Abstract
Background
Patients with asthma are comparatively susceptible to respiratory viral infections and more likely to develop severe symptoms than people without asthma. During the coronavirus disease 2019 (COVID-19) pandemic, it is necessary to adequately evaluate the characteristics and outcomes of the population with asthma in the population tested for and diagnosed as having COVID-19.
Objective
To perform a study to assess the impact of asthma on COVID-19 diagnosis, presenting symptoms, disease severity, and cytokine profiles.
Methods
This was an analysis of a prospectively collected cohort of patients suspected of having COVID-19 who presented for COVID-19 testing at a tertiary medical center in Missouri between March 2020 and September 2020. We classified and analyzed patients according to their pre-existing asthma diagnosis and subsequent COVID-19 testing results.
Results
Patients suspected of having COVID-19 (N = 435) were enrolled in this study. The proportions of patients testing positive for COVID-19 were 69.2% and 81.9% in the groups with asthma and without asthma, respectively. The frequencies of relevant symptoms were similar between the groups with asthma with positive and negative COVID-19 test results. In the population diagnosed as having COVID-19 (n = 343), asthma was not associated with several indicators of COVID-19 severity, including hospitalization, admission to an intensive care unit, mechanical ventilation, death due to COVID-19, and in-hospital mortality after multivariate adjustment. Patients with COVID-19 with asthma exhibited significantly lower levels of plasma interleukin-8 than patients without asthma (adjusted P = .02).
Conclusion
The population with asthma is facing a challenge in preliminary COVID-19 evaluation owing to an overlap in the symptoms of COVID-19 and asthma. However, asthma does not increase the risk of COVID-19 severity if infected.
Introduction
Coronavirus disease 2019 (COVID-19) is caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and leads to multiple respiratory symptoms, among a myriad of other symptoms.1 COVID-19 has been characterized as a pandemic by the World Health Organization and has affected more than 73 million people worldwide at the time of this writing according to the COVID-19 dashboard at Johns Hopkins University. People with asthma are generally more susceptible to respiratory viral infections than people without asthma owing to an abnormal airway epithelium and less effective or deficient antiviral innate immune responses.2 , 3 In the setting of respiratory viral infections, which include human rhinovirus, respiratory syncytial virus, seasonal coronaviruses, and many others, patients with asthma usually exhibit more severe and longer durations of respiratory symptoms than healthy individuals and may experience asthma exacerbations.4, 5, 6
Researchers have postulated that patients with asthma may be both at high risk of being infected with SARS-CoV-2 and experiencing more severe outcomes when infected.7 However, these assumptions have not been clearly demonstrated in numerous studies to date.10, 11, 12, 8, 9 Early findings from Wuhan, China, revealed that asthma was underrepresented in patients infected with COVID-19, with the prevalence of asthma only at 0.9% among 548 patients with COVID-19, whereas the overall prevalence of asthma was 6.4% in the adult Wuhan population.13 Similarly, COVID-19 infection was noted to be comparatively low in patients in the Severe Asthma Network in Italy, and patients with asthma also had comparatively low mortality rates if infected.14 Even though the prevalence of asthma among patients with COVID-19 in Europe and the United States has been substantially higher, ranging from approximately 8% to 17%, asthma was not observed to be associated with an increased risk of adverse outcomes such as hospitalization, intensive care unit (ICU) transfer, intubation, hospital readmissions, and mortality in patients with COVID-19.8 , 15, 16, 17 Several explanations for the surprisingly equivalent or favorable infection rates and outcomes of COVID-19 in patients with asthma have been proposed, including decreased epithelial angiotensin-converting enzyme 2 expression in asthma.18 , 19 However, interestingly, a large cohort study from England identified patients with asthma with recent oral corticosteroid use as being at increased risk for death due to COVID-19, whereas other studies found that asthma prolonged endotracheal intubation times.20, 21, 22, 23
Although most COVID-19–related research in asthma to date has focused on associations with morbidity and mortality, less focus has been placed on assessing 2 other pressing issues. First, are there difficulties in differentiating between the symptoms of COVID-19 and other infections and those of asthma? Most of the patients who are diagnosed as having COVID-19 report nonspecific signs and symptoms, including dyspnea, cough, wheezing, fever, fatigue, anosmia, and ageusia. Of note, there is an overlap between the common symptoms of COVID-19 and other viral infections and asthma exacerbations.24 These shared symptoms may cause considerable confusion among people with asthma and their providers regarding the need for COVID-19 testing, isolation recommendations, and disease management. Second, are there any differences in the presenting signs and immune responses between patients with COVID-19 with and without asthma? To date, no studies have described and compared the presenting characteristics between patients with COVID-19 with and without pre-existing asthma. Clues to the presenting symptoms could help providers better understand the common presentation of COVID-19 infection in patients with asthma and risk-stratify them.
To this end, we conducted an analysis using a prospectively collected cohort of patients who presented with COVID-19–like symptoms and received testing at a medical center. We evaluated the characteristics of patients with and without pre-existing asthma in this population suspected of having COVID-19 and, subsequently, within the population diagnosed as having COVID-19. Furthermore, we compared the cytokine profiles between patients diagnosed as having COVID-19 with and without pre-existing asthma and assessed the associations between asthma and subsequent COVID-19 outcomes.
Methods
Patient Recruitment
This study was approved by the institutional review board at Washington University in St Louis (approval number: 202003085). Patients who presented with symptoms consistent with COVID-19 at 2 urban medical centers within the BJC HealthCare system in St Louis, Missouri, and for whom a health care provider requested SARS-CoV-2 testing were eligible for enrollment in this study from March 2020 through September 2020. Patients who were interested in the study provided written consent and were asked about their symptoms, which were collected through participant interviews, with additional medical data extracted from medical records. SARS-CoV-2 positivity was determined using polymerase chain reaction–based testing performed on nasopharyngeal specimens.
Definition of Variables
Asthma and other comorbidities were identified on the basis of the International Classification of Diseases, Tenth Revision codes noted in health records. Patients were defined as having a pre-existing diagnosis of asthma if their health records contained an International Classification of Diseases, Tenth Revision code beginning with J45. Obesity was defined by a body mass index (BMI) equal to or greater than 30 kg/m2 found in the electronic medical record. Race and presenting symptoms were self-reported by participants at the interview.
Cytokine Quantification
Plasma samples from a subset of participants were analyzed using cytokine quantification as described previously.25 Plasma was analyzed using a human magnetic cytokine panel providing parallel measurement of 35 cytokines (Thermo Fisher Scientific, Waltham, Massachusetts). Each sample was performed in duplicate and then analyzed on a FLEXMAP 3D instrument (Luminex Corporation, Austin, Texas).
Statistical Analysis
Only adults aged above 18 years were included in this analysis. All statistical analysis, graphs, and figures were produced using RStudio (RStudio, PBC, Boston, Massachusetts). A P value < .05 was the standard used to determine if the statistical tests and models were significant. Demographics, major comorbidities, symptoms, and clinical outcomes were compared using the nonparametric Mann-Whitney U test for continuous variables and the χ2 test for categorical variables. Multivariable logistic regression models were used to evaluate whether asthma was a predictor of COVID-19 severe outcomes (including the individual outcomes of hospitalization, ICU admission, mechanical ventilation, death due to COVID-19, and in-hospital mortality) after adjustment for sex, age, race, chronic obstructive pulmonary disease (COPD), and obesity.
Results
Among the Population Suspected of Having COVID-19, Patients With Asthma Had Lower Rates of Testing Positive for COVID-19 Than Those Without Asthma
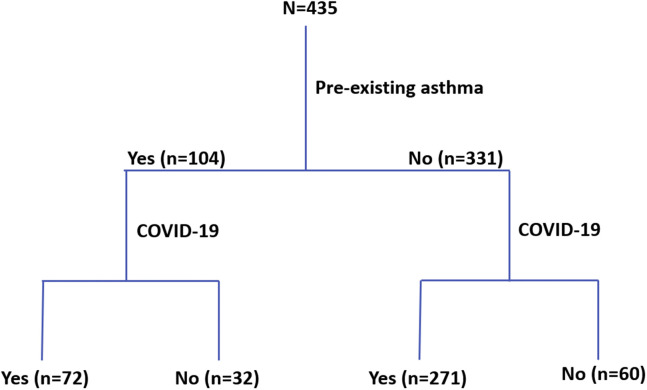
There were 435 patients in this cohort who were suspected of having COVID-19 by a health care provider and subsequently tested. The algorithm for placing patients into different categories is noted in Figure 1 . Among the 435 patients, 104 (23.9%) had a pre-existing diagnosis of asthma, and 331 (76.1%) had no asthma diagnosis. Among the participants with an asthma diagnosis, 72 of the 104 patients (69.2%) subsequently tested positive for COVID-19, whereas among the participants without asthma, 271 of the 331 patients (81.9%) subsequently tested positive for COVID-19. Patients with a diagnosis of asthma who were suspected of having COVID-19 had significantly lower rates of receiving positive test results than patients without asthma suspected of having COVID-19 (P = .006, χ2 test).
Figure 1.
Algorithm for identifying patients with asthma and coronavirus disease 2019. Patients with pre-existing asthma were identified by the International Classification of Disease, Tenth Revision codes and confirmed by chart review. Patients with coronavirus disease 2019 were identified using polymerase chain reaction–based testing on nasopharyngeal specimens. COVID-19, coronavirus disease 2019.
We compared different characteristics between patients with COVID-19 positive and negative test results stratified by asthma (Table 1 ). Regardless of asthma status, the group with COVID-19 was older (P = .0014 in the group with asthma and P < .0001 in the group without asthma) and had a higher percentage of underlying comorbidities (hypertension [P = .001 in the group with asthma and P < .0001 in the group without asthma] and diabetes [P = .02 in the group with asthma and P < .0001 in the group without asthma]). More men were in the group with COVID-19, but this was only significant for the patients without asthma (P < .001) . The participants who were diagnosed as having COVID-19 were more likely to report their race as Black in the group without asthma (P < .0001), but this did not reach statistical significance in the group with asthma (P = .053). In addition, the participants diagnosed as having COVID-19 had a substantially higher BMI (P = .0001) and percentage of obesity (P = .0055) than patients without COVID-19, but statistical significance was only observed in the population with asthma.
Table 1.
Characteristics of Patients Suspected of Having COVID-19 With and Without Asthma
| Variable | Asthma (n = 104) |
Nonasthma (n = 331) |
||||
|---|---|---|---|---|---|---|
| SARS-CoV-2 positive (n = 72) | SARS-CoV-2 negative (n = 32) | P value | SARS-CoV-2 positive (n = 271) | SARS-CoV-2 negative (n = 60) | P value | |
| Age (y), mean (SD) | 55.42 (15.66) | 44.72 (15.36) | .001a | 62.07 (15.71) | 49.71 (17.98) | <.001a |
| Sex: Male,b n (%) | 23 (31.9) | 10 (31.2) | .94 | 169 (62.6) | 23 (38.3) | <.001a |
| Race: Black,b n (%) | 56 (77.8) | 19 (59.4) | .053 | 186 (68.9) | 22 (36.7) | <.001a |
| BMI, mean (SD) | 36.20 (12.12) | 28.86 (7.48) | <.001a | 31.03 (8.58) | 30.39 (8.18) | .63 |
| Obesity, n (%) | 48 (66.7) | 12 (37.5) | .006a | 129 (47.6) | 26 (45.6) | .78 |
| COPD, n (%) | 27 (37.5) | 8 (25.0) | .21 | 43 (15.9) | 6 (10) | .25 |
| Hypertension, n (%) | 60 (83.3) | 17 (53.1) | .001a | 202 (74.5) | 29 (48.3) | <.001a |
| Diabetes, n (%) | 43 (59.7) | 11 (34.4) | .02a | 137 (50.6) | 12 (20) | <.001a |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory disease coronavirus 2.
NOTE. Obesity was defined as BMI equal to or greater than 30 kg/m2. Significance was evaluated on the basis of the Mann-Whitney U test or χ2 test.
P < .05;
Having missing data.
Patients With Asthma Exhibited Similar Symptoms Regardless of COVID-19 Test Results
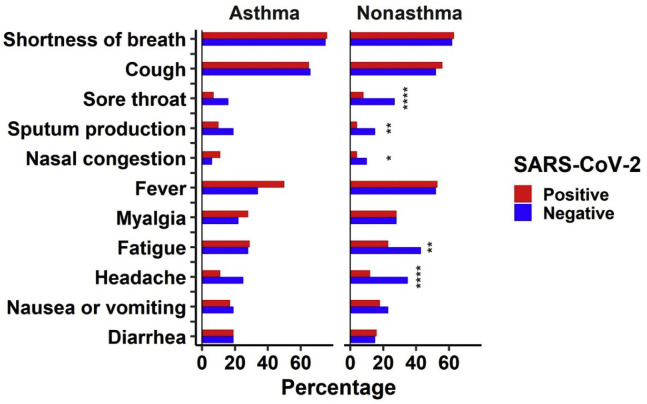
To examine whether people with positive and negative COVID-19 tests exhibited different symptoms at enrollment, we compared the frequencies of major symptoms stratified by asthma diagnosis. Among patients with asthma, no significant differences regarding the frequency of any of the presenting symptoms were observed between those who eventually tested positive for COVID-19 and those who did not (Fig 2 , eTable 1), suggesting that patients with asthma suspected of having COVID-19 have very similar symptoms regardless of subsequently testing positive for SARS-CoV-2 infection or not. However, interestingly, in people without an asthma diagnosis, we identified substantial differences among the eventual COVID-19 diagnoses: patients who tested negative for COVID-19 experienced more frequent symptoms of sore throat, sputum production, nasal congestion, fatigue, and headache than those who tested positive (Fig 2, eTable 2).
Figure 2.
Differences in symptoms between patients who tested positive and negative for coronavirus disease 2019 stratified by asthma. Symptoms were self-reported by patients at enrollment. Significance was evaluated on the basis of χ2 tests. The asterisk indicates P < .05; the double asterisk indicates P < .005; and the triple asterisk indicates P < .0001. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Among the Population Diagnosed as Having COVID-19, Asthma Was Not Associated With Increased Risks for Severe Outcomes
Next, we focused specifically on patients who tested positive for COVID-19 (n = 343) to evaluate whether those with asthma have distinct symptoms and cytokine profiling compared with those without asthma. The basic characteristics of these participants are shown in Table 2 . Patients with COVID-19 with asthma (n = 72) were older than those without asthma (n = 271; P = .00066). Men were more prevalent in the group without asthma (62.6%) than women, but this was not the case in the group with asthma (31.9%). The BMI was higher in the group with asthma than in the group without asthma (P <= .00038). The presence of obesity (P = .0040) and COPD (P < .001) was higher among patients with asthma, but no significant differences in the prevalence of hypertension and diabetes existed between the 2 groups. There were also no significant differences with known COVID-19 exposure before illness.
Table 2.
Characteristics of Patients With Coronavirus Disease 2019 With and Without Asthma
| Variable | Asthma (n = 72) | Nonasthma (n = 271) | P value |
|---|---|---|---|
| Age (y), mean (SD) | 55.42 (15.66) | 62.07 (15.71) | <.001a |
| Sex: Male,b n (%) | 23 (31.9) | 169 (62.6) | <.001a |
| Race: Black,b n (%) | 56 (77.8) | 186 (68.9) | .14 |
| BMI, mean (SD) | 36.2 (12.12) | 31.03 (8.58) | <.001a |
| Obesity, n (%) | 48 (66.7) | 129 (47.6) | .004a |
| COPD, n (%) | 27 (37.5) | 43 (15.9) | <.001a |
| Hypertension, n (%) | 60 (83.3) | 202 (74.5) | .12 |
| Diabetes, n (%) | 43 (59.7) | 137 (50.6) | .17 |
| Known COVID-19 exposure,b n (%) | 40 (56.3) | 130 (48.5) | .24 |
| Current smoker, n (%) | 9 (12.5) | 33 (12.2) | .94 |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019.
NOTE. Obesity was defined as BMI equal to or greater than 30 kg/m2. Significance was evaluated on the basis of the Mann-Whitney U test or χ2 test.
P < .05;
Having missing data.
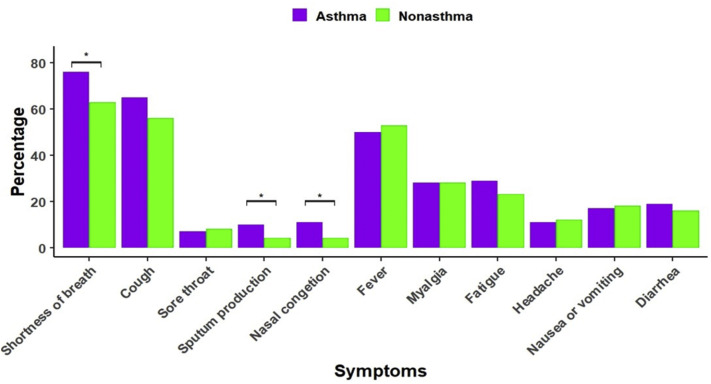
When comparing symptoms, patients with COVID-19 with asthma had a higher percentage of respiratory symptoms, including shortness of breath (P = .03), sputum production (P = .04), and nasal congestion (P = .01), than patients with COVID-19 without an asthma diagnosis (eFig 1). However, there were no substantial differences in systemic and gastrointestinal symptoms (eFig 1).
To evaluate the associations between pre-existing asthma and COVID-19 severity, multivariable logistic regression models were used and included age, sex, race (Black, not Black), COPD, and obesity as covariates. We did not find statistically significant relationships between an asthma diagnosis and several indicators of COVID-19 severity, including hospitalization, ICU admission, mechanical ventilation, death due to COVID-19, and in-hospital mortality (Table 3 ).
Table 3.
Multivariable Logistic Regression Models of Asthma and Coronavirus Disease 2019 Severe Outcomes
| COVID-19 severity indicator1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 | OR (95% CI) | P value |
|---|---|---|
| Hospitalization | 1 (0.34-3.28) | >.99 |
| ICU admission | 0.59 (0.31-1.08) | .01 |
| Mechanical ventilation | 1.10 (0.56-2.12) | .77 |
| Death due to COVID-19 | 0.73 (0.30-1.64) | .46 |
| In-hospital mortality | 0.72 (0.31-1.57) | .42 |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; ICU, intensive care unit; OR, odds ratio.
NOTE. Multivariable regression models adjusted for age, race (Black or not Black), sex, chronic obstructive pulmonary disease, and obesity.
Patients With COVID-19 With Asthma Exhibited Lower Levels of Plasma Interleukin-8 Than Those Without Asthma
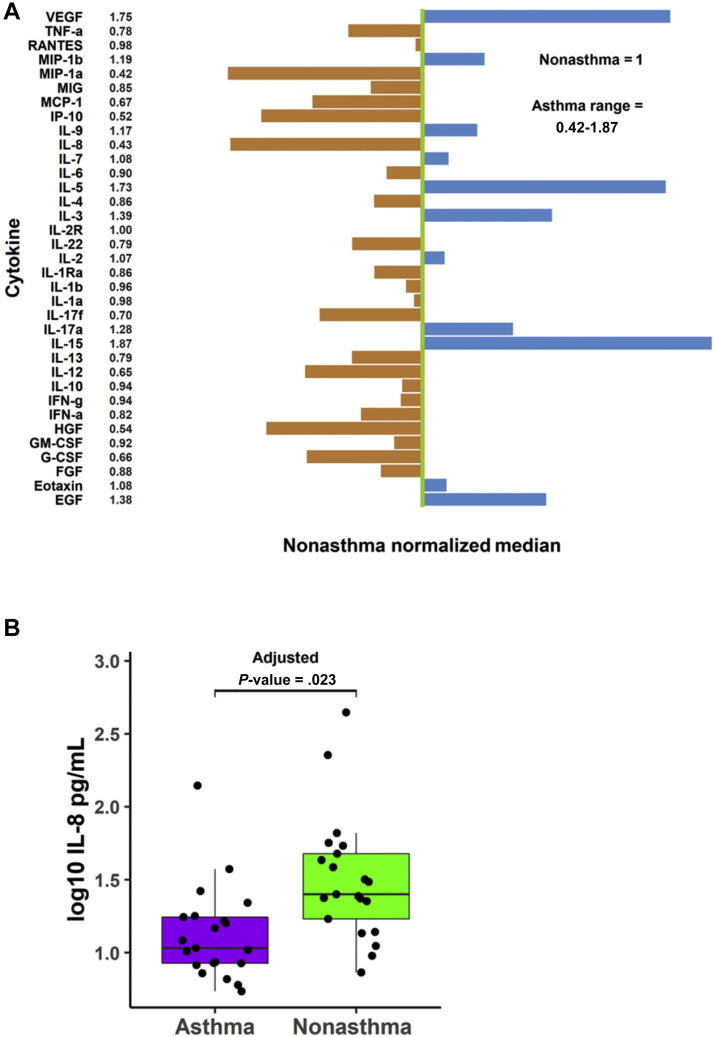
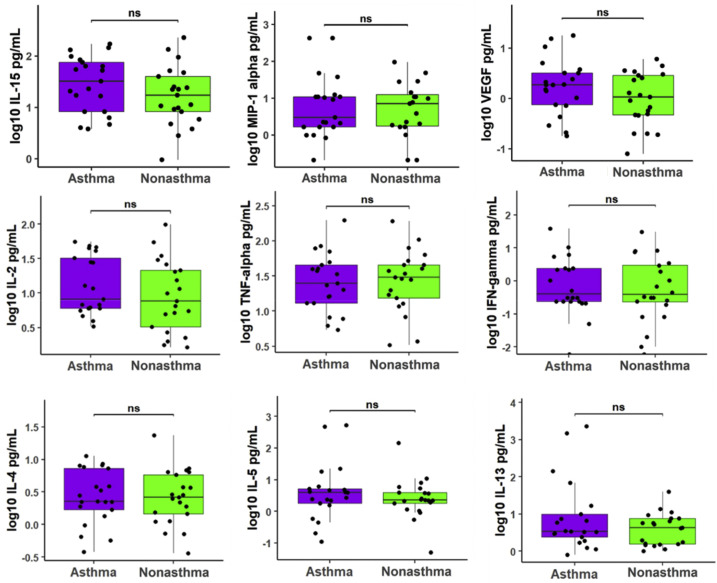
To assess the differences in the underlying immune responses between patients with COVID-19 with and without asthma, we measured 35 plasma cytokine levels from 42 patients, including 21 asthma-control pairs matched by age (within a 5-year interval) and sex. We found that for 23 out of 35 cytokines, patients with asthma had lower median cytokine levels than patients without asthma, and for 11 out of 35 cytokines, patients with asthma had higher median cytokine levels (Fig 3 A). A direct comparison further revealed that patients with COVID-19 with asthma exhibited significantly reduced levels of interleukin-8 (IL-8) compared with those without asthma (Bonferroni adjusted P = .02, Fig 3B), but no other measured cytokines demonstrated statistical differences between these 2 groups. A comparison of selective cytokines (vascular endothelial growth factor, macrophage inflammatory protein–1 alpha, IL-2, IL-4, IL-5, IL-13, IL-15, tumor necrosis factor alpha, and interferon gamma) is displayed in eFigure 2.
Figure 3.
Cytokine concentrations in patients with coronavirus disease 2019 with and without asthma. A, Cytokine relative abundance. Green line: normalized median cytokine level in patients without asthma. Each bar: median cytokine level in patients with asthma relative to the green line. B, Interleukin-8 values plotted on log10 scale. P values from Mann-Whitney U test (Bonferroni adjusted). EGF, epidermal growth factor; FGF, fibroblast growth factor; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; HGF, hepatocyte growth factor; IFN-a, interferon alpha; IFN-g, interferon gamma; IL, interleukin; IP, inducible protein; MIG, monokine induced by interferon gamma; MIP, macrophage inflammatory protein; RANTES, regulated on activation, normal T cell expressed and secreted; TNF-a, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor.
Discussion
We performed an analysis on a prospectively collected cohort of patients suspected of having COVID-19 and presenting to 2 BJC HealthCare hospitals in St Louis, Missouri. Our results showed that among patients suspected of having COVID-19, those with asthma were more likely to test negative for COVID-19 than those without asthma, which is consistent with results from a large retrospective cohort study in Israel.26 Furthermore, we found that the presenting symptoms were similar, regardless of subsequent COVID-19 diagnosis among patients with and without asthma, and this confirms the sometimes vague nature of COVID-19 and the similarities in symptoms with other respiratory infections. Because people with asthma are generally more susceptible to various respiratory viral infections or environmental allergens and subsequently develop asthma symptoms, they are at increased risk for exhibiting symptoms that are similar to those of COVID-19, but they may not necessarily have COVID-19. Therefore, patients with asthma may be confused about their symptoms, and their health care providers face a great challenge in risk-stratifying them for COVID-19 testing—a problem that may be only worsened as the winter season begins. The proclivity for respiratory symptoms in patients with asthma may explain their lower rate for eventual positive COVID-19 tests because they are more likely to present with concerning symptoms, but they may not necessarily have COVID-19. Of note, patients with asthma may nevertheless be at increased risk of being exposed to COVID-19 just by presenting to the hospital owing to confusion about their symptoms.8 Thus, there urgently needs to be more research on how to find better and quicker ways to differentiate an asthma exacerbation from a COVID-19 infection and perform testing safely in outpatient or home settings.
Among the population suspected of having COVID-19 without asthma, we found that patients who tested positive for COVID-19 showed fewer prominent upper airway signs such as sore throat and nasal congestion, as well as other symptoms, including sputum production, headache, and fatigue, than people who tested negative for COVID-19. This finding is consistent with previous reports and suggests that SARS-CoV-2 may have distinct infection mechanisms compared with previous common respiratory viruses.1 , 27 , 28 Interestingly, this unique symptom pattern related with COVID-19 was not observed in patients with asthma, reflecting a possibly different modulation of respiratory infections, including SARS-CoV-2, in patients with asthma and greater difficulties for patients with asthma to differentiate other infections from a SARS-CoV-2 infection solely on the basis of their symptoms.
In our study, asthma was not a risk factor for severe COVID-19 outcomes, which strengthens the belief that asthma may not be a relevant risk factor for COVID-19 severity, as found in other studies.15 , 16 , 29 A novel aspect of our study was the comparison of symptoms and cytokine profiles in patients with COVID-19 with and without asthma. Although more patients with COVID-19 with asthma reported respiratory symptoms (shortness of breath, sputum production, and nasal congestion) than patients without asthma, the frequency of systemic symptoms (fever, myalgia, fatigue, and headache) remained the same, regardless of pre-existing asthma. Furthermore, the plasma cytokine patterns were similar between the patients diagnosed as having COVID-19 with and without asthma at enrollment, except for IL-8. Patients with COVID-19 with asthma expressed reduced levels of IL-8 compared with patients without asthma. As a proinflammation chemokine, IL-8 up-regulation is robustly associated with COVID-19 infection and critical outcomes.30, 31, 32 IL-8 is well known to trigger the activation and migration of neutrophils and is a key mediator in innate immune responses and systemic inflammation.33 The reduced levels of IL-8 in the population with asthma on SARS-CoV-2 infection might be a mechanism underlying favorable COVID-19 outcomes. It is worth noting that a previous study found that patients with COVID-19 with comorbid COPD had a higher risk of developing severe outcomes and had a remarkedly higher serum IL-8 than patients with COVID-19 with asthma.34
Some limitations of this study need to be considered. First, the self-reported symptoms might introduce under- and overestimation of symptom association with COVID-19 or asthma. Second, a pre-existing asthma diagnosis was determined solely on the basis of the patients’ health records without diagnostic testing. Therefore, the possibility exists that asthma may be systemically over- or underestimated in this study. Third, a sudden loss of smell or taste has been remarkably prevalent among people who test positive for COVID-19.35 , 36 However, these 2 symptoms were not listed in our data because our study started in March when loss of smell or taste was less recognized as a prominent symptom of COVID-19 infection. Therefore, our comparison of symptoms could be improved if loss of smell or taste is included in future studies.
In conclusion, this study examined the characteristics and outcomes of patients with suspected and diagnosed COVID-19 stratified by an asthma diagnosis. This study raised a particular area of concern for patients with asthma during the COVID-19 pandemic: patients with asthma are facing a great challenge in differentiating between symptoms of COVID-19 and other respiratory infections and routine asthma exacerbations. As some asthma-related testing and care have been disrupted during the pandemic, this difficulty with differentiating symptoms could pose a great risk for asthma management and COVID-19 prevention among the population with asthma. It is critical to continue investigations into the relationship between asthma and COVID-19, better characterize the unique features of COVID-19 in the population with asthma and identify safe and reliable testing mechanisms.
Acknowledgments
We appreciate the critical scientific advice from Dr Gregory Storch (Washington University School of Medicine in St Louis) and the cytokine data from Dr Ali Ellebedy (Washington University School of Medicine in St Louis).
Footnotes
Disclosures: The authors have no conflicts of interest to report.
Funding: This work was supported by the National Institutes of Health (NIH) (grant number 5R21AI139649-02). This study used samples obtained from the Washington University School of Medicine in St Louis’s coronavirus disease biorepository, which is supported by the Barnes-Jewish Hospital Foundation; the Siteman Cancer Center (grant number P30 CA091842 from the National Cancer Institute of the NIH); and the Washington University Institute of Clinical and Translational Sciences (grant number UL1TR002345 from the National Center for Advancing Translational Sciences of the NIH). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.anai.2021.02.020.
Supplementary Data
eTable 1.
Symptoms Reported at Enrollment by Patients With Asthma Stratified by SARS-CoV-2 Test Results
| Symptom | SARS-CoV-2 positive (N = 72) | SARS-CoV-2 negative (N = 32) | P value |
|---|---|---|---|
| Shortness of breath | 55 (76.4) | 24 (75.0) | .88 |
| Cough | 47 (65.3) | 21 (65.6) | .97 |
| Sore throat | 5 (6.9) | 5 (15.6) | .17 |
| Sputum production | 7 (9.7) | 6 (18.8) | .20 |
| Nasal congestion | 8 (11.1) | 2 (6.2) | .44 |
| Fever | 36 (50.0) | 11 (34.4) | .14 |
| Myalgia | 20 (27.8) | 7 (21.9) | .53 |
| Fatigue | 21 (29.2) | 9 (28.1) | .91 |
| Headache | 8 (11.1) | 8 (25.0) | .070 |
| Nausea or vomiting | 12 (16.7) | 6 (18.8) | .80 |
| Diarrhea | 14 (19.4) | 6 (18.8) | .93 |
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
NOTE. Value format is n (%). Symptoms were self-reported at enrollment. Significance was evaluated on the basis of χ2 tests.
eTable 2.
Symptoms Reported at Enrollment by Patients Without Asthma Stratified by SARS-CoV-2 Test Results
| Symptom | SARS-CoV-2 positive (N = 271) | SARS-CoV-2 negative (N = 60) | P value |
|---|---|---|---|
| Shortness of breath | 171 (63.1) | 37 (61.7) | .84 |
| Cough | 152 (56.1) | 31 (51.7) | .53 |
| Sore throat | 22 (8.1) | 16 (26.7) | <.0001a |
| Sputum production | 10 (3.7) | 9 (15.0) | .00065a |
| Nasal congestion | 10 (3.7) | 6 (10.0) | .039a |
| Fever | 144 (53.1) | 31 (51.7) | .84 |
| Myalgia | 77 (28.4) | 17 (28.3) | .99 |
| Fatigue | 61 (22.5) | 26 (43.3) | .00091a |
| Headaches | 32 (11.8) | 21 (35.0) | <.0001a |
| Nausea or vomiting | 50 (18.5) | 14 (23.3) | .39 |
| Diarrhea | 43 (15.9) | 9 (15) | .87 |
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
NOTE. Values are expressed as n (%). Symptoms were self-reported at enrollment. Significance was evaluated on the basis of χ2 tests.
P < .05.
eFigure 1.
Comparison of symptom frequencies reported at enrollment between patients with coronavirus disease 2019 with and without asthma. Significance was evaluated on the basis of χ2 tests. The asterisk indicates P value of < .05.
eFigure 2.
Selective cytokine raw values plotted on a log10 scale. P values were calculated using the Mann-Whitney U test and were adjusted for multiple comparisons using the Bonferroni method. IFN, interferon; IL, interleukin; MIP, macrophage inflammatory protein; ns, not significant; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busse W.W., Lemanske R.F., Jr., Gern J.E. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376(9743):826–834. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritchie A.I., Jackson D.J., Edwards M.R., Johnston S.L. Airway epithelial orchestration of innate immune function in response to virus infection. A focus on asthma. Ann Am Thorac Soc. 2016;13(suppl 1):S55–S63. doi: 10.1513/AnnalsATS.201507-421MG. [DOI] [PubMed] [Google Scholar]
- 4.Message S.D., Laza-Stanca V., Mallia P. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008;105(36):13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darveaux J.I., Lemanske R.F., Jr. Infection-related asthma. J Allergy Clin Immunol Pract. 2014;2(6):658–663. doi: 10.1016/j.jaip.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gern J.E. How rhinovirus infections cause exacerbations of asthma. Clin Exp Allergy. 2015;45(1):32–42. doi: 10.1111/cea.12428. [DOI] [PubMed] [Google Scholar]
- 7.Johnston S.L. Asthma and COVID-19: is asthma a risk factor for severe outcomes? Allergy. 2020;75(7):1543–1545. doi: 10.1111/all.14348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartmann-Boyce J., Gunnell J., Drake J. Asthma and COVID-19: review of evidence on risks and management considerations [e-pub ahead of print]. BMJ Evid Based Med. https://doi.org/10.1136/bmjebm-2020-111506 [DOI] [PubMed]
- 9.Liu S., Zhi Y., Ying S. COVID-19 and asthma: reflection during the pandemic. Clin Rev Allergy Immunol. 2020;59(1):78–88. doi: 10.1007/s12016-020-08797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto K., Saito H. Does asthma affect morbidity or severity of COVID-19? J Allergy Clin Immunol. 2020;146(1):55–57. doi: 10.1016/j.jaci.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halpin D.M.G., Faner R., Sibila O., Badia J.R., Agusti A. Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respir Med. 2020;8(5):436–438. doi: 10.1016/S2213-2600(20)30167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broadhurst R., Peterson R., Wisnivesky J.P. Asthma in COVID-19 hospitalizations: an overestimated risk factor? Ann Am Thorac Soc. 2020;17(12):1645–1648. doi: 10.1513/AnnalsATS.202006-613RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X., Xu S., Yu M. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heffler E., Detoraki A., Contoli M. COVID-19 in Severe Asthma Network in Italy (SANI) patients: clinical features, impact of comorbidities and treatments [e-pub ahead of print]. Allergy. https://doi.org/10.1111/all.14532 [DOI] [PMC free article] [PubMed]
- 15.Chhiba K.D., Patel G.B., Vu T.H.T. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146(2):307–314.e4. doi: 10.1016/j.jaci.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovinsky-Desir S., Deshpande D.R., De A. Asthma among hospitalized patients with COVID-19 and related outcomes. J Allergy Clin Immunol. 2020;146(5):1027–1034.e4. doi: 10.1016/j.jaci.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemus Calderon J.A., Beneyto Martin P., Guzman Rodriguez R., Caligaris Cataldi H.S., Senent Sánchez C.J. Differentiating characteristics of patients with asthma in the severe acute respiratory syndrome coronavirus 2 infection. Ann Allergy Asthma Immunol. 2021;126(1):92–93. doi: 10.1016/j.anai.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson D.J., Busse W.W., Bacharier L.B. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020;146(1):203–206.e3. doi: 10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters M.C., Sajuthi S., Deford P. COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020;202(1):83–90. doi: 10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williamson E.J., Walker A.J., Bhaskaran K. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schultze A., Walker A.J., MacKenna B. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8(11):1106–1120. doi: 10.1016/S2213-2600(20)30415-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahdavinia M., Foster K.J., Jauregui E. Asthma prolongs intubation in COVID-19. J Allergy Clin Immunol Pract. 2020;8(7):2388–2391. doi: 10.1016/j.jaip.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenthal J.A., Awan S.F., Fintzi J., Keswani A., Ein D. Asthma is associated with increased risk of intubation but not hospitalization or death in coronavirus disease 2019. Ann Allergy Asthma Immunol. 2021;126(1):93–95. doi: 10.1016/j.anai.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abrams E.M., ‘t Jong G.W., Yang C.L. Asthma and COVID-19. CMAJ. 2020;192(20):E551. doi: 10.1503/cmaj.200617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mudd P.A., Crawford J.C., Turner J.S. Distinct inflammatory profiles distinguish COVID-19 from influenza with limited contributions from cytokine storm. Sci Adv. 2020;6(50) doi: 10.1126/sciadv.abe3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green I., Merzon E., Vinker S., Golan-Cohen A., Magen E. COVID-19 susceptibility in bronchial asthma. J Allergy Clin Immunol Pract. 2021;9(2):684–692.e1. doi: 10.1016/j.jaip.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Chen J., Chen W. Does asthma increase the mortality of patients with COVID-19?: A systematic review and meta-analysis. Int Arch Allergy Immunol. 2021;182(1):76–82. doi: 10.1159/000510953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galani I.E., Rovina N., Lampropoulou V. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat Immunol. 2021;22(1):32–40. doi: 10.1038/s41590-020-00840-x. [DOI] [PubMed] [Google Scholar]
- 31.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghazavi A., Ganji A., Keshavarzian N., Rabiemajd S., Mosayebi G. Cytokine profile and disease severity in patients with COVID-19. Cytokine. 2021;137:155323. doi: 10.1016/j.cyto.2020.155323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moser B., Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2(2):123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 34.Song J., Zeng M., Wang H. Distinct effects of asthma and COPD comorbidity on disease expression and outcome in patients with COVID-19. Allergy. 2021;76(2):483–496. doi: 10.1111/all.14517. [DOI] [PubMed] [Google Scholar]
- 35.Menni C., Valdes A.M., Freidin M.B. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26(7):1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glezer I., Bruni-Cardoso A., Schechtman D., Malnic B. Viral infection and smell loss: the case of COVID-19 [e-pub ahead of print]. J Neurochem. https://doi.org/10.1111/jnc.15197 [DOI] [PMC free article] [PubMed]