Abstract
Nerve growth factor (NGF), a prototypical neurotrophic factor essential for neuronal cell proliferation and survival, has been implicated as a marker of tumor progression, as well as a potential target for novel therapeutic approaches in cancer. To investigate the functional potential of NGF in liver cancer in the present study, a stable NGF-overexpressing HepG2 cell line was generated. The scratch-wound assay was used to investigate cell motility and polarity. Western blotting was performed to evaluate the expression levels of epithelial-mesenchymal transition (EMT)-related proteins, including E-cadherin, N-cadherin and vimentin. Moreover, immunofluorescence was performed to investigate the arrangement of the actin cytoskeleton. Cell anoikis resistance was examined using a suspension culture model and cell apoptosis was examined via flow cytometry. The present results indicated that NGF overexpression in HepG2 cells disrupted HepG2 cell polarity and promoted cell motility. Furthermore, NGF overexpression induced EMT and actin cytoskeleton rearrangement in HepG2 cells, as well as enhanced anoikis resistance and prevented cellular apoptosis. Notably, a tropomyosin receptor kinase A receptor inhibitor blocked NGF-induced cell motility and apoptosis. Therefore, it was suggested that NGF serves a critical role in the invasion and metastasis of liver cancer. The use of NGF as a biomarker or potential new target could lead to the development of novel factors for diagnosis or for improving therapeutic strategies in liver cancer.
Keywords: NGF, cell polarity, EMT, actin cytoskeleton, anoikis, liver cancer
Introduction
Liver cancer is the sixth most common types of cancer and the fourth leading cause of cancer-associated morality worldwide (1,2). China had the highest number of primary liver cancer cases globally, with an incidence rate of 17.8 cases/100,000 inhabitants in 2014 (3). Despite significant progress in liver cancer therapeutics, the recognition of cancer cell invasion into the surrounding environment and metastatic spread remains a major research challenge and clinical problem. Moreover, the underlying molecular mechanisms that initiate cancer cell invasion and metastases remain poorly understood. It has been reported that cell polarity defects, which are associated with cell viability, motility and adhesion ability, can serve as initiators of cancer cell invasion and metastatic spread (4–8). Furthermore, epithelial-mesenchymal transition (EMT), which can mutually interact with the actin cytoskeleton and cell polarity, is critical during the early steps of metastasis and invasion (9–12). These processes appear to be associated with altered expression of adhesion molecules and dysregulation of growth factor signaling.
Nerve growth factor (NGF) is a prototypical neurotrophic factor that is essential for neuronal cell growth and survival (13,14). NGF can interact with its receptor tropomyosin receptor kinase A (TrkA) with a high affinity, whereas it interacts with p75 neurotrophin receptor (p75NTR) with a low affinity (15). Binding of NGF to TrkA results in intracellular signaling and leads to cell differentiation and survival. Conversely, the interaction of NGF with p75NTR activates Jun-N-terminal kinase and ceramide to promote apoptosis (16,17). Although NGF is undetectable in adult and developing livers, its expression is markedly elevated in liver cancer (18,19). In recent years, several studies have reported that NGF, together with TrkA and p75NTR, are involved in aspects of tumor biology, including growth, invasion and metastasis (20–23). NGF has also been implicated as a marker of tumor progression and is a potential target for novel therapeutic approaches in cancer (24,25).
HepG2 cells are non-tumorigenic cells with high proliferation rates and have been used to evaluate cell polarity and motility as an in vitro model in several studies (26,27). The present study generated a NGF-overexpressing HepG2 cell line to investigate the functional potential of NGF in liver cancer, and subsequently examined the regulatory mechanism of NGF on cell motility, polarity and EMT, as well as the underlying effects on cytoskeleton rearrangements and apoptosis. The present results could elucidate the possible role of NGF in hepatic carcinogenesis and provide novel insights into the treatment of liver cancer.
Materials and methods
Cell culture
The HepG2 cell line used in this study was purchased from the China Center for Type Culture Collection and was authenticated by short tandem repeat profiling. The cells were cultured in DMEM containing 10% FBS (Thermo Fisher Scientific, Inc.), 100 U/ml penicillin G and 100 µg/ml streptomycin at 37°C with 5% CO2 and 95% O2.
Plasmid transfections
Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) was used to transfect cells according to the manufacturer's protocol. In brief, 4 µg pcDNA3 vector (pcDNA3-control) or pcDNA3-NGF plasmid (gift from Professor Philip Barker, McGill University, Montreal, Canada) was mixed with 10 µl Lipofectamine for 20 min at room temperatures and the mixture was transfected into 90% confluent HepG2 cells for 1 h. The transfected cells were cultured at 37°C in DMEM for 6 h, and then in DMEM with 10% FBS for 48 h at 37°C with 5% CO2 and 95% O2. To select stable transfectants, cells were cultured at 37°C in DMEM with 600 µg/ml G418 for 4 weeks to generate a stable NGF-overexpressing HepG2 cell line. After a single colony of stable cells was selected for further culture, the concentration of G418 was subsequently reduced by half and maintained in cultivation. One pcNA3-control and two different pcDNA3-NGF stable cell lines were selected for subsequent studies.
Wound healing assays
In brief, cultured cells with DMEM containing 10% FBS were grown to 100% confluence on plastic dishes or coverslips (for microscopic studies) and scratched using a 10 µl pipette tip. Debris was removed from the wound and washed out with PBS. The cells were then cultured with DMEM containing 10% FBS and the images were acquired at 0, 24 and 48 h using an inverted light microscope (IX83; Olympus Corporation; magnification, ×10) after cells were wounded. Wound closure was quantitatively analyzed using ImageJ Fiji software (version 1.53g 4; National Institutes of Health). Each test was performed in triplicate. A total of 10 mg/l CEP701 (Sigma-Aldrich Merck KGaA) was added after cells were scratched and maintained in the culture medium at 37°C until images at different time points were acquired.
Golgi reorientation polarity assays
As previously described (28,29), the wounded cells were fixed with cold 4% paraformaldehyde at 4°C for 10 min and stained with the cis-Golgi matrix protein of 130 kDa (GM130) to visualize Golgi positioning after 16 h. A total of 7 µg/ml anti-GM130 antibody (cat. no. ab169276; Abcam) were incubated with cells at 4°C overnight. The appropriate secondary antibody conjugated with rhodamine were incubated for 1 h at room temperature. Then, 4′6-diamidino-2-phenyl-indole (DAPI) staining was performed as previously described (29). Cell images were acquired using a Nikon TE2000S fluorescence microscope (magnification, ×20) and were analyzed using ImageJ Fiji software (version 1.53g 4; National Institutes of Health). Cell orientation was determined only for cells at the wound edge. The cell was divided into three 120° regions, with one region facing the wound edge. The cell was recognized to possess an aligned Golgi only when its Golgi realigned to the 120° region facing the wound edge. The cell positioning angle was calculated between a line along the long axis of the nucleus and a line tracing the wound front. For example, cells aligned perpendicular to the leading edge demonstrated a nearly 90° orientation, whereas cells aligned parallel to the wound front had a 0° orientation. For each experiment, ≥20 cells were examined.
Western blot analysis
Whole-cell protein was extracted with cell lysis buffer (Cell Signaling Technology, Inc.). Protein concentrations were determined via bicinchoninic acid protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). A total of 50 µg protein from whole-cell lysates were solubilized in SDS sample buffer and separated on SDS 12.5% polyacrylamide gels. The proteins were transferred to polyvinylidene difluoride membranes and incubated with blocking solution (Tris buffer containing 0.1% Tween-20 and 5% non-fat dry milk) at room temperature for 1 h. The membrane was then incubated with the primary antibody at 4°C overnight and the secondary antibody at room temperature for 1 h. The primary antibodies against NGF (1:1,000; cat. no. sc32300; Santa Cruz Biotechnology Inc.), E-cadherin (1:1,000; cat. no. 14472; Cell Signaling Technology, Inc.), N-cadherin (1:1,000; cat. no. 4061; Cell Signaling Technology, Inc.), vimentin (1:1,000; cat. no. 3932; Cell Signaling Technology, Inc.), F-actin (1:1,000; cat. no. ab130935; Abcam) and β-actin (1:1,000; cat. no. sc69879; Santa Cruz Biotechnology Inc.) were used for different proteins with horseradish peroxidase (HRP)-conjugated secondary antibodies (all 1:2,000; cat. nos. ab205718, ab205719 and ab205720; all Abcam). β-actin protein was detected as a loading control for whole-cell protein. An enhanced chemiluminescent substrate for detection of HRP (cat. no. 32209; Thermo Fisher Scientific, Inc.) was used to visualize the bands with ChemiDoc imaging system (Bio-Rad Laboratories, Inc.). The bands were analyzed with ImageJ Fiji software (version 1.53g 4; National Institutes of Health).
Immunofluorescence and confocal imaging
Briefly, HepG2 and HepG2-NGF cells were plated onto sterile coverslips and incubated in a humidified chamber at 37°C. A total of 10 mg/l CEP701 (Sigma-Aldrich; Merck KGaA) was added at 37°C 24 h before fixation. After 24 h, the cells were washed, fixed with cold 4% paraformaldehyde at 4°C for 10 min and permeabilized with 0.2% Triton X-100. After each experiment, cells were washed three times for 5 min in PBS, then blocked with 5% BSA (Abcam) in PBS for 30 min at room temperature and incubated with anti-NGF (1:250; cat. no. ab52918; Abcam) or 5 µg/ml anti-F-actin antibody (cat. no. ab130935; Abcam) at 4°C overnight. Cells were then treated with 10 µg/ml corresponding secondary antibody conjugated with FITC (cat. no. F-2765) or rhodamine (cat. no. R-6393; both Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h at room temperature. Nuclear staining was performed by incubating cells with 0.4 µmol/l DAPI for 2 min at room temperature. Subsequently, cells were examined under a confocal microscope with 10× or 60× oil objectives (Olympus Corporation).
Anoikis assay
The anoikis assay was performed as described by Frisch and Francis (30) by plating cells into ultra-low attachment plates. Cells were plated at a density of 100×106 cells, onto 60-mm polyHEMA (10 mg/ml)-coated Petri dishes. After culturing for 24 h, images were obtained using an inverted light microscope (cat. no. IX83; Olympus Corporation; magnification, ×10) and cells were collected for flow cytometry (Attune NxT; Thermo Fisher Scientific, Inc.). BD FACS Diva software 6.0 (BD Biosciences) was used to analysis the apoptosis ratio. In order to investigate the effect of CEP701, the cells were treated at 37°C with 10 mg/l CEP701 for 24 h and images were acquired.
Statistical analysis
Data are presented as the mean ± SEM (n≥3) P-values were calculated using an ordinary one-way ANOVA, which was followed by a Tukey's test. P<0.05 was considered to indicate a statistically significant difference.
Results
NGF expression in HepG2-pcDNA3-NGF cells
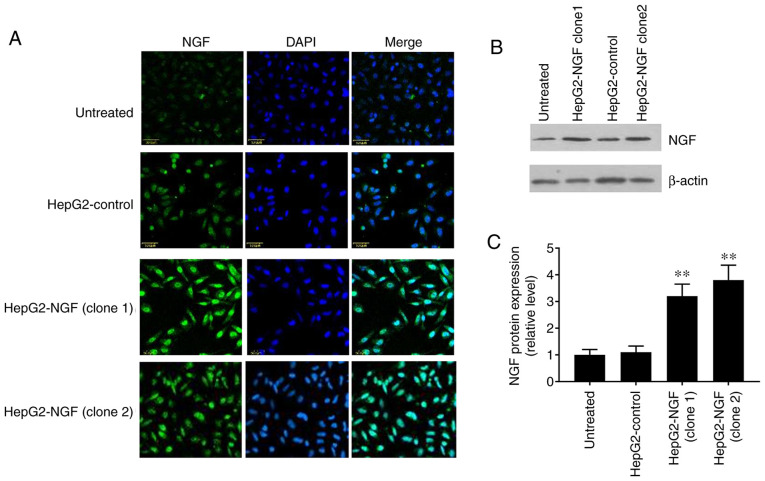
The pcDNA3-control and pcDNA3-NGF were stably transfected into HepG2 cells and NGF expression was detected via western blotting and immunofluorescence. The fluorescence intensity level of NGF was notably higher in the two NGF-overexpressing HepG2 clones (HepG2-NGF clone 1 and clone 2) compared with that in the uninfected cells or pcDNA3-control cells (HepG2-control) (Fig. 1A). Furthermore, western blotting demonstrated that the NGF protein expression level in HepG2-NGF cells was increased by >3 fold when compared with that observed in the control group (HepG2-control) (Fig. 1B and C). These results indicated that the NGF was successfully transfected into HepG2 cells.
Figure 1.
Overexpression of NGF in HepG2 cells. (A) HepG2 cells were transfected with pcDNA3-control (HepG2-control) and pcDNA3-NGF (HepG2-NGF), immunolabelled with NGF (green) and then imaged with confocal microscopy. Nuclei are stained with DAPI (blue). (B) Representative western blotting image for NGF protein expression with β-actin loading control. (C) Normalized NGF protein signals were analyzed using one-way ANOVA. Scale bar, 50 mm. Data are presented as the mean ± SEM, n=5. **P<0.01 vs. HepG2-control and untreated groups. NGF, nerve growth factor.
Effect of NGF regulation on cell motility and polarity
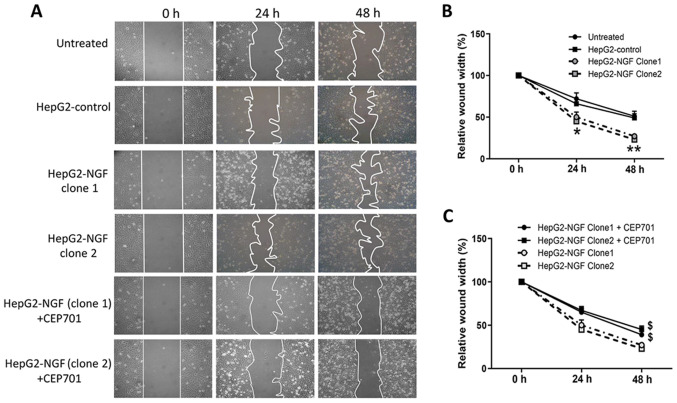
The effects of NGF on cell motility and polarity were subsequently examined after establishing the HepG2-NGF stable cell line. As presented in Fig. 2, 48 h after the cells were scratched, the relative wound width of control cells was ~50% of the original scratch width, compared with 27 and 23% in the two different HepG2-NGF clones, indicating that NGF overexpression in both HepG2-NGF cell clones can significantly promote HepG2 cell motility.
Figure 2.
Effect of NGF on cell motility. Wound healing assay were used to examine the cell motility. (A) Images were acquired at 0, 24 and 48 h using an inverted microscope (10× magnification) after the confluent cells were wounded by scratching cell sheets with a 10 µl pipette tip. CEP701 was added at 0 h. (B) The wound closure was quantitatively analyzed using ImageJ software by outlining and assessing the unhealed area in the wound images. Data are presented as the mean ± SEM, n=4. *P<0.05, **P<0.01 vs. HepG2-control and untreated group (one-way ANOVA). (C) Effect of CEP701 on cell motility. Data are presented as the mean ± SEM, n=4. $P<0.05 vs. HepG2-NGF cells without CEP701 treatment (one-way ANOVA). NGF, nerve growth factor.
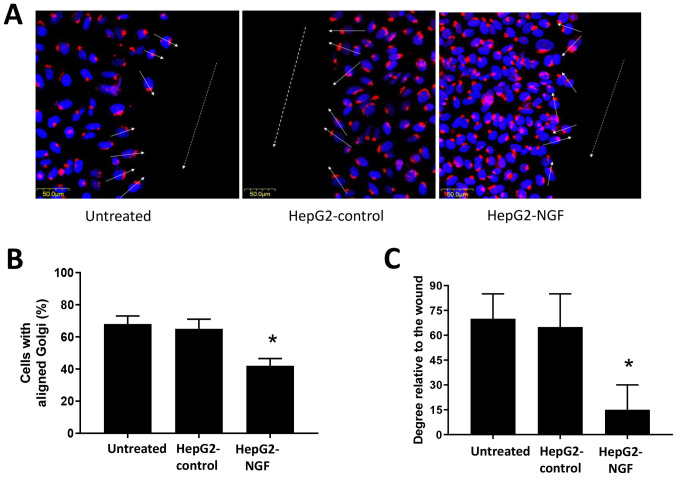
The Golgi serves an important role in protein trafficking to the leading cell edge and can function as a cell polarization marker (29,31,32). Therefore, Golgi reorientation was examined in HepG2-NGF cells when cell polarity was stimulated. A total of 16 h after cells were scratched, they were fixed and stained for the protein GM130. The majority of untreated HepG2 cells and pcDNA3-control cells were polarized in a direction perpendicular to the wound (the average orientation was nearly 70° to the wound). Moreover, ~70% of untreated HepG2 cells and pcDNA3-control cells demonstrated proper orientation (reoriented in front of the nucleus) and were realigned to the 120° region facing the direction of movement after scratching was performed (Fig. 3). By contrast, HepG2-NGF cells presented only 42% of cells with proper Golgi positioning and only a 30° orientation relative to the wound, indicating defective cell polarity after NGF overexpression in these cells (Fig. 3).
Figure 3.
Effect of NGF on cell apical-basal polarity. (A) Golgi (red) and DAPI (blue) immunofluorescence staining in HepG2 cells. Arrows: Golgi positioning relative to the wound designated with a dashed line. (B) Bar graph presenting the percentage of cells with aligned Golgi (n=20 cells per experimental group). (C) Bar graph depicting cell positioning in degrees relative to the wound (n=20 cells per experimental group). Scale bar, 50 µm. Data are presented as the mean ± SEM, n=4 experiments. *P<0.05 vs. HepG2-control and untreated group (one-way ANOVA). NGF, nerve growth factor.
NGF overexpression initiates EMT
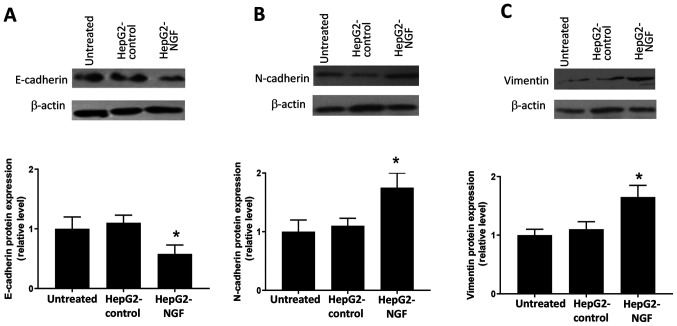
In cancer cells, loss of the apical-basal polarity and acquisition of the migratory phenotype is considered a subtype of EMT, which is suggested to promote cancer cell migration and invasion (9–11). Based on the results from Figs. 2 and 3, it was considered that there may be a potential relationship between NGF and the EMT process. Hence, the effects of NGF on EMT were subsequently examined. Western blotting results indicated that NGF overexpression induced the loss of E-cadherin (Fig. 4A), as well as the production of N-cadherin (Fig. 4B) and vimentin (Fig. 4C) in HepG2 cells. These results suggested that NGF could induce a cadherin switch and initiate EMT in HepG2 cells.
Figure 4.
Western blot analyses of the protein expression levels of E-cadherin, N-cadherin and vimentin. (A) E-cadherin, (B) N-cadherin and (C) vimentin expression levels were normalized to β-actin expression levels. Data were obtained from ≥3 different experiments. Data are presented as the mean ± SEM. *P<0.05 vs. HepG2-control and untreated group (one-way ANOVA). NGF, nerve growth factor.
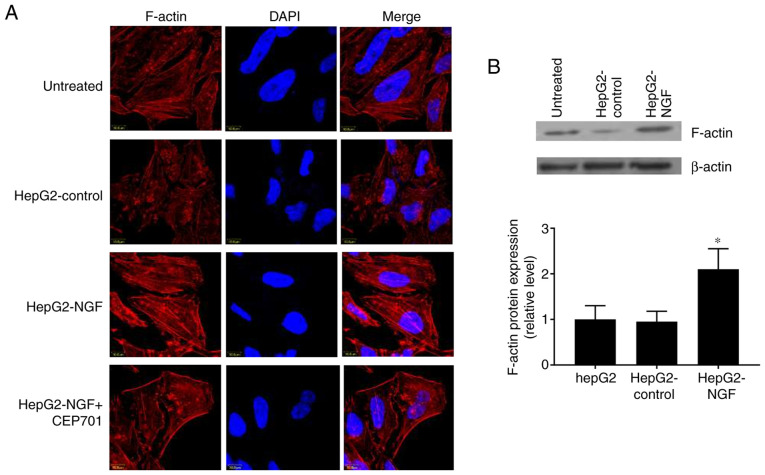
Rearrangement of the actin cytoskeleton in NGF-overexpressing HepG2 cells
In addition to disrupting cell-cell adhesions and the overall loss of epithelial homeostasis, the altered functions of the polarity determinants can result in cytoskeleton rearrangements and regulate actin dynamics (33,34). Herein, it was detected whether NGF can affect the F-actin cytoskeletal arrangement and protein expression. In control cells, F-actin was organized in a circular pattern and formed circumferential bundles with visible slim central fibers, as visualized using immunofluorescence and confocal laser microscopy (Fig. 5A). However, in HepG2-NGF cells, F-actin was redistributed into strong central fibers (stress fibers) and these stress fibers were arranged parallel to the elongated shape of a cell. Furthermore, the F-actin protein expression level was increased in NGF-overexpressing HepG2 cells (Fig. 5B). These results indicated that NGF overexpression can change the actin cytoskeleton arrangement in HepG2 cells, even in the absence of stress or stretch induction.
Figure 5.
Effect of NGF on F-actin arrangement and expression. (A) Immunofluorescence staining of F-actin (red) in NGF transfected HepG2 cells. Nuclei were stained with DAPI (blue). (B) Western blot analyses of protein expression of F-actin. F-actin expression levels were normalized to β-actin expression levels. Data were obtained from three different experiments. Scale bar, 10 µm. Data are presented as the mean ± SEM. *P<0.05 vs. HepG2-control and untreated group (one-way ANOVA). NGF, nerve growth factor.
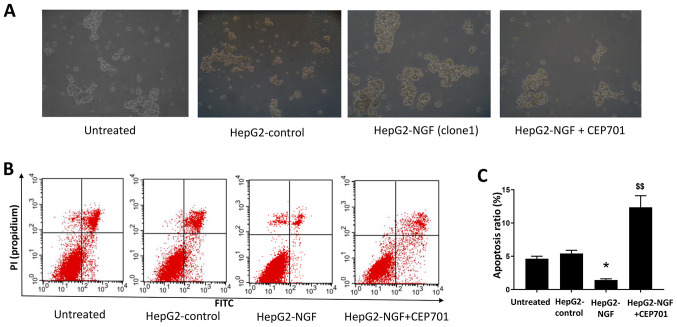
Effect of NGF overexpression on anoikis resistance and apoptosis
In a previous study, NGF signaling was reported to alter cell death and survival in various cancer cells (15,35). Therefore, the effect of NGF on cell anoikis resistance and apoptosis was examined in HepG2 cells cultured in a suspension culture model. As presented in Fig. 6, compared with HepG2-control cells, after culturing for 24 h in ultra-low attachment plates, the diameters of HepG2-NGF cell colonies were considerably larger and the apoptosis ratios were lower in HepG2-NGF cells. This indicated that NGF overexpression could enhance anoikis resistance and prevent apoptosis in HepG2 cells.
Figure 6.
Effect of NGF on anoikis resistance and apoptosis. (A) HepG2 cells cultured in suspension culture model. Images were acquired with an inverted microscope (10× magnification) (B) Flow cytometry of HepG2 cells. (C) Apoptosis ratio of HepG2 cells. Data are presented as the mean ± SEM. *P<0.05 vs. HepG2-control and untreated group. $$P<0.01 vs. HepG2-NGF cells without CEP701 treatment (one-way ANOVA). NGF, nerve growth factor.
Effects of the TrkA receptor inhibitor CEP701 on cell motility and apoptosis
To determine whether NGF regulates cell motility by interacting with its receptors, the TrkA receptor inhibitor CEP701 (10 mg/l) was used in wound healing assays in which cells were scratched, and cell motility was evaluated. A total of 48 h after cells were wounded, the relative wound width of both HepG2-NGF clones was considerably higher compared with that of untreated cells, suggesting that CEP701 can prevent NGF-promoted cell motility (Fig. 2). Moreover, NGF overexpression-induced F-actin rearrangement can be prevented by CEP701 (Fig. 5). The effect of CEP701 on cell anoikis resistance and apoptosis was further examined in HepG2-NGF cells cultured in the suspension culture model. Notably, it was identified that 10 mg/l CEP701 prevented anoikis resistance and increased the cell apoptosis ratio (Fig. 6).
Discussion
The primary aim of the present study was to determine the association of NGF, which is reportedly involved in breast and prostate cancer cell death and survival (15,35), with liver cancer progression. In the present study, it was observed that NGF overexpression in HepG2 cells could disrupt cell polarity and promote cell motility. Additionally, NGF overexpression could induce EMT and actin cytoskeleton rearrangement in HepG2 cells. Furthermore, NGF could enhance anoikis resistance and prevent the apoptosis of HepG2 cells. Collectively, these data support the hypothesis that NGF signaling serves a critical role in the invasion and metastasis of liver cancer.
Cell polarization is required for several cellular processes, including differentiation, migration, morphogenesis and motility (33,36). Disruption of cell polarity can disrupt normal cell behavior, resulting in the cancer initiation and progression (11,33). Furthermore, disruption of cell polarity can initiate EMT, which is required for cancer cell migration and invasion (12,36). Conversely, EMT can alter the function of polarity complexes and induce the loss of epithelial polarity (37–39). Similar to numerous other growth factors and cytokines (40–42), the current in vitro model data obtained from scratch-induced migration experiments demonstrated that NGF overexpression in HepG2 cells could promote cell motility and induce defective cell polarity. Consistent with these findings, NGF could induce a cadherin switch and vimentin expression in HepG2 cells, indicating that NGF can initiate EMT. To the best of our knowledge, the present study was the first to report that NGF was involved in liver cancer progression, especially in cancer cell invasion and metastasis, in addition to cell death and survival.
Previously, it has been reported that cell polarity proteins can regulate actin dynamics and cytoskeleton organization (33,34). Conversely, increasing evidence suggests that the cytoskeleton can regulate cell polarity and provide the structural design and mechanical strength necessary for EMT (38,39). Consistent with the polarity defect and EMT initiation in NGF-overexpressing HepG2 cells, the present study demonstrated that NGF overexpression could also induce F-actin redistribution and actin cytoskeleton development from circumferential bundles (circular actin pattern) to a system of parallel stress fibers. This rearrangement of the actin cytoskeleton is a prerequisite for cancer cell migration and invasion (39).
The mechanism via which NGF overexpression in HepG2 cells induces the loss of the apical-basal polarity and acquires the migratory phenotype remains unknown. Reportedly, neurotrophins, their receptors Trk and p75NTR and related signaling pathways serve an important role in the development of digestive cancer types (19,24). Moreover, Zhou et al (43) revealed that NGF receptor knockdown can elevate the expression level of endogenous p53 and result in hepatoprotective effects in HepG2 cells, while Indo5, which can inhibit the kinase activities of TrkA and TrkB in HepG2 cells, can suppress the growth of liver cancer (44). Although the expression of NGF is undetectable in healthy hepatocytes, NGF and Trk mRNA expression levels are significantly elevated in the liver tissue of the majority of patients with liver cancer, as well as in metastatic liver cancer cell lines, compared with those in healthy tissues or cell lines (18,25). In agreement with previous studies (45,46), the present study observed that a TrkA receptor inhibitor can prevent NGF-induced cell motility, F-actin rearrangement and anoikis resistance, thus supporting an autocrine role for NGF signaling via its receptors in hepatocytes (47). However, the present study only used one exogenous overexpression system HepG2 cell line to investigate the role of NGF and its receptor in liver cancer progression. Due to this limitation, to use a different liver cancer cell line or primary cultured hepatocytes as another experimental model, to knockdown NGF or transfect a mutated NGF in HepG2 cell line or to study the detailed signaling downstream of NGF/Trk will help to clarify the role of NGF in regulating the cell motility and polarity.
In conclusion, the present study demonstrated that NGF overexpression could induce defective liver cancer cell polarity, EMT initiation and cell cytoskeleton rearrangement, which are required for tumor progression. The use of NGF as a biomarker or potential new target could lead to the development of new factors for diagnosis or for improving therapeutic strategies in liver cancer.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- NGF
nerve growth factor
- EMT
epithelial-mesenchymal transition
- Trk
tropomyosin receptor kinase
- p75NTR
p75 neurotrophin receptor
Funding Statement
This study received funding from the Scientific and Technological Innovation Foundation of Yantian District of Shenzhen City (grant no. 20190104).
Funding
This study received funding from the Scientific and Technological Innovation Foundation of Yantian District of Shenzhen City (grant no. 20190104).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
HL was involved in study conceptualization, funding and design, biochemical experiments, data analysis and interpretation, and study coordination and manuscript preparation. HH was involved in experimental design, data interpretation and analysis, and manuscript preparation. YY, WC, SZ and YZ were involved in the biochemical experiments, and data interpretation and analysis. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA, WHO Classification of Tumours Editorial Board The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–188. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Zheng R, Qu C, Zhang S, Zeng H, Sun K, Gu X, Xia C, Yang Z, Li H, Wei W, et al. Liver cancer incidence and mortality in China: Temporal trends and projections to 2030. Chin Jl Cancer Res. 2018;30:571–579. doi: 10.21147/j.issn.1000-9604.2018.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003;5:590–597. doi: 10.1016/S0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- 5.Woodham EF, Machesky LM. Polarised cell migration: Intrinsic and extrinsic drivers. Chin J Cancer Res. 2014;30:25–32. doi: 10.1016/j.ceb.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Royer C, Lu X. Epithelial cell polarity: A major gatekeeper against cancer? Cell Death Differ. 2011;18:1470–1477. doi: 10.1038/cdd.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung HY, Fattet L, Tsai JH, Kajimoto T, Chang Q, Newton AC, Yang J. Apical-basal polarity inhibits epithelial-mesenchymal transition and tumour metastasis by PAR-complex-mediated SNAI1 degradation. Nat Cell Biol. 2019;21:359–371. doi: 10.1038/s41556-019-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee M, Vasioukhin V. Cell polarity and cancer-cell and tissue polarity as a non-canonical tumor suppressor. J Cell Sci. 2008;121:1141–1150. doi: 10.1242/jcs.016634. [DOI] [PubMed] [Google Scholar]
- 9.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: Role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 10.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Weinberg RA. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 13.Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 14.Hetman M, Xia Z. Signaling pathways mediating anti-apoptotic action of neurotrophins. Acta Neurobiol Exp (Wars) 2000;60:531–545. doi: 10.55782/ane-2000-1374. [DOI] [PubMed] [Google Scholar]
- 15.Bradshaw RA, Pundavela J, Biarc J, Chalkley RJ, Burlingame AL, Hondermarck H. NGF and ProNGF: Regulation of neuronal and neoplastic responses through receptor signaling. Adv Biol Regul. 2015;58:16–27. doi: 10.1016/j.jbior.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon SO, Casaccia-Bonnefil P, Carter B, Chao MV. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J Neurosci. 1998;18:3273–3281. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frade JM, Rodríguez-Tébar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- 18.Tokusashi Y, Asai K, Tamakawa S, Yamamoto M, Yoshie M, Yaginuma Y, Miyokawa N, Aoki T, Kino S, Kasai S, et al. Expression of NGF in hepatocellular carcinoma cells with its receptors in non-tumor cell components. Int J Cancer. 2005;114:39–45. doi: 10.1002/ijc.20685. [DOI] [PubMed] [Google Scholar]
- 19.Kishibe K, Yamada Y, Ogawa K. Production of nerve growth factor by mouse hepatocellular carcinoma cells and expression of TrkA in tumor-associated arteries in mice. Gastroenterology. 2002;122:1978–1986. doi: 10.1053/gast.2002.33581. [DOI] [PubMed] [Google Scholar]
- 20.Garrido MP, Torres I, Avila A, Chnaiderman J, Valenzuela-Valderrama M, Aramburo J, Oróstica L, Durán-Jara E, Lobos-Gonzalez L, Romero C. NGF/TRKA decrease miR-145-5p levels in epithelial ovarian cancer cells. Int J Mol Sci. 2020;21:7657. doi: 10.3390/ijms21207657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faulkner S, Griffin N, Rowe CW, Jobling P, Lombard JM, Oliveira SM, Walker MM, Hondermarck H. Nerve growth factor and its receptor tyrosine kinase TrkA are overexpressed in cervical squamous cell carcinoma. FASEB Bioadv. 2020;2:398–408. doi: 10.1096/fba.2020-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blondy S, Christou N, David V, Verdier M, Jauberteau MO, Mathonnet M, Perraud A. Neurotrophins and their involvement in digestive cancers. Cell Death Dis. 2019;10:123. doi: 10.1038/s41419-019-1385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu X, Liu Z, Hou R, Nie Y, Chen R. Nerve growth factor and its receptors on onset and diagnosis of ovarian cancer. Oncol Lett. 2017;14:2864–2868. doi: 10.3892/ol.2017.6527. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Demir IE, Tieftrunk E, Schorn S, Friess H, Ceyhan GO. Nerve growth factor & TrkA as novel therapeutic targets in cancer. Biochim Biophys Acta. 2016;1866:37–50. doi: 10.1016/j.bbcan.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Berretta M, Cavaliere C, Alessandrini L, Stanzione B, Facchini G, Balestreri L, Perin T, Canzonieri V. Serum and tissue markers in hepatocellular carcinoma and cholangiocarcinoma: Clinical and prognostic implications. Oncotarget. 2017;8:14192–14220. doi: 10.18632/oncotarget.13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han P, Fu Y, Liu J, Wang Y, He J, Gong J, Li M, Tan Q, Li D, Luo Y, et al. Netrin-1 promotes cell migration and invasion by down-regulation of BVES expression in human hepatocellular carcinoma. Am J Cancer Res. 2015;5:1396–1409. [PMC free article] [PubMed] [Google Scholar]
- 27.Yan W, Han P, Zhou Z, Tu W, Liao J, Li P, Liu M, Tian D, Fu Y. Netrin-1 induces epithelial-mesenchymal transition and promotes hepatocellular carcinoma invasiveness. Dig Dis Sci. 2014;59:1213–1221. doi: 10.1007/s10620-013-3016-z. [DOI] [PubMed] [Google Scholar]
- 28.Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- 29.Zhang S, Schafer-Hales K, Khuri FR, Zhou W, Vertino PM, Marcus AI. The tumor suppressor LKB1 regulates lung cancer cell polarity by mediating cdc42 recruitment and activity. Cancer Res. 2008;68:740–748. doi: 10.1158/0008-5472.CAN-07-2989. [DOI] [PubMed] [Google Scholar]
- 30.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yadav S, Puri S, Linstedt AD. A primary role for golgi positioning in directed secretion, cell polarity, and wound healing. Mol Biol Cell. 2009;20:1728–1736. doi: 10.1091/mbc.e08-10-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravichandran Y, Goud B, Manneville JB. The Golgi apparatus and cell polarity: Roles of the cytoskeleton, the Golgi matrix, and Golgi membranes. Curr Opin Cell Biol. 2019;62:104–113. doi: 10.1016/j.ceb.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Piroli ME, Blanchette JO, Jabbarzadeh E. Polarity as a physiological modulator of cell function. Front Biosci (Landmark Ed) 2019;24:451–462. doi: 10.2741/4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elias BC, Das A, Parekh DV, Mernaugh G, Adams R, Yang Z, Brakebusch C, Pozzi A, Marciano DK, Carroll TJ, Zent R. Cdc42 regulates epithelial cell polarity and cytoskeletal function during kidney tubule development. J Cell Sci. 2015;128:4293–4305. doi: 10.1242/jcs.164509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melck D, De Petrocellis L, Orlando P, Bisogno T, Laezza C, Bifulco M, Di Marzo V. Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology. 2000;141:118–126. doi: 10.1210/endo.141.1.7239. [DOI] [PubMed] [Google Scholar]
- 36.Gandalovičová A, Vomastek T, Rosel D, Brábek J. Cell polarity signaling in the plasticity of cancer cell invasiveness. Oncotarget. 2016;7:25022–25049. doi: 10.18632/oncotarget.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuertes-Alvarez S, Maeso-Alonso L, Villoch-Fernandez J, Wildung M, Martin-Lopez M, Marshall C, Villena-Cortes AJ, Diez-Prieto I, Pietenpol JA, Tissir F, et al. p73 regulates ependymal planar cell polarity by modulating actin and microtubule cytoskeleton. Cell Death Dis. 2018;9:1183. doi: 10.1038/s41419-018-1205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lomakin AJ, Lee KC, Han SJ, Bui DA, Davidson M, Mogilner A, Danuser G. Competition for actin between two distinct F-actin networks defines a bistable switch for cell polarization. Nat Cell Biol. 2015;17:1435–445. doi: 10.1038/ncb3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olson MF, Sahai E. The actin cytoskeleton in cancer cell motility. Clin Exp Metastasis. 2009;26:273–287. doi: 10.1007/s10585-008-9174-2. [DOI] [PubMed] [Google Scholar]
- 40.Witsch E, Sela M, Yarden Y. Roles for growth factors in cancer progression. Physiology (Bethesda) 2010;25:85–101. doi: 10.1152/physiol.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okamoto M, Koma YI, Kodama T, Nishio M, Shigeoka M, Yokozaki H. Growth differentiation factor 15 promotes progression of esophageal squamous cell carcinoma via TGF-β type II receptor activation. Pathobiology. 2020;87:100–113. doi: 10.1159/000504394. [DOI] [PubMed] [Google Scholar]
- 42.West NR, McCuaig S, Franchini F, Powrie F. Emerging cytokine networks in colorectal cancer. Nat Rev Immunol. 2015;15:615–629. doi: 10.1038/nri3896. [DOI] [PubMed] [Google Scholar]
- 43.Zhou X, Hao Q, Liao P, Luo S, Zhang M, Hu G, Liu H, Zhang Y, Cao B, Baddoo M, et al. Nerve growth factor receptor negates the tumor suppressor p53 as a feedback regulator. Elife. 2016;5:e15099. doi: 10.7554/eLife.15099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo T, Zhang SG, Zhu LF, Zhang F, Li W, Zhao K, Wen XX, Yu M, Zhan YQ, Chen H, et al. A selective c-Met and Trks inhibitor Indo5 suppresses hepatocellular carcinoma growth. J Exp Clin Cancer Res. 2019;38:130. doi: 10.1186/s13046-019-1104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lagadec C, Meignan S, Adriaenssens E, Foveau B, Vanhecke E, Romon R, Toillon RA, Oxombre B, Hondermarck H, Le Bourhis X. TrkA overexpression enhances growth and metastasis of breast cancer cells. Oncogene. 2009;28:1960–1970. doi: 10.1038/onc.2009.61. [DOI] [PubMed] [Google Scholar]
- 46.Festuccia C, Muzi P, Gravina GL, Millimaggi D, Speca S, Dolo V, Ricevuto E, Vicentini C, Bologna M. Tyrosine kinase inhibitor CEP-701 blocks the NTRK1/NGF receptor and limits the invasive capability of prostate cancer cells in vitro. Int J Oncol. 2007;30:193–200. [PubMed] [Google Scholar]
- 47.Tsai MS, Lee PH, Sun CK, Chiu TC, Lin YC, Chang IW, Chen PH, Kao YH. Nerve growth factor upregulates sirtuin 1 expression in cholestasis: A potential therapeutic target. Exp Mol Med. 2018;50:e426. doi: 10.1038/emm.2017.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.