Abstract
目的
探讨miR-600是否可通过HIF-1α信号通路调控宫颈癌HeLa细胞的增殖及对Cyclin D1和血管内皮生长因子(VEGF)表达的影响。
方法
采用miR-600 mimic和Plasmid-HIF-1α转染HeLa细胞增加和诱导miR-600和HIF-1α的表达。qPCR和Western blot检测转染6 h后Plasmid-HIF-1α对于HeLa细胞HIF-1α表达的影响。将HeLa细胞分为空白对照组(无特殊处理)、miR-600 mimic组(转染miR-600拟似物miR-600 mimic)、Plasmid-HIF-1α组(P-HIF-1α组,转染Plasmid-HIF-1α)和miR-600 mimic+Plasmid-HIF-1α组(miR-600 mimic+P-HIF-1α组,同时转染miR-600 mimic和Plasmid-HIF-1α)。在转染6 h后,使用MTT法检测细胞活性,采用qPCR和Western blot测定VEGF、Cyclin D1和HIF-1α mRNA和蛋白的表达水平。
结果
在转染6 h后,miR-600 mimic和Plasmid-HIF-1α对HeLa细胞活性无显著影响(P均 < 0.05)。但转染Plasmid-HIF-1α 6 h后,细胞HIF-1α表达水平显著增加。在转染24 h和48 h,与对照组相比较,miR-600 mimic组细胞活性呈时间依赖性下降,Plasmid-HIF-1α组和miR-600 mimic+Plasmid-HIF-1α组细胞活性呈时间依赖性增加,且Plasmid-HIF-1α组较miR-600 mimic+Plasmid-HIF-1α组细胞活性时间依赖性增加更加显著(P均 < 0.05)。转染6 h后,与对照组相比较,miR-600 mimic组VEGF、Cyclin D1和HIF-1α表达均明显下降,Plasmid-HIF-1α组和miR-600 mimic+Plasmid-HIF-1α组VEGF、Cyclin D1和HIF-1α表达均明显增加,且Plasmid-HIF-1α组较miR-600 mimic+Plasmid-HIF-1α组增加更明显(P均 < 0.05)。
结论
在HeLa细胞中miR-600可以通过抑制HIF-1α信号通路下调Cyclin D1和VEGF的表达,从而抑制肿瘤细胞的增殖和分化。
Keywords: HeLa细胞, miR-600, HIF-1α, 细胞增殖, 血管内皮生长因子
Abstract
Objective
To determine whether miR-600 suppresses the proliferation of HeLa cells by inhibiting hypoxia-inducible factor-1α (HIF-1α) signaling pathway and its effect on expressions of cyclin D1 and vascular endothelial growth factor (VEGF).
Methods
HeLa cells were transfected with miR-600 mimic and plasmid-HIF-1α, either alone or in combination, to up-regulate miR-600 and HIF-1α expressions in the cells. Six hours after the transfection, the cell viability was assessed using MTT assay, and the mRNA and protein expressions of VEGF, cyclin D1, and HIF-1α were analyzed with qPCR and Western blotting.
Results
The viability of HeLa cells showed no obvious changes 6 h after transfection with miR-600 mimic or Plasmid-HIF-1α. At 24 h and 48 h, the cells transfected with miR-600 mimic showed a time-dependent reduction of cell viability, while the cells transfected with Plasmid-HIF-1α alone and with both miR-600 mimic and Plasmid-HIF-1α showed increased cell viability. The cell viabilities in Plasmid-HIF-1α group were significantly higher than those in miR-600 mimic+Plasmid-HIF-1α group at 24 h and 48 h. Six hours after transfection with miR-600 mimic, the cells exhibited significantly decreased expressions of VEGF, cyclin D1, and HIF-1α, which were all significantly up-regulated in Plasmid-HIF-1α group and miR-600 mimic+Plasmid-HIF-1α group. VEGF, cyclin D1, and HIF-1α expressions were significant higher in Plasmid-HIF-1α group than in miR-600 mimic+ Plasmid-HIF-1α group.
Conclusions
miR-600 suppresses the proliferation of HeLa cells and down-regulate the expressions of cyclin D1 and VEGF by inhibiting HIF-1α signaling pathway.
Keywords: HeLa cells, miR-600, hypoxia-inducible factor-1α, cell proliferation, vascular endothelial growth factor
宫颈癌是最常见的妇科恶性肿瘤之一,在我国其发病率仅次于乳腺癌位居第2[1-2],在世界范围内位居第3[3-4]。流行病学研究发现中国的宫颈癌的发病率和死亡率分别为7.5和3.4每10万人,同时HPV的感染率约为16.8%[4]。miRNA属于非编码小RNA类,其主要通过转录后调控机制靶向调控基因的表达。近期,研究发现miR-600对部分恶性肿瘤如直肠癌和非小细胞肺癌的增殖和分化具有重要的调控作用。实验证实上调miR-600的表达可有效抑制直肠肿瘤细胞和非小细胞肺癌细胞的增殖、分化和转移过程[5-6]。进一步研究显示miR-600主要通过下调低氧诱导因子-1α(HIF-1α)信号通路抑制非小细胞肺癌A549细胞的增殖和分化[6]。但miR-600对于宫颈癌生物学特征的影响尚不清楚。因此,在本研究中将采用miR-600 mimic和Plasmid-HIF-1α转染HeLa细胞诱导miR-600和HIF-1α的表达,探讨miR-600对于宫颈癌细胞系HeLa细胞增殖和分化的调控作用,以及其潜在的信号通路和分子机制。
1. 材料和方法
1.1. 研究对象
HeLa细胞株由中国科学院上海细胞库提供;MTT试剂盒(Promega);鼠抗人HIF-1α抗体、鼠抗人VEGF抗体、鼠抗人Cyclin D1抗体和鼠抗人β-actin抗体(Santa Cruz);miR-NC (空白miR,作为miR-600 mimic的阴性对照)、miR-600 mimic、Plasmid-NC(空白Plasmid,作为Plasmid-HIF-1α的阴性对照)和PlasmidHIF-1α(上海吉玛制药技术有限公司);DMEM培养基(Gibco);Lipofectamine 2000(Invitrogen);RNA提取试剂盒和qPCR试剂盒(Qiagen);其余试剂均为分析纯。
1.2. 细胞培养
根据本实验室既往研究方法培养HeLa细胞[7]。简介如下,HeLa细胞常规接种在含10%胎牛血清,100 g/L青霉素、100 g/L链霉素的RPMI 1640培养液中,置于37 ℃,5 % CO2,孵箱内培养。每48 h换液、传代1次,取对数生长期细胞用于实验。第1步:实验时利用Lipofectamine 2000将终浓度为50 nmol/L的miR-NC(miR-NC对细胞任何miR无影响,作为miR-600 mimic的对照)、miR-600 mimic(增加细胞miR-600表达)、Plasmid-NC(Plasmid空载体,不改变细胞任何基因的表达,作为Plasmid-HIF-1α的对照)和Plasmid-HIF-1α(诱导细胞HIF-1α表达)对细胞进行转染。在转染(干预)6 h后先使用台盼蓝(Trypan Blue)染色观察细胞后,收集细胞,分离mRNA和蛋白进行qPCR和Western blot检测。第2步:检测细胞在转染Plasmid-HIF-1α 6 h后HIF-1α的表达水平。将细胞分为Control组、PlasmidNC组(转染Plasmid-NC)和Plasmid-HIF-1α组(转染Plasmid-HIF-1α)。在转染6 h后收集细胞,对HIF-1α mRNA和蛋白水平采用qPCR和Western blot检测,明确Plasmid-HIF-1α对HeLa细胞HIF-1α表达的影响。因为miR-600 mimic是miR-600的拟似物故无需使用qPCR进行检测。第3步:将细胞分为4组:Control组(空白对照组,无特殊处理)、miR-600 mimic组(转染miR-600拟似物miR-600 mimic增加细胞miR-600水平)、Plasmid-HIF-1α组(P-HIF-1α组,转染Plasmid-HIF-1α上调细胞HIF-1α表达)和miR-600 mimic+Plasmid-HIF-1α组(miR-600 mimic+P-HIF-1α组,同时转染miR-600 mimic和Plasmid-HIF-1α增加细胞miR-600水平和HIF-1α表达)。在干预(给予miR-600 mimic和/或转染Plasmid-HIF-1α)0 h、6 h、24 h和48 h后使用台盼蓝(Trypan Blue)染色观察细胞。同时,收集转染6 h后的细胞,分离mRNA和蛋白进行qPCR和Western blot检测。以探讨HeLa细胞中miR-600对于HIF-1α、Cyclin D1和VEGF表达的影响。
1.3. MTT法检测细胞活性
取对数生长期细胞消化制成单细胞悬液,接种于96孔培养板中,每孔接种100 μL约含5×103细胞。使用MTT比色试验对转染后不同时间点(0 h、6 h、24 h、48 h)的细胞生长状态进行测定,实验重复5次。细胞活性(%)=(A干预组/A对照组0 h)×100(%)[7-8]。
1.4. qPCR法检测细胞中血管内皮生长因子、Cyclin D1和HIF-1α mRNA表达检测
干预6 h后,按照qPCR试剂盒说明书提取细胞总RNA。β-actin作为内参照。引物:VEGF正义5'-TACCTCCACCATGCCAAGTG-3',反义5'-ATGATTCTGCCCTCCTCCTTC-3';Cyclin D1正义5'-CGTGGGCTCTAAGATGAAGG-3',反义5'-TGCGGATGATCTGTTTGTTC-3';HIF-1α正义5'-CGTTCCTTCGATCAGTTGTC-3',反义5'-TCAGTGGTGGCAGTGGTAGT-3';β-actin正义:5'-CATGTACGTTGCTATCCAGGC-3',反义:5'-CTCCTTAATGTCACGCACGAT-3'。按照试剂盒说明书介绍进行反转录和扩增实验。使用2-ΔΔCt法对目标mRNA表达水平进行测定,ΔΔCt=(Ct,目标−Ct,内参照)干预组(-Ct,目标−Ct,内参照)对照组[9-10]。
1.5. Western blot法检测细胞中VEGF、Cyclin D1和HIF-1α蛋白表达
干预6 h后,按照试剂盒操作要求,首先提取细胞总蛋白,并进行聚丙烯酰胺凝胶电泳(SDS-PAGE),然后转移至硝酸纤维素滤膜上,用脱脂奶粉封闭1 h,分别加入鼠抗人单克隆抗体VEGF(1:1200)、Cyclin D1(1: 1000)、HIF-1α(1:1000)和β-actin(1:1000),4 ℃孵育过夜,洗膜后加入相应的辣根过氧化物酶标记的二抗(1:2000),用ECL进行显色,用凝胶成像分析系统进行扫描。
1.6. 统计学方法
计量资料以均数±标准差表示。采用SPSS 22.0进行单因素方差分析,两样本均数多重比较采用LSD法,P < 0.05为差异有统计学意义。
2. 结果
2.1. 细胞转染miR-NC、miR-600 mimic、Plasmid-NC和Plasmid-HIF-1α 6 h对于HeLa细胞活性的影响
细胞转染miR-NC、miR-600 mimic、Plasmid-NC和Plasmid-HIF-1α 6 h后使用MTT法分析HeLa细胞的活性(表 1)。与空白对照相比较,给予miR-NC、miR-600 mimic、Plasmid-NC和Plasmid-HIF-1α 4种试剂干预HeLa细胞6 h后细胞活性无显著差异(P均>0.05),各组细胞形态无明显差别(图 1)。因此,选取干预6 h作为检测HIF-1α、Cyclin D1和VEGF表达的时间点,以消除因为细胞数量不同而产生基因表达差异的情况。
1.
miR-600 mimic和Plasmid-HIF-1α转染6 h对于HeLa细胞活性的影响
Effects of miR-600 and Plasmid-HIF-1α overexpression on viability of HeLa cells at 6 h (n=5, Mean±SD)
| Group | 0h | 6 h |
| *P < 0.05 compared with control at the corresponding time points. | ||
| Control | 100 | 99.1±1.2 |
| miR-NC | 97.6±3.8 | 103.2±4.3 |
| miR-600 mimic | 99.8±2.2 | 96.7±1.7 |
| P-NC | 98.4±3.1 | 102.5±3.4 |
| P-HIF-1α | 94.6±2.9 | 102.2±5.3 |
1.
对照组、miR-NC组、miR-600 mimic组、P-NC组和P-HIF-1α组6 h HeLa细胞形态
Morphology of HeLa cells in control, miR-NC, miR-600 mimic, P-NC, and P-HIF-1α groups at 6 h after transfection (Original magnification: ×400).
2.2. Plasmid-HIF-1α对HeLa细胞HIF-1α表达的影响
为明确Plasmid-HIF-1α转染细胞6 h后对于HIF-1α表达的影响。将细胞分为对照组、Plasmid-NC组和Plasmid-HIF-1α组。在转染6 h后与空白对照组(对照组)相比较,Plasmid-NC组HIF-1α表达无明显差异(P均>0.05),Plasmid-HIF-1α组HIF-1α表达明显增加(P < 0.05,图 2)。
2.
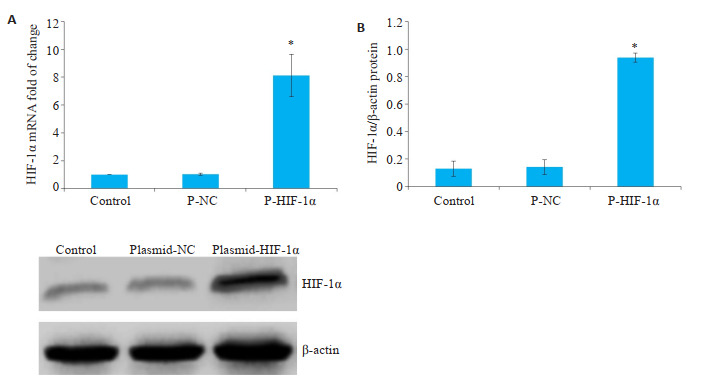
转染6 h,对照组、Plasmid-NC组和PlasmidHIF-1α组HIF-1α的表达
HIF-1α expression in control, Plasmid-NC, and Plasmid-HIF-1α groups at 6 h after transfection. A: HIF-1α mRNA expression detected by qPCR. B: HIF-1α protein expression detected by Western blotting.*P < 0.05 vs with control group.
2.3. 转染6 h、24 h和48 h对于HeLa细胞活性的影响
在转染6 h后与对照组比较,miR-600 mimic组、PHIF-1α组和miR-600 mimic+P-HIF-1α组细胞活性无显著差异(P均>0.05),且各组细胞形态无明显差别(表 2、图 3)。在转染24 h和48 h,与对照组相比较,miR-600 mimic组细胞活性呈时间依赖性下降,P-HIF-1α组和miR-600 mimic+P-HIF-1α组细胞活性呈时间依赖性增加,且P-HIF-1α组较miR-600 mimic+P-HIF-1α组细胞活性时间依赖性增加更加显著(P均 < 0.05,表 2)。
2.
不同时间点各组HeLa细胞活性
Viability of HeLa cells at different time points after transfection (n=5, Mean±SD)
| Group | 0 h | 6 h | 24 h | 48 h |
| *P < 0.05 vs control group at the corresponding time points; #P < 0.05 vs miR-600 mimic group at the corresponding time points; †P < 0.05 vs miR-600 mimic+P-HIF-1α group at the corresponding time points. | ||||
| Control | 100 | 98.7±3.2 | 133.6±7.6 | 169.3+10.4 |
| miR-600 mimic | 98.9±2.8 | 97.2±5.l | 89.7±5.3* | 79.6±7.9* |
| P-HIF-la | 96.5±4.4 | 106.2±2.8 | 163.5±8.3*#† | 231.6±11.4*#† |
| miR-600 mimic+P-HIF-lα | 102.3±3.7 | 105.8±5.3 | 144.6±10.3*# | 188.7±13.1*# |
3.
Control组、miR-600 mimic组、P-HIF-1α组和miR-600 mimic+P-HIF-1α组6 h后 HeLa细胞形态
Morphology of HeLa cells in control, miR-600 mimic, P-HIF-1α and miR-600 mimic+P-HIF-1α groups at 6 h after transfection (×400).
2.4. 转染6 h对于HeLa细胞VEGF、Cyclin D1和HIF-1α表达的影响
在转染6 h后与对照组比较,miR-600 mimic组VEGF、Cyclin D1和HIF-1α表达均明显下降,P-HIF-1α组和miR-600 mimic+P-HIF-1α组VEGF、Cyclin D1和HIF-1α表达均明显增加,且P-HIF-1α组较miR-600 mimic+P-HIF-1α组增加更明显(P均 < 0.05,图 4)。
4.
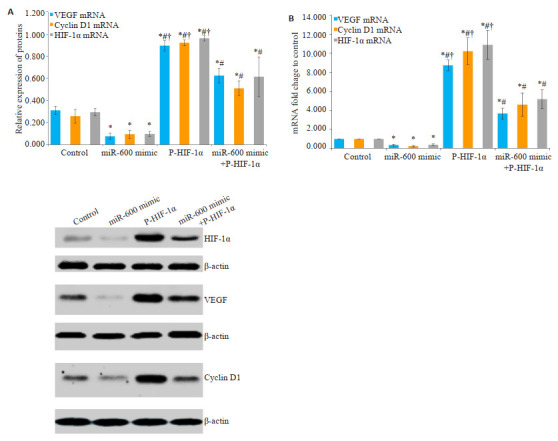
转染6h,Control组、miR-600 mimic组、PHIF-1α组和miR-600 mimic+P-HIF-1α组VEGF、Cyclin D1和HIF-1α的表达
HIF-1α expression in control, miR-600 mimic, P-HIF-1α and miR-600 mimic+P-HIF-1α groups at 6 h after transfection. A: mRNA expression by qPCR. B: Protein expression by Western blotting. *P < 0.05 vs control group; #P < 0.05 vs miR-600 mimic group; †P < 0.05 vs miR-600 mimic+P-HIF-1α group
3. 讨论
低氧诱导因子-1(HIF-1)作为一种广泛表达的核转录因子(TF),可通过结合靶基因启动子(promoter)区域的特定序列-缺氧反应元件(HRE)诱导基因的表达[11-13]。研究发现Cyclin D1和VEGF基因转录调控区存与HRE序列[11-13]。HIF-1与HRE结合可诱导该基因的转录。大量研究显示下调或阻断HIF-1α信号通路对多种肿瘤细胞的增殖和分化具有抑制作用[11-15]。Wei等[14]的实验表明在缺氧状态下,地高辛主要可以通过下调HIF-1α相关信号通路抑制A549细胞的增殖和分化。同时,吴维光等[15]的研究显示转染shRNA-HIF-1α质粒后HeLa细胞增殖能力较对照组明显下降,表明下调HIF-1α可有效抑制HeLa细胞的增殖和分化。因此推测诱导宫颈癌细胞株HeLa细胞HIF-1α过表达亦可导致肿瘤细胞的过度增殖。首先我们发现在干预6 h时miR-600 mimic和Plasmid-HIF-1α对HeLa细胞活性无显著影响。因此在此时间点检测mRNA和蛋白表达量可排除由于细胞数量差异导致基因表达差异而产生的影响。进一步我们发现在干预24 h和48 h时,Plasmid-HIF-1α时间依赖性的上调了HeLa细胞的数量。该结果表明HIF-1α过度表达可有效促进HeLa细胞的增殖和分化。因此,结合既往研究[15]和本实验结果提示HIF-1α是调控宫颈癌细胞增殖和细胞周期的关键分子。
研究显示miR-600对结直肠癌和肺癌等多种肿瘤细胞增殖有显著的抑制作用[16-17]。Zhang等[5]的研究显示上调miR-600可有效抑制人结肠癌SW480细胞、SW620细胞和DLD-1细胞的增殖和分化。进一步研究表明miR-600主要通过调控HIF-1α mRNA的转录后修饰控制肿瘤细胞的增殖过程。Chi等[6]通过离体和在体(裸鼠移植瘤)实验证明对于A549细胞等多个肿瘤细胞系miR-600可通过下调HIF-1α的合成有效抑制肿瘤的增殖、分化和转移过程,表明对于非小细胞肺癌的增殖miR-600是HIF-1α的关键调控因子。但目前miR-600对于宫颈癌生物学特征是否也具有重要的调控作用尚不清楚。因此,本研究试探讨上调miR-600表达对于HeLa细胞增殖和分化的影响,以及与HIF-1α信号通路的关系。我们的结果显示上调miR-600水平导致HeLa细胞活性呈时间依赖性下降,并有效抑制了PlasmidHIF-1α诱导的HIF-1α表达。该结果表明在HeLa细胞中miR-600主要是通过下调HIF-1α合成抑制肿瘤的增殖和分化,提示宫颈癌细胞中miR-600对于HIF-1α具有重要负性调控作用。
为了进一步验证HIF-1a信号通路是miR-600调控HeLa细胞增殖和分化的关键环节及相关的分子机制。我们对HeLa细胞中受HIF-1α调控的与肿瘤增殖和分化相关的下游分子进行了分析。在宫颈癌和肺癌等多种肿瘤细胞中HIF-1α对VEGF和Cyclin D1的表达具有关键的调控作用,VEGF和Cyclin D1基因启动子序列存在缺氧反应元件(HRE),上调HIF-1α可促进VEGF和Cyclin D1基因的转录[14, 18-20]。其中,Cyclin D1是细胞周期调控的关键因子[21]。Cyclin D1的过度表达是诱导肿瘤细胞异常增殖的关键因素之一[20]。研究表明下调Cyclin D1可减缓肿瘤细胞的增殖速度[21-23]。Siveen[24]的研究显示二甲双胍可通过下调Cyclin D1的表达抑制HeLa细胞的增殖,并促进肿瘤细胞凋亡[22]。VEGF是促进肿瘤血管生成和转移的关键分子。Zhao等[18]的研究表明在宫颈癌HeLa细胞和SiHa细胞中丙戊酸可通过下调HIF-1α/VEGF信号通路抑制肿瘤血管生成。在本研究中我们发现在HeLa细胞中miR-600 mimic可有效抑制Plasmid-HIF-1α诱导的VEGF和Cyclin D1表达。该结果表明在HeLa细胞中miR-600可通过抑制HIF-1α通路下调VEGF和Cyclin D1的表达。提示宫颈癌细胞中miR-600主要通过HIF-1α信号通路参与了肿瘤细胞的增殖和分化的调控。
综上所述,本研究首次证明在HeLa细胞中miR-600可以通过抑制HIF-1α信号通路下调Cyclin D1和VEGF的表达,从而抑制肿瘤细胞的增殖和分化。但HeLa中miR-600调控HIF-1α的具体分子仍需要进一步分析和研究。
Biography
周晓霞,硕士,主治医师,E-mail: zhouxiaoxia2013@126.com
Funding Statement
国家自然科学基金青年科学基金(81600388)
Supported by Youth Program of National Natural Science Foundation of China (81600388)
Contributor Information
周 晓霞 (Xiaoxia ZHOU), Email: zhouxiaoxia2013@126.com.
王 嘉佳 (Jiajia WANG), Email: herongcdjq2010@163.com.
References
- 1.乔 友林, 赵 宇倩. 宫颈癌的流行病学现状和预防. 中华妇幼临床医学杂志:电子版. 2015;11(2):1–6. doi: 10.3877/cma.j.issn.1673-5250.2015.02.001. [乔友林, 赵宇倩.宫颈癌的流行病学现状和预防[J].中华妇幼临床医学杂志:电子版, 2015, 11(2): 1-6.] [DOI] [Google Scholar]
- 2.Shrestha AD, Neupane D, Vedsted P, et al. Cervical cancer prevalence, incidence and mortality in low and middle income countries: a systematic review. Asian Pac J Cancer Prev. 2018;19(2):319–24. doi: 10.22034/APJCP.2018.19.2.319. [Shrestha AD, Neupane D, Vedsted P, et al. Cervical cancer prevalence, incidence and mortality in low and middle income countries: a systematic review[J]. Asian Pac J Cancer Prev, 2018, 19 (2): 319-24.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F, Ren JS, Masuyer E, et al. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132(5):1133–45. doi: 10.1002/ijc.27711. [Bray F, Ren JS, Masuyer E, et al. Global estimates of cancer prevalence for 27 sites in the adult population in 2008[J]. Int J Cancer, 2013, 132(5): 1133-45.] [DOI] [PubMed] [Google Scholar]
- 4.Zhao FH, Lewkowitz AK, Hu SY, et al. Prevalence of human papillomavirus and cervical intraepithelial neoplasia in China: a pooled analysis of 17 population-based studies. Int J Cancer. 2012;131(12):2929–38. doi: 10.1002/ijc.27571. [Zhao FH, Lewkowitz AK, Hu SY, et al. Prevalence of human papillomavirus and cervical intraepithelial neoplasia in China: a pooled analysis of 17 population-based studies[J]. Int J Cancer, 2012, 131(12): 2929-38.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang PL, Zuo ZG, Wu AH, et al. miR-600 inhibits cell proliferation, migration and invasion by targeting p53 in mutant p53-expressing human colorectal cancer cell lines. Oncol Lett. 2017;13(3):1789–96. doi: 10.3892/ol.2017.5654. [Zhang PL, Zuo ZG, Wu AH, et al. miR-600 inhibits cell proliferation, migration and invasion by targeting p53 in mutant p53-expressing human colorectal cancer cell lines[J]. Oncol Lett, 2017, 13(3): 1789-96.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chi YB, Luo QC, Song YT, et al. Circular RNA circPIP5K1A promotes non-small cell lung cancer proliferation and metastasis through miR-600/HIF-1α regulation. J Cell Biochem. 2019;120(11):19019–30. doi: 10.1002/jcb.29225. [Chi YB, Luo QC, Song YT, et al. Circular RNA circPIP5K1A promotes non-small cell lung cancer proliferation and metastasis through miR-600/HIF-1α regulation[J]. J Cell Biochem, 2019, 120 (11): 19019-30.] [DOI] [PubMed] [Google Scholar]
- 7.周 晓霞, 王 智彪. 曲格列酮上调PPAR-γ抑制宫颈癌HeLa细胞ICAM-1和MMP-9表达. http://www.j-smu.com/oa/pdfdow.aspx?Sid=2014111693. 南方医科大学学报. 2014;34(11):1693–6, 1701. doi: 10.3969/j.issn.1673-4254.2014.11.29. [周晓霞, 王智彪.曲格列酮上调PPAR-γ抑制宫颈癌HeLa细胞ICAM-1和MMP-9表达[J].南方医科大学学报, 2014, 34(11): 1693-6, 1701.] [DOI] [PubMed] [Google Scholar]
- 8.Zhu T, Li CY, Zhang X, et al. GLP-1 analogue liraglutide enhances SP-A expression in LPS-induced acute lung injury through the TTF-1 signaling pathway. Mediators Inflamm. 2018;2018:3601454. doi: 10.1155/2018/3601454. [Zhu T, Li CY, Zhang X, et al. GLP-1 analogue liraglutide enhances SP-A expression in LPS-induced acute lung injury through the TTF-1 signaling pathway[J]. Mediators Inflamm, 2018, 2018: 3601454.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu T, Chen ZH, Chen GH, et al. Curcumin attenuates asthmatic airway inflammation and mucus hypersecretion involving a PPARγ-dependent NF-κB signaling pathway in vivo and in vitro. Mediat Inflamm. 2019;2019:4927430. doi: 10.1155/2019/4927430. [Zhu T, Chen ZH, Chen GH, et al. Curcumin attenuates asthmatic airway inflammation and mucus hypersecretion involving a PPARγ-dependent NF-κB signaling pathway in vivo and in vitro[J]. Mediat Inflamm, 2019, 2019: 4927430.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu T, Zhang W, Feng SJ, et al. Emodin suppresses LPS-induced inflammation in RAW264.7 cells through a PPARγ-dependent pathway. Int Immunopharmacol. 2016;34:16–24. doi: 10.1016/j.intimp.2016.02.014. [Zhu T, Zhang W, Feng SJ, et al. Emodin suppresses LPS-induced inflammation in RAW264.7 cells through a PPARγ-dependent pathway[J]. Int Immunopharmacol, 2016, 34: 16-24.] [DOI] [PubMed] [Google Scholar]
- 11.Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5(5):378–89. doi: 10.1016/j.apsb.2015.05.007. [Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy[J].Acta Pharm Sin B, 2015, 5(5): 378-89.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albadari N, Deng SS, Li W. The transcriptional factors HIF-1 and HIF-2 and their novel inhibitors in cancer therapy. Expert Opin Drug Discov. 2019;14(7):667–82. doi: 10.1080/17460441.2019.1613370. [Albadari N, Deng SS, Li W. The transcriptional factors HIF-1 and HIF-2 and their novel inhibitors in cancer therapy[J]. Expert Opin Drug Discov, 2019, 14(7): 667-82.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serocki M, Bartoszewska S, Janaszak-Jasiecka A, et al. miRNAs regulate the HIF switch during hypoxia: a novel therapeutic target. Angiogenesis. 2018;21(2):183–202. doi: 10.1007/s10456-018-9600-2. [Serocki M, Bartoszewska S, Janaszak-Jasiecka A, et al. miRNAs regulate the HIF switch during hypoxia: a novel therapeutic target [J].Angiogenesis, 2018, 21(2): 183-202.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei D, Peng JJ, Gao H, et al. Digoxin downregulates NDRG1 and VEGF through the inhibition of HIF-1α under hypoxic conditions in human lung adenocarcinoma A549 cells. Int J Mol Sci. 2013;14(4):7273–85. doi: 10.3390/ijms14047273. [Wei D, Peng JJ, Gao H, et al. Digoxin downregulates NDRG1 and VEGF through the inhibition of HIF-1α under hypoxic conditions in human lung adenocarcinoma A549 cells[J]. Int J Mol Sci, 2013, 14 (4): 7273-85.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.吴 维光, 陈 亚琼, 曹 秀琴. RNA干扰沉默HIF-1R基因对宫颈癌HeLa细胞定植和侵袭的影响. https://www.cnki.com.cn/Article/CJFDTOTAL-JCYL201003022.htm. 基础医学与临床. 2010;30(3):284–8. [吴维光, 陈亚琼, 曹秀琴. RNA干扰沉默HIF-1R基因对宫颈癌HeLa细胞定植和侵袭的影响[J].基础医学与临床, 2010, 30(3): 284-8.] [Google Scholar]
- 16.Wei WW, Huo BS, Shi XL. miR-600 inhibits lung cancer via downregulating the expression of METTL3. Cancer Manag Res. 2019;11:1177–87. doi: 10.2147/CMAR.S181058. [Wei WW, Huo BS, Shi XL. miR-600 inhibits lung cancer via downregulating the expression of METTL3[J]. Cancer Manag Res, 2019, 11: 1177-87.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun JF, Hu JY, Wang GJ, et al. LncRNA TUG1 promoted KIAA1199 expression via miR-600 to accelerate cell metastasis and epithelialmesenchymal transition in colorectal cancer. J Exp Clin Cancer Res. 2018;37(1):106. doi: 10.1186/s13046-018-0771-x. [Sun JF, Hu JY, Wang GJ, et al. LncRNA TUG1 promoted KIAA1199 expression via miR-600 to accelerate cell metastasis and epithelialmesenchymal transition in colorectal cancer[J]. J Exp Clin Cancer Res, 2018, 37(1): 106.] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Zhao Y, You W, Zheng J, et al. Valproic acid inhibits the angiogenic potential of cervical cancer cells via HIF-1α/VEGF signals. Clin Transl Oncol. 2016;18(11):1123–30. doi: 10.1007/s12094-016-1494-0. [Zhao Y, You W, Zheng J, et al. Valproic acid inhibits the angiogenic potential of cervical cancer cells via HIF-1α/VEGF signals[J]. Clin Transl Oncol, 2016, 18(11): 1123-30.] [DOI] [PubMed] [Google Scholar]
- 19.Zhang WQ, Xiong ZG, Wei TQ, et al. Nuclear factor 90 promotes angiogenesis by regulating HIF-1α/VEGF-A expression through the PI3K/Akt signaling pathway in human cervical cancer. Cell Death Dis. 2018;9(3):276. doi: 10.1038/s41419-018-0334-2. [Zhang WQ, Xiong ZG, Wei TQ, et al. Nuclear factor 90 promotes angiogenesis by regulating HIF-1α/VEGF-A expression through the PI3K/Akt signaling pathway in human cervical cancer[J]. Cell Death Dis, 2018, 9(3): 276.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang B, Li YL, Zhao JL, et al. Hypoxia-inducible factor-1 promotes cancer progression through activating AKT/Cyclin D1 signaling pathway in osteosarcoma. Biomedecine Pharmacother. 2018;105:1–9. doi: 10.1016/j.biopha.2018.03.165. [Zhang B, Li YL, Zhao JL, et al. Hypoxia-inducible factor-1 promotes cancer progression through activating AKT/Cyclin D1 signaling pathway in osteosarcoma[J]. Biomedecine Pharmacother, 2018, 105: 1-9.] [DOI] [PubMed] [Google Scholar]
- 21.Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med. 2016;94(12):1313–26. doi: 10.1007/s00109-016-1475-3. [Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment[J]. J Mol Med, 2016, 94(12): 1313-26.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yudhani RD, Astuti I, Mustofa M, et al. Metformin modulates cyclin D1 and P53 expression to inhibit cell proliferation and to induce apoptosis in cervical cancer cell lines. Asian Pac J Cancer Prev. 2019;20(6):1667–73. doi: 10.31557/APJCP.2019.20.6.1667. [Yudhani RD, Astuti I, Mustofa M, et al. Metformin modulates cyclin D1 and P53 expression to inhibit cell proliferation and to induce apoptosis in cervical cancer cell lines[J]. Asian Pac J Cancer Prev, 2019, 20(6): 1667-73.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao JH, Yu H, Guo WK, et al. The anticancer effects of ferulic acid is associated with induction of cell cycle arrest and autophagy in cervical cancer cells. Cancer Cell Int. 2018;18:102. doi: 10.1186/s12935-018-0595-y. [Gao JH, Yu H, Guo WK, et al. The anticancer effects of ferulic acid is associated with induction of cell cycle arrest and autophagy in cervical cancer cells[J]. Cancer Cell Int, 2018, 18: 102.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siveen KS, Prabhu K, Krishnankutty R, et al. Vascular endothelial growth factor (VEGF) signaling in tumour vascularization: potential and challenges. Curr Vasc Pharmacol. 2017;15(4):339–51. doi: 10.2174/1570161115666170105124038. [Siveen KS, Prabhu K, Krishnankutty R, et al. Vascular endothelial growth factor (VEGF) signaling in tumour vascularization: potential and challenges[J]. Curr Vasc Pharmacol, 2017, 15(4): 339-51.] [DOI] [PubMed] [Google Scholar]


