Abstract
Objective
To assess the predictors and outcomes of acute kidney injury (AKI) among patients with coronavirus disease 2019 (COVID-19).
Methods
This retrospective observational study was conducted among patients with a confirmed diagnosis of COVID-19 admitted to Hankou Hospital between January, 5 and March 8, 2020. We evaluated the association of AKI with the demographic and biochemical parameters and clinical outcomes of the patients using univariate regression analysis.
Results
Atotal of 287 COVID-19 patients, including 55 with AKI and 232 without AKI, were included in the analysis. Compared with the patients without AKI, the patients with AKI were older, predominantly male, and were more likely to have hypoxia and pre-existing hypertension and cerebrovascular diseases. The patients with AKI also had higher levels of white blood cells, D-dimer, aspartate aminotransferase, total bilirubin, creatine kinase, lactate dehydrogenase, procalcitonin, C-reactive protein, a higher prevalence of hyperkalemia, lower lymphocyte counts, and higher chest computed tomographic scores. The incidence of stage 1 AKI was 14.3% and that of stage 2 or 3 AKI was 4.9%. The patients with AKI had much higher mortality rate than those without AKI.
Conclusions
AKI is an important complication of COVID-19. An older age, a male gender, multiple pre- existing comorbidities, lymphopenia, increased infection indicators, elevated D-dimer, and impaired heart and liver functions are all potential risk factors ofAKI. COVID- 19 patients with AKI that progresses into stages 2 or 3 AKI have a high mortality rate. Prevention of AKI and monitoring kidney function is critical in the care of COVID-19 patients.
Keywords: coronavirus disease 2019, acute kidney injury, kidney function
Abstract
目的
COVID-19可能累及肾脏,本研究评估了COVID-19患者中急性肾损伤(AKI)的预测因素和预后。
方法
本研究为单中心回顾性观察性研究。纳入2020年1月5日~3月8日在武汉汉口医院住院的临床确诊COVID-19患者。采用单因素回归分析评估AKI和COVID-19疾病的发病率变化与临床结果之间的关系。
结果
共有287例患者进入分析,55例AKI患者和232例非AKI患者。与没有AKI的患者相比,AKI患者年龄更大,以男性为主,更有可能出现缺氧,合并高血压和脑血管疾病。此外,AKI患者的白细胞、D-二聚体、天冬氨酸氨基转移酶、总胆红素、肌酸激酶、乳酸脱氢酶、降钙素原、C反应蛋白水平较高,高钾血症患病率较高,淋巴细胞计数较低,胸部CT评分较高。1期AKI发生率为14.3%,2~3期AKI发生率为4.9%。AKI患者的死亡率明显较高。
结论
AKI是COVID-19的重要并发症。高龄、男性、合并多种疾病、淋巴细胞减少、感染指标增高、D-二聚体升高、心、肝功能受损可能是AKI的危险因素。进展到2期或3期AKI的COVID-19患者死亡率较高。
Keywords: COVID-19, 急性肾损伤, 肾功能
INTRODUCTION
Since its first outbreak in December, 2019, coronavirus disease 2019 (COVID-19) has rapidly evolve into a worldwide pandemic with clinical presentations greatly resembling viral pneumonia [1]. The clinical spectrum of COVID-19 pneumonia ranges from non-severe to severe cases [2]. Previous studies have described the general epidemiological findings, clinical manifestations, and clinical outcomes of COVID-19 patients [3-8]. The latest data suggest that apart from the lungs, the kidney is the most vulnerable organ under attack by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and up to 37.5% of the fatal cases of COVID-19 showed signs of acute kidney injury (AKI) [3]. However, currently no studies have been reported to examine the risk factors of AKI in COVID-19 patients or how they affect the patients' outcomes. In this study, we investigated AKI in COVID-19 patients admitted to Hankou Hospital in Wuhan, China. We analyzed the factors associated with AKI in the hospitalized patients to identify the factors that can be potentially used for early identification of COVID-19 patients who are at high risk of developing AKI, so that they can be provided with timely intensive care to reduce the mortality and morbidity associated with AKI.
PATIENTS AND METHODS
Study design and participants
This single-center, retrospective, observational study was conducted at Hankou Hospital in Wuhan, China, which is a designated hospital of COVID-19 patients. The diagnostic criteria for suspected and confirmed COVID-19 cases were based on the criteria established by WHO [7, 8], and only COVID-19 patients who met the WHO criteria were included in this study. Patients who were younger than 18 years, who had undergone renal replacement therapy (RRT) before admission, or whose entire stay lasted for less than 48 h were excluded from the analysis. The primary variable of interest was the incidence of AKI, and the secondary outcome variables included 28-day mortality (calculated from the day of admission) and the length of hospital stay. This study was approved by the National Health Commission of China and the Ethics Commission of Hankou Hospital (hkyy2020-005). The Ethics Commission of Hankou Hospital waived the requirement for obtaining informed consent from the patients.
Data collection and definitions
We obtained epidemiological, demographic, clinical, laboratory, management, and outcome data from the patients' medical records using standardized data collection forms. The clinical outcomes of the patients were followed up to March 8, 2020. To analyze the relationship between AKI staging and clinical outcomes, a minimum follow-up time of 14 days was required, which also applies to the patients who were discharged before March 8, 2020. If the relevant data were missing from the records or clarification was needed, the researchers directly contacted the patients or their families to ascertain the epidemiological and symptom data. All the data were examined by two physicians. The initial investigations included complete blood cell counts, coagulation profile, and serum biochemical tests of renal and liver function, creatine kinase, lactate dehydrogenase (LDH), electrolytes, and 10 common respiratory pathogens. All the patients received treatment procedures and measurements following the protocols published previously [7].
All the patients underwent chest computed tomography (CT) scan on admission. All the CT images were reviewed independently by two fellowship-trained cardiothoracic radiologists with approximately 5 years of experience, using the Electronic Medical Record System of Hankou Hospital, and the final decisions were made by consensus. In case of disagreement over image interpretations between the two radiologists, a third fellowship-trained cardiothoracic radiologist with 10 years of experience adjudicated on the final decision. No negative control cases were included and no blinding was used in this study. The radiologists evaluated the initial CT scans and assigned quantitative scores for the characteristics using a recently published scoring system [9]. The total CT score was the sum of lung involvement: score 1-5 for each of the 5 lobes, 0 for none, and the maximum total score was 25 [10]
AKI was identified and classified on the basis of the highest serum creatinine (Cr) level according to the Kidney Disease Improving Global Outcomes (KDIGO) definition and staging system [11]. However, urine output was not routinely monitored in our hospital, which rendered this approach infeasible. We thus used creatinine increment alone for the diagnosis and staging of AKI. In addition, baseline serum creatinine measurement data were missing in some patients before admission. Two alternative methods were used to estimate the baseline creatinine level to make the diagnosis of AKI: the average serum creatinine method used the data from non-AKI patients to calculate the average serum creatinine value based on age and sex [12], and the estimated glomerular filtration rate (eGFR) method used an Modification of Diet in Renal Disease (MDRD) equation to determine the eGFR based on age and sex (75 mL·min-1·1.73 m-2) [11, 13]. Briefly, the diagnostic criteria of AKI are: an increase in serum creatinine level by ≥26.5 μmol/L within 48 h, or an increase in serum creatinine level to ≥1.5 times the baseline level in < 7 days, using a known or presumed baseline level. The criteria for AKI staging are: stage 1, an increase in serum creatinine to 1.5 to 1.9 folds of the baseline, or an increase in serum creatinine by ≥26.5 μmol/L; stage 2, an increase in serum creatinine to 2.0 to 2.9 folds of the baseline; stage 3, an increase in serum creatinine to 3.0 folds of the baseline or beyond, or an increase in serum creatinine to ≥353.6 μmol/L, or the initiation of renal replacement therapy.
Statistical analysis
Statistical analysis was performed using SPSS software for Windows, version 20.0 (IBM Corp, Armonk, NY, USA). Descriptive analyses were performed for the demographic, clinical, and laboratory data. Univariate regression analysis was used to assess the association of AKI status with different parameters of interest. The continuous data such as white blood cell count were converted into categorical variables (normal, low, or high levels). Multivariate analysis was not performed due to the small sample size. The odds ratio (OR) obtained by logistic regression was used to measure the strength of the association of each factor with AKI. A P value < 0.05 was considered to indicate a statistically significant difference. The date of symptom onset was considered as the starting date. We conducted a death certificate search of the medical records to determine whether any patients who were alive on discharge died subsequently, and included these data in the analysis when applicable.
RESULTS
During the study period, a total of 287 patients who met the case definition of clinically confirmed COVID-19 and other inclusion criteria were included. The characteristics of the overall patients on admission and their AKI status are shown in Tab. 1. Fifty-five patients were diagnosed with AKI and 232 patients did not have AKI. In these patients, the incidence of AKI was 19.2% according to the average serum creatinine method. Compared to the patients without AKI, the patients with AKI were more likely to be male, have an older age, and have underlying comorbidities including chronic renal insufficiency, hypertension, and cerebrovascular disease. The patients with AKI tended to have more severe pneumonia, and were more likely to have hypoxia and tachypnea. Tab. 2 shows the results of laboratory tests of the patients performed on admission. Compared with the patients without AKI, the patients with AKI had higher white blood cell and neutrophil counts, higher levels of D-dimer, aspartate aminotransferase, total bilirubin, creatine kinase, LDH, procalcitonin (PCT), and C-reactive protein (CRP), and had a higher incidence of hyperkalemia; they also had lower lymphocyte counts and lower serum albumin levels.
1.
Characteristics of COVID-19 patients on admission according to acute kidney injury status
| Characteristics | Total | AKI | No AKI | Odds ratio | 95% CI | P |
| Data are presented as number of cases with percentage in parenthesis unless otherwise specified. AKI: Acute kidney injury; IQR: Interquartile range. 1Other symptoms include shivering, headache, chest pain, wheezing, hemoptysis, sore throat, vomiting, and diarrhea. 2Hypoxia is defined as an oxygen saturation ≤93%. 3Tachypnea is defined as a respiratory rate ≥30 min-1 at rest. 4Disease severity is graded according to the American Thoracic Society and Infectious Diseases Society of America criteria. | ||||||
| No. of patients | 287(100.0) | 55(19.0) | 232 (81.0) | - | - | - |
| Sex | ||||||
| Male | 160 (55.7) | 38 (69.1) | 122 (52.6) | 1.0 (ref.) | - | - |
| Female | 127 (44.3) | 17(30.9) | 110(47.4) | 0.50 | 0.27-0.93 | 0.03 |
| Age [year, median (IQR)] | 62 (51-70) | 66 (57-74) | 60 (49-69) | 1.03 | 1.01-1.05 | 0.01 |
| Weight (kg, Mean±SD) | 64.2±11.7 | 68.1±9.3 | 63.0±12.1 | 1.04 | 0.99-1.10 | 0.16 |
| Body mass index (Mean±SD) | 23.6±3.3 | 24.2±2.4 | 23.4±3.5 | 1.08 | 0.90-1.29 | 0.43 |
| Pre-existing comorbidities | ||||||
| Chronic obstructive pulmonary disease | 16(6.0) | 6(11.0) | 10(4.0) | 2.72 | 0.94-7.83 | 0.06 |
| Chronic renal insufficiency | 5 (2.0) | 3 (5.0) | 2(1.0) | 6.64 | 1.08-40.72 | 0.04 |
| Cardiovascular disease | 33(12.0) | 10(18.0) | 23 (10.0) | 2.02 | 0.90-4.54 | 0.08 |
| Hypertension | 87 (30.0) | 23 (42.0) | 64 (28.0) | 1.89 | 1.03-3.47 | 0.04 |
| Diabetes mellitus | 45(16.0) | 12(22) | 33 (14.0) | 1.68 | 0.80-3.52 | 0.16 |
| Cerebrovascular disease | 23 (8.0) | 9(16.0) | 14 (6.0) | 3.05 | 1.24-7.46 | 0.02 |
| Chronic liver disease | 10(4.0) | 1 (2.0) | 9 (4.0) | 0.46 | 0.06-3.70 | 0.73 |
| Cancer | 8 (3.0) | 3 (5.0) | 5(2.0) | 2.62 | 0.61-11.31 | 0.38 |
| Onset to admission time [day, median (IQR)] | 10(6-14) | 10 (6-14) | 9(5-13) | 1.02 | 0.97-1.07 | 0.54 |
| Signs and symptoms | ||||||
| Fever | 223 (78.0) | 40 (73.0) | 183 (79.0) | 0.71 | 0.37-1.40 | 0.32 |
| Cough | 209 (73.0) | 41 (75.0) | 168 (72.0) | 1.17 | 0.57-2.18 | 0.75 |
| Dyspnea | 139(48.0) | 29 (53.0) | 110(47.0) | 1.24 | 0.69-2.23 | 0.48 |
| Myalgia or fatigue | 137(48.0) | 25 (45.0) | 112(48.0) | 0.89 | 0.50-1.61 | 0.71 |
| Other symptoms1 | 138(48.0) | 24 (44.0) | 114(49.0) | 0.80 | 0.44-1.45 | 0.46 |
| Hypoxia2 | 99 (35.0) | 27 (49.0) | 72 (31.0) | 2.14 | 1.18-3.89 | 0.01 |
| Tachypnea3 | 21 (7.0) | 10(18.0) | 11 (5.0) | 4.47 | 1.79-11.14 | 0.01 |
| Family cluster | 23 (8.0) | 3 (6.0) | 20 (9.0) | 0.61 | 0.18-2.14 | 0.62 |
| Disease severity4 | ||||||
| Non-severe | 163 (57.0) | 21 (38.0) | 142 (61.0) | 1.00 (ref.) | - | - |
| Severe | 124(43.0) | 34 (62.0) | 90 (39.0) | 2.54 | 1.40-4.68 | 0.01 |
| PCR result | ||||||
| Negative | 131 (45.6) | 25 (45.5) | 106(45.7) | 1.0 (ref.) | - | - |
| Positive | 156 (54.4) | 30 (54.5) | 126 (54.3) | 1.01 | 0.56-1.82 | 0.98 |
2.
Laboratory results of COVID-19 patients on admission according to acute kidney injury status
| Parameter | Normal range | Total (n=287) | AKI (n=55) | No AKI (n=232) | P |
| Data are presented as median with interquartile range (IQR) in parenthesis unless specified otherwise. AKI: Acute kidney injury. CT: Computed tomography. NA: Not applicable. 1By PCR tests for influenza A virus, influenza B virus, rhinovirus/enterovirus, respiratory syncytial virus A/B, adenovirus, Mycoplasma pneumoniae, parainfluenza viruses, Bordetella pertussis, Legionella pneumophila, and Chlamydophila pneumoniae. 2For each patient, the initial CT scans were evaluated and a quantitative score was assigned based on characteristics reported in recently published literature[9]. | |||||
| White blood cell count (×109/L) | 4.0-10.0 | 5.0 (3.8-7.2) | 5.8 (4.3-9.5) | 4.9 (3.6-6.7) | < 0.01 |
| < 4 [n(%)] | - | 129(45) | 9(16) | 120 (52) | |
| 4-10 [n(%)] | - | 131 (46) | 34 (62) | 97 (42) | < 0.01 |
| >0 [n(%)] | - | 27(9) | 12(22) | 15(6) | |
| Neutrophil count (×109/L), | 1.8-6.3 | 3.6 (2.3-5.5) | 4.5 (3.0-8.5) | 3.4 (2.2-5.1) | < 0.01 |
| Lymphocyte count (×109/L), | 1.1-3.2 | 0.8 (0.6-1.2) | 0.7 (0.4-1.0) | 0.9 (0.6-1.2) | < 0.01 |
| Hemoglobin (g/L, Mean±SD) | 120-150 | 128.3±17.1 | 125±20.0 | 129.1±16.3 | 0.11 |
| Prothrombin time (s, Mean±SD) | 11.0-16.0 | 15.3±5.8 | 17.1±11.7 | 14.9±2.8 | 0.11 |
| Activated partial thromboplastin time (s, Mean±SD) | 27.0-45.0 | 35.8±7.3) | 37.7±10.5 | 35.4±6.3 | 0.05 |
| D-dimer (mg/mL) | 0.0-0.5 | 0.6 (0.2-2.0) | 0.9 (0.2-6.6) | 0.5 (0.2-1.6) | < 0.01 |
| Alanine aminotransferase (U/L) | 0-40 | 25(17-38) | 27(18-43) | 24(16-37) | 0.18 |
| Aspartate aminotransferase (U/L) | 0-40 | 27(20-41) | 35 (23-50) | 26(19-40) | < 0.01 |
| Total bilirubin (μmol/L) | 3.4-20.5 | 8.9(6.6-12.0) | 11.0(8.1-16.0) | 8.6(6.3-11.38) | < 0.01 |
| Albumin (g/L, Mean±SD) | 34.0-54.0 | 33.4±4.8 | 31.6±5.6 | 33.8±4.5 | < 0.01 |
| Creatine kinase (U/L) | 30.0-310.0 | 100.0(58.0-172.0) | 118.0(69.0-223.0) | 99.0(51.25-154.0) | 0.03 |
| Lactate dehydrogenase (U/L) | 120-150 | 255(188-359) | 344 (234-469) | 238(183-333) | < 0.01 |
| Glucose>11.1 mmol/L [n(%)] | 3.9-6.1 | 44(15.0) | 12(22.0) | 32(14.0) | 0.14 |
| Procalcitonin (ng/mL) | < 0.05 | 0.07(0.05-0.16) | 0.15(0.09-0.41) | 0.07 (0.04-0.13) | < 0.01 |
| C-reactive protein (mg/L) | ≤5.0 | 23.4(4.1-38.0) | 34.6(10.6-71.7) | 19.5(4.0-37.0) | 0.02 |
| Hyperkalemia (>5.0 mmol/L) [n(%)] | ≤5 | 22 (8.0) | 10(18.0) | 12(5.0) | < 0.01 |
| Other respiratory pathogens1 [n(%)] | NA | 4(1.0) | 0(0.0) | 4(2.0) | >0.99 |
| CT score2 | NA | 7(5-12) | 9(6-15) | 7(5-11) | < 0.01 |
A nucleic acid assay for 10 respiratory pathogens antibodies detected respiratory syncytial virus A/B infection in 2 patients, Mycoplasma pneumoniae infection in 1 patient, and Chlamydophila pneumoniae coinfection in 1 patient. None of these 4 patients with respiratory coinfections developed AKI (Tab. 2). Fig. 1 shows the typical CT images of a patient taken at different time points during hospitalization (from the initial onset of symptoms to full recovery). The patients with AKI had a significantly higher median chest CT score than those without AKI (Tab. 2).
1.
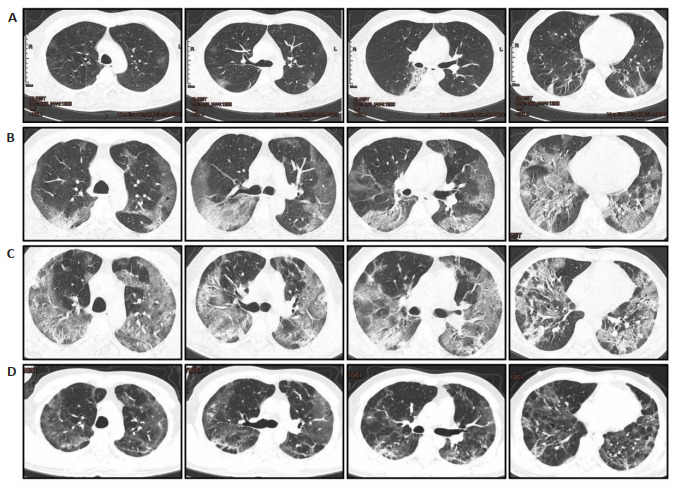
Transverse chest computed tomography (CT) images of the lungs of a 47-year-old man with COVID-19.
Of the 287 patients included in the study, 65.5% were discharged by March 8, 2020 (the date on which follow-up was censored). The mortality rate was 6.6% (Tab. 3). The incidence of stage 1 and stages 2/3 AKI was 14.3% and 4.9%, respectively. The AKI stage was related to the clinical endpoint of the patient. Analysis of the outcomes of the patients in relation to AKI status and stage showed that patients with stage 1 and stages 2/3 AKI had significantly lower discharge rates than the non-AKI patients (41.5% and 14.3% vs 72.4%, P < 0.01) with also significantly higher mortality rates (7.3% and 64.3% vs 3.0%, P < 0.01). The median length of hospital stay of non-AKI, AKI stage 1, and AKI stages 2/3 patients was 18.0, 22.5, and 12.0 days, respectively.
3.
Clinical outcomes of COVID-19 patients at data cutoff
| Outcome | All patients (n=287) | NoAKI (n=232) | AKI stage 1 (n=41) | AKI stages 2 & 3 (n= 14) | P |
| Clinical outcomes [n(%)] | < 0.01 | ||||
| Discharge from hospital | 188 (65.5) | 168 (72.4) | 17(41.5) | 2(14.3) | |
| Hospitalization | 80 (27.9) | 57 (24.6) | 21 (51.2) | 3(21.4) | |
| Death | 19 (6.6) | 7(3.0) | 3(7.3) | 9 (64.3) | |
| Hospital stay [day, median (IQR)] | 18.0 (12.0-23.0) | 18.0(13.0-23.0) | 22.5 (8.3-28.0) | 12.0(4.5-17.8) | 0.02 |
DISCUSSION
Our results show that an older age, severe pneumonia, and pre-existing cardiovascular and renal disease are all associated with an increased risk of AKI in COVID-19 patients. A recent seminal work suggested that the COVID-19 uses the angiotensin converting enzyme Ⅱ (ACE2) as a cell entry receptor, a cellular mechanism identical to that of the SARS-CoV [14, 15]. In addition, a gain-of-function experiment found that the ACE2 could be used by a wildtype coronavirus of a bat origin to gain entry into cells [15, 16]. Of note, the expression of ACE2 is almost 100-fold higher in the kidney tissue than in the respiratory organs, suggesting that the renal cells may be the target of infection by SARS-CoV and SARS-CoV-2. The study by Li et al [15]on kidney function in patients with COVID-19 showed that 63% of the patients exhibited proteinuria, with an elevated level of plasma creatinine and blood urea nitrogen. CT scans also showed radiographic abnormalities of the kidneys in COVID-19 patients [15], and most importantly, SARS-CoV-2 nucleic acids were found in the urine of the patients [2]. Taken together, these evidences indicate that kidney impairment may be one of the major causes of morbidity and may contribute to multiorgan failure and death in COVID-19 patients.
We found that among the 287 patients included in this study, the incidence of AKI was substantially higher than the overall incidence of 0.5%-7% reported in previous studies [2, 7] and was comparable to that in critically ill patients hospitalized with other illnesses (29%) [3]. The high incidence of AKI observed in this study was presumably attributed to several reasons. First, most of the COVID-19 patients included in this study had severe disease. At the time of admission, 43% of the patients had severe disease. Although many patients had low pulse oxygen saturation at admission, they appeared to have mild symptoms of dyspnea, possibly as a result of gradual deterioration of the condition to allow for adaption and compensation. The very high CT scores and rapid deterioration after admission in many patients also suggest the severe condition of these patients. Second, the methods that we used to diagnose and stage AKI might cause overestimation of the incidence and severity of AKI. In the early stage of COVID-19 outbreak, the medical resources was overwhelmed and many patients did not have a baseline serum creatinine test prior to admission or in the previous year, so that we could only estimate the baseline serum creatinine according to the patient's sex, weight, medical history and other related parameters. The incidence of AKI was determined based on baseline values and serum creatinine levels within 7 days of admission. In order to reduce the bias of the estimation, we used two methods for AKI diagnosis in these patients, namely the average serum creatinine method [11] and eGFR 75 method [17], by which the incidence of AKI was 25.8% and 19.2%, respectively (Tab. 4). The estimated baseline serum creatinine levels by the eGFR75 method differed significantly from the test results of some of the patients whose baseline serum creatinine results were available. The inaccuracies in the estimated baseline serum creatinine level might have caused an overestimation of the incidence of AKI in our study.
4.
Accuracy of AKI diagnosis between average SCr method and eGFR 75 method [n(%)]
| Diagnosis | Average SCr1 | eGFR 752 |
| 1Baseline serum creatinine level was estimated from average levels of SCr based on age and gender using the data of non-AKI patients. 2Estimated baseline SCr was back-calculated from the eGFR value fixed by age and gender (75 mL·min-1·1.73 m-2) using the MDRD equation. | ||
| Non-AKI | 213(74.2) | 232(80.8) |
| AKI | 74(25.8) | 55(19.2) |
| Stage 1 AKI | 55(19.2) | 41(14.3) |
| Stage 2-3AKI | 19(6.6) | 14(4.9) |
However, even the overestimated incidence of AKI does not directly affect our investigation of the risk factors of AKI. We found that an older age, multiple pre-existing comorbidities, an increased white blood cell count, low lymphocyte count, and increased levels of PCT and CRP were all risk factors for AKI in patients with COVID-19. The prevalence of several pre-existing comorbidities, including chronic lung diseases and diabetes mellitus, did not differ significantly between the patients with and without AKI, although this might be due to the relatively small number of cases of AKI in this study. Lymphopenia indicates that decreased immunity might occur in patients with COVID-19, and increased levels of PCT and CRP suggest that some patients may have secondary bacterial infections that caused excessive inflammatory responses. Unfortunately, we did not conduct bacterial or fungal cultures for the patients with respiratory coinfections detected by PCR due to the shortage of medical staff, and were unable to test the patients' inflammatory factor profiles because no test kits were available in the local hospital. Previous studies [2-4, 7] have shown that patients with COVID-19 have significantly increased D-dimer levels, multiple organ damage, and electrolyte disturbances. Our study showed that these abnormalities were more pronounced in patients with AKI. The occurrence and development of AKI often leads to the imbalance of blood volume and electrolytes, accumulation of metabolites, and aggravation of multiple organ dysfunction, thus creating a vicious cycle. The majority of patients with stage 1 AKI recovered and were discharged, but those who progressed into stage 2/3 AKI had an extremely high mortality rate, possibly due to the limited treatment conditions in the local facility. Early intervention of AKI is thus important in these patients, and continuous renal replacement should be considered to prevent the progression of AKI.
This study has several limitations. First, due to the large number of patients and the shortage of medical staff and equipment, we did not perform blood gas analysis or blood lactate measurement in most patients. Instead, we indirectly evaluated oxygenation of the patients based on their chief complaints, pulse oxygen saturation monitoring and CT. Second, we did not explore the impact of treatment on AKI, because our treatments were based on WHO's standardized protocols [18], in which the antiviral drugs, antibacterial drugs and hormones have little effect on renal function. Third, we did not exclude patients with chronic renal insufficiency. Five of the patients had chronic renal insufficiency, including 3 in AKI group and 2 in the non-AKI group. Two of the patients had hypertension and 3 were diabetic at admission, but they all had normal creatinine level in the previous year. Fourth, the urgency in data collection and short follow-up time of the patients (the shortest hospital stay of the patients was only 14 days) may affect the final prognostic evaluation of the patients to cause bias in survival time analysis. Fifth, we did not analyze such comorbidities as shock and adult respiratory distress syndrome (ARDS), because we aimed primarily to analyze the risk factors of AKI in COVID-19 patients on admission to provide evidence for the development of COVID-19 prevention and treatment guidelines.
Conclusion
The kidney is a primary target organ of SARS-CoV-2 and the incidence of AKI is high in hospitalized patients with COVID-19. Deterioration of kidney function aggravates other organ damage. An older age, severe pneumonia, and pre-existing cardiovascular and renal diseases are potential risk factors for AKI in patients with COVID-19. Although the incidence of stage 1 AKI is high in critically ill patients, most of the patients can have favorable outcomes; but progression into stage 2/3 AKI is associated with a very high mortality rate, and the prevention of AKI and monitoring of kidney function is thus important in clinical management of COVID-19. Future studies of AKI in COVID-19 patients should use more accurate baseline creatinine levels and urine volume measurements as the basis for AKI diagnosis. Larger, multi-centered studies are needed to obtain a better understanding of AKI for its prevention and management in COVID-19 patients.
Biographies
肖冠华,硕士,副主任医师,E-mail: nanfang_xiao@vip.163.com
胡鸿彬,博士,E-mail: hobewoos@163.com
吴凤,博士,副主任医师,E-mail:wishuhappy@126.com
Funding Statement
Supported by National Natural Science Foundation of China (81871604), Natural Science Foundation of Guangdong Province(2020A151501361, 2017A030313590)
国家自然科学基金(81871604);广东省自然科学基金(2020A151501361,2017A030313590)
Contributor Information
Guanhua XIAO (肖 冠华), Email: nanfang_xiao@vip.163.com.
Hongbin HU (胡 鸿彬), Email: hobewoos@163.com.
Feng WU (吴 凤), Email: wishuhappy@126.com.
Zhongqing CHEN (陈 仲清), Email: zhongqingchen2008@163.com.
Shumin CAI (蔡 淑敏), Email: smallsnail_cherry@163.com.
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33. doi: 10.1056/NEJMoa2001017. [Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019[J]. N Engl J Med, 2020, 382(8): 727-33.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. doi: 10.1056/NEJMoa2002032. [Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China[J]. N Engl J Med, 2020, 382(18): 1708-20.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a singlecentered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81. doi: 10.1016/S2213-2600(20)30079-5. [Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a singlecentered, retrospective, observational study[J]. Lancet Respir Med, 2020, 8(5): 475-81.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9. doi: 10.1001/jama.2020.1585. [Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China[J]. JAMA, 2020, 323(11): 1061-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-toperson transmission: a study of a family cluster. Lancet. 2020;395(10223):514–23. doi: 10.1016/S0140-6736(20)30154-9. [Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-toperson transmission: a study of a family cluster[J]. Lancet, 2020, 395 (10223): 514-23.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–74. doi: 10.1016/S0140-6736(20)30251-8. [Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding[J]. Lancet, 2020, 395(10224): 565-74.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China[J]. Lancet, 2020, 395 (10223): 497-506.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13. doi: 10.1016/S0140-6736(20)30211-7. [Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study[J]. Lancet, 2020, 395(10223): 507-13.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295(1):202–7. doi: 10.1148/radiol.2020200230. [Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV)[J]. Radiology, 2020, 295(1): 202-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295(3):715–21. doi: 10.1148/radiol.2020200370. [Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19)[J]. Radiology, 2020, 295(3): 715-21.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84. doi: 10.1159/000339789. [Khwaja A. KDIGO clinical practice guidelines for acute kidney injury [J]. Nephron Clin Pract, 2012, 120(4): c179-84.] [DOI] [PubMed] [Google Scholar]
- 12.Hatakeyama Y, Horino T, Nagata K, et al. Evaluation of the accuracy of estimated baseline serum creatinine for acute kidney injury diagnosis. Clin Exp Nephrol. 2018;22(2):405–12. doi: 10.1007/s10157-017-1481-y. [Hatakeyama Y, Horino T, Nagata K, et al. Evaluation of the accuracy of estimated baseline serum creatinine for acute kidney injury diagnosis[J]. Clin Exp Nephrol, 2018, 22(2): 405-12.] [DOI] [PubMed] [Google Scholar]
- 13.De Rosa S, Samoni S, Ronco C. Creatinine-based definitions: from baseline creatinine to serum creatinine adjustment in intensive care. Crit Care. 2016;20:69. doi: 10.1186/s13054-016-1218-4. [De Rosa S, Samoni S, Ronco C. Creatinine-based definitions: from baseline creatinine to serum creatinine adjustment in intensive care [J]. Crit Care, 2016, 20: 69.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–54. doi: 10.1038/nature02145. [Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus[J]. Nature, 2003, 426(6965): 450-54.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Wu M, Yao JW, et al. Kidney dysfunctions of COVID-19 patients: a multi-centered, retrospective, observational study. SSRN Electronic Journal. 2020 doi: 10.2139/ssrn.3556634. [Li Z, Wu M, Yao JW, et al. Kidney dysfunctions of COVID-19 patients: a multi-centered, retrospective, observational study[J]. SSRN Electronic Journal, 2020, 10.2139/ssrn.3556634] [DOI] [Google Scholar]
- 16.Menachery VD, Yount BL Jr, Debbink K, et al. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat Med. 2015;21(12):1508–13. doi: 10.1038/nm.3985. [Menachery VD, Yount BL Jr, Debbink K, et al. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence [J]. Nat Med, 2015, 21(12): 1508-13.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kork F, Balzer F, Krannich A, et al. Back-calculating baseline creatinine overestimates prevalence of acute kidney injury with poor sensitivity. Acta Physiol (Oxf) 2017;219(3):613–24. doi: 10.1111/apha.12763. [Kork F, Balzer F, Krannich A, et al. Back-calculating baseline creatinine overestimates prevalence of acute kidney injury with poor sensitivity[J]. Acta Physiol (Oxf), 2017, 219(3): 613-24.] [DOI] [PubMed] [Google Scholar]
- 18.WHO. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. 28 January, 2020. <a href="https://www.who.int/publications-detail/clinincal-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected" target="_blank">https://www.who.int/publications-detail/clinincal-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected</a>.


