Dear Editor,
To date, spinal cord involvement in association with COVID-19 has been poorly characterized. However, its incidence could have been underestimated, due to the difficulties in performing complete neurological, neurophysiological and neuroimaging tests in a highly contagious, intensive-care setting. Here we report on three cases of COVID-19-related acute myelopathies, as further evidence endorsing SARS-CoV-2 neurotropic potential.[1] Table 1 provides further details to the following case descriptions.
Table 1.
Clinical, laboratory and neuroimaging features in COVID-19 patients with acute myelopathies.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Subject demographics | Female, 25 y-o | Female, 50 y-o | Male, 69 y-o |
| Medical history | – | Essential tremor | Hypertension, dyslipidemia |
| COVID-19 symptoms | Mild (fever, anosmia, dysgeusia) | Mild (fever) | Moderate (fever, asthenia, interstitial pneumonia) |
| Exposure to proven COVID-19 cases | Untraceable | Workmates | Wife |
| COVID-19 treatment | Acetaminophen | HCQ 200 mg bid for 7 days, azithromycin 500 mg for 5 days, amoxicillin/clavulanate 875/125 mg bid for 7 days and celecoxib | Amoxicillin/clavulanate 875/125 mg bid for 2 days |
| CNS Neurological features | Sub-acute paraplegia, sensory impairment with upper thoracic level, bladder dysfunction | Sub-acute sensorimotor impairment to the lower limbs with gait ataxia | Acute flaccid paraplegia, sensory impairment with mid thoracic level, bladder and bowel dysfunction |
| Latency/Overlap between infectious and neurological onset | 15 days/No | 15 days/No | 3 days/Yes |
| Lab tests (at admission) | Normal | Normal | WBC 6,6/mmc Ly 1,2/mmc CRP 151 mg/l Ferritin 1422 ng/ml LDH 592 U/L |
| Chest imaging during hospitalization | Negative | Negative | Interstitial pneumonia |
| SARS-CoV-2 RT-PCR (swab) – performed after symptom onset: | Positive | Negative (not performed during the infectious course) | Positive |
|
20 days | 30 days | 3 days |
|
5 days | 15 days | <1 day |
| Brain neuroimaging | MRI: Normal findings | MRI: Single periventricular lesion (Fig. 1, II) | MRI: Single lesion in the left superior cerebellar peduncle (Fig. 1, III) |
| Spine neuroimaging | MRI: Multiple lesions (cervical and thoracic spinal cord) (Fig. 1, I) | MRI: Two lesions (one extending from the bulbo-medullary junction down to C6, one located at the conus) (Fig. 1, II) | MRI: Multiple lesions (cervical and upper thoracic spinal cord, LETM extending from mid-thoracic down to the epiconus) (Fig. 1, III) |
| CSF findings | Slight lymphomonocytic pleocytosis (8 cells/mmc), no OCBs | Moderate lymphomonocytic pleocytosis (65 cells/mmc), hyperproteinorrachia (161.2 mg/dl), slight hypoglycorrachia (41 mg/dl), no OCBs | Marked neutrophilic pleocytosis (672 cells/mmc), hyperproteinorrachia (90 mg/dl), normal glucose, “mirror pattern” OCBs. Control CSF (after 5 days): reduced pleocytosis (26 cells/mmc) and hyperproteinorrachia (58 mg/dl), normal glucose, no OCBs |
| Neurophysiology | Motor evoked potentials: temporal dispersion, normal central conduction times. EMG/ENG: normal. |
Somatosensory evoked potentials: delayed latency for cortical response with lower limbs stimulation. EMG/ENG: normal. |
Motor evoked potentials: no response for lower limbs. EMG/ENG: “polio-like syndrome” in lower limbs (reduced amplitude of compound motor action potentials, reduced recruitment pattern, presence of denervation potentials on needle EMG, absent F wave responses) |
| SARS-CoV-2 RT-PCR on CSF | Negative | N.P. | Negative |
| Diagnosis | Post-infectious demyelinating myelitis | Post-infectious encephalomyelitis | Para-infectious demyelinating and polio-like encephalomyelitis |
| Treatment | M-P 1 g OD for 7 days | Ceftriaxone 2 g x 2 for 5 days, Ampicillin 2 g x 6 for 5 days, Acyclovir 750 mg x 3 for 3 days M-P 1 g OD for 5 days, 500 mg OD for 3 days, 250 mg OD for 3 days |
Ceftriaxone 2 g x 2 for 10 days, acyclovir 750 mg x 3 for 5 days, lopinavir/ritonavir 400/100 mg and HCQ 200 mg x 2 for 14 days M-P 1 g OD for 7 days; IVIg 0.4 g/kg OD for 5 days |
| Clinical follow up | After 2 weeks; discharged to rehab with paraplegia and sensory gait ataxia, able to walk with a walker After 3 months: Discharged home, able to walk with a walker, mild sensory gait ataxia |
After 2 weeks: discharged to rehab with moderate gait ataxia After 3 month: Slight gait ataxia, able to walk without devices |
After 3 weeks: discharged to rehab with severe paraplegia, unable to stand After 3 months: Still in rehab, moderate improvement of strength, able to walk with a walker and assistanc |
Abbreviations: y-o, year-old; F, female; M, male; CNS, central nervous system; Ly, lymphocytes; CSF, cerebrospinal fluid; OCBs, oligoclonal bands; LETM, longitudinal elongated transverse myelitis; N.P., not performed; OD, omne in die; bid, bis in die; M-P, methylprednisolone; HCQ, hydroxychloroquine; IVIg, intravenous immunoglobulins.
Case 1. In March 2020, in Pavia (Lombardy, Italy), a 25 year-old woman reported mild COVID-19 symptoms for a few days. Fifteen days later, she sub-acutely developed paraplegia, mid-thoracic hypesthesia and incomplete bladder voiding. MRI revealed multiple white matter cervical and thoracic lesions, without post-contrast enhancement [Fig. 1, I ]. Cerebrospinal fluid (CSF) showed mild lymphomonocytic pleocytosis without oligoclonal bands (OCBs). Extensive screening for autoantibodies and infections tested negative on blood and CSF. RT-PCR for SARS-CoV-2 RNA was positive on nasopharyngeal swab (NPS) but negative on CSF. Neurophysiological studies supported central damage with no peripheral abnormalities. The patient was treated with i.v. steroids, with dramatic improvement. After rehabilitation, she is able to move with a walker for persisting sensory gait ataxia.
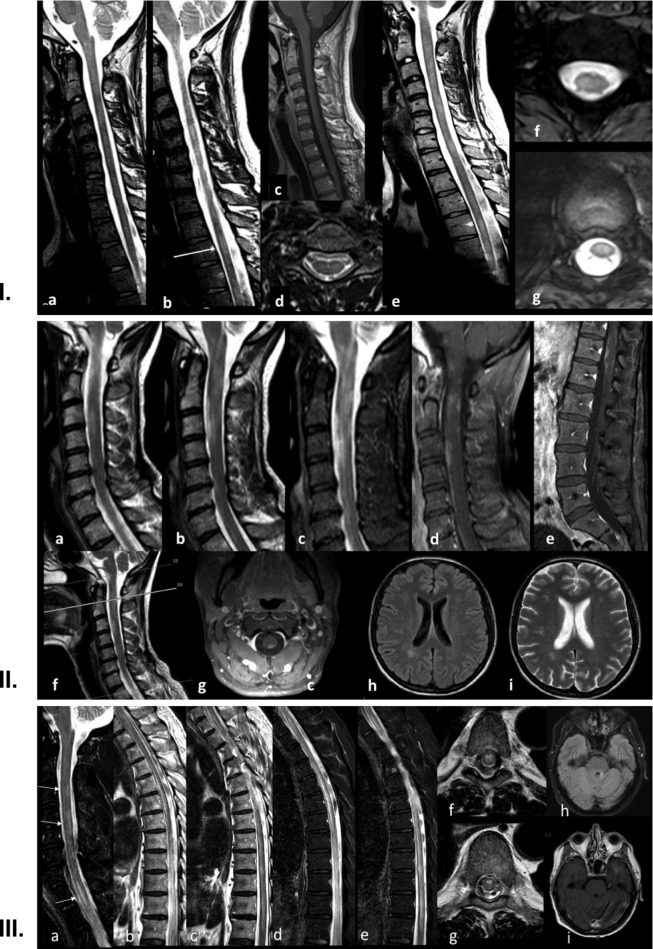
Fig. 1.
I.Case 1: Neuroimaging. Spine MRI performed in the acute phase (a–d) from C1 to D5 with sagittal (a), parasagittal (b) and axial (d) T2 Turbo Spin Echo (TSE) weighted images and post gadolinium sagittal T1-Spin Echo (SE) weighted images (c). Follow-up MRI performed 1-month later (e–g), with sagittal T2-SE (e) and axial T2-Fast Field Echo (FFE) sequences (f, g).
In the acute phase, a slight diffuse multifocal hyperintensity at the cervical level (a, b) and a blurred hyperintensity at D3 level (b, white arrow, and d) were detectable. No enhancement was evident after gadolinium injection (c). Follow-up MRI better showed multifocal cervical lesions at C3-C5 and C6-C7 (e), mainly involving the cervical lateral (f) and dorsal centro-medullary region (g).
II. Case 2: Neuroimaging. Spine MRI with sagittal T2 (a, b, f), sagittal T2-STIR sequences (c), post-contrast sagittal (d) and axial (g) T1 sequences of the cervical tract and post-contrast T1 sequence (e) of the lumbosacral tract. Cervical sequences detected a diffuse T2-and STIR-hyperintensity extending from the bulbo-medullary junction down to C6 (a–c, f), with peripheral enhancement and a relative sparing of the centro-medullary area (d, g). A marked post-contrast enhancement was noted in the conus, preeminently involving the anterior and posterior columns with a relative central sparing (e). Brain MRI with FLAIR (h) and T2 (i) sequences, showing a single right posterior periventricular lesion.
III. Case 3: Neuroimaging. Spine MRI with sagittal T2-Spin Echo (SE, a–c) and T2-STIR sequences (d, e) and axial T2-Fast Field Echo (FFE) sequences (f, g). Brain MRI (h, i) with axial FLAIR (h) and post–contrast T1-SE (i).
Spine MRI (a) shows multiple, predominantly posterior hyperintense cervical lesions at C3, C4-C5 level (arrows) and in the upper dorsal region (T1-T2, arrows), while a more extensive alteration is evident from T5 down to the epiconus (b–e). Axial slices (f, g) detect an “H-shaped” grey matter involvement (f), predominantly affecting the anterior horns (g). Brain MRI shows one single hyperintense lesion in the left superior cerebellar peduncle (h), without contrast enhancement (i).
Case 2. In April 2020, in Milan (Lombardy, Italy), a 50 year-old woman experienced mild COVID-19. Two weeks later, she sub-acutely developed a painful sensorimotor impairment in the lower limbs. RT-PCR for SARS-CoV-2 tested negative on NPS. MRI revealed one periventricular lesion and multiple lesions affecting the cervical spinal cord and the conus [Fig. 1, II]. CSF showed moderate lymphomonocytic pleocytosis and hyperproteinorrachia, without OCBs. Microbiological tests for neurotropic pathogens were negative. Broad-spectrum antibiotics and antiviral were administered. Screening tests for autoimmune disorders were negative. Neurophysiological assessment excluded overt signs of peripheral involvement. The patient received i.v. steroids for 11 days, with strength improvement. After rehab, she is able to walk unassisted, with persisting mild sensory impairment in the lower limbs and the perineal area.
Case 3. In March 2020, in Alessandria (Piedmont, Italy), a 69 year-old man presented for urinary retention, fever and asthenia in the last three days. Microbiological urinalysis was normal. RT-PCR for SARS-CoV-2 on NPS was positive. Interstitial pneumonia was detected by chest imaging, however no signs of respiratory failure occurred. During observation, he acutely developed flaccid paraplegia with areflexia and anesthesia with a mid-thoracic level. MRI revealed one cerebellar lesion, multiple cervical and thoracic lesions, and an elongated lesion extending from the mid-thoracic level down to the epiconus, preeminently involving the anterior horn grey matter [Fig. 1, III]. CSF disclosed a marked neutrophilic pleocytosis, hyperproteinorrachia, and “mirror pattern” OCBs. Extensive screening on blood and CSF for endemic neurotropic pathogens resulted negative. SARS-CoV-2 RNA was undetectable on CSF. Broad-spectrum antibiotics and antivirals were started. Systemic and neurologic autoimmune and vascular diseases were ruled out. Treatment with i.v. steroids was started within 48 hours from onset and continued for 7 days, with significant sensory improvement. Follow up MRI showed a notable reduction in white matter lesions size, and only slight changes as for the multimetameric dorsal lesion. Subsequent treatment with i.v. immunoglobulin 0.4 g/kg daily for 5 days determined a slight motor improvement. Neurophysiology results were consistent with the clinical and neuroimaging findings. After rehab, he regained ability to stand and walk with a walker.
In Case 1 and Case 2 we described a multifocal myelitis and a encephalomyelitis with pre-eminent spinal involvement occurring soon after clinical recovery from COVID-19. The prototypical post infectious neurological syndrome is represented by acute disseminated encephalomyelitis (ADEM), which typically follows a recent (from 1 to 6 weeks prior) viral infection or vaccination and is featured by multifocal demyelinating lesions [2]. Differently from our cases, ADEM lesions usually affect the brain to a greater extent than the spinal cord, although cases of post-infectious site-restricted isolated myelitis have already been reported [2]. In both cases, infectious and neurological course were temporally separated, as outlined by the lack of symptoms or indexes of systemic infection or inflammation at admission tests. Systemic and neurological chronic autoimmune disorders were excluded. CSF lymphomonocytosis, the disseminated pattern of lesions with major white matter involvement, and the dramatic improvement after steroid treatment are consistent with an immune-mediated, inflammatory damage that was reasonably triggered by the previous infection [2].
Conversely, in Case 3 we described an extensive spinal cord damage in a COVID-19 patient where the overlapping infectious and neurological symptoms point to a para-infectious mechanism. The absence of SARS-CoV-2 RNA in the CSF samples, although it does not support a direct pathogenic role of the virus, is consistent with recent reports of COVID-19 patients with CNS involvement [3]. Neuroimaging was distinctive: multiple disseminated lesions without contrast enhancement, mostly affecting the spinal cord, including a dorsal longitudinal elongated transverse myelitis (LETM) with a central “H-shaped” pattern and preeminent involvement of the anterior horns. Interestingly, both LETM and anterior horn myelitis are known to occur also as para-infectious phenomena [4]. The major involvement of the spinal grey matter points to a direct viral damage and is often labeled as “polio-like viral related syndrome”, since its similarity with the classical poliomyelitis.
The main pathways played by COVID-19 while affecting the CNS are likely represented by a combination of direct viral pathogenicity, immune-mediated tissue damage, inflammatory vascular involvement and intravascular coagulation [1,5]. It is important acknowledging that the temporal relationship between COVID-19 and acute myelopathies is not sufficient to prove causation and further data are warranted. To this effect, we encourage searching signs of spinal cord involvement in COVID-19 patients, whose incidence could have been underestimated but could play a major role in determining post-acute functional outcome.
Authors’ contribution
Isabella Canavero*: study conception and authors' coordination, clinical data collection, data interpretation, literature search, writing.
Francesca Valentino*: study conception and authors' coordination, clinical data collection, data interpretation, literature search, writing.
Elena Colombo: clinical data collection, data interpretation, literature search, writing.
Diego Franciotta: data collection, data analysis (CSF), data interpretation, literature search, writing.
Delfina Ferrandi: clinical data collection, data interpretation, literature search, writing.
Marco Mussa: clinical data collection, data interpretation, literature search, writing.
Rodolfo Schizzi: neuroimaging data collection and analysis, data interpretation, figures, literature search, writing.
Kalliopi Marinou: clinical data collection, data interpretation, literature search, writing.
Carla Zanferrari: clinical data collection, data interpretation, literature search, writing.
Pietro Businaro: clinical data collection, data interpretation, literature search, writing.
Sabrina Ravaglia: clinical and neurophysiological data interpretation, literature search, writing.
Paolo Prunetti: neurophysiological data collection and analysis, data interpretation, figures, literature search, writing.
Giuseppe Cosentino: neurophysiological data collection and analysis, data interpretation, literature search, writing.
Lisa Maria Farina: neuroimaging data collection and analysis, data interpretation, figures, literature search, writing.
Elisa Rognone: neuroimaging data collection and analysis, data interpretaton, figures, literature search, writing.
Anna Pichiecchio: study conception, neuroimaging data collection, analysis and interpretation, figures, literature search, writing, revision.
Giuseppe Micieli: study conception, clinical data collection, data interpretation, literature search, writing, revision.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The corresponding author had full access to all the data in the study and takes full and final responsibility for the decision to submit for publication.
Ethics and patient consent
All clinical procedures were performed in accordance with the national and international ethical standards. Written informed consent was obtained from all the patients.
Declaration of competing interest
None to declare.
Footnotes
Dedicated to the loving memory of Dr. Giovanni Canavero, Family Doctor and Infectious Disease Specialist, COVID-19 victim.
References
- 1.Natoli S., Oliveira V., Calabresi P., et al. Does SARS-Cov-2 invade the brain? Translational lessons from animal models. Eur J Neurol. 2020 doi: 10.1111/ene.14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tenembaum S., Chitnis T., Ness J., Hahn J.S., International pediatric MS study group. Acute disseminated encephalomyelitis. Neurology. 2007;68(16 Suppl 2):S23–S36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 3.Helms J., Kremer S., Merdji H., et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montalvo M., Cho T.A. Infectious Myelopathies. Neurol Clin. 2018;36(4):789–808. doi: 10.1016/j.ncl.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Reichard R.R., Kashani K.B., Boire N.A., et al. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020:1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]