Abstract
Mild traumatic brain injury (mTBI) is often characterized by deficits in response inhibition, which can contribute to marked social and occupational dysfunction. mTBI often occurs in the context of psychologically traumatic events. This can cause posttraumatic stress disorder (PTSD), which also impedes response inhibition. The overlap or distinction in these inhibitory deficits in mTBI and PTSD is unclear. This study aimed to assess behavioral, neurophysiological, and neuroimaging indices of response inhibition in mTBI by also assessing these parameters in healthy controls (HC) and PTSD participants. Participants with mTBI (without PTSD) (n = 46), PTSD (without mTBI) (n = 41), and HC (n = 40) were assessed during a response inhibition task (the Go/NoGo task) during neuropsychological testing and separate functional magnetic imaging and event-related potentials sessions. PTSD symptom severity was assessed with the Clinician-Administered PTSD Scale. Both mTBI and PTSD participants performed more omission errors on the Go/NoGo task and were associated with greater N2 amplitude, greater left inferior parietal activation and reduced connectivity of the left inferior parietal cluster and left angular gyrus compared to HC. There were no differences between mTBI and PTSD on any of these measures. These findings highlight that both mTBI and PTSD contribute to neural dysfunction during response inhibition, and arguably these occur due to distinct mechanisms. In the context of the common comorbidity between these two conditions, strategies to address response inhibition deficits in mTBI may need to consider causative factors underpinning neurological insult of mTBI and psychological effects associated with PTSD.
Keywords: Mild traumatic brain injury, Posttraumatic stress disorder, Response inhibition, Functional magnetic resonance imaging, Evoked response potential
1. Introduction
The capacity to implement cognitive functions in a changing environment requires adequate inhibitory control over irrelevant responses. There has been considerable attention in recent years on the neural substrates of response inhibition, which involves inhibiting a prepotent response. In terms of neural activation during response inhibition, it has been postulated that the lateral prefrontal cortex (PFC) exerts top-control control over sensory and motor processing (Miller and Cohen, 2001). More specifically, it is thought that response inhibition involves the lateral and medial PFC (Picton et al., 2007; Swick et al., 2008). Arguably the most commonly used experimental paradigms to measure response inhibition is the Go/NoGo task, which requires participants to respond to a specified stimulus but withhold the response when the stimulus is different. One meta-analysis of response inhibition studies in healthy individuals found that the major regions activated during inhibition were the right anterior insula and the pre-supplementary motor area; the Go/NoGo task particularly engaged a fronto-parietal control network (Swick et al., 2011). Using electroencephalography (EEG), studies have measured the temporal patterns associated with response inhibition by indexing specific event-related potentials (ERPs); response inhibition in healthy individuals is typically associated with a N200 (a negative component elicited approximately 200 ms after attempted response inhibition) and a P300 (a positive component elicited approximately 300 ms after attempted response inhibition) (Huster et al., 2013; Smith et al., 2008).
Sustaining a mild traumatic brain injury (mTBI) can significantly impair inhibitory control, reflected in reduced executive functions, impulsive verbal tendencies and behavioural disinhibition (Karr et al., 2014). One meta-analysis of studies addressing this issue in TBI found a small to moderate effect of impaired inhibitory control in TBI patients relative to healthy controls (Dimoska-Di Marco et al., 2011). There have been numerous investigations of neural activation during response inhibition in mTBI, with evidence of hypoactivation in prefrontal, precuneus, and inferior parietal regions (McAllister et al., 1999, 2001), including one study that used a variant of the Go/NoGo task (Stop Signal Task, Fischer et al., 2014). Using the Stop Signal Task, there is also evidence of differing activation in the default mode network (DMN; comprising the ventromedial prefrontal cortex, posterior cingulate/precuneus, and inferior parietal regions) in mTBI (Bonnelle et al., 2012; Fischer et al., 2014) and of increased posterior cerebellum activation in children with mTBI during a task that involved inhibitory control (Krivitzky et al., 2011). Another study found that during an inhibitory control task mTBI patients had greater activation than controls in the left thalamus, right putamen, and right cerebellum on non-inhibition trials but no differences between groups during inhibition (Xu et al., 2017). These investigators suggested that mTBI patients may have had difficulty switching between stimuli, causing them to excessively recruit inhibitory control networks on non-inhibitory trials. It is also likely that a failure to deactivate the DMN during goal directed tasks could lead to lapses in attention and interfere with their ability to successfully exhibit inhibitory control (Sours et al., 2013). The DMN is a network of the brain that focuses on internal mental states and is anti-correlated with task positive brain networks. The abnormalities related to the DMN have been reported previously during both resting state as well as response inhibition in mTBI (Zhou et al., 2012), which suggest it could be a potential mechanism underlying poor inhibitory control in mTBI. Studies of mTBI have also investigated response inhibition using EEG and generally found mixed patterns of evoked responses, with normal, reduced, or increased ERPs relative to healthy controls (Larson et al., 2011, 2012; Maki-Marttunen, 2015).
A core challenge in understanding the nature of response inhibition in mTBI is its potential overlap with posttraumatic stress disorder (PTSD). A significant proportion of mTBI patients sustain their injury in psychologically traumatic circumstances, including motor vehicle accidents, assaults, and traumatic falls (Dewan et al., 2018). Moreover, there is convergent evidence that sustaining a mTBI increases the likelihood of developing PTSD (Bryant et al., 2010; Hoge et al., 2008), which can explain the common comorbidity between the two conditions. Further, the symptoms of PTSD and common sequelae of mTBI can overlap, such as sleep difficulties, concentration deficits, irritability, and alterations in mood (Bryant, 2011). This is relevant for the issue of response inhibition in mTBI because PTSD is also characterized by inhibitory deficits (Olff et al., 2014; Vasterling et al., 1998, 2012). Moreover, there is reduced PFC activation during response inhibition on the Go/NoGo task in PTSD relative to controls (Carrion et al., 2008; Falconer et al., 2008) as well as on other response inhibition tasks (Jovanovic et al., 2013). PTSD is also associated with longer P3 latency (Shucard et al., 2008; Wu et al., 2015) and shorter N2 latency (Wu et al., 2010) during the Go/NoGo task. These findings highlight that in addition to the clinical overlap between mTBI and PTSD, there are commonalities in neural functioning during response inhibition in both conditions.
Although there have been a number of studies addressing the differential neural profiles of mTBI and PTSD (Spadoni et al., 2017; Spielberg et al., 2015), there is a dearth of studies directly disentangling the neural processes underpinning response inhibition in the two conditions. One study that focused on behavioural responses found no differences on the Go/NoGo task between PTSD and mTBI participants (Swick et al., 2012). Another Go/NoGo study found that in veterans with comorbid PTSD and mTBI, greater impairment in response inhibition was associated with smaller amygdala volume (Depue et al., 2014), however this study did not differentiate between mTBI and PTSD. One relevant study compared veterans with mTBI who either did or did not have comorbid PTSD during EEG recording on an inhibitory control task (Shu et al., 2014); comborbid patients had greater N200 response during inhibition than those without PTSD.
The aim of this study was to disentangle the neural processes implicated in response inhibition in mTBI in a way that recognizes the role of PTSD. To this end, we assessed participants with mTBI (without PTSD), PTSD (without mTBI), and healthy controls. The comparison groups were used to remove potential overlap between mTBI and PTSD, and to investigate the effects of mTBI without the confound of PTSD. There is also a need to understand the neural connections between brain networks during response inhibition because there is limited evidence regarding network connectivity during inhibition in both mTBI and PTSD (Sadeh et al., 2015; Stephens et al., 2018). Further, in recognition that a comprehensive assessment of response inhibition requires both spatial and temporal indices of neural response, we assessed participants on a Go/NoGo task during separate functional magnetic resonance imaging (fMRI) and evoked response potential (ERP) recording sessions. On the basis of evidence that PTSD is associated with greater inhibitory ERPs and mTBI is linked with normal or attenuated inhibitory ERPs, we hypothesized that mTBI participants would have reduced inhibitory ERPs relative to controls and PTSD participants. We also hypothesized that mTBI participants would also display less activation of the PFC and cognitive control brain circuitry relative to controls; as hypoactivation of the PFC has also been shown in PTSD, no clear hypotheses were made in relation to mTBI relative to PTSD.
2. Methods
2.1. Participants
127 participants of mean age 42.2 ± 12.3 years were recruited from public advertisements. The sample comprised of 46 mTBI participants without PTSD (31 males, 15 females) who had a self-reported head injury, loss of consciousness of less than 30 min, and post-traumatic amnesia of less than 24 h; these parameters of mTBI were classified via clinical interview. The PTSD group comprised 41 PTSD participants with no history of mTBI (22 males, 19 females) who satisfied DSM-IV criterion for PTSD (based on symptoms in the past month and anchored to an index trauma identified through clinical interview) as diagnosed by clinical psychologists using the Clinician Administered PTSD Scale (CAPS (Blake et al., 1995); and 40 non-trauma-exposed controls (HC) (22 males, 18 females) who had never experienced a Criterion A stressor and did not currently have an Axis I disorder as assessed using the Mini International Neuropsychiatric Interview (MINI version 5.5 (Sheehan et al., 1998)). Participants with both mTBI and PTSD, a history of neurological disorder, psychosis, or current substance dependence were excluded. Participants were permitted to be taking prescribed selective serotonin uptake inhibitors (SSRIs) if they were on a stable dosage for at least two months prior to the scan; SSRIs were used by 24 participants (19%). The groups were matched for age and gender. To accommodate subsequent genetic analyses, all participants were of European Australians. Table 1 presents the participant characteristics.
Table 1.
Participant characteristics.
| mTBI |
PTSD |
Healthy controls |
|
|---|---|---|---|
| (n = 45) | (n = 40) | (n = 40) | |
| Age, mean (SD) | 43.6 (12.2) | 40.3 (11.3) | 42.7 (13.5) |
| Male, n (%) | 31 (69) | 22 (55) | 22 (55) |
| CAPS Score, mean (SD) | 14.4 (20.0) | 70.3 (16.1) | – |
| Time since Trauma, months, mean (SD) | 140 (132) | 21 (15) | – |
| Index Trauma | |||
| Road accident | 30 (65.2) | 6 (14.6) | |
| Assault | 14 (30.4) | 14 (34.2) | |
| Police duties | – | 15 (36.6) | |
| Domestic violence | – | 6 (14.6) | |
| Industrial accident | 2 (4.4) | – | |
| Prescribed SSRI, n (%) | 5 (11.1) | 19 (47.5) | – |
| Major Depressive Disorder, n (%) | 2 (4.3) | 27 (69.2) | – |
| Social Phobia n (%) | 2 (4.3) | 18 (45) | – |
| Panic Disorder, n (%) | 1 (2.2) | 7 (18.4) | – |
| Agoraphobia, n (%) | 3 (7.7) | 24 (64.9) | – |
| Generalized Anxiety Disorder, n (%) | 8 (17.4) | 15 (37.5) | – |
| Obsessive Compulsive Disorder, n (%) | 1 (2.2) | 5 (14.7) | – |
Abbreviations: CAPS, clinician administered PTSD scale; mTBI, mild traumatic brain injury; PTSD, post-traumatic stress disorder; SD, standard deviation; SSRI, selective serotonin reuptake inhibitor medications.
2.2. Procedure
The Western Sydney Area Health Service Human Research Ethics Committee approved this study, and all participants gave written consent to partake in the study. Following assessment of primary diagnosis, clinical psychologists used the MINI to assess for current major depressive episode, generalized anxiety disorder, social phobia, panic disorder, agoraphobia, obsessive-compulsive disorder and substance use disorder. Participants underwent clinical and lab (EEG and MRI) assessments.
2.3. Go/No-Go task
The Go/No-Go task assesses response inhibition in a manner that restricts a prepotent response. On this task participants were instructed to respond by pressing a button as quickly as possible to the ‘Go’ trials, which were indicated by the word “PRESS” in green writing; participants were also instructed to withhold a response on ‘No-Go’ trials, which were the word “PRESS” in red writing; these stimuli were presented in different colors to ensure that they were readily discernible for participants. Each stimulus was presented for 500 ms, with a 750 ms interstimulus interval. There were 180 Go stimuli and 60 No-Go stimuli presented in a pseudorandom order to ensure that the No-Go stimulus did not occur more than three times in a row. The Go/No-Go task was repeated three times for each participant. Performance was assessed in terms of commission errors (failing to withhold a response), omission errors (failing to correctly respond), and reaction time. The first task was conducted without neural recordings. The task was then repeated whilst continuous electroencephalogram (EEG) data was being recorded and subsequently during a magnetic resonance imaging scan.
2.4. EEG acquisition and analyses
Electrophysiological recordings were obtained from 32 EEG channels at 500 Hz with a skin resistance of <5 kOhms, using a Quick Cap and NuAmps DC system (Neuroscan). 26 cephalic sites, 4 electro-oculogram (EOG) sites, an orbicularis oculus site, and a masseter site comprised the 32 channels. Electrodes were placed 1.5 cm lateral to the outer canthus of each eye to monitor horizontal eye movement. To record vertical eye movement, electrodes were placed 3 mm above the left eyebrow and 1.5 cm below the left lower eyelid. The online reference was the AFz electrode, and the data was re-referenced offline to the average of A1 and A2 electrodes located on the mastoids. Artefact rejection was set at 100 μV. Event-related potential epochs were filtered using a low-pass Tukey filter. This attenuated any frequencies above 25 Hz using a cosine ramp from 1 down to 0.5 as an envelope between 25 Hz and 35 Hz. Eye movement artifacts were corrected using an established procedure (EMCP – eye movement correction procedure (Gratton et al., 1983)). This procedure uses a regression method to estimate the propagation factors to calculate the relationship between the EOG channels and each EEG channel and uses them to correct both blinks and eye movements from the raw EEG data. The main benefit of this approach is that all trials in the experiment can be retained irrespective of ocular artefacts. The pre-stimulus baseline value was set to 100 ms and a fixation cross was viewed between stimuli. ERPs were time-locked to stimulus onset and were recorded from −100 ms (pre-stimulus) to 600 ms (post-stimulus).
NoGo trials were averaged together to form ERP waves, and were hand-scored to determine the peak-value and latency of the N2 and P3 peaks using the individual-participant method to determine latency; these were examined for the Fz, FCz, and Cz electrodes for N2, and additionally the Pz electrode was included for P3. These waveforms and electrodes were selected based on prior studies of response inhibition (Huster et al., 2013; Nguyen et al., 2016). The N2 peak amplitude was defined as the negative peak within the 160–280 ms period. The P3 peak amplitude was the positive peak value occurring between 220 and 450 ms. We conducted 3x1 and 4x1 repeated-measures ANOVAs to investigate differences in electrophysiological response during processing NoGo trials between the mTBI, PTSD and healthy control groups. Four sets of candidate measures (N2-amplitude, N2-latency, P3-amplitude, and P3-latency) were entered as a within-subjects variable. Electrodes in significant sets were subject to further posthoc ANOVAs and t-tests. Given our focus to evaluate abnormalities associated with mTBI, we performed a step-wise analysis where we first evaluated differences between the mTBI and HC groups. Then to evaluate if these alterations were associated with PTSD, we tested by comparing these measures relative to PTSD.
2.5. fMRI acquisition and analyses
All functional MRIs were conducted on a 3.0T GE Signa Twinspeed HDx scanner and an eight-channel head coil, using an echo planar imaging protocol. There were 120 T2*-weighted functional volumes acquired in the task run, and three dummy scans were collected before the sequence to ensure magnetisation had stabilised. Each volume comprised 40 axial slices parallel to the intercommissural line, with 3.5 mm thickness, 2.5 s TR, 27.5 TE, and 90° flip angle. The field of view was 24 × 24 cm2 and the matrix size was 64 x 64. A T1-weighted anatomical image with 1 mm3 isotropic voxel resolution was also obtained to normalise the fMRI data to standard space. This was produced using a 3D spoiled gradient echo sequence in the sagittal plane with the following parameters: TR = 8.3 ms, TE = 3.2 ms, flip angle = 11°, TI = 500 ms, NEX = 1, ASSET = 1.5, S/I frequency direction, 256 x 256 matrix size, and 180 contiguous 1 mm slices.
Statistical Parametric Mapping (SPM8, Wellcome Department of Neurology, London) software running on MATLAB 2014b was used to realign, normalise (into standardised MNI space), and smooth the MRI data. fMRI images were realigned and unwarped to the initial image for the task run to correct for participant motion. We used the FMRIB linear registration tool to co-register the functional data to the T1 anatomical scan in order to normalise data into stereotactic MNI space, and used the FMRIB nonlinear registration tool to normalise the weighted 3D spoiled gradient echo sequence. A mask covering the ventricles and white matter was used to estimate their corresponding signal and correct for any physiological noise. We conducted smoothing on all fMRI data using an 8 mm Gaussian kernel.. For first-level analysis, the BOLD response was modelled using the canonical hemodynamic response function (HRF) within the general linear model (GLM) framework. Each participant's first-level GLM included two experimental condition regressors (for Go and NoGo, respectively) as well as the motion regressors. The onset times for each trial within each condition across the whole run were specified for the respective regressor and were used to model the hemodynamic response function for each condition. Contrasts were then derived using these estimated regressors.
We identified scans with excessive movement or signal variations using quality control diagnostics. Three translational and three rotational motion parameters were estimated during realignment and used to identify problematic volumes within a scan. Motion artifacts were defined as a volume frame with greater than 0.5 mm displacement from the previous frame in the x, y or z direction. A volume was also defined as an artifact if the global mean intensity in that volume was further than 3 standard deviations away from the mean image intensity for the entire scan. Problematic volumes were included as regressors in first-level analysis to remove motion artifacts. Typically, participants with more than 25% of volumes designated as artifacts are rejected from further analysis. In our analysis, none of the participants had more than 25% problematic volumes and there were no group differences for number of problematic volumes.
To index neural responses on the Go/NoGo task, contrast images for response inhibition were determined by comparing the No-Go versus Go conditions. We focused on brain regions making up the default mode network (DMN) and the cognitive control network (CCN) because previous meta-analyses and studies have indicated the role of these networks in response inhibition processes (Niendam et al., 2012). Regions of interest (ROIs) were defined using 10 mm radius spheres combined into a single network specific mask. The DMN consisted of the medial prefrontal cortex, the posterior cingulate cortex and precuneus. The CCN consisted of the dorsolateral prefrontal cortex, dorsal anterior cingulate cortex, inferior parietal and superior parietal cortices. Exploratory analyses were also conducted at the whole-brain level to evaluate effects beyond our pre-defined ROIs (reported in the supplementary section). All analyses were conducted voxel-wise and judged significant at a family-wise-error-corrected p-value of 0.05.
As done with the EEG data, we first evaluated group differences in activation between the mTBI and healthy control groups by conducting an independent samples t-test on the contrast images of each group. We then evaluated any significant effects to compare the mTBI group to the PTSD group and for PTSD relative to HC.
We ran a generalized psychophysiological interaction (gPPI) analysis to evaluate alterations in functional connectivity between groups, using significant clusters from activation analyses as seed regions in the gPPI. The pre-processed data from the activation analysis was used as input for the gPPI models. The gPPI model included 5 regressors (in addition to the motion regressor described above): a psychological regressor of onset times for each condition (Go and NoGo); the physiological regressor which is the time series of the seed region; and a psychophysiological regressor for each condition which is an interaction term consisting of the product of the condition regressor multiplied by the time-series regressor. The psychophysiological term is modelled against the time course of other brain regions to assess task-modulated connectivity. The psychophysiological interaction for the Go condition was subtracted from the NoGo interaction to generate the first-level contrast. As done for the activation data, both ROI and exploratory whole brain connectivity analyses for the selected seeds were performed (whole brain analyses reported in supplementary).
To assess beyond the significant seeds, we also assessed connectivity between all nodes of the DMN and CCN during the Go/No-Go task in a correlational psychophysiological interaction (cPPI) analysis. A functional time series was extracted from each node and correlated with every other node to create an 11x11 connectivity matrix for each participant. Functional task-based connectivity for each group was assessed using the Network Based Statistic (NBS (Zalesky, 2010)). As above, we conducted two-sample comparisons between the mTBI and HC groups to determine differences in connectivity. A primary threshold of p < 0.05 was set to identify a set of candidate connections. The size of each supra-threshold connection was computed, and statistical significance assessed by comparing to an empirically generated null distribution. Connections with a component-wise corrected (family wise correction) p < 0.05 were identified as part of significant networks.
Finally, to evaluate whether the PTSD symptoms in the mTBI group could explain behavioural, ERP and fMRI alterations associated with mTBI and also to evaluate any confounds of duration since experienced trauma, we evaluated correlations of CAPS symptom scores and time since trauma with the significant measures within the mTBI group. We also tested these correlations independently in the PTSD group.
3. Results
3.1. Participant characteristics
The demographic and clinical characteristics for the three groups are described in Table 1. The three groups were matched for age and gender. As expected, the PTSD group had greater CAPS severity than the mTBI group (t(78) = 16.049, p < 0.001). The mTBI group had a higher average time since trauma than the PTSD group (t(74) = 4.253, p < 0.001) and an overall smaller proportion of participants currently prescribed with SSRI medications (χ2(1, 80) = 13.306, p < 0.001).
The PTSD group had a greater proportion of patients with MDD (χ2(1,87) = 41.688, p < 0.001), Panic Disorder (χ2(1,87) = 9.824, p = 0.007), Agoraphobia (χ2(1,87) = 27.752, p < 0.001), Social Phobia (χ2(1,87) = 20.915, p < 0.001) and OCD (χ2(1,87) = 12.881, p = 0.002) compared to the mTBI group. The two groups did not differ in rates of Generalized Anxiety Disorder.
3.2. Go/No-Go task performance
We conducted a repeated measures ANOVA (within subject repeated measures collected across the three task runs with participant group as a between subjects variable) to compare commission errors, omission errors and reaction time measures. There were significant main effects of task run across all three measures [greater commission errors (F = 58.329, p < 0.001) and omission errors (F = 4.385, p = 0.041) during the fMRI task run, but shorter reaction time during the EEG task run (F = 27.241, p < 0.001) compared to the task run without neural recordings] but no run*group interactions. Planned contrasts revealed a significant difference between the mTBI and HC group only for omission errors (p = 0.017; mTBI > HC). There were no differences in omission errors between mTBI and PTSD, but the PTSD group also committed more omission errors than HC (p = 0.013). There were no significant correlations between omission errors and PTSD symptom severity or time since trauma for the mTBI or PTSD group. There were no differences in commission errors or reaction time between the three groups.
3.3. EEG results
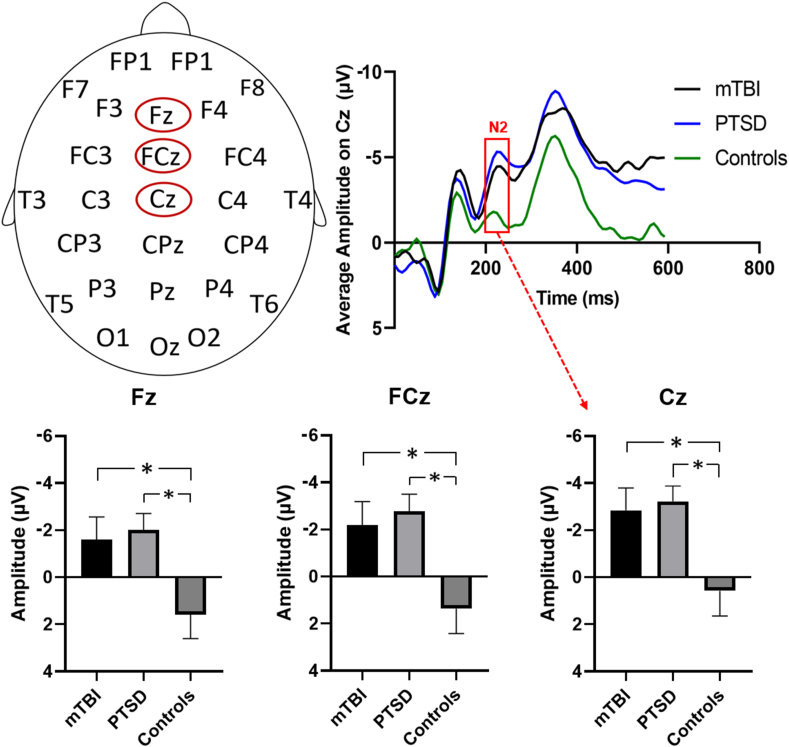
We observed a significant group difference for the peak amplitude of the N2 wave with higher amplitude for mTBI relative to HC (F = 5.42, p = 0.025). Posthoc analyses on individual electrodes within this set (Fz, FCz, and Cz) found a significant difference on all three electrodes (Fz: t = 2.28, p = 0.028, FCz: t = 2.415, p = 0.021, Cz: t = 2.404, p = 0.021) (See Fig. 1). There were no differences between the mTBI and PTSD group on the N2 wave whereas significant differences were also observed for PTSD relative to HC, with a higher amplitude for PTSD relative to HC (F = 10.69, p = 0.002). All electrodes tested showed higher amplitude for the PTSD group relative to HC on the N2 wave (Fz: t = 2.92, p = 0.007, FCz: t = 3.32, p = 0.002, Cz: t = 3.04, p = 0.005).
Fig. 1.
EEG responses to NoGo trials. Top left: Schematic diagram showing the positions of the three electrodes (Fz, FCz, Cz) displaying a difference between mTBI, PTSD and healthy controls. Top right: ERP waveforms for the mTBI, PTSD, and control groups for the Cz electrode. Red box highlights difference between groups on the N2 wave. Bottom: Barplots of peak amplitude on the N2 wave for the mTBI, PTSD and control groups for the Fz, FCz and Cz electrodes. For all three electrodes, N2 peak amplitude is significantly lower in the controls compared to both the mTBI and PTSD groups. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
There were no significant correlations for N2 amplitude with the CAPS score or time since trauma for the mTBI or PTSD group. There were no differences between mTBI and HC for amplitude of P3 or latency for both N2 and P3 components.
3.4. fMRI results
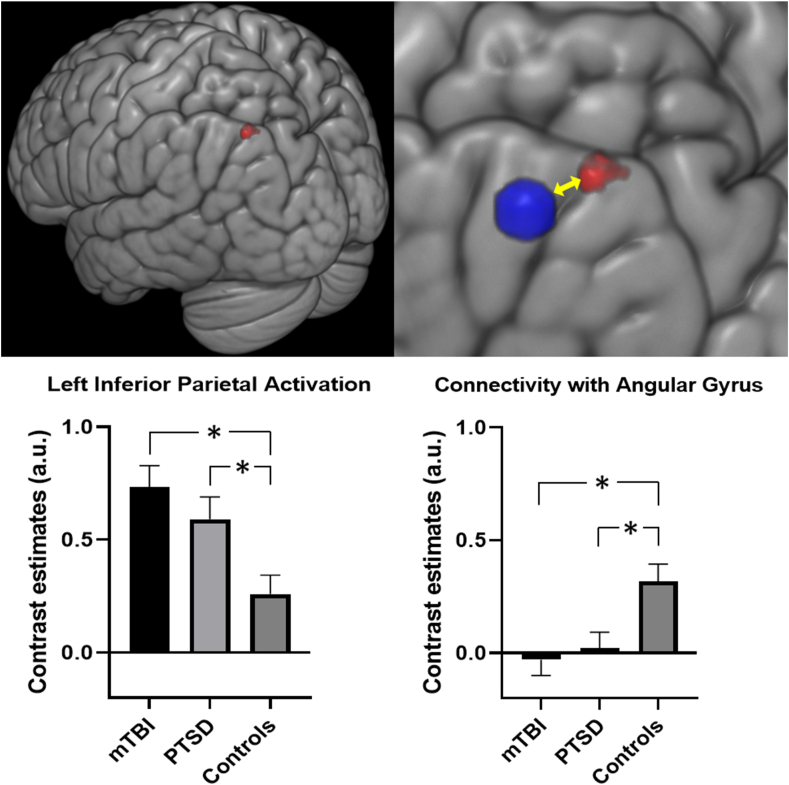
For fMRI activation for response inhibition (NoGo vs Go), we observed significantly greater activity in the left inferior parietal cortex for the mTBI sample as compared to HC (pFWE = 0.024; Supplementary Table S6). As with the EEG data, we did not find any significant activation differences between the mTBI and PTSD groups, but PTSD also demonstrated greater activity within this region relative to HC (Fig. 2, pFWE = 0.033).
Fig. 2.
Activation and connectivity for NoGo – Go contrast. Top left: Cluster of voxels in the left inferior parietal cortex (in red) displaying significantly greater activation in the mTBI and PTSD groups compared to the healthy controls. Top Left: The inferior parietal cluster displayed reduced connectivity to the left Angular Gyrus (in blue) for mTBI and PTSD compared to controls. Bottom left: Barplot displaying left inferior parietal activation values for the three groups. Bottom right: Barplot displaying connectivity values in angular gyrus using left inferior parietal seed for the three groups. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
We used a gPPI analysis to investigate functional connectivity differences between the mTBI and healthy control groups, using the left inferior parietal cluster as a seed. We found reduced connectivity between this seed and the left angular gyrus in the mTBI group relative to the controls (pFWE = 0.018; Fig. 2 & Supplementary Table S9). The mTBI group did not differ from the PTSD group, and the PTSD group also exhibited reduced connectivity compared to healthy controls (pFWE = 0.025). There were no further significant connections within or between the DMN-CCN brain networks which differentiated the mTBI group from healthy controls. Neither activation nor connectivity were significantly correlated with CAPS scores or with time since trauma for the mTBI or PTSD group.
4. Discussion
This study aimed to refine our understanding of the neural processes involved in response inhibition in mTBI by also considering response inhibition patterns in PTSD. Overall, we found that both mTBI and PTSD demonstrated abnormalities in response inhibition with more omission errors during the task, greater N2 ERP amplitude, greater fMRI activation in the left inferior parietal cortex and reduced connectivity for this region relative to healthy controls. There were no differences between mTBI and PTSD. These findings indicate commonalities in neural mechanisms responsible for response inhibition difficulties in both conditions.
mTBI is often followed by a range of functional, cognitive and emotional problems collectively known as postconcussive syndrome. However, it is unknown how much of this syndrome is attributed to psychological trauma experienced by the individual (Meares et al., 2008, 2011). Comparing response inhibition in individuals with mTBI with those with PTSD may help distangle these underlying neural mechanisms as both conditions are characterized by impaired inhibitory capacity (Olff et al., 2014; Vasterling et al., 2012). The pattern of overlap observed here highlights that future studies of response inhibition in mTBI should be considering the potential influence of PTSD. The relevance of this conclusion is underscored by the wealth of evidence of the comorbidity of mTBI with PTSD, especially in people who sustain their mTBI in the context of war, assaults, and traumatic accidents (Bryant, 2001).
Our study found that mTBI and PTSD participants produced more omission errors than healthy controls, but did not differ on commission errors or reaction time. The finding that both mTBI and PTSD participants displayed greater omission errors accords with considerable evidence of attentional deficits in both conditions, as has been shown in studies of patients with each condition separately as well as when they are comorbidly present (Etkin et al., 2013; Vasterling et al., 2018). Commission errors are an index of inhibitory control which suggest that both patient groups had overall normal performance in this domain, whereas there were difficulties in sustained attention in both cohorts as reflected by omission errors in our task.
mTBI and PTSD participants displayed greater N2 amplitude during response inhibition than HC participants. Greater amplitude of the N2 is regarded as a reflection of increased need for cognitive control (Folstein and Van Petten, 2008). It is possible that the greater N2 amplitude in mTBI reflects the greater cognitive effort these individuals needed during response inhibition; this need could arise from deficits that have been widely documented in mTBI in cognitive control (Dimoska-Di Marco et al., 2011). This result contrasts with our hypothesis and some prior findings of reduced N2 during response inhibition in mTBI patterns with healthy participants (Zhao, 2018) but it should be noted that most of these studies have employed tasks other than the Go/NoGo task (Larson et al., 2011, 2012).
Similar to the ERP finding, we observed greater activation in the inferior parietal region of the cognitive control brain network for mTBI and PTSD participants relative to healthy individuals. This again corresponds to greater recruitment effort of the cognitive brain regions by mTBI and PTSD individuals during response inhibition. Increased activation in cognitive control brain regions has been previously reported in mTBI and has been regarded as a compensatory mechanism due to an increased demand on neural resources to maintain task performance (Scheibel, 2017).
In our study, the mTBI (and PTSD) cohort had matched performance to HC on commission errors, an index of inhibitory control. This may be because the Go/NoGo task is a relatively easy task of response inhibition. It has been theorized that in an efficient neural system, easier tasks require fewer cognitive resources (Dunst et al., 2014). Consequently, healthy individuals, relative to mTBI or PTSD, may not need to engage in effortful cognitive processing for a simple task, especially as they become more familiar with the task. Additionally, lower recruitment of the inferior parietal brain regions has been previously associated with better executive function in healthy individuals (Breukelaar et al., 2018), yet over-activation in mTBI has been associated with improving cognitive control performance (Scheibel et al., 2009). This latter finding can be understood in relation to the current observation of greater activation of the right inferior parietal cortex in mTBI and PTSD as supporting the interpretation that these participants may have needed to engage greater cognitive effort during inhibition.
In our study, we also investigated the DMN to evaluate if inability to deactivate the task negative processes (e.g. focus on internal attention) could underlie response inhibition problems in mTBI. Failure to regulate the DMN brain regions has been associated with impairment of inhibitory control in mTBI (Bonnelle et al., 2012). However, we did not observe any differences in activation patterns in the regions of the DMN for mTBI vs HCs. Instead we found reduced connectivity between the inferior parietal CCN region with the angular gyrus region of the DMN in mTBI. The inferior parietal region within the CCN is considered to control top-down attentional orienting that is necessary during cognitive performance (Shomstein, 2012). Although we cannot infer causality from our connectivity data, this reduced connectivity could reflect inability of this brain region to dampen interference from task negative processes. The observed reduced connectivity is consistent with the greater omission errors in mTBI and PTSD participants, and may reflect a common underlying deficit that these individuals have in sustained attention (Vasterling et al., 2012).
Despite the fact that there were no neural differences between mTBI and PTSD and both groups had similar abnormalities as compared to HC, the neural alterations could not be explained by PTSD symptom severity in the mTBI cohort. One reason for this could be that our mTBI cohort did not have comorbid PTSD, as reflected by their lower CAPS scores. It is possible that these correlations may be more pronounced in those with existing PTSD symptoms. To test this hypothesis, we evaluated correlations of symptom measures with neural measures in the PTSD cohort and also found no significant correlations. It is possible that no associations were found because the Go/NoGo task employed in this study was not sufficiently difficult to be sensitive to the problems that may be attributed to PTSD symptoms.
We note some limitations of this study. First, we assessed mTBI retrospectively via self-report. As participants to this study were recruited by advertising, we were not able to access medical records of the exact severity or duration of the mTBI. Relatedly, we retrospective assessed mTBI via self-report accounts of mTBI rather than using a structured measure of mTBI (e.g. the Ohio State University TBI Identification Method; Corrigan and Bogner, 2007) which could have provided more information about repeated mTBIs or mTBIs that occur soon after each other; this information is important because of evidence that cognitive (Manley et al., 2017) and neural (Karr et al., 2014) disturbance is more evident in people who have been repeatedly experienced mTBIs. Second, although the sample size is larger than most studies of neural indices of inhibition in mTBI, we acknowledge that a larger sample size would permit analysis of sub-groups of participants (e.g. types of injury). The sample in this study comprised patients with diffuse mTBIs rather than focal injuries, and future studies should explore the neural profiles of patients with specific injuries, such as following blows or gunshots. Finally, despite noise correction techniques, the effect of noise cannot be completely eliminated and there is still the possibility of residual noise in the data which may impact the findings.
5. Conclusions
These limitations notwithstanding, these findings provide novel insight into the impact of mTBI on response inhibition and its overlap with PTSD. The comparable deficits observed in mTBI and PTSD participants relative to HC suggests that whereas these two conditions share a common neural dysfunction underpinning response inhibition, the mechanisms may be distinct. Although mTBI is characterized by neurological insult, PTSD is a psychological disorder. Many mTBI patients are exposed to psychologically traumatic events as part of their injury. The nature of PTSD typically results in increased arousal and demands on working memory (Vasterling et al., 2009), which is reflected in evidence that performance on response inhibition tasks is negative associated with severity of intrusive memories that can impede one's cognitive capacity to perform executive functions (Swick et al., 2012). It appears that mTBI is associated with altered neural processes during response inhibition, possibly as a means of compensating for deficits by reflecting greater effort in relevant neural networks. These deficits may be compounded by the contributing role of PTSD symptoms. If future research clarifies this possibility, there remains a possibility that some of the deficits associated with mTBI may be addressed by strategies used to reduce PTSD symptoms. Future research should endeavour to standardize the response inhibition tasks employed across studies to permit greater generalizability of findings, which would clarify the distinct and common neural substrates of inhibition in mTBI and PTSD.
Funding
This study was supported by the National Health and Medical Research Council Program Grant (1073041). The study sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
CRediT authorship contribution statement
Mayuresh S. Korgaonkar: designed the study, coordinated data collection, Formal analysis, Writing - original draft. Thomas Williamson: conducted analyses, Writing - original draft, and. Richard A. Bryant: designed the study, coordinated data collection, Formal analysis, Writing - original draft, Funding acquisition.
Declaration of competing interest
No authors are declaring a conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100308.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S., Keane T.M. The development of a Clinician-administered PTSD Scale. J. Trauma. Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bonnelle V., Ham T.E., Leech R., Kinnunen K.M., Mehta M.A., Greenwood R.J., Sharp D.J. Salience network integrity predicts default mode network function after traumatic brain injury. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109:4690–4695. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breukelaar I.A., Williams L.M., Antees C., Grieve S.M., Foster S.L., Gomes L., Korgaonkar M.S. Cognitive ability is associated with changes in the functional organization of the cognitive control brain network. Hum. Brain Mapp. 2018;39:5028–5038. doi: 10.1002/hbm.24342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R.A. Posttraumatic stress disorder and traumatic brain injury: can they co-exist? Clin. Psychol. Rev. 2001;21:931–948. doi: 10.1016/s0272-7358(00)00074-x. [DOI] [PubMed] [Google Scholar]
- Bryant R. Post-traumatic stress disorder vs traumatic brain injury. Dialogues Clin. Neurosci. 2011;13:251–262. doi: 10.31887/DCNS.2011.13.2/rbryant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R.A., Creamer M., O'Donnell M., Silove D., Clark C.R., McFarlane A.C. The psychiatric sequelae of traumatic injury. Am. J. Psychiatr. 2010;167:312–320. doi: 10.1176/appi.ajp.2009.09050617. [DOI] [PubMed] [Google Scholar]
- Carrion V.G., Garrett A., Menon V., Weems C.F., Reiss A.L. Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth. Depress. Anxiety. 2008;25:514–526. doi: 10.1002/da.20346. [DOI] [PubMed] [Google Scholar]
- Corrigan J.D., Bogner J. Initial reliability and validity of the Ohio state university TBI identification method. J. Head Trauma Rehabil. 2007;22:318–329. doi: 10.1097/01.HTR.0000300227.67748.77. [DOI] [PubMed] [Google Scholar]
- Depue B.E., Olson-Madden J.H., Smolker H.R., Rajamani M., Brenner L.A., Banich M.T. Reduced amygdala volume is associated with deficits in inhibitory control: a voxel- and surface-based morphometric analysis of comorbid PTSD/mild TBI. Biomed. Res. International. 2014;2014:691505. doi: 10.1155/2014/691505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan M.C., Rattani A., Gupta S., Baticulon R.E., Hung Y.C., Punchak M., Agrawal A., Adeleye A.O, Shrime M.G., Rubiano A.M., Rosenfeld J.V., Park K.B. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2019;130:1080–1097. doi: 10.3171/2017.10.JNS17352. [DOI] [PubMed] [Google Scholar]
- Dimoska-Di Marco A., McDonald S., Kelly M., Tate R., Johnstone S. A meta-analysis of response inhibition and Stroop interference control deficits in adults with traumatic brain injury (TBI) J. Clin. Exp. Neuropsychol. 2011;33:471–485. doi: 10.1080/13803395.2010.533158. [DOI] [PubMed] [Google Scholar]
- Dunst B., Benedek M., Jauk E., Bergner S., Koschutnig K., Sommer M., Ischebeck A., Spinath B., Arendasy M., Bühner M., Heribert Freudenthaler H., Neubauer A.C. Neural efficiency as a function of task demands. Intelligence. 2014;42:22–30. doi: 10.1016/j.intell.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Gyurak A., O’Hara R. A neurobiological approach to the cognitive deficits of psychiatric disorders. Dialogues. Clin. Neurosci. 2013;15:419–429. doi: 10.31887/DCNS.2013.15.4/aetkin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer E., Bryant R., Felmingham K.L., Kemp A.H., Gordon E., Peduto A., Olivieri G., Williams L.M. The neural networks of inhibitory control in posttraumatic stress disorder. J. Psychiatr. Neurosci. 2008;33:413–422. [PMC free article] [PubMed] [Google Scholar]
- Fischer B.L., Parsons M., Durgerian S., Reece C., Mourany L., Lowe M.J., Beall E.B., Koenig Katherine A., Koenig K.A., Jones S.E., Newsome M.R., Scheibel R.S., Wilde E.A., Troyanskaya M., Merkley T.L., Walker M., Harvey S., Levin H.S., Rao S.M. Neural activation during response inhibition differentiates blast from mechanical causes of mild to moderate traumatic brain injury. J. Neurotrauma. 2014;31:169–179. doi: 10.1089/neu.2013.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein J.R., Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G., Coles M.G., Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., Castro C.A. Mild traumatic brain injury in US Soldiers returning from Iraq. N. Engl. J. Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Huster R.J., Enriquez-Geppert S., Lavallee C.F., Falkenstein M., Herrmann C.S. Electroencephalography of response inhibition tasks: functional networks and cognitive contributions. Int. J. Psychophysiol. 2013;87:217–233. doi: 10.1016/j.ijpsycho.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Jovanovic T., Ely T., Fani N., Glover E.M., Gutman D., Tone E.B., Norrholm S.D., Bradley B., Ressler K.J. Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex. 2013;49:1884–1891. doi: 10.1016/j.cortex.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr J.E., Areshenkoff C.N., Duggan E.C., Garcia-Barrera M.A. Blast-related mild traumatic brain injury: a Bayesian random-effects meta-analysis on the cognitive outcomes of concussion among military personnel. Neuropsychol. Rev. 2014;24:428–444. doi: 10.1007/s11065-014-9271-8. [DOI] [PubMed] [Google Scholar]
- Krivitzky L.S., Roebuck-Spencer T.M., Roth R.M., Blackstone K., Johnson C.P., Gioia G. Functional magnetic resonance imaging of working memory and response inhibition in children with mild traumatic brain injury. J. Int. Neuropsychol. Soc. 2011;17:1143–1152. doi: 10.1017/S1355617711001226. [DOI] [PubMed] [Google Scholar]
- Larson M.J., Clayson P.E., Farrer T.J. Performance monitoring and cognitive control in individuals with mild traumatic brain injury. J. Int. Neuropsychol. Soc. 2012;18:323–333. doi: 10.1017/S1355617711001779. [DOI] [PubMed] [Google Scholar]
- Larson M.J., Farrer T.J., Clayson P.E. Cognitive control in mild traumatic brain injury: conflict monitoring and conflict adaptation. Int. J. Psychophysiol. 2011;82:69–78. doi: 10.1016/j.ijpsycho.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Maki-Marttunen V., Kuusinen V., Brause M., Perakyla J., Polvivaara M., dos Santos Ribeiro R., Ohman J., Hartikainen K.M. Enhanced attention capture by emotional stimuli in mild traumatic brain injury. J. Neurotrauma. 2015;32:272–279. doi: 10.1089/neu.2014.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley G., Gardner A.J., Schneider K.J., Guskiewicz K.M., Bailes J., Cantu R.C., Castellani R.J., Turner M., Jordan B.D., Randolph C., Dvořák J., K Alix Hayden K.A., Tator C.H., McCrory P., Iverson G.L. A systematic review of potential long-term effects of sport-related concussion. Br. J. Sports Med. 2017;51:969–977. doi: 10.1136/bjsports-2017-097791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister T.W., Saykin A.J., Flashman L.A., Sparling M.B., Johnson S.C., Guerin S.J., Mamourian A.C., Weaver J.B., Yanofsky N. Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology. 1999;53:1300–1308. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- McAllister T.W., Sparling M.B., Flashman L.A., Guerin S.J., Mamourian A.C., Saykin A.J. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14:1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- Meares S., Shores E.A., Taylor A.J., Batchelor J., Bryant R.A., Baguley I.J., Chapman J., Gurka J., Dawson K., Capon L., Marosszeky J.E. Mild traumatic brain injury does not predict acute postconcussion syndrome. J. Neurol. Neurosurg. Psychiatr. 2008;79:300–306. doi: 10.1136/jnnp.2007.126565. [DOI] [PubMed] [Google Scholar]
- Meares S., Shores E.A., Taylor A.J., Batchelor J., Bryant R.A., Baguley I., Chapman J., Gurka J., Marosszeky J.E. The prospective course of postconcussion syndrome: the role of mild traumatic brain injury. Neuropsychology. 2011;25:454–465. doi: 10.1037/a0022580. [DOI] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Nguyen A.T., Moyle J.J., Fox A.M. N2 and P3 modulation during partial inhibition in a modified go/nogo task. Int. J. Psychophysiol. 2016;107:63–71. doi: 10.1016/j.ijpsycho.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Niendam T.A., Laird A.R., Ray K.L., Dean Y.M., Glahn D.C., Carter C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognit. Affect Behav. Neurosci. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff M., Polak A.R., Witteveen A.B., Denys D. Executive function in posttraumatic stress disorder (PTSD) and the influence of comorbid depression. Neurobiol. Learn. Mem. 2014;112:114–121. doi: 10.1016/j.nlm.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Picton T.W., Stuss D.T., Alexander M.P., Shallice T., Binns M.A., Gillingham S. Effects of focal frontal lesions on response inhibition. Cerebr. Cortex. 2007;17:826–838. doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- Sadeh N., Spielberg J.M., Miller M.W., Milberg W.P., Salat D.H., Amick M.M., Fortier C.B., McGlinchey R.E. Neurobiological indicators of disinhibition in posttraumatic stress disorder. Hum. Brain. Mapp. 2015;36:3076–3086. doi: 10.1002/hbm.22829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel R.S. Functional magnetic resonance imaging of cognitive control following traumatic brain injury. Front. Neurol. 2017;8:352. doi: 10.3389/fneur.2017.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel R.S., Newsome M.R., Troyanskaya M., Steinberg J.L., Goldstein F.C., Mao H., Levin H.S. Effects of severity of traumatic brain injury and brain reserve on cognitive-control related brain activation. J. Neurotrauma. 2009;26:1447–1461. doi: 10.1089/neu.2008.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Harnett-Sheehan K., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G. The Mini International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview. J. Clin. Psychiatr. 1998;59(Suppl. 20):22–33. [PubMed] [Google Scholar]
- Shomstein S. Cognitive functions of the posterior parietal cortex: top-down and bottom-up attentional control. Front. Integr. Neurosci. 2012;6:38. doi: 10.3389/fnint.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu I.W., Onton J.A., O'Connell R.M., Simmons A.N., Matthews S.C. Combat veterans with comorbid PTSD and mild TBI exhibit a greater inhibitory processing ERP from the dorsal anterior cingulate cortex. Psychiatr. Res. 2014;224:58–66. doi: 10.1016/j.pscychresns.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Shucard J.L., McCabe D.C., Szymanski H. An event-related potential study of attention deficits in posttraumatic stress disorder during auditory and visual Go/NoGo continuous performance tasks. Biol. Psychol. 2008;79:223–233. doi: 10.1016/j.biopsycho.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.L., Johnstone S.J., Barry R.J. Movement-related potentials in the Go/NoGo task: the P3 reflects both cognitive and motor inhibition. Clin. Neurophysiol. 2008;119:704–714. doi: 10.1016/j.clinph.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Sours C., Zhuo J., Janowich J., Aarabi B., Shanmuganathan K., Gullapalli R.P. Default mode network interference in mild traumatic brain injury - a pilot resting state study. Brain Res. 2013;1537:201–215. doi: 10.1016/j.brainres.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoni A.D., Huang M., Simmons A.N. Emerging approaches to neurocircuits in PTSD and TBI: imaging the interplay of neural and emotional trauma. Curr. Top. Behav. Neurosci. 2018;38:163–192. doi: 10.1007/7854_2017_35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg J.M., McGlinchey R.E., Milberg W.P., Salat D.H. Brain network disturbance related to posttraumatic stress and traumatic brain injury in veterans. Biol. Psychiatr. 2015;78:210–216. doi: 10.1016/j.biopsych.2015.02.013. [DOI] [PubMed] [Google Scholar]
- Stephens J.A., Liu P., Lu H., Suskauer S.J. Cerebral blood flow after mild traumatic brain injury: Associations between symptoms and post-injury perfusion. J. Neurotrauma. 2018;35:241–248. doi: 10.1089/neu.2017.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D., Ashley V., Turken A.U. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D., Ashley V., Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Swick D., Honzel N., Larsen J., Ashley V., Justus T. Impaired response inhibition in veterans with post-traumatic stress disorder and mild traumatic brain injury. J. Int. Neuropsychol. Soc. 2012;18:917–926. doi: 10.1017/S1355617712000458. [DOI] [PubMed] [Google Scholar]
- Vasterling J.J., Brailey K., Constans J.I., Sutker P.B. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- Vasterling J.J., Brailey K., Proctor S.P., Kane R., Heeren T., Franz M. Neuropsychological outcomes of mild traumatic brain injury, post-traumatic stress disorder and depression in Iraq-deployed US Army soldiers. Br. J. Psychiatry. 2012;201:186–192. doi: 10.1192/bjp.bp.111.096461. [DOI] [PubMed] [Google Scholar]
- Vasterling J.J., Verfaellie M., Sullivan K.D. Mild traumatic brain injury and posttraumatic stress disorder in returning veterans: perspectives from cognitive neuroscience. Clin. Psychol. Rev. 2009;29:674–684. doi: 10.1016/j.cpr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Wu J., Ge Y., Shi Z., Duan X., Wang L., Sun X., Zhang K. Response inhibition in adolescent earthquake survivors with and without posttraumatic stress disorder: a combined behavioral and ERP study. Neurosci. Lett. 2010;486:117–121. doi: 10.1016/j.neulet.2010.07.040. [DOI] [PubMed] [Google Scholar]
- Wu J., Yuan Y., Cao C., Zhang K., Wang L., Zhang L. The relationship between response inhibition and posttraumatic stress symptom clusters in adolescent earthquake survivors: an event-related potential study. Sci. Rep. 2015;5:8844. doi: 10.1038/srep08844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Sandrini M., Levy S., Volochayev R., Awosika O., Butman J.A., Pham D.L., Cohen L.G. Lasting deficit in inhibitory control with mild traumatic brain injury. Sci. Rep. 2017;7:14902. doi: 10.1038/s41598-017-14867-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Milham M.P., Lui Y.W., Miles L., Reaume J., Sodickson D.K., Grossman R.I., Ge Y. Default-mode network disruption in mild traumatic brain injury. Radiology. 2012;265:882–892. doi: 10.1148/radiol.12120748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Wu R., Wang S., Qi H., Qian Y., Wang S. Behavioral and neurophysiological abnormalities during cued continuous performance tasks in patients with mild traumatic brain injury. Brain Behav. 2018;8 doi: 10.1002/brb3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.