar, c protein, d lipid. Data represents data of 3 replicates. Data with the same letters are not significantly different (Tukey’s HSD multiple comparisons at p ≤ 0.05). Significant differences between session 2018 and 2019 plants are indicated by symbol ‘*’ as obtained from unpaired t test analysis
Other primary metabolites like protein and lipid contents also varied significantly with nanopriming. Protein and lipid contents also followed similar trends. The protein and lipid contents varied quadratically with nZVI concentrations and the peak reached after treatment with 20 mg l−1 nZVI (Fig. 9c and d).
The secondary metabolite, phenol content, and thiamine, riboflavin, ascorbic acid (vitamin B1, B2, and C) levels were also estimated in the grains obtained after harvesting from the control and nanoprimed seedlings (Fig. 10a–d). Nanopriming could significantly modulate the grain secondary metabolite levels. The highest levels of phenol and all the vitamins were obtained in the 20 mg l−1 nZVI treatment sets.
Fig. 10.
Influence of seed priming with different concentrations of nZVI on secondary metabolite levels in rice grains a phenol, b riboflavin, c thiamine, d Ascorbic acid. Data represents data of 3 replicates. Data with the same letters are not significantly different (Tukey’s HSD multiple comparisons at p ≤ 0.05). Significant differences between session 2018 and 2019 plants are indicated by symbol ‘*’ as obtained from unpaired t test analysis
Plant Mineral Content and Partition Coefficient Analysis
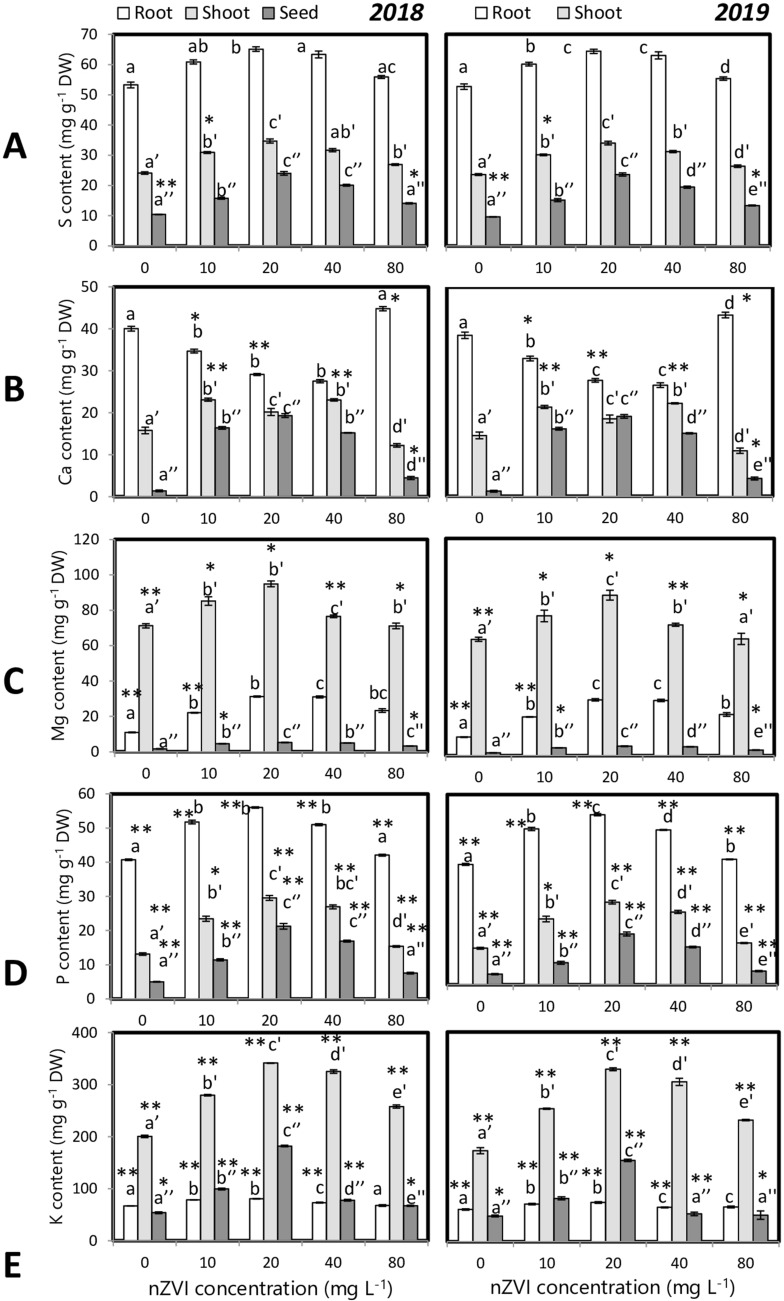
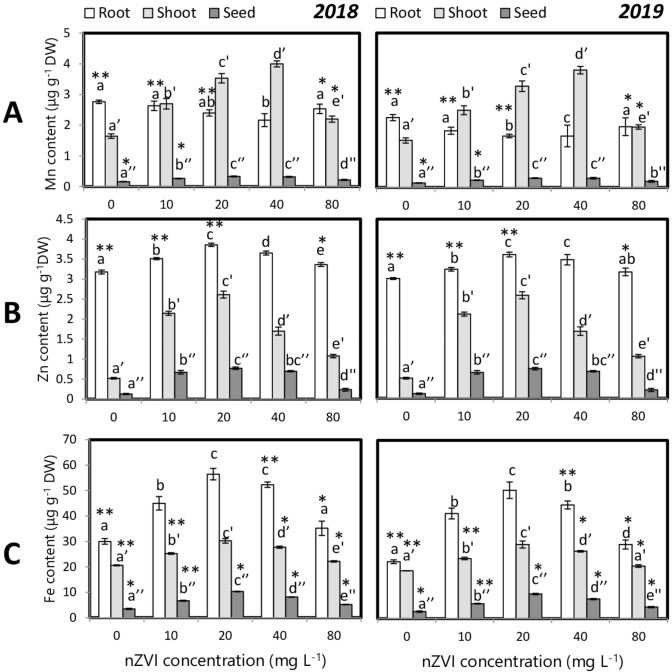
Macronutrients (P, K, Ca, Mg, S) and micronutrient (Fe, Mn, Zn) concentrations were estimated from a plant root, shoot, and panicles. nZVI priming had an impact on mineral distribution and accumulation in different plant organs (Figs. 11 and 12). Nanopriming treatments were responsible for higher S, Ca, Mg, P, K, Mn, Zn, and Fe levels. Maximum grain mineral accumulation was obtained in the grains of 20 mg l−1 nZVI primed seedlings. Nanopriming could significantly alter the proportional mineral content in an organ relative to the proportional dry weight of that organ as reflected by partition coefficient analysis (Table S1). It was found that the PQ value for root declined in the nanoprimed set whereas shoot and grain PQ value increased significantly.
Fig. 11.
Influence of seed priming with different concentrations of nZVI on macronutrient levels in rice grains a S, b Ca, c Mg, d P and e K. Data represent data of 3 replicates. Data with the same letters are not significantly different (Tukey’s HSD multiple comparisons at p ≤ 0.05). Significant differences between session 2018 and 2019 plants are indicated by symbol ‘*’ as obtained from unpaired t test analysis
Fig. 12.
Influence of seed priming with different concentrations of nZVI on macronutrient levels in rice grains a Mn, b Zn, and c Fe. Data represents data of 3 replicates. Data with the same letters are not significantly different (Tukey’s HSD multiple comparisons at p ≤ 0.05). Significant differences between session 2018 and 2019 plants are indicated by symbol ‘*’ as obtained from unpaired t test analysis
Correlation Analysis
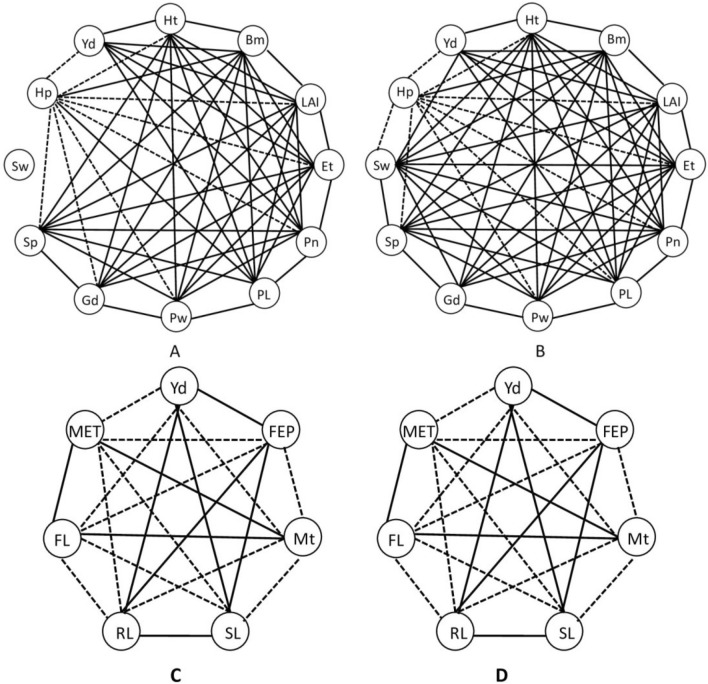
Pearson’s correlation analyses were performed separately to find out relationships among 12 agronomic parameters and another correlation study was carried out to investigate the relationship among the early seedling characteristics, flowering and maturity period, and crop yield.
Among the agronomic traits positive correlations were observed among Plant height (Ht), biomass (Bm), leaf area (LA), effective tiller number (Et), Total panicle number per m−2 (Pn), panicle length (PL), panicle weight (Pw), Grain density (Gd), number of seed per panicle (Sp) seed weight (Sw) and total yield (Yd). However, the results obtained from the 2018 study showed that seed weight was not significantly correlated to any of the agronomic traits but, results from 2019 reflected positive correlations. Negative correlations were found only for Hollow grain percentage (Hp) (Fig. 13a, b).
Fig. 13.
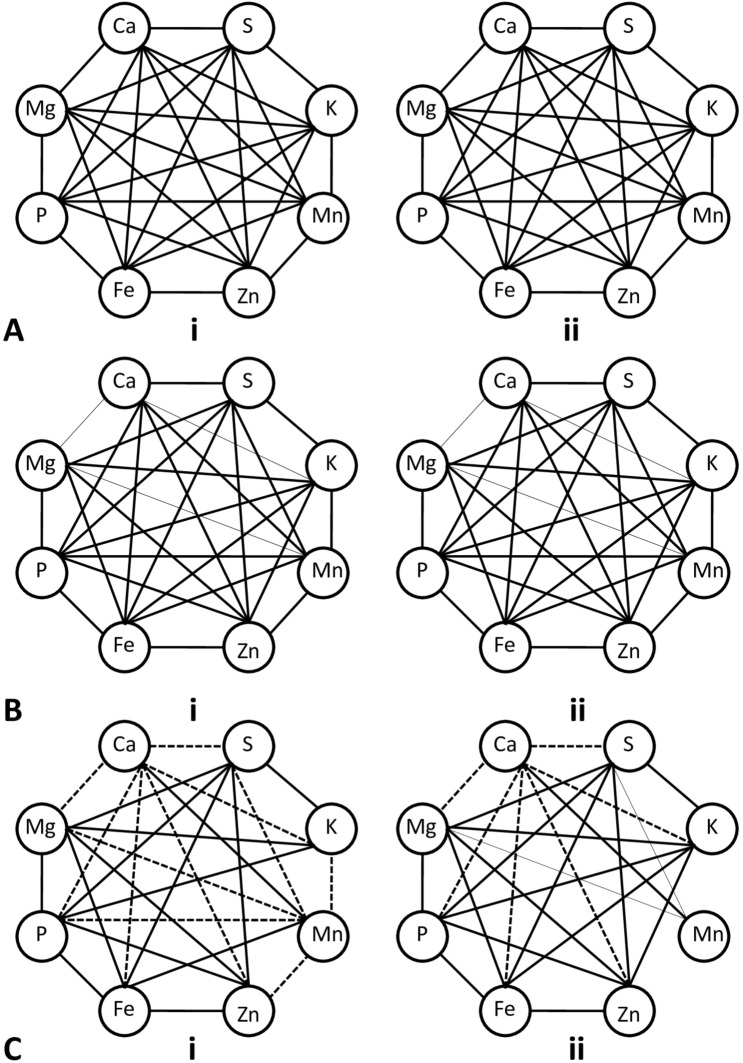
Agronomic traits correlation analyses. a, b Pearson’s correlation analysis of 12 agronomic characters obtained after maturity for session 2018 and 2019 respectively. C-D. Pearson’s correlation analysis of 7 agronomic characters obtained from seedlings for session 2018 and 2019 respectively for rice plants cultivated after nanopriming seeds with different concentrations of nZVI. Solid lines represent a significant positive correlation and dashed lines represent a significant negative correlation. Thinner lines indicate significance at the 0.05 level and thicker lines indicate significance at the 0.01 level (Ht: Plant height, Bm: biomass, LA: leaf area, Et: effective tiller number, Pn: total panicle number per m−2, PL: panicle length, Pw: panicle weight, Gd: grain density, Sp: number of seed per panicle, Sw: seed weight, FEP: final emergence percentage, RL: root length, SL: shoot length. MET: mean emergence time, FL: flowering time, Mt: maturity time, and Yd: total yield)
Seedling characteristics were directly correlated with crop yield (Yd). There was a positive correlation between Yd and final emergence percentage (FEP), root (RL), and shoot length (SL) of seedlings. On the other hand, mean emergence time (MET), flowering time (FL), and maturity time (Mt) were negatively correlated with Yd (Fig. 13c, d).
Pearson’s correlation analysis was performed to evaluate the relationship between macro and micro-nutrient levels of seedlings after priming with different concentrations of nZVI. Negative correlations among the minerals were observed only in the root. Calcium was positively correlated with Mn and for other minerals, it had a negative correlation (Fig. 14a–c).
Fig. 14.
Pearson’s correlation analysis of 8 mineral concentrations in grains (a i–ii), shoot (b i–ii), and root (c i–ii), of rice plants grown for session 2018 and 2019 respectively after nanopriming seeds with different concentrations of nZVI. Solid lines represent a significant positive correlation and dashed lines represent a significant negative correlation. Thinner lines indicate significance at the 0.05 level and thicker lines indicate significance at the 0.01 level
Discussion
In the context of sustainable agriculture, the application of nanoparticles is regarded to be one of the promising approaches due to its vast potential to revolutionize global agriculture and food production. Fe plays a significant role during seed germination. Deficiency of Fe in seeds can cause dormancy as well as impairs seed longevity (Murgia and Morandini 2017). In the present study, we found that seed priming with nZVI, significantly improved seedling establishment, growth, morphology and yield-related traits, kernel quality, and nutrient uptake capacity of rice.
According to our previous report, seed priming with nZVIcan up-regulate hydrolytic enzyme activities which accounted for increases in seedling vigor (Guha et al. 2018). Rapid and regulated utilization of metabolites improved seedling establishment in the primed sets (Basra et al. 2005). From the present results of the field study, we found that seed priming treatment could significantly reduce the mean emergence time (MET) and increase the final emergence percentage (FEP) of seedlings. Lower MET indirectly results in uniform emergence of seedlings in the nursery (Hampton and Tekrony 1995). The growth of nursery seedlings was increased significantly with nanopriming shown in Fig. S3. From the PPB stained images, it was confirmed that upon nZVI priming, the iron levels in the seeds increased in a dose-dependent manner. Although iron levels were found to be higher in the seeds treated with 40–80 mg l−1 nZVI, maximum growth enhancement was found in 20 mg l−1 nZVI primed sets. This is chiefly attributed to the ROS concentration window. Since nZVI reacted with dissolved oxygen in the water and generate ROS (such as H2O2 and OH−), it plays an important role in modulating the ROS status of the seed (Keenan et al. 2009).ROS are key players in regulating seed germination and longevity and seed responses are strictly dependent upon ROS levels. If ROS concentration is below a certain threshold, the seed remains in a dormant stage and at the same time, if the ROS level is too high the seeds begin to lose viability because of oxidative stress. Thus upon treatment with 20 mg l−1 nZVI, the ROS concentration threshold was found to be optimum and hence maximum seed germination rate was found in the 20 mg l−1 nZVI treated set. SEM analysis revealed that nZVI induced no morphological damage on embryonic root tissue. RAPD banding also confirmed that low concentrations of nZVI do not induce DNA damage. Duration of flowering and maturity of nanoprimed seedlings were also reduced as a result of healthy and more vigorous seedling establishment. A positive correlation was observed between the growth of nursery seedlings and the yield obtained after maturity of field-grown plants, which is congruent with the findings of Farooq et al. (2007).
Plant architectural traits like, plant height, tiller number, and canopy characteristics are important agronomic traits that determine crop performance in the field and yield in rice (Mathan et al. 2016). Tillering is a key component of crop yield since it governs the number of panicles per plant (Xing and Zhang 2010), and tiller number in a plant is reported to be directly related to the vigor of nursery seedlings (Reddy 2004). Nanoprimed seedlings had a higher number of effective tillers per plant and increased plant height, biomass, and leaf area. A similar result was found upon priming spinach seeds with FeS2 nanoparticles where an increase in plant biomass and leaf area was observed upon seed treatment (Srivastava et al. 2014).
Due to the increase in effective tiller number, the total panicle number per field area (in m2) was enhanced significantly. nZVI priming could also improve other yield-related characteristics such as panicle length, panicle weight, seed per panicle, hollow grain percentage. Seed priming treatment also enhanced grain yield which is also directly linked to healthier and vigorous seedling growth as proved by correlation analysis.
It was also observed that during the growth session of 2018, the maximum increase in yield and other agronomic characters were noted. This is mainly due to higher rainfall in session 2019 during September to October i.e. the reproductive phase. According to Abbas and Mayo (2020), increased rainfall during the flowering stage shows a negative relationship with rice production. This is mainly due to the effects of sediments in muddy water which block the pores and hampers respiration and photosynthesis, thus resulting in reduced production of the crop (Balasubramanian et al. 2004). However, it was found that nanoprimed seedlings could overcome the negative effects of heavy rainfall during reproductive stages and still showed higher crop yield compared to the control sets.
Plant growth parameters such as absolute growth rate (AGR), crop growth rate (CGR), and net assimilation rate (NAR) were estimated in different time points which reflect plant growth rates in different stages. AGR is a function of changes in plant height over a given time- period and it is directly dependent on environmental factors. AGR values were higher in nanoprimed seedlings compared to that of the control set during stem elongation and tillering stages. This suggests that healthier nursery seedlings from nanoprimed sets had the capacity for enhanced and earlier resource capture as compared to that of the control set (Farooq et al. 2007). However, CGR which indicates the change of dry weight over time was maximum during the flowering and maturity stages of the nanoprimed seedlings since they had higher yield capacity. NAR, on the other hand, correlates dry matter accumulation and leaf area of the plant which is an indicator of the plant’s net photosynthetic effectiveness in capturing light, CO2 assimilation, and storage of photo-assimilates. NAR value was also higher during tiller and shoot-elongation stages but it declined during maturity because leaf area expansion rate generally reduces during this stage. However,nanoprimed seedlings had higher NAR value in all the 3-time points compared to the control plants which reflects enhanced photosynthetic efficiency.
According to Morales-Díaz et al. (2017), nanomaterials because of their unique physico-chemical properties can boost plant metabolism and crop nutritional quality. Hence, we further evaluated the grain nutrient status. In addition to starch, sugar, lipids, and proteins, Gobindobhog rice grains are rich in various essential phytochemicals such as phenol, ascorbic acid, thiamine, and riboflavin (Bhattacharyya and Roy, 2018). These phytochemicals are produced from secondary metabolism and are important bioactive compounds with antioxidant properties. Grains obtained from nanoprimed sets had higher phytochemical reserves compared to the control sets due to higher NAR value which indicates increased photo assimilation, and its translocation and partitioning to the grains (Farooq et al. 2007).
From ICP-AES results it was also found that nanopriming with different concentrations of nZVI could result in enhanced mineral uptake which resulted in higher mineral contents in grains. Similar results were also found by Srivastava et al. (2014), where nanopriming spinach seeds with FeS2 NPs could significantly increase the nutrient uptake capacity of plants. Significant increase in Ca, Mn, and Zn contents were reported in the nanoprimed plants which are congruent with our observations. The mineral uptake potential is mainly due to enhancement in root metabolism which was previously proved by Mahakham et al. (2017). However, the increase in mineral uptake such as Ca, Mg, S, P, and K lead to enhanced plant vigor. In addition to this increase in micronutrient content such as Zn had an immense role in increasing crop yield, Mn contributed in increasing photosynthesis and Fe also helped to boost plant metabolism and yield due to its role in embryogenesis. Significant correlations were observed among the mineral contents in the root, shoot, and panicles of nanoprimed sets. In most of the cases, positive correlations were observed due to the chemical similarity between the ions which allows competition for the site of absorption and transport in plants (Sperotto et al. 2012). Among all the minerals Ca in root showed a negative correlation which is similar to the observations of Sperotto et al. (2012). However, Ca content in shoot and grain showed positive correlations with other minerals.
Since any increase of mineral in a sink tissue (i.e. grain) must have been caused as a result of enhanced mineral uptake potential of the plant or from remobilization of minerals from one organ to another; the partition quotient (PQ) of each mineral was calculated. The PQ value allowed us to explore the changes in mineral distribution dynamics in nanoprimed sets. A decrease in PQ value in the root is generally associated with an increase in the remobilization of minerals to the aerial parts (Roriz et al. 2014). For Ca, K, P, and Mn decrease in PQ value in root were observed in the nanoprimed sets due to remobilization of these minerals from the root to aerial parts. According to Silveira et al. (2007), Ca and K concentrations in root generally decrease when Fe levels are sufficient. Hence, we found a decrease in the PQ value of Ca and K in roots along with an increase in PQ value in rice shoots and grains. Several reports also claim that P remobilization occurs during later stages of growth if sufficient P has been absorbed by the plants during early growth stages (Grant et al. 2001). Thus it can be inferred that nanoprimed seedlings had proper P nutrition during the early stages, hence at maturity, root PQ value declined due to P remobilization. Mn is also an essential trace element that has a major role in photosynthesis (Socha and Guerinot 2014). Hence increased remobilization of root Mn to aerial parts of nanoprimed plants played an important role in increasing plant photosynthetic efficiency.
PQ value for other minerals like, Mg, S, Fe, and Zn was higher in all the plant organs of nanoprimed sets compared to that of control. Thus it is evident that nutrient uptake from soil was also enhanced due to nanopriming. An increase in Mg uptake can be explained by the positive interaction between P and Mg has been reported by Fageria (2001). Mg is an activator of kinase enzymes and activates phosphate transfer reactions. Nanopriming with nZVI enhanced the uptake of both Fe and S. It can be said that the external supply of nZVI helped to maintain Fe homeostasis which also stimulates the S uptake as proposed by Zuchi et al. (2015). The demand for the biosynthesis of Fe-S clusters in the organelles produces a feedback signal that regulates the uptake of both Fe and S (Balk and Pilon 2011). Our study also showed an increase in Zn uptake which is in accordance with the finding of Silveira et al. (2007) which also showed a positive correlation between Fe and Zn. Thus it can be said that nanopriming helped in the enrichment of all the essential macro- and micronutrients which resulted in improved crop performances.
Lastly, from our results, it can be suggested that a low concentration of nZVI acted as a suitable priming agent of the seeds. Priming with 20 mg l−1 nZVI had the maximum potential to increase crop yield. According to Rout and Sahoo (2015), iron is a prosthetic group for several enzymes (like cytochrome, catalase, and peroxidase) and thus plays various roles in metabolic processes like DNA synthesis, respiration, and photosynthesis. Iron also plays a significant role in the germination and development of the embryo as a result of which germination rate escalated in the nanoprimed sets. Nanopriming up-regulated plant photosynthetic efficiency and metabolism which is evident from high NAR values. Since iron plays an important role in chlorophyll synthesis, an increase in photosynthetic pigment content was found in nZVI primed seed as reported earlier by us (Guha et al. 2018). Due to the up-regulation of photosynthetic efficiency and plant metabolism, rice grains also had higher concentrations of phytochemicals which increased their nutritive value. The elemental analysis also proved that nZVI promoted nutrient uptake which played a major role in increasing crop yield. Thus, it can be concluded that seed priming with nZVI can increase rice yield compared to that of hydroprimed seeds without the addition of NPK fertilizers. Seed priming induced synchronized germination of seedlings and enhanced seedling vigor. Enhancement of metabolic activities of seeds before their journey in the soil also improved crop performance, nutrient assimilation capacity in later stages. This seed treatment process will help to cut down the fertilizer usage to some extent since crop yield was increased significantly with a seed treatment. Thus nZVI has a promising prospect in agriculture as a nano-priming agent or ‘pro-fertilizer’. The application of nZVI can reduce the fertilizer footprints on our planet and prevent soil and water pollution due to fertilizer leaching. However, further field researches on the application of nZVI as a vehicle for pesticide or insecticide delivery and the role of nZVI in crop protection, stress tolerance are yet to be explored.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
CAS, DST-FIST Department of Botany, Calcutta University, supported this research by providing infrastructural and instrumentation facilities. The authors are thankful to the University of Calcutta for the instrumentation facilities and for providing plots for field study. The authors also acknowledge IIT Bombay for ICP/AES analysis. TG thanks CSIR, Government of India for her fellowship.
Author Contributions
Conceptualization, TG, AM and RK; methodology, TG; investigation, TG; writing—original draft, TG; writing—review & editing, TG, AM and RK; funding acquisition, RK; resources, AM and RK; supervision, AM and RK.
Data Availability
All data generated or analyzed during this study are included in this published article.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbas S, Mayo ZA. Impact of temperature and rainfall on rice production in Punjab, Pakistan. Environ Dev Sustain. 2020;25:1–23. doi: 10.1007/s10668-020-00647-8. [DOI] [Google Scholar]
- Anand KV, Anugraga AR, Kannan M, Singaravelu G, Govindaraju K. Bio-engineered magnesium oxide nanoparticles as nano-priming agent for enhancing seed germination and seedling vigour of green gram (Vigna radiata L.) Mater Lett. 2020 doi: 10.1016/j.matlet.2020.127792. [DOI] [Google Scholar]
- Atienzar FA, Jha AN. The random amplified polymorphic DNA (RAPD) assay and related techniques applied to genotoxicity and carcinogenesis studies: a critical review. Mutat Res. 2006;613:76–102. doi: 10.1016/j.mrrev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Balasubramanian MK, Bi E, Glotzer M. Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells. Curr Biol. 2004;14(18):R806–818. doi: 10.1016/j.cub.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Balk J, Pilon M. Ancient and essential: the assembly of iron–sulfur clusters in plants. Trends Plant Sci. 2011;16(4):218–226. doi: 10.1016/j.tplants.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Basra SMA, Farooq M, Tabassum R. Physiological and biochemical aspects of seed vigour enhancement treatments in fine rice (Oryza sativaL.) Seed Sci Technol. 2005;33:623–628. doi: 10.15258/sst.2005.33.3.09. [DOI] [Google Scholar]
- Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H. Seeds. Physiology of development, germination and dormancy. 3. New York: Springer; 2013. [Google Scholar]
- Bhattacharyya S, Roy S. Qualitative and quantitative assessment of bioactive phytochemicals in gobindobhog and black rice, cultivated in West Bengal, India. Int J Pharm Sci Res. 2018;9(9):3845–3851. doi: 10.13040/IJPSR.0975-8232.9(9).3845-51. [DOI] [Google Scholar]
- Das B, Sahoo RN, Pargal S, et al. Quantitative monitoring of sucrose, reducing sugar and total sugar dynamics for phenotyping of water-deficit stress tolerance in rice through spectroscopy and chemometrics. Spectrochim Acta B. 2018;192:41–51. doi: 10.1016/j.saa.2017.10.076. [DOI] [PubMed] [Google Scholar]
- Das CK, Jangir H, Kumar J, et al. Nano-pyrite seed dressing: a sustainable design for NPK equivalent rice production. Nanotechnol Environ Eng. 2018;3(1):14. doi: 10.1007/s41204-018-0043-1. [DOI] [Google Scholar]
- Delfani M, Firouzabadi MB, Farrokhi N, Makarian H. Some physiological responses of black-eyed pea to iron and magnesium nanofertilizers. Commun Soil Sci Plant Anal. 2014;45(4):530–540. doi: 10.1080/00103624.2013.863911. [DOI] [Google Scholar]
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19:11–15. [Google Scholar]
- Ellis RA, Roberts EH. The quantification of ageing and survival in orthodox seeds. Seed Sci Technol. 1981;9:373–409. [Google Scholar]
- Fageria VD. Nutrient interactions in crop plants. J Plant Nutr. 2001;24(8):1269–1290. doi: 10.1081/PLN-100106981. [DOI] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) (2009) How to feed the world in 2050. Proceedings of the expert meeting on how to feed the world in 2050, 24–26 June 2009. FAO Headquarters, Rome
- Farooq MSMA, Basra SMA, Ahmad N. Improving the performance of transplanted rice by seed priming. Plant Growth Regul. 2007;51(2):129–137. doi: 10.1007/s10725-006-9155-x. [DOI] [Google Scholar]
- Ghule PL, Dahiphale VV, Jadhav JD, et al. Absolute growth rate, relative growth rate, net assimilation rate as influenced on dry matter weight of Bt cotton. Int Res J Agric Econ Stat. 2013;4(1):42–46. [Google Scholar]
- Gimeno-García E, AndreuV BR. Heavy metals incidence in the application of inorganic fertilizers and pesticides to rice farming soils. Environ Pollut. 1996;92(1):19–25. doi: 10.1016/0269-7491(95)00090-9. [DOI] [PubMed] [Google Scholar]
- Grant CA, Flaten DN, TomasiewiczDJ SSC. The importance of early season phosphorus nutrition. Can J Plant Sci. 2001;81(2):211–224. doi: 10.4141/P00-093. [DOI] [Google Scholar]
- Grillet L, Ouerdane L, Hoang MTT, et al. Ascorbate efflux as a new strategy for iron reduction and transport in plants. J Biol Chem. 2013;289(5):2515–2525. doi: 10.1074/jbc.M113.514828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha T, Ravikumar KV, Mukherjee A, Kundu R. Nanopriming with zero valent iron (nZVI) enhances germination and growth in aromatic rice cultivar (Oryza sativa cv. Gobindabhog L.) Plant Physiol Biochem. 2018;127:403–413. doi: 10.1016/j.plaphy.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Hampton JG, Tekorny DM. Handbook of ISTA vigour test methods. 3. Zurich: ISTA; 1995. [Google Scholar]
- Hunt R. Plant growth analysis: the rationale behind the use of the fitted mathematical function. Ann Bot. 1979;43(2):245–249. doi: 10.1093/oxfordjournals.aob.a085632. [DOI] [Google Scholar]
- Itroutwar PD, Govindaraju K, Tamilselvan S, et al. Seaweed-based biogenic ZnO nanoparticles for improving agro-morphological characteristics of rice (Oryza sativa L.) J Plant Growth Regul. 2019;5:1–2. doi: 10.1007/s00344-019-10012-3. [DOI] [Google Scholar]
- Keenan CR, Goth-Goldstein R, Lucas D, et al. Oxidative stress induced by zero-valent iron nanoparticles and Fe(II) in human bronchial epithelial cells. Environ Sci Technol. 2009;43:4555–4560. doi: 10.1007/s11051-008-9446-4. [DOI] [PubMed] [Google Scholar]
- Li X, Yang Y, Gao B, et al. Stimulation of peanut seedling development and growth by zero-valent iron nanoparticles at low concentrations. PLoS ONE. 2015;10(4):e0122884. doi: 10.1371/journal.pone.0122884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- Mahakham W, Sarmah AK, Maensiri S, et al. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci Rep. 2017;7(1):1–21. doi: 10.1038/s41598-017-08669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathan J, Bhattacharya J, Ranjan A. Enhancing crop yield by optimizing plant developmental features. Development. 2016;143(18):3283–3294. doi: 10.1242/dev.134072. [DOI] [PubMed] [Google Scholar]
- Mirshekari B. Physical seed treatment techniques may influence stand establishment and yield of wheat in delayed cropping. Idesia. 2015;33(3):49–54. doi: 10.4067/S0718-34292015000300008. [DOI] [Google Scholar]
- Morales-Díaz AB, Ortega-Ortíz H, Juárez-Maldonado A, et al. Application of nanoelements in plant nutrition and its impact in ecosystems. Adv Nat Sci-Nanosci Nanotechnol. 2017;8(1):013001. doi: 10.1088/2043-6254/8/1/013001. [DOI] [Google Scholar]
- Mukherjee AK, Tripathi S, Mukherjee S, et al. Effect of integrated nutrient management in sunflower (Helianthus annuus L.) on alluvial soil. Curr Sci. 2019;117(8):1364. doi: 10.18520/cs/v117/i8/1364-1368. [DOI] [Google Scholar]
- Murgia I, Morandini P. Iron deficiency prolongs seed dormancy in Arabidopsis plants. Front Plant Sci. 2017;8:2077. doi: 10.3389/fpls.2017.02077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem F, Farooq M, Ullah A, et al. Influence of Zn nutrition on the productivity, grain quality and grain biofortification of wheat under conventional and conservation rice-wheat cropping systems. Arch Agron Soil Sci. 2019 doi: 10.1080/03650340.2019.1652273. [DOI] [Google Scholar]
- Nandagoapalan V, Doss A, Marimuthu C. Phytochemical analysis of some traditional medicinal plants. Biosci Discov. 2016;7:17–20. [Google Scholar]
- Okolie NP, Falodun A, Davids O. Evaluation of the antioxidant activity of root extract of pepper fruit (Dennetiatripetala), and it’s potential for the inhibition of lipid peroxidation. Afr J Tradit Complem Altern Med. 2014;11(3):221–227. doi: 10.4314/ajtcam.v11i3.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero N, Vitoria L, Soler A, et al. Fertilisercharacterisation: major, trace and rare earth elements. Appl Geochem. 2005;20(8):1473–1488. doi: 10.1016/j.apgeochem.2005.04.002. [DOI] [Google Scholar]
- Palaniswamy KM, Gomez KA. Length-width method for estimating leaf area of rice. Agron J. 1974;66(3):430–433. doi: 10.2134/agronj1974.00021962006600030027x. [DOI] [Google Scholar]
- Paparella S, Araújo SS, Rossi G, et al. Seed priming: state of the art and new perspectives. Plant Cell Rep. 2015;34:1281–1293. doi: 10.1007/s00299-015-1784-y. [DOI] [PubMed] [Google Scholar]
- Poudel PB, Poudel MR, Gautam A, et al. COVID-19 and its global impact on food and agriculture. J Biol Today's World. 2020;9(5):221. [Google Scholar]
- Ravikumar KVG, Dubey S, Pulimi M, et al. Scale-up synthesis of zero-valent iron nanoparticles and their applications for synergistic degradation of pollutants with sodium borohydride. J Mol Liq. 2016;224:589–598. doi: 10.1016/j.molliq.2016.10.040. [DOI] [Google Scholar]
- Reddy S. Agronomy of field crops. New Delhi: Kalyani Publishers; 2004. [Google Scholar]
- Rehman HU, Basra SMA, Farooq M. Field appraisal of seed priming to improve the growth, yield, and quality of direct seeded rice. Turk J Agric For. 2011;35(4):357–365. doi: 10.3906/tar-1004-954. [DOI] [Google Scholar]
- Roriz M, Carvalho SM, Vasconcelos MW. High relative air humidity influences mineral accumulation and growth in iron deficient soybean plants. Front Plant Sci. 2014;5:726. doi: 10.3389/fpls.2014.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschzttardtz H, Conèjèro G, Divol F, et al. New insights into Fe localization in plant tissues. Front Plant Sci. 2013;4:350. doi: 10.3389/fpls.2013.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout GR, Sahoo S. Role of iron in plant growth and metabolism. Rev Agric Sci. 2015;3:1–24. doi: 10.7831/ras.3.1. [DOI] [Google Scholar]
- Rui M, Ma C, Hao Y, et al. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachishypogaea) Front Plant Sci. 2016;7:815. doi: 10.3389/fpls.2016.00815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde S, Paralikar P, Ingle AP, Rai M. Promotion of seed germination and seedling growth of Zea mays by magnesium hydroxide nanoparticles synthesized by the filtrate from Aspergillus niger. Arab J Chem. 2020;13(1):3172–3182. doi: 10.1016/j.arabjc.2018.10.001. [DOI] [Google Scholar]
- Silveira VCD, Oliveira APD, Sperotto RA, et al. Influence of iron on mineral status of two rice (Oryza sativa L.) cultivars. Braz J Plant Physiol. 2007;19(2):127–139. doi: 10.1590/S1677-04202007000200005. [DOI] [Google Scholar]
- Socha AL, Guerinot ML. Mn-euvering manganese: the role of transporter gene family members in manganese uptake and mobilization in plants. Front Plant Sci. 2014;5:106. doi: 10.3389/fpls.2014.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperotto RA, Vasconcelos MW, Grusak MA, et al. Effects of different Fe supplies on mineral partitioning and remobilization during the reproductive development of rice (Oryza sativa L.) Rice. 2012;5(1):27. doi: 10.1186/1939-8433-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava G, Das CK, Das A, et al. Seed treatment with iron pyrite (FeS2) nanoparticles increase the production of spinach. RSC Adv. 2014;4:58495–58504. doi: 10.1039/C4RA06861K. [DOI] [Google Scholar]
- Su L, Yin JJ, Charles D, et al. Total phenolic contents, chelating capacities, and radical-scavenging properties of black peppercorn, nutmeg, rosehip, cinnamon and oregano leaf. Food Chem. 2007;100(3):990–997. doi: 10.1016/j.foodchem.2005.10.058. [DOI] [Google Scholar]
- UN (United Nations Department of Economic and Social Affairs, Population Division) (2013) World Population Prospects: the 2012 Revision
- Wang Z, Cuschieri A. Tumour cell labelling by magnetic nanoparticles with determination of intracellular iron content and spatial distribution of the intracellular iron. Int J Mol Sci. 2013;14:9111–9125. doi: 10.3390/ijms14059111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters BM, Grusak MA. Whole-plant mineral partitioning throughout the life cycle in Arabidopsis thaliana ecotypes Columbia, Landsbergerecta, Cape Verde Islands, and the mutant line ysl1ysl3. New Phytol. 2008;177(2):389–405. doi: 10.1111/j.1469-8137.2007.02288.x. [DOI] [PubMed] [Google Scholar]
- Xing Y, Zhang Q. Genetic and molecular bases of rice yield. Annu Rev Plant Biol. 2010;61:421–442. doi: 10.1146/annurev-arplant-042809-112209. [DOI] [PubMed] [Google Scholar]
- Yoon H, Kang YG, Chang YS, et al. Effects of zerovalent iron nanoparticles on photosynthesis and biochemical adaptation of soil-grown Arabidopsis thaliana. Nanomaterials. 2019;9(11):1543. doi: 10.3390/nano9111543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yugandhar P, Savithramma N. Green synthesis of calcium carbonate nanoparticles and their effects on seed germination and seedling growth of Vigna mungo (L.) Hepper. Int J Adv Res. 2013;1(8):89–103. [Google Scholar]
- Zuchi S, Watanabe M, Hubberten HM, et al. The interplay between sulfur and iron nutrition in tomato. Plant Physiol. 2015;169(4):2624–2639. doi: 10.1104/pp.15.00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.