Abstract
Background:
While immune-based therapies have been approved for extensive-stage small cell lung cancer, there is limited data on the efficacy of immunotherapy in patients with limited-stage disease.
Methods:
We used the National Cancer Database to first evaluate factors associated with the inclusion of immunotherapy as part of the initial therapeutic course in patients diagnosed with limited-stage small cell lung cancer (LS-SCLC). Consequently, we evaluated the impact of this immunotherapy on 2-year and 5-year overall survival (OS). We did this by performing 1:1 matching for controls that did not receive immunotherapy, and comparing survival between cohorts using the Kaplan–Meier method.
Results:
A total of 98 patients with LS-SCLC received immunotherapy as part of their initial therapeutic regimen. Age and facility type were the only significant predictors of the use of immunotherapy. There was no statistically significant difference between matched case-control cohorts in median OS (p = 0.985), 2-year OS (p = 0.747), and 5-year OS (p = 0.934).
Conclusion:
In this study using a large national database, we found that the inclusion of immunotherapy as part of the initial systemic therapy regimen was not significantly associated with improved OS in a cohort of LS-SCLC patients.
Keywords: immunotherapy, limited-stage, small cell lung cancer, survival
Introduction
Lung cancer is a leading cause of death in the United States (US), contributing over 240,000 new cases and 145,000 deaths in 2019.1 Immune-based therapies have transformed the treatment of non-small cell lung cancer (NSCLC) over the course of the last decade. However, advances in therapeutic options for small cell lung cancer (SCLC) have been less radical. Approximately 15% of lung cancer cases in the US are characterized as SCLC.2 As the most aggressive form of lung cancer, patients with this disease exhibit extremely poor outcomes, with 5-year overall survival (OS) rates of less than 10%.2 Historically, systemic therapy for both limited- and extensive-stage SCLC has been restricted to chemotherapy with a platinum agent plus etoposide.
After decades of minimal progress, the use of checkpoint inhibitors has led to significant progress in the treatment of extensive-stage small cell lung cancer (ES-SCLC). In 2016, the National Comprehensive Cancer Network (NCCN) guidelines included the recommendation of immunotherapy with nivolumab (anti-PD-1) alone, or in combination with ipilimumab (anti-CTLA-4) for relapsed SCLC, based on results from the CheckMate 032 trial.3 Subsequently, in March 2019, the US Food and Drug Administration (FDA) approved atezolizumab, in combination with carboplatin and etoposide, for the first-line treatment of adult patients with ES-SCLC based on the based on the IMpower133 trial.4 Currently, there is no recommendation to administer immunotherapy to patients with limited-stage small cell lung cancer (LS-SCLC) based on a lack of data in this population.5 In this study, we sought to use data from a large, national registry to explore trends in survivorship among patients with LS-SCLC, who received the off-label use of immunotherapy as part of their first-line treatment modality.
Materials and methods
Patient data
We extrapolated data from patients with SCLC, diagnosed between 2004 and 2016, based on a National Cancer Database (NCDB) Participant User File (PUF) award granted to the principal investigator (R.A.). The NCDB is an extensive registry of cancer outcomes, estimated to include 70% of all cancer patients in the US.6 Supported jointly by the Commission on Cancer (CoC) and the American College of Surgeons (ACS), the information in this database is collected from over 1500 medical institutions.6
LS-SCLC patients who received immunotherapy as part of their “first-course therapy” were included for analysis in this study.7 Although the NCDB does not document which specific agent was used, it defines immunotherapy as “biological or chemical agents that alter the immune system, or change the host’s response to tumor cells” and reports whether patients have received immunotherapy at any of the treating facilities.7
Statistical analysis
Data analysis was conducted using the Statistical Package for Social Sciences (SPSS), version 27.0 (IBM Corp, Armonk, NY, USA). Descriptive statistics, namely frequency and proportion, were performed to describe independent clinicopathologic factors in all LS-SCLC cases. Univariate chi-squared analyses were then used to evaluate for a significant association between sociodemographic or clinicopathologic factors and the use of the immunotherapy. Significant variables (p <0.05) were subsequently included in a multivariate logistic regression model for predictors of the use of immunotherapy. All p values for the multivariate analysis reflect comparison with a reference variable [indicated by “(ref)” in Table 1]. We performed 1:1 case matching between patients who received first-line immunotherapy and those who did not, using the following parameters: age, sex, race, Charlson/Deyo comorbidity index and the use of surgery, chemotherapy, time to chemotherapy (days), radiation therapy, time to radiotherapy (days), and chemoradiation sequence (concurrent versus sequential). Chi-squared analyses assessed for significant differences between the use of immunotherapy and 2- or 5-year OS. Finally, Kaplan–Meier survival analysis, and the log-rank test, were used to compare survival curves between these cohorts.
Results
Patient characteristics
From 50,527 patients with LS-SCLC registered in the NCDB, first-line immunotherapy was administered to n = 98 patients, for which descriptive statistics can be found in Table 1. Of these, the majority were under 70 years old (71.4%), female (57.1%), White (92.9%), and had a Charlson/Deyo comorbidity score of 0 (90.8%). The predominant insurance type was Medicare (56.1%), followed by private insurance (30.6%), and Medicaid or other governmental insurance (7.1%). Only 3.1% of patients were uninsured. The most common facility type associated with the treatment of this patient cohort was comprehensive community cancer programs (46.9%), followed by academic/research cancer programs (39.8%), integrated network cancer programs (11.2%), and community cancer programs (5.1%).
Table 1.
Characteristics of limited-stage SCLC cohort and multivariate model for predictors of the use of immunotherapy.
| Variable | Immunotherapy | Multivariate OR (95% CI)** | p-value | |
|---|---|---|---|---|
| No (n = 50,429) | Yes (n = 98) | |||
| Age | ||||
| <70 (ref) | 28,075 (55.6%) | 70 (71.4%) | 1 | |
| >70 | 22,452 (44.4%) | 28 (28.6%) | 0.513 (0.331–0.797) | 0.003 |
| Sex | ||||
| Male (ref) | 21,904 (43.4%) | 42 (42.9%) | 1 | |
| Female | 38,623 (56.6%) | 56 (57.1%) | 1.012 (0.678–1.511) | 0.954 |
| Race | ||||
| White (ref) | 46,927 (92.9%) | 91 (92.9%) | 1 | |
| Black | 3600 (7.1%) | 7 (7.1%) | 0.920 (0.425–1.992) | 0.833 |
| Charlson/Deyo score | ||||
| 0–1 (ref) | 52,587 (84.3%) | 89 (90.8%) | 1 | |
| 2–3 | 7940 (15.7%) | 9 (9.2%) | 0.584 (0.293–1.160) | 0.125 |
| Insurance* | ||||
| Uninsured | 1215 (2.5%) | 3 (3.2%) | ||
| Private | 13,276 (26.8%) | 30 (31.6%) | ||
| Medicaid or other governmental insurance | 4054 (8.2%) | 7 (7.4%) | NA | NA |
| Medicare | 21,041 (62.6%) | 55 (57.9%) | ||
| Facility type | ||||
| Community CP (ref) | 6140 (12.2%) | 5 (5.1%) | 1 | |
| Comprehensive community CP | 24,086 (47.8%) | 46 (46.9%) | 2.397 (0.952–6.036) | 0.064 |
| Academic/research CP | 13,123 (26.0%) | 36 (36.7%) | 3.320 (1.301–8.473) | 0.012 |
| Integrated network CP | 7032 (14.0%) | 11 (11.2%) | 1.966 (0.683–5.665) | 0.210 |
Insurance was not included in the multivariable model due to insignificant p value at univariate level.
p values reflect comparison with reference [indicated by “(ref)”]
CI, confidence interval; CP, cancer program; OR, odds ratio.
Use of immunotherapy
In a multivariate model for predictors of the use of immunotherapy (Table 1), the only significant variables were age and facility type. Patients older than 70 years were less likely to receive immunotherapy [odds ratio (OR): 0.513, 95% confidence interval (CI): 0.331–0.797, p = 0.003]. Patients who were treated at academic/research cancer programs were 3.3 times more likely to receive immunotherapy compared with those treated at community cancer programs (OR: 3.320, 95% CI: 1.301–8.473, p = 0.012).
Cohort matching
Patients that received first-line immunotherapy (n = 98 cases) were matched to patients that did not receive immunotherapy (n = 96 controls). Table 2 describes both cohorts, and indicates no significant difference (p > 0.05) between them with respect to the following variables: age, sex, race, Charlson/Deyo comorbidity score, use of surgery, use of chemotherapy, use of radiotherapy, and chemoradiation sequence (i.e., concurrent versus sequential). Details on the timing of these therapeutic modalities can also be found in Table 2. The median time to initiation of immunotherapy was 40.0 (IQR 22.0–93.0) days from diagnosis. In this cohort of patients receiving immunotherapy, the median time to chemotherapy was 25.5 [interquartile range (IQR) 15–38.5] days; while the median time to radiotherapy was 48.5 (IQR 31.5–87.5) days. There was no difference (p > 0.05) in time to chemotherapy and time to radiotherapy between both cohorts.
Table 2.
Characteristics of 1:1 matching between no immunotherapy and immunotherapy cohorts.
| Variable | No immunotherapy (n = 96) | Immunotherapy (n = 98) | *p value |
|---|---|---|---|
| Age, | |||
| Mean ± SD | 65.3 ± 10.1 | 65.1 ± 10.2 | >0.05 |
| Sex | |||
| Male | 42 (21.6%) | 42 (21.6%) | >0.05 |
| Female | 54 (27.8%) | 56 (28.9%) | |
| Race | |||
| White | 90 (46.4%) | 91 (46.9%) | >0.05 |
| Black | 6 (3.1%) | 7 (3.6%) | |
| Charlson/Deyo score | |||
| 0–1 (ref) | 87 (44.8%) | 89 (45.9%) | >0.05 |
| 2–3 | 9 (4.6%) | 9 (4.6%) | |
| Surgery | |||
| No | 94 (48.7%) | 95 (49.2%) | >0.05 |
| Yes | 2 (1.0%) | 2 (1.0%) | |
| Chemotherapy | |||
| No | 3 (1.5%) | 4 (2.1%) | >0.05 |
| Yes | 93 (47.9%) | 94 (48.5) | |
| Radiotherapy | |||
| No | 30 (15.5%) | 30 (15.5%) | >0.05 |
| Yes | 66 (34.0%) | 68 (35.1%) | |
| Chemoradiation | |||
| Concurrent | 44 (34.6%) | 41 (32.3%) | >0.05 |
| Sequential | 20 (15.7%) | 22 (17.3%) | |
| Time to chemotherapy (days) | |||
| Median (IQR) | 25.0 (15.0–32.0) | 25.5 (15.0–38.5) | >0.05 |
| Time to radiotherapy (days) | |||
| Median (IQR) | 53.0 (36.0–79.0) | 48.5 (31.5–87.5) | >0.05 |
| Time to immunotherapy (days) | |||
| Median (IQR) | – | 40.0 (22.0–93.0) | – |
Calculated using chi-square testing for categorical variables and non-parametric, Mann–Whitney U Test for continuous variables (time to chemotherapy, time to radiotherapy and time to immunotherapy).
IQR, interquartile range; SD, standard deviation.
Survival analysis
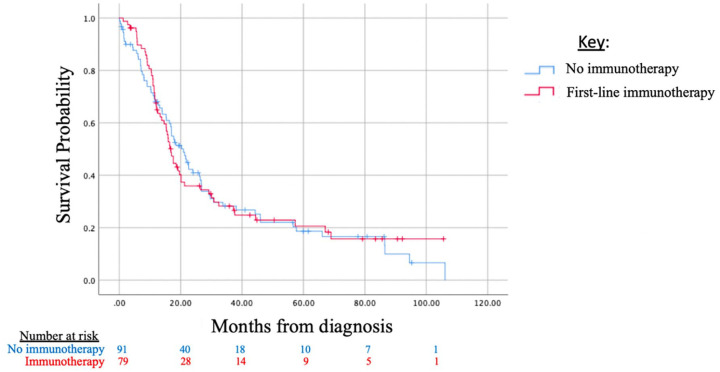
Survival data were not available for all patients in the NCDB dataset, as it was not collected for patients diagnosed in 2016. Survival could be assessed in n = 79 patients who had received immunotherapy, compared with a matched cohort of n = 91 patients who did not receive immunotherapy. There was no difference between these cohorts with respect to the following survival parameters: median OS (p = 0.985), 2-year OS (p = 0.747), and 5-year OS (p = 0.934) (Table 3). Survival curves are depicted in Figure 1 in a Kaplan–Meier analysis of cases to matched cohorts.
Table 3.
Median, 2-year and 5-year overall survival between no immunotherapy and immunotherapy cohorts.
| Variable | Median OS | 2-year OS | 5-year OS | |||
|---|---|---|---|---|---|---|
| Months (95% CI) | p value | n (%) | p value | n (%) | p value | |
| *Immunotherapy | ||||||
| No (n = 91) | 20.9 (16.3–25.5) | 0.985 | 31 (34.1%) | 0.747 | 10 (11.0%) | 0.934 |
| Yes (n = 79) | 16.8 (14.8–18.8) | 25 (31.6%) | 9 (11.4%) | |||
Cases were not included at this level of analysis if they did not include temporal survival data.
CI, confidence interval; OS, overall survival.
Figure 1.
Kaplan–Meier survival analysis for limited-stage, SCLC by use of immunotherapy (includes cases and matched controls, see Table 2).
SCLC, small cell lung cancer.
Discussion
In this study, using a large national database, we found that the use of immunotherapy as part of “first-course therapy” was not significantly associated with improved OS in a cohort of LS-SCLC patients, when compared with a case-matched cohort.
Up to 30% of checkpoint inhibitor drug use may be implemented as an “off-label” treatment modality.8 This may occur in patients with poor performance status or those who decline aggressive chemotherapy.9 In our study, however, we found that patients were more likely to be administered first-course immunotherapy if they were younger than 70 years and if they were treated at academic/research institutions. The comorbidity index, and the proportion of patients receiving chemotherapy and radiation, did not differ significantly between the two cohorts analyzed in this study. This would suggest that immunotherapy was given in addition to standard treatments, yet still did not have a significant impact on OS in this cohort of patients.
Limitations of this study include those typically noted in registry-based, retrospective analyses. The NCDB registry does not document the specific immunologic-based therapy used in these patients, or the duration of this therapy. We only know that included patients have received at least one infusion. Thus, it is not possible to know whether the lack of association with improved OS may be due to limited treatment times. Furthermore, the registry lacks information regarding whether tumor biomarker testing was performed, such as expression of PD-L1, tumor mutational burden (TMB) or microsatellite instability (MSI) status. These molecular markers are often used to predict response to immune checkpoint inhibitor therapy.10 Therefore, it is not possible to know whether patients in this cohort would have been deemed good candidates for immunotherapy based on these biomarker assays. The strengths of this study include the relatively large size of this atypical group of patients, as well as the ability to strictly case-match to a control group in order to decrease the likelihood of confounding biases. The quality of data in the NCDB is of high fidelity, having undergone a rigorous internal verification process. All programs reporting to the NCDB are accredited by the American College of Surgeons Commission on Cancer, which participates in annual practices of quality assurance and quality control.11 Additionally, all cases are coded into the registry under the supervision of certified tumor registrars (CTRs).
Our knowledge of the utility of immunologic-based therapies in SCLC is still growing. The CheckMate 032 trial supporting the use of nivolumab alone or in combination with ipilimumab for relapsed disease did not support the use of PD-L1 as a biomarker in SCLC.3 Additionally, in contrast to NSCLC, PD-L1 expression in SCLC is uncommon – estimated to be present in less than 20% of patients.12,13 PD-L1 expression also differs by stage, with higher expression seen in LS-SCLC (stage I–III) versus metastatic cases.14 The IMpower133 trial exploring the benefit of atezolizumab in ES-SCLC did not show significant predictive utility of blood-based tumor mutational burden (bTMB),4 another predictive biomarker in NSCLC.
Results from prospective, controlled trials – two of which were actively recruiting at the time of this analysis15,16 – are eagerly awaited to verify significant biomarkers of response to therapy, and to assess whether immune-based therapies can confer a significant survival benefit in the treatment of LS-SCLC.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics approval and consent to participate: Ethical approval was obtained from the Cleveland Clinic Institutional Review Board (IRB) prior to conducting this study (FLA 20-005). All patient data was strictly de-identified and provided, with approval, from the American College of Surgeons as part of the National Cancer Database.
Consent for publication: No individual person’s data included; all data is reported in an aggregate manner.
Availability of data and material: The data that support the findings of this study are available from the American College of Surgeons/American Cancer Society but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the American College of Surgeons/American Cancer Society.
ORCID iD: Nadeem Bilani  https://orcid.org/0000-0002-7335-9319
https://orcid.org/0000-0002-7335-9319
Contributor Information
Nadeem Bilani, Department of Hematology-Oncology, Cleveland Clinic Florida, 2950 Cleveland Clinic Blvd, Weston, FL 33331, USA.
Evan Alley, Department of Hematology-Oncology, Maroone Cancer Center, Cleveland Clinic Florida, Weston, FL, USA.
Leah Elson, Department of Hematology-Oncology, Maroone Cancer Center, Cleveland Clinic Florida, Weston, FL, USA.
Zeina Nahleh, Department of Hematology-Oncology, Maroone Cancer Center, Cleveland Clinic Florida, Weston, FL, USA.
Rafael Arteta-Bulos, Department of Hematology-Oncology, Maroone Cancer Center, Cleveland Clinic Florida, Weston, FL, USA.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7–34. [DOI] [PubMed] [Google Scholar]
- 2. Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer 2015; 121: 664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016; 17: 883–895. [DOI] [PubMed] [Google Scholar]
- 4. Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018; 379: 2220–2229. [DOI] [PubMed] [Google Scholar]
- 5. National Comprehensive Cancer Network. Small Cell Lung Cancer NCCN Evidence Blocks (Version 1). (2020). https://www.nccn.org/professionals/physician_gls/pdf/sclc_blocks.pdf.
- 6. Bilimoria KY, Stewart AK, Winchester DP, et al. The national cancer data base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008; 15: 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Cancer Data Base Participant User File (PUF). Data dictionary. Version. PUF, 2016. https://www.facs.org/-/media/files/quality-programs/cancer/ncdb/puf_data_dictionary.ashx
- 8. De Souza JA, Duong YY. Off-label immunotherapy prescription: financial implications for payers and patients. J Clin Oncol 2017; 35(Suppl. 8): 6.27870565 [Google Scholar]
- 9. Fojo T. Desperation oncology. Semin Oncol 2018; 45: 105–106. [DOI] [PubMed] [Google Scholar]
- 10. Nakamura Y. Biomarkers for immune checkpoint inhibitor-mediated tumor response and adverse events. Front Med (Lausanne) 2019; 6: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American College of Surgeons. Optimal resources for cancer care, https://www.facs.org/quality-programs/cancer/coc/standards/2020 (accessed 21 October 2020)
- 12. Hellmann MD, Ott PA, Zugazagoitia J, et al. Nivolumab (nivo)±ipilimumab (ipi) in advanced small-cell lung cancer (SCLC): first report of a randomized expansion cohort from CheckMate 032. J Clin Oncol 2017; 35(Suppl. 15): 8503. [Google Scholar]
- 13. Hellmann MD, Callahan MK, Awad MM, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 2018; 33: 853–861.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonanno L, Pavan A, Dieci MV, et al. The role of immune microenvironment in small-cell lung cancer: Distribution of PD-L1 expression and prognostic role of FOXP3-positive tumour infiltrating lymphocytes. Eur J Cancer 2018; 101: 191–200. [DOI] [PubMed] [Google Scholar]
- 15. Senan S, Okamoto I, Lee GW, et al. Design and rationale for a phase III, randomized, placebo-controlled trial of durvalumab with or without tremelimumab after concurrent chemoradiotherapy for patients with limited-stage small-cell lung cancer: the ADRIATIC study. Clin Lung Cancer 2020; 21: e84–e88. [DOI] [PubMed] [Google Scholar]
- 16. Clinical trials.gov. Chemoradiation with or without atezolizumab in treating patients with limited stage small cell lung cancer, https://clinicaltrials.gov/ct2/show/NCT03811002 .