Abstract
Background:
The clinical and economic burden of pulmonary arterial hypertension (PAH) is poorly understood outside the United States. This retrospective database study describes the characteristics of patients with PAH in England, including their healthcare resource utilisation (HCRU) and associated costs.
Methods:
Data from 1 April 2012 to 31 March 2018 were obtained from the National Health Service (NHS) Digital Hospital Episode Statistics database, which provides full coverage of patient events occurring in NHS England hospitals. An adult patient cohort was defined using an algorithm incorporating pulmonary hypertension (PH) diagnosis codes, PAH-associated procedures, PH specialist centre visits and PAH-specific medications. HCRU included inpatient admissions, outpatient visits and Accident and Emergency (A&E) attendances. Associated costs, calculated using national tariffs inflation-adjusted to 2017, did not include PAH-specific drugs on the High Cost Drugs list.
Results:
The analysis cohort included 2527 patients (68.4% female; 63.6% aged ⩾50 years). Mean annual HCRU rates ranged from 2.9 to 3.2 for admissions (21–25% of patients had ⩾5 admissions), 9.4–10.3 for outpatient visits and 0.8–0.9 for A&E attendances. Costs from 2013 to 2017 totalled £43.2M (£33.9M admissions, £8.3M outpatient visits and £0.9M A&E attendances). From 2013 to 2017, mean cost per patient decreased 13% (from £4400 to £3833) for admissions and 13% (from £1031 to £896) for outpatient visits, but increased 52% (from £81 to £123) for A&E attendances.
Conclusion:
PAH incurs a heavy economic burden on a per-patient basis, highlighting the need for improved treatment strategies able to reduce disease progression and hospitalisations.
The reviews of this paper are available via the supplemental material section.
Keywords: burden of illness, healthcare resource utilisation, hospital episode statistics, pulmonary arterial hypertension, real-world data, retrospective database analysis
Introduction
Pulmonary arterial hypertension (PAH) is a rare and debilitating chronic disease, characterised by vascular proliferation and remodelling of the small pulmonary arteries, ultimately leading to right heart failure and death.1,2 Current treatment guidelines recognise that PAH imposes a heavy clinical burden, with high morbidity and mortality and the need for intensive management by specialists.3 However, there is limited real-world evidence available on the burden of illness outside the United States (US).
Estimating the burden of PAH has been hampered by the lack of specific diagnosis codes distinguishing PAH from other forms of pulmonary hypertension (PH) in the current clinical classification.4,5 Consequently, defining patients with PAH in administrative databases relies on algorithms incorporating various codes indicative of this disease. To date, no single standard algorithm has been developed.5,6
No previous published study has estimated the overall healthcare resource utilisation and associated costs among patients with PAH in England. This represents an important data gap for decision-makers such as hospital trust managers, especially given the high cost of managing PAH documented in other countries.7,8 Understanding the typical healthcare resource utilisation of PAH patients in England should increase the ability to identify key areas where interventions would be most impactful. Identifying the specialities currently managing PAH patients, and the distribution of treatment between inpatient, outpatient and emergency settings, could help to optimise the standard of care.
The present retrospective database study was therefore conducted to assess the burden of PAH in England, using a real-world, population-level administrative database.
Methods
Data source
Data were obtained from the National Health Service (NHS) Digital Hospital Episode Statistics (HES) database, which contains administrative data collected to allow hospitals to document and be paid for the care they deliver.9 The HES database includes details of all inpatient admissions, outpatient visits and Accident and Emergency (A&E) attendances (equivalent to emergency department visits in other countries) at all NHS hospital trusts in England. The HES database is the best available source for addressing our study objectives as it is representative of the overall patient population in England.
All HES data are subject to strict statistical disclosure control in accordance with the NHS Digital protocol, to ensure maintenance of patient confidentiality.9 The HES database is compliant with the European Union General Data Protection Regulation (GDPR).9 All data used in the present study were anonymised.
Study design
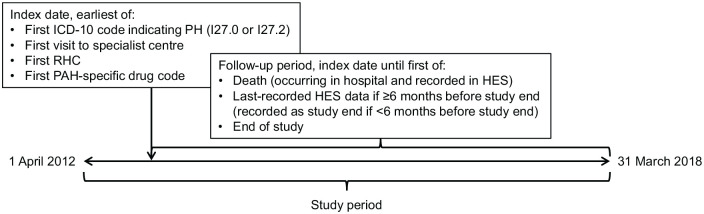
The study design, cohort definition algorithm and data analysis plan were developed in collaboration with clinicians and experts in the field of PAH. The study period encompassed the entire period of the dataset obtained from HES, from 1 April 2012 to 31 March 2018 (Figure 1). Within this study period, each patient’s index date was defined as the earliest of the following: the first recorded International Classification of Diseases, Tenth Revision (ICD-10) diagnosis code for PH [either I27.0 (Primary pulmonary hypertension) or I27.2 (Other secondary pulmonary hypertension)]; the first recorded event in the HES database within a specialist referral centre (an event was defined as any interaction of a patient with an NHS hospital in England); the first recorded right heart catheterisation (RHC); or the date of dispensation of the first recorded PAH-specific drug.
Figure 1.
Study design.
HES, Hospital Episode Statistics; ICD-10, International Classification of Diseases; Tenth Revision; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; RHC, right heart catheterisation.
Patients were followed from their index date until death (if occurred in hospital and recorded in the HES database), last contact date [if the last date recorded in HES occurred ⩾6 months (180 days) before end of study (31 March 2018), in which case the patient was considered lost to follow up], or the study end date (if the last date recorded in HES occurred within 6 months of end of study).
Patients with an index date more than 1 year after the start of the dataset were considered to be incident patients, assuming that there would have been at least one PAH-related healthcare activity within a year if PAH had been diagnosed before the index date. Patients with an index date within 1 year after the start of the study period were considered to be prevalent patients, as their medical history prior to the start date of the dataset is unknown; this method could have resulted in some incident patients being miscategorised as prevalent.
Cohort selection
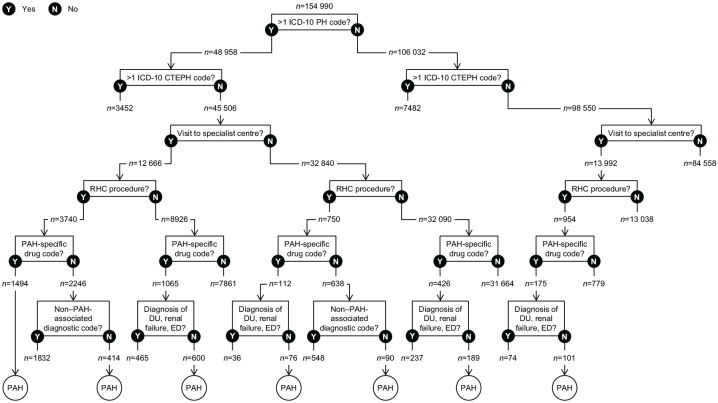
The diagnosis coding system used in the HES database, ICD-10, lacks a unique code for PAH.4,5 The ICD-10 code I27.0 for primary PH corresponds to idiopathic PAH in the current PAH classification,10–12 but there are no ICD-10 codes that differentiate the other PAH subgroups from non-PAH forms of PH. Therefore, patients with PAH were defined using an algorithm incorporating not only PH diagnosis codes but also procedures associated with PAH, PAH-specific medications and visits to a PH specialist centre (because the NHS England PH service specification states that all patients receiving disease-targeted therapy should be reviewed at least once a year by a visiting PH specialist or at a PH specialist centre).13 The algorithm combined these criteria in different ways depending on presence or absence of diagnosis codes.
Eligible patients were aged 18–120 years at index date. Eligibility was further defined by different combinations of criteria as shown in Figure 2, accounting for limitations of the HES data. For example, although most patients had more than one record with a diagnosis code for PH, outpatient services are not required to list ICD-10 codes for the HES reimbursement system, and thus approximately 90% of outpatient visits were missing ICD-10 codes. Therefore, the algorithm did not require an ICD-10 code for PH if a patient had other strong indicators of PAH, namely a combination of RHC (identified by procedure codes), a visit to an NHS England-designated PH specialist centre (identified by trust codes), and a PAH-specific drug (identified by drug codes). Conversely, since RHC could have occurred prior to the study period, a record of RHC was not required if a patient had more than one record with a diagnosis code for PH in addition to a PAH-specific drug. Similarly, because a PAH patient might not have a recorded visit to a PH specialist centre (e.g. if they receive visits from a specialist and/or their local physician consults a PH centre), a visit to a specialist centre was not required if a patient had more than one record with a diagnosis code for PH in addition to either a PAH-specific drug or RHC with no non–PAH-associated diagnosis. All codes are listed in Supplemental Table S1.
Figure 2.
Identification of patients with PAH.
CTEPH, chronic thromboembolic pulmonary hypertension; DU, digital ulcer; ED, erectile dysfunction; ICD-10, International Classification of Diseases, Tenth Revision; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; RHC, right heart catheterisation.
PAH-specific drugs, considered to be proxies for PAH, comprised prostacyclin-pathway agents, endothelin receptor antagonists (ERAs), phosphodiesterase type 5 inhibitors (PDE-5is) and the soluble guanylate cyclase stimulator riociguat. As most of these drugs are specific to PAH as recommended by the ESC/ERS guidelines for PH,3 a confirmed dispensation of these drugs supports a PAH diagnosis. Furthermore, off-label prescribing of these drugs is unlikely in the United Kingdom (UK), where the use of PAH-specific medication is well controlled and the National Audit has ensured that essentially 100% of patients receiving these drugs have a recorded diagnosis.14 However, because HES does not capture dispensations from retail pharmacies (where PDE-5is, mandated as first-line therapy by NHS England,15 are mainly dispensed) these drugs are likely under-represented in the database. We used codes from the Office of Population Censuses and Surveys (OPCS) High Cost Drugs list, but inpatient records may have missing OPCS codes because drugs administered during inpatient visits may already be covered within other reimbursement codes, and generic PDE-5is are not captured as high-cost drugs. Therefore, absence of a recorded PAH-specific drug code did not disqualify a patient if they had a combination of other PAH indicators.
PAH-specific drugs could be used off-label or for other indicated conditions [e.g. the ERA bosentan for digital ulcers, PDE-5is for erectile dysfunction, epoprostenol for renal dialysis and riociguat for chronic thromboembolic pulmonary hypertension (CTEPH)]. To exclude such use, PAH-specific drugs were considered indicative of PAH only in the presence of an OPCS code (X82.1–.4) reserved for treating PAH according to coding standards,16 and only in the absence of ICD-10 codes indicative of a diagnosis of digital ulcers, erectile dysfunction or renal failure.
Exclusion criteria included: an ICD-10 code for CTEPH (I26) at any time; missing age at index date or missing gender; and patients with a clear indication of corrupted or unreliable data (e.g. index date outside of the study period, multiple ages, multiple hospital recordings after the recorded date of death, etc.).
Outcomes
To align to Office for National Statistics population figures, prevalence of PAH in England was estimated for each year as the number of patients with PAH living in England on 1 June of that year. Annual incidence was calculated as a rate over the total population in England in each year, based on national statistics.17
Healthcare resource utilisation was quantified, considering inpatient admissions, outpatient visits, and A&E attendances. Length of hospitalisation for inpatient admissions was calculated as the number of days between a patient’s admission and discharge dates in the same spell, which was defined as a continuous period spent as an inpatient from admission until the earlier of discharge or study exit. If there was a linked A&E attendance 24 h prior to the inpatient admission, this was added to the length of hospitalisation.
Associated costs were inflation-adjusted to 2017 using Bank of England yearly inflation factors. Costing used Healthcare Resource Group tariffs, which include standard treatments and drugs, but not drugs on the High Cost Drugs List, which are reimbursed separately. Therefore, most PAH-specific drugs were not included in healthcare resource utilisation or cost calculations.
Healthcare resource utilisation and costs were classified as PAH-related if the event in question had an ICD-10 code for PH, or non-PAH-related if the event had no PH ICD-10 code.
Statistical analysis
As this descriptive study was not designed to test hypotheses, no statistical tests were conducted, and thus sample-size calculations were not required. Continuous variables are reported as mean ± standard deviation (SD) and categorical variables are reported as frequency distribution n (%). All analyses were performed using R version 3.4.3.18
Results
Patient prevalence and incidence
After the application of all eligibility criteria, 2527 patients were included in the analysis cohort (Figure 2). The number of patients each year ranged from 1370 to 1852, while annual incidence ranged from 3.6 to 6.3 patients per million person-years in England (Table 1).
Table 1.
Patient prevalence and incidence rate of PAH in 2013–2017.
| 2013 | 2014 | 2015 | 2016 | 2017 | Total individual patients 2013–2017 | |
|---|---|---|---|---|---|---|
| Number of patientsa | 1370 | 1605 | 1774 | 1850 | 1825 | 2527 |
| Incidence, n per million person-yearsb | 5.0 | 6.3 | 5.5 | 3.8 | 3.6 | — |
Based on all patients.
Based on incident patients, defined as patients with an index date >1 year after the start of the dataset.
PAH, pulmonary arterial hypertension.
Patient characteristics
At the index date, approximately two-thirds (68.4%) of all patients were female. Overall, 63.6% of patients were at least 50 years of age at the index date and the percentage of patients in each age category increased from the 18–29-year-old group to the ⩾70-year-old group (Figure 3). For incident patients, mean ± SD age at first PAH event was 58.0 ± 16.8 years.
Figure 3.
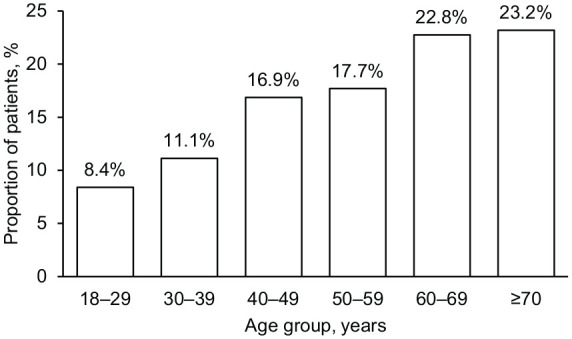
Age distribution of patients with PAH (n = 2527), 2013–2017.
PAH, pulmonary arterial hypertension.
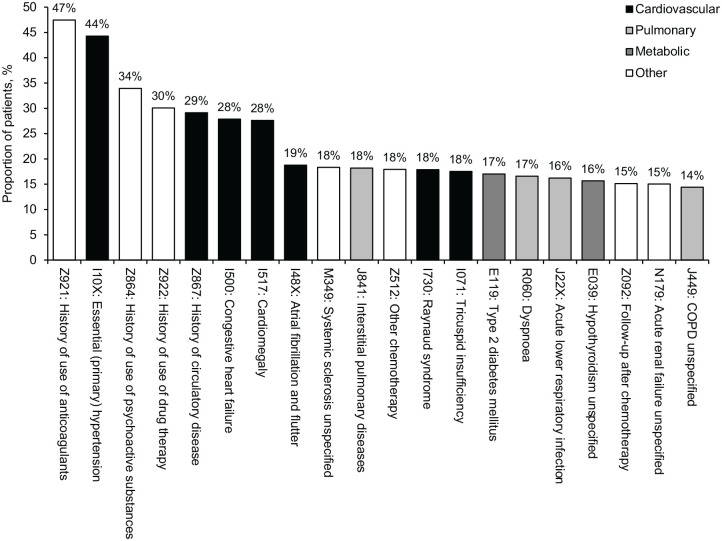
The most common associated diagnosis codes in all records after the index date in the overall cohort were related to cardiovascular, pulmonary and metabolic diseases (Figure 4). Based on ICD-10 codes, 44.3% of patients had essential hypertension, 29.1% had a history of cardiovascular disease and 27.9% had congestive heart failure.
Figure 4.
Top 20 ICD-10 codes in patients with PAH (n = 2527), 2013–2017.
COPD, chronic obstructive pulmonary disease; ICD-10, International Classification of Diseases, Tenth Revision; PAH, pulmonary arterial hypertension.
Healthcare resource utilisation
The mean number of inpatient, outpatient and A&E events was generally consistent across calendar years from 2013 to 2017 (Table 2). The mean number of events per year ranged from 2.9 to 3.2 for inpatient admissions, 9.4–10.3 for outpatient attendances and 0.8–0.9 for A&E attendances.
Table 2.
Inpatient, outpatient and A&E hospital events in patients with PAH by year, 2013–2017.
| 2013 (n = 1370) | 2014 (n = 1605) | 2015 (n = 1774) | 2016 (n = 1850) | 2017 (n = 1825) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Inpatient admissions | Total | 3.2 | 2.8 | 3.0 | 2.7 | 2.9 | 2.6 | 3.0 | 3.8 | 2.9 | 4.3 |
| PAH-related | 2.1 | 2.5 | 1.9 | 2.3 | 2.0 | 2.6 | 2.0 | 2.6 | 2.0 | 3.1 | |
| Non–PAH-related | 1.1 | 2.9 | 1.0 | 3.0 | 0.9 | 2.6 | 1.0 | 4.6 | 0.9 | 5.2 | |
| Outpatient visits | Total | 9.8 | 8.1 | 9.4 | 7.4 | 9.9 | 7.5 | 10.3 | 7.6 | 10.0 | 7.4 |
| A&E attendances | Total | 0.8 | 1.2 | 0.8 | 1.2 | 0.8 | 1.2 | 0.9 | 1.2 | 0.8 | 1.0 |
| PAH-related | 0.3 | 0.8 | 0.3 | 0.8 | 0.3 | 0.9 | 0.3 | 0.8 | 0.3 | 0.9 | |
| Non–PAH-related | 0.5 | 1.5 | 0.5 | 1.5 | 0.5 | 1.8 | 0.5 | 1.5 | 0.5 | 1.1 | |
A&E, Accident and Emergency; PAH, pulmonary arterial hypertension; SD, standard deviation.
The proportion of patients with inpatient admissions was generally consistent across calendar years, ranging from 79.9% to 83.6% (Supplemental Table S2). The proportion of patients with an outpatient visit was also relatively constant, ranging from 96.0% to 98.0%. The proportion of patients with an A&E attendance was more variable, ranging from 32.2% to 41.0%.
For incident patients, the highest level of healthcare resource utilisation occurred in the first year following the index date, with reductions from year 1 to year 2 post-index of 41%, 17% and 27% for mean number of inpatient admissions, outpatient visits and A&E attendances, respectively (Supplemental Table S3). For incident patients, most inpatient admissions were for PAH-related events rather than non-PAH-related events (mean 5.5 versus 2.1). Mean number of A&E attendances was similar for PAH-related and non-PAH-related events (1.0 and 1.1, respectively). The previously noted large proportion of outpatient records missing ICD-10 codes precludes drawing meaningful conclusions about the results for PAH-related versus non-PAH-related outpatient visits, and thus stratified results are not reported for outpatient visits (Supplemental Table S3).
Inpatient admissions were common for the overall cohort, with 24–30% of patients having one admission per calendar year, 21–24% having two, 16–18% having three, 10–11% having four and 21–25% having five or more admissions (Supplemental Table S4). The median length of hospitalisation per stay was 1 day in every year in the study period, while the mean length of hospitalisation per stay ranged from 3.3 to 3.8 days (Table 3). In each year, the mean duration of inpatient hospital stays was longer for PAH-related events than for non-PAH-related events. For incident patients, the median length of hospitalisation per stay was 1 day in every year following the index date, but the mean length of hospitalisation per stay decreased from year 1 to year 3 post-index (Supplemental Table 5).
Table 3.
Length of hospital stays in patients with PAH by year, 2013–2017.
| Days per inpatient spella | 2013 (n = 1370) | 2014 (n = 1605) | 2015 (n = 1774) | 2016 (n = 1850) | 2017 (n = 1825) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Mean | SD | Median | Mean | SD | Median | Mean | SD | Median | Mean | SD | Median | Mean | SD | |
| Total | 1.0 | 3.6 | 8.9 | 1.0 | 3.7 | 8.7 | 1.0 | 3.8 | 8.9 | 1.0 | 3.6 | 7.4 | 1.0 | 3.3 | 7.6 |
| PAH-related | 1.0 | 4.1 | 8.4 | 1.0 | 4.3 | 9.9 | 1.0 | 4.4 | 10.0 | 1.0 | 4.0 | 8.1 | 1.0 | 3.8 | 8.1 |
| Non–PAH-related | 1.0 | 2.7 | 9.5 | 1.0 | 2.5 | 5.9 | 1.0 | 2.6 | 5.5 | 1.0 | 2.6 | 5.7 | 1.0 | 2.3 | 6.2 |
PAH, pulmonary arterial hypertension; SD, standard deviation.
Defined as a continuous period spent as an inpatient, from admission until the earlier of discharge or study exit, plus any linked A&E attendance 24 h prior to the inpatient admission.
Healthcare costs
For the 5-year period 2013–2017, total cost was £43.2M, comprising 79% (£33.9M) for inpatient admissions, 19% (£8.3M) for outpatient visits and 2% (£0.9M) for A&E attendances (Table 4). From 2013 to 2017, mean costs per patient decreased by 13% (from £4400 to £3833) for inpatient admissions and by 13% (from £1031 to £896) for outpatient visits, but increased by 52% (from £81 to £123) for A&E attendances (Figure 5). From 2013 to 2017, the mean cost per event decreased by 4% (from £1394 to £1336) for inpatient admissions and by 15% (from £105 to £90) for outpatient visits but increased by 43% (from £102 to £146) for A&E attendances.
Table 4.
Total economic burden for patients with PAH by year, 2013–2017.
| Cost, £M | 2013 | 2014 | 2015 | 2016 | 2017 | |
|---|---|---|---|---|---|---|
| Inpatient admissions | Total | 6.03 | 6.69 | 7.05 | 7.21 | 7.00 |
| PAH-related | 4.61 | 5.01 | 5.32 | 5.48 | 5.72 | |
| Non–PAH-related | 1.42 | 1.68 | 1.73 | 1.73 | 1.27 | |
| Outpatient visits | Total | 1.41 | 1.50 | 1.77 | 1.98 | 1.63 |
| A&E attendances | Total | 0.11 | 0.17 | 0.20 | 0.23 | 0.20 |
| PAH-related | 0.05 | 0.07 | 0.09 | 0.10 | 0.10 | |
| Non–PAH-related | 0.06 | 0.10 | 0.10 | 0.13 | 0.12 | |
A&E, Accident and Emergency; PAH, pulmonary arterial hypertension.
Figure 5.
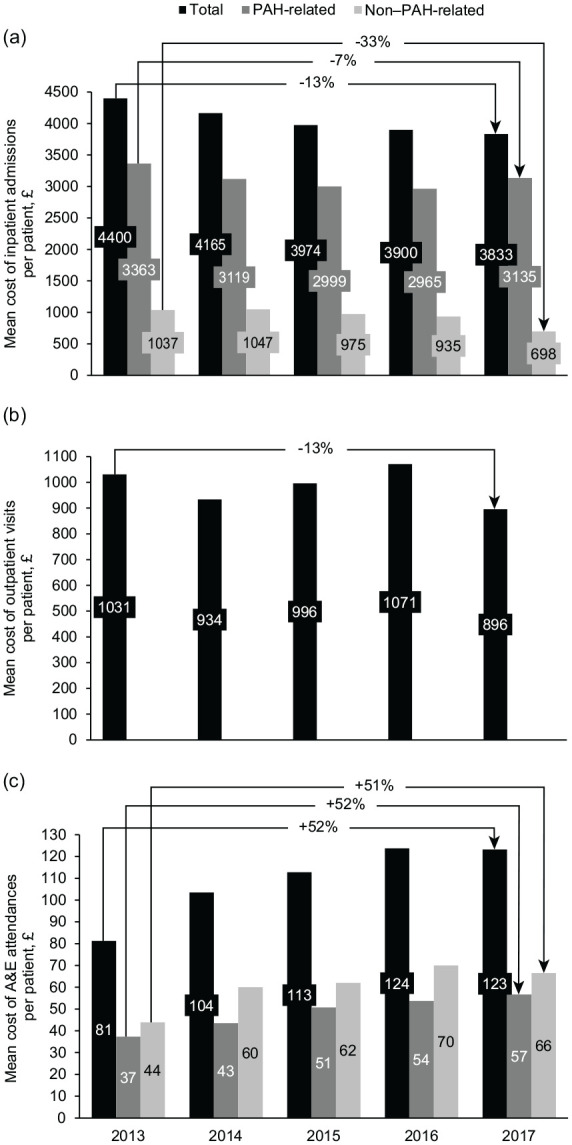
Annual mean cost per patient with PAH for (a) inpatient admissions, (b) outpatient visits and (c) A&E attendances, 2013–2017.
A&E, Accident and Emergency; PAH, pulmonary arterial hypertension.
Top 20% of patients in terms of healthcare expenditure
The top 20% of patients in terms of healthcare expenditure accounted for 52% (£17.5M) of the inpatient admission costs, 37% (£3.1M) of the outpatient visit costs and 44% (£0.4M) of the A&E attendance costs of the overall cohort. This subgroup had a similar gender and age distribution to the overall cohort (female: male ratio, 70%:30% versus 68%:32%, respectively; percentage ⩾50 years old, 66% versus 64%, respectively).
The most common associated ICD-10 codes occurred more frequently in the top 20% most costly patients than in the overall cohort. These included essential hypertension (64% versus 44% in the overall PAH population), history of cardiovascular disease (45% versus 29%) and congestive heart failure (48% versus 28%) (Supplemental Figure S1).
Compared with the overall cohort, the top 20% most costly patients had 137% higher mean number of inpatient admissions per patient, 83% higher mean number of outpatient visits and 36% higher mean number of A&E attendances (Supplemental Figure S2 and Supplementary Table S6). The mean cost per inpatient admission was 8% higher in the top 20% most costly patients than in the overall cohort, while mean costs per outpatient and A&E events were similar to those in the overall cohort (Supplemental Figure S3).
Discussion
This is the first published study using the HES database to assess the overall economic and clinical burden of PAH in England. The only previous PAH study using the HES database examined the healthcare resource utilisation of patients with idiopathic PAH prior to diagnosis, finding high levels of healthcare activity for several years before diagnosis.19
Gender and age distributions of patients defined in our study using HES data were consistent with those reported in previous UK studies based on patients with confirmed PAH diagnoses,20–22 increasing confidence that our algorithm accurately detected patients with PAH.
However, the total of 2527 PAH patients in our cohort from 2013 to 2017 is lower than the number of PAH patients included in the UK National Audit of Pulmonary Hypertension, which reported that, on 31 March 2015 there were 5776 patients being followed at PH specialist centres in England, of whom 46% (i.e. approximately 2657) had PAH.23 This could indicate that our algorithm missed some PAH patients, likely reflecting the previously mentioned difficulties in defining patients with PAH in a real-world database, as well as the difference between our use of the HES database and the National Audit’s use of data submitted by PH specialist centres. For instance, while our algorithm excluded patients with digital ulcers, erectile dysfunction or renal failure to increase specificity for PAH, this would fail to capture patients with PAH who had these conditions as comorbidities.
A main difference between our cohort definition algorithm and most others in the PAH literature is that we employed a decision tree with different combinations of three types of evidence: diagnosis, drug and procedure codes. Less complex algorithms of this type were used in US studies by Sikirica et al. and Dufour et al.8,24 In contrast, most previous researchers have either used ICD and drug codes associated with PAH,25,26 ICD and procedure codes,27 or ICD, drug and procedure codes that were required to be present for all included patients.28–30 Although requiring all three lines of evidence decreases false positives, this also increases the chance of missing some patients with PAH.31 We believe that the algorithm we used represents an effective balance between these two goals. However, several issues were encountered when applying the algorithm to HES data, including lack of precision in detecting drugs of interest, and missing diagnosis codes for outpatient visits. The inclusion of visits to a PH centre in the algorithm does not distinguish PAH from other forms of PH, which account for more than half of cases seen in these centres.23 Additional insight into the accuracy of the algorithm would be gained by applying it to different databases and validating it against patients with PAH directly confirmed by chart review, as recommended for other algorithms.6
Inpatient admissions accounted for the majority of costs despite comprising a much smaller proportion of healthcare events than outpatient visits. Furthermore, we found that PAH-related admissions were nearly three-fold costlier on average than admissions for other reasons, demonstrating the increased healthcare burden of hospitalisations for PAH. The exacerbation of this burden by a high rate of readmission after an initial PAH-related hospitalisation has been documented in the US REVEAL registry of patients with PAH,32 and in a retrospective claims database analysis of PAH patients in the US.25 Hospitalisation events are also known to be an important prognostic factor for survival; a post hoc analysis of data from the GRIPHON trial of selexipag in PAH patients demonstrated that hospitalisation for worsening of PAH was associated with a significantly increased risk for subsequent mortality.33 Similarly, all-cause hospitalisation within the last 6 months was included as a prognostic variable for mortality by Benza et al. in the REVEAL Risk Score Calculator 2.0.34 The reason all-cause rather than PAH-related hospitalisation was included in this risk equation was that only all-cause hospitalisation data were collected for the entire REVEAL cohort, but Benza et al. noted that since many admissions are triggered by PAH-related comorbidities, all-cause hospitalisation may be a reasonable proxy for PAH-related hospitalisation in this patient population.34 These findings highlight why it is important to look beyond drug acquisition costs and to appropriate treatment strategies that minimise PAH-related hospitalisations for newly diagnosed patients to reduce overall societal disease burden. As previously explained, a study limitation was that the database did not include most PAH-specific drugs; consequently, we were unable to compare the cost of pharmaceuticals with other healthcare components.
Substantial increases were seen in the per-patient and per-event costs of A&E attendances from 2013 to 2017. Although not all A&E attendances for patients with PAH are related to PAH,35 the observed trend of increasing costs suggests there is scope for improved PAH management to reduce these visits. Further research is warranted to explain the drivers of increased A&E costs and to determine the extent to which this may be addressed by optimising care (e.g. by reducing unwarranted practice variation).36
Our observation that mean hospitalisation length was more than three-fold higher than median hospitalisation length points to the skewed healthcare resource utilisation in the PAH patient population, with a subset of patients accounting for a disproportionate share. The top 20% most costly patients had more frequent healthcare resource utilisation and more costly inpatient admissions. They had higher rates of cardiovascular, respiratory and renal disease as indicated by ICD-10 codes, which suggests that targeted review of medical histories could help physicians to identify patients with PAH who are likely to show more intensive healthcare activity. Due to the limitations in the clinical variables available in the HES database, we were unable to assess the impact on the top 20% most costly patients of many well-established prognostic variables, such as exercise capacity, functional class and PAH aetiology.34 Similarly, limited records for high-cost drugs in the database meant that we could not evaluate the number and type of PAH-specific therapies received, precluding assessment of the potential role of under-treatment in driving higher healthcare resource use and hospitalisation in this subgroup (e.g. failure to initiate combination therapy and/or prostacyclin therapy for high-risk patients).
In conclusion, patients with PAH in England incur a heavy burden of healthcare resource utilisation and associated costs. This burden has changed over time, with decreasing average expenditures for inpatient admissions and outpatient visits, contrasting with an increase in the average cost of A&E attendances. Multiple admissions are common in patients with PAH, highlighting the importance of focussing not only on drug acquisition costs but also on improving management to reduce inpatient hospitalisation.
Supplemental Material
Supplemental material, sj-pdf-1-tar-10.1177_1753466621995040 for Burden of pulmonary arterial hypertension in England: retrospective HES database analysis by Fernando Exposto, Ruben Hermans, Åsa Nordgren, Luke Taylor, Sanam Sikander Rehman, Robert Ogley, Evan Davies, Amina Yesufu-Udechuku and Amélie Beaudet in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_1753466621995040 for Burden of pulmonary arterial hypertension in England: retrospective HES database analysis by Fernando Exposto, Ruben Hermans, Åsa Nordgren, Luke Taylor, Sanam Sikander Rehman, Robert Ogley, Evan Davies, Amina Yesufu-Udechuku and Amélie Beaudet in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_1753466621995040 for Burden of pulmonary arterial hypertension in England: retrospective HES database analysis by Fernando Exposto, Ruben Hermans, Åsa Nordgren, Luke Taylor, Sanam Sikander Rehman, Robert Ogley, Evan Davies, Amina Yesufu-Udechuku and Amélie Beaudet in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_1753466621995040 for Burden of pulmonary arterial hypertension in England: retrospective HES database analysis by Fernando Exposto, Ruben Hermans, Åsa Nordgren, Luke Taylor, Sanam Sikander Rehman, Robert Ogley, Evan Davies, Amina Yesufu-Udechuku and Amélie Beaudet in Therapeutic Advances in Respiratory Disease
Acknowledgments
Medical writing and editorial support were provided by W. Mark Roberts, Montréal, Québec, Canada, funded by Actelion Pharmaceuticals Ltd. Additional editorial input was provided by Viviane Sprecher and Rafael Sauter, Actelion Pharmaceuticals Ltd, Allschwil, Switzerland.
Footnotes
Author contribution(s): Fernando Exposto: Conceptualization; Methodology; Writing–original draft; Writing–review & editing.
Ruben Hermans: Methodology; Formal analysis; Writing–original draft; Writing–review & editing.
Åsa Nordgren: Methodology; Formal analysis; Writing–original draft; Writing–review & editing.
Luke Taylor: Methodology; Writing–review & editing.
Sanam Sikander Rehman: Methodology; Writing–original draft; Writing–review & editing.
Robert Ogley: Methodology; Writing–original draft; Writing–review & editing.
Evan Davies: Methodology; Writing–original draft; Writing–review & editing.
Amina Yesufu-Udechuku: Conceptualization; Methodology; Writing–original draft; Writing–review & editing.
Amélie Beaudet: Conceptualization; Methodology; Funding acquisition; Project administration; Supervision; Writing–original draft; Writing–review & editing.
Conflict of interest statement: Fernando Exposto, Ruben Hermans, Åsa Nordgren, Luke Taylor, Sanam Sikander Rehman, and Robert Ogley are employees of IQVIA; IQVIA received funding from Actelion Pharmaceuticals Ltd in connection with this research. Evan Davies and Amélie Beaudet are employees of Actelion Pharmaceuticals Ltd. Amélie Beaudet holds stock options in Johnson & Johnson. Amina Yesufu-Udechuku was an employee of Actelion Pharmaceuticals UK Ltd when this research was conducted (current affiliation: Takeda UK Limited).
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Actelion Pharmaceuticals Ltd, Allschwil, Switzerland. Actelion Pharmaceuticals Ltd was involved in study design; in the interpretation of data; in the writing of the paper; and in the decision to submit the paper for publication.
Ethics approval and informed consent: This retrospective study did not involve any interventional research requiring ethics committee review.
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Fernando Exposto, IQVIA, 210 Pentonville Road, London, N1 9JY, UK.
Ruben Hermans, IQVIA, London, UK.
Åsa Nordgren, IQVIA, London, UK.
Luke Taylor, IQVIA, London, UK.
Sanam Sikander Rehman, IQVIA, London, UK.
Robert Ogley, IQVIA, London, UK.
Evan Davies, Actelion Pharmaceuticals Ltd, Allschwil, Basel-Landschaft, Switzerland.
Amina Yesufu-Udechuku, Actelion Pharmaceuticals UK Ltd, High Wycombe, UK.
Amélie Beaudet, Actelion Pharmaceuticals Ltd, Allschwil, Basel-Landschaft, Switzerland.
References
- 1. Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30: 2493–2537. [DOI] [PubMed] [Google Scholar]
- 2. Humbert M, Guignabert C, Bonnet S, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 2019; 53: 1801887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 4. Link J, Glazer C, Torres F, et al. International classification of diseases coding changes lead to profound declines in reported idiopathic pulmonary arterial hypertension mortality and hospitalizations: implications for database studies. Chest 2011; 139: 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gillmeyer KR, Lee MM, Link AP, et al. Accuracy of algorithms to identify pulmonary arterial hypertension in administrative data: a systematic review. Chest 2019; 155: 680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mathai SC, Hemnes AR, Manaker S, et al. Identifying patients with pulmonary arterial hypertension (PAH) using administrative claims algorithms. Ann Am Thorac Soc 2019; 16: 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilkens H, Grimminger F, Hoeper M, et al. Burden of pulmonary arterial hypertension in Germany. Respir Med 2010; 104: 902–910. [DOI] [PubMed] [Google Scholar]
- 8. Dufour R, Pruett J, Hu N, et al. Healthcare resource utilization and costs for patients with pulmonary arterial hypertension: real-world documentation of functional class. J Med Econ 2017; 20: 1178–1186. [DOI] [PubMed] [Google Scholar]
- 9. NHS Digital. Hospital Episode Statistics (HES), https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics (2019, accessed 11 May 2019).
- 10. Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009; 54: S43–S54. [DOI] [PubMed] [Google Scholar]
- 11. Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–D41. [DOI] [PubMed] [Google Scholar]
- 12. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. NHS England. 2013/14 NHS standard contract for pulmonary hypertension centres (adult). Particulars, Schedule 2 - The services, A - Service specifications, https://www.england.nhs.uk/wp-content/uploads/2018/08/Pulmonary-hypertension-centres-adult.pdf (2013, accessed 11 May 2019).
- 14. NHS Digital. National audit of pulmonary hypertension: Great Britain, 2018-19. Tenth annual report, https://digital.nhs.uk/data-and-information/publications/statistical/national-pulmonary-hypertension-audit/2019 (2019, accessed 1 November 2020).
- 15. NHS England. Commissioning policy: targeted therapies for use in pulmonary hypertension in adults, https://www.england.nhs.uk/wp-content/uploads/2018/07/Targeted-therapies-for-use-in-pulmonary-hypertension-in-adults.pdf (2015, accessed 4 July 2019).
- 16. NHS Digital. High cost drugs clinical coding standards OPCS-4, https://hscic.kahootz.com/gf2.ti/f/762498/30488101.2/PDF/-/High_Cost_Drugs_Clinical_Coding_StandardOPCS4v2.pdf (2018, accessed 13 May 2019).
- 17. Office for National Statistics. Dataset: estimates of the population for the UK, England and Wales, Scotland and Northern Ireland, https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland (2018, accessed 11 May 2019).
- 18. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2017. [Google Scholar]
- 19. Bergemann R, Allsopp J, Jenner H, et al. High levels of healthcare utilization prior to diagnosis in idiopathic pulmonary arterial hypertension support the feasibility of an early diagnosis algorithm: the SPHInX project. Pulm Circ 2018; 8: 2045894018798613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peacock AJ, Murphy NF, McMurray JJ, et al. An epidemiological study of pulmonary arterial hypertension. Eur Respir J 2007; 30: 104–109. [DOI] [PubMed] [Google Scholar]
- 21. Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012; 186: 790–796. [DOI] [PubMed] [Google Scholar]
- 22. Hurdman J, Condliffe R, Elliot CA, et al. ASPIRE registry: assessing the spectrum of pulmonary hypertension identified at a REferral centre. Eur Respir J 2012; 39: 945–955. [DOI] [PubMed] [Google Scholar]
- 23. National Health Service. National audit of pulmonary hypertension 2015, http://www.content.digital.nhs.Uk/catalogue/PUB20043/nati-pulm-hype-audi-2015-rep.pdf (2016, accessed 2 June 2017).
- 24. Sikirica M, Iorga SR, Bancroft T, et al. The economic burden of pulmonary arterial hypertension (PAH) in the US on payers and patients. BMC Health Serv Res 2014; 14: 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burke JP, Hunsche E, Régulier E, et al. Characterizing pulmonary hypertension-related hospitalization costs among Medicare Advantage or commercially insured patients with pulmonary arterial hypertension: a retrospective database study. Am J Manag Care 2015; 21(Suppl. 3): s47–s58. [PubMed] [Google Scholar]
- 26. Studer S, Hull M, Pruett J, et al. Treatment patterns, healthcare resource utilization, and healthcare costs among patients with pulmonary arterial hypertension in a real-world US database. Pulm Circ 2019; 9: 2045894018816294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kirson NY, Birnbaum HG, Ivanova JI, et al. Excess costs associated with patients with pulmonary arterial hypertension in a US privately insured population. Appl Health Econ Health Policy 2011; 9: 293–303. [DOI] [PubMed] [Google Scholar]
- 28. Copher R, Cerulli A, Watkins A, et al. Treatment patterns and healthcare system burden of managed care patients with suspected pulmonary arterial hypertension in the United States. J Med Econ 2012; 15: 947–955. [DOI] [PubMed] [Google Scholar]
- 29. Johnson S, Delate T, Boka A, et al. Characterizing the financial burden of pulmonary arterial hypertension within an integrated healthcare delivery system. J Med Econ 2013; 16: 1414–1422. [DOI] [PubMed] [Google Scholar]
- 30. Burger CD, Ozbay AB, Lazarus HM, et al. Treatment patterns and associated health care costs before and after treatment initiation among pulmonary arterial hypertension patients in the United States. J Manag Care Spec Pharm 2018; 24: 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Papani R, Sharma G, Agarwal A, et al. Validation of claims-based algorithms for pulmonary arterial hypertension. Pulm Circ 2018; 8: 2045894018759246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burger CD, Long PK, Shah MR, et al. Characterization of first-time hospitalizations in patients with newly diagnosed pulmonary arterial hypertension in the REVEAL registry. Chest 2014; 146: 1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McLaughlin VV, Hoeper MM, Channick RN, et al. Pulmonary arterial hypertension-related morbidity is prognostic for mortality. J Am Coll Cardiol 2018; 71: 752–763. [DOI] [PubMed] [Google Scholar]
- 34. Benza RL, Gomberg-Maitland M, Elliott CG, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest 2019; 156: 323–337. [DOI] [PubMed] [Google Scholar]
- 35. Wilcox SR, Kabrhel C, Channick RN. Pulmonary hypertension and right ventricular failure in emergency medicine. Ann Emerg Med 2015; 66: 619–628. [DOI] [PubMed] [Google Scholar]
- 36. Ryan JJ, Butrous G, Maron BA. The heterogeneity of clinical practice patterns among an international cohort of pulmonary arterial hypertension experts. Pulm Circ 2014; 4: 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tar-10.1177_1753466621995040 for Burden of pulmonary arterial hypertension in England: retrospective HES database analysis by Fernando Exposto, Ruben Hermans, Åsa Nordgren, Luke Taylor, Sanam Sikander Rehman, Robert Ogley, Evan Davies, Amina Yesufu-Udechuku and Amélie Beaudet in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_1753466621995040 for Burden of pulmonary arterial hypertension in England: retrospective HES database analysis by Fernando Exposto, Ruben Hermans, Åsa Nordgren, Luke Taylor, Sanam Sikander Rehman, Robert Ogley, Evan Davies, Amina Yesufu-Udechuku and Amélie Beaudet in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_1753466621995040 for Burden of pulmonary arterial hypertension in England: retrospective HES database analysis by Fernando Exposto, Ruben Hermans, Åsa Nordgren, Luke Taylor, Sanam Sikander Rehman, Robert Ogley, Evan Davies, Amina Yesufu-Udechuku and Amélie Beaudet in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_1753466621995040 for Burden of pulmonary arterial hypertension in England: retrospective HES database analysis by Fernando Exposto, Ruben Hermans, Åsa Nordgren, Luke Taylor, Sanam Sikander Rehman, Robert Ogley, Evan Davies, Amina Yesufu-Udechuku and Amélie Beaudet in Therapeutic Advances in Respiratory Disease