Abstract
The most investigated conducting polymer (CP) is polyaniline (PANI)), a promising polymer due to its excellent environmental stability, simplicity of synthesis, and high electrical conductivity [1], [2], [3], [4]. In corrosion protection applications, the PANI film has shown promising potential in protecting active metals such as iron by acting as physical barrier coatings, as a primer layer and as component in a multi-layer coating system [5]. The PANI has an excellent potential to replace the toxic metal, such as chromates, in corrosion protection and is considered a green anti-corrosion candidate [5], [6], [7]. The electrochemical synthesis of PANI coatings on active metals is accomplished by the dissolution of the metal at a potential lower than the monomer oxidation potential [8], [9]. Therefore, electrochemical synthesis of PANI coatings on active metal requires a proper choice of the electrolyte and solvent that should strongly passivate the metal without hindering the electropolymerization process [10], [11]. The data reported here are obtained while the anodic polarization of mild steel (MS) is carried out in succinic acid, sulphanilic acid, sodium orthophosphate, sodium potassium tartrate (Na-K tartrate), and benzoic acid in 3:1 alcohol-water (BAW) solutions [11]. However, the results of electrolytes sodium-potassium tartrate (Na-K tartrate) and benzoic acid in alcohol-water (BAW) are reported for the polymerization of aniline onto MS [11]. The SEM image of MS sample polarized in 0.3 M oxalic acid solution and 0.1 M aniline in 0.3 M oxalic acid is reported as a dataset or a supplementary material of the main manuscript ‘The Effect of Electrolytes on the Coating of Polyaniline on Mild Steel by Electrochemical Methods and Its Corrosion Behaviour [11].’
Keywords: Electropolymerization, Mild Steel, Benzoic acid, Sodium-potassium tartrate, Polyaniline
Specifications Table
| Subject | Electrochemistry |
| Specific subject area | Conducting polymer |
| Type of data | Graphs and Images |
| How data were acquired | Polarization curve using a Hokuto Denko HA-151 potentiostat controlled by a self-made LabVIEW software interfaced with an IBM computer [12] and SEM image by a JEM-1200EX electron microscope. |
| Data format | Raw Analyzed |
| Parameters for data collection | Open circuit potential (OCP) was recorded for 30 min at an interval of 2 min using a 3-electrode setup. Anodic polarization was then carried out in electrolytes from OCP to 2 V with a 1 mV/sec scan rate, and SEM image of MS surface after polarization was acquired at 10 kV at a working distance of 10.3 mm. |
| Description of data collection | Polarization was done in a 3-electrode system using MS specimen as a working electrode, a saturated calomel electrode (SCE) as a reference electrode, and a graphite rod as a counter electrode. OCP was recorded for 30 min before each anodic polarization to get a steady-state condition. Prior to electrochemical measurements, the MS surface was abraded with SiC paper till #1200 grits and ultrasonicated in ethanol, rinsed with distilled water and dried with air stream. SEM image was taken after PANI formation on the MS surface. |
| Data source location | Central Department of Chemistry, Tribhuvan University, Kirtipur, Nepal and CSIR- Central Salt and Marine Chemical Research Institute (CSMCRI), Bhavnagar, Gujarat, India |
| Data accessibility | With Article |
| Related research article | Dipak Kumar Gupta, Shova Neupane, Sanjay Singh, Nabin Karki, Amar Prasad Yadav, The effect of electrolytes on the coating of polyaniline on mild steel by electrochemical methods and its corrosion behavior, Progress in organic coating, 152 (2021) 106,127. https://doi.org/10.1016/j.porgcoat.2020.106127 |
Value of the Data
-
•
The polyaniline coating is extensively used to protect metals and alloys as a single layer coating, multi-layer coating, or primer. Therefore, the presented data provide valuable input for industries working on corrosion protection coating of PANI on active metals used in various environments.
-
•
The acquired data reveals that succinic acid, sulphanilic acid, and sodium orthophosphate only passivate the MS surface without electropolymerization of aniline. On the other hand, Na-K tartrate and BAW both passivated the MS surface and help in the electropolymerization of aniline to polyaniline onto the MS surface and act as a corrosion inhibitor.
-
•
Electropolymerization of aniline in BAW results in suppressing iron dissolution so that low contamination of electrolyte occurs.
1. Data Description
The shared data were recorded to select proper electrolytes that cause passivation of mild steel (MS) and subsequently electropolymerization of aniline using a Hokuto Denko HA-151 potentiostat controlled by a self-made LabVIEW software interfaced with an IBM computer [12]. Open circuit potential (OCP) of MS was recorded as a function of time in various electrolytes, and anodic polarization was recorded as a function of the electrolyte composition. The MS passivated surface morphology in oxalic acid and PANI film formed on MS was ascertained by SEM using a JEM-1200EX electron microscope (JEOL, Tokyo, Japan).
Fig. 1 depicts the variation of OCP of MS in different electrolytes. A shift of OCP to positive value indicates faster passivation of MS surface by forming compound with iron, e.g., sodium orthophosphate. In the case of a negative shift of OCP, the dissolution of iron occurs, followed by passivation of MS due to the formation of iron-salt, e.g., sodium-potassium tartrate. In succinic acid, sulphanilic acid, and sodium orthophosphate, OCP remains constant throughout the immersion period, indicating rapid passive layer formation on MS. The raw data in the attached zip archive present the complete acquired data range.
Fig. 1.
Variation of open circuit potential (OCP) of MS specimen immersed in 0.5 M succinic acid, 0.05 M sulphanilic acid, 0.5 M sodium orthophosphate, 0.2 M Na-K Tartrate, and 0.04 M BAW solutions. The OCP was recorded in the interval of every 2 min for 30 min.
Fig. 2 shows the anodic polarization of MS in succinic acid, sulphanilic acid, and sodium orthophosphate solutions. After OCP remained constant for 30 min, anodic polarization was then carried out in 0.5 M succinic acid, 0.05 M sulphanilic acid and 0.5 M sodium orthophosphate from OCP to 2 V a scan rate of 1 mV/sec. In the whole anodic potential range, there is no change of current due to dissolution of iron and breakdown of the passivation layer, which are prerequisites for polymerization of aniline to polyaniline. Therefore, these three electrolytes cannot be used for electropolymerization of aniline on MS, unlike sodium-potassium tartrate and BAW [11]
Fig. 2.
Anodic polarization of MS specimen in 0.5 M succinic acid, 0.05 M sulphanilic acid, and 0.5 M sodium orthophosphate solutions after 30 min immersion in respective solution.
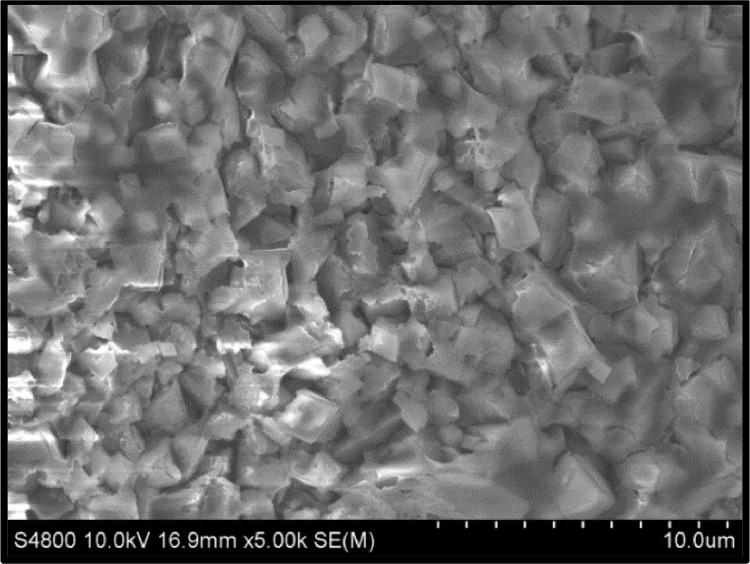
Fig. 3 shows the SEM micrograph of MS specimen anodically polarized in 0.3 M aqueous oxalic acid up to 0.1 V vs. SCE so that only passive layer is formed, and Fig. 4 shows the SEM micrograph of PANI deposited on MS sample in 0.3 M aqueous oxalic acid solution containing 0.1 M aniline by anodic polarization up to 1.6 V. In Fig. 3, the formation of Fe-oxalate takes place, giving a granular structure. The formation of granular and some niddle shaped PANI is obvious in Fig. 4 when the polarization is carried out till 1.6 V in the presence of aniline solution.In the case of Na-K tartrate and BAW, the formation of very fine morphology took place [11].
Fig. 3.
The SEM image of MS sample polarized in 0.3 M oxalic acid solution.
Fig. 4.
SEM image of MS sample polarized in 0.1 M aniline in 0.3 M oxalic acid solution showing the formation of granular PANI coating.
2. Experimental Design, Materials and Methods
The electrochemical measurements were performed by using a Hokuto Denko HA-151 potentiostat controlled by self-made LabVIEW software interfaced with an IBM computer. First of all, a mild steel (MS) sample with dimensions 3 cm x 3 cm x 0.15 cm was taken as working electrode, abraded on SiC paper till 1200 grits. It was ultrasonicated in ethanol, rinsed with distilled water and dipped into electrolytic solution (0.3 M oxalic acid containing 0.1 M aniline monomer) and graphite electrode as a counter electrode in a cell coupled with saturated calomel electrode as reference electrode. Aqueous solution of aniline was prepared after distillation of as purchased aniline. Oxalic acid was reagent grade and used without any treatments. Before the potentiodynamic polarization, OCP was recorded for 30 min at the interval of 2 min. Anodic polarization was then carried out in electrolytes from OCP to 2 V with a 1 mV/sec scan rate. The analysis of data was performed using the Microsoft Excel program. SEM images were recorded using a JEM-1200EX electron microscope after the anodic polarization in the potential limit specific for passive layer formation and PANI formation.
Ethics Statement
The experiments were performed in the Central Department of Chemistry laboratory, Tribhuvan University, Nepal, and all the relevant references have been cited. Data reproducibility was confirmed by repeating several measurements.
CRediT Author Statement
Dipak Kumar Gupta: Data collection, Analysis, and Original draft preparation; Shova Neupane: Reviewing the obtained data and manuscript editing; Sanjay Singh: Helped in experimental setup; Nabin Karki: Draft review; Amar Prasad Yadav: Conceptualization and Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships which have or could be perceived to have influenced the work reported in this article.
Acknowledgments
The authors would like to thank Central Salt and Marine Chemical Research Institute (CSIR), Bhavnagar, Gujarat, India, for providing SEM facilities. D.K. Gupta would also like to acknowledge the Center for Co-operation in Science and Technology among Developing Societies (CCSTDS), DST, India for providing India Science and Research Fellowship (ISRF) and Nepal Academy of Science and Technology (NAST) for the partial Ph.D. financial support to carry out this study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.dib.2021.106875.
Appendix. Supplementary materials
References
- 1.Sazou D., Kourouzidou M., Pavlidou E. Potentiodynamic and potentiostatic deposition of polyaniline on stainless steel: electrochemical and structural studies for a potential application to corrosion control. Electrochim. Acta. 2007;52:4385–4397. doi: 10.1016/j.electacta.2006.12.020. [DOI] [Google Scholar]
- 2.Popović M.M., Grgur B.N. Electrochemical synthesis and corrosion behavior of thin polyaniline-benzoate film on mild steel. Synth. Met. 2004;143:191–195. doi: 10.1016/j.synthmet.2003.12.022. [DOI] [Google Scholar]
- 3.Masdarolomoor F., Hajizadeh S., Arab Chamjangali M., Innis P.C. Novel approach to the synthesis of polyaniline possessing electroactivity at neutral pH. Synth. Met. 2019;250:121–130. doi: 10.1016/j.synthmet.2019.03.011. [DOI] [Google Scholar]
- 4.Abdel-Gaber A.M., Abd-El-Nabey B.A., Khamis E., Salman R.M., Rahal H.T., El Morr Z. Electrochemical synthesis and corrosion behaviour of polyaniline on stainless steel in sodium hydroxide solutions. Chem Eng. Commun. 2020:1–10. doi: 10.1080/00986445.2019.1710493. [DOI] [Google Scholar]
- 5.Zhu A., Wang H., Sun S., Zhang C. The synthesis and antistatic, anticorrosive properties of polyaniline composite coating. Progr. Org. Coat. 2018;122:270–279. doi: 10.1016/j.porgcoat.2018.06.004. [DOI] [Google Scholar]
- 6.Wang M., Yun H., Tan K., Guo A., Ling J., Jiang F., Shen X., Xu Q. One-step electrochemical synthesis of poly(vinyl pyrrolidone) modified polyaniline coating on stainless steel for high corrosion protection performance. Progr. Organic Coat. 2020;149 doi: 10.1016/j.porgcoat.2020.105908. [DOI] [Google Scholar]
- 7.Boshkova N., Tabakova N., Atanassova G., Boshkov N. Electrochemical obtaining and corrosion behavior of Zinc-Polyaniline (Zn-PANI) hybrid coatings. Coatings. 2019;9:487. doi: 10.3390/coatings9080487. [DOI] [Google Scholar]
- 8.Pawar P., Gaikawad A.B., Patil P.P. Electrochemical synthesis of corrosion protective polyaniline coatings on mild steel from aqueous salicylate medium. Sci. Technol. Adv. Mater. 2006;7:732–744. doi: 10.1016/j.stam.2006.09.014. [DOI] [Google Scholar]
- 9.Ogurtsov N.A., Pud A.A., Kamarchik P., Shapoval G.S. Corrosion inhibition of aluminum alloy in chloride mediums by undoped and doped forms of polyaniline. Synth. Met. 2004:5. [Google Scholar]
- 10.Chaudhari S., Patil P.P. Inhibition of nickel coated mild steel corrosion by electrosynthesized polyaniline coatings. Electrochim. Acta. 2011;56:3049–3059. doi: 10.1016/j.electacta.2010.12.096. [DOI] [Google Scholar]
- 11.Kumar Gupta Dipak, Neupane Shova, Singh Sanjay, Karki Nabin, Prasad Yadav Amar. The effect of electrolytes on the coating of polyaniline on mild steel by electrochemical methods and its corrosion behaviour. Progr. Org. Coat. 2021;152 doi: 10.1016/j.porgcoat.2020.106127. [DOI] [Google Scholar]
- 12.Neupane S. Tribhuvan University; Nepal: 2013. Development of a Windows Based Program to Control the Analogue Potentiostat in Combination with an ADA Convertor, M.Sc. Disertation, Tri-Chandra Multiple Campus. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.