Abstract
Background and Aim:
Serum prostate-specific-antigen (PSA) guided systematic transrectal ultrasound (TRUS)-guided biopsies are known to have a low predictive value in detection of primary prostate carcinomas (PCa). Our aim was to evaluate the accuracy of gallium-68 (Ga-68) prostate-specific membrane antigen positron emission tomography/computed tomography (PSMA PET/CT) for the detection of PCa with serum PSA <50 ng/ml.
Patients and Methods:
We retrospective analyzed prebiopsy Ga-68 PSMA PET/CT's of all patients with suspected PCa from October 2019 to March 2020. Several quantitative clinical and PET/CT variables were compared in benign and malignant groups and assessed for significance using an independent t-test. Diagnostic performance of PSMA PET/CT for detection of cancer was evaluated and compared with the diagnostic performance of cancer risk predicting calculator (European Randomized Study for Screening of Prostate Cancer [ERSPC3]). The standard of reference was 12-core TRUS-guided biopsies.
Results:
Sixty-four patients were included with mean age 70 years (range 48–94 years); mean PSA 15.67 ng/ml (range 1.74–44), mean PSA density 0.32 ng/ml2 (range 0.01–0.99) and mean prostate volume 54.55 cc (range 16.5–182). 64% (n = 41/64) patients had benign histology and 36% (n = 23/64) had carcinoma. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of PSMA PET/CT for detecting PCa reported using the prostate cancer molecular imaging standardized evaluation (PROMISE) was 74%, 92%, 85%, 86%, and 86%, respectively. Mean prostate maximum standardized uptake value (SUVmax) was significantly higher in PCa versus Benign lesions (19.56 ± 18.11 vs. 4.21 ± 1.5, P = 0.00001), in patients with PSA >20 ng/ml versus PSA <20 ng/ml (19.1 ± 20.6 vs. 6.01 ± 5.4, P-0.0052), and in patients with Gleason's score (GS) score >7 versus GS ≤7 (28.1 ± 20.3 vs. 10.2 ± 8.9, P-0.010). SUVmax cutoff value of 5.6 on PSMA PET/CT showed a sensitivity of 95% and specificity of 90.9% (area under the curve 0.990, P < 0.0001).
Conclusion:
Ga-68 PSMA PET/CT can differentiate benign and malignant lesions of the prostate with very high accuracy and when used alongside with ERSPC3 calculator and magnetic resonance imaging, could potentially reduce painful and often unnecessary prostate biopsies.
Keywords: Biopsy, calculator, cancer, carcinoma, European Randomized study for Screening of Prostate Cancer, gallium-68, hyperplasia, magnetic resonance imaging, positron emission tomography/computed tomography, prostate, prostatitis, prostate specific membrane antigen, transrectal ultrasound
Introduction
Prostate cancer is the leading cause of cancer in men worldwide, and the burden of the disease continues to grow due to an increase in life expectancies. In India too, most population-based cancer registries have shown a significant increase in the incidence of prostate cancers over the past three decades, and currently estimates is about 10/1,00,000 population[1,2]. With increased awareness for growing prostate cancer in developing countries like India, widespread serum prostate-specific antigen (PSA) screening is also expected to increase. PSA-based systematic biopsies are associated with a high incidence of false-positive results (about 60%–75% reported in major trials) and also carries minimal risk for biopsy-related complications such as pain, fever, and bleeding[3]. To reduce unnecessary biopsies, European Association of Urology strongly recommends the use of cancer predicting risk calculators such as European Randomized Study for Screening of Prostate Cancer (ERSPC3) or advanced imaging such as multi-parametric magnetic resonance imaging (mpMRI), especially in asymptomatic patients with the normal digital rectal examination and PSA between 2 and 10 ng/ml.[4]
Another emerging diagnostic tool for predicting prostate cancer before biopsy is molecular imaging using gallium-68 labeled prostate-specific membrane antigen positron emission tomography/computed tomography (PSMA PET/CT).[5,6] PSMA is a transmembrane protein with significantly increased expression in the cells and metastases of prostate cancer (PCa) about four times compared with normal prostate (PN).[7] A study done by Woythal et al. showed that prostate PSMA uptake on PET/CT strongly correlates with PSMA expression on immunohistochemistry, thereby supporting and validating the potential diagnostic use of PSMA PET in primary PCa detection.[8]
Our primary aim of this study was to evaluate the diagnostic accuracy of prebiopsy 68-Ga-PSMA PET/CT for the detection of primary PCa in patients with PSA range 0.4–50 ng/ml. In addition, we aimed to compare the diagnostic performance of PSMA PET/CT with ERSPC3 and also compared findings of PSMA PET/CT and bi-parametric MRI (Bip-MRI) in a small sub-cohort of patients (n = 25).
Patients and Methods
This was a retrospective study analyzing the results of all Ga-68 PSMA PET/CT, which was done before biopsy for clinically suspected cancer cases with raised PSA and/or positive digital rectal examination. Patients with PSA outside the range of that used in the ERSPC3 calculator (0.4–50 ng/ml), prior treatment (hormonal, radiotherapy, or surgery) or without biopsy evidence was excluded from the study [Figure 1]. The study was done after receiving clearance from the institutional ethical committee.
Figure 1.
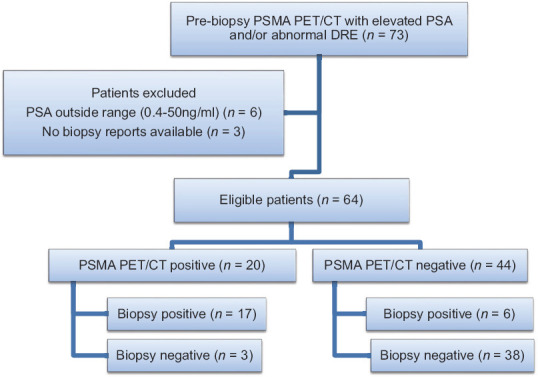
Flow chart of patients included in the study
Radiopharmaceutical for imaging was gallium-68 labeled PSMA 11 (Ga-68 PSMA), synthesized using computer run fully automated synthesizer iQS-TS system (ITM Isotopen Technologien München AG, Germany). Quality control of radiopharmaceuticals was done to ensure 95% radio-labeling before injecting to patients. The total synthesis time was about 20 min. About 2–2.2 MBq/kg of synthesized Ga-68 PSMA-11 was injected intravenously (IV) injected in the arm, and scans were acquired after 60 min and another delayed scan of pelvis post 20 mg furosemide IV at 120 min. Imaging was performed on a GE 5 ring PET/CT system Discovery IQ 5 Ring block detectors PET/CT (General Electric, Milwaukee, WI, USA), combining bismuth germanium oxide (BGO)-based PET crystal and 16-slice CT components. Noncontrast CT and PET data were acquired from mid-thigh level to the top of the skull with the arms raised. PET emission counts were collected over 2.5 min per table position, acquired in a three-dimensional mode with standard Vue Point HD (VPHD) reconstruction (filter 5.5 mm, subsets 12, 4 iterations, order 4) or Q. clear algorithm (beta value 350). No adverse events were reported in any patient post PET/CT scans.
PET/CT scans were interpreted by two separate experienced Nuclear Medicine Physicians. PSMA PET/CT was reported as per the prostate cancer molecular imaging standardized evaluation (PROMISE) criteria for quantifying PSMA expression on the prostate (mi-PSMA ES). Mi-PSMA ES Score 0-PSMA uptake below mediastinal blood pool, mi-PSMA ES Score 1: PSMA uptake above blood poo but less than liver uptake, mi-PSMA ES Score 2: Uptake more than a liver activity but less than parotid uptake and mi-PSMA ES Score 3: Uptake more than parotid uptake. Score 0 and 1 were considered PET/CT negative for malignancy, and Score 2 and 3 were considered PET/CT positive for malignancy. BipMRI using the only T2-weighted and diffusion-weighted imaging sequences (DWI) were used to prebiopsy lesion localization. No dynamic contrast-enhanced sequences (DCE) were acquired in any patient. Images were reported as per the prostate imaging and reporting data system version 2 (PI-RADS). PI-RADS 4 or 5 were regarded as positive, and PI-RADS=/< 3 regarded as negative. Gold standard for evaluation of PET/CT findings was 12 core transrectal ultrasound (TRUS) guided biopsies.
Diagnostic performance of PSMA PET/CT, ERSPC3 and MRI was evaluated. Independent t-test was used to compare the mean values of the quantifiable variable between the malignant and benign groups. The same test was used to assess the difference in maximum standardized uptake value (SUVmax) values between tumors with high-risk features (PSA >20 ng/ml, PSA density >0.15 and Gleason's score [GS] >7) and low-risk features in patients with biopsy proven malignancies (PSA =/< 20 ng/ml, PSA density =/< 0.15 and GS =/< 7). Receiver operating characteristic curve (ROC) analysis was done to determine the best cutoff and area under the curve for ERSPC3 and PSMA PET/CT. P ≤ 0.05 were regarded as indicating statistical significance.
Results
Overall 64 patients were recruited in the study with mean age 70 years (range 48–94 years), mean PSA 15.67 ng/ml (range 1.74–44), mean PSA density 0.32 (range 0.01–0.99) and mean prostate volume was 54.55 cc (range 16.5–182) [Figure 1 and Table 1]. About 64% (n = 41) patients had benign histology and 36% (n = 23) had carcinoma. Out of 23 patients, 9% (n = 2) patients had GS 6, 40% (n = 9) patients had GS 7, 17% (n = 4) patients had GS 8, 17% (n = 4) patients had GS9 and 17% (n = 4) patients had GS 10. Of 23 carcinoma cases, 65.2% of the cases were organ confined, 17.4% of cases were N1 (stage IVA) and 17.4% were M1 (stage IVB) [Table 1]. Mean blood pool, liver, and parotid SUVmax (±SD), calculated from 41 patients with normal PSMA PET/CT was 2.04 (±0.84), 6.48 (±1.7), and 14.49 (±5.8).
Table 1.
Patient demographics
| n=64 | Value (Range) |
|---|---|
| Mean Age | 70 years (48-94) |
| Mean PSA | 13.7ng/ml (1.74-44) |
| Mean Prostate Volume | 54.55cc (16.5-182) |
| Mean PSA density | 0.32 ng/ml2 (0.01-0.99) |
| Digital rectal examination | |
| Normal | 24 |
| Abnormal | 40 |
| ERSPC risk calculator 3 | |
| <20% risk | 15 |
| >/= 20% risk | 49 |
| PSMA PET/CT | 43 |
| Negative | 21 |
| Positive | |
| Benign cases | 41 |
| Benign Hyperplasia | 28 |
| Prostatitis | 13 |
| Carcinomas | 23 |
| =/<7 Gleason’s score | 11 |
| >7 Gleason’s score | 12 |
| Clinical stage of Carcinomas | |
| Organ confined | 15 |
| IVA | 4 |
| IVB | 4 |
Comparison of the variables between the malignant and benign groups showed that the mean prostate SUVmax was significantly higher in PCa vs. benign lesions (19.56 ± 18.11 vs. 4.21 ± 1.5, P = 0.00001) [Figure 2]. Mean PSA, PSA density, and prostate to liver SUVmax ratio were also significantly higher in the malignant group compared to benign, except prostate volume, which was not significantly different between the two groups [Table 2].
Figure 2.
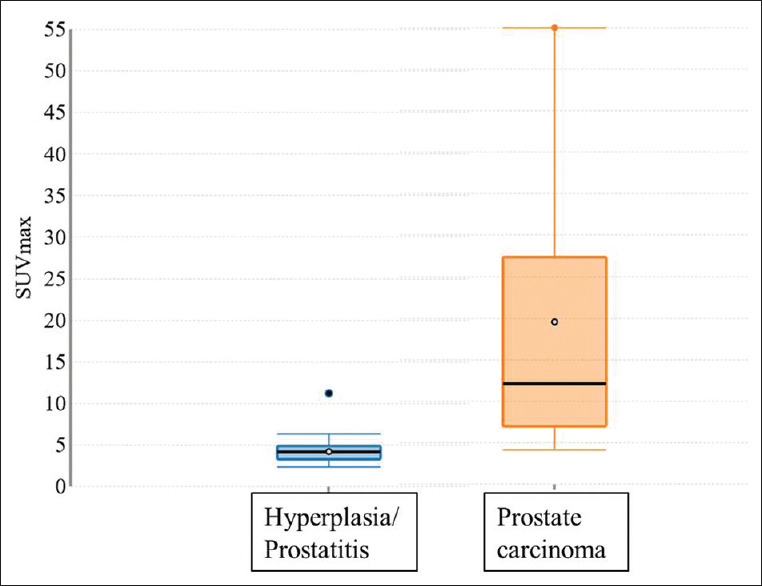
Box plot to show relation between prostate maximum standardized uptake value and biopsy results
Table 2.
Difference in mean values of various parameters in benign and malignant prostate lesions using independent t-test
| Variables | Benign (Mean+/- SD) | Malignant (Mean+/- SD) | P |
|---|---|---|---|
| Mean Age (years) | 69.9+/-8.38 | 70.7+/-9.9 | 0.348 |
| Mean PSA (ng/ml) | 13.16+/-10.77 | 20.16+/-12.4 | 0.010 |
| Mean Prostate volume (cc) | 56.29+/-22.9 | 51.47+/-35.7 | 0.256 |
| Mean PSA density (ng/ml2) | 0.26 +/-0.23 | 0.44 +/-0.27 | 0.0039 |
| Mean Prostate SUVmax | 4.21+/-1.5 | 19.56+/-18.11 | 0.00001 |
| Mean Prostate to liver SUVmax ratio | 0.83+/-0.70 | 3.01 +/-2.89 | 0.00001 |
Comparison of the high and low risk groups showed that mean prostate SUVMax was significantly higher in patients with PSA >20 ng/ml versus PSA =/< 20 ng/ml (19.1 ± 20.6 vs. 6.01 ± 5.4, P-0.0052), in patients in PSA density >0.15 versus PSA density ≤0.15 (11.8 ± 15.4 vs. 5.4 ± 2.89, P-0.038) and in patients with GS score >7 versus GS ≤7 (28.1 ± 20.3 vs. 10.2 ± 8.9, P-0.010) [Figure 3 and Table 3]. There was a weak positive correlation of SUV wax with GS (r = 0.316), PSA value (r = 0.497), PSA density (r = 0.257), and prostate volume (r = 0.28).
Figure 3.
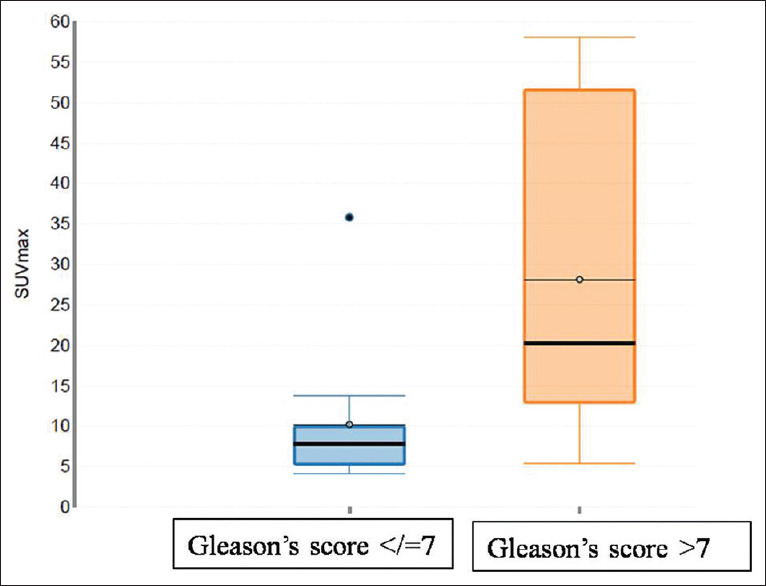
Box plot to show relation between prostate maximum standardized uptake value and Gleason's scores
Table 3.
Difference in mean prostate maximum standardized uptake value values between high-risk and low-risk groups using independent t-test
| Variables | Mean SUVmax+/- SD | P |
|---|---|---|
| PSA =/<20ng/ml PSA >20 ng/ml |
6.01+/-5.4 19.1+/-20.6 |
0.00007 |
| PSA density =/<0.15ng/ml2
PSA density >0.15ng/ml2 |
5.4+/-2.89 11.8+/-15.4 |
0.038 |
| GS score </=7 GS score >7 |
10.2+/-8.9 28.1+/-20.3 |
0.006 |
Using standardized PROMISE criteria, PSMA PET/CT was negative (defined as score miPSMA expression score 0 and 1) in 69% (n = 44) patients and positive (defined as score miPSMA expression score 2 or 3) in 31% patients (n = 20). Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of PSMA PET/CT for detecting prostate cancer using PROMISE criteria was 74%, 92%, 85%, 86%, and 86% respectively with six false-negatives and three false-positives on PSMA PET/CT. Details of these patients are summarized in [Tables 4 and 5].
Table 4.
Patients with false positive PSMA PET/CT
| Age (yrs) | PSA (ng/ml) | MRI | SUVmax | mi-PSMA score | Biopsy |
|---|---|---|---|---|---|
| 68 | 12.8 | N/A | 5.74 | 2 | Benign hyperplasia |
| 94 | 7.31 | N/A | 11.22 | 2 | Benign hyperplasia |
| 75 | 45 | N/A | 5.32 | 2 | Benign hyperplasia |
Table 5.
Patients with false-negative prostate-specific membrane antigen positron emission tomography/ computed tomography
| Age (yrs) | PSA (ng/ml) | MRI | SUVmax | mi-PSMA score | Biopsy | Gleason’s score |
|---|---|---|---|---|---|---|
| 52 | 18.66 | N/A | 4.26 | 1 | Carcinoma | 6 |
| 68 | 15 | PIRADS5 | 6.4 | 1 | Carcinoma | 9 |
| 68 | 6.77 | N/A | 6.3 | 1 | Carcinoma | 6 |
| 69 | 7.4 | PIRADS5 | 4.2 | 1 | Carcinoma | 7 |
| 66 | 17.5 | PIRADS1 | 4.12 | 1 | Carcinoma | 7 |
| 58 | 22.3 | PIRADS5 | 5.47 | 1 | Carcinoma | 10 |
On ROC analysis, SUVmax cutoff value of 5.6 on PSMA PET/CT showed a sensitivity of 95% and specificity of 90.9% (area under the curve [AUC] 0.990, P < 0.0001). The cutoff value of the ERSPC3 calculator at a 40% threshold showed a sensitivity of 65% and specificity of 54.5% (AUC 0.669, P-0.031) [Table 6 and Figure 4].
Table 6.
Diagnostic performance of prostate-specific membrane antigen positron emission tomography/computed tomography compared to the European randomized study for prostate cancer risk calculator on receiver operating characteristic analysis
| n=64 | Sensitivity (%) | Specificity (%) | Area Under curve (AUC) |
|---|---|---|---|
| PSMA PET/CT (Using SUVmax threshold 5.6) | 95 | 90 | 0.99 |
| ERSPC3 calculator (40% risk probability threshold) | 57 | 80 | 0.67 |
Figure 4.
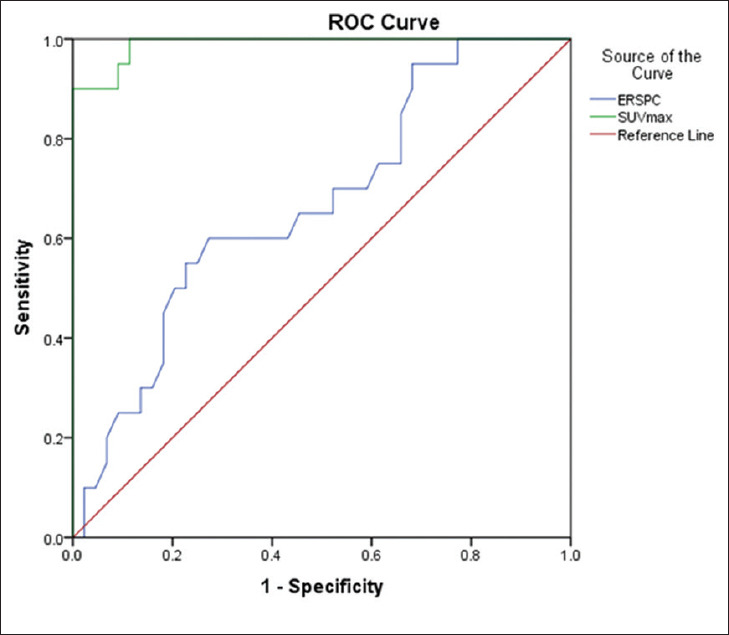
Receiver-operator characteristic curve of the European Randomized study for Screening of Prostate Cancer and maximum standardized uptake value for prediction of prostate cancer
Bip-MRI was available in 39% (n = 25) patients. MRI was positive (PI-RAD 4/5) in 18 patients and negative (PI-RADS 1/2/3) in 6 patients. Findings of PSMA PET/CT and Bi-MRI were concordant in 15 cases and discordant in 10 cases [Figures 5 and 6]. All these discordant cases were seen in patients with positive MRI findings (PI-RADS 4–5) and negative PSMA PET/CT (mi-PSMA ES <2) and 70% (n = 7/10) of these patients were biopsy negative. The details of these PSMA-MRI discordant cases are summarized in Table 7.
Figure 5.
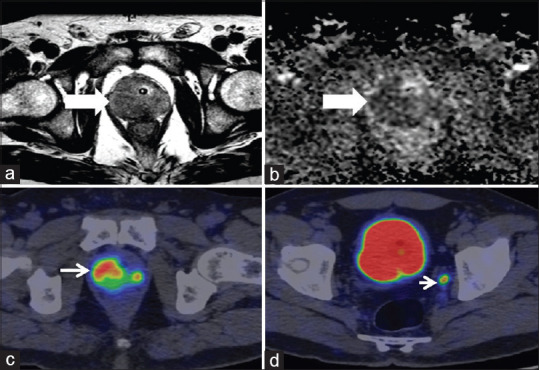
Concordant magnetic resonance imaging and prostate-specific membrane antigen positron emission tomography findings. Hypointense signal noted in the right peripheral zone of the prostate on T2-weighted and apparent diffusion coefficient map, PIRADS 5 (a and b, bold white arrows, respectively), intense prostate specific membrane antigen uptake (mi-prostate-specific membrane-antigen score 3) noted in the right peripheral zone, (thin white arrow, c) and 5mm left internal iliac node missed on magnetic resonance imaging (white arrow head, d). Biopsy was suggestive of adenocarcinoma Gleason's score 9
Figure 6.
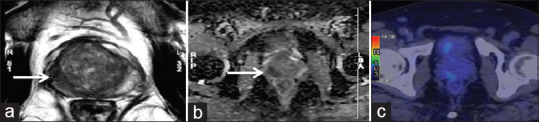
Discordant magnetic resonance imaging and prostate-specific membrane antigen positron emission tomography/computed tomography. Hypointense lesion on T2-weighted and apparent diffusion coefficient map in the right peripheral zone of the prostate (PIRADS 5) showing no focal abnormal prostate specific membrane antigen uptake on the transaxial fused positron emission tomography/computed tomography image (mi-prostate-specific membrane antigen score 1). Biopsy was suggestive of prostatitis
Table 7.
Patients with discordant magnetic resonance imaging and prostate-specific membrane antigen positron emission tomography/computed tomography findings
| Age (yrs) | PSA (ng/ml) | MRI | SUVmax | mi-PSMA score | Biopsy | Gleason’s score |
|---|---|---|---|---|---|---|
| 65 | 6.9 | PI-RADS5 | 3.25 | 1 | Prostatitis | N/A |
| 72 | 8.1 | PI-RADS4 | 3.28 | 1 | Hyperplasia | N/A |
| 69 | 7.4 | PI-RADS5 | 4.2 | 1 | Carcinoma | 7 |
| 69 | 5.85 | PI-RADS4 | 3.06 | 1 | Prostatitis | N/A |
| 48 | 8.57 | PI-RADS4 | 3.5 | 1 | Prostatitis | N/A |
| 80 | 12.6 | PI-RADS4 | 4.3 | 1 | Prostatitis | N/A |
| 69 | 11.87 | PI-RADS5 | 4.1 | 1 | Hyperplasia | N/A |
| 58 | 22.3 | PI-RADS5 | 5.47 | 1 | Hyperplasia | N/A |
| 68 | 15 | PI-RADS5 | 6.48 | 1 | Carcinoma | 9 |
| 52 | 18.66 | PI-RADS4 | 4.26 | 1 | Carcinoma | 6 |
Discussion
Serum PSA is burdened by low diagnostic accuracy because it is an organ-specific rather than tumor-specific biomarker, and it can be elevated not just in cancer but also in benign lesions such as prostatic hyperplasia and prostatitis. Positive predictive value for cancer detection using PSA value >50 ng/ml is almost 100%, whereas <30 ng/ml it reduces to 73.6%, and it is seen that about 14% of the prostate cancer cases occur below the globally accepted PSA level of 4 ng/ml.[9,10] Hence, our study of using PSMA PET/CT for cancer prediction was primarily aimed at patients with total PSA between 0.4 and 50 ng/ml, a range within which clinical diagnosis of prostate cancer is most often uncertain. The results of our study show that within this PSA range, PSMA PET/CT not just showed a very high diagnostic accuracy for prebiopsy primary prostate cancer prediction but also had better discriminatory value than cancer risk calculators and Bip-MRI.
Diagnostic performance of PSMA PET/CT in predicting prostate cancer prior to biopsy in similar PSA range (0.4–50 ng/ml) was previously reported by Zhang et al. in 58 patients.[11] The sensitivity and specificity of PSMA PET/CT were 91.6 and 81.2% in their study. The higher sensitivity reported in their study could be probably because their study had a higher percentage of patients who were biopsy positive (64% of patients), compared to our study (36% patients). Another difference is that in their study, positive PET/CT was visual estimation defined based on any prostate uptake more than local background (i.e., uptake in gluteal muscle). In this study, we instead scoring system as per the PROMISE criteria, a recently formulated standardized framework for reporting PSMA ligand-based PET/CTs.[12] Out of the 23 biopsy positive cases in our study, mi-PSMA ES score 1 was seen in 26% (n = 6/23), and 74% (n = 17/23) were either mi-PSMA ES score 2/3. Using this system, our sensitivity and specificity were 74% and 92%, respectively. A similar scoring system was used in a study done by Liu et al. in patients with prior negative biopsy and elevated PSA, and they reported sensitivity and specificity of PSMA PET/CT as 89% and 71%, respectively.[13] Their study found that 12.9% (4 out of 31) were falsely positive; in our study, we found 4.6% (n = 3/64) cases to be false-positive (all with mi-PSMA-ES score 2) and 9.3% of cases (n = 6/64) as false negative (all with mi-PSMA ES 1, i.e., uptake less than liver uptake) on PSMA PET/CT [Tables 4 and 5]. None of our patients with mi-PSMA-ES score 3 had benign biopsy results. Diagnostic accuracy of PSMA PET/CT results using SUVmax cutoff value seems to be better than using the standardized PROMISE criteria. We must understand the fact that mi PSMA-ES scoring compares the PSMA uptake in prostate relative to uptake in the blood pool, liver, or parotid uptake. Mean blood pool, liver, and parotid SUVmax in our study were 2.04 ± 0.84, 6.48 ± 1.7, and 14.49 ± 5.8, respectively. Compared to blood pool uptake (variance 0.7), liver and parotid uptake show higher individual variation (variance 3.10 and 34.4, respectively) and hence probably are not reliable standards of reference uptake when reporting PSMA ligand PET/CTs.[14] We believe using SUVmax cutoff value instead may not just have higher diagnostic accuracy but would probably have a better reproducibility and inter-observer agreement than comparing relative organ uptakes. Large prospective studies with inter-observer agreement evaluation are needed to validate our claim.
Individualized screening using established prostate cancer predicting algorithms such as ERSPC or prostate cancer prevention trial risk calculators have been used to reduce unnecessary biopsies and its associated complications.[15] The ERSPC risk calculator uses three categories total PSA value (within the range 0.4–50 ng/ml), digital rectal examinations, and TRUS findings to predict the probability of prostate cancer risk. As per the ERSPC-RC3 guidelines, prostate biopsy is indicated if the probability of cancer risk by the calculator is >20%[16]. We compared PSMA PET and ERSPC3 calculator with high threshold probability (>40% probability risk) and found that PET/CT is more accurate in cancer prediction than the risk calculator on the ROC analysis [Figure 4 and Table 6]. Additionally, when results of PSMA PET/CT were used along with that of the risk calculator, we found that biopsy could have been avoided in up to 50% of our study patients. Similarly, in the study done by Zhang et al. showed that when PSMA results were taken into account before biopsy, about 19% of the biopsies could have been avoided.[11] Hence, we recommend that PSMA PET/CT, given its higher predictive ability, should be used along with cancer risk calculators to improve patient selection before biopsy.
Our results show a statistically significant difference (P = 0.00001) in the mean SUVmax between benign lesions (mean SUVmax-4.21 ± 1.5) and that of PCa (mean SUVmax-19.56 ± 18.11) [Figure 2]. Rahbar et al. documented a significant difference (P < 0.001) in median SUVmax between PCa (11.0 ± 7.8) and PN (2.7 ± 0.9), and these were correlated with histology map reconstructed from the radical prostatectomy specimens.[17] Woythal et al. reported mean SUVmax values of 14.06 ± 15.56 and 2.43 ± 0.63 in PCa and PN, respectively.[8] A study done by Uprimny et al. (n = 90) also showed a median difference in SUVmax between PCa and PN (12.5 vs. 3.9).[18] These results prove that although hyperplasia or inflammation of prostate shows low-grade PSMA uptake, it is at least 3–4 times less compared to the PSMA uptake in prostate cancer, giving PSMA PET/CT a very high discriminatory value. This can be very useful in accurate lesion localization and can aid TRUS or MRI-guided biopsy, where targeting sites with high PSMA avidity may help in diagnosis, especially in patients with repeated negative biopsies.
It is well established now that we diagnose clinically significant prostate cancer instead of overdiagnosing/over-treating low-risk disease. As previously reported by Bravaccini et al. that immunohistochemistry PSMA expression correlates with GS scores in both biopsy and prostatectomy specimen, with a lower PSMA expression in GS3 pattern vs. GS pattern 4.[19] Few other pathological studies reported that high PSMA expression in the primary tumor is independently associated with disease recurrence.[20,21] In this regard, we need to ascertain whether in vivo-PSMA expression, as seen by PET/CT correlates with high-risk features on pathological evaluation and can predict long-term outcomes. In our study, we found higher median prostate SUVmax in malignant lesions with high-risk features such as Gleason with GS score >7 (P-0.0107) and in patients total PSA levels >20 ng/ml (P-0.005) [Figure 3]. Few other studies which reported higher prostate SUVmax with higher GS, do not, however, show similar statistically significant correlation[8,22]. Larger multi-centric studies are needed to validate this promising prospect of using quantification data obtained through PSMA PET/CT for risk stratification (active observation vs. surveillance vs. treatment) and planning appropriate therapy.
Multi-parametric magnetic resonance imaging (mpMRI) is used to localize the primary tumor (especially with prior negative biopsy), local staging of cancer and to plan nerve-preserving radical prostatectomy. There is now level IA indication to perform MRI before systematic biopsy and level 2a evidence to perform the biopsy in PI-RADS=/>[4]. However, a meta-analysis showed that there is a wide variation in reported diagnostic accuracies (44%–87%) for MRI in the detection of clinically significant PCa.[23] A study done by Chen et al. showed that using a PI-RADS cutoff of 4 or more, MRI missed 24.2% of the clinically significant PCa and 66.7% of clinically significant PCa in PI-RADS 3. Other limitations of MRI include low inter-observer agreement, lower specificity in the identification of low-grade tumors, lower accuracy in transitional zone tumors or prostatitis (which are known to have overlapping with cancer) and instances where MRI is contraindicated (claustrophobia, patients with metallic implants and patients with renal failure where gadolinium is contraindicated).[24,25,26] We compared PSMA PET CT findings with Bip-MRI in 25 patients and found concordant findings [Figure 5] in 60% of patients (n = 15/25) and discordant findings in 40% of patients (n = 10/25) [Table 7]. In our study, 24% of patients (n = 6/25) reported as PI-RADS 4/5 MRI were negative on biopsy. 66.6% of these (n = 4/6) patients were related to prostatitis and 33.3% (n = 2/6) related to benign hyperplasia. All these 6 cases were negative on PET/CT (mean SUVmax 3.25, and all with PROMISE score 1, i.e., uptake less than the liver) [Figure 6]. It has been reported before that diffusion-weighted MRI may incorrectly identify prostatitis as malignancy as both of these usually have overlapping apparent diffusion coefficient values.[27] Hence, it appears probably that PSMA PET/CT scan has a higher diagnostic value than MRI if there is a high degree of clinical suspicion of prostatitis.
Recent studies show a higher diagnostic accuracy for the detection of primary prostate malignancy for PSMA PET/CT compared to mpMRI. Donato et al. compared the diagnostic accuracy of PSMA PET/CT and MRI against radical prostatectomy whole gland histopathology and found that PSMA PET/CT had better sensitivity for the detection of index lesion, bilateral and multi-focal disease (94%, 42%, and 32%, respectively) compared to mpMRI (90%, 21%, and 19%).[28] One Indian study done in 15 patients showed that the accuracy of PSMA PET/CT was higher than mpMRI for PSA 4–20 ng/ml (80% Vs. 66.6%) for the detection of primary prostate cancer.[6] However, one multi-centric retrospective study done by Kalapara et al. showed no significant difference with PSMA PET/CT and MRI in localization of all index prostate tumors, clinical significant index tumors or transitional zone tumors.[29] Further studies are needed to ascertain whether combined PSMA PET-MRI with higher accuracy can indeed be a one shop detection of prostate cancer and whether it can completely replace systematic prostate biopsies.[30]
The limitations of our study are small sample size and retrospective design. Furthermore, we did not have long-term follow-up or repeat biopsies of patients with benign histology. All 23 cases in our studies (except 1 case) were clinically significant prostate cancer. Hence, we are unable to determine the accuracy of PSMA PET/CT in diagnosing clinically insignficant prostate cancer and significant prostate cancer separately. We did not compare the PSMA PET/CT imaging findings against the radical prostatectomy (most of our patients opted for radiotherapy), which would be a better gold standard than TRUS-guided biopsy specimens. As TRUS guided biopsies are known to miss tumors in lateral most peripheral zones and apex of the prostate, this might alter the overall diagnostic accuracy of PSMA PET/CT. All the 25 MRI prostate scans included in this study were bi-parametric (T2 and DWI sequences) without DCE sequences. Bip-MRI is not the standard of care as far as prostate cancer imaging is concerned, and a comparison of PSMA PET findings ideally should be have been done with multi-parametric MRI. However, a systematic review and meta-analysis study showed comparable diagnostic accuracy for bi-parametric and multi-para-metric MRI for the diagnosis of prostate cancer.[31]
Conclusions
In addition to the well-established re-staging and staging indications, molecular imaging using Ga-68 PSMA PET/CT appears to have a promising role in differentiating benign and malignant lesions of the prostate with high diagnostic accuracy. Used along with cancer predicting risk calculators such as ERSPC3 and MRI, PSMA PET/CT has the potential to significantly reduce the painful and often unnecessary prostate biopsies. Larger prospective studies are needed to validate our results.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Jain S, Saxena S, Kumar A. Epidemiology of prostate cancer in India. Meta Gene. 2014;2:596–605. doi: 10.1016/j.mgene.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hariharan K, Padmanabha V. Demography and disease characteristics of prostate cancer in India. Indian J Urol. 2016;32:103–8. doi: 10.4103/0970-1591.174774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenton JJ, Weyrich MS, Durbin S, Liu Y, Bang H, Melnikow J. Prostate-specific antigen–based screening for prostate cancer: evidence report and systematic review for the US preventive services task force. JAMA. 2018;319:1914–31. doi: 10.1001/jama.2018.3712. [DOI] [PubMed] [Google Scholar]
- 4.European Association of Urology Guideline on Prostate Cancer. European Association of Urology. [Last accessed on 2020 Apr 15]. Available from: http://uroweborg/guideline/prostate-cancer .
- 5.Sasikumar A, Joy A, Pillai AM, Oommen KE, Somarajan S, Raman VK, et al. Gallium 68-PSMA PET/CT for lesion characterization in suspected cases of prostate carcinoma. Nucl Med Commun. 2018;39:1013–21. doi: 10.1097/MNM.0000000000000906. [DOI] [PubMed] [Google Scholar]
- 6.Kumar N, Yadav S, Kumar S, Saurav K, Prasad V, Vasudeva P. Comparison of percentage free PSA, MRI and GaPSMA PET scan for diagnosing cancer prostate in men with PSA between 4 and 20 ng/ml. Indian J Urol. 2019;35:202–7. doi: 10.4103/iju.IJU_91_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad V, Steffen IG, Diederichs G, Makowski MR, Wust P, Brenner W. Biodistribution of 68Ga-PSMA-HBED-CC in patients with prostate cancer: Characterization of uptake in normal organs and tumour lesions. Mol Imaging Biol. 2016;18:428–36. doi: 10.1007/s11307-016-0945-x. [DOI] [PubMed] [Google Scholar]
- 8.Woythal N, Arsenic R, Kempkensteffen C, Miller K, Janssen JC, Huang K, et al. Immunohistochemical validation of PSMA expression measured by (68) Ga-PSMA PET/CT in primary prostate cancer. J Nucl Med. 2018;59:238–43. doi: 10.2967/jnumed.117.195172. [DOI] [PubMed] [Google Scholar]
- 9.Gerstenbluth RE, Seftel AD, Hampel N, Oefelein MG, Resnick MI. The accuracy of the increased prostate specific antigen level (greater than or equal to 20 ng/ml) in predicting prostate cancer: Is biopsy always required. J Urol. 2002;168:1990–3. doi: 10.1016/S0022-5347(05)64279-6. [DOI] [PubMed] [Google Scholar]
- 10.Shao Y, Albertsen PC, Roberts CB, Lin Y, Mehta AR, Stein MN, et al. Risk profiles and treatment patterns among men diagnosed as having prostate cancer and a prostate-specific antigen level below 4.0 ng/mL. Arch Int Med. 2010;170:1256–61. doi: 10.1001/archinternmed.2010.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Shao S, Wu P, Liu D, Yang B, Han D, et al. Diagnostic performance of 68Ga-PSMA PET/CT in the detection of prostate cancer prior to initial biopsy: Comparison with cancer-predicting nomograms. Eur J Nucl Med Mol Imaging. 2019;46:908–20. doi: 10.1007/s00259-018-4255-1. [DOI] [PubMed] [Google Scholar]
- 12.Eiber M, Herrmann K, Calais J, Hadaschik B, Giesel FL, Hartenbach M, et al. Prostate cancer molecular imaging standardized evaluation (PROMISE): Proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59:469–78. doi: 10.2967/jnumed.117.198119. [DOI] [PubMed] [Google Scholar]
- 13.Liu C, Liu T, Zhang Z, Zhang N, Du P, Yang Y, et al. PSMA PET/CT and standard plus PET/CT-Ultrasound fusion targeted prostate biopsy can diagnose clinically significant prostate cancer in men with previous negative biopsies. J Nucl Med. 2020 doi: 10.2967/jnumed.119.235333. pii: Jnumed 119235333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fendler WP, Schmidt DF, Wenter V, Thierfelder KM, Zach C, Stief C, et al. 68Ga-PSMA PET/CT detects the location and extent of primary prostate cancer. J Nucl Med. 2016;57:1720–5. doi: 10.2967/jnumed.116.172627. [DOI] [PubMed] [Google Scholar]
- 15.Louie KS, Seigneurin A, Cathcart P, Sasieni P. Do prostate cancer risk models improve the predictive accuracy of PSA screening? A meta-analysis. Ann Oncol. 2015;26:848–64. doi: 10.1093/annonc/mdu525. [DOI] [PubMed] [Google Scholar]
- 16.Roobol MJ, Steyerberg EW, Kranse R, Wolters T, van den Bergh RC, Bangma CH, et al. A risk-based strategy improves prostate-specific antigen-driven detection of prostate cancer. Eur Urol. 2010;57:79–85. doi: 10.1016/j.eururo.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Rahbar K, Weckesser M, Huss S, Semjonow A, Breyholz H, Schrader A, et al. Correlation of intraprostatic tumor extent with 68Ga-PSMA distribution in patients with prostate cancer. J Nucl Med. 2016;57:563–7. doi: 10.2967/jnumed.115.169243. [DOI] [PubMed] [Google Scholar]
- 18.Uprimny C, Kroiss AS, Decristoforo C, Fritz J, von Guggenberg E, Kendler D, et al. 68Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging. 2017;44:941–9. doi: 10.1007/s00259-017-3631-6. [DOI] [PubMed] [Google Scholar]
- 19.Bravaccini S, Puccetti M, Bocchini M, Ravaioli S, Celli M, Scarpi E, et al. PSMA expression: A potential ally for the pathologist in prostate cancer diagnosis. Sci Rep. 2018;8:4254. doi: 10.1038/s41598-018-22594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross JS, Sheehan CE, Fisher HA, Kaufman RP, Jr, Kaur P, Gray K, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9:6357–62. [PubMed] [Google Scholar]
- 21.Perner S, Hofer MD, Kim R, Shah RB, Li H, Möller P, et al. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum Pathol. 2007;38:696–701. doi: 10.1016/j.humpath.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Ceci F, Uprimny C, Nilica B, Geraldo L, Kendler D, Kroiss A, et al. 68Ga-PSMA PET/CT for restaging recurrent prostate cancer: Which factors are associated with PET/CT detection rate? Eur J Nucl Med Mol Imaging. 2015;42:1284–94. doi: 10.1007/s00259-015-3078-6. [DOI] [PubMed] [Google Scholar]
- 23.Fütterer JJ, Briganti A, De Visschere P, Emberton M, Giannarini G, Kirkham A, et al. Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol. 2015;68:1045–53. doi: 10.1016/j.eururo.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Chen M, Zhang Q, Zhang C, Zhao X, Marra G, Gao J, et al. Combination of 68Ga-PSMA PET/CT and multiparametric MRI improves the detection of clinically significant prostate cancer: A lesion-by-lesion analysis. J Nucl Med. 2019;60:944–9. doi: 10.2967/jnumed.118.221010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bratan F, Niaf E, Melodelima C, Chesnais AL, Souchon R, Mège-Lechevallier F, et al. Influence of imaging and histological factors on prostate cancer detection and localisation on multiparametric MRI: A prospective study. Eur Radiol. 2013;23:2019–29. doi: 10.1007/s00330-013-2795-0. [DOI] [PubMed] [Google Scholar]
- 26.Dianat SS, Carter HB, Macura KJ. Performance of multiparametric magnetic resonance imaging in the evaluation and management of clinically low-risk prostate cancer. Urol Oncol. 2014;32:39e1–10. doi: 10.1016/j.urolonc.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagel KN, Schouten MG, Hambrock T, Litjens GJ, Hoeks CM, ten Haken B, et al. Differentiation of prostatitis and prostate cancer by using diffusion-weighted MR imaging and MR-guided biopsy at 3 T. Radiology. 2013;267:164–72. doi: 10.1148/radiol.12111683. [DOI] [PubMed] [Google Scholar]
- 28.Donato P, Roberts MJ, Morton A, Kyle S, Coughlin G, Esler R, et al. Improved specificity with 68Ga PSMA PET/CT to detect clinically significant lesions “invisible” on multiparametric MRI of the prostate: A single institution comparative analysis with radical prostatectomy histology. Eur J Nucl Med Mol Imaging. 2019;46:20–30. doi: 10.1007/s00259-018-4160-7. [DOI] [PubMed] [Google Scholar]
- 29.Kalapara AA, Nzenza T, Pan HY, et al. Detection and localisation of primary prostate cancer using 68 Ga-PSMA PET/CT compared with mpMRI and radical prostatectomy specimens [published online ahead of print, 2019 Jul 1] BJU Int. 2019 doi: 10.1111/bju.14858. 101111/bju14858. [DOI] [PubMed] [Google Scholar]
- 30.Hicks RM, Simko JP, Westphalen AC, Nguyen HG, Greene KL, Zhang L, et al. Diagnostic accuracy of 68Ga-PSMA-11 PET/MRI compared with multiparametric MRI in the detection of prostate cancer. Radiology. 2018;289:730–7. doi: 10.1148/radiol.2018180788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woo s, Suh C, Kim S, Cho J, Kim S, Moon M. Head-to-head comparison between biparametric and multiparametric MRI for the diagnosis of prostate cancer: A systematic review and meta-analysis. Am J Roentgenol. 2018;211:W226–41. doi: 10.2214/AJR.18.19880. [DOI] [PubMed] [Google Scholar]


