Abstract
Non-small cell lung cancer (NSCLC)1 is one of the most common malignancies in the western world [1]. Despite multiple therapeutic and diagnostic advances, the overall survival is low and recurrence of NSCLC is a common problem with different treatment regimens. The inclusion of 18fluorine fluorodeoxyglucose (18FDG) positron emission tomography (PET) in combination with computed tomography (CT) in clinical practice was revolutionary for the staging of NSCLC [2]. 18FDG-PET/CT provides morphological, functional, and metabolic information about the tumor, which is usually highly, metabolically active. Due to the increased glucose uptake, 18FDG is actively accumulatedin the tumor tissues, resulting in an increased standardized uptake value (SUV). The tumor tissue itself consists of neoplastic cells, extracellular matrix, fibroblasts, and various immune cells. These immune cells include tumor-infiltrating lymphocytes, regulatory T cells, and macrophages. Macrophages have different activation patterns and play an essential role in inflammation and cancer. In particular, tumor-associated macrophages (TAMs) are a specialized group of alternatively activated or M2 macrophages. TAMs release several chemokines that are different from those released by classically activated macrophages found in an inflammatory environment. One of the most important chemokines released by TAMs is CC-chemokine ligand 18 (CCL18). Although CCL18 is present in healthy subjects, its levels are significantly elevated in the serum of patients with NSCLC. It correlates with overall survival and tumor stage in several malignant diseases [3,4]. A recurring problem is that increased glucose metabolism can be found in the inflammatory tissue, which can also lead to an increased SUV in 18FDG PET/CT, lowering its oncological specificity [5]. In a previous study, we demonstrated that serum CCL18 levels can be used to differentiate between patients with NSCLC and healthy subjects [3]. Hence, we investigated the correlation between serum CCL18 levels and the maximum standardized uptake value (SUVmax) of the primary tumor using 18FDG-PET/CT. We found a significant correlation between the SUVmax of the primary tumor and the serum CCL18 level. The data are important because they can be used to draw conclusions about immunometabolism. Furthermore, they can serve as basis for future prospective clinical studies.
Keywords: CCL18, NSCLC, Theranostics, Alternatively activated macrophages, M2, PARC
Specifications Table
| Subject | Surgery |
| Specific subject area | Thoracic Surgery |
| Type of data | Table and Figures |
| How data were acquired | In this prospective observational study, the patients’ clinical data were collected from their medical records. The study was approved by the local ethics committee (16–6966-BO) and was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. All patients provided written informed consent. |
| Data format | Raw baseline data |
| Parameters for data collection | Baseline clinical parameters serum CCL18 levels, maximum standardized uptake value (SUVmax) |
| Description of data collection | Data were prospectively recorded from the patients’ written and electronic clinical records. CCL18 was measured using ELISA, while SUVmax was measured using 18FDG PET |
| Data source location | City/Town/Region: Ruhrlandklinik, University Hospital Essen, University Duisburg-Essen, Essen, Germany Country: Germany |
| Data accessibility | The raw data can be accessed through Mendeley Data http://dx.doi.org/10.17632/yv4f7n2k7y.1 |
Value of the Data
-
•
Our observational data may be useful for clinicians and researchers working in the fields of lung cancer and M2 macrophages. Our study shows that serum CCL18 levels, indirectly indicative of the amount of TAMs, increases with the increasing SUVmax.
-
•
The data provide insights into immunometabolism and may be beneficial for translational researchers working on macrophages and other researchers interested in the clinical application of the 18FDG PET.
-
•
The dataset provides a valuable starting point for further prospective studies in the field of theranostics. Serum CCL18 levels are easy to measure and may help identify 18FDG-positive pulmonary nodules (such as post-inflammatory residue from malignancy). Therefore, we believe that these data are valuable for study planning and patient number calculation.
1. Data Description
This dataset contains the clinical data on patients with lung cancer who underwent surgery. We included 22 patients with non-small cell lung cancer (NSCLC) (10 patients with squamous carcinoma [SQC] and 12 patients with adenocarcinoma [AC]) in our investigation. Table 1 shows the characteristics of the different patient groups. The SCQ group consisted of nine male patients and one female patient, with a median age of 72 years (range, 59–81 years). According to the postoperative tumor classification, especially T stage, two cases were classified as pT1a, three cases were classified as pT1b, and five cases were classified as pT2a. All patients (AC and SQC) were node negative (pN0) and had no evidence of distant metastasis (cM0). In the SQC group, the median serum CCL18 level was 179 ng/mL (range, 65–862 ng/mL) and the median SUVmax was 9 (range, 3.6–27). The median forced expiratory volume in 1 s (FEV1) predicted was 74% (range, 24%–100%).
Table 1.
Patients characteristics.
| Value | AC (n = 12) | SQC (n = 10) | C (n = 11) | p-value |
|---|---|---|---|---|
| Sex | Male 10 Female 2 |
Male 9 Female 1 |
Male 7 Female 4 |
p = 0.2 |
| Median age in years | 61 (range, 42–72) | 72 (range, 59–81) | 56 (range, 17–80) | |
| pT* | pT1a 7 pT1b 1 pT2a 4 |
pT1a 2 pT1b 3 pT2a 5 |
Hamartochondroma 4 Aspergilloma 1 Bronchogenic cyst 1 Intrapulmonary lymph node 1 Pectus excavatum 2 Diaphragmatic hernia 1 Lung infarction 1 |
p = 0.1 |
| SUVmax⁎⁎ | 5.2 (range, 2–15.8) | 9 (range, 3.6–27) | Not applicable | p = 0.1 |
| FEV1⁎⁎⁎ | 72% (range, 55%–100%) | 74% (range, 24%–100%) | 94% (range, 59%–100%) | p = 0.1 |
| CCL18⁎⁎⁎⁎ | 95 (range, 47–233) | 179 (range, 65–862) | 47 (range, 28–98) | p<0.01 |
Based on the classification of the Union for International Cancer Control 8th Edition.
Median SUVmax of the primary tumor.
Median FEV1 percent predicted.
Median serum CCL18 level in ng/mL.
In contrast, the AC group consisted of 10 male patients and two female patients, with a median age of 61 years (range, 47–72 years). Seven cases were classified as pT1a, one case was classified as pT1b, and four cases were classified as pT2a. The median serum CCL18 level was 95 ng/mL (range, 47–233 ng/mL), and the median FEV1 predicted was 72% (range, 55%–100%). The median SUVmax was 5.2 (range, 2–15.8).
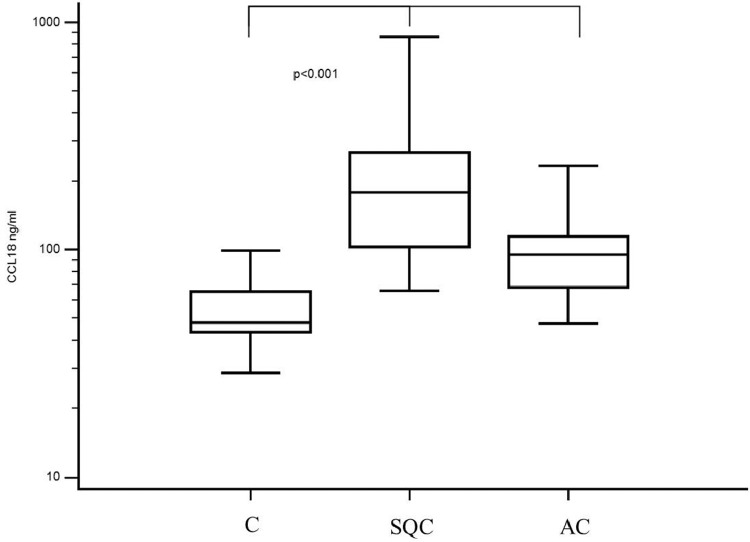
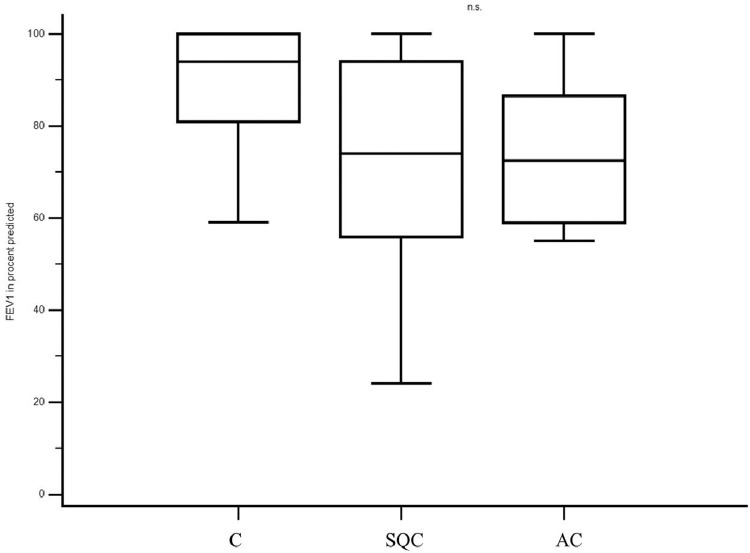
We also included 11 patients (seven male patients and four female patients) in the control group (C). Among them, four patients were diagnosed with hamartochondroma, one patient was diagnosed with aspergilloma, one patient was diagnosed with a bronchogenic cyst, one patient was diagnosed with an intrapulmonary lymph node, two patients were diagnosed with pectus excavatum, one with suspected lung cancer (final histology revealed a pulmonary embolism), and one patient was diagnosed with diaphragmatic hernia. The median patient age in the control group was 56 years (range, 17–80 years), and the median serum CCL18 level was 47 ng/mL (range, 28–98 ng/mL) (Fig. 1). 18FDG-PET CT was performed only in the patient who was preoperatively suspected to have lung cancer; the postoperative diagnosis was a pulmonary embolus. In this case, the median SUVmax was 3.5, and the median serum CCL18 level was 73 ng/mL. The median FEV1 predicted was 94% (range 59%–100%) (Fig. 2).
Fig. 1.
Median CCL18 serum level shown along the different histological subgroups. The median CCL18 was higher in the group of patients with malignancy compared to the control group (patients with benign disease) (Median CCL 18 Level was 47 ng/ml (C) vs 95 ng/ml (AC) vs. 179 ng/ml (SQC); p<0.0001) (C = Control group with benign disease, AC= adenocarcinoma, SQC= squamous carcinoma).
Fig. 2.
The preoperative median FEV1 percent predicted differed not significantly in the various groups (Median FEV1 percent predicted was 74% (SQC) vs. 72% (AC) vs. 94% (C)). (C = Control group with benign disease, AC= adenocarcinoma, SQC= squamous carcinoma).
Multivariate analysis revealed that FEV1 and the largest tumor diameter were not significantly different between the study groups. In addition, the SUVmax did not differ significantly between the SQC and AC groups. However, the median serum CCL18 level was significantly more elevated in the SCQ (179 ng/mL; range, 65–862 ng/mL) and AC groups (95 ng/mL; range, 47–233 ng/ml) than that in the control group (median 47 ng/ml, range 28–98.9 ng/ml) (p<0.0001).
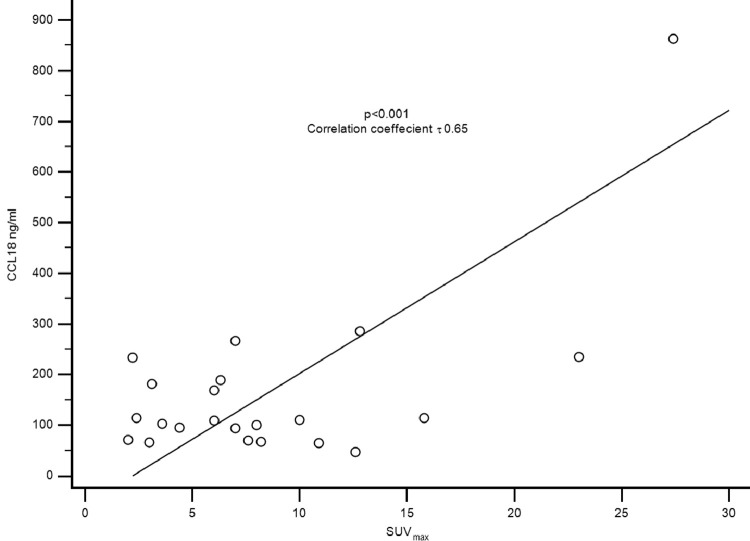
The serum CCL18 level was significantly correlated with the SUVmax in all patients with NSCLC (correlation coefficient r = 0.6; p<0.002; Fig. 3). However, the SUVmax did not correlate with the largest tumor diameter and the FEV1 predicted (Correlation coefficient r = 0.1; p = 0.5).
Fig. 3.
The median CCL18 serum level correlates posetively with the SUVmax (p<0.01; r 0.65).
2. Experimental Design, Materials and Methods
We evaluated the prospectively collected preoperative blood samples from patients treated at our department. We included patients with a pulmonary node or a pulmonary mass that had not been histologically diagnosed prior to surgery. Patients with benign lesions were included in the control group (Table 1). Patients with organ fibrosis (especially lung fibrosis), immunosuppressive disease, autoimmune disease, or other pre-existing malignancies or those taking immunosuppressive medications were excluded. 18FDG-PET/CT was performed as part of the routine staging procedure according to the current guidelines or as indicated by the treating physician. In line with the guidelines of the European Respiratory Society and the European Society of Thoracic Surgery, all patients underwent lung function evaluation prior to surgery. The staging was supplemented by preoperative endobronchial ultrasound-guided transbronchial needle aspiration and head CT or magnetic resonance imaging. All cases were discussed with different disciplines in our institutional thoracic oncology board before and after the operation. All clinical data (including SUVmax) were collected from our medical database. Data on the largest tumor diameter, estimated by a pathologist, was collected from the histological report. In the case of NSCLC, we classified the tumors according to the 8th edition of the Union for International Cancer Control. Venous blood (7.5 mL) was sampled before surgery through the routine collection process. All samples were rested for 20 min and then centrifuged. After collecting the serum, the samples were stored at −80 °C. Serum CCL18 levels were determined using the DuoSet ELISA Development System Kit (R&D Systems Europe, Wiesbaden, Germany). All samples were measured in duplicate with an intra-assay coefficient of variation of 10%. An inter-assay coefficient of variation of 20% was also accepted. The lower detection limit of the assay was 7 pg/mL. As described by Ploenes et al., we used a cutoff value of 83 ng/mL for CCL18 levels to detect malignancy [3].
Patients underwent PET/CT imaging with 18FDG after fasting for at least 6 h. All patients were euglycemic at the beginning of the investigation (mean glucose level, 106 ± 13 mg/dL). After 61 ± 6 min of 277 ± 49 MBq injection, the 18FDG scans were acquired from the base of the skull to the mid-thigh using Biograph mCT128 (Siemens, Erlangen Germany). The emission time per bed position was 90 s. In addition, a CT scan was acquired for anatomical imaging and attenuation correction. PET data were corrected for attenuation and reconstructed using the time-of-flight technology and the point spread function algorithm. Finally, the SUVmax was calculated using the commercially available software syngo.via provided by Siemens.
For statistical analyses, we used MedCalc version 19.2.1 (MedCalc Software, Belgium). CCL18 levels are presented as median (range) and shown as box plots. Correlations were analyzed using the Spearman rank correlation. Comparisons between the patient groups were performed using analysis of variance and the Bonferroni–Dunn test for multiple comparisons. A p value of <0.05 was considered statistically significant.
Ethics Statement
The local institutional review board approved this study (16–6966-BO). All participants provided informed consent.
CRediT Author Statement
Tugba Dönmez: Resources; Kerstin Höhne, Gernot Zissel: Investigation; Ken Herrmann, Hubertus Hautzel: Investigation; Clemens Aigner, Balazs Hegedüs: Writing – Review & Editing; Till Ploenes: Conceptualization, Formal analysis, Writing – Original Draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships which have or could be perceived to have influenced the work reported in this article.
Acknowledgments
We acknowledge support from the Open Access Publication Fund of the University of Duisburg-Essen.
Footnotes
NSCLC: Non-small cell lung cancer; 18FDG: 18fluorine fluorodeoxyglucose; PET: positron emission tomography; CT: computed tomography; SUV: standardized uptake value; TAM: tumor-associated macrophage; CCL18: CC-chemokine ligand 18; SUVmax: maximum standardized uptake value; SQC: squamous carcinoma; AC: adenocarcinoma; FEV1: forced expiratory volume in 1 s;
References
- 1.R.L. Siegel, K.D. Miller, A. Jemal, Cancer statistics, 2019, C.A. Cancer J. Clin. 69(1) (2019) 7–34, doi: 10.3322/caac.21551. [DOI] [PubMed]
- 2.Lardinois D., Weder W., Hany T.F., Kamel E.M., Korom S., Seifert B., von Schulthess G.K., Steinert H.C. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N. Engl. J. Med. 2003;348(25):2500–2507. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- 3.Plönes T., Krohn A., Burger M., Veelken H., Passlick B., Müller-Quernheim J., Zissel G. Serum level of CC-chemokine ligand 18 is increased in patients with non-small-cell lung cancer and correlates with survival time in adenocarcinomas. PLoS ONE. 2012;7(7):e41746. doi: 10.1371/journal.pone.0041746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang H., Li J., Hu W.J., Chen C., Luo H.Q., Tang X.D., Zhou K.Y., Zhong W.T., Li X.Y. The serum level of CC chemokine ligand 18 correlates with the prognosis of non-small cell lung cancer. Int. J. Biol. Markers. 2019;34(2):156–162. doi: 10.1177/1724600819829758. [DOI] [PubMed] [Google Scholar]
- 5.Rahman W.T., Wale D.J., Viglianti B.L., Townsend D.M., Manganaro M.S., Gross M.D., Wong K.K., Rubello D. The impact of infection and inflammation in oncologic (18)F-FDG PET/CT imaging. Biomed. Pharmacother. 2019;117 doi: 10.1016/j.biopha.2019.109168. [DOI] [PMC free article] [PubMed] [Google Scholar]