Abstract
Despite accelerating progress towards schistosomiasis control in Africa, several age groups have been eclipsed by current treatment and monitoring strategies that mainly focus on school-aged children. As schistosomiasis poses a threat to people of all ages, unfortunate gaps exist in current treatment coverage and associated monitoring efforts, preventing subsequent health benefits to preschool-aged children as well as certain adolescents and adults. Expanding access to younger ages through forthcoming paediatric praziquantel formulation and improving treatment coverage in older ages is essential. This should occur alongside formal inclusion of these groups in large-scale monitoring and evaluation activities. Current omission of these age groups from treatment and monitoring exacerbates health inequities and has long-term consequences for sustainable schistosomiasis control.
Keywords: schistosomiasis, health inequities, preschool-aged children, adults, mass drug administration, praziquantel
Successes and remaining challenges in African schistosomiasis control
Schistosomiasis is a water-borne parasitic disease with over 220 million people infected in sub-tropical and tropical regions across the globe, the vast majority of whom live in sub-Saharan Africa [1]. For the last decade, the World Health Organization’s (WHO) goal has been to reduce schistosomiasis morbidity by this year (2020), and eliminate schistosomiasis as a public health burden by 2025 ([2]; Box 1). The primary method for achieving these goals is preventive chemotherapy (PC, see Glossary) through mass drug administration (MDA) with the anthelminthic praziquantel. These ambitious goals are underpinned by targets that require countries to reach at least 75% treatment coverage of school-aged children (SAC) and at-risk adults, with MDA schedules and target groups depending on schistosomiasis endemicity [3] (Figure 1). Data collated by WHO demonstrate that 46.3% of all people eligible and requiring treatment globally in 2017 were reached, while coverage of just SAC was higher at 70.8% [4]. Despite this progress, it is clear that access to praziquantel for SAC still falls short and preschool-aged children (PSAC), certain adolescents, and adults are commonly omitted from MDA campaigns.
Box 1: Neglected Tropical Disease (NTD) Roadmap.
Neglected Tropical Diseases are a diverse group of infections that impact the world’s poorest communities. The WHO first convened Global Partner Meetings in 2007 to help integrate strategies, goals and targets across disease control programmes. The roadmap includes specific targets for each of the twenty NTDs. For schistosomiasis, the goal is to control the disease by 2020 (defined as <5% heavy infection intensities across sites) and eliminate the disease as a public health problem by 2025 (defined as <1% heavy infection intensity across sites). To achieve these goals, the WHO originally set a target of 75% treatment coverage of both SAC by 2025. A draft of the NTD Roadmap for 2021–2030 has just been published by the WHO and partners for further consultations [12]. Currently, extending “extending MDA to all populations in need and ensuring access to the necessary drugs” is critical action for schistosomiasis alongside more integrated control methods. These actions will be necessary for achieving the ambitious goal of eliminating schistosomiasis as a public health problem (defined as < 1% proportion of heavy intensity infections) in all endemic countries by 2030). In this opinion we argue that preschool-aged children, adolescents, pregnant women, and adults across many communities urgently require treatment to reduce individual morbidity and improve MDA efficacy.
Figure 1. Age transitions across lifetime and treatment interventions for individuals in schistosome endemic regions in sub-Saharan Africa.

(A) Infants (<1 year) and preschool-aged children (1-4 years) are currently not included any mass drug administration (MDA). At risk adults (>14 years) are only included in MDA if the prevalence in school-aged children is >50% (see endemicity). (B) Estimates of the proportion of school aged children (here shown as 5-14 years) in Sub-Saharan African countries requiring schistosomiasis preventive chemotherapy (data from [7, 14]). Red points indicate countries that include SAC in MDA in 2017 and/or 2018, the most recent reporting years. All countries(C) Estimates of the proportion of adults (here shown as 15 years and older) in sub-Saharan African countries requiring schistosomiasis preventive chemotherapy. Red points indicate countries that give MDA to adults in 2017 and/or 2018, and white indicates no MDA programmes that include adults. Absence of points and shading indicate data not available (or outside sub-Saharan Africa).
Models show that high PC coverage in both SAC and at-risk adults are required for morbidity control, particularly in high endemic areas [5, 6]. Current programmes do not always have complete geographic coverage across endemic schistosomiasis regions (Figure 1B–C; [4, 7]). Despite this, empirical work demonstrates that WHO goals can be achieved in as little as a single MDA round in low endemicity settings [8] but large scale operational studies have also shown that persistent transmission hotspots are a common occurrence [9, 10]. Although progress is being made, here we focus on highlighting the unique challenges to the control and management of schistosomiasis in age groups beyond SAC with a focus on preschool-aged children and adults, but with implications for control of schistosomiasis across all ages.
Currently, schistosomiasis control in endemic regions relies on regular MDA and monitoring of infections in SAC (Figure 1A–B). Over-emphasis of treatment and evaluation in this limited age group eclipses other high-risk groups and may lead to undetected bounce backs later on [11]. As we enter the new decade, the WHO and partners have put forward a draft of an updated Neglected Tropical Disease (NTD) roadmap for 2021–2030 [12], which includes new goals for schistosomiasis control and elimination as a public health problem (Box 1). Despite heightened control efforts over the past decade, the roadmap indicates that critical action is required for expanding MDA to all populations at risk. In order to succeed across wide geographic areas, we argue that both control and monitoring strategies should extend to all age groups such that no one with schistosomiasis is systematically excluded. Here, we provide an evaluation of the epidemiological and clinical manifestations of schistosomiasis in age groups beyond SAC, discuss their current inclusion in control strategies, and highlight consequences of existing policies on health inequities.
Epidemiology of schistosomiasis and age structures in the era of MDA
Prevalence of schistosomiasis among SAC is used to establish endemicity and inform control strategies [3]. This was initially justified by a series of studies that demonstrated that schistosomiasis in SAC, particularly ages 9-12 year olds, positively correlated with prevalence and intensity across communities in a diversity of geographical and epidemiological settings [13]. However, many of these studies were conducted before national MDA programmes began, and as the burden across a community is unevenly altered by ongoing control interventions a re-appraisal of individuals at risk, those requiring treatment, and those contributing to transmission is needed.
The age structure, or relative abundance of individuals in each age class, varies between countries and is dependent on birth and death rates. In many of the sub-Saharan African countries which are highly endemic for schistosomiasis, high birth rates mean that PSAC, 1-4 years old, can comprise up to 25% of the population ([14]; Figure 2 A,B). Even in these rapidly growing populations, adults are still the most abundant age group. Age prevalence and intensity curves vary across endemicity areas, and are also likely altered by treatment history, particularly where that treatment has repeatedly targeted only an age-specific subsection of the population.
Figure 2. Distribution of populations and schistosomiasis burden across age groups in sub-Saharan Africa.
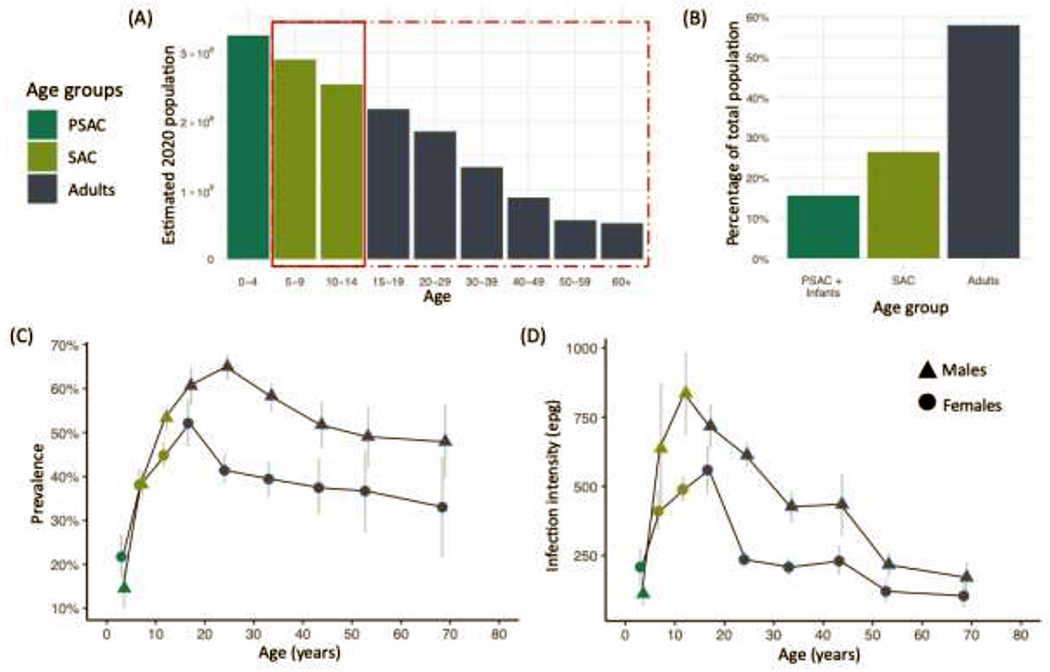
(A) Growing populations in sub-Saharan Africa means that the population, on average, is very young [14], the solid red rectangle indicates individuals eligible for school-based mass drug administration (MDA), whereas the dot-dash rectangle indicates those currently included in community-based MDA. (B) The majority of the population are in the adult age group (ages 15 and above). (C) Prevalence of Schistosoma mansoni across major age groups in a survey of 10,000 in Uganda (data from [15]). Peak prevalence in females occurs between 15-19 years, whereas males’ peak prevalence occurs from 20-29 years, and then falls in both sexes as they grow older. (D) Schistosoma mansoni infection intensities from the same population, showing infection intensities peak at approximately the same age (or even older for females) and then decline more rapidly than prevalence.
Community members that aren’t covered in school-based MDA, including infants, PSAC, non-enrolled school-aged children, and/or adults, could be important contributors to schistosome transmission [15, 16] (Figure 2 C,D). Of the approximately 120 million children affected by schistosomiasis worldwide, it is estimated that approximately 50 million are PSAC [17–19]. Children below five years of age are not regularly included in MDA campaigns [20], and drug licensing of praziquantel only applies to individuals aged four and above. In addition, certain adolescents and adults, particularly aged 16 – 25 years, can have higher mean infection intensities than SAC [21–23], most commonly when their livelihoods put them in daily contact with infectious water (e.g. car washing, fishing, housework in regions with limited clean water access) [24, 25]. A recent systematic review of studies on MDA found that combined MDA (community-based MDA supplemented with school-based MDA) had the highest overall coverage in SAC, including unenrolled SAC, better than school-based MDA which had the lowest overall coverage in SAC, which was exacerbated in unenrolled SAC [26]. Because MDAs are targeted at a subset of the population, this selective treatment can cause an age shift in prevalence and intensity of infection, meaning that the burden of schistosomiasis accumulates at older ages with increasing school-based MDA or higher coverage in SAC during community based MDA [5], though the effect at larger scales still needs to be investigated. Regardless, theoretical work has underscored the importance of quantifying infections across all community members to design the most effective control and monitoring programs for schistosomiasis [6].
Preschool-aged children are still excluded from mass drug administrations
Despite recommendations by the WHO in 2010 to treat 2- and 3- year old PSAC with praziquantel in off-licence settings [17], guidelines are difficult to implement. Praziquantel is only formally licensed for 4 year olds and above, and the global community continues to discuss the best tools and approaches for targeting this age group. Reasons for PSAC exclusion from MDA are wide ranging and include operational difficulties in access and diagnosis, inadequate knowledge about risk and infection burden, and lack of a paediatric formulation of the drug praziquantel for treatment of this age group [27] (Box 2).
Box 2: Paediatric Praziquantel Consortium.
Praziquantel is an existing drug approved for treatment of schistosomiasis and is currently licensed for ages 4 and older, although off-label informal for younger children with infections is advised by WHO. To improve formal access to treatment, the Consortium’s goal is to make an orally dispersible tablet (ODT) of praziquantel that is suitable for use and dosing in children 3 months to 6 years. Clinical trials phase I & II are complete and the Phase III trials of safety and efficacy of the developed of a mono-isomeric praziquantel formulation in ODT (150mg) for subsequent dosing at a 50mg/kg is ongoing. The trials are comparing the new ODT to crushed or broken praziquantel tablets in the target age group. Pending success in these trials which will allow formal change in licencing of praziquantel, this paediatric praziquantel formulation will help alleviate the current health inequity in schistosomiasis control.
We now know that schistosome infection can occur with water contact immediately after birth [20, 28–30], and that the intensity of infection continues to accumulate until a child’s first praziquantel treatment at age 5 or older during MDA. This age group can have prevalence rates exceeding 50%, and intensities of over 400 eggs per gram of stool have been detected in children as young as 2 years [31, 32]. Such heavy infections in PSAC reduce physical and cognitive development and, when persistently untreated, can lead to severe morbidity, and in serious cases, mortality [33]. Given the opportunity to influence overall future health in infants and young children, repeated treatment in these groups could provide lasting health benefits [34]. Praziquantel treatment has been shown to significantly reduce schistosome-associated proteinuria, albuminuria and microhaematuria in PSAC [34]. Children 5 years old and below can develop microhaematuria as soon as three months after exposure and existing tools are suitable for diagnosis [35]. Promisingly, this early morbidity resolves within three months of a single dose of praziquantel treatment [35]. More chronic effects on growth and development, for example stunting, may be irreversible if infection is left untreated for longer periods. To help mitigate this, experimental modelling suggests that repetitive treatment before reaching school age (~6 years old) can facilitate “catch-up growth” [36]. However, current exclusion of this age group from MDA programmes creates a serious health inequity, particularly in high endemicity areas [27, 37, 38]. By leaving this age group untreated, PSAC are systematically excluded from the benefits of treatment, leading to accumulation of morbidity that can become irreversible, an unacceptable health deficit unparalleled in any other parasitic disease.
A well-known deficit of praziquantel is that treatment does not prevent reinfection(s), which upon subsequent water contact and exposure leads to recurring infection and progressive morbidity [39, 40]. However, treatment of schistosome infections can induce protective immune responses against reinfection [41–43], and an increase in post-praziquantel immune responses are associated with reduced reinfection levels in PSAC [44]. In addition, evidence from experimental studies suggest a that short infections that are treated may provide greater levels of protection against reinfection compared to immunity developed through treatment of longer, more chronic infections [45, 46]. Therefore, early treatment of schistosome infections in PSAC can be further justified to reduce reinfections and associated long-term morbidity.
Opportunities for interventions in PSAC
If we are to deliver sustainable schistosomiasis elimination programmes, treatment of PSAC needs to be prioritized. There are major unanswered questions: how do we quantify infection in this age group, and more importantly, unlike SAC who typically are easily reached through school-based programmes, how do we access these younger children and at what frequency [17, 47]? Following a “test and treat” approach for PSAC [48, 49]— pending roll-out of the paediatric praziquantel formulation - could significantly reduce infection and disease burden in this age group. If PSAC treatment falls within MDA, it will be important to estimate infection burden in PSAC to identify focal areas where treatment is needed most [48]. The frequency of treatment could follow guidelines set by the WHO, but whether or not endemicity of an area (determined by SAC prevalence [19]) is an accurate estimate of PSAC burden is not fully understood and requires more research.
Studies in some endemic areas have shown that existing health systems can be tapped to improve access to PSAC and non-enrolled SAC at primary and/or secondary school levels. For example, studies in Zimbabwe have utilised the local primary health centres as a means to access PSAC, showing high compliance even in follow-up studies [35, 39, 50]. In other countries, Child Health Days offer a potential opportunity for easily reaching PSAC [17], as demonstrated in Uganda for albendazole treatments [51]. Another approach is to empower health workers to identify and treat non-specific signs of suspected clinical cases of schistosomiasis in PSAC [48]. The caveat here, however, will be the potential of providing praziquantel treatment for non-specific clinical symptoms due to other conditions aside from schistosomiasis. Of critical importance will be to monitor and evaluate any changes and constraints that these strategies may cause to health systems already implementing other intervention programmes. PSAC are currently not included in national or regional metrics on schistosomiasis, and they should be included in formal monitoring and evaluation across a diversity of endemicities to address this critical gap in knowledge.
Improvements in treatment uptake among adults is urgently needed to combat chronic schistosomiasis
In contrast to PSAC, at-risk adults in high endemicity areas are currently eligible to receive treatment annually during community-based MDA, but coverage of adults is significantly lower than that of SAC [4]. A minority (43.8%) of the population eligible for schistosomiasis PC in sub-Saharan Africa (does not include PSAC) were successfully treated in 2017, similar to global levels described in the introduction – the majority were SAC (coverage 69.4%); only 13.4% of adults requiring PC were treated [4]. Adults are not included in several national control programmes, despite a high proportion of adults requiring PC across the region (Figure 1C). Notwithstanding insufficient stocks of praziquantel, barriers to adult uptake of MDA include community perceptions of schistosomiasis as a children’s disease, hesitancy to report symptoms of schistosomiasis due to fear of stigma, failure to offer drugs to adults during MDA, non-specific or mild symptoms, fear of side effects, and difficulty seeking MDA if it requires time away from work [52–56]. Pregnant women are frequently excluded from MDA, despite studies demonstrating the safety and efficacy of praziquantel during pregnancy and lactation [57, 58]. Many health practitioners and national policy makers are reticent to support treatment of pregnant women [59]. With an average of 4.5 live births per female across sub-Saharan Africa [14], a significant portion of the crucial years of a female’s adult life can be spent excluded from MDA. These gaps in treatment coverage, especially if individuals are systemically untreated, have significant clinical impacts, as discussed below, and can play a major role in maintaining transmission even after decades of MDA [60, 61]. Monitoring efforts to measure treatment uptake, but also parasitological prevalence and intensity, are necessary to improve effectiveness of current community-based MDA. Additionally, we believe a re-evaluation of current MDA guidelines to ensure adolescents and adults that are infected across a range of endemicities receive treatment.
Morbidity in adults
Female genital schistosomiasis (FGS) and male genital schistosomiasis (MGS) are gender specific manifestations of schistosomiasis, associated with pathology caused by the migration of schistosome eggs through genital tissue. While most commonly observed with Schistosoma haematobium infection, cases associated with S. mansoni infection have also been described [62, 63].
In approximately half of girls and women with S. haematobium infection, or an estimated 40 million girls and women in Africa [64], FGS causes gynaecological morbidity marked by symptoms including post-coital bleeding, genital itch, genital discharge, and infertility. The infection additionally has been associated with stigma and often goes untreated due to misperceptions of FGS as a sexually transmitted infection and lack of awareness of healthcare workers about this debilitating condition [55]. Even when FGS is suspected, confirmatory laboratory testing is limited by poor sensitivity [65]. Another broken connection is the interplay of FGS and menstrual hygiene management [66]. In addition to direct gynaecological effects, FGS has also been associated with increased prevalence of HIV infection [67, 68], and S. haematobium infection with increased hazard of HIV acquisition in women [69]. Evidence suggests that early, repeated praziquantel treatment in girls may have the potential to reverse these gynaecological abnormalities [70] and potentially to decrease the risk of HIV acquisition in at-risk girls and women.
MGS was first described in 1911 by Madden in Egypt [71]. It has been found in 43% of participants in a small epidemiological study [72] and up to 58 % of autopsy patients infected with Schistosoma haemotobium [73], but the majority of published research on MGS is case studies [74]. MGS causes genital or ejaculatory pain, abnormal ejaculate content, haemospermia, infertility, enlarged genital organs, granulomatous infiltration, fibrosis and calcifications [63, 72, 75, 76]. Though praziquantel resolves some of the symptoms, the reversibility of chronic pathologies is not yet known, and more frequent doses may be more efficacious [77]. The distribution and full extent of MGS is unknown as there are challenges in the diagnosis - semen microscopy in particular has low sensitivity and is difficult to carry out in standard health care practice [78]. Other prevalent co-occurring diseases, such as sexually transmitted infections and tuberculosis, present similar symptomatology and pathologies as MGS, further complicating diagnosis. While early studies suggested a relationship between MGS and HIV, more research is required to understand MGS’s role in HIV acquisition and onward transmission [69, 79–82].
For both MGS and FGS, awareness of these conditions, improved diagnosis, and timely treatment are needed to alleviate the clinical burden of chronic infections and to address co-morbidities that they facilitate. Further operational and implementation research studies on FGS and MGS are critically needed in order to address these neglected forms of schistosomiasis and understand how improved uptake and frequency of treatment can prevent and reverse morbidity. Regular inclusion of adults in monitoring activities will improve understanding of the scope and distribution of schistosomiasis in communities and help target gender-specific interventions.
Opportunities for strengthening interventions in adults
Numerous opportunities exist to improve access of MDA to adults [83], and local data documenting the most significant barriers may be useful in designing the most effective strategies for improvement in specific communities or regions. Some interventions likely to be successful in many sites include optimising selection and training for community drug distributors [84], providing health education about schistosomiasis [85] focussing on adults who have lived in villages for prolonged periods [86], and targeting young adults in college or other youth settings [52, 87]. Repeated MDA campaigns can lead to treatment fatigue [86] and low compliance may be low due to non-specific symptoms. Therefore, targeted treatment, including ‘test and treat’, may be a useful tool in encouraging drug uptake in communities where and has been used successfully in low endemic settings [88]. Ensuring reliable availability of praziquantel in health centres will also facilitate access to treatment outside of the often-narrow time window of MDA.
Reinfection still occurs even after treatment in adults. Even successfully-delivered annual praziquantel treatment may be insufficient; 40% of adult women in an endemic region who had schistosome infections treated at baseline were found to be schistosome-infected at least once during 12 months of follow-up [56]. These findings are supported by other studies in communities with high water contact in adults [24, 25], although reinfection occurs more often in SAC and younger groups [10], potentially due to an increase in adult immunity towards reinfection. Although treatment will not completely prevent morbidity due to reinfection, community-based MDA is more cost-effective than school-based MDA in a range of settings [89, 90] and there is a reduced likelihood of reinfection in adults [25].
Treatment as a compliment to integrated methods for interventions
Identifying communities with persisting high prevalence of infection, determining the best approaches to deliver drugs outside of the school environment, building capacity within health centres, ensuring sufficient praziquantel availability, and targeted approaches to undertreated age groups are all ways to improve existing MDA programmes [26]. However, treatment with praziquantel will not be sufficient for the sustainable control and elimination of schistosomiasis in many areas [91]. Improved water, sanitation and hygiene (WASH), behaviour change, targeted education and snail control are all fundamental facets to ensuring that schistosome transmission will reduce, and ultimately eliminated, in endemic communities [92] and are cost-effective [93]. It is important that these additional interventions be deployed equitably, so that benefits are achieved throughout the lifespan of individuals. Precision mapping provides an innovative way to better target interventions and allocate resources to focal transmission areas and the specific species of Schistosoma to target [94]. Strengthening deployment while reducing the global burden disease burden will improve the financial sustainability of control programmes.
Concluding remarks
At any age, coming into contact with freshwater harbouring cercariae can lead to schistosome infection. Indeed, schistosomiasis can be pervasive and affects the world’s poor, who are commonly dependent on local water bodies and are exposed, sometimes on a daily basis. Whilst water contact patterns and immune status may change throughout life, there is an urgent need to ensure that treatment is available to all age groups (see Outstanding Questions). MDA with praziquantel, which is inexpensive and has a low side-effect profile, has focused primarily on SAC but as we strive for elimination, it is clear that widening this approach to include the entire population at risk will be essential to success, and we should leave no one behind.
Outstanding Questions Box.
What are the most effective methods to improve access to information and treatment in groups already eligible for MDA, including pregnant women and unenrolled SAC?
How can infected individuals receive treatment outside of MDA programmes?
Can we identify characteristics of communities where it is essential to integrate control in PSAC and adult age groups?
Can we include monitoring of all age groups in schistosomiasis programming, so that PSAC and adults are systematically included in the evaluation of control effectiveness and used to inform future control strategies?
Pending roll-out of the paediatric formulation of the anthelminthic praziquantel, what is the best way to quantify infection burden in PSAC to inform control strategies? How can we leverage existing health systems to improve treatment access to this age group?
What mechanisms cause FGS and MGS and what interventions can prevent these conditions?
If treatment is expanded to include all age groups at risk, will there be sufficient praziquantel available? And if not, how can we produce and access the higher numbers of praziquantel tablets needed for this wider treatment campaign?
Highlights.
To date in Africa, schistosomiasis treatment and monitoring focuses on school-aged children, but much less attention has been given to preschool-aged children and adults.
Here, we summarize the distribution and morbidity of schistosomiasis across these age groups and highlight gaps in current control strategies.
Preschool-aged children incur significant morbidity but are systematically excluded from current treatment programmes; inclusion in control programmes could have both short- and long-term health benefits.
Mass drug administration must be improved to reach at-risk adults. Low coverage has cascading implications for health, including morbidities specific to reproductive ages, and can facilitate infection with other diseases such as HIV.
Control and elimination of schistosomiasis will require future integration of all age groups into treatment programmes and evaluation of progress.
Acknowledgement
CLF and PHLL were supported by a European Research Council Starting Grant (SCHISTO_PERSIST_680088) to PHLL. PHLL is also funded by a GCRF MRC Foundation award (MR/P025447/1) and an EPSRC grant (EP/R01437X/1). DMNO acknowledges support from the Darwin Trust of Edinburgh, Thrasher Research Fund (12440), Wellcome Trust (108061/Z/15/Z), and the National Institute for Health Research (NIHR) Global Health Research programme (16/136/33) using UK Aid from the UK Government. JAD is supported by an NIH K23 (AI110238). We are also grateful for the Global Schistosomiasis Alliance’s role in organizing a symposium on ’Schistosomiasis Control Through the Ages’ during the 2019 European Congress on Tropical Medicine and International Medicine that sparked discussion to form the basis of this opinion piece.
Glossary
- Age structure
The age structure of a country is defined by the proportion of the population in different age classes. The age structure of a country’s population is determined by mean birth and death rates at each age.
- At-risk adults
Adults that are frequently exposed to unsafe water. This can range from a subset of a community that engages in economic and domestic activities that put them in contact with water to all adults in a community living close to an infested water body. We use at-risk adults and adults interchangeably in this piece.
- Endemicity
Categories of schistosomiasis risk determined at the community or regional level. The WHO gives guidelines for three categories of risk and the MDA schedules for each, which is based off prevalence in school-aged children determined by parasitological methods:
- Low endemicity
<10%. Recommend treating all school-aged children twice during primary school and having praziquantel available at dispensaries and clinics.
- Moderate endemicity
≥10% but <50%. Recommend treating school-age children and adults at risk once every two years.
- High endemicity
≥ 50%. Recommend treating school-age children and all adults at risk once a year
- Geographic coverage
The proportion of endemic country implementation units (usually districts or states) that are reached in a given round of MDA.
- Health Inequity
A systematic difference in health based on demographics or social status (such as age group).
- Infants
Usually defined as children under 1 year of age. Not the focus of this paper as there are currently limited schistosomiasis treatment options, even in the pipeline, for this age category.
- Intensity of infection
The infection intensity of schistosome infections is indirectly measured by counting the number of eggs excreted in faeces (expressed as eggs per gram, epg) or in urine (expressed as eggs per 10 ml of urine), depending on the species. Infection intensity of intestinal schistosomiasis is defined as light intensity (0 to 99 epg), moderate intensity (100 to 399 epg) or heavy intensity (≥ 400 epg). Infection intensity of urogenital schistosomiasis is defined as light intensity (1 to 50 eggs per 10 ml) or heavy intensity (> 50 eggs per 10 ml).
- Mass drug administration (MDA)
the administration of treatment (praziquantel for schistosomiasis) to every member of a defined population, irrespective of individual infection status.
- School-based MDA
Praziquantel (for schistosomiasis) only distributed at schools (usually primary schools, but sometimes also secondary schools).
- Community-based MDA
Praziquantel (for schistosomiasis) distributed to community members deemed at risk – usually all SAC and at-risk adults (water contact occupations). Recommended door-to-door administration but can also be, and usually is, distributed centrally.
- Combined MDA
Praziquantel (for schistosomiasis) distributed to community members deemed at risk and at schools.
- Preventive chemotherapy (PC)
The use of safe, effective drugs to treat populations at risk to prevent morbidity.
- Preschool-aged children (PSAC)
Individuals that are here defined as children between 1 and 4 years of age, but the specific range can vary by location (sometimes 1–5 years old). We include toddlers (1-2 years of age) in PSAC, as these ages are included in the paediatric praziquantel formulation (see Box 1).
- School-aged children (SAC)
Individuals that are usually defined as children between 5 and 14 years, but the specific range can vary by location. These children are usually eligible to attend primary school. However, all children in this age range – both enrolled and unenrolled in school- are part of this group regardless of their school enrolment or attendance. Non-enrolled SAC is also often neglected in school-based MDA campaigns.
- Treatment coverage
The proportion of eligible individuals (above the age of 5 and/or SAC based on MDA guidelines) that take praziquantel in a given round of MDA.
References
- 1.World Health Organization, Schistosomiasis: Fact Sheet, World Health Organization, Geneva, Switzerland, 2019. [Google Scholar]
- 2.World Health Organization, Accelerating work to overcome the global impact of Neglected Tropical Diseases. A roadmap for implementation. , Geneva, 2012. [Google Scholar]
- 3.World Health Organization, Preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers., World Health Organization, Geneva, 2006. [Google Scholar]
- 4.World Health Organization (2018) Schistosomiasis and soiltransmitted helminthiases: numbers of people treated in 2017. Weekly Epidemiological Record 50 (93), 681–692. [Google Scholar]
- 5.Turner HC et al. (2017) Evaluating the variation in the projected benefit of community-wide mass treatment for schistosomiasis: Implications for future economic evaluations. Parasites & vectors 10 (1), 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toor J et al. (2018) The design of schistosomiasis monitoring and evaluation programmes: The importance of collecting adult data to inform treatment strategies for Schistosoma mansoni. PLoS neglected tropical diseases 12 (10), e0006717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization, PCT databank, 2020. [Google Scholar]
- 8.Deol A, Fleming F, Calvo-Urbano B, Walker M, Bucumi V, Gnandou I, Tukahebwa EM, Jemu S, Mwingira UJ, Alkohlani A, Traore M, SCI, Basáñez M-G, French MD, Webster JP (2019) Schistosomiasis – assessing progress towards the 2020 and 2025 goals. N Engl J Med 381 (26), 2519–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kittur N et al. (2017) Defining persistent hotspots: areas that fail to decrease meaningfully in prevalence after multiple years of mass drug administration with praziquantel for control of schistosomiasis. Am J Trop Med Hyg 97 (6), 1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiegand RE et al. (2017) A Persistent Hotspot of Schistosoma mansoni Infection in a Five-Year Randomized Trial of Praziquantel Preventative Chemotherapy Strategies. The Journal of Infectious Diseases 216 (11), 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell KM et al. (2014) Predicted impact of mass drug administration on the development of protective immunity against Schistosoma haematobium. PLoS Neglected Tropical Diseases 8 (7), e3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization, Ending the Neglect to Attain the Sustainable Development Goals: A road map for neglected tropical diseases 2021-2030, World Health Organization, Geneva, 2020. [Google Scholar]
- 13.Mwinzi PN et al. (2015) Predictive value of school-aged Children’s schistosomiasis prevalence and egg intensity for other age groups in western Kenya. The American Journal of Tropical Medicine and Hygiene 93 (6), 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.United Nations, D.o.E.a.S.A., Population Division, (2019) World Population Prospects 2019. https://population.un.org/wpp/, (accessed 11/02/20).
- 15.Kabatereine NB et al. (2004) Epidemiology and geography of Schistosoma mansoni in Uganda: implications for planning control. Tropical Medicine & International Health 9 (3), 372–380. [DOI] [PubMed] [Google Scholar]
- 16.Njenga SM et al. (2011) Adult population as potential reservoir of NTD infections in rural villages of Kwale district, Coastal Kenya: implications for preventive chemotherapy interventions policy. Parasites & vectors 4 (1), 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization, Report of a meeting to review the results of studies on the treatment of schistosomiasis in preschool-age children, World Health Organization, Geneva, 2010. [Google Scholar]
- 18.Mutapi F (2015) Changing policy and practice in the control of pediatric schistosomiasis. Pediatrics 135 (3), 536–44. [DOI] [PubMed] [Google Scholar]
- 19.WHO Expert Committee (2002) Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organ Tech Rep Ser 912, i–vi, 1-57, back cover. [PubMed] [Google Scholar]
- 20.Stothard JR and Gabrielli AF (2007) Schistosomiasis in African infants and preschool children: to treat or not to treat? Trends Parasitol 23 (3), 83–6. [DOI] [PubMed] [Google Scholar]
- 21.Black CL et al. (2009) Impact of intense, longitudinal retreatment with praziquantel on cure rates of schistosomiasis mansoni in a cohort of occupationally exposed adults in western Kenya. Tropical Medicine & International Health 14 (4), 450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira APB et al. (2010) The prevalence of schistosomiasis in school-aged children as an appropriate indicator of its prevalence in the community. Memórias do Instituto Oswaldo Cruz 105 (4), 563–569. [DOI] [PubMed] [Google Scholar]
- 23.French MD et al. (2010) Observed reductions in Schistosoma mansoni transmission from large-scale administration of praziquantel in Uganda: a mathematical modelling study. PLOS Neglect Trop D 4 (11), e897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabatereine NB et al. (2004) Epidemiology and morbidity of Schistosoma mansoni infection in a fishing community along Lake Albert in Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene 98 (12), 711–718. [DOI] [PubMed] [Google Scholar]
- 25.Karanja DM et al. (2002) Resistance to reinfection with Schistosoma mansoni in occupationally exposed adults and effect of HIV-1 co-infection on susceptibility to schistosomiasis: a longitudinal study. The Lancet 360 (9333), 592–596. [DOI] [PubMed] [Google Scholar]
- 26.Burnim M et al. (2017) Systematic review of community-based, school-based, and combined delivery modes for reaching school-aged children in mass drug administration programs for schistosomiasis. PLoS neglected tropical diseases 11 (10), e0006043–e0006043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osakunor DNM et al. (2018) Paediatric schistosomiasis: What we know and what we need to know. PLoS Negl Trop Dis 12 (2), e0006144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ekpo UF et al. (2012) Schistosomiasis in infants and pre-school-aged children in sub-Saharan Africa: implication for control. Parasitology 139 (7), 835–841. [DOI] [PubMed] [Google Scholar]
- 29.Nalugwa A et al. (2015) Intestinal schistosomiasis among preschool children along the shores of Lake Victoria in Uganda. Acta tropica 142, 115–121. [DOI] [PubMed] [Google Scholar]
- 30.Woolhouse ME et al. (2000) Exposure, infection and immune responses to Schistosoma haematobium in young children. Parasitology 120 ( Pt 1), 37–44. [DOI] [PubMed] [Google Scholar]
- 31.Ruganuza DM et al. (2015) Schistosoma mansoni among pre-school children in Musozi village, Ukerewe Island, North-Western-Tanzania: prevalence and associated risk factors. Parasites & Vectors 8 (1), 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stothard JR et al. (2011) Schistosoma mansoni infections in young children: when are schistosome antigens in urine, eggs in stool and antibodies to eggs first detectable? PLoS Neglected Tropical Diseases 5 (1), e938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freer JB et al. (2018) Schistosomiasis in the first 1000 days. Lancet Infect Dis 18 (6), e193–e203. [DOI] [PubMed] [Google Scholar]
- 34.Wami WM et al. (2016) Comparative assessment of health benefits of praziquantel treatment of urogenital schistosomiasis in preschool and primary school-aged children. BioMed research international 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osakunor DNM et al. (2018) Dynamics of paediatric urogenital schistosome infection, morbidity and treatment: a longitudinal study among preschool children in Zimbabwe. BMJ Glob Health 3 (2), e000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurarie D et al. (2011) Modeling the effect of chronic schistosomiasis on childhood development and the potential for catch-up growth with different drug treatment strategies promoted for control of endemic schistosomiasis. The American journal of tropical medicine and hygiene 84 (5), 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stothard JR et al. (2013) Schistosomiasis in African infants and preschool children: let them now be treated! Trends Parasitol 29 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stothard JR et al. (2011) Closing the praziquantel treatment gap: new steps in epidemiological monitoring and control of schistosomiasis in African infants and preschool-aged children. Parasitology 138 (12), 1593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mutsaka-Makuvaza MJ et al. (2018) Reinfection of urogenital schistosomiasis in pre-school children in a highly endemic district in Northern Zimbabwe: a 12 months compliance study. Infect Dis Poverty 7 (1), 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chandiwana SK et al. (1991) Factors affecting the intensity of reinfection with Schistosoma haematobium following treatment with praziquantel. Parasitology 102 Pt 1, 73–83. [DOI] [PubMed] [Google Scholar]
- 41.Black CL et al. (2010) Increases in levels of schistosome-specific immunoglobulin E and CD23(+) B cells in a cohort of Kenyan children undergoing repeated treatment and reinfection with Schistosoma mansoni. J Infect Dis 202 (3), 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bourke CD et al. (2013) Integrated analysis of innate, Th1, Th2, Th17, and regulatory cytokines identifies changes in immune polarisation following treatment of human schistosomiasis. J Infect Dis 208 (1), 159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Black CL et al. (2010) Influence of exposure history on the immunology and development of resistance to human Schistosomiasis mansoni. PLoS Negl Trop Dis 4 (3), e637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rujeni N et al. (2013) Immunological consequences of antihelminthic treatment in preschool children exposed to urogenital schistosome infection. J Trop Med 2013, 283619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Riet E et al. (2007) Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology 212 (6), 475–490. [DOI] [PubMed] [Google Scholar]
- 46.Behnke JM and Robinson M (1985) Genetic control of immunity to Nematospiroides dubius: a 9-day anthelmintic abbreviated immunizing regime which separates weak and strong responder strains of mice. Parasite immunology 7 (3), 235–253. [DOI] [PubMed] [Google Scholar]
- 47.Sousa-Figueiredo JC et al. (2012) Performance and safety of praziquantel for treatment of intestinal schistosomiasis in infants and preschool children. PLoS Negl Trop Dis 6 (10), e1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bustinduy AL et al. (2016) Expanding praziquantel (PZQ) access beyond mass drug administration programs: paving a way forward for a pediatric PZQ formulation for schistosomiasis. PLoS neglected tropical diseases 10 (9), e0004946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization, Report of a meeting to review the results of studies on the treatment of schistosomiasis in preschool-age children, World Health Organization,, Geneva, 2011. [Google Scholar]
- 50.Mutapi F et al. (2011) Schistosoma haematobium treatment in 1-5 year old children: safety and efficacy of the antihelminthic drug praziquantel. PLoS Negl Trop Dis 5 (5), e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alderman H et al. (2006) Effect on weight gain of routinely giving albendazole to preschool children during child health days in Uganda: cluster randomised controlled trial. BMJ 333 (7559), 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adriko M et al. (2018) Low Praziquantel Treatment Coverage for Schistosoma mansoni in Mayuge District, Uganda, Due to the Absence of Treatment Opportunities, Rather Than Systematic Non-Compliance. Trop Med Infect Dis 3 (4), 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coulibaly J et al. (2018) A Rapid Appraisal of Factors Influencing Praziquantel Treatment Compliance in Two Communities Endemic for Schistosomiasis in Côte d’Ivoire. Tropical Medicine and Infectious Disease 3 (2), 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knopp S et al. (2016) Praziquantel coverage in schools and communities targeted for the elimination of urogenital schistosomiasis in Zanzibar: a cross-sectional survey. Parasites & Vectors 9 (1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kukula VA et al. (2019) A major hurdle in the elimination of urogenital schistosomiasis revealed: Identifying key gaps in knowledge and understanding of female genital schistosomiasis within communities and local health workers. PLOS Neglected Tropical Diseases 13 (3), e0007207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mishra P et al. (2019) Insufficiency of annual praziquantel treatment to control Schistosoma mansoni infections in adult women: A longitudinal cohort study in rural Tanzania. PLOS Neglected Tropical Diseases 13 (11), e0007844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ndibazza J et al. (2010) Effects of deworming during pregnancy on maternal and perinatal outcomes in Entebbe, Uganda: a randomized controlled trial. Clinical Infectious Diseases 50 (4), 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.World Health Organization, Report of the WHO Informal Consultation on the use of praziquantel during pregnancy lactation and albendazole/mebendazole in children under 24 months., World Health Organization, Geneva, 2003. [Google Scholar]
- 59.Friedman JF et al. (2018) Praziquantel for the treatment of schistosomiasis during human pregnancy. Bulletin of the World Health Organization 96 (1), 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farrell SH and Anderson RM (2018) Helminth lifespan interacts with non-compliance in reducing the effectiveness of anthelmintic treatment. Parasites & Vectors 11 (1), 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dyson L et al. (2017) Measuring and modelling the effects of systematic non-adherence to mass drug administration. Epidemics 18, 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poggensee G et al. (2001) Presence of Schistosoma mansoni eggs in the cervix uteri of women in Mwanga District, Tanzania. Transactions of the Royal Society of Tropical Medicine and Hygiene 95 (3), 299–300. [DOI] [PubMed] [Google Scholar]
- 63.Gelfand M et al. (1970) Schistosomiasis of the Male Pelvic Organs: Severity of Infection as Determined by Digestion of Tissue and Histologic Methods in 300 Cadavers. American Journal of Tropical Medicine and Hygiene 19 (5), 779–784. [PubMed] [Google Scholar]
- 64.Hotez PJ et al. (2019) Female Genital Schistosomiasis. New England Journal of Medicine 381 (26), 2493–2495. [DOI] [PubMed] [Google Scholar]
- 65.Galappaththi-Arachchige HN et al. (2018) Evaluating diagnostic indicators of urogenital Schistosoma haematobium infection in young women: A cross sectional study in rural South Africa. PLOS ONE 13 (2), e0191459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stothard R et al. (2020) Connecting female genital schistosomiasis and menstrual hygiene initiatives. Trends in Parasitology 36, 410–412. [DOI] [PubMed] [Google Scholar]
- 67.Kjetland EF et al. (2006) Association between genital schistosomiasis and HIV in rural Zimbabwean women. Aids 20 (4), 593–600. [DOI] [PubMed] [Google Scholar]
- 68.Downs JA et al. (2011) Urogenital schistosomiasis in women of reproductive age in Tanzania’s Lake Victoria region. The American journal of tropical medicine and hygiene 84 (3), 364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wall KM et al. (2018) Schistosomiasis is associated with incident HIV transmission and death in Zambia. PLOS Neglected Tropical Diseases 12 (12), e0006902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kjetland EF et al. (2008) Prevention of gynecologic contact bleeding and genital sandy patches by childhood anti-schistosomal treatment. The American journal of tropical medicine and hygiene 79 (1), 79–83. [PubMed] [Google Scholar]
- 71.Madden FC (1911) Two Rare Manifestations of Bilharziosis. The Lancet 178 (4593), 754–755. [Google Scholar]
- 72.Leutscher P et al. (2000) Community-based study of genital schistosomiasis in men from Madagascar. The Lancet 355 (9198), 117–118. [DOI] [PubMed] [Google Scholar]
- 73.Elem B and Patil P (1987) Haemospermia: observations in an area of endemic bilharziasis. British journal of urology 60 (2), 170–173. [DOI] [PubMed] [Google Scholar]
- 74.Kayuni S et al. (2019) A systematic review with epidemiological update of male genital schistosomiasis (MGS): A call for integrated case management across the health system in sub-Saharan Africa. Parasite Epidemiology and Control 4, e00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vilana R et al. (1997) Schistosomiasis of the male genital tract: transrectal sonographic findings. J Urol 158 (4), 1491–3. [PubMed] [Google Scholar]
- 76.Squire B and Stothard JR (2014) Schistosomiasis In Tropical Medicine: Lecture notes (7th edn) (Beeching N and Gill G eds), pp. 151–162, Wiley-Blackwell. [Google Scholar]
- 77.Lang R et al. (2017) Hematospermia in a returned traveler. Canadian Urological Association Journal 11 (1-2), E41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kayuni SA et al. (2019) How can schistosome circulating antigen assays be best applied for diagnosing male genital schistosomiasis (MGS): An appraisal using exemplar MGS cases from a longitudinal cohort study among fishermen on the south shoreline of Lake Malawi. Parasitology, 1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leutscher PDC et al. (2005) Increased prevalence of leukocytes and elevated cytokine levels in semen from Schistosoma haematobium-infected individuals. Journal of Infectious Diseases 191 (10), 1639–1647. [DOI] [PubMed] [Google Scholar]
- 80.Stecher CW et al. (2015) Considering treatment of male genital schistosomiasis as a tool for future HIV prevention: a systematic review. Int J Public Health 60 (7), 839–48. [DOI] [PubMed] [Google Scholar]
- 81.Midzi N et al. (2017) Decrease in Seminal HIV-1 RNA Load After Praziquantel Treatment of Urogenital Schistosomiasis Coinfection in HIV-Positive Men—An Observational Study. Open Forum Infectious Diseases 4 (4), ofx199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Furch BD et al. (2020) Interactions of Schistosoma and HIV in Sub-Saharan Africa: A Systematic Review. The American Journal of Tropical Medicine and Hygiene 102 (4), 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deardorff KV et al. (2018) Strategies to improve treatment coverage in community-based public health programs: A systematic review of the literature. PLoS neglected tropical diseases 12 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chami GF et al. (2017) Community-directed mass drug administration is undermined by status seeking in friendship networks and inadequate trust in health advice networks. Social Science & Medicine 183, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tuhebwe D et al. (2015) Uptake of Mass Drug Administration Programme for Schistosomiasis Control in Koome Islands, Central Uganda. PLOS ONE 10 (4), e0123673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chami GF et al. (2015) Profiling nonrecipients of mass drug administration for schistosomiasis and hookworm infections: a comprehensive analysis of praziquantel and albendazole coverage in community-directed treatment in Uganda. Clin Infect Dis 62 (2), 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Korir H et al. (2018) Young Adults in Endemic Areas: An Untreated Group in Need of School-Based Preventive Chemotherapy for Schistosomiasis Control and Elimination. Tropical medicine and infectious disease 3 (3), 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barkia H et al. (2014) Contribution of Mobile Teams to Efforts to Eliminate Schistosomiasis at Schistosoma haematobium in Morocco- Narrative Review Article. Iranian journal of public health 43 (9), 1167–1175. [PMC free article] [PubMed] [Google Scholar]
- 89.Lo NC et al. (2015) Comparison of community-wide, integrated mass drug administration strategies for schistosomiasis and soil-transmitted helminthiasis: a cost-effectiveness modelling study. The Lancet Global Health 3 (10), e629–e638. [DOI] [PubMed] [Google Scholar]
- 90.Lo NC et al. (2016) Assessment of global guidelines for preventive chemotherapy against schistosomiasis and soil-transmitted helminthiasis: a cost-effectiveness modelling study. The Lancet Infectious Diseases 16 (9), 1065–1075. [DOI] [PubMed] [Google Scholar]
- 91.Secor WE and Colley DG (2018) When should the emphasis on schistosomiasis control move to elimination? Tropical medicine and infectious disease 3 (3), 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sokolow SH et al. (2016) Global Assessment of Schistosomiasis Control Over the Past Century Shows Targeting the Snail Intermediate Host Works Best. PLOS Neglected Tropical Diseases 10 (7), e0004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lo NC et al. (2018) Impact and cost-effectiveness of snail control to achieve disease control targets for schistosomiasis. Proceedings of the National Academy of Sciences 115 (4), E584–E591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tchuem Tchuenté L-A et al. (2018) Precision mapping: An innovative tool and way forward to shrink the map, better target interventions, and accelerate toward the elimination of schistosomiasis. PLOS Neglected Tropical Diseases 12 (8), e0006563. [DOI] [PMC free article] [PubMed] [Google Scholar]


