Abstract
Background
Cardiac manifestations in multisystem inflammatory syndrome in children (MIS-C) occur in ∼80% of patients. Left ventricular (LV) systolic dysfunction is the most frequent cardiac finding.
Methods
In this single-centre, retrospective cohort study, we report on detailed assessment of LV function in MIS-C patients using strain and strain rate analysis. We compare those with normal peak systolic strain z-scores (both longitudinal and circumferential strain) to those with abnormal peak systolic strain z-scores (decreased circumferential and/or longitudinal strain).
Results
Among 25 patients, 14 (56%) were male, 20 (80%) were Black or Hispanic, 13 (52%) were overweight/obese, and the median age was 11.4 years (interquartile range: 7.5 to 16). Median ejection fraction (EF) was 55.2% (interquartile range: 48.3% to 58%), with the abnormal strain patients having a lower EF (P < 0.01). Demographics were similar between groups. The abnormal strain patients had more organ systems involved and were more likely to require inotropic support. In a comparison of MIS-C patients with normal EF (n = 15) to controls, MIS-C patients had lower peak systolic strain as well as lower early diastolic strain rates. In patients with initially depressed function, EF normalized in 8 of 10 (80%), but 4 of 11 (36%) patients had persistently abnormal systolic strain after discharge.
Conclusions
LV systolic dysfunction is common in the acute phase of MIS-C, and detection may be improved with strain imaging. Longitudinal cardiac follow-up is imperative, as some patients may be at risk for persistent LV dysfunction.
Résumé
Contexte
Des manifestations cardiaques sont observées chez environ 80 % des patients atteints du syndrome inflammatoire multisystémique de l'enfant (SIM-E). La dysfonction systolique ventriculaire gauche est le problème cardiaque observé le plus fréquemment.
Méthodologie
Dans cette étude de cohorte rétrospective et unicentrique, nous rapportons les résultats d'une évaluation détaillée de la fonction ventriculaire gauche chez des patients atteints du SIM-E sous l'angle de l'étude des contraintes et des taux de contrainte. Nous comparons les patients dont les écarts z des pics de contrainte systolique sont normaux (contraintes tant longitudinales que circonférentielles) et ceux dont les écarts z des pics de contrainte systolique sont anormaux (réduction de la contrainte circonférentielle ou longitudinale).
Résultats
Sur 25 patients, 14 (56 %) étaient de sexe masculin, 20 (80 %) étaient noirs ou hispaniques, 13 (52 %) étaient en surpoids ou obèses, et l'âge médian était de 11,4 ans (intervalle interquartile : de 7,5 à 16). La fraction d'éjection (FE) médiane était de 55,2 % (intervalle interquartile : de 48,3 % à 58 %), et était moins élevée chez les patients présentant une contrainte anormale (p < 0,01). Les caractéristiques démographiques étaient comparables dans tous les groupes. Les patients chez lesquels la contrainte était anormale présentaient un plus grand nombre d'organes atteints et étaient plus susceptibles de nécessiter un soutien inotrope. Comparativement au groupe témoin, les patients SIM-E ayant une FE normale (n = 15) présentaient un pic de contrainte systolique moins élevé et des taux de contrainte diastolique précoce plus faibles. Chez les patients dont la fonction était déprimée à l'origine, la FE s'est normalisée chez huit patients sur 10 (80 %), mais quatre sur 11 (36 %) présentaient une contrainte systolique persistant après leur sortie de l'hôpital.
Conclusions
La dysfonction systolique ventriculaire gauche est fréquente dans la phase aiguë du SIM-E, et son repérage pourrait être amélioré par l'imagerie permettant de visualiser les contraintes. Un suivi cardiaque longitudinal est impératif, car certains patients peuvent être à risque de souffrir d'une dysfonction ventriculaire gauche persistante.
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has resulted in a global pandemic, and although morbidity and mortality in adults with acute COVID-19 has been high, children mostly have been spared.1, 2, 3, 4, 5 Recently, however, following multiple case series, a newly recognized condition termed multisystem inflammatory syndrome in children (MIS-C) has been described, occurring 3-6 weeks after COVID-19 exposure, characterized by immune dysregulation, severe inflammation, cardiac dysfunction, and features of other inflammatory diseases including Kawasaki disease, toxic shock syndrome, and macrophage activation syndrome.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19
Involvement of the cardiovascular system in MIS-C is common, occurring in ∼80% of cases.11, 12, 13, 14,17 In the case series published to date, 38%-58% have presented with depressed left ventricular (LV) systolic function based on ejection fraction (EF), and a significant number have presented with cardiogenic shock.6,11, 12, 13,17, 18, 19, 20, 21 Notably, the mechanism of LV dysfunction is yet to be fully elucidated. The trajectory of recovery of systolic function and the response to anti-inflammatory medications are also not fully described, although initial reports suggest that most patients have normalization of systolic function in the initial 7-10 days after presentation.6,13,14,18, 19, 20, 21
Detailed analysis of LV function in MIS-C has been limited in this newly described condition. Two-dimensional speckle-tracking echocardiography, an emerging tool in pediatric cardiology, is an angle-independent method for assessing myocardial strain and deformation, and has been shown to reveal abnormalities in children with acute Kawasaki disease and acute myocarditis; it may be more sensitive in detection of ventricular dysfunction compared with conventional parameters such as LVEF.22, 23, 24, 25 To characterize cardiac manifestations in patients with MIS-C, we performed a detailed echocardiographic assessment of LV function. We hypothesized that (i) myocardial strain is a more sensitive indicator of LV dysfunction in MIS-C, and (ii) in MIS-C patients with preserved EF, myocardial function as measured by peak systolic longitudinal and circumferential strain would be abnormal.
Methods
Study population and case definition
We conducted a retrospective review of clinical data of patients diagnosed with MIS-C admitted to Boston Children’s Hospital from March 1, 2020 to June 31, 2020. The study was approved by the Boston Children’s Hospital Institutional Review Board, which waived the need for informed consent for this study.
Demographic, clinical, and laboratory data were obtained from review of the medical record. All patients met the Centers for Disease Control and Prevention (CDC) diagnostic criteria for MIS-C, which included any individual aged < 21 years with all the following: evidence of severe illness requiring hospitalization; fever (body temperature > 38 °C or subjective fever for > 24 hours); laboratory evidence of inflammation; multisystem (≥ 2) organ involvement; no alterative plausible diagnosis; and evidence of current or recent SARS-CoV-2 infection.5 Evidence of SARS-CoV-2 infection included any of the following: positive reverse transcription polymerase chain reaction, positive serology, and exposure to a suspected or confirmed COVID-19 case within the 4 weeks preceding onset of symptoms.5 A multidisciplinary institutional adjudication committee reviewed all cases of possible MIS-C for case reporting to the state. Patients were excluded if they had preexisting congenital heart disease or cardiomyopathy, if no echocardiogram was performed, or if an echocardiogram was performed at low temporal resolution.
Echocardiograms
All patients underwent transthoracic 2-dimensional (2D) and Doppler echocardiograms at the time of diagnosis using EPIQ 7 ultrasound (Philips Healthcare, Andover, MA). Follow-up echocardiograms were routinely performed at 7-10 days and 3-6 weeks postdiagnosis, with more frequent echocardiograms per clinician discretion. Functional and anatomic measurements apart from LVEF and strain measurements were collected from clinical reports produced at the time the study was performed, including LV end-diastolic volume (LV), LV mass:volume, coronary artery measurements, and qualitative grade of valvular regurgitation and pericardial effusion size. Z-scores indexed to body surface area were calculated. Measurements of LVEF were performed by a single individual (KGF) and compared with those from the clinical reports to assess for interobserver variability. An LVEF z-score of < 2 was considered abnormal.25 We defined and classified coronary artery abnormalities, according to American Heart Association guidelines, as follows: dilation (z-score = 2.0-2.49); small aneurysm (z-score = 2.5-4.9); moderate aneurysm (z-score = 5-9.9); and large/giant aneurysm (z-score ≥10).26
In addition to standard 2D and Doppler assessment, LV images were acquired in apical 4-chamber and/or short axis mid-papillary level in a Philips native data format (also known as the “acquisition frame rate”), providing high temporal resolution images suitable for speckle-tracking analysis. QLAB v.10.8 (Philips Healthcare, Andover, MA) was used to provide longitudinal (apical 4-chamber) and/or circumferential (short axis mid-papillary level) peak systolic strain, peak systolic strain rate, peak early diastolic strain rate, and peak late diastolic strain rate, based on endocardial layer speckle-tracking. All measurements were performed by a single individual (AMF) with over 3 years of experience with the strain software, using images obtained at the time of the clinical echocardiogram. The index echocardiogram was the first study following diagnosis in which native data images were acquired.
We divided patients into normal systolic strain and abnormal systolic strain groups based on peak systolic strain z-scores. Those with normal longitudinal and circumferential peak systolic strain (z-score >–2) were considered normal, and those with abnormal circumferential peak systolic strain (z-score <–2) and/or abnormal longitudinal peak systolic strain (z-score <–2) were considered abnormal.27 As there are no published pediatric normative data for systolic and diastolic strain rates, we performed 3:1 matching with healthy age-matched patients. These controls were matched by age within 1 year of the MIS-C patients, and they were outpatients with structurally normal hearts and no significant medical history who underwent screening echocardiograms for indications such as murmur, chest pain, syncope, or palpitations.
Statistical analysis
Demographic, clinical, laboratory, and echocardiographic data were summarized using medians and interquartile ranges (IQRs) for continuous variables, and frequencies and percentages for categorical variables. Comparisons across the MIS-C patient groups were performed using exact Kruskal-Wallis tests for continuous variables, and Fisher’s exact tests for categorical variables. Comparisons of echocardiographic findings between MIS-C and control patients were performed with the Wilcoxon rank sum test. Spearman rank correlation coefficients were used to identify relationships between lab values and LV systolic function. A P-value of <0.05 was considered statistically significant. Analyses were performed using SPSS v21.1 (IBM, Armonk, NY).
Results
Thirty patients were identified among whom 25 were included in the study. One patient was excluded because of previously diagnosed complex congenital heart disease, and 4 were excluded due to lack of sufficient echocardiographic imaging to perform strain measurements. Among the 25 included patients, the median age was 11.4 years (IQR: 7.5 to 16), and 14 (56%) were male (Table 1). Eighteen (72%) patients had no significant past medical history. The most common preexisting medical histories included asthma (n = 3) and previously diagnosed Kawasaki disease (n = 2). Thirteen (52%) patients were overweight or obese. Fifteen (60%) patients were Hispanic or Latino; 5 (20%) were Black, non-Hispanic; 3 (12%) were Asian; and 2 (8%) were White, non-Hispanic.
Table 1.
Demographic and clinical data and early treatment comparison for patients with normal vs abnormal LVEF; patients with normal longitudinal and circumferential peak systolic strain vs patients with abnormal circumferential peak systolic strain and/or longitudinal peak systolic strain
| All (n = 25) | Normal LVEF (n = 15) | Abnormal LVEF (n = 10) | P | Normal strain (n = 13) | Abnormal strain (n = 12) | P | |
|---|---|---|---|---|---|---|---|
| Age (years) | 11.4 [7.5,1 6] | 12 [10, 16.4] | 8.2 [2.2, 14] | 0.09 | 13.3 [8, 17] | 10.4 [5.8, 13] | 0.19 |
| Male Sex | 14 (56) | 8 (53) | 6 (60) | 0.74 | 7 (54) | 7 (58) | 0.57 |
| Ethnicity | 0.67 | 0.35 | |||||
| White, Non-Hispanic | 2 (8) | 1 (7) | 1 (10) | 1 (8) | 1 (8) | ||
| Hispanic or Latino | 15 (60) | 10 (67) | 5 (50) | 7 (54) | 8 (67) | ||
| Black | 5 (20) | 1 (7) | 4 (40) | 2 (15) | 3 (25) | ||
| Asian | 3 (12) | 3 (20) | 0 (0) | 3 (23) | 0 (0) | ||
| BMI (kg/m2) | 22.3 [17.5, 28] | 22 [17.5, 28.7] | 22.2 [20.2, 26] | 0.97 | 22.3 [17.5, 27] | 22.7 [17.2, 30] | 0.63 |
| BMI (%ile) | 87 [31, 98] | 87 [25, 97.9] | 71.3 [43.4, 96.8] | 0.89 | 63 [31, 92] | 89.2 [35.4, 99] | 0.50 |
| Overweight/obese | 13 (52) | 8 (53) | 5 (50) | 0.87 | 6 (46) | 7 (58) | 0.42 |
| Pre-existing condition | 7 (28) | 4 (27) | 3 (30) | 0.86 | 3 (23) | 4 (33) | 0.45 |
| Total days of fever | 6 [4.5, 8] | 6 [4.3, 6] | 7 [5, 8] | 0.40 | 6 [4.5, 6] | 7 [5, 8] | 0.49 |
| Organ system involvement | |||||||
| Hypotension/shock | 10 (40) | 4 (27) | 6 (60) | 0.10 | 3 (23) | 7 (58) | 0.07 |
| Cardiac | 17 (68) | 9 (60) | 8 (80) | 0.29 | 8 (62) | 9 (75) | 0.47 |
| Renal | 1 (4) | 1 (7) | 0 (0) | -- | 1 (8) | 0 (0) | 0.33 |
| Respiratory | 9 (36) | 4 (27) | 5 (50) | 0.23 | 3 (23) | 6 (50) | 0.16 |
| Hematologic | 20 (80) | 11 (73) | 9 (90) | 0.31 | 9 (69) | 11 (92) | 0.19 |
| Gastrointestinal | 17 (68) | 11 (73) | 6 (60) | 0.48 | 7 (54) | 10 (83) | 0.20 |
| Dermatologic | 13 (52) | 7 (47) | 6 (60) | 0.51 | 5 (38) | 8 (67) | 0.24 |
| Neurologic | 1 (4) | 0 (0) | 1 (10) | -- | 0 (0) | 1 (8) | 0.29 |
| Total systems | 3 [3, 4] | 3 [2, 4] | 4 [3, 5] | 0.06 | 3 [2, 3] | 4 [3, 4] | < 0.01 |
| COVID testing & diagnosis | |||||||
| PCR+ | 12 (48) | 6 (40) | 6 (60) | 0.32 | 7 (54) | 6 (50) | 0.58 |
| Serology+ | 14 (56) | 9 (60) | 5 (50) | 0.62 | 6 (46) | 8 (67) | 0.43 |
| COVID+ exposure | 4 (16) | 2 (13) | 2 (20) | 0.66 | 2 (15) | 2 (17) | 0.93 |
| Length of hospitalization | 7 [3, 12] | 5 [3, 13] | 9 [6, 10] | 0.41 | 5 [2, 12] | 8 [6, 11] | 0.18 |
| ICU admission | 12 (48) | 5 (33) | 7 (70) | 0.07 | 4 (31) | 8 (67) | 0.73 |
| Inotropic support | 6 (24) | 1 (7) | 5 (50) | 0.01 | 1 (8) | 5 (42) | 0.05 |
| Positive pressure ventilation | 5 (20) | 2 (13) | 3 (30) | 0.31 | 1 (8) | 4 (33) | 0.16 |
| Intubation | 1 (4) | 0 (0) | 1 (10) | -- | 0 (0) | 1 (8) | 0.29 |
| AV block on telemetry | 4 (16) | 1 (7) | 3 (30) | 0.12 | 1 (8) | 3 (25) | 0.32 |
| Laboratories | |||||||
| Troponin T (ng/mL) | 0.01 [0.01, 0.04] | 0.01 [0.01, 0.01] | 0.06 [0.03, 0.14] | 0.01 | 0.01 [0.01, 0.03] | 1.1 [0.01, 0.06] | 0.79 |
| BNP (pg/mL) | 101 [10, 346] | 10 [10, 118] | 395 [105, 786] | 0.01 | 88.5 [10,112] | 297 [78, 730] | 0.04 |
| WBC (cells/μL) | 9.9 [7, 13] | 8 [6.3, 11.3] | 10.1 [9.2, 13.9] | 0.17 | 9.8 [7.4, 11] | 10.1 [6.9, 14.9] | 0.57 |
| Hemoglobin (g/dL) | 11.5 [10.4, 12] | 12 [11.5, 12.3] | 11.1 [8.9, 11.9] | 0.22 | 12.3 [11.4, 14] | 11.4 [9.4, 11.8] | 0.07 |
| Laboratories | |||||||
| Platelets (cells/μL) | 189 [130, 246] | 194 [129, 271] | 165 [136, 203] | 0.70 | 220 [140, 293] | 155 [129, 200] | 0.32 |
| ANC (cells/μL) | 7.1 [5.3, 9] | 6.5 [4.9, 9.2] | 8.4 [6.1, 11.3] | 0.29 | 6.5 [5.3, 9] | 8.4 [5.5, 12] | 0.25 |
| ALC (cells/μL) | 1 [0.63, 2] | 0.8 [0.7, 1.5] | 1.2 [0.7, 1.8] | 0.58 | 1.2 [0.7, 2] | 0.9 [0.6, 1.3] | 0.25 |
| ESR (mm/hr) | 45 [25, 72] | 50 [26, 58] | 45 [28, 81] | 0.75 | 56 [18, 59] | 44 [30, 82] | 0.83 |
| CRP (mg/mL) | 13.1 [8.8, 18] | 13.1 [9.1, 16.7] | 14.8 [6.1, 24.9] | 0.78 | 13 [5.2, 16] | 16.9 [9.3, 24] | 0.21 |
| Procalcitonin (ng/mL) | 1.2 [0.4, 9] | 0.7 [0.2, 1.8] | 10.8 [6.4, 17.9] | 0.01 | 0.7 [0.1, 2] | 3.7 [1.1, 12.3] | 0.05 |
| Interleukin 6 (pg/mL) | 9.5 [2.9, 19] | 3.5 [2, 5.8] | 32.7 [14.8, 57] | 0.01 | 4.7 [2, 5] | 12.6 [8, 44] | 0.08 |
| D-dimer (μg/mL) | 2.7 [1.5, 3] | 2.4 [1.4, 3.3] | 2.8 [1.9, 3.3] | 0.64 | 1.7 [1.3, 3] | 3.1 [2.3, 5] | 0.08 |
| Ferritin (ng/mL) | 537 [240, 1475] | 537 [239, 1473] | 708 [247, 1410] | 0.87 | 345 [236, 1471] | 1094 [259, 1630] | 0.47 |
| Albumin (g/dL) | 3.6 [3, 4] | 3.8 [3.6, 4] | 3.1 [2.8, 3.3] | 0.01 | 3.7 [3.6, 4] | 3.1 [2.8, 3.6] | 0.06 |
| Creatinine (mg/dL) | 1.5 [0.4, 1] | 0.5 [0.4, 0.7] | 0.5 [0.4, 0.8] | 0.96 | 0.5 [0.4, 1] | 0.6 [0.4, 0.9] | 0.94 |
| ALT (unit/L) | 40 [23, 61] | 41 [23, 60] | 31 [24, 62] | 0.98 | 37 [21, 42] | 49 [26, 61] | 0.50 |
| Treatment | |||||||
| IVIg | 19 (76%) | 10 (67%) | 9 (90%) | 0.18 | 7 (54%) | 12 (100%) | < 0.01 |
| Steroids | 15 (60%) | 8 (53%) | 7 (70%) | 0.40 | 5 (38%) | 10 (83%) | 0.02 |
| Anakinra | 5 (20%) | 3 (20%) | 2 (20%) | 0.99 | 3 (23%) | 2 (17%) | 0.69 |
| Remdesivir | 7 (28%) | 3 (20%) | 4 (40%) | 0.28 | 3 (23%) | 4 (33%) | 0.57 |
| Aspirin | 16 (64%) | 8 (53%) | 8 (80%) | 0.17 | 7 (54%) | 9 (75%) | 0.27 |
| Enoxaparin | 16 (64%) | 9 (60%) | 7 (70%) | 0.61 | 7 (54%) | 9 (75%) | 0.27 |
| Apixaban | 10 (40%) | 5 (33%) | 5 (50%) | 0.40 | 4 (31%) | 6 (50%) | 0.33 |
Values are number (percent) or median [interquartile range]. Bold values indicate P ≤ 0.05.
ALC, absolute lymphocyte count; ALT, alanine transaminase; ANC, absolute neutrophil count; AV, atrioventricular; BMI, body mass index; BNP, B-type natriuretic peptide; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ICU, intensive care unit; IVIg, intravaneous immunoglobulin; LVEF, left ventricular ejection fraction; PCR, polymerase chain reaction; WBC, white blood cell count.
Of the 25 patients, 13 (52%) had normal longitudinal and circumferential peak systolic strain, and 12 (48%) had abnormalities in circumferential and/or longitudinal peak systolic strain. Among the patients with abnormal strain, 11 of 12 had abnormal peak circumferential strain, with 5 concurrently demonstrating abnormal peak longitudinal systolic strain. Only 1 patient had depressed peak longitudinal systolic strain with normal peak circumferential systolic strain. In total, 10 patients (40%) demonstrated depressed LVEF. Among the 15 patients with normal initial EF, 27% had depressed peak systolic circumferential strain. Comparison of the demographic, clinical, and laboratory data for the MIS-C groups is shown in Table 1. Demographics were similar between groups. MIS-C patients with abnormal systolic strain had higher brain natriuretic peptide (BNP) and procalcitonin levels compared to those of patients with normal strain values. There was no significant difference in troponin level between the 2 groups.
Across all patients, there was a median of 3 organ systems involved at presentation. Patients with abnormal strain had a higher number of organ systems involved (4) compared to those with normal peak systolic strain (3) and were more likely to require inotropic support. In total, 12 (48%) patients required admission to the intensive care unit (ICU), 5 (20%) required positive pressure ventilation, and 1 (4%) was intubated. Patients with both depressed peak longitudinal and circumferential strain (n = 5) demonstrated higher illness severity as indicated by hypotension/shock on presentation (P = 0.02) and need for ICU admission (P = 0.02), inotropic support (P < 0.01), and positive pressure ventilation (P = 0.04). No patients were placed on extracorporeal membrane oxygenation, and there were no deaths. Treatments are summarized in Table 1.
All patients underwent at least one echocardiogram during the hospital admission (median 2; IQR: 2 to 3). An index echocardiogram was performed a median of 2 days after admission (IQR: 1 to 4). Compared to age-matched controls, MIS-C patients universally had lower systolic and diastolic strain and strain rate (Table 2). Furthermore, when comparing MIS-C patients with normal initial EF (n = 15) to controls, we found evidence of impaired systolic and diastolic function, with MIS-C patients having lower peak circumferential systolic strain (–24.1 [–20.1, –32.7] vs –30.6 [–27.3, –33.4], P < 0.01) and lower peak circumferential early diastolic strain rate (4.1 [1.8, 5.3] vs 4.7 [4.1, 5.5], P = 0.029). Among the 5 patients with normal EF requiring ICU admission, 2 of 5 (40%) had abnormal peak systolic circumferential strain.
Table 2.
Ventricular strain and strain rate in patients with multisystem inflammatory syndrome in children, compared to controls
| Strain/strain rate | Cases | Controls (n = 90) | P |
|---|---|---|---|
| Circumferential | n = 22 | ||
| Peak systolic strain (%) | –23.2 [–20.1, –26.2] | –30.6 [–27.3, –33.4] | < 0.01 |
| Peak systolic strain rate | –2.9 [–2.2, –3.3] | –3.2 [2.9, –3.6] | < 0.01 |
| Peak early diastolic strain rate | 4.1 [3.3, 4.8] | 4.7 [4.1, 5.5] | < 0.01 |
| Peak late diastolic strain rate | 1 [0.9, 1.6] | 1.3 [1, 2] | < 0.01 |
| Longitudinal | n = 24 | ||
| Peak systolic strain (%) | –19 [–17.2, –22.2] | –21 [–19.2, –23.3] | < 0.01 |
| Peak systolic strain rate | –2.2 [–1.7, –2.5] | –2.1 [–2, –2.5] | < 0.01 |
| Peak early diastolic strain rate | 3.2 [2.5, 4.9] | 3.8 [3.2, 4.5] | < 0.01 |
| Peak late diastolic strain rate | 1.1 [0.4, –1.6] | 1.4 [1.1, 1.6] | < 0.01 |
Values are median [interquartile range]. Rates are per second.
Echocardiographic findings in the MIS-C groups are summarized in Table 3. Median EF at presentation was 55.2% (IQR: 48.3 to 58; Table 3). Ten (40%) patients had an EF of <55% on index echocardiogram. Only 2 patients had severe LV systolic dysfunction (EF <40%). Notably, one patient with normal circumferential and longitudinal strain had an EF of <55%. Seven of the 12 (58.3%) patients admitted to the ICU had an EF of <55% on index echocardiogram. Among those with an EF of <55%, ventricular dysfunction was initially identified at a median of 5 days after onset of symptoms (IQR: 2.5 to 8). Median EF in the normal systolic strain group was 57.2%, compared to a median EF of 48.3% in the abnormal systolic strain group. LV end-diastolic volume, coronary artery z-scores, presence of valvular dysfunction, and pericardial effusion were similar between groups. There was not a significant difference in longitudinal peak systolic strain between the 2 groups. There was a trend toward lower circumferential early diastolic strain rate between groups that did not meet statistical significance. The interobserver variability coefficient for LVEF was 0.96.
Table 3.
Echocardiographic data
| Data | All (n = 25) | Normal peak systolic strain (n = 13) | Abnormal peak systolic strain (n = 12) | P |
|---|---|---|---|---|
| Basic echo data | ||||
| LVEF (%) | 55.2 [48.3, 58] | 57.2 [55.2, 59.5] | 48.3 [44.8, 58] | 0.04 |
| LVEF < 55% | 9 (36) | 1 (8) | 8 (67) | < 0.01 |
| LV EDV (mL) | 101 [77, 121] | 105 [84, 150] | 96 [67, 128] | 0.69 |
| LV EDV (z-score) | 0.2 [–0.6, 1.5] | –0.3 [–0.8, 1.4] | 0.3 [–0.5, 1] | 0.83 |
| LV mass:vol (g/mL) | 0.8 [0.7, 0.9] | 0.8 [0.7, 0.9] | 0.8 [0.7, 0.9] | 0.43 |
| LV mass:vol (z-score) | –0.5 [–1.5, 0.1] | –0.4 [–1.8, 0.1] | –0.6 [0.3, 1] | 0.53 |
| LMCA (z-score) | 0.3 [–0.1, 1] | 0.02 [–0.6, 0.3] | 0.6 [0.3, 1.2] | 0.02 |
| Proximal RCA (z-score) | 0.7 [–0.4, 1.3] | 0.4 [–0.3, 1.4] | 0.9 [–0.4, 1.3] | 0.75 |
| Proximal LAD (z-score) | 0.2 [–0.6, 0.8] | –0.5 [–1.1, 0.7] | 0.6 [0.2, 0.8] | 0.35 |
| CA z-score 2-2.5 | 2 (8) | 1 (8) | 1 (8) | 0.95 |
| CA z-score >2.5 | 4 (16) | 2 (15) | 2 (17) | 0.93 |
| Valve regurgitation > mild | 1 (4) | 0 (0) | 1 (8) | 0.29 |
| Pericardial effusion | 0 (0) | 0 (0) | 0 0) | 1.0 |
| Strain and strain rate | ||||
| Circumferential | ||||
| Peak systolic (%) | –23.2 [–20.1, –26.2] | –26.7 [–24.2, –28.5] | –20.2 [–23.1, –16.9] | < 0.01 |
| Systolic rate | –2.9 [–2.2, –3.3] | –3.3 [–2.7, –3.6] | –2.4 [–3, –2] | 0.04 |
| Early diastolic rate | 4.1 [3.3, 4.8] | 4.8 [4, 4.8] | 3.7 [2.8, 4.2] | 0.07 |
| Late diastolic rate | 1 [0.9, 1.6] | 1 [1, 1.6] | 1 [0.5, 1.5] | 0.54 |
| Longitudinal | ||||
| Peak systolic (%) | –19 [–17.2, –22.2] | –18.6 [–22.1, –18] | –19.4 [–21.7, –13.5] | 0.36 |
| Systolic rate | –2.2 [–2.5, –1.8] | –2.3 [–1.9, 2.6] | –2 [–2.3, –1.3] | 0.06 |
| Early diastolic rate | 3.2 [2.5, 4.9] | 4 [2.6, 5] | 3.1 [2.2, 4] | 0.23 |
| Late diastolic rate | 1.1 [0.8, 1.7] | 1 [0.8, 1.4] | 1.1 [0.9, 1.8] | 0.54 |
Values are number (percentage) or median [interquartile range]. Rates are per second. Bold values indicate P ≤ 0.05.
CA, coronary artery; EDV, end-diastolic volume; LAD, left anterior descending artery; LMCA, left main coronary artery; LV, left ventricular; LVEF, left ventricular ejection fraction; RCA, right coronary artery; Vol, volume.
LVEF normalized to ≥ 55% in 7 of 9 (78%) patients who had a depressed EF on index echocardiogram, which occurred at a median of 6 days postdiagnosis (IQR: 3 to 8 days). Two patients had a mildly depressed EF at most recent follow-up (55 and 47 days postdischarge) among the 23 patients who had echocardiograms after discharge. However, among the 11 patients with available strain measurements following discharge, 4 of 11 (36%) had abnormal peak systolic strain (2 abnormal longitudinal, 1 abnormal circumferential, 1 both longitudinal and circumferential) at latest follow-up (median 8 days postdischarge).
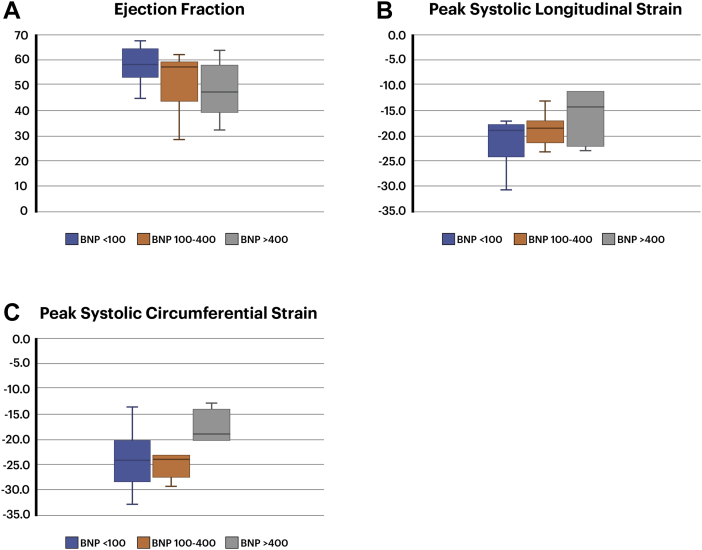
At presentation, 9 of 23 (39%) patients had elevated troponin levels. Using previously published BNP-level cutoff points, 13 of 23 (57%) had a mildly elevated BNP level— between 100 and 400—and 6 of 23 (26%) had a significantly elevated BNP level of > 400.28 A BNP level of > 400 was associated with significantly lower peak systolic circumferential and longitudinal strain, and to a lesser extent, with a lower EF compared to patients with a BNP level of 100-400 or < 100 (Fig. 1). Initial BNP levels were moderately correlated with EF (correlation coefficient = 0.52, P = 0.015), peak longitudinal systolic strain (r = 0.50, P = 0.022), and peak circumferential systolic strain (r = 0.54, P = 0.015).
Figure 1.
Box plots depicting (A) ejection fraction, (B) peak systolic longitudinal strain, and (C) peak systolic circumferential strain in 3 groups: patients with brain natriuretic peptide (BNP) level < 100, patients with BNP level of 100-400, and patients with BNP level > 400.
Discussion
There is increasing recognition of the frequency and severity of cardiac involvement in both COVID-19 and MIS-C. In MIS-C, although a broad range of organ inflammation has been described, cardiac involvement is common and plays a large role in both acute management and prognosis.5, 6, 7, 8, 9, 10, 11, 12, 13, 14 The most common cardiac manifestation of MIS-C is depressed myocardial function.6,11, 12, 13,17, 18, 19, 20, 21 In this single-centre cohort study, impaired LV systolic function was common, with 40% of patients having a depressed EF, and 48% demonstrating a decrease in at least one component of systolic strain. Notably, even among MIS-C patients with a normal EF, strain assessment showed evidence of impaired LV systolic and diastolic function compared with that of healthy controls. We also found that LV dysfunction persists in some patients beyond the acute phase.
Strain imaging may allow for improved recognition of MIS-C patients with myocardial involvement. Most published MIS-C case series have assessed for myocardial function with LVEF alone and have reported depressed EF in 38%-58% of patients.6,11, 12, 13,17, 18, 19, 20, 21 We found that an additional 8% of patients were identified as having impaired LV systolic function, using strain measurements vs EF alone. Among MIS-C patients with normal initial EF, 27% of patients had depressed peak circumferential systolic strain, and in the setting of the high incidence of elevated troponin and BNP levels, the percentage of patients with myocardial involvement in MIS-C may be higher than what has been reported using EF alone. This finding is consistent with results of prior studies of Kawasaki disease, wherein peak strain and strain rates have been noted to be abnormal despite normal EF.22,23
In our population, preserved longitudinal and diminished circumferential systolic strain was noted in multiple patients, which is a pattern distinct from many other disease processes and forms of myocarditis and has not been noted in other studies of children with MIS-C.18,22, 23, 24, 25,28, 29, 30, 31, 32, 33, 34, 35, 36 The underlying myocardial mechanism for this finding is unclear. Although limited, magnetic resonance imaging data in children with MIS-C have demonstrated both diffuse and sub-epicardial myocardial edema patterns.19,21 Although subendocardial myocardial damage in ischemic heart disease has been associated with early reduction in longitudinal strain, it is possible that the unique inflammatory milieu and pattern of myocardial damage result in greater reduction in circumferential shortening, as approximately 60% of circumferentially oriented myofibers are located in the mid-muscular layer.30,34 This possibility highlights the importance of further evaluation of the etiology of myocardial dysfunction in this new disease process and of the potential utility of acquiring both longitudinal and circumferential strain in this population.
Underscoring the importance of cardiac dysfunction in MIS-C, we demonstrate that the presence and degree of abnormality in peak systolic longitudinal and circumferential strain are strongly associated with indices of severity of illness including extent of organ system involvement, presence of hypotension/shock, need for ICU admission, need for inotropic support, and need for positive pressure ventilation.
The few MIS-C case series to date with longitudinal LVEF measurements have reported rapid improvement in EF, with normalization in virtually all patients within a range of days to a few weeks.6,13,14,17 We also found that the majority of patients have prompt improvement in LV systolic function. But a concerning finding, based on limited follow-up data, is that at >1 month from discharge, 2 patients had persistent LV dysfunction as measured by EF, and 4 of 11 patients with available strain imaging had continued evidence of abnormal peak systolic strain. These findings emphasize that MIS-C patients require longitudinal cardiac follow-up, as the natural history of myocardial involvement is not fully understood and some patients have persistent myocardial abnormalities. As return-to-play guidelines are formulated and assessed in this population, serial strain measurements may be helpful in identifying patients with ongoing myocardial disease.
Although there is minimal histologic data describing the etiology of myocardial dysfunction in MIS-C, myocardial inflammation related to the systemic inflammatory milieu is the proposed mechanism of cardiac dysfunction. In other forms of myocarditis, activation of the host viral immune response and infiltration of natural killer cells, macrophages, and virus-specific T cells result in damage to myocardial cells, which in turn affects both systolic and diastolic function.37
Further understanding of the natural history of cardiac dysfunction in MIS-C will require comprehensive assessment of LV systolic and diastolic function in a large, multi-institution cohort followed longitudinally. Cardiac magnetic resonance imaging, cardiac catheterization with endomyocardial biopsy, and examination of postmortem pathology may also help in elucidating the underlying pathology and mechanisms of myocardial involvement in MIS-C, potentially inform prognosis, and help in tailoring therapies in the future. Furthermore, continued evaluation of the impact of strain imaging in exploring subtle ventricular function abnormalities in patients with MIS-C and preserved EF is warranted.
This is a single-centre study with multiple limitations including its retrospective design, small sample size, and limited follow-up period. Further, the majority of the echocardiograms used in this study were obtained under rigorous precautions against infection, with limited imaging performed in order to minimize exposure and contact. This approach precluded further ventricular assessment with other parameters including mitral inflow and tissue Doppler as well as other aspects of global strain on a routine basis. Patients with imaging inadequate for strain analysis were excluded from the study. The MIS-C cohort has differences in baseline characteristics (eg, ethnicity and weight percentile), compared to the cohort used as controls, that have mild effects on strain parameters.
Conclusions
LV systolic dysfunction is common in the acute phase of MIS-C, and detection may be improved with use of strain imaging. Longitudinal cardiac follow-up is imperative, as the natural history of LV dysfunction in MIS-C is uncertain and patients may be at risk for persistent ventricular dysfunction beyond the acute phase.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The study was approved by the Boston Children’s Hospital Institutional Review Board, which waived the need for informed consent for this study.
See page 886 for disclosure information.
References
- 1.Goyal P., Choi J.J., Pinheiro L.C., et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong Y., Mo X., Hu Y., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman P., Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39:355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19) https://www.cdc.gov/mis-c/hcp/ Available at:
- 6.Belhadjer Z., Méot M., Bajolle F., et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 7.Chiotos K., Bassiri H., Behrens E.M., et al. Multisystem inflammatory syndrome in children during the COVID-19 pandemic: a case series. J Pediatric Infect Dis Soc. 2020;9:393–398. doi: 10.1093/jpids/piaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riphagen S., Gomez X., Gonzalez-Martinez C., et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verdoni L., Mazza A., Gervasoni A., et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toubiana J., Poirault C., Corsia A., et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dufort E.M., Koumans E.H., Chow E.J., et al. Multisystem inflammatory syndrome in children in New York State. N Engl Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldstein L.R., Rose E.B., Horwitz S.M., et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capone C.A., Subramony A., Sweberg T., et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory disease of childhood (MIS-C) associated with SARS-CoV-2 infection. J Pediatr. 2020;224:141–145. doi: 10.1016/j.jpeds.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimaud M., Starck J., Levy M., et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10:69. doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin C., Zhou L., Hu Z., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whittaker E., Bamford A., Kenny J., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsubara D., Kauffman H.L., Wang Y., et al. Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID-19 in the United States. J Am Coll Cardiol. 2020;76:1947–1961. doi: 10.1016/j.jacc.2020.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theocharis P., Wong J., Pushparajah K., et al. Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur Heart J Cardiovasc Imaging. 2020 doi: 10.1093/ehjci/jeaa212. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaitonde M., Ziebell D., Kelleman M.S., et al. COVID-19-related multisystem inflammatory syndrome in children affects left ventricular function and global strain compared with Kawasaki disease. J Am Soc Echocardiogr. 2020;33:1285–1287. doi: 10.1016/j.echo.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valverde I., Singh Y., Sanchez-de-Toledo J., et al. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation. 2021;143:21–32. doi: 10.1161/CIRCULATIONAHA.120.050065. [DOI] [PubMed] [Google Scholar]
- 22.Xu Q.Q., Ding Y.Y., Lv H.T., et al. Evaluation of left ventricular systolic strain in children with Kawasaki disease. Pediatr Cardiol. 2014;35:1191–1197. doi: 10.1007/s00246-014-0915-5. [DOI] [PubMed] [Google Scholar]
- 23.McCandless R.T., Minich L.L., Wilkinson S.E., et al. Myocardial strain and strain rate in Kawasaki disease. Eur Heart J Cardiovasc Imaging. 2013;14:1061–1068. doi: 10.1093/ehjci/jet041. [DOI] [PubMed] [Google Scholar]
- 24.Kostakou P.M., Kostopoulos V.S., Tryfou E.S., et al. Subclinical left ventricular dysfunction and correlation with regional strain analysis in myocarditis with normal ejection fraction. A new diagnostic criterion. Int J Cardiol. 2018;259:116–121. doi: 10.1016/j.ijcard.2018.01.058. [DOI] [PubMed] [Google Scholar]
- 25.Colan S.D. In: Echocardiography in Pediatric and Congenital Heart Disease. Lai W.W., Cohen M.S., Geva T., Mertens L., editors. Wiley-Blackwell; West Sussex, UK: 2009. Normal echocardiographic values for cardiovascular structures. [Appendix 1] 765-785. [Google Scholar]
- 26.Gursu H.A., Cetin, Azak E., et al. The assessment of treatment outcomes in patients with acute viral myocarditis by speckle tracking and tissue Doppler methods. Echocardiography. 2019;36:1666–1674. doi: 10.1111/echo.14449. [DOI] [PubMed] [Google Scholar]
- 27.McCrindle B.W., Rowley A.H., Newburger J.W., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 28.Adar A., Ghelani S.J., Sleeper L.A., et al. Normal values for left ventricular strain and synchrony in children based on speckle tracking echocardiography. Am J Cardiol. 2019;123:1546–1554. doi: 10.1016/j.amjcard.2019.01.044. [DOI] [PubMed] [Google Scholar]
- 29.Gaggin H.K., Januzzi J.L. Cardiac biomarkers and heart failure. https://www.acc.org/latest-in-cardiology/articles/2015/02/09/13/00/cardiac-biomarkers-and-heart-failure Available at: Accessed January 30, 2021.
- 30.Farzaneh-Far A., Romano S. Imaging and impact of myocardial strain in myocarditis. JACC Cardiovasc Imaging. 2020;13:1902–1905. doi: 10.1016/j.jcmg.2020.05.028. [DOI] [PubMed] [Google Scholar]
- 31.Weigand J., Nielsen J.C., Sengupta P.P., et al. Feature tracking-derived peak systolic strain compared to late gadolinium enhancement in troponin-positive myocarditis: a case-control study. Pediatr Cardiol. 2016;37:696–703. doi: 10.1007/s00246-015-1333-z. [DOI] [PubMed] [Google Scholar]
- 32.Thavendiranathan P., Poulin F., Lim K.D., et al. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751–2768. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 33.Voigt J.U., Cvijic M. 2- and 3-dimensional myocardial strain in cardiac health and disease. JACC Cardiovasc Imaging. 2019;12:1849–1863. doi: 10.1016/j.jcmg.2019.01.044. [DOI] [PubMed] [Google Scholar]
- 34.Potter E., Marwick T.H. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging. 2018;11:260–274. doi: 10.1016/j.jcmg.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Sengeløv M., Jørgensen P.G., Jensen J.S., et al. Global longitudinal strain is a superior predictor of all-cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging. 2015;8:1351–1359. doi: 10.1016/j.jcmg.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Wisotzkey B.L., Soriano B.D., Albers E.L., et al. Diagnostic role of strain imaging in atypical myocarditis by echocardiography and cardiac MRI. Pediatr Radiol. 2018;48:835–842. doi: 10.1007/s00247-017-4061-0. [DOI] [PubMed] [Google Scholar]
- 37.Fung G., Honglin L., Qiu Y., et al. Myocarditis. Circ Res. 2016;118:496–514. doi: 10.1161/CIRCRESAHA.115.306573. [DOI] [PubMed] [Google Scholar]